- 1Programa de Pós-graduação em Ecologia e Conservação, Universidade do Estado de Mato Grosso – UNEMAT, Nova Xavantina, Brazil
- 2Departamento de Zoologia, Universidade Federal do Paraná – UFPR, Curitiba, Brazil
- 3Independent Researcher, Toronto, ON, Canada
The Amazon comprises many of the largest rivers in the planet and also houses some of the richest bat communities in the world. Rivers are important geographic barriers for the dispersal and distribution of different taxa worldwide and, particularly in the Amazon region, they form the conceptual and empirical bases for the recognition of the so-called Areas of Endemism of terrestrial vertebrates. Despite the vast literature on the role of rivers on vertebrate community structure in the Amazon Forest, this process has never been investigated using a comprehensive dataset of Neotropical bat communities in the region. In this study, we aimed at: (1) evaluating the patterns of bat endemism across the Amazon Forest; (2) testing for the relationship between the distribution of bat species in the Amazon and the interfluve Areas of Endemism as currently recognized, and; (3) analyzing the importance of major Amazonian rivers in bat beta-diversity (turnover and nestedness) in the Amazon. Our results indicate that rivers are not major barriers for the current distribution of most bat species, and bat community composition breaks were divided into two clusters separating the east and west regions, and a third cluster in northern Amazon. In addition, there was no significant overlap among species distribution limits and the interfluve Areas of Endemism. Interestingly, the geographic patterns that we found for bat communities composition breaks highly resembles the one recovered using bird communities, suggesting that similar ecological and historical drivers might be acting to determine the distribution of flying vertebrates in the Amazon. Moreover, Amazonian bat distribution and endemism patterns were likely shaped by factors other than rivers, such as species interactions and the current environmental conditions. In conclusion, our results highlight the importance of modern analytical approaches to investigate large scale ecological patterns in the Neotropical region, and also challenge the widely recognized role of rivers on the determination of community structure and endemism patterns in the Amazon Forest, at least for bats.
Introduction
Rivers have long been hypothesized to be ecological and geographical barriers for the dispersal and colonization of new habitats for different taxa (Wallace, 1854; Napier, 1976; Hershkovitz, 1977). Not surprisingly, rivers represent one of the most important drivers of species distribution and endemism worldwide (Harcourt and Wood, 2011; Ramachandran et al., 2017; Mahulu et al., 2021). The Amazon region is not only one of the most biologically diverse regions on the planet, but also comprises the largest network of rivers (Junk, 1997). Four of the 10 largest rivers and 20 of the 34 largest tropical rivers are located in the Amazon region (Latrubesse et al., 2005). The role of the vast Amazonian hydric system on the diversification patterns and community assembly processes of Neotropical organisms has been investigated for many animal groups, such as primates (Ayres and Clutton-Brock, 1992; da Silva and Oren, 1996), amphibians and lizards (Moraes et al., 2016), butterflies (Hall and Harvey, 2002), comparatively among different taxonomic groups (Santorelli et al., 2018) and, in particular, using birds as model organisms (Ribas et al., 2005, 2012; Ferreira et al., 2017; Oliveira et al., 2017; Naka and Brumfield, 2018).
The Areas of Endemism (AoE) hypothesis, initially proposed by Cracraft in 1985 using birds as model organisms (Cracraft, 1985), gained extensive support in the scientific literature in the following decades, with the evidence mostly arising from historical biogeography studies (Fernandes et al., 2012; Ribas et al., 2012; d’Horta et al., 2013; Lutz et al., 2013). In this scenario, distinct AoEs would be located at the interfluves of major Amazonian rivers (Figure 1), which are believed to harbor unique species communities (Ayres and Clutton-Brock, 1992; Gascon et al., 2000; Silva et al., 2005). However, the role of Amazonian rivers on actual endemism patterns of birds was only quantitatively tested using a large distribution dataset and community ecology regionalization methods by Oliveira et al. (2017). These authors showed that the AoEs had no support from an actual test of biotic regionalization using a dataset that covered a broad geographic area in lowland Amazon. Moreover, many studies have failed to provide evidence that supports the role of major Amazonian rivers as geographic limits for terrestrial species (see Aleixo, 2004; Fernandes et al., 2013; Fecchio et al., 2018; Santorelli et al., 2018; Dambros et al., 2020), questioning the current knowledge and definitions of AoE in the Amazon. Nonetheless, the role of large Amazonian rivers acting as dispersal barriers is usually undisputed, and this has been demonstrated using both distribution and divergence patterns as inferred by genetic data (Maldonado-Coelho et al., 2013; Weir et al., 2015; Pirani et al., 2019).
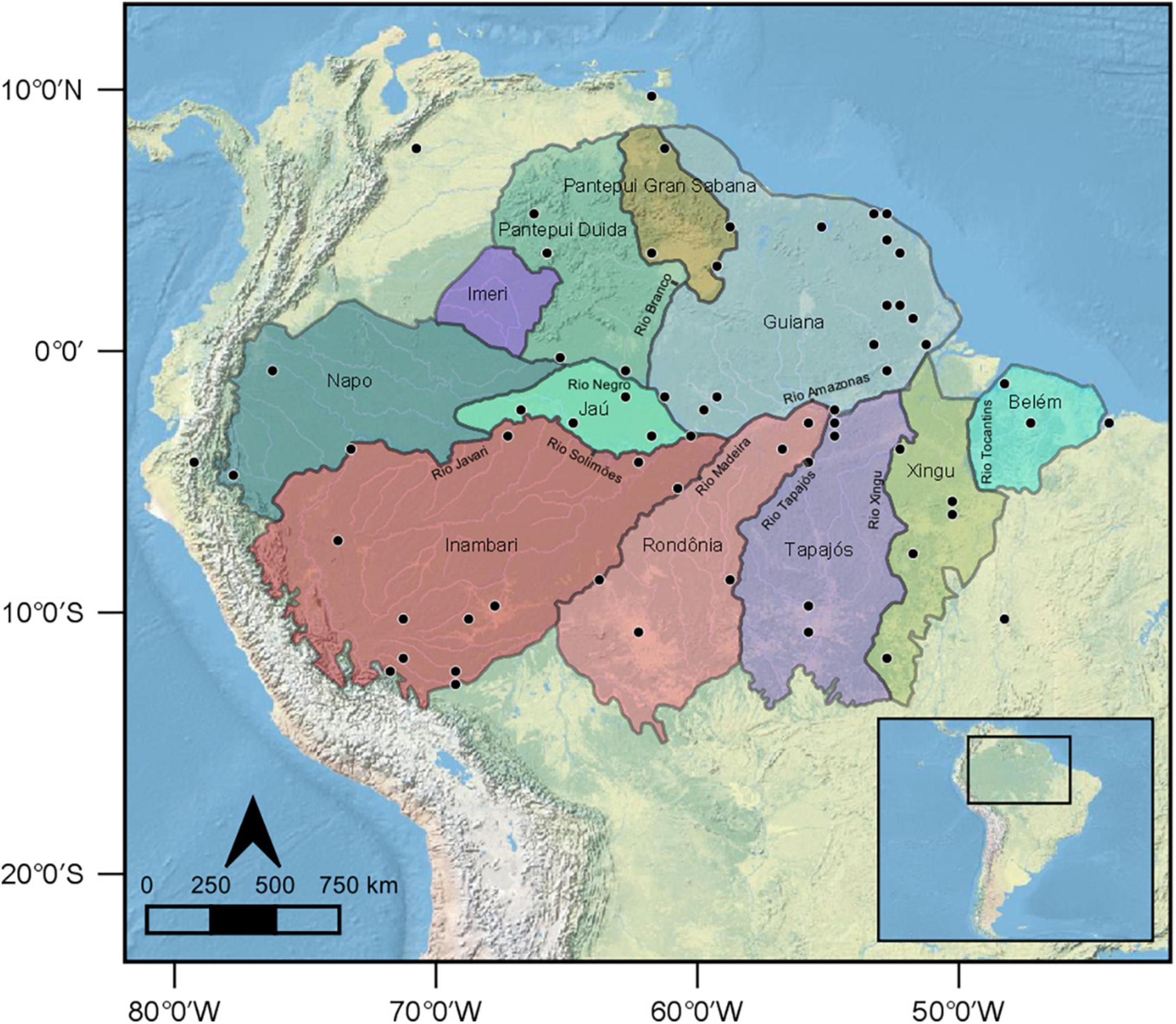
Figure 1. Geographic location of the 64 bat communities used in this study (black circles). For reference, the figure depicts the interfluve hypothesis of lowland Amazonian vertebrates Areas of Endemism. This currently accepted Areas of Endemism classification was proposed by Cracraft (1985) and subsequently modified by da Silva and Bates (2002), Naka (2011), and Borges and da Silva (2012).
The Amazon region (6.9 million km2) covers more than a third of the total Neotropical region area, harboring more than 170 bat species, which represents over 10% of the world’s bat diversity, and more than 100 species might be recorded in a single locality (Medlin et al., 2010; Burgin et al., 2018; Santos et al., 2019). Even though some bat species are able to make long-distance dispersals (Arnone et al., 2016; Esbérard et al., 2017), many Neotropical species have small home ranges and specific ecological requirements, which can particularly limit their distribution ranges and contribute to the origin and maintenance of local endemism patterns (Meyer et al., 2005; Henry and Kalko, 2007; Voss et al., 2016). Surprisingly, and considering that the AoE hypothesis was proposed using flying vertebrates as models, no large-scale study has ever tested if the Amazon rivers might be defining species distribution limits and, hence, influencing the maintenance of AoEs for bats in the Amazon Forest.
The few studies that analyzed the effect of Amazonian rivers on bat distribution showed that rivers were not an important barrier for the dispersal of bat species (Santorelli et al., 2018), with climate showing to be a more important variable to predict similarity in species composition than geographic distance (Dambros et al., 2020). In addition, endemism areas were responsible for explaining less than 10% of the observed difference in species composition among sites (Dambros et al., 2020). Nonetheless, three AoEs in the Amazon showed significantly different species compositions among them (Dambros et al., 2020). These are local-scale studies that sampled a relatively small geographic area within the Amazon, and only included a small number of bat species, i.e., ∼60 bat species (Dambros et al., 2020) and 76 species (Santorelli et al., 2018) from a total of more than 170 bat species known to occur in the biome (Santos et al., 2019). Noteworthy, the study of Tavares et al. (2017) analyzed 26 Amazon localities, with 161 sampled bat species, and proposed that the composition of Amazon bat species can be divided into three zones: eastern Brazilian Amazon, Guiana Shield, and western Amazon. However, this study did not use spatially explicit analyses, rather basing its geographic interpretations on the composition distance among bat assemblages. Thus, a more comprehensive evaluation on the impact of rivers as dispersal barriers for bats and the importance of AoEs to explain differences in species composition within the Amazon rainforest is still warranted.
Recently proposed endemism metrics that integrate information on geographic rarity and phylogenetic divergence among the biota of a particular region have a stronger explanatory power in the detection of endemism patterns, compared to traditional methods (Crisp et al., 2001; Rosauer et al., 2009). Moreover, advances in the identification and differences in the phylogenetic composition of AoEs can also help in exploring the evolutionary significance of such areas, as made possible by the Categorical Analysis of Neo and Paleo-endemism (CANAPE, Mishler et al., 2014). The CANAPE is capable of distinguishing areas containing significant concentrations of rare long branches (paleo-endemism), rare short branches (neo-endemism), or mixed endemism. Taken together, these metrics allow for the identification of complementary areas of biodiversity that have unique evolutionary histories and characteristics and, therefore, should be taken into account in studies investigating geographic regionalization patterns (Mishler et al., 2014; González-Orozco et al., 2015; Scherson et al., 2017; López-Aguirre et al., 2018; Veron et al., 2019; Azevedo et al., 2020).
Considering the above-mentioned methodological advances, our main goal was to understand patterns of beta diversity and bat endemism in cis-Andean Amazonia and their relationships with the large river system of the Amazon. Specifically, we aimed at:
(1) Inferring patterns of bat endemism in cis-Andean Amazonia using species richness corrected endemism metrics and inferring the location of areas with evidence of neo-, paleo-, and mixed endemism; If the interfluve AoE hypothesis is indeed a strong predictor of terrestrial vertebrate endemism patterns in the Amazon, it is expected that rivers would play an important role on bat endemism patterns. However, as previously shown for birds (Oliveira et al., 2017), this hypothesis might not hold true for flying vertebrates. Hence, we predict that (i) endemism patterns will not be correlated with the geography of interfluve AoEs as currently proposed (Scherson et al., 2017).
(2) Investigating if there is a relationship between the distribution of bat species in the Amazon and areas of endemism as traditionally proposed (interfluves AoE). If the interfluves AoE hypothesis is true, each bat species should be mainly distributed within one or, at least, a few AoEs. Nevertheless, many bat species are able to fly long distances and over large rivers, meaning that rivers should not be strong geographic barriers and would not be a process influencing endemism patterns for these organisms. Thus, we predict that (ii) the limits of the distribution ranges of each bat species will not depict a high fit to the interfluve AoEs limits, as also shown for birds (Oliveira et al., 2017).
(3) Estimating patterns of bat beta diversity and its decomposed components (turnover and nestedness) in order to understand the relationship between species assemblage breaks and the geography of major Amazon rivers, and verifying the biotic similarity among communities using phylogenetic measures of beta-diversity. Again, if the interfluve AoE hypothesis holds true, rivers should play a clear role in the variation of bat species composition across the Amazonian landscape. However, evidence suggests that processes other than the effects of rivers in the Amazonian geography should be responsible for the variation in species composition (Silva et al., 2019; Dambros et al., 2020). Hence, we predict that, (iii) there will be no direct correlation among geographic breaks on species composition and the main Amazonian riverine system. Finally, if the interfluve AoEs define a general community structure pattern for bats, we would expect that communities within each AoE would be more similar to each other compared to communities in other AoEs. Hence, and following the same reasoning of our previous expectations in relation to the lack of influence of the interfluve AoEs on bat biogeograhic patterns in the Amazon, we predict that, (iv) beta-diversity among communities within each AoE will not be smaller than beta-diversity among communities in different AoEs, and that (v) communities within each AoE will not be more similar to each other in terms of phylogenetic community structure (Scherson et al., 2017).
Materials and Methods
Communities and Phylogenetic Dataset
We used a previous compilation by Santos et al. (2019) that assembled data for 44 bat communities in the Amazon as a starting point for our dataset. In addition, we included another eight communities available in the literature (Peracchi et al., 1984; Martins et al., 2006, 2011; Patterson et al., 2006; Peters et al., 2006; Rex et al., 2008; Carvalho et al., 2018; Carrasco-Rueda et al., 2021). Finally, we supplemented our database by extracting information on the distribution of individual bat species in the Amazon from Aguiar et al. (2020). Because of recent taxonomic changes, we removed all records of the species Pteronotus parnellii from the dataset and only included data for undisputed P. rubiginosus and P. alitonus records (Pavan et al., 2018). Because we are using community and individual species records, we defined bat communities and eliminated duplicate data by using a grid with 0.50° × 0.50° (∼50 km) cells, and created a binary matrix with species occurrence in each community (i.e., presence and absence data for each grid cell). To reduce biases related to differences in sampling effort and methodology from different sources, we applied a threshold of a minimum of 20 species for each cell to be included in the final communities dataset, which should represent a well-sampled community in the Amazon region (e.g., Tavares et al., 2017). All species identified only at the genus level were excluded from the dataset. We checked all records to verify the presence of misidentified species based on species ranges as provided by Gardner (2008) and Rojas et al. (2018), and used current species names as available in the Mammal Diversity database.1
As most of the data described above derives from studies that used mist nets, and this sampling method is not particularly efficient for non-Phyllostomidae bat species in the Neotropical region (Marques et al., 2016), the presence of some rarely sampled species might bias our general inferences. In order to evaluate the extent of such possible issue, we used two different datasets to run the analyses. First, we used all species that comprised the final assembled dataset (as explained above), considering all bat families present in this study. Second, we only used species from the Phyllostomidae family, as they are better represented in mist net surveys. Since our main findings did not change by using the two different datasets, we graphically report results of analyses using the first complete dataset with all species, while results for Phyllostomidade can be found in Supplementary Material.
We used a consensus phylogenetic tree to represent evolutionary relationships among all species in our dataset from the most recent species-level mammal phylogeny (Upham et al., 2019). If a particular species was not present in the phylogeny, we used its sister-species or, when this was not possible, its closest known relative as a substitute in the phylogeny (Cisneros et al., 2015; de Carvalho et al., 2019). We only had to replace seven species (3.9% of all species in the dataset, Supplementary Figure 1), and since phylogenetic placement itself is not used in our analyses, but phylogenetic distance only, this approach should not influence our results. The species names on the phylogeny were updated using their most recent valid synonyms, according to recent taxonomic arrangements for Neotropical bats (Garbino et al., 2020). The tree was pruned to only include the species in our dataset using the package ape (Paradis and Schliep, 2019) in R version 3.6.2 (R Core Team, 2019; Supplementary Figure 1).
Statistical Analyses
To identify areas of endemism of bat communities in the Amazon and test our first prediction, we used two metrics available in the Biodiverse software version 3.1 (Laffan et al., 2010). First, we calculated the corrected weighted endemism (CWE), which is less biased by species richness and measures the proportion of endemism in each community (Crisp et al., 2001). Second, we used the CANAPE protocol to measure phylogenetic endemism (PE; Rosauer et al., 2009) and relative phylogenetic endemism (RPE; Mishler et al., 2014). The values of RPE are dependent on species richness because the PE of a set of species naturally increases when new species are added to the phylogeny. To circumvent this problem, we compared the actual PE and RPE values of each grid cell to 999 values of a null distribution (Mishler et al., 2014; Laffan et al., 2016). The p-values were estimated from a bi-tailed distribution of values to identify areas with higher (>0.975) or smaller (< 0.025) PE and RPE compared to the null distribution. A PE/RPE ratio higher or smaller than the null distribution indicates, respectively, paleo or neo endemism in a given community. Mixed endemism occurs when PE is significantly higher than the null distribution, but presents intermediate RPE values (i.e., indicating communities with high levels of paleo and neo endemism). We followed Azevedo et al. (2020) to include communities (grid cells) below the 0.01 significance level as mixed endemism, instead of using the term “super endemism” as suggested by Mishler et al. (2014). The final classification of metrics was done in R using a custom script modified from https://github.com/NunzioKnerr/biodiverse_pipeline.
To test our second prediction and infer whether the distribution of each bat species overlaps with interfluve AoEs, we quantified the percentage of area overlap between each species distribution and interfluve AoEs by considering a grid with cells of 0.50° × 0.50° (∼50 km) covering the whole Amazon region. We measured the species distribution fit to interfluve AoE distribution using an index proposed by Oliveira et al. (2017). The index varies from 0 to 1 (1 = total fit) where higher values indicate that the species is very restricted to the AoE and occupies a large part of its area. For this analysis, we did not apply the threshold used to define communities (as detailed above). In turn, we used all available geographic coordinates for each species, in order to have the best representation of each species distribution. We only considered species with 90% of their distribution within each AoE as a way to exclude species that are not restricted to each particular AoE (Oliveira et al., 2017). Since this analysis demanded the most comprehensive information on species distributions, and not communities (grid cells) as previously defined, we used our whole distribution dataset (i.e., all available individual species records from the above-mentioned data sources, except those outside the AoEs).
To investigate species composition breaks and test our third prediction, we described spatial variation in species composition using a Bray-Curtis dissimilarity matrix transformed into linear values using Non-Metric Multidimensional Scaling (NMDS). These values are, then, geographically interpolated on a map using a Bayesian technique, assuming spatial autocorrelation among the values (Oliveira et al., 2017), which provides a surface map indicating species composition variation. To identify the components that influence bat beta-diversity variation throughout the Amazon, we partitioned beta diversity into turnover and nestedness components (Baselga, 2010). The similarity between the results of each component was measured through a Pearson correlation analysis for each NMDS axis. The values of beta diversity were geographically interpolated with three axes using the Nearest Neighbor technique. This interpolator uses Voronoi polygons to calculate the area of influence around the samples, and all intermediate points are calculated by averaging the neighboring polygons. We chose this interpolation because it does not depend on spatial autocorrelation, like most interpolators. All beta-diversity analyses were performed using the BioDinamica toolkit (Oliveira et al., 2019).
We compared beta diversity between and within each interfluve AoE (fourth prediction) using an univariate ANOVA (Quinn and Keough, 2002). We tested the normality of the mean of the NMDS axes (Shapiro-Wilk test) and homogeneity of variance (Levene test) to satisfy test assumptions. Differences were considered significant when the p-value was < 0.05 after Tukey’s post-hoc test for unequal sample sizes (Zar, 2010). To test our fifth prediction and investigate phylogenetic beta-diversity among all communities in our dataset, while also inferring general similarity patterns among them, we used a clustering analysis implemented in Biodiverse, using the Unweighted Pair Group Method with Arithmetic Mean Averaging (UPGMA). This inference is based on the phylogenetic turnover among communities, which we calculated using the phylogenetic range-weighted turnover index (PhyloRWTurnover). This index measures phylogenetic turnover taking the branch lengths from the phylogenetic tree in consideration while weighting for the shared taxa among the communities, and ultimately down-weights the influence of widespread species with large range sizes (Laffan et al., 2016).
Results
Geographic Patterns of Bat Endemism in the Amazon (CWE, PE, RPE, and CANAPE)
In total, we obtained 5,100 locality records for 182 species belonging to nine families of the order Chiroptera (Supplementary Figure 2). After cleaning and excluding duplicate species within each grid cell, our final dataset comprised 3,236 unique species records, totaling 64 communities with 177 bat species. For the Phyllostomidae family, we had a total of 2,317 unique species records, totaling 61 communities with 101 species (Figure 1 and Supplementary Table 1). We report the following results using the geographic locations of interfluve AoEs to facilitate comparisons and interpretation, but we urge readers not to directly interpret our mentions to interfluve AoEs as evidence of support to this hypothesis. In relation to the endemism patterns (CWE, PE and CANAPE) used to test our first prediction, the corrected weighted endemism indicated 13 communities with significant endemism values (Figure 2A, in red and blue). We found significant values in the Solimões/Negro/Javari and Tapajós/Amazonas interfluvial zones (Figure 2A). In addition to these, in the southern region of Inambari (in blue) and north of Guyana (in red) there are communities with high significant values of endemism. Finally, high values of endemism were also found in communities at the Andean region and north of the Amazon, which lie outside the interfluve AoEs boundaries. The CWE using the Phyllostomidae dataset identified eight communities with significant values in the regions of Inambari, Pantepui Duida, Rondônia (in blue), Xingu, Tapajós and Guyana (in red) (Supplementary Figure 3A).
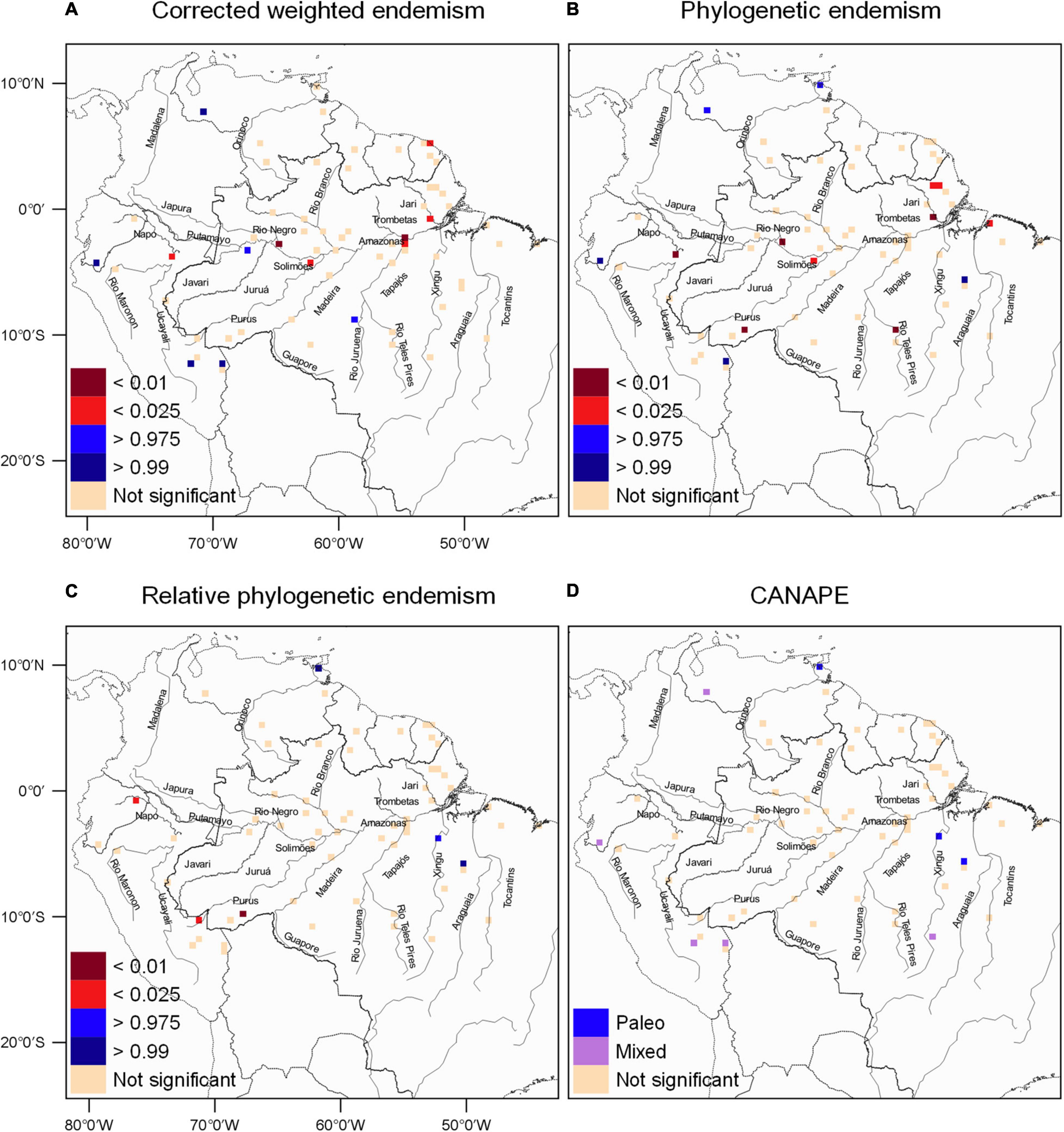
Figure 2. Bat endemism patterns in the Amazon region. (A) Results for corrected weighted endemism (CWE). Significance was estimated using two-tailed randomization tests (see section “Materials and Methods”). Beige cells are not significant, red shaded cells depict communities with significantly less CWE than expected, and blue cells depict communities with significantly more CWE than expected. (B) Results for phylogenetic endemism (PE). Significance was estimated using two-tailed randomization tests. Beige cells are not significant, red cells contain significantly lower PE than expected, and blue cells contain significantly higher PE than expected. (C) Results for relative phylogenetic endemism (RPE). Significance was estimated using two-tailed randomization tests. Beige cells are not significant, red cells contain significantly lower RPE than expected (phylogenetic branch lengths in the grid cells are shorter than expected), and blue cells contain significantly higher RPE (branch lengths in the grid cells are longer than expected). (D) Phylogenetic endemism centers derived from the CANAPE analysis. Beige cells are not significant, blue cells indicate centers of paleo-endemism and purple cells are a mix of neo- and paleo-endemism.
We found Amazonian bat communities where phylogenetic endemism was significantly high (>0.99 and >0.975) in western Amazonia in the Inambari region, in the Xingu region and communities at the Andean region and north of the Amazon, whereas significantly low (<0.01 and < 0.025) where present in the regions of Guiana (Amazon interfluvial zone), Belém, Tapajós, Inambari (Solimões interfluvial zone), Jaú (Negro interfluvial zone), and Napo (Javari interfluvial zone) (Figure 2B). The phylogenetic endemism of Phyllostomidae species was significantly high (>0.99 and >0.975) in the south in the Inambari region, Xingu region (Xingu interfluvial zone), communities at the Andean region and north of the Amazon. Significant low PE (<0.01 and <0.025) in the regions of Guyana (Amazonas interfluvial zone) and Napo (Javari interfluvial zone) (Supplementary Figure 3B). Significantly high RPE communities included one case in northern Amazonia and in Xingu, while significantly low RPE communities were found in the region of Inambari and Napo (Figure 2C). For the Phyllostomidae family, the significantly high RPE is found in the Xingu region and in the Amazonas/Tapajós/Solimões interfluvial zone (in blue), whereas the significantly low RPE was found in the Rondônia and Inambari regions (Supplementary Figure 3C). The CANAPE analysis indicated that most communities are composed by mixed endemism (significantly high PE; Figure 2B and non-significant RPE; Figure 2C), that is, high in paleo and neo endemism, concentrated in the western, east, and northern regions of the Amazon and in Inambari and Xingu (Figure 2D). In addition, two communities in the Xingu and another in the northern Amazon region were dominated by paleo-endemisms (Figure 2D). The CANAPE results for Phyllostomidae indicated two additional endemism areas, a community dominated by neoendemism in Rondônia and a mixed community in Pantepui Duida (Supplementary Figure 3D). Overall, our endemism results seem to be robust considering the inclusion of all species, while also capturing the information from more ancient splits in the bat tree of life.
Bat Species Fit to Interfluve Areas of Endemisms
The fit between the distribution ranges of the species and the interfluve AoEs (Prediction 2) was generally low (mean 0.50) (Supplementary Table 2). Of the total 177 species, only 20 were fully inserted within only one interfluve AoE range; ten of these were exclusive to Inambari AoE, six to Guiana, two to Napo, and one species each to Belém and Rondônia. All species with 100% of the occurrence within one AoE occupied at most 4% of each area. Furthermore, Jaú, Xingu, Tapajós, Pantepui Gran Sabana, and Pantepui Duida did not have exclusive species. In the analysis with the species of the Phyllostomidae family, 12 species were fully inserted within the interfluvial AoEs. This result shows that the Phyllostomidae are responsible for 60% of the species completely inserted within some of the interfluvial AoEs (Supplementary Table 2).
Beta-Diversity of Bats in the Amazon: Geographic Patterns of Turnover and Nestedness
The spatial variation in composition breaks of bat species in the Amazon was divided into three clusters (Prediction 3): two clusters separating the east and west regions and a third in northern Amazonia (Figures 3A–D). The Phyllostomidae dataset also presented three clusters and, while the overall pattern is similar to the complete dataset, the clusters are predominantly divided in a west to east fashion (Supplementary Figures 4A,B). The NMDS analysis showed a high correlation between the observed distance and the ordering distance (non-metric adjustment R2 = 0.97, linear adjustment R2 = 0.91), and even more prominent in the Phyllostomidae dataset (0.99, 0.98). The same was observed for the beta-diversity partitioning in the complete dataset turnover (0.93, 0.54) and nestedness (0.99, 0.98), and Phyllostomidae turnover (0.94, 0.61) and nestedness (0.99, 0.99), indicating that the analyses satisfactorily represented the Bray-Curtis distance matrices. The first NMDS axis indicated a division between the western end of the Amazon, along the Maranon and Madalena rivers, and the eastern region contemplating the extension of the Amazon River in the results of both datasets (Supplementary Figures 5A, 6A). The second NMDS axis showed a composition break between the northern region of the Orinoco River and the southern region around the Madeira River (Supplementary Figures 5B, 6B). The third NMDS axis indicated greater dissimilarity in the composition of species from the central Amazonian region around the Madeira and Negro rivers, outwardly to the other regions (Supplementary Figures 5C, 6C). The variation in the composition of bat species in the Amazon was related to both turnover and nestedness but with a higher contribution of the turnover component for both datasets (Supplementary Figures 5D–F, 6D–F). The correlation between the axes of the complete dataset (1, 2, and 3) of the total beta diversity and turnover were 0.80, 0.82, and −0.04, respectively (Supplementary Figures 6D–F), whereas the correlation between the axes of total beta diversity and nestedness were 0.62, −0.64, and −0.37 (Supplementary Figures 5G–I). The correlation between the axes for Phyllostomidae (1, 2, and 3) of the total beta diversity and turnover were −0.76, −0.30, and 0.69, respectively (Supplementary Figures 6D–F), whereas the correlation between the axes of total beta diversity and nestedness were −0.13, −0.28, and 0.42 (Supplementary Figures 6G–I).
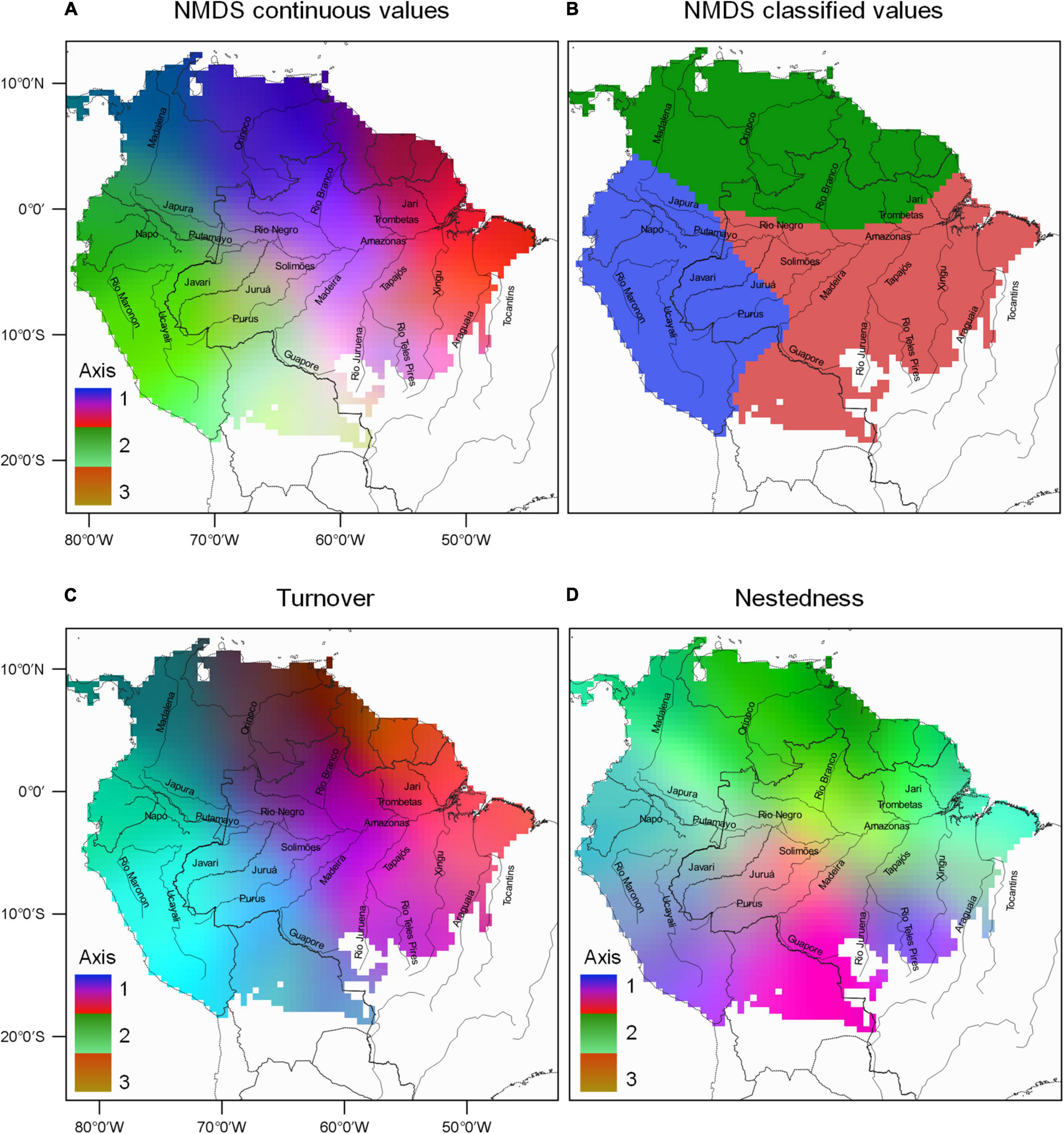
Figure 3. Spatial variation in bats communities composition throughout the Amazon. (A) Species composition were obtained by the interpolation of NMDS scores (three axis represented by a RGB scale). (B) Classification of the first three breaks (most significant in search order) in species composition. (C) Spatial turnover of bat species composition throughout the Amazon. (D) Spatial nestedness of bat species composition throughout the Amazon.
There was no difference in the NMDS scores of the total beta diversity among the interfluvial AoEs (Prediction 4) (F = 1.593, df = 9, p > 0.05, ANOVA) (Figure 4). The clustering analysis using the PhyloRWTurnover index (Prediction 5) indicated that most lowland Amazonian communities share similar branches of the phylogenetic tree (Supplementary Figure 7A). This phylogenetic turnover analysis did not depict a community geographic structure that could be correlated to the interfluvial AoEs (Supplementary Figure 7A). The largest cluster is distributed over all sampled interfluvial AoEs, and the few smaller clusters are found in Inambari, two communities in northern Amazonia (outside the interfluvial AoEs limits) and one Andean community. A similar result was obtained with the Phyllostomidae dataset, the main difference being a cluster in the Pantepui Duida and Inambari regions (Supplementary Figure 7B).
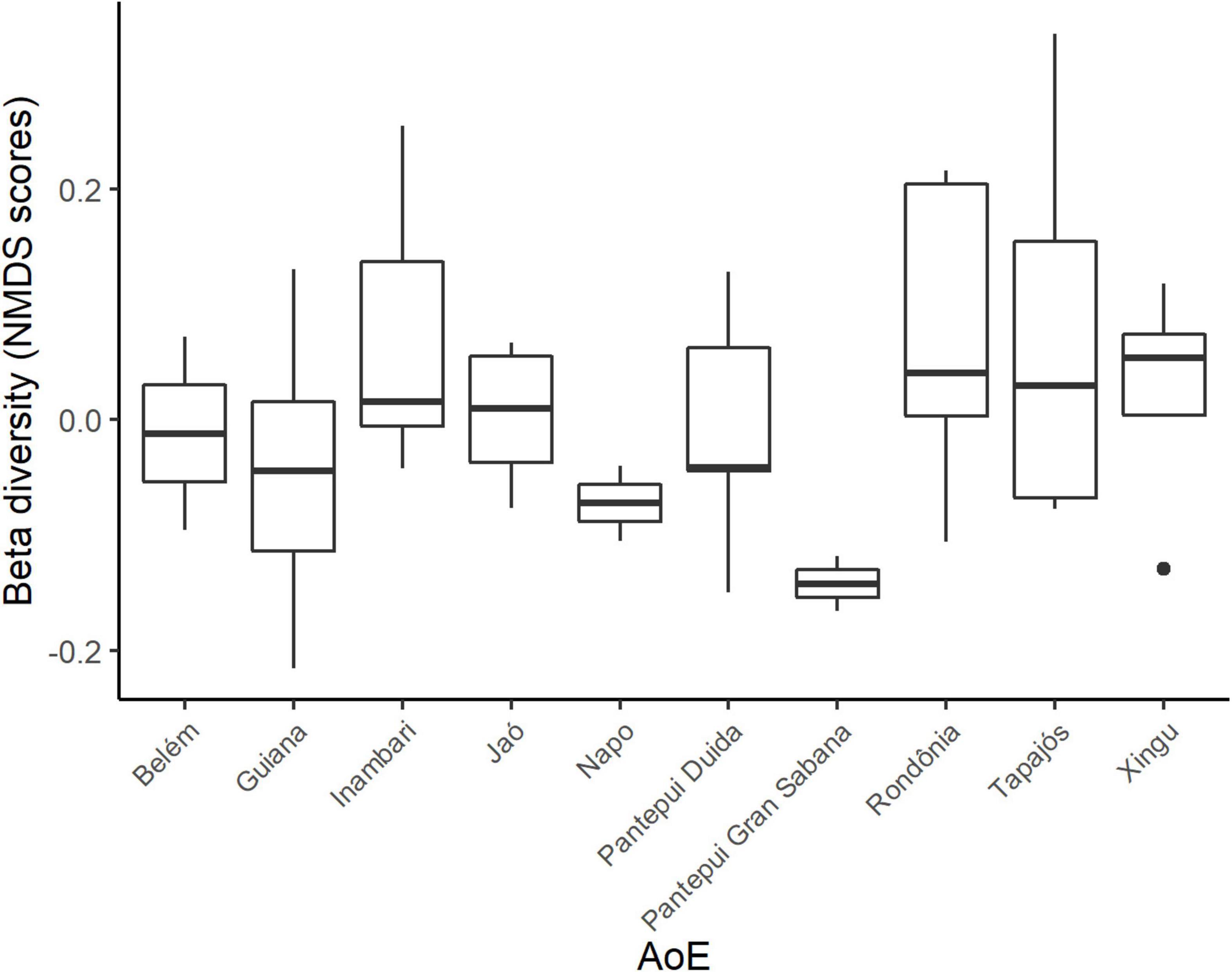
Figure 4. Comparison of total beta diversity (NMDS scores) among interfluvial AoEs. No significant difference was found (ANOVA, p > 0.05), meaning that beta-diversity among communities within each interfuvial AoE is not smaller than beta-diversity among communities in different AoEs.
Discussion
In this study, we simultaneously assessed the importance of the currently recognized interfluve areas of endemism (AoE) and the patterns of beta-diversity and endemism (taxonomic and phylogenetic) for the assembly of bat communities in cis-Andean Amazon. We applied modern statistical approaches that improved our capacity to identify unique areas of evolutionary history (i.e., neo and paleo endemism) in order to better understand the importance of the Amazonian riverine system in structuring bat communities. Our results lend little to no support to the interfluve AoE hypothesis considering the complete dataset with 177 species and also the dataset with 101 phyllostomid species (which represents a very high species richness for one study, including close to 45% of all existing Phyllostomidae species). Furthermore, we did not find a clear geographic pattern pertaining to the influence of rivers in the distribution of phylogenetic and taxonomic endemism of bat communities. Our results also depicted a lack of fit of bat species to each interfluvial AoE, and also no community structure that could be associated to the interfluvial AoEs.
Because the traditional interfluve AoE hypothesis was mainly supported by historical biogeography evidence, no causality is necessarily expected among this body of evidence and processes determining community assembly in Amazonian bats, as recently shown for birds (Oliveira et al., 2017). In other words, there is no strong evidence that rivers are functioning as important ecological or evolutionary processes behind bat endemism patterns in the Amazon forest, which is supported by the main patterns we found following our hypothesis-testing framework. Furthermore, the evidence presented by Ruokolainen et al. (2019) indicate that the courses of many Amazonian rivers have gone through important changes in recent times (Pleistocene and Holocene), calling into question the use of current riverine arrangements to infer biogeographic patterns based on data on the distribution of organisms. Our results reinforce this interpretation, and suggest a geographic regionalization similar to that proposed by Tavares et al. (2017), separating the Amazonian bat biota into three groups that are not consistent with the current distribution of Amazonian rivers: Eastern Brazilian Amazon, Guiana Shield, and Western Amazon. Additionally, a recent comparative phylogeography study (Silva et al., 2019) tested alternative diversification models, including the interfluve AoE hypothesis, and concluded that a geomorphological model consisting of roughly these same three geographic groups, as well as climatic history, better describes diversification patterns among upland “terra firme” Amazonian birds. The evidence, thus, indicates that other additional abiotic factors, such as temperature and climate history, and biotic interactions such as competition, might also play important roles in explaining bat diversity patterns in cis-Andean Amazon (Santorelli et al., 2018; Dambros et al., 2020).
Our main hypothesis, that Amazonian rivers are not an important ecological or evolutionary process behind bat endemism patterns, was corroborated. Although some rivers seem to be important in determining geographic patterns of species composition, our first prediction was corroborated and the results did not depict a clear influence of interfluvial AoEs in the geographic distribution of unbiased taxonomic endemism (CWE), phylogenetic endemism (PE-RPE), and paleo and neo endemism (Figure 2). Nonetheless, we found two occasions where CWE regions geographically close to each other were separated by rivers, the Amazon and Tapajós rivers (Figure 2A), and one occasion where PE regions were separated by the Amazon river (Figure 2B). Thus, our results on bat endemism and distribution do not support the interfluvial AoE hypothesis as currently recognized (Borges and da Silva, 2012).
Our endemism analysis that took into consideration the branch lengths of the species in the phylogeny (CANAPE) showed that communities are mostly composed of mixed endemism, which is defined by the co-occurrence of short and long rare branches narrowly distributed in the landscape (Mishler et al., 2014; López-Aguirre et al., 2018; Azevedo et al., 2020). These communities are mainly distributed south of the Amazon River (Figure 2D), and this region is also home to a high proportion of endemic species found on very long branches (paleo-endemism), indicating that they are the current local survivors of clades supposedly older than the current landscape configuration. The ecological processes behind this pattern deserve further investigation, but the historical maintenance of suitable habitats and the species coexistence abilities might play important roles in species persistence in the landscape (Grandcolas et al., 2014; Veron et al., 2019). Sole neo-endemic communities were also less frequent for other taxonomic groups (Mishler et al., 2014; Scherson et al., 2017; Veron et al., 2019), and for New World bat superfamilies (López-Aguirre et al., 2018). Hence, the lack of neo-endemism in our data highlights the Amazon rainforest contribution to long-term species persistence in the landscape in the Neotropical region (Smith et al., 2014; Antonelli et al., 2018). Interestingly, the overall low amount of endemism in our bat communities (paleo, neo or mixed) supports the idea that even though species richness is quite high in the Amazon (Delgado-Jaramillo et al., 2020), most species inhabiting this area are geographically widespread and come from a relatively even sampling of the bat tree of life, with phylogenetic branches that are neither particularly long nor short (Mishler et al., 2014; Scherson et al., 2017).
Another important result corroborating our hypothesis, and in accordance with our second prediction, was the lack of congruence between the distribution limits of species within interfluvial AoEs. Few species occupied only a very small portion (∼4%) of the interfluvial AoEs, and most species (∼70%) occurred in five or more AoEs, a very similar pattern to what was found for the Amazonian avifauna (Oliveira et al., 2017). Moreover for the 22 species that did have 100% of their distribution in only one interfluvial AoEs, 30% are classified as data deficient (DD-six species) by the IUCN (Supplementary Table 2), which suggests that their distribution are still largely unknown and can extend far beyond one interfluvial AoE. Thus, their distribution might not necessarily be limited by rivers, considering that our results might be relatively biased by the lack of sampling efforts. Bat species have a high dispersal capacity, and foraging is influenced by the vertical availability of resources in the forest, which makes it difficult to collect species that forage above the canopy (Kalko and Handley, 2001; Carvalho et al., 2013; Farneda et al., 2019). We expected that individual bat species would not be restricted to interfluvial AoEs, due to their mobility and high dispersion capacity, but our results still suggest that larger rivers might act as dispersal barriers for a few species, which was also true for a few Amazonian birds (Oliveira et al., 2017).
Corroborating our third prediction, bat community composition breaks were divided into three clusters separating the east and west regions, with the third cluster in northern Amazonia (Figure 3B). Interestingly, the three clusters resemble those estimated for birds by Oliveira et al. (2017), indicating that major biotic regionalization processes might be at play for flying vertebrates in the Amazon (Santorelli et al., 2018; Dambros et al., 2020, but also see Ritter et al., 2019). Although no clear evidence for the role of rivers in the variation of bat communities composition was found, the general compositional changes over the landscape might fit a pattern of rivers acting as dispersal barriers for at least a few bat species. This finding may be related to the persistence of the species and its ability to disperse in the landscape matrix (Smith et al., 2014). In general, flying vertebrates have a large home range, and it would be expected that these species would only be affected by isolation at larger geographic scales (e.g., Trevelin et al., 2013; Aguiar et al., 2014; Arnone et al., 2016). Our results showed that compositional variation is more related to species turnover, which may indicate that some species ranges are indeed influenced by a high dispersion capacity, ultimately leading to lower regional endemism signals (e.g., Peixoto et al., 2014; Varzinczak et al., 2018). In addition, bat nestedness was mostly restricted to northern Amazon (Figure 3D), and species composition breaks in this region might be related to the distinct biogeographic history of the Guiana Shield (Fouquet et al., 2012), as well as to historical reasons related to the contraction of the Southern portion of the forest during the last glacial cycles (Rull, 2004; Rull and Nogué, 2007). Moreover, and corroborating our fifth prediction, the phylogenetic turnover analysis indicated that most communities share close related branches in the phylogenetic tree. Hence, this and our previous results indicate that bat communities in the Amazon are not geographically structured when phylogenetic history is taken into account, reinforcing the lack of evidence supporting the interfluvial AoEs hypothesis (Supplementary Figure 7).
Finally, we conclude that bat distribution and endemism in the Amazon are likely limited by factors other than rivers. Intraspecific and interspecific interactions, differences in environmental conditions among the regions, and other historical reasons might be more important drivers of bat distribution and endemism in this vast part of the Neotropical region. Here, we show the importance of analytical strategies that take into account the evolutionary history of the species (CANAPE) for inferring community-wide biogeographic patterns. In the future, the data assembled in this paper can also be used to investigate specific community assembly processes of each community based on the putative interaction among species (Ruffley et al., 2019), and the role of the dynamics of the Amazon biogeographic region and its intricate contribution to the long-term persistence of species based on ecological preferences (Crouch et al., 2019). This type of inference might be done in association with information on the biogeographic history of the Amazon, for example considering the Plio-Pleistocene climate change patterns (Hoorn et al., 2010, 2017; Nogueira et al., 2013; Rossetti et al., 2014), which might have promoted limits to dispersion for some species (Naka and Brumfield, 2018), particularly considering that phylogenetic endemism for mammals is mainly associated with energy availability and post-Last Glacial Maximum climate variability (Rosauer and Jetz, 2015). The bioregionalization pattern we found closely resembles that found for birds (Oliveira et al., 2017), and provides further evidence for the pattern suggested by Tavares et al. (2017). It is possible that this pattern reflects geological processes that are older than the formation of the rivers putatively structuring the interfluvial AoEs (Albert et al., 2021; Méndez-Camacho et al., 2021). Furthermore, the fact that we applied metrics that allow for the identification of complementary areas of biodiversity that have unique evolutionary histories, bring into light important geographic locations that might be taken into account in decision-making for conservation policies (Mishler et al., 2014).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DS, HO, and FD conceived the main ideas, questions, and study design. DS and FD developed specific aims and hypotheses and decided on analytical approaches and methodology. DS and PZ collected the data. DS analyzed the data and led the writing of the manuscript. All authors critically contributed to the drafts and gave final approval for publication.
Funding
DS acknowledges Programa de Pós-graduação em Ecologia e Conservação, Universidade do Estado de Mato Grosso—UNEMAT and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)-Finance Code 001 for a Ph.D. scholarship. HO acknowledges the Universidade Federal do Paraná (UFPR) Capes-PRINT program for a Young Talent Fellowship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor FW declared a past co-authorship with the author FD.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Ubirajara Oliveira for sharing an updated interfluve Areas of Endemism shapefile.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.774083/full#supplementary-material
Footnotes
References
Aguiar, L. M. S., Bernard, E., and Machado, R. B. (2014). Habitat use and movements of Glossophaga soricina and Lonchophylla dekeyseri (Chiroptera?: Phyllostomidae) in a Neotropical savannah. Zoologia 31, 223–229. doi: 10.1590/S1984-46702014000300003
Aguiar, L. M. S., Pereira, M. J. R., Zortéa, M., and Machado, R. B. (2020). Where are the bats? An environmental complementarity analysis in a megadiverse country. Divers. Distrib. 26, 1510–1522. doi: 10.1111/ddi.13137
Albert, J. S., Bernt, M. J., Fronk, A. H., Fontenelle, J. P., Kuznar, S. L., and Lovejoy, N. R. (2021). Late neogene megariver captures and the great Amazonian biotic interchange. Glob. Planet. Change 205:103554. doi: 10.1016/j.gloplacha.2021.103554
Aleixo, A. (2004). Historical diversification of a terra-firme forest bird superspecies: a phylogeographic perspective on the role of different hypotheses of Amazonian diversification. Evolution 58, 1303–1317. doi: 10.1111/J.0014-3820.2004.TB01709.X
Antonelli, A., Zizka, A., Carvalho, F. A., Scharn, R., Bacon, C. D., Silvestro, D., et al. (2018). Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. U. S. A. 115, 6034–6039. doi: 10.1073/PNAS.1713819115
Arnone, I. S., Trajano, E., Pulchério-Leite, A., Passos, F., and de, C. (2016). Long-distance movement by a great fruit-eating bat, Artibeus lituratus (Olfers, 1818), in Southeastern Brazil (Chiroptera, Phyllostomidae): evidence for migration in Neotropical bats? Biota Neotrop. 16, 1–6. doi: 10.1590/1676-0611-BN-2015-0026
Ayres, J. M., and Clutton-Brock, T. H. (1992). River boundaries and species range size in Amazonian primates. Am. Nat. 140, 531–537. doi: 10.1086/285427
Azevedo, J. A. R., Guedes, T. B., Nogueira, C., de, C., Passos, P., Sawaya, R. J., et al. (2020). Museums and cradles of diversity are geographically coincident for narrowly distributed Neotropical snakes. Ecography 43, 328–339. doi: 10.1111/ecog.04815
Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143. doi: 10.1111/j.1466-8238.2009.00490.x
Borges, S. H., and da Silva, J. M. C. (2012). A new area of endemism for Amazonian birds in the rio negro basin. J. Ornithol. 124, 15–23. doi: 10.1676/07-103.1
Burgin, C. J., Colella, J. P., Kahn, P. L., and Upham, N. S. (2018). How many species of mammals are there? J. Mammal. 99, 1–14. doi: 10.1093/JMAMMAL/GYX147
Carrasco-Rueda, F., Zavala, D. J., Alcarraz, Y., Carrasco-Escudero, L., and Zamora, H. T. (2021). Noteworthy records of bats (Mammalia, chiroptera) from Southeastern Peru. Check List 17, 171–180. doi: 10.15560/17.1.171
Carvalho, F., Fabián, M. E., and Menegheti, J. O. (2013). Vertical structure of an assemblage of bats (Mammalia: Chiroptera) in a fragment of Atlantic forest in Southern Brazil. Zoologia 30, 491–498. doi: 10.1590/S1984-46702013000500004
Carvalho, W. D., Gomes, L. A. C., De Castro, I. J., Martins, A. C., Esbérard, C. E. L., and Mustin, K. (2018). Beyond the Amazon forest: richness and abundance of bats in the understory of savannahs, campinaranas and terra firme forest. Acta Chiropterol. 20, 407–419. doi: 10.3161/15081109ACC2018.20.2.011
Cisneros, L. M., Fagan, M. E., and Willig, M. R. (2015). Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers. Distrib. 21, 523–533. doi: 10.1111/ddi.12277
Cracraft, J. (1985). Historical biogeography and patterns of differentiation within the south American avifauna: areas of endemism. Ornithol. Monogr. 36, 49–84. doi: 10.2307/40168278
Crisp, M. D., Laffan, S., Linder, H. P., and Division, A. M. (2001). Endemism in the Australian flora. J. Biogeogr. 28, 183–198. doi: 10.1046/j.1365-2699.2001.00524.x
Crouch, N. M. A., Capurucho, J. M. G., Hackett, S. J., and Bates, J. M. (2019). Evaluating the contribution of dispersal to community structure in Neotropical passerine birds. Ecography 42, 390–399. doi: 10.1111/ecog.03927
d’Horta, F. M., Cuervo, A. M., Ribas, C. C., Brumfield, R. T., and Miyaki, C. Y. (2013). Phylogeny and comparative phylogeography of Sclerurus (Aves: Furnariidae) reveal constant and cryptic diversification in an old radiation of rain forest understorey specialists. J. Biogeogr. 40, 37–49. doi: 10.1111/J.1365-2699.2012.02760.X
da Silva, J. M. C., and Bates, J. M. (2002). Biogeographic patterns and conservation in the South American Cerrado: a tropical savanna hotspot. Bioscience 52, 225–223. doi: 10.1641/0006-3568(2002)052[0225:bpacit]2.0.co;2
da Silva, J. M. C., and Oren, D. C. (1996). Application of parsimony analysis of endemicity in Amazonian biogeography: an example with primates. Biol. J. Linn. Soc. 59, 427–437. doi: 10.1111/J.1095-8312.1996.TB01475.X
Dambros, C., Zuquim, G., Moulatlet, G. M., Costa, F. R. C., Tuomisto, H., Ribas, C. C., et al. (2020). The role of environmental filtering, geographic distance and dispersal barriers in shaping the turnover of plant and animal species in Amazonia. Biodivers. Conserv. 29, 3609–3634. doi: 10.1007/s10531-020-02040-3
de Carvalho, W. D., Palmeirim, J. M., Martins, M. A., and Esbérard, C. E. L. (2019). Traits that allow bats of tropical lowland origin to conquer mountains?: bat assemblages along elevational gradients in the South American Atlantic Forest. J. Biogeogr. 46, 316–331. doi: 10.1111/jbi.13506
Delgado-Jaramillo, M., Aguiar, L. M. S., Bernard, E., and Machado, R. B. (2020). Assessing the distribution of a species-rich group in a continental-sized megadiverse country?: bats in Brazil. Divers. Distrib. 26, 632–643. doi: 10.1111/ddi.13043
Esbérard, C. E. L., Godoy, M. S. M., Renovato, L., and Carvalho, W. D. (2017). Novel long-distance movements by Neotropical bats (Mammalia: Chiroptera: Phyllostomidae) evidenced by recaptures in southeastern Brazil. Stud. Neotrop. Fauna Environ. 52, 75–80. doi: 10.1080/01650521.2016.1273751
Farneda, F. Z., Grelle, C. E. V., Rocha, R., Ferreira, D. F., López-Baucells, A., and Meyer, C. F. J. (2019). Predicting biodiversity loss in island and countryside ecosystems through the lens of taxonomic and functional biogeography. Ecography 43, 97–106. doi: 10.1111/ecog.04507
Fecchio, A., Pinheiro, R., Felix, G., Faria, I. P., Pinho, J. B., Lacorte, G. A., et al. (2018). Host community similarity and geography shape the diversity and distribution of haemosporidian parasites in Amazonian birds. Ecography 41, 505–515. doi: 10.1111/ECOG.03058
Fernandes, A. M., Gonzalez, J., Wink, M., and Aleixo, A. (2013). Multilocus phylogeography of the Wedge-billed Woodcreeper Glyphorynchus spirurus (Aves, Furnariidae) in lowland Amazonia: widespread cryptic diversity and paraphyly reveal a complex diversification pattern. Mol. Phylogenet. Evol. 66, 270–282. doi: 10.1016/J.YMPEV.2012.09.033
Fernandes, A. M., Wink, M., and Aleixo, A. (2012). Phylogeography of the chestnut-tailed antbird (Myrmeciza hemimelaena) clarifies the role of rivers in Amazonian biogeography. J. Biogeogr. 39, 1524–1535. doi: 10.1111/J.1365-2699.2012.02712.X
Ferreira, M., Aleixo, A., Ribas, C. C., and Santos, M. P. D. (2017). Biogeography of the Neotropical genus Malacoptila (Aves: Bucconidae): the influence of the Andean orogeny, Amazonian drainage evolution and palaeoclimate. J. Biogeogr. 44, 748–759. doi: 10.1111/jbi.12888
Fouquet, A., Noonan, B. P., Rodrigues, M. T., Pech, N., Gilles, A., and Gemmell, N. J. (2012). Multiple quaternary refugia in the Eastern Guiana Shield revealed by comparative phylogeography of 12 frog species. Syst. Biol. 61, 461–461. doi: 10.1093/SYSBIO/SYR130
Garbino, G. S. T., Gregorin, R., Lima, I. P., Loureiro, L., Moras, L. M., Moratelli, R., et al. (2020). Updated Checklist of Brazilian Bats: versão 2020. Com. da List. Morcegos do Bras. Soc. Bras. para o Estud. Quirópteros. Available online at: https://www.sbeq.net/lista-de-especies (accessed February 23, 2021).
Gardner, A. L. (2008). Mammals of South America: Marsupials, Xenarthrans, Shrews, and Batsed. Chicago, IL: The University of Chicago Press.
Gascon, C., Malcolm, J. R., Patton, J. L., Da Silva, M. N. F., Bogart, J. P., Lougheed, S. C., et al. (2000). Riverine barriers and the geographic distribution of Amazonian species. Proc. Natl. Acad. Sci. U. S. A. 97, 13672–13677. doi: 10.1073/pnas.230136397
González-Orozco, C. E., Mishler, B. D., Miller, J. T., Laffan, S. W., Knerr, N., Unmack, P., et al. (2015). Assessing biodiversity and endemism using phylogenetic methods across multiple taxonomic groups. Ecol. Evol. 5, 5177–5192. doi: 10.1002/ece3.1747
Grandcolas, P., Nattier, R., and Trewick, S. (2014). Relict species: a relict concept? Trends Ecol. Evol. 29, 655–663. doi: 10.1016/J.TREE.2014.10.002
Hall, J. P. W., and Harvey, D. J. (2002). The phylogeography of Amazonia revisited: new evidence from riodinid butterflies. Evolution 56, 1489–1497. doi: 10.1111/J.0014-3820.2002.TB01460.X
Harcourt, A. H., and Wood, M. A. (2011). Rivers as barriers to primate distributions in Africa. Int. J. Primatol. 33, 168–183. doi: 10.1007/S10764-011-9558-Z
Henry, M., and Kalko, E. K. V. (2007). Foraging strategy and breeding constraints of Rhinophylla pumilio (Phyllostomidae) in the Amazon Lowlands. J. Mammal. 88, 81–93. doi: 10.1644/06-MAMM-A-001R1.1
Hershkovitz, P. (1977). Living New World Monkeys (Platyrrhini)?: with an Introduction to Primates. Chicago: University of Chicago Press.
Hoorn, C., Bogotá-A, G. R., Romero-Baez, M., Lammertsma, E. I., Flantua, S. G. A., Dantas, E. L., et al. (2017). The Amazon at sea: onset and stages of the Amazon River from a marine record, with special reference to Neogene plant turnover in the drainage basin. Glob. Planet. Change 153, 51–65. doi: 10.1016/J.GLOPLACHA.2017.02.005
Hoorn, C., Wesselingh, F. P., Ter Steege, H., Bermudez, M. A., Mora, A., Sevink, J., et al. (2010). Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931. doi: 10.1126/science.1194585
Junk, W. J. (1997). The Central Amazon Floodplain?: Ecology of a Pulsing System. Heidelberg: Springer.
Kalko, E. K. V., and Handley, C. J. (2001). Neotropical bats in the canopy: diversity, community structure, and implications for conservation. Plant Ecol. 153, 319–333. doi: 10.1093/jmammal/gyz023
Laffan, S. W., Lubarsky, E., and Rosauer, D. F. (2010). Biodiverse, a tool for the spatial analysis of biological and related diversity. Ecography 33, 643–647. doi: 10.1111/j.1600-0587.2010.06237.x
Laffan, S. W., Rosauer, D. F., Di Virgilio, G., Miller, J. T., González-Orozco, C. E., Knerr, N., et al. (2016). Range-weighted metrics of species and phylogenetic turnover can better resolve biogeographic transition zones. Methods Ecol. Evol. 7, 580–588. doi: 10.1111/2041-210X.12513
Latrubesse, E. M., Stevaux, J. C., and Sinha, R. (2005). Tropical rivers. Geomorphology 70, 187–206. doi: 10.1016/J.GEOMORPH.2005.02.005
López-Aguirre, C., Hand, S. J., Laffan, S. W., and Archer, M. (2018). Phylogenetic diversity, types of endemism and the evolutionary history of New World bats. Ecography 41, 1955–1966. doi: 10.1111/ecog.03260
Lutz, H. L., Weckstein, J. D., Patané, J. S. L., Bates, J. M., and Aleixo, A. (2013). Biogeography and spatio-temporal diversification of Selenidera and Andigena Toucans (Aves: Ramphastidae). Mol. Phylogenet. Evol. 69, 873–883. doi: 10.1016/J.YMPEV.2013.06.017
Mahulu, A., Stelbrink, B., Van Bocxlaer, B., Riedel, F., and Albrecht, C. (2021). Going with the flow? Diversification of gastropods reflects drainage evolution in Africa. J. Biogeogr. 48, 1579–1593. doi: 10.1111/JBI.14096
Maldonado-Coelho, M., Blake, J., Silveira, L., Batalha-Filho, H., and Ricklefs, R. (2013). Rivers, refuges and population divergence of fire-eye antbirds (Pyriglena) in the Amazon Basin. J. Evol. Biol. 26, 1090–1107. doi: 10.1111/JEB.12123
Marques, J. T., Ramos Pereira, M. J., and Palmeirim, J. M. (2016). Patterns in the use of rainforest vertical space by Neotropical aerial insectivorous bats: all the action is up in the canopy. Ecography 39, 476–486. doi: 10.1111/ecog.01453
Martins, A. C. M., Bernard, E., and Gregorin, R. (2006). Rapid biological surveys of bats (Mammalia, Chiroptera) in three conservation units in Amapá, Brasil. Rev. Bras. Zool. 23, 1175–1184.
Martins, A. C. M., Bernard, E., Gregorin, R., and da Silva, W. A. S. (2011). Filling data gaps on the diversity and distribution of Amazonian bats (Chiroptera): the case of Amapá, easternmost Brazil. Zoologia 28, 177–185. doi: 10.1590/S1984-46702011000200004
Medlin, R. E., Connior, M. B., Gaines, K. F., and Risch, T. S. (2010). Responses of bats to forest fragmentation in the Mississippi River Alluvial Valley, Arkansas, USA. Diversity 2, 1146–1157. doi: 10.3390/d2101146
Méndez-Camacho, K., Leon-Alvarado, O., and Miranda-Esquivel, D. R. (2021). Biogeographic evidence supports the Old Amazon hypothesis for the formation of the Amazon fluvial system. PeerJ 9:e12533. doi: 10.7717/PEERJ.12533
Meyer, C. F. J., Weinbeer, M., and Kalko, E. K. V. (2005). Home-range size and spacing patterns of Macrophyllum macrophyllum (Phyllostomidae) foraging over water. J. Mammal. 86, 587–598. doi: 10.1644/1545-1542(2005)86[587:hsaspo]2.0.co;2
Mishler, B. D., Knerr, N., González-Orozco, C. E., Thornhill, A. H., Laffan, S. W., and Miller, J. T. (2014). Phylogenetic measures of biodiversity and neo-and Paleo-endemism in Australian acacia. Nat. Commun. 5:4473. doi: 10.1038/ncomms5473
Moraes, L. J. C. L., Pavan, D., Barros, M. C., and Ribas, C. C. (2016). The combined influence of riverine barriers and flooding gradients on biogeographical patterns for amphibians and squamates in south-eastern Amazonia. J. Biogeogr. 43, 2113–2124. doi: 10.1111/jbi.12756
Naka, L. N. (2011). Avian distribution patterns in the Guiana Shield: implications for the delimitation of Amazonian areas of endemism. J. Biogeogr. 38, 681–696. doi: 10.1111/J.1365-2699.2010.02443.X
Naka, L. N., and Brumfield, R. T. (2018). The dual role of Amazonian rivers in the generation and maintenance of avian diversity. Sci. Adv. 4:eaar8575. doi: 10.1126/sciadv.aar8575
Napier, P. H. (1976). Catalogue of primates in the British Museum (Natural History). I. Families Callitrichidae and Cebidae. British Museum (Natural History). London: British Museum.
Nogueira, A. C. R., Silveira, R., and Guimarães, J. T. F. (2013). Neogene-Quaternary sedimentary and paleovegetation history of the eastern Solimões Basin, central Amazon region. J. South Am. Earth Sci. 46, 89–99. doi: 10.1016/j.jsames.2013.05.004
Oliveira, U., Soares-Filho, B., Leitão, R. F. M., and Rodrigues, H. O. (2019). BioDinamica: a toolkit for analyses of biodiversity and biogeography on the Dinamica-EGO modelling platform. PeerJ 7:e7213. doi: 10.7717/PEERJ.7213
Oliveira, U., Vasconcelos, M. F., and Santos, A. J. (2017). Biogeography of Amazon birds: rivers limit species composition, but not areas of endemism. Sci. Rep. 7:2992. doi: 10.1038/s41598-017-03098-w
Paradis, E., and Schliep, K. (2019). APE 5.0: analyses of phylogenetics and evolution in R language. Bioinformatics 35, 526–528. doi: 10.1093/bioinformatics/btg412
Patterson, B. D., Stotz, D. F., and Solari, S. (2006). Mammals and birds of the manu biosphere reserve, Peru. Fieldiana Zool. 2006:110.
Pavan, A. C., Bobrowiec, P. E. D., and Percequillo, A. R. (2018). Geographic variation in a South American clade of mormoopid bats, Pteronotus (Phyllodia), with description of a new species. J. Mammal. 99, 624–645. doi: 10.1093/jmammal/gyy048
Peixoto, F. P., Braga, P. H. P., Cianciaruso, M. V., Diniz-Filho, J. A. F., and Brito, D. (2014). Global patterns of phylogenetic beta diversity components in bats. J. Biogeogr. 41, 762–772. doi: 10.1111/jbi.12241
Peracchi, A. L., Raimundo, S. D. L., and Tannure, A. M. (1984). Quirópteros do Território Federal do Amapá. Brasil (Mammalia, Chiroptera). Arq. Univ. Fed. Rur. Rio Janeiro 7, 89–100.
Peters, S. L., Malcolm, J. R., and Zimmerman, B. L. (2006). Effects of selective logging on bat communities in the southeastern Amazon. Conserv. Biol. 20, 1410–1421. doi: 10.1111/j.1523-1739.2006.00526.x
Pirani, R. M., Werneck, F. P., Thomaz, A. T., Kenney, M. L., Sturaro, M. J., Ávila-Pires, T. C. S., et al. (2019). Testing main Amazonian rivers as barriers across time and space within widespread taxa. J. Biogeogr. 46, 2444–2456. doi: 10.1111/jbi.13676
Quinn, G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511806384
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramachandran, V., Robin, V. V., Tamma, K., and Ramakrishnan, U. (2017). Climatic and geographic barriers drive distributional patterns of bird phenotypes within peninsular India. J. Avian Biol. 48, 620–630. doi: 10.1111/JAV.01278
Rex, K., Kelm, D. H., Wiesner, K., Kunz, T. H., and Voigt, C. C. (2008). Species richness and structure of three Neotropical bat assemblages. Biol. J. Linn. Soc. 94, 617–629. doi: 10.1111/j.1095-8312.2008.01014.x
Ribas, C. C., Aleixo, A., Nogueira, A. C. R., Miyaki, C. Y., and Cracraft, J. (2012). A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proc. R. Soc. B Biol. Sci. 279, 681–689. doi: 10.1098/rspb.2011.1120
Ribas, C. C., Gaban-Lima, R., Miyaki, C. Y., and Cracraft, J. (2005). Historical biogeography and diversification within the Neotropical parrot genus Pionopsitta (Aves: Psittacidae). J. Biogeogr. 32, 1409–1427. doi: 10.1111/j.1365-2699.2005.01289.x
Ritter, C. D., Faurby, S., Bennett, D. J., Naka, L. N., ter Steege, H., Zizka, A., et al. (2019). The pitfalls of biodiversity proxies: differences in richness patterns of birds, trees and understudied diversity across Amazonia. Sci. Rep. 91:19205. doi: 10.1038/s41598-019-55490-3
Rojas, D., Moreira, M., Ramos Pereira, M. J., Fonseca, C., and Dávalos, L. M. (2018). Updated distribution maps for neotropical bats in the superfamily Noctilionoidea. Ecology 99:2131. doi: 10.1002/ECY.2404/SUPPINFO
Rosauer, D. F., and Jetz, W. (2015). Phylogenetic endemism in terrestrial mammals. Glob. Ecol. Biogeogr. 24, 168–179. doi: 10.1111/GEB.12237
Rosauer, D., Laffan, S. W., Crisp, M. D., Donnellan, S. C., and Cook, L. G. (2009). Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 18, 4061–4072. doi: 10.1111/j.1365-294X.2009.04311.x
Rossetti, D. F., Cohen, M. C. L., Bertani, T. C., Hayakawa, E. H., Paz, J. D. S., Castro, D. F., et al. (2014). Late quaternary fluvial terrace evolution in the main Southern Amazonian tributary. Catena 116, 19–37. doi: 10.1016/J.CATENA.2013.11.021
Ruffley, M., Peterson, K., Week, B., Tank, D. C., and Harmon, L. J. (2019). Identifying models of trait-mediated community assembly using random forests and approximate Bayesian computation. Ecol. Evol. 9, 13218–13230. doi: 10.1002/ece3.5773
Rull, V. (2004). An evaluation of the Lost World and vertical displacement hypotheses in the Chimantá Massif, Venezuelan Guayana. Glob. Ecol. Biogeogr. 13, 141–148. doi: 10.1111/J.1466-882X.2004.00073.X
Rull, V., and Nogué, S. (2007). Potential migration routes and barriers for vascular plants of the Neotropical Guyana Highlands during the Quaternary. J. Biogeogr. 34, 1327–1341. doi: 10.1111/J.1365-2699.2006.01602.X
Ruokolainen, K., Moulatlet, G. M., Zuquim, G., Hoorn, C., and Tuomisto, H. (2019). Geologically recent rearrangements in central amazonian river network and their importance for the riverine barrier hypothesis. Front. Biogeogr. 11:e45046. doi: 10.21425/F5FBG45046
Santorelli, S., Magnusson, W. E., and Deus, C. P. (2018). Most species are not limited by an Amazonian river postulated to be a border between endemism areas. Sci. Rep. 8:2294. doi: 10.1038/s41598-018-20596-7
Santos, T. C. M., Lopes, G. P. L., Rabelo, R. M. R., and Giannini, T. C. G. (2019). Bats in three protected areas of the Central Amazon Ecological Corridor in Brazil. Acta Chiropterol. 21, 425–442. doi: 10.3161/15081109ACC2019.21.2.017
Scherson, R. A., Thornhill, A. H., Urbina-Casanova, R., Freyman, W. A., Pliscoff, P. A., and Mishler, B. D. (2017). Spatial phylogenetics of the vascular flora of Chile. Mol. Phylogenet. Evol. 112, 88–95. doi: 10.1016/j.ympev.2017.04.021
Silva, J. M. C., Rylands, A. B., and da Fonseca, G. A. B. (2005). The fate of the Amazonian areas of endemism. Conserv. Biol. 19, 689–694. doi: 10.1017/s0031182000064751
Silva, S. M., Townsend Peterson, A., Carneiro, L., Burlamaqui, T. C. T., Ribas, C. C., Sousa-Neves, T., et al. (2019). A dynamic continental moisture gradient drove Amazonian bird diversification. Sci. Adv. 5:eaat5752. doi: 10.1126/SCIADV.AAT5752/SUPPL_FILE/AAT5752_TABLE_S2.XLSX
Smith, B. T., McCormack, J. E., Cuervo, A. M., Hickerson, M. J., Aleixo, A., Cadena, C. D., et al. (2014). The drivers of tropical speciation. Nature 515, 406–409. doi: 10.1038/nature13687
Tavares, V., da, C., Nobre, C. C., Palmuti, C. F., de, S., Nogueira, E., et al. (2017). The Bat Fauna from Southwestern Brazil and its affinities with the fauna of Western Amazon. Acta Chiropterol. 19, 93–106. doi: 10.3161/15081109ACC2017.19.1.007
Trevelin, L. C., Silveira, M., Port-Carvalho, M., Homem, D. H., and Cruz-Neto, A. P. (2013). Use of space by frugivorous bats (Chiroptera: Phyllostomidae) in a restored Atlantic forest fragment in Brazil. For. Ecol. Manage. 291, 136–143. doi: 10.1016/J.FORECO.2012.11.013
Upham, N. S., Esselstyn, J. A., and Jetz, W. (2019). Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17:e3000494. doi: 10.1371/journal.pbio.3000494
Varzinczak, L. H., Lima, C. S., Moura, M. O., and Passos, F. C. (2018). Relative influence of spatial over environmental and historical processes on the taxonomic and phylogenetic beta diversity of Neotropical phyllostomid bat assemblages. J. Biogeogr. 45, 617–627. doi: 10.1111/jbi.13150
Veron, S., Haevermans, T., Govaerts, R., Mouchet, M., and Pellens, R. (2019). Distribution and relative age of endemism across islands worldwide. Sci. Rep. 9:11693. doi: 10.1038/s41598-019-47951-6
Voss, R. S., Fleck, D. W., Strauss, R. E., Velazco, P. M., and Simmons, N. B. (2016). Roosting ecology of Amazonian bats: evidence for guild structure in hyperdiverse mammalian communities. Am. Museum Novit. 2016, 1–43. doi: 10.1206/3870.1
Wallace, A. R. (1854). On the monkeys of the Amazon. Ann. Mag. Nat. Hist. 14, 451–454. doi: 10.1080/037454809494374
Weir, J. T., Faccio, M. S., Pulido-Santacruz, P., Barrera-Guzmán, A. O., and Aleixo, A. (2015). Hybridization in headwater regions, and the role of rivers as drivers of speciation in Amazonian birds. Evolution 69, 1823–1834. doi: 10.1111/EVO.12696
Keywords: beta-diversity, biogeography, Chiroptera, South America, geographic barriers
Citation: Silva DC, Oliveira HFM, Zangrandi PL and Domingos FMCB (2022) Flying Over Amazonian Waters: The Role of Rivers on the Distribution and Endemism Patterns of Neotropical Bats. Front. Ecol. Evol. 10:774083. doi: 10.3389/fevo.2022.774083
Received: 10 September 2021; Accepted: 30 March 2022;
Published: 27 April 2022.
Edited by:
Fernanda P. Werneck, National Institute of Amazonian Research (INPA), BrazilReviewed by:
Paulo Estefano Bobrowiec, National Institute of Amazonian Research (INPA), BrazilAlexandre Aleixo, University of Helsinki, Finland
Copyright © 2022 Silva, Oliveira, Zangrandi and Domingos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daiana C. Silva, ZGF5YW5uYV9jc0Bob3RtYWlsLmNvbQ==
 Daiana C. Silva
Daiana C. Silva Hernani F. M. Oliveira
Hernani F. M. Oliveira Priscilla L. Zangrandi3
Priscilla L. Zangrandi3 Fabricius M. C. B. Domingos
Fabricius M. C. B. Domingos