- 1College of Plant Protection, Hunan Agricultural University, Changsha, China
- 2Hunan Province Tobacco Company, Changsha, China
- 3Hunan Changsha Municipal Tobacco Company, Changsha, China
- 4Hunan Forestry Academy, Changsha, China
- 5Hunan Yongzhou Municipal Tobacco Company, Yongzhou, China
- 6College of Shaoyang Urban and Rural Construction, Shaoyang, China
Background: Arma custos Fallou (Hemiptera: Asopinae) is an important predatory insect native to China, South Korea, and Mongolia. It is important to understand the evolution of egg cannibalism in A. custos to evaluate the biocontrol potential of this species. However, few reports have suggested egg cannibalism in A. custos, and whether hungry adult A. custos males and females prey on their eggs remains unknown. Here, we investigated the effects of the parental sex of A. custos adults on egg cannibalism of parental and non-parental eggs (kinship) under no-choice and free-choice conditions, along with the effects of predator and egg density on egg cannibalism under starvation conditions.
Results: Females frequently visited and cannibalized a higher proportion of eggs, whereas males almost did not participate in egg cannibalism (less than 17% males showed egg cannibalism behavior). Moreover, regardless of their relationship with the egg, neither male nor female adults consumed all available eggs even in the absence of an alternative food source, and >70% of eggs remained unconsumed. In contrast, cannibalistic males and females did not discriminate between parental and non-parental egg types. Meanwhile, cannibalism rates were similar when adults were offered 30 eggs or more. However, when offered fewer than 30 eggs, cannibalism rates declined disproportionally, suggesting that limited egg availability reduced cannibalism. Additionally, the lifespan of A. custos adult females increased significantly with increasing number of consumed eggs (p < 0.05).
Conclusion: Arma custos females exhibit a higher tendency for egg cannibalism than males. Neither male or female A. custos discriminated between parental and non-parental egg types. Cannibalism enhances survival in that a starved individual who predates on eggs survives similarly to a well-fed individual. These findings provide a model to study the evolution and biological significance of egg cannibalism in A. custos and also contribute to the efficient mass rearing and realization of A. custos for biological control.
Introduction
Cannibalism, the consumption of conspecifics, is a behavioral trait observed in several animal species (Fox, 1975; Elgar and Crespi, 1992). Egg cannibalism, an important mechanism for self-regulating population size (Fox, 1975; Polis, 1981), is widespread in insects (Dobler and Kölliker, 2010; Parsons et al., 2013; Schultner et al., 2013; Bayoumy and Michaud, 2015; Jacobs and Stigall, 2019), including Hippodamia convergens (Guerin-Meneville) (Bayoumy and Michaud, 2015) and Tribolium confusum (du Val) (Parsons et al., 2013) of the order Coleoptera and Forficula auricularia L. (Dermaptera) (Dobler and Kölliker, 2010), Formica aquilonia Yarr. (Hymenoptera) (Schultner et al., 2013), Rhinocoris tristis Stal (Thomas and Manica, 2003), and Callicorixa producta Reut. of the order Hemiptera (Zalom, 1978). Although egg cannibalism reduces population size in insect species (Hamilton, 1964; Pfennig, 1997), it can be beneficial in the following ways: (1) by serving as the source of nutrients under starvation conditions (Pizzatto and Shine, 2008; Dobler and Kölliker, 2010); (2) by improving fitness, as predation on unrelated eggs decreases intraspecific competition (Fox, 1975; Polis, 1981; Vickery et al., 1988; Ichikawa, 1991); (3) by eliminating parasitized and infected eggs to improve offspring survival and development (Rohwer, 1978); (4) by increasing lifespan and boosting reproductive rate (Rohwer, 1978; Manica, 2002a).
Sex of the predator (Revynthi et al., 2018a,b) and kinship (Samu et al., 1999; Hoffman, 2012; Parsons et al., 2013) affect egg cannibalism in insects. The effect of the gender of the preying adult on egg cannibalism is species-specific. For instance, adult females of H. convergens (Bayoumy and Michaud, 2015), Coccinella undecimpunctata L. (Bayoumy et al., 2016), and Adalia bipunctata L. (Agarwala and Dixon, 1992) are more cannibalistic than conspecific males, whereas adult males of F. aquilonia (Schultner et al., 2013) are more cannibalistic than conspecific females. Moreover, some species avoid consuming their own eggs when non-parental eggs are available (Dobler and Kölliker, 2010, 2011; Parsons et al., 2013). For example, female red flour beetles (T. confusum) are more likely to consume non-parental eggs than their own eggs (Parsons et al., 2013), and European earwigs (F. auricularia) delay preying upon their juvenile offspring when unrelated juveniles are available (Dobler and Kölliker, 2010, 2011).
Arma custos (Fallou) (Hemiptera: Pentatomidae: Asopinae) (Zhao et al., 2018) is an important predaceous insect species native to China, Korea, and Mongolia (Rider and Zheng, 2002), which preys upon lepidopterans, coleopterans, hymenopterans, and hemipterans (Zheng and Chen, 1992; Gao et al., 2011; Zou et al., 2012, 2015). There are three stages in the lifecycle of A. custos, including egg, nymph (with five instars), and the adult (Zou et al., 2012). The developmental time of eggs, instar nymphs, and male and female adults are 6.43, 20.66, 37.25, and 44.18 days, respectively, and adult A. custos females oviposit approximately 20–30 eggs at one time with 90% egg hatchability (Zou et al., 2012). The first-instar nymphs of A. custos require only water, while other instar nymphs and adults require live insect larvae or pupae for growth and development and suck the body fluids of their prey using their proboscis (Wu et al., 2019, 2020). Notably, several organisms also feed upon the same prey item (Zou et al., 2012).
Many hemipteran insects, such as Arizona backswimmers (Frank) (Zalom, 1978), Triatoma sordida (Stal) and Triatoma infestans (Klug) (Ryckman, 1951), Arctocorisa carinata and Callicorixa producta (Pajunen and Pajunen, 1991), Xylocoris flavipes (Reuter) (Arbogast, 1979), Macrolophus pygmaeus (Ranmbur), and Dicyphus cerastii (Wagner), are known for their cannibalistic behavior, and adults prey on immature offspring (Duarte et al., 2021). Most previous studies have focused on the predation ability and predation behavior of A. custos (Gao et al., 2019; Li et al., 2020; Wang et al., 2020). Furthermore, A. custos could efficiently control Ambrostoma quadriimpressum (Motschulsky), Cnidocampa flavescens (Walk.) and Spodoptera exigua (Hübner) with an A. custos-to-pest ratio of 1:15 (Gao et al., 2019; Li et al., 2020; Wang et al., 2020). However, to the best of our knowledge, there have been no studies on egg cannibalism in A. custos. Moreover, whether the species exhibits significant egg cannibalistic behavior remains unknown.
In the laboratory, some A. custos adults, mostly females, prey on the eggs of other insects, suggesting that this species exhibits egg cannibalism with significant differences between males and females that may be shaped by some selection pressure. Furthermore, it may be difficult for the insect to find a suitable prey to survive in the wild. Thus, it is important to understand how male and female adults of this species select eggs (parental versus non-parental) for feeding and what proportion of eggs is consumed under starvation conditions. Additionally, the effects of gender and kinship on egg cannibalism have not been studied in this species. Therefore, further investigations will not only allow us to elucidate the mechanism of egg cannibalism in this species but also help to evaluate its suitability as a long-term pest biocontrol agent. It would also provide a model to study the evolution and biological significance of egg cannibalism in insects.
In view of the above observations, we hypothesized the following: (1) starved A. custos adults would cannibalize a significant proportion of the eggs provided; (2) adult females would exhibit significant egg cannibalism, whereas males would not; (3) A. custos would distinguish between parental and non-parental eggs; and (4) egg cannibalism would increase the survival rate of A. custos. To verify these hypotheses, we performed the following experiments: (1) Evaluation of the probability of cannibalism exhibited by A. custos male and female adults on parental and non-parental eggs under selection and non-selective conditions; (2) Evaluation of the probability of egg cannibalism in adult males and females under different prey and predator densities; (3) Comparison of lifespan of cannibalistic and non-cannibalistic adults.
Materials and Methods
Insect Rearing and Experimental Conditions
Arma custos adults were collected from the wild in Langfang, Hebei Province, China, in 2018 and reared in artificial climate boxes in the laboratory, as previously described by Pan et al. (2019). The insects were fed with Chinese oak silk moth, Antherea pernyi (Guérin-Méneville) (Lepidoptera: Saturniidae) pupae purchased from a supermarket in Liaoning, China. After hatching, first-instar A. custos nymphs from individual egg mass were placed in transparent plastic dishes (10 ×1.5 cm2) and fed only with water, using a piece of moist absorbent cotton. Chinese oak silk moth pupae were provided to second-instar nymphs, which were replenished every 4–5 days (Pan et al., 2019).
Newly emerged adults (<6 h old) were paired, placed in 6 ×10 cm2 Petri dishes containing one Chinese oak silk moth pupa and lined with a paper tube (diameter: 1 cm; height: 6 cm), and allowed to mate. After 4–5 days, the eggs laid in the paper tube were used for the experiments within 24 h, and the remnants of the moth pupa were removed.
We assessed the cannibalistic behavior of adult A. custos under laboratory conditions by placing freshly laid eggs (<24 h old) in small plastic dishes (10 ×1.5 cm2) covered with an insect-proof screen (80-μm mesh) for ventilation. All adults used in the experiments were 8 days old and starved for 24 h. The females used in the experiments had laid eggs, and the males had mated. All experiments were performed at 25 ± 2°C, 60 ± 10% RH, under a photoperiod of 16:8 h (L:D). No additional food was supplied to the adults during the observation period.
Adult females of A. custos typically lay batches of 20–40 eggs, and the findings of a preliminary test indicated that the adults of this species consume a daily average of 1 s-instar beet armyworm larva, the weight of which is equivalent to that of 30 eggs. We accordingly conducted free-choice and no-choice experiments using a predator-to-egg ratio of 1:30 and used the same proportions to analyze the effects of different egg and predator densities on egg cannibalism in A. custos.
Observation of Egg Cannibalism in Arma custos
To establish whether A. custos adults simply break eggshells or break eggshells and consume the egg contents, we monitored the duration of male and female cannibalism on parental and non-parental eggs based on 30 replicate video observations (Sony, FDR-AX700, Japan). Eggs with broken shells were considered to have been cannibalized, and the number of replicates with cannibalized eggs was used to determine the extent of cannibalism.
Egg Cannibalism in Arma custos Under No-Choice and Free-Choice Conditions
For the food-choice experiments, eggs were monitored under a 40× magnifying glass for 5 min post-release and every 15 min thereafter, during the first 48 h. After 48 h, the eggs were monitored every 3 h for 7 days. The number of consumed and unconsumed eggs (broken eggshells) was counted. The number of replicates with evident egg cannibalism were used to determine the probability of egg-residual behavior in male and female adults, and the number of eggs consumed by A. custos under each food choice condition were used to estimate the strength of female and male egg cannibalism.
In addition, the number of replicates with cannibalized eggs and the number of consumed eggs after every 6 h were used to assess the effects of the incubation period on egg cannibalism in A. custos. Subsequently, the remaining eggs not eaten by male or female adults were recorded.
No-Choice Test
To determine whether A. custos adults feed on eggs regardless of kinship (parental or non-parental) with the eggs, 30 eggs were placed in each plastic dish, and no-choice tests were conducted using the following solitary adults: parental female, parental male, non-parental female, and non-parental male. In the parental treatments, the eggs were the parental offspring of the predatory adults, whereas in the non-parental treatments, the eggs were non-parental to the predatory adults.
Free-Choice test
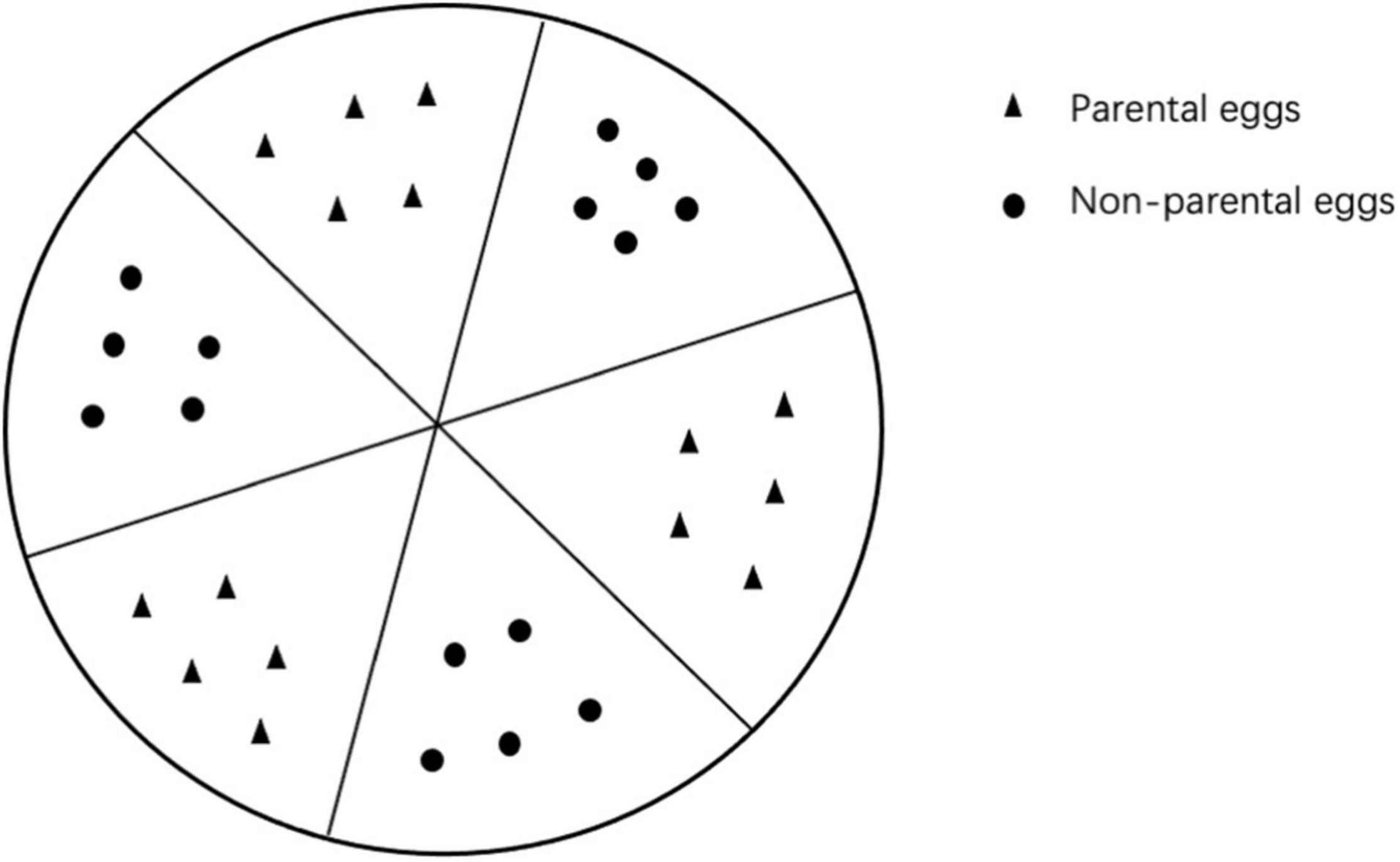
At the beginning of the free-choice experiments, we drew six flabellate grids on the Petri dishes (Figure 1), which divided the dishes into six equal parts. To test whether A. custos preferentially preyed on its parental eggs when allowed to freely choose between them and non-parental eggs, either a male or a female adult was placed in a plastic dish containing 15 parental and 15 non-parental eggs. The eggs from the same parent were evenly divided into three groups of five eggs each and then placed on non-adjacent sections of the grid. Similarly, 15 eggs from a different set of parents were placed on non-adjacent grid sections in groups of five, as shown in Figure 1. Each treatment combination was replicated 30 times and consisted of one adult, male or female, exposed to 30 conspecific eggs of mixed kinship.
Density-Dependent Egg Cannibalism in Arma custos
We previously observed that A. custos adults do not consume all available eggs and always cannibalize the same number of eggs irrespective of the number of eggs available to feed on. Therefore, we tested the following two hypotheses. (1) Egg cannibalism in A. custos adults does not change with varying number of eggs; (2) Egg cannibalism in A. custos improves with increasing number of adults.
Varying Number of Eggs Test
Each female A. custos lays 10–60 eggs at one time; therefore, the egg-density experiments comprised 14 treatments (i.e., a 2 × 7 factorial design), wherein one female or one male was placed in the experimental Petri dish arena and exposed to either 1, 10, 20, 30, 40, 50, or 60 non-parental eggs. All the adults used in these experiments were used only once and observed for 7 days.
Varying Number of Adult Arma custos Test
In nature, multiple adults may randomly attack the eggs at one place at the same time. Hence, in the predator-density experiments, 1, 2, 4, 6, 8, or 10 A. custos females or males were placed in plastic dishes containing 30 non-parental eggs, and egg cannibalism was observed under artificial conditions. Each density treatment consisted of 30 replicates, and each dish was considered an experimental replicate. All the adults used in these experiments were used only once and observed for 7 days.
For both the density experiments, the number of eggs cannibalized by A. custos adults in each replicate were used to evaluate the effect of egg or predator density on egg cannibalism in A. custos. Moreover, the number of eggs consumed by A. custos were recorded to reveal whether the number of eggs consumed by the adults increases linearly with the increase in egg or predator density. Subsequently, the remaining eggs from the male and female adult tests were recorded.
Effect of Egg Cannibalism on the Lifespan of Arma custos
We assumed that egg cannibalism would supply energy for A. custos to understand its effect on the lifespan of A. custos. Furthermore, we assumed that the lifespan of A. custos who cannibalized eggs would be longer than those that did not. Therefore, we observed the lifespan of female and male A. custos under the following conditions: (1) Supplying 1-day food for female or male A. custos; (2) Female or male A. custos does not cannibalize eggs; and (3) Female or male A. custos cannibalize eggs. Each treatment involved 30 replicates with 30 different adults.
Supplying One-Day Food Test
One female or one male was placed in the experimental Petri dish arena after being starved for 24 h. Chinese oak silk moth was provided as food for 1 day. Then, food was not supplied to female or male A. custos until they died. The lifespan of female or male A. custos was recorded. Each treatment involved 30 replicates with 30 different adults.
Non-cannibalized and Cannibalized Test
One female or one male was placed in the experimental Petri dish arena after being starved for 24 h. Then, 30 normal eggs were provided as food until female or male A. custos died. If yes, it becomes part of the cannibalized group; If no, it becomes part of the non-cannibalized group. The lifespan of female or male A. custos were recorded. Each treatment involved 30 replicates with 30 different adults. Meanwhile, most female or male A. custos did not cannibalize eggs, but we want to know the lifespan of female or male A. custos, respectively, under the cannibalized group and the non-cannibalized group in 30 replicates. So, we only observed 30 times for each treatment. Although, more female or male A. custos did not cannibalize eggs when we observed the lifespan of the cannibalized group, we no longer record their lifespans, because we have got 30 replicates.
Statistical Analyses
Chi-square analyses were performed to compare the cannibalistic behavior of adult males and females and test whether kinship affected egg cannibalism in the two genders under different conditions. An analysis of variance was performed to assess the differences in egg consumption, the ratio of remaining eggs, and the lifespan of male and female A. custos. Bartlett’s test was used to test the homogeneity of variances, and the square root transformation (sqrt) was calculated to analyze datasets when the variance was heterogeneous, with p < 0.05 being significant. Multiple comparisons of the lifespan of cannibalistic and non-cannibalistic male and female A. custos adults were performed using Tukey’s honest significant difference (HSD) tests between male and female A. custos egg consumption and egg emergence ratio. Benjamini–Hochberg adjustment was used to control false discovery rates (FDRs) for such multiple comparisons, with q < 0.05 being significant (Lee, 2016; Lee and Lee, 2018). Furthermore, a generalized linear model (GLM) using Poisson’s distribution was used to evaluate the relationships of male and female A. custos lifespans with the number of consumed eggs. All statistical analyses were performed using R v.3.3.3 (R Development Core Team, 2017).
Results
Egg Cannibalism in Arma custos
We observed that the egg-puncturing behavior exhibited by A. custos adults was followed by egg cannibalism, which continued for 65 s (Figures 2, 3). The adults used their proboscis and not their legs or other body parts to puncture the eggshell (Figure 2). After puncturing, eggs protein were eaten and a silver-white eggshell was left out (Figure 2). The normal eggs were integral, round, smooth, and yellow (Figure 3A) and the cannibalized eggs were tattered, dim, shrunken, and silver-white (Figure 3B). Moreover, egg cannibalism in the adults was neither affected by kinship nor by the sex of the predator (Tukey’s HSD test, p > 0.4; Figure 4).

Figure 2. Arma custos adult consuming eggs. After being cannibalized by the proboscis of A. custos, eggs protein were eaten and a silver-white eggshell was left out.
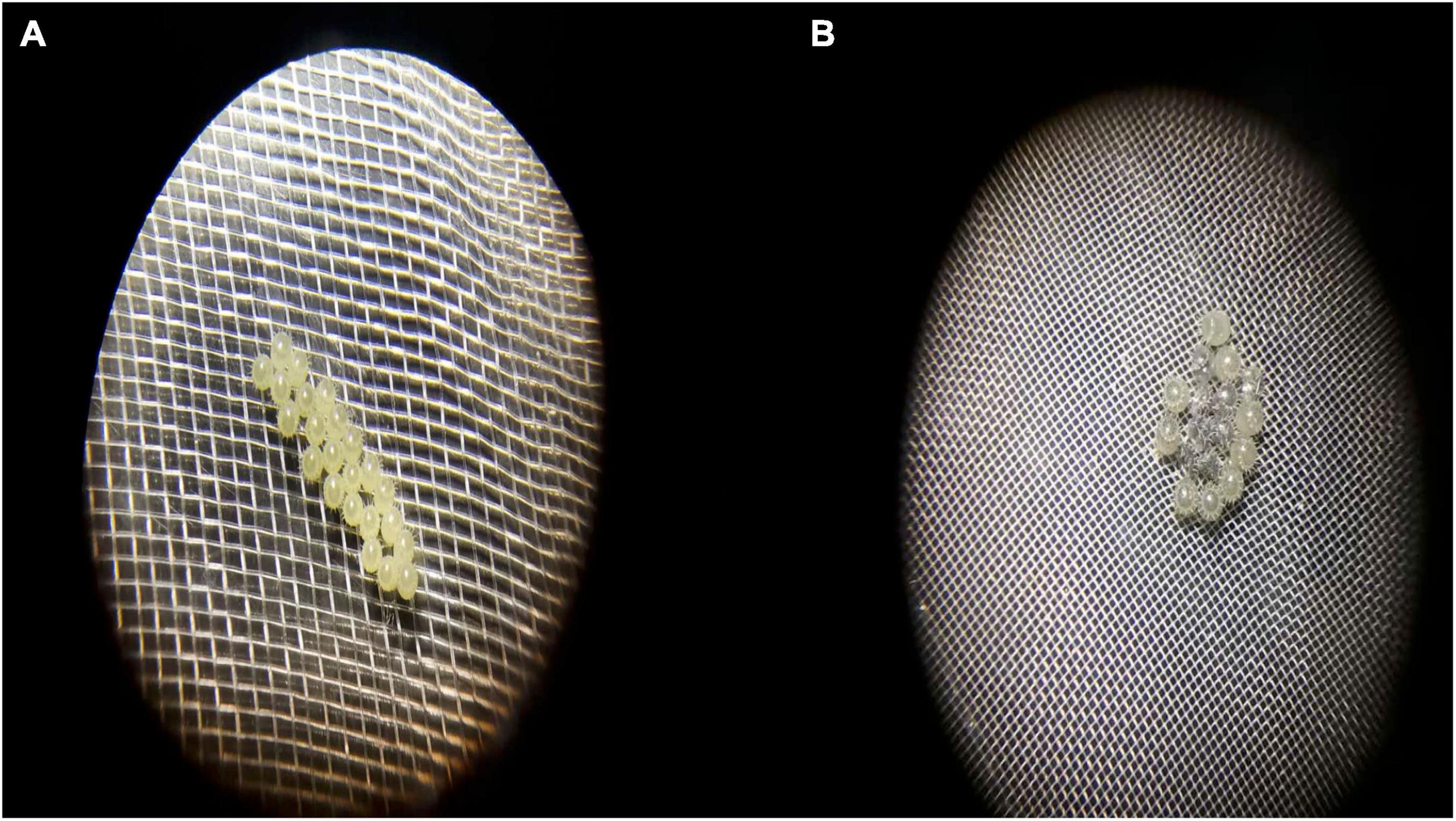
Figure 3. Normal eggs (A) and cannibalized eggs (B). The normal eggs were integral, round, smooth, and yellow, the cannibalized eggs were tattered, dim, shrunken, and silver-white.
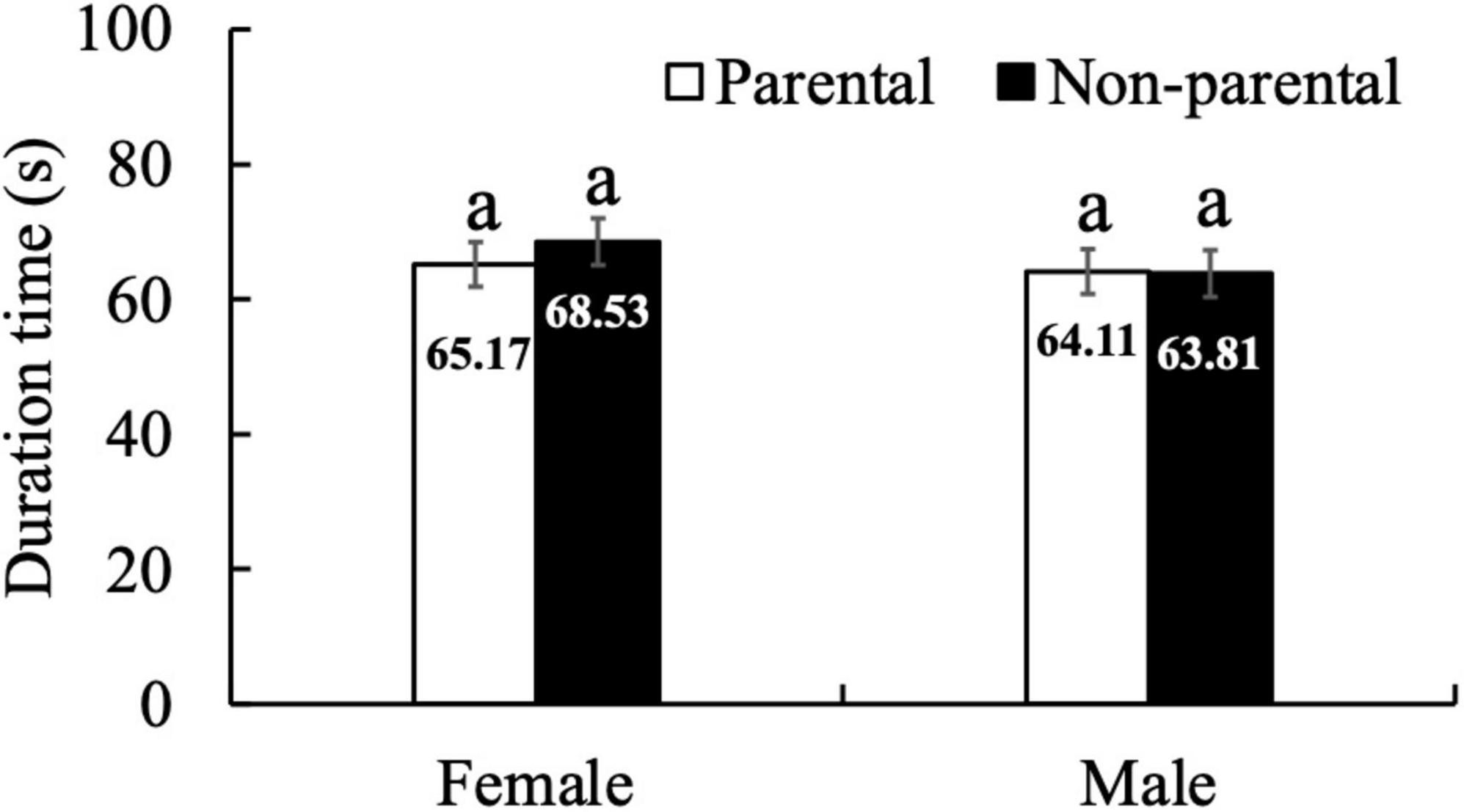
Figure 4. Duration times of egg cannibalism in A. custos adults upon incubation with parental and non-parental eggs. Lowercase letters indicate significant differences (p < 0.05). Thirty replicates were used for each experimental condition.
Egg Cannibalism in Arma custos Under No-Choice and Free-Choice Conditions
Kinship had no effect on egg cannibalism exhibited by A. custos adults (Figure 5). Of the 30 no-choice experimental replicates, adult females preyed on kin eggs in 16 replicates and on non-parental eggs in 18 replicates (Figure 5A), whereas, adult males preyed upon kin eggs in four experimental replicates and on non-kin eggs in four replicates (Figure 5A). Chi-square analysis showed that the differences observed for kin and non-kin eggs were not significant in both males (c2 = 0, df = 1, p = 1) and females (c2 = 0.07, df = 1, p = 0.79). Similarly, in the 30 free-choice experimental replicates, the number of replicates in which female adults preyed upon kin eggs (15) was not significantly different from the number of replicates in which the females preyed upon the non-kin eggs (16) (c2 = 0, df = 1, p = 1; Figure 5B). Moreover, there was no significant difference between the number of replicates wherein adult females preyed upon kin eggs (5) and those in which adult males preyed on non-parental eggs (4) (c2 = 0, df = 1, p = 1; Figure 5B).
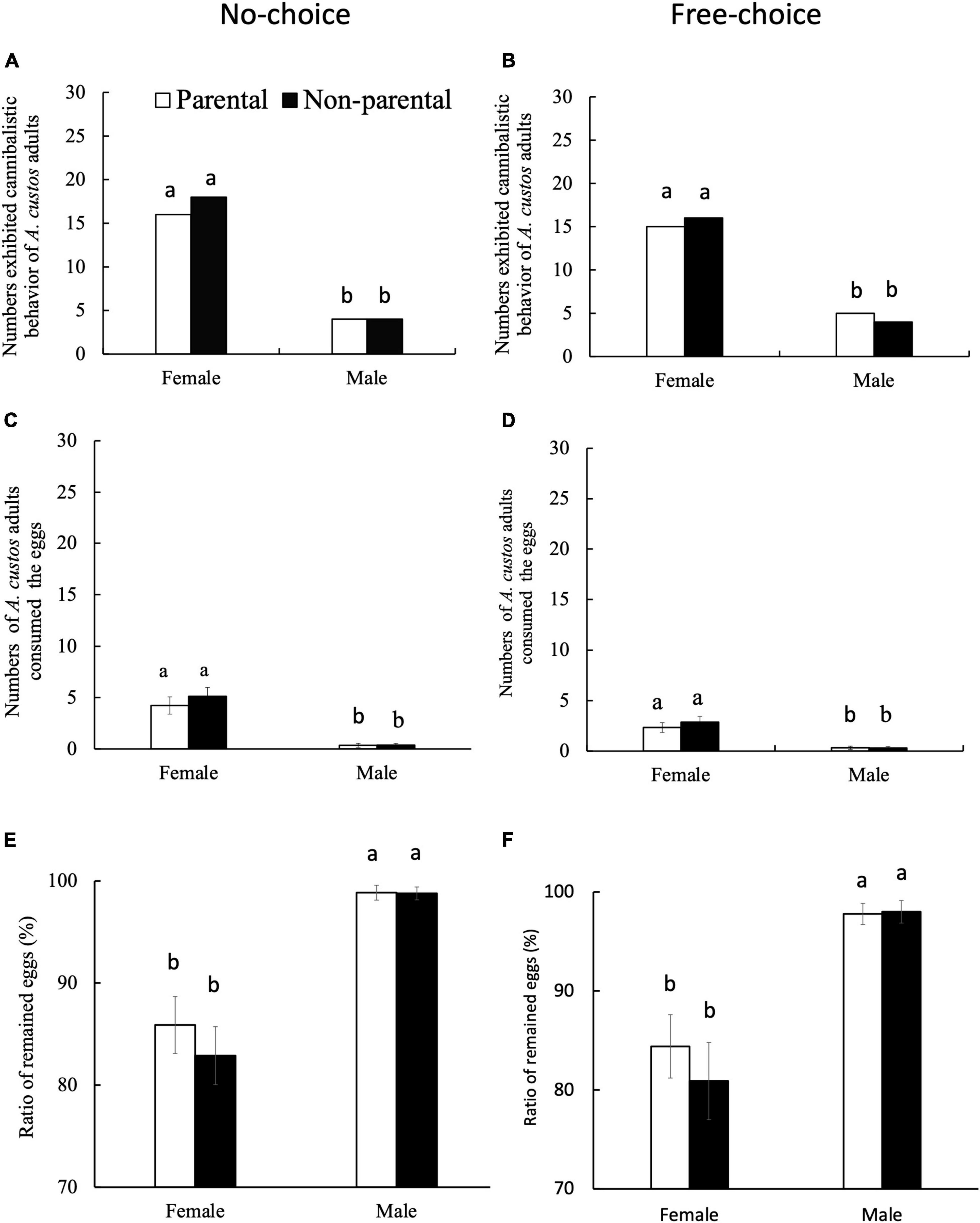
Figure 5. Egg cannibalism exhibited by female and male A. custos adults incubated under no-choice and free-choice conditions for 7 days. Under the no-choice condition, each A. custos adult was either incubated with 30 parental or 30 non-parental eggs, whereas under the free-choice condition, each adult was simultaneously incubated with 15 parental and 15 non-parental eggs. Different lowercase letters on pillars indicate significant differences (p < 0.05). A total of 30 replicates were used for each food-choice experiment. Numbers exhibited cannibalistic behavior of A. custos adults in No-choice (A) and Free-choice (B) conditions. Numbers of A. custos adults consumed the eggs in No-choice (C) and Free-choice (D) conditions. Ratio of remained eggs in No-choice (E) and Free-choice (F) conditions.
Chi-square analysis revealed that sex-based differences in predator behavior were significant between parental and non-parental egg types under both the food choices (Figures 5A,B), wherein A. custos females more frequently preyed upon eggs than conspecific males under no-choice (parental eggs: c2 = 5.81, df = 1, p < 0.05; non-parental eggs: c2 = 4.31, df = 1, p < 0.05) and free-choice (parental eggs: c2 = 4.69, df = 1, p < 0.05; non-parental eggs: c2 = 7.33, df = 1, p < 0.05) conditions (Figures 5A,B).
Moreover, out of 30 males, only four exhibited cannibalism (13.33%) of either parental or non-parental origin (Figures 5A,B); out of 30 females, only 16 in the parental (53%) test and 18 (60%) in the non-parental test exhibited cannibalism (Figures 5A,B). Egg cannibalism by neither sex was altered by the origin of the eggs (Figure 5). In addition to frequent predation, adult females consumed significantly more eggs (4.23–5.13) than adult males (0.34–0.37) under no-choice conditions, irrespective of egg origin (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figures 5C,D). Similarly, under free-choice conditions, adult females consumed a significantly larger number of eggs (2.33–2.86) than adult male (0.30–0.33), irrespective of origin (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 5B). However, <20% of the available eggs were consumed by both sexes, and >80% of the available eggs remained under both no-choice and free-choice conditions with female and male adults (Figures 5E,F). Furthermore, the ratios of remaining eggs with males were significantly higher than those with females under both no-choice and free-choice conditions (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figures 5E,F).
Under both the no-choice and free-choice conditions, no differential preference for parental and non-parental eggs was observed over the study period at any moment (Chi-square: All, p > 0.05; Figures 6A,B). Meanwhile, the number of consumed parental eggs was also not significantly different from that of non-parental eggs under the experimental conditions at any moment (Tukey’s HSD test: p > 0.05; Figures 6C,D).
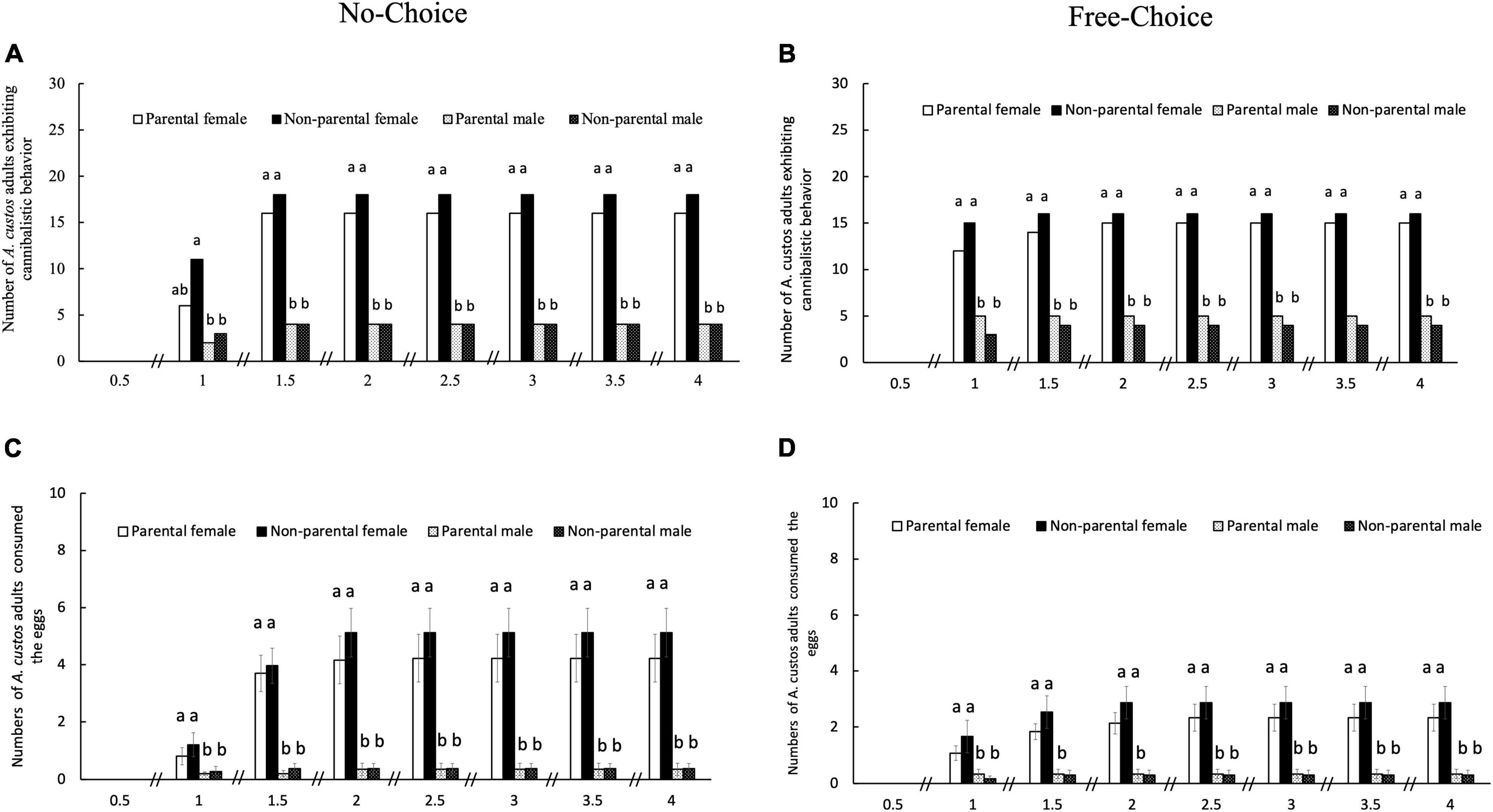
Figure 6. Real-time egg cannibalistic behavior observed in male and female A. custos adults for 4 days at different times. Cannibalistic behavior did not change after 4 days of incubation with eggs. Under the no-choice condition, each A. custos adult was incubated with 30 parental or 30 non-parental eggs, whereas under the free-choice condition, each adult was simultaneously incubated with 15 parental and 15 non-parental eggs. A total of 30 replicates were used for each food choice. Different lowercase letters on pillars indicate significant differences (p < 0.05). A total of 30 replicates were used for each food-choice experiment. Numbers of A. custos adults exhibiting cannibalistic behavior in no-choice (A) and free-choice (B) condition. Numbers of A. custos adults consumed the eggs in no-choice (C) and free-choice (D) condition.
Furthermore, under both no-choice and free-choice conditions, egg cannibalism in adults was observed to be more prominent within the initial 48 h (2 day) of incubation with eggs. No adult preyed eggs in the first 12 h (0.5 day) of all tests. Both female and male adults preyed on eggs during 12–36 h (0.5–1.5 day) of no-choice tests [Chi-square of 12 vs. 36 h (0.5 vs. 1.5 days): Parental female, c2 = 19.18, df = 1, p < 0.05; Nonparental female, c2 = 22.94, df = 1, p < 0.05; Parental male, c2 = 2.41, df = 1, p = 0.12; Nonparental male, c2 = 2.41, df = 1, p = 0.12; Figures 7A,B] and free-choice tests [Chi-square of 12 vs. 36 h (0.5 vs. 1.5 days): Parental female, c2 = 15.75, df = 1, p < 0.05; Nonparental female, c2 = 19.18, df = 1, p < 0.05; Parental male, c2 = 3.49, df = 1, p = 0.06; Nonparental male, c2 = 2.41, df = 1, p = 0.12; Figures 7A,B]. None of the adults consumed eggs after 48 h (2 day) under both no-choice and free choice conditions (Tukey’s HSD test: All, p > 0.05; Figures 7C,D).
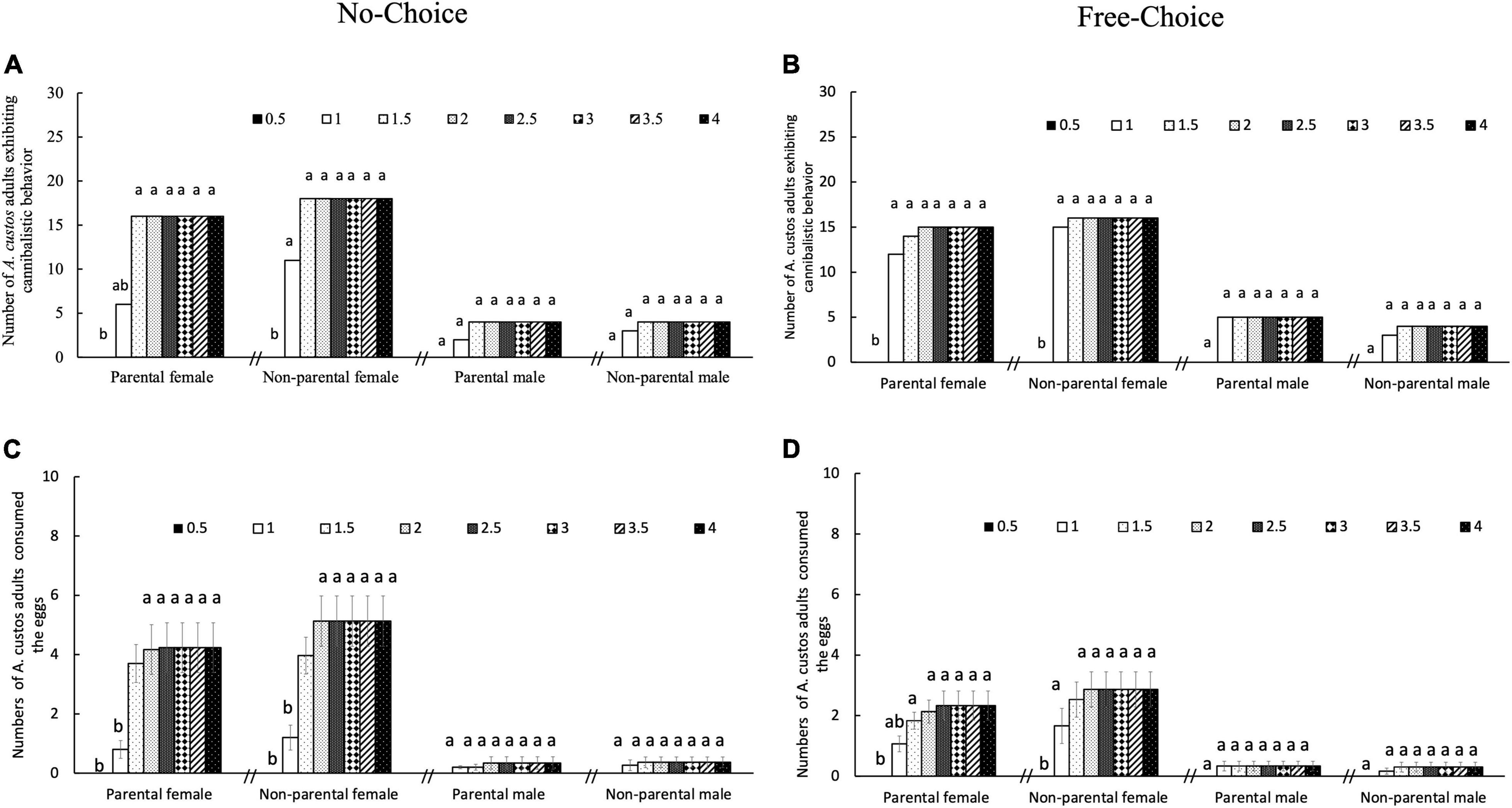
Figure 7. Egg cannibalistic behavior of female and male A. custos adults under no-choice and free-choice conditions at different times for 4 days. Cannibalistic behavior did not change after 4 days of incubation with eggs. Under the no-choice condition, each A. custos adult was incubated with 30 parental or 30 non-parental eggs, whereas under the free-choice condition, each adult was simultaneously incubated with 15 parental and 15 non-parental eggs. A total of 30 replicates were used for each food choice. Different lowercase letters on pillars indicate significant differences (p < 0.05). A total of 30 replicates were used for each food choice experiment. Numbers of A. custos adults exhibiting cannibalistic behavior in no-choice (A) and free-choice (B) condition. Numbers of A. custos adults consumed the eggs in no-choice (C) and free-choice (D) condition.
Density-Dependent Egg Cannibalism in Arma custos
We also studied the effects of different densities of non-parental eggs and predators on egg cannibalism in A. custos (Figure 8). We observed no significant differences in the number of replicates in which females exhibited egg cannibalism (c2 = 0, df = 1, p = 1) and the number of consumed eggs (Tukey’s HSD test: p > 0.05) between the replicates with 30 and >30 non-parental eggs (Figures 8A,C). Similar results were obtained when adult males were incubated with >30 non-parental eggs (Figures 8A,C). However, when the number of eggs was reduced, egg cannibalism exhibited by A. custos adults decreased significantly, especially when the number of eggs was reduced from 30 to 10 (for all 30 replicates), wherein only eight females showed egg cannibalism (c2 = 4.39, df = 1, p < 0.05) and the average number of eggs consumed was 1.4 (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figures 8A,C).
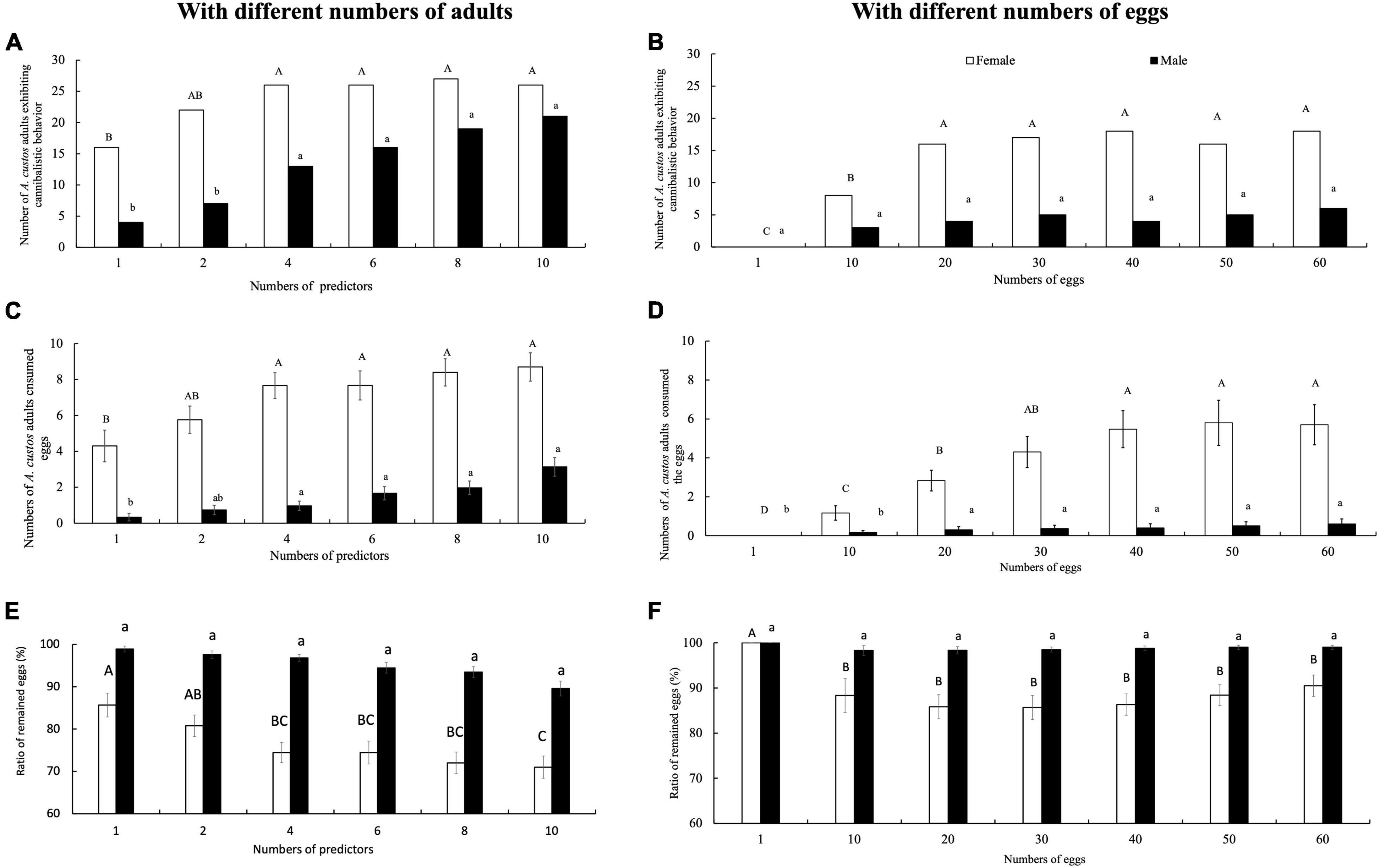
Figure 8. Egg cannibalistic behavior exhibited by A. custos adults incubated at different egg or predator densities. Different uppercase letters indicate significant differences among females (p < 0.05), different lowercase letters indicate significant differences among males (p < 0.05). Panels (C,D) indicate the number of consumed eggs overall individual. A total of 30 replicates were used for each experiment. Numbers of A. custos adults exhibiting cannibalistic behavior in No-choice (A) and Free-choice (B) condition. Numbers of A. custos adults consumed eggs in No-choice (C) and Free-choice (D) condition. Ratio of remained eggs in No-choice (E) and Free-choice (F) condition.
Similarly, when only one egg was supplied, neither the adult males nor females showed egg cannibalism throughout the study period (7 days), the eggs remained intact, and 93% of them hatched into first-instar nymphs (Figures 8A,C), suggesting that limited egg availability reduces egg cannibalism in this species. In contrast, despite the greater availability of eggs, no more than 14.0 and 2.6% of the available eggs were consumed by A. custos females and males, respectively, and more than 85% of the available eggs remained (Figure 8E). Moreover, the numbers of eggs consumed by adult predators was significantly lower than the number of unconsumed eggs (p < 0.05), irrespective of the number of available eggs (Figure 8E).
When the number of predators was increased, the number of replicates with egg cannibalism also increased (Figure 8B). Chi-square analysis revealed that when the number of predators increased four times, there were significant differences in egg cannibalism in both males (c2 = 3.89, df = 1, p < 0.05) and females (c2 = 6.43, df = 1, p < 0.05; Figure 8B). Meanwhile, when the number of predators increased four times, the number of consumed eggs was also significantly different in both males (Tukey’s HSD test: 1 vs. 4 adults, p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05) and females (Tukey’s HSD test: 1 vs. 4 adults, p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 8D). However, when the number of predators increased six, eight, and 10 times, there were no significant differences in egg cannibalism in both males (Chi-square of 6, 8, and 10 adults, respectively, with four adults: All, p < 0.05) and females (Chi-square of 6, 8, and 10 adults, respectively, with four adults: All, p < 0.05; Figure 8D). Furthermore, the residual food intake in females at densities of 4, 5, 6, 8, and 10 was significantly higher than that of a single A. custos female (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 8D). Similarly, the residual food intake by males at densities of 4, 5, 6, 8, and 10 was also significantly higher than that of a single A. custos male (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 8D).
In addition, despite the increasing number of predators and the subsequent increasing chances of egg cannibalism, the number of consumed eggs was consistently low for both males (9.5%) and females (28.7%), and more than 70% of the available eggs remained (Figure 8F). These findings suggest that high-density groups decrease egg cannibalism exhibited by a solitary A. custos adult.
Effect of Egg Cannibalism on the Lifespan of Arma custos
Egg cannibalistic behavior significantly affected the lifespan of male and female A. custos adults (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 9A). Linear regression analysis revealed that the number of consumed eggs positively correlated with the lifespan of adult A. custos (males: F = 215.73, p < 0.05; females: F = 394.60, p < 0.05; Figure 9B). However, there was no significant difference in the lifespan of non-cannibalistic adult males (10.08 days) and females (10.59 days) (Tukey’s HSD test: p = 0.99; Figure 9A), whereas the lifespan of cannibalistic A. custos adults were significantly increased (Figure 9B) and females’ lifespan were much longer than that of males (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 9A). Furthermore, the lifespan of cannibalistic males and females were not significantly different from those of the adults provided with 1-day food (Tukey’s HSD test: p < 0.05; Benjamini–Hochberg adjustment of FDR: q < 0.05; Figure 9A), indicating that egg availability contributed to the longevity of male and female A. custos adults.
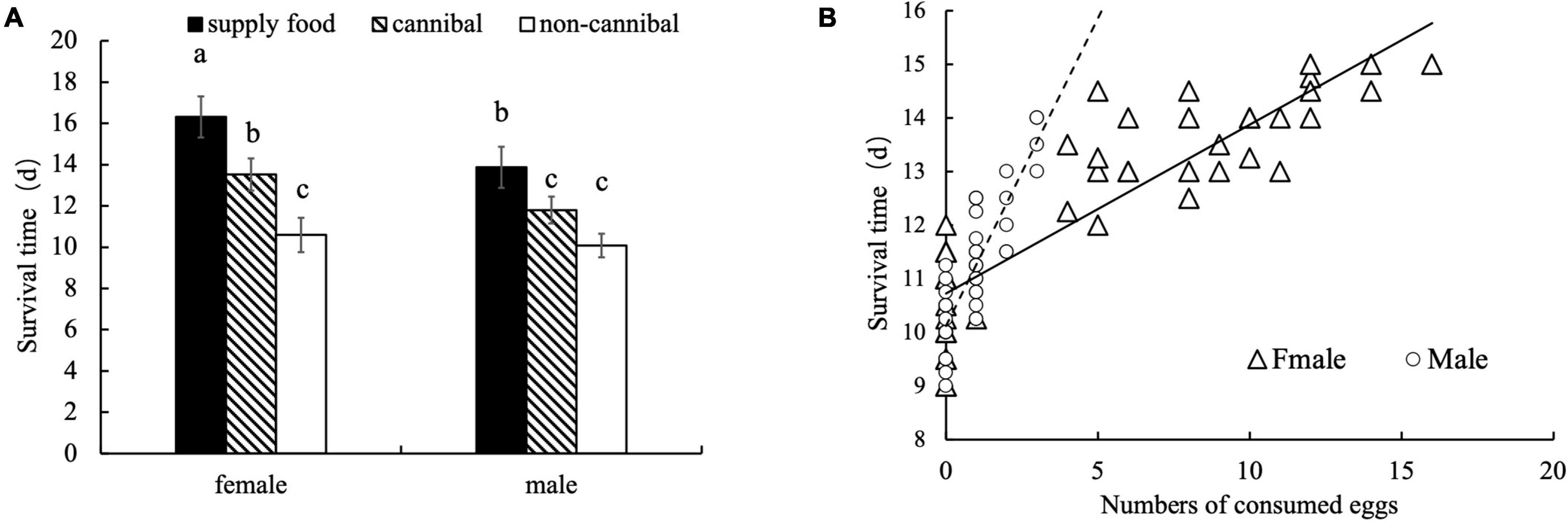
Figure 9. Effect of egg cannibalism on the lifespan of male and female A. custos adults provided with sufficient eggs (number of eggs = 30). Different lowercase letters indicate significant differences (p < 0.05). Survival time of female and male A. custos adults in supply food, cannibal and non-cannibal conditions (A). Survival time of female and male A. custos adults as numbers of consumed eggs (B).
Discussion
Although A. custos is widely used for biocontrol of pests, egg cannibalistic behavior of this species has not yet been investigated. Therefore, we comprehensively and systematically studied the effects of gender of the predator and kinship on egg cannibalism in A. custos and showed that kinship does not affect egg cannibalism, while egg cannibalism is significantly affected by the gender of the predatory adult. We also showed that egg cannibalism in A. custos is markedly affected by the number of eggs and predators present at one location.
We observed that only a small proportion of A. custos adults exhibited egg cannibalism, which was not significant; 86.77% of male adults did not consume eggs of either parental or non-parental origin, 40% and 47% of female adults did not consume eggs of parental and non-parental origin, respectively, and the number of eggs consumed by A. custos was insignificant. This may be because the eggs release hormones or substances that inhibit insect feeding (Narasimha et al., 2019) or because adults regulate their diets (Polis, 1981; Smith and Reay, 1991; Manica, 2002b). The small number of adults exhibiting egg cannibalism also indicates the existence of such a regulatory mechanism.
Thomas and Manica (2003) reported that although R. tristis cannibalizes its own eggs, it can selectively prey on parasitized eggs. However, in the present study, no parasitized eggs were used. Moreover, Okada et al. (2015) reported that hungry Andrias japonicus preys on unfertilized and dead eggs; however, in our study, only two-third of the females exhibited egg cannibalism under similar conditions. Therefore, these studies could not explain the results of the present study. It is unclear whether A. custos adults only prey on unfertilized eggs. Zou et al. (2012) observed that virgin insect females also lay eggs, indicating that the eggs eaten by the adults may either be vegetative, which cannot develop into adults (Perry and Roitberg, 2006) or unfertilized eggs (Mrowka, 1987). For example, mouthbrooding females of Pseudocrenilabrus multicolor consume unfertilized eggs for the first few days after spawning (Mrowka, 1987). However, we do not know whether the eggs consumed by the female and male A. custos were unfertilized. In future, unfertilized and fertilized eggs should be used to test the egg cannibalism behavior of female and male A. custos toward fertilized eggs, which would help us to further identify whether A. custos adults only prey on unfertilized eggs.
Rohwer (1978) showed that males prey on eggs for nutrition and longer lifespan, thereby, taking care of the subsequent generation, and providing their offspring with better conditions for growth and development. Rohwer (1978) has suggested that insects selectively consume a small number of eggs to obtain sufficient nutrition and better opportunities for reproduction, which tends to be consistent with the findings of the present study, wherein we observed that adults cannibalized their own eggs by using their mouthparts, and that females consuming a larger number of eggs are generally characterized by a longer lifespan, thereby enhancing reproductive opportunities. Furthermore, A. custos females lay eggs multiple times during their lifetime (Zou et al., 2012), and we observed that cannibalistic females of this species lived longer than their non-cannibalistic counterparts.
The evolution of cannibalism is driven by the balance between its benefits and costs (Hamilton, 1964; Rudolf et al., 2010). One evident benefit of egg cannibalism is starvation avoidance (Pizzatto and Shine, 2008; Dobler and Kölliker, 2010). Moreover, selective cannibalism provides an alternative food source for adult insects, while ensuring the survival of most of their offspring and maintaining the population size (Polis, 1981; Smith and Reay, 1991; Manica, 2002b). Our study revealed that hungry A. custos adults do not prey on all the available eggs (>70% of the eggs were unconsumed under high predator density) irrespective of the predator or egg density, and adult males exhibit minimal egg cannibalism. Moreover, when the egg density is extremely low, the egg cannibalistic behavior exhibited by A. custos reduces significantly; none of the adults exhibited egg cannibalism when only one egg was available.
Although more A. custos adults participated in egg cannibalism under high predator density, the percentage of eggs consumed by A. custos adults was less than 30%. Previous studies suggest that a solitary male or female A. custos adult can consume 1 s-instar beet armyworm larva per day, and adult A. custos remain alive for at least 7 days. In the present study, although A. custos adults were hungry for the first 48 h, all adults did not consume eggs after 48 h, suggesting that A. custos exhibits a cannibalism-regulating mechanism irrespective of the selection pressure to ensure the survival of most of its eggs and maintain the population size (Hamilton, 1964; Pfennig, 1997). Meanwhile, it is also possible that adults interfere with each other as predator density increases, and this may limit cannibalism in some way. In future, we will study the relationship between density and cannibalism in A. custos adults.
Kin-killing behavior is observed when the nutritional profits outweigh the costs of decreased inclusive fitness (Pfennig, 1997). Although strategies for avoiding cannibalism have been studied in many taxa (Samu et al., 1999; Siegel et al., 2007; Widdig, 2007; Edenbrow and Croft, 2012; Parsons et al., 2013; Bayoumy and Michaud, 2015; Garza and Waldman, 2015; Ringler et al., 2017), little is known about the mechanisms regulating the predatory behavior of hemipterans (Arbogast, 1979; Pajunen and Pajunen, 1991; Agarwala and Dixon, 1992). Most arthropods avoid consuming their own kin when given the choice (Dobler and Kölliker, 2010, 2011; Parsons et al., 2013). Female C. undecimpunctata avoided cannibalizing their own eggs and preferentially consumed non-filial eggs in a choice-based study (Bayoumy and Michaud, 2015). Female A. bipunctata recognize and avoid eating their own eggs (Dumont et al., 2020), and female H. convergens recognize their own egg clusters, sometimes even non-kin eggs added to them, and preferentially cannibalize non-filial clusters (Bayoumy and Michaud, 2015). However, the present study showed that kinship did not play a significant role in egg cannibalism exhibited by A. custos under free-choice or no-choice conditions, which coincides with the observations in Phytoseiulus persimilis Athias-Henriot (a phytoseiid mite) (Revynthi et al., 2018b). However, we could not determine whether A. custos recognized parental and non-parental eggs.
In the present study, adult females exhibited a higher predation frequency than adult males. This is concordant with the observations in H. convergens (Bayoumy and Michaud, 2015), C. undecimpunctata (Bayoumy et al., 2016), and A. bipunctata (Agarwala and Dixon, 1992) but is in contrast to the observations in F. aquilonia, wherein males reduce mating competition using egg cannibalism (Schultner et al., 2013). The difference between the predation frequencies of males and females may be attributed to the high food demand of females during spawning (Neff, 2003; Harshman and Zera, 2007; Miller and Zink, 2012).
Previous studies have shown that developmental stage, including the ages of the adults, nymph and eggs, and food quality (King and Dawson, 1972) also influence egg cannibalistic behavior (Ho and Dawson, 1966; Pajunen and Pajunen, 1991; Parsons et al., 2013; Schultner et al., 2013; Bayoumy and Michaud, 2015). King and Dawson (1972) showed that improving food quality reduced cannibalism rates in Tribolium, while Pajunen and Pajunen (1991) showed that female rock-pool corixids cannibalized new eggs at a greater frequency than 1-day old eggs. Moreover, in some species, such as T. confusum (Parsons et al., 2013), C. undecimpunctata (Bayoumy et al., 2016), and male H. convergens (Bayoumy and Michaud, 2015), virgin adults are more cannibalistic than mated adults, whereas mated females of H. convergens (Bayoumy and Michaud, 2015) and some mite species are more cannibalistic than virgin females (Schausberger, 2003).
In the present study, we observed that adults exhibited egg cannibalism during the first 48 h of incubation, but its cause could not be determined. It would be noteworthy to determine the effect on egg cannibalism when older eggs are replaced with fresh eggs (<24 h old) every day or when A. custos adults have a choice between fresh and older eggs. In addition, it remains unknown whether virgin males and females cannibalize eggs, and whether different aged nymph cannibalize eggs. Hence, further studies are required to determine whether the egg cannibalistic behavior of A. custos depends on developmental stage, including the ages of the eggs, nymphs, and adults. The lifespan of insects with higher appetite was longer in the present study than those with lower appetite, and the lifespan of male and female predators increased with the increasing number of cannibalized eggs. These findings are similar to the observations made in the ant F. aquilonia (Schultner et al., 2013).
Egg cannibalism offers insect species a means to avoid starvation and prolong lifespan by providing an alternative source of nutrition and energy (Polis, 1981; Smith and Reay, 1991; Pizzatto and Shine, 2008; Okada et al., 2015). However, under field conditions, whether A. custos would exhibit the same behavior, possibly avoiding egg cannibalism by protecting at least some of its eggs and instead searching for other prey, is still unknown (Revynthi et al., 2018b). Furthermore, egg cannibalistic behavior has also been reported in the nymphs of F. aquilonia (Schultner et al., 2013) and T. castaneum (Ho and Dawson, 1966). Given that compared with laboratory conditions, nymphs generally have little difficulty in locating eggs in the wild, it will be instructive to investigate egg cannibalism among A. custos nymphs, as well as egg cannibalism as a whole, under natural conditions. However, our observations were conducted under confined conditions, and it remains to be determined whether A. custos adult would exhibit the same behavior at more extensive spatial scales.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SW, WZ, JL, YL, XL, LT, HC, and ZZ conceived and designed the experiments. SW, WZ, JL, ML, XL, WD, and ZZ performed the experiments. SW, WZ, YL, and ZZ analyzed the data and wrote the manuscript. All authors have read and approved the final manuscript for submission.
Funding
This study was funded by the Natural Science Foundation of Hunan Province (Nos. 2020JJ5290 and 2018JJ2364), Natural Enemies Breeding Project supported by the Science Foundation of Hunan (Nos. 18–21 Aa06, HN2020KJ12, and 110201901040), Major Program for Science and Technology of Hunan Province (No. 2020NK2034), and Double First-Class Construction Project of Hunan Agricultural University (No. SYL 2019029), and Hunan Forestry Science and Technology Innovation Funding Project (No. XLK202104-1).
Conflict of Interest
WZ and HC were employed by the Hunan Changsha Municipal Tobacco Company. SW, ML and ZZ were employed by the Hunan Province Tobacco Company. JL was employed by the Hunan Yongzhou Municipal Tobacco Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Changhua Zhang and Fangzhao Jia for providing the insects and Anfang Zhu for feeding the insects.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.758587/full#supplementary-material
References
Agarwala, B. K., and Dixon, A. F. G. (1992). Laboratory study of cannibalism and interspecific predation in ladybirds. Ecol. Entomol. 17, 303–309. doi: 10.1111/j.1365-2311.1992.tb01062.x
Arbogast, R. T. (1979). Cannibalism in Xylocoris flavipes (Hemiptera: Anthocoridae), a predator of stored-product insects. Entomol. Exp. Appl. 25, 128–135. doi: 10.1111/j.1570-7458.1979.tb02862.x
Bayoumy, M. H., Abou-Elnaga, A. M., Ghanim, A. A., and Mashhoot, G. A. (2016). Egg cannibalism potential benefits for adult reproductive performance and offspring fitness of Coccinella undecimpunctata L. (Coleoptera: Coccinellidae). Egypt. J. Biol. Pest Control 26, 35–42.
Bayoumy, M. H., and Michaud, J. P. (2015). Egg cannibalism and its life history consequences vary with life stage, sex, and reproductive status in Hippodamia convergens (Coleoptera: Coccinellidae). J. Econ. Entomol. 108, 1665–1674. doi: 10.1093/jee/tov148
Dobler, R., and Kölliker, M. (2010). Kin-selected siblicide and cannibalism in the European earwig. Behav. Ecol. 21, 257–263. doi: 10.1093/beheco/arp184
Dobler, R., and Kölliker, M. (2011). Influence of weight asymmetry and kinship on siblicidal and cannibalistic behaviour in earwigs. Anim. Behav. 82, 667–672. doi: 10.1016/j.anbehav.2011.06.017
Duarte, A. G., Caldas, F., Pechirra, A. Borges da Silva, E., and Figueiredo, E. (2021). Intraguild predation and cannibalism 1190 among Dicyphini: Dicyphus cerastii vs. two commercialized 1191 species. Entomol. Exp. Appl. 169, 90–96. doi: 10.1111/eea.12943
Dumont, F., Lucas, É., and Alomar, O. (2020). Oviposition behaviour of the mirid Macrolophus pygmaeus under risk of intraguild predation and cannibalism. Insect Sci. 28, 224–230. doi: 10.1111/1744-7917.12752
Edenbrow, M., and Croft, D. P. (2012). Kin and familiarity influence association preferences and aggression in the mangrove killifish Kryptolebias marmoratus. J. Fish Biol. 80, 503–518. doi: 10.1111/j.1095-8649.2011.03181.x
Elgar, M. A., and Crespi, B. J. (1992). Cannibalism: Ecology and Evolution Among Diverse Taxa. Oxford: Oxford University Press.
Fox, L. R. (1975). Factors influencing cannibalism, a mechanism of population limitation in the predator Notonecta hoffmanni. Ecology 56, 933–941. doi: 10.2307/1936303
Gao, Q., Wang, D., Zhang, W. H., Liu, Y. D., Meng, F. C., Wu, B., et al. (2019). Study on predatory function of Arma chinensis on Spodoptera litura (Fabricius). Chin. Tob. Sci. 40, 55–59.
Gao, Z., Wang, X., Zhang, L., Sun, Y., Fan, J., and Wang, G. (2011). Biological characteristic of Arma chinensis. J. Eng. 2, 72–77.
Garza, S., and Waldman, B. (2015). Kin discrimination in polyphenic salamander larvae: trade-offs between inclusive fitness and pathogen transmission. Behav. Ecol. Sociobiol. 69, 1473–1481. doi: 10.1007/s00265-015-1959-0
Hamilton, W. D. (1964). The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. doi: 10.1016/0022-5193(64)90039-6
Harshman, L. G., and Zera, A. J. (2007). The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86. doi: 10.1016/j.tree.2006.10.008
Ho, F. K., and Dawson, P. S. (1966). Egg cannibalism by Tribolium larvae. Ecology 47, 318–322. doi: 10.2307/1933784
Hoffman, C. R. (2012). Kinship and Familiarity Affect Recognition and Foraging in the Wolf Spider, Pardosa milvina (Araneae: Lycosidae). Honors thesis. Oxford, OH: Miyami University.
Ichikawa, N. (1991). Egg mass destroying and guarding behaviour of the giant water bug, Lethocerus deyrollei Vuillefroy (Heteroptera: Belostomatidae). J. Ethol. 9, 25–29. doi: 10.1007/BF02350293
Jacobs, A. C., and Stigall, T. (2019). Paternity and egg cannibalism in the ringlegged earwig Euborellia annulipes (Dermaptera: Anisolabididae). Entomol. Sci. 22, 250–257. doi: 10.1111/ens.12363
King, C. E., and Dawson, P. S. (1972). Population biology and the Tribolium model. Evol. Biol. 5, 133–227. doi: 10.1007/978-1-4757-0256-9-5
Lee, S. (2016). Avoiding negative reviewer comments: common statistical errors in anesthesia journals. Korean J. Anesthesiol. 69, 219–226. doi: 10.4097/kjae.2016.69.3.219
Lee, S., and Lee, D. K. (2018). What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 71, 353–360. doi: 10.4097/kja.d.18.00242
Li, F. Q., Li, B. P., and Meng, L. (2020). Effects of plant structure on searching and predation behaviors on Spodoptera litura caterpillars by Arma chinensis (Hemiptera: Pentatomidae) across life stages. Chin. J. Biol. Control 37, 464–471. doi: 10.16409/j.cnki.2095-039x.2021.01.006
Manica, A. (2002a). Filial cannibalism in teleost fish. Biol. Rev. Camb. Philos. Soc. 77, 261–277. doi: 10.1017/s1464793101005905
Manica, A. (2002b). Alternative strategies for a father with a small brood: mate, cannibalise or care. Behav. Ecol. Sociobiol. 51, 319–323. doi: 10.1007/s00265-001-0444-0
Miller, J. S., and Zink, A. G. (2012). Parental care trade-offs and the role of filial cannibalism in the maritime earwig, Anisolabis maritima. Anim. Behav. 83, 1387–1394. doi: 10.1016/j.anbehav.2012.03.006
Mrowka, W. (1987). Filial cannibalism and reproductive success in the maternal mouthbrooding cichlid fish Pseudocrenilabrus multicolor. Behav. Ecol. Sociobiol. 21, 257–265. doi: 10.1007/BF00292507
Narasimha, S., Nagornov, K. O., Menin, L., Mucciolo, A., Rohwedder, A., Humbel, B. M., et al. (2019). Drosophila melanogaster cloak their eggs with pheromones, which prevents cannibalism. PLoS Biol. 17:e2006012. doi: 10.1371/journal.pbio.2006012
Neff, B. D. (2003). Paternity and condition affect cannibalistic behavior in nest-tending bluegill sunfish. Behav. Ecol. Sociobiol. 54, 377–384. doi: 10.1007/s00265-003-0645-9
Okada, S., Fukuda, Y., and Takahashi, M. K. (2015). Paternal care behaviors of Japanese giant salamander Andrias japonicus in natural populations. J. Ethol. 33, 1–7. doi: 10.1007/s10164-014-0413-5
Pajunen, V. I., and Pajunen, I. (1991). Oviposition and egg cannibalism in rock-pool corixids (Hemiptera: Corixidae). Oikos 60, 83–90. doi: 10.2307/3544996
Pan, M. Z., Zhang, H., Zhang, L., and Chen, H. (2019). Effects of starvation and prey availability on predation and dispersal of an omnivorous predator Arma chinensis Fallou. J. Insect Behav. 32, 134–144. doi: 10.1007/s10905-019-09718-9
Parsons, W., Zhong, W., and Rudolf, V. H. W. (2013). Mating status and kin recognition influence the strength of cannibalism. Anim. Behav. 85, 365–369. doi: 10.1016/j.anbehav.2012.11.006
Perry, J. C., and Roitberg, B. D. (2006). Trophic egg laying: hypotheses and tests. Oikos 112, 706–714. doi: 10.1111/j.0030-1299.2006.14498.x
Pizzatto, L., and Shine, R. (2008). The behavioral ecology of cannibalism in cane toads (Bufo marinus). Behav. Ecol. Sociobiol. 63, 123–133. doi: 10.1007/s00265-008-0642-0
Polis, G. A. (1981). The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 12, 225–251. doi: 10.1146/annurev.es.12.110181.001301
R Development Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Revynthi, A. M., Egas, M., Janssen, A., and Sabelis, M. W. (2018a). Prey exploitation and dispersal strategies vary among natural populations of a predatory mite. Ecol. Evol. 8, 10384–10394. doi: 10.1002/ece3.4446
Revynthi, A. M., Janssen, A., and Egas, M. E. (2018b). Gender-specific differences in cannibalism between a laboratory strain and a field strain of a predatory mite. Exp. Appl. Acarol. 74, 239–247. doi: 10.1007/s10493-018-0232-4
Rider, D. A., and Zheng, L. Y. (2002). Checklist and nomenclatural notes on the Chinese Pentatomidae (Heteroptera) I. Asopinae. Entomotaxonomia 24, 107–115. doi: 10.1520/STP11985S
Ringler, E., Barbara Beck, K., Weinlein, S., Huber, L., and Ringler, M. (2017). Adopt, ignore, or kill? Male poison frogs adjust parental decisions according to their territorial status. Sci. Rep. 7:43544. doi: 10.1038/srep43544
Rohwer, S. (1978). Parent cannibalism of offspring and egg raiding as a courtship strategy. Am. Nat. 112, 429–440. doi: 10.1086/283284
Rudolf, V. H. W., Kamo, M., and Boots, M. (2010). Cannibals in space: the coevolution of cannibalism and dispersal in spatially structured populations. Am. Nat. 175, 513–524. doi: 10.1086/651616
Ryckman, R. E. (1951). Recent observations of cannibalism in Triatoma (Hemiptera: Reduviidae). J. Parasitol. 37, 433–434. doi: 10.2307/3273249
Samu, F., Toft, S., and Kiss, B. (1999). Factors influencing cannibalism in the wolf spider Pardosa agrestis (Araneae, Lycosidae). Behav. Ecol. Sociobiol. 45, 349–354. doi: 10.1007/s002650050570
Schausberger, P. (2003). Cannibalism among phytoseiid mites: a review. Exp. Appl. Acarol. 29, 173–191. doi: 10.1023/A:1025839206394
Schultner, E., d’Ettorre, P., and Helanterä, H. (2013). Social conflict in ant larvae: egg cannibalism occurs mainly in males and larvae prefer alien eggs. Behav. Ecol. 24, 1306–1311. doi: 10.1093/beheco/art067
Siegel, S. M., Lee, J. W., and Oaklander, A. L. (2007). Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth. Analg. 105, 1820–1829. doi: 10.1213/01.ane.0000295234.21892.bc
Smith, C., and Reay, P. (1991). Cannibalism in teleost fish. Rev. Fish Biol. Fish. 1, 41–64. doi: 10.1007/BF00042661
Thomas, L. K., and Manica, A. (2003). Filial cannibalism in an assassin bug. Anim. Behav. 66, 205–210. doi: 10.1006/anbe.2003.2202
Vickery, W. L., Whoriskey, F. G., and FitzGerald, G. J. (1988). On the evolution of nest raiding and male defensive behaviour in sticklebacks (Pisces: Gasterosteidae). Behav. Ecol. Sociobiol. 22, 185–193. doi: 10.1007/BF00300568
Wang, L., Meng, L., and Li, B. P. (2020). Effects of diets with Harmonia axyridis pupae on growth and development performance of predatory stinkbug Arma chinensis. J. Nanjing Agric. Univ. 43, 645–649.
Widdig, A. (2007). Paternal kin discrimination: the evidence and likely mechanisms. Biol. Rev. Camb. Philos. Soc. 82, 319–334. doi: 10.1111/j.1469-185X.2007.00011.x
Wu, H., Coudron, T. A., Zhang, L., Aldrich, J. R., Xu, W., Xu, J., et al. (2019). Identification and field verification of aggregation-sex pheromone from the predaceous bug, Arma chinensis. Chemoecology 29, 235–245. doi: 10.1007/s00049-019-00292-2
Wu, S. L., Deng, W., Cai, H. L., Yang, J. S., Zeng, W. A., Zhou, Z. C., et al. (2020). The occurrence period and effect of intraspecific cannibalism behavior of Arma chinensis under starvation. Chin. J. Biol. 36, 169–174. doi: 10.16409/j.cnki.2095-039x.2020.03.001
Zalom, F. G. (1978). Backswimmer prey selection with observations on cannibalism (Hemiptera: Notonectidae). Southwest. Nat. 23, 617–622. doi: 10.2307/3671183
Zhao, Q., Wei, J., Bu, W., Liu, G., and Zhang, H. (2018). Synonymize Arma chinensis as Arma custos based on morphological, molecular and geographical data. Zootaxa 4455, 161–176. doi: 10.11646/zootaxa.4455.1.7
Zheng, Z. Y., and Chen, Y. W. (1992). Experiments on the use of Arma custos (Fabricius) [Hem.: Pentatomidae] to control forest pests. Chin. J. Biol. Control 8, 155–156.
Zou, D., Wang, M., Zhang, L., Zhang, Y., Zhang, X., and Chen, H. (2012). Taxonomic and bionomic notes on Arma chinensis (Fallou). Zootaxa 3382, 41–52. doi: 10.11646/zootaxa.3382.1.4
Zou, D. Y., Coudron, T. A., Wu, H. H., Gu, X. S., Xu, W. H., Zhang, L. S., et al. (2015). Performance and cost comparisons for continuous rearing of Arma chinensis (Hemiptera: Pentatomidae: Asopinae) on a zoophytogenous artificial diet and a secondary prey. J. Econ. Entomol. 108, 454–461. doi: 10.1093/jee/tov024
Keywords: Arma custos Fallou, cannibalistic behavior, predatory, egg cannibalism, kinship difference
Citation: Wu S, Zeng W, Deng W, Li J, Li M, Tan L, Cai H, Li X, Li Y and Zhou Z (2022) Parental Sex and Not Kinship Determines Egg Cannibalism in Arma custos Fallou (Hemiptera: Pentatomidae: Asopinae). Front. Ecol. Evol. 10:758587. doi: 10.3389/fevo.2022.758587
Received: 14 August 2021; Accepted: 12 January 2022;
Published: 10 March 2022.
Edited by:
Sasha Raoul Xola Dall, University of Exeter, United KingdomReviewed by:
Anindita Bhadra, Indian Institute of Science Education and Research Kolkata, IndiaBram Kuijper, University of Exeter, United Kingdom
Copyright © 2022 Wu, Zeng, Deng, Li, Li, Tan, Cai, Li, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youzhi Li, bGl5b3V6aGlAaHVuYXUuZWR1LmNu; Zhicheng Zhou, emhvdXpjaG55Y0AxMjYuY29t
†These authors have contributed equally to this work
 Shaolong Wu1,2†
Shaolong Wu1,2† Wan Deng
Wan Deng Youzhi Li
Youzhi Li