- State Key Laboratory of Herbage Improvement and Grassland Agro-ecosystems, College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou, China
Nitrogen (N) and phosphorus (P) are the main restrictive elements in terrestrial ecosystems, which have an important role in determining the community composition of plants and soil microorganisms. However, there is still a lack of understanding about whether plant and soil microbes respond synchronously to external N and P addition deposition, particularly on a short time scale (< 1 year). Here, we conducted a short-term experiment (3 months) involving control, N addition, P addition, and N + P addition in an alpine grassland on the Qinghai-Tibetan Plateau. Responses of plant and soil microbial (bacterial and fungal) communities were analyzed using the quadrat method and high-throughput sequencing, respectively. N addition significantly increased aboveground biomass and changed the plant community composition, but had no significant effect on soil microbes. Thus, microbial and plant processes were asynchronous following the resource availability in this alpine meadow. According to our research, the plant community may react to short-term nutrient deposition more quickly than the soil microbial community.
Introduction
In terrestrial ecosystems, nitrogen (N) and phosphorus (P) are two crucial nutrients for organisms and have an impact on a variety of biogeochemical processes. Due to several recent worldwide changes (such as climate warming, nitrogen deposition, etc.) caused by human activity, the availability of these nutrients in the environment has dramatically changed (Wang et al., 2020). This change triggered an imbalance between the availability of resources and organisms. On a worldwide scale, numerous studies have been carried out to investigate how plants or bacteria react to simulated N and P addition (Stiles et al., 2017; Li et al., 2020). These studies considerably contribute to our understanding of biogeochemical cycles in the context of global change (Ling et al., 2022). However, combined field experimental studies about both plant and microbial responses to N and P additions are still comparatively underutilized. In order to comprehend terrestrial ecosystem responses to nutrient deposition more thoroughly, both plant and soil communities should be taken into account at the same time.
Naturally, the aboveground and belowground ecosystems are closely connected (Wardle et al., 2004), and both plant and soil microbial communities could be affected by environmental factors (Dahl et al., 2017; Ma et al., 2018; Adair et al., 2019). The interactions between plants and soil bacteria make it more difficult to distinguish how environmental change affects a single population (Rudgers et al., 2020). On the other hand, little is known about whether these communities respond similarly or synchronously to environment change (Classen et al., 2015). It will be necessary to fill information gaps regarding the sequential or concurrent responses of plant and soil microbial populations to environmental change. In order to achieve this, short-term trials (less than a year) may present the chance to screen out the ephemeral responses brought on by the stepwise change, which are crucial for comprehending and identifying the tipping points (De Boeck et al., 2015).
The Qinghai-Tibetan Plateau is the plateau with the highest altitude and the largest area on earth, known as the “third pole of the world.” Affected by global change, the climate in this region has undergone drastic changes, and the annual average temperature, annual precipitation, and total nitrogen deposition rate have increased to varying degrees (Chen et al., 2013; IPCC, 2018). Under such a background, it is necessary to investigate the response mode mechanism of plant and soil microbial organisms to resource availability in this region. Here, we conducted a 3 months experiment with increasing soil fertility (nitrogen and phosphorus addition), the response of species composition of plant and soil microbes under different treatments were measured simultaneously. The following questions were specifically addressed by this study in an effort to provide answers: (1) How do the assemblages of plant and soil microbes respond to temporary external nutrient addition? (2) Do soil microbial and plant communities react simultaneously to resource availability?
Materials and methods
Study site
This experiment was carried out on a flat field located at the Haibei Alpine Grassland Ecosystem Research Station (37°36′N, 101°19′E, c. 3,215 m a.s.l.), located on the northeastern Qinghai-Tibetan Plateau in Qinghai Province, China. The site is a typical continental climate, where the annual average precipitation is 488 mm, and the annual average temperature is –1.1°C. The growing season in this region is from May to September. The vegetation is dominated by Kobresia humilis, Elymus nutans, and Stipa aliena.
Experimental design
Experimental treatments began in June 2021. A two-way factorial design with N and P addition was implemented involving four treatments: (1) control, CK; (2) nitrogen addition, N; (3) phosphorus addition, P; and (4) combined N and P addition, NP. With NH4NO3 and Ca(H2PO4)2, respectively, 10 g N m–2 and 5 g P m–2 of N and P addition were added to the meadow’s surface. The fertilizer was manually spread in solid form manually on drizzly day. Each treatment was replicated four times, resulting in a total of 16 plots. These plots (2.5 m × 2.5 m) were arranged in a complete randomized block design and each plot was separated by a 2-m buffer zone to minimize nutrient exchange between plots.
Sampling
After 3 months, we collected samples from all plots in the middle of August 2021. The plant species richness was determined for each plot as the total number of species in one 0.5 m × 0.5 m quadrat at the center of each plot. In each plot, three 0.16 m × 0.16 m quadrats were further randomly selected to measure the plant biomass. All individual plants in each quadrat were clipped to the soil surface and used to measure the shoot biomass after drying at 80°C for 48 h. Three soil cores with a depth of 0–10 cm and a diameter of 3.5 cm were randomly collected and mixed as one sample. Fine live roots were carefully separated from each soil sample, washed cleanly, and used for the determination of belowground biomass. The remaining soil samples were transported in sterile plastic bags on dry ice to the laboratory and stored at –20°C until the start of the extraction.
Sequencing and bioinformatics
Total DNA was extracted from soils using a PowerSoil® DNA Isolation Kit (Qiagen, USA), following the manufacturer’s instructions. Universal primers were used to amplify the 16S rRNA genes of bacteria (338F and 806R) and ITS genes of fungi (ITS1 and ITS2). Amplicons were purified and sequenced by Magigene (Guangzhou, China) on a NovaSeq6000 platform (Illumina Inc., San Diego, USA). The resulting sequences were first merged using FLASh v1.2.11. Afterward, the primer sequences were removed by the command of “fastq_filter” in VSEARCH 2.14.2. The filtered sequences were assigned to zero-radius operational taxonomic units (zOTUs) using UNOISE3 algorithm. The command of “usearch_global” was then used to map the fastq file into an zOTU table. The representative sequences were annotated for their taxonomic information based on the RDP database version 11.5 for bacteria and UNITE database release version 8.2 for fungi, respectively. All non-bacteria or non-fungi sequences and singletons were removed from the analysis. To correct for sampling effort, the number of sequences per sample was rarefied to the minimum number for the bacterial and fungal community, respectively. Raw sequence data is deposited to the Genome Sequence Archive (GSA) in National Genomics Data Center, China National Center for Bioinformation (CNCB) under the BioProject id PRJCA011844.
Statistical analysis
The data analyses were conducted using various packages in R v4.0.2. Before analysis, the data were tested for normality and loge(x + 1)-transformed when needed. The effects of treatments on plant and soil microbial richness were analyzed using mixed linear models with block as a random effect. The analysis was performed in R using the “lmer” command in the “lme4” package. The corresponding F and P values were derived from the “anova” function in the “lmerTest” package. Based on the linear mixed-effects models, the differences of each variable between treatments were tested by post hoc pairwise comparisons (Tukey method) using “glht” function of multcomp package. The principal coordinates analysis (PCoA) was performed to visualize differences in the plant or microbial community composition using “pcoa” function of the ade4 package in R. Significant differences in the plant or microbial community among treatments were further analyzed by Permutational multivariate ANOVA (PERMANOVA) with constraining permutations within blocks using “adonis2” function of the vegan package.
Results
The N addition significantly increased shoot biomass (F = 21.59, P < 0.001). Compared with unfertilized soils, the shoot biomass was increased by 17.91 and 31.94% under N and NP treatments, respectively (Figure 1A). Besides, the shoot biomass was marginally affected by P addition (F = 3.63, P = 0.08) and not affected by the interaction between N and P addition (F = 0.95, P = 0.35). On the other hand, we did not observe significant effects of our treatments on the root biomass (all P > 0.05, Figure 1B). We recorded 56 plant species belonging to 41 genera and 18 families over the course of the experiment. The richness of plant communities was not significantly affect by N addition (F = 0.01, P = 0.93), P addition (F = 0.07, P = 0.78), or their interaction (F = 1.43, P = 0.25; Figure 1C). PCoA ordinations of plant communities revealed that the species composition was highly changed by nitrogen addition (Figure 1D), which was confirmed by PERMANOVA analysis (F = 1.71, P = 0.01). With an increase in N availability, fast-growing grasses like E. nutans are more abundant relatively.
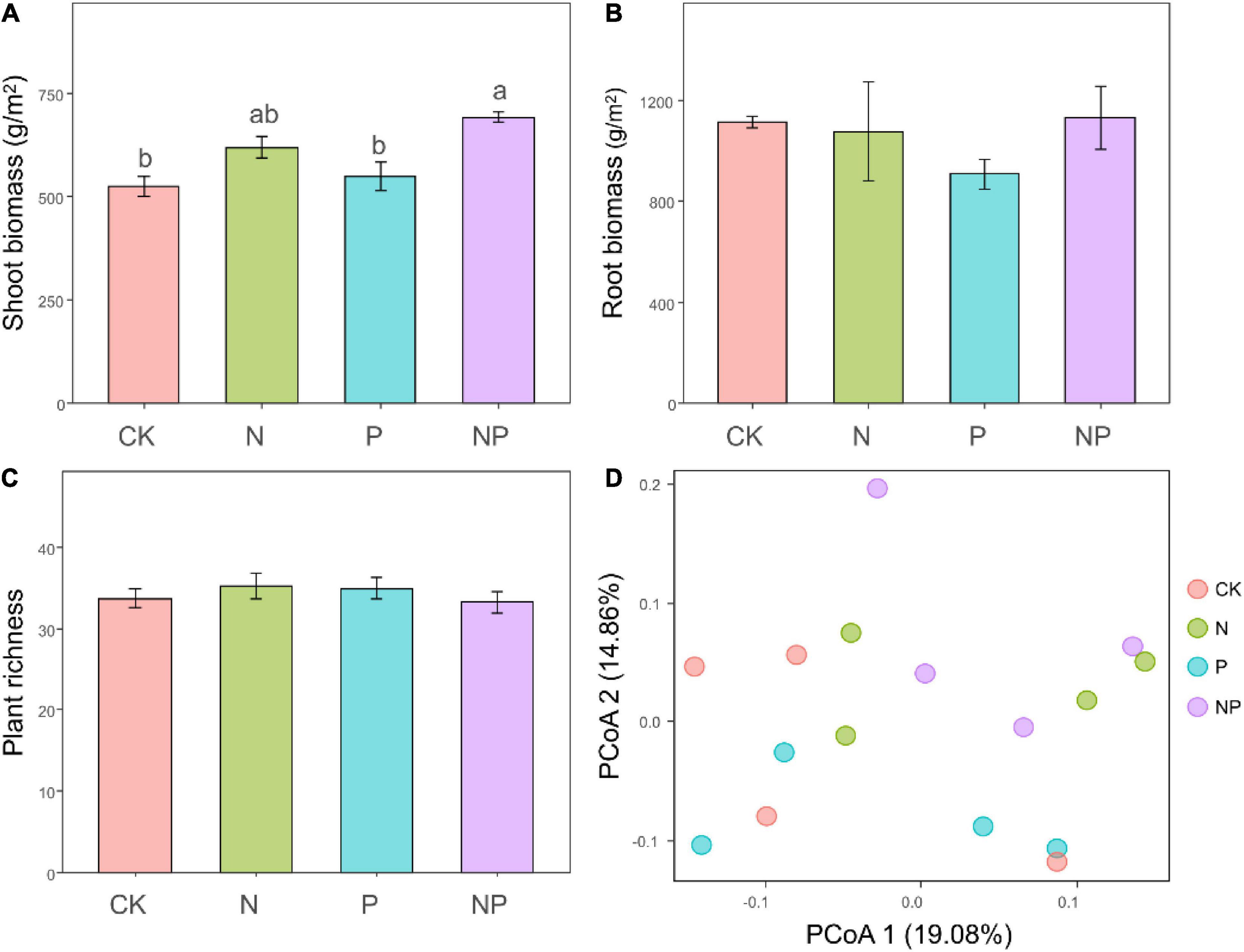
Figure 1. Shoot (A), root (B) biomass and plant richness (C) under different treatments. Bars represent means ± SEs. Significant differences of each variable among treatments are indicated by dissimilar letters above boxes (post hoc Tukey’s test). Principal coordinate analysis ordination (PCoA) plot based on a Bray–Curtis dissimilarity matrix of plant communities under different treatments (D).
Illumina NovaSeq paired-end sequencing of bacterial V3–V4 and fungal ITS1 region yielded 412,048 and 688,592 clean reads, respectively. These sequences were grouped into 7,016 bacterial zOTUs and 2,142 fungal zOTUs. The bacterial zOTUs were classified into 21 phyla, Proteobacteria (35.89%) and Acidobacteria (16.04%) were the two predominant phyla (Figure 2A), followed by Actinobacteria (15.48%) and Bacteroidetes (9.17%). The fungal communities were mainly dominated by Ascomycota with an average of 34.04% reads, followed by Basidiomycota with 26.94% of reads (Figure 2B). We did not observe any significant effect of our treatment on bacterial or fungal richness (all P > 0.05, Figures 3A,B). The PCoA based on Bray–Curtis dissimilarities was conducted to reflect the soil bacterial and fungal beta diversity among different treatments. The results showed that our treatment did not influence the bacterial or fungal community composition (Figures 3C,D), which was confirmed by the PERMANOVA analysis (all P > 0.05). Therefore, we speculate that in an alpine meadow on the Qinghai-Tibetan Plateau, the soil microbial community may react to resource availability more slowly than plants.

Figure 2. Relative abundances (%, proportion of sequencing reads) of bacteria (A) and fungi (B) at the phylum level in this study.
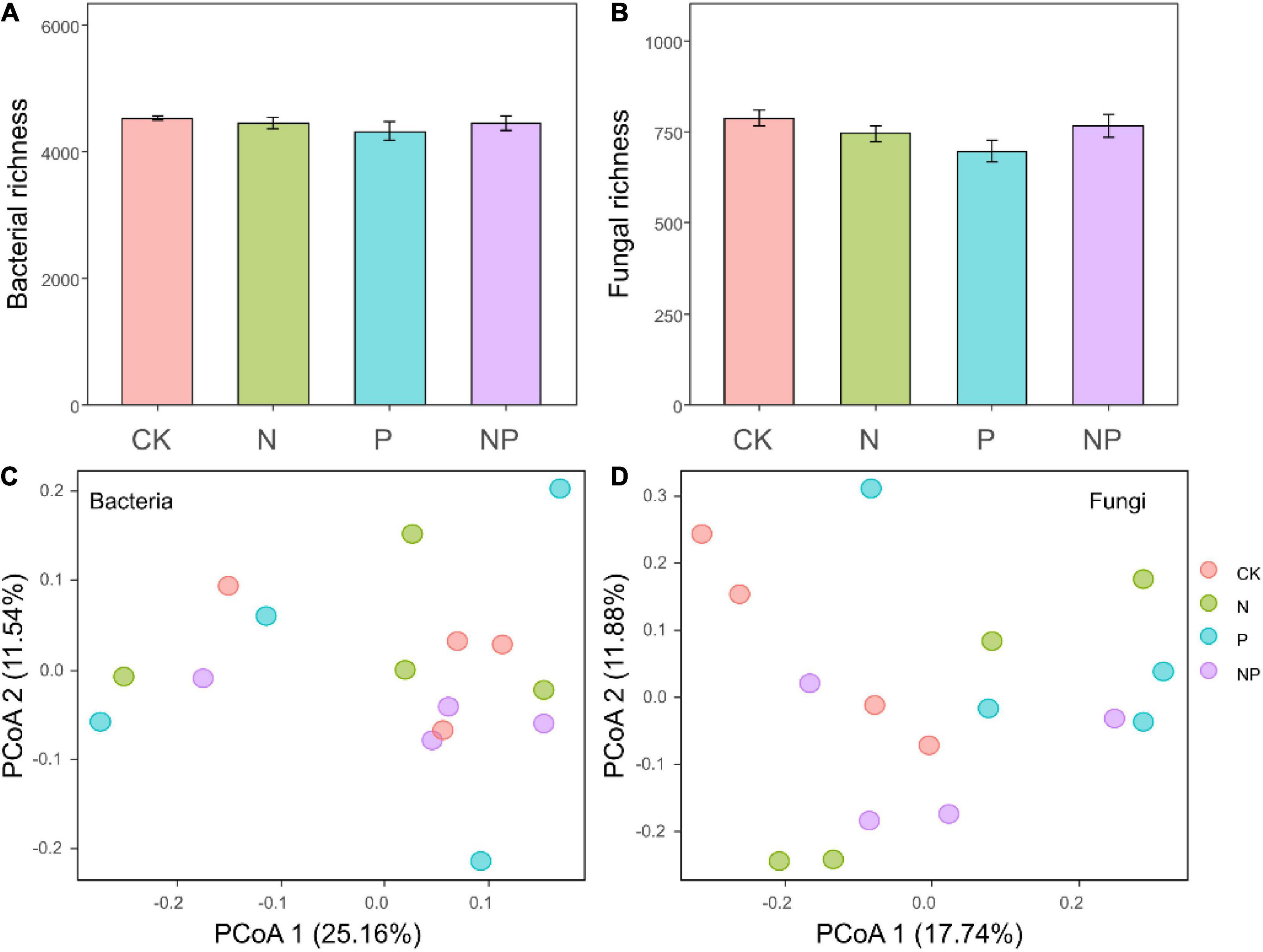
Figure 3. Bacterial richness (A) and fungal richness (B) under different treatments. Bars represent means ± SEs. Principal coordinate analysis ordination (PCoA) plot based on a Bray–Curtis dissimilarity matrix of bacterial communities (C) and fungal communities (D) under different treatments.
Discussion
By examining the effect of nutrient addition on the plant and soil microbial community structure, our results provide strong evidence that plant and soil microbes responded asynchronously to short-term nutrient deposition on the Qinghai-Tibetan Plateau.
Our results demonstrated that the N addition significantly increased aboveground biomass and altered the composition of plant community. The results are in line with the findings of previous studies, which shown that additional N increases aboveground biomass (Gao et al., 2018) and changed plant community composition (Stiles et al., 2017). However, neither the aboveground biomass nor the plant community composition was substantially influenced by P addition in our study. Therefore, it is possible that N was the predominant limiting nutrient in the alpine grassland on the Qinghai-Tibetan Plateau (Jiang et al., 2018; Li et al., 2021). In contrast to most previous findings, we did not observe any significant effect of nutrient addition on plant richness. In alpine meadows, nutrient addition typically resulted in the loss of some sedges and forbs (Sun et al., 2016). The explanation for the not significant decline of plant richness in response to nutrient enrichment in our study might be the relatively short-term experimental duration (Humbert et al., 2016). So, instead of the loss of species, our data also implied that the response of plants to nitrogen addition is firstly caused by changes in community composition.
Soil microorganisms are commonly thought to respond quickly to short-term events due to their rapid generation rates, high population densities, and diverse communities (Chase et al., 2021). Contrary to what we expected, the addition of fertilizer had no obvious influence on the diversity or composition of the soil microbial community. The following potential reasons might be responsible for the observations. First, soil microbial communities are insensitive to nutrient addition in our ecosystem, because previous study also reported that soil microbial biomass and community structure showed minor responses to 4 years of nitrogen and phosphorus addition (Chen et al., 2019). Since the root biomass was the major determinant of soil microbial community (Rinnan et al., 2005), the insensitivity of soil microbes could be related to the stability of root biomass in this study. The limitation of our study design is that we did not analyze the soil properties from soil. Therefore, we could not conclude whether the stability of soil microbial communities was also related to the soil properties.
Second, soil microbial composition transition may need a longer time than plant community to show up, since many studies reported changes in both plant and soil microbial community composition after long-term scale (Dahl et al., 2017; Ma et al., 2018). An apparent lack of responses in the short term may progress or cascade through the system, leading to long-term changes and adaptations (Knapp et al., 2012). In this case, the changes of soil microbial communities maybe triggered by plant community or changes in plant resource stoichiometry (Yuan et al., 2016; Liu et al., 2020; Wang et al., 2022). This finding corroborates the previous study which also reported that N deposition has greater effects on the plant community than on the soil microbial community (Wu et al., 2013). However, given that long-term time scale (8 years) used in their study, our current result may relate to different mechanism. Further dynamic monitoring is needed to illustrate the possible effects and mechanisms by which resource availability alter plant and soil microbial development at multiple time points.
In our study, fertilization addition affected plant shoot biomass and community composition while having no effect on the composition of the soil microbial community, demonstrating that, on a short-term time scale, resource availability has a greater impact on the plant community than the soil microbial community. This research contributes to our understanding of how above- and below-ground microbial communities are impacted by short-term environmental change, and the knowledge acquired can be applied to forecast long-term implications in alpine grassland ecosystems.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/structure, PRJCA011844.
Author contributions
SJ designed the research. JD and TT performed the field survey and data analysis. SJ wrote the first draft, with inputs from JD and TT. All authors read and approved the final manuscript.
Funding
This work was supported by the Youth Science and Technology Fund program of Gansu Province (No. 22JR5RA522).
Acknowledgments
We are grateful to the Haibei National Field Research Station in the Alpine Grassland Ecosystem for maintaining the experimental facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adair, K. L., Lindgreen, S., Poole, A. M., Young, L. M., Bernard-Verdier, M., Wardle, D. A., et al. (2019). Above and belowground community strategies respond to different global change drivers. Sci. Rep. 9:2540. doi: 10.1038/s41598-019-39033-4
Chase, A. B., Weihe, C., and Martiny, J. B. (2021). Adaptive differentiation and rapid evolution of a soil bacterium along a climate gradient. Proc. Natl. Acad. Sci. U.S.A. 118:e2101254118. doi: 10.1073/pnas.2101254118
Chen, H., Zhu, Q., Peng, C., Wu, N., Wang, Y., Fang, X., et al. (2013). The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Change Biol. 19, 2940–2955. doi: 10.1111/gcb.12277
Chen, X., Hao, B., Jing, X., He, J.-S., Ma, W., and Zhu, B. (2019). Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant Soil 444, 21–37. doi: 10.1007/s11104-019-04250-3
Classen, A. T., Sundqvist, M. K., Henning, J. A., Newman, G. S., Moore, J. A., Cregger, M. A., et al. (2015). Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6:130. doi: 10.1890/ES15-00217.1
Dahl, M. B., Priemé, A., Brejnrod, A., Brusvang, P., Lund, M., Nymand, J., et al. (2017). Warming, shading and a moth outbreak reduce tundra carbon sink strength dramatically by changing plant cover and soil microbial activity. Sci. Rep. 7:16035. doi: 10.1038/s41598-017-16007-y
De Boeck, H. J., Vicca, S., Roy, J., Nijs, I., Milcu, A., Kreyling, J., et al. (2015). Global change experiments: challenges and opportunities. BioScience 65, 922–931. doi: 10.1093/biosci/biv099
Gao, Y., Cooper, D. J., and Zeng, X. (2018). Nitrogen, not phosphorus, enrichment controls biomass production in alpine wetlands on the Tibetan Plateau, China. Ecol. Eng. 116, 31–34. doi: 10.1016/j.ecoleng.2018.02.016
Humbert, J. Y., Dwyer, J. M., Andrey, A., and Arlettaz, R. (2016). Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: a systematic review. Glob. Chang. Biol. 22, 110–120. doi: 10.1111/gcb.12986
IPCC (2018). Climate change: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC.
Jiang, S., Liu, Y., Luo, J., Qin, M., Johnson, N. C., Öpik, M., et al. (2018). Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytol. 220, 1222–1235. doi: 10.1111/nph.15112
Knapp, A. K., Smith, M. D., Hobbie, S. E., Collins, S. L., Fahey, T. J., Hansen, G. J., et al. (2012). Past, present, and future roles of long-term experiments in the LTER network. BioScience 62, 377–389. doi: 10.1525/bio.2012.62.4.9
Li, C., Li, Y., Li, X., Ma, L., Xiao, Y., and Zhang, C. (2021). Differential responses of plant primary productivity to nutrient addition in natural and restored Alpine Grasslands in the Qinghai Lake Basin. Front. Plant Sci. 12:792123. doi: 10.3389/fpls.2021.792123
Li, L., Li, X., Liu, B., Lei, J., Yue, Z., and Li, C. (2020). Imbalanced stoichiometric patterns in foliar nutrient resorption response to N and P addition in grazing alpine grassland. Acta Oecol. 102:103505. doi: 10.1016/j.actao.2019.103505
Ling, N., Wang, T., and Kuzyakov, Y. (2022). Rhizosphere bacteriome structure and functions. Nat. Commun. 13:836. doi: 10.1038/s41467-022-28448-9
Liu, X., Lamb, E. G., and Zhang, S. (2020). Nitrogen addition impacts on soil microbial stoichiometry are driven by changes in plant resource stoichiometry not by the composition of main microbial groups in an alpine meadow. Biol. Fertil. Soils. 56, 261–271. doi: 10.1007/s00374-019-01423-1
Ma, S., Verheyen, K., Props, R., Wasof, S., Vanhellemont, M., Boeckx, P., et al. (2018). Plant and soil microbe responses to light, warming and nitrogen addition in a temperate forest. Funct. Ecol. 32, 1293–1303. doi: 10.1111/1365-2435.13061
Rinnan, R., Keinänen, M. M., Kasurinen, A., Asikainen, J., Kekki, T. K., Holopainen, T., et al. (2005). Ambient ultraviolet radiation in the Arctic reduces root biomass and alters microbial community composition but has no effects on microbial biomass. Glob. Change Biol. 11, 564–574. doi: 10.1111/j.1365-2486.2005.00933.x
Rudgers, J. A., Afkhami, M. E., Bell-Dereske, L., Chung, Y. A., Crawford, K. M., Kivlin, S. N., et al. (2020). Climate disruption of plant-microbe interactions. Annu. Rev. Ecol. Evol. Syst. 51, 561–586. doi: 10.1146/annurev-ecolsys-011720-090819
Stiles, W. A., Rowe, E. C., and Dennis, P. (2017). Long-term nitrogen and phosphorus enrichment alters vegetation species composition and reduces carbon storage in upland soil. Sci. Total Environ. 593, 688–694. doi: 10.1016/j.scitotenv.2017.03.136
Sun, X., Yu, K., Shugart, H. H., and Wang, G. (2016). Species richness loss after nutrient addition as affected by N: C ratios and phytohormone GA3 contents in an alpine meadow community. J. Plant Ecol. 9, 201–211. doi: 10.1093/jpe/rtv037
Wang, P., Yang, F., Chen, X., Li, J., Zhou, X., and Guo, H. (2022). Long-term fertilization effects on soil biotic communities are mediated by plant diversity in a Tibetan alpine meadow. Plant Soil. 474, 525–540. doi: 10.1007/s11104-022-05356-x
Wang, Y., Ren, Z., Ma, P., Wang, Z., Niu, D., Fu, H., et al. (2020). Effects of grassland degradation on ecological stoichiometry of soil ecosystems on the Qinghai-Tibet Plateau. Sci. Total Environ. 722:137910. doi: 10.1016/j.scitotenv.2020.137910
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setala, H., Van Der Putten, W. H., and Wall, D. H. (2004). Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633. doi: 10.1126/science.109487
Wu, J., Liu, W., Fan, H., Huang, G., Wan, S., Yuan, Y., et al. (2013). Asynchronous responses of soil microbial community and understory plant community to simulated nitrogen deposition in a subtropical forest. Ecol. Evol. 3, 3895–3905. doi: 10.1002/ece3.750
Keywords: soil microbes, fertilization, alpine meadow, asynchronous response, Qinghai-Tibetan Plateau
Citation: Du J, Tan T and Jiang S (2022) Divergent responses of plant and soil microbial community to short-term nutrient addition in alpine grassland on the Qinghai-Tibetan Plateau. Front. Ecol. Evol. 10:1056111. doi: 10.3389/fevo.2022.1056111
Received: 28 September 2022; Accepted: 24 October 2022;
Published: 04 November 2022.
Edited by:
Hui Zhang, Hainan University, ChinaReviewed by:
Jiabao Li, Chengdu Institute of Biology (CAS), ChinaQiongfen Qiu, Ningbo University, China
Copyright © 2022 Du, Tan and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengjing Jiang, amlhbmdzaGoxM0BsenUuZWR1LmNu
†These authors have contributed equally to this work
 Juan Du
Juan Du Tianyuan Tan
Tianyuan Tan Shengjing Jiang
Shengjing Jiang