- 1Functional Ecology, Institute of Zoology, Universität Hamburg, Hamburg, Germany
- 2Mention Zoologie et Biodiversité Animale, Faculté des Sciences, Université d’Antananarivo, Antananarivo, Madagascar
- 3School of Biological and Environmental Sciences, Liverpool John Moores University, Liverpool, United Kingdom
Rapid environmental changes are challenging for endothermic species because they have direct and immediate impacts on their physiology by affecting microclimate and fundamental resource availability. Physiological flexibility can compensate for certain ecological perturbations, but our basic understanding of how species function in a given habitat and the extent of their adaptive scope is limited. Here we studied the effect of acute, experimental microclimate change on the thermal physiology of two populations of the widespread Malagasy bat, Macronycteris commersoni. Populations of this species are found roosting under contrasting conditions, i.e., in a constant hot and humid cave or below foliage unprotected from fluctuations in ambient conditions. We exposed free-ranging individuals of each population to the respective opposite condition and thus to novel microclimate within an ecologically realistic scope while measuring metabolic rate and skin temperature. Cave bats in forest setting had a limited capacity to maintain euthermia to the point that two individuals became hypothermic when ambient temperature dropped below their commonly experienced cave temperature. Forest bats on the other hand, had difficulties to dissipate heat in the humid cave set-up. The response to heat, however, was surprisingly uniform and all bats entered torpor combined with hyperthermia at temperatures exceeding their thermoneutral zone. Thus, while we observed potential for flexible compensation of heat through “hot” torpor, both populations showed patterns suggestive of limited potential to cope with acute microclimate changes deviating from their typically occupied roosts. Our study emphasizes that intraspecific variation among populations could be misleading when assessing species’ adaptive scopes, as variation may arise from genetic adaptation, developmental plasticity or phenotypic flexibility, all of which allow for compensatory responses at differing time scales. Disentangling these mechanisms and identifying the basis of variation is vital to make accurate predictions of species’ chances for persisting in ever rapidly changing habitats and climates.
Introduction
Rapid environmental alteration has an immediate impact on the ecological stability of habitats and their inhabitants. Extreme weather events and habitat destruction can affect prevailing habitat structure, microclimatic condition and/or resource availability and may thus disrupt species’ physiological functioning (Schmidt-Nielsen, 1997; Seebacher and Franklin, 2012; Tattersall et al., 2012). Certain environmental variation, however, can be mitigated by species‘ physiological flexibility since the physiological scope determines species’ tolerance to abiotic factors (Canale and Henry, 2010; Bozinovic et al., 2011). Indeed, many endotherms living in unpredictable or highly seasonal habitats or those that are widespread and face heterogeneous environmental conditions, have evolved considerable flexibility in traits associated with thermal maintenance, and these may vary among and within populations (e.g., Stawski and Geiser, 2010; Glanville et al., 2012; Noakes et al., 2016; van Jaarsveld et al., 2021). For example, populations inhabiting arid habitats often have lower water turnover rates and mechanisms to retain water more efficiently than their conspecifics from more mesic habitats (e.g., Klüg-Baerwald and Brigham, 2017; Cooper et al., 2018; Gearhart et al., 2020). Quantifying such intraspecific variation can aid our understanding of the physiological compensatory capacity of a species and ultimately its general ecological resilience.
Intraspecific physiological variation may arise from genetic divergence across generations in response to different local selection pressures and may even imply incipient speciation (Angiletta et al., 2010; Violle et al., 2012; Richardson et al., 2014). Especially widespread species may face contrasting environmental conditions throughout their distribution and distinct populations may be locally adapted (Tracy and Walsberg, 2000). Thus, variation observed among populations does not necessarily entail that each population can take advantage of the range of flexibility seen in a species. Phenotypic flexibility by contrast allows fast and reversible adjustments to a changing environment, e.g., through acclimatization (Piersma and Drent, 2003). For example, in zebra finches (Taeniopygia guttata) even short-term previous experience with high ambient temperature (Ta) confers a more favorable physiological response during subsequent heat exposure (Cooper et al., 2020). Also, juvenile European rabbit (Oryctolagus cuniculus) express uncoupling protein 1 (essential for the metabolism of brown adipose tissue to generate body heat) depending on their thermal position in the litter huddle (e.g., warmer center or cooler periphery; Bautista et al., 2013). Such flexible responses are considered key for species’ survival in the long-term, as genetic-based traits are not necessarily adaptive in short term (Ghalambor et al., 2007; Canale and Henry, 2010; Boyles et al., 2011; Huey et al., 2012). However, even the acclimatization of physiological traits within weeks may be too slow for some species given the increasing frequency of extreme weather events associated with climate change. Coping mechanisms also include acute responses (Huey and Bennett, 1990; Angilletta et al., 2006). Mongolian gerbils (Meriones unguiculatus), for example, flexibly remodel mitochondrial membrane lipids depending on acute heat or cold exposure (Pan et al., 2014), and zebra finches adjust the lipid composition of stratum corneum within hours after water deprivation (Menon et al., 1996). Disentangling acute, plastic and genetic mechanisms and identifying the underpinnings of variation is therefore vital when assessing species’ and populations’ scopes for coping with rapid environmental perturbations but also when managing conservation action such as defining suitable refugia or translocating wildlife to new habitat (Boyles et al., 2011; Tarszisz et al., 2014; Madliger and Love, 2015; Cooper et al., 2018).
Heterothermic species may have a pre-adaptive advantage for responding flexibly and efficiently to short-term changes through their ability to temporally abandon a euthermic life-style and enter energy- and water-saving torpor, with a significant, but controlled reduction of metabolic rate (MR; Herreid and Schmidt-Nielsen, 1966; Geiser, 2004; Heldmaier et al., 2004; Cooper et al., 2005; Levin et al., 2015). Torpor bout duration, frequency and/or level of metabolic depression may be finely adjusted depending on environmental pressures and individual constraints (Stawski and Geiser, 2011; Turner et al., 2012; Lovegrove et al., 2014). Thereby, torpor has also proven to be a powerful response for enduring acute disturbances caused by extreme weather events such as droughts, heatwaves, storms, fires, and flooding (Doucette et al., 2012; Bondarenco et al., 2014; Nowack et al., 2015, 2016a; Stawski et al., 2015; Barak et al., 2019).
The tropical Commerson’s leaf-nosed bat Macronycteris commersoni, makes extensive use of torpor in an exposed foliage roost, and torpor timing as well as duration are related to daytime heat (Reher and Dausmann, 2021). This species is one of the most widely distributed bat species in Madagascar (Goodman, 2011). In addition to exposed foliage, it also roosts in larger colonies in caves, which offer buffered, more stable microclimates (Reher et al., 2018), and torpor patterns, i.e., frequency, duration and timing, vary depending on respective roost condition (Reher et al., 2022). Both cave and forest populations are vulnerable to several threats: cave populations in southwestern Madagascar have already declined substantially due to ongoing unsustainable hunting and individuals may no longer be able to use the caves when disturbance persists (Goodman, 2006; Andriafidison et al., 2008). At the same time, forest populations are increasingly confronted with habitat destruction (Zinner et al., 2014) as well as global warming and its concomitants, i.e., higher temperature in the remaining fragments, more frequent heat waves and droughts (Tuff et al., 2016; Nematchoua et al., 2018). It is therefore of major importance to determine the extent to which these bats can potentially benefit from flexible compensatory mechanisms enabling them to sustain in, and adapt to, new habitat conditions.
We studied M. commersoni’s acute coping mechanisms by identifying the effects of experimental microhabitat change on the metabolic responses of a forest and a cave population. We manipulated the bats’ roosting microclimate to resemble the respective opposite conditions (cave vs. forest) and examined their thermal physiology to determine the bats’ physiological flexibility. Although these manipulated environmental conditions were outside the range normally experienced by the particular population, they were within the normal range faced by individuals of the same species and thus within their realistic ecological scope.
Materials and Methods
Study Species and Study Sites
To study intraspecific flexibility on a population-level, we worked with the endemic insectivorous Commerson’s leaf-nosed bat Macronycteris commersoni (Hipposideridae). This species is widespread across various vegetation formations and climatic regimes in Madagascar (only absent from the central highlands) and uses different types of diurnal roosts within its range (Goodman, 2011). For example, in Tsimanampetsotse National Park in south-western Madagascar, a dry spiny forest habitat, a population of M. commersoni roosts in a humid hot cave year-round (“cave habitat”; Reher et al., 2019), while another population roosts in the open vegetation among branches in dry deciduous forest ∼380 km further north, in Kirindy Forest/CNFEREF (“forest habitat”; Reher and Dausmann, 2021). The hot cave is well buffered and offers constant conditions of 32°C and 98% relative humidity at bat height (Reher et al., 2018), whereas the bats roosting in exposed foliage without any insulation have to cope with daily ambient fluctuations and weather extremes (Reher and Dausmann, 2021). Both habitats are situated in Madagascar’s drier western formations and highly seasonal, with a pronounced dry season without any rainfall for up to 9 months and reduced resource availability. In the dry spiny forest rain may even be absent for several years (Ratovonamana et al., 2013). Ta fluctuates on a daily basis between 12.6 and 32.5°C (mean daily minimum and maximum; cave habitat, outside the cave) as well as 13.3 and 34.0°C (forest habitat) in the dry season. During the wet season, resources are more abundant and particularly night-time Ta is higher, with daily fluctuations between 24.4 and 38.5°C (cave habitat) as well as 23.6 and 36.0°C (forest habitat).
Trapping and Handling
We trapped bats in June/July 2016 (dry season) and February/March 2017 (wet season) in the cave habitat and in February/March as well as July/August 2018 in the forest habitat (wet season and dry season, respectively). Depending on habitat structure and bats’ roosting behavior, we used different trapping methods. In the cave habitat during the dry season, we trapped the bats with a hand net in the early morning hours between 07:00 and 09:00 because this species is inactive during this time of the year. In the wet season, we erected a harp trap (two-bank 4.2 m2 harp trap; Faunatech∼Austbat, Bairnsdale, Australia) in front of the same cave’s entrance. In the forest habitat, two to three mist nets (3 m high × 6 m long, mesh size 19 mm; Ecotone, Sopot, Poland) per trapping night were installed blocking main flight corridors in the local grid systems N5, CS5, CS6, and CS7 in both seasons. The trap and nets remained open for the first 3 h after sunset and were checked every 10–20 min depending on season. We transferred a maximum of two bats into separate cloth bags and released additional adults and juveniles immediately at the capture site. None of the females were reproductively active during data collection.
We manipulated the bats at the point of capture. They were weighed to the nearest of 1 g, sexed and the forearm length was measured. We removed a patch of fur between the shoulder blades using a razor blade and attached a temperature-sensitive radio transmitter (∼ 0.9 g; Biotrack, Wareham, United Kingdom) with medical skin glue (Osto-Bond, Montreal, Canada; or Manfred Sauer GmbH, Lobbach, Germany), whereby the thermal sensor was placed directly on the skin. The proportion of transmitter mass to the lowest bat’s body mass was 2.7% (mean = 1.8 ± 0.43%, N = 70 bats) and thus well below recommended maxima of 5–10% (e.g., Aldridge and Brigham, 1988; Rojas et al., 2010). All transmitters were calibrated in a water bath at seven intervals over a temperature range of 3–45°C against a precision thermometer traceable to a national standard. Skin temperature (Tskin) obtained via external transmitters provide a non-invasive and reliable proxy of body temperature (Tb), particularly in small mammals (Audet and Thomas, 1996; Dausmann, 2005; Langer and Fietz, 2014; but see Willis and Brigham, 2003). We marked all bats with an individual, three-digit wing tattoo using non-toxic ink (Hauptner-Herberholz, Solingen, Germany) after locally anesthetizing the membrane (EMLA, AstraZeneca, Wedel, Germany). Animal handling lasted about 10 min per individual and never exceeded 15 min.
Study Set-Up
To study the effects of sudden environmental changes on the metabolic responses of the respective populations, we exposed individuals of each population to the respective contrasting roosting conditions, i.e., cave bats had to cope with fluctuating conditions outside the cave and the forest population with constant hot and humid conditions. In the cave habitat, measurements (N = 10 dry season, N = 13 wet season) were conducted in a shaded enclosure near the camp without any further buffer to ambient extremes. In the forest habitat, two modified incubators (Exo Terra PT2445; HAGEN, Holm, Germany) served as climate chambers to mimic cave conditions and were calibrated to maintain constant Ta = 32.1 ± 0.3°C and ambient relative humidity (RHa) = 88.9 ± 2.4%. RHa was increased by passing the air entering the climate chamber through a humidified sponge and by adding a dish with water to the floor of the climate chamber. Although 88.9% does not reflect cave relative humidity precisely (i.e., 98%), it never dropped below 82% and was as close as we could get under field conditions. We placed the respirometry chamber into the climate chamber and connected it to the oxygen analyzer (see below), whose pump drew the humidified air from the climate chamber through the respirometry chamber and finally the excurrent dried air to the analyzer. Since we did not trap any bats in the forest during the dry season, we only ran these measurements in the wet season (N = 10). The data from these experimental set-up were then compared to the physiological responses of bats resting in their native roost type, which are summarized and analyzed in an ecological context, i.e., focusing on habitat and roosting conditions but not acute microclimate changes, in Reher et al. (2022).
Respirometry Run and Temperature Measurements
We used open-flow respirometry in pull mode to measure the rate of oxygen consumption (V̇O2). We individually transferred the bats into 2 L plastic metabolic chambers, which were equipped with a net for the bats to hold onto, right after preparing them at their respective capture sites (i.e., attaching tag etc., see above). The chamber was connected to a portable gas analyzer (OxBox; T. Ruf and T. Paumann, University of Veterinary Medicine Vienna, Austria) with an integrated electro-chemical oxygen cell (Bieler & Lang, Achern, Germany). The oxygen sensor was calibrated in the laboratory before and after each field trip using room air and calibration gases generated by a gas mixing pump (19.9, 20.3, and 21% O2 content; 2KM300/a, Wösthoff Messtechnik GmbH, Bochum, Germany). During the measurements, ambient air was pulled through the respirometry chamber at a constant flow of 50 L h–1 using a diaphragm pump, dried with silica gel and filtered before passing through a flowmeter integrated in the OxBox. An aliquot thereof was then drawn through the gas analyzer. The oxygen content of the sample air was measured every 10 s for 55 min; reference air from outside the metabolic chamber was also dried with silica gel, filtered and its oxygen content was measured every 10 s for the remaining 5 min to control for drifts in the oxygen sensor. These data were used to correct the oxygen traces (Clampfit v10.3.1.4, Molecular Devices, Sunnyvale, United States).
The measurements started between 21:00 and 23:00 h in the wet season and 09:00 and 11:00 hr in the dry season owing to different trapping times. We recorded data for 20–32 h and ended the respirometry runs with the beginning of the bats’ usual active phase between 17:30 and 18:30 h to ensure access to immediate foraging possibilities upon release. We used the first MR values right after introducing the bats to the metabolic chambers as indicators of individual stress levels. If there was no marked decrease in these initial values within 30 or 60 min (depending on the inactive or active phase, respectively), the bats were immediately released at the capture site. Before we released them at their capture site, they were weighed a second time to calculate mean body mass (BM) and again offered food and fresh water (see Reher et al., 2018). During each respirometry run, we recorded Ta and RHa inside each respirometry chamber (resolution: 0.0625°C, 0.04% RH; Hygrochron iButtons, Maxim integrated, San Jose, United States), and Tskin using a remote receiver/logger placed next to the setup (DataSika SRX-800-D, Biotrack, Wareham, United Kingdom). All temperature data were recorded at 5-min intervals.
Data Processing and Analyses
In total, we analyzed data of 70 bats, none of which was a recapture (Table 1). We processed all data using Cran R (R Development Core Team, 2018) in the “RStudio” environment (RStudio Team, 2016) with support of the packages “plyr” (Wickham, 2011), “dplyr” (Wickham et al., 2020), “readxl” (Wickham and Bryan, 2017), “lubridate” (Grolemund and Wickham, 2011), “zoo” (Zeileis and Grothendieck, 2005), “stringr” (Wickham, 2019), “tidyr” (Wickham, 2020), “ggplot2” (Wickham, 2016), “ggpubr” (Kassambara, 2020a), and “cowplot” (Wilke, 2020).
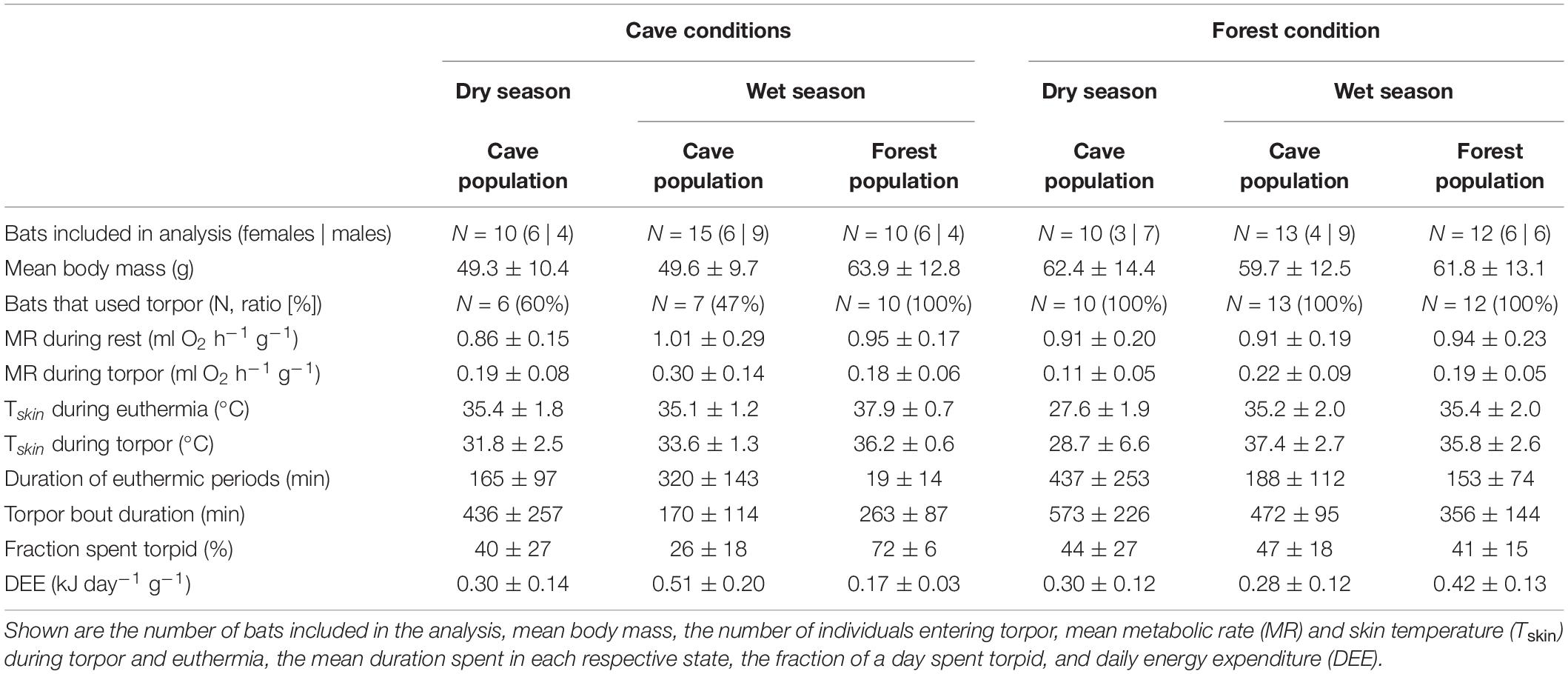
Table 1. Overview of the different populations in the dry and wet seasons exposed to either cave or forest conditions, and their key physiological variables.
Of all data sets, including those collected under natural conditions that sometimes lasted several days (Reher et al., 2022), only the first day of a measurement (from earliest 21:00 to ∼ 30 min before sunset) was analyzed. Moreover, because the forest environment is somewhat milder and both, warm (cloudy, rainy) and very hot days, can occur during the rainy season, we only included data from forest bats measured on hot days (N = 12 bats), which better reflected the southern regular weather.
We calculated individual MR (ml V̇O2 h–1), using the rate of oxygen consumption (V̇O2) as ml O2 h–1 corrected to STPD with equation 11.2 given by Lighton (2008) specifically for this set-up, assuming an average respiratory quotient of 0.85 (oxidation of 50% fat and 50% carbohydrate, Dausmann et al., 2009). To account for effects of differences in BM among individuals on MR, we performed an initial regression analysis showing that resting MR (RMR) was better explained by BM as a linear function through the origin (likelihood ratio test: Chi2 = −939.1, P = 0.023). For comparability in further analysis and data reporting, we therefore divided V̇O2 by mean BM for mass-specific MR calculations (ml V̇O2 h–1 g–1). We defined different physiological states via visual inspection of MR patterns following Reher and Dausmann (2021). Thereby, a drop in MR by at least 50% compared to RMR was considered to constitute torpor, which lies within the range of higher metabolic reductions seen during torpor in warm environments (25–84%; Song et al., 1997; Dausmann et al., 2009; Grimpo et al., 2013; Kobbe et al., 2014). We removed torpor arousal and torpor entry phases and continued with a subset of MR data to determine RMR and torpid MR (TMR). For RMR, we only included the lowest 50% of resting data per hour and per individual within the bats’ usual resting phase, i.e., from sunrise to 30 min before sunset to ensure that variations owing to disturbance or minor activity were excluded (Bethge et al., 2017; Reher and Dausmann, 2021; Rodgers and Franklin, 2021). For TMR, we included the lowest 70% of data per hour and per individual. Macronycteris commersoni is known to enter torpor bouts of varying lengths, including micro-torpor bouts lasting 12–20 min on average (Reher and Dausmann, 2021; Reher et al., 2022). However, the bats rarely entered micro-bouts in the manipulated conditions, and we therefore only included torpor bouts lasting ≥ 1 h for downstream analysis. We allocated RMR and TMR to different bins of Ta by rounding Ta to the nearest integer and assigning individual means of the different metabolic states to the respective Ta to avoid pseudo-replication.
To obtain Tskin, we converted the recorded transmitter signals from beat per minutes into temperature data using second-order polynomial regressions obtained from the calibration curves (all R2 ≥ 0.99). Tskin was used as a proxy for Tb to complement already defined torpor states through MR.
Forest Bats Exposed to Experimental Cave Conditions (Wet Season)
The natural cave and experimentally created cave conditions were highly constant in the wet season. We therefore used t-tests adjusted for unequal variances if necessary or Wilcoxon signed-rank tests (package “rstatix”; Kassambara, 2020b) to compare RMR, TMR, Tskin, Tskin-Ta differential, time spent euthermic and the fraction of the measurement duration spent torpid between forest-dwelling bats exposed to experimental cave conditions and native cave-dwelling bats. To determine whether the timing of torpor entry differed between populations, we applied Watson two-tailed tests (package “circular,” Jammalamadaka and Sengupta, 2001). Time is given as circular mean ± standard deviation.
Cave Bats Exposed to Experimental Forest Conditions (Wet Season)
To analyze the cave bats’ physiological responses in fluctuating forest conditions in the wet season, we explored the effect of Ta, population as well as the interaction of Ta and population on different physiological variables. We fitted separate generalized linear mixed effect models (GLMMs; package “lme4,” Bates et al., 2015) for TMR and Tskin, accounting for repeated measures by including bat ID as random effect. We fitted a similar GLMM for RMR below the thermal neutral zone (TNZ, i.e., the Ta-range at which no active thermoregulatory support is needed to maintain euthermic Tb) to compare the respective slopes and used a Wilcoxon-test to identify potential differences in RMR within the TNZ between native forest bats and cave bats exposed to experimental forest conditions. For the forest population, the TNZ has already been determined in Reher and Dausmann (2021) and we therefore followed the same approach for the cave population. We estimated the lower and upper limit using broken-stick regression, i.e., iterative fitting of linear models combined with a bootstrap restarting approach, making the algorithm less sensitive to the estimated starting value, to determine whether the observed RMR distribution could be explained by multiple linear segments (Wood, 2001; Muggeo, 2008). We did not fit separate models for torpid Tskin below the TNZ despite obvious variation between populations owing to the small sample size (Nforest = 2 at Ta ≤ 31°C). Furthermore, RHa was excluded from the analyses in the fluctuating environmental set-ups because it is not independent from Ta. We also did not include body conditions and pooled the data of females and males, as both were not important predictors when modeling RMR or TMR during longer torpor bouts in M. commersoni (Reher et al., 2022). Data exploration and model validation were done following Zuur et al. (2009), Zuur et al. (2010) and Zuur and Ieno (2016).
Cave Bats Exposed to Experimental Forest Conditions (Dry Season)
We did not trap any M. commersoni in the dry season in the forest habitat. Thus, we reported the cave-dwelling bats’ physiological responses when exposed to fluctuating conditions outside their cave descriptively and opportunistically analyzed single variables compared to all other bats, i.e., torpor bout duration and TMR, using t-tests or Wilcoxon signed-rank tests. We compared energetic costs imposed by the unfamiliar roosting conditions compared to what the respective populations usually consume in their natural roosting environment. Energy expenditure was calculated as daily energy expenditure (DEE, in kJ) from 22:00 to 18:00 extrapolated to 24 h based on per-minute metabolic rate values using an oxycalorific equivalent of 20.37 kJ/L O2 (Schmidt-Nielsen, 1997). All data are shown as mean ± standard deviation and range if appropriate; N represents the number of individuals.
Results
Forest Bats Exposed to Experimental Cave Conditions (Wet Season)
The forest population naturally roosted in a fluctuating environment with daily variation in Ta between on average 23.6 ± 1.2°C and 36.0 ± 2.2°C (min/max: 21.2/41.7°C) as well as daily variation in RHa between on average 47.6 ± 10.6% and 98.5 ± 2.2% (min/max: 29.0/101.2%). When exposing individuals from this population to constant 32.1 ± 0.3°C and 88.9 ± 2.4% RHa (i.e., experimental cave conditions), all bats entered torpor within the first hour of measurement. Individuals were torpid for more than 70% of the total measurement duration (mean torpor bout duration: 263 ± 87 min; Table 1) despite several arousals within 24 h. They were euthermic for an average of only 19 ± 14 min at a time and never > 57 min (Figure 1A). This was in clear contrast to the torpor pattern observed in the native population as only 60% of native cave bats entered torpor and for a much smaller fraction of the total measurement duration (26%; Table 1; W = 1, P < 0.001). In comparison, native cave bats showed ∼17-fold longer euthermic phases (320 ± 143 min; W = 144, P < 0.001), usually entered torpor in the late morning and aroused shortly before their active phase (Figure 1C).
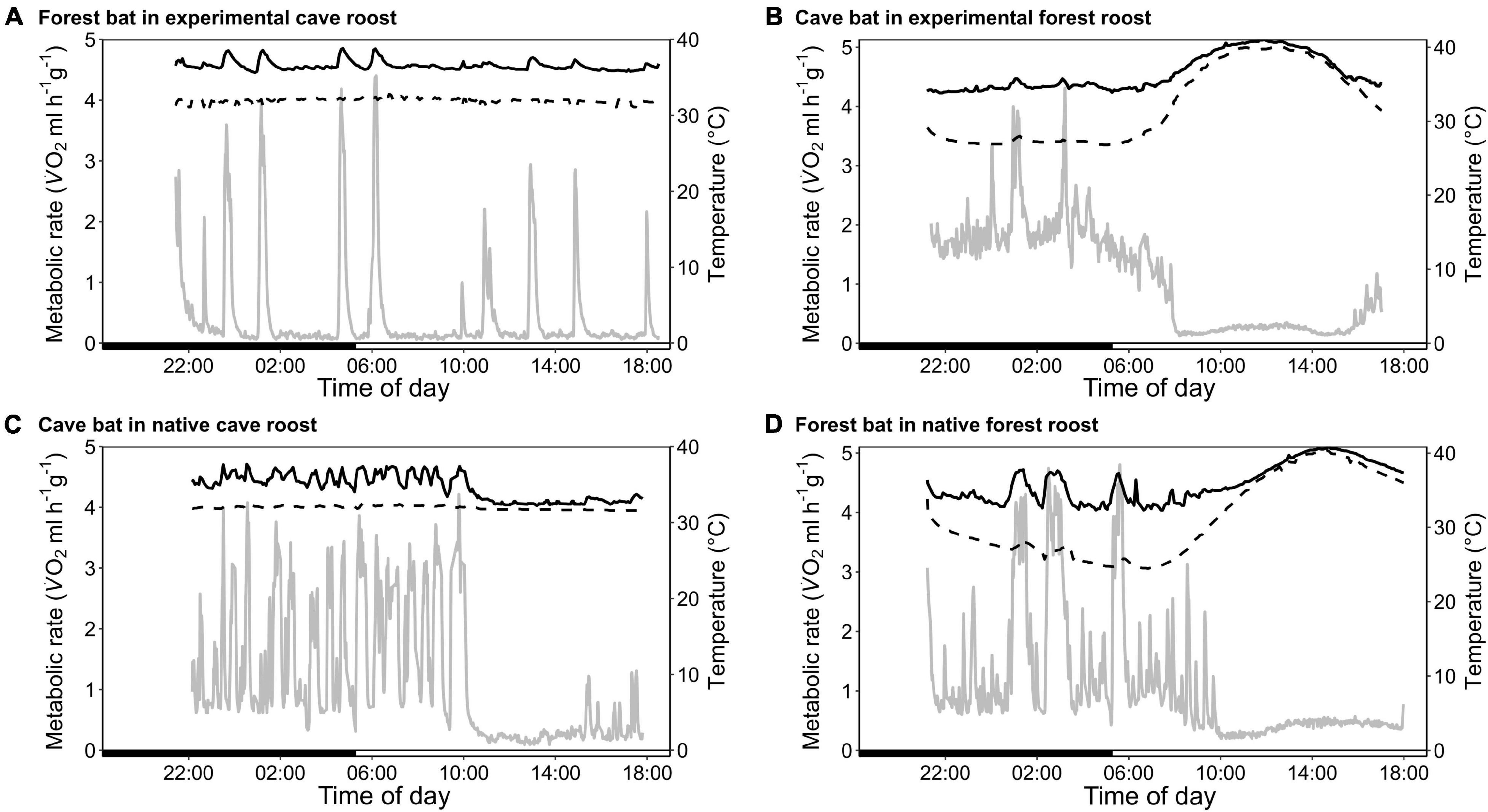
Figure 1. Variation in metabolic rate as V̇O2 (MR, ml h–1 g–1; gray; solid line), skin temperature (Tskin,°C; black; solid line) and ambient temperature (Ta,°C; black; dashed line) over the course of the day. (A) A forest-dwelling bat exposed to experimental cave conditions; (C) a cave-dwelling bat in its natural cave microhabitat; (B) a cave-dwelling bat exposed to experimental forest conditions, and (D) a forest-dwelling bat in its commonly experienced forest habitat.
We found no difference in RMR between the two populations (Table 1; t22.8 = 0.497, P = 0.624) but TMR was lower in the forest bats exposed to cave conditions than the native cave population (Table 1; W = 91, P = 0.010). Despite lower TMR, forest bats had higher Tskin (Table 1; t13 = −5.08, P < 0.001) and maintained a greater Tskin-Ta differential while torpid under cave conditions than native cave bats (2.3 ± 1.0°C vs. 4.2 ± 0.6°C; t16.2 = −5.21, P < 0.001). Simultaneously, during the short euthermic periods Tskin increased to 37.9 ± 0.7°C in the forest population, which was significantly higher than euthermic Tskin in the native cave population (35.1 ± 1.2°C; t19.3 = −4.65, P < 0.001), as was the Tskin-Ta differential (native cave population: 3.0 ± 1.0°C vs. experimental cave conditions: 5.2 ± 0.6°C; t22.3 = −4.72, P < 0.001).
Cave Bats Exposed to Experimental Forest Conditions (Wet Season)
The cave population roosts at constant 32 ± 0.1°C and 98 ± 0.5% RHa at bat height (Reher et al., 2022). When we exposed cave bats to experimental forest conditions in the wet season, i.e., Ta fluctuating on a daily basis between on average 24.4 ± 1.7°C and 38.5 ± 3.1°C (min/max: 22.4/43.0°C) as well as RHa fluctuating between on average 37.0 ± 8.4% and 85.6 ± 7.0% (min/max: 25.8/96.6%), all individuals entered torpor. One individual was torpid for almost the complete measuring duration while the other twelve bats remained euthermic during their usual active phase and entered torpor in the morning (Figure 1B). Similar to the experimental forest bats, all individuals from the native forest population entered torpor on hot days, i.e., days on which Ta exceeded its euthermic Tskin (Table 1 and Figure 1D; Reher and Dausmann, 2021). Individuals from the cave population in forest conditions entered torpor at high Ta earlier in the day (cave bats entry: 09:02 ± 0:40 h vs. native forest bats entry: 10:48 ± 1:06 h; x = 0.272, P < 0.01) and at lower Ta than individuals from the native forest population, at 32.1 ± 1.8°C and 34.8 ± 1.4°C, respectively (t19.4 = −3.98, P < 0.001).
We determined a TNZ for the cave bats in experimental forest between 31 and 34°C, which was slightly lower and narrower than the TNZ in the native forest population (32–36°C; Reher and Dausmann, 2021; Figure 2A). Within the TNZ, there was no significant difference in RMR between cave bats and native forest bats (0.67 ± 0.09 ml O2 h–1 g–1 vs. 0.77 ± 0.20 ml O2 h–1 g–1, respectively, W = 258, P = 0.057; Figure 2A). Below the respective TNZ, RMR increased with decreasing Ta (t = −10.51, P < 0.001), whereby the slope was steeper in cave bats measured under forest conditions (t = 2.41, P = 0.016; Supplementary Figure 1). In all bats, TMR increased with increasing Ta, which was especially apparent at higher Tas (t = 11.53, P < 0.001).
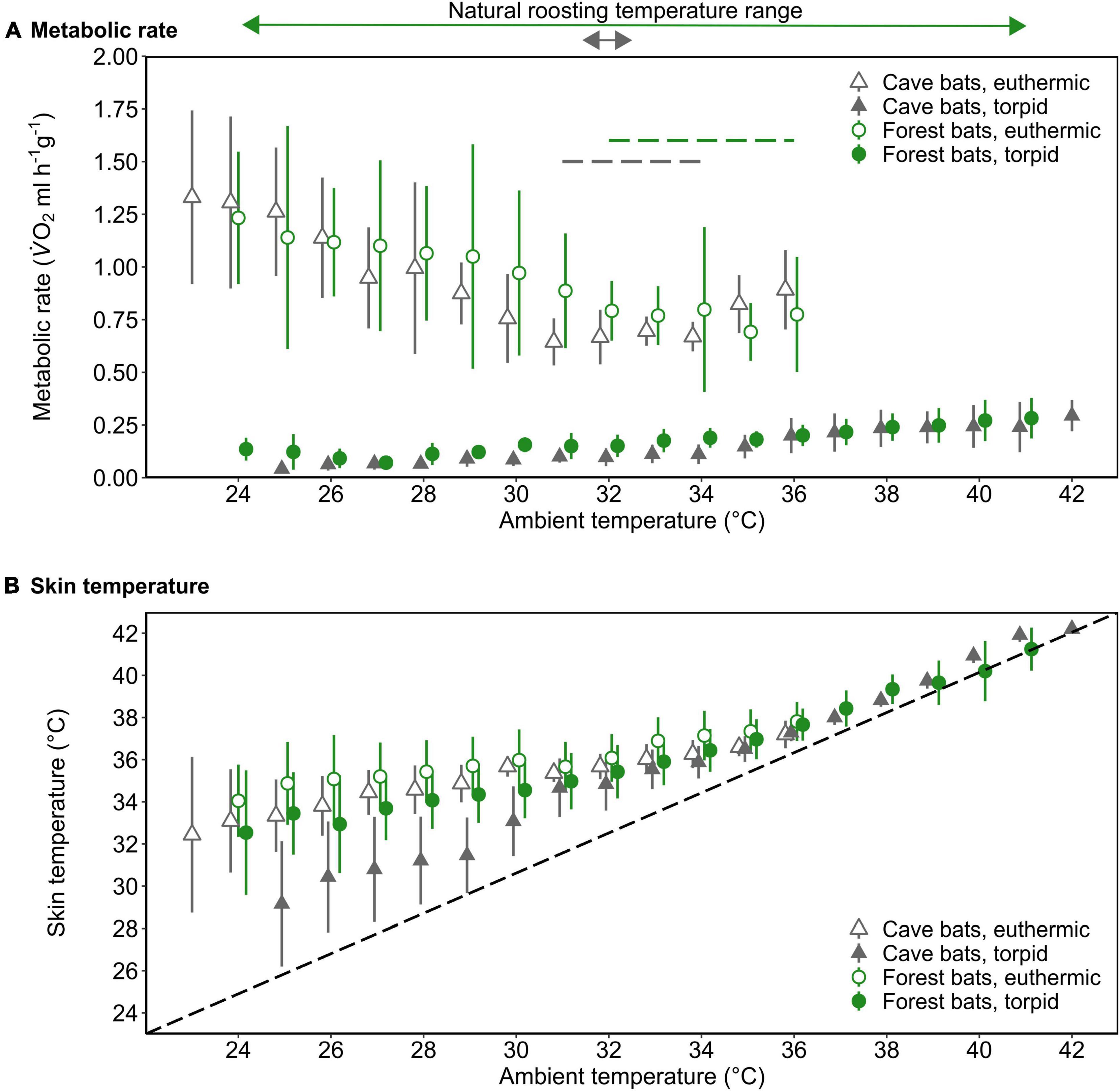
Figure 2. The mass-specific metabolic rate (A; MR, ml V̇O2 h–1 g–1) and skin temperature (B; Tskin,°C) as a function of ambient temperature (Ta,°C) of forest-dwelling bats (green circles) as well as cave-dwelling bats (gray triangles) during euthermia (open) and torpor (filled) when roosting in a forest environment in the wet season. The dashed lines in A indicate the thermoneutral zone for each population (colors correspond with population); the dashed line in B illustrates Tskin = Ta. The arrows above the upper panel indicate the typical roosting Ta range and colors correspond with bat population. Please note that at Ta ≤ 29°C, Nforest bats = 2 for torpid individuals. Error bars represent standard deviation; points are jittered for illustrative purposes.
Tskin during both, torpor and euthermia increased with increasing Ta (torpor, t = 37.51, P < 0.001; euthermia, t = 12.74, P < 0.001; Figure 2B), but the slope of this increase was steeper in the cave population in experimental forest (torpor, t = −8.17, P < 0.001; euthermia, t = −2.28, P = 0.022; Supplementary Figure 2). At lower Tas, there was a noticeable difference in cooling rates in torpid individuals, with cave bats under forest conditions losing heat more quickly than their native forest conspecifics (Figure 2B).
Cave Bats Exposed to Experimental Forest Conditions (Dry Season)
In the dry season, the cave population roosted at constant 31.4 ± 0.1°C and 98.1 ± 2.8% RHa at bat height. All individuals entered torpor when we exposed cave bats to experimental forest dry season conditions, i.e., fluctuating Ta between on average 16.3 ± 1.9°C and 32.5 ± 2.6°C (min/max: 6.9/36.4°C) as well as RHa fluctuating between on average 31.5 ± 8.4% and 86.9 ± 7.6% (min/max: 17.8/99.1%). One individual was torpid for the entire measurement duration and did not arouse before it was returned to the cave, while all other individuals entered torpor either at night (02:18 ± 1 h 22 min, 14.9 ± 3.4°C; N = 4; Supplementary Figure 3A) or in the morning when Ta had increased (11:13 ± 32 min, 31.3 ± 1.0°C, N = 5). Only three bats were able to defend a low, but fairly stable Tskin when Ta decreased at night (Tskin: 29.3 ± 1.0°C; Ta: 17.5 ± 2.5°C; Figure 3A), with a daily amplitude of 27.7 ± 0.5–34.3 ± 2.4°C.
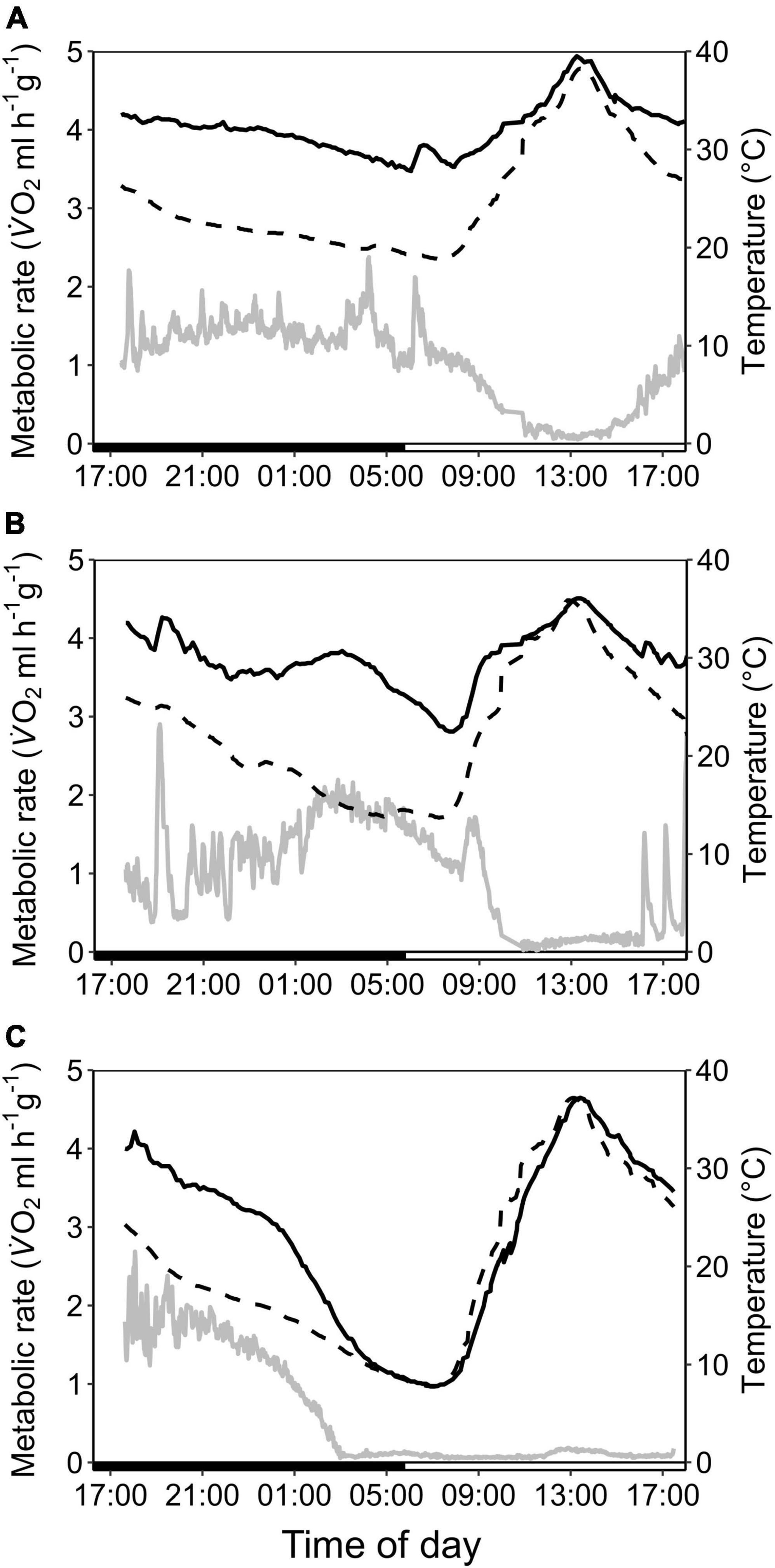
Figure 3. Variation in metabolic rate as V̇O2 (ml h–1 g–1; gray; solid line), skin temperature (Tskin,°C; black; solid line) and ambient temperature (Ta,°C; black; dashed line) over the course of the day in cave-dwelling bats exposed to forest habitat in the cooler dry season. (A) Example of an individual defending a low euthermic Tskin at night and entering torpor in the morning when Ta increased (note the hyperthermic Tskin); (B) example of an individual failing to defend a stable euthermic Tskin at night and entering torpor in the morning when Ta increased; (C) example of an individual possibly becoming hypothermic (see Supplementary Figure 3).
Mean euthermic Tskin in cave bats exposed to experimental forest dry season conditions was lower than that of bats under all other conditions (W = 2; P < 0.001; Table 1). In six bats, Tskin decreased with decreasing Ta (Figure 4B) although they increased maximum MR up to twofold RMR between Ta of 20–30°C (2.02 ± 0.60 ml O2 h–1 g–1) and up to threefold RMR below Ta of 20°C (2.94 ± 0.15 ml O2 h–1 g–1; Figure 4A), with an individual maximum of 6.46 ml O2 h–1 g–1, i.e., > 6-fold RMR. Four of these six bats eventually entered torpor with a considerable drop in MR and reached a steady-state TMR within ∼1 h (55 min ± 17 min; Figure 3B). Tskin at torpor entry was 29.6 ± 1.1°C and torpid Tskin ranged from 7.8 to 38.4°C, depending on time of day and thus Ta. The other two bats slowly decreased MR and Tskin over several hours (3 h 38 min ± 49 min; Figure 3C), a pattern resembling hypothermia and not torpor as suggested by reversed hysteresis of MR and Tskin (Geiser et al., 2014; Supplementary Figure 3). Nonetheless, mean MR during torpor and potential hypothermia (excluding entry and arousal phases) were comparable (torpor 0.08 ± 0.05 ml O2 h–1 g–1 vs. potential hypothermia 0.07 ± 0.04 ml O2 h–1 g–1).
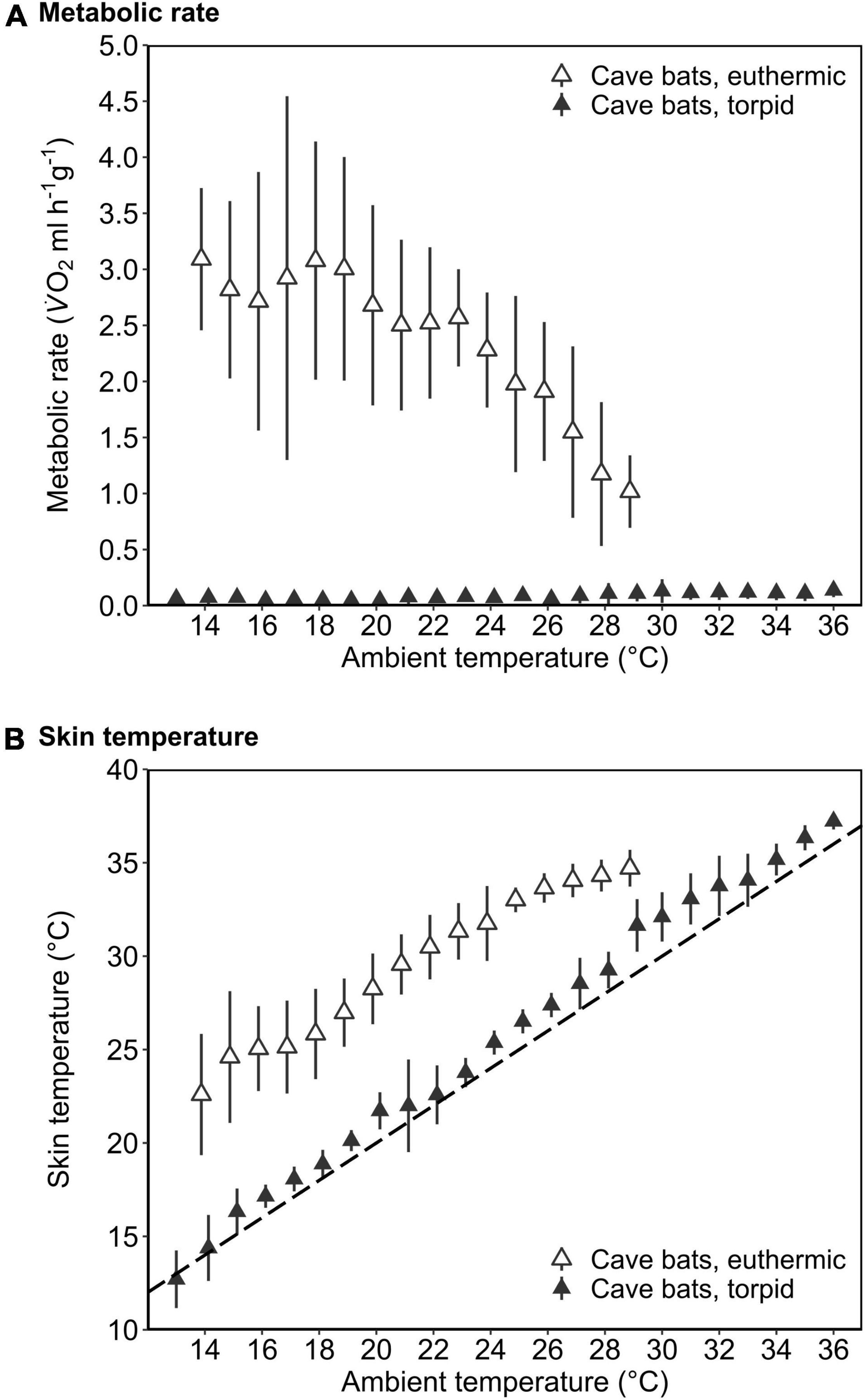
Figure 4. The mass-specific metabolic rate (A; MR, ml V̇O2 h–1 g–1) and skin temperature (B; Tskin,°C) as a function of ambient temperature (Ta,°C) of cave-dwelling bats when defending euthermia (i.e., maximum MR; open triangles) and when torpid (filled triangles) roosting in a forest environment in the dry season. The dashed line in B illustrates Tskin = Ta. Error bars represent standard deviation. Please note that the MR during euthermia shown here corresponds to the respective maximum MR.
The cave bats exposed to experimental forest dry season conditions entered the longest torpor bouts (W = 282; P = 0.039; Table 1) with lowest mean TMR of all bats (W = 80; P < 0.001; Table 1).
Energetic Costs of a Change in Microclimatic Conditions
Interestingly, roosting in unfamiliar environmental conditions did not lead to increased energy expenditure under any of the conditions (Table 1). In the wet season, both—cave bats exposed to the forest as well as forest bats exposed to experimental cave conditions—consumed less energy than their conspecifics in their natural roost (cave bats in forest microhabitat: W = 162, P = 0.002; forest bats in cave microhabitat: W = 1, P < 0.001). In the dry season, the cave bats had a similar energy expenditure when roosting in the experimental forest environment or in their native cave (W = 42, P = 0.842) but spent 84% of their energy budget at night defending euthermia, much more than they usually do in the cave during this time of the year (55%; t17 = −5.276, P < 0.001; Supplementary Figure 4).
Discussion
We exposed individuals of two populations of Commerson’s leaf-nosed bats M. commersoni resting in either a cave or a forest roost to the respective contrasting roosting conditions to examine the effects of experimental microhabitat changes on metabolic patterns and energy expenditure. While the majority of bats showed potential to cope with the new conditions through torpor, certain observed responses such as hypothermia are potentially fatal and may not sustain survival for an extended period.
Cave Bats Exposed to Experimental Forest Conditions (Dry Season)
Bats usually roosting in constant cave conditions could not defend euthermia when Ta decreased below their TNZ under experimental forest conditions. While three individuals defended a stable, albeit low, Tskin (∼29°C), six bats failed maintaining it constant below a Ta of ∼30°C: Tskin decreased with decreasing Ta despite maximal MR increases of sixfold RMR. Most bats eventually entered torpor to compensate for increased energy demands during this extensive upregulation of metabolic heat production. Two cave individuals, however, showed hypothermia-like responses in forest conditions, indicated by a noticeable slow torpor entry with reversed hysteresis and negligible Tskin-Ta differentials. Additionally, although these bats later passively rewarmed to almost euthermic Tskin (∼34.9°C, Supplementary Figure 3), they did not arouse spontaneously but only upon disturbance. These patterns resemble hypothermia rather than controlled torpor (Tomlinson et al., 2007; Geiser et al., 2014) and thus, reflect the bats’ limited capacity to generate sufficient heat at Tas below the TNZ. Simultaneously though, MR was substantially reduced and as low as MR in bats that had entered torpor. While we cannot specify the exact mechanism, we suggest that these bats entered torpor involuntarily with a hypothermic descent and subsequent transition into torpor.
Small mammals usually acclimatize to cold conditions by increasing their capacity for non-shivering thermogenesis (NST) in skeletal muscle or brown adipose tissue (Cannon and Nedergaard, 2004; Bal et al., 2012, 2016; Nowack et al., 2017a). Given the lack of cold exposure though, the cave bats likely have only limited amounts of brown adipose tissue and only limited capacity for muscle NST (Heldmaier and Hoffmann, 1974; Bal et al., 2016). This population overwinters in a hot cave offering thermal neutral 32°C year-round and does not leave the cave for the entire winter season, when they potentially could face low Ta of ∼7°C outside the cave (Rakotoarivelo et al., 2007; Raharinantenaina et al., 2008; Goodman, 2011; Reher et al., 2019). In the wet season by contrast, Ta outside the cave may drop to ∼20°C, but bats only experience this comparatively low Ta while foraging. Flapping flight is costly, and MR may increase up to 8–15-fold during flight, thereby generating substantial amounts of heat, which helps to maintain a stable body temperature despite the cold air (Carpenter, 1986; Speakman and Thomas, 2003; Voigt and Lewanzik, 2011). Tb regulation during the active phase is further supported by digestive heat production (MacArthur and Campbell, 1994; Beale et al., 2018), and thus, the native cave individuals may rarely ever have to actively increase heat production for thermoregulatory purposes.
Importantly, the attempts to defend euthermia at low Ta enforced a shift in the allocation of energy. In experimental forest microhabitat, cave bats spent 84% of their energy expenditure at night, compared to 55% under natural cave conditions. Interestingly, cave bats under forest dry season conditions entered longer and deeper torpor bouts during the day than all other individuals, by which they compensated for the increased night-time energy expenditure and maintained a similar energy expenditure as under native cave conditions. Although flexible torpor use mitigated the energetic costs, the cave population severely struggled defending euthermic Tb when Ta dropped below their TNZ, suggesting adaption to high cave Ta.
Forest Bats Exposed to Experimental Cave Conditions (Wet Season)
All forest bats exposed to constant experimental cave conditions remained torpid for a large fraction of the measurement duration and the occasional euthermic periods were the shortest recorded within this study. RMR during those short euthermic bouts was comparable to RMR of the native cave individuals, although resting Tskin was higher in forest bats in cave conditions than in cave bats and increased to a hyperthermic maximum of 39.4°C in one individual. The forest population usually experiences considerable daily fluctuations in both Ta and RHa (total range: 21.2–41.7°C, 29.0–101.2%; Reher et al., 2022), but in the forest highest Ta always coincides with lowest RHa. Many endotherms inhabiting warm and dry environments have developed adaptations to conserve water (Heldmaier et al., 2013; Fuller et al., 2016; Clarke, 2017). For example, different bird (Muñoz-Garcia and Williams, 2005; Champagne et al., 2012) and bat species (Muñoz-Garcia et al., 2012; Ben-Hamo et al., 2016) from arid habitats have a modified skin lipid composition with higher proportions of waxy lipids compared to their conspecifics from mesic habitat, which reduces the skin’s permeability and thus the rate of cutaneous water loss (Haugen et al., 2003). If the forest population, which is roosting under rather dry conditions, did have such adaptations, its evaporative cooling capacity would be limited. In addition, high RHa near saturation experienced by the bats under (experimental) cave conditions reduces the gradient that drives evaporation and thus, the amount of heat an animal can dissipate (Gerson et al., 2014). Cooling through evaporation is possible at high RHa when the water vapor pressure (WVP), e.g., on the skin surface, is higher than that in the air (Schmidt-Nielsen, 1997; Withers et al., 2016). Indeed, individuals may lick their forearms when heat stressed (Reher and Dausmann, 2021). This observation, and the high Tskin we measured during short euthermic periods, suggest that the forest bats were attempting to maintain cooling mechanisms by increasing both skin temperature and WVP on the skin’s surface. A higher Tskin-Ta differential additionally allows for passive pathways of heat loss through convection, conduction and radiation (Tattersall et al., 2012; Mitchell et al., 2018). Interestingly, torpid forest bats in the experimental cave had a higher Tskin than their torpid native cave conspecifics despite lower TMR and thus, lower metabolic heat production. During torpor however, vasomotor regulation is considered less functional (Muñoz-Garcia et al., 2016), suggesting that the high Tskin was not necessarily actively upregulated by vasodilation but that the patterns reflect difficulties in efficient heat dissipation. Thus, the excessive use of torpor in forest bats measured under hot cave conditions was probably driven by heat stress, a response also observed in the same population in their natural forest environment but at higher Ta (Reher and Dausmann, 2021). Although Ta of the imitated cave was within the forest bats’ TNZ, we suggest that the combination of high Ta with high RHa may have overwhelmed their thermoregulatory system (Cooper and Withers, 2008; Gerson et al., 2014). This particular population appears to rely on a certain water vapor gradient to dissipate excess heat, and this gradient was compromised when the bats were exposed to the highly humid conditions mimicking the experimental cave.
Cave Bats Exposed to Experimental Forest Conditions (Wet Season)
When we exposed the cave bats to experimental forest wet season conditions, we found a lower thermal inertia below the TNZ and higher cooling rates during torpor in cave bats than native forest bats. This is consistent with the dry season results discussed previously and indicates that this population lacks traits for coping with Ta below their commonly experienced 32°C in the cave. However, the bats’ responses to Ta exceeding their TNZ (up to 42.7°C) were surprisingly homogenous. At daytime Ta higher than usually experienced in the cave, all individuals responded by entering hyperthermic (“hot”) torpor, thus showing the same response that native forest bats use in their forest environment (Reher and Dausmann, 2021). Interestingly, bats from both populations did not use torpor in the early morning hours to benefit from lower Ta and enhance energetic savings like other tropical bats do (Geiser et al., 2000; Bondarenco et al., 2013), but entered torpor when Ta approached or exceeded their respective upper TNZ limit. Torpor at high Ta reduces metabolic heat production, allowing higher rates of heat from the environment to be stored in the body via facultative hyperthermia and reduces water lost during evaporative cooling when Ta exceeds Tb (Lovegrove et al., 2014; Welman et al., 2017; Mitchell et al., 2018; Reher and Dausmann, 2021). Although the general patterns were intriguingly similar, cave bats measured under forest conditions entered torpor at lower Ta, indicating that they were more heat stressed at lower Ta than the native forest bats, which is also reflected by their slightly lower TNZ. Since the cave bats roost in a very specific microclimate of high Ta and high RHa year-round, it is likely that they are adapted to their roosting conditions rather than the larger-scale, dry and hot climate of southwestern Madagascar. Water is scarce here, which could entail that outside the cave, at a higher WVP gradient and without sufficient access to water, this population may not necessarily be able to uphold cooling mechanisms (Mitchell et al., 2018) and the bats’ water budget could deplete quickly. Thus, entering torpor may have been the only physiological option when roosting outside the cave (Bondarenco et al., 2014; Reher and Dausmann, 2021). However, tropical bats experiencing high Ta in their diurnal roosts appear to have a great capacity for coping with heat (Cory Toussaint and McKechnie, 2012; Bondarenco et al., 2013, 2014; Czenze et al., 2020, 2022; Noakes et al., 2021). Indeed, torpor combined with hyperthermia never was observed in the native cave population roosting under constant cave conditions, which indicates that this particular population has a broader physiological repertoire than it commonly uses in its natural environment and thus, certain phenotypic flexibility in the context of heat exposure.
Potential Costs and Benefits of the Observed Patterns
Outside the respective populations’ commonly experienced environmental range, most bats showed patterns that aid overcoming acute perturbations. However, we have also seen responses suggesting limited physiological compensation of acute microclimate change. Forest bats, for example, had difficulties to dissipate heat at high RHa to the point that they were hyperthermic during short euthermic periods, which may have severe physiological consequences when Tb exceeds certain thresholds during tropical cyclones or extreme rainfall events, adding humidity to high Ta (Schmidt-Nielsen, 1997; Lepock, 2003). The cave population exposed to Ta below their TNZ could not maintain a high and constant euthermic Tskin despite expending more than 80% of their energy budget and two individuals had intriguing torpor entries similar to hypothermia. Hypothermia is potentially fatal (Tomlinson et al., 2007; Geiser et al., 2014) and the high night-time energy consumption may become problematic in the long-term when torpor has to be traded off with social interactions like reproduction to avoid depleting energy reserves in the dry season. While the response to heat resembled the forest bats’ response, cave bats were heat stressed at lower Ta than forest bats. It remains to be confirmed whether longer term responses to changed conditions mirror those of our study with a time-limited scope. Endotherms may adapt to novel conditions within hours, weeks or generations depending on their scope for phenotypic plasticity (Menon et al., 1996; Piersma and Drent, 2003; Heldmaier et al., 2013; Noakes and McKechnie, 2020). To take advantage of these processes, however, acute coping mechanisms are crucial to overcome interim time spans. Accordingly, the narrow capacity of the cave population to cope with conditions unlike those specific to hot caves is of particular concern given that human-induced disturbance is already evident in this population (Goodman, 2006), and no further hot caves are currently known to exist in the region (Reher et al., 2018).
Interestingly, increased torpor use appeared to be the common response for coping with unfamiliar microclimatic conditions, as reflected in the high ratio of bats entering torpor in the experimental set-ups. In this particular bat species though, multi-hour torpor is usually avoided when the populations roost in their natural conditions unless energy savings are vital or the bats are heat stressed (Reher and Dausmann, 2021; Reher et al., 2022). This may be due to certain costs associated with torpor (see Landes et al., 2020 for review). Especially the ecological and behavioral consequences may be critical for M. commersoni. Reduced sensory and motor capabilities during torpor can increase predation risk and limit social opportunities (Choi et al., 1998; Mzilikazi and Lovegrove, 2002; Nowack et al., 2016b). Nonetheless, torpor is a powerful emergency response to withstand acute disturbances such as droughts, heatwaves, storms, fires and flooding (Doucette et al., 2012; Bondarenco et al., 2014; Nowack et al., 2015, 2016a; Stawski et al., 2015; Barak et al., 2019). Importantly, torpor allows individuals to drastically reduce internal heat production if the water budget does not allow for extensive evaporative cooling or to finely balance the energy budget if unanticipated environmental changes have necessitated a short-term increase in energy expenditure (Herreid and Schmidt-Nielsen, 1966; Geiser, 2004; Heldmaier et al., 2004; Cooper et al., 2005; Nowack et al., 2017b; Reher and Dausmann, 2021). This is advantageous to endure unfavorable environmental conditions in general, but potentially also to bridge the time needed for adaptive mechanisms to take effect. None of these are options for homeothermic species, which likely face dramatic challenges when their environment changes rapidly.
Conclusion
This study is an important step to understand the impact of environmental change on tropical bats, and provides the basis for future studies (1) to estimate the bats’ capacity for phenotypic flexibility and (2) to disentangle plasticity and local adaptation with controlled acclimation or common garden experiments. Here, we looked into responses to acute microclimatic change that are predicted to become both more frequent and more intense concomitant with climate change. Based on our results, we argue that a wide distribution over broad environmental ranges and intraspecific variation in physiological traits reflecting differences in local microhabitat, as seen in M. commersoni (Reher et al., 2022), may be misleading when assessing adaptive scopes of species. Distinct populations may be locally adapted (Violle et al., 2012; Richardson et al., 2014), and physiological flexibility observed within a species is thus not necessarily transferable among individual populations. Quantifying the extent of intraspecific variation and particularly understanding the mechanism behind this variation is therefore vital when managing conservation actions, including translocating or reintroducing species to new habitat, and when predicting wildlife’s chances for persisting in ever rapidly changing environments and climates.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved under the “Accord de Collaboration” between Université d’Antananarivo (Département de Biologie Animale), Madagascar National Parks and Universität Hamburg. We thank these authorities and the Ministère de l’Environnement, de l’Ecologie et des Forêts for support and project authorization. The research was approved by the Directeur du Système des Aires Protégées, Ministère de l’Environnement, Antananarivo (Autorisation de recherche no. 90/ 16-, 003/ 17-, 296/17, 158/18- and 08/19/MEEF/SG/DGF/DSAP/SCB.Re) and all described procedures comply with the current ethical regulations and laws of Madagascar as well as the ethical guidelines of the Institute for Zoology, Universität Hamburg (statement no. 03/2016) for the care and use of animals.
Author Contributions
SR and KD designed the study. SR and HR carried out fieldwork. SR analyzed the data and wrote the first draft of the manuscript. SR, KD, and JN discussed and interpreted the results, revised, and edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
The German Research Foundation (DA 1013/7-1) and IDEA WILD (REHEMADA1116) supported this work financially.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Robert Jean Niry, Claude Jean Randrianasolo, Arne Wulff and the team of the association Analasoa for their constant help during fieldwork and Jörg Ganzhorn for permission to use the research Camp Andranovao in Tsimanampetsotse National Park. We are grateful to Peter Kappeler and Claudia Fichtel for allowing us to operate from, and use the facilities of, the field station of the German Primate Centre (DPZ) in Kirindy. The Centre National de Formation d’Etude et de Recherche en Environment et Foresterie (CNFEREF) supported our fieldwork in Kirindy. We highly appreciate the help of Jacques Rakotondranary and Yedidia Rakotomalala Ratovonamana with the organization of logistics and authorizations, Steve Goodman’s expertise in a variety of Malagasy bat-related topics and Janina Bethge’s feedback on an earlier draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.779381/full#supplementary-material
References
Aldridge, H. D. J. N., and Brigham, R. M. (1988). Load carrying and maneuverability in an insectivorous bat: a test of the 5% “rule” of radio-telemetry. J. Mammal. 69, 379–382. doi: 10.2307/1381393
Andriafidison, D., Cardiff, S., Goodman, S., Hutson, A., Jenkins, R., Kofoky, A., et al. (2008). Hipposideros commersoni. IUCN Red List Threat. Species 2008, e–T10120A3168011.
Angiletta, M. J., Cooper, B. S., Schuler, M. S., and Boyles, J. G. (2010). The evolution of thermal physiology in endotherms. Front. Biosci. 2:861–881. doi: 10.2741/e148
Angilletta, M. J., Bennett, A. F., Guderley, H., Navas, C. A., Seebacher, F., and Wilson, R. S. (2006). Coadaptation: a unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 79, 282–294. doi: 10.1086/499990
Audet, D., and Thomas, D. W. (1996). Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can. J. Zool. 74, 1778–1781. doi: 10.1139/z96-196
Bal, N. C., Maurya, S. K., Singh, S., Wehrens, X. H. T., and Periasamy, M. (2016). Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J. Biol. Chem. 291, 17247–17257. doi: 10.1074/jbc.M116.728188
Bal, N. C., Maurya, S. K., Sopariwala, D. H., Sahoo, S. K., Gupta, S. C., Shaikh, S. A., et al. (2012). Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579. doi: 10.1038/nm.2897
Barak, O., Geiser, F., and Kronfeld-Schor, N. (2019). Flood-induced multiday torpor in golden spiny mice (Acomys russatus). Aust. J. Zool. 66, 401–405. doi: 10.1071/ZO19061
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bautista, A., Castelán, F., Pérez-Roldán, H., Martínez-Gómez, M., and Hudson, R. (2013). Competition in newborn rabbits for thermally advantageous positions in the litter huddle is associated with individual differences in brown fat metabolism. Physiol. Behav. 118, 189–194. doi: 10.1016/j.physbeh.2013.05.035
Beale, P. K., Marsh, K. J., Foley, W. J., and Moore, B. D. (2018). A hot lunch for herbivores: physiological effects of elevated temperatures on mammalian feeding ecology. Biol. Rev. 93, 674–692. doi: 10.1111/brv.12364
Ben-Hamo, M., Muñoz-Garcia, A., Larrain, P., Pinshow, B., Korine, C., and Williams, J. B. (2016). The cutaneous lipid composition of bat wing and tail membranes: a case of convergent evolution with birds. Proc. R. Soc. B 283:20160636. doi: 10.1098/rspb.2016.0636
Bethge, J., Wist, B., Stalenberg, E., and Dausmann, K. (2017). Seasonal adaptations in energy budgeting in the primate Lepilemur leucopus. J. Comp. Physiol. B. 187, 827–834. doi: 10.1007/s00360-017-1082-9
Bondarenco, A., Körtner, G., and Geiser, F. (2013). Some like it cold: summer torpor by freetail bats in the Australian arid zone. J. Comp. Physiol. B 183, 1113–1122. doi: 10.1007/s00360-013-0779-7
Bondarenco, A., Körtner, G., and Geiser, F. (2014). Hot bats: extreme thermal tolerance in a desert heat wave. Sci. Nat. 101, 679–685. doi: 10.1007/s00114-014-1202-2
Boyles, J. G., Seebacher, F., Smit, B., and McKechnie, A. E. (2011). Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690. doi: 10.1093/icb/icr053
Bozinovic, F., Calosi, P., and Spicer, J. I. (2011). Physiological correlates of geographic range in animals. Annu. Rev. Ecol. Evol. Syst. 42, 155–179. doi: 10.1146/annurev-ecolsys-102710-145055
Canale, C., and Henry, P. (2010). Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim. Res. 43, 135–147. doi: 10.3354/cr00897
Cannon, B., and Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 227–359. doi: 10.1152/physrev.00015.2003
Carpenter, R. E. (1986). Flight physiology of intermediate-sized fruit bats (Pteropodidae). J. Exp. Biol. 120, 79–103. doi: 10.1242/jeb.120.1.79
Champagne, A. M., Muñoz-Garcia, A., Shtayyeh, T., Tieleman, B. I., Hegemann, A., Clement, M. E., et al. (2012). Lipid composition of the stratum corneum and cutaneous water loss in birds along an aridity gradient. J. Exp. Biol. 215, 4299–4307. doi: 10.1242/jeb.077016
Choi, I., Cho, Y., Oh, Y. K., Jung, N., and Shin, H. (1998). Behavior and muscle performance in heterothermic bats. Physiol. Zool. 71, 257–266. doi: 10.1086/515915
Clarke, A. (2017). Principles of Thermal Ecology: Temperature, Energy, and Life. Oxford: Oxford University Press.
Cooper, C. E., Hurley, L. L., and Griffith, S. C. (2020). Effect of acute exposure to high ambient temperature on the thermal, metabolic and hygric physiology of a small desert bird. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 244, 110684. doi: 10.1016/j.cbpa.2020.110684
Cooper, C. E., McAllan, B. M., and Geiser, F. (2005). Effect of torpor on the water economy of an arid-zone marsupial, the stripe-faced dunnart (Sminthopsis macroura). J. Comp. Physiol. B 175, 323–328. doi: 10.1007/s00360-005-0488-y
Cooper, C. E., and Withers, P. C. (2008). Allometry of evaporative water loss in marsupials: implications of the effect of ambient relative humidity on the physiology of brushtail possums (Trichosurus vulpecula). J. Exp. Biol. 211, 2759–2766. doi: 10.1242/jeb.019463
Cooper, C. E., Withers, P. C., Munns, S. L., Geiser, F., and Buttemer, W. A. (2018). Geographical variation in the standard physiology of brushtail possums (Trichosurus): implications for conservation translocations. Conserv. Physiol. 6:coy042. doi: 10.1093/conphys/coy042
Cory Toussaint, D., and McKechnie, A. E. (2012). Interspecific variation in thermoregulation among three sympatric bats inhabiting a hot, semi-arid environment. J. Comp. Physiol. B 182, 1129–1140. doi: 10.1007/s00360-012-0683-6
Czenze, Z. J., Naidoo, S., Kotze, A., and McKechnie, A. E. (2020). Bat thermoregulation in the heat: limits to evaporative cooling capacity in three southern African bats. J. Therm. Biol. 89:102542. doi: 10.1016/j.jtherbio.2020.102542
Czenze, Z. J., Smit, B., van Jaarsveld, B., Freeman, M. T., and McKechnie, A. E. (2022). Caves, crevices and cooling capacity: roost microclimate predicts heat tolerance in bats. Funct. Ecol. 36, 38–50. doi: 10.1111/1365-2435.13918
Dausmann, K. H. (2005). Measuring body temperature in the field—evaluation of external vs. implanted transmitters in a small mammal. J. Therm. Biol. 30, 195–202. doi: 10.1016/j.jtherbio.2004.11.003
Dausmann, K. H., Glos, J., and Heldmaier, G. (2009). Energetics of tropical hibernation. J. Comp. Physiol. B 179, 345–357. doi: 10.1007/s00360-008-0318-0
Doucette, L. I., Brigham, R. M., Pavey, C. R., and Geiser, F. (2012). Prey availability affects daily torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Oecologia 169, 361–372. doi: 10.1007/s00442-011-2214-7
Fuller, A., Mitchell, D., Maloney, S. K., and Hetem, R. S. (2016). Towards a mechanistic understanding of the responses of large terrestrial mammals to heat and aridity associated with climate change. Clim. Change. Resp. 3:10. doi: 10.1186/s40665-016-0024-1
Gearhart, C., Adams, A. M., Pinshow, B., and Korine, C. (2020). Evaporative water loss in Kuhl’s pipistrelles declines along an environmental gradient, from mesic to hyperarid. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 240:110587. doi: 10.1016/j.cbpa.2019.110587
Geiser, F. (2004). Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. doi: 10.1146/annurev.physiol.66.032102.115105
Geiser, F., Currie, S. E., O’Shea, K. A., and Hiebert, S. M. (2014). Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1324–R1329. doi: 10.1152/ajpregu.00214.2014
Geiser, F., Holloway, J. C., Körtner, G., Maddocks, T. A., Turbill, C., and Brigham, R. M. (2000). “Do patterns of torpor differ between free-ranging and captive mammals and birds?,” in Life in the Cold, eds G. Heldmaier and M. Klingenspor (Berlin, Heidelberg: Springer Berlin Heidelberg), 95–102. doi: 10.1007/978-3-662-04162-8_10
Gerson, A. R., Krabbe Smith, E., Smit, B., McKechnie, A. E., and Wolf, B. O. (2014). The impact of humidity on evaporative cooling in small desert birds exposed to high air temperatures. Physiol. Biochem. Zool. 87, 782–795. doi: 10.1086/678956
Ghalambor, C. K., McKAY, J. K., Carroll, S. P., and Reznick, D. N. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. doi: 10.1111/j.1365-2435.2007.01283.x
Glanville, E. J., Murray, S. A., and Seebacher, F. (2012). Thermal adaptation in endotherms: climate and phylogeny interact to determine population-level responses in a wild rat. Funct. Ecol. 26, 390–398. doi: 10.1111/j.1365-2435.2011.01933.x
Goodman, S. M. (2006). Hunting of Microchiroptera in south-western Madagascar. Oryx 40, 225–228. doi: 10.1017/S0030605306000354
Goodman, S. M. (2011). Les Chauves-Souris De Madagascar: Guide De Leur Distribution, Biologie et Identification. Antananarivo: Association Vahatra.
Grimpo, K., Legler, K., Heldmaier, G., and Exner, C. (2013). That’s hot: golden spiny mice display torpor even at high ambient temperatures. J. Comp. Physiol. B 183, 567–581. doi: 10.1007/s00360-012-0721-4
Grolemund, G., and Wickham, H. (2011). Dates and times made easy with lubridate. J. Stat. Softw. 40, 1–25. doi: 10.18637/jss.v040.i03
Haugen, M. J., Tieleman, B. I., and Williams, J. B. (2003). Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J. Exp. Biol. 206, 3581–3588. doi: 10.1242/jeb.00596
Heldmaier, G., and Hoffmann, K. (1974). Melatonin stimulates growth of brown adipose tissue. Nature 247, 224–225. doi: 10.1038/247224a0
Heldmaier, G., Neuweiler, G., and Rössler, W. (2013). Vergleichende Tierphysiologie, 2nd Edn. Berlin Heidelberg: Springer Spektrum.
Heldmaier, G., Ortmann, S., and Elvert, R. (2004). Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329. doi: 10.1016/j.resp.2004.03.014
Herreid, C., and Schmidt-Nielsen, K. (1966). Oxygen consumption, temperature, and water loss in bats from different environments. Am. J. Physiol. 211, 1108–1112. doi: 10.1152/ajplegacy.1966.211.5.1108
Huey, R. B., and Bennett, A. F. (1990). “Physiological adjustments to fluctuating thermal environments: An ecological and evolutionary perspective,” in Stress Proteins in Biology and Medicine, eds A. Morimoto, A. Tissieres, and C. Georgopoulus (Spring: Cold Spring Harbor Laboratory Press), 37–59.
Huey, R. B., Kearney, M. R., Krockenberger, A., Holtum, J. A. M., Jess, M., and Williams, S. E. (2012). Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. doi: 10.1098/rstb.2012.0005
Jammalamadaka, S. R., and Sengupta, A. (2001). Topics in Circular Statistics. Singapore: World Scientific Publishing.
Kassambara, A. (2020a). ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.4.0. Available online at: https://CRAN.R-project.org/package=ggpubr.
Kassambara, A. (2020b). rstatix: pipe-friendly framework for basic statistical tests. R package version 0.6.0. Available online at: https://CRAN.R-project.org/package=rstatix.
Klüg-Baerwald, B. J., and Brigham, R. M. (2017). Hung out to dry? Intraspecific variation in water loss in a hibernating bat. Oecologia 183, 977–985. doi: 10.1007/s00442-017-3837-0
Kobbe, S., Nowack, J., and Dausmann, K. H. (2014). Torpor is not the only option: seasonal variations of the thermoneutral zone in a small primate. J. Comp. Physiol. B 184, 789–797. doi: 10.1007/s00360-014-0834-z
Landes, J., Pavard, S., Henry, P.-Y., and Terrien, J. (2020). Flexibility is costly: hidden physiological damage from seasonal phenotypic transitions in heterothermic species. Front. Physiol. 11:985. doi: 10.3389/fphys.2020.00985
Langer, F., and Fietz, J. (2014). Ways to measure body temperature in the field. J. Therm. Biol. 42, 46–51. doi: 10.1016/j.jtherbio.2014.03.002
Lepock, J. R. (2003). Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int. J. Hyperthermia 19, 252–266. doi: 10.1080/0265673031000065042
Levin, E., Plotnik, B., Amichai, E., Braulke, L. J., Landau, S., Yom-Tov, Y., et al. (2015). Subtropical mouse-tailed bats use geothermally heated caves for winter hibernation. Proc. R. Soc. B 282:20142781. doi: 10.1098/rspb.2014.2781
Lighton, J. R. B. (2008). Measuring Metabolic Rates: A Manual for Scientists. Oxford: Oxford University Press.
Lovegrove, B. G., Canale, C., Levesque, D., Fluch, G., R̆eháková-Petrů, M., and Ruf, T. (2014). Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 87, 30–45. doi: 10.1086/673313
MacArthur, R. A., and Campbell, K. L. (1994). Heat increment of feeding and its thermoregulatory benefit in the muskrat (Ondatra zibethicus). J. Comp. Physiol. B 164, 141–146. doi: 10.1007/BF00301656
Madliger, C. L., and Love, O. P. (2015). The power of physiology in changing landscapes: considerations for the continued integration of conservation and physiology. Integr. Comp. Biol. 55, 545–553. doi: 10.1093/icb/icv001
Menon, G. K., Maderson, P. F. A., Drewes, R. C., Baptista, L. F., Price, L. F., and Elias, P. M. (1996). Ultrastructural organization of avian stratum corneum lipids as the basis for facultative cutaneous waterproofing. J. Morphol. 227, 1–13. doi: 10.1002/(SICI)1097-4687(199601)227:1<1::AID-JMOR1>3.0.CO;2-F
Mitchell, D., Snelling, E. P., Hetem, R. S., Maloney, S. K., Strauss, W. M., and Fuller, A. (2018). Revisiting concepts of thermal physiology: Predicting responses of mammals to climate change. J. Anim. Ecol. 87, 956–973. doi: 10.1111/1365-2656.12818
Muggeo, V. M. R. (2008). Modeling temperature effects on mortality: multiple segmented relationships with common break points. Biostatistics 9, 613–620. doi: 10.1093/biostatistics/kxm057
Muñoz-Garcia, A., Larraín, P., Ben-Hamo, M., Cruz-Neto, A., Williams, J. B., Pinshow, B., et al. (2016). Metabolic rate, evaporative water loss and thermoregulatory state in four species of bats in the Negev desert. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 191, 156–165. doi: 10.1016/j.cbpa.2015.10.010
Muñoz-Garcia, A., Ro, J., Reichard, J. D., Kunz, T. H., and Williams, J. B. (2012). Cutaneous water loss and lipids of the stratum corneum in two syntopic species of bats. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 161, 208–215. doi: 10.1016/j.cbpa.2011.10.025
Muñoz-Garcia, A., and Williams, J. B. (2005). Cutaneous water loss and lipids of the stratum corneum in house sparrows Passer domesticus from arid and mesic environments. J. Exp. Biol. 208, 3689–3700. doi: 10.1242/jeb.01811
Mzilikazi, N., and Lovegrove, B. G. (2002). Reproductive activity influences thermoregulation and torpor in pouched mice. Saccostomus campestris. J. Comp. Physiol. B 172, 7–16. doi: 10.1007/s003600100221
Nematchoua, M. K., Ricciardi, P., Orosa, J. A., and Buratti, C. (2018). A detailed study of climate change and some vulnerabilities in Indian Ocean: a case of Madagascar island. Sustain. Cities Soc. 41, 886–898. doi: 10.1016/j.scs.2018.05.040
Noakes, M. J., and McKechnie, A. E. (2020). Phenotypic flexibility of metabolic rate and evaporative water loss does not vary across a climatic gradient in an Afrotropical passerine bird. J. Exp. Biol 223:jeb.220137. doi: 10.1242/jeb.220137
Noakes, M. J., McKechnie, A. E., and Brigham, R. M. (2021). Interspecific variation in heat tolerance and evaporative cooling capacity among sympatric temperate-latitude bats. Can. J. Zool. 99, 480–488. doi: 10.1139/cjz-2020-0276
Noakes, M. J., Wolf, B. O., and McKechnie, A. E. (2016). Seasonal and geographical variation in heat tolerance and evaporative cooling capacity in a passerine bird. J. Exp. Biol. 219, 859–869. doi: 10.1242/jeb.132001
Nowack, J., Cooper, C. E., and Geiser, F. (2016a). Cool echidnas survive the fire. Proc. R. Soc. B 283:20160382. doi: 10.1098/rspb.2016.0382
Nowack, J., Delesalle, M., Stawski, C., and Geiser, F. (2016b). Can hibernators sense and evade fires? Olfactory acuity and locomotor performance during deep torpor. Sci. Nat. 103:73. doi: 10.1007/s00114-016-1396-6
Nowack, J., Giroud, S., Arnold, W., and Ruf, T. (2017a). Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 8:889. doi: 10.3389/fphys.2017.00889
Nowack, J., Stawski, C., and Geiser, F. (2017b). More functions of torpor and their roles in a changing world. J. Comp. Physiol. B 187, 889–897. doi: 10.1007/s00360-017-1100-y
Nowack, J., Rojas, A. D., Körtner, G., and Geiser, F. (2015). Snoozing through the storm: torpor use during a natural disaster. Sci. Rep. 5:11243. doi: 10.1038/srep11243
Pan, Q., Li, M., Shi, Y. L., Liu, H., Speakman, J. R., and Wang, D. H. (2014). Lipidomics reveals mitochondrial membrane remodeling associated with acute thermoregulation in a rodent with a wide thermoneutral zone. Lipids 49, 715–730. doi: 10.1007/s11745-014-3900-0
Piersma, T., and Drent, J. (2003). Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. doi: 10.1016/S0169-5347(03)00036-3
R Development Core Team (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raharinantenaina, I. M. O., Kofoky, A. F., Mbohoahy, T., Randrianandrianina, F., and Ramilijaona, O. R. (2008). Hipposideros commersoni (E. Geoffory, 1813, Hipposideridae) roosting in trees in littoral forest, south-eastern Madagascar. Afr. Bat. Conserv. News 15, 2–3.
Rakotoarivelo, A. A., Ranaivoson, N., Ramilijaona, O. R., Kofoky, A. F., Racey, P. A., and Jenkins, R. K. B. (2007). Seasonal food habits of five sympatric forest Microchiropterans in western Madagascar. J. Mammal. 88, 959–966. doi: 10.1644/06-MAMM-A-112R1.1
Ratovonamana, R. Y., Rajeriarison, C., Roger, E., Kiefer, I., and Ganzhorn, J. U. (2013). Impact of livestock grazing on forest structure, plant species composition and biomass in southwestern Madagascar. Scr. Bot. Belgica 50, 82–92.
Reher, S., and Dausmann, K. H. (2021). Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc. R. Soc. B. 288:20202059. doi: 10.1098/rspb.2020.2059
Reher, S., Ehlers, J., Rabarison, H., and Dausmann, K. H. (2018). Short and hyperthermic torpor responses in the Malagasy bat Macronycteris commersoni reveal a broader hypometabolic scope in heterotherms. J. Comp. Physiol. B 188, 1015–1027. doi: 10.1007/s00360-018-1171-4
Reher, S., Rabarison, H., and Dausmann, K. (2019). Seasonal movements of insectivorous bat species in southwestern Madagascar. Malagasy Nat. 13, 117–124.
Reher, S., Rabarison, H., Montero, B. K., Turner, J. M., and Dausmann, K. H. (2022). Disparate roost sites drive intraspecific physiological variation in a Malagasy bat. Oecologia 198, 35–52. doi: 10.1007/s00442-021-05088-2
Richardson, J. L., Urban, M. C., Bolnick, D. I., and Skelly, D. K. (2014). Microgeographic adaptation and the spatial scale of evolution. Trends Ecol. Evol. 29, 165–176. doi: 10.1016/j.tree.2014.01.002
Rodgers, E., and Franklin, C. E. (2021). Aerobic scope and climate warming: testing the “plastic floors and concrete ceilings” hypothesis in the estuarine crocodile (Crocodylus porosus). J. Exp. Zool. A Ecol. Integr. Physiol. 335, 108–117. doi: 10.1002/jez.2412
Rojas, A. D. Körtner, G., and Geiser, F. (2010). Do implanted transmitters affect maximum running speed of two small marsupials? J. Mammal. 91, 1360–1364. doi: 10.1644/10-MAMM-A-052.1
Schmidt-Nielsen, K. (1997). Animal Physiology: Adaptation and Environment. Cambridge: Cambridge University Press.
Seebacher, F., and Franklin, C. E. (2012). Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos. Trans. R. Soc. B 367, 1607–1614. doi: 10.1098/rstb.2012.0036
Song, X., Körtner, G., and Geiser, F. (1997). Thermal relations of metabolic rate reduction in a hibernating marsupial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R2097–R2104. doi: 10.1152/ajpregu.1997.273.6.R2097
Speakman, J. R., and Thomas, D. W. (2003). “Physiological ecology and energetics of bats,” in Bat Ecology, eds T. H. Kunz and M. B. Fenton (Chicago and London: University of Chicago Press).
Stawski, C., and Geiser, F. (2010). Seasonality of torpor patterns and physiological variables of a free-ranging subtropical bat. J. Exp. Biol. 213, 393–399. doi: 10.1242/jeb.038224
Stawski, C., and Geiser, F. (2011). Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R542–R547. doi: 10.1152/ajpregu.00792.2010
Stawski, C., Körtner, G., Nowack, J., and Geiser, F. (2015). The importance of mammalian torpor for survival in a post-fire landscape. Biol. Lett. 11:20150134. doi: 10.1098/rsbl.2015.0134
Tarszisz, E., Dickman, C. R., and Munn, A. J. (2014). Physiology in conservation translocations. Conserv. Physiol 2:cou054. doi: 10.1093/conphys/cou054
Tattersall, G. J., Sinclair, B. J., Withers, P. C., Fields, P. A., Seebacher, F., Cooper, C. E., et al. (2012). Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr. Physiol. 2, 2151–2202. doi: 10.1002/cphy.c110055
Tomlinson, S., Withers, P. C., and Cooper, C. (2007). Hypothermia versus torpor in response to cold stress in the native Australian mouse Pseudomys hermannsburgensis and the introduced house mouse Mus musculus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 148, 645–650. doi: 10.1016/j.cbpa.2007.08.013
Tracy, R. L., and Walsberg, G. E. (2000). Prevalence of cutaneous evaporation in Merriam’s kangaroo rat and its adaptive variation at the subspecific level. J. Exp. Biol. 203, 773–781. doi: 10.1242/jeb.203.4.773
Tuff, K. T., Tuff, T., and Davies, K. F. (2016). A framework for integrating thermal biology into fragmentation research. Ecol. Lett. 19, 361–374. doi: 10.1111/ele.12579
Turner, J. M., Körtner, G., Warnecke, L., and Geiser, F. (2012). Summer and winter torpor use by a free-ranging marsupial. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 162, 274–280. doi: 10.1016/j.cbpa.2012.03.017
van Jaarsveld, B., Bennett, N. C., Kemp, R., Czenze, Z. J., and McKechnie, A. E. (2021). Heat tolerance in desert rodents is correlated with microclimate at inter- and intraspecific levels. J. Comp. Physiol. B 191, 575–588. doi: 10.1007/s00360-021-01352-2
Violle, C., Enquist, B. J., McGill, B. J., Jiang, L., Albert, C. H., Hulshof, C., et al. (2012). The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. doi: 10.1016/j.tree.2011.11.014
Voigt, C. C., and Lewanzik, D. (2011). Trapped in the darkness of the night: thermal and energetic constraints of daylight flight in bats. Proc. R. Soc. B 278, 2311–2317. doi: 10.1098/rspb.2010.2290
Welman, S., Tuen, A. A., and Lovegrove, B. G. (2017). Using thermoregulatory profiles to assess climate change vulnerability in an arboreal tropical bat: heterothermy may be a pre-adaptive advantage. Clim. Res. 74, 161–170. doi: 10.3354/cr01496
Wickham, H. (2011). The split-apply-combine strategy for data analysis. J. Stat. Softw. 40, 1–29. doi: 10.18637/jss.v040.i01
Wickham, H. (2019). stringr: Simple, Consistent Wrappers for Common String Operations. R Package Version 1.4.0. Available online at: https://CRAN.R-project.org/package=stringr
Wickham, H. (2020). tidyr: Tidy Messy Data. R Package Version 1.1.2. Available online at: https://CRAN.R-project.org/package=tidyr
Wickham, H., and Bryan, J. (2017). readxl: Read excel files. R package version 1.3.1. Available online at: https://CRAN.R-project.org/package=readxl.
Wickham, H., François, R., Henry, L., and Müller, K. (2020). dplyr: A Grammar of Data Manipulation. R package version 1.0.2. Available online at: https://CRAN.R-project.org/package=dplyr.
Wilke, C. O. (2020). Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R package version 1.1.0. Available online at: https://CRAN.R-project.org/package=cowplot.
Willis, C. K. R., and Brigham, R. M. (2003). Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J. Comp. Physiol. B 173, 379–389. doi: 10.1007/s00360-003-0343-y
Withers, P. C., Cooper, C. E., Maloney, S. K., Bozinovic, F., and Neto, A. P. C. (2016). Ecological and Environmental Physiology of Mammals. Oxford, New York: Oxford University Press.
Wood, S. N. (2001). Minimizing model fitting objectives that contain spurious local minima by bootstrap restarting. Biometrics 57, 240–244. doi: 10.1111/j.0006-341X.2001.00240.x
Zeileis, A., and Grothendieck, G. (2005). zoo: S3 infrastructure for regular and irregular time series. J. Stat. Soft. 14, 1–27. doi: 10.18637/jss.v014.i06
Zinner, D., Wygoda, C., Razafimanantsoa, L., Rasoloarison, R. T., Andrianandrasana, H., Ganzhorn, J. U., et al. (2014). Analysis of deforestation patterns in the central Menabe, Madagascar, between 1973 and 2010. Reg. Environ. Change 14, 157–166. doi: 10.1007/s10113-013-0475-x
Zuur, A. F., and Ieno, E. N. (2016). A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 7, 636–645. doi: 10.1111/2041-210X.12577
Zuur, A. F., Ieno, E. N., and Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. doi: 10.1111/j.2041-210X.2009.00001.x
Keywords: bats, hyperthermia, hypothermia, intraspecific variation, Madagascar, physiological flexibility, torpor, tropics
Citation: Reher S, Rabarison H, Nowack J and Dausmann KH (2022) Limited Physiological Compensation in Response to an Acute Microclimate Change in a Malagasy Bat. Front. Ecol. Evol. 10:779381. doi: 10.3389/fevo.2022.779381
Received: 18 September 2021; Accepted: 31 January 2022;
Published: 07 March 2022.
Edited by:
Robyn Sheila Hetem, University of the Witwatersrand, South AfricaReviewed by:
Andrew McKechnie, University of Pretoria, South AfricaLin Zhang, Hubei University of Chinese Medicine, China
R. Mark Brigham, University of Regina, Canada
Copyright © 2022 Reher, Rabarison, Nowack and Dausmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie Reher, stephanie.reher@uni-hamburg.de
 Stephanie Reher
Stephanie Reher Hajatiana Rabarison
Hajatiana Rabarison Julia Nowack
Julia Nowack Kathrin H. Dausmann
Kathrin H. Dausmann