
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 01 December 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1057023
This article is part of the Research Topic Adaptations to Subterranean Environments View all 14 articles
Caves in temperate regions are characterized by food scarcity compared to surface habitats. Therefore, hypotheses on adaptation to cave life suggest that many characteristics, such as resistance to starvation, reduced energy demand, and increased food-finding ability, have evolved among cave dwellers to cope with food frugality. To test the hypothesis involving increased food-finding ability, the prey detection performances of three surface and three subterranean populations of the Pyrenean newt, Calotriton asper, were compared. First, the rapidity of surface individuals in capturing prey at short distances inside a restricted aquarium under dark and light conditions and their score for capturing prey in a larger aquarium under similar conditions were measured. Simultaneously the scores obtained from individuals in cave populations in darkness were compared. Individuals from one of the surface population were maintained in darkness for approximately five years and retested. The surface C. asper individuals captured prey faster at short distances under light conditions than in total darkness; however, the differences were not significant. The scores of the surface C. asper individuals in the large aquarium were significantly better in darkness than in light conditions. In addition, the scores of cave populations at short distances in darkness were better than those of epigean populations in light conditions; however, the differences were not significant. Finally, the scores of surface individuals maintained in darkness for five years improved. To explain these results, it should be noted that surface C. asper populations forage at night using non-visual cues (e.g., chemical and mechanical cues), further suggesting that vision may somewhat inhibit other senses. This ability to forage at night may be favorable for cave colonization. Moreover, some hypogean populations, when the use of prey-detecting non-visual senses has developed, may start to exhibit adaptivity.
In temperate regions, caves are characterized by food scarcity, among other features, compared to surface habitats (Juberthie and Decu, 1994; Hüppop, 2000; Culver and Pipan, 2009; Romero, 2009). To cope with frugality, theories on adaptation to cave life suggest that many characteristics have evolved in cave dwellers, such as increased food-finding ability, resistance to starvation, and reduced energy demand (Hervant and Renault, 2002). The Pyrenean newt, Calotriton asper, inhabits surface brooks and cave rivers. Previous studies have correlated a fasting adaptation (Issartel et al., 2010), a lower metabolic level in cave populations, and a decreased surface population with their acclimation to cave conditions for years (Guillaume et al., 2020). However, few studies on their food-finding ability have been conclusive. Calotriton asper can develop efficient foraging activity in darkness; however, this has only been observed in captivity and not in nature (Uiblein et al., 1992), although the species may exhibit nocturnal activity, as established by some observations reported in the gray literature (for instance Nicol, 1990; Dalibard, 2021). Previous preliminary observations made on some individuals of unknown origin (Uiblein et al., 1992) showed that C. asper could detect prey using only chemical and/or mechanical cues but concluded that vision dominates the foraging behavior of C. asper at short distances with improved performance in light conditions than in darkness. This study investigated the possible variation in the food-feeding ability between surface and cave populations and its possible improvement when surface C. asper populations are maintained in cave conditions for years.
Adult Calotriton asper individuals were captured from three surface and three hypogean populations (the locations of these populations are indicated in Table 1). They were placed in the “Station d’Ecologie Théorique et Expérimentale, SETE” (Ariège, France) cave in aquaria filled with water from the cave river (10.13 ± 0.19 ppm O2, 11.5 ± 0.25°C), and marked using Nanotec transponders. Individuals from surface populations were exposed to alternating 12 h day/night photic cycles, whereas those from cave populations were maintained in total darkness. They were fed live blood worms (prey usually found in the diet of C. asper) ad libitum once a week (Clergue-Gazeau, 1969; Montori, 1991). The first series of tests was performed up to 3 weeks after capture with twenty individuals for each population. The twenty individuals from surface population 1 were maintained in total darkness for five years and retested. These were identified by their transponder so their scores at the first series and the second one five years later could have been individually compared.
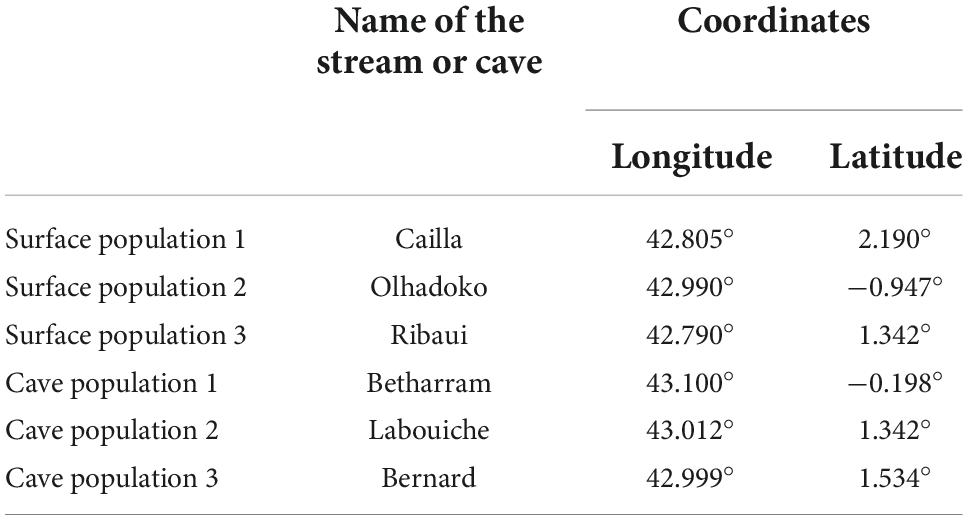
Table 1. Name and location of the three surface and three cave C. asper populations where individuals used in the study were obtained.
Individuals were subjected to a one-week fast before testing. They were isolated in a small 30 × 15 × 20 cm aquarium filled with 4.5 L water. Following a 2 h acclimatization period, one live blood worm was introduced to the center of the aquarium, and observations were done remotely with a camera connected to a recorder. The time to worm capture was recorded; failure to capture the prey after 2 h was also recorded. Then, the newts were fed ad libitum and were replaced with others. Twenty individuals from each population were tested successively. Tests were repeated after with the same individuals identified by their transponders inside a larger 80 × 30 × 20 cm aquarium filled with 22.5 L water. Finally, each individuals from surface populations was tested successively (after a one-week fast between each test) four time (one in a small aquarium in light, one in a small aquarium in darkness, one in a larger aquarium in light, one in a larger aquarium in darkness) except for individuals from the surface population & who were tested again two more time (one in a small aquarium in dark five years later, one in a larger aquarium in dark five years later), and each individuals from cave populations, was tested two times (one in a small aquarium in dark, one in a larger aquarium in dark). For individuals from surface populations, tests under the light condition (spectrum daylight fluorescent lamps True-light ®Natural Daylight 5500 K/Color Rendering 1 A, Ra > 90) were conducted during the day, and those in darkness were conducted at night. Observations in darkness were made using infrared light [420TVL, 0.5 lux/f2.0, 0, Lux (15m IR ON), 24 LED’s IR].
Twenty individuals from each population were examined. The variations in time required for prey capture under various conditions (aquarium size, in darkness or light conditions) were not normally distributed, and considering the limited sample size, data were thus analyzed using non-parametric tests. The scores of the same identified animals tested under various conditions were compared using the Wilcoxon unilateral test (XLstat software). Differences between populations were analyzed using the Friedman test (XLstat software). P < 0.05 were considered statistically significant.
All the required French permits for animal experimentation on the species used in this study were obtained from the Animal Experimentation Accreditation: n°A09583 for the lab, n° A09-1 (2001)–A09-2 (2007)–A09-3(2011)–A09-5(2013) for the experimenters, and n° 09-19, 09-273, 09-295 for the animal caretakers and handlers for the use for wildlife for scientific purposes.
All the required French permits (permit no. 2017-04, 2007-11-1342, and 2016-s-01) for the capture, marking, transport, detention, use, and release of protected amphibian species, and animal experimentation were obtained. The project was approved by the National Council for Nature Protection on March 19, 2007, and the Regional Scientific Council for the Natural Heritage of the Region Languedoc-Roussillon-Midi-Pyrénées on April 5, 2016.
For the three surface populations, the time to prey capture in the small aquarium was shorter under light conditions than in darkness, although the differences were not significant (Figure 1). In contrast, the time to prey capture in the larger aquarium was significantly longer under light conditions than in darkness for the three surface populations (population 1, P = 0.32; population 2, P = 0.021; population 3, P = 0.022).
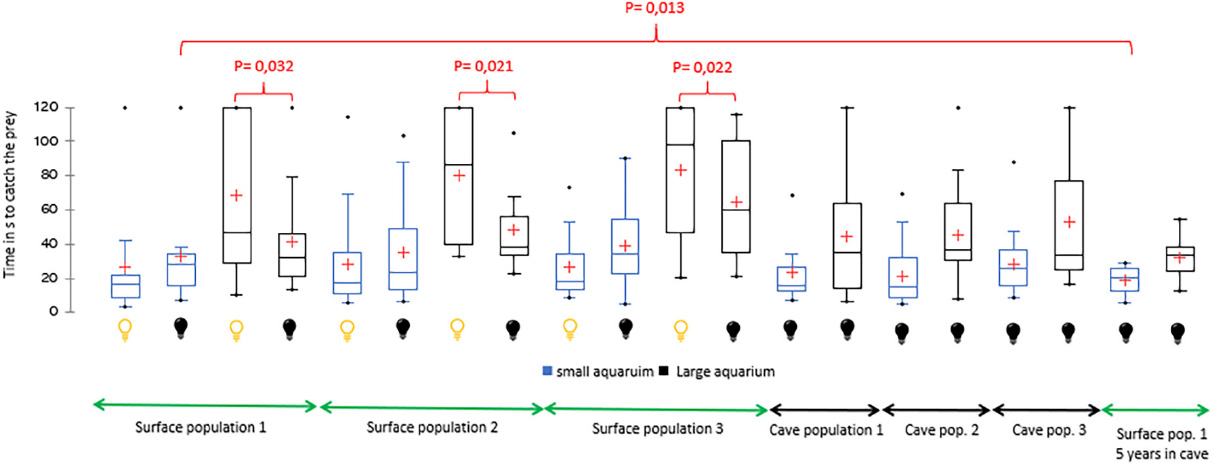
Figure 1. Time (in seconds) to prey capture by individuals (N = 20) from surface and cave populations in the small aquarium (4.5 L), large aquarium (22.5 L), in light conditions ( ), and darkness (
), and darkness ( ). The red crosses represent the means. The central horizontal bars represent the medians. The lower and upper limits of the box represent the first and third quartiles, respectively. The minimum is shown at the far bottom of the whisker, and the maximum is shown at the far top. P-values are indicated when significant.
). The red crosses represent the means. The central horizontal bars represent the medians. The lower and upper limits of the box represent the first and third quartiles, respectively. The minimum is shown at the far bottom of the whisker, and the maximum is shown at the far top. P-values are indicated when significant.
The scores of cave populations in the small aquarium in darkness were better than those of the epigean populations in light and darkness; however, in all cases, the differences were not significant.
The scores of the three surface populations in the large aquarium in darkness did not differ from those of the cave populations.
The scores of surface population 1 subjected to darkness for five years improved compared to the previous tests: (i) a significant difference was observed in the small aquarium in darkness (P = 0.013), (ii) an insignificant difference was observed in the small aquarium in light conditions, and (iii) an insignificant difference was observed in the large aquarium in darkness.
The study findings herein confirmed that surface C. asper individuals could develop efficient foraging activity in darkness at night; however, they exhibited an improved speed for prey capture in light at a short distance than in darkness. Nevertheless, these values were not higher than those of individuals from cave populations in darkness. Moreover, surface C. asper individuals captured prey more rapidly in total darkness at a long distance. In another study with individuals from epigean populations, vision was highly dominant, considerably inhibiting extra-optical prey perception (Uiblein et al., 1995). These results prompted a question: given that multimodal sensory information for foraging is widespread throughout the animal kingdom, can vision blur other senses in C. asper and decrease efficiency when combined? Relatively little evidence of such processes exists. For instance, in the pit viper, visual and infrared information is effective in prey targeting; however, interference between the two modalities can occur, causing a reduction in performance (Chen et al., 2012). Moreover, Uiblein et al. (1995) hypothesized that the change in the foraging style of surface C. asper was related to the prey encountered in darkness. Similar behavior has been observed in fire salamanders, where larvae from cave-breeding populations have a better predation performance in complete darkness than in light conditions (Manenti and Ficetola, 2013); they exhibit a more active search strategy in cave conditions, giving up the standard sit-and-wait strategy of epigeans, which is less efficient in caves due to prey scarcity and difficulty in detecting preys (Manenti et al., 2013). In surface C. asper populations, the shift toward an efficient strategy to forage in the dark may be favored by a predisposition to night foraging. The present study was conducted only with adults, and similar experiments on youngers also could have been interesting to really assess the potential of the species to survive in caves, however, in the majority of the populations, larvae and juveniles are rarely observed, especially among caves populations, and the spontaneous reproduction of this species in captivity is still hard to obtain (Clergue-Gazeau, 1971, 1976; Montori, 1988; Guillaume, 2000). Another important finding in this study was that surface C. asper individuals had an improved ability to capture in the dark when maintained in cave conditions for years. The mechanism by which this is achieved is unclear; however, some species, such as zebrafish (Danio rerio), have been found to learn to forage in the dark (Carrillo and McHenry, 2016). Foraging plasticity is considered a facilitating trait for colonization and adaptation to new habitats (Crispo, 2008), especially when experiencing challenging pressures, such as in caves (Manenti et al., 2013). Furthermore, plasticity might be a dominant process in the first steps of colonization because it contributes to the persistence of settlers, allowing for rapid adaptive responses, which can, in a subsequent phase, accompany local adaptations (Crispo, 2007). A strong genotypic differentiation exists between the surface and cave C. asper populations, suggesting a lack of gene flow between them for a long time and the existence of local adaptive phenotypic divergence for multiple traits (Milá et al., 2010; Valbuena-Ureña et al., 2018; Lucati et al., 2020). However, the contribution of plasticity in the processes of genotypic diversification should be clarified. In particular, the question of whether adaptive evolution proceeds through the genetic fixation of plastic phenotypes must be addressed. Finally, this study supports the hypothesis that surface C. asper individuals can colonize caves through plastic traits and that surface and cave populations may have fragmented and diverged during the postglacial climatic warming where conditions in low valleys were unfavorable, except in caves (Guillaume, 2001).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This animal study was reviewed and approved by Ministére de L’Enseignement Supérieur et de la Recherche N°068.
OG conceived and designed the experiments, collected the urodelan species, performed the experiments, analyzed the data, prepared figure and table, authored, and reviewed drafts of the manuscript.
This study was funded by the program PEPS (First Support for Exploratory Projects) from the Institute of Ecology and Environment of the French National Center for Scientific Research, and the Interreg POCTEFA Project ECTOPYR (No. EFA031/15).
This study is set within the framework of the “Laboratoires d’Excellences (LABEX)” “TULIP (ANR-10-LABX-41)”. We thank editage (www.editage.com) for English language editing.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RM declared a past collaboration with the author OG.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Carrillo, A., and McHenry, M. J. (2016). Zebrafish learn to forage in the dark. J. Exp. Biol. 219, 582–589. doi: 10.1242/jeb.128918
Chen, Q., Deng, H., Brauth, S. E., Ding, L., and Tang, Y. (2012). Reduced performance of prey targeting in pit vipers with contralaterally occluded infrared and visual senses. PLoS One 7:e34989. doi: 10.1371/journal.pone.0034989
Clergue-Gazeau, M. (1971). L’Euprocte pyrénéen. Conséquence de la vie cavernicole sur son développement et sa reproduction. Ann. Spéléol. 25, 825–960.
Clergue-Gazeau, M. (1976). Reproduction des urodèles. Perturbations apportées à la reproduction de l’espèce Euproctus asper épigée par sa mise en élevage à la grotte de Moulis. II. Euproctus asper femelle. Ann. Spéléol. 31, 163–168.
Crispo, E. (2007). The Baldwin effect and genetic assimilation: Revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479. doi: 10.1111/j.1558-5646.2007.00203.x
Crispo, E. (2008). Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J. Evol. Biol. 21, 1460–1469. doi: 10.1111/j.1420-9101.2008.01592.x
Culver, D. C., and Pipan, T. (2009). Biology of caves and other subterranean habitats. Oxford: Oxford University Press, 254.
Dalibard, M. (2021). Étude de l’habitat du Calotriton des Pyrénées (Calotriton asper): De l’échelle de son aire de distribution en France à celle d’un cours d’eau [thesis]. Toulouse: University of Toulouse.
Guillaume, O. (2000). Importance des communications chimiques dans le comportement social des Urodèles cavernicoles. Comparaison entre un cavernicole strict (Proteus anguinus, Proteidae) et un cavernicole facultatif (Euproctus asper, Salamandridae) [thesis]. Lyon: University of Claude Bernard.
Guillaume, O. (2001). Données et hypothèses sur la chronologie de l’enfouissement et l’isolement des populations souterraines d’euprocte des pyrénées (Euproctus asper) en Ariège. Mém. Biospéol. 27, 80–83.
Guillaume, O., Deluen, M., Raffard, A., Calvez, O., and Trochet, A. (2020). Reduction in the metabolic levels due to phenotypic plasticity in the Pyrenean newt, Calotriton asper, during cave colonization. Ecol. Evol. 10, 12983–12989. doi: 10.1002/ece3.6882
Hervant, F., and Renault, D. (2002). Long-term fasting and realimentation in hypogean and epigean isopods: A proposed adaptive strategy for groundwater organisms. J. Exp. Biol. 205, 2079–2087. doi: 10.1242/jeb.205.14.2079
Hüppop, K. (2000). “How do cave animals cope with the food scarcity in caves?,” in Subterranean Ecosystems. Ecosystems of the world, Vol. Volume 30, eds H. Wilkens, D. C. Culver, and W. F. Humphreys (Amsterdam: Elsevier), 159–188.
Issartel, J., Voituron, Y., Guillaume, O., Clobert, J., and Hervant, F. (2010). Selection of physiological and metabolic adaptations to food deprivation in the Pyrenean newt Calotriton asper during cave colonisation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 155, 77–83. doi: 10.1016/j.cbpa.2009.10.002
Juberthie, C., and Decu, V. (1994). “Structure et diversité du domaine souterrain; particularités des habitats et adaptations des espèces,” in Encyclopaedia biospeologica, Vol. Volume I, eds C. Juberthie and V. Decu (Moulis: Société Internationale de Biospéologie), 5–22.
Lucati, F., Poignet, M., Miró, A., Trochet, A., Aubret, F., Barthe, L., et al. (2020). Multiple glacial refugia and contemporary dispersal shape the genetic structure of an endemic amphibian from the Pyrenees. Mol. Ecol. 29, 2904–2921. doi: 10.1111/mec.15521
Manenti, R., and Ficetola, G. F. (2013). Salamanders breeding in subterranean habitats: Local adaptations or behavioural plasticity? J. Zool. 289, 182–188. doi: 10.1111/j.1469-7998.2012.00976.x
Manenti, R., Denoël, M., and Ficetola, G. F. (2013). Foraging plasticity favours adaptation to new habitats in fire salamanders. Anim. Behav. 86, 375–382. doi: 10.1016/j.anbehav.2013.05.028
Milá, B., Carranza, S., Guillaume, O., and Clobert, J. (2010). Marked genetic structuring and extreme dispersal limitation in the Pyrenean brook newt Calotriton asper (Amphibia: Salamandridae) revealed by genome-wide AFLP but not mtDNA. Mol. Ecol. 19, 108–120. doi: 10.1111/j.1365-294X.2009.04441.x
Montori, A. (1988). Estudio sobre la biología y ecología del tritón pirenaico Euproctus asper (Dugès, 1852) en la Cerdanya [thesis]. Barcelona: University of Barcelona.
Montori, A. (1991). Alimentacion de los adultos de Euproctus asper (Dugès 1852) en la montaña media del prepirineo Catalan (España). Rev. Esp. Herp. 5, 23–36.
Uiblein, F., Durand, J. P., Juberthie, C., and Parzefall, J. (1992). Predation in caves: The effects of the prey immobility and darkness on the foraging behaviour of two salamanders, Euproctus asper and Proteus anguinus. Behav. Proc. 28, 33–40. doi: 10.1016/0376-6357(92)90046-G
Uiblein, F., Engelke, S., and Parzefall, J. (1995). Trade-off between visual detectability and nutrient content in the patch choice of the Pyrenean salamander Euproctus asper. Ethology 101, 39–45. doi: 10.1111/j.1439-0310.1995.tb00343.x
Keywords: newts, food-feeding, detection performance, cave, darkness
Citation: Guillaume O (2022) Surface newt Calotriton asper acclimation to cave conditions improved their foraging ability in darkness. Front. Ecol. Evol. 10:1057023. doi: 10.3389/fevo.2022.1057023
Received: 29 September 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Enrico Lunghi, University of L’Aquila, ItalyReviewed by:
Raoul Manenti, University of Milan, ItalyCopyright © 2022 Guillaume. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Guillaume, b2xpdmllci5ndWlsbGF1bWVAc2V0ZS5jbnJzLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.