- 1School of Biological Sciences, Seoul National University, Seoul, South Korea
- 2Honam National Institute of Biological Resources, Incheon, South Korea
- 3Biodiversity Research Center, Academia Sinica, Taipei, Taiwan
- 4National Marine Biodiversity Institute of Korea, Seocheon, South Korea
- 5Department of Fisheries Science Graduate School, Kunsan National University, Gunsan, South Korea
Loggerhead and green turtles inhabit all oceans except the polar regions. External surfaces of sea turtles are often colonized by epibiotic chelonibiid barnacles. Barnacle taxonomy studies in Korea began in 1985, but until present, no turtle barnacles were recorded. This suggests that either the diversity and frequency of occurrence of turtle barnacles in Korean waters are low or the turtle barnacles have been understudied. This study complies with data collected over 6 years of sea turtle stranding events in Korea (2015–2020). We examined the diversity, frequency, and intensity of turtle barnacle occurrence. Of the 55 recorded strandings, loggerhead turtles were the most common (58%), followed by green turtles (33%). Only one species of barnacle, Chelonibia testudinaria, was found on both loggerhead and green turtles. The frequency of barnacle occurrence on loggerhead turtles was 28%, with an intensity of 2.4 ± 2.7 barnacles per turtle. Notably, 11% of green turtles had barnacles, with an average of one individual per turtle. The frequency and intensity of barnacle occurrence on green turtles analyzed in this study were five times lower than that on green turtle populations in Okinawan, Bornean, and Australian waters in the Indo-Pacific. Based on these new data and the available literature, we speculated that the barnacle larval pools in cold, high-latitude Korean waters are smaller than those occurring in other locations in the Indo-Pacific. The frequency and intensity of occurrence of barnacles on loggerhead turtles in Korea fall within the range recorded in other Indo-Pacific locations. The longer migratory routes of loggerhead turtles allow them to pass through different larval pools in the Indo-Pacific water, exposing them to higher barnacle abundances.
Introduction
The loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) are latitudinally and longitudinally widespread marine reptiles (FitzSimmons and Limpus, 2014). Carcasses of loggerhead and green turtles often carry epibiotic assemblages, including algae, meiofauna, decapods, and barnacles (Pfaller et al., 2008; Silver-Gorges et al., 2021). Chelonibiid barnacles are common epibionts on both loggerhead and green turtles (Zardus, 2021). Currently, sixteen confirmed species of turtle-associated barnacles are known (Zardus, 2021). The examples include the commonly occurring Chelonibia testudinaria attaching on the turtle carapaces (Zardus et al., 2014). Chelolepas cheloniae, in turn, bores into turtle carapaces, and Platylepas spp. attach to the skin (Hayashi, 2012; Zardus, 2021).
The diversity of epibiotic barnacle species on turtles in the Indo-Pacific has been studied in specific regions, including the Pacific coast of Japan (10 species, Hayashi, 2012), Malaysia (one species, Lim et al., 2021), Taiwan (two species, Chan et al., 2009), and China (nine species, Liu and Ren, 2007). These studies indicate a considerable diversity of species. Korean waters cover both the subtropical region around Jejudo Island and temperate waters around the Korean Peninsula, which is at the northern limit of the range of green turtles in the Western Pacific. Sea turtles have most often been recorded in Jejudo Island (Kim et al., 2017) and the east coast of Korea, in the East Sea (Sea of Japan).
Barnacle fauna of Korea have been comprehensively described by Kim (2011) and Kim H. K. et al. (2020), but there are no reports of turtle-associated barnacles, suggesting that either the diversity and occurrence rate of turtle barnacles in Korea is low or the local turtle barnacles have been understudied. It is also possible that commensal barnacles on green turtles inhabiting the northern edge of their range may show lower frequency and intensity of occurrence when compared to tropical turtle populations. This study reports the diversity and occurrence of turtle-associated barnacles based on the stranding records (2015–2020) of loggerhead and green turtles in Korean waters. We tested the hypothesis that the frequency and intensity of barnacle occurrence on sea turtles are lower in Korean populations than other previously investigated sea turtle populations in the Indo-Pacific.
Materials and Methods
Korean Marine Ecoregions
There are three distinct ecoregions within the marine environment around the Korean Peninsula: the Yellow Sea ecoregion on the west coast, the East China Sea ecoregion along the southern coast and outlying islands, including Jejudo Island, and the East Sea ecoregion on the east coast, facing the East Sea. The Yellow Sea ecoregion is affected by the Korean Coastal Current which has opposite flow directions in summer and winter (Figure 1). The East China Sea ecoregion is affected by the Cheju and Tsushima Warm Currents (Figure 1). The East Sea ecoregion is influenced by the North Korean Cold Currents and the Tsushima Warm Current (Kim H. K. et al., 2020). The ecoregions differ mainly in winter water temperature (Figure 1). Waters in the Yellow Sea ecoregion have the lowest winter temperatures (∼2°C), followed by those in the East Sea ecoregion (∼10°C). The South Sea ecoregion is the warmest, with winter water temperature ranging from 12°C to 15°C (Figures 1B,C; Kim H. K. et al., 2020).
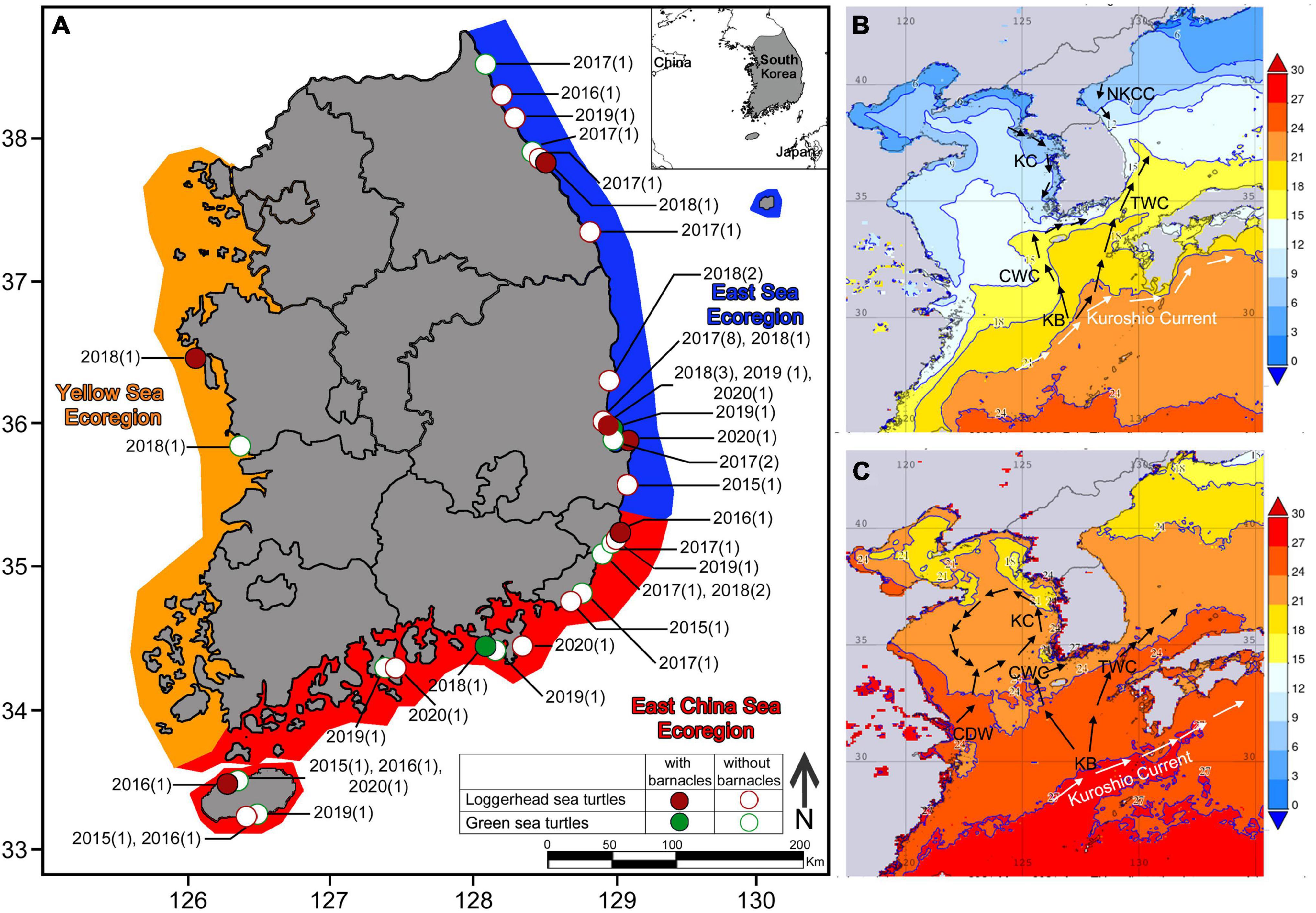
Figure 1. (A) Records of stranded loggerhead and green sea turtles in Korea (Orange: Yellow Sea ecoregion, Red: East China Sea ecoregion, Blue: East Sea ecoregion). Locations of a loggerhead sea turtle with barnacles are represented by red circles. Locations of a loggerhead sea turtle without barnacles are represented by open red circles. Locations of a green sea turtle with barnacles are represented by green circles. Locations of a green sea turtle without barnacles are represented by open green circles. In each location, the year and number of strandings are stated in brackets; (B) Averaged winter surface seawater temperature (November 2020–February 2021) and oceanographic currents in Korean and adjacent waters; (C) Averaged summer surface seawater temperature (May–August 2021) and oceanographic currents in Korean and adjacent waters. Sea surface temperature map created from NASA Giovanni database version 4.36. KC, Korean Coastal Current; KB, Branch of Kuroshio Current; TWC, Tsushima Warm Current; CWC, Cheju Warm Current; CDW, Changjiang Diluted Water; NKCC, North Korean Coastal Current.
Stranded Turtles and Epibiotic Barnacles
Stranded sea turtle carcasses were collected from the Korean coastal regions from 2015 to 2020 by the National Marine Biodiversity Institute of Korea (MABIK). For each stranding, we recorded ecoregion, specific location, and turtle species. The curved carapace length of turtles was measured using tape rulers to the nearest millimeter. Barnacles were collected from the sea turtle head, plastron, and carapace using a stainless steel hand scraper. They were subsequently identified and counted.
Comparison of the Frequency and Intensity of Occurrence of C. testudinaria on Sea Turtles in the Indo-Pacific
The frequency of occurrence of barnacles on loggerhead and green turtles in this study was calculated as the ratio between the stranded turtles with barnacles and the total number of stranded turtles (2015–2020). The intensity of barnacle occurrence on a given sea turtle species was expressed as the average number of barnacles per turtle.
The frequency and intensity of C. testudinaria in other Indo-Pacific locations were extracted from the previously published studies conducted in Okinawa (Hayashi and Tsuji, 2008), Australia (Limpus et al., 1994; Doell et al., 2017), Aldabra Atoll, Seychelles (Frazier, 1971), Mabul Island in N. Borneo (Lim et al., 2021), Kyushu, Japan (Matsuura and Nakamura, 1993), and Natal, South Africa (Hughes, 1974; Supplementary Table 1). Although Hughes (1974) identified the barnacles observed on loggerheads as Chelonibia sp., we included his observations in the current dataset due to the general scarcity of distribution data for C. testudinaria on loggerhead turtles in the Indo-Pacific region (also refer to the literature cited by Zardus, 2021). To study the variation in sea surface temperature among the studied regions of C. testudinaria in the Indo-Pacific, a time-averaged overlay sea surface temperature map of the Indo-Pacific region was created using the NASA Giovanni Database version 4.361.
Results
Frequency and Intensity of Barnacle Occurrence
From 2015 to 2020, we recorded 55 sea turtle stranding events. The following sea turtles were observed (Figures 1, 2): 32 loggerheads, 18 green turtles, two leatherbacks, two olive ridleys, and one hawksbill (Figure 2). Loggerhead and green turtle strandings were recorded in all three ecoregions: East Sea (23 and 5, respectively), East China Sea (8 and 12, respectively), and Yellow Sea (1 and 1, respectively) (Figure 1). Most of the stranded sea turtle carcasses were relatively fresh with no signs of decomposition. Most of the strandings occurred from May to October (Supplementary Table 2).
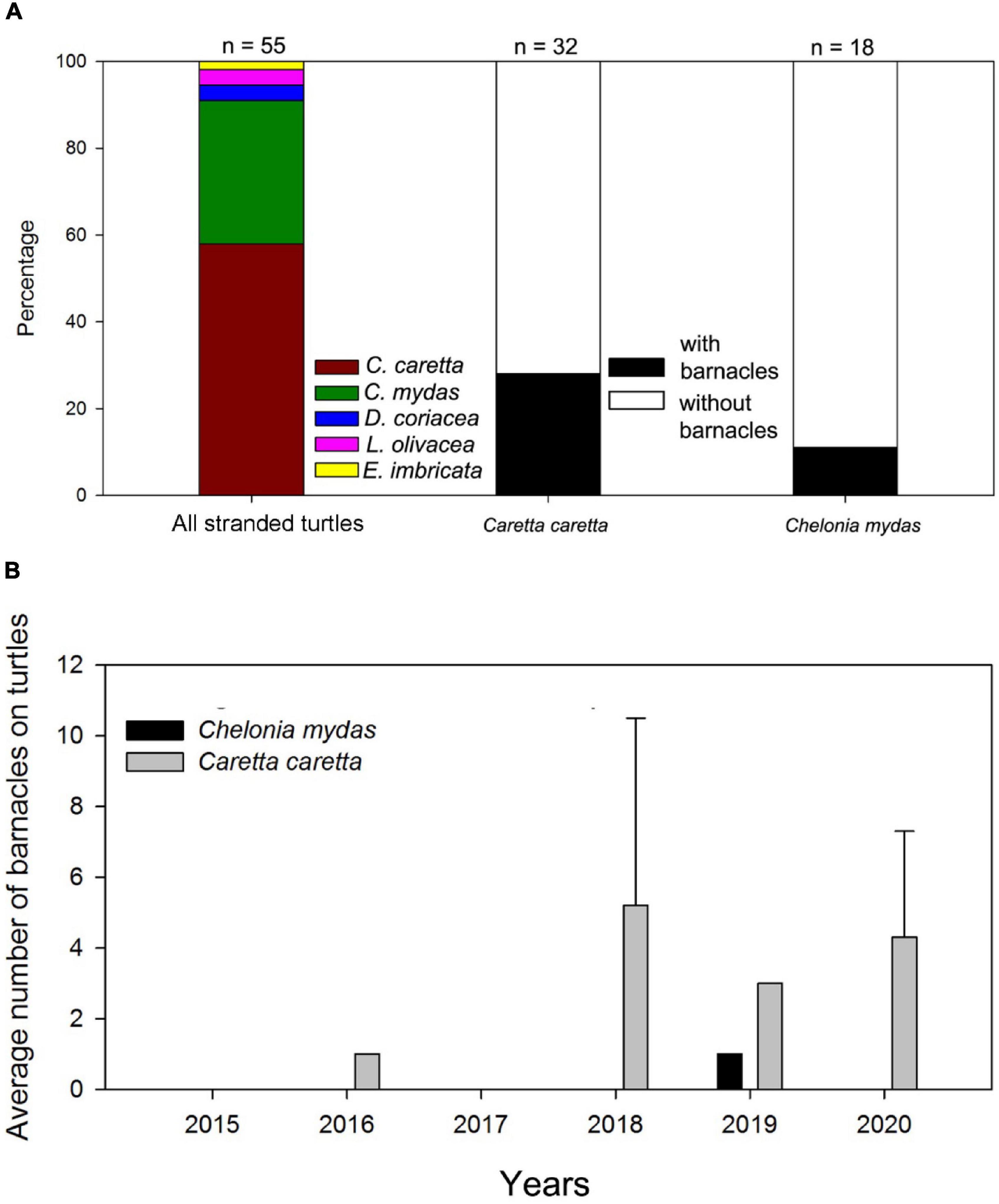
Figure 2. Stranded sea turtles collected from 2015 to 2020 in Korea. (A) Percentage of stranded turtles collected from 2015 to 2020 and percentage of loggerhead and green sea turtles with barnacles; (B) Density (average number of barnacles per turtle) on stranded loggerhead and green sea turtles from 2015 to 2020.
Loggerhead and green turtles hosted barnacles, while leatherback, olive ridley, and hawksbill turtles had no barnacles. C. testudinaria on stranded loggerhead turtles was mainly found in the East China Sea and East Sea ecoregions, with one record in the Yellow Sea ecoregion (Figure 1). On loggerhead turtles, the frequency of occurrence was 28%, and the intensity of occurrence was 2.4 ± 2.7 barnacles per turtle. Among the recorded barnacles, 82% of C. testudinaria were found on the carapace and 18% on the plastron (Figure 2). The curved carapace length of loggerhead turtles with barnacles ranged from 588 to 861 mm (subadults to adults).
Stranded green turtles with barnacles were only found in the East China Sea and East Sea ecoregion (Figure 1). In green turtles, the frequency of occurrence was 11%, and the intensity of occurrence was 1.0 ± 0.2 barnacles per turtle (Figure 2). The carapace lengths of green turtles with barnacles on their carapaces ranged from 460 to 740 mm (one juvenile and one subadult) (Figure 3). Stranded adult green turtles carried no barnacles (Figure 3).
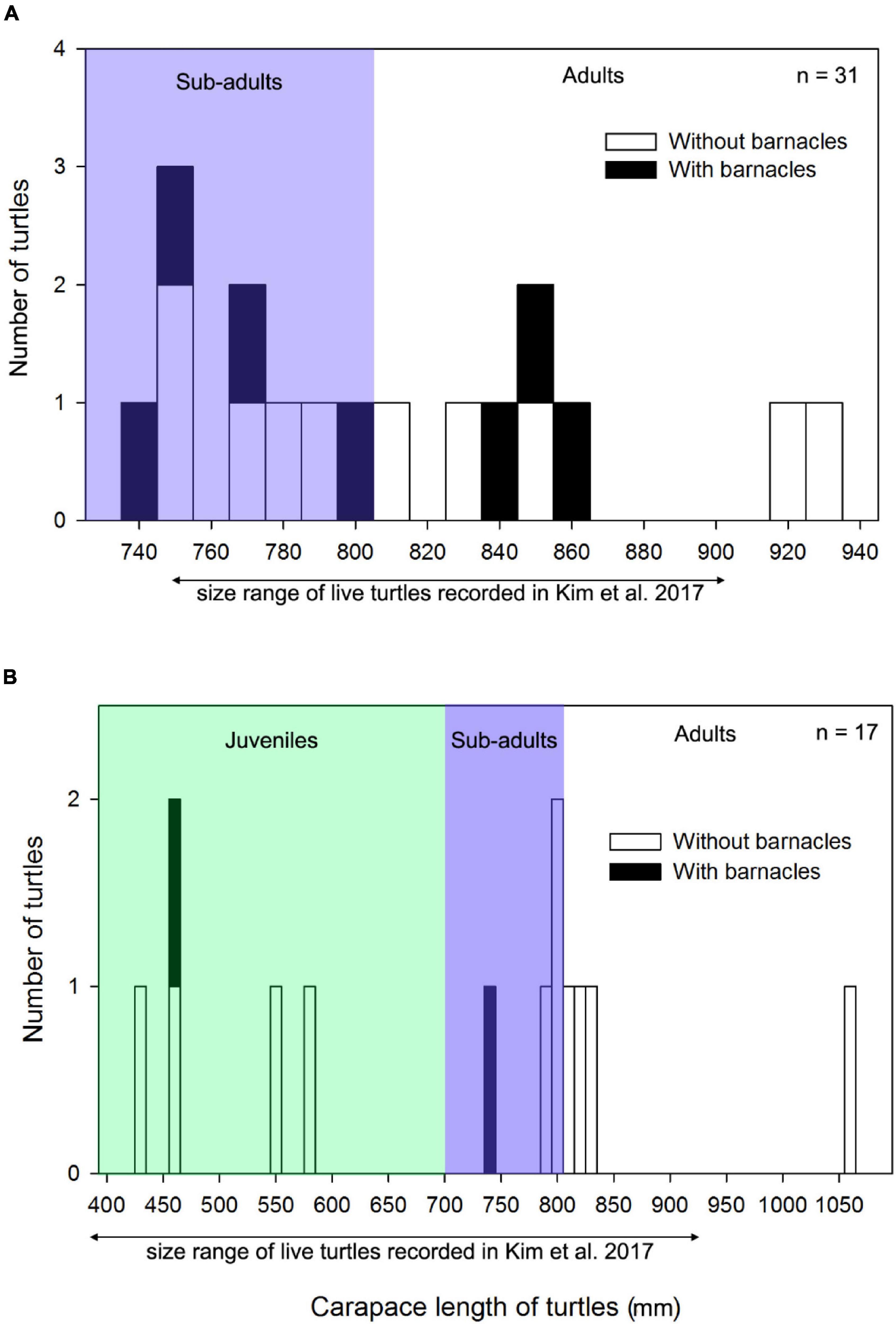
Figure 3. Frequency histograms of carapace length (mm) of (A) Loggerhead sea turtles and (B) Green sea turtles, with (black bars) and without (white bars) barnacles in Korea. The size range of live loggerhead and green sea turtles recorded in Korea in the study by Kim et al. (2017) is provided for comparison with the stranded turtles in this study. The definition of juveniles, subadults, and adults is based on the size range defined in the study by Hughes (1974).
Literature-Based Comparison of Frequency and Intensity of Occurrence of C. testudinaria on Sea Turtles in the Indo-Pacific
Based on the available data, we were able to calculate the following parameters. The frequency of occurrence of C. testudinaria on loggerheads from South Africa ranged from 18% (juvenile specimens from Natal) to 73% (Supplementary Table 1 and Figure 4). In green turtles, the frequency of occurrence exceeded 50% in all analyzed populations (Supplementary Table 1 and Figure 4).
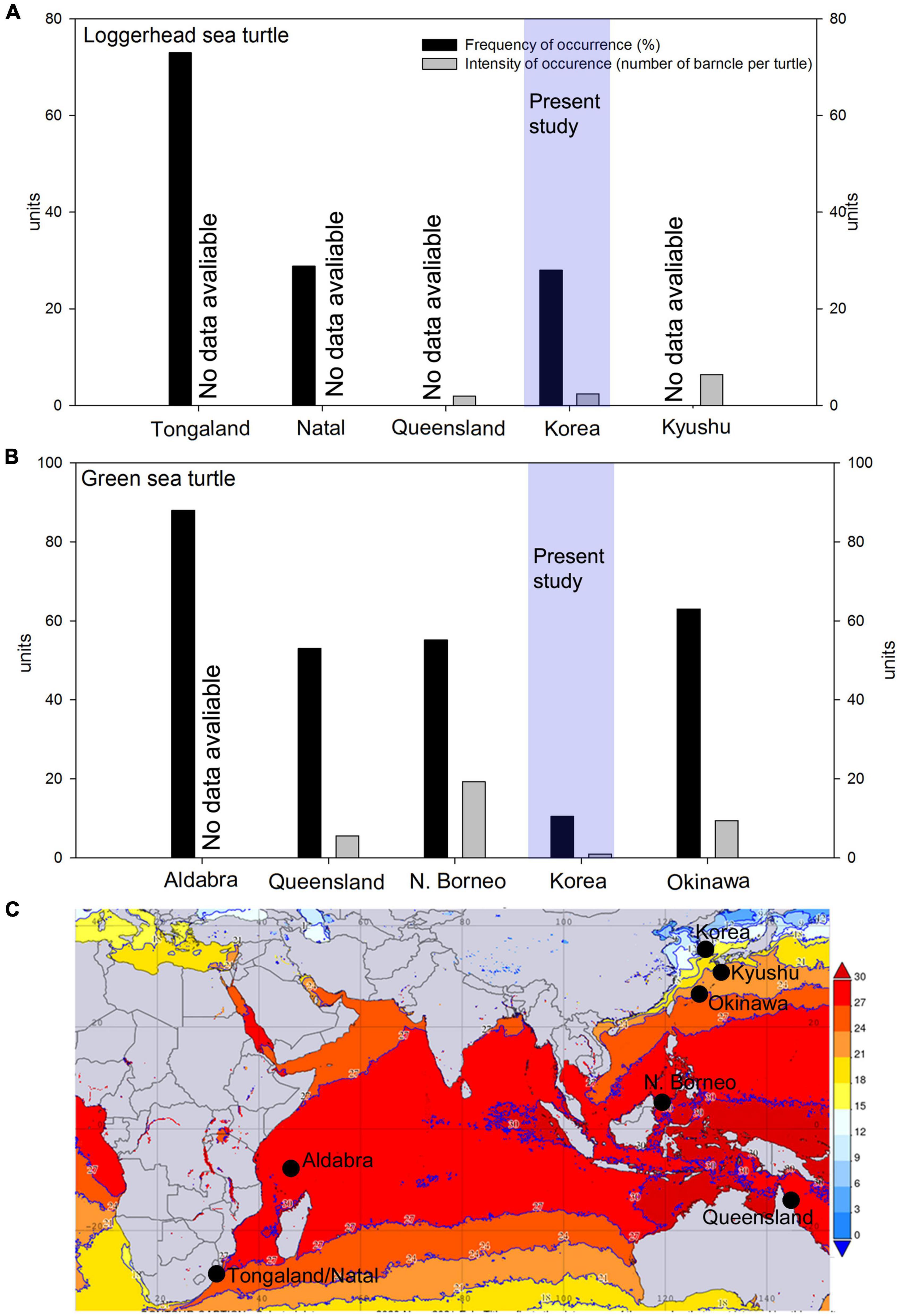
Figure 4. Comparison of the frequency of occurrence (%, black bar) and intensity of occurrence (number of barnacles per turtle, gray bars) in the Indo-Pacific of (A) Loggerhead sea turtles and (B) Green sea turtles in Korea. Data sources from geographical population: Okinawa (Hayashi and Tsuji, 2008), Queensland in Australia (Limpus et al., 1994; Doell et al., 2017), Aldabra Atoll (Frazier, 1971), Mabul Island in N. Borneo (Lim et al., 2021), and Natal and Tongland (Hughes, 1974). The frequency of occurrence of barnacles on loggerhead sea turtles on Natal and the frequency of occurrence and intensity of occurrence of barnacles in green sea turtles in N. Borneo were averaged from the data on juveniles, subadults, and adults. For details, refer to Supplementary Table 1; (C) Averaged winter sea surface temperature map of the Indo-Pacific (November 2020–February 2021), generated from NASA Giovanni database version 4.36. Black dots show the locations in panels (A,B).
The intensity of occurrence of C. testudinaria on loggerheads from Kyushu and Australia was 6.4 and 1.9 barnacles per turtle, respectively (Supplementary Table 1). In green turtles, the intensity of occurrence was 2.6 barnacles per turtle in Australia, 8.4 in Okinawa, and 19.3 in North Borneo. In the latter location, up to 43 barnacles per turtle were recorded in adult female populations (Supplementary Table 1 and Figure 4).
Discussion
Only one barnacle species, i.e., C. testudinaria, was found for the first time as a turtle-associated barnacle in Korean waters. Prior to this study, this species had not been recorded in Korea, although the taxonomic studies of Korean barnacles began in 1985 (Kim, 2011). Among the 13 sea turtle barnacles reported from the Indo-Pacific region (Zardus, 2021), Platylepas hexastylos was recorded from a “stuffed turtle specimen” kept in a university museum without any information on the collection site (Kim, 2011) and thus may not be of Korean origin. It has been suggested that the diversity of epibiotic turtle-associated barnacles in Korea was likely lower than that in adjacent regions, including the Pacific coast of Japan (Hayashi, 2012). Thus, C. testudinaria is not expected to be common in Korean sea turtle populations. Some of the sea turtle populations from the adjacent Pacific waters may be separated from Korea, resulting in differences in the diversity of turtle-associated barnacles among regions (Jang et al., 2018). The relatively low temperature of Korean waters, as compared to the adjacent regions, may limit the diversity of turtle barnacles in Korea.
The frequency and intensity of occurrence of C. testudinaria on Korean loggerhead turtles (28%, 2.4 barnacles per turtle) fell within the ranges estimated for other Indo-Pacific populations (18–73%, 1.9–6.4 barnacles per turtle). Both the frequency and intensity of occurrence of C. testudinaria on green turtles (11%, one barnacle per turtle), in turn, were several times lower than the values calculated for other Indo-Pacific populations. Another study investigating Korean green turtles (Moon et al., 2009) presented a photograph of a wild turtle carrying a single barnacle on its carapace. Although the specimen was identified as a Balanus species, the photograph clearly indicates that the taxon in question was C. testudinaria (refer to Figure 8 in Moon et al., 2009). Observations made by us over the years (the personal observations of Y. N. Choi, C. Yi, I.-H. Kim, and Y. N. Choi), as well as photographs provided by other authors (Moon et al., 2009; Kim et al., 2017, 2019; Kim I. H. et al., 2020), suggest that green turtles in Korea (but also hawksbills and olive ridleys) rarely carry any barnacles.
Satellite tracking studies of green turtles in Korea and Japan indicate that these animals can stay in waters surrounding Jejudo Island for up to 1 year and migrate between Kyushu, the Korean Peninsula, and Jejudo Island in the East Sea region (Jang et al., 2018). As previously suggested (Kim H. K. et al., 2020), lower water temperatures in this region may limit the larval pools of C. testudinaria and other barnacles. For example, in Malaysia, leatherback, olive ridley, and hawksbill turtles are often colonized by C. testudinaria (Lim et al., 2021). In Korean waters, these sea turtle species have not yet been observed to host C. testudinaria.
We speculated that the local loggerhead turtles do not follow this trend due to their longer migratory routes (Bowen et al., 1995). Satellite tracking of juvenile loggerheads revealed that they are restricted to the pelagic feeding grounds located in the Central North Pacific, where they stay for up to 1–2 years (Briscoe et al., 2016). During non-reproductive periods, numerous loggerheads are also observed in the East China Sea enclosed by Korea, eastern China, Taiwan, and Kyushu (Kobayashi et al., 2011). Therefore, loggerhead turtles in the West Pacific would have had to pass through the more diverse and possibly larger larval pools than green turtles during their migrations. Considering the life span of C. testudinaria (up to 2 years; Doell et al., 2017), barnacles present on Korean loggerheads might have settled on these animals in different geographical regions.
It has to be highlighted that this study analyzed barnacles that present solely on the carcasses of stranded sea turtles, and it is possible that such data would not accurately reflect the actual trends in living sea turtle populations. However, based on the low degree of decomposition of the carcasses used in this study, we are confident that all animals resided in Korean waters. Somewhat in support of this statement, the distribution patterns of the Korean sea turtles shown by Kim et al. (2017) largely agree with the stranding locations (this study). Similarly, the size range of sea turtles recorded in Korean waters (Kim et al., 2017) overlaps with that of the analyzed carcasses.
We acknowledged the relatively small sample size of 55 individual sea turtles, as well as the general scarcity of similar studies in the Indo-Pacific region, and emphasized that our results should be interpreted with caution. It is likely that the local sea turtle barnacle diversity remains underestimated, and more studies (preferably including living turtles) are necessary to further investigate the correctness of some of our assumptions. Given the possible significant effect of water temperature on sea turtle barnacle abundance and ranges, in the future, more barnacle species may be observed in Korea due to global warming. The analyses of preserved sea turtle carcasses and carapaces may help detect such temperature-related changes over different time scales.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HKK and BKKC conceived and designed the study. CY and I-HK collected the field data. BKKC, HKK, I-HK, and CY wrote the manuscript and prepared the figures. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Marine Biodiversity Institute of Korea (2021M00300) and the Honam National Institute of Biological Resources (HNIBR202101104). BKKC was supported by the Senior Investigator Award in Academia Sinica, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JZ declared a past co-authorship with one of the authors, BKKC, to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the associate editor RM and the reviewers for their editing and comments to further improve the quality of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.785692/full#supplementary-material
Footnotes
References
Bowen, B. W., Abreu-Grobois, F. A., Balazs, G. H., Kamezaki, N., Limpus, C., and Ferl, R. J. (1995). Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl. Acad. Sci. U.S.A. 92, 3731–3734. doi: 10.1073/pnas.92.9.3731
Briscoe, D. K., Parker, D. M., Bograd, S., Hazen, E., Scales, K., Balazs, G. H., et al. (2016). Multi-year tracking reveals extensive pelagic phase of juvenile loggerhead sea turtles in the North Pacific. Mov. Ecol. 4:23. doi: 10.1186/s40462-016-0087-4
Chan, B. K. K., Prabowo, R. E., and Lee, K. S. (2009). Crustacean Fauna of Taiwan: Barnacles, Volume 1: Cirripedia: Thoracica Excluding the Pyrgomatidae and Acastinae. Taiwan: National Taiwan Ocean University Press.
Doell, S. A., Connolly, R. M., Limpus, C. J., and Pearson, R. M. (2017). Using growth rates to estimate age of the sea turtle barnacle Chelonibia testudinaria. Mar. Biol. 164:222. doi: 10.1007/s00227-017-3251-5
FitzSimmons, N. N., and Limpus, C. J. (2014). Marine turtle genetic stocks of the Indo-Pacific: identifying boundaries and knowledge gaps. Indian Ocean Turt. Newsl. 20, 2–18.
Frazier, J. (1971). Observations on sea turtles at Aldabra Atoll. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 260, 373–410. doi: 10.1098/rstb.1971.0019
Hayashi, R. (2012). Atlas of the barnacles on marine vertebrates in Japanese waters including taxonomic review of superfamily Coronuloidea (Cirripedia: Thoracica). J. Mar. Biol. Assoc. U.K. 92, 107–127. doi: 10.1017/S0025315411000737
Hayashi, R., and Tsuji, K. (2008). Spatial distribution of turtle barnacles on the green sea turtle, Chelonia mydas. Ecol. Res. 23, 121–125. doi: 10.1007/s11284-007-0349-0
Hughes, G. R. (1974). The sea turtles of South-East Africa. II. The biology of the tongaland loggerhead turtle, Caretta caretta L. with comments on the Leatherback Turtle Dermochelys coriacea L. and the green turtle Chelonia mydas L. in the study region. Ocean Res. Inst. Invest. Rep. 36, 1–96.
Jang, S., Balazs, G. H., Parker, D. M., Kim, B. Y., Kim, M. Y., Ng, C. K. Y., et al. (2018). Movements of green sea turtles (Chelonia mydas) rescued from pound nets near Jeju Island, republic of Korea. Chelonian Conserv. Biol. 17, 236–244. doi: 10.2744/CCB-1279.1
Kim, H. K., Chan, B. K. K., Lee, S. K., and Kim, W. (2020). Biogeography of intertidal and subtidal native and invasive barnacles in Korea in relation to oceanographic current ecoregions and global climatic changes. J. Mar. Biol. Assoc. U.K. 100, 1079–1091. doi: 10.1017/S0025315420001009
Kim, I. H., Yi, C. H., Han, D. J., Park, D., Park, J., Cho, I. Y., et al. (2020). First record of the hawksbill turtle (Eretmochelys imbricata, Reptilia: Testudines: Cheloniidae) from South Korea. J. Asia Pac. Biodivers. 13, 151–155. doi: 10.1016/j.japb.2020.02.006
Kim, I. H. (2011). Cirripedia, symbiotic Copepoda, and pycnogonida. Illus. Encycl. Fauna Flora Korea 38:1038.
Kim, I. H., Moon, D. Y., Cho, I. Y., Kim, M. S., An, Y. R., Han, D., et al. (2017). Occurrence of sea turtles in the Korean waters and the morphological characteristics of two major species. Fish. Aquatic. Sci. 50, 311–318. doi: 10.5657/KFAS.2017.0311
Kim, I. H., Yi, C. H., Lee, J. H., Park, D., Cho, I. Y., Han, D. J., et al. (2019). First record of the olive ridley sea turtle Lepidochelys olivacea (Reptilia: Testudines: Cheloniidae) from South Korea. Curr. Herpetol. 38, 153–159. doi: 10.5358/hsj.38.153
Kobayashi, D. R., Cheng, I. J., Parker, D. M., Polovina, J. J., Kamezaki, N., and Balaza, G. H. (2011). Loggerhead turtle (Caretta caretta) movement off the coast of Taiwan: characterization of a hotspot in the East China Sea and investigation of mesoscale eddies. ICES J. Mar. Sci. 68, 707–718. doi: 10.1093/icesjms/fsq185
Lim, K. K., Hussein, M. A. S., and Palaniappan, P. (2021). Abundance, placement and sexual identity of the epizoic barnacle Chelonibia testudinaria relative to the size and species of host turtles in Mabul Island, Malaysia. J. Mar. Biol. Assoc. U.K. 100, 1299–1309. doi: 10.1017/S0025315420001198
Limpus, C. J., Couper, P. J., and Read, M. A. (1994). The green turtle, Chelonia mydas, in Queensland: population structure in a warm temperature feeding area. Mem. Queensl. Mus. 35, 139–154.
Liu, R. Y., and Ren, X. Q. (2007). Fauna Sinica. Invertebrata. Volume 42 Crustacea Cirripedia Thoracica. Beijing: Science Press.
Matsuura, I., and Nakamura, K. (1993). Attachment pattern of the turtle barnacle Chelonibia testudinaria on carapace of nesting loggerhead turtle Caretta caretta. Nippon Suisan Gakkaishi 59:1803. doi: 10.2331/suisan.59.1803
Moon, D. Y., Jung, M. M., An, Y. R., Choi, S. G., Oh, B. S., Kim, Z. G., et al. (2009). Distribution and strandings of endangered sea turtles in Korean waters. Fish. Aquatic. Sci. 42, 657–663. doi: 10.5657/KFAS.2009.42.6.657
Pfaller, J. B., Bjorndal, K. A., Reich, K. J., Williams, K. L., and Frick, M. G. (2008). Distribution patterns of epibionts on the carapace of loggerhead turtles, Caretta caretta. Mar. Biodivers. Rec. 1:e36. doi: 10.1017/S1755267206003812
Silver-Gorges, I., Ingels, J., dos Santos, G. A. P., Valdes, Y., Pontes, L. P., Silva, A. C., et al. (2021). Epibionts reflect spatial and foraging ecology of gulf of Mexico loggerhead turtles (Caretta caretta). Front. Ecol. Evol. 9:696412. doi: 10.3389/fevo.2021.696412
Zardus, J. D. (2021). A global synthesis of the correspondence between epizoic barnacles and their sea turtle hosts. Integr. Org. Biol. 3:obab002. doi: 10.1093/iob/obab002
Keywords: stranding, turtle barnacles, green sea turtles, loggerhead sea turtles, Indo-Pacific
Citation: Kim HK, Chan BKK, Yi C, Kim I-H and Choi YN (2022) Barnacle Epibiosis on Sea Turtles in Korea: A West Pacific Region With Low Occurrence and Intensity of Chelonibia testudinaria (Cirripedia: Chelonibiidae). Front. Ecol. Evol. 10:785692. doi: 10.3389/fevo.2022.785692
Received: 29 September 2021; Accepted: 04 January 2022;
Published: 15 February 2022.
Edited by:
Roksana Majewska, North-West University, South AfricaReviewed by:
John Zardus, The Citadel, Military College of South Carolina, United StatesThomas George Bornman, South African Environmental Observation Network (SAEON), South Africa
Copyright © 2022 Kim, Chan, Yi, Kim and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benny K. K. Chan, Y2hhbmtrQGdhdGUuc2luaWNhLmVkdS50dw==
 Hyun Kyong Kim
Hyun Kyong Kim Benny K. K. Chan
Benny K. K. Chan Changho Yi
Changho Yi Il-Hun Kim
Il-Hun Kim Yu Na Choi
Yu Na Choi