- 1College of Life Sciences, Zhejiang Normal University, Jinhua, China
- 2College of Life Sciences and Agricultural Engineering, Nanyang Normal University, Nanyang, China
- 3State Key Laboratory of Vegetation and Environmental Change, Zhejiang Qianjiangyuan Forest Biodiversity National Observation and Research Station, Institute of Botany, The Chinese Academy of Sciences, Beijing, China
- 4Center of Ecology and Resources, Qianjiangyuan National Park, Kaihua, China
Understory herbaceous plants are an important component of forest ecosystems, playing important roles in species diversity and forest dynamics in forests. However, the current understanding of the biodiversity of forest communities is mostly from woody plants, and knowledge of community structure and species diversity for understory herbaceous plants remains scarce. In a subtropical forest in China, we investigated understory vascular herbaceous diversity from 300 plots (5 × 5 m) in the main growing season. In this study, we analyzed the community structure and diversity pattern of the understory herbaceous community and linked the species diversity pattern to both abiotic and biotic environments. We found a rich diversity of understory herbaceous communities in this forest (81 species belonging to 55 genera), and floristic elements at the genus level were dominated by tropical elements, followed by temperate elements. The diversity pattern of the understory herbaceous showed a significant habitat preference, with the highest diversity in the lowland valleys and then followed by in middle slopes. In addition, herbaceous diversity was significantly affected by both abiotic factors (such as terrain convexity) and biotic factors (such as the diversity of surrounding woody plants). Our study indicated that species diversity of understory herbaceous showed a remarkable habitat preference, such as lowland valleys, and highlighted the importance of both abiotic and biotic environments in driving herbaceous diversity patterns in the subtropical forest understory.
1. Introduction
Understory herbaceous plants are important components in forest ecosystems worldwide (Murphy et al., 2016), which are the most species-rich plant growth forms, even accounting for 80% of all vascular plant species in temperate forests (Gilliam, 2007). Understory herbaceous communities affect forest regeneration patterns through species interactions with woody plant seedlings (Holeksa, 2003), playing important roles in ecosystem function and biodiversity conservation (Landuyt et al., 2019). In addition, understory herbaceous plants are small in stature and have less-persistent aboveground structures than most woody plants, and their diversity is more sensitive to both spatial and temporal environmental changes (Garg et al., 2022). To date, the majority of our knowledge of forest biodiversity comes from woody plants (Murphy et al., 2016), which only represent a small fraction of biodiversity in the forest community. However, as an important component in forests, knowledge of community structure and species diversity for understory herbaceous communities remains scarce (Wang et al., 2021).
Previous studies showed that the diversity pattern and species coexistence mechanism of herbaceous plants might be quite different from woody plants (Spicer et al., 2021). On the one hand, herbaceous plants and woody plants show quite differences in the root system (Cicuzza et al., 2013), leaves, and stature, which may lead to a significant difference in responding to light resources and available nutrition (Siebert, 2002; Ramadhanil et al., 2008), and ultimately present a different diversity pattern. On the other hand, local environmental factors, such as elevation, slope (Wiharto et al., 2021), soil (Beck and Givnish, 2021; Mao et al., 2021), community edge (De Pauw et al., 2021), thinning intensity (Wang et al., 2021), and climate change (Cacciatori et al., 2022), also have been shown to have significant effects on the diversity of understory herbaceous plants. However, the effect of habitat filtration may be different between woody and herbaceous plants. For example, elevation showed opposite effects on the diversity of woody plants and herbaceous plants (Cicuzza et al., 2013; Xu et al., 2019). Therefore, a more comprehensive analysis of possible mechanisms of understory herbaceous diversity is needed, as are studies on woody plants.
However, these present studies mainly focus on tropical forests and cold temperate forests and usually do not take subtropical understory into consideration (Wang et al., 2021; Garg et al., 2022). Understory herbaceous plants in different climatic zones, such as tropical and temperate forests, may show different species richness and biodiversity (Gilliam, 2007). Moreover, almost no study has been conducted to investigate the effects of both abiotic and biotic environments on understory herbaceous diversity patterns in subtropical forests at the same time. Thus, the assessment of the community structure and diversity pattern of the understory herbaceous community and linked the species diversity pattern to abiotic and biotic environments will further improve our understanding of the ecology of understory herbaceous plant diversity (Lagomarsino et al., 2016).
The subtropical evergreen broad-leaved forest is a typical zonal vegetation type with a vast area in China (Zhu et al., 2008). Its rich vegetation diversity is much higher than that of other evergreen broad-leaved forests, and its species richness is second only to tropical rainforests (Song et al., 2005). However, the research data on herbaceous plant communities in China are limited, especially in the typical subtropical evergreen broad-leaved forest plots (Song et al., 2015).
Thus, in order to understand the community structure and diversity pattern of the understory herbaceous community and the potential contribution of abiotic and biotic environments to the species diversity pattern, we analyzed the community structure and diversity pattern of the understory herbaceous community and linked the species diversity pattern to abiotic and biotic environments in the Gutianshan 24-ha subtropical forest dynamic plot. Specifically, we focused on the following questions:
(1) What is the community structure and diversity pattern of the understory herbaceous community in the subtropical evergreen broad-leaved forest?
(2) To what extent do the species diversity pattern of herbaceous plants in the subtropical understory driven by both abiotic and biotic environments?
2. Materials and methods
2.1. Study area and herbaceous plants survey
The Gutianshan National Nature Reserve (29°10′19.4″–29°17′41.4″N, 118°03′49.7″–118°11′12.2″E) is located in Kaihua County, Zhejiang Province in China, with a total area of 8,107 ha and a typical mid-subtropical evergreen broad-leaved forest (Supplementary Figure 1A; Legendre et al., 2009). According to the ForestGEO plot construction standard (Condit, 1998; Davies et al., 2021), the 24-ha (600 m × 400 m) Gutianshan Forest Dynamics Plot (GFDP) was established in 2005 by selecting typical mid-subtropical evergreen broad-leaved forest with dominant species of Castanopsis eyrei and Schima superba (Legendre et al., 2009). The plot ranges from 446.3 to 714.9 m in elevation, ranges from 12.8 to 62.0° in slope, and ranges from 93.9 to 269.2° in aspect (Ren et al., 2022). The first tree census in the 24-ha forest plot was conducted in 2005 and re-censused at 5-year intervals. All free-standing woody stems ≥1 cm diameter at breast height (d.b.h., 1.3 m) were tagged, measured, mapped, and identified (Legendre et al., 2009; Wang et al., 2020). According to the first survey, there were 140,700 trees in total, with 159 species belonging to 49 families and 103 genera.
In order to meet the necessary systematic investigation scope and area of systematic investigation and minimize human interference to the plot, on the basis of the original 24-ha plot in Gutianshan, we divided 600 20-m × 20-m unit quadrats (as the basic stations) into two parts evenly in July 2011. Therefore, we conducted a systematic investigation of herbaceous plants in 300 stations with a 5 m × 5 m area. The position of these survey quadrats was relatively fixed in the 20-m × 20-m unit plots; that is, the east of the unit plot was the second 5-m × 5-m quadrat to the north (Supplementary Figure 1B). The herbaceous plants in the stations were systematically investigated from August to October in the main growing season in 2011. The species composition of herbaceous vascular plants in each quadrat, the number of individuals of each species (Poaceae and Cyperaceae record the number of clusters, and ferns that cannot count the number of clusters record the number of members), the average height, the coverage of each species, and the total coverage of all herbaceous vascular plants were all recorded.
2.2. Community structure of understory herbaceous
According to the existing research on the distribution types and floristic division of seed plants (Wu et al., 2003; Li, 2008; Zuo et al., 2017) and ferns (Lu, 2017), the distribution types of herbaceous genera in the plot were divided. We calculated the important value (IV) of each constituent species of the herbaceous plant community in the 24-ha plot by using the survey data of 300 5-m × 5-m quadrats:
Thus, the relative density refers to the percentage of the number of individuals of one species to the number of individuals of all species in the whole plot.
2.3. Herbaceous diversity and habitat types
We counted the number of herb species (S) in each 5-m × 5-m survey quadrat and calculated the Shannon–Wiener index (H) and Pielou’s evenness index (E) (Whittaker, 1972; Whitney and Foster, 1988):
where , N0 is the number of individuals of all observed species, Ni is the number of individuals of species i, and S is the species richness.
Based on the multivariate regression tree (MRT) analysis results constructed on the topography, soil, and the first survey data of the plot (Chen et al., 2010), the habitats of the GFDP were divided into the following five types: low valley, low ridge, mid-slope, high slope, and high ridge (Supplementary Figure 2). Low ridge and low valley are the two most extensive habitat types among them.
According to the sampling principle of rarefaction (Hurlbert, 1971; Heck et al., 1975), we observed the characteristics of the change curve of community species richness as the sampling number of individual herbaceous plants in the plot increased. We divided herbaceous community individuals based on their habitat types and performed the rarefaction repeated sampling. We compared the abundance of herbaceous plants distributed in different habitats and the differences in the species richness of herbaceous plants in different habitat types under the same sampling amount. We also analyzed the distribution of species diversity index of herbaceous plants in different habitat types by variance analysis and compared the differences in herbaceous plant diversity among different habitat types by the Tukey honestly significant difference (HSD) test.
2.4. Abiotic and biotic drivers of diversity pattern of understory herbaceous
We performed correlation analysis on the total herb coverage, the Shannon–Wiener index (Hherb), Pielou’s evenness index (Eherb), and the main environmental factors of the plot, then calculated the Pearson correlation coefficient matrix among the variables and performed the significance test. Environmental variables that affect the growth of herbaceous plants can reflect environmental factors, such as forest vegetation community coverage, terrain, and light conditions in the quadrat. The variables selected in this study are as follows: terrain concavity and convexity, elevation, slope, and aspect where the plot is located, canopy closure, tree community diversity in the upper and surrounding plots, and the sum of cross-sectional area at breast height (dm2) during environmental variables that affect the growth of herbaceous plants can reflect environmental factors, such as forest vegetation community coverage, terrain, and light conditions in the quadrat. The four topographic factors are calculated as the same as the 20-m × 20-m plot in which the survey plot is located (Lai et al., 2009); we defined trees on the upper level of the quadrat and surrounding and living individuals of woody plants with DBH ≥1 cm within a radius of 10 m from the center of the survey plot (including dead, broken and lodging individuals, the data are from the first review of the plot in 2010). The Shannon–Wiener index was used to represent the tree diversity, which is more comprehensive and suitable for tree communities (taking into account both abundance and evenness).
Based on the correlation coefficient test results, we used the distribution patterns of herbaceous plant diversity indices (richness S, Shannon–Wiener index H, and Pielou’s evenness index E) in each survey quadrat as fitting variables, performed multiple linear regression fitting with factors that are significantly related to a diversity index as explanatory variables, analyzed the fitting of the model to the variation of the diversity pattern, and compared the effects of each explanatory variable. Then, we further explored the relative importance of each variable to herbaceous plant diversity.
All analyses were performed with R (version 4.1.3): The species diversity index and rarefaction species richness comparison were calculated with the vegan package (Jost, 2007). Tukey HSD test was calculated with the agricolae package (Hsu, 1996), and the fitted line for scatter graph between variables was performed with lowess function (Cleveland, 1979, 1981). The relative importance analysis based on l mg and pmvd methods was implemented by the calc.relimp function in the relaimpo package (Groemping, 2006).
3. Results
3.1. Species composition and flora of understory herbaceous
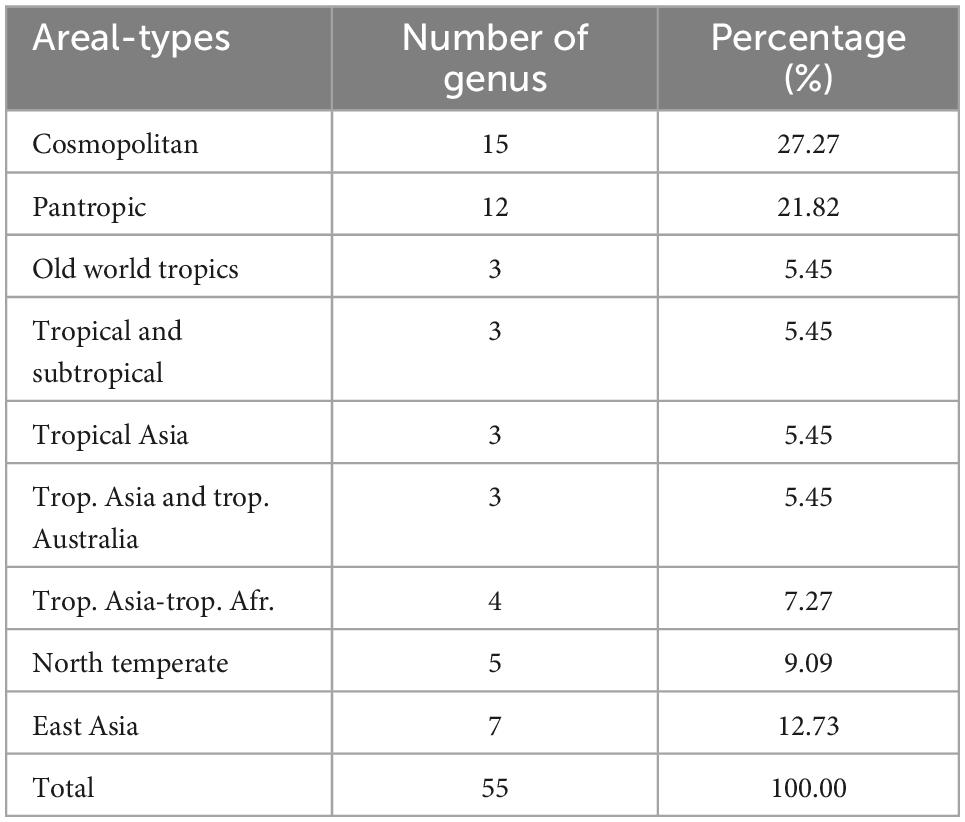
We recorded a total of 81 species, 31 families, and 55 genera of herbaceous vascular plants in the 24-ha Gutianshan plot (Supplementary Table 1). The results of the floristic analysis showed that the world distribution types accounted for the largest proportion of herbaceous community genera, including 15 genera, such as Dryopteris, followed by pantropical genera with 12 genera, including local common herbaceous groups, such as Diplopterygium, and more genera are widely distributed in Northern hemisphere and East Asia. There are 28 genera of tropical types, accounting for 50.91% of the total, indicating that the herbaceous groups living under the subtropical evergreen broad-leaved forest in Gutianshan are mainly tropical species, while temperate and world distributions account for 49.09% of the total, making it an important constituent group of the herbaceous community (Table 1).
The important values of each species are shown in Supplementary Table 1; the important value of Diplopterygium glaucum is the largest. There are a large number of D. glaucum in a total of 162 quadrats, and their rhizomes walk underground with huge leaves, with a wide distribution in the plot. Nearly 39,482 clonal component units were counted, accounting for 64.70% of the total number of individuals in the herbaceous plant community. The relative frequency and relative coverage are 11.54 and 81.90%, and the important value is 48.69%, which indicates its extensive distribution and dominant position in the plot. The important values of Dicranopteris pedata and Carex sp. are also relatively high.
The top 15 important values of herbaceous plants account for 92.19% of the total. These species are the most common in the plot, and their density and coverage are relatively large. In the survey, 24 species of the herbaceous plant appeared in only one quadrat, accounting for 29.63% of the total number of species, and 44 species appeared in no more than five quadrats, accounting for 54.32% of the total species, and the sum of their important values accounted for 2.18%. These reflect the imbalance in the composition pattern of the understory herbaceous plant community in the plots and the differences in the species composition in different areas. When the dominant species status is clear, there are more rare species coexisting in the community, and this structural feature can also be observed in frequency statistics for species (Figure 1A).
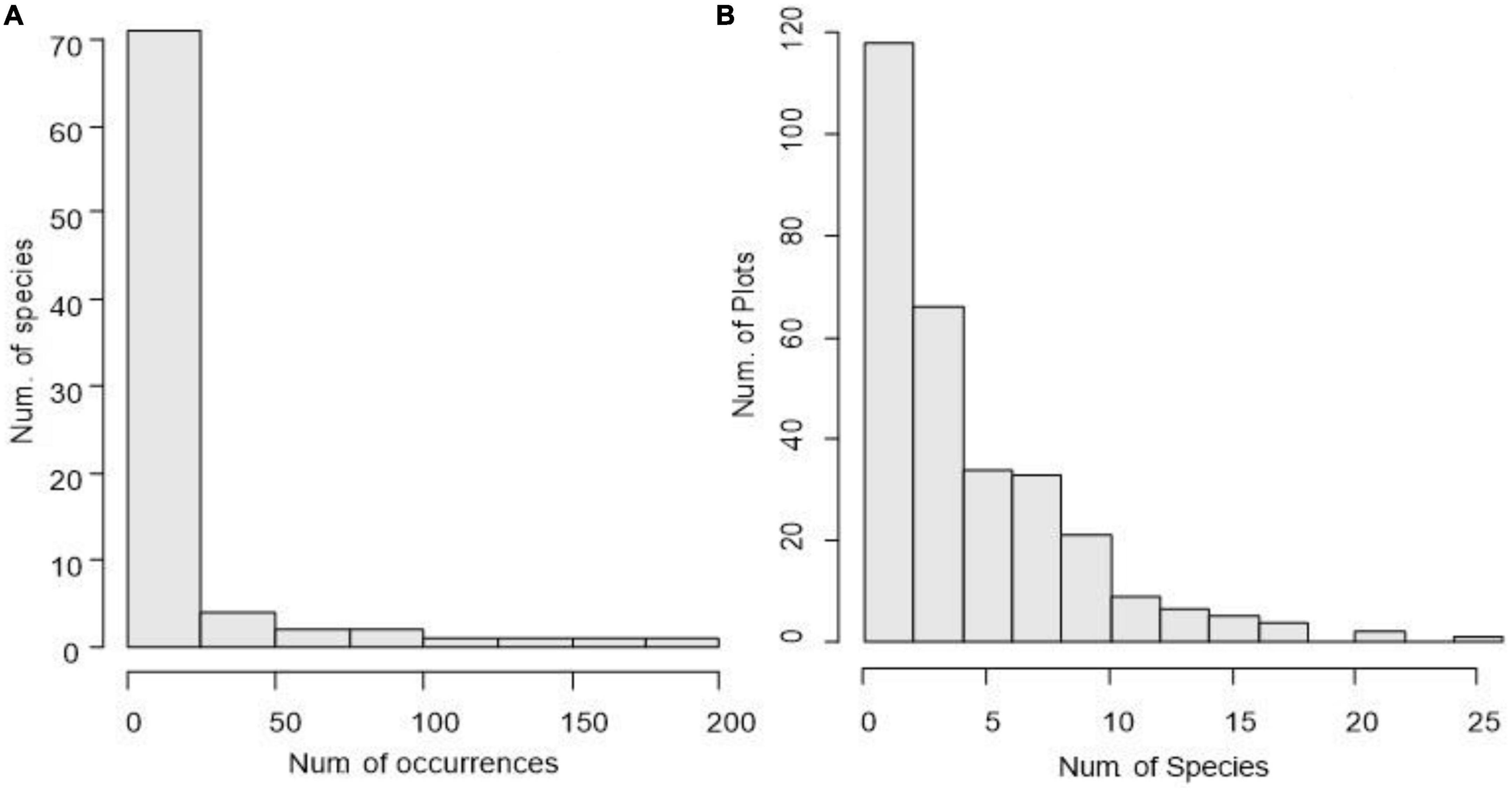
Figure 1. Species occurrences (A) and species richness distribution of herbaceous plants (B) in Gutianshan subtropical understory.
The coverage of herbaceous plants varies greatly in different plots. In some plots, herbaceous plants cover the entire understory, while there are no herbaceous plants at all in others (Figure 2A). There is no particularly obvious regularity in the distribution of the coverage in Gutianshan.
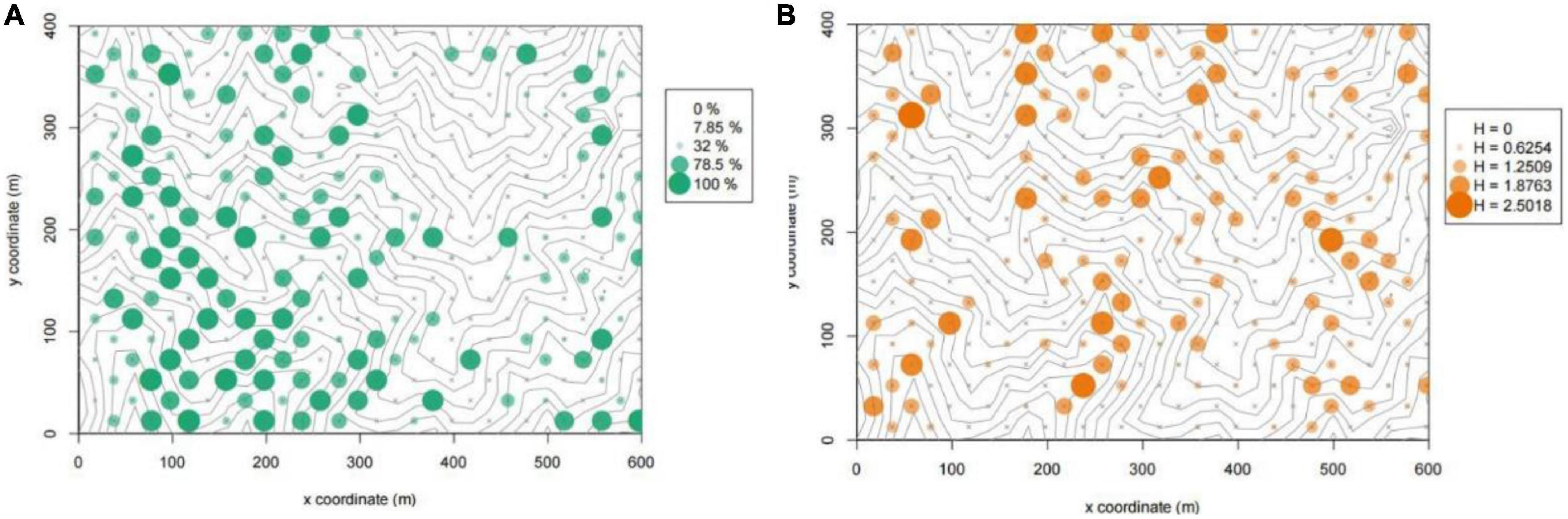
Figure 2. Coverage (A) and diversity (B) distribution of herbaceous plants in Gutianshan subtropical understory. “×” shows the locations of investigation plots, and the size of “•” represents the coverage or diversity index values in each plot.
3.2. Herbaceous diversity pattern within habitat types
There are differences in species richness among the 300 survey quadrats, with the number of species ranging from 0 to 25. Only a few quadrats have high species richness (Figure 1B), which might be related to the occurrence of specific environmental conditions, and the number of species in most quadrats is less than 10. The range of the Shannon–Wiener index of each quadrat is 0.00–2.50. The diversity of the ridge zone of the quadrats is generally low, while the quadrats with higher diversity index tend to be distributed along the valley (Figure 2B). This shows that the relatively shady and humid environmental conditions in the valley area are conducive to the coexistence of more understory herbaceous plants.
With the increase of rarefaction sampling, the trend of species richness increase in various habitat types is obviously different (Figure 3, five curves representing different habitats are significantly distinguished). The low valley has the fastest increase, followed by the mid-slope, indicating that this habitat type is conducive to maintaining high diversity of herbaceous plants. However, the high ridge rises the slowest, indicating that many herbaceous plants are not suitable for surviving in this habitat. Similarly, although many herbaceous plants are distributed in the low ridge, the species richness is very limited. Even if the number of samples is increased, the potential to find more coexisting species is very low.
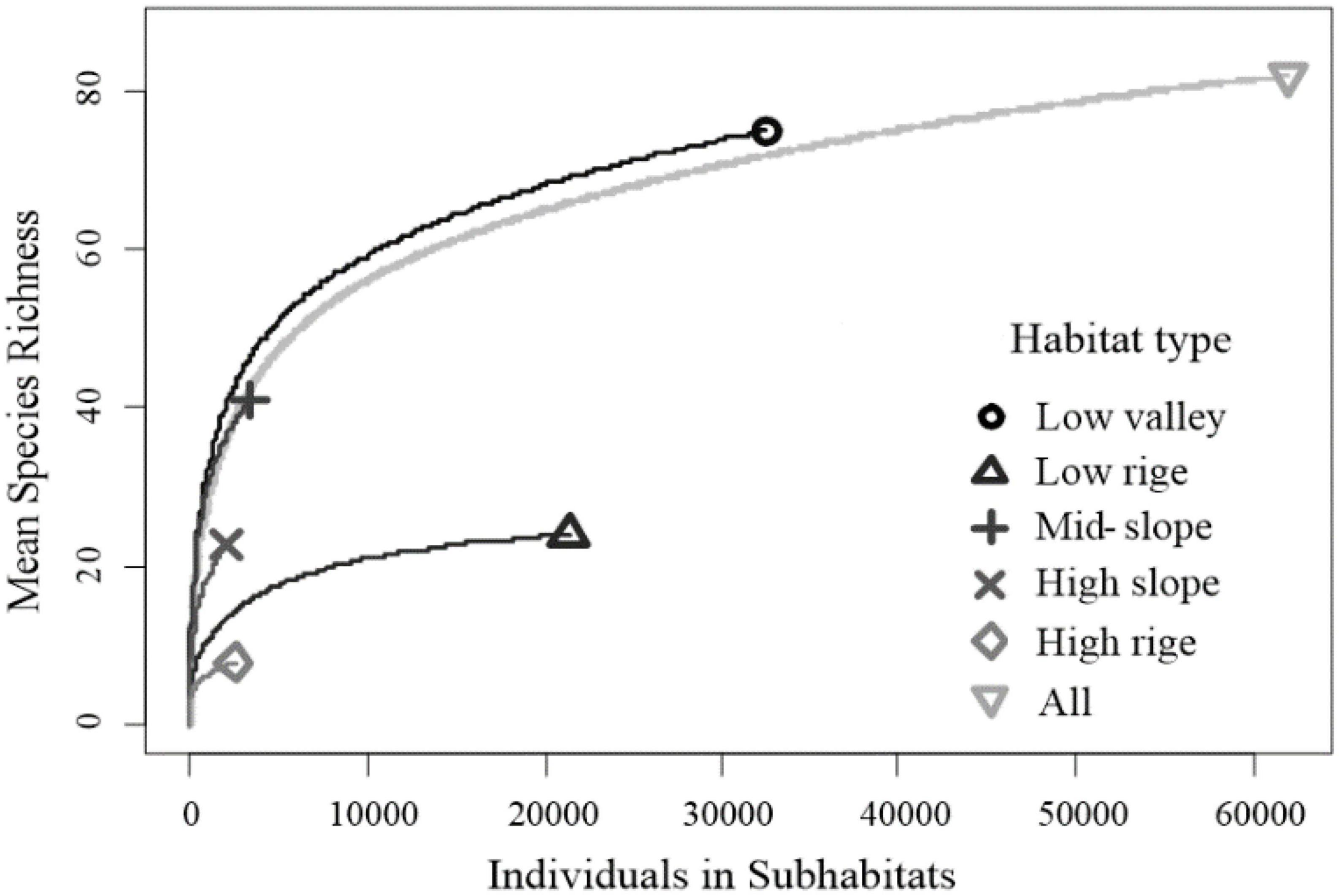
Figure 3. Rarefied species richness of herbaceous plants within different habitat types in Gutianshan subtropical understory.
The Shannon–Wiener diversity index of herbaceous plants varies greatly among habitat types, with significant differences among different habitats. The highest diversity lies in the low valley and mid-slope, and the lowest in the low ridge and high ridge. The difference between the high slope and other habitat types is not significant (Table 2), and the variation of evenness among different habitats is relatively weak. Compared with different habitats, the low valley was the highest, the low ridge, mid-slope, and high slope were the second, and the high ridge was the lowest.
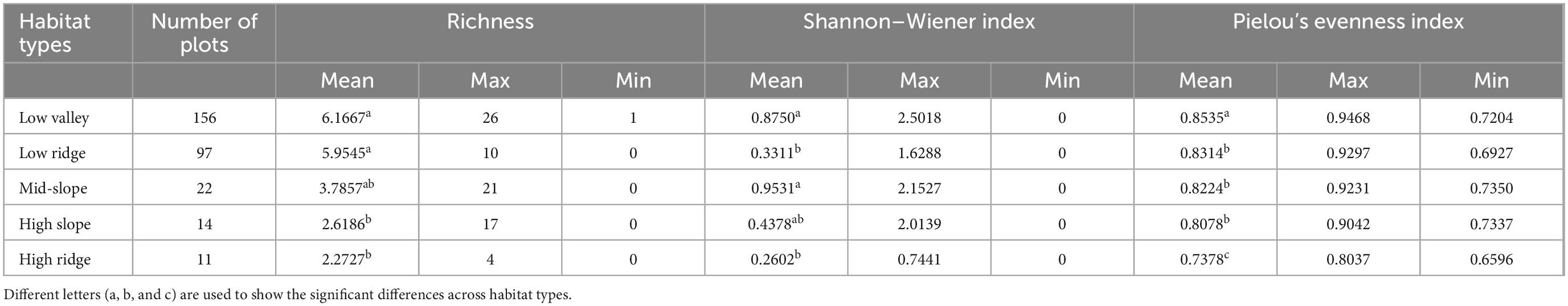
Table 2. Tukey honestly significant difference (HSD) test of richness, herbaceous plant diversity, and evenness between different habitat types in Gutianshan subtropical understory.
3.3. Abiotic and biotic drivers of understory herbaceous diversity
According to the results of correlation analysis, Hherb is significantly negatively correlated with coverage as a whole, while Eherb has a significant positive correlation with coverage (Figure 4). Except for the aspect factor, other abiotic factors were significantly correlated with at least one of the three characteristic variables of the herbaceous plant community, and the diversity of the tree community (Htrees) was significantly positively correlated with all three herbal community variables, in particular, the linear fitting degree with the evenness of herbaceous plants is very high, and the linear regression analysis of the two is up to 0.56. The canopy density was significantly negatively correlated with the three herbaceous plant community variables. The tree breast height basal area of the tree and convexity was significantly negatively correlated with Hherb and Eherb. The elevation was significantly negatively correlated with the Eherb and coverage of herbaceous plants. The slope was positively correlated with the coverage of herbaceous plants but was not significantly related to the Hherb and Eherb (Figure 4).
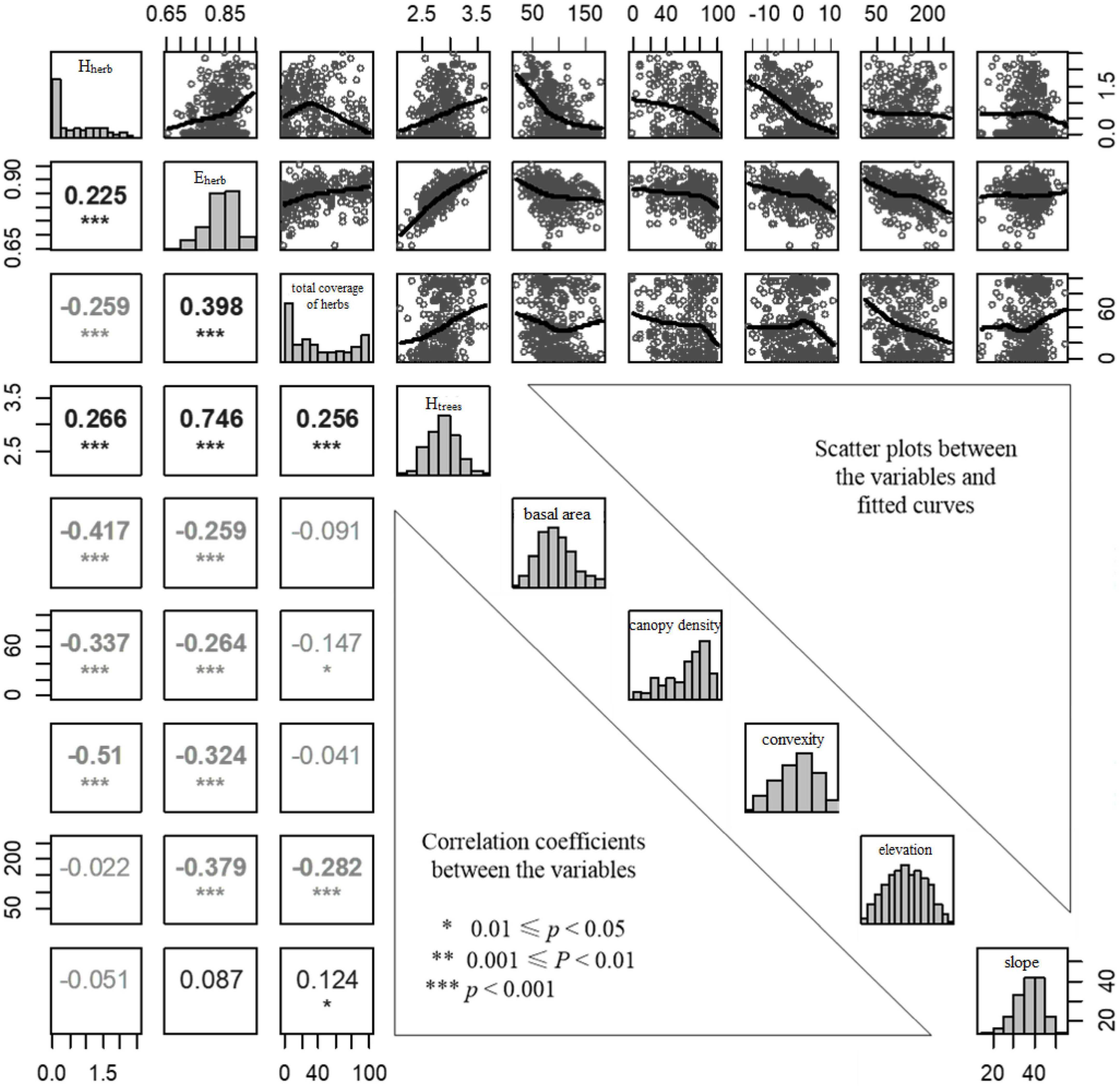
Figure 4. Correlation matrix of nine herbaceous plant community and environment variables in Gutianshan subtropical understory. Histograms of variables were shown on the diagonal, scatter plots between the variables were shown on the upper panel, and the lower panel showed Pearson correlation coefficients, where gray represents negative correlation.
The multiple linear regression analysis of the herbaceous community diversity index showed that the effect of each influencing factor on community diversity was consistent with the results of correlation coefficient analysis which includes R2 of species richness (S), Shannon–Wiener index (H), and Pielou’s evenness index (E) model is 0.46, 0.47 and 0.61 (Table 3). The results show that the selected explanatory variables can well explain the various characteristics of herbaceous plant community diversity distribution patterns.
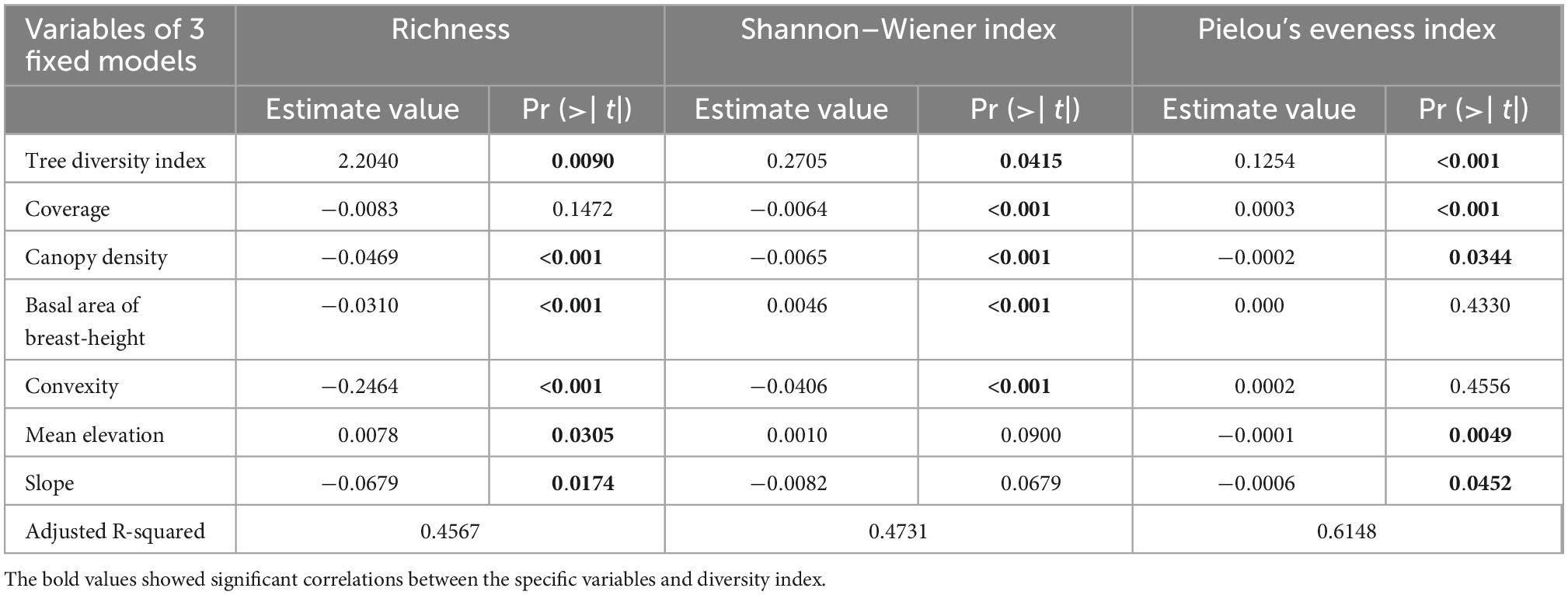
Table 3. Multivariable linear regression models of three diversity indices of herbaceous plant community to seven environment factors in Gutianshan subtropical understory.
The analysis of the relative importance of various factors showed that tree diversity was the main factor affecting the evenness of understory herbaceous plants, and the concavity and basal area of trees made the greatest contribution to the explanation of herbaceous richness and the Shannon–Wiener index variation (Figure 5).
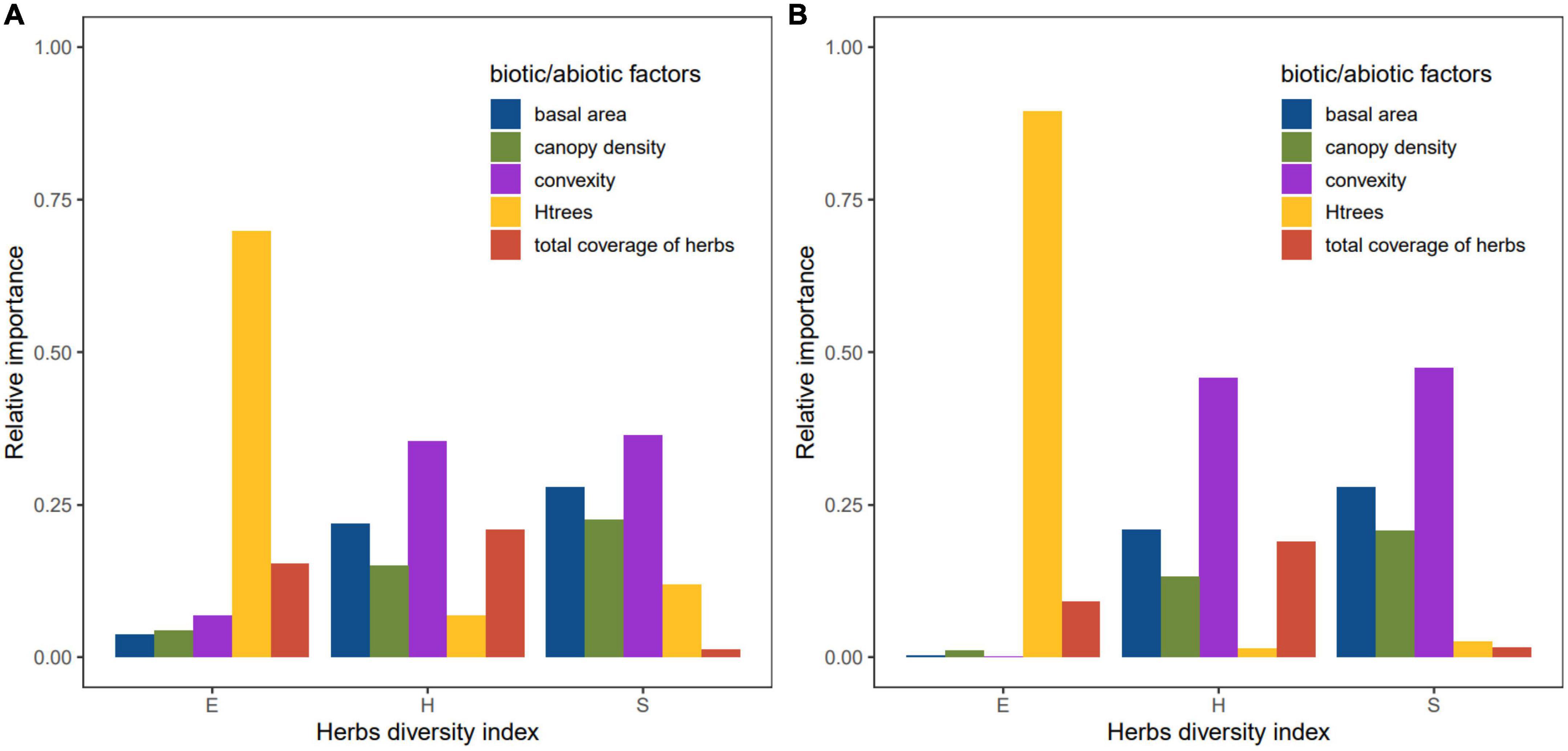
Figure 5. Relative importance of biotic/abiotic factors based on lmg (A) and pmvd (B) to the diversity of understory herbaceous plants.
4. Discussion
4.1. Species composition and flora of herbaceous in the subtropical understory
The herbaceous plants in Gutianshan 24-ha plot are dominated by tropical areal types, with both cosmopolitan areal type and temperate characteristics, which is the same as the general trend indicated by the results of the floristic analysis of trees in the plot (Zhu et al., 2008) and the floristic analysis of the middle subtropical evergreen broad-leaved forest in China (Chen, 2006). The species composition of herbaceous plants is rich (81 species, 31 families, and 55 genera). Compared with other plots located in different climatic zones in China, it is found that the species richness of evergreen broad-leaved forest understory herbaceous plants in Gutianshan are less than those in 20-ha Parashorea chinensis forest in Xishuangbanna (115 species, 41 families, and 76 genera) (Chen, 2008), which is a tropical forest, and the 25-ha broad-leaved Korean pine forest in Changbai Mountain (102 species, 40 families, and 84 genera) (Li et al., 2008), which is a temporal forest. However, the species richness of Gutianshan is close to that of 90 species in the Longyan forest zone, which is also in the subtropical monsoon climate zone (Su et al., 2022). It shows that there is a different richness of forest herbaceous plants in different climatic zones.
The coverage of herbaceous plant communities varies greatly in different areas, among which ferns are numerous and widely distributed. Ferns are strongly dependent on natural environmental conditions, and their species diversity changes are closely related to environmental conditions (Lwanga et al., 1998). They can respond to changes in environmental conditions with a significant indicative effect (Karst et al., 2005; Gasper et al., 2021). The prosperity of understory ferns may be related to their physiological characteristics. Some studies have suggested that the shade tolerance of ferns is related to their photosynthetic mechanism, so it is suitable for the understory environment (Kawai et al., 2003; Nuccio et al., 2020). D. glaucum and D. pedata occupy a lot of space in the understory and have high coverage, which is the absolutely dominant species of herbaceous plant community and an important indication of vegetation types in the understory of this area, which may be related to the climatic conditions, light and heat environment, forest succession types, and acidic soil properties of the plots (da Costa et al., 2019; Oseguera-Olalde et al., 2022).
4.2. Herbaceous diversity pattern related to habitat types
Among the five habitat types of the plot, the low valley and mid-slope could maintain higher herbaceous species richness, while the low ridge had the lowest species richness (Figure 3 and Table 2). This is similar to the individual tree distribution pattern and woody seedling regeneration pattern in the plot affected by habitat heterogeneity (Lai et al., 2009). The heterogeneous habitats of the Gutianshan plot also had a significant impact on the diversity distribution pattern of understory herbaceous plant communities. D. glaucum is the dominant species with high density in the plot, which mostly occurs in the lowland ridge. It is speculated that the low species richness in the ridge (Figure 3) is due to D. glaucum being too luxuriant in the herbaceous plant community, and the strong competitive exclusion makes it difficult for other herbaceous species to obtain living space, despite the fact that the light conditions in the valley of the plot are not as good as those in the ridge. However, the competitive pressure from the dominant species is less, and the water resources are good, so there may be more species growth (Figures 2B, 3). The proportion of rare species will also be higher. This is also consistent with the negative effect of terrain convexity on herbaceous plant diversity.
4.3. Abiotic and biotic drivers of understory herbaceous diversity
The Shannon–Wiener index and Pielou’s evenness index of understory herbaceous plant community are opposite to the coverage of herbaceous plants. Further analysis shows that the Shannon–Wiener index has a better fitting relationship with the quadratic formula of coverage; it shows a unimodal relationship (Figure 4). It is speculated that the environmental stress and filtering effect may be stronger in the areas with too low coverage, so the biomass and richness of herbaceous plant communities are limited (Grace, 1999; Fornara and Tilman, 2009; Chase, 2010). In contrast, areas with too large coverage are too dense to compete and repel and do not take advantage of the maintenance of richness. The Pielou’s evenness index increases monotonously with the increase of coverage, which may be because the richness of the herbaceous community with low coverage is small and the random variability of species is very large, so the abundance of each species is more inconsistent, that is, the evenness is the lowest. While in the herbaceous plant community with high coverage, because environmental factors are suitable for the growth of more herbaceous species, and the structural composition of the dominant species of the community and even associated species is relatively stable, so the evenness is the highest.
In tropical and temperate forests, the community structure and diversity of herbaceous plants are affected by temperature (Cicuzza et al., 2013), elevation, slope (Wiharto et al., 2021), soil (Mao et al., 2021), canopy (Ford et al., 2000), and other factors. The results of correlation analysis and multiple regression analysis between the diversity of subtropical herbaceous plants and the main biotic/abiotic factors showed that elevation, concavity, basal tree area, tree diversity, and canopy density had certain effects on the diversity pattern of understory herbaceous plants in the subtropical forest. However, this effect showed different significance under different indexes.
Generally speaking, in subtropical forests, the increase of tree diversity is beneficial to maintain the evenness of herbaceous plants, which, in turn, can improve the species diversity of the whole forest vegetation. At the same time, the areas with fewer individuals of big trees will have higher understory herbaceous plant diversity, which may be because the fewer individuals of big trees, the smaller the canopy density of the community, and the more available light quantity will be obtained by understory herbaceous plants, and ultimately lead to showing higher richness (Facciano et al., 2023). Our results further confirmed the relationship between canopy density and herbaceous diversity under the forest. Therefore, we speculate that the diversity pattern of vegetation in the canopy and herb layer of the subtropical forest community is closely related: In the community with a high degree of coexistence of canopy trees, there will be an under-forest environment suitable for the stable coexistence of more herbaceous species. At the same time, the increase of herbaceous plant diversity can produce a ground cover environment suitable for seedling planting and renewal of multi-tree species, which, in turn, increases the diversity of woody plants (Rawlik et al., 2018; Cheng et al., 2020). Elevation was negatively correlated with Pielou’s evenness index and total coverage of herbaceous communities, which was different from the conclusion that there was a unimodal relationship between herbaceous plant diversity and elevation in tropical forests (Gómez-Díaz et al., 2017). In addition, there is no significant relationship between slope and herbaceous community diversity, which may be affected by canopy openness and surface soil (Wiharto et al., 2021).
5. Conclusion
The findings reported here in the community structure and diversity pattern of understory herbaceous, as well as a link between species diversity pattern and abiotic and biotic environments, provide insight into the community structure and biodiversity of herbaceous plants in the subtropical understory. We found that understory herbaceous species diversity is a rich component in the Gutianshan forest, with approximately accounting half of species of woody plant species in this subtropical forest. Understory herbaceous diversity prefers in some specific habitats, which was highest in the lowland valleys, followed by middle slopes. Moreover, we found that herbaceous diversity was driven by both abiotic and biotic environments. Among them, tree diversity and terrain convexity are the main important factors in driving the diversity of understory herbaceous plants. Our results suggest that understory herbaceous communities are a non-negligible and important component of biodiversity in a subtropical forest. Our study also indicates that in order to better protect herbaceous biodiversity in the subtropical understory, more attention should be paid to some special habitats, such as low valleys and mid-slopes.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KT, YW, and JC conceived and designed the study and collected herbaceous data. KT and PC performed statistical analyses and wrote the first draft with substantial input from YW and JC. LC, HQ, SC, XM, HR, and KM provided data and contributed to the development of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (32001300), the Zhejiang Provincial Natural Science Foundation of China (Y5100361 and LQ22C030001), the State Key Laboratory of Vegetation and Environmental Change (LVEC2010-01), and the key specialized research and development breakthrough program in Henan province (222102320289).
Acknowledgments
We acknowledge the Zhejiang Qianjiangyuan Forest Biodiversity National Observation and Research Station and the Center of Ecology and Resources of Qianjiangyuan National Park for the support provided to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1113742/full#supplementary-material
References
Beck, J. J., and Givnish, T. J. (2021). Fine-scale environmental heterogeneity and spatial niche partitioning among spring-flowering forest herbs. Am. J. Bot. 108, 63–73. doi: 10.1002/ajb2.1593
Cacciatori, C., Bacaro, G., Chećko, E., Zaremba, J., and Szwagrzyk, J. (2022). Windstorm effects on herbaceous vegetation in temperate forest ecosystems: Changes in plant functional diversity and species trait values along a disturbance severity gradient. For. Ecol. Manag. 505:119799. doi: 10.1016/j.foreco.2021.119799
Chase, J. M. (2010). Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391. doi: 10.1126/science.1187820
Chen, L., Mi, X., Comita, L. S., Zhang, L., Ren, H., and Ma, K. (2010). Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol. Lett. 13, 695–704. doi: 10.1111/j.1461-0248.2010.01468.x
Chen, W. (2006). Floristic phytogeography of evergreen broad-leaved forest (EBLF) in mid-subtropical China. Shanghai: MS. East China Normal University.
Chen, Z. (2008). Study on the diversity and distribution pattern of herbaceous plants under Parashorea forest of Xishuangbanna. Shanghai: MS. Chinese Academy of Sciences.
Cheng, J., Shi, X., Fan, P., Zhou, X., Sheng, J., and Zhang, Y. (2020). Relationship of species diversity between overstory trees and understory herbs along the environmental gradients in the Tianshan Wild Fruit Forests, Northwest China. J. Arid Land 12, 618–629. doi: 10.1007/s40333-020-0055-0
Cicuzza, D., Krömer, T., Poulsen, A. D., Abrahamczyk, S., Delhotal, T., Piedra, H. M., et al. (2013). A transcontinental comparison of the diversity and composition of tropical forest understory herb assemblages. Biodivers. Conserv. 22, 755–772. doi: 10.1007/s10531-013-0447-y
Cleveland, W. S. (1979). Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74:829. doi: 10.1080/01621459.1979.10481038
Cleveland, W. S. (1981). Lowess: A program for smoothing scatterplots by robust locally weighted regression. Am. Stat. 35:54. doi: 10.1080/00031305.1981.10479306_3
da Costa, L. E. N., Arnan, X., de Paiva Farias, R., and Barros, I. C. L. (2019). Community responses to fine-scale environmental conditions: Ferns alpha and beta diversity along Brazilian Atlantic forest remnants. Acta Oecol. 101:103475. doi: 10.1016/j.actao.2019.103475
Davies, S. J., Abiem, I., Abu Salim, K., Aguilar, S., Allen, D., Alonso, A., et al. (2021). ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol. Conserv. 253:108907. doi: 10.1016/j.biocon.2020.108907
De Pauw, K., Meeussen, C., Govaert, S., Sanczuk, P., Vanneste, T., Bernhardt-Römermann, M., et al. (2021). Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 109, 2629–2648. doi: 10.1111/1365-2745.13671
Facciano, L., Sasal, Y., and Suarez, M. L. (2023). How do understory trees deal with small canopy openings? The case of release in growth following drought-induced tree mortality. For. Ecol. Manag. 529:120692. doi: 10.1016/j.foreco.2022.120692
Ford, W. M., Odom, R. H., Hale, P. E., and Chapman, B. R. (2000). Stand-age, stand characteristics, and landform effects on understory herbaceous communities in southern Appalachian cove-hardwoods. Biol. Conserv. 93, 237–246. doi: 10.1016/S0006-3207(99)00126-3
Fornara, D. A., and Tilman, D. (2009). Ecological mechanisms associated with the positive diversity–productivity relationship in an N-limited grassland. Ecology 90, 408–418. doi: 10.1890/08-0325.1
Garg, S., Joshi, R. K., and Garkoti, S. C. (2022). Effect of tree canopy on herbaceous vegetation and soil characteristics in semi-arid forests of the Aravalli hills. Arid Land Res. Manag. 36, 224–242. doi: 10.1080/15324982.2021.1953634
Gasper, A. L. D., Grittz, G. S., Russi, C. H., Schwartz, C. E., and Rodrigues, A. V. (2021). Expected impacts of climate change on tree ferns distribution and diversity patterns in subtropical Atlantic Forest. Perspect. Ecol. Conserv. 19, 369–378. doi: 10.1016/j.pecon.2021.03.007
Gilliam, F. S. (2007). The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 57, 845–858. doi: 10.1641/B571007
Gómez-Díaz, J. A., Krömer, T., Kreft, H., Gerold, G., Carvajal-Hernández, C. I., and Heitkamp, F. (2017). Diversity and composition of herbaceous angiosperms along gradients of elevation and forest-use intensity. PLoS One 12:e0182893. doi: 10.1371/journal.pone.0182893
Grace, J. B. (1999). The factors controlling species density in herbaceous plant communities: An assessment. Perspect. Plant Ecol. Evol. Syst. 2, 1–28. doi: 10.1078/1433-8319-00063
Groemping, U. (2006). Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 17, 1–27. doi: 10.18637/jss.v017.i01
Heck, K. L. Jr., van Belle, G., and Simberloff, D. (1975). Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 56, 1459–1461. doi: 10.2307/1934716
Holeksa, J. (2003). Relationship between field-layer vegetation and canopy openings in a Carpathian subalpine spruce forest. Plant Ecol. 168, 57–67. doi: 10.1023/A:1024457303815
Hurlbert, S. H. (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586. doi: 10.2307/1934145
Jost, L. (2007). Partitioning diversity into independent alpha and beta components. Ecology 88, 2427–2439. doi: 10.1890/06-1736.1
Karst, J., Gilbert, B., and Lechowicz, M. J. (2005). Fern community assembly : The roles of chance and the environment at local and intermediate scales. Ecology 86, 2473–2486. doi: 10.1890/04-1420
Kawai, H., Kanegae, T., Christensen, S., Kiyosue, T., Sato, Y., Imaizumi, T., et al. (2003). Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421, 287–290. doi: 10.1038/nature01310
Lagomarsino, L. P., Condamine, F. L., Antonelli, A., Mulch, A., and Davis, C. C. (2016). The abiotic and biotic drivers of rapid diversification in A ndean bellflowers (Campanulaceae). New Phytol. 210, 1430–1442. doi: 10.1111/nph.13920
Lai, J., Mi, X., Ren, H., and Ma, K. (2009). Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 20, 415–423. doi: 10.1111/j.1654-1103.2009.01065.x
Landuyt, D., De Lombaerde, E., Perring, M. P., Hertzog, L. R., Ampoorter, E., Maes, S. L., et al. (2019). The functional role of temperate forest understorey vegetation in a changing world. Glob. Change Biol. 25, 3625–3641. doi: 10.1111/gcb.14756
Legendre, P., Mi, X., Ren, H., Ma, K., Yu, M., Sun, I. F., et al. (2009). Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 90, 663–674. doi: 10.1890/07-1880.1
Li, B., Zhang, J., Yao, X., Ye, J., Wang, X., and Hao, Z. (2008). Seasonal dynamics and spatial distribution patterns of herbs diversity in broadleaved Korean Pine (Pinus koraiensis) mixed forest in Changbai Mountains. Chin. J. Appl. Ecol. 19, 467–473.
Lwanga, J. S., Balmford, A., and Badaza, R. (1998). Assessing fern diversity: Relative species richness and its environmental correlates in Uganda. Biodivers. Conserv. 7:1387. doi: 10.1023/A:1008865518378
Mao, Q., Chen, H., Gurmesa, G. A., Gundersen, P., Ellsworth, D. S., Gilliam, F. S., et al. (2021). Negative effects of long-term phosphorus additions on understory plants in a primary tropical forest. Sci. Total Environ. 798:149306. doi: 10.1016/j.scitotenv.2021.149306
Murphy, S. J., Salpeter, K., and Comita, L. S. (2016). Higher β-diversity observed for herbs over woody plants is driven by stronger habitat filtering in a tropical understory. Ecology 97, 2074–2084. doi: 10.1890/15-1801.1
Nuccio, E. E., Starr, E., Karaoz, U., Brodie, E. L., Zhou, J., Tringe, S. G., et al. (2020). Niche differentiation is spatially and temporally regulated in the rhizosphere. ISME J. 14, 999–1014. doi: 10.1038/s41396-019-0582-x
Oseguera-Olalde, T. K., Bonilla-Valencia, L., Fonseca, R. M., Martínez-Orea, Y., Lorea-Hernández, F., and Castillo-Argüero, S. (2022). Fern diversity in altitude and anthropogenic gradients in a temperate forest in Mexico City, Mexico. Trees Forests People 10:100345. doi: 10.1016/j.tfp.2022.100345
Ramadhanil, R., Tiltrosoedirdjo, S., and Setiadi, D. (2008). Structure and composition of understory plant assemblages of six land use types in the Lore Lindu National Park, Central Sulawesi, Indonesia. Bangladesh J. Plant Taxon. 15, 1–12. doi: 10.3329/bjpt.v15i1.911
Rawlik, M., Kasprowicz, M., Jagodziński, A. M., Kaźmierowski, C., Łukowiak, R., and Grzebisz, W. (2018). Canopy tree species determine herb layer biomass and species composition on a reclaimed mine spoil heap. Sci. Total Environ. 635, 1205–1214. doi: 10.1016/j.scitotenv.2018.04.133
Ren, H., Svenning, J.-C., Mi, X., Lutz, J. A., Zhou, J., and Ma, K. (2022). Scale-dependent species–area relationship: Niche-based versus stochastic processes in a typical subtropical forest. J. Ecol. 110, 1883–1895. doi: 10.1111/1365-2745.13924
Siebert, S. F. (2002). From shade- to sun-grown perennial crops in Sulawesi, Indonesia: Implications for biodiversity conservation and soil fertility. Biodivers. Conserv. 11, 1889–1902. doi: 10.1023/A:1020804611740
Song, Y., Chen, X., and Wang, X. (2005). Studies on evergreen broad-leaved forests of China: A retrospect and prospect. J. East China Norm. Univ. Nat. Sci. 1, 1–8.
Song, Y., Yan, E., and Song, K. (2015). Synthetic comparison of eight dynamics plots in evergreen broadleaf forests, China. Biodivers. Sci. 23:139. doi: 10.17520/biods.2014140
Spicer, M. E., Radhamoni, H. V. N., Duguid, M. C., Queenborough, S. A., and Comita, L. S. (2021). Herbaceous plant diversity in forest ecosystems: Patterns, mechanisms, and threats. Plant Ecol. 223, 117–129. doi: 10.1007/s11258-021-01202-9
Su, X., Zheng, G., and Chen, H. Y. H. (2022). Understory diversity are driven by resource availability rather than resource heterogeneity in subtropical forests. For. Ecol. Manag. 503:119781. doi: 10.1016/j.foreco.2021.119781
Wang, G., Sun, Y., Zhou, M., Guan, N., Wang, Y., Jiang, R., et al. (2021). Effect of thinning intensity on understory herbaceous diversity and biomass in mixed coniferous and broad-leaved forests of Changbai Mountain. For. Ecosyst. 8:53. doi: 10.1186/s40663-021-00331-x
Wang, Y., Cadotte, M. W., Chen, J., Mi, X., Ren, H., Liu, X., et al. (2020). Neighborhood interactions on seedling survival were greatly altered following an extreme winter storm. For. Ecol. Manag. 461:117940. doi: 10.1016/j.foreco.2020.117940
Whitney, G. G., and Foster, D. R. (1988). Overstorey composition and age as determinants of the understorey flora of woods of central New England. J. Ecol. 76, 867–876. doi: 10.2307/2260578
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon 21, 213–251. doi: 10.2307/1218190
Wiharto, M., Wijaya, M., Hamka, L., and Syamsiah. (2021). The understory herbaceous vegetation at tropical mountain forest of mount Bawakaraeng, South Sulawesi. J. Phys. 1899:12002. doi: 10.1088/1742-6596/1899/1/012002
Wu, Z., Zhou, Z., Li, D., Peng, H., and Sun, H. (2003). The areal-types of the world families of seed plants. Plant Divers. 25, 1–3. doi: 10.1111/j.1744-7909.2008.00711.x
Xu, J., Dang, H., Wang, M., Chai, Y., Guo, Y., Chen, Y., et al. (2019). Is phylogeny more useful than functional traits for assessing diversity patterns under community assembly processes? Forests 10:1159. doi: 10.3390/f10121159
Zhu, Y., Zhao, G., Zhang, L., Shen, G., Mi, X., Ren, H., et al. (2008). Community composition and structure of Gutianshan forest dynamic plot in a mid-subtropical evergreen broad-leaved forest, East China. Chin. J. Plant Ecol. 32, 262–273.
Keywords: vascular herbaceous plants, community structure, species diversity, Gutianshan, subtropical forest
Citation: Tian K, Chai P, Wang Y, Chen L, Qian H, Chen S, Mi X, Ren H, Ma K and Chen J (2023) Species diversity pattern and its drivers of the understory herbaceous plants in a Chinese subtropical forest. Front. Ecol. Evol. 10:1113742. doi: 10.3389/fevo.2022.1113742
Received: 01 December 2022; Accepted: 16 December 2022;
Published: 09 January 2023.
Edited by:
Xiang Liu, Lanzhou University, ChinaReviewed by:
Shuai Fang, Institute of Applied Ecology (CAS), ChinaFengwei Xu, Chinese Academy of Forestry, China
Copyright © 2023 Tian, Chai, Wang, Chen, Qian, Chen, Mi, Ren, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunquan Wang,  eXF3YW5nQHZpcC4xMjYuY29t; Jianhua Chen,
eXF3YW5nQHZpcC4xMjYuY29t; Jianhua Chen,  c2t5NzhAempudS5jbg==
c2t5NzhAempudS5jbg==
†These authors have contributed equally to this work
 Kai Tian
Kai Tian Pengtao Chai
Pengtao Chai Yunquan Wang
Yunquan Wang Lei Chen3
Lei Chen3 Xiangcheng Mi
Xiangcheng Mi Haibao Ren
Haibao Ren Keping Ma
Keping Ma