- 1Department of Entomology, University of Georgia, Athens, GA, United States
- 2Philip E. Marucci Center for Blueberry and Cranberry Research, Department of Entomology, Rutgers University, New Brunswick, NJ, United States
Since its first appearance in California in 2008 and subsequent spread across the continental United States, the spotted-wing drosophila, Drosophila suzukii Matsumura, has become an economically damaging pest of multiple stone and soft-skinned fruits in the United States. The adjuvant ACTTRA SWD, when mixed with a suitable insecticide, constitutes an innovative attract-and-kill tactic that can be applied as a sprayable bait to manage D. suzukii. As an adjuvant, growers can mix ACTTRA SWD with any insecticide recommended for D. suzukii management in a specific crop; however, to achieve this, the efficacy of this adjuvant incorporated with various insecticides needs testing. This research aims to test the suitability of nine insecticides added to two ACTTRA SWD formulations (named OR1 and TD) to maintain the formulation’s attractiveness to D. suzukii adults and in resulting mortality. We conducted a series of two-choice bioassays to test the relative attraction of D. suzukii to ACTTRA SWD formulations prepared with and without a specific insecticide. Additionally, we tested the efficacy of ACTTRA SWD formulations mixed with insecticides in managing D. suzukii by using no-choice efficacy bioassays. Adding Mustang Maxx (zeta-cypermethrin) to ACTTRA SWD OR1 significantly improved D. suzukii adult attraction to the formulation, while Azera (azadirachtin + pyrethrins) significantly reduced attraction to both ACTTRA SWD formulations. Among the insecticides tested, we identified Danitol (fenpropathrin), Exirel (cyantraniliprole), Malathion (malathion), Mustang Maxx, and Entrust (spinosad) as suitable insecticide additives for both ACTTRA SWD formulations. The results from this study will assist growers in selecting proper insecticide components when preparing attract-and-kill formulations of the new adjuvant ACTTRA SWD.
Introduction
The spotted-wing drosophila (SWD), Drosophila suzukii Matsumura (Diptera: Drosophilidae), is an invasive species that has become an economically damaging pest of multiple stone and soft-skinned fruits in the United States. For fresh market fruits, there is a zero-tolerance for fruit infestation by D. suzukii (Cuthbertson et al., 2014). Consequently, multiple chemical, physical, biological, cultural, behavioral, and genetic management methods have been developed for this pest (Bruck et al., 2011; Cormier et al., 2015; Renkema et al., 2016; Buchman et al., 2018; Klick et al., 2019; Rendon et al., 2020; Wang et al., 2020; Fanning et al., 2021). Despite this, low tolerance for fruit infestation by D. suzukii in small fruit crops, along with the low efficacy, high cost, or practical difficulties associated with the implementation of certain management options, limit the use of many non-chemical measures as a stand-alone strategy for managing this pest (Rhodes et al., 2018; Rendon and Walton, 2019; Schöneberg et al., 2020). Instead, for small fruit growers, several conventional and a few organic insecticide options that are effective and economically viable are available for D. suzukii management (Farnsworth et al., 2017). Thus, prophylactic insecticide applications are the primary management method employed by fruit growers in conventional and organic small fruit production systems (Sial et al., 2019). Moreover, repeated insecticide applications, often 6–9 applications per fruit growing season, are required to manage this pest (Van Timmeren and Isaacs, 2013). This heavy reliance on insecticides can lead to insecticide resistance evolution, adverse environmental effects, and insecticide residue contamination in the harvested fruits (Diepenbrock et al., 2016; Gress and Zalom, 2019; Sarkar et al., 2020; Spaulding, 2020). To mitigate this, multiple resident pupal parasitoids and one introduced larval parasitoid, Ganaspis brasiliensis (Ihering), have been identified as potential biological control agents for managing D. suzukii in North America and Europe (Wang et al., 2021). A permit to release G. brasiliensis in the United States has been approved, and the preparation for the large-scale release of these parasitoids has been underway. Additionally, augmentation and conservation biological control plans utilizing the resident pupal parasitoids are being developed. However, the current D. suzukii management programs that largely depend on frequent insecticide cover sprays are not likely compatible with these biological control efforts. Thus, the development of integrated pest management (IPM) strategies that limit non-target impacts of insecticides and are compatible with D. suzukii parasitoids and other natural enemies is getting increased attention.
Recently, a few behavior-modifying strategies have been tested to reduce D. suzukii infestation (Cloonan et al., 2018; Tait et al., 2021). A promising novel behavioral-based D. suzukii management strategy that can reduce the overall insecticide inputs in a field season is the implementation of attract-and-kill (A&K) management tactics based on the ACTTRA SWD formulations (ISCA Technologies, Inc., Riverside, CA, United States) (Klick et al., 2019). Compared with the traditional insecticide cover sprays, A&K formulations prepared with ACTTRA SWD are applied at discrete sites across the field. Thus, only a portion of the total field area is exposed to the attractant + insecticide mixture, reducing the overall insecticide use per unit area while minimizing the non-target impacts and insecticide drift (Mafra-Neto et al., 2014; Klick et al., 2019). The ACTTRA SWD formulations are based on SPLAT (Specialized Pheromone & Lure Application Technology, ISCA Technologies Inc., Riverside, CA, United States), emulsions that control the release of incorporated semiochemicals over an extended period under field conditions (Mafra-Neto et al., 2014). These formulations consist of a blend of adult D. suzukii olfactory attractants, phagostimulants, and a pink coloration for insect visual attraction (Mafra-Neto et al., 2014). Two ACTTRA SWD adjuvants are currently being considered for registration: ACTTRA SWD OR1 (hereafter referred to as “OR1”) and ACTTRA SWD TD (hereafter referred to as “TD”). Both of the above formulations contain adult D. suzukii attractant blends but lack a killing/insecticide component. Moreover, while the OR1 and TD are adjuvants (lacking added insecticide), the HOOK SWD is an experimental formulation containing the OR1 adjuvant as an attractant/phagostimulant component, with the technical grade spinosad (99% purity) as a killing component incorporated during the manufacturing process. A recent study with HOOK SWD formulations suggests that the blueberry crop treated with HOOK SWD formulation reduced D. suzukii fruit infestation by 2–8 times than the untreated control (Klick et al., 2019). Additionally, weekly or biweekly HOOK SWD applications preceded by a single insecticide cover spray resulted in 2–5 times less D. suzukii infestation in the raspberry field than the standard grower cover spray alone (Klick et al., 2019). These results indicate the potential of A&K formulations based on ACTTRA SWD as a D. suzukii management option in small fruit IPM.
Behavior-modifying chemicals that attract insects to an insecticide and/or improve the insecticide uptake by serving as a phagostimulant can enhance the efficacy of the insecticide (Cowles et al., 2015). On the other hand, while preparing an A&K formulation, the addition of insecticide as a killing component should be done with care as the selection of insecticide can reduce the overall attractiveness of the final A&K product to the target organisms. The constituents of a commercial insecticide formulation, either the active ingredient(s) or other formulation components, can interact with the attractants, reducing formulation attractiveness or even causing a net repellency on the target organism (Lin et al., 1993; El-Sayed et al., 2009). Since both OR1 and TD formulations are envisioned to be marketed as an adjuvant, i.e., without an insecticide, growers can potentially mix these two ACTTRA SWD formulations with any insecticide recommended for D. suzukii management in a specific crop when preparing an attract-and kill formulation. Selecting a suitable insecticide as a killing component of an A&K mixture should be based on multiple desirable characteristics of the insecticide that include high efficacy, lack of repellence or deterrence, an excellent residual activity that matches the life of the attractants, a limited non-target effect of insecticide, and rapid killing capability of the toxicant (Gregg et al., 2018). Previous research has recognized several insecticides that elicit specific insect behavior responses, including repellency, feeding, or oviposition deterrence (Kumar and Chapman, 1984; Ross and Cochran, 1992; Lin et al., 1993). For instance, volatiles from the active ingredient and other formulation components in certain permethrin formulations is known to affect the diamondback moth, Plutella xylostella (L.) larval behavior response to the formulations (Lin et al., 1993). Similarly, azadirachtin, the active ingredient in many commercial insecticide formulations, has strong antifeedant, oviposition deterrent, and repellent effects on multiple insect species (Mordue and Blackwell, 1993). Thus, identifying appropriate insecticide formulations that growers can mix with ACTTRA SWD without compromising the formulation’s capabilities to attract adult D. suzukii while providing a desirable level of pest suppression needed investigation.
Presently, spinosad (Entrust™ 22.5SC, Dow AgroSciences LLC, Indianapolis, IN, United States) is the default insecticide component for the A&K formulations prepared using ACTTRA SWD adjuvants. Since organic growers have only a few insecticide options for managing D. suzukii in small fruit crops, and spinosad is the most effective insecticide option, D. suzukii management in the organic production system heavily relies on this insecticide. So, adopting ACTTRA SWD-based A&K technique that uses spinosad as the killing component could increase the grower’s reliance on spinosad for D. suzukii management and increase the selection pressure of this insecticide in this pest and accelerate resistance evolution. Accordingly, a recent study by Gress and Zalom (2019) confirmed that spinosad resistance is emerging in specific D. suzukii populations in California. Additionally, post-season D. suzukii populations screened for insecticide tolerance in 2019 in Georgia exhibited higher resistance ratios for spinosad than the population collected in the 2018 field season and the 2019 pre-season (Spaulding, 2020). Thus, clearly deploying an A&K strategy for managing D. suzukii entirely based on the spinosad is unsustainable. There are multiple effective insecticide options available for the conventional system, and a few promising insecticides listed by the Organic Materials Review Institute (OMRI) are available for the organic system for managing D. suzukii in small fruit crops. Despite that, none of these insecticides have been tested for their compatibility and effectiveness with ACTTRA SWD adjuvants as an A&K formulation for managing D. suzukii in small fruit IPM.
Thus, the objective of our research was to test the suitability of nine selected insecticides, both organic and conventional insecticides, added to two ACTTRA SWD formulations (named OR1 and TD) to maintain the resulting formulation’s attractiveness to D. suzukii adults and toxicity resulting in mortality. We conducted a series of two-choice bioassays to test the D. suzukii relative attraction to the ACTTRA SWD formulations prepared with and without a specific insecticide. Additionally, by using no-choice bioassays, we tested the efficacy of ACTTRA SWD formulations mixed with insecticides in managing D. suzukii. Results from this study will help identify appropriate insecticides that can be added to an ACTTRA SWD formulation before deploying the product as an A&K tactic to manage D. suzukii infestation in small fruit crops.
Materials and Methods
Insects
Drosophila suzukii adults were reared from a laboratory colony maintained at the University of Georgia, Athens, GA, United States. This colony was established from a population raised from blueberries collected from Clarke Co., GA, in 2013. Flies were reared in 117 mL square-bottom polypropylene bottles (Genesee Scientific, San Diego, CA, United States). Each bottle was provided with ≈37 mL of standard fly diet (Reed et al., 2010; Jaramillo et al., 2015), added with a pinch of active baker’s yeast. Rearing bottles were capped with cellulose acetate plugs and maintained in a growth chamber (Percival Scientific, Perry, IA, United States), held at 24 ± 2°C, 60% RH, and 14:10 L:D period.
ACTTRA SWD Formulations
We tested two ACTTRA SWD formulations in our experiments, OR1 and TD (ISCA Technologies, Inc., Riverside, CA, United States). Both the above formulations and a related product, HOOK SWD, an A&K formulation from the same company, contained adult D. suzukii attractants and phagostimulants mixed with a pink dye in a SPLAT matrix. The HOOK SWD had the insecticide spinosad already incorporated in the formulation at a 0.5% active ingredient concentration by weight. In contrast, the TD and OR1 formulations lacked the insecticide, and growers would need to mix a suitable insecticide with these adjuvants before deploying the resulting formulation in the field as an A&K management strategy. The only difference between the TD and OR1 formulations is the number of components in the attractive blend; the TD blend has more components than the OR1 blend and is relatively more attractive to adult D. suzukii (Babu et al., 2022). The authors are unable to disclose detailed compositions of the tested A&K formulations because they constitute proprietary technologies. Both the OR1 and TD adjuvants were kindly provided by ISCA Technologies, Inc. (Riverside, CA, United States).
Insecticides
Insecticide commercial formulations used in this study, active ingredients in these formulations, and their manufacturers are listed in Table 1. Commercial insecticide formulations are a blend of one or more active ingredients and other ingredients with no direct biocidal action (Nagy et al., 2020). Therefore, any change in the behavioral response of the insects to the A&K formulation added with a commercial insecticide formulation observed in this study may not only depend on the type and concentration of the active ingredient(s) in the formulation, but could also depend on the other ingredients and its concentration. Therefore, during the subsequent result and discussion, when appropriate, we focused on the specific insecticide commercial name and formulation tested over the specific active ingredient(s) in the formulation.
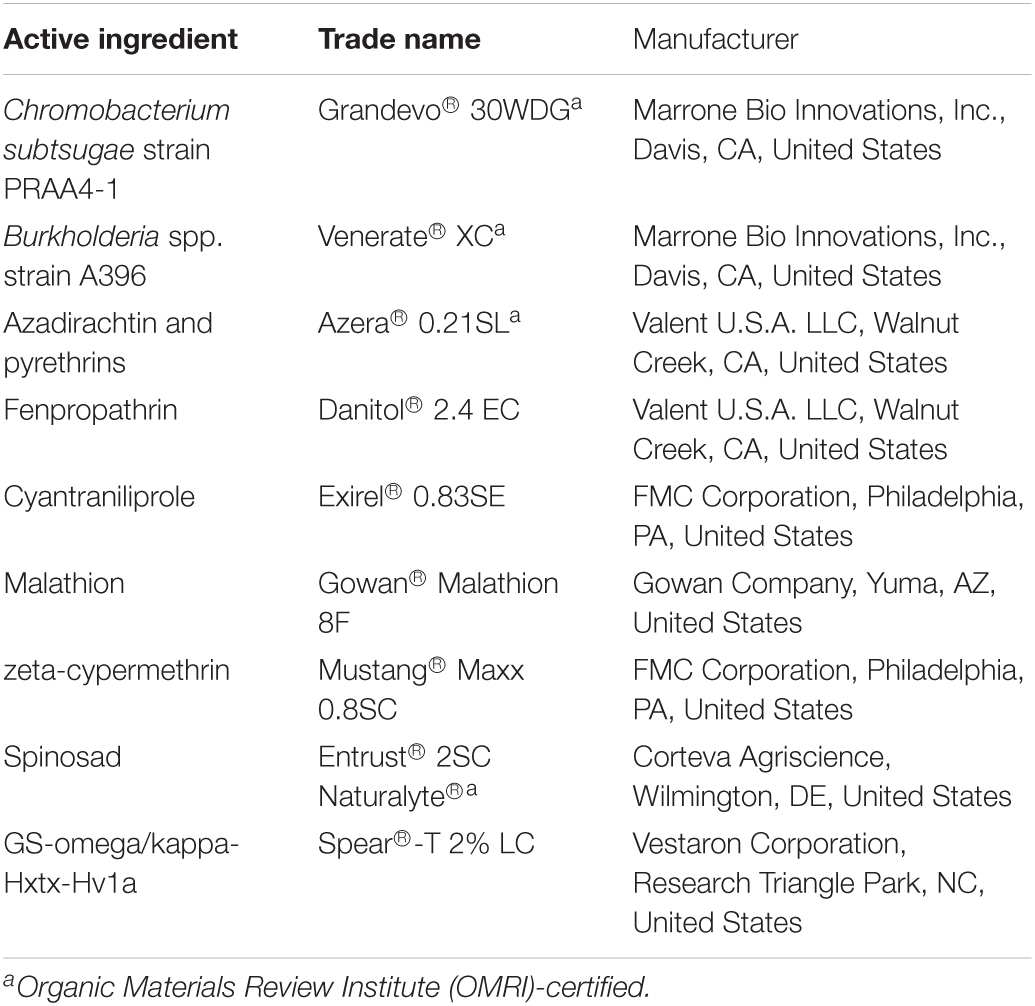
Table 1. List of active ingredients, trade name, manufacturer of insecticide formulations used in this study.
Behavioral Response of Drosophila suzukii to ACTTRA SWD Formulations Mixed With Selected Insecticides
We conducted a series of two-choice laboratory assays with TD and OR1 formulations to evaluate the behavioral response of adult D. suzukii to ACTTRA SWD formulations when mixed with a specific insecticide compared with the corresponding blank adjuvant formulation. For the two-choice bioassays, a clear 1839 mL polyethylene container (Dart Container Corporation, Mason, MI, United States) of 19.7 × 20.6 × 7.8 cm dimension, fitted with a matching lid, served as an assay chamber (Figure 1). The chamber lid had two circular openings at the center, each 2 cm in diameter, one secured with a mesh covering to permit air circulation. The other opening, sealed with a cotton ball, served as an insect release point before the assay. Two custom-made traps, each made with 59 mL clear plastic portion cups (Fabri-Kal Corp., Kalamazoo, MI, United States) with a fitting lid, were glued diagonally inside the chamber bottom. The trap lid had a single 1.6 cm diameter hole at the center, fitted with an inverted rubber cap taken from a floral water pick (DL 3805, Diamond line containers, Akron, OH, United States). This rubber cap had a single 3-mm diameter opening in the center to serve as the D. suzukii entry point to the traps. The water pick cap was inserted into the trap lid such that the upper surface of the cap projected into the trap while the bottom edge of the rubber cap was in line with the upper surface of the trap lid. Three circular cotton pads (Swisspers, Gastonia, NC, United States) moisturized with a total of 8 mL of distilled water was arranged inside the arena, diagonally between the traps, to maintain high humidity (≈90%) inside the assay chambers. Additionally, a floral tube (Juvo Plus Inc., Monrovia, CA, United States) filled with distilled water and secured with a cotton ball placed in the bottom center of the arena served as the additional water source for flies during the assay.

Figure 1. The two-choice trap bioassay arena used in this study. (A) View from the top. (B) Side view. Components of bioassay arena include (1) a clear 1839 mL polyethylene container with lid, (2) moisturized cotton pads, (3) custom-made traps with treatment inside, (4) mesh-covered opening for air circulation, (5) a Drosophila suzukii water source, and (6) insect release point sealed with a cotton ball.
We aimed to test the suitability of nine insecticides (Table 1) added to two ACTTRA SWD formulations (OR1 and TD) to maintain the formulation’s attractiveness to D. suzukii adults. We tried a range of application volumes of blank TD and OR1 to standardize our two-choice assay methods during previous research. The purpose was to determine an optimum treatment volume in the two-choice assay that will maximize the overall insect response rate to treatment traps during the assay (Babu et al., 2022). Based on the results, we identified 80 μL ACTTRA SWD/choice trap as the standard treatment volume for all the subsequent two-choice assays. Thus, for all the two-choice assays described in this manuscript, 80 μL of ACTTRA SWD, either TD or OR1 with and without a specific insecticide, served as choices. All ACTTRA SWD treatments, including ACTTRA SWD blank controls, had an effective ACTTRA SWD concentration of 87.5%. Depending on the treatment, the rest of the formulation volume was made up of the commercial insecticide formulation at 0.25% active ingredient (A.I.) by volume and distilled water. Treatments in a treatment pair were applied randomly to traps to avoid positional bias between the choices during the assay. The bioassays were conducted in a laboratory maintained at 21 ± 2°C, and 24 h light. The relative humidity inside the two-choice arena was held at 90 ± 2% throughout the assay. Twenty adult D. suzukii of 5–7-day old (1: 1 sex ratio), starved for ≈2 h, were released in each arena. Each treatment pair was replicated eight times. All the two-choice assays with a specific ACTTRA SWD formulation (either OR1 or TD) were carried out on the same day. The number of D. suzukii in each trap that responded to a specific treatment in a two-choice assay was counted 24 h after the insect release. The percentage of total insects released into the arena that responded to each trap was calculated.
Efficacy of ACTTRA SWD Formulations Mixed With Selected Insecticides
Two separate no-choice bioassays, one trial each for OR1 and TD adjuvant, were conducted to test the efficacy of adjuvants incorporated with nine selected insecticides (see Table 1) in managing D. suzukii adults. Each bioassay consisted of 12 treatments, including nine insecticides + ACTTRA SWD (either OR1 or TD) treatments, two blank adjuvants without any added insecticide as positive controls (ACTTRA SWD TD control and ACTTRA SWD OR1 control), and untreated control (Tables 1–3). All ACTTRA SWD treatments, including ACTTRA SWD blank controls, had an effective ACTTRA SWD concentration of 87.5%. Depending on the treatment, the rest of the formulation volume was made up of the commercial insecticide formulation at 0.25% A.I. by volume and distilled water. The bioassay arena consisted of a 946 mL clear plastic container (Fabri-Kal®, Kalamazoo, MI, United States) with a matching lid, and with a single 5–7 cm long blueberry terminal with 4–5 leaves inserted into a water pick (DL 3805, Diamond Line, Akron, OH, United States) filled with distilled water, and was fitted through a circular hole in the bottom center of the chamber (Figure 2). This bioassay is commonly used to assess toxicity of insecticides against D. suzukii (Van Timmeren and Isaacs, 2013; Roubos et al., 2019; Fanning et al., 2021). Each assay chamber was supplied with 15 ripe organic blueberries (Simple Truth Organic, Kroger Co., Cincinnati, OH, United States) to serve as a food source and oviposition substrate during the assay. A floral tube (Juvale, Juvo Plus Inc., Monrovia, CA) filled with distilled water and plugged with moist cotton was provided as a water source. Except for the untreated check, each chamber received a single 0.2 mL drop of one ACTTRA SWD treatment, applied to the adaxial surface of the topmost leaf. Bioassay chambers were then arranged in a randomized complete block design, and treatments were replicated eight times. Five male and five female adult D. suzukii of 5–7 days old and starved for ≈2 h were released into the bioassay chambers ≈2 h after treatment application. The containers were then kept in a laboratory maintained at 21°C, 14:10 L:D, and 80 ± 10% RH for 6 days. Six days after treatment (6 DAT), blueberries were removed from the bioassay containers and transferred to a ventilated 237 mL deli container (Fabri-Kal®, Kalamazoo, MI, United States) with a cotton round (Swisspers, Gastonia, NC, United States) in the bottom, and incubated in the same laboratory environment for 14 days for adult D. suzukii progeny emergence. Treatment efficacy was determined based on the adult D. suzukii mortality, and the number of progenies developed from the infested blueberry fruits. Adult D. suzukii mortality was counted at 1, 3, and 6 days after the treatments and the percentage mortality of D. suzukii flies in each bioassay chamber was calculated. Additionally, the number of adult progenies that emerged from the blueberries was counted at 20 DAT.

Figure 2. The no-choice bioassay arena used in this study. The components of the no-choice bioassay arena include (1) a 5–7 cm long blueberry terminal with 4–5 leaves inserted into (2) a water pick, (3) 15 ripe organic blueberries, and (4) a Drosophila suzukii water source.
Statistical Analyses
Data from all the two-choice behavioral assays were subjected to Student’s two-tailed paired t-test (GraphPad Prism V.9, GraphPad Software, San Diego, CA, United States) at α = 0.05. Before the parametric test, the Shapiro-Wilk statistics were calculated to ensure that the difference between the paired observations comes from a normally distributed population (Horton, 1995). The D. suzukii adult mortality and progeny count data from the no-choice efficacy bioassays were subjected to analysis of variance (ANOVA) (PROC GLIMMIX, SAS v. 9.4) with data fitted to a normal distribution. The replication (block) was considered as a random effect. Mean percent adult mortality and progeny counts were separated post ANOVA using Tukey-Kramer (P ≤ 0.05) test.
Results
Behavioral Response of Adult Drosophila suzukii to ACTTRA SWD Formulations Mixed With Selected Insecticides
OR1 + Insecticides
Among the various insecticide + OR1 formulations tested, the addition of Azera 0.21SL containing azadirachtin and pyrethrins as active ingredients, and Mustang Maxx 0.8SC containing zeta-cypermethrin as the active ingredient, resulted in a change in the relative attractiveness of OR1 formulation to adult D. suzukii individuals (Figure 3). Compared with the blank OR1 formulation, the addition of Azera 0.21SL to the OR1 formulation significantly reduced the overall attractiveness of the formulation to adult D. suzukii. Contrary, the addition of Mustang Maxx 0.8SC to the OR1 formulation significantly enhanced the attractiveness of the formulation to adult D. suzukii individuals.
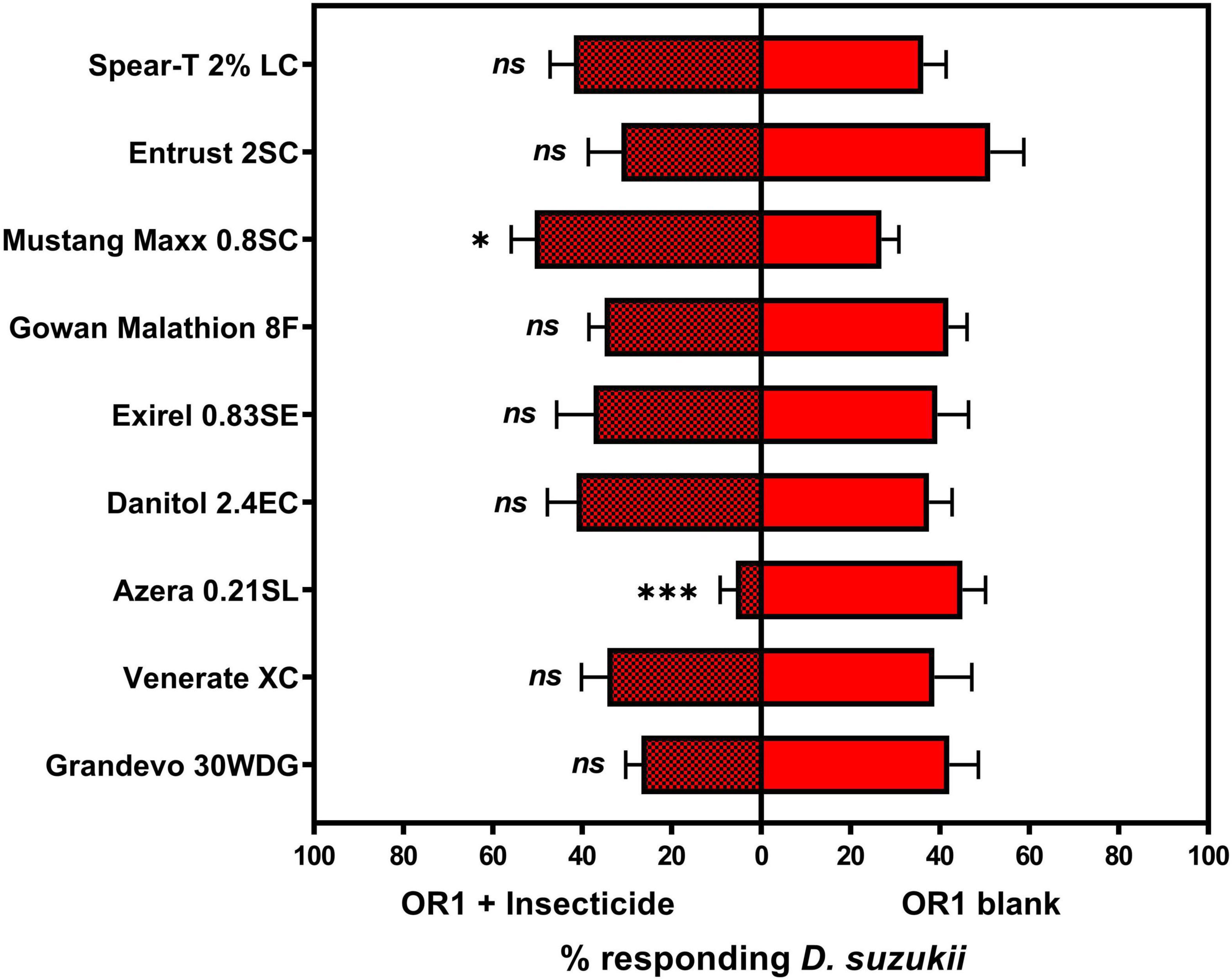
Figure 3. Mean (±SEM) percent of total Drosophila suzukii released in an arena captured inside the traps contains an ACTTRA SWD OR1 + insecticide treatment vs. trap containing blank ACTTRA SWD OR1. “ns,” no statistical difference (paired t-test; α = 0.05); significant difference, *P < 0.05 and ***P < 0.001. For OR1 + Grandevo 30WDG vs. OR1 blank: N = 8, df = 7, t = 1.732, P = 0.1268; OR1 + Venerate vs. OR1 blank: N = 8, df = 7, t = 0.351, P = 0.7362; OR1 + Azera 0.21SL vs. OR1 blank: N = 8, df = 7, t = 6.323, P < 0.0004; OR1 + Danitol 2.4EC vs. OR1 blank: N = 8, df = 7, t = 0.330, P = 0.7512; OR1 + Exirel 0.83SE vs. OR1 blank: N = 8, df = 7, t = 0.132, P = 0.8985; OR1 + Gowan Malathion 8F vs. OR1 blank: N = 8, df = 7, t = 0.925, P = 0.3860; OR1 + Mustang Maxx 0.8SC vs. OR1 blank: N = 8, df = 7, t = 3.397, P = 0.0115; OR1 + Entrust 2SC vs. OR1 blank: N = 8, df = 7, t = 1.402, P = 0.2037; and OR1 + Spear-T 2% LC vs. OR1 blank: N = 8, df = 7, t = 0.583, P = 0.5785.
TD + Insecticides
For the TD adjuvant, among the various insecticide + TD formulations tested, the addition of only Azera 0.21SL resulted in a change in formulation’s relative attractiveness to adult D. suzukii individuals (Figure 4). Similar to the OR1 formulation, the addition of Azera 0.21SL into TD formulation also resulted in a significant reduction in the formulation’s attractiveness to D. suzukii adults.
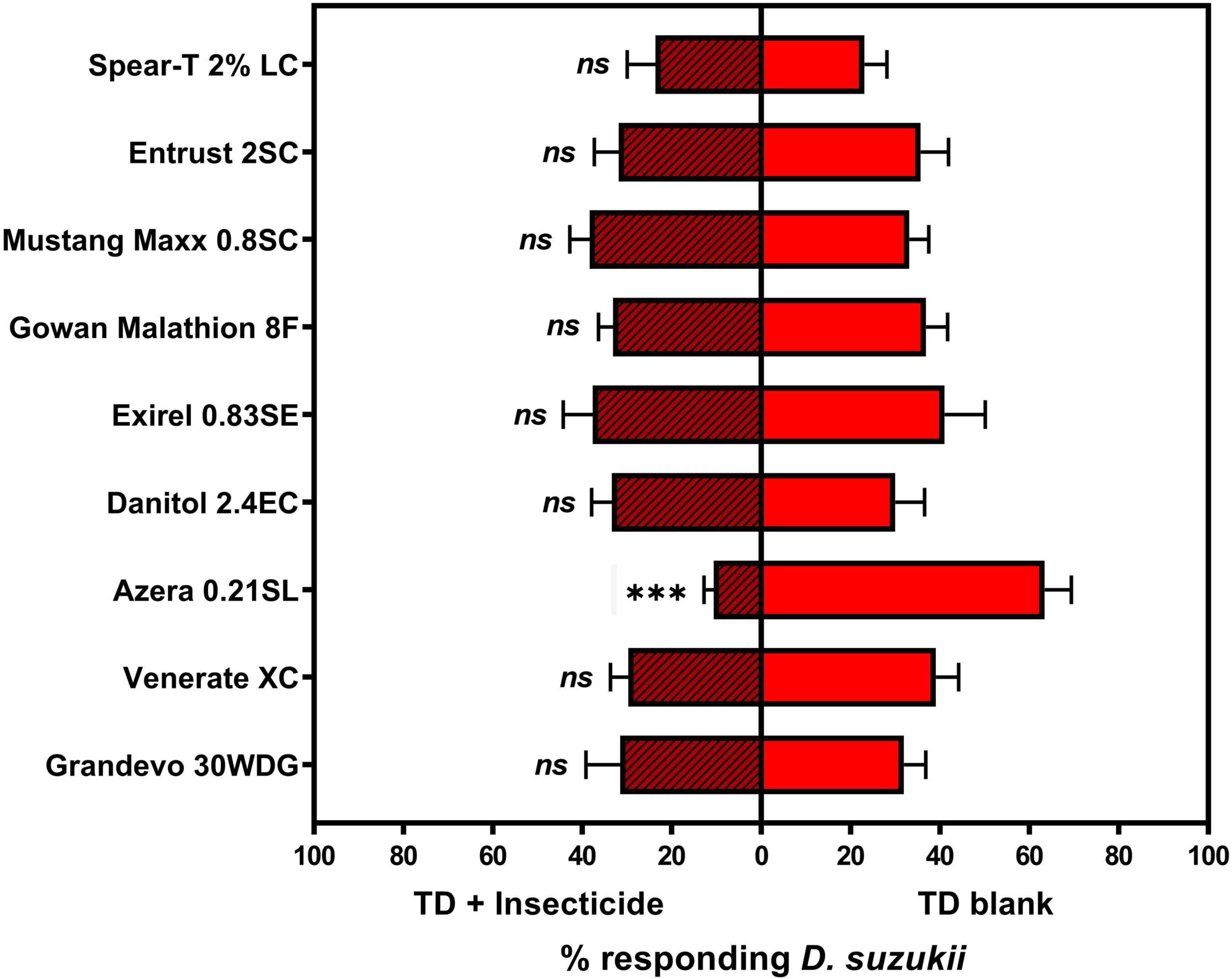
Figure 4. Mean (±SEM) percent of total Drosophila suzukii released in an arena captured inside the traps contains an ACTTRA SWD TD + insecticide treatment vs. trap containing blank ACTTRA SWD TD. “ns,” no statistical difference (paired t-test; α = 0.05); significant difference, ***P < 0.001. For TD + Grandevo 30WDG vs. TD blank: N = 8, df = 7, t = 0.040, P = 0.9692; TD + Venerate vs. TD blank: N = 8, df = 7, t = 1.644, P = 0.1443; TD + Azera 0.21SL vs. TD blank: N = 8, df = 7, t = 7.121, P = 0.0002; TD + Danitol 2.4EC vs. TD blank: N = 8, df = 7, t = 0.343, P = 0.7415; TD + Exirel 0.83SE vs. TD blank: N = 7, df = 6, t = 0.213, P = 0.8385; TD + Gowan Malathion 8F vs. TD blank: N = 8, df = 7, t = 0.541, P = 0.6051; TD + Mustang Maxx 0.8SC vs. TD blank: N = 8, df = 7, t = 0.775, P = 0.4635; TD + Entrust 2SC vs. TD blank: N = 8, df = 7, t = 0.346, P = 0.7397; and TD + Spear-T 2% LC vs. TD blank: N = 8, df = 7, t = 0.067, P = 0.9485.
Efficacy of ACTTRA SWD Formulations Mixed With Selected Insecticides
OR1 + Insecticides
No significant interaction of treatments with sex was observed for adult mortality in any of the observation dates (1 DAT, F = 1.52; df = 11, 154; P = 0.128; 3 DAT, F = 1.46; df = 11, 154; P = 0.154; 6 DAT, F = 1.30; df = 11, 155; P = 0.232). Additionally, no significant difference in male and female mortality was observed in any observation dates (1 DAT, F = 0.30; df = 1, 166; P = 0.582; 3 DAT, F = 0.63; df = 1, 165; P = 0.430; 6 DAT, F = 0.81; df = 1, 166; P = 0.368). However, a significant difference in SWD adult mortality was observed among the treatments on 1, 3, and 6 DAT (Table 2). At 1, 3, and 6 DAT, ACTTRA SWD OR1 formulation with Danitol 2.4EC (fenpropidin), Exirel 0.83SE (cyantraniliprole), Gowan Malathion 8F (malathion), Mustang Maxx 0.8SC, and Entrust 2SC Naturalyte (spinosad) resulted in significantly higher SWD mortality compared with the untreated control (Table 2). Additionally, on 6 DAT, Azera 0.21SL and ACTTRA SWD TD positive control resulted in significantly higher mortality than the untreated control.
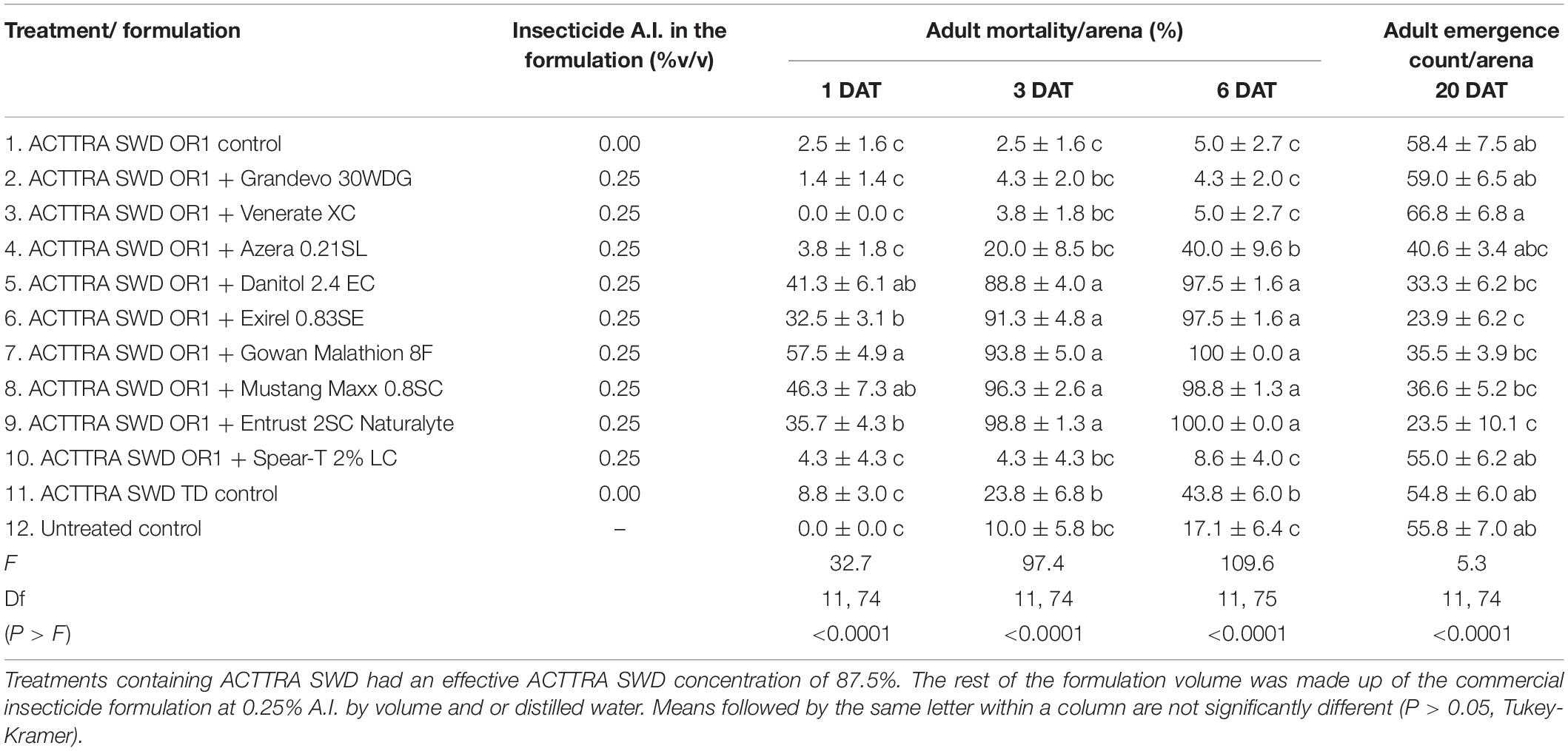
Table 2. Percent adult Drosophila suzukii mortality (mean ± SEM), 1, 3, and 6 days after exposing the insects to various ACTTRA SWD OR1 + insecticide formulations and controls treatments and the mean (±SEM) counts of adult D. suzukii emerged 20 DAT, from the blueberries incubated from the bioassay arena.
Similar to adult mortality, no significant interaction of treatments with sex was observed for adult progeny emergence (F = 0.54; df = 11, 155; P = 0.876). Averaged across the treatments, 16% more adult female progenies were produced from blueberries than male progenies (male = 20.8, female = 24.2; F = 6.29; df = 1, 166; P = 0.013). Moreover, a significant difference in D. suzukii adult emergence was observed among the treatments (Table 2) and compared with the untreated control; significantly fewer D. suzukii adults emerged from blueberries placed in the arena with ACTTRA SWD OR1 incorporated with either Exirel 0.83SE or Entrust 2SC Naturalyte.
TD + Insecticides
No significant interaction of treatments with sex was observed for adult mortality in any of the observation dates (1 DAT, F = 1.09; df = 11, 166; P = 0.374; 3 DAT, F = 0.68; df = 11, 159; P = 0.756; 6 DAT, F = 0.36; df = 11, 166; P = 0.970). However, significantly higher male mortality was observed at 1 DAT (male = 18.9%, female = 12.2%; F = 8.37; df = 11, 166; P = 0.004). A significant difference in D. suzukii adult mortality was observed among the treatments at 1, 3, and 6 DAT (Table 3). At 1, 3, and 6 DAT, ACTTRA SWD TD with Danitol 2.4EC, Exirel 0.83SE, Gowan Malathion 8F, and Mustang Maxx 0.8SC resulted in significantly higher SWD mortality compared with the untreated control (Table 3). Additionally, on 3 and 6 DAT, Entrust 2SC Naturalyte, and on 6 DAT, Azera 0.21SL and Venerate XC (Burkholderia spp.) resulted in significantly higher mortality than the untreated control but not higher than the ACTTRA SWD TD positive control. At 6 DAT, among the OMRI-approved insecticides tested, only Entrust 2SC Naturalyte incorporated into ACTTRA SWD TD caused more than 80% mortality.
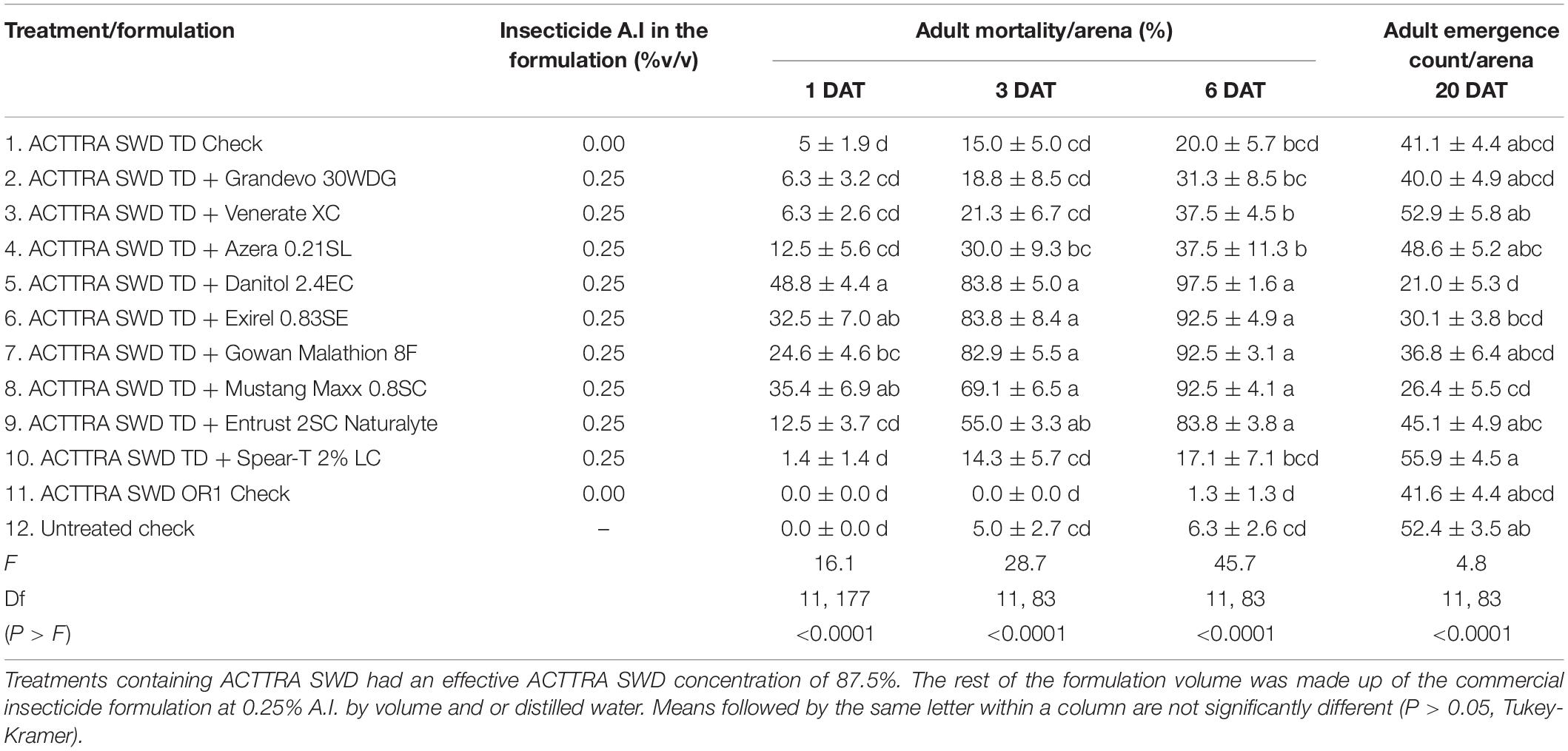
Table 3. Percent adult Drosophila suzukii mortality (mean ± SEM), 1, 3, and 6 days after exposing the insects to various ACTTRA SWD TD + insecticide formulations and controls treatments and the mean (±SEM) counts of adult D. suzukii emerged 20 DAT, from the blueberries incubated from the bioassay arena.
Similar to the adult mortality data, no significant interaction of treatments with sex was observed for adult progeny emergence (F = 1.36; df = 11, 166; P = 0.197). However, averaged across the treatments, 25% more adult female progenies were produced than male progenies (male = 18.2, female = 22.7; F = 16.18; df = 1, 177; P < 0.0001). A significant difference in D. suzukii adult emergence was observed among the treatments (Table 3), and compared with untreated check, application of the ACTTRA SWD TD formulation with Danitol 2.4 EC and Mustang Maxx 0.8SC resulted in significantly reduced D. suzukii adult emergence.
Discussion
Presently, the experimental ACTTRA SWD-based A&K system solely relies on spinosad (Entrust) as a killing component (Disi and Sial, 2019; Klick et al., 2019; LaTora, 2019). This study identifies several additional insecticide formulations that are suitable for mixing with both OR1 and TD formulations. The selection of suitable insecticides for ACTTRA SWD was based on: (1) the formulation’s efficacy in managing D. suzukii as an A&K formulation after mixing ACTTRA SWD with selected insecticide and (2) the resulting formulation’s attractiveness to the target insect. Among the nine insecticides tested, we identified Danitol 2.4EC, Exirel 0.83SE, Gowan Malathion 8F, Mustang Maxx 0.8SC, and Entrust 2SC as suitable insecticide additives for both ACTTRA SWD formulations. Additionally, adding Mustang Maxx to OR1 at 0.25% A.I. by volume resulted in a significant improvement (≈1.9 fold) in formulation capability in attracting D. suzukii adults. On the contrary, adding Azera 0.21SL to either the TD or the OR1 formulation at 0.25% A.I. by volume resulted in a significant decrease in the formulation’s attractiveness to adult D. suzukii. The addition of Azera to the OR1 and TD formulations resulted in a 10.7- and 5.7-fold decrease, respectively, in insect attraction than the corresponding blank ACTTRA SWD formulation, suggesting that Azera is unsuitable for mixing with either the OR1 or the TD adjuvants when preparing A&K formulations to manage D. suzukii.
In our two-choice studies, the treatments were placed inside an insect trap that prevents flies from directly contacting the treatment before entering the trap. Therefore, the flies’ decision to choose a treatment over another in a two-choice situation was based on the olfactory cues emitted from the treatments. Based on results from the two-choice assay with OR1 formulation mixed with and without Mustang Maxx, volatiles from the OR1 with the insecticide were significantly more attractive to D. suzukii adults than from the blank OR1 formulation. A commercial insecticide formulation is a blend of one or more active ingredients and multiple other components with no direct biocidal action (Nagy et al., 2020). Thus, it is unclear whether the volatiles from the active ingredient in the Mustang Maxx, i.e., zeta-cypermethrin, or any other components present in the formulation resulted in this change in attraction. A similar behavioral response was observed in the Western cherry fruit fly, Rhagoletis indifferens Curran, to cherry fruits treated with Mustang Maxx at the highest field recommended rate. Compared with the water-treated fruits, R. indifferens landed more frequently on fruits treated with Mustang Maxx in a no-choice situation. Interestingly, R. indifferens spent significantly less time on Mustang Maxx-treated fruits than the water-treated ones, and the frequent landing of insects on insecticide-treated fruits was attributed to the insect’s response to toxicity and irritant effects of the insecticide (Yee and Alston, 2012).
Contrary to the effect of Mustang Maxx with OR1, the addition of Azera 0.21SL in TD or OR1 formulation resulted in a significant decrease in the formulation attractiveness to adult D. suzukii. This is not surprising as Azera contains two active ingredients, azadirachtin and pyrethrins, both known to elicit repellency or deterrence effects in some insects. For instance, odors from the technical grade permethrin and various commercial permethrin formulations were shown to elicit a repellent effect in Plutella xylostella (L.) larvae (Lin et al., 1993). Similarly, azadirachtin is a known deterrent, antifeedant, oviposition deterrent, and insect growth regulator for several insect species (Mordue and Blackwell, 1993, and citations therein). While pyrethroids are known to elicit an olfactory response prior to contact in some insects through volatile cues (Lin et al., 1993; Joseph, 2020), the antifeedant and insect growth regulation effects of pure azadirachtin are predominantly attributed to post-contact effects (Mordue and Blackwell, 1993). Moreover, the pure azadirachtin is non-volatile (Morgan, 2009). Therefore, the pre-contact repellent effect observed by our study when ACTTRA SWD was mixed with Azera is less likely due to azadirachtin and more likely due to the pyrethrins. However, other components in the Azera formulation could also be responsible for the observed reduction in attraction. Further experiments are needed with pure active ingredients to confirm the repellency of pyrethrins to D. suzukii adults (Zalucki and Furlong, 2017).
Interestingly, among the OR1 and TD formulations tested with Mustang Maxx, the addition of insecticide with OR1, not with TD, resulted in an enhanced attraction of D. suzukii adults than the corresponding blank adjuvants. Compared with the OR1 formulation, TD is eight times more attractive to adult D. suzukii (Babu et al., unpublished). Additionally, previous research has shown that TD is more competitive in attracting D. suzukii in the presence of external competing volatiles like host fruit odors (Babu et al., 2022). It is, therefore, possible that the positive influence of Mustang Maxx that enhances the attractiveness of adult D. suzukii to the adjuvant in the A&K formulation is relatively weak and manifested only when mixed with the adjuvant that is a relatively weak attractant. Alternatively, changes in attractiveness can also result from an interaction of components in the adjuvant with the components in the insecticide formulation (El-Sayed et al., 2009).
Notably, the addition of most of the insecticides tested in this study with the ACTTRA SWD formulations did not significantly change the formulation’s attractiveness to D. suzukii adults. Maintaining formulation attractiveness to the target organism after mixing with an insecticide is essential for insects being attracted to the A&K point source and subsequently interacting with the formulation containing an insecticide. In addition to this, the efficacy of an A&K product containing an insecticide in managing the target organism depends on multiple insecticide and insect related factors. This includes the target insect acquiring adequate insecticide dose while interacting with the A&K formulation and ensuring a high level of mortality or physiological and behavioral changes after the insect acquired the insecticide, leading to population suppression (El-Sayed et al., 2009). Therefore, based on the insecticide’s ability to maintain or enhance the formulation attractiveness and due to high levels of mortality (>80%) during the 6 days exposure period, we conclude that Danitol 2.4EC, Exirel 0.83SE, Gowan Malathion 8F, Mustang Maxx 0.8SC, and Entrust 2SC are suitable insecticide additives for both ACTTRA SWD formulations. However, it should be noted that the behavioral response and insect mortality observed in our study depend on the specific concentration of active ingredient(s) and possibly other formulation components blended in the A&K product. For instance, the same insecticide product that did not influence the behavioral response of target insects, when mixed at a lower concentration, can lead to an overall change in an A&K formulation attractivity at a higher concentration (Lin et al., 1993). Additionally, the concentration of insecticide-active ingredients in the A&K formulation could be further fine-tuned to enhance the mortality of target insects. This is especially relevant for certain organic insecticides resulting in suboptimum mortality levels at lower doses. Thus, to identify the optimum concentration of commercial insecticide products, we encourage further studies testing the effect of insecticide concentrations in the ACTTRA SWD-based A&K formulations on the D. suzukii behavioral response and mortality.
In summary, our results expand the choices of insecticide additives when deploying ACTTRA SWD-based A&K system for managing D. suzukii in small fruit crops. The identification of multiple suitable insecticides choices is a significant improvement from the previous ACTTRA SWD-based A&K system that depends on the spinosyn insecticides spinetoram (Delegate) or spinosad (Entrust) as the only killing component. Additionally, identifying multiple insecticide options compatible with conventional management will avoid the dependency on a single insecticide for deploying ACTTRA SWD-based A&K technologies. However, only a limited chemical control option is available for the organic growers for D. suzukii management, and spinosad is the primary insecticide that provides a satisfactory control. In accordance with previous research (e.g., Disi and Sial, 2019; Klick et al., 2019; LaTora, 2019), our study confirmed that spinosad is a suitable insecticide component for the development of A&K formulations for organic farming. Further research is encouraged to verify our findings using field trials, and to identify additional OMRI-listed insecticides that can be suitable for ACTTRA SWD A&K formulations to relax the selection pressure on spinosad when managing D. suzukii in small fruit organic farms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AB performed the laboratory assays and prepared a draft manuscript. All authors conceived the research idea, carried out the data analyses, contributed to the writing and reviewing of the manuscript before submission, and approved the submitted version.
Funding
This research was supported by funding from the USDA Specialty Crops Research Initiative (Award # 2015-51181-24252 and 2020-51181-32140), the USDA Organic Agriculture Research and Extension Initiative (Award # 2018-51300-28434), Extension Implementation Program (Award # 2017-70006-27202), Georgia Berry Exchange, and Georgia Agricultural Commodity Commission for Blueberries.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Rosan Adhikari, Subin Neupane, Rupinder Signh, and Caroline Polacheck for technical assistance. In addition, we thank ISCA Technologies, Inc. (Riverside, CA, United States) for providing ACTTRA SWD formulations for research.
References
Babu, A., Rodriguez-Saona, C., and Sial, A. A. (2022). Factors influencing the efficacy of novel attract-and-kill (ACTTRA SWD) formulations against Drosophila suzukii. J. Econ. Entomol. 2022:273. doi: 10.1093/jee/toab273
Bruck, D. J., Bolda, M., Tanigoshi, L., Klick, J., Kleiber, J., DeFrancesco, J., et al. (2011). Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag. Sci. 67, 1375–1385. doi: 10.1002/ps.2242
Buchman, A., Marshall, J. M., Ostrovski, D., Yang, T., and Akbari, O. S. (2018). Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. Proc. Natl. Acad. Sci. U.S.A. 115, 4725–4730. doi: 10.1073/pnas.1713139115
Cloonan, K. R., Abraham, J., Angeli, S., Syed, Z., and Rodriguez-Saona, C. (2018). Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 44, 922–939. doi: 10.1007/s10886-018-1000-y
Cormier, D., Veilleux, J., and Firlej, A. (2015). Exclusion net to control spotted wing Drosophila in blueberry fields. IOBC-WPRS Bull 109, 181–184.
Cowles, R. S., Rodriguez-Saona, C., Holdcraft, R., Loeb, G. M., Elsensohn, J. E., and Hesler, S. P. (2015). Sucrose improves insecticide activity against Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 108, 640–653. doi: 10.1093/jee/tou100
Cuthbertson, A. G. S., Collins, D. A., Blackburn, L. F., Audsley, N., and Bell, H. A. (2014). Preliminary screening of potential control products against Drosophila suzukii. Insects 5, 488–498. doi: 10.3390/insects5020488
Diepenbrock, L. M., Rosensteel, D. O., Hardin, J. A., Sial, A. A., and Burrack, H. J. (2016). Season-long programs for control of Drosophila suzukii in southeastern US blueberries. Crop. Prot. 81, 76–84. doi: 10.1016/j.cropro.2015.12.012
Disi, J. O., and Sial, A. A. (2019). Efficacy of HOOK SWD attract-and-kill SPLAT for management of spotted-wing drosophila in Georgia rabbiteye blueberry, 2018. Arthropod Manag. Tests 44, 1–2.
El-Sayed, A. M., Suckling, D. M., Byers, J. A., Jang, E. B., and Wearing, C. H. (2009). Potential of “lure and kill” in long-term pest management and eradication of invasive species. J. Econ. Entomol. 102, 815–835. doi: 10.1603/029.102.0301
Fanning, P., Lanka, S., Mermer, S., Collins, J., Van Timmeren, S., Andrews, H., et al. (2021). Field and laboratory testing of feeding stimulants to enhance insecticide efficacy against spotted-wing drosophila, Drosophila suzukii (Matsumura). J. Econ. Entomol. 114, 1638–1646. doi: 10.1093/jee/toab084
Farnsworth, D., Hamby, K. A., Bolda, M., Goodhue, R. E., Williams, J. C., and Zalom, F. G. (2017). Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag. Sci. 73, 1083–1090. doi: 10.1002/ps.4497
Gregg, P. C., Del Socorro, A. P., and Landolt, P. J. (2018). Advances in attract-and-kill for agricultural pests: beyond pheromones. Annu. Rev. Entomol. 63, 453–470. doi: 10.1146/annurev-ento-031616-035040
Gress, B. E., and Zalom, F. G. (2019). Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manag. Sci. 75, 1270–1276. doi: 10.1002/ps.5240
Horton, D. R. (1995). Statistical considerations in the design and analysis of paired–choice assays. Environ. Entomol. 24, 179–192. doi: 10.1093/ee/24.2.179
Jaramillo, S. L., Mehlferber, E., and Moore, P. J. (2015). Life-history trade-offs under different larval diets in Drosophila suzukii (Diptera: Drosophilidae). Physiol. Entomol. 40, 2–9. doi: 10.1111/phen.12082
Joseph, S. V. (2020). Repellent effects of insecticides on Stephanitis pyrioides Scott (Hemiptera: Tingidae) under laboratory conditions. Crop. Prot. 127:104985. doi: 10.1016/j.cropro.2019.104985
Klick, J., Rodriguez-Saona, C. R., Cumplido, J. H., Holdcraft, R. J., Urrutia, W. H., Da Silva, R. O., et al. (2019). Testing a novel attract-and-kill strategy for Drosophila suzukii (Diptera: Drosophilidae) management. J. Insect Sci. 19, 1–6. doi: 10.1093/jisesa/iey132
Kumar, K., and Chapman, R. B. (1984). Sublethal effects of insecticides on the diamondback moth Plutella xylostella (L.). Pestic. Sci. 15, 344–352.
LaTora, A. G. (2019). Innovations in spotted wing drosophila (Drosophila suzukii Matsumura) monitoring and attract-and-kill for development of more targeted integrated pest management programs. MS Thesis. Gainesville, Fl: University of Florida.
Lin, H., Hoy, C. W., and Head, G. (1993). Olfactory response of larval diamondback moth (Lepidoptera: Plutellidae) to permethrin formulations. Environ. Entomol. 22, 1096–1102. doi: 10.1093/ee/22.5.1096
Mafra-Neto, A., Fettig, C. J., Munson, A. S., Rodriguez-Saona, C., Holdcraft, R., Faleiro, J. R., et al. (2014). “Development of specialized pheromone and lure application technologies (SPLAT®) for management of coleopteran pests in agricultural and forest systems,” in Biopesticides: state of the art and future opportunities. Symposium Series 1172, eds A. D. Gross, J. R. Coats, S. O. Duke, and J. N. Seiber (Washington, DC: American Chemical Society), 211–242. doi: 10.1021/bk-2014-1172.ch015
Mordue, A. J., and Blackwell, A. (1993). Azadirachtin: an update. J. Insect Physiol. 39, 903–924. doi: 10.1016/0022-1910(93)90001-8
Morgan, E. D. (2009). Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 17, 4096–4105. doi: 10.1016/j.bmc.2008.11.081
Nagy, K., Duca, R. C., Lovas, S., Creta, M., Scheepers, P. T., Godderis, L., et al. (2020). Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 181:108926. doi: 10.1016/j.envres.2019.108926
Reed, L. K., Williams, S., Springston, M., Brown, J., Freeman, K., DesRoches, C. E., et al. (2010). Genotype-by-diet interactions drive metabolic phenotype variation in Drosophila melanogaster. Genetics 185, 1009–1019. doi: 10.1534/genetics.109.113571
Rendon, D., Hamby, K. A., Arsenault-Benoit, A. L., Taylor, C. M., Evans, R. K., Roubos, C. R., et al. (2020). Mulching as a cultural control strategy for Drosophila suzukii in blueberry. Pest Manag. Sci. 76, 55–66. doi: 10.1002/ps.5512
Rendon, D., and Walton, V. M. (2019). Drip and overhead sprinkler irrigation in blueberry as cultural control for Drosophila suzukii (Diptera: Drosophilidae) in Northwestern United States. J. Econ. Entomol. 112, 745–752. doi: 10.1093/jee/toy395
Renkema, J. M., Wright, D., Buitenhuis, R., and Hallett, R. H. (2016). Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 6, 1–10. doi: 10.1038/srep21432
Rhodes, E. M., Avery, P. B., and Liburd, O. E. (2018). Efficacy of entomopathogenic fungal products for biological control of spotted wing drosophila (Diptera: Drosophilidae) under laboratory conditions. Fla. Entomol. 101, 526–528. doi: 10.1653/024.101.0329
Ross, M. H., and Cochran, D. G. (1992). Strain differences in the response of German cockroaches (Dictyoptera: Blattellidae) to emulsifiable concentrates. J. Econ. Entomol. 85, 1201–1208. doi: 10.1093/jee/85.4.1201
Roubos, C. R., Gautam, B. K., Fanning, P. D., Van Timmeren, S., Spies, J., Liburd, O. E., et al. (2019). Impact of phagostimulants on effectiveness of OMRI-listed insecticides used for control of spotted-wing drosophila (Drosophila suzukii Matsumura). J. Appl. Entomol. 143, 609–625. doi: 10.1111/jen.12620
Sarkar, N., Rhodes, E. M., Spies, J., Roubos, C. R., Little, B. A., Sial, A. A., et al. (2020). Evaluation of non-target effects of OMRI-listed insecticides for management of Drosophila suzukii Matsumura in berry crops. J. Appl. Entomol. 144, 12–25. doi: 10.1111/jen.12713
Schöneberg, T., Arsenault-Benoit, A., Taylor, C. M., Butler, B. R., Dalton, D. T., Walton, V. M., et al. (2020). Pruning of small fruit crops can affect habitat suitability for Drosophila suzukii. Agr. Ecosyst. Environ. 294:106860. doi: 10.1016/j.agee.2020.106860
Sial, A. A., Roubos, C. R., Gautam, B. K., Fanning, P. D., Van Timmeren, S., Spies, J., et al. (2019). Evaluation of organic insecticides for management of spotted-wing drosophila (Drosophila suzukii) in berry crops. J. Appl. Entomol. 143, 593–608. doi: 10.1111/jen.12629
Spaulding, N. R. (2020). Investigation of the state of insecticide resistance in Georgia populations of Drosophila suzukii. MS thesis. Athens, GA: University of Georgia.
Tait, G., Mermer, S., Stockton, D., Lee, J., Avosani, S., Abrieux, A., et al. (2021). Drosophila suzukii (Diptera: Drosophilidae): a decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 114, 1950–1974. doi: 10.1093/jee/toab158
Van Timmeren, S., and Isaacs, R. (2013). Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop. Prot. 54, 126–133. doi: 10.1016/j.cropro.2013.08.003
Wang, X., Daane, K. M., Hoelmer, K. A., and Lee, J. C. (2021). “Biological control of spotted-wing Drosophila: an update on promising agents,” in Drosophila suzukii Management, Chap. 8, ed. F. R. M. Garcia (Cham: Springer), 143–168. doi: 10.1007/978-3-030-62692-1_8
Wang, X., Lee, J. C., Daane, K. M., Buffington, M. L., and Hoelmer, K. A. (2020). Biological control of Drosophila suzukii. CAB Rev. 2020:15.
Yee, W. L., and Alston, D. G. (2012). Behavioral responses, rate of mortality, and oviposition of western cherry fruit fly exposed to malathion, zeta-cypermethrin, and spinetoram. J. Pest Sci. 85, 141–151. doi: 10.1007/s10340-011-0388-8
Keywords: spotted-wing drosophila, behavioral management, attract-and-kill, choice tests, SPLAT, adjuvant
Citation: Babu A, Rodriguez-Saona C and Sial AA (2022) Comparative Adult Mortality and Relative Attractiveness of Spotted-Wing Drosophila (Diptera: Drosophilidae) to Novel Attract-and-Kill (ACTTRA SWD) Formulations Mixed With Different Insecticides. Front. Ecol. Evol. 10:846169. doi: 10.3389/fevo.2022.846169
Received: 30 December 2021; Accepted: 04 March 2022;
Published: 30 March 2022.
Edited by:
Gianfranco Anfora, Fondazione Edmund Mach, ItalyReviewed by:
Valerio Mazzoni, Fondazione Edmund Mach, ItalyFelipe Andreazza, Duke University, United States
Copyright © 2022 Babu, Rodriguez-Saona and Sial. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashfaq A. Sial, YXNoc2lhbEB1Z2EuZWR1
 Arun Babu
Arun Babu Cesar Rodriguez-Saona
Cesar Rodriguez-Saona Ashfaq A. Sial
Ashfaq A. Sial