
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 14 March 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.820915
 Yanwen Fu1,2,3
Yanwen Fu1,2,3 Mengyu Tan1,2,3
Mengyu Tan1,2,3 Yinan Gong1,2,3
Yinan Gong1,2,3 Guojing Zhao1,2,3
Guojing Zhao1,2,3 Jianping Ge1,2,3
Jianping Ge1,2,3 Haitao Yang1,2,3*
Haitao Yang1,2,3* Limin Feng1,2,3
Limin Feng1,2,3Geopolitical borderlands are politically sensitive areas and biodiversity hotspots, strictly controlled by the government and military. How to ensure political security, while protecting the biodiversity in borderlands is a problem for ecologists and governments. In this study, the nest site selection of the wild boar Sus scrofa was a case study in the Sino-Russia borderland to understand the survival strategy of wild life under anthropogenic pressure. We investigated (a) how the spatial distribution of anthropogenic pressure and wild boar nests in the borderland and (b) how anthropogenic pressure and the border influence on the wild boars’ nest site selection. The Getis-Ord Gi* analysis was used to analyze the distribution patterns of wild boar nest sites and anthropogenic pressures in the borderland, the Structural Equation Models was used to explore the influence of border, roads, settlements, agricultural land, grassland and anthropogenic pressure on wild boars’ nest site selection. The results indicated that wild boar nest sites are close to the border, roads and agricultural land and away from settlements and grassland. Regardless of the combination of anthropogenic pressure, wild boars make the most advantageous choice and prefer to be closer to the borderland. We speculated that military control played a vital role in borderlands for animal protection under anthropogenic pressure. Wild boars benefit from the prohibition of anthropogenic persecution due to military control. Compared with existing measures, we suggest a different protection/wildlife management strategy, what we need to do may be to prohibit anthropogenic persecution rather than perform other human interventions to protect animals. However, for a species with trouble potential, we need to base our conservation strategies on the recovery of top predators, and play the community control role of top predators to avoid the occurrence of trouble.
Most borderlands, which are politically sensitive areas under strict military control, overlap with biodiversity hotspot (Macdonald et al., 2020), contain important ecosystems, and provide critical habitats for endangered species. For example, the Amur leopard (Panthera pardus orientalis) is only home to the borderland between China and Russia (Vitkalova et al., 2018), and the demilitarized zone between North Korea and South Korea is a narrow strip with high biodiversity (Lee and Miller-Rushing, 2014). However, geopolitical boundaries artificially fracture biomes and their associated ecosystems (Liu et al., 2020), and the negative impact on biodiversity loss in borderlands can be significant if the borderlands experience excessive interference due to anthropogenic disturbances, such as border barriers (Flesch et al., 2010; Pokorny et al., 2017; Peters et al., 2018). Due to the particularities of borderlands, how to protect the biodiversity while ensure the needs of political security in borderlands is an urgent problem which need more concern by the ecologists and the government (Liu et al., 2020).
As anthropogenic pressure continues to increase in magnitude, instances and extent (Venter et al., 2016; Watson et al., 2016; Halpern et al., 2019), the impact of anthropogenic pressure on animal behaviors become a hot spot in conservation biology and wildlife management in recent years (Gaynor et al., 2018; Samia et al., 2019; Suraci et al., 2019). These diverse anthropogenic impacts can induce changes in animal behavior by altering the conditions under which animals make behavioral decisions (Wilson et al., 2020). The direct anthropogenic presence and indirect impacts on an animal’s surroundings can alter behavior via changes in population densities, top-down effects, bottom-up effects and changes in the physical environment (Wilson et al., 2020). In response, flexible animals can adapt to hunting and other anthropogenic pressures by changing their habitat use or rhythm (Laguna et al., 2021a,b; Recio et al., 2021). Therefore, the impact of anthropogenic pressure on animals may lead animals to move toward areas where has higher suitability, such as the borderland (Llaneza et al., 2012; Holbrook et al., 2019). This indicate that these borderlands can act as refuge areas (Amici et al., 2012).
Wildlife living along borderlands benefit from the quality habitat and the enforcement by the military or government (Vitkalova et al., 2018; Liu et al., 2020). Conversation-friendly borderland can be divided into two categories: remote and uninhabited regions and regions under military control (Liu et al., 2020). First, many borderlands lie in remote and uninhabited regions, with low anthropogenic pressure. For example, due to the lack of human habitations, large roadless regions exist along the borderland between Sweden and Finland as well as among Russia and Eastern European countries, greatly reducing the impact of anthropogenic pressure on animals (Leblond et al., 2013; Psaralexi et al., 2017; Fedorca et al., 2019). Second, borderlands are characterized by tightened military control (Liu et al., 2020). Previous studies have speculated that due to the tightened military controls at the borderlands, which limit public access, borderlands have developed habitats with low anthropogenic disturbance and improved habitat suitability (Vitkalova et al., 2018). These zones can also have importance as corridors, connecting different patches (Hosseini et al., 2019). Reinhardt et al. (2019) point out that military training areas facilitating the spreading of wolf (Canis lupus) territories in the surrounding landscape may be linked to a low level of anthropogenic mortality. However, there is likely to be no less anthropogenic pressure in borderlands under military control than in demilitarized zones due to the large amount of logistical and military activity caused by the presence of troops. In fact, we do not know the distribution of anthropogenic pressure in militarily controlled borderlands, and likewise, we do not know the effect of borderlands on animal behavior under anthropogenic pressure. Therefore, we speculated that anthropogenic pressure is not rare in militarily controlled borderlands, and under anthropogenic pressure, the borderland still has a positive effect on animal behavior.
What causes the high biodiversity of military controlled borderland? Could the management of military controlled borderland be applied to the protection and management of wildlife in other area? Thus, we used the wild boars’ selection of nest sites in the military controlled borderland between China and Russia as a case study to investigate the speculation. The wild boar belongs to the Suidae family and is widely distributed in Europe, Asia and Africa (Zhao et al., 2019). Wild boars have a strong ability to adapt to environmental changes (Luskin et al., 2017). In Hoge Kempen National Park, wild boars have adapted to the existence of humans in suburban areas and have survived stably in areas with greater anthropogenic pressure by changing their rhythm (Wevers et al., 2020). Among wild boar behaviors, the selection of nest sites is highly sensitive to environmental pressure (Fernández-Llario, 2004). We first quantified the different types of anthropogenic pressure in the study area and then explored (a) how is the spatial distribution of anthropogenic pressure and wild boar nests in the borderland and (b) how anthropogenic pressure and the border influence on the wild boars’ nest site selection? Finally, we discussed the significance of prohibition of anthropogenic persecution in military control borderland areas for wildlife protection and management.
We conducted this study in the northeastern section of Jilin Province in an area of 6,501 km2 (130.165609 E- 131.317653 E and 42.614041 N–43.553505 N) that is part of the Northeast Tiger and Leopard National Park in Northeast China (Figure 1). The highest elevation is located in the core region at 1,477 m (Yang et al., 2019). Because the area borders southwestern Primorsky Krai, Russia, a fire barrier, which was grassland more than 200 km long and at least 50 m wide, has been built along the border. In addition, approaching the border, the force of the military control effect was enhanced. Over the past decade, this region has been exposed to increasing levels of agricultural and industrial development, particularly mining and the building of new roads (Wang et al., 2018). Settlements and agricultural land were usually along the main roads. With the implementation of the Natural Forest Conservation Program (NFCP) and Grain-to-Green Program (GTGP) since 1999 (Liu et al., 2008; Yang et al., 2013), the commercial logging of natural forests has been halted. The main commercial activities in rural areas were free-range cattle grazing, edible ferns, ginseng farms, and frog farming. Thus, the land cover in the study area included settlement areas, agricultural land, grassland, water bodies and forests.
This region has high biodiversity. However, the Amur tiger (Panthera tigris altaica) and Amur leopard density were low in this area (Wang et al., 2016). The ungulate prey species of the tiger and leopard included the sika deer (Cervus nippon), Siberian roe deer (Capreolus pygargus) and musk deer (Moschus moschiferus). The Asiatic black bear (Ursus thibetanus) and brown bear (Ursus arctos) were also found in this area, along with other carnivores, such as the Asian badger (Meles leucurus), raccoon dog (Nyctereutes procyonoides), red fox (Vulpes vulpes) and leopard cat (Prionailurus bengalensis) (Yang et al., 2018a).
About 30,000 camera trapping were fixed in Northeast Tiger and Leopard National Park. The area in this study is the main distribution area of the Amur tiger and Amur leopard in China (Wang et al., 2016; Vitkalova et al., 2018), therefore, about 13,000 camera trappings were fixed in this area. The camera trapping sites are systematically designed based the grids (the grid size is 1 km2). In each grid, mostly 2 camera traps (Ltl-6511-4G, Zhuhai, China, more than 500 m apart) were placed along roads, trails or ridges which were natural routes for the wild animals tend to use. The cameras were fastened trees, 40–80 cm above the ground, and were programmed to shoot 10-s videos with a 1-min interval between consecutive events, the sensitive level of cameras was set to low level. The camera traps operated 24-h per day throughout the year.
The nest survey took place during the installation of camera trappings in study area. We conducted the survey of wild boar nests between April and November 2020. When building a nest, wild boars gnawed off branches around them to build a conspicuous structure (Fernández-Llario, 2004). This provides us with the identification of the nest, stacked structure, surrounded by a large number of gnawed branches. Rangers searched for wild boar nests in the corresponding grid when visiting to the camera sites. Please note that the survey is based on the camera placement process, which implies that no human intervention is involved in the nest’s finding. Once a wild boar’s nest was found, rangers recorded the location with a global positioning system (GPS), took a picture of the nest and recorded data regarding the area (e.g., land cover type) around the nest (10 m × 10 m). Infrared cameras captured videos of the construction and use of wild boar nests, lasting for about 22 days (Supplementary Video 1).
Then, we used the land cover data to determine the distribution of anthropogenic pressure, such as agricultural land and grassland. We acquired remote sensing satellite imagery from the Geospatial Data Cloud1 and USGS (United States Geography Survey2). First, we chose images with a spatial resolution of 30 m during the growing season (Apr–Nov), corresponding with the timing of the field survey to avoid misinterpreting habitat aspects (e.g., snow and seasonal aspects). Second, we conducted geometric corrections, noise corrections, and image enhancement (haze reduction) on the study area’s primary images before classification. According to characteristics of the wild boar habitat and the field survey findings, the study area was classified into 4 major land cover types: forest, agricultural land, grassland, and water (Hearn et al., 2016). Although shrub is an important habitat for wild boars, we found no typical shrub distribution in the study area by referring to globalland30 published in China (Jun et al., 2014; Alexander et al., 2016). For each type, we drew > 10 initial training polygons based on data from vegetation surveys and visual analysis of locations on high-resolution Google Maps (Google, Mountain View, CA, United States). We performed maximum likelihood supervised classification in ENVI 5.3. To improve classification accuracy, we removed training polygons with confusing spectral signatures and created new polygons until we obtained a perfect classification template through accuracy assessment (Fonji and Taff, 2014). After classification, we randomly selected 50 points of each type to carry out an accuracy assessment. The overall accuracy of the classification of the study area land cover was 85.72%, and the kappa coefficient was 0.82; both coefficients indicated strong agreement and accuracy between the classification of the study area and the true ground reference information (Manandhar et al., 2009). In addition, we acquired the full sampling of settlements and roads through artificial recognition based on high-resolution Google Maps (Google, Mountain View, CA, United States).
To explore the relationship between the spatial distribution patterns of anthropogenic pressure and wild boar nests in the borderland, we first vectorized all factors and turned them into feature points. Then, we established the Euclidean distance (in m) to the borderline, and we assigned the distance from the borderline to the point element. According to the first law of geography, near things in space are more similar (spatially autocorrelated) than things that are farther apart (Tobler, 1970). Getis-Ord Gi* analysis was used to investigate the relationships of the borderland with the spatial distribution patterns of the manifest variables, including wild boar nests, grassland, agricultural land, roads and settlements. Economic activities in the study area included free-range cattle grazing, which frequently invaded grasslands, meaning that grasslands brought anthropogenic pressure to wildlife (Wang et al., 2018). Getis-Ord Gi* is a local index of spatial association (LISA) test that is often used for “hot spot” analysis (Getis and Ord, 1992) and computes z-scores and P-values, specifying where those feature patterns are located (Wong and Lee, 2005). For significantly positive z-scores, the larger the z-score is, the greater the clustering of high values (hot spot); for negative z-scores, the smaller the z-score is, the greater the clustering of low values (cold spot) (Medland et al., 2020). This indicates aggregation far from the borderline (hot spots) and aggregation close to the borderline (cold spots) of anthropogenic pressure factors and wild boar nests within the study area.
The number of wild boar nests within 1 km2 was calculated as the nest density through neighborhood analysis at the location of the nest site as the degree of selection by the wild boars in a given area. The Euclidean distance (in m) between the wild boar nest site and the borderline was calculated as a borderland effect indicator, and agricultural land, grassland, roads and settlements were calculated as anthropogenic pressure indicators. Then, we analyzed the impact of indicators on wild boars’ nest site selection (number of nests within 1 km2) (Sobral et al., 2017). A structural equation model (SEM) was used to estimate and test the relationships among manifest variables, including the density of nest (Den), distance from the borderland (Bor), distance from grassland (Gras), distance from agricultural land (Agri), distance from roads (Road) and distance from settlements (Sett). To fit the model, a theoretically important set of relationships was used to design the a priori model. However, the fit of the initial a priori model was not good; according to the guidance of modification indices (MIs), we respecified the model by adding new relationships. We sequentially added new relationships with biological meaning until an adequate model fit was achieved (Santos et al., 2020). After fitting the final model, we tested the goodness of fit of the models by means of maximum-likelihood estimation on the variance-covariance matrix. The chi-squared P-value of the chosen solution was 0.181 (χ2 statistic, 3.417; d.f.,2; a non-significant goodness of fit test indicated that the model provided a good description of the observed covariance among the variables) (Santos et al., 2020). All data were standardized in R 3.6.2 (R Development Core Team, 2014), and SEM was conducted with AMOS 20 software (IBM SPSS, Inc.).
We acquired a total of 241 wild boar nest sites. The results from Getis-Ord Gi* analysis indicated that wild boar preferred areas close to the borderline for building nests (48.13% of wild boar nests were gathered in cold spots close to the borderline), rather than gathering far away from the borderline (32.78% of wild boar nests were far away from the borderline) (Figure 2). A total of 34.10% of the settlements gathered in cold spots close to the borderline, and 12.90% of the settlements gathered in hot spots far away from the borderline. Although 53.00% of the settlements had no significant tendency to gather near or far from the borderline, the other three anthropogenic disturbances related to settlements had an effect of borderline aggregation, similar to the nest selection of wild boar. More grassland and agricultural land gathered near the borderline than far away from the borderline (49.21 VS 30.56%, 53.23 VS 17.74%, respectively, for grassland and agricultural land). The distribution of roads in the study area was fairly uniform (43.70% gathered close to the borderline, 45.67% gathered far away from the borderline).
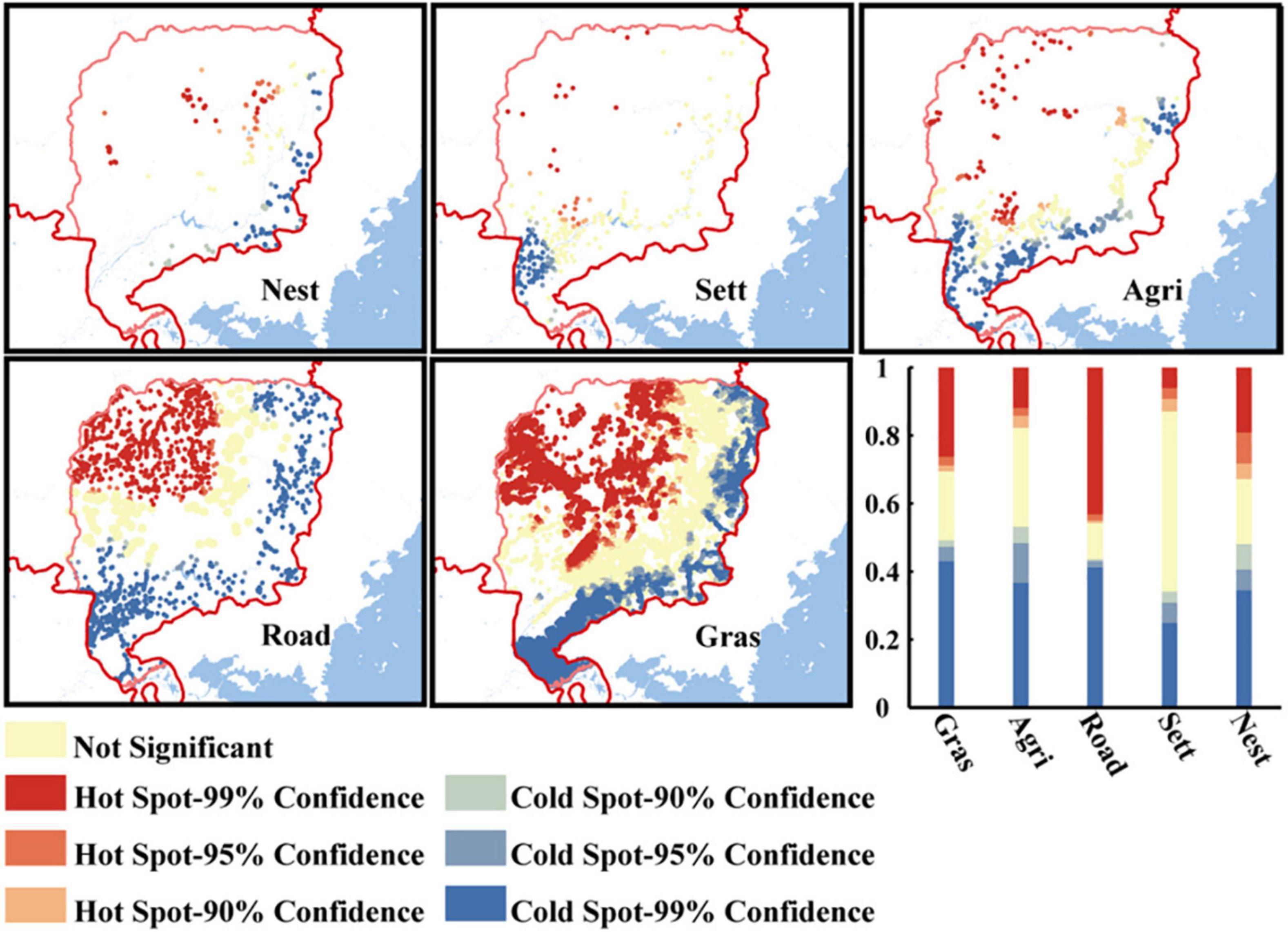
Figure 2. The number of hot and cold spots of anthropogenic pressure. This analysis judges the characteristics of cold spots and hot spots at the three confidence levels of 99, 95, and 90%. Hot spot means aggregation far from the borderline and cold spot means aggregation close to the borderline of anthropogenic pressure factors and wild boar nests within study area.
Structural equation model indicated that the nest site selection of wild boar still showed a significant trend toward the borderland under anthropogenic pressure (Figure 3). Furthermore, there might be a trade-off in wild boar nest selection between being far away from settlements and being close to the borderland (Figure 3 and Table 1). Except for the impact of the borderland and settlements, the nest selection of wild boar was likely to be close to roads and agricultural land and away from grassland (Figure 3 and Table 1). The model suggested that even if a nest site had to be close to the grassland, it would still be close to the borderland due to the stronger influence of the borderland (Figures 2, 3 and Table 1). When agricultural land and settlements became the selection conditions, due to the stronger negative impact of settlements, wild boar would select to build the nest far away from agricultural land and settlements (Figure 3 and Table 1). A similar situation also occurred in the combinations of settlements and grassland, roads and grassland, and roads and agricultural land. Regardless of the combination, wild boar always made the most advantageous choice and preferred to be close to the borderland (Figures 2, 3 and Table 1).
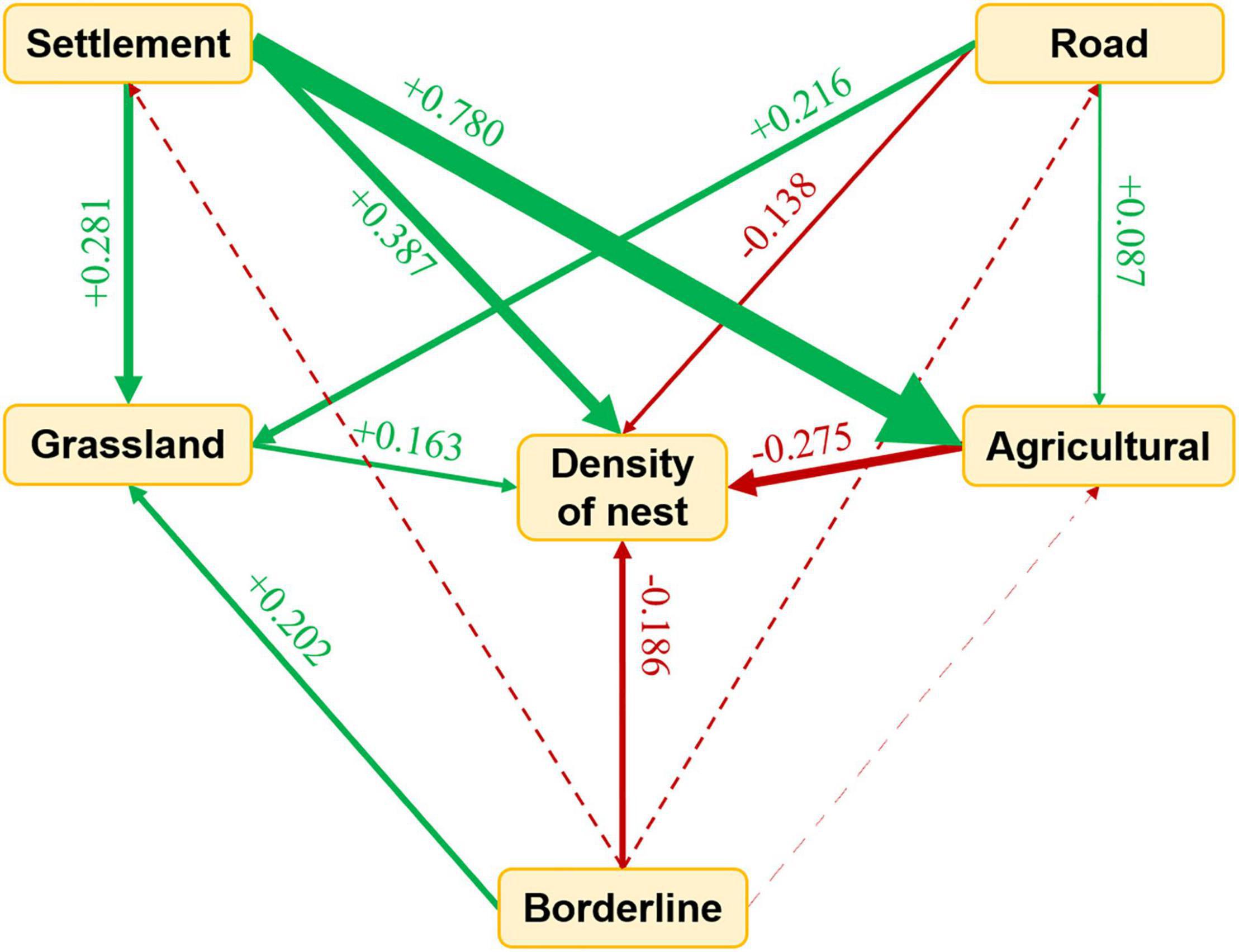
Figure 3. Results of the structural equation model analyzing the relationship of distance from borderline, distance from grassland, distance from agricultural land with distance from road, density of wild boar’s nest, distance from settlements, p < 0.05. For all paths of the final path model, arrow width is proportional to the relative strength of standardized path coefficients, (P = 0.181, χ2 = 3.417, d.f. = 2). Red and green arrows indicate opposite and consistent trends, respectively. The consistent trends between borderline, grassland, agricultural land, road, settlements with the density of nest indicated that the closer the distance from variable was, the lower the density of nest was. The opposite trends means that the closer the distance from variable was, the higher the density of nest was. The consistent trends between variables means that the closer the nest to variable 1, the closer the nest to variable 2. The opposite trends means that the closer the nest to variable 1, the further the nest to variable 2. Interrupted lines indicate the relationship none significant.
With the increasing spatiotemporal overlap between humans and wildlife, it is critical to understand how anthropogenic pressure modifies animal behavior depending on the spatial and temporal distribution of anthropogenic pressure, which will deepen the knowledge of ecosystem structure and function (Wilson et al., 2020). The influences of anthropogenic pressures on wildlife are complex, and anthropogenic pressure can directly or indirectly impact wildlife through top-down effects. For example, anthropogenic pressure can alter how and where prey perceive risk by modifying the populations of their natural predators (Suraci et al., 2019). Anthropogenic disturbance can also change the availability and distribution of resources to impact animals through bottom-up effects (Monk et al., 2018). Another example of this anthropic pressure is ecological traps. Artificially altered environments can lead organisms to misjudge environmental suitability, for example, grassland birds nesting in agricultural fields: Individuals settle in agricultural fields or dry grasslands that seem suitable, but their nests are destroyed during farming activities (Schlaepfer et al., 2002). Anthropogenic pressure, such as grazing and structural modifications, is probably one of the most important causes of species loss (D’Eon and Glenn, 2005). We also cannot deny that not all human activities have negative impacts on wildlife; for example, anthropogenic pressure in borderlands is more specific than that in inland areas (Hanski, 2005), and human activity in borderlands (e.g., military activity) may be an important factor in the survival of species (Vitkalova et al., 2018). Thus, it is necessary to understand the distribution of anthropogenic pressures in borderlands for biodiversity conservation.
The study area in this study is proximal to the border with Russia and the DPRK, and a highway runs through the whole study area along the borderline (Figure 1). Thus, there is a large human population along the borderland, with most people being engaged in agriculture, forestry or the military (Wang et al., 2017, 2018). As a result of the existence of the agricultural population in the borderland, farming and grazing activities related to agriculture are carried out, and poaching increases accordingly. Roads are built not only to meet the needs of agricultural and forestry production activities, but also more importantly, to meet the needs of regular patrols by troops. The increase in roads facilitates anthropogenic activities but also exacerbates the anthropogenic influence on wildlife (Samia et al., 2019; Suraci et al., 2019). The result of Getis-Ord Gi* analysis confirm the above intuition: 49.21% of grassland, 52.23% of agricultural land, 43.70% of roads and 34.10% of settlements were clustered and distributed close to the borderline (Figure 2). Getis-Ord Gi* analysis indicated that the borderland might not be the ideal nesting site for wild boar (Stolba and Woodgush, 1984; Jensen, 1989; Dellmeier and Friend, 1991), but 48.13% of wild boar nests were still clustered and distributed close to the borderland, which indicated that wild boar preferred to select nest sites near the borderland even under intense anthropogenic pressure. We speculate that this may be related to the prohibit on hunting. Hunting can have direct and indirect effects on animals. A typical example is either a direct reduction in the animals population or an indirect effect on the social structure of the population by eliminating key individuals (Leclerc et al., 2017). Along the borderland, where hunting is prohibited, which could change the trade-offs of animals (Wilson et al., 2020). Finally, they choose to nest near the borderland.
The SEM results were similar to the results of Getis-Ord Gi* analysis, indicating that the nest site selection of wild boar approached the borderland but was away from settlements (Figure 3 and Table 1). However, wild boar made the choice to nest close to the borderland and therefore face to anthropogenic pressure and predation pressure (Yang et al., 2018b,2019). The borderland includes agricultural land, grassland and roads, as well as Amur tiger and leopard populations (Figure 3 and Table 1), which is in conflict with the habitat selection of wildlife. The indirect ecological effects of anthropogenic pressure on wildlife, such as farming, grazing and other related activities, are much more intensive than the direct effects of human presence on wildlife (Ripple and Beschta, 2004; Madin et al., 2010; Wilson et al., 2020). Negative effects of farming and grazing on wild animals have been demonstrated in several studies (Liu et al., 1999, 2007; Zhang et al., 2017). The negative impacts of roads on wildlife conservation have been proven in studies of snow leopard (Panthera uncial), brown bear, caribou (Rangifer tarandus), and many other wild animals (Leblond et al., 2013; Fedorca et al., 2019). Studies have indicated that animals are also active near agricultural land and roads, which require animals to be constantly attentive to spatiotemporal activity to avoid risk (Yang et al., 2019; Zhao et al., 2019; Wevers et al., 2020). For the free-living wild boar, nests are built to promote piglet survival during the first several days after birth (Fernández-Llario, 2004). Therefore, to understand why wild boars select nest sites near the borderland, we need to investigate not only the trade-offs made by the wild boar to achieve a higher survival rate or fitness but also the control strategies in the borderland, particularly in terms of their role in limiting anthropogenic disturbances (Vitkalova et al., 2018; Mazaris et al., 2019; Liu et al., 2020; Mason et al., 2020). For the wild boar, the construction of nests in protected places seems to be of great importance to reduce losses due to anthropogenic disturbances and predation (Spitz and Janeau, 1995; Fernández-Llario, 2004). A previous study in this area indicated that wild boars are active around human settlements due to the agricultural land that is usually situated near the settlements (Zhao et al., 2019). In this study, we took settlements and agricultural land in anthropogenic pressure, respectively, and found that wild boars preferred agricultural land and avoided settlements (Figure 3 and Table 1). Intensive anthropogenic disturbances are usually present around settlements, such as poaching, domestic dogs (hunting dogs), grazing, night lights, heavy traffic and other factors, which have adverse effects on wild boars (Liu et al., 1999, 2007; Leblond et al., 2013; Zhang et al., 2017; Fedorca et al., 2019). Agricultural land can indeed provide additional energy supplements to wild boars, especially in the breeding season (February to June) (Wu et al., 1999). Food during the breeding season is scarce, and female wild boars have to produce milk to feed piglets (Fernández-Llario, 2004). The remaining corn and soybean in agricultural land are very attractive to wild boars in this period. However, under intensive anthropogenic pressure, wild boars have to make a trade-off; when settlements and agricultural land exist in the same area, wild boars prefer to be far from both settlements and agricultural land (Figures 2, 3 and Table 1). In this study, wild boars chose only the agricultural land that was far away from settlements, which was usually located in the borderland (Figures 2, 3 and Table 1). Abundant vegetation cover is also very important for the nest selection of wild boars (Fernández-Llario, 2004). Thus, grasslands are not suitable areas for wild boar nests due to the high visibility of predators. In this study area, the main causes of grassland formation in the borderland include (1) logging, (2) cattle grazing and (3) fire barriers built by the government and troops. Logging has been banned for years, especially in the borderland (Wang et al., 2017, 2018). However, grazing is severe throughout the study area (Vitkalova et al., 2018; Yang et al., 2018b). The presence of cattle not only poses a threat to the young wild boar, such as by trampling and destroying wild boar nests, but also further reduces the limited food resources of wild boars and other ungulates. Therefore, wild boars select nest sites far from the grassland (Figure 3 and Table 1).
Borderlands are located in environmentally heterogeneous areas, such as mountainous terrain or other geologically complex landscapes. On the other hand, such areas are often located in politically sensitive areas and controlled by military limitations, while parts of the borderland are not controlled by militarily, public access is also off limits; thus, borderlands naturally support high richness and endemism, especially conservation-friendly borderlands (e.g., the borderland between China and Russia in this study) (Lee and Miller-Rushing, 2014; Wang et al., 2017; Vitkalova et al., 2018; Liu et al., 2020). In this study, the borderland is completely controlled by the military, and all activities in the borderland require military permission. However, there are usually settlements in the borderland for consolidating national defense, accompanied by agriculture-related activities, including farming and grazing. These activities are restricted within specific areas, and settling in the hinterland of the borderland is still prohibited; thus, the indirect and direct impacts of human activities on animals are reduced, which provides good nesting conditions and supplementary food for the wild boar. Roads (all dirt roads) near the borderland facilitate the military control of the area (e.g., patrolling), and residents are strictly restricted. The difference between the military and the residents is that the military strictly observes animal protection regulations and has a certain periodicity. Thus, the roads in the borderland have no significant negative impact on the wild boar, and the presence of a road reduces the movement resistance of wild animals. Due to mandatory control, wildlife has to make a trade-off between the different degrees of anthropogenic pressure, preferring the mandatory control area with relatively weak anthropogenic impacts (Vitkalova et al., 2018). Not only do wild boar tend to congregate in the borderland, but the Amur tiger, Amur leopard and Sika deer also show this trend (Wang et al., 2017; Yang et al., 2018b,2019).
The case study about the nest site selection of wild boar in this study area reveals that, under the existing wildlife protection and management strategies, the more important strategy may be the prohibition of direct anthropogenic persecution, rather than performing other human intervention activities to protect animals in an area. For example, the Yalu River demarcates the international border between China and North Korea on the northwestern fringes of the Korean Peninsula. Due to the political sensitivity of the area, public access is prohibited, and there are no human intervention activities. Although food resources are reduced, many birds still return to the area to inhabit (Zhang et al., 2018). The same situation has occurred in the demilitarized zone between North Korea and South Korea, a narrow strip with a width of approximately 4 km. More than 4,400 species have been recorded, 68 of which are endangered species in South Korea, including the Eurasian otter (Lutra Lutra) and Siberian musk deer (Moschus moschiferus). This area also provides an important wintering habitat for migratory birds (Higuchi and Pierre, 2005; Lee and Miller-Rushing, 2014). In other borderlands, the restoration of species has been promoted by controlling anthropogenic pressure; for example, at the borderland between China and Myanmar, the sun bear (Helarctos malayanus) was rediscovered after an absence of 45 years. In Sweden and Norway, gray wolves were considered functionally extinct by the late 1960s, and yet in recent decades, their populations have rapidly recovered and currently exceed 260 individuals (Boitani and Ciucci, 2009; Chapron et al., 2014; Li et al., 2017). These examples and our case study illustrated that, compared with the usual human intervention protection and management strategies, by controlling the activity authority of public in protected areas, the possibility of direct anthropogenic persecution is reduced, which would promote the survival of wildlife.
In fact, the growth of wild boars’ population is causing a global problem. But in our study area, wild boars, the main prey of tigers, have not caused a great deal of trouble. In addition, the population size of wild boar is crucial to the spread of the Amur tiger in China. At present, the population of the Amur tiger in China is gradually recovering, so we should build suitable habitat for the Amur tiger from the bottom-up, including the protection of key prey, to prevent the decline of the Amur tiger population due to the decline of prey population. During the recovery process, the role of the Amur tiger as the top predator in the ecosystem would be expected to gradually emerge, and the community will gradually stabilize. The problems that follow are unforeseeable. An increase of wild boar and tiger numbers may lead to an increase of conflicts with humans because of damages to human activities, in absence of adequate measures to prevent/mitigate crop damage and livestock depredation. This could put more strain on conservation efforts. The park has taken some preventive measures, such as adding protective fences to farming areas, gradually relocating grazing areas, and providing proper economic compensation and income transformation measures for residents. The implementation of these measures provides a favorable guarantee for the protection of animals, particularly when top predator habitats are still vulnerable.
We compare the anthropogenic pressure distribution in the borderland between China and Russia with the response of the wild boar to anthropogenic pressure. We suspect that wild boars’ nest site selection strategy is affected by the military control that prohibits the anthropogenic persecution of animals, which changes the trade-off strategy of wild boar when selecting nest sites. Compared with existing measures, we suggest a different protection/wildlife management strategy, what we need to do may be to prohibit anthropogenic persecution rather than perform other human interventions to protect animals. However, for a species with trouble potential, we need to base our conservation strategies on the recovery of top predators, and play the community control role of top predators to avoid the occurrence of trouble.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because this research has used non-invasive field methods of wild animals. We guarantee that this research is carried out in accordance with international, national and institutional rules, taking into account the rights of animals to be free from harm, to live freely, to be free from fear and discomfort, and to biodiversity.
HY, LF, and JG conceived the ideas and designed the methodology. YF, MT, YG, and GZ collected the data. YF analyzed the data. YF and HY led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
This work was supported by the National Scientific and Technical Foundation Project of China (Grant Numbers 2012FY112000, 2019FY101700), the National Natural Science Foundation of China (Grant Number 31200410), and Cyrus Tang Foundation (Grant Number 2016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge the Chinese National Forestry and Grassland Administration, Northeast Tiger and Leopard National Park, all rangers with the field work for their assistance in field sampling, measurements, and data input. We also thank the editors, reviewers, and Jiajia Liu for their helpful comments on a draft manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.820915/full#supplementary-material
Alexander, N. S., Massei, G., and Wint, W. J. O. H. D. (2016). The European distribution of Sus scrofa. model outputs from the project described within the poster Where are All the Boars? An attempt to gain a continental perspective. Open Health Data 4:e1.
Amici, A., Serrani, F., Rossi, C. M., and Primi, R. (2012). Increase in crop damage caused by wild boar (Sus scrofa L.): the “refuge effect”. Agron. Sustain. Dev. 32, 683–692. doi: 10.1007/s13593-011-0057-6
Boitani, L., and Ciucci, P. (2009). “Wolf management across Europe: species conservation without boundaries,” in A New Era for Wolves and People: Wolf Recovery, Human Attitudes, and Policy, eds L. Boitani, M. Musiani, and P. C. Paquet (Calgary: University of Calgary Press), 15–40.
Chapron, G., Kaczensky, P., Linnell, J. D. C., von Arx, M., Huber, D., Andren, H., et al. (2014). Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519. doi: 10.1126/science.1257553
Dellmeier, G. R., and Friend, T. H. (1991). Behavior and extensive management of domestic sows (Sus scrofa) and litters. Appl. Anim. Behav. Sci. 29, 327–341. doi: 10.1016/0168-1591(91)90258-y
D’Eon, R. G., and Glenn, S. M. (2005). The influence of forest harvesting on landscape spatial patterns and old-growth-forest fragmentation in southeast British Columbia. Landsc. Ecol. 20, 19–33. doi: 10.1007/s10980-004-0286-z
Fedorca, A., Russo, I.-R. M., Ionescu, O., Ionescu, G., Popa, M., Fedorca, M., et al. (2019). Inferring fine-scale spatial structure of the brown bear (Ursus arctos) population in the Carpathians prior to infrastructure development. Sci. Rep. 9:9494. doi: 10.1038/s41598-019-45999-y
Fernández-Llario, P. (2004). Environmental correlates of nest site selection by wild boar Sus scrofa. Acta Theriol. 49, 383–392. doi: 10.1007/bf03192536
Flesch, A. D., Epps, C. W., Cain, J. W., Clark, M., Krausman, P. R., and Morgart, J. R. (2010). Potential effects of the United States-Mexico border fence on wildlife. Conserv. Biol. 24, 171–181. doi: 10.1111/j.1523-1739.2009.01277.x
Fonji, S. F., and Taff, G. N. (2014). Using satellite data to monitor land-use land-cover change in North-eastern Latvia. Springerplus 3:61. doi: 10.1186/2193-1801-3-61
Gaynor, K. M., Hojnowski, C. E., Carter, N. H., and Brashares, J. S. (2018). The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235. doi: 10.1126/science.aar7121
Getis, A., and Ord, J. K. (1992). The analysis of spatial association by use of distance statistics. Geogr. Anal. 24, 189–206. doi: 10.1111/j.1538-4632.1992.tb00261.x
Halpern, B. S., Frazier, M., Afflerbach, J., Lowndes, J. S., Micheli, F., O’Hara, C., et al. (2019). Recent pace of change in human impact on the world’s ocean. Sci. Rep. 9:11609. doi: 10.1038/s41598-019-47201-9
Hanski, I. (2005). Landscape fragmentation, biodiversity loss and the societal response: the longterm consequences of our use of natural resources may be surprising and unpleasant. EMBO Rep. 6, 388–392. doi: 10.1038/sj.embor.7400398
Hearn, A. J., Ross, J., Macdonald, D. W., Bolongon, G., Cheyne, S. M., Mohamed, A., et al. (2016). Predicted distribution of the Sunda clouded leopard Neofelis diardi (Mammalia: Carnivora: Felidae) on Borneo. Raffles Bull. Zool. 33, 149–156.
Higuchi, H., and Pierre, J. P. (2005). Satellite tracking and avian conservation in Asia. Landsc. Ecol. Eng. 1, 33–42. doi: 10.1007/s11355-005-0002-4
Holbrook, J. D., Olson, L. E., DeCesare, N. J., Hebblewhite, M., Squires, J. R., and Steenweg, R. (2019). Functional responses in habitat selection: clarifying hypotheses and interpretations. Ecol. Appl. 29:e01852. doi: 10.1002/eap.1852
Hosseini, M., Farashi, A., Khani, A., and Farhadinia, M. S. (2019). Landscape connectivity for mammalian megafauna along the Iran-Turkmenistan-Afghanistan borderland. J. Nat. Conserv. 52:125735. doi: 10.1016/j.jnc.2019.125735
Jensen, P. (1989). Nest site choice and nest building of free-ranging domestic pigs due to farrow. Appl. Anim. Behav. Sci. 22, 13–21. doi: 10.1016/0168-1591(89)90076-2
Jun, C., Ban, Y., and Li, S. (2014). Open access to Earth land-cover map. Nature 514, 434–434. doi: 10.1038/514434c
Laguna, E., Barasona, J. A., Vicente, J., Keuling, O., and Acevedo, P. (2021a). Differences in wild boar spatial behaviour among land uses and management scenarios in Mediterranean ecosystems. Sci. Total Environ. 796:148966. doi: 10.1016/j.scitotenv.2021.148966
Laguna, E., Carpio, A. J., Vicente, J., Barasona, J. A., Triguero-Ocana, R., Jimenez-Ruiz, S., et al. (2021b). The spatial ecology of red deer under different land use and management scenarios: protected areas, mixed farms and fenced hunting estates. Sci. Total Environ. 786:147124. doi: 10.1016/j.scitotenv.2021.147124
Leblond, M., Dussault, C., and Ouellet, J. P. (2013). Avoidance of roads by large herbivores and its relation to disturbance intensity. J. Zool. 289, 32–40. doi: 10.1111/j.1469-7998.2012.00959.x
Leclerc, M., Frank, S. C., Zedrosser, A., Swenson, J. E., and Pelletier, F. (2017). Hunting promotes spatial reorganization and sexually selected infanticide. Sci. Rep. 7:45222. doi: 10.1038/srep45222
Lee, S.-D., and Miller-Rushing, A. J. (2014). Degradation, urbanization, and restoration: a review of the challenges and future of conservation on the Korean Peninsula. Biol. Conserv. 176, 262–276. doi: 10.1016/j.biocon.2014.05.010
Li, F., Zheng, X., Jiang, X.-L., and Chan, B. P. L. (2017). Rediscovery of the sun bear (Helarctos malayanus) in Yingjiang County, Yunnan Province, China. Zool. Res. 38, 206–207. doi: 10.24272/j.issn.2095-8137.2017.044
Liu, J., Yong, D. L., Choi, C.-Y., and Gibson, L. (2020). Transboundary frontiers: an emerging priority for biodiversity conservation. Trends Ecol. Evol. 35, 679–690. doi: 10.1016/j.tree.2020.03.004
Liu, J. G., Dietz, T., Carpenter, S. R., Alberti, M., Folke, C., Moran, E., et al. (2007). Complexity of coupled human and natural systems. Science 317, 1513–1516. doi: 10.1126/science.1144004
Liu, J. G., Li, S. X., Ouyang, Z. Y., Tam, C., and Chen, X. D. (2008). Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. U.S.A. 105, 9477–9482. doi: 10.1073/pnas.0706436105
Liu, J. G., Ouyang, Z., Taylor, W. W., Groop, R., Tan, K. C., and Zhang, H. M. (1999). A framework for evaluating the effects of human factors on wildlife habitat: the case of giant pandas. Conserv. Biol. 13, 1360–1370. doi: 10.1046/j.1523-1739.1999.98418.x
Llaneza, L., Lopez-Bao, J. V., and Sazatornil, V. (2012). Insights into wolf presence in human-dominated landscapes: the relative role of food availability, humans and landscape attributes. Divers. Distrib. 18, 459–469. doi: 10.1111/j.1472-4642.2011.00869.x
Luskin, M. S., Brashares, J. S., Ickes, K., Sun, I. F., Fletcher, C., Wright, S. J., et al. (2017). Cross-boundary subsidy cascades from oil palm degrade distant tropical forests. Nat. Commun. 8:2231. doi: 10.1038/s41467-017-01920-7
Macdonald, D. W., Chiaverini, L., Bothwell, H. M., Kaszta, Z., Ash, E., Bolongon, G., et al. (2020). Predicting biodiversity richness in rapidly changing landscapes: climate, low human pressure or protection as salvation? Biodivers. Conserv. 29, 4035–4057. doi: 10.1007/s10531-020-02062-x
Madin, E. M. P., Gaines, S. D., Madin, J. S., and Warner, R. R. (2010). Fishing indirectly structures macroalgal assemblages by altering herbivore behavior. Am. Nat. 176, 785–801. doi: 10.1086/657039
Manandhar, R., Odeh, I. O. A., and Ancev, T. (2009). Improving the accuracy of land use and land cover classification of Landsat data using post-classification enhancement. Remote Sens. 1, 330–344. doi: 10.3390/rs1030330
Mason, N., Ward, M., Watson, J. E. M., Venter, O., and Runting, R. K. (2020). Global opportunities and challenges for transboundary conservation. Nat. Ecol. Evol. 4, 694–701. doi: 10.1038/s41559-020-1160-3
Mazaris, A. D., Kallimanis, A., Gissi, E., Pipitone, C., Danovaro, R., Claudet, J., et al. (2019). Threats to marine biodiversity in European protected areas. Sci. Total Environ. 677, 418–426. doi: 10.1016/j.scitotenv.2019.04.333
Medland, S., Shaker, R., Forsythe, K., Mackay, B., and Rybarczyk, G. (2020). A multi-criteria wetland suitability index for restoration across Ontario’s Mixedwood Plains. Sustainability 12:9953. doi: 10.3390/su12239953
Monk, C. T., Barbier, M., Romanczuk, P., Watson, J. R., Alos, J., Nakayama, S., et al. (2018). How ecology shapes exploitation: a framework to predict the behavioural response of human and animal foragers along exploration-exploitation trade-offs. Ecol. Lett. 21, 779–793. doi: 10.1111/ele.12949
Peters, R., Ripple, W. J., Wolf, C., Moskwik, M., Carreon-Arroyo, G., Ceballos, G., et al. (2018). Nature divided, scientists united: US-Mexico border wall threatens biodiversity and binational conservation. Bioscience 68, 740–743. doi: 10.1093/biosci/biy063
Pokorny, B., Flajsman, K., Centore, L., Krope, F. S., and Sprem, N. (2017). Border fence: a new ecological obstacle for wildlife in Southeast Europe. Eur. J. Wildl. Res. 63, 1–6. doi: 10.1007/s10344-016-1074-1
Psaralexi, M. K., Votsi, N.-E. P., Selva, N., Mazaris, A. D., and Pantis, J. D. (2017). Importance of roadless areas for the European Conservation Network. Front. Ecol. Evol. 5:2. doi: 10.3389/fevo.2017.00002
R Development Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.r-project.org/
Recio, M. R., Knauer, F., Molinari-Jobin, A., Huber, D., Filacorda, S., and Jerina, K. (2021). Context-dependent behaviour and connectivity of recolonizing brown bear populations identify transboundary conservation challenges in Central Europe. Anim. Conserv. 24, 73–83. doi: 10.1111/acv.12624
Reinhardt, I., Kluth, G., Nowak, C., Szentiks, C. A., Krone, O., Ansorge, H., et al. (2019). Military training areas facilitate the recolonization of wolves in Germany. Conserv. Lett. 12:e12635. doi: 10.1111/conl.12635
Ripple, W. J., and Beschta, R. L. (2004). Wolves and the ecology of fear: Can predation risk structure ecosystems? Bioscience 54, 755–766.
Samia, D. S. M., Bessa, E., Blumstein, D. T., Nunes, J. A. C. C., Azzurro, E., Morroni, L., et al. (2019). A meta-analysis of fish behavioural reaction to underwater human presence. Fish Fish. 20, 817–829. doi: 10.1111/faf.12378
Santos, A. M. C., Cianciaruso, M. V., Barbosa, A. M., Bini, L. M., Diniz-Filho, J. A. F., Faleiro, F. V., et al. (2020). Current climate, but also long-term climate changes and human impacts, determine the geographic distribution of European mammal diversity. Glob. Ecol. Biogeogr. 29, 1758–1769. doi: 10.1111/geb.13148
Schlaepfer, M. A., Runge, M. C., and Sherman, P. W. (2002). Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. doi: 10.1016/S0169-5347(02)02580-6
Sobral, M., Silvius, K. M., Overman, H., Oliveira, L. F. B., Raab, T. K., and Fragoso, J. M. V. (2017). Mammal diversity influences the carbon cycle through trophic interactions in the Amazon. Nat. Ecol. Evol. 1, 1670–1676. doi: 10.1038/s41559-017-0334-0
Spitz, F., and Janeau, G. (1995). Daily selection of habitat in wild boar (Sus scrofa). J. Zool. 237, 423–434. doi: 10.1111/j.1469-7998.1995.tb02772.x
Stolba, A., and Woodgush, D. G. M. (1984). The identification of behavioural key features and their incorporation into a housing design for pigs. Ann. Rech. Vet. 15, 287–302.
Suraci, J. P., Clinchy, M., Zanette, L. Y., and Wilmers, C. C. (2019). Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol. Lett. 22, 1578–1586. doi: 10.1111/ele.13344
Tobler, W. R. (1970). A Computer movie simulating urban growth in the detroit region. Econ. Geogr. 46, 234–240.
Venter, O., Sanderson, E. W., Magrach, A., Allan, J. R., Beher, J., Jones, K. R., et al. (2016). Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7:12558. doi: 10.1038/ncomms12558
Vitkalova, A. V., Feng, L., Rybin, A. N., Gerber, B. D., Miquelle, D. G., Wang, T., et al. (2018). Transboundary cooperation improves endangered species monitoring and conservation actions: a case study of the global population of Amur leopards. Conserv. Lett. 11:e12574. doi: 10.1111/conl.12574
Wang, T., Feng, L., Mou, P., Wu, J., Smith, J. L. D., Xiao, W., et al. (2016). Amur tigers and leopards returning to China: direct evidence and a landscape conservation plan. Landsc. Ecol. 31, 491–503. doi: 10.1007/s10980-015-0278-1
Wang, T., Feng, L., Yang, H., Han, B., Zhao, Y., Juan, L., et al. (2017). A science-based approach to guide Amur leopard recovery in China. Biol. Conserv. 210, 47–55. doi: 10.1016/j.biocon.2016.03.014
Wang, T., Royle, J. A., Smith, J. L. D., Zou, L., Lu, X., Li, T., et al. (2018). Living on the edge: opportunities for Amur tiger recovery in China. Biol. Conserv. 217, 269–279. doi: 10.1016/j.biocon.2017.11.008
Watson, J. E. M., Shanahan, D. F., Di Marco, M., Allan, J., Laurance, W. F., Sanderson, E. W., et al. (2016). Catastrophic declines in wilderness areas undermine global environment targets. Curr. Biol. 26, 2929–2934. doi: 10.1016/j.cub.2016.08.049
Wevers, J., Fattebert, J., Casaer, J., Artois, T., and Beenaerts, N. (2020). Trading fear for food in the Anthropocene: how ungulates cope with human disturbance in a multi-use, suburban ecosystem. Sci. Total Environ. 741:140369. doi: 10.1016/j.scitotenv.2020.140369
Wilson, M. W., Ridlon, A. D., Gaynor, K. M., Gaines, S. D., Stier, A. C., and Halpern, B. S. (2020). Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 23, 1522–1536. doi: 10.1111/ele.13571
Wong, D. W. S., and Lee, J. (2005). Statistical Analysis of Geographic Information with ArcView GIS and ArcGIS. Hoboken, NJ: John Wiley.
Wu, S., Chen, H., and Cai, X. (1999). Preliminary study on the population structures and reproductive habit in wild boar(Sus scrofe) in Dawuling Natural Reserve. Acta Theriol. Sin. 20, 151–156.
Yang, H., Dou, H., Baniya, R. K., Han, S., Guan, Y., Xie, B., et al. (2018a). Seasonal food habits and prey selection of Amur tigers and Amur leopards in Northeast China. Sci. Rep. 8:6930. doi: 10.1038/s41598-018-25275-1
Yang, H., Xie, B., Han, S., Wang, T., and Feng, L. (2018b). Seasonal spatial pattern of abundance in manchurian sika deer and influcing factors in Hunchun National Nature Reserve, Jilin Province. J. Beijing Norm. Univ. 4, 498–505. doi: 10.16360/j.cnki.jbnuns.2018.04.012
Yang, H., Han, S., Xie, B., Mou, P., Kou, X., Wang, T., et al. (2019). Do prey availability, human disturbance and habitat structure drive the daily activity patterns of Amur tigers (Panthera tigris altaica)? J. Zool. 307, 131–140. doi: 10.1111/jzo.12622
Yang, W., Liu, W., Vina, A., Luo, J. Y., He, G. M., Ouyang, Z. Y., et al. (2013). Performance and prospects of payments for ecosystem services programs: evidence from China. J. Environ. Manage. 127, 86–95. doi: 10.1016/j.jenvman.2013.04.019
Zhang, M., Gouveia, A., Qin, T., Quan, R., and Nijman, V. (2017). Illegal pangolin trade in northernmost Myanmar and its links to India and China. Glob. Ecol. Conserv. 10, 23–31. doi: 10.1016/j.gecco.2017.01.006
Zhang, S.-D., Ma, Z., Choi, C.-Y., Peng, H.-B., Bai, Q.-Q., Liu, W.-L., et al. (2018). Persistent use of a shorebird staging site in the Yellow Sea despite severe declines in food resources implies a lack of alternatives. Bird Conserv. Int. 28, 534–548. doi: 10.1017/s0959270917000430
Keywords: anthropogenic pressure, borderland, China, military control, protection strategy, wild boar
Citation: Fu Y, Tan M, Gong Y, Zhao G, Ge J, Yang H and Feng L (2022) Wild Boar Survives in a Landscape That Prohibits Anthropogenic Persecution. Front. Ecol. Evol. 10:820915. doi: 10.3389/fevo.2022.820915
Received: 23 November 2021; Accepted: 21 February 2022;
Published: 14 March 2022.
Edited by:
Emmanuel Serrano Ferron, Universitat Autònoma de Barcelona, SpainReviewed by:
Francesco Ferretti, University of Siena, ItalyCopyright © 2022 Fu, Tan, Gong, Zhao, Ge, Yang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Yang, eWh0OTBoQGJudS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.