
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 08 December 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1058584
This article is part of the Research Topic Behavioral Ecological Insights into Organismal Responses to Anthropogenic Environmental Change: A Multi-Stress Perspective View all 8 articles
The urban environment is associated with a multitude of challenges and stressors for populations of wild species from the surrounding natural environment. Among those, habitat fragmentation and noise pollution are suspected to have negative effects on the behavior and physiology of free-living birds in urban areas. Exposure in early life and chronic exposure to anthropogenic noise could be particularly deleterious, with short-and long-term consequences. In this study, we investigated if noise levels in city parks affect the distribution and reproductive success of two common bird species in the urban environment, the great tit (Parus major) and the blue tit (Cyanistes caeruleus) and if vegetation cover could mitigate those effects. We predicted that high noise levels might correlate with a decreased nest-box occupancy rate, a delayed laying date or a decreased clutch size, hatching, and fledging success. On the contrary, vegetation cover was expected to correlate positively with nest occupancy rate, advanced laying date, increased clutch size, hatching, and fledging success. We used data from population monitoring collected between 2012 and 2019 in parks and green public spaces in the city center and suburbs of Paris, France, and did not find any correlation between nest occupancy rates and noise levels or vegetation cover for both species. Laying date was not significantly related to anthropogenic noise in any species but was delayed with increasing vegetation cover in the great tit, while we did not find any association with clutch size. Hatching success in blue tits negatively correlated with increasing noise levels, and positively with increasing vegetation coverage. Finally, we did not find any correlation between anthropogenic noise or vegetation cover and the clutch size or fledging success in both species. In this study, two closely related species that share a common environment show a different sensibility to environmental parameters during reproduction, a key period for population maintenance. It also highlights the importance of considering multiple parameters when studying wild populations living in the urban environment.
Urbanization can be defined as the process characterized by city growth and associated structures to the detriment of rural spaces (Pickett et al., 2011). Urbanization negatively affects avian evolutive and taxonomic diversity because some species are excluded from urban areas (Chace and Walsh, 2006; McKinney, 2006), and because the density of bird species in cities is often lower compared to other areas (Aronson et al., 2014). However, other studies found that avian functional diversity is higher in the urban than in rural or semi-natural environments, due to potentially higher habitat diversity (Hagen et al., 2017). On the other hand, species that have been living in cities for a long time show relatively larger population densities in urban as compared to rural habitats (Møller et al., 2012). However, even if numerous avian species conquered the urban environment, it is a challenging environment that differently affects species depending on their ecological and life history traits. The question of the urban environment as a suitable habitat for bird species remains unresolved. Indeed, theory suggests that animals should select their breeding habitat such that fitness is maximized. Nonetheless, numerous studies found a mismatch between habitat preferences and fitness outcomes (Chalfoun and Schmidt, 2012). Urban environments tend to act as ecological traps, with features favorable to adult survival like warmer weather and milder winters in the city than in surrounding rural habitats (Haggard, 1990; Lepczyk et al., 2017) and higher availability of resources (food and nesting opportunities) (Isaksson, 2018; Burt et al., 2021). The study of paired populations of birds has shown that urban populations are characterized by an advanced reproductive phenology and reduced reproductive success due to smaller clutch size, lighter fledgling body mass and fewer fledged offspring (reviewed in Chamberlain et al., 2009).
Urbanization changes habitat and increases habitat fragmentation which particularly impacts bird species when native vegetation is replaced by crops or urban structures creating barriers to species dispersion (Marzluff and Ewing, 2001; Crooks et al., 2004). In the remaining vegetation patches, native species are often replaced by exotic decorative species (Marzluff and Ewing, 2001). The change in vegetation composition has consequences on food quantity and quality available for insectivore species as it induces a change in insect communities (Helden et al., 2012; Pollock et al., 2017). Together with anthropogenic sources of food (Burt et al., 2021) this altered food quality and quantity in urban environments may lead to compensatory changes in parental investment in nestling feeding (Isaksson and Andersson, 2007), which may have consequences on adult survival and reproductive success. The total-foliage hypothesis also suggests that vegetation is an important feature in the habitat for birds because it should reduce predation, a denser vegetation coverage providing better protection from predators (Martin and Roper, 1988; Jackson et al., 2012; Jara et al., 2020). A recent study found that a higher proportion of grass cover and a higher tree density in the city were beneficial and increased diversity and abundance of birds over seasons (Mühlbauer et al., 2021). Habitat modifications observed in urbanized areas may thus affect species through resources and interspecific interaction, but green patches could help maintain avian biodiversity in cities.
Urbanization is also characterized by several and simultaneous environmental stress factors that have consequences at behavioral and physiological levels for species living in cities: chemical pollution, artificial light at night, noise pollution and human disturbances (Isaksson, 2018). Indeed, urban populations exhibit an altered response to stress compared to forest populations (Partecke et al., 2006). Among these stress factors, noise pollution is of particular interest because of its ubiquitous nature, as it results from any anthropogenic noise caused by human activities: road traffic, rail traffic, air traffic, and industrial and commercial activities. Assessing the effects of noise pollution on wildlife is challenging, but growing scientific research provided evidence that anthropogenic noise is detrimental to natural ecosystems and wildlife, despite different sensitivity across taxa (Shannon et al., 2016).
Anthropogenic noise in the urban environment is often characterized by an increased number of high-intensity noise events and elevated and homogenized background sound levels. It can disrupt avian populations at different biological levels, including behavior. It has been reported for example that exposure to peak noise levels near an airport increased antipredator vigilance behavior and decreased the time spent on foraging activities, altering the birds’ time, and thus potentially energetic, budget (Klett-Mingo et al., 2016). Noise pollution induces a change in song patterns and vocal communication, with great tits (Parus major) singing with a higher minimum frequency (Slabbekoorn and Peet, 2003), shorter and faster songs (Slabbekoorn and den Boer-Visser, 2006) in urban areas compared to rural areas. Similar observations were done in white-crowned sparrows (Zonotrichia leucophrys nuttalli) populations (Derryberry et al., 2016). These modifications in song patterns are hypothesized to counteract the masking of song and vocal communication by the low-frequency background noise in cities, but some studies found that it is not enough for alarm calls to be heard over traffic noise (Templeton et al., 2016). In addition to impaired sexual selection and antipredator behavior, this disruption of acoustic communication could have further consequences for bird species providing biparental care and living in the urban environment. Indeed, vocal communication between male and female is known to be crucial for pair synchronization, during incubation and nestling feeding activities, and to affect breeding success (Mariette and Griffith, 2012; Bebbington and Hatchwell, 2016; Boucaud et al., 2016a,b; Boucaud et al., 2017; Kavelaars et al., 2019). Noise pollution may therefore have direct negative effects on avian reproduction through behavioral changes or impairments.
Noise pollution also has adverse effects on birds’ body condition and physiology, with indirect consequences on reproductive success. Chronic exposure to low-frequency noise in house sparrows (Passer domesticus) reduced fitness as a result of producing fewer fledglings, of lower body mass and lower probability to recruit in the population (Schroeder et al., 2012). In another study, however, experimental exposure to traffic noise during growth resulted in reduced nestling telomere lengths in the absence of any effect on corticosterone levels, body condition or fledging success (Meillère et al., 2015). In tree swallow (Tachycineta bicolor) populations, exposure to traffic noise positively correlated with increased basal cortisol levels and with greater telomere attrition, potentially reducing post-fledging survival (Injaian et al., 2019). In a study of three passerine species, an increase in noise exposure was associated with a decrease in baseline plasma corticosterone in females and nestlings, and an increase in stress-induced nestling corticosterone response (Kleist et al., 2018). Finally, in great tits, increasing background noise levels were associated with an increase in nestlings’ plasma haptoglobin levels, an indicator of physiological condition and health, reflecting increased inflammatory processes (Raap et al., 2017), and with a reduction in telomere length in small brood members (Grunst et al., 2020). The fact that these negative effects of anthropogenic noise on bird body condition, physiology and reproductive success can be detected at the community level in different species suggests that noise pollution acts as a chronic and unavoidable stressor (Ware et al., 2015; Kleist et al., 2018). Noise pollution is therefore likely to have short-and long-term consequences on free-living bird populations in cities.
Although the number of urban ecology studies investigating the effects of habitat quality and pollutions on birds is increasing, the vast majority of them contrast urban versus rural populations, and seldom investigate how varying levels of habitat quality and environmental stressors affect reproductive life history traits and reproductive success within the urban habitat. In addition, the potential interactive effects of habitat quality and environmental stressors are rarely investigated. In this study, we investigated if noise pollution in city parks affects the distribution and reproductive phenology, investment and success and if vegetation cover could mitigate these effects in two model species widely studied in evolutionary and environmental research that are common in the urban environment, the great tit (Parus major) and the blue tit (Cyanistes caeruleus). Previous studies comparing urban and nearby rural populations across Europe reported that urbanization has negative effects on the reproductive success of these species (Solonen, 2001; Bailly et al., 2016; Glądalski et al., 2017; de Satgé et al., 2019) and drive phenotypic differences (Biard et al., 2017) when compared to rural populations. However, which environmental factors underlie these patterns and at which stage of reproduction they act is not precisely known. In particular, studies investigating how the degree of vegetation cover within a territory may influence breeding success in the urban habitat are lacking. One study investigated the relationship between two stressors in the urban environment (noise and artificial light at night) and birds’ physiology (Raap et al., 2017). Other studies experimentally investigated the combined effect of two stressors (noise and artificial light at night) on activity patterns (Dominoni et al., 2020b), or of a stressor (artificial light at night) and a mitigating factor (spring temperature) on the timing of reproduction (Dominoni et al., 2020a), but they were conducted in captivity or in a forest environment, respectively.
In a first step toward addressing these issues, we used a correlative approach using population surveys conducted in city parks and green spaces over 8 consecutive reproductive seasons, and measures of noise pollution and vegetation cover surrounding the nest boxes. We hypothesized that high anthropogenic noise levels in the city could impair breeding phenology, and reproductive investment and success, and that a high vegetation coverage should positively affect reproduction, and might moderate the effects of noise, mainly by providing an environment with more food resources. We predicted that nest boxes in noisier areas would present lower occupancy rates. If pairs do not avoid high noise level nest boxes, we expected a delayed laying date, reduced clutch size, and reduced reproductive success (hatching and fledging probability and success) for pairs breeding and raising nestlings at chronic, high noise levels. The great tit and the blue tit are closely related passerine species, but they differ in their foraging ecology and reproductive investment (Gosler, 1993; Stenning, 2018), and may therefore exhibit different environmental sensitivities. In this case, we would expect the effects of noise pollution and vegetation cover to differ between the two species and the reproductive stage considered.
Data were collected from 2012 to 2019, in urban populations of great tits and blue tits, breeding in the Paris city area; in city parks and cemeteries of Paris (48°51′N, 02°20′E; six green spaces) and Rueil-Malmaison (48°52′N, 02°11′E; three green spaces). These study sites contained 69 and 29 nest boxes respectively (Schwegler wood-concrete nest boxes 2M, hole diameter 32 mm), whose coordinates were recorded and did not change during the study (Figure 1A, Supplementary Figure 1). Nests were regularly visited from late March to June to determine the laying date (the date the first egg in a clutch was laid, in Julian day from January 1st), incubation date, clutch size (number of eggs laid), the number of unhatched eggs (used to determine the number of successfully hatched eggs and calculate hatching success), the number of fledglings (brood size 14–16 days after hatching), and finally the number of dead nestlings in the nest after fledging (used to determine the number of successfully fledged nestlings and calculate fledging success). Data presented here only include first clutches of each reproductive season.
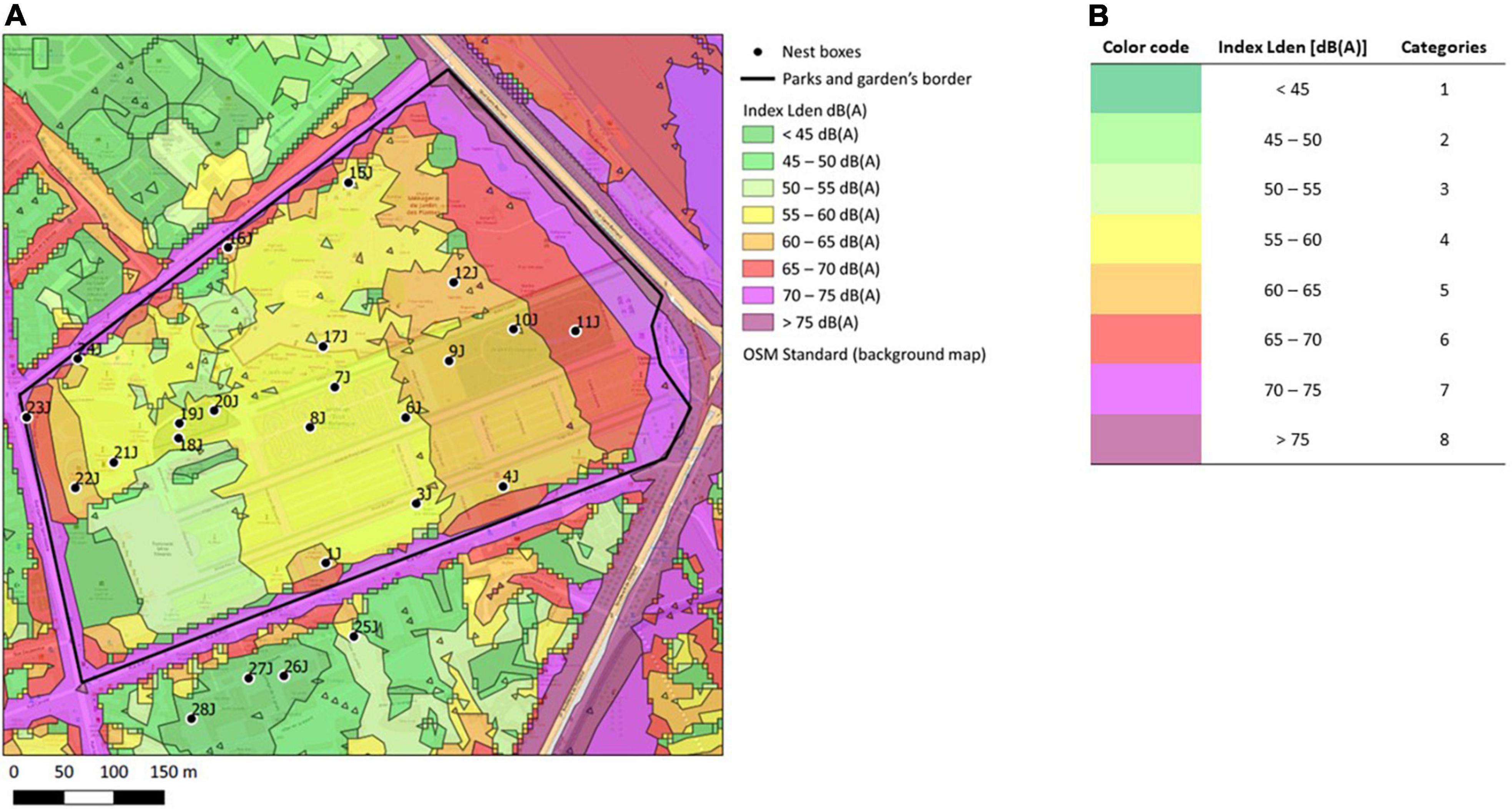
Figure 1. Estimation of noise level around the nest-boxes. (A) Extract of the map obtained after overlaying Bruitparif SIG data of cumulative noise levels (road, trains, and airplanes traffic) (Bruitparif, under open license) (available at https://carto.bruitparif.fr/) and nest-boxes maps. The extract shows the Jardin des Plantes, Muséum National d’Histoire Naturelle, in Paris, France (48500380 0N, 02210370 0E) (for the other green spaces included in this study, see Supplementary Figure 1). Nest-boxes are represented by a dot and their identification code. Background map by OpenStreetMap France @OpenStreetMap contributors (available at http://www.openstreetmap.fr/fonds-de-carte/). (B) Corresponding Lden index in decibels with A-weighting [dB(A)] from Bruitparif data and associated noise level categories for statistical analysis.
Noise pollution levels were assessed from Bruitparif open access data [Bruitparif. Cartes stratégiques du bruit (CSB1) (Bruitparif, 2019)]. The main sources of noise around or within city parks did not substantially change over the course of the study (roads and car traffic, railway; C.B. pers. obs.). We extracted the noise level index Lden (Level-day-evening-night) for each nest by overlaying our nest maps and Bruitparif SIG data (Figure 1A, Supplementary Figure 1). This index is calculated with equivalent noise levels (LAeq) of the three periods of a day: day (6 am to 6 pm, Lday index), evening (6 pm to 10 pm, Levening index), and night (10 pm to 6 am, Lnight index). For evening and night, an additional 5 dB(A) and 10 dB(A) are respectively added to measures to account for different noise sensibilities between day and night (Bruitparif2). The Lden index hence accounts that at night, the same noise will be more disturbing than during the day. It should be noted that this index is defined and used for regulatory purposes and takes human sensibility into account, which likely differs from that of birds (e.g., noise may be more disturbing during dawn chorus than during evening or night). However, the raw Lday and Levening data were not available from Bruitparif and we could not adjust the calculation of Lden. For statistical analyses, we chose to order noise levels into 8 categories (Figure 1B). Due to the correlative approach in our study, noise levels may be correlated to light pollution at night, if nest-boxes closer to the edges are more exposed to artificial light at night than nest-boxes closer to the center of a park (Figure 1A, Supplementary Figure 1). However, noise levels and light pollution were only weakly positively correlated in our data set (τKendall = 0.25, z = 3.25, p-value = 0.001; light pollution data derived from opendata.paris.fr and completed by our own census of street lamps in Rueil-Malmaison, unpub.), which may be explained by the presence of street lamps within some of our city parks.
Vegetation coverage is a measure of the area covered by vegetation (grass, bushes, trees) in a 50 m radius around each nest, representing the mean size of great tits and blue tits territories in urban habitats (Hedblom and Söderström, 2012). This measure was performed using Qgis software (version 2.18, Las Palmas) to quantify overlaps between vegetation areas and a 50 m radius buffer around each nest on satellite images. From this measure of vegetation coverage in square meters, we calculated the percentage of vegetation coverage around each nest by dividing the vegetation coverage value by the total area of the buffer. There has not been any substantial change in trees and lower vegetation management and cover around our nest-boxes since the start of the study (C.B. pers.obs.).
All statistical analyses were performed using R software version 3.6.2 (R Core Team 2019). To assess whether noise levels variations between day and night were consistent for all nests, we ran a linear regression between Lden (Level day-evening-night) and Ln (Level night) indexes (lm function, package stats). We found that those two indexes were significantly correlated (n = 99; r2 = 0.756; t-value = 17.27; df = 1; p < 0.001). We can consider that noise variations between day and night are similar for all nest boxes. In the following, we ran either linear or generalized linear mixed models [lmer or glmer functions respectively, R package: lme4 (RRID:SCR_015654)] except if otherwise mentioned.
We investigated if the probability a nest box is occupied in a park correlates with noise levels or vegetation cover. We used a generalized linear mixed model for data with a binomial distribution on nest boxes occupancy (value 0 or 1 for each year) (Nnestboxes.year = 760), with noise level, vegetation coverage and interaction between these two predictors as fixed effects. We included nest box identification code as a random effect. This analysis was run on great tits (244 broods over 8 years) and blue tits (85 broods over 8 years) populations separately because a nest-box can only be occupied by one species at a time.
We then investigated variation in breeding parameters using either linear or generalized linear mixed-effects models depending on the response variable. We included park identity as a random intercept effect in our models because micro-environments associated with each location differed from another. Some individuals in our population were found breeding in the same park several consecutive years. However, since adults are captured when feeding nestlings, the identity of the parents are not known for breeding events that failed before or just after hatching. In addition, we did not succeed in capturing all adults each year. As a result of missing data (about 50% of breeding events lacked male or female identity, or both) individual identity of the parents could not be included in the models as additional random effects to take pseudoreplication into account. Finally, year could be included as a random intercept effect to account for differences in breeding conditions among years. However, residuals of these models did not meet normality and equivariance for some dependent variables. As it did not qualitatively change any of the results presented here, we chose to run the models without year in the random structure.
Variation in reproductive phenology, measured by laying date, was investigated using a linear mixed model for data with Gaussian distribution. Due to differences in laying dates among years and between species, prior analysis, laying dates were scaled (zLD, centered and standardized laying date) by subtracting the mean and dividing by the standard deviation for each species, each year. Variation in clutch size was analyzed using a generalized linear mixed-effects model for data with a Poisson distribution. In order to investigate variation in reproductive success (i) we first modeled the probability that at least one chick hatched or fledged (subsequently referred to as Hatching or Fledging probability), compared with that of complete reproductive failure and/or clutch or brood desertion. We used a binary measure of success as a response variable (0: no chick hatched or fledged; 1: at least one chick hatched or fledged), and a generalized linear mixed model for data with binomial distribution (ii) We then focused our analysis on pairs that hatched or fledged at least one nestling and analyzed hatching or fledging success as a proportion (subsequently referred to as Hatching or Fledging success) using vectors as dependent variables (total number of incubated eggs and number of hatchlings, or brood size and number of successfully fledged nestlings, respectively). We used generalized linear mixed models with a binomial distribution to analyze hatching success, and with quasi-binomial distribution for fledging success due to data overdispersion [glmmPQL function, Modern Applied Statistics with S (RRID:SCR_019125)].
In all cases, the starting full model included species, noise level, vegetation coverage and their two-and three-way interactions as fixed effects. Noise levels and vegetation cover were weakly, but significantly, positively correlated (τKendall = 0.17, z = 2.16, p = 0.03). For all models, we verified that there was no collinearity between predictors [vif function, Companion to Applied Regression (RRID:SCR_022137)]. Models were simplified by removing non-significant interactions one at a time. In case of a significant interaction involving species, separate models including the same other effects were subsequently run separately for each species. Assumptions regarding normality and equivariance of residuals were verified using the plotresid function [R package: RVAideMemoire (RRID:SCR_015657)]. Due to multiple testing on the same data set, we corrected the obtained p-values for false discovery rates (p.adjust function with “fdr” method, stats package).
Estimates (slopes or differences between species with C. caeruleus as reference group) are given ± s.e. Figures illustrating the results were generated using package ggplot2 (RRID:SCR_014601) (Figures 2–4, 6) and package plot3D (Figure 5).
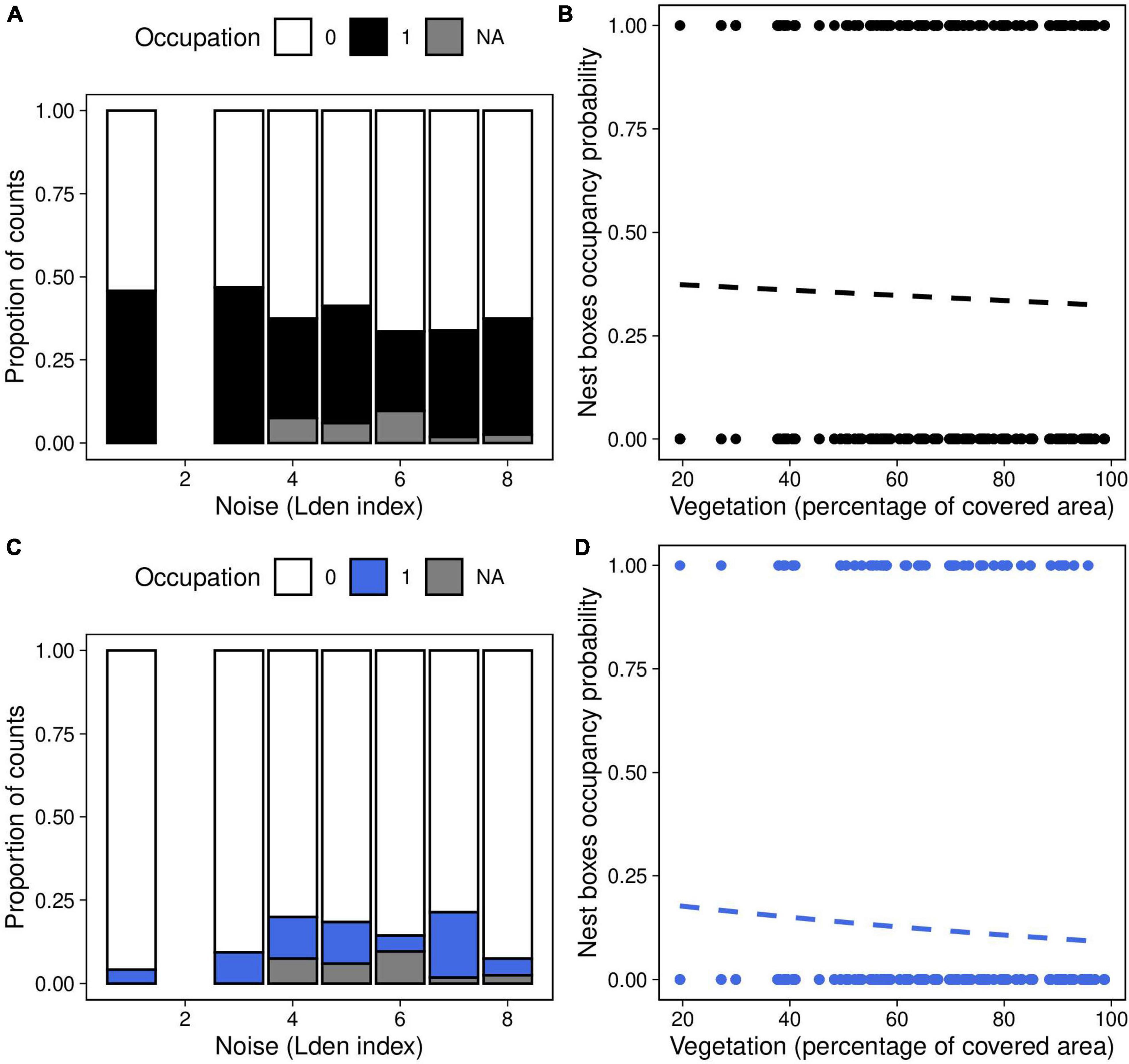
Figure 2. Nest-boxes occupancy probability as a function of (A,C) noise level (categorized Lden index) and (B,D) vegetation coverage around the nest box (percentage of covered area in a radius of 50 m around each nest) in great tits (panels A,B) and blue tits (panels C,D). Dashed lines of best fit represent the predictions from the generalized mixed linear models, the effects of noise and vegetation were not significant (Table 1).
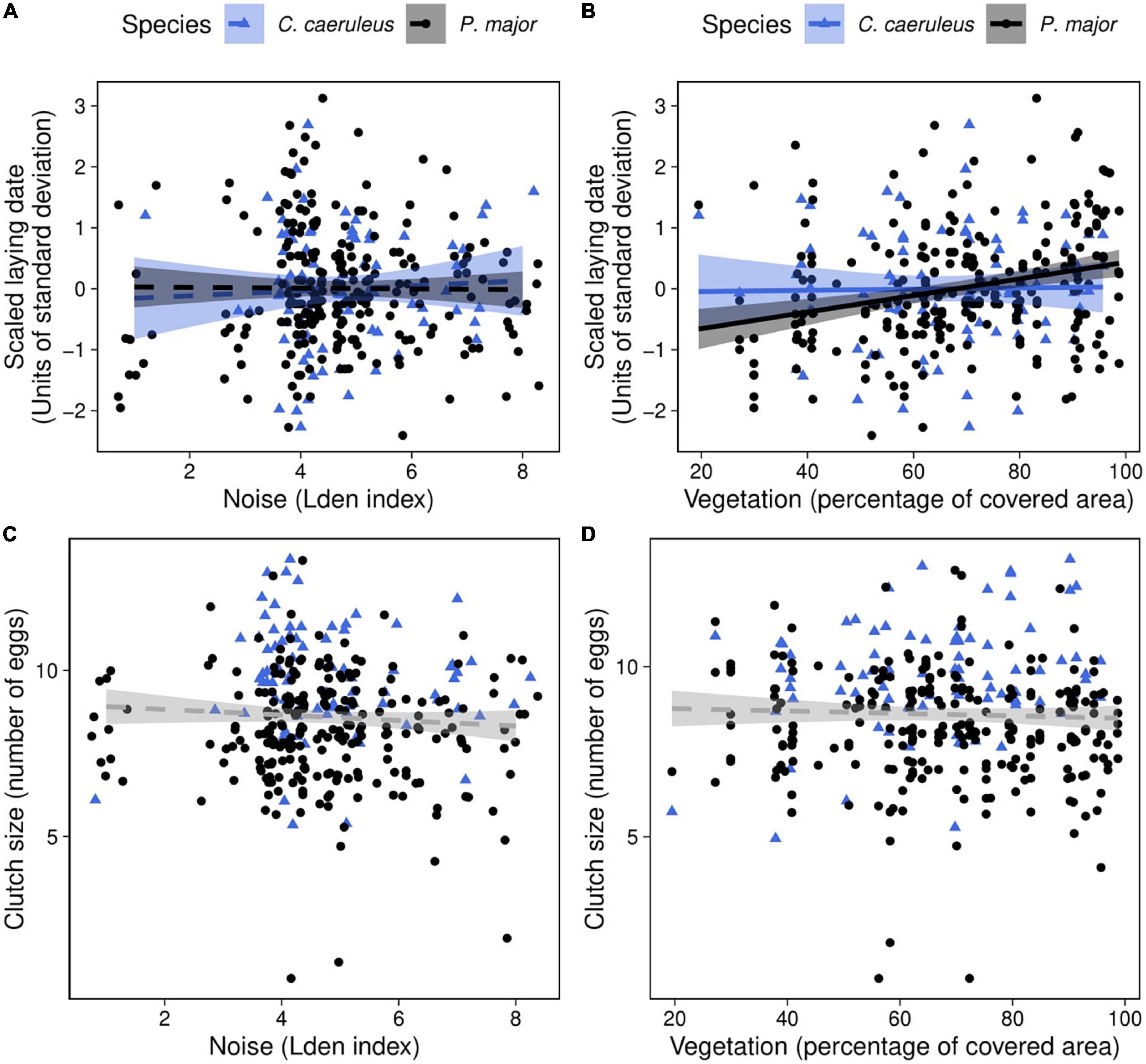
Figure 3. Observed laying date (in units of standard deviation; laying date was centered and standardized for each species, each year) as a function of (A) noise level (categorized Lden index), (B) vegetation coverage around the nest box (percentage of covered area in a radius of 50 m around each nest), in blue tits (blue triangles) and great tits (black dots). Lines of best fit represent the predictions from the simplified mixed linear models, and shaded areas the corresponding 95% confidence intervals (Table 2B). Vegetation coverage significantly and positively correlated with laying date in great tits: when vegetation coverage increased around nests, birds lay their first egg later. Observed clutch size (number of eggs) as a function of (C) noise level and (D) vegetation coverage around the nest box in blue tits (blue triangles) and great tits (black dots). Dashed lines of best fit represent the predictions from the generalized mixed linear model (Table 3). Neither noise level nor vegetation coverage were significantly associated with clutch size in both species.
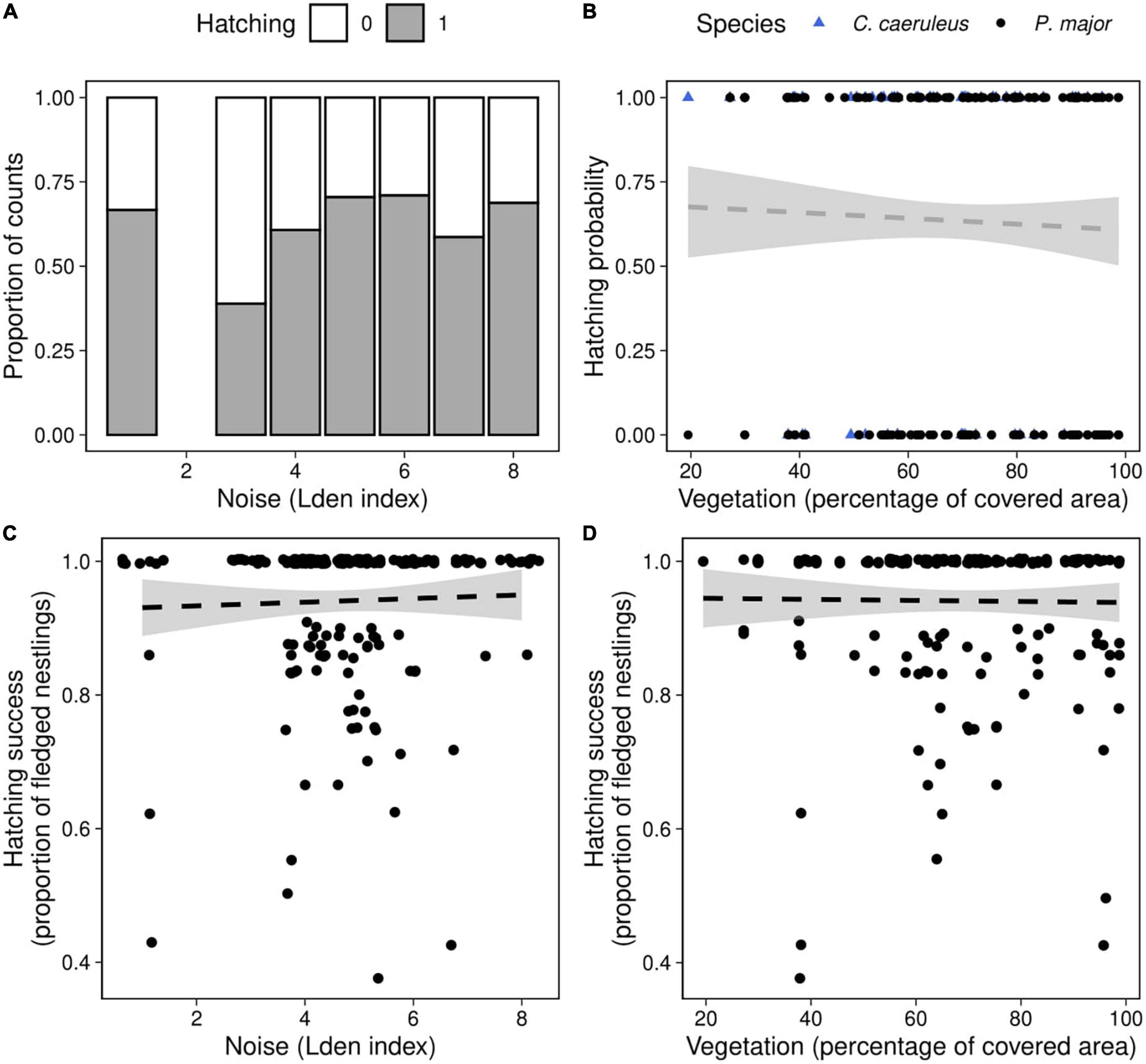
Figure 4. Hatching probability as a function of (A) noise level (categorized Lden index, proportion of counts) in both species, (B) vegetation coverage around the nest box (percentage of covered area in a radius of 50 m around each nest, data points represent the observed fledging outcomes), in great tits (black dots) and blue tits (blue triangles). Hatching success as a function of (C) noise level, (D) vegetation coverage in great tits, data points represent the observed proportion of hatched eggs. Dashed lines of best fit represent the predictions from the generalized mixed linear models, and shaded areas the corresponding 95% confidence intervals [(panels A,B): Table 4; (panels C,D): Table 5B]. Neither noise level nor vegetation coverage were significantly associated with hatching probability in both species or with hatching success of great tits.
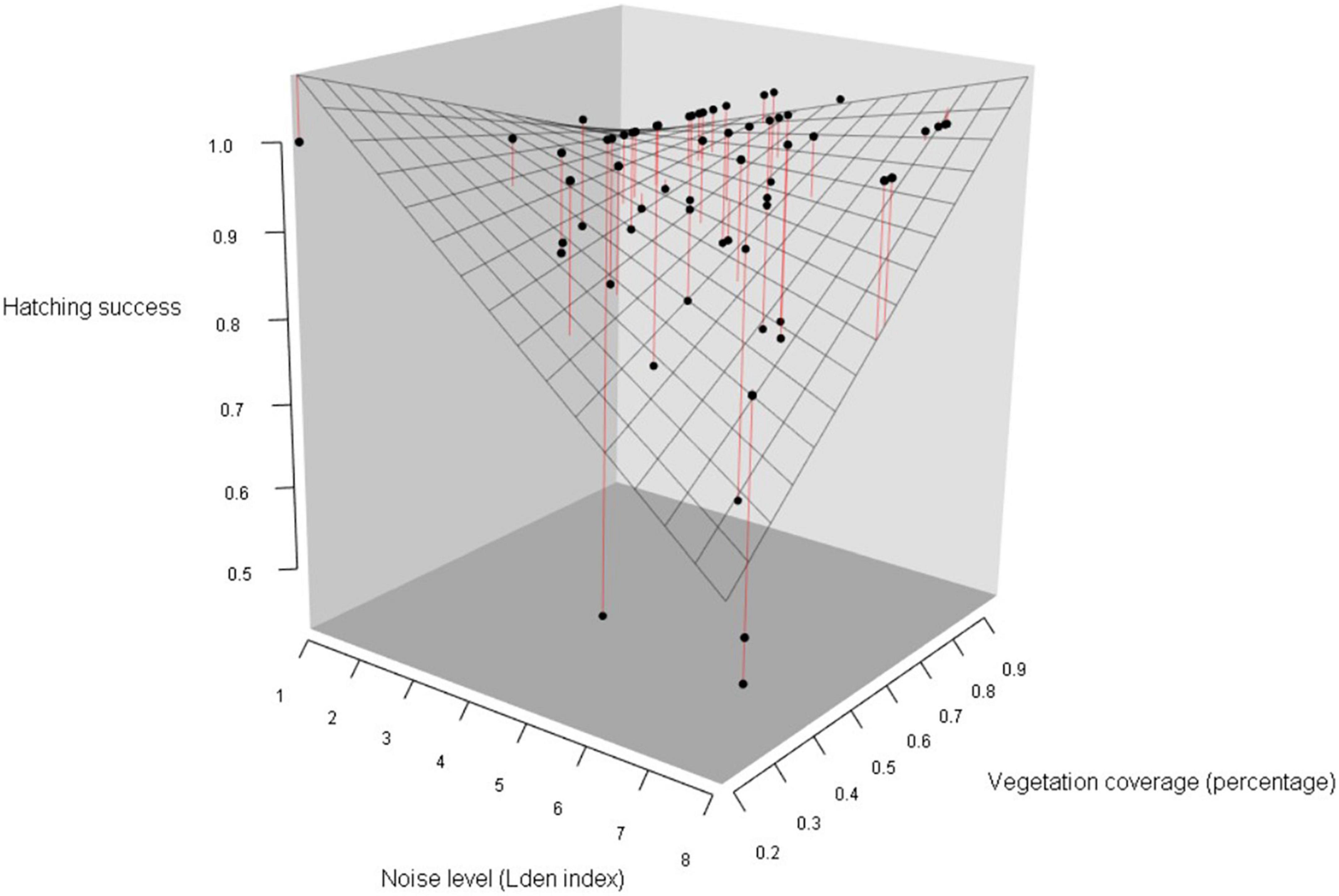
Figure 5. Hatching success in blue tits as a function of noise level (categorized Lden index) and vegetation coverage around the nest box (percentage of covered area in a radius of 50 m around each nest). Data points represent the observed proportion of hatched eggs, mesh surface represents the predictions of the simplified generalized linear mixed model, and red lines figure distances between observed values and the prediction surface (Table 5B). As noise level increased and vegetation coverage decreased, the probability of hatching success decreased.
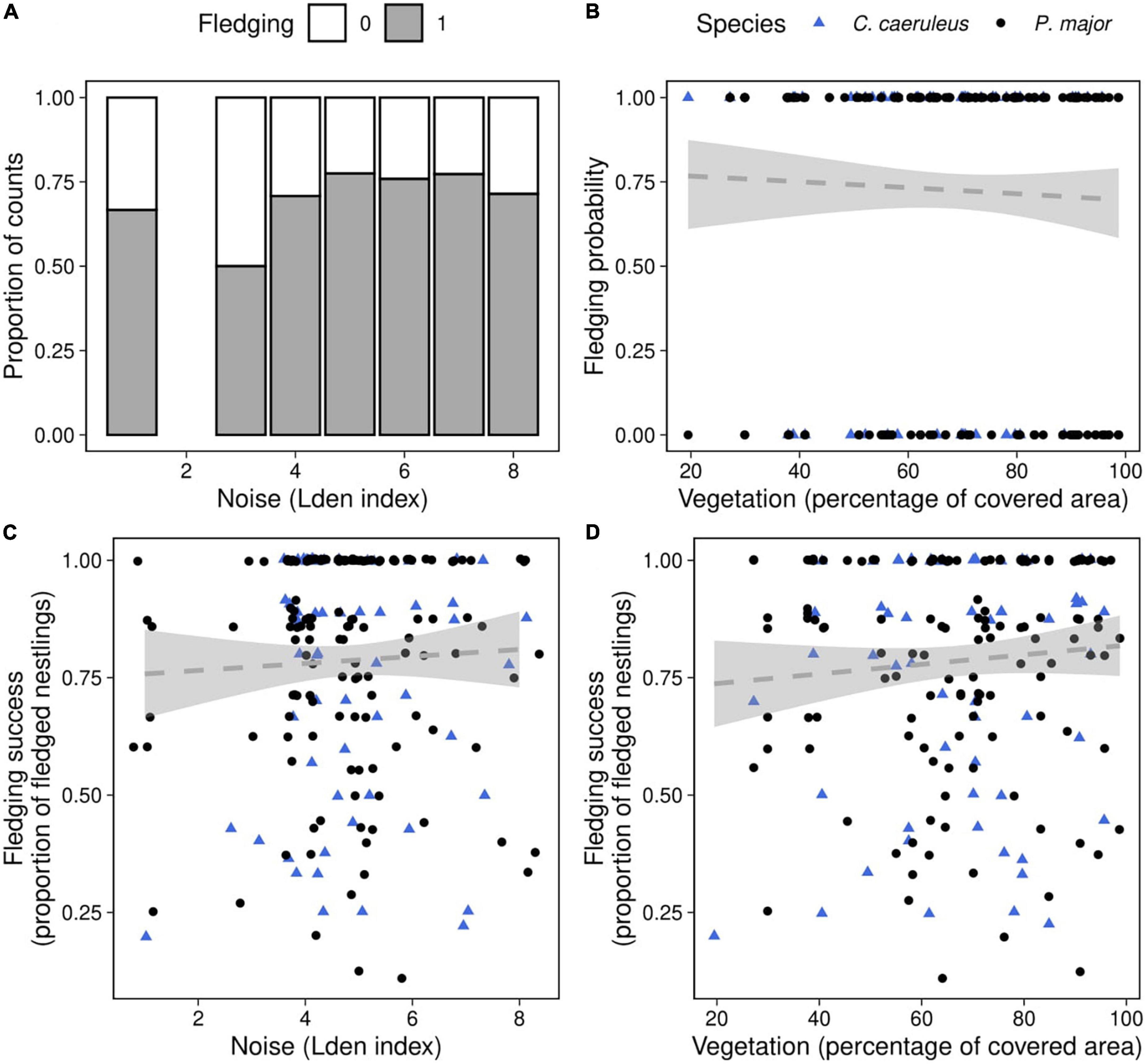
Figure 6. Fledging probability as a function of (A) noise level (categorized Lden index, proportion of counts) in both species, (B) vegetation coverage around the nest box (percentage of covered area in a radius of 50 m around each nest, data points represent the observed fledging outcomes), in great tits (black dots) and blue tits (blue triangles). Fledging success as a function of (C) noise level, (D) vegetation coverage in great tits (black dots) and blue tits (blue triangles), data points represent the observed proportion of fledged nestlings. Dashed lines of best fit represent the predictions from the generalized mixed linear models, and shaded areas the corresponding 95% confidence intervals [(panels A,B): Table 6; (panels C,D): Table 7]. Neither noise level nor vegetation coverage were significantly associated with fledging success, in both species.
This work conforms to French legal requirements, including those relating to the capture and handling of protected species, conservation, and welfare. It was conducted under authorizations from the CRBPO (Muséum National d’Histoire Naturelle; research program # 537, bird ringing authorization #1454), and dispensations to the ban to capture protected species (DRIEE-2012-31à38, 2019 DRIEE-IF/033; 2019 DRIEE-IF/046). The protocol was approved by Charles Darwin n°005 ethical committee (APAFIS#19941-2019032516275025 v5).
The probability that a nestbox was occupied did not significantly correlate with noise level or vegetation coverage, either in interaction or as main effects, in great tits and in blue tits (Table 1, Figures 2A–D).
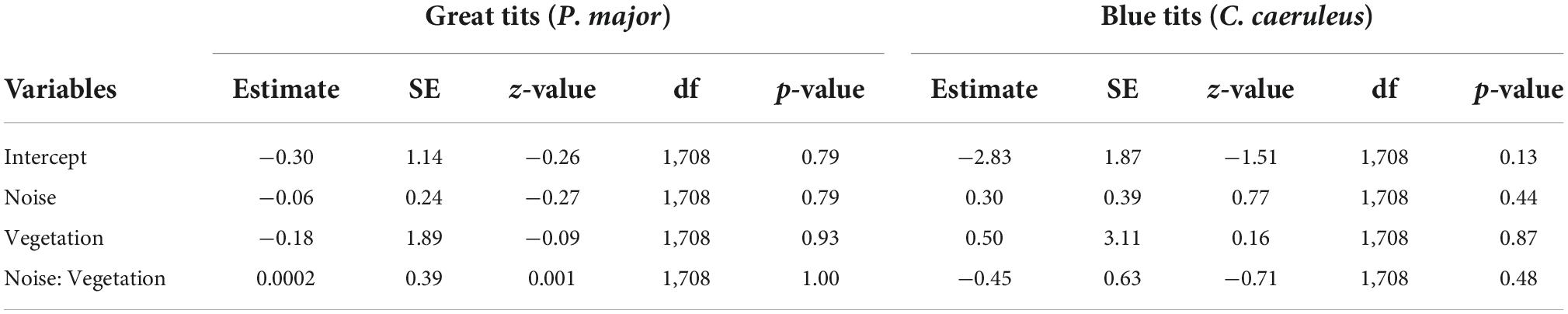
Table 1. Results from the generalized mixed linear model investigating the probability a nest-box is occupied (binomial distribution) by great tits or blue tits as a function of noise level (Noise), vegetation coverage (Vegetation) and their interaction as fixed effects (*p < 0.05; **p < 0.01; and ***p < 0.001). The identity of the nest-box was entered as random effect.
In the full model investigating variation in laying dates, there was a marginally significant interaction between species and vegetation coverage (Table 2A), which was significant when running the model without other, non-significant, interaction terms (Species: Vegetation: 1.43 ± 0.70, t-value = 2.04, p-value = 0.042). Therefore, we subsequently ran analyses separately for great tits and blue tits. In great tits, laying date did not vary with noise level (Figure 3A; Table 2B), but laying date was positively and significantly related to vegetation coverage: when vegetation coverage increased around nests, great tits laid their first egg later (Figure 3B; Table 2B). In blue tits, there was no significant relationship between laying date and noise level or vegetation coverage (Figures 3A,B; Table 2B).
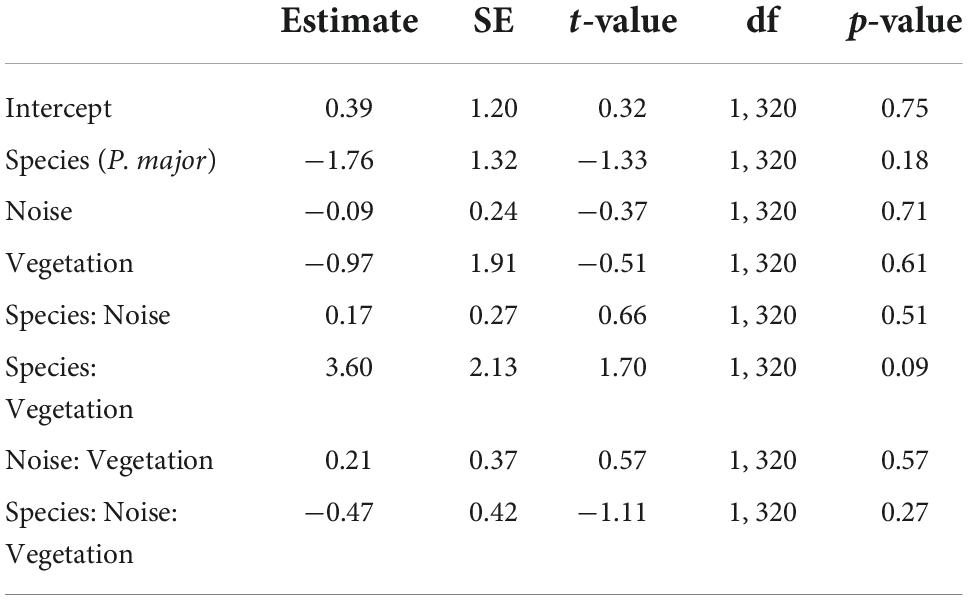
Table 2A. Results from the mixed linear model investigating variation in scaled laying date (for each species, each year; gaussian distribution) in great tits and blue tits as a function of species (C. caeruleus as the reference group), noise level (Noise), vegetation coverage (Vegetation), and their interactions as fixed effects (*p < 0.05; **p < 0.01; ***p < 0.001), with city park identity as random effect.
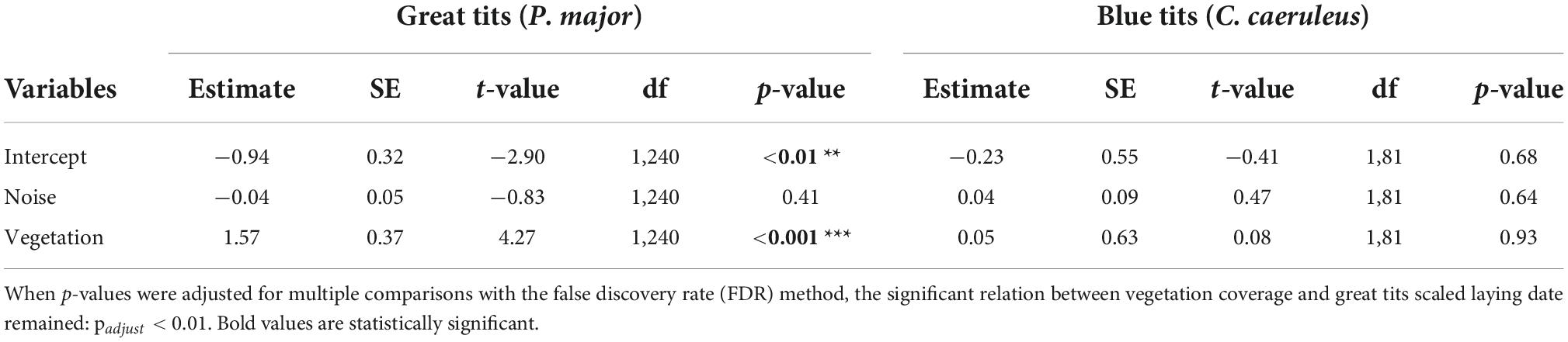
Table 2B. Results from the mixed linear model investigating variation in scaled laying date (for each specie, each year) (gaussian distribution) separately for great tits or blue tits as a function of noise level (Noise), vegetation coverage (Vegetation) as fixed effects (p-values in bold are statistically significant, *p < 0.05; **p < 0.01; and ***p < 0.001), with city park identity as random effect.
Clutch size did not significantly vary with any interaction between noise level, vegetation coverage or species (Figures 3C,D; Table 3). In the final model, only species significantly explained variation in clutch size, with great tits laying and incubating less eggs than blue tits [great tit: 8.21 ± 1.63 eggs, blue tit: 9.71 ± 1.60 eggs; Species (P. major): −0.17 ± 0.04, z-value = −4.05, p-value < 0.001; Noise level: −0.01 ± 0.01, z-value = −0.80, p-value = 0.42; Vegetation cov.: 0.002 ± 0.11, z-value = 0.02, p-value = 0.99].
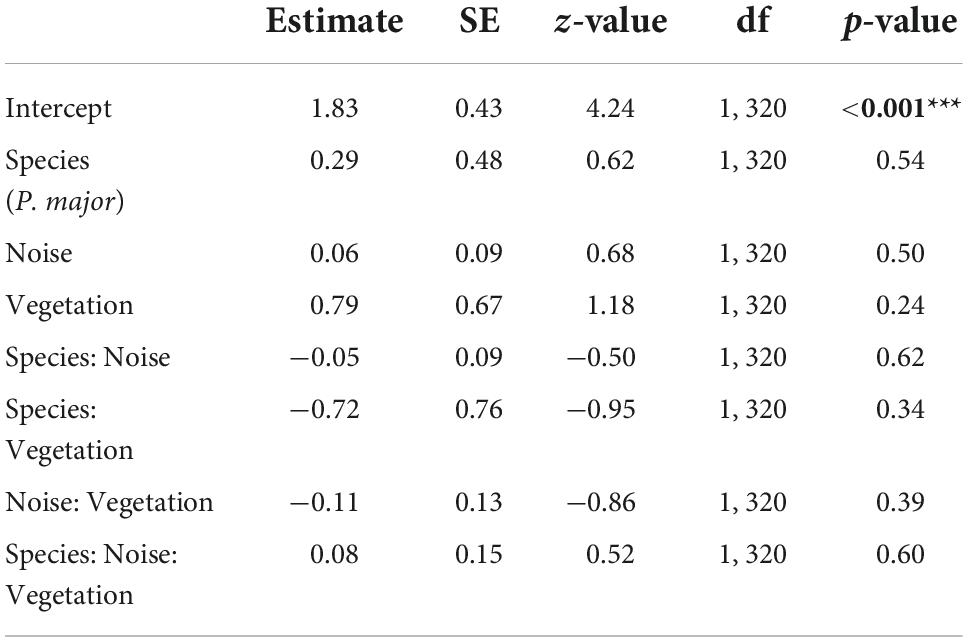
Table 3. Results from the generalized mixed linear model investigating the association between clutch size (Poisson distribution) in great tits and blue tits, and noise level (Noise), vegetation coverage (Vegetation), species (C. caeruleus as reference group), and their interactions as fixed effects (p-values in bold are statistically significant, *p < 0.05; **p < 0.01; ***p < 0.001), with city park identity as random effect.
Hatching probability (the probability that at least one chick hatched) was not significantly related to any interaction between anthropogenic noise, vegetation coverage and species (Table 4). After removing non-significant interaction terms, hatching probability was not related to noise level (Noise level: 0.07 ± 0.10, z-value = 0.79, p-value = 0.43) or to vegetation cover (Vegetation cov.: −0.31 ± 0.74, z-value = −0.42, p-value = 0.68) (Figures 4A,B). However, we found a significant difference between both species [Intercept: 1.15 ± 0.69, z-value = 1.67, p-value < 0.10, Species (P. major): −0.89 ± 0.31, z-value = −2.92, p-value < 0.01]: at a fixed value of noise pollution or vegetation coverage, the predicted probability for a blue tit to hatch at least one chick was exp (1.15)/[1 + exp (1.15)] = 0.76, and for a great tit exp (1.15–0.89)/[1 + exp (1.15–0.89)] = 0.56.
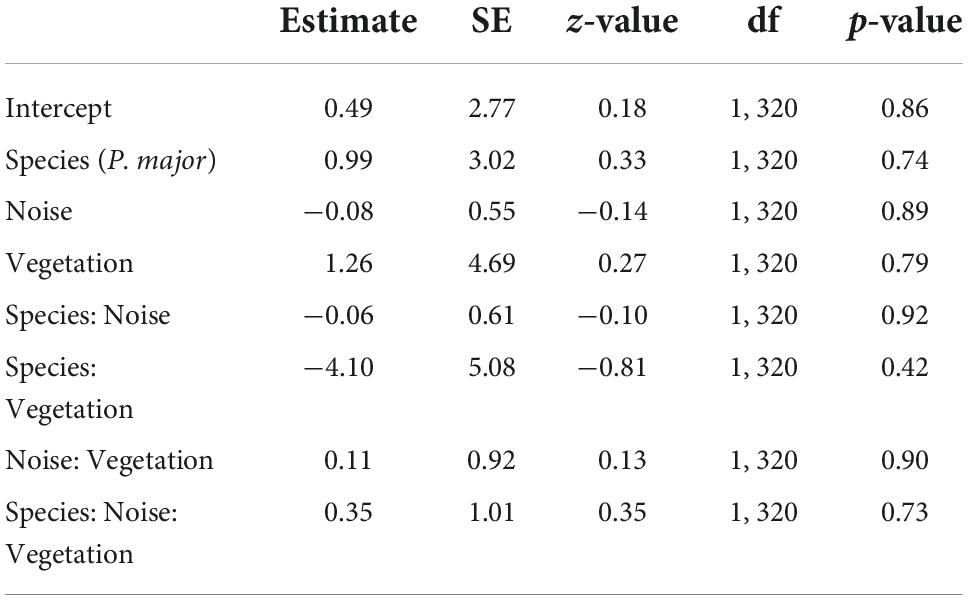
Table 4. Results from the generalized mixed linear model investigating variation in hatching probability (probability to hatch at least one nestling, binomial distribution) for great tits and blue tits as a function of noise levels (Noise), vegetation coverage (Vegetation), species (C. caeruleus as reference group), and their interactions as fixed effects (*p < 0.05; **p < 0.01; ***p < 0.001), with city park identity as random effect.
We then investigated if noise level and vegetation coverage were associated with hatching success, measured as the proportion of hatched eggs, and found significant interactions between the three predictors and between noise pollution and species (Table 5A). Therefore, we performed separate analyses for the two species. Hatching success in great tits did not significantly vary with noise level and vegetation coverage or their interaction (Figures 4C,D; Table 5B). In the model testing the relationship between hatching success in blue tits and noise level and vegetation coverage, we found a significant interactive effect for both these predictors: when noise level increased, hatching success decreased, and on the contrary, when vegetation cover increased around nest boxes, hatching success of blue tits increased (Figure 5; Table 5B).
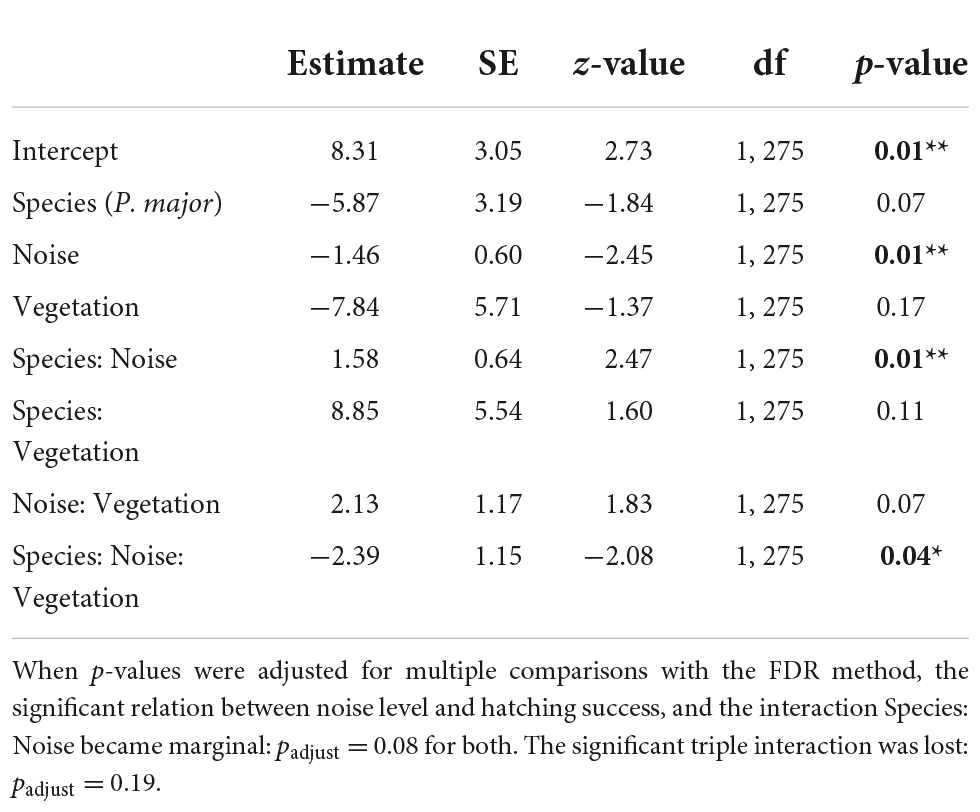
Table 5A. Results from the generalized mixed linear model investigating the association between hatching success (proportion of successfully hatched eggs, binomial distribution) for great tits and blue tits, as a function of noise level (Noise), vegetation coverage (Vegetation), species (C. caeruleus as reference group), and their interactions as fixed effects, with city park identity as random effect (p-values in bold are statistically significant, *p < 0.05; **p < 0.01; ***p < 0.001).

Table 5B. Results from the generalized mixed linear model investigating the association between hatching success (proportion of successfully hatched eggs, binomial distribution) separately by species, as a function of noise level (Noise), vegetation coverage (Vegetation) and their interaction as fixed effects (p-values in bold are statistically significant, *p < 0.05; **p < 0.01; and ***p < 0.001), with city park identity as random effect.
We investigated if fledging probability (the probability that at least one nestling fledged) was related to noise level and vegetation cover (Table 6). After removing non-significant interactions, we did not detect significant effects of noise level (Noise level: 0.16 ± 0.11, z-value = 1.39, p-value = 0.16), vegetation coverage (Vegetation cov.: −0.65 ± 0.86, z-value = −0.76, p-value = 0.45) (Figures 6A,B), nor differences between species in the probability of fledging at least one chick [Species (P. major): −0.40 ± 0.32, z-value = −1.24, p-value = 0.22].
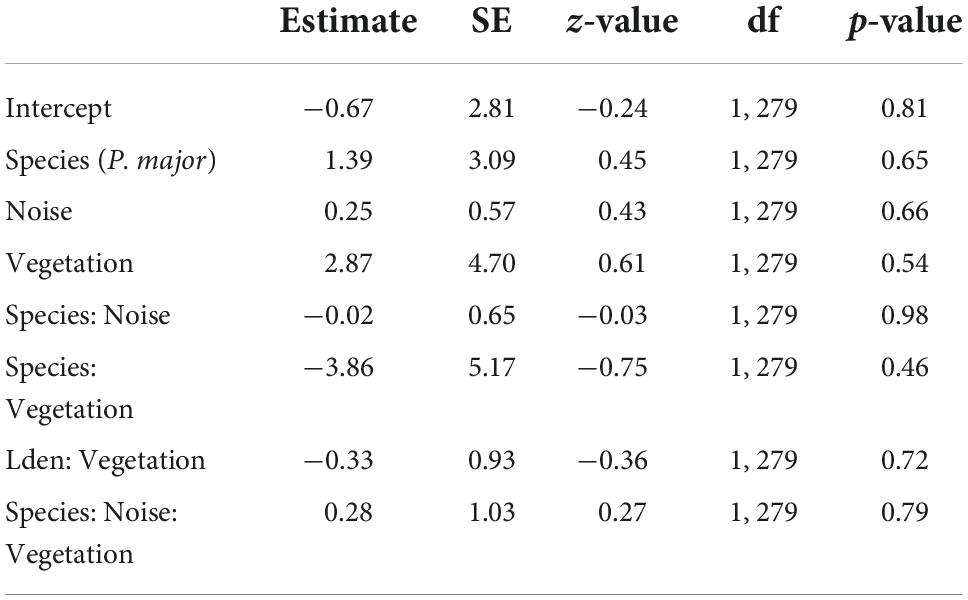
Table 6. Results from the generalized mixed linear model investigating fledging probability (probability to fledge at least one nestling, binomial distribution) for great tits and blue tits as a function of noise level (Noise), vegetation coverage (Vegetation), species (C. caeruleus as reference group), and their interactions as fixed effects (*p < 0.05; **p < 0.01; ***p < 0.001), with city park identity as random effect.
Finally, we investigated the relationship between noise level, vegetation coverage and fledging success for both species. We did not find any significant effect of the different predictors, either as interaction or as main effects (Figures 6C,D; Table 7).
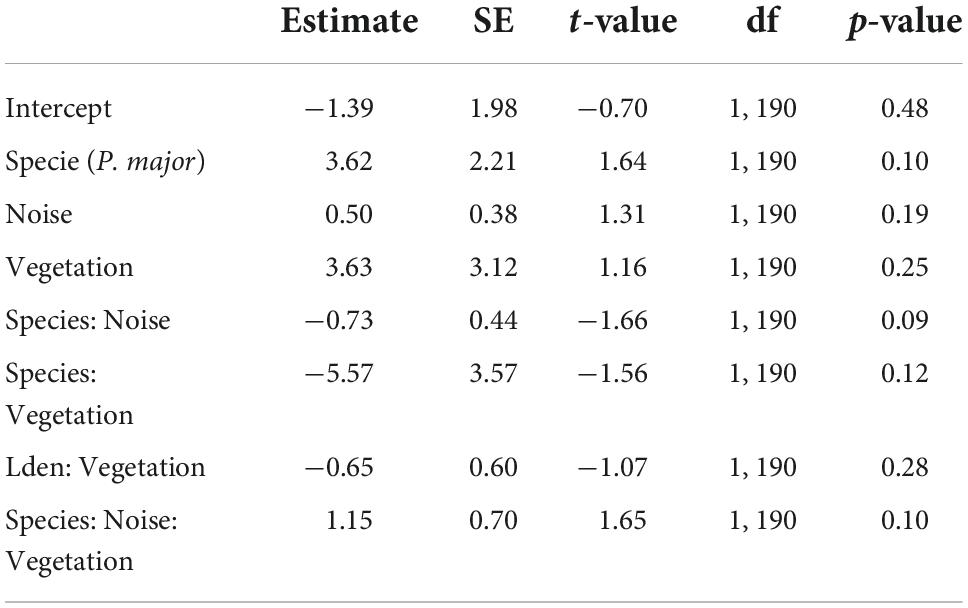
Table 7. Results from the generalized mixed linear model investigating the association between the fledging success (proportion of successfully fledged nestlings, binomial distribution) for great tits and blue tits as a function of noise level (Noise), vegetation coverage (Vegetation), species (C. caeruleus as reference group), and their interactions as fixed effects (*p < 0.05; **p < 0.01; ***p < 0.001), with city park identity as random effect.
In this study, we aimed to assess if noise levels in city parks affect the distribution and reproductive success of two common bird species, the great tit, and the blue tit, and if vegetation coverage could mitigate these effects. By analyzing data on nest box occupancy, breeding phenology, reproductive investment, and success over 8 reproductive seasons with estimates of noise level and vegetation coverage at each nest box, we first found that neither of those factors can predict the pairs’ nest box choice at our study sites. According to our predictions, we found that increased anthropogenic noise level and decreased vegetation coverage are associated with a decreased hatching success in blue tits. Interestingly, in great tits, only vegetation coverage was associated with laying date, birds laying their first egg later with increasing vegetation around the nest. However, we did not find any association between noise level or vegetation coverage and clutch size or fledging success in both species.
We first hypothesized that breeding pairs would avoid nest boxes exposed to high noise levels or prefer nest boxes with a higher vegetation coverage in the surroundings. We did not find any significant association between the probability that a nest box is occupied and both these factors, for the two studied species. Other studies on the contrary identified that when great tits and tree swallows have the choice in their environment between next boxes exposed to more or less background noise, they avoid high-noise level nest boxes (Halfwerk et al., 2016; Injaian et al., 2018). An explanation for our result might be that in some of the studied parks, noise levels are rather similar and elevated at each nest box. A lack of within-park variability in noise levels likely has reduced the power of our study design to detect differences in occupancy, as individuals living in and around these parks may not have the choice between noisy and quiet nest boxes. Moreover, our study sites are separated by a dense urban matrix and relatively far from each other, and we did not observe reproductive dispersal between them.
Concerning reproductive phenology, we did not find any significant association with noise pollution in either species, but for great tits, laying date was delayed with increasing vegetation coverage around the nest boxes. A higher vegetation coverage may provide a cooler and more humid microenvironment, locally reducing the heat island effect common in urban environments. It is known that temperature at the nest box is correlated to laying date, in particular night temperatures (Bleu et al., 2017). In the blue tit, we did not find any association between laying date and noise pollution or vegetation coverage. Similarly, for clutch size there was no significant association with noise level or vegetation coverage for both species. This is consistent with the absence of a relationship between anthropogenic noise and clutch size in great tits in another population (Grunst et al., 2020). Two previous experimental studies yielded contrasting results on the effects of anthropogenic noise on laying date and clutch size; the first one identified that anthropogenic noise delays laying date, and reduces clutch size in great tits exposed to higher levels of traffic noise, although that result could be due to birds of lower quality settling at noisier nest boxes (Injaian et al., 2018). The other one did not detect any effect of gas compressor noise on clutch size in tree swallows (MacLeod et al., 2022). It was found for both species that increased spring temperatures advance laying date, while increased winter temperatures reduce clutch size (Møller et al., 2020). Furthermore, in that study, there was an interaction between the density of great tit and winter temperatures on laying date of both species, while the density of blue tit interacted with spring temperature on laying date in great and blue tit. The potential effects of noise levels and vegetation cover might also be masked by, or interact with, factors that we did not consider here, like population density, interactions between great and blue tits who use the same nest sites, and interactions with other species, climate and seasonality.
We predicted that pairs breeding in noisier environments would present a reduced breeding success (hatching and fledging probability and success), while a higher vegetation coverage would moderate the negative effect of noise pollution. The probability that at least one chick hatched did not vary with noise level or vegetation cover in either species. However, hatching probability in great tits was lower than that of blue tit, which suggests a higher probability of clutch desertion, and/or a decreased capacity to maintain optimal incubation conditions, resulting in complete hatching failure. According to our prediction, in blue tits we found a decreased hatching success with higher noise level and lower vegetation coverage, while pairs breeding in noisy environments with higher vegetation coverage were still successful. Such a negative relationship between hatching success and noise levels varying within the same range as in our study has been previously demonstrated experimentally in western bluebirds (Kleist et al., 2018), while exposure to higher levels of noise did not affect hatching success in tree swallows (MacLeod et al., 2022). An increased vegetation coverage could provide higher quantity and quality resources for that species, as the blue tits’ diet is mainly composed of seeds and insects all year long, but also buds, sap, pollen and nectar from trees just before Lepidoptera larvae emergence in the spring (Isenmann, 1996; Stenning, 2018). Moreover, blue tits forage mostly in tree branches, while great tits tend to find more resources by foraging on the ground too (Stenning, 2018). In both species during incubation, the female uses specific vocal communications to signal her energetic needs to the male, who feeds her at the nest (Eens and Gorissen, 2005; Boucaud et al., 2016a,b). The negative relationship between hatching success and noise level and the positive effect of vegetation coverage are consistent with the hypothesis that urban noise disrupts within-pair communication, which may alter the female time budget, i.e., time spent incubating versus in foraging trips if the male fails to fulfill the female needs. In territories where vegetation cover is more important, the female may need less time to find food, which could mitigate the adverse noise effect. In great tits, hatching success was however not related to noise or vegetation cover. Noise levels occurring at our study sites may not reach the threshold at which within-pair communication is compromised in this species, or the ability of great tit to complement their diet with resources found on the ground may offset any effect of noise. A previous correlative study found that incubation duration was positively associated with the distance to the nearest road in great tits, and negatively with the distance to the nearest pedestrian path in blue tits (Corsini et al., 2017). Traffic noise and human-associated noise and perturbation may thus differentially affect reproductive investment in incubation in the two species, which would require further study.
Regarding results on fledging probability and fledging success, contrary to our predictions we did not find any association with noise pollution or vegetation coverage in either species. Previous studies yielded contrasting results: Similarly as in our study, noise pollution was not associated with brood size or fledging success in great tits (Corsini et al., 2017; Grunst et al., 2020), and experimental exposure to anthropogenic noise did not affect fledging success in tree swallows (MacLeod et al., 2022), while in others, anthropogenic noise was associated with a decrease in the number of fledged offspring or their body condition in the same two passerine species (Halfwerk et al., 2011; Injaian et al., 2019, respectively). However, no relationship between anthropogenic noise and fledgling body mass was found in great tits (Raap et al., 2017; Grunst et al., 2020).
These results suggest that during breeding, urban blue tits might be more sensitive to noise pollution than great tits and that habitat quality, as measured by vegetation cover, affects both species, albeit on different stages of reproduction: delayed laying date in the great tit and increased hatching success in the blue tit. However, noise and vegetation cover were not associated with the other reproductive traits or measures of success studied here. Two previous correlative studies investigating whether variation in noise levels within the urban habitat was associated with reproductive life history traits and success in great and blue tits did not report any significant relationship either, while detecting negative associations between noise levels and telomere length and carotenoid-based plumage coloration in nestlings (Corsini et al., 2017; Grunst et al., 2020). In addition, physiological effects of anthropogenic noise have been repeatedly reported (reviewed in Introduction). This suggests that blue and great tits might be able to compensate for these deleterious effects to some extent, maintaining reproductive investment and success. This would however be traded against other life history traits, and further investigations of adult survival and offspring recruitment rates would be needed to better understand the mechanisms involved. In addition, here we considered parameters relevant to breeding success, but considering measures of adult or nestling body and physiological condition in future studies might allow us to detect more subtle, potentially interactive, effects at the individuals’ level. It has been suggested that considering variation in occupants’ quality would improve the predictions of habitat effects on individual or population fitness (Germain and Arcese, 2014). Indeed, noise variation levels were associated with a reduction in telomere length in smaller nestlings (Grunst et al., 2020), indicating that environmental stressors may differently affect individuals depending on their quality. Another, not mutually exclusive hypothesis, would be that the bird’s response to noise is not linear, and that the threshold at which effects can be detected was not passed. However, this threshold may be challenging to identify at moderate levels of noise, which are hypothesized to induce stress responses indirectly (Kleist et al., 2018), and may also vary depending on the species and reproductive stage considered, as shown by the inconsistencies in effects reported in previous studies in which noise levels varied in the same range as in ours [40 to 80 dB(A): Halfwerk et al., 2011; Ware et al., 2015; Raap et al., 2017; Kleist et al., 2018; Injaian et al., 2019] or were higher [85db(C): MacLeod et al., 2022]. It also has to be acknowledged that in our study, nest-boxes placement design was not initially planned to test the effects of noise or vegetation cover. There was 20 nest-boxes at high-levels [65 to more than 75 dB(A)] and only 3 at very low levels [< 45 dB(A)] out of 98. Therefore the majority of nest-boxes were exposed to medium levels of noise, with lack of representation of low noise areas, and our gradient of noise may not have been wide enough and lacked power.
In this study, measures of anthropogenic noise level and vegetation cover were obtained from map layers accessible in open data. Concerning noise level, this presents both advantages and disadvantages: Lden indexes from these maps result from several measures, over several years, and are calculated taking into account landscape elements to account for attenuation (see text footnote 2). But it might be an extrapolation compared to the perceived noise levels at nest boxes, with potential annual variations. Also, vegetation cover might not on its own accurately reflect vegetation quality nor the abundance and quality of arthropod food sources for tits such as caterpillars or spiders. Vegetation structure (grass, bushes, trees), height of deciduous trees and tree species may influence arthropod community. In addition, likely due to environmental stress, urban tree leaves have been found to be of lesser quality, which further impacts the diet quality of insectivorous birds (Isaksson, 2009). A future direction will be to more precisely characterize environments around nest boxes, by repeatedly measuring noise levels over the breeding season, and describing vegetation structure and composition in addition to coverage at each nest box. We only considered two variables to describe habitat quality at each nest box, but other aspects of habitat quality such as chemical pollution, light pollution and food resources might be important drivers too in the complex urban environment. Interactive effects between artificial light at night and noise on great tits’ activity patterns have indeed been experimentally demonstrated: while noise exposure tends to disturb activity patterns, artificial light at night increases activity. Both disturbances have a synergistic effect on total activity and night activity, while having an antagonistic effect on daytime activity (Dominoni et al., 2020b). These results suggest complex interactions between different factors, divergent effects depending on the biological levels studied and co-variables considered. In addition, given their known influence on birds’ breeding phenology and success, and their potential to interact with habitat quality, it would be necessary to measure temperature variation in the nest box over the breeding season, and also take winter and spring temperatures into account. It is indeed important for future studies on avian populations in the urban environment to characterize its complexity and not only consider stress factors, but also factors that may explain how species compensate and thrive in cities.
In this correlative study in Paris and its surroundings, breeding pairs of blue tits and great tits did not seem to avoid high noise level nest boxes. Anthropogenic noise levels did not correlate with the laying date for both species, but great tits laid their first egg later when vegetation coverage increased around the nest box. Hatching success for blue tits was reduced at high noise levels and low vegetation coverage nest boxes, while for great tits there was no association with either anthropogenic noise or vegetation coverage. Finally, there was no association between fledging success and noise levels and vegetation coverage in either species. However, these results unveiled a difference in species sensibility to environmental parameters during reproduction, and highlight the importance of considering multiple parameters when studying free-living birds’ populations in the urban environment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Charles Darwin n°005 ethical committee.
CB and CV contributed to the design of the study. CB managed the project, fieldwork, and provided the raw data. EM ran the analyses with input from CB and CV. EM drafted and revised the manuscript with input from FJ, CV, and CB. All authors contributed to the article and approved the submitted version.
We acknowledge Sorbonne Université (grant Émergence-UPMC EME 1110 and recurrent research funding) for funding this research.
We would like to acknowledge the support of the cities of Paris and Rueil-Malmaison, of the Muséum National d’Histoire Naturelle, and of count Stefan Czarnecki at the Château de la Petite Malmaison, in allowing us to survey tit populations breeding in their green spaces and private properties, respectively. We also thank all students who participated in fieldwork each breeding season.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1058584/full#supplementary-material
Aronson, M. F. J., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). ‘A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers’. Proc. R. Soc. B Biol. Sci. 281:20133330. doi: 10.1098/rspb.2013.3330
Bailly, J., Scheifler, R., Berthe, S., Clément-Demange, V.-A., Leblond, M., Pasteur, B., et al. (2016). ‘From eggs to fledging: Negative impact of urban habitat on reproduction in two tit species’. J. Ornithol. 157, 377–392. doi: 10.1007/s10336-015-1293-3
Bebbington, K., and Hatchwell, B. J. (2016). ‘Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird’. Behav. Ecol. 27, 652–659. doi: 10.1093/beheco/arv198
Biard, C., Brischoux, F., Meillère, A., Michaud, B., Nivière, M., Ruault, S. F., et al. (2017). Growing in cities: An urban penalty for wild birds? A study of phenotypic differences between urban and rural great tit chicks (Parus major). Front. Ecol. Evol. 5:79. doi: 10.3389/fevo.2017.00079
Bleu, J., Agostini, S., and Biard, C. (2017). ‘Nest-box temperature affects clutch size, incubation initiation, and nestling health in great tits’. Behav. Ecol. 28, 793–802. doi: 10.1093/beheco/arx039
Boucaud, I. C. A., Aguirre Smith, M. L. N., Valère, P. A., and Vignal, C. (2016a). ‘Incubating females signal their needs during intrapair vocal communication at the nest: A feeding experiment in great tits’. Anim. Behav. 122, 77–86. doi: 10.1016/j.anbehav.2016.09.021
Boucaud, I. C. A., Perez, E. C., Ramos, L. S., Griffith, S. C., and Vignal, C. (2017). ‘Acoustic communication in zebra finches signals when mates will take turns with parental duties’. Behav. Ecol. 28, 645–656. doi: 10.1093/beheco/arw189
Boucaud, I. C. A., Valère, P. A., Smith, M. L., Doligez, B., Doligez, B., Cauchard, L., et al. (2016b). Interactive vocal communication at the nest by parent great tits parus major. IBIS 158, 630–644. doi: 10.1111/ibi.12374
Bruitparif (2019). Cartes stratégiques du bruit (CSB). Available online at: https://carto.bruitparif.fr/ (accessed on February 17, 2020).
Burt, S. A., Imamura, M., Uchida, T., Ohya, K., Morio, K., Fujino, H., et al. (2021). ‘Nutritional implications of feeding free-living birds in public urban areas’. J. Anim. Physiol. Anim. Nutr. 105, 385–393. doi: 10.1111/JPN.13441
Chace, J. F., and Walsh, J. J. (2006). ‘Urban effects on native avifauna: A review’. Landsc. Urban Plan. 74, 46–69. doi: 10.1016/j.landurbplan.2004.08.007
Chalfoun, A. D., and Schmidt, K. A. (2012). ‘Adaptive breeding-habitat selection: Is it for the Birds?’. Auk 129, 589–599. doi: 10.1525/AUK.2012.129.4.589
Chamberlain, D. E., Cannon, A. R., Toms, M., Leech, D., Hatchwell, B., and Gaston, K. (2009). ‘Avian productivity in urban landscapes: A review and meta-analysis’. IBIS 151, 1–18. doi: 10.1111/j.1474-919X.2008.00899.x
Corsini, M., Dubiec, A., Marrot, P., and Szulkin, M. (2017). ‘Humans and tits in the city: Quantifying the effects of human presence on great tit and blue tit reproductive trait variation’. Front. Ecol. Evol. 5:82. doi: 10.3389/FEVO.2017.00082/BIBTEX
Crooks, K. R., Suarez, A. V., and Bolger, D. T. (2004). Avian assemblages along a gradient of urbanization in a highly fragmented landscape. Biol. Conserv. 115, 451–462. doi: 10.1016/S0006-3207(03)00162-9
de Satgé, J., Strubbe, D., Elst, J., de Laet, J., Adriaensen, F., and Matthysen, E. (2019). Urbanisation lowers great tit Parus major breeding success at multiple spatial scales. J. Avian Biol. 50:e02108. doi: 10.1111/JAV.02108
Derryberry, E. P., Danner, R. M., Danner, J. E., Derryberry, G. E., Phillips, J. N., and Lipshutz, S. E. (2016). ‘Patterns of song across natural and anthropogenic soundscapes suggest that white-crowned sparrows minimize acoustic masking and maximize signal content’. PLoS One 11:e0154456. doi: 10.1371/journal.pone.0154456
Dominoni, D. M., Kjellberg Jensen, J., de Jong, M., Visser, M. E., and Spoelstra, K. (2020a). Artificial light at night, in interaction with spring temperature, modulates timing of reproduction in a passerine bird. Ecol. Appl. 30:e02062. doi: 10.1002/eap.2062
Dominoni, D. M., Smit, J. A. H., Visser, M. E., and Halfwerk, W. (2020b). ‘Multisensory pollution: Artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major)’. Environ. Pollut. 256:113314. doi: 10.1016/j.envpol.2019.113314
Eens, M., and Gorissen, L. (2005). ‘Complex female vocal behaviour of great and blue tits inside the nesting cavity’. Behaviour 142, 489–506. doi: 10.1163/1568539054012056
Germain, R. R., and Arcese, P. (2014). ‘Distinguishing individual quality from habitat preference and quality in a territorial passerine’. Ecology 95, 436–445. doi: 10.1890/13-0467.1
Glądalski, M., Bańbura, M., Kaliński, A., Markowski, M., Skwarska, J., Wawrzyniak, J., et al. (2017). ‘Differences in the Breeding Success of Blue Tits Cyanistes caeruleus between a Forest and an Urban Area: A Long-Term Study’. Acta Ornithol. 52, 59–68. doi: 10.3161/00016454AO2017.52.1.006
Grunst, M. L., Grunst, A. S., Pinxten, R., and Eens, M. (2020). ‘Anthropogenic noise is associated with telomere length and carotenoid-based coloration in free-living nestling songbirds’. Environ. Pollut. 260:114032. doi: 10.1016/j.envpol.2020.114032
Hagen, E. O., Hagen, O., Ibáñez-Álamo, J. D., Petchey, O. L., and Evans, K. L. (2017). Impacts of urban areas and their characteristics on avian functional diversity. Front. Ecol. Evol. 5:84. doi: 10.3389/fevo.2017.00084
Haggard, W. H. (1990). ‘Urban weather’. Int. J. Environ. Stud. 36, 73–82. doi: 10.1080/00207239008710584
Halfwerk, W., Bot, S., Buikx, J., van der Velde, M., Komdeur, J., ten Cate, C., et al. (2011). Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl. Acad. Sci. U.S.A. 108, 14549–14554. doi: 10.1073/pnas.1109091108
Halfwerk, W., Both, C., and Slabbekoorn, H. (2016). Noise affects nest-box choice of 2 competing songbird species, but not their reproduction. Behav. Ecol. 7, 1592–1600. doi: 10.1093/beheco/arw095
Hedblom, M., and Söderström, B. (2012). ‘Effects of urban matrix on reproductive performance of Great Tit (Parus major) in urban woodlands’. Urban Ecosyst. 15, 167–180. doi: 10.1007/s11252-011-0204-5
Helden, A. J., Stamp, G. C., and Leather, S. R. (2012). ‘Urban biodiversity: Comparison of insect assemblages on native and non-native trees’. Urban Ecosyst. 15, 611–624. doi: 10.1007/s11252-012-0231-x
Injaian, A. S., Gonzalez-Gomez, P. L., Taff, C. C., Bird, A. K., Ziur, A. D., Patricelli, G. L., et al. (2019). ‘Traffic noise exposure alters nestling physiology and telomere attrition through direct, but not maternal, effects in a free-living bird’. Gen. Comp. Endocrinol. 276, 14–21. doi: 10.1016/j.ygcen.2019.02.017
Injaian, A. S., Poon, L. Y., and Patricelli, G. L. (2018). ‘Effects of experimental anthropogenic noise on avian settlement patterns and reproductive success’. Behav. Ecol. 29, 1181–1189. doi: 10.1093/beheco/ary097
Isaksson, C. (2009). ‘The chemical pathway of carotenoids: From plants to birds’. Ardea 97, 125–128. doi: 10.5253/078.097.0116
Isaksson, C. (2018). “Impact of urbanization on birds,” in Bird species: How they arise, modify and vanish, ed. D. T. Tietze (Cham: Springer), 235–257. doi: 10.1007/978-3-319-91689-7_13
Isaksson, C., and Andersson, S. (2007). ‘Carotenoid diet and nestling provisioning in urban and rural great tits ’Parus major. J. Avian Biol. 38, 564–572. doi: 10.1111/j.2007.0908-8857.04030.x
Jackson, A. K., Froneberger, J. P., and Cristol, D. A. (2012). ‘Habitat near nest boxes correlated with fate of eastern bluebird fledglings in an urban landscape’. Urban Ecosyst. 16, 367–376. doi: 10.1007/S11252-012-0265-0
Jara, R. F., Crego, R. D., Samuel, M. D., Rozzi, R., and Jiménez, J. E. (2020). ‘Nest-site selection and breeding success of passerines in the world’s southernmost forests’. PeerJ 8:e9892. doi: 10.7717/peerj.9892
Kavelaars, M. M., Lens, L., and Müller, W. (2019). ‘Sharing the burden: On the division of parental care and vocalizations during incubation’. Behav. Ecol. 30, 1062–1068. doi: 10.1093/beheco/arz049
Kleist, N. J., Guralnick, R. P., Cruz, A., Lowry, C. A., and Francis, C. D. (2018). ‘Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community’. Proc. Natl. Acad. Sci. U.S.A. 115, 648–E657. doi: 10.1073/PNAS.1709200115/SUPPL_FILE/PNAS.201709200SI.PDF
Klett-Mingo, J. I., Pavón, I., and Gil, D. (2016). ‘Great tits, Parus major, increase vigilance time and reduce feeding effort during peaks of aircraft noise. Anim. Behav. 115, 29–34. doi: 10.1016/J.ANBEHAV.2016.02.021
Lepczyk, C. A., Aronson, M. F., Evans, K., Goddard, M. A., Lerman, S., and MacIvor, S. J. (2017). ‘Biodiversity in the City: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation’. BioScience 67, 799–807. doi: 10.1093/BIOSCI/BIX079
MacLeod, K. J., Naugle, L., Brittingham, M. C., and Avery, J. D. (2022). ‘Gas compressor noise does not influence tree swallow nestling condition or immune response’. J. Zool. 318, 1–9. doi: 10.1111/JZO.12997
Mariette, M. M., and Griffith, S. C. (2012). ‘Nest visit synchrony is high and correlates with reproductive success in the wild Zebra finch Taeniopygia guttata’. J. Avian Biol. 43, 131–140. doi: 10.1111/j.1600-048X.2012.05555.x
Martin, T. E., and Roper, J. J. (1988). ‘Nest predation and nest-site selection of a western population of the hermit thrush’. Condor 90, 51–57. doi: 10.2307/1368432
Marzluff, J. M., and Ewing, K. (2001). ‘Restoration of fragmented landscapes for the conservation of birds: A general framework and specific recommendations for urbanizing landscapes’. Restor. Ecol. 9, 280–292. doi: 10.1007/978-0-387-73412-5_48
McKinney, M. L. (2006). ‘Urbanization as a major cause of biotic homogenization’. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
Meillère, A., Brischoux, F., Ribout, C., and Angelier, F. (2015). Traffic noise exposure affects telomere length in nestling house sparrows. Biol. Lett. 11:20150559. doi: 10.1098/rsbl.2015.0559
Møller, A. P. J., Balbontín, A. A., Dhondt, F., Adriaensen, A., Artemyev, J., and Bańbura, et al. (2020). ‘Interaction of climate change with effects of conspecific and heterospecific density on reproduction’. Oikos 129, 1807–1819. doi: 10.1111/OIK.07305
Møller, A. P., Diaz, M., Flensted-Jensen, E., Grim, T., Ibáńez-Álamo, J. D., Jokimäki, J., et al. (2012). ‘High urban population density of birds reflects their timing of urbanization’. Oecologia 170, 867–875. doi: 10.1007/S00442-012-2355-3
Mühlbauer, M., Weisser, W., Müller, N., and Meyer, S. (2021). ‘A green design of city squares increases abundance and diversity of birds’. Basic Appl. Ecol. 56, 446–459. doi: 10.1016/J.BAAE.2021.05.003
Partecke, J., Schwabl, I., and Gwinner, E. (2006). Stress and the city: Urbanization and its effects on the stress physiology in European Blackbirds. Ecology 87, 1945–1952. doi: 10.1890/0012-9658200687[1945:SATCUA]2.0.CO;2
Pickett, S. T. A., Cadenasso, M. L., Grove, J. M., Boone, C. G., Groffman, P. M., Irwin, E., et al. (2011). ‘Urban ecological systems: Scientific foundations and a decade of progress’. J. Environ. Manag. 92, 331–362. doi: 10.1016/j.jenvman.2010.08.022
Pollock, C. J., Capilla-Lasheras, P., McGill, R. A. R., Helm, B., and Dominoni, D. M. (2017). Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyanistes caeruleus). Sci. Rep. 7:5014. doi: 10.1038/S41598-017-04575-Y
Raap, T., Pinxten, R., Casasole, G., Dehnhard, N., and Eens, M. (2017). Ambient anthropogenic noise but not light is associated with the ecophysiology of free-living songbird nestlings. Sci. Rep. 7:2754. doi: 10.1038/s41598-017-02940-5
Schroeder, J., Nakagawa, S., Cleasby, I. R., and Burke, T. (2012). ‘Passerine birds breeding under chronic noise experience reduced fitness’. PLoS One 7:e39200. doi: 10.1371/journal.pone.0039200
Shannon, G. M. F., Mckenna, L. M., Angeloni, K. R., Crooks, K. M., Fristrup, E., and Brown. (2016). ‘A synthesis of two decades of research documenting the effects of noise on wildlife’. Biol. Rev. 91, 982–1005. doi: 10.1111/brv.12207
Slabbekoorn, H., and den Boer-Visser, A. (2006). ‘Cities change the songs of birds’. Curr. Biol. 16, 2326–2331. doi: 10.1016/j.cub.2006.10.008
Slabbekoorn, H., and Peet, M. (2003). ‘Birds sing at a higher pitch in urban noise’. Nature 424:267. doi: 10.1038/424267a
Solonen, T. (2001). ‘Breeding of the great tit and blue tit in urban and rural habitats in Southern Finland’. Ornis Fennica 78, 49–60.
Templeton, C. N., Zollinger, S. A., and Brumm, H. (2016). Traffic noise drowns out great tit alarm calls. Curr. Biol. 26, R1173–R1174. doi: 10.1016/j.cub.2016.09.058
Keywords: blue tit, great tit, anthropogenic stressors, laying date, clutch size, hatching success, fledging success, urban environment
Citation: Monniez E, Jiguet F, Vignal C and Biard C (2022) Differential effects of anthropogenic noise and vegetation cover on the breeding phenology and success of two urban passerines. Front. Ecol. Evol. 10:1058584. doi: 10.3389/fevo.2022.1058584
Received: 30 September 2022; Accepted: 17 November 2022;
Published: 08 December 2022.
Edited by:
Melissa Lin Grunst, University of La Rochelle, FranceReviewed by:
Lotte Schlicht, Max Planck Institute for Organismal Biology, GermanyCopyright © 2022 Monniez, Jiguet, Vignal and Biard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clotilde Biard, Y2xvdGlsZGUuYmlhcmRAc29yYm9ubmUtdW5pdmVyc2l0ZS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.