- College of Plant Protection, Henan Agricultural University, Zhengzhou, China
The cucurbit chlorotic yellows virus (CCYV) has caused serious damage to melon crops in many countries in recent years. This plant virus is exclusively transmitted by the whitefly Bemisia tabaci (Gennadius) in a semi-persistent mode. Previous studies have shown that both persistent and non-persistent viruses can affect the orientation and performance of insect vectors, through changing host phenotype or interacting with insect vectors directly to facilitate the spread of viruses. However, how CCYV affects host-plant selection by B. tabaci has not been reported. In this study, we investigated the visual and olfactory preferences of B. tabaci between healthy and CCYV-infected host plants Cucumis sativus (Cucurbitaceae). Volatile profiles of healthy and CCYV-infected C. sativus plants were analyzed using gas chromatography-mass spectrometry (GC-MS). In the choice assay, whiteflies preferred to settle on CCYV-infected C. sativus seedlings. However, the concentrations of total volatiles and terpenes in C. sativus plants decreased after CCYV infection. Interestingly, in the Y-tube assay and vision preference test, whitefly B. tabaci adults showed significant visual preference to CCYV-infected host but showed olfactory preference to healthy plants. These results indicated that CCYV infection in plants differently affected the visual and olfactory-mediated orientation behaviors of vector whiteflies and implied that visual cues could play a more important role than olfactory cues in whiteflies in locating CCYV-infected host plants.
Introduction
More than 80% of plant viruses are dependent on vectors for spread (Bak et al., 2017). Based on the retention sites and period of virions within insect vectors, plant viruses are classified into persistent, semi-persistent, and non-persistent transmission modes (Mauck et al., 2018). The persistent viruses are retained in salivary glands, the semi-persistent viruses are retained in the foregut, while the non-persistent viruses bind to stylets (Ng and Falk, 2006; Jia et al., 2018). Cucurbit chlorotic yellows virus (CCYV, genus Crinivirus, family Closteroviridae) mainly infects cucurbit plants, such as Cucumis melo and Cucumis sativus, and seriously decreases crop production in many Asian and some American countries (Huang et al., 2010; Okuda et al., 2010; Gu et al., 2011; Hernandez et al., 2021). CCYV is transmitted by the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) in the semi-persistent mode (Li et al., 2016). Whitefly B. tabaci transmits more than 200 plant viruses (Chen et al., 2019; Henrique et al., 2019; Chi et al., 2020). Currently, Q biotype (MED, Mediterranean) and B biotype (MEAM1, Middle East-Asia Minor 1) are the two biotypes of B. tabaci dominating in China (Wu et al., 2002; Chu et al., 2010; Teng et al., 2010).
Numerous studies have indicated that plant viruses can affect the behaviors of insect vectors directly and/or indirectly to promote virus transmission (Whitfield et al., 2015). For example, viruses influence the orientation, feeding behaviors, and physiology of insect vectors indirectly by modifying the color, morphology, odor, and other qualities of host plants (Eigenbrode et al., 2002; Ingwell et al., 2012; He et al., 2015; Wan et al., 2020; Wang S. F. et al., 2020). Interestingly, the different transmission modes of viruses modified the physiology of the host plant differently. Therefore, the feeding behaviors of insect vectors were differentially affected (Mauck et al., 2012; Lu et al., 2017, 2019). The cucumber mosaic virus (CMV), transmitted by aphids in the non-persistent mode with a short acquisition-access period (AAP), reduced the palatability of host plants to force vector aphids to move to new hosts for CMV transmission (Mauck et al., 2010). On the contrary, the persistent viruses require insect vectors to continuously ingest phloem sap on the virus-infected plants for virion acquisition or inoculation (Su et al., 2015; Jhan et al., 2019; Moeini et al., 2020). Thus, persistent viruses enhance the host plant quality to attract insect vectors for long-term feeding on virus-infected plants. Moreover, plant viral particles can directly interact with insect vectors and affect the mating and feeding behaviors of their insect vectors (He et al., 2015; Wan et al., 2020; Wang S. F. et al., 2020). Wang X. R. et al. (2020) reported that tomato yellow leaf curl virus (TYLCV), which can enter and hijack the machinery components of the cell of the insect vector, induced sensory defects by directly interacting with vector whitefly. However, CCYV, which binds at the foregut and cannot circulate and replicate within the whitefly, may have less direct effects on the sensory system of the whitefly. Therefore, we pay more attention to the CCYV-induced indirect effects on the orientation behaviors of whitefly B. tabaci in this study.
Both the vision and olfaction of herbivore insects are involved in locating host plants (Song and Lee, 2018). The olfactory mechanism is employed by insects to perceive the volatiles from the host plant (Bruce and Pickett, 2011), while the visual mechanism is the ability to perceive the physical stimuli and receive the information of size and color that help insects to discriminate between host and non-host plants (Prokopy and Owens, 1983). As known, plant viruses can interfere with host metabolism and induce phenotype changes of host plants, like color and odor. Those changes affect the orientation behavior of insects. However, how those CCYV-induced changes affect the vector orientation to host plants has received limited attention. Previous studies revealed that persistent viruses can alter plant volatiles and color to attract insect vectors (Fereres et al., 2016; Chen et al., 2017; Mwando et al., 2018), as Chen et al. (2017) indicated that TYLCV greatly reduced host volatiles (e.g., o-xylene), resulting in attracting insect vectors to feed and lay eggs. Wang et al. (2019) found that mikania micrantha wilt virus (MMWV) notably changed the volatile profiles of host plants and insect vectors preferred to settle on virus-infected host plants. Therefore, we explored whether the volatiles and plant leaf symptoms induced by CCYV will affect the orientation behaviors of the vector whitefly. Studies on the effects of CCYV on the visual and olfactory orientation of insect vectors would help researchers to understand the transmission mechanisms of CCYV and other semi-persistent viruses and may contribute to the implementation of new strategies for the control of plant viruses and their insect vectors.
Materials and Methods
Plants and Insects
Cucumis sativus (var. Bojie-107) cucumber plants were cultivated in plastic pots (10 cm in diameter, 12 cm in height), with 1 plant in each pot, and were maintained in a greenhouse with photoperiod 16:8 (light:dark), temperature 27 ± 3°C, and relative humidity: 70 ± 5%. Seedlings with 3–4 leaves were used for the experiments.
A colony of B. tabaci biotype Q (Mediterranean, MED) was maintained on healthy cucumber plants. The genetic purity of B. tabaci biotype Q was monitored every three generations using the random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) technique combined with the sequencing of mtCO1 gene (Li et al., 2016). Non-viruliferous B. tabaci adults were transferred onto CCYV-infected cucumber plants for a 3-day AAP. Then, 50 pairs of viruliferous adults of B. tabaci were transferred into clip cages and kept on the leaves of healthy plants for 3 days (Li et al., 2016). Finally, the insects (including adults, eggs, and nymphs) were removed with a small brush. The virus infection status of plants and whiteflies was detected by RT-PCR (Zang et al., 2005). Leaves of healthy and CCYV-infected plants were harvested separately at 10, 20, and 30 days post-inoculation (dpi) and kept in liquid nitrogen, and then, the virus titers were determined.
Quantification of Viral Titers in Plants
Leaves were sampled from healthy and CCYV-infected plants. Each plant was sampled for one time per dpi time point. Total RNA was extracted from 100 mg plant leaves with TRIzol reagent according to the instructions of the manufacturer (Takara Bio, Shiga, Japan). Total RNA was treated with RNase-free DNase I for 2 min at 42°C to remove residual DNA. The RNA concentration was determined using microspectrophotometry (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, United States). Total RNA (1 μg) was used with PrimerScript RT reagent kit (Takara) for reverse transcription according to the instructions.
Primers were designed by Primer 5.0 software according to the CCYV coat protein coding sequence (Accession number: QVG59408) (the forward primer: 5′-GCGACCATCATCTACAGGCA-3′, nucleotide positions 548–567; the reverse primer: 5′-CCGACTTGTTCCTTTCAGAGC-3′; nucleotide positions 679–699). The qRT-PCR assays were performed using TB Green Premix Ex Taq™ II (Takara, Code No. RR820A). Reactions were carried out in a total volume of 20:10 μl of TB Green Premix Ex Taq™ II, 1 μl cDNA or plasmid dilutions, 0.8 μl 10 μM of each primer (the forward primer and the reverse primer), 0.4 μl of ROX Reference Dye II, and 7 μl of ddH2O. Amplification reactions were performed as follows: 94°C for 2 min; 40 cycles of 94°C for 15 s, 60°C for 20 s, and 72°C for 20 s (Li et al., 2016). For the standard curve, serial 10-fold dilutions of plasmid (1.49 × 103 to 1.49 × 109 copies/μl) were used as templates for RT-PCR, and the standard curve equation is Y = −3.222lgX + 35.909, R2 = 1. Finally, virulence titers were calculated using the standard curve.
Choice Assay
The choice assay was conducted under white light at 50 μmol/m2⋅s. A pair of CCYV-infected seedling at 20 dpi and healthy seedling at the same stage was placed in an insect cage (60 cm × 60 cm × 60 cm) with 45 cm apart. Then, 50 pairs of male and female B. tabaci adults, after 30 min starvation, were released into the middle area of the cage. The number of whitefly adults on each plant was counted at intervals of 1, 3, 5, and 24 h. The viruliferous and non-viruliferous whiteflies were tested separately. To eliminate the environmental influence, we exchanged positions of two groups of plants between replications. Four replications were conducted.
Collection and Analysis of Plant Volatiles
Headspace volatiles of the CCYV-infected and healthy host plants were collected using the aeration sampling system as described by Mwando et al. (2018). The plant was contained in an odorless polyethylene terephthalate (PET) bag (50 cm × 55 cm) and connected to a vacuum pump with an inlet flow rate of 0.3 L/min. Volatiles of the host plant were collected by passing the outlet air through a PoraPak Q (60 mg, mesh 50–80, Supelco, Bellefonte, PA, United States) cartridge at the rate of 0.1 L/min. Before use, PoraPak Q filters were washed sequentially with hexane, acetone, and diethyl ether three times and dried by a stream of nitrogen gas. After 8 h collection, the filters were eluted with 500 μl hexane, and then, the eluates were stored at −80°C for the gas chromatography-mass spectrometry (GC-MS) analysis. The collection of volatiles was conducted from 10:00 a.m to 06:00 p.m. Five pairs of CCYV-infected and healthy plants were used in this experiment.
Collected volatiles samples were analyzed using the GC-MS (Agilent 7890B coupled to a 5977 mass spectral detector, Agilent Technologies, United States) equipped with a non-polar HP-5 MS column (30 m × 0.25 mm, 0.25 μm film). One microliter sample was injected with helium as a carrier gas at a flow rate of 1.0 ml/min. The temperature program was 40°C for 2 min to 180°C at 5°C/min and finally to 250°C at 15°C/min. Chemical spectra were recorded at 70 eV in the electron impact (EI) ionization mode. Some chemicals, such as α-pinene, β-ocimene, nonanal, and α-farnesene, were identified by comparing the retention time and mass spectrogram with standard chemicals; while other chemicals were identified by comparing with mass spectral data library (NIST 14.0) on Masshunter software. The standard chemical nonyl acetate was diluted with hexane to 6 concentrations, and the external standard curve was established based on the different concentrations and peak areas (R2 = 0.9976). The concentrations of chemicals were calculated using the standard curve.
Y-Tube Assay
The CCYV-infected and healthy cucumber plants were separately contained in two odorless PET bags (50 cm × 55 cm) and connected to a vacuum pump with an inlet flow rate of 0.3 L/min. The flows were separately introduced into the two arms of the Y-tube (10 cm length of the base tube or Y-arms, 2 cm in tube internal diameter, and 60° angle between the two arms). Adult whiteflies were released individually from the end of the base tube. A choice for one of the two odor sources was recorded when the whitefly crossed one-third of a choice arm within 3 min (Chen et al., 2017). Twenty whiteflies were set as one replication, and four replications were carried out. We exchanged the positions of two arms of the Y-tube between replications to eliminate the environmental effects.
When we tested the olfactory response of B. tabaci to standard chemicals (i.e., α-pinene, β-ocimene, nonanal, and α-farnesene) (purity > 99%, TRC, Canada), standard chemicals were diluted with paraffin oil (v/v = 1: 999; α-pinene: 0.860 ng/μl; β-ocimene: 0.818 ng/μl; α-farnesene: 0.862 ng/μl; nonanal: 0.827 ng/μl), and paraffin oil was used as control. The four chemicals were tested because they were significantly decreased in the plant emissions after CCYV infection. Two hundred microliters of diluted chemicals were added onto the 3 × 2 cm filter paper. Then, the two pieces of the cartridge paper were separately placed into two bottles connected to a Y-tube. The flow of the odor was blown into the arm tubes at 0.3 L/min. When an insect crossed 1/3 of an arm within 3 min, it was regarded as a choice. Twenty adults were tested individually and set as one replication. Three replications were conducted.
Whitefly Bemisia tabaci Visual Preference Assay
The visual preference assay apparatus is made of colorless glass (Figure 1). The middle layer is a removable partition. The two tubes and the middle partition are an integrated structure. CCYV-infected seedlings at 20 dpi (CCYV in richest titers inducing notable yellowing symptoms) and healthy C. sativus seedlings at the same stage were used in this study. A pair of healthy and CCYV-infected plants was placed in the lower area of the box. Then, the glass plate and the box can be embedded together. To ensure there was no airflow, the connection part was sealed by parafilm. One hundred adult insects, after starvation for 30 min, were released into the center of the release point. Visual preference assay was conducted under white light at 50 μmol/m2⋅s. Whiteflies can detect visual cues through the colorless glass. The number of insects in both tubes was counted after 1 h. Experiments were repeated seven times. Positions of two treatments of plants were exchanged, and the tubes were cleaned with absolute ethanol between replications. Thus, the environmental influence could be eliminated. Seven pairs of healthy and CCYV-infected plants were used in this test.
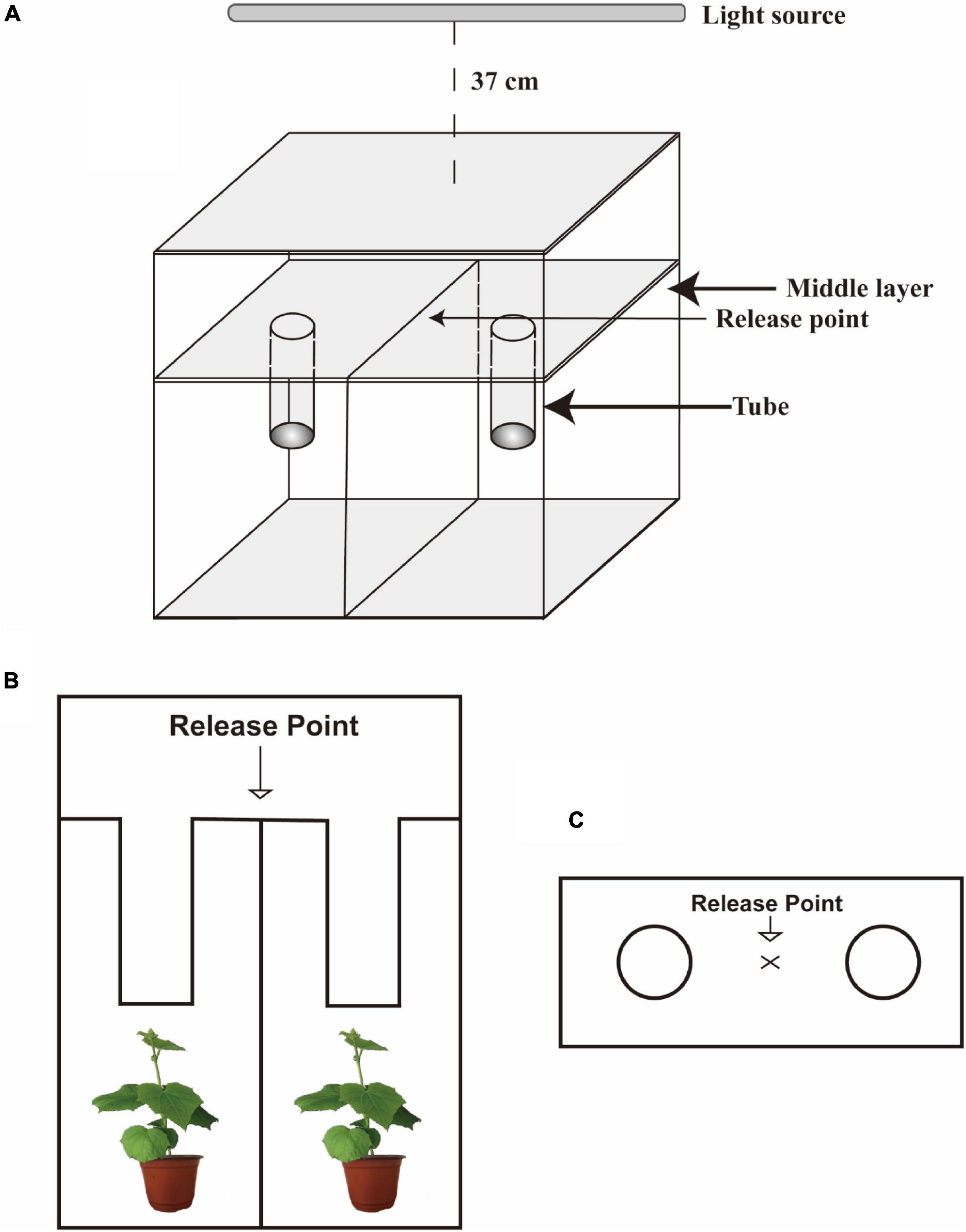
Figure 1. The apparatus for B. tabaci visual preference assay 3-dimensional drawing (A), the front view (B), and the top view (C) of the visual selection apparatus. The apparatus is made of colorless glass (60 × 45 × 20 cm).
Data Analysis
SPSS 22.0 was used to analyze the differences between the treatment and the control groups. In the choice assay, the number of B. tabaci responding to one treatment was transferred into a percentage. Before difference analysis, the data were transformed with arcsine transformation. One-sample t-test was used to analyze the difference based on the normality assumption of the data. In the analysis of plant volatile compounds, the quantity difference of volatiles between CCYV-infected and healthy plants was calculated using independent-sample t-test. In the virus titer determination, one-way ANOVA was used to analyze the difference of virus titers at different dpi time points (Tamiru et al., 2011). In the Y-tube assay and visual preference test, the number of B. tabaci responding to one treatment was transferred into a percentage and transformed with arcsine transformation for difference analysis.
Results
Virus Titer Determination in Plants
The virus titers varied significantly at different stages of plants. At 10 days after CCYV infection, the coat protein gene of CCYV was 1.40 × 102 copies, 2.94 × 106 copies after 20 days, and 2.33 × 106 copies at 30 days. No copies were detected in healthy cucumber leaves (Figure 2). With the increase of virulence titers, the yellowing symptoms became more obvious.
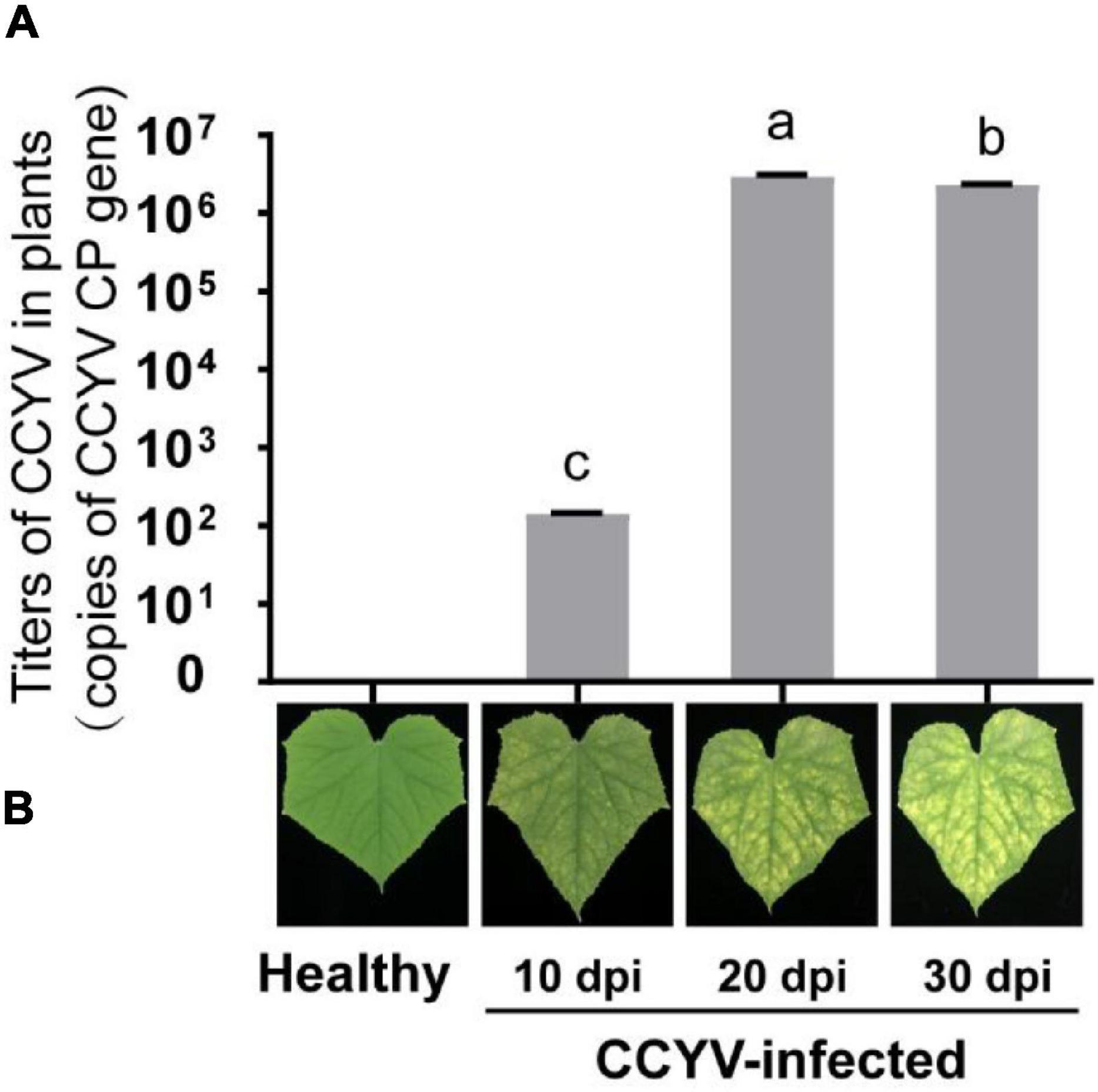
Figure 2. Virus titers (A) and symptoms (B) in CCYV-infected plants at different stages. Virus titers in CCYV-infected cucumber leaves on 10, 20, and 30 dpi were detected by qRT-PCR. Columns show mean ± standard error. Y-axis represents the copies of the CCYV coat protein gene. Differences among incubation periods were analyzed by a one-way ANOVA. Means with the same letter were not significantly different (p < 0.05).
Choice Assay of Whiteflies
Choice assay of whitefly B. tabaci showed that, 5 h after insect releasing, the percentages of B. tabaci selecting CCYV-infected plants were significantly higher than that for healthy plants (Figure 3), and both viruliferous and non-viruliferous B. tabaci adults preferred to settle on CCYV-infected host plants (viruliferous: 1 h, t = 2.100, p = 0.127; 3 h, t = 2.122, p = 0.124; 5 h, t = 5.126, p = 0.014; 24 h, t = 3.747, p = 0.033, df = 3; non-viruliferous: 1 h, t = 0.659, p = 0.557; 3 h, t = 1.379, p = 0.262; 5 h, t = 4.316, p = 0.023; 24 h, t = 3.993, df = 3, p = 0.028).
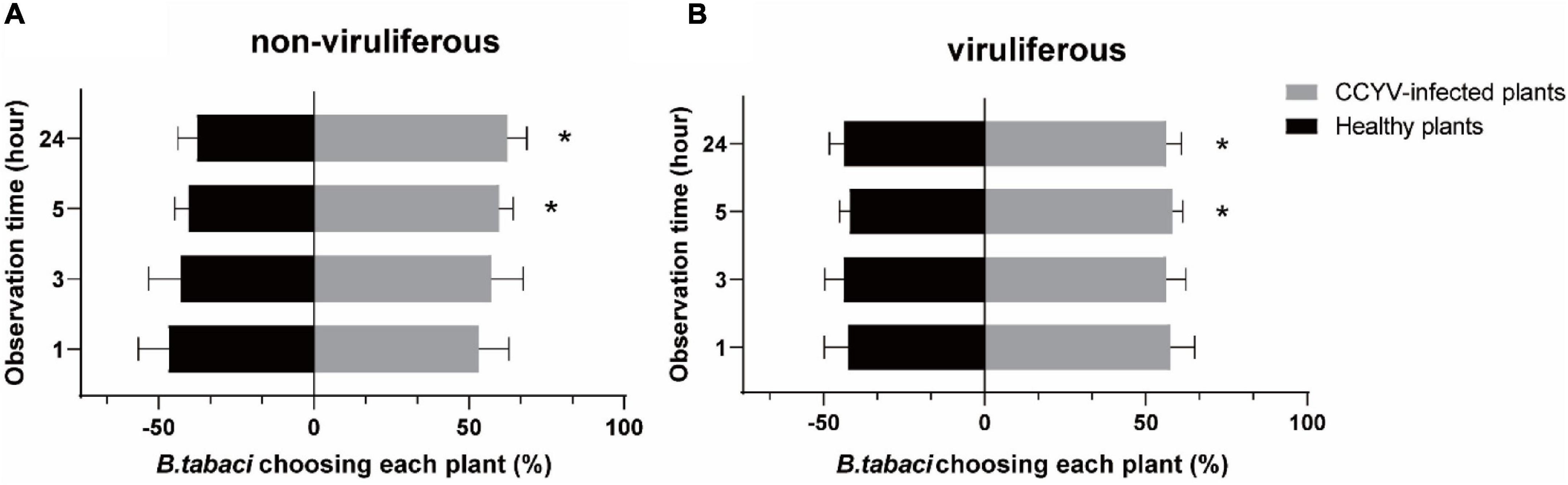
Figure 3. Behavioral response of non-viruliferous (A) and viruliferous (B) B. tabaci to CCYV-infected and healthy plants. This experiment was conducted in an insect cage (60 × 60 × 60 cm), and B. tabaci adults were released in the center. “*” means p < 0.05. Percentage was transformed with arcsine before difference analysis. The one-sample t-test was used to analyze the difference (p < 0.05). The difference between the percentage and the hypothesis of 50%.
Chemical Analysis of Plant Volatiles
Plant volatile profile changed greatly after CCYV infection, with more and higher quantities of volatiles in the healthy plants (Figure 4 and Table 1). Some chemicals, such as 3,3-dimethyl-1-butanol, benzyl alcohol, methyl salicylate, nonanal, and α-farnesene, found in healthy plants were not detected in CCYV-infected plants. After the statistical analysis of the detected volatiles, we found that the total concentration of volatiles and terpenes from CCYV-infected plants was remarkably lower than that in healthy plants (Figure 5) (total: t = 5.183, df = 8, p = 0.001; terpenes: t = 3.302, df = 8, p = 0.011). In particular, the monoterpenes α-pinene and β-ocimene decreased after the CCYV infection (Figure 5).
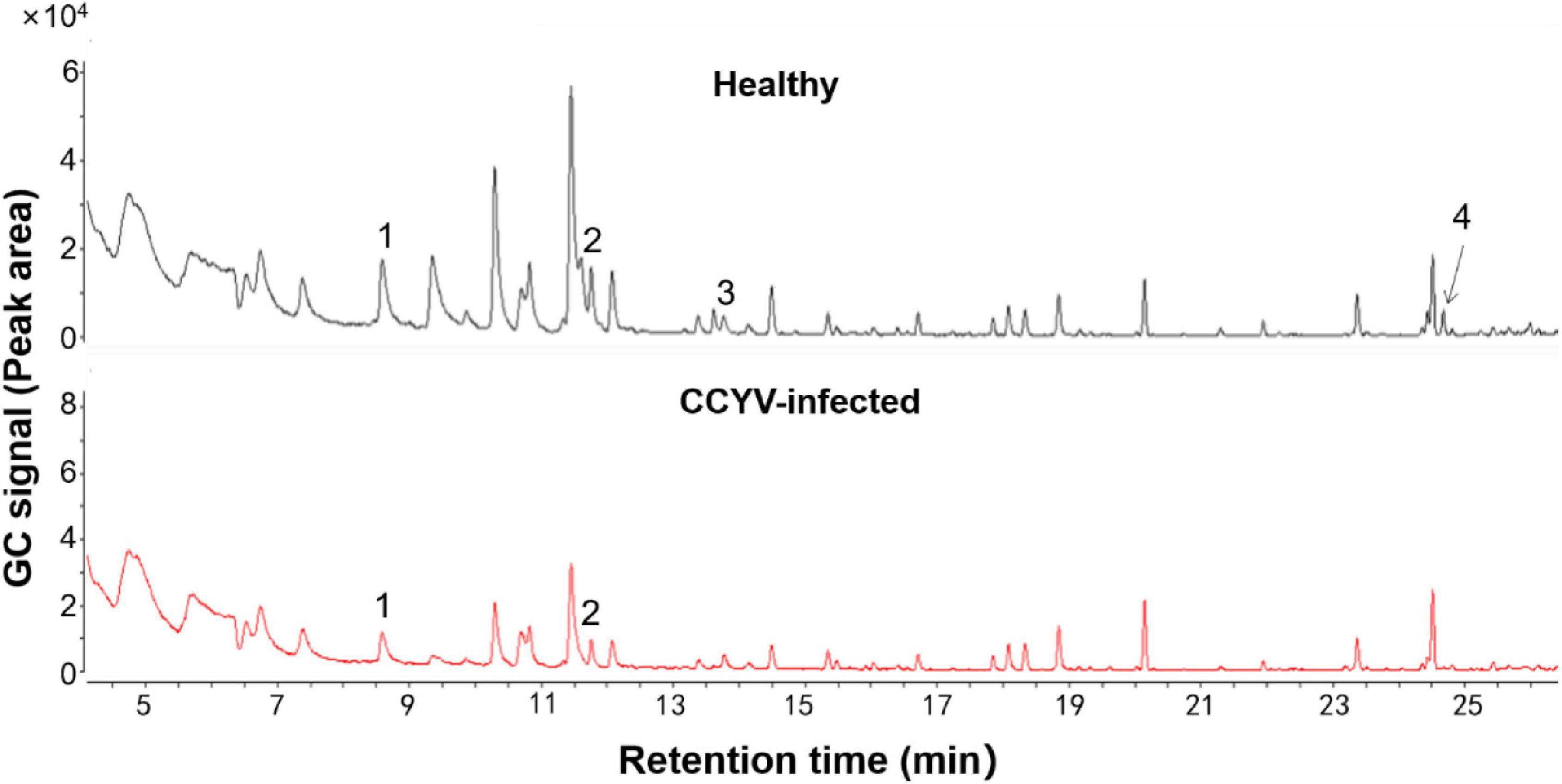
Figure 4. Gas chromatography profiles of headspace volatiles from healthy and CCYV-infected Cucumis sativus seedlings. 1: α-pinene, 2: β-ocimene, 3: nonanal, and 4: α-farnesene.
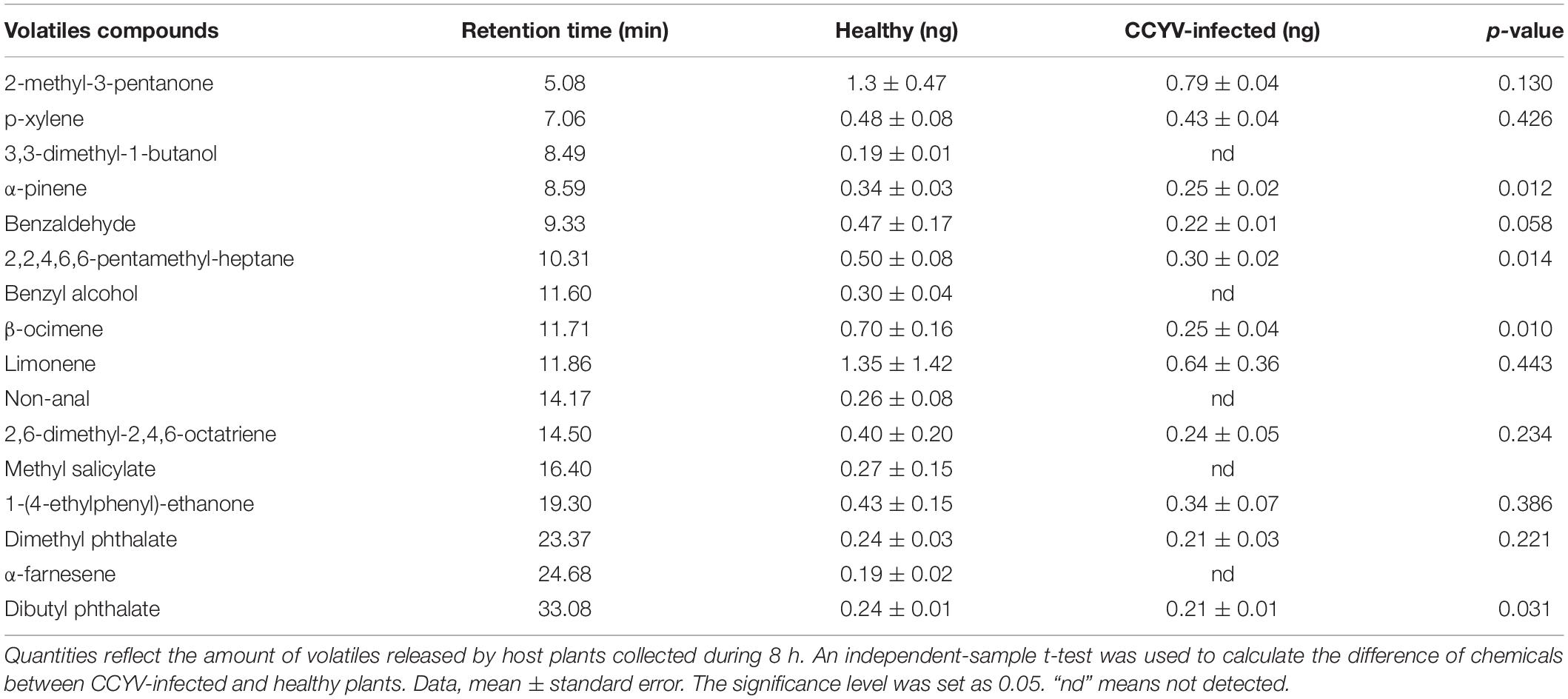
Table 1. Quantification of major volatile organic compounds from healthy and CCYV-infected Cucumis sativus seedlings.
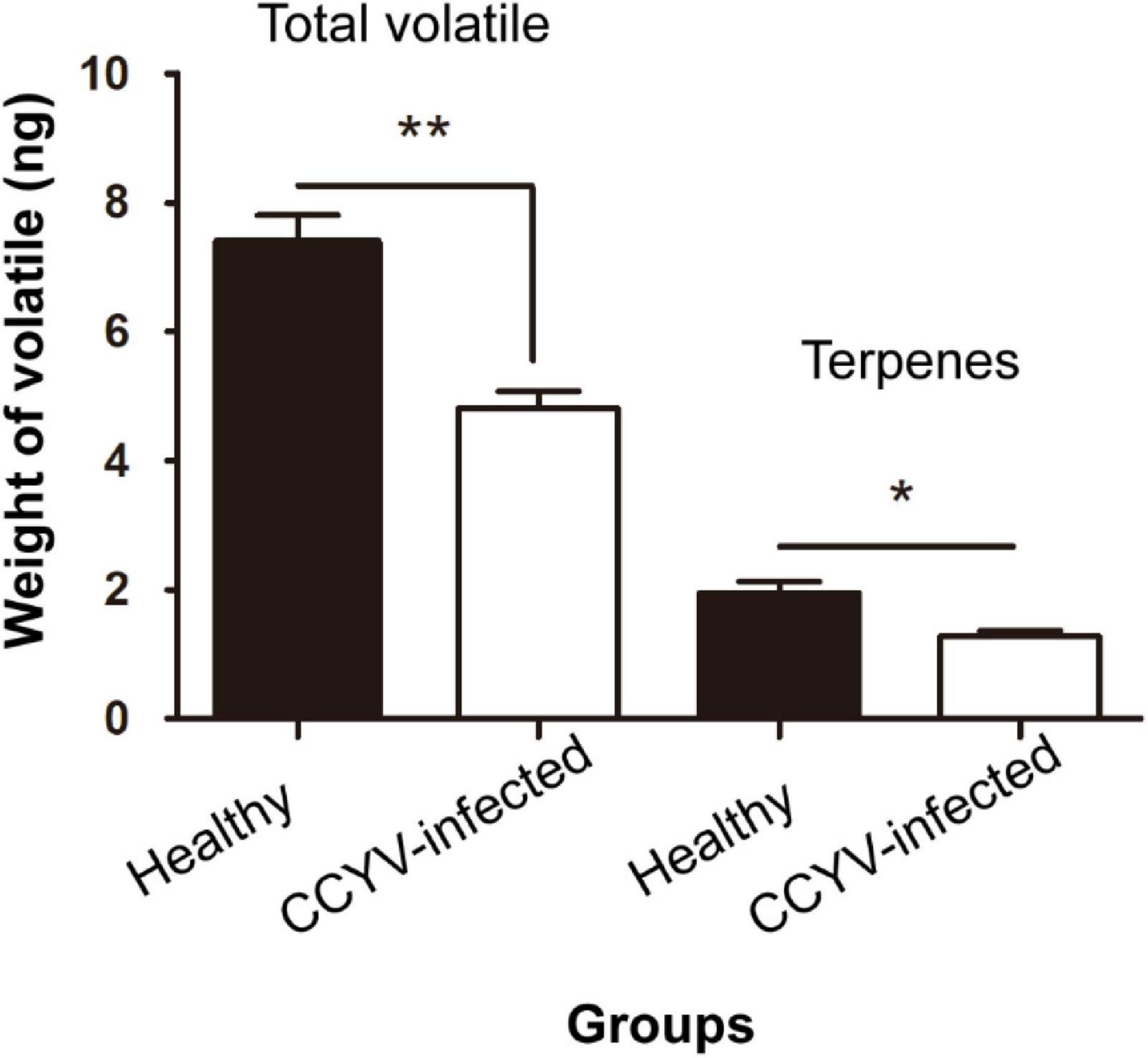
Figure 5. Total amounts of volatiles and terpenes from healthy and CCYV-infected Cucumis sativus seedlings. Columns show mean ± standard error. “*” means p < 0.05, “**” means p < 0.01, the independent-sample t-test was used to calculate the difference.
Y-Tube Assay of Bemisia tabaci to the Odor of Plants and Standard Chemicals
To investigate how whiteflies respond to the odor of healthy and CCYV-infected plants, we conducted a Y-tube assay. Whiteflies showed olfactory preference to the odor of healthy plants (Figure 6A) (t = 5.098, df = 4, p = 0.007). Furthermore, we studied whether such olfactory preference to healthy cucumber plants was due to the significantly changed chemicals, and four chemicals (i.e., α-pinene, β-ocimene, nonanal, and α-farnesene) were selected. Those chemicals have been commonly reported in the volatiles of host plants damaged by whiteflies (Mercke et al., 2004; Silva et al., 2018). In the Y-tube assay, they were all attractive to whiteflies (Figure 6B) (α-farnesene: t = 4.33, df = 2, p = 0.049; β-ocimene: t = 5.5, df = 2, p = 0.032; nonanal: t = 9.5, df = 2, p = 0.011; α-pinene: t = 13.856, df = 2, p = 0.005). It means that B. tabaci shows preference to the odors of healthy plants.
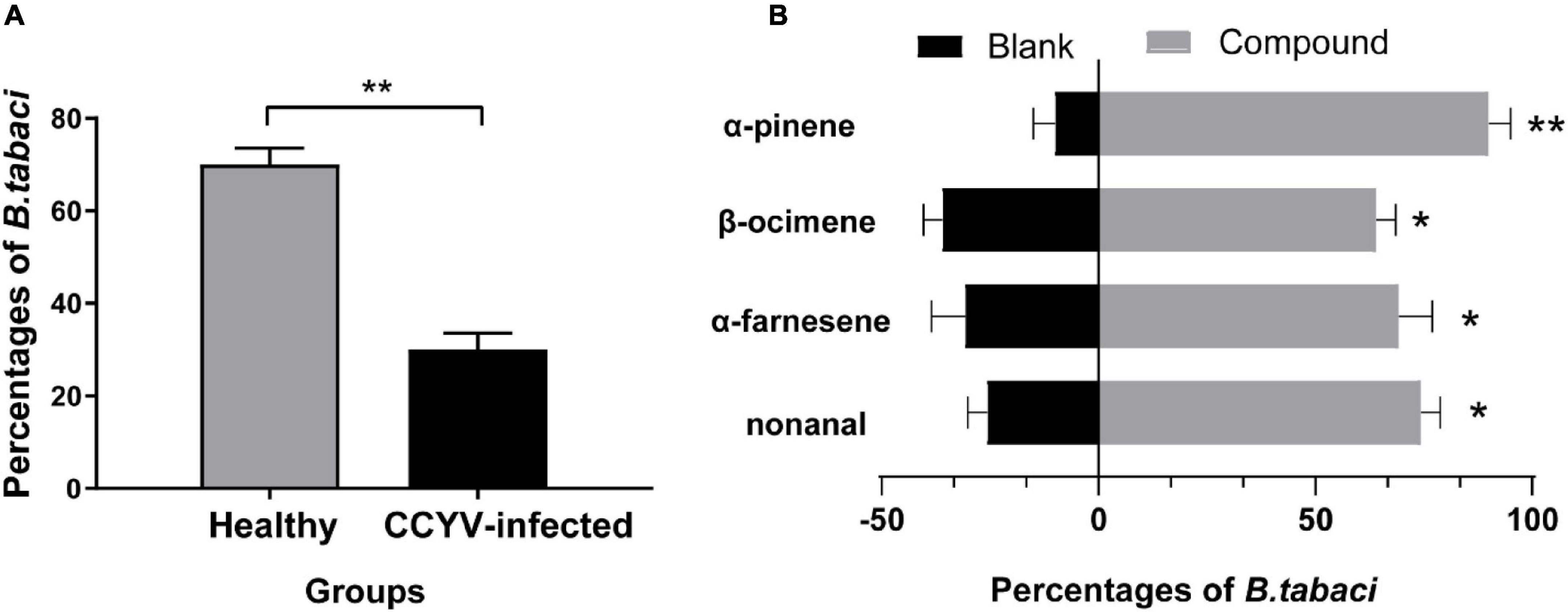
Figure 6. Y-tube assay of B. tabaci to the odor of CCYV-infected and healthy Cucumis sativus plants (A) as well as to standard chemicals (B). Columns show mean ± standard error (A); 0.1% diluted chemicals (α-pinene: 0.860 ng/μl; β-ocimene: 0.818 ng/μl; α-farnesene: 0.862 ng/μl; nonanal: 0.827 ng/μl) were used in the Y-tube assay. Three replicates of 20 individuals were tested in each replication. In the olfactory assay of whiteflies to the odor of CCYV-infected and healthy cucumber plants, five replications were carried out. The number of B. tabaci was transferred into percentage and transformed with arcsine for analyzing difference under the one-sample t-test. “*” means p < 0.05, “**” means p < 0.01. We determined if the percentage differed significantly from the hypothesis of 50%.
Visual Preference Assay to the Infected and Healthy Plants
In the visual selection assay, we analyzed the difference between the arcsine value of B. tabaci selecting CCYV-infected plant (20 dpi) and the hypothesis value 50% with the one-sample t-test. The proportion of whiteflies preferred to CCYV-infected plants was significantly higher than that to healthy plants (Figure 7) (t = −2.654, df = 13, p = 0.038). In this test, only the visual cues can be detected by whitefly. It indicates that CCYV-induced symptoms affect the orientation behavior and enhance the visual preference of whiteflies to CCYV-infected host plants.
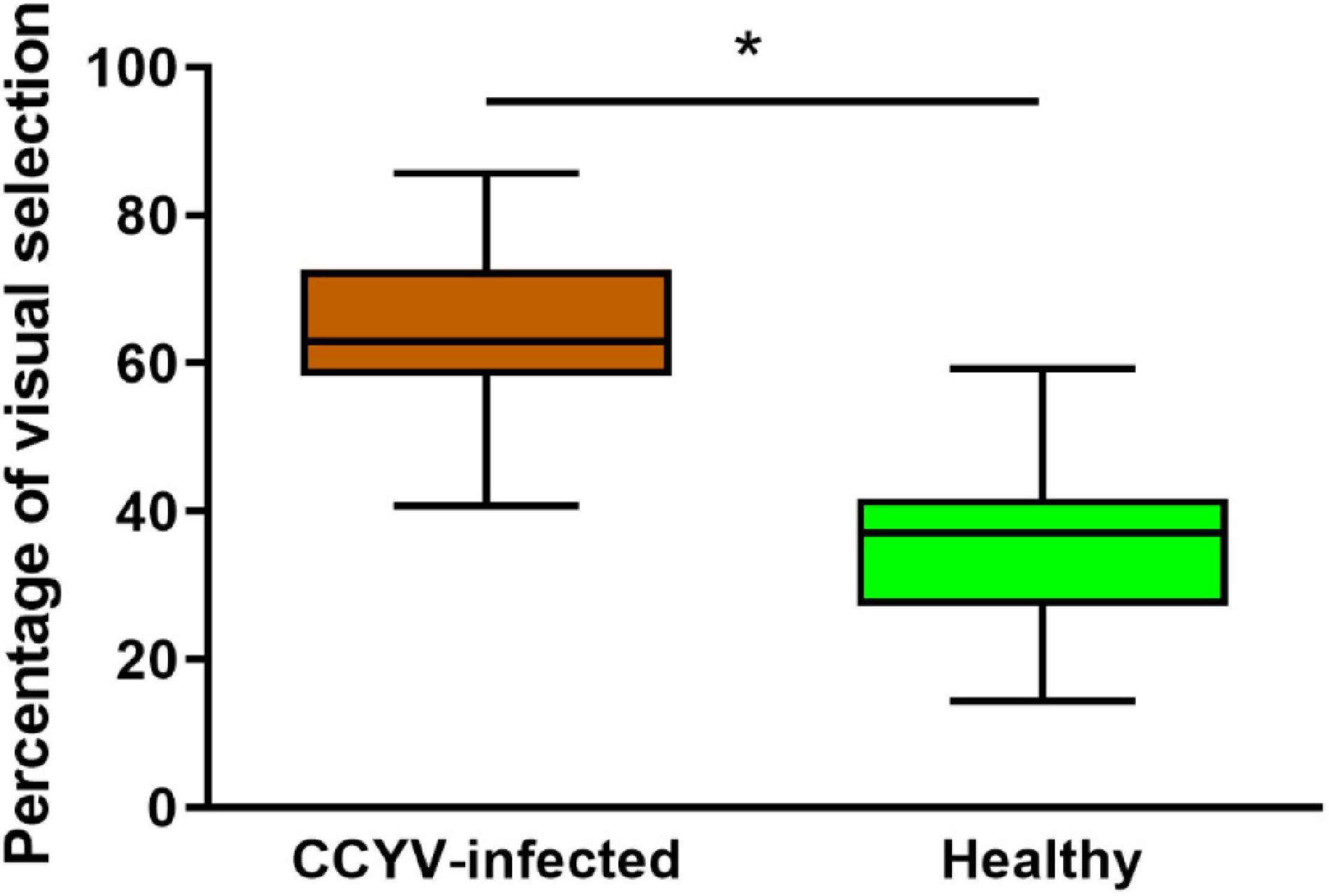
Figure 7. Visual selection preference of B. tabaci between CCYV-infected and healthy plants. Data = mean ± standard error. One hundred adult insects were tested, and seven replicates were carried out. The percentage of B. tabaci was transferred with arcsine transformation for analyzing the difference using the one-sample t-test (hypothesis value: 50%, “*” < 0.05).
Discussion
In this study, we found that visual cues play dominant roles in B. tabaci orientation to CCYV-infected cucumber plants. A previous study proved that BYDV-aphids preferred non-infected wheat plants, while BYDV-free aphids preferred the BYDV-infected wheat plants (Ingwell et al., 2012). In this study, we found that both viruliferous and non-viruliferous whiteflies preferred to settle on CCYV-infected plants in the choice assay. The result means that the preference of whiteflies to CCYV-infected plants is not related with whether whiteflies acquired CCYV or not. Unlike persistent viruses, semi-persistent viruses do not enter the cell of the insect vector for circulation and replication (Whitfield et al., 2015), so they may cause less effects on the physiology of insect vectors. The choice assay showed that there was no statistical difference within 5 h. Instead, the proportion of whiteflies settling on CCYV-infected cucumber plants was significantly higher after 5 h (Figure 3). We suspected that if CCYV enhanced the palatability of cucumber plants, whiteflies tended to settle on CCYV-infected plants, since whiteflies could move between CCYV-infected and healthy cucumber plants within the first 3 h. However, another study in our group found that CCYV infection decreased the nutrition of cucumber plants (Zhang et al., 2021), which suggested that CCYV infection in plants would negatively influence the feeding behavior of whiteflies. So, whiteflies preferring to settle on CCYV-infected plants may not be a gustatory-mediated behavior. Moreover, only some persistent viruses with long AAP, such as TYLCV, were reported to enhance the quality of host plants to attract insect vectors to feed on infected plants continuously (Chen et al., 2017). As for the semi-persistent viruses, like CCYV, whose AAP is shorter than the persistent viruses (Li et al., 2016), enhancing the quality of the host plant seems unfavorable for CCYV transmission.
Visual, olfactory cues are also crucial for herbivore insects when they are making the foraging decisions (Fereres et al., 2016; Wang et al., 2019). Plant volatiles act as a signal for herbivore insects in locating their hosts at long distance, providing insects with information about the species and location of plants (Bruce and Pickett, 2011). Some viruses have been reported increasing the volatile emissions of host plants that affect the behavior of insect vectors, such as cucumber mosaic virus (CMV) and tomato spotted wilt virus (TSWV) (Mauck et al., 2010; Peñaflor et al., 2016; Chen et al., 2017), while some other viruses suppressed the plant emissions, such as tomato severe rugose virus transmitted by the whitefly (Fereres et al., 2016). CCYV infection decreased the volatile emissions of the host plant, especially the terpenes (Figure 5) that were regarded as the most important signals for insect–plant interaction (Huang and Osbourn, 2019). The decreased plant emissions made infected plants less attractive than healthy plants in odor (Figure 6A). Our observation is consistent with previous studies that insects prefer plants with higher volatile emissions (Mauck et al., 2012; Turlings and Erb, 2018). The α-pinene, β-ocimene, nonanal, and α-farnesene, which have been commonly found in the volatiles of other plant species damaged by whiteflies, significantly decreased the emissions after CCYV infection. Importantly, whiteflies showed olfactory preference to the four chemicals (Figure 6B). This result suggests that those chemicals may contribute to the olfactory attraction of whiteflies to healthy plants. As known, viruses can suppress the plant defensive response to protect their insect vectors (Abe et al., 2012; Wang et al., 2019; Patton et al., 2020). In the coevolution, some natural enemies, such as predators or parasitoids, may use plant volatiles, such as terpenes, to locate their prey (Block et al., 2019; Boncan et al., 2020). It has been reported previously that plant viruses protect their insect vectors from enemies (Belliure et al., 2005, 2008). Luan et al. (2013) proved that the begomovirus tomato yellow leaf curl China virus suppressed the terpenes biosynthesis of host plants to improve the mutualisms with its vector whitefly. Hu et al. (2019) also found that a plant virus repressed the production of plant volatile to protect its insect vector. Therefore, we assume that the reduction of terpenes might be a strategy employed by CCYV to protect vector whiteflies from natural enemies, thus improving the CCYV spread. Different changes in volatiles induced by viruses were possibly linked to the varied infection mechanisms within host plants.
In the visual preference assay, whiteflies showed a significant preference to CCYV-infected cucumber plants (Figure 7). In the apparatus of visual preference assay, only visual cues could be detected by whiteflies, so we speculate that CCYV-induced yellowing symptoms may affect the orientation behavior of whiteflies. With the virus titer increased, the CCYV-induced yellowing symptoms became more obvious. Whitefly B. tabaci is sensitive to yellow–blue color (500–580 nm), especially yellow color is more attractive than the others (Mound, 1962; Prokopy and Owens, 1983). The phenomenon that viruses increased the visual attraction of host plant has been reported in studies about tomato chlorosis virus (ToCV), tomato severe rugose virus (TSRV), and TYLCV (Fereres et al., 2016; Johnston and Martini, 2020). These three viruses above mentioned viruses are all transmitted by B. tabaci and cause yellowing and chlorosis symptoms. Those studies implied that plant viruses-induced symptoms on host plants could be a vital factor that affects the selection of insect vectors between healthy and viruses-infected plants.
Manipulating the behaviors of insect vectors by plant viruses directly and indirectly to facilitate their transmission has been commonly reported in three transmission modes of plant viruses (Mauck et al., 2010; Wang S. F. et al., 2020). In this study, we found that CCYV also changed the phenotype of host plants to recruit insect vector, improving the acquisition and transmission of the virus. Interestingly, CCYV-induced plant changes differently affected the visual and olfactory cues of whiteflies to plants. This study highlights the importance of visual cues for insect vector B. tabaci when selecting host plants. Future studies include how CCYV causes such effects, and to be more specific, which proteins encoded by CCYV play roles in impacting the preference of B. tabaci to infected host, and how CCYV interacts with host factors to attract insect vector.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
FY and JL: conceptualization, validation, and funding acquisition. ZZ and HH: methodology. ZZ: software, formal analysis, data curation, and writing—original draft preparation. ZZ, BZ, MY, and HH: investigation. FY: resources and supervision. JL: project administration. All authors read and approved the final manuscript and contributed to the writing—review and editing.
Funding
This research was supported by the National Natural Foundation of China (Project Nos. 31871973, 31471776, and 31901886).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Cuihong Huang for the suggestions on performing this research.
References
Abe, H., Tomitaka, Y., Shimoda, T., Seo, S., Sakurai, T., Kugimiya, S., et al. (2012). Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 53, 204–212. doi: 10.1093/pcp/pcr173
Bak, A., Cheung, A. L., Yang, C., Whitham, S. A., and Casteel, C. L. (2017). A viral protease relocalizes in the presence of the vector to promote vector performance. Nat. Comm. 8:14493. doi: 10.1038/ncomms14493
Belliure, B., Janssen, A., Maris, P. C., Peters, D., and Sabelis, M. W. (2005). Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79.
Belliure, B., Janssen, A., and Sabelis, M. W. (2008). Herbivore benefits from vectoring plant virus through reduction of period of vulnerability to predation. Oecologia 156, 797–806. doi: 10.1007/s00442-008-1027-9
Block, A. K., Vaughan, M. M., Schmelz, E. A., and Christensen, S. A. (2019). Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 249, 21–30. doi: 10.1007/s00425-018-2999-2
Boncan, D. A. T., Tsang, S. S. K., Li, C., Lee, I. H. T., Lam, H. M., Chan, T. F., et al. (2020). Terpenes and Terpenoids in plants: interactions with environment and insects. Internat. J. Mole. Sci. 21:7382. doi: 10.3390/ijms21197382
Bruce, T. J., and Pickett, J. A. (2011). Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 72, 1605–1611. doi: 10.1016/j.phytochem.2011.04.011
Chen, G., Su, Q., Shi, X., Liu, X., Peng, Z., Zheng, H., et al. (2017). Odor, not performance, dictates Bemisia tabaci’s selection between healthy and virus infected plants. Front. Physiol. 8:146. doi: 10.3389/fphys.2017.00146
Chen, T., Saeed, Q., He, Z., and Lu, L. (2019). Transmission efficiency of Cotton leaf curl Multan virus by three cryptic species of Bemisia tabaci complex in cotton cultivars. PeerJ 7:e7788. doi: 10.7717/peerj.7788
Chi, Y., Pan, L. L., Bouvaine, S., Fan, Y. Y., Liu, Y. Q., Liu, S. S., et al. (2020). Differential transmission of Sri Lankan cassava mosaic virus by three cryptic species of the whitefly Bemisia tabaci complex. Virology 540, 141–149. doi: 10.1016/j.virol.2019.11.013
Chu, D., Wan, F. H., Zhang, Y. J., Zhang, Y. J., and Brown, J. K. (2010). Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Env. Entomol. 39, 1028–1036. doi: 10.1603/EN09161
Eigenbrode, S. D., Ding, H., Shiel, P., and Berger, P. H. (2002). Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B-Biol. Sci. 269, 455–460. doi: 10.1098/rspb.2001.1909
Fereres, A., Peñaflor, M. F., Favaro, C. F., Azevedo, K. E., Landi, C. H., Maluta, N. K., et al. (2016). Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 8:225. doi: 10.3390/v8080225
Gu, Q. S., Liu, Y. H., Wang, Y. H., Huangfu, W. G., Gu, H. F., Xu, L., et al. (2011). First report of Cucurbit chlorotic yellows virus in cucumber, melon, and watermelon in China. Plant Dis. 95:73. doi: 10.1094/pdis-07-10-0550
He, W. B., Li, J., and Liu, and S. S. (2015). Differential profiles of direct and indirect modification of vector feeding behaviour by a plant virus. Sci. Rep. 5:7682. doi: 10.1038/srep07682
Henrique, B. V., Maranho, W. L. F., and Rosa, S. B. (2019). Evidence for increased efficiency of virus transmission by populations of Mediterranean species of Bemisia tabaci with high Hamiltonella prevalence. Phytoparasitica 47, 293–300.
Hernandez, R. N., Isakeit, T., Al Rwahnih, M., Hernandez, R., and Alabi, O. J. (2021). First report of Cucurbit chlorotic yellows virus infecting cantaloupe (Cucumis melo L.) in Texas. Plant Dis. 2021:378. doi: 10.1094/PDIS-02-21-0378-PDN
Hu, K., Qiu, L., Ding, W., He, H., and Li, Y. (2019). Evaluation of reference genes and expression of key genes involved in the isoprenoid metabolic pathway of rice leaves after infection by the Southern rice black-streaked dwarf virus. Mole. Biol. Rep. 46, 3945–3953. doi: 10.1007/s11033-019-04841-4
Huang, A. C., and Osbourn, A. (2019). Plant terpenes that mediate below-ground interactions: prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 75, 2368–2377. doi: 10.1002/ps.5410
Huang, L. H., Tseng, H. H., Li, J. T., and Chen, T. C. (2010). First report of Cucurbit chlorotic yellows virus infecting cucurbits in Taiwan. Plant Dis. 94:1168. doi: 10.1094/PDIS-94-9-1168B
Ingwell, L. L., Eigenbrode, S. D., and Bosque-Perez, N. A. (2012). Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2:578.
Jhan, P. K., Shim, J. K., Lee, S., and Lee, K. Y. (2019). Differential responses between a vector species Bemisia tabaci and a nonvector species Trialeurodes vaporariorum following ingestion of tomato yellow leaf curl virus. Archiv. Insect Biochem. Physiol. 100:e21503. doi: 10.1002/arch.21517
Johnston, N., and Martini, X. (2020). The Influence of visual and olfactory cues in host selection for Bemisia tabaci biotype B in the presence or absence of Tomato yellow leaf curl virus. Insects. 11:115. doi: 10.3390/insects11020115
Jia, D., Chen, Q., Mao, Q., Zhang, X., Wu, W., Chen, H., et al. (2018). Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 2018, 127–132. doi: 10.1016/j.coviro.2017.12.004
Li, J. J., Liang, X. Z., Wang, X. L., Shi, Y., Gu, Q. S., Kuo, Y. W., et al. (2016). Direct evidence for the semipersistent transmission of cucurbit chlorotic yellows virus by a whitefly vector. Sci. Rep. 6:36604. doi: 10.1038/srep36604
Lu, S. H., Chen, M. S., Li, J. J., Shi, Y., Gu, Q. S., and Yan, F. M. (2019). Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by cucurbit chlorotic yellows virus. Virol. J. 16:106. doi: 10.1186/s12985-019-1215-8
Lu, S. H., Li, J. J., Wang, X. L., Song, D. Y., Bai, R. E., Shi, Y., et al. (2017). A semipersistent plant virus differentially manipulates feeding behaviors of different sexes and biotypes of its whitefly vector. Viruses 9:4. doi: 10.3390/v9010004
Luan, J. B., Yao, D. M., Zhang, T., Walling, L. L., Yang, M., Wang, Y. J., et al. (2013). Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16, 390–398. doi: 10.1111/ele.12055
Mauck, K. E., Bosque-Pérez, N. A., Eigenbrode, S. D., Moraes, C. M. D., and Mescher, M. C. (2012). Transmission mechanisms shape pathogen effects on host-vector interactions: evidence from plant viruses. Funct. Ecol. 26, 1162–1175.
Mauck, K. E., Chesnais, Q., and Shapiro, L. R. (2018). Evolutionary determinants of host and vector manipulation by plant viruses. Adv. Virus Res. 101, 189–250. doi: 10.1016/bs.aivir.2018.02.007
Mauck, K. E., De Moraes, C. M., and Mescher, M. C. (2010). Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. U S A 107, 3600–3605. doi: 10.1073/pnas.0907191107
Mercke, P., Kappers, I. F., Verstappen, F. W., Vorst, O., Dicke, M., and Bouwmeester, H. J. (2004). Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol. 135, 2012–2024. doi: 10.1104/pp.104.048116
Moeini, P., Afsharifar, A., Izadpanah, K., Sadeghi, S. E., and Eigenbrode, S. D. (2020). Maize Iranian mosaic virus (family Rhabdoviridae) improves biological traits of its vector Laodelphax striatellus. Archiv. Virol. 165, 169–178. doi: 10.1007/s00705-019-04450-3
Mound, L. A. (1962). Studies on the olfaction and colour sensitivity of Bemisia tabaci (genn.) (Homoptera,Aleyrodidae). Entomol. Exp. Appl. 5, 99–104. doi: 10.1111/j.1570-7458.1962.tb00571.x
Mwando, N. L., Tamiru, A., Nyasani, J. O., Obonyo, M. A. O., Caulfield, J. C., Bruce, T. J. A., et al. (2018). Maize chlorotic mottle virus induces changes in host plant volatiles that attract vector thrips species. J. Chem. Ecol. 44, 681–689. doi: 10.1007/s10886-018-0973-x
Ng, J. C. K., and Falk, B. W. (2006). Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44, 183–212. doi: 10.1146/annurev.phyto.44.070505.143325
Okuda, M., Okazaki, S., Yamasaki, S., Okuda, S., and Sugiyama, M. (2010). Host range and complete genome sequence of Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology 100, 560–566. doi: 10.1094/PHYTO-100-6-0560
Patton, M. F., Bak, A., Sayre, J. M., Heck, M. L., and Casteel, C. L. (2020). A polerovirus, potato leafroll virus, alters plant-vector interactions using three viral proteins. Plant Cell Env. 43, 387–399. doi: 10.1111/pce.13684
Peñaflor, M. F. G. V., Mauck, K. E., Alves, K. J., De Moraes, C. M., and Mescher, M. C. (2016). Effects of single and mixed infections of Bean pod mottle virus and Soybean mosaic virus on host-plant chemistry and host–vector interactions. Funct. Ecol. 30, 1648–1659.
Prokopy, R. J., and Owens, E. D. (1983). Visual detection of plants by herbivorous insects. Ann. Rev. Entomol. 28, 337–364.
Silva, D. B., Bueno, V. H. P., Van Loon, J. J. A., Peñaflor, M. F. G. V., Bento, J. M. S., and Van Lenteren, J. C. (2018). Attraction of three mirid predators to tomato infested by both the tomato leaf mining moth Tuta absoluta and the Whitefly Bemisia tabaci. J. Chem. Ecol. 44, 29–39. doi: 10.1007/s10886-017-0909-x
Song, B. M., and Lee, C. H. (2018). Toward a mechanistic understanding of color vision in insects. Front. Neur. Circ. 12:16. doi: 10.3389/fncir.2018.00016
Su, Q., Preisser, E. L., Zhou, X. M., Xie, W., Liu, B. M., Wang, S. L., et al. (2015). Manipulation of host quality and defense by a plant virus improves performance of whitefly vectors. J. Econ. Entomol. 108, 11–19. doi: 10.1093/jee/tou012
Tamiru, A., Bruce, T. J., Woodcock, C. M., Caulfield, J. C., Midega, C. A., Ogol, C. K., et al. (2011). Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett. 14, 1075–1083. doi: 10.1111/j.1461-0248.2011.01674.x
Teng, X., Wan, F. H., and Chu, D. (2010). Bemisia tabaci biotype Q dominates other biotypes across China. Florida Entomol. 2010, 363–368.
Turlings, T. C. J., and Erb, M. (2018). Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Ann. Rev. Entomol. 63, 433–452. doi: 10.1146/annurev-ento-020117-043507
Wan, Y., Hussain, S., Merchant, A., Xu, B., Xie, W., Wang, S., et al. (2020). Tomato spotted wilt orthotospovirus influences the reproduction of its insect vector, western flower thrips, Frankliniella occidentalis, to facilitate transmission. Pest Manag. Sci. 76, 2406–2414. doi: 10.1002/ps.5779
Wang, R. L., Zhu-Salzman, K., Elzaki, M. E. A., Huang, Q. Q., Chen, S., Ma, Z. H., et al. (2019). Mikania micrantha wilt virus alters insect vector’s host preference to enhance its own spread. Viruses 11:336. doi: 10.3390/v11040336
Wang, S. F., Guo, H. J., Ge, F., and Sun, Y. C. (2020). Apoptotic neurodegeneration in whitefly promotes the spread of TYLCV. eLife 9:e56168. doi: 10.7554/eLife.56168
Wang, X. R., Wang, C., Ban, F. X., Ghanim, M., Pan, L. L., Qian, L. X., et al. (2020). Apoptosis in a whitefly vector activated by a Begomovirus enhances viral transmission. mSystems 5, e433–e420. doi: 10.1128/mSystems.00433-20
Whitfield, A. E., Falk, B. W., and Rotenberg, D. (2015). Insect vector-mediated transmission of plant viruses. Virology 479-480, 278–289. doi: 10.1016/j.virol.2015.03.026
Wu, X. X., Hu, D. X., Li, Z. X., and Shen, Z. R. (2002). Using RAPD-PCR to distinguish biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) in China. Insect Sci. 9, 1–8. doi: 10.1111/j.1744-7917.2002.tb00147.x
Zang, L. S., Liu, Y. Q., and Liu, S. S. (2005). A new type of small leaf clip-cage for rearing whiteflies in experimental studies. Chin. Bull. Entomol. 42, 329–331.
Keywords: cucurbit chlorotic yellows virus, Bemisia tabaci, Cucumis sativus, volatile, vision, olfactory
Citation: Zhang Z, Zhang B, He H, Yan M, Li J and Yan F (2022) Changes in Visual and Olfactory Cues in Virus-Infected Host Plants Alter the Behavior of Bemisia tabaci. Front. Ecol. Evol. 10:766570. doi: 10.3389/fevo.2022.766570
Received: 29 August 2021; Accepted: 07 January 2022;
Published: 22 February 2022.
Edited by:
Adam Steinbrenner, University of Washington, United StatesReviewed by:
Alvin Simmons, Alvin Simmons, United StatesJustin George, Agricultural Research Service, United States Department of Agriculture (USDA), United States
George Broufas, Democritus University of Thrace, Greece
Freddy Ibanez-Carrasco, Texas A&M AgriLife Research and Extension Center at Weslaco, United States
Copyright © 2022 Zhang, Zhang, He, Yan, Li and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Li, ampsaUBoZW5hdS5lZHUuY24=; Fengming Yan, Zm15YW5AaGVuYXUuZWR1LmNu
 Zelong Zhang
Zelong Zhang Beibei Zhang
Beibei Zhang Fengming Yan
Fengming Yan