- 1Grupo de I+D+i EVOADAPTA (Evolución Humana y Adaptaciones Económicas y Ecológicas durante la Prehistoria), Departamento Ciencias Históricas, Universidad de Cantabria, Santander, Spain
- 2Ungar Lab, Department of Anthropology, University of Arkansas, Fayetteville, AR, United States
- 3Department of Anthropology, Loyola University Chicago, Chicago, IL, United States
Introduction: Reconstructing the dietary and behavioral strategies of our hominin ancestors is crucial to understanding their evolution, adaptation, and overall way of life. Teeth in general, and dental microwear specifically, provide a means to examine these strategies, with posterior teeth well positioned to tell us about diet, and anterior teeth helping us examine non-dietary tooth-use behaviors. Past research predominantly focused on strategies of adult individuals, leaving us to wonder the role children may have played in the community at large. Here we begin to address this by analyzing prehistoric and historic children through dental microwear texture analysis of deciduous anterior teeth.
Materials and Methods: Four sample groups were used: Neandertals (N = 8), early modern humans (N = 14), historic Egyptians from Amarna (N = 19) and historic high-Arctic Inuit from Point Hope, Alaska (N = 6). Anterior deciduous teeth were carefully cleaned, molded, and cast with high-resolution materials. Labial surfaces were scanned for dental microwear textures using two white-light confocal microscopes at the University of Arkansas, and a soft filter applied to facilitate data comparisons.
Results and Discussion: Results show that dental microwear texture analysis successfully differentiated the samples by all texture variables examined (anisotropy, complexity, scale of maximum complexity, and two variants of heterogeneity). Interestingly, the Neandertal and Point Hope children had similar mean values across all the texture variables, and both groups were significantly different from the Amarna, Egyptian children. These differences suggest diversity in abrasive load exposure and participation in non-dietary anterior tooth-use behaviors. Further analyses and an expanded sample size will help to strengthen the data presented here, but our results show that some prehistoric and historic children took part in similar behaviors as their adult counterparts.
1. Introduction
Dental microwear texture analysis (DMTA) is widely recognized as a useful method to highlight differences in both dietary and behavioral strategies of fossil and modern hominins (Scott et al., 2005, 2006; Ungar et al., 2008, 2010, 2012; El Zaatari, 2010; Krueger and Ungar, 2010, 2012; El Zaatari et al., 2011; Estalrrich et al., 2017). While molar microwear have demonstrated to be especially valuable as a dietary proxy (e.g., Scott et al., 2005; El Zaatari, 2007; Ungar et al., 2008, 2010), incisor microwear texture analyses are useful in understanding behavioral and dietary strategies, as well as abrasive load exposure (Krueger, 2006; Krueger and Ungar, 2010, 2012).
The majority of dental microwear research has focused on adult individuals using permanent enamel. Only a few examples have examined children and their deciduous dentition (Bullington, 1991; Toussaint et al., 2010; Hlusko et al., 2013; El Zaatari et al., 2014; Mahoney et al., 2016; Bas et al., 2020; Kelly et al., 2020). Examinations of children’s diet and behavior are usually limited to weaning and other types of dietary stress, as shown by skeletal indicators of malnutrition, dental enamel defects, and other feeding-practice studies (Skinner, 1997; Lewis, 2007; Prowse et al., 2008; Clement and Freyne, 2012). Even basic dental macrowear analyses in children are limited and are then only used for age estimation or social status purposes (Lewis, 2007; Dawson and Brown, 2013). Why is this the case?
The first difference is the number of teeth, with fewer deciduous than permanent teeth. This is important when considering available sample sizes between child and adult remains. Another distinction is the composition of deciduous and permanent enamel. Deciduous enamel is not only less mineralized than permanent enamel (92% vs. 96%), but also has a higher water content (De Menezes Oliveira et al., 2010). These composition variations make deciduous enamel softer. Moreover, the mean thickness of deciduous enamel is less than half that of its permanent counterpart (1.14 mm vs. 2.58 mm, De Menezes Oliveira et al., 2010). Collectively, these differences cause greater susceptibility to fracture, chipping, and wear in deciduous teeth. Add the limited sample size to these other differences, and it is unsurprising that research has focused on the dietary and behavior reconstruction of adult individuals.
However, there is another important reason dietary and behavioral reconstructions have favored adults and their permanent teeth: the under-representation of children in the archaeological and paleoanthropological record. This is due not only to the lower mortality rates in children, but also to taphonomic processes, which affect the preservation of fragile sub-adult bones and teeth (Lewis, 2007; McFadden et al., 2021). For example, a child’s body skeletonizes faster, becomes readily disarticulated, and the smaller size makes them more attractive to scavengers, allowing for dispersal of body parts (Lewis, 2007). Due to these phenomena, analyses of sub-adult bones and teeth are not as common as in adult individuals, and are limited to those specific, unique sites where children are present, and preservation is exceptional.
Challenges in studying deciduous teeth (and children in general) exist; however, there is evidence that significant information can be gleaned from what is preserved in the archaeological and fossil record. For example, a recent study on the anterior tooth-use behavior of Paleolithic children (Estalrrich and Marín-Arroyo, 2021) revealed comparable behavioral patterns as their adult counterparts, despite these known differences between the deciduous and permanent enamel. These data, along with those demonstrating the efficacy of microwear textures in differentiating hominin anterior tooth-use behaviors in different ecological zones (Krueger et al., 2017, 2019), show we need to push the boundaries of what we know – or thought we could know – about children in the past. The goal of this paper is to present and analyze the largest microwear texture dataset of deciduous anterior teeth of both fossil (Neandertals and early modern humans) and recent individuals (Amarna Egyptians and Point Hope Inuit), and, ultimately, to better recognize the role these children played in daily life.
2. Materials and methods
2.1. Materials
Statistical analyses have previously indicated that microwear textures do not differ significantly across anterior permanent dentition (Krueger et al., 2017). Thus, we included all anterior tooth types to maximize the sample. We analyzed a sample of 47 deciduous incisor and canine teeth, including, based on their cultural context, Neandertal (N = 8); early modern humans (N = 14), and recent modern humans from the historic Egyptians from Amarna (N = 19), and historic high-Arctic Inuit from Point Hope, Alaska (N = 6). Supplementary Table S1 provides details of the studied samples. All the samples studied here are samples curated at different museums, and each museum complies with the ethical issues addressed by each country. By us accessing those samples in order to make the molds and the study, we signed and agreed to follow the required ethical issues.
2.2. Dental microwear texture analysis
The high-resolution replicas were used for analysis of both the fossil and recent human comparative samples. All molds and casts were prepared following standard microwear analysis protocols (Bromage, 1987; Teaford and Oyen, 1989). The labial surface of each specimen was gently cleaned with acetone using cotton swabs. President Jet regular body polysiloxane (Coltene-Whaledent) and Epotek 301 epoxy base and hardener (Epoxy Technologies) were used as the mediums for mold and cast production, respectively. Each tooth was examined for antemortem microwear on the labial surface, next to the incisal edge, using a Sensofar Plμ white-light confocal profiler, Connie (Solarius Development Inc., Sunnyvale, California) and Sensofar Plμ Neox confocal profiler, Wall-e, (Sensofar, Barcelona, Spain) both found at the Department of Anthropology of the University of Arkansas in Fayetteville.
With the Sensofar Plμ white-light confocal profiler four adjacent scans of the enamel surface were taken using a 100x objective lens, yielding a lateral point spacing of 0.18 mm and individual fields of view of 138 × 102 μm, following Scott et al. (2006). We also used Sensofar Plμ Neox confocal profiler in white-light mode with a 100x objective to analyze some specimens. A stitched point cloud of 242 × 181 μm with a lateral spacing of 0.17 μm and a published vertical resolution <1 nm was obtained for each surface.
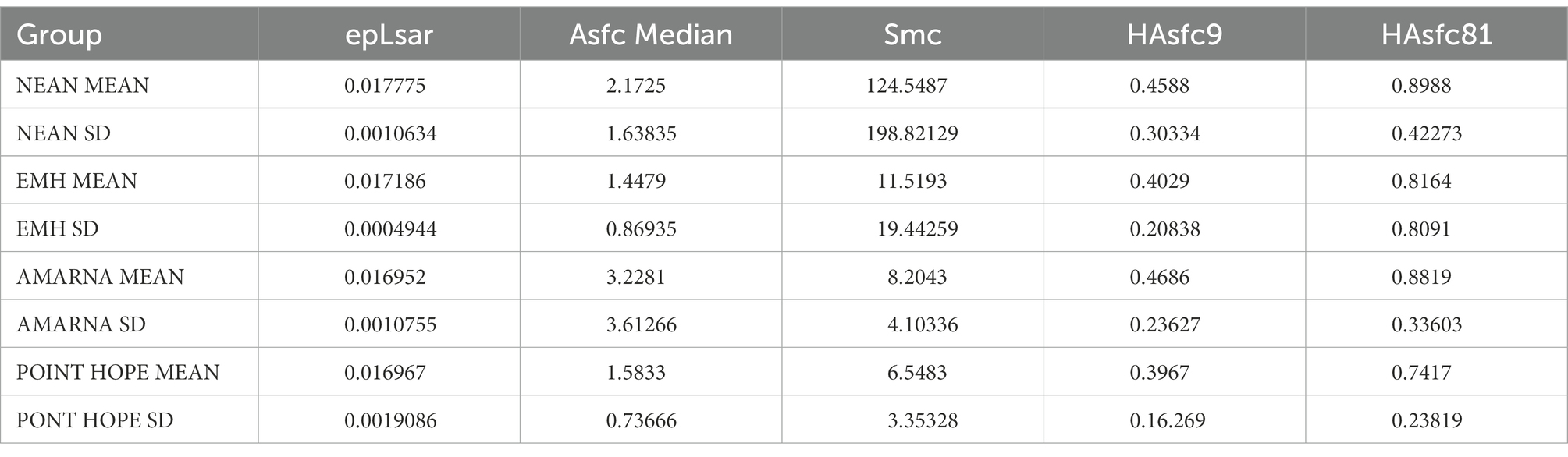
Data from each specimen were then imported to MountainsMap software version 8 (DigitalSurf, Besançon, France), where the scans were processed and calibrated applying the soft filter (Arman et al., 2016) to ensure a standard data collection across different profilers. After this, the scale-sensitive fractal variables were calculated with the same software. Briefly, the variables considered are complexity, scale of maximum complexity, anisotropy, and two variants of heterogeneity (Scott et al., 2006). Complexity or area-scale fractal complexity (Asfc) measures the change in surface roughness at different scales. Scale of maximum complexity (Smc) measures the fine scale limit of the steepest part of the curve described for the Asfc measure. Surfaces dominated by large features on a microscopic scale would have a high Smc. Anisotropy (epLsar) measures the degree of directionality in surface roughness at a fine scale. Heterogeneity of area-scale fractal complexity (HAsfc) reflects variability of complexity across the surface. More heterogeneous surfaces will have higher values. Two forms of this variable are used here: HAsfc 3 × 3 (HAsfc9) and HAsfc 9 × 9 (HAsfc81).
2.3. Statistical analyses
Independent-Samples Kruskal-Wallis tests were completed with the four groups (Neandertal, early modern humans, and recent modern humans from Amarna Egyptians, and Point Hope Inuit) as independent variables and microwear texture variables (epLsar, Asfc, Smc, HAsfc9 and HAsfc81) as dependent. Non-parametric pairwise comparisons to find sources of significant differences in the Kruskal-Wallis tests were then completed. Significance values have been adjusted by the Bonferroni correction for multiple comparisons tests in Supplementary Tables S5a–e. These non-parametric tests were selected as they do not assume normality, are less sensitive to outliers, and appropriate given our limited sample sizes (G. Matthews, pers. comm.). It is important to note we found the same results with both parametric and non-parametric tests.
3. Results
Photosimulations of the occlusal surfaces of selected teeth are shown in Figure 1. Kruskal-Wallis and pairwise comparisons are represented visually in Figure 2. Descriptive statistics for each group are provided in Table 1. Individual microwear texture values are provided in Supplementary Table S2, as well as the test for normality of the microwear data (Supplementary Table S3), Kruskal–Wallis results (Supplementary Table S4), and pairwise comparisons (Supplementary Tables S5a–e).
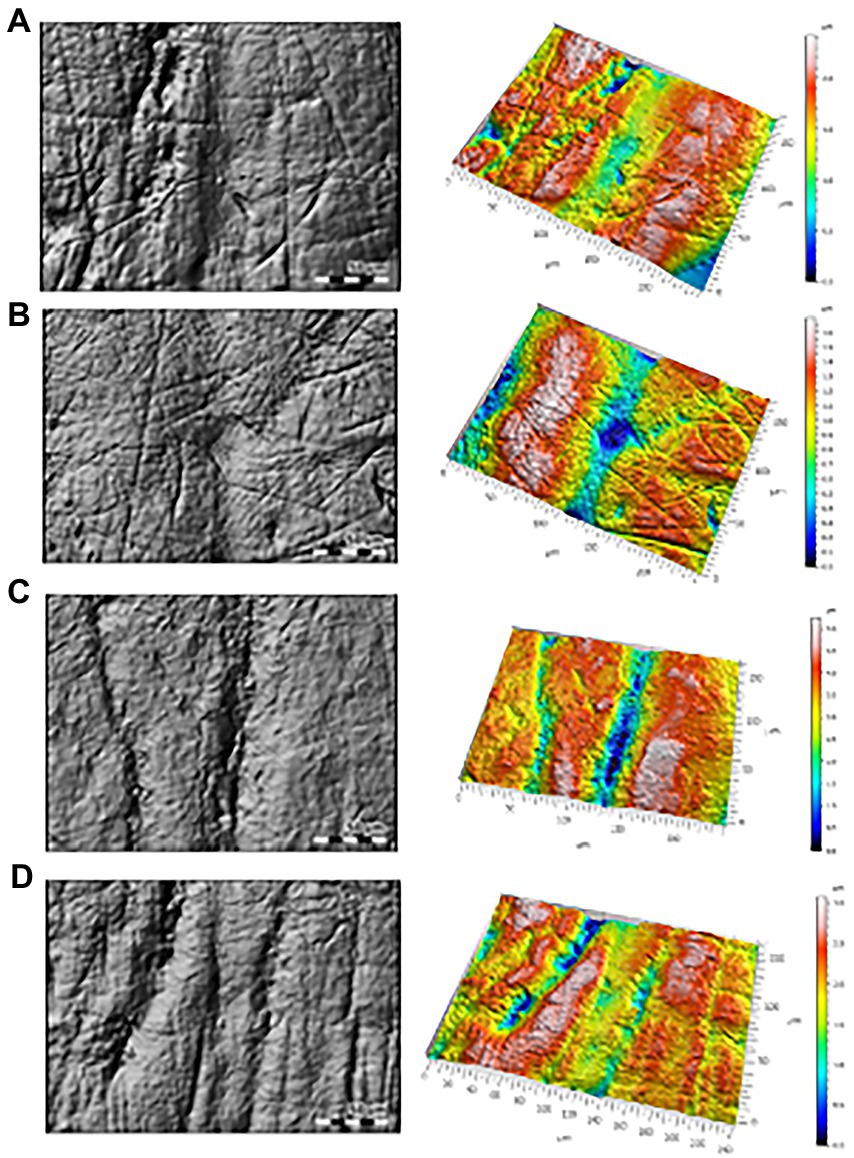
Figure 1. Photosimulation (left) and 3D view (right) of (A) Neandertal Krapina 13, a deciduous left lower lateral incisor; (B) Early modern human from Saint Germain 1970-7-4, a deciduous incisor; (C) Recent modern human from Amarna Egyptian SK304, a left deciduous central incisor; (D) Recent modern human from Point Hope 108, an upper left deciduous central incisor.
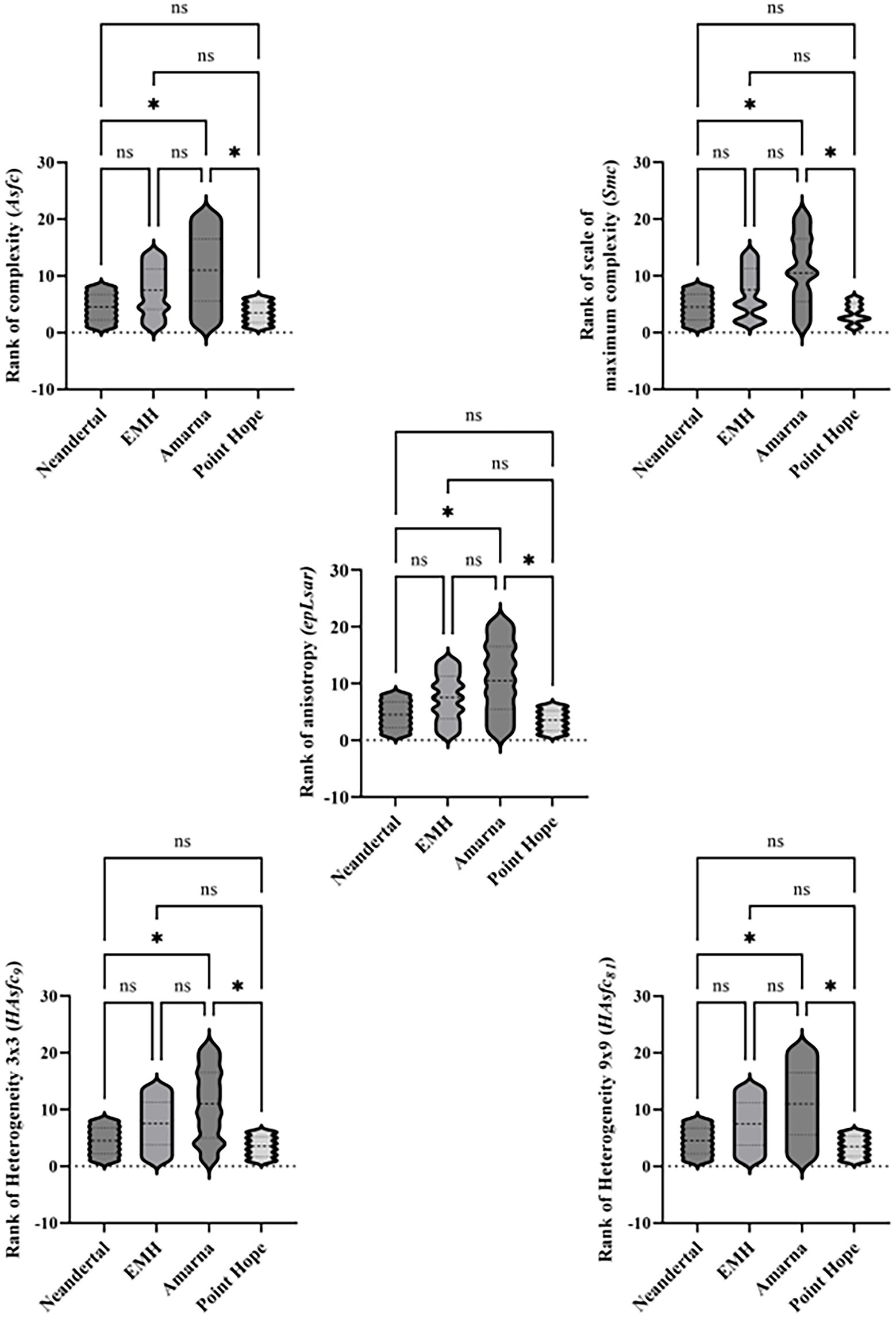
Figure 2. Violin plots with pairwise comparisons of ranked microwear data by variable. Two dotted lines and one single dashed line within each violin plot represent quartiles and median, respectively. * = significant difference and ns = no significant difference between the two groups.
Tests for normality were completed, and except for anisotropy, the microwear texture data were not normally distributed (Supplementary Table S3). As a result, nonparametric tests were used. The Independent-Samples Kruskal–Wallis tests found statistically significant differences at the 0.05 level among the groups in all five microwear texture variables (Figure 2; Supplementary Table S4).
In every microwear texture variable analyzed here, the Neandertal and Point Hope children were significantly different from their Amarna counterparts (Figure 2; Supplementary Tables S5a–S5e).
4. Discussion and conclusions
This study examined a large sample of prehistoric and historic deciduous teeth to better understand the role children played within society. Were children behaving like their adult counterparts? If so, can we glean what those teeth-as-tools behaviors could have been? If not, at what age were they expected to contribute to the community at large? While this study has answered some of these questions, we also need to continue searching for more evidence.
The samples studied here include Neandertal and early modern human children from various sites and a wider time range, whereas the historic Point Hope and Amarna samples are from the same site and time (see Supplementary Table S1). We would expect more variability within the fossil microwear values, as they are geographically and temporally distinct. Interestingly, our data show that when analyzing these groups, every dental microwear texture variable used here distinguish the Neandertal and Point Hope children from those of the Amarna Egyptians.
This certainly is not the first time that Neandertal and high-Arctic aboriginal samples have been El Zaatari et al., 2011. Indeed, decades of research, especially regarding anterior tooth-use behaviors, heavily associated Neandertals and their unique anterior tooth wear patterns with Arctic groups who used their anterior teeth as a clamp or third hand during animal hide processing (Brace, 1967, 1975, 1979; Brace and Molnar, 1967; Ryan, 1980; Brace et al., 1981). However, all these analyses focus on adult individuals. This is the first that links similar microwear textures between Neandertal and high-Arctic children. This suggests that the Neandertal and Point Hope Inuit children, at least those sampled here, were completing similar anterior tooth-use behaviors. Whether that means they were eating similar dietary items, had similar abrasive loads, and/or were completing tooth-use behaviors requires a deeper look at the values.
The microwear texture values presented here were collected using two different white-light confocal profilers, and a filter was applied to make these data comparable (Arman et al., 2016); however, we have not applied that filter to other published microwear texture datasets. Even if we did, there are limited available datasets of deciduous teeth from which to make comparisons. A confounding issue is understanding if microwear forms similarly or differently between permanent and deciduous enamel, as studies have found conflicting results (Krueger, 2016; Mahoney et al., 2016; Kelly et al., 2020). Therefore, our interpretations should be viewed with caution, and seen as preliminary until these issues are resolved.
Largely viewed within the realm of “hunter-gatherers,” Neandertals relied on a mixed diet and were highly dependent on the ecogeographic setting in which they lived (El Zaatari et al., 2011; Fiorenza et al., 2011). It is parsimonious to assume that their children relied on a similar diet and were also constrained by their environment. Indeed, stable isotope research of prehistoric juveniles in California suggests some were foraging independently, in addition to parent-provided resources, especially during high-stress times associated with social or climate change (Greenwald et al., 2016; Fournier et al., 2022). It is not unreasonable to assume that Neandertal children were subsisting on similar diets as their adult counterparts, and perhaps were even able to forage independently when the need arose.
Neandertal adults were found to perform different non-dietary anterior tooth-use behaviors based on their habitat (Krueger et al., 2017). Using a comparative approach, it was found that Neandertals in cold, open environments had similar microwear textures to high-Arctic Alaskan aboriginal groups who used their anterior teeth in clamping and grasping behaviors related to animal hide preparation for clothing production. Other Neandertals in more mixed environments were using their anterior teeth for other behaviors, such as wood softening or cordage production (Krueger et al., 2017). Interestingly, a preliminary study of Pleistocene deciduous teeth from northern Spain indicated they too showed the characteristic dental wear features associated to para-masticatory or cultural-related dental wear, including toothpick use (Estalrrich and Marín-Arroyo, 2021). When previous analyses on diet and tooth-use behaviors are considered, they suggest that Neandertal adults and children were subsisting on similar dietary and behavioral strategies that are heavily influenced by their eco-geographic setting.
The Point Hope Inuit were also considered “hunter-gatherers,” and their diet largely consisted of land and sea mammals (especially caribou, whale, walrus, and seal), fish, and edible plants (Larsen and Rainey, 1948; Lester and Shapiro, 1968; Dabbs, 2009; Brubaker et al., 2010; El Zaatari, 2014). They took part in non-dietary anterior tooth-use behaviors in the form of wood softening, clamping and grasping tasks related to hide preparation, and sinew cord production, and were, at times, subjected to high abrasive loads (Burch, 1981; Foote, 1992). Some of these individuals lived seasonally at Point Hope, while others lived there year-round, which is located 125 miles north of the Arctic Circle (Larsen and Rainey, 1948; Dabbs, 2009).
On the other hand, the Amarna Egyptians were not “hunter-gatherers,” but were excavated from the non-elite South Tombs Cemetery and date from 3,300 to 3,280 BP (Rose, 2006). This cemetery is composed of an estimated 5,000 individuals from different occupations and/or socio-economic positions but did not hold elite or royal status (Dabbs et al., 2015). The excavated individuals showed high rates of subadult death, workload stress, trauma, and nutritional deficiencies (Rose and Zabecki, 2009; Dabbs et al., 2015). Adult microwear analysis suggests this sample was reliant on tough food, most likely bread, and the desert environment at Amarna would make sand a likely adherent abrasive (Krueger and Scott, in press).
The Neandertal and Point Hope children, both from “hunter-gatherer” groups, had significantly lower complexity (Asfc), scale of maximum complexity (Smc) and heterogeneity (HAsfc9 and HAsfc81) than their Amarna counterparts (Table 1, Supplementary Table S2, Figure 2). While these variables are not often used in texture analyses of anterior teeth, they are useful for molar analyses, especially regarding the fracture properties of foods and abrasive loads. Here, we propose that the significantly lower values of these three variables for the Neandertal and Point Hope Inuit children show differences in abrasive loads from the Amarna children. While the Amarna children were subjected to a desert environment with little-to-no tree cover, their higher values may indicate their increased exposure to diverse types of abrasives that were adherent to their food. On the other hand, the lower values of the Neandertal and Point Hope Inuit children suggest more limited exposure to abrasives, which could be due to their reliance on a mixed diet.
The Neandertal and Point Hope children had significantly lower anisotropy (epLsar) than their Amarna counterparts. This variable is more heavily used in anterior tooth texture analyses and indicates the use of these teeth in non-dietary behaviors (e.g., clamping, grasping, tool retouching, etc.; Krueger and Ungar, 2012; Krueger et al., 2017, 2019). These results suggest both Neandertal and Point Hope children were taking part in non-dietary anterior tooth-use behaviors, while the Amarna children were not. While we are hesitant to suggest what specific types of behaviors in which these children may have been engaging, perhaps it was related to clamping and grasping behaviors like those found in their adult counterparts; however, an expanded sample size and comparative datasets are necessary to strengthen this idea.
It is worth noting that no statistically significant differences were found between the early modern human children and neither the Neandertal nor historic modern human counterparts (see Supplementary Tables S5a–S5e). Perhaps this is simply a reflection of our limited sample size, and building this dataset is necessary to recognize potential differences. Or, perhaps this reflects a more diverse diet, abrasive load exposure, or landscape in which these children lived. However, these data provide the largest dataset from which to work in the future, and we look forward to continued analyses to reinforce or refute the ideas posited here.
In conclusion, these datasets provide a crucial pathway to understanding the role children played in the Paleolithic and beyond. Perhaps this is a starting point to investigating complex issues like independent foraging in Paleolithic children, especially considering the stress that climate change may have had on their dietary and behavioral strategies. It also helps us understand how teaching and learning may have transpired between adults and children, especially if the latter are performing non-dietary anterior tooth-use behaviors similar to their adult counterpart. We hope this creates a larger platform for additional analyses surrounding Paleolithic children, so that we may better recognize an entire community’s contribution to survival.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AE and KK designed the research, analyzed data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the AE is supported by H2020-MSCA-IF project No. 891529 (3DFOSSILDIET).
Acknowledgments
We are sincerely grateful to Peter S. Ungar for allowing us the use of the microscope facilities at the Department of Anthropology in the University of Arkansas and encouraging discussions on microwear and diet. We also acknowledge Greg Matthews for his advice on statistical analyses and Emily Hallett and Jacopo Cerasoni for their input on data visualization. Thank you to Jean-Jacques Hublin, Manuel R. González Morales, David Frayer, Antonio Rosas, Sireen El Zaatari, F. Igor Gutiérrez Zugasti, Cristina Vega Maeso and Borja González Rabanal for access to some of the fossils sampled here. We thank Barry Kemp and Anna Stevens for permission to mold the Amarna individuals, Museo de Prehistoria y Arqueología de Cantabria (Spain) for permission to mold the El Castillo tooth, and the American Museum of Natural History for permission to mold the Point Hope sample.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1066680/full#supplementary-material
References
Arman, S. D., Ungar, P. S., Brown, C. A., DeSantis, L. R. G., Schmidt, C., and Prideaux, G. J. (2016). Minimizing inter-microscope variability in dental microwear texture analysis. Surf. Topogr. Metrol. Prop. 4:024007. doi: 10.1088/2051-672X/4/2/024007
Bas, M., Le Luyer, M., Kanz, F., Rebay-Salisbury, K., Queffelec, A., Souron, A., et al. (2020). Methodological implications of intra-and inter-facet microwear texture variation for human childhood paleo-dietary reconstruction: insights from the deciduous molars of extant and medieval children from France. J. Archaeol. Sci. Rep. 31:102284. doi: 10.1016/j.jasrep.2020.102284
Brace, C. L. (1967). Environment, tooth form and size in the Pleistocene. J. Dent. Res. 46, 809–816. doi: 10.1177/00220345670460053501
Brace, C. L. (1975). Comment on: did La Ferrassie I use his teeth as a tool? Curr. Anthropol. 16, 396–397.
Brace, C. L. (1979). Krapina, “classic” Neanderthals, and the evolution of the European face. J. Hum. Evol. 8, 527–550. doi: 10.1016/0047-2484(79)90043-5
Brace, C. L., and Molnar, S. (1967). Experimental studies in human tooth wear: I. Am. J. Phys. Anthropol. 27, 213–221. doi: 10.1002/ajpa.1330270210
Brace, C. L., Ryan, A. S., and Smith, B. H. (1981). Comment: tooth wear in La Ferrassie man. Curr. Anthropol. 22, 426–430.
Bromage, T. (1987). The scanning electron microscopy replica technique and recent applications to the study of fossil bone. Scanning Microsc. 1, 607–613.
Brubaker, M., Berner, J., Bell, J., Warren, J., and Rolin, A. (2010). Climate Change in Point Hope, Alaska: Strategies for Community Health ANTHC Center for Climate and Health, 1–40.
Bullington, J. (1991). Deciduous dental microwear of prehistoric juveniles from the lower Illinois River valley. Am. J. Phys. Anthropol. 84, 59–73. doi: 10.1002/ajpa.1330840106
Burch, E. S. (1981). The Traditional Eskimo Hunters of Point Hope, Alaska : 1800–1875. North Slope Borough, Barrow, AK.
Clement, A. F., and Freyne, A. (2012). A revised method for assessing tooth wear in deciduous dentition. in Proceedings of the twelfth annual conference of the British Association for Biological Anthropology, 119–129.
Dabbs, G. R. (2009). Health and nutrition at prehistoric point Hope, Alaska: Application and critique of the Western hemisphere health index. Ph.D. Dissertation. Fayetteville, AR: University of Arkansas.
Dabbs, G. R., Rose, J. C., and Zabecki, M. (2015). “The bioarchaeology of Akhenaten: unexpected results from a capital city,” in Egyptian Bioarchaeology: Humans, Animals, and the Environment. eds. S. Ikram, J. Kaiser, and R. Walker (Leiden: Sidestone Press), 43–52.
Dawson, H., and Brown, K. R. (2013). Exploring the relationship between dental wear and status in late medieval subadults from England. Am. J. Phys. Anthropol. 150, 433–441. doi: 10.1002/ajpa.22221
De Menezes Oliveira, M. A. H., Torres, C. P., Gomez-Silva, J. M., Chinelatti, M. A., De Menezes, F. C. H., Palma-Dibb, R. G., et al. (2010). Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 73, 572–577. doi: 10.1002/jemt.20796
El Zaatari, S. (2007). Ecogeographic variation in Neandertal dietary habits: Evidence from microwear texture analysis. [Ph.D. Dissertation]. [New York, NY]. Stony Brook University.
El Zaatari, S. (2010). Occlusal microwear texture analysis and the diets of historical/prehistoric hunter-gatherers. Int. J. Osteoarchaeol. 20, 67–87.
El Zaatari, S. (2014). “The diets of the Ipiutak and Tigara (point Hope, Alaska): evidence from occlusal molar microwear texture analysis,” in The foragers of point Hope: Bioarcheology on the edge of the Alaskan Arctic. eds. C. E. Hilton, B. M. Auerbach, and L. W. Cowgill (Cambridge: Cambridge University Press), 120–137. doi: 10.1017/CBO9781139136785.011
El Zaatari, S., Grine, F. E., Ungar, P. S., and Hublin, J. J. (2011). Ecogeographic variation in Neandertal dietary habits: evidence from occlusal microwear texture analysis. J. Hum. Evol. 61, 411–424. doi: 10.1016/j.jhevol.2011.05.004
El Zaatari, S., Krueger, K. L., and Hublin, J. J. (2014). “Dental microwear texture analysis and the diet of the Scladina child,” in The Juvenile Neanderthal Facial Remains from Scladina cave (Belgium). eds. M. Tousaint and D. Bonjean (Liège (Belgium): Etudes et Recherches Archeologiques de l'Université de Liège), 363–368.
Estalrrich, A., El Zaatari, S., and Rosas, A. (2017). Dietary reconstruction of the El Sidròn Neandertal familial group (Spain) in the context of other Neandertal and modern hunter-gatherer groups. A molar microwear texture analysis. J. Hum. Evol. 104, 13–22. doi: 10.1016/j.jhevol.2016.12.003
Estalrrich, A., and Marín-Arroyo, A. B. (2021). Evidence of habitual behavior from non-alimentary dental wear on deciduous teeth from the middle and upper Paleolithic Cantabrian region, Northern Spain. J. Hum. Evol. 158:103057. doi: 10.1016/j.jhevol.2021.103047
Fiorenza, L., Benazzi, S., Tausch, J., Kullmer, O., Bromage, T. G., and Schrenk, F. (2011). Molar macrowear reveals Neanderthal eco-geographic dietary variation. PLoS One 6:e14769. doi: 10.1371/journal.pone.0014769
Foote, B. A. (1992). The Tigara Eskimos and Their Environment. North Slope Borough, Commission on Iñupiat history, Language, and Culture, Point Hope.
Fournier, N. A., Thornton, E. K., Arellano, M. W., and Leventhal, A. (2022). Stable isotope reconstruction of weaning and childhood diet during times of change: an examination of life history and health of san Franscisco Bay Area juveniles. J. Archaeol. Sci. Rep. 44:103495
Greenwald, A. M., Eerkens, J. W., and Bartelink, E. J. (2016). Stable isotope evidence of juvenile foraging in prehistoric Central California. J. Archaeol. Sci. Rep. 7, 146–154. doi: 10.1016/j.jasrep.2016.04.003
Hlusko, L., Carlson, J. P., Guatelli-Steinberg, D., Krueger, K. L., Mersey, B., Ungar, P. S., et al. (2013). Neanderthal teeth from Moula-Guercy, Ard eche, France. Am. J. Phys. Anthropol. 151, 477–491. doi: 10.1002/ajpa.22291
Kelly, C. D., Schmidt, C. W., and D’Anastasio, R. (2020). Dental microwear texture analysis in deciduous teeth. In: Dental Wear in Evolutionary and Biocultural Contexts. 169–186.
Krueger, K. L. (2006). Incisal dental microwear of the prehistoric point Hope communities: A dietary and cultural synthesis. MA Thesis. Kalamazoo, MI: Western Michigan University.
Krueger, K. L. (2016). Dental microwear texture differences between permanent and deciduous enamel. Am. J. Phys. Anthropol. 159 (S62), 196–197.
Krueger, K. L., and Scott, J. R. (in press). “Dietary and behavioral trends in the south tombs cemetery individuals at Amarna,” in Amarna: The South Tombs Cemetery. eds. G. R. Dabbs, J. C. Rose, and A. Stevens, Vol. 2 (Egypt Exploration Society: Cairo)
Krueger, K. L., and Ungar, P. S. (2010). Incisor microwear textures of five bioarchaeological groups. Int. J. Osteoarchaeol. 20, 549–560. doi: 10.1002/oa.1093
Krueger, K. L., and Ungar, P. S. (2012). Anterior dental microwear texture analysis of the Krapina Neandertals. Cent. Eur. J. Geosci. 4, 651–662.
Krueger, K. L., Ungar, P. S., Guatelli-Steinberg, D., Hublin, J.-J., Pérez-Pérez, A., Trinkaus, E., et al. (2017). Anterior dental microwear textures show habitat-driven variability in Neandertal behavior. J. Hum. Evol. 105, 13–23. doi: 10.1016/j.jhevol.2017.01.004
Krueger, K. L., Willman, J. C., Matthews, G. J., Hublin, J. J., and Pérez-Pérez, A. (2019). Anterior tooth-use behaviors among early modern humans and Neandertals. PLoS One 14:e0224573. doi: 10.1371/journal.pone.0224573
Larsen, H., and Rainey, F. (1948). Ipiutak and the Arctic whale hunting culture. Anthropological Papers of the American Museum of Natural History, vol. 42, New York: Nabu Press.
Lester, C. W., and Shapiro, H. L. (1968). Vertebral arch defects in the lumbar vertebrae of pre-historic American Eskimos. Am. J. Phys. Anthropol. 28, 43–47. doi: 10.1002/ajpa.1330280113
Mahoney, P., Schmidt, C. W., Deter, C., Remy, A., Slavin, P., Johns, S. E., et al. (2016). Deciduous enamel 3D microwear texture analysis as an indicator of childhood diet in medieval Canterbury, England. J. Archaeol. Sci. 66, 128–136. doi: 10.1016/j.jas.2016.01.007
McFadden, C., Muir, B., and Oxenham, M. F. (2021). Determinants of infant mortality and representation in bioarchaeological samples: a review. Am. J. Bio. Anthropol. 177, 196–206. doi: 10.1002/ajpa.24406
Prowse, T. L., Saunders, S. R., Schwarcz, H. P., Garnsey, P., Macchiarelli, R., and Bondioli, L. (2008). Isotopic and dental evidence for infant and young child feeding practices in an imperial roman skeletal sample. Am. J. Phys. Anthropol. 137, 294–308. doi: 10.1002/ajpa.20870
Rose, J. C. (2006). Paleopathology of the commoners at tell Amarna, Egypt, Akhenaten’s capital city. Mem. Inst. Oswaldo Cruz 101, 73–76. doi: 10.1590/S0074-02762006001000013
Rose, J. C., and Zabecki, M. (2009). “The commoners of tell el-Amarna,” in Beyond the horizon: Studies in Egyptian art, archaeology and history in honour of Barry J. eds. S. Ikram and A. Dodson, Kemp, vol. 2 (Cairo: Supreme Council of Antiquities Press), 408–422.
Ryan, A. S. (1980). Anterior dental microwear in hominid evolution: Comparisions with human and nonhuman primates. PhD Dissertation. Ann Arbor (MI): University of Michigan.
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Childs, B. E., Teaford, M. F., et al. (2006). Dental microwear texture analysis: technical considerations. J. Hum. Evol. 51, 339–349. doi: 10.1016/j.jhevol.2006.04.006
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Grine, F. E., Teaford, M. F., et al. (2005). Dental microwear texture analysis reflects diets of living primates and fossil hominins. Nature 436, 693–695. doi: 10.1038/nature03822
Skinner, M. (1997). Dental wear in immature late Pleistocene hominines. J. Archaeol. Sci. 24, 677–700. doi: 10.1006/jasc.1996.0151
Teaford, M. F., and Oyen, O. (1989). Live primates and dental replication: new problems and new techniques. Am. J. Phys. Anthropol. 80, 73–81. doi: 10.1002/ajpa.1330800109
Toussaint, M., Olejniczak, A. J., El Zaatari, S., Cattelain, P., and Pirson, S. (2010). The Neandertal lower right deciduous second molar from the “Trou de l'Abîme” at Couvin, Belgium. J. Hum. Evol. 58, 56–67. doi: 10.1016/j.jhevol.2009.09.006
Ungar, P. S., Grine, F. E., and Teaford, M. F. (2008). Dental microwear and diet of the PlioPleistocene hominin Paranthropus boisei. PLoS One 3, 1–6. doi: 10.1371/journal.pone.0002044
Ungar, P. S., Krueger, K. L., Blumenschine, R. J., Njau, J., and Scott, R. S. (2012). Dental microwear texture analysis of hominins recovered by the Olduvai landscape paleoanthropology project, 1995–2007. J. Hum. Evol. 63, 429–437. doi: 10.1016/j.jhevol.2011.04.006
Keywords: labial surface, deciduous enamel, dietary reconstruction, prehistoric children, historic populations, Neandertal
Citation: Estalrrich A and Krueger KL (2022) Behavioral strategies of prehistoric and historic children from dental microwear texture analysis. Front. Ecol. Evol. 10:1066680. doi: 10.3389/fevo.2022.1066680
Edited by:
Maciej Tomasz Krajcarz, Institute of Geological Sciences (PAN), PolandReviewed by:
Paweł Dąbrowski, Wroclaw Medical University, PolandYuichi Naito, Central Research Institute of Electric Power Industry (CRIEPI), Japan
Copyright © 2022 Estalrrich and Krueger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Almudena Estalrrich,  YWVzdGFscnJpY2hhbGJvQGdtYWlsLmNvbQ==
YWVzdGFscnJpY2hhbGJvQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Almudena Estalrrich
Almudena Estalrrich Kristin L. Krueger
Kristin L. Krueger