
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol., 08 February 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.810156
This article is part of the Research TopicAdvances in EcoacousticsView all 12 articles
 Miles J. G. Parsons1*
Miles J. G. Parsons1* Tzu-Hao Lin2
Tzu-Hao Lin2 T. Aran Mooney3
T. Aran Mooney3 Christine Erbe4
Christine Erbe4 Francis Juanes5
Francis Juanes5 Marc Lammers6
Marc Lammers6 Songhai Li7
Songhai Li7 Simon Linke8
Simon Linke8 Audrey Looby9,10
Audrey Looby9,10 Sophie L. Nedelec11
Sophie L. Nedelec11 Ilse Van Opzeeland12,13
Ilse Van Opzeeland12,13 Craig Radford14
Craig Radford14 Aaron N. Rice15
Aaron N. Rice15 Laela Sayigh3,16
Laela Sayigh3,16 Jenni Stanley17
Jenni Stanley17 Edward Urban18
Edward Urban18 Lucia Di Iorio19,20
Lucia Di Iorio19,20Aquatic environments encompass the world’s most extensive habitats, rich with sounds produced by a diversity of animals. Passive acoustic monitoring (PAM) is an increasingly accessible remote sensing technology that uses hydrophones to listen to the underwater world and represents an unprecedented, non-invasive method to monitor underwater environments. This information can assist in the delineation of biologically important areas via detection of sound-producing species or characterization of ecosystem type and condition, inferred from the acoustic properties of the local soundscape. At a time when worldwide biodiversity is in significant decline and underwater soundscapes are being altered as a result of anthropogenic impacts, there is a need to document, quantify, and understand biotic sound sources–potentially before they disappear. A significant step toward these goals is the development of a web-based, open-access platform that provides: (1) a reference library of known and unknown biological sound sources (by integrating and expanding existing libraries around the world); (2) a data repository portal for annotated and unannotated audio recordings of single sources and of soundscapes; (3) a training platform for artificial intelligence algorithms for signal detection and classification; and (4) a citizen science-based application for public users. Although individually, these resources are often met on regional and taxa-specific scales, many are not sustained and, collectively, an enduring global database with an integrated platform has not been realized. We discuss the benefits such a program can provide, previous calls for global data-sharing and reference libraries, and the challenges that need to be overcome to bring together bio- and ecoacousticians, bioinformaticians, propagation experts, web engineers, and signal processing specialists (e.g., artificial intelligence) with the necessary support and funding to build a sustainable and scalable platform that could address the needs of all contributors and stakeholders into the future.
Aquatic (i.e., marine, brackish, and freshwater) environments encompass the world’s most extensive habitats, rich with sounds produced by a diverse range of animals. Advances in data acquisition, storage and processing that enable increased recording durations at reduced costs, and easier logistics of sensor deployment and retrieval, have made passive acoustic monitoring (PAM) a more accessible and feasible tool than ever before (Lindseth and Lobel, 2018; Chapuis et al., 2021; Wall et al., 2021). Combined with an increasing appreciation of the ecological importance of acoustic cues to almost all aquatic fauna, these advances have expanded the field of underwater bioacoustic and ecoacoustic research to increasing numbers of researchers and organizations (Lindseth and Lobel, 2018). The result has been an almost exponential increase in the volume of aquatic PAM data being collected around the world, in conjunction with increases in soundscape research (Lindseth and Lobel, 2018; Mooney et al., 2020; Duarte et al., 2021). Researchers now routinely collect substantially more PAM data, on an increasing number of taxa, and in more locations than ever before, from freshwater to marine, from shallow waters to the deep, and from tropical to polar regions (Wall et al., 2021). Higher sampling frequencies and longer deployment durations mean that datasets may now easily exceed terabytes in size and years in duration, potentially containing millions of sounds and hundreds of different types (Waddell et al., 2021; Wall et al., 2021). This makes manual classification of underwater sounds by experts—the traditional method of verifying call presence and source identification—increasingly difficult (Mooney et al., 2020; Waddell et al., 2021).
PAM is already used for a multitude of biological applications. Examples include monitoring, characterizing and delineating underwater soundscapes, and investigating aquatic communities (e.g., Desjonquères et al., 2015; Erbe et al., 2015; Menze et al., 2017; Mooney et al., 2020; Stanley et al., 2021); documenting the distribution and migration patterns of the great whales (e.g., Risch et al., 2014; Tsujii et al., 2016; Davis et al., 2020; Warren et al., 2021); characterizing the spatial and temporal responses of fish choruses to environmental drivers like temperature, salinity, lunar phase, tide, and time of sunset (e.g., Barrios, 2004; Rountree et al., 2006; Parsons, 2010; Straight et al., 2015; Rice et al., 2016; McWilliam et al., 2017; Parsons et al., 2016; Karaconstantis et al., 2020; Linke et al., 2020); understanding how animals change their behavior and distribution in response to climate change (Gordon et al., 2018), anthropogenic noise sources (e.g., Thompson et al., 2013; Cerchio et al., 2014; Erbe et al., 2019; Meekan et al., 2021), algal blooms (e.g., Rycyk et al., 2020) and extreme weather events like hurricanes (e.g., Locascio and Mann, 2005; Fandel et al., 2020; Boyd et al., 2021; Schall et al., 2021); understanding how prey change their sound production rates or behaviors with the presence of predators (e.g., Luczkovich and Keusenkothen, 2007; Hughes et al., 2014; Bailey et al., 2019; Burnham and Duffus, 2019); and how noise and propagation conditions can affect communication spaces (e.g., Alves et al., 2016; McKenna et al., 2021). This wide range of uses for PAM is expanding with developments in technology, providing a great volume of easily accessible data on aquatic life.
With an increase in the use of PAM, there is increasing awareness of the impacts the acoustic environment (i.e., frequency-dependent propagation loss) can have on characteristics of recorded sound. For example, the same humpback whale song may produce different received spectra in two spatially separated recordings, depending on propagation conditions, and appear as two different types of calls. Sound production mechanisms (e.g., directionality of the source signal) can also influence recorded sound characteristics, such as the azimuth-dependent received spectra of some odontocete calls (Lammers and Au, 2003). Together with the impact of signal-to-noise ratio (SNR) on the clarity of a sound sample, these factors all affect the reproducibility of signals, which needs to be considered when assessing the sound samples provided to, and by, a sound library.
Underpinning much of this work is the ability to identify or characterize sound sources either to assess them individually or understand their contribution to the overall soundscape (Mooney et al., 2020; McKenna et al., 2021). We are beginning to understand how these biological sounds, together with anthropogenic and geophysical sounds that make up the local soundscape (Schafer, 1969, 1977; Southworth, 1969; Krause, 2008; Hildebrand, 2009), can collectively provide information on physical habitats, biodiversity, and aquatic ecosystem health (Mooney et al., 2020). PAM is proving to be one of the most effective ways to monitor visually elusive but vocal species in aquatic environments, which can potentially aid in more effective conservation management, such as spatio-temporal zoning measures found in marine park areas or fishery closures (Coquereau et al., 2017; Nikolich et al., 2021). At a time when global biodiversity is in significant decline (Sala and Knowlton, 2006; Worm et al., 2006; Marques, 2020) and increasingly impacted by climate change (e.g., Poloczanska et al., 2013; Sydeman et al., 2015), there is a need to document and understand as many sound sources in the ocean as possible, potentially before they disappear.
There are 126 marine mammal species, approximately 35,000 known species of fish, and nearly 250,000 documented species of marine invertebrates in the world (Froese and Pauly, 2021; World Register of Marine Species, 2021), and the number of known soniferous (actively sound-producing) species underwater is consistently increasing (see Figure 1 for spectrograms of example sounds). There are even a handful of reports of sound produced by birds underwater (e.g., Thiebault et al., 2019). It is thought that all aquatic mammal species exhibit soniferous behavior underwater and reports have so far confirmed this trait for almost all of them (e.g., Mellinger and Clark, 2006; Richardson et al., 2013). Calls of many marine mammal species are often distinctive and can even show significant variability among individuals (e.g., Janik and Sayigh, 2013; McCordic et al., 2016; Bailey et al., 2021). Additionally, as comparatively large and charismatic species, mammals can often be verified as the source of a sound with nearby surface sightings or from studies of animals in human care (e.g., Rogers et al., 1996).
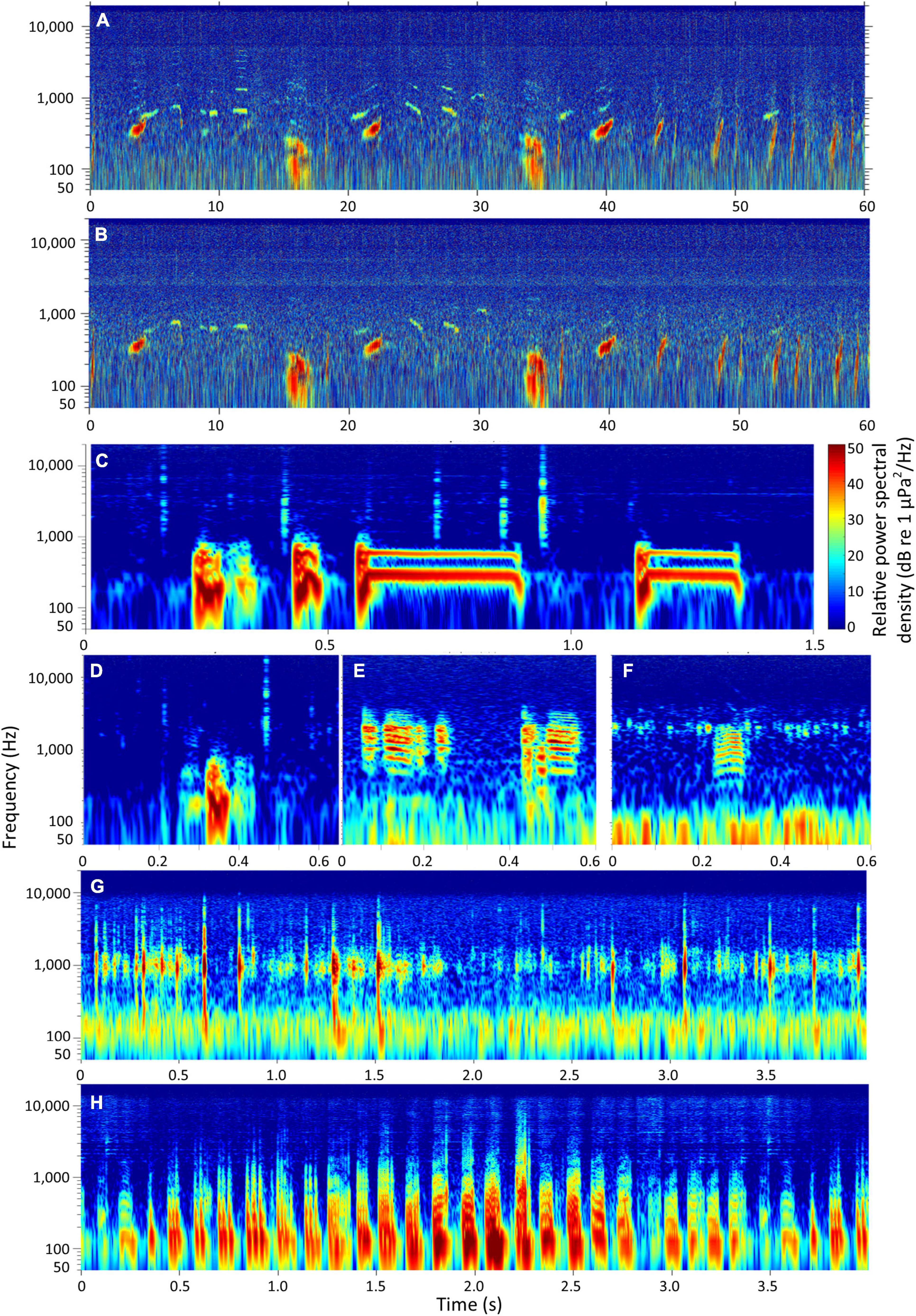
Figure 1. Example spectrograms produced (1,024 point-long Hanning window, 0.9 overlap, frequency display 50–20,000 Hz, relative received levels) from: two simultaneous recordings of a humpback whale (Megaptera novaeangliae) song in (A) 20 m and (B) 40 m depth waters off Okinawa, Japan, (recording locations separated by ≈500 m, note the lack of high-frequency energy in B); (C) a complex call and (D) a single grunt sound from gulf toadfish (Opsanus beta); (E) two sounds from a 20–30 cm-long sooty grunter (Hephaestus fuliginosus); (F) one sound from a 7 cm-long spangled grunter (Leiopotherapon unicolor); (G) 4 s of sounds made a crawling kina urchin (Evechinus chloroticus) and (H) 4 s of sounds produced by a New Zealand paddle crab (Ovalipes catharus). Power spectral density axes in each spectrogram are relative and span 50 dB re 1 μPa2/Hz. Spectrograms are for comparative purposes and, as such, recording conditions and methods are not provided. All recordings sampled at 44.1 ksps except that producing panel (E), which was sampled at 48 ksps.
Overall, validated sounds have been attributed to a much lower proportion of species from the speciose groups of aquatic invertebrates and fishes, than for marine mammals. Whereas almost all marine mammals are confirmed to produce sounds underwater, this behavior has been validated for fewer than 100 species of aquatic invertebrates (e.g., Popper et al., 2001; Coquereau et al., 2016) and approximately 1,000 fish species (Kaatz, 2002; Parmentier et al., 2017; Bolgan et al., 2020a; Looby et al., 2021; Rice et al., 2022); however, the former includes members of Alpheidae, the “snapping shrimp” family with over 500 species and the latter represents over two-thirds of fish families, implying many more species are soniferous (Parmentier et al., 2021). Fishes and invertebrates are typically more difficult to validate in the field than mammals (e.g., Sprague and Luczkovich, 2001; Riera et al., 2017), though visual confirmation is on occasion achieved (e.g., Lobel, 1992, 1996, 1998, 2001; Allen and Demer, 2003; Lobel et al., 2010; Parsons et al., 2013a) or inferred by weight-of-evidence from the species present at the time of recording and their behavior (e.g., Tricas and Boyle, 2014; Pyć et al., 2021), or by localization (e.g., Parsons et al., 2009; Mouy et al., 2018). Sound travels much farther than light underwater and efficiently through turbid waters that often prohibit visual source validation more than a few meters from an observer or camera, or even ranges of centimeters in turbid environments (Harvey et al., 2004; Jones et al., 2019). This is particularly problematic when the source in question is “small and cryptic,” found within an assemblage of several species’, at great depth, or within complex habitat. Moreover, many fish and invertebrate species are predominantly nocturnal, rendering simultaneous visual and audio observations arduous or impossible (e.g., Spence, 2017). Thus, while some sources have been confirmed, the majority of fish and invertebrate sounds and choruses remain anonymous, uncharacterized and largely unreported, as they do not comprise sounds of a project’s target species. Recordings taken under controlled conditions (e.g., within tanks or aquaria) can provide confirmation of species’ sound production (e.g., Sprague and Luczkovich, 2001) as well as other information on sound-producing behavior that could be challenging to collect in the field (e.g., Montie et al., 2017; Riera et al., 2018); however, assessment of the acoustic characteristics and behavioral context of these sounds requires additional consideration. The material, dimensions and background noise within a constrained environment, for example, affect the received signal (e.g., Akamatsu et al., 2002). Additionally, soniferous behavior may be affected by captivity, such as the acclimation time, surroundings and number of other individuals within the environment, among other factors (e.g., Holt and Johnston, 2014). The nature and extent of the effects of captivity has on recorded sounds and overall acoustic behavior may vary between species and potentially even individuals (e.g., Bolgan et al., 2020b,c).
Although substantial work has been conducted on freshwater species, predominantly on fishes and initially in aquaria (Gerald, 1971; Desjonquères et al., 2020; Grabowski et al., 2020; Linke et al., 2020; Roca et al., 2020; Rountree and Juanes, 2020; Higgs and Beach, 2021), the majority of efforts to record aquatic biological sounds have historically focused on the marine environment (Greenhalgh et al., 2020). Freshwater recordings present a variety of complexities that are less common in the marine environment, such as terrestrial, aerial and water-surface sounds from birds, insects, and road or air traffic (Erbe et al., 2018; Linke et al., 2020; Rountree et al., 2020; Leon-Lopez et al., 2021).
In addition to the difficulties in identifying soniferous species, there is also potential variability in sound types and characteristics of sound production for a given species (e.g., McIver et al., 2014; Parsons and McCauley, 2017; Bolgan et al., 2020c). There are very few species where the entire suite of calls has been captured and even at a single location, full repertoires are rarely confirmed or reported. Further, numerous taxa are cosmopolitan, either as wide-roaming individuals, such as the great whales, or as broadly distributed species, such as many fishes. Some of these global (and regional) travelers exhibit dialects, or completely different signal structures among regions, several of which evolve over time (e.g., Parmentier et al., 2005; Garland et al., 2011; Figure 1).
Alongside active sound production for the purported purpose of communication, many aquatic species produce “passive sounds” as a by-product of other life-functions, such as eating, swimming, and crawling (e.g., Fish, 1948; Moulton, 1958, 1960, 1963, 1964; Uno and Konagaya, 1960; Mallekh et al., 2003; Radford et al., 2008; Rountree et al., 2018; Ajemian et al., 2021; Tricas and Boyle, 2021; Figure 1). These passive sounds may be less acoustically complex or distinct than active sounds; however, they still provide important contributions to the soundscape and have demonstrated ecological signal potential in select circumstances (Banner, 1972; Connor et al., 2000; Tricas and Boyle, 2014; Rountree et al., 2018). Thus, while collating global records of known sound production may be feasible to accomplish (e.g., for fishes; Looby et al., 2021), because of the variation in sound within and among species and individuals, the effort required to collect and maintain representative sounds for every species is a continuous and laborious process. Further, even when unidentifiable biological sounds are described in detail, there remains no global system with which to attempt to characterize or identify them (Anderson et al., 2008; Rountree et al., 2020).
Although some of these sources are obvious, pervasive, and readily observed in long-term recordings, many more are rare and of lower amplitude, often going undetected unless the observer is specifically searching for them (Mooney et al., 2020). Many studies are conducted with single- or limited-species objectives, although these recordings are often filled with a great diversity of sounds. Collectively there are now multi-millions of recording hours around the world that could potentially be assessed for a plethora of both known and, to date, unidentified biological sounds. Only recently have studies begun to address the groupings of such sounds, in the field of acoustic community ecology (Desiderà et al., 2019; Bolgan et al., 2020a; Di Iorio et al., 2021).
The provision of audio samples is an important activity as it is often difficult for a researcher to confirm that a sound they have recorded is the same as one that has been previously identified, based on a description in a journal or website. This is particularly true if the two were recorded under different environmental conditions. A library provides first-hand examples for comparison, preferably with a spectrogram that has clear annotations describing the specific time and frequency range of the target signals, along with sufficient metadata to facilitate comparison between user and library samples, to maximize the use of the library. The audio-visual combination provides the user with a good understanding of the call type (under the recorded conditions). This combination can be particularly important for high-biodiversity systems such as coral reefs, where even a short recording can pick up multiple animal sounds.
Several independent libraries of biological sounds, many of which either contain aquatic examples, or have an underwater focus, have been established around the world (see Table 1 for selected examples). Existing libraries often focus on species of interest that are targeted by the host institute’s researchers and are often recorded from a particular phylum or more restricted taxon, with a smaller selection of opportunistically recorded species. A few libraries describe many known sound sources from a region as the basis for an article describing reported species distribution in the region, including standardized characteristics of each sound type for the species, with a link to a website where the sounds can be downloaded (e.g., Erbe et al., 2017). Other libraries are national and may be incrementally expanded by contributions of a handful of researchers with associated papers outlining sounds as they are observed (Table 1). The FishSounds website project, for example, began with a systematized, global review of fish species examined for sound production (with or without documented sonifery) in the peer-reviewed and gray literature, which is now being expanded to include representative recordings of fish sounds contributed by researcher donations of known and unknown fish species (Looby et al., 2021). This is a significant step; however, this library currently only accepts recordings of fish sounds that can be associated with some form of published reference.
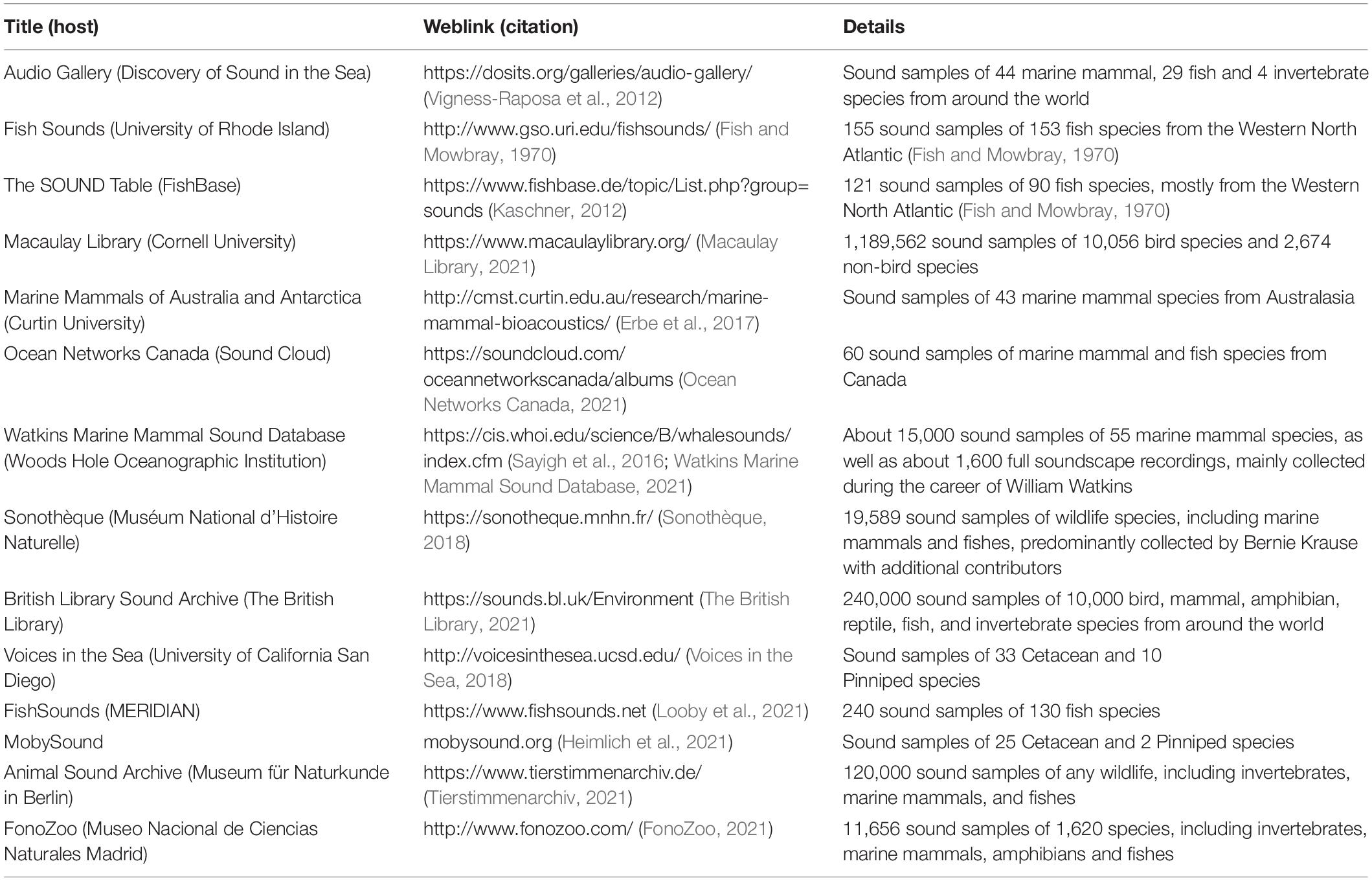
Table 1. Example biological sound libraries with recordings of mammal, fish, avian and invertebrate sounds.
In general, existing libraries are “silos”—lacking the cohesiveness that a taxa-independent global library or network could provide. Moreover, PAM is not a traditional method of categorizing or preserving information on diversity. Thus, keeping such libraries up to date has not been a focus and, in recent years, many libraries have lagged in their updates. Sustainability and accessibility of a sound collection is critical, particularly when it is tied to a single researcher, rather than a host institution.
Finally, few libraries identify what is missing from their catalogs. While this is a more complex task for fish and invertebrates, examples like Cornell University’s Macaulay Library have a list of target species for which they have fewer than 10 recordings. As our list of confirmed sources and known soniferous species increases, so does the ease with which the unconfirmed sources can be identified via a “weight of evidence” approach.
Here, we provide justification for the creation of a global bioacoustics platform that integrates and expands on existing libraries by describing five critical characteristics of such a program and what its extensions can bring to acoustic research and monitoring. The benefits of a global sound library include: (1) a full inventory of known underwater sound sources; (2) a baseline of unidentified biological sounds; (3) the foundation for a training platform for detection and classification algorithms (at both a source and soundscape level); (4) standardized metadata for understanding how, when, and where the recordings were made; and (5) an open-access (including for citizen science/public users) database to make aquatic biological sounds more accessible to the general public and allow them to upload sounds and add to the dataset (see Figure 2, for a conceptual diagram of such a potential integrated library). In addition to these benefits, the global sharing of such an expansive database—from potentially numerous contributors—holds the potential for multiple broadscale collaborations on regional and international trends of PAM detections. Similar efforts have been achieved in related fields, like acoustic telemetry (e.g., Hussey et al., 2015; Sequeira et al., 2019; Lédée et al., 2021; Matley et al., 2021), and visual censusing of marine fauna (e.g., Langlois et al., 2020), fostered by open forums and working groups to develop such research. We also discuss some of the technical challenges in developing this platform, historical hurdles that may have prevented previous attempts at such global data sharing environments, and a potential way forward for building this resource.
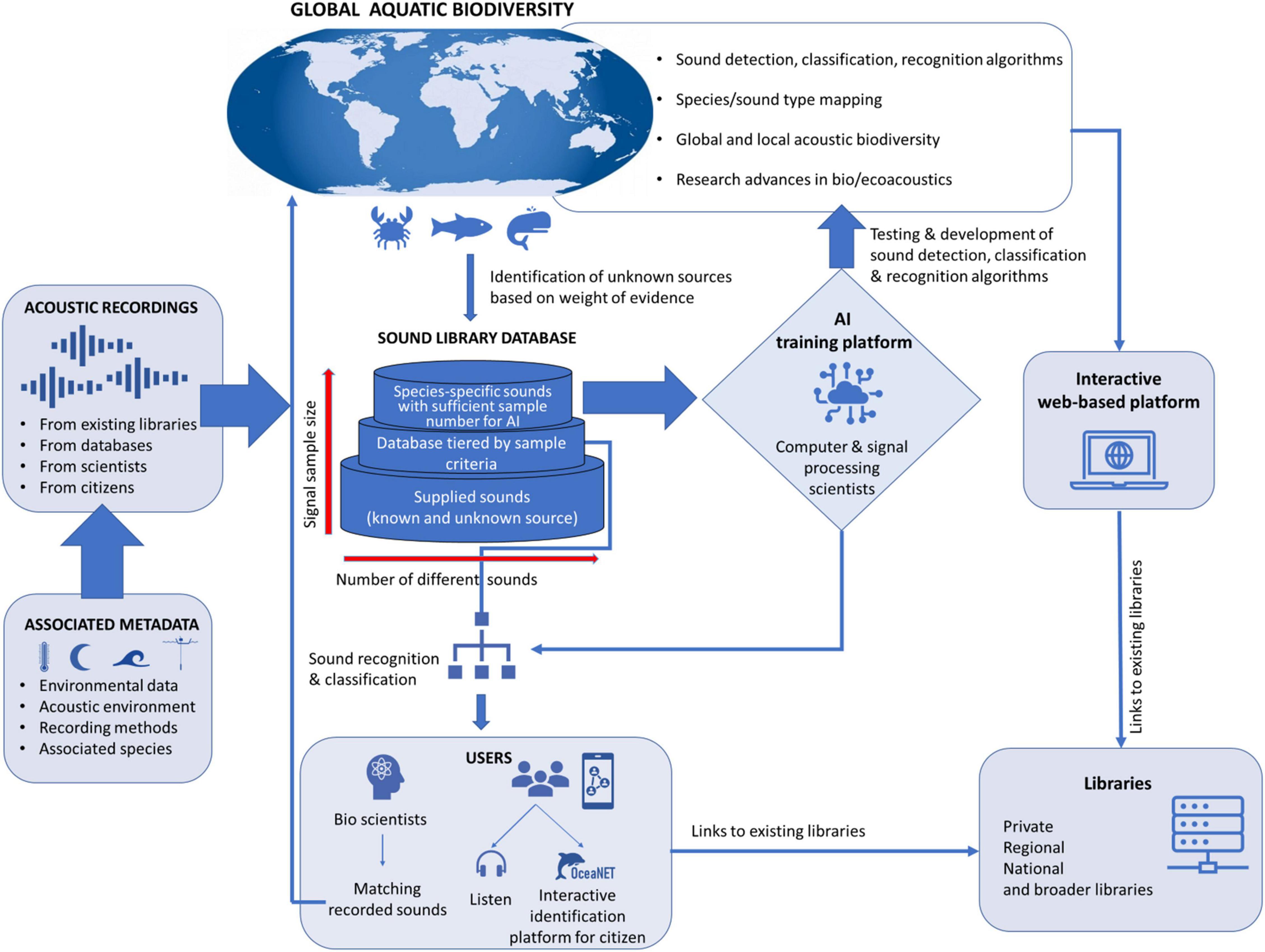
Figure 2. Conceptual diagram of the basic structure and data flow within a potential global integrated library and database sharing platform.
The discussion presented in this paper originated within the “Working Group on Acoustic Measurement of Ocean Biodiversity Hotspots” of the International Quiet Ocean Experiment (Boyd et al., 2011), an international program of research, observation and modeling formed to better characterize and understand ocean sound fields and the effects of sound on marine life. This collaboration was then expanded to include authors that are involved in the development, presentation and maintenance of existing underwater bioacoustics repositories; i.e., an overall partnership that represents various stakeholder groups—including bio- and ecoacousticians, and research specialists of a variety of taxa and ecosystems. All of these authors are aware of the benefits a global library of underwater biological sounds offers to the scientific, environmental management and public sectors. This paper, however, is not meant to dictate the exact form such a global underwater biological sounds library should take, but is meant to renew and revitalize discussion on the topic, present some of the many considerations such an effort would require, and describe a possible path forward for us or others to undertake as opportunities and interests arise. A more detailed discussion of such a program, involving a wider network of contributors, is planned through upcoming stakeholder engagement and scoping workshops.
Creating a reference library of aquatic sounds from known origins will broaden our reference list for confirming the sources of sounds that appear in recordings and help expand our knowledge of aquatic acoustic diversity, as well as our understanding of taxonomic biodiversity and ecology. Bringing known sounds together in a unified depository or single platform with links to multiple existing databases facilitates easy comparison among species, locations, species repertoires, and recording methodologies.
Studies often focus on a single or a limited number of species and, therefore, so does a project’s data analysis. Although the advent of multi-species automated detection platforms has brought significant advances in the terrestrial environment (Potamitis, 2014; Sueur and Farina, 2015; Farina et al., 2018; Kahl et al., 2021), such analyses are currently lagging behind in the underwater environment. The potential to process each dataset for sources beyond the focal species is rarely undertaken due to the funding and effort required as well as a lack of individual knowledge about all the different sounds and sources that exist. Easing this burden requires a collective effort to detect, identify, characterize, and collate sources. A global reference library of underwater biological sounds would increase the ability for more researchers in more locations to broaden the number of species assessed within their datasets and to identify sounds they personally do not recognize. Such access would ultimately lead to a description and catalog of acoustic biodiversity around the globe, and an increased understanding of acoustic ecology. A global database could serve broader questions, like determining universal trends in underwater sound production, while individual, specialized repositories could continue to inform and detail other topics, such as documenting the presence of soniferous species in a particular region.
The expansion of PAM data collection has increased our understanding of spatiotemporal patterns of individual species’ presence and acoustic behavior. As a result, reported distributions of these species are being expanded. Even some of the great whales are being found in places they were not expected (Allen et al., 2021), and occasionally a new species (Rosel et al., 2021) or a new sound (Rice et al., 2014; Cerchio et al., 2020) is discovered. This fact could prove vital for soniferous fauna, as our ever-changing climate ensures that many species are modifying their distributions and broadening or reducing their ranges (e.g., Scheinin et al., 2011; Ramirez et al., 2017; Bonebrake et al., 2018). Biologically important areas can be mapped; spawning grounds, essential fish habitat, and migration pathways can be delineated (e.g., Luczkovich et al., 1999; Rountree et al., 2006; Mann et al., 2009; Morano et al., 2012a; Schärer et al., 2014; Bertucci et al., 2015; Lammers and Munger, 2016; Karaconstantis et al., 2020); the timing of reproductive activities can be associated with the environment (e.g., Mann and Grothues, 2008; Parsons, 2010; McWilliam et al., 2017; Zarada et al., 2019); and displacement from preferred habitats due to anthropogenic activities and noise, such as that from shipping lanes and exploration surveys, can be mapped (e.g., Tyack, 2008; Castellote et al., 2012; Rako et al., 2013). These and other questions can be queried on broader scales if we have a global catalog of sounds.
Comparison of sounds from a single species across broad areas and times provides the ability to understand signal diversity and evolution, and to gain insights into species ecology (e.g., Tellechea et al., 2010). Fin whale (Balaenoptera physalus) calls, for example, differ among populations (Delarue et al., 2009) between the Northern and Southern hemispheres (Gedamke and Robinson, 2010; Širović et al., 2013, 2017; Aulich et al., 2019), as well as over seasons (Morano et al., 2012b). Pilot whales (Globicephala melas), on the other hand, produce similar call types across the hemispheres even though populations’ home ranges do not (or no longer) cross the equator, raising interesting questions about their acoustic ecology and evolution (Courts et al., 2020). Fishes may also develop “dialects,” such as the different acoustic characteristics of agonistic sounds produced by the skunk anemonefish (Amphiprion akallopisos) in Madagascar compared with those in Indonesia (Parmentier et al., 2005). Cultural evolution of humpback whale (Megaptera novaeangliae) song has been observed across ocean basins, providing greater understanding of population interactions across the Pacific Ocean (Garland et al., 2011) and around the coast of Australia (Allen et al., 2018). Call structure, and source spectra of blue (Balaenoptera musculus) and pygmy blue whales (Balaenoptera musculus brevicauda) around the world evolve through time, with peak frequencies changing each year (e.g., McDonald et al., 2009; Gavrilov and McCauley, 2012), such that detection algorithms developed in 1 year may not be successful some years later; thus, keeping libraries up to date aids classification efforts. Sound production between similar species within a taxonomic family can also be compared, such as those of mulloway (Argyrosomus japonicus) and black jewfish (Protonibea diacanthus) in Australia (Parsons et al., 2012, 2013b,2016) with those of French meagre (A. regius) in Europe (Lagardère and Mariani, 2006; Bolgan et al., 2020b), or those of various species of toadfishes in the Pacific, Indian, and Atlantic oceans (Thorson and Fine, 2002; Rice and Bass, 2009; Mosharo and Lobel, 2012; Alves et al., 2016; Staaterman et al., 2018; Pyć et al., 2021), to better understand the variation within families.
There is increasing evidence that the study of acoustic communities, based on acoustic characteristics of the sounds emitted by animal communities, provides ecologically relevant information (Francis et al., 2009; Farina and James, 2016; Desiderà et al., 2019; Mooney et al., 2020). Soundscapes provide unique opportunities to investigate the biodiversity and community of soniferous species, frequency and temporal niche partitioning, and organism-environment relationships (Ruppe et al., 2015; Di Iorio et al., 2021; McKenna et al., 2021). However, this field is in its infancy and requires a catalog of identified sounds to develop reliable and time-efficient classification techniques that will be necessary for describing the acoustic communities and relating them to the underlying animal assemblages (Mooney et al., 2020).
Anthropogenic noise, such as that from vessels, exploration, construction, and aerial vehicles (Reine et al., 2014; Newhall et al., 2016; Pangerc et al., 2016; Erbe et al., 2018; Chion et al., 2019; McCauley et al., 2021; Parsons et al., 2021), is a growing pollutant in the underwater environment and high ambient noise levels, such as those found in areas of intense human activity, inhibit signal detection (Hildebrand, 2009; Duarte et al., 2021). If the observer knows a target species’ signal characteristics, these sounds may be more easily detected, but without prior knowledge of either presence or structure of sounds, listening through the noise can be difficult. This has been highlighted by the recent COVID “anthropause” experienced at various aquatic locations around the world (e.g., Bates et al., 2021; De Clippele and Risch, 2021; Dunn et al., 2021; Gabriele et al., 2021; Ryan et al., 2021), where removal of the anthropogenic component of some soundscapes has provided an opportunity to observe sounds (and therefore presence) of marine fauna that might otherwise be lost in the noise (e.g., Pine et al., 2021). However, it is not just anthropogenic noise that limits acoustic detection of marine fauna. The ocean is naturally noisy and geophysical noise (such as from wind and ice) exceeds anthropogenic noise in many regions and seasons (e.g., Farcas et al., 2020; Erbe et al., 2021; Sertlek, 2021). The number and intensity of storms and extreme weather events are expected to increase with climate change (e.g., Cheal et al., 2017), inevitably contributing further noise to the underwater environment (e.g., Zhao et al., 2014; Ashokan et al., 2015; Zhang et al., 2018). A reference library of sounds, as well as detection algorithms, would significantly ease the detection of sounds in low SNR environments.
A sound catalog can provide a reference for comparison with unknown sounds to assist in their source identification, potentially through an online tool, within the library. The associated metadata that accompanies recordings (discussed below) may also contribute to a weight-of-evidence approach in identifying sound sources. Sainburg et al. (2020), demonstrated the use of unsupervised learning to assemble acoustic signals displaying similar spectral-temporal modulation features into groups. Such exploratory data analysis tools will assist in identification of sound sources; however, their design and functionality may be dependent on the amount of data (general and source-specific acoustic data and validation data), the distribution of the source signal and potential sources, and the characteristics of the signal, among other factors. In selected species, where sound production has not yet been confirmed, it may be possible to use signals reported from a closely related species to assist in detecting sounds or choruses from the targeted species. Calls produced by terapontid fish species, for example, are often similar, but not all species within the family have been reported to produce sound (Parmentier et al., 2016; Looby et al., 2021). Although not definitive, sound production by related species can provide evidence toward soniferous behavior, though caution is warranted as species that appear morphologically similar can be acoustically different, such as Ophidiformes (Mann et al., 1997; Parmentier et al., 2006, 2010).
A library of reference sounds requires only a handful of examples for each individual sound type. In contrast, a dataset for training artificial intelligence (AI) requires a far larger number of signals, ideally several thousands of examples (e.g., Madhusudhana et al., 2020, used > 11,000 replicates of the same call type to build robust detectors). The library itself can be of benefit as it provides the basis from which the AI datasets can be developed either through providing numerous examples for directly training models, or for facilitating event mining from previously collected data streams (Xie et al., 2008; Zhang et al., 2013). For example, Miller et al. (2021) initiated an open-access library of annotated recordings to train and evaluate automated detectors of Antarctic blue whale and fin whale (B. physalus) calls. The library was designed to include recordings from a broad range of instruments, locations, environmental conditions, and years, to ensure that robust detectors can be developed and tested across a suite of recording conditions. However, given the scope of this library initiated through the Southern Ocean Research Partnership Fin and Blue Whale Acoustics Group (Van Opzeeland et al., 2014), it is unlikely to be extended to other regions and taxa.
As the number of samples of a given sound type reaches critical mass, and recordings of them are also sufficiently rich in signals recorded under different conditions (e.g., SNR, acoustic environment, recording methods), that sound type can be flagged as available for the development of detection algorithms. Indeed, presenting the information on current sample numbers within each sound type could promote contributors to target them, increasing the likelihood of collectively bringing the dataset up to the required level. If it can be achieved, a library that includes an entire species’ sound repertoire will assist in validating detection algorithms and provide the ability to expand these algorithms to datasets where the call type was not the original target for analysis, and conduct this on a global, rather than local scale. For species that produce sounds that change with time, historical data and continual updating of the library could assist in predicting future evolution (Gavrilov and McCauley, 2012).
A database of unidentified sounds is, in some ways, as important as one for known sources; as the field progresses, new unidentified sounds will be collected, and more unidentified sounds can be matched to species. These sounds and the times and locations of their recording can form a basis for future identification and ease mapping of the species’ distribution once the source has been confirmed. Given the increasing rate of data collection, it is better to start building a map of these sounds as soon as possible. The library can also provide evidence to help test hypotheses of source species for unknown sounds if there are sufficient recording locations that can be compared to distribution maps of potential source species (e.g., those produced from catch data or visual census).
Although the analysis of acoustic communities benefits from a baseline of cataloged sounds, most sound sources that contribute to the soundscape remain uncertain and most libraries only archive signals with known species’ identity. In addition, we know more about the sounds of endangered or commercially important species than those of commonly encountered species (Luczkovich et al., 2008; Popper and Hawkins, 2019). This knowledge gap has impeded effective use of underwater soundscapes in monitoring marine biodiversity, but much information on acoustic ecology can still be gleaned from categorized sound types of unknown origin (Le Bot et al., 2015; Rountree et al., 2019; Bertucci et al., 2020; Bolgan et al., 2020a; Di Iorio et al., 2021). A library to archive unknown sounds and their recording times and locations will be crucial for guiding future studies of marine bioacoustics and biodiversity. This is especially important in areas that are rarely investigated or where source identification is particularly problematic, such as the twilight and midnight zones, where a description of unknown sounds can give us insights on biodiversity in the deep ocean (e.g., Mann and Jarvis, 2004; Rountree et al., 2012; Lin et al., 2019).
Signal- and image-processing techniques have been used in the temporal and spectral domains to automatically detect and quantify fauna sounds. For example, click detectors operating on waveforms have been applied to recordings of dolphins and porpoises (e.g., Sostres and Nuuttila, 2015), belugas (Le Bot et al., 2015), sperm whales (Madhusudhana et al., 2015), beaked whales (e.g., Yack et al., 2010; Le Bien, 2017), and snapping shrimp (e.g., Bohnenstiehl et al., 2016; Du et al., 2018). Matched-filtering of spectrograms has been used to detect highly stereotypical sounds of some whales and fishes (e.g., Mellinger and Clark, 1997; Ricci et al., 2017; Madhusudhana et al., 2020; Ogundile and Versfeld, 2020). Information entropy detectors have been applied to aberrant (non-stereotypical) tonal sounds (Erbe and King, 2008). Detectors are further applied to wavelets and the cepstral domain as well (e.g., Alias et al., 2016; Noda et al., 2016; Malfante et al., 2018). Machine and deep learning (AI) methods have been increasingly used to classify and detect sources in multiple applications (e.g., Lin et al., 2017; MacAodha et al., 2018; Bergler et al., 2019; Stowell et al., 2019).
There have been considerable advances in the fields of facial and voice recognition that have advanced public use of phone-based apps to identify music, plants, and the calls of frogs and birds (Kahl et al., 2021). However, this success has been largely due to the enormity of the respective databases from which AI algorithms can be trained, a goal that has only recently become possible in the aquatic environment and only for selected call types. The sheer enormity of data now collected in many underwater acoustic studies, together with the myriad of signals often present and the extreme amount of time required to search these records in more “standard” methods, means there is a clear opportunity for AI to improve efficiency and extract more information from these datasets. Increasingly, neural networks and other AI methods are being used to detect marine mammals in historical recordings such as of humpback whales across the Hawaiian archipelago (Allen et al., 2021), and multiple cetacean species along the west coasts of Canada and Australia (Mellinger and Clark, 2006). Detections of pulse trains, typically based on previously identified or grouped inter-pulse timing (the time between pulses of sound) are being successfully applied to count the number of echolocating individuals and fish sounds within datasets (Bahoura and Simard, 2010; Le Bot et al., 2015; Ibrahim et al., 2018).
Many machine learning techniques have been developed under the framework of AI (e.g., Shamir et al., 2014). Application of these techniques has begun to coalesce and recent studies now extract multiple features to detect the different types of signals, such as Malfante et al. (2018), who tested 84 extracted features to detect and characterize four general classes of fish sounds: (1) impulsive (pulses with signal separation > 1 s); (2) trains of > 15 pulses audible as either a single tone or a series of knocks in quick succession; (3) wide-band signals of 10–30 s in duration; and (4) short signals with harmonic structure. Each of these sound types required the detection of different features and individual machine learning. The recent development of deep learning has significantly reduced the work required in feature extraction (Shiu et al., 2020; Kahl et al., 2021; Waddell et al., 2021) and even made end-to-end learning feasible, in which AI models automatically learn features from raw audio to perform signal detection and classification. Successful examples include avian calls (Bravo Sanchez et al., 2021), odontocete clicks (Luo et al., 2019; Roch et al., 2021), and frog calls (Xie et al., 2020). Hand-selected features, such as peak frequency and frequency bandwidth measured manually or automatically, are no longer a necessity.
Developing a global database that can assist in modifying or developing algorithms in the underwater environment holds significant potential for detecting, classifying, and quantifying spatiotemporal distribution and abundance of aquatic fauna. Such a global database of known and unknown sound sources can benefit both supervised and unsupervised machine learning. Supervised machine learning is effective when training data of detection/classification targets are available. However, most underwater biodiversity assessments are unable to make sure all sound sources are already covered in the training database. Unsupervised machine learning may help discover the structure of sound categories from a substantial number of unlabeled recordings and reduce the effort required in manually annotating signal types and characteristics (Frasier et al., 2017; Phillips et al., 2018; Lin et al., 2021; Ozanich et al., 2021). Deep-learning models that have learned the structure of labeled and unlabeled recordings archived in this global database will be adaptable to other applications, via transfer learning (Yosinski et al., 2014). Therefore, this global library will benefit the detection and quantification of signal types, which may be added to a suite of acoustic metrics, to be used collectively as scene classifiers, to routinely characterize the soundscape.
Any AI model must learn the difference between target signals and background noise. Voice recognition techniques often begin with clean recordings and synthesize training data by adding noise to the known reference (Lu et al., 2013; Xu et al., 2015). These are then used to train models to extract speech from recordings that contain real noise and target signals in long-term recordings. Such data augmentation techniques have proven effective in improving the performance of AI models and have been widely applied in speech and music enhancement models (see review in Lin and Tsao, 2020). Recordings of biological sounds with high SNR will therefore be crucial to the development of a marine fauna AI database. The ultimate goal of an AI algorithm is that it can accurately classify sounds through any or all recordings of any duration and noise level.
An audio database for training AI models requires large numbers of recordings for one species or sound type of unknown origin. The goal of building such a database is to train a model that can effectively recognize species-specific or sound type acoustic features. This requires signals that have been recorded and processed to a certain set of criteria. Although it is difficult to assess how many recordings will be needed, in general the greater the number of sound samples and the higher the sound quality, the more reliable and precise the automatic classification becomes, as the algorithm learns and improves its performance with increasing data availability (Zhong et al., 2020). For species with more complex vocal repertoires, greater amounts of training data further improve classification. Thus, the prerequisite to apply these techniques is a robust and representative training dataset, which is what the library we propose here could provide.
AI has facilitated the development of many highly popular image-based animal, plant and music recognition applications (apps). Possibly the best known is iNaturalist,1 though other more taxonomically focused applications are emerging (e.g., Merlin BirdID,2 WikiAves).3 The iNaturalist app started as a crowd-sourced community, where people uploaded animal or plant photos to be identified by other users, and has become a place where images are identified by artificial intelligence. In the biological sounds space, FrogID4 and BirdNet5 have shown the possibility of using machine learning with signal processing to allow researchers and citizen scientists alike to identify frogs and birds by recording calls with a phone (Rowley et al., 2019; Kahl et al., 2021).
Much like BirdNet and FrogID, a library of underwater biological sounds and any automated detection algorithms would be useful not only for the scientific, industry and marine management communities, but also for users with a general interest. Acoustic technology has reached the stage where a hydrophone can be connected to a mobile phone so people can listen to fishes and whales in the rivers and seas around them. Therefore, sound libraries are becoming invaluable to citizen scientists and the general public, with signal-processing automated detection algorithms supporting the decision networks behind apps like FrogID and BirdNet for someone to record a sound and identify the source. FrogID has over 50,000 recordings uploaded for the > 240 species of frogs in Australia, and the Cornell Lab of Ornithology’s BirdNet app has been downloaded over 1,000,000 times and has records of 3,000 species of bird calls across 40 countries (Kahl et al., 2021). As evidence of this type of application moving into the underwater world, the River Listening app,6 which began in Australia (Barclay et al., 2018), encourages the general public to record sounds in rivers and coastal waters to listen to the sounds of fishes. Further, Chapuis et al. (2021) showed the utility of waterproof recreational recording systems (such as GoPros) to collect information on underwater soundscapes and in particular, recording and cataloging biological sounds, while Lamont et al. (2022) highlighted how low-cost alternative hydrophones and recording systems (such as the Hydromoth) are becoming increasingly available to scientists and the general public. These types of systems can provide valuable PAM data; however, the calibration, variability in sensitivity and directionality, and low signal-to-noise ratios mean additional considerations must be made, to be able to use the data within the library, in particular, for sound analysis purposes.
Increased sampling efforts from citizen scientists could be invaluable for the detection of vocal fauna in coastal and inland waters. For example, FishBase7 uses community input to provide information on each species (Froese and Pauly, 2021), while Redmap (Range Extension Database & Mapping project)8 is more explicit, inviting the general public to spot, log, and map marine species that are uncommon in Australia, or along particular parts of the coast to monitor changes in species distribution. Future online libraries, such as the Open Portal to Underwater Soundscapes (OPUS)9 would be expected to facilitate public contributions, similar to WhaleFM,10 a citizen science project that focused on categorization of call types produced by two cetacean species.
Creating a library with established metadata and information criteria will help standardize the format in which signals are reported, optimize use of the library, and ease future classifications of sounds (Frazao et al., 2019). It is important to provide guidelines on all the pertinent information that could be provided by a person collecting the original recording and that should be included when it is presented, for example, as a static spectrogram on a library website (Parsons, 2010; Warren et al., 2018; Frazao et al., 2019; Looby et al., 2021; Miller et al., 2021). Such metadata standardization can also build confidence in the library’s utility and attract support from national bodies for its application, such as the ADEON noise reporting standards (e.g., Ainslie et al., 2017). Each criterion may not be required for entry of a sample into the library, but the level of information supplied determines the level of potential use of a sample within the database. There are three criteria that determine how useful a recording could be to the database and how it could fit with known information about the species and its soniferous behavior around the world. These relate to the information available about the recording and the source species:
• Metadata pertaining to the specific recording (e.g., recording equipment and pre-amplifier used, calibration, model, and sensitivity; recording settings such as gain, sampling rate, number of bits and duty cycle; recording methodology such as deployment configuration; environmental conditions such as depth and bottom characteristics; location and timing), and how it is presented (e.g., un-/calibrated waveform, spectrum with specified window lengths, resolution, FFT/DFT size, and overlap, or the code and settings associated with a plugin automatically generating visualizations of the recording). Recordings taken under controlled conditions (e.g., within tanks or aquaria) have additional acoustic and behavioral considerations and therefore require additional metadata, such as the tank material and dimensions, acclimation time and number of other individuals present.
• Information about the source species in general, such as recording-specific information (e.g., behavioral context if concurrent visual observations were made); more general information may be supplied by the contributor or updated by the host, through continued review of literature (e.g., known distribution, auditory ability, known sound types and their characteristics, and sound production mechanism and typical behavioral contexts associated with sound production, if known). This information provides context to place calls into a species’ known behaviors.
• Associated information about the location relative to the broader region (e.g., description of community species composition, habitat, and local soundscape information). This information provides a broader picture of how the local environment may have affected the animal producing the sound.
Defining these requirements, their level of detail, and the final platform design requires the collective expertise of biologists (who have experience related to the potential numbers and types of sounds a species may exhibit), acousticians (who appreciate the impact that propagation losses, sampling methods, and processing techniques may have on the characteristics of the audio clips), signal processing experts (who develop and apply detection, classification, and recognition algorithms, and who can detail the needs of turning example signals into a database for automated detection), and data scientists/database developers (who can develop a scalable and searchable database that can be effectively used and accessed by a broader user community).
In such a way, data are optimized at a quality that is useful for future applications, such as AI development or global-scale meta-analyses and reviews of sound production. Users would benefit from not only the sounds themselves, but the associated metadata about the sounds (Teixeira et al., 2019; Kahl et al., 2021; Lin et al., 2021) and, if there are multiple recordings, a classification of the sound type in which it fits (Sainburg et al., 2020). This information can assist in categorization of an unknown sound and provide context around the recording from an environmental, methodological, or behavioral perspective. However, the level of data made available in the library for each species and each recording depends on the information provided by the contributor, and researchers from fields with different objectives, backgrounds, and experiences, who typically report information in different ways. The most common example in bioacoustics is the classification of signals by phonetic description (onomatopoeia), such as the “thwop,” “muah,” and “boop” of humpback whale social sounds (Recalde-Salas et al., 2020), and various onomatopoeic descriptions of species-specific and unidentified fish sounds (e.g., Tavolga, 1971; Thorson and Fine, 2002; Staaterman et al., 2018; Waddell et al., 2021). Such calls may also be reported in a physics-focused context that include categories of frequency- or amplitude- modulated or continuous-wave signals (Erbe et al., 2017).
Finally, the library and its potential as an AI database would benefit from a scalable design that allows frequent expansion and a web platform that can be continually updated. The database would include a user-friendly interface to investigate data and upload sounds, with automated quality control. Presentation of the data is also a consideration, not only with respect to the variety of signals, but also individual recording locations and their temporal distributions. Data portals, such as the interactive maps of the North West Atlas in Australia,11 allow viewers to choose any study site in a map and view a synopsis of species composition and a video snapshot of the site, along with environmental data. This could also be achieved from an acoustic perspective. For example, the data portal of the Integrated Marine Observing System12 allows viewers to peruse long-term spectrograms of recording sites for a user-defined period. An interactive map that can incorporate all these options becomes a user platform for the acoustic data. OPUS is a recent initiative driven by the International Quiet Ocean Experiment that is currently under development and includes some of these functions. This program was created to share underwater soundscapes through audio and synchronized spectral visualizations at staggered temporal resolution. It allows viewers to select locations from a map and explore local soundscapes while also logging events of interest, thereby inviting the public to participate in creating overall logs with acoustic events that can support further processing of the data.
This is not the first time a global approach to data sharing has been suggested in underwater acoustics research. In recent years, multiple international research and blue economy-focused workshops have repeatedly identified the need for global sharing of data, technology, and best practices, to grow techniques and ensure that economic, environmental, and social benefits, developed through the application of knowledge, are realized to the benefit of all (e.g., World Wildlife Fund [WWF], 2017; European Commission, 2018). Most recently, the emergence of COVID-19 has provided a perfect example of the need for international transparency and collaboration to maximize research opportunities and rapidly respond to urgent needs, and it has highlighted how achievable this approach is with modern technology (Apuzzo and Kirkpatrick, 2020). In an acoustics forum, among other workshops, the special sessions of the Acoustical Society of America [ASA] (2018) identified that there are “an increasing number of applications of machine learning methods in ocean acoustics, particularly when working with large data sets” and discussions focused on data access, code-sharing, and reproducible research.
An integrated sound sharing platform begins with three main areas of development to focus and pool efforts: large-scale archives of annotated and unannotated audio data, the open-access reference library of identified and unidentified sound sources, and data mining processes, including AI algorithms. Acoustic repositories and data portals, such as OPUS and IMOS, are becoming increasingly common. Importantly, initiatives such as the allocation of 15 petabytes for a passive acoustic data portal by the National Center for Environmental Information (NCEI) of the National Oceanic and Atmospheric Administration (NOAA; United States of America) illustrate the growing appreciation and realization of this need at the national scale (Wall et al., 2021).
Reference libraries have existed on various scales for many years and advances in technology are quickly increasing their ability to expand and integrate user contributions. The Detection Classification, Localization and Density Estimation (DCLDE) and the Detection and Classification of Acoustic Scenes and Events (DCASE) workshop series (2003–2022 and 2013–2022, respectively) have focused on data mining and analytical approaches. These groups have been the main producers of public datasets to advance machine learning applications of biological sounds in the ocean (Frazao et al., 2019) and the workshops regularly provide training sets to test detection algorithms under different conditions (e.g., various frequency-dependent SNR and propagation losses).13 Their outputs have shown what is achievable from data-sharing of comparatively “small” platforms (previously up to 10 TB), which complement the sharing of open-source code that individuals are increasingly providing with the publication of analytical works (e.g., Bergler et al., 2019; Bermant et al., 2019; Madhusudhana et al., 2020; Lin et al., 2021). Together these activities highlight the potential for applications of data-sharing of acoustic information to be applied to larger repositories that are now more achievable with cloud-based options, such as AI for Social Good,14 or government supported platforms, such as the NCEI.
Although the development of a global integrated and open-access underwater sound reference library, repository and sharing platform has been suggested previously, despite these discussions and the increasing appearance and support for individual components of such a program, it has not been fully realized on an international level. The main barrier to creating an international database of aquatic bioacoustics may be as simple as sourcing adequate funding to achieve such a sizable task, due to a lack of awareness of the value and importance of the product among organizations with the financial resources to support its creation and continuation.
To make a global underwater sound library a success, broader engagement, buy-in, and support of the scientific community will be needed, as well as providing incentives for individuals to contribute their sounds and algorithms to the library (e.g., Bradbury et al., 1999; Gaunt et al., 2005). There are several non-trivial hurdles to establishing this buy-in. Firstly, researchers often need to be convinced about the value of open and accessible science that may counterbalance more individualistic benefits associated with their intellectual property and therefore encourage contribution of recorded sounds to a repository; this parallels the ongoing broader scientific cultural change toward promoting data sharing and accessibility. A repository that provides a way to have example sounds as citable data (such as through providing a DOI number) further motivates individuals to contribute by ensuring they receive appropriate future credit for their original recordings; however, this is matched with the consideration that in some cases, contribution may require signing over copyright and access rights for that acknowledgment. Secondly, a repository needs to reduce burdens for individuals to contribute sounds and provide a system that can easily ingest audio and relevant metadata (Bradbury et al., 1999). A third challenge is raising the awareness that many individual archives are not as permanent as individuals think; analog media often degrades over time (Gaunt et al., 2005) and hard drives are not immune from failure, so depositing sounds in a sustainable repository is an urgent need, particularly for older recordings. One example of such archiving is the recovery and digitization of the fish sound recordings taken by Fish and Mowbray (1970), as described in Rountree et al. (2002). Launching a new library is particularly taxing as it requires building the interest of potential contributors to maximize their donations, while having limited outputs to offer initially. This could be alleviated by integrating efforts from existing libraries and archives, rather than initiating an entirely new database, which will also increase the library’s appeal to potential funding sources.
There may also be more nebulous factors that have limited the provision of appropriate funding, including the likely duration of the program (i.e., including long-term planning and on-going resources to maintain the platform) and facilitating the repeated meeting of numerous global partners needed to identify and agree on its structure and criteria. Securing the longevity of the program is vital to the usefulness of the platform as libraries that are not scalable, well-maintained, and continually updated can quickly become redundant or outdated. The world’s increasing awareness around the environmental costs of data storage and processing mean that consideration of carbon neutrality will also be a key factor in the design and longevity of the program.
Passive acoustic research is now, it appears, rapidly approaching a nexus point. The changing environment and decreasing biodiversity are compelling the documentation of baseline acoustic observations. Technical advances associated with data collection and an increasing number of researchers and institutes collecting PAM data are providing the ability to create bioacoustic databases. Concurrently, awareness of the importance of acoustic cues to aquatic fauna, the impacts of noise on them and the potential for acoustic communities to provide an indication of ecosystem health has reached a stage where PAM is becoming appreciated as a mainstream data source across more species and ecosystems than ever. Finally, public interest and access to user applications means citizen scientists can drive widespread knowledge sharing. Now is the time to facilitate that progress by gathering the acoustic, ecological, and bioinformatic community together to realize an aquatic-sounds sharing platform.
The development of an international platform for sharing acoustic data is non-trivial and requires identifying and describing a number of inter-dependent factors including: (1) sources and protocols for securing and maintaining significant funding at national and international levels; (2) global interdisciplinary collaboration and stakeholder consultation to develop and agree on criteria for data supply and reporting and system configuration that produce the most useful, yet user-friendly, environment; (3) an appropriate scalable platform on which the facility can be hosted; (4) an open forum to facilitate open access and common development of AI algorithms; (5) continual system management and quality assurance; (6) establishment and agreement on the use of data and metadata standards; and (7) on-going promotion and engagement to ensure maximum use, such as open working groups to foster international collaborations focused on global spatiotemporal trends in detected aquatic fauna. These are multidisciplinary tasks requiring input from bio- and eco-acousticians, bioinformatics experts, AI engineers, web engineers, and stakeholders. To begin our journey along this shared pathway, we recommend a multi-disciplinary workshop to detail all the requirements for developing an appropriate library/database to fulfill the needs of all that may wish to access it and to detail the resources needed to support the work. Such an effort is critical and timely as we enter the UN Decade of Ocean Science for Sustainable Development.
MP, THL, TAM, CE, FJ, SoL, SiL, ML, AL, SLN, IVO, CR, ANR, LS, JS, EU, and LDI contributed to the conceptualization, writing, preparation, review, and editing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Support for the initial author group to meet, discuss, and build consensus on the issues within this manuscript was provided by the Scientific Committee on Oceanic Research, Monmouth University Urban Coast Institute, and Rockefeller Program for the Human Environment. The U.S. National Science Foundation supported the publication of this article through Grant OCE-1840868 to the Scientific Committee on Oceanic Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The IQOE Science Committee created the Working Group (WG) on Acoustic Measurement of Ocean Biodiversity Hotspots to address the examination of marine acoustic diversity. We thank them for their initiative and support. We would also like to thank the reviewers for their time and constructive comments in consideration of this manuscript.
Acoustical Society of America [ASA] (2018). 176th Meeting of the Acoustical Society of America and 2018 Acoustics Week in Canada. J. Acoust. Soc. Am. 144, A1–A2016. doi: 10.1121/2.0000948
Ainslie, M. A., Miksis-Olds, J., Martin, B., Heaney, K., de Jong, C. A. F., von Benda-Beckmann, A. M., et al. (2017). ADEON Soundscape and Modeling Metadata Standard. Version 2.0. DRAFT. TNO for ADEON Prime Contract No. M16PC00003. Available online at: https://adeon.unh.edu/sites/default/files/user-uploads/WEB-DRAFTADEONSoundscapeSpecification_V2.pdf [Accessed October 28, 2021].
Ajemian, M. J., Lamboy, C., Ibrahim, A., DeGroot, B. C., Bassos-Hull, K., Mann, D. A., et al. (2021). Capturing shell-crushing by large mobile predators using passive acoustics technology. J. Exp. Mar. Biol. Ecol. 535:151497. doi: 10.1016/j.jembe.2020.151497
Akamatsu, T., Okumura, T., Novarini, N., and Yan, H. Y. (2002). Empirical refinements applicable to the recording of fish sounds in small tanks. J. Acoust. Soc. Am. 112, 3073–3082. doi: 10.1121/1.1515799
Alias, F., Socoró, J. C., and Sevillano, X. (2016). A review of physical and perceptual feature extraction techniques for speech, music and environmental sounds. Appl. Sci. 6:143. doi: 10.3390/app6050143
Allen, A. N., Harvey, M., Harrell, L., Jansen, A., Merkens, K. P., Wall, C. C., et al. (2021). A convolutional neural network for automated detection of humpback whale song in a diverse, long-term passive acoustic dataset. Front. Mar. Sci. 8:607321. doi: 10.3389/fmars.2021.607321
Allen, J., Garland, E. C., Dunlop, R. A., and Noad, M. J. (2018). Cultural revolutions reduce complexity in the songs of humpback whales. Proc. Royal Soc. B 285:20182088. doi: 10.1098/rspb.2018.2088
Allen, S., and Demer, D. A. (2003). Detection and characterization of yellowfin and bluefin tuna using passive-acoustical techniques. Fish. Res. 63, 393–403. doi: 10.1016/S0165-7836(03)00096-1
Alves, D., Amorim, M. C. P., and Fonseca, P. J. (2016). Assessing acoustic communication active space in the Lusitanian toadfish. J. Exp. Biol. 219, 1122–1129. doi: 10.1242/jeb.134981
Anderson, K. A., Rountree, R. A., and Juanes, F. (2008). Soniferous fishes in the Hudson River. T. Am. Fish. Soc. 137, 616–626. doi: 10.1577/t05-220.1
Apuzzo, M., and Kirkpatrick, D. D. (2020). Covid-19 Changed How the World does Science, Together. New York: New York Times.
Ashokan, M., Latha, G., and Ramesh, R. (2015). Analysis of rain noise in shallow waters of Bay of Bengal during cyclonic storm. Indian J. Mar. Sci. 44, 795–799.
Aulich, M. G., McCauley, R. D., Saunders, B. J., and Parsons, M. J. G. (2019). Fin whale (Balaenoptera physalus) migration in Australian waters using passive acoustic monitoring. Sci. Rep. 9:8840. doi: 10.1038/s41598-019-45321-w
Bahoura, M., and Simard, Y. (2010). Blue whale calls classification using short-time Fourier and wavelet packet transforms and artificial neural network. Digit. Signal Process. 20, 1256–1263. doi: 10.1016/j.dsp.2009.10.024
Bailey, H., Fandel, A. D., Silva, K., Gryzb, E., McDonald, E., Hoover, A. L., et al. (2021). Identifying and predicting occurrence and abundance of a vocal animal species based on individually specific calls. Ecosphere 12:e03685. doi: 10.1002/ecs2.3685
Bailey, H., Lyubchich, V., Wingfield, J., Fandel, A., Garrod, A., and Rice, A. N. (2019). Empirical evidence that large marine predator foraging behavior is consistent with area-restricted search theory. Ecology 100:e02743. doi: 10.1002/ecy.2743
Banner, A. (1972). Use of sound in predation by young lemon sharks, Negaprion brevirostris (Poey). Bull. Mar. Sci. 22, 251–283.
Barclay, L., Gifford, T., and Linke, S. (2018). River Listening: acoustic ecology and aquatic bioacoustics in global river systems. Leonardo 51, 298–299. doi: 10.1162/leon_a_01516
Barrios, A. T. (2004). Use of Passive Acoustic Monitoring to Resolve Spatial and Temporal Patterns of Spawning Activity for Red Drum, Sciaenops Ocellatus, in the Neuse River Estuary, North Carolina. USA: North Carolina State University.
Bates, A. E., Primack, R. B., Biggar, B. S., Bird, T. J., Clinton, M. E., Command, R. J., et al. (2021). Global COVID-19 lockdown highlights humans as both threats and custodians of the environment. Biol. Conserv. 263:109175. doi: 10.1016/j.biocon.2021.109175
Bergler, C., Schröter, H., Cheng, R. X., Barth, V., Weber, M., Nöth, E., et al. (2019). ORCA-SPOT: an automatic killer whale sound detection toolkit using deep learning. Sci. Rep. 9:10997. doi: 10.1038/s41598-019-47335-w
Bermant, P. C., Bronstein, M. M., Wood, R. J., Gero, S., and Gruber, D. F. (2019). Deep machine learning techniques for the detection and classification of sperm whale bioacoustics. Sci. Rep. 9:12588. doi: 10.1038/s41598-019-48909-4
Bertucci, F., Lejeune, P., Payrot, J., and Parmentier, E. (2015). Sound production by dusky grouper Epinephelus marginatus at spawning aggregation sites. J. Fish Biol. 87, 400–421. doi: 10.1111/jfb.12733
Bertucci, F., Maratrat, K., Berthe, C., Besson, M., Guerra, A. S., Raick, X., et al. (2020). Local sonic activity reveals potential partitioning in a coral reef fish community. Oecologia 193, 125–134. doi: 10.1007/s00442-020-04647-3
Bohnenstiehl, D. R., Lillis, A., and Eggleston, D. B. (2016). The curious acoustic behavior of estuarine snapping shrimp: temporal patterns of snapping shrimp sound in sub-tidal oyster reef habitat. PLoS One 11:e0143691. doi: 10.1371/journal.pone.0143691
Bolgan, M., Gervaise, C., Di Iorio, L., Lossent, J., Lejeune, P., Raick, X., et al. (2020a). Fish biophony in a Mediterranean submarine canyon. J. Acoust. Soc. Am. 147, 2466–2477. doi: 10.1121/10.0001101
Bolgan, M., Pereira, B. P., Crucianelli, A., Mylonas, C. C., Pousão-Ferreira, P., Parmentier, E., et al. (2020b). Vocal repertoire and consistency of call features in the meagre Argyrosomous regius (Asso, 1801). PLoS One 15:e0241792. doi: 10.1371/journal.pone.0241792
Bolgan, M., Crucianelli, A., Mylonas, C. C., Henry, S., Flaguière, J. C., and Parmentier, E. (2020c). Calling activity and calls’ temporal features inform about fish reproductive condition and spawning in three cultured Sciaenidae species. Aquaculture 524:735243. doi: 10.1016/j.aquaculture.2020.735243
Bonebrake, T. C., Brown, C. J., Bell, J. D., Blanchard, J. L., Chauvenet, A., Champion, C., et al. (2018). Managing consequences of climate-driven species redistribution requires integration of ecology, conservation and social science. Biol. Rev. 93, 284–305. doi: 10.1111/brv.12344
Boyd, A. D., Gowans, S., Mann, D. A., and Simard, P. (2021). Tropical Storm Debby: soundscape and fish sound production in Tampa Bay and the Gulf of Mexico. PLoS One 16:e0254614. doi: 10.1371/journal.pone.0254614
Boyd, I. L., Frisk, G., Urban, E., Tyack, P., Ausubel, J., Seeyave, S., et al. (2011). An International Quiet Ocean Experiment. Oceanography 24, 174–181. doi: 10.5670/oceanog.2011.37
Bradbury, J., Budney, G. F., Stemple, D. W., and Kroodsma, D. E. (1999). Organizing and archiving private collections of tape recordings. Anim. Behav. 57, 1343–1344. doi: 10.1006/anbe.1999.1113
Bravo Sanchez, F. J., Hossain, M. R., English, N. B., and Moore, S. T. (2021). Bioacoustic classification of avian calls from raw sound waveforms with an open-source deep learning architecture. Sci. Rep. 11:15733. doi: 10.1038/s41598-021-95076-6
Burnham, R. E., and Duffus, D. A. (2019). Acoustic predator-prey reaction: gray whales’ (Eschrichtius robustus) acoustic response to killer whales (Orcinus orca). Aquat. Mamm. 45, 340–348. doi: 10.1578/AM.45.3.2019.340
Castellote, M., Clark, C. W., and Lammers, M. O. (2012). Acoustic and behavioural changes by fin whales (Balaenoptera physalus) in response to shipping and airgun noise. Biol. Conserv. 147, 115–122. doi: 10.1016/j.biocon.2011.12.021
Cerchio, S., Strindberg, S., Collins, T., Bennett, C., and Rosenbaum, H. (2014). Seismic surveys negatively affect humpback whale singing activity off Northern Angola. PLoS One 9:e86464. doi: 10.1371/journal.pone.0086464
Cerchio, S., Willson, A., Leroy, E. C., Muirhead, C., Harthi, S. A., Baldwin, R., et al. (2020). A new blue whale song-type described for the Arabian Sea and Western Indian Ocean. Endanger. Species Res. 43, 495–515. doi: 10.3354/esr01096
Chapuis, L., Williams, B., Gordon, T. A. C., and Simpson, S. D. (2021). Low-cost action cameras offer potential for widespread acoustic monitoring of marine ecosystems. Ecol. Indic. 129:107957. doi: 10.1016/j.ecolind.2021.107957
Cheal, A. J., MacNeil, M. A., Emslie, M. J., and Sweatman, H. (2017). The threat to coral reefs from more intense cyclones under climate change. Glob. Change Biol. 23, 1511–1524. doi: 10.1111/gcb.13593
Chion, C., Dominic, L., and Jérôme, D. (2019). A meta-analysis to understand the variability in reported source levels of noise radiated by ships from opportunistic studies. J. Front. Mar. Sci. 6:714. doi: 10.3389/fmars.2019.00714
Connor, R. C., Heithaus, M. R., Berggren, P., and Miksis, J. L. (2000). “Kerplunking”: surface fluke-splashes during shallow-water bottom foraging by bottlenose dolphins. Mar. Mamm. Sci. 16, 646–653. doi: 10.1111/j.1748-7692.2000.tb00959.x
Coquereau, L., Grall, J., Chauvaud, L., Gervaise, C., Clavier, J., Jolivet, A., et al. (2016). Sound production and associated behaviours of benthic invertebrates from a coastal habitat in the north-east Atlantic. Mar. Biol. 163:127. doi: 10.1007/s00227-016-2902-2
Coquereau, L., Lossent, J., Grall, J., and Chauvaud, L. (2017). Marine soundscape shaped by fishing activity. R. Soc. Open Sci. 4:160606. doi: 10.1098/rsos.160606
Courts, R., Erbe, C., Wellard, R., Boisseau, O., Jenner, K. C., and Jenner, M.-N. (2020). Australian long-finned pilot whales (Globicephala melas) emit stereotypical, variable, biphonic, multi-component, and sequenced vocalisations, similar to those recorded in the northern hemisphere. Sci. Rep. 10:20609. doi: 10.1038/s41598-020-74111-y
Davis, G. E., Baumgartner, M. F., Corkeron, P. J., Bell, J., Berchok, C., Bonnell, J. M., et al. (2020). Exploring movement patterns and changing distribution of baleen whales in the western North Atlantic using a decade of passive acoustic data. Glob. Chang. Biol. 26, 4812–4840. doi: 10.1111/gcb.15191
De Clippele, L. H., and Risch, D. (2021). Measuring sound at a cold-water coral reef to assess the impact of COVID-19 on noise pollution. Front. Mar. Sci. 8:674702. doi: 10.3389/fmars.2021.674702
Delarue, J., Todd, S. K., Van Parijs, S. M., and Di Iorio, L. (2009). Geographic variation in Northwest Atlantic fin whale (Balaenoptera physalus) song: implications for stock structure assessment. J. Acoust. Soc. Am. 125, 1774–1782. doi: 10.1121/1.3068454
Desiderà, E., Guidetti, P., Panzalis, P., Navone, A., Valentini-Poirrier, C., Boissery, P., et al. (2019). Acoustic fish communities: sound diversity of rocky habitats reflects fish species diversity and beyond? Mar. Ecol. Prog. Ser. 608, 183–197.
Desjonquères, C., Rybak, F., Depraetere, M., Gasc, A., Le Viol, I., Pavoine, S., et al. (2015). First description of underwater acoustic diversity in three temperate ponds. PeerJ. 3:e1393. doi: 10.7717/peerj.1393
Desjonquères, C., Rybak, F., Ulloa, J. S., Kempf, A., Bar-Hen, A., and Sueur, J. (2020). Monitoring the acoustic activity of an aquatic insect population in relation to temperature, vegetation and noise. Fresh. Biol. 65, 107–116. doi: 10.1111/fwb.13171
Di Iorio, L., Audax, M., Deter, J., Holon, F., Lossent, J., Gervaise, C., et al. (2021). Biogeography of acoustic biodiversity of NW Mediterranean coralligenous reefs. Sci. Rep. 11:16991. doi: 10.1038/s41598-021-96378-5
Du, X., Youssef, A., Wang, Y., Padfield, N., and Matthews, D. (2018). Detection of snapping shrimp using machine learning. Austr. Acoust. Soc. Conf. Proc. 2018, 451–452.
Duarte, C. M., Chapuis, L., Collin, S. P., Costa, D. P., Devassy, R. P., Eguiluz, V. M., et al. (2021). The Ocean Soundscape of the Anthropocene. Science 371:581. doi: 10.1126/science.aba4658
Dunn, C., Theriault, J., Hickmott, L., and Claridge, D. (2021). Slower ship speed in the Bahamas due to COVID-19 produces a dramatic reduction in ocean sound levels. Front. Mar. Sci. 8:673565. doi: 10.3389/fmars.2021.673565
Erbe, C., and King, A. R. (2008). Automatic detection of marine mammals using information entropy. J. Acoust. Soc. Am. 124, 2883–2840. doi: 10.1121/1.2982368
Erbe, C., Dunlop, R., Jenner, K. C. S., Jenner, M.-N. M., McCauley, R. D., Parnum, I., et al. (2017). Review of underwater and in-air sounds emitted by Australian and Antarctic marine mammals. Acoust. Aust. 45, 179–241. doi: 10.1007/s40857-017-0101-z
Erbe, C., Marley, S. A., Schoeman, R. P., Smith, J. N., Trigg, L. E., and Embling, C. B. (2019). The effects of ship noise on marine mammals—a review. Front. Mar. Sci. 6:606. doi: 10.3389/fmars.2019.00606
Erbe, C., Schoeman, R. P., Peel, D., and Smith, J. N. (2021). It often howls more than it chugs: wind versus ship noise under water in Australia’s maritime regions. J. Mar. Sci. Engin. 9:5. doi: 10.3390/jmse9050472
Erbe, C., Verma, A., McCauley, R., Gavrilov, A., and Parnum, I. (2015). The marine soundscape of the Perth Canyon. Prog. Oceanogr. 137, 38–51. doi: 10.1016/j.pocean.2015.05.015
Erbe, C., Williams, R., Parsons, M., Parsons, S. K., Hendrawan, I. G., and Dewantama, I. M. I. (2018). Underwater noise from airplanes: an overlooked source of ocean noise. Mar Pollut. Bull. 137, 656–661. doi: 10.1016/j.marpolbul.2018.10.064
European Commission (2018). Declaration of the Sustainable Blue Economy Finance Principles. Available online at: https://wwfeu.awsassets.panda.org/downloads/sbefp_declaration___6_aug_2018.pdf [Accessed October 25, 2021].
Fandel, A. D., Garrod, A., Hoover, A. L., Wingfield, J. E., Lyubchich, V., Secor, D. H., et al. (2020). Effects of intense storm events on dolphin movements and foraging behavior. Sci. Rep. 10:19247. doi: 10.1038/s41598-020-76077-3
Farcas, A., Powell, C. F., Brookes, K. L., and Merchant, N. D. (2020). Validated shipping noise maps of the Northeast Atlantic. Sci. Total Environ. 735:139509. doi: 10.1016/j.scitotenv.2020.139509
Farina, A., and James, P. (2016). The acoustic communities: definition, description and ecological role. Biosystems 147, 11–20. doi: 10.1016/j.biosystems.2016.05.011
Farina, A., Gage, S. H., and Salutari, P. (2018). Testing the ecoacoustics event detection and identification (EEDI) approach on Mediterranean soundscapes. Ecol. Ind. 85, 698–715. doi: 10.1016/j.ecolind.2017.10.073
Fish, M. P. (1948). Sonic Fishes of the Pacific. Woods Hole: Woods Hole Oceanographic Institution. doi: 10.1575/1912/2767
Fish, M. P., and Mowbray, W. H. (1970). Sounds of Western North Atlantic fishes, A Reference File of Biological Underwater Sounds. London: John Hopkins.
FonoZoo. (2021). Fonoteca Zoológica, Museo Nacional de Ciencias Naturales de Madrid (Spain). Available online at: http://www.fonozoo.com/index.php [Accessed October 25, 2021]
Francis, C. D., Ortega, C. P., and Cruz, A. (2009). Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. doi: 10.1016/j.cub.2009.06.052
Frasier, K. E., Roch, M. A., Soldevilla, M. S., Wiggins, S. M., Garrison, L. P., and Hildebrand, J. A. (2017). Automated classification of dolphin echolocation click types from the Gulf of Mexico. PLoS Comput. Biol. 13:e1005823. doi: 10.1371/journal.pcbi.1005823
Frazao, F., Padovese, B., and Kirsebom, O. S. (2019). Workshop Report: detection and Classification in Marine Bioacoustics with Deep Learning. Available online at: https://arxiv.org/abs/2002.08249 [Accessed November 4, 2021].
Froese, R., and Pauly, D. (eds) (2021). FishBase. World Wide Web electronic publication. Available online at: www.fishbase.org. version (08/2021)
Gabriele, C., Ponirakis, D. W., and Klinck, H. (2021). Underwater sound levels in Glacier Bay during reduced vessel traffic due to the COVID-19 pandemic. Front. Mar. Sci. 8:736. doi: 10.3389/fmars.2021.674787
Garland, E. C., Goldizen, A. W., Rekdahl, M. L., Constantine, R., Garrigue, C., Hauser, N. D., et al. (2011). Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 21, 687–691. doi: 10.1016/j.cub.2011.03.019
Gaunt, S. L. L., Nelson, D. A., Dantzker, M. S., Budney, G. F., and Bradbury, J. W. (2005). New directions for bioacoustics collections. Auk 122, 984–987. doi: 10.1093/auk/122.3.984
Gavrilov, A., and McCauley, R. D. (2012). Steady inter and intra-annual decrease in the vocalization frequency of Antarctic blue whales. J. Acoust. Soc. Am. 131, 4476–4480. doi: 10.1121/1.4707425
Gedamke, J., and Robinson, S. M. (2010). Acoustic survey for marine mammal occurrence and distribution of East Antarctica (30-80°E) in January-February 2006. Deep-Sea Res. II 57, 968–981. doi: 10.1016/j.dsr2.2008.10.042
Gerald, J. W. (1971). Sound production during courtship in six species of sunfish (Centrarchidae). Evolution 25, 75–87. doi: 10.2307/2406500
Gordon, T. A. C., Harding, H. R., Wong, K. E., Merchant, N. D., Meekan, M. G., McCorkmick, M. I., et al. (2018). Habitat degradation negatively affects auditory settlement behavior of coral reef fishes. Proc. Nat. Acad. Sci. 115, 5193–5198. doi: 10.1073/pnas.1719291115
Grabowski, T., Young, S. P., and Cott, P. A. (2020). Looking for love under the ice: using passive acoustics to detect burbot (Lota lota: Gadidae) spawning activity. Freshw. Biol. 65, 37–44. doi: 10.1111/fwb.13314
Greenhalgh, J. A., Genner, M. J., Jones, G., and Desjonquères, C. (2020). The role of freshwater bioacoustics in ecological research. WIREs Water. 7:e1416. doi: 10.1002/wat2.1416
Harvey, E., Fletcher, D., Shortis, M. R., and Kendrick, G. A. (2004). A comparison of underwater visual distance estimates made by scuba divers and a stereo-video system: implications for underwater visual census of reef fish abundance. Mar. Freshw. Res. 55, 573–580. doi: 10.1071/MF03130
Heimlich, S., Mellinger, D., and Klicnk, H. (2021). MobySound. Available online at: http://www.mobysound.org/ [Accessed October 22, 2021]
Higgs, D. M., and Beach, R. K. (2021). Ecoacoustic monitoring of lake sturgeon (Acipenser fulvescens) spawning and its relation to anthropogenic noise. J. App. Ichthy. 37, 816–825. doi: 10.1111/jai.14269
Hildebrand, J. A. (2009). Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20. doi: 10.3354/Meps08353
Holt, D. E., and Johnston, C. E. (2014). Sound production and associated behaviours in blacktail shiner Cyprinella venusta: a comparison between field and lab. Environ. Biol. Fishes 97, 1207–1219. doi: 10.1007/s10641-013-0208-5
Hughes, A. R., Mann, D. A., and Kimbro, D. L. (2014). Predatory fish sounds can alter crab foraging behaviour and influence bivalve abundance. Proc. R. Soc. B. 281:20140715. doi: 10.1098/rspb.2014.0715
Hussey, N. E., Kessel, S. T., Aaarestrup, K., Cooke, S. J., Cowley, P. D., Fisk, A. T., et al. (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1221. doi: 10.1126/science.1255642
Ibrahim, A. K., Zhuang, H., Chérubin, L. M., Schärer-Umpierre, M. T., and Erdol, N. (2018). Automatic classification of grouper species by their sounds using deep neural networks. J. Acoust. Soc. Am. 144, EL196–EL202. doi: 10.1121/1.5054911
Janik, V. M., and Sayigh, L. S. (2013). Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A 199, 479–489. doi: 10.1007/s00359-013-0817-7
Jones, R. E., Griffin, R. A., Rees, S. C., and Unsworth, R. K. (2019). Improving visual biodiversity assessments of motile fauna in turbid aquatic environments. Limnol. Oceanogr. Methods 17, 544–554. doi: 10.1002/lom3.10331
Kaatz, I. (2002). Multiple sound-producing mechanisms in teleost fishes and hypotheses regarding their behavioural significance. Bioacoustics 12, 230–233. doi: 10.1080/09524622.2002.9753705
Kahl, S., Wood, C. M., Eibl, M., and Klinck, H. (2021). BirdNET: a deep learning solution for avian diversity monitoring. Ecol. Inform. 61:101236. doi: 10.1016/j.ecoinf.2021.101236
Karaconstantis, C., Desjonquères, C., Gifford, T., and Linke, S. (2020). Spatio-temporal heterogeneity in river sounds: Disentangling micro- and macro-variation in a chain of waterholes. Freshw. Biol. 65, 96–106. doi: 10.1111/fwb.13439
Kaschner, K. (2012). “The SOUNDS table in FishBase” in World Wide Web Electronic Publication. eds R. Froese and D. Pauly. Available online at: www.fishbase.org, version (08/2012)
Krause, B. L. (2008). Anatomy of the soundscape: evolving perspectives. J. Audio Eng. Soc. 56, 73–80.
Lagardère, J. P., and Mariani, A. (2006). Spawning sounds in meagre Argyrosomus regius recorded in the Gironde estuary. France. J. Fish Biol. 69, 1697–1708. doi: 10.1111/j.1095-8649.2006.01237.x
Lammers, M. O., and Au, W. W. L. (2003). Directionality in the whistles of Hawaiian spinner dolphins (Stenella longirostris): a signal feature to cue direction of movement? Mar. Mamm. Sci. 19, 249–264. doi: 10.1111/j.1748-7692.2003.tb01107.x
Lammers, M. O., and Munger, L. M. (2016). “From Shrimp to Whales: biological Applications of Passive Acoustic Monitoring on a Remote Pacific Coral Reef” in Listening in the Ocean. Modern Acoustics and Signal Processing. eds W. W. Au and N. Lammers (New York: Springer). doi: 10.1007/978-1-4939-3176-7_4
Lamont, T. A. C., Chapuis, L., Williams, B., Dines, S., Gridley, T., Frainer, G., et al. (2022). HydroMoth: testing a Prototype Low-cost Acoustic Recorder for Aquatic Environments. London: Zoological Society of London. doi: 10.1002/rse2.249
Langlois, T., Goetze, J., Bond, T., Monk, J., Abesamis, R. A., Asher, J., et al. (2020). A field and video annotation guide for baited remote underwater stereo video surveys of demersal fish assemblages. Methods Ecol. Evol. 11, 1401–1409. doi: 10.1111/2041-210X.13470
Le Bien, J. (2017). Automated Species Classification Methods for Passive Acoustic Monitoring of Beaked Whales. New Orleans: University of New Orleans.
Le Bot, O., Mars, J. I., Gervaise, C., and Simard, Y. (2015). Rhythmic analysis for click train detection and source separation with examples on beluga whales. Appl. Acoust. 95, 37–49. doi: 10.1016/j.apacoust.2015.02.005
Lédée, E. J. I., Heupel, M. R., Taylor, M. D., Harcourt, R. G., Jaine, F. R. A., Huveneers, C., et al. (2021). Continental-scale acoustic telemetry and network analysis reveal new insights into stock structure. Fish Fish. 22, 987–1005. doi: 10.1111/faf.12565
Leon-Lopez, B., Romero-Vivas, E., and Viloria-Gomora, L. (2021). Reduction of roadway noise in a coastal city underwater soundscape during COVID-19 confinement. J. Acoust. Soc. Am. 149, 652–659. doi: 10.1121/10.0003354
Lin, T. H., Fang, S. H., and Tsao, Y. (2017). Improving biodiversity assessment via unsupervised separation of biological sounds from long-duration recordings. Sci. Rep. 7:4547. doi: 10.1038/s41598-017-04790-7
Lin, T.-H., Akamatsu, T., and Tsao, Y. (2021). Sensing ecosystem dynamics via audio source separation: a case study of marine soundscapes off northeastern Taiwan. PLoS Comput. Biol. 17:e1008698. doi: 10.1371/journal.pcbi.1008698
Lin, T.-H., and Tsao, Y. (2020). Source separation in ecoacoustics: a roadmap towards versatile soundscape information retrieval. Remote Sens. Ecol. Conserv. 6, 236–247. doi: 10.1002/rse2.141
Lin, T.-H., Chen, C., Watanabe, H. K., Kawagucci, S., Yamamoto, H., and Akamatsu, T. (2019). Using soundscapes to assess deep-sea benthic ecosystems. Trends Ecol. Evol. 34, 1066–1069. doi: 10.1016/j.tree.2019.09.006
Lindseth, A. V., and Lobel, P. S. (2018). Underwater soundscape monitoring and fish bioacoustics: a review. Fishes 3:36. doi: 10.3390/fishes3030036
Linke, S., Decker, E., Gifford, T., and Desjonquères, C. (2020). Diurnal variation in freshwater ecoacoustics: implications for site-level sampling design. Freshw. Biol. 65, 86–95. doi: 10.1111/fwb.13227
Lobel, P. S. (1992). Sounds produced by spawning fishes. Environ. Biol. Fishes. 33, 351–358. doi: 10.1007/BF00010947
Lobel, P. S. (1996). Spawning sound of the trunkfish, Ostracion meleagris (Ostraciidae). Biol. 191, 308–309. doi: 10.1086/BBLv191n2p308
Lobel, P. S. (1998). Possible species specific courtship sounds by two sympatric cichlid fishes in Lake Malawi. Africa. Environ. Biol. Fishes. 52, 443–452. doi: 10.1023/A:1007467818465
Lobel, P. S. (2001). Fish bioacoustics and behavior: passive acoustic detection and the application of a closed-circuit rebreather for field study. Mar. Technol. Soc. J. 35, 19–28. doi: 10.4031/002533201788001884
Lobel, P. S., Kaatz, I. M., and Rice, A. N. (2010). “Acoustical behavior of coral reef fishes” in Reproduction and Sexuality in Marine Fishes: evolutionary Patterns and Innovations. ed. K. S. Cole (San Diego: Elsevier Academic Press). 307–386. doi: 10.1525/9780520947979-013
Locascio, J. V., and Mann, D. A. (2005). Effects of Hurricane Charley on fish chorusing. Biol. Lett. 1, 362–365. doi: 10.1098/rsbl.2005.0309
Looby, A., Riera, A., Vela, S., Cox, K., Rountree, F., Juanes, F., et al. (2021). FishSounds, version 1.0. Available online at: http://www.fishsounds.net [Accessed on October 17, 2021]
Lu, X., Tsao, Y., Matsuda, S., and Hori, C. (2013). Speech enhancement based on deep denoising autoencoder. Interspeech 2013, 436–440.
Luczkovich, J. J., and Keusenkothen, M. (2007). “Behavior and sound production by longspine squirrelfish Holocentrus rufus during-playback of Predator and conspecific sounds” in Diving for Science 2007 Proceedings of the American Academy of Underwater Sciences 26th Symposium. eds N. W. Pollock and J. M. Godfrey (Dauphin Island: AAUS). 127–134.
Luczkovich, J. J., Mann, D., and Rountree, R. (2008). Passive acoustics as a tool in fisheries science. Trans. Am. Fish. Soc. 137, 533–541. doi: 10.1577/T06-258.1
Luczkovich, J. J., Sprague, M. W., Johnson, S. E., and Pullinger, C. (1999). Delimiting spawning areas of weakfish Cynoscion regalis (family Sciaenidae) in Pamlico Sound, North Carolina using passive hydroacoustic surveys. Bioacoustics 10, 143–160. doi: 10.1080/09524622.1999.9753427
Luo, W., Yang, W., and Zhang, Y. (2019). Convolutional neural network for detecting odontocete echolocation clicks. J. Acoust. Soc. Am. 145, EL7–EL12. doi: 10.1121/1.5085647
MacAodha, O., Gibb, R., Barlow, K. E., Browning, E., and Firman, M. (2018). Bat detective—deep learning tools for bat acoustic signal detection. PLoS Comput. Biol. 14:e1005995. doi: 10.1371/journal.pcbi.1005995
Macaulay Library (2021). Available online at: https://www.macaulaylibrary.org [Accessed October 20, 2021].
Madhusudhana, S., Gavrilov, A. N., and Erbe, C. (2015). Automatic detection of echolocation clicks based on a Gabor model of their waveform. J. Acoust. Soc. Am. 137, 3077–3086. doi: 10.1121/1.4921609
Madhusudhana, S., Murray, A., and Erbe, C. (2020). Automatic detectors for low-frequency vocalizations of Omura’s whales, Balaenoptera omurai: a performance comparison. J. Acoust. Soc. Am. 147, 3078–3090. doi: 10.1121/10.0001108
Malfante, M., Mars, J. I., Dalla Mura, M., and Gervaise, C. (2018). Automatic fish sounds classification. J. Acoust. Soc. Am. 143, 2834–2846. doi: 10.1121/1.5036628
Mallekh, R., Lagardère, J. P., Eneau, J. P., and Cloutour, C. (2003). An acoustic detector of turbot feeding activity. Aquaculture 221, 481–489. doi: 10.1016/s0044-8486(03)00074-7
Mann, D. A., and Grothues, T. M. (2008). Short-term upwelling events modulate fish sound production at a mid-Atlantic Ocean observatory. Mar. Ecol. Prog. Ser. 375, 65–71. doi: 10.3354/meps07720
Mann, D. A., and Jarvis, S. M. (2004). Potential sound production by a deep-sea fish. J. Acoustic. Soc. Am. 115, 2331–2333. doi: 10.1121/1.1694992
Mann, D. A., Bowers-Altman, J., and Rountree, R. A. (1997). Sounds produced by the striped cusk-eel Ophidion marginatum (Ophidiidae) during courtship and spawning. Copeia 1997, 610–612. doi: 10.2307/1447568
Mann, D. A., Locascio, J. V., Coleman, F. C., and Koenig, C. C. (2009). Goliath grouper Epinephelus itajara sound production and movement patterns on aggregation sites. Endanger. Species Res. 7, 229–236. doi: 10.3354/esr00109
Marques, L. (2020). Collapse of Biodiversity in the Aquatic Environment. Switzerland: Springer. 275–301. doi: 10.1007/978-3-030-47527-7_11
Matley, J. K., Klinard, N. V., Barbosa-Martins, A. P., Thostad, E. B., Vandergoot, C. S., Fisk, A. T., et al. (2021). Global trends in aquatic animal tracking with acoustic telemetry. Trends Ecol. Evol. 37, 79–94. doi: 10.1016/j.tree.2021.09.001
McCauley, R. D., Meekan, M. G., and Parsons, M. J. G. (2021). Acoustic pressure, particle motion, and induced ground motion signals from a commercial seismic survey array and potential implications for environmental monitoring. J. Mar. Sci. Eng. 9:571 doi: 10.3390/jmse9060571
McCordic, J. A., Root-Gutteridge, H., Cusano, D. A., Denes, S. L., and Parks, S. E. (2016). Calls of North Atlantic right whales Eubalaena glacialis contain information on individual identity and age class. Endanger. Species Res. 30, 157–169. doi: 10.3354/esr00735
McDonald, M. A., Hildebrand, J. A., and Mesnick, S. (2009). Worldwide decline in tonal frequencies of blue whale songs. Endang. Species Res. 9, 13–21. doi: 10.3354/esr00217
McIver, E. L., Marchaterre, M. A., Rice, A. N., and Bass, A. H. (2014). Novel underwater soundscape: acoustic repertoire of plainfin midshipman fish. J. Exp. Biol. 217, 2377–2389. doi: 10.1242/jeb.102772
McKenna, M. F., Baumann-Pickering, S., Kok, A. C. M., Oestreich, W. K., Adams, J. D., Barkowski, J., et al. (2021). Advancing the interpretation of shallow water marine soundscapes. Front. Mar. Sci. 8:719258. doi: 10.3389/fmars.2021.719258
McWilliam, J. N., McCauley, R. D., Erbe, C., and Parsons, M. J. G. (2017). Patterns of biophonic periodicity on coral reefs in the Great Barrier Reef. Sci. Rep. 7:17459. doi: 10.1038/s41598-017-15838-z
Meekan, M. G., Speed, C. W., McCauley, R. D., Fisher, R., Birt, M. J. Currey-Randall, L. M., et al. (2021). A large-scale experiment finds no evidence that a seismic survey impacts a demersal fish fauna. Proc. Natl. Acad. Sci. U.S.A. 118:e2100869118. doi: 10.1073/pnas.2100869118
Mellinger, D. K., and Clark, C. W. (1997). Methods for automatic detection of mysticete sounds. Mar. Freshw. Behav. Physiol. 29, 163–181. doi: 10.1080/10236249709379005
Mellinger, D. K., and Clark, C. W. (2006). MobySound: a reference archive for studying automatic recognition of marine mammal sounds. Appl. Acoust. 67, 1226–1242. doi: 10.1016/j.apacoust.2006.06.002
Menze, S., Zitterbart, D. P., van Opzeeland, I., and Boebel, O. (2017). The influence of sea ice, wind speed and marine mammals on Southern Ocean ambient sound. Roy. Soc. Open Sci. 4:160370. doi: 10.1098/rsos.160370
Miller, B. S., The Iwc-Sorp/Soos Acoustic Trends Working Group, Balcazar, N., Nieukirk, N. S., Leroy, E. C., Aulich, M., et al. (2021). An open access dataset for developing automated detectors of Antarctic baleen whale sounds and performance evaluation of two commonly used detectors. Sci. Rep. 11:806. doi: 10.1038/s41598-020-78995-8
Montie, E. W., Hoover, M., Kehrer, C., Yost, J., Brenkert, K., O’Donnell, T., et al. (2017). Acoustic monitoring indicates a correlation between calling and spawning in captive spotted seatrout (Cynoscion nebulosus). PeerJ. 5:e2944. doi: 10.7717/peerj.2944
Mooney, T. A. C., Di Iorio, L., Lammers, M., Lin, T. H., Nedelec, S. L., Parsons, M. J. G., et al. (2020). Listening forward: approaching marine biodiversity assessments using acoustic methods. Roy. Soc. Open Sci. 7:201287. doi: 10.1098/rsos.201287
Morano, J. L., Rice, A. N., Tielens, J. T., Estabrook, B. J., Murray, A., Roberts, B., et al. (2012a). Acoustically detected year-round presence of right whales in an urbanized migration corridor. Conserv. Biol. 26, 698–707. doi: 10.1111/j.1523-1739.2012.01866.x
Morano, J. L., Salisbury, D. P., Rice, A. N., Conklin, K. L., Falk, K. L., and Clark, C. W. (2012b). Seasonal changes in fin whale song in the Western North Atlantic Ocean. J. Acoust. Soc. Am. 132, 1207–1212. doi: 10.1121/1.4730890
Mosharo, K. K., and Lobel, P. S. (2012). Acoustic signals of two toadfishes from Belize: Sanopus astrifer and Batrachoides gilberti (Batrachoididae). Environ. Biol. Fishes 94, 623–638. doi: 10.1007/s10641-011-9969-x
Moulton, J. M. (1958). The acoustical behavior of some fishes in the Bimini area. Biol. Bull. 114, 357–374. doi: 10.2307/1538991
Moulton, J. M. (1960). Swimming sounds and the schooling of fishes. Biol. Bull. 119, 210–223. doi: 10.2307/1538923
Moulton, J. M. (1963). “Acoustic behaviour of fishes” in Acoustic Behaviour of Animals. ed. R. G. Busnel (Amsterdam: Elsevier). 655–693.
Mouy, X., Rountree, R. A., Dosso, S. E., and Juanes, F. (2018). Cataloging fish sounds in the wild using combined acoustic and video recordings. J. Acoust. Soc. Am. 143, EL333–EL339. doi: 10.1121/1.5037359
Newhall, A. E., Ying, L., Miller, J., Potty, G., Vigness-Rapose, K., Frankel, A., et al. (2016). Monitoring the acoustic effects of pile driving for the first offshore wind farm in the United States. J. Acoust. Soc. Am. 139:2181. doi: 10.1121/1.4950483
Nikolich, K., Halliday, W. D., Pine, M. K., Cox, K., Black, M., Morris, C., et al. (2021). The sources and prevalence of anthropogenic noise in Rockfish Conservation Areas with implications for marine reserve planning. Mar. Pollut. Bull. 164:112017. doi: 10.1016/j.marpolbul.2021.112017
Noda, J. J., Travieso, C. M., and Sánchez-Rodríguez, D. (2016). Automatic taxonomic classification of fish based on their acoustic signals. Appl. Sci. 6:443. doi: 10.3390/app6120443
Ocean Networks Canada (2021). Ocean Networks Canada SoundCloud. Available online at: https://soundcloud.com/oceannetworkscanada [Accessed October 21, 2021].
Ogundile, O. O., and Versfeld, D. J. J. (2020). Analysis of template-based detection algorithms for inshore Bryde’s whale short pulse calls. IEEE Access 8, 14377–14385. doi: 10.1109/ACCESS.2020.2966254
Ozanich, E., Thode, A., Gerstoft, P., Freeman, L. A., and Freeman, S. (2021). Deep embedded clustering of coral reef bioacoustics. J. Acoust. Soc. Am. 49, 2587–2601. doi: 10.1121/10.0004221
Pangerc, T., Theobald, P. D., Wang, L. S., Robinson, S. P., and Lepper, P. A. (2016). Measurement and characterisation of radiated underwater sound from a 3.6 MW monopile wind turbine. J. Acoust. Soc. Am. 140, 2913–2922. doi: 10.1121/1.4964824
Parmentier, E., Bertucci, F., Bolgan, M., and Lecchini, D. (2021). How many fish could be vocal? An estimation from a coral reef (Moorea Island). Belg. J. Zool. 151, 1–29. doi: 10.26496/bjz
Parmentier, E., Bouillac, G., Dragičević, B., Dulčić, J., and Fine, M. (2010). Call properties and morphology of the sound-producing organ in Ophidion rochei (Ophidiidae). J. Exp. Biol. 213, 3230–3236. doi: 10.1242/jeb.044701
Parmentier, E., Diogo, R., and Fine, M. L. (2017). Multiple exaptations leading to fish sound production. Fish Fish. 18, 958–966. doi: 10.1111/faf.12217
Parmentier, E., Fine, M. L., and Mok, H.-K. (2016). Sound production by a recoiling system in the Pempheridae and Terapontidae. J. Morphol. 277, 717–724. doi: 10.1002/jmor.20529
Parmentier, E., Fontenelle, N., Fine, M., Vandewalle, P., and Henrist, C. (2006). Functional morphology of the sonic apparatus in Ophidion barbatum (Teleostei. Ophidiidae). J. Morphol. 267, 1461–1468. doi: 10.1002/jmor.10496
Parmentier, E., Lagardere, J. P., Vandewalle, P., and Fine, M. L. (2005). Geographical variation in sound production in the anemonefish Amphiprion akallopisos. Proc. Royal Soc. B Biological Sci. 272, 1697–1703. doi: 10.1098/rspb.2005.3146
Parsons, M. J. G. (2010). An Investigation into Active and Passive Acoustic Techniques to Study Aggregating Fish Species. Australia: Curtin University.
Parsons, M. J. G., and McCauley, R. D. (2017). Sound production by mulloway (Argyrosomus japonicus) and variation within individual calls. Acoust. Aust. 45, 261–272. doi: 10.1007/s40857-017-0112-9
Parsons, M. J. G., Erbe, C., Meekan, M. G., and Parsons, S. K. (2021). A review and meta-analysis of underwater noise radiated by small (<25 m length) vessels. J. Mar. Sci. Eng. 9:827. doi: 10.3390/jmse9080827
Parsons, M. J. G., Longbottom, S., Lewis, P., McCauley, R. D., and Fairclough, D. V. (2013a). Sound production by the West Australian dhufish (Glaucosoma hebraicum). J. Acoust. Soc. Am. 134, 2701–2709. doi: 10.1121/1.4818775
Parsons, M. J. G., McCauley, R. D., and Mackie, M. C. (2013b). Characterisation of mulloway (Argyrosomus japonicus) advertisement sounds. Acoust. Aust. 41, 196–201.
Parsons, M. J. G., McCauley, R. D., Mackie, M. C., Siwabessy, P. J., and Duncan, A. J. (2012). In situ source levels of mulloway (Argyrosomus japonicus) calls. J. Acoust. Soc. Am. 132, 3559–3568. doi: 10.1121/1.4756927
Parsons, M. J. G., McCauley, R. D., Mackie, M. C., Siwabessy, P., and Duncan, A. J. (2009). Localization of individual mulloway (Argyrosomus japonicus) within a spawning aggregation and their behaviour throughout a diel spawning period. ICES J. Mar. Sci. 66, 1007–1014. doi: 10.1093/icesjms/fsp016
Parsons, M. J. G., Salgado-Kent, C. P., Marley, S. A., Gavrilov, A. N., and McCauley, R. D. (2016). Characterizing diversity and variation in fish choruses in Darwin Harbour. ICES J. Mar. Sci. 73, 2058–2074. doi: 10.1093/icesjms/fsw037
Phillips, Y. F., Towsey, M., and Roe, P. (2018). Revealing the ecological content of long-duration audio-recordings of the environment through clustering and visualisation. PLoS One 13:e0193345. doi: 10.1371/journal.pone.0193345
Pine, M. K., Wilson, L., Jeffs, A. G., McWhinnie, L., Juanes, F., Scuderi, A., et al. (2021). A Gulf in lockdown: how an enforced ban on recreational vessels increased dolphin and fish communication ranges. Glob. Change Biol. 27, 4839–4848. doi: 10.1111/gcb.15798
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925. doi: 10.1038/nclimate1958
Popper, A. N., and Hawkins, A. D. (2019). An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 94, 692–713. doi: 10.1111/jfb.13948
Popper, A., Salmon, M., and Horch, K. (2001). Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A 187, 83–89. doi: 10.1007/s003590100184
Potamitis, I. (2014). Automatic classification of a taxon-rich community recorded in the wild. PLoS One 9:e96936. doi: 10.1371/journal.pone.0096936
Pyć, C. D., Vallarta, J. H., Rice, A. N., Zeddies, D. G., Maxner, E. E., and Denes, S. L. (2021). Vocal behavior of the endangered splendid toadfish and potential masking by anthropogenic noise. Cons. Sci. Prac. 3:e352. doi: 10.1111/csp2.352
Radford, C., Jeffs, A., Tindle, C., and Montgomery, J. C. (2008). Resonating sea urchin skeletons create coastal choruses. Mar. Ecol. Prog. Ser. 362, 37–43. doi: 10.3354/meps07444
Rako, N., Fortuna, C. M., Holcer, D., Mackelworth, P., Nimak-Wood, M., Pleslić, G., et al. (2013). Leisure boating noise as a trigger for the displacement of the bottlenose dolphins of the Cres–Lošinj archipelago (northern Adriatic Sea, Croatia). Mar. Pollut. Bull. 68, 77–84. doi: 10.1016/j.marpolbul.2012.12.019
Ramirez, F., Afan, I., Davis, L. S., and Chiaradia, A. (2017). Climate impacts on global hot spots of marine biodiversity. Sci. Adv. 3:e1601198. doi: 10.1126/sciadv.1601198
Recalde-Salas, A., Erbe, C., Salgado Kent, C., and Parsons, M. (2020). Non-song vocalizations of humpback whales in Western Australia. Front. Mar. Sci. 7:141. doi: 10.3389/fmars.2020.00141
Reine, K. J., Clarke, D., and Dickerson, C. (2014). Characterization of underwater sounds produced by hydraulic and mechanical dredging operations. J. Acoust. Soc. Am. 135, 3280–3294. doi: 10.1121/1.4875712
Ricci, S. W., Bohnenstiehl, D. R., Eggleston, D. B., Kellogg, M. L., and Lyon, R. P. (2017). Oyster toadfish (Opsanus tau) boatwhistle call detection and patterns within a large-scale oyster restoration site. PLoS One 12:e0182757. doi: 10.1371/journal.pone.0182757
Rice, A. N., and Bass, A. H. (2009). Novel vocal repertoire and paired swimbladders of the three-spined toadfish, Batrachomoeus trispinosus: insights into the diversity of the Batrachoididae. J. Exp. Biol. 212, 1377–1391. doi: 10.1242/jeb.028506
Rice, A.N., Farina, S.C., Makowski, A.J., Kaatz, I.M., Lobel, P.S., Bemis, W.E., and Bass, A.H. (2022). Evolutionary patterns in sound production across fishes. Ichthyol. Herpetol. 110, 1–12. doi: 10.1643/i2020172
Rice, A. N., Morano, J. L., Hodge, K. B., and Muirhead, C. A. (2016). Spatial and temporal patterns of toadfish and black drum chorusing activity in the South Atlantic Bight. Environ. Biol. Fishes. 99, 705–716. doi: 10.1007/s10641-016-0511-z
Rice, A. N., Palmer, K. J., Muirhead, C. A., Tielens, J. T., and Clark, C. W. (2014). Potential Bryde’s whale (Balaenoptera edeni) calls recorded in the Northern Gulf of Mexico. J. Acoust. Soc. Am. 135, 3066–3076. doi: 10.1121/1.4870057
Richardson, W. J., Greene, C. R. Jr., Malme, C. I., and Thomson, D. H. (2013). Marine Mammals and Noise. New York: Academic Press.
Riera, A., Rountree, R. A., and Juanes, F. (2017). Auditioning fish for sound production in captivity to contribute to a catalogue of known fish sounds to inform regional passive acoustic studies. J. Acoust. Soc. Am. 141:3862. doi: 10.1121/1.4988628
Riera, A., Rountree, R. A., Pine, M. K., and Juanes, F. (2018). Sounds of Arctic cod (Boreogadus saida) in captivity: a preliminary description. J. Acoust. Soc. Am. 143, EL317–EL321. doi: 10.1121/1.5035162
Risch, D., Castellote, M., Clark, C. W., Davis, G. E., Dugan, P. J., Hodge, L. E. W., et al. (2014). Seasonal migrations of North Atlantic minke whales: novel insights from large-scale passive acoustic monitoring networks. Mov. Ecol. 2:24. doi: 10.1186/s40462-014-0024-3
Roca, I. T., Magnan, P., and Proulx, R. (2020). Use of acoustic refuges by freshwater fish: theoretical framework and empirical data in a three-species trophic system. Freshw. Biol. 65, 45–54. doi: 10.1111/fwb.13077
Roch, M. A., Lindeneau, S., Aurora, G. S., Frasier, K. E., Hildebrand, J. A., Glotin, H., et al. (2021). Using context to train time-domain echolocation click detectors. J. Acoust. Soc. Am. 149, 3301–3310. doi: 10.1121/10.0004992
Rogers, T. L., Cato, D. H., and Bryden, M. M. (1996). Behavioral significance of underwater vocalizations of captive leopard seals, Hydurga leptonyx. Mar. Mam. Sci. 12, 414–427. doi: 10.1111/j.1748-7692.1996.tb00593.x
Rosel, P. E., Wilcox, L. A., Yamada, T. K., and Mullin, K. D. (2021). A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Mar. Mam. Sci. 37, 577–610. doi: 10.1111/mms.12776
Rountree, R. A., and Juanes, F. (2020). Potential for use of passive acoustic monitoring of piranhas in the Pacaya–Samiria National Reserve in Peru. Freshw. Biol. 65, 55–65. doi: 10.1111/fwb.13185
Rountree, R. A., Bolgan, M., and Juanes, F. (2019). How can we understand freshwater soundscapes without fish sound descriptions? Fisheries. 44, 137–143. doi: 10.1002/fsh.10190
Rountree, R. A., Gilmore, R. G., Goudey, R. G., Goudey, C. A., Hawkins, A. D., Luczkovich, J. J., et al. (2006). Listening to fish: applications of passive acoustics to fisheries science. Fisheries 31, 433–446. doi: 10.1577/1548-8446(2006)31[433:ltf]2.0.co;2
Rountree, R. A., Juanes, F., and Bolgan, M. (2018). Air movement sound production by alewife, white sucker, and four salmonid fishes suggests the phenomenon is widespread among freshwater fishes. PLoS One 13:e0204247. doi: 10.1371/journal.pone.0204247
Rountree, R. A., Juanes, F., and Bolgan, M. (2020). Temperate freshwater soundscapes: a cacophony of undescribed biological sounds now threatened by anthropogenic noise. PLoS One 15:e0221842. doi: 10.1371/journal.pone.0221842
Rountree, R. A., Juanes, F., Goudey, C. A., and Ekstrom, K. E. (2012). “Is biological sound production important in the deep sea?” in The Effects of Noise on Aquatic Life. eds A. N. Popper and A. Hawkins (New York: Springer). 181–183. doi: 10.1007/978-1-4419-7311-5_41
Rountree, R. A., Perkins, P. J., Kennery, R. D., and Hinga, K. R. (2002). Sounds of western north Atlantic fishes – data rescue. Bioacoustics 12, 242–244. doi: 10.1080/09524622.2002.9753710
Rowley, J. J. L., Callaghan, C. T., Cutajar, T., Portway, C., Potter, K., Mahony, S., et al. (2019). FrogID: citizen scientists provide validated biodiversity data on frogs of Australia. Herpetol. Conserv. Biol. 14, 155–170. doi: 10.3897/zookeys.912.38253
Ruppe, L., Clement, G., Herrel, A., Ballesta, L., Decamps, T., Kever, L., et al., (2015). Environmental constraints drive the partitioning of the soundscape in fishes. Proc. Nat. Acad. Soc. PNAS, 112, 6092–6097, doi: 10.1073/pnas.1424667112
Ryan, J. P., Joseph, J. E., Margolina, T., Hatch, L. T., Azzara, A., Reyes, A., et al. (2021). Reduction of low-frequency vessel noise in Monterey Bay National Marine Sanctuary during the COVID-19 pandemic. Front. Mar. Sci. 8:656566. doi: 10.3389/fmars.2021.656566
Rycyk, A. M., Tyson Moore, R. B., Wells, R. S., McHugh, K. A., McCabe, E. J., and Mann, D. A. (2020). Passive acoustic listening stations (PALS) show rapid onset of ecological effects of harmful algal blooms in real time. Sci. Rep. 10:17863. doi: 10.1038/s41598-020-74647-z
Sainburg, T., Thielk, M., and Gentner, T. Q. (2020). Finding, visualizing, and quantifying latent structure across diverse animal vocal repertoires. PLoS Comput. Biol. 16:e1008228. doi: 10.1371/journal.pcbi.1008228
Sala, E., and Knowlton, N. (2006). Global marine biodiversity trends. Annu. Rev. Environ. Resour. 31, 93–122. doi: 10.1146/annurev.energy.31.020105.100235
Sayigh, L., Daher, M. A., Allen, J., Gordon, H., Joyce, K., Stuhlmann, C., et al. (2016). The Watkins marine mammal sound database: an online, freely accessible resource. Proc. Mtgs. Acoust. 27:040013. doi: 10.1121/2.0000358
Schafer, R. M. (1969). The New Soundscape: a Handbook for the Modern Music Teacher. Canada: Berandol Music.
Schafer, R. M. (1977). The sounds: our Sonic Environment and the Tuning of the World. Rochester: Destiny Books.
Schall, E., Thomisch, K., Boebel, O., Gerlach, G., Woods, S. M., El-Gabbas, A., et al. (2021). Multi-year presence of humpback whales in the Atlantic sector of the Southern Ocean but not during El Niño. Commun. Biol. 4:790. doi: 10.1038/s42003-021-02332-6
Schärer, M. T., Nemeth, M. I., Rowell, T. J., and Appeldoorn, R. S. (2014). Sounds associated with the reproductive behavior of the black grouper (Mycteroperca bonaci). Mar. Biol. 161, 141–147. doi: 10.1007/s00227-013-2324-3
Scheinin, A., Kerem, D., MacLeod, C., Gazo, M., Chicote, C., and Castellote, M. (2011). Gray whale (Eschrichtius robustus) in the Mediterranean Sea: anomalous event or early sign of climate-driven distribution change? Mar. Biodivers. Rec. 4:E28. doi: 10.1017/S1755267211000042
Sequeira, A. M. M., Heupel, M. R., Lea, M.-A., Eguíluz, V. M., Duarte, C. M., Meekan, M. G., et al. (2019). The importance of sample size in marine megafauna tagging studies. Ecol. Appl. 29:e01947. doi: 10.1002/eap.1947
Sertlek, H. Ö (2021). Hindcasting Soundscapes before and during the COVID-19 Pandemic in Selected Areas of the North Sea and the Adriatic Sea. J. Mar. Sci. Engin. 9:702. doi: 10.3390/jmse9070702
Shamir, L., Yerby, C., Simpson, R., von Benda-Beckmann, A. M., Tyack, P., Samarra, F., et al. (2014). Classification of large acoustic datasets using machine learning and crowdsourcing: application to whale calls. J. Acoust. Soc. Am. 135, 953–962. doi: 10.1121/1.4861348
Shiu, Y., Palmer, K. J., Roch, M. A., Fleishman, E., Liu, X., Nosal, E.-M., et al. (2020). Deep neural networks for automated detection of marine mammal species. Sci. Rep. 10:607. doi: 10.1038/s41598-020-57549-y
Širović, A., Oleson, E. M., Buccowich, J., Rice, A., and Bayless, A. R. (2017). Fin whale song variability in southern California and the Gulf of California. Sci. Rep. 7:10126. doi: 10.1038/s41598-017-09979-4
Širović, A., Williams, L. N., Kerosky, S. M., Wiggins, S. M., and Hildebrand, J. A. (2013). Temporal separation of two fin whale call types across the eastern North Pacific. Mar. Biol. 160, 47–57. doi: 10.1007/s00227-012-2061-z
Sonothèque (2018). La Sonothèque du Muséum National d’Histoire Naturelle, Résultats des Espèces. Available online at: https://sonotheque.mnhn.fr/?q=&tbs=qr:e [Accessed October 21, 2021].
Sostres, A., and Nuuttila, H. (2015). Detection rates of wild harbour porpoises and bottlenose dolphins using static acoustic click loggers vary with depth. Bioacoustics 24, 101–110. doi: 10.1080/09524622.2014.980319
Southworth, M. (1969). The sonic environment of cities. Environ. Behav. 1, 49–70. doi: 10.1177/001391656900100104
Spence, H. R. (2017). Passive acoustic monitoring of nocturnal fish sounds in Quintana Roo. Mexico. Bull. Mar. Sci. 93, 641–652. doi: 10.5343/bms.2016.1041
Sprague, M. W., and Luczkovich, J. J. (2001). Do striped cusk-eels Ophidion marginatum (Ophidiidae) produce the “chatter” sound attributed to weakfish Cynoscion regalis (Sciaenidae)? Copeia 2001, 854–859. doi: 10.1643/0045-8511(2001)001[0854:dsceom]2.0.co;2
Staaterman, E., Brandl, S. J., Hauer, M., Casey, J., Gallagher, A. J., and Rice, A. N. (2018). Individual voices in a cluttered soundscape: acoustic ecology of the Bocon toadfish, Amphichthys cryptocentrus. Environ. Biol. Fishes 101, 979–995. doi: 10.1007/s10641-018-0752-0
Stanley, J. A., Van Parijs, S. M., Davis, G. E., Sullivan, M., and Hatch, L. T. (2021). Monitoring spatial and temporal soundscape features within ecologically significant U.S. National Marine Sanctuaries. Ecol. Appl. 31:e02439. doi: 10.1002/eap.2439
Stowell, D., Wood, M. D., Pamuła, H., Stylianou, Y., and Glotin, H. (2019). Automatic acoustic detection of birds through deep learning: the first Bird Audio Detection challenge. Methods Ecol. Evol. 10, 368–380. doi: 10.1111/2041-210X.13103
Straight, C. A., Jackson, C. R., Freeman, B. J., and Freeman, M. C. (2015). Diel patterns and temporal trends in spawning activities of robust redhorse and river redhorse in Georgia, assessed using passive acoustic monitoring. Trans. Am. Fish. Soc. 144, 563–576. doi: 10.1080/00028487.2014.1001040
Sueur, J., and Farina, A. (2015). Ecoacoustics: the ecological investigation and interpretation of environmental sound. Biosemiotics 8, 493–502. doi: 10.1007/s12304-015-9248-x
Sydeman, W. J., Poloczanska, E., Reed, T. E., and Thompson, S. A. (2015). Climate change and marine vertebrates. Science 350, 772–777. doi: 10.1126/science.aac9874
Teixeira, D., Maron, M., and van Rensburg, B. J. (2019). Bioacoustic monitoring of animal vocal behavior for conservation. Conserv. Sci. Prac. 1:e72. doi: 10.1111/csp2.72
Tellechea, J. S., Norbis, W., Olsson, D., and Fine, M. L. (2010). Calls of the black drum (Pogonias cromis: Sciaenidae): geographical differences in sound production between northern and southern hemisphere populations. J. Exp. Zool. 315A, 48–55. doi: 10.1002/jez.651
The British Library (2021). Environment & Nature Sounds. The British Library. Available online at: https://sounds.bl.uk/Environment [Accessed October 21, 2021].
Thiebault, A., Charrier, I., Aubin, T., Green, D., and Pistorius, P. (2019). First evidence of underwater vocalisations in hunting penguins. PeerJ. 7:e8240. doi: 10.7717/peerj.8240
Thompson, P. M., Brookes, K. L., Graham, I. M., Barton, T. R., Needham, K., Bradbury, G., et al. (2013). Short-term disturbance by a commercial two-dimensional seismic survey does not lead to long-term displacement of harbour porpoises. Proc. R. Soc. B. 280:20132001. doi: 10.1098/rspb.2013.200
Thorson, R. F., and Fine, M. L. (2002). Crepuscular changes in emission rate and parameters of the boatwhistle advertisement call of the Gulf toadfish. Opsanus beta. Environ. Biol. Fishes 63, 321–331. doi: 10.1023/A:1014334425821
Tierstimmenarchiv (2021). The Animal Sound Archive (Tierstimmenarchiv) at the Museum für Naturkunde in Berlin. Available online at: https://www.tierstimmenarchiv.de/ [Accessed October 21, 2021]
Tricas, T. C., and Boyle, K. S. (2014). Acoustic behaviors in Hawaiian coral reef fish communities. Mar. Ecol. Prog. Ser. 511, 1–16. doi: 10.3354/meps10930
Tricas, T. C., and Boyle, K. S. (2021). Parrotfish soundscapes: implications for coral reef management. Mar. Ecol. Prog. Ser. 666, 149–169. doi: 10.3354/meps13679
Tsujii, K., Otsuki, M., Akamatsu, T., Matsuo, I., Amakasu, K., Kitamura, M., et al. (2016). The migration of fin whales into the southern Chukchi Sea as monitored with passive acoustics. ICES J. Mar. Sci. 73, 2085–2092. doi: 10.1093/icesjms/fsv271
Tyack, P. L. (2008). Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mammal. 89, 549–558. doi: 10.1644/07-MAMM-S-307R.1
Uno, M., and Konagaya, T. (1960). Studies on the swimming noise of the fish. Nippon Suisan Gakkaishi 26, 1069–1073. doi: 10.2331/suisan.26.1069
Van Opzeeland, I., Samaran, F., Stafford, K. M., Findlay, K., Gedamke, J., Harris, D., et al. (2014). Towards collective circum-Antarctic passive acoustic monitoring: the Southern Ocean Hydrophone Network (SOHN). Polarforschung 83, 47–61.
Vigness-Raposa, K. J., Scowcroft, G., Miller, J. H., and Ketten, D. (2012). “Discovery of sound in the sea: an online resource” in The Effects of Noise on Aquatic Life, Advances in Experimental Medicine and Biology. eds A. N. Popper and A. Hawkins (New York: Springer). 135–138. doi: 10.1007/978-1-4419-7311-5_30
Voices in the Sea (2018). Voices in the Sea. Available online at: http://voicesinthesea.ucsd.edu/index.html [Accessed October 21, 2021]
Waddell, E., Rasmussen, J. H., and Širović, A. (2021). Applying artificial intelligence methods to detect and classify fish calls from the Northern Gulf of Mexico. J. Mar. Sci. Eng. 9:1128. doi: 10.3390/jmse9101128
Wall, C. C., Haver, S. M., Hatch, L. T., Miksis-Olds, J., Bochenek, R., Dziak, R. P., et al. (2021). The next wave of passive acoustic data management: how centralized access can enhance science. Front. Mar. Sci. 8:703682. doi: 10.3389/fmars.2021.703682
Warren, J. D., Ainslie, M. A., Miksis-Olds, J. L., Martin, B., and Heaney, K. D. (2018). “ADEON Calibration and Deployment Good Practice Guide. Version 1.0” in Technical Report by Stony Brook University for ADEON Prime Contract No. M16PC00003. (NY: Stony Brook University).
Warren, V. E., Širović, A., McPherson, C., Goetz, K. T., Radford, C. A., and Constantine, R. (2021). Passive acoustic monitoring reveals spatio-temporal distributions of Antarctic and pygmy blue whales around central New Zealand. Front. Mar. Sci. 7:575257. doi: 10.3389/fmars.2020.575257
Watkins Marine Mammal Sound Database (2021). Watkins Marine Mammal Sound Database, Woods Hole Oceanographic Institution and the New Bedford Whaling Museum. Available online at: https://cis.whoi.edu/science/B/whalesounds/index.cfm [Accessed October 21, 2021].
World Register of Marine Species (2021). Species Databases. Available online at: https://www.marinespecies.org/ [Accessed October 25, 2021]
World Wildlife Fund [WWF] (2017). Roadmap for the Development of a Sustainable Blue Economy Protocol. Available onlien at: http://ocean.panda.org.s3.amazonaws.com/media/Draft+Sust+Blue+Economy+Protocol+Feb+2017+WWF.pdf [Accessed September 25, 2021]
Worm, B., Barber, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Xie, J., Hu, K., Zhu, M., and Guo, Y. (2020). Bioacoustic signal classification in continuous recordings: syllable-segmentation vs sliding-window. Expert Syst. Appl. 152:113390. doi: 10.1016/j.eswa.2020.113390
Xie, L., Sundaram, H., and Campbell, M. (2008). Event mining in multimedia streams. Proc. IEEE 96, 623–647. doi: 10.1109/JPROC.2008.916362
Xu, Y., Du, J., Dai, L.-R., and Lee, C.-H. (2015). A regression approach to speech enhancement based on deep neural networks. IEEE/ACM Transac. Aud. Speech Lang. Process. 23, 7–19. doi: 10.1109/TASLP.2014.2364452
Yack, T. M., Barlow, J., Roch, M. A., Klinck, H., Martin, S., Mellinger, D. K., et al. (2010). Comparison of beaked whale detection algorithms. App. Acoust. 71, 1043–1049. doi: 10.1016/j.apacoust.2010.04.010
Yosinski, J., Clune, J., Bengio, Y., and Lipson, H. (2014). How transferable are features in deep neural networks? Adv. Neural Inform. Process. Syst. 27, 3320–3328.
Zarada, K., Burnsed, W. S., Bickford, J., Ducharme-Barth, N., Ahrens, M., and Lowerre-Barbieri, S. (2019). Estimating site-specific spawning parameters for a spawning aggregation: an example with spotted seatrout. Mar. Ecol. Prog. Ser. 624, 117–129. doi: 10.3354/meps13016
Zhang, J., Huang, K., Cottman-Fields, M., Truskinger, A., Roe, P., Duan, S., et al. (2013). “Managing and analysing big audio data for environmental monitoring” in 2013 IEEE 16th International Conference on Computational Science and Engineering. (United States: IEEE). 997–1004. doi: 10.1109/CSE.2013.146
Zhang, S., Yang, T. C., and Chi, W. C. (2018). “Estimating the Acoustic Intensity of Typhoon Shanshan at Very Low Frequencies” in 2018 OCEANS - MTS/IEEE Kobe Techno-Oceans. 1–4. doi: 10.1109/OCEANSKOBE.2018.8558879
Zhao, Z., D’asaro, E. A., and Nystuen, J. A. (2014). The sound of tropical cyclones. Bull. Am. Meteorol. Soc. 44, 2763–2788. doi: 10.1175/JPO-D-14-0040.1
Keywords: soundscape, bioacoustics database, artificial intelligence, biodiversity, passive acoustic monitoring, ecological informatics
Citation: Parsons MJG, Lin T-H, Mooney TA, Erbe C, Juanes F, Lammers M, Li S, Linke S, Looby A, Nedelec SL, Van Opzeeland I, Radford C, Rice AN, Sayigh L, Stanley J, Urban E and Di Iorio L (2022) Sounding the Call for a Global Library of Underwater Biological Sounds. Front. Ecol. Evol. 10:810156. doi: 10.3389/fevo.2022.810156
Received: 06 November 2021; Accepted: 11 January 2022;
Published: 08 February 2022.
Edited by:
Almo Farina, University of Urbino Carlo Bo, ItalyReviewed by:
Andre Fiebig, Technical University of Berlin, GermanyCopyright © 2022 Parsons, Lin, Mooney, Erbe, Juanes, Lammers, Li, Linke, Looby, Nedelec, Van Opzeeland, Radford, Rice, Sayigh, Stanley, Urban and Di Iorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miles J. G. Parsons, bS5wYXJzb25zQGFpbXMuZ292LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.