- 1Department of Geological Sciences and Engineering and Global Water Center, University of Nevada Reno, Reno, NV, United States
- 2Centro Regional Universitario Bariloche (CRUB)-CONICET, Departamento de geología y petróleo, Universidad Nacional del Comahue, San Carlos de Bariloche, Argentina
- 3Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC, United States
- 4Department of Biological and Environmental Sciences, Georgia College & State University, Milledgeville, GA, United States
- 5Department of Biology and Global Water Center, University of Nevada Reno, Reno, NV, United States
Periphyton assemblages from the nearshore environment of the west (California) side of Lake Tahoe, were analyzed to determine their taxonomic composition and community structure across habitats and seasons. Lake Tahoe is the second deepest lake in the US and an iconic oligotrophic subalpine lake with remarkable transparency. It has experienced offshore cultural eutrophication since the 1960s with observations of nuisance nearshore algal growth since the mid 2000s attributed to anthropogenic stressors. Samplings from November 2019–September 2020 provide useful snapshots against which older monitoring may be contextualized. A voucher flora, complete with descriptions, photo-documentation and referencing to species concepts employed, was created as a method of providing reproducible identification and enumeration of algal species, and more seamless reconciliation of detailed taxonomic data with future monitoring projects. The eulittoral zone (0–2 m) is seasonally dominated by elongate araphid (Synedra, Ulnaria) and stalked or entubed diatoms (Gomphonema, Cymbella, Encyonema). The sublittoral zone (>2 m) is dominated by a nitrogen-fixing Epithemia-cyanobacteria assemblage with less seasonal changes in dominance and composition that expanded to impinge on the 2 m depths of the eulittoral zone in the Fall. Sublittoral epipsammic samples, despite their proximity to rocks, had a very distinct diatom composition and high species dominance, similar to what was seen in the Fall eulittoral samples, with high numbers of Staurosirella chains and small biraphid diatoms. The deeper samples at 30 and 50 m contained high numbers of live Epithemia, and indicate a thriving sublittoral assemblage at these greater depths, but with less biomass. The 2019–20 data show many of the same diatom taxa observed in the 1970’s and 1980’s but with changes in species dominance. Notably, there was less of the green alga Mougeotia, when compared to the 1970’s data, and a higher dominance by nitrogen fixing Epithemia in the sublittoral zone, persisting year-round. These new data show roughly double the algal species biodiversity that had been documented previously in the Lake Tahoe nearshore, and is largely attributed to the methods employed. Adopting these new methods in future monitoring efforts should improve harmonization of taxonomic data and help advance our knowledge of the contributions to nearshore cultural eutrophication.
1. Introduction
Known for its remarkable water clarity, Lake Tahoe is an iconic subalpine oligotrophic lake located in the Sierra Nevada at an elevation of 1900 m.a.s.l., straddling the state line between California and Nevada. It is a large, steep-sided deep lake (maximum depth of 501 m) with a littoral zone encompassing ~19% of the lake’s total surface area (Goldman, 1974). Progressive, offshore cultural eutrophication has been documented since the 1960’s with a reduction in clarity by 10 m, and has resulted in changes to the lake benthos including alterations to the amount of bottom algal production (Chandra et al., 2005). In recent decades, local residents have observed nearshore, nuisance algal blooms that has been attributable to the introduction of species, changes in the timing of groundwater flow, and warmer temperatures (Naranjo et al., 2019; Vadeboncoeur et al., 2021). Since the 1970’s a large body of research has characterized many aspects of the limnology and investigated causes for water quality decline (TERC, 2022), with some research suggesting little or decreasing change in eulittoral biomass (Atkins et al., 2021). Few published studies address species-level algal biodiversity and/or taxonomy in Lake Tahoe, especially with respect to the periphyton. Herein, the term periphyton, ingrained in the Tahoe literature, simply refers to benthic algae.
Species-level enumeration of periphyton, especially diatom taxa from lakes and rivers, is an important component of water quality monitoring. Documenting project-specific morphological species concepts enables harmonization with existing and future monitoring data, and is vital to maintain informative long-term records and maximize data use (Potapova et al., 2022). Given the high species diversity and degree of endemism in freshwater diatom communities, as well as rapid developments in freshwater diatom taxonomy, there are no single taxonomic references that may be satisfactorily employed for species-level identification. Thus, the development of localized taxonomic references becomes essential in documenting the biodiversity and community structure. In Lake Tahoe, taxonomic surveys of periphyton were conducted in the 1960’s through 1980’s (Goldman, 1974; Goldman and De Amezaga, 1975; Van Landingham, 1987), and investigations of community composition of the periphyton in eulittoral and sublittoral zones additionally provide limited composition data (Aloi et al., 1988; Atkins et al., 2021). These resources contain only species lists, lacking photo-documentation, reference citation information, specimen accession numbers, and species notes affirming the taxonomic concepts that were employed. Taxonomically based work is sparse for Tahoe diatom periphyton, limited to select species of gomphonemoids (Kociolek and Stoermer, 1988), and as such the earlier species lists are difficult to harmonize with modern taxonomic usage.
In the context of a project with support from the California Lahontan Water Quality Control Board that serves to examine factors effecting nuisance algal growth in the nearshore, a diatom voucher flora was constructed to serve as the taxonomic identification resource for the west shore of Lake Tahoe. Voucher floras, as discussed by Bishop et al. (2017) and Alers-García et al. (2021), serve as working laboratory documents that can provide taxonomic consistency and quality control for algal enumeration during a project lifetime. They are devised as in-house taxonomic references for a specific project that provides a consistent method for analyzing large sample sets that often employ multiple analysts (Tyree et al., 2020a), and may be conducted over an extended period of years. Voucher floras differ from traditional monographic descriptions of diatom floras in that they document both well-established species, and also unidentified taxa whose taxonomic affinity to published scientific names is unresolved for a variety of reasons. Herein, we provide a diatom voucher flora and apply this method to enumeration of seasonal data from epilithic (=rock substrate) and epipsammic (=sand substrate) habitats in order to best characterize the community composition and structure. We additionally use soft algal community analysis to determine relative abundances of all algal groups in the nearshore environment from 0.5 to 50 m depth, including live diatoms, through observation of physiologically active chloroplasts within diatom frustules. The taxonomic survey and identification reference appended in the Supplementary material can serve as a foundation for future biodiversity work in the Lake Tahoe basin, and provide support for understanding why the nearshore environment in pristine lakes are undergoing enhanced algal growth and degrading water quality conditions.
2. Materials and methods
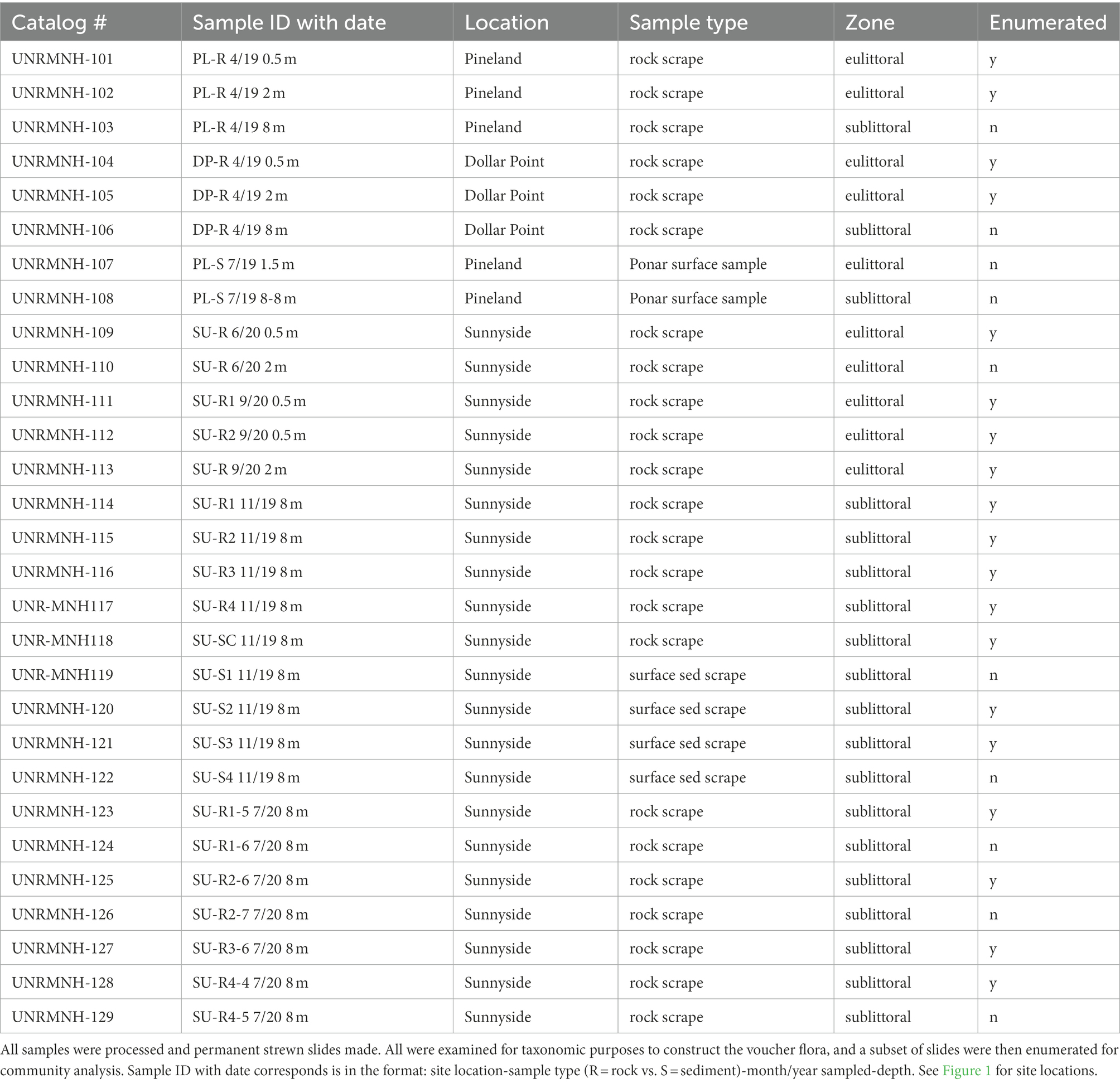
The diatom voucher flora constructed for the Lake Tahoe nearshore area contains images of the taxa encountered in the examination of 29 samples collected from rock scrapings and surface sediment from 0.5 m to 8 m water depth in the vicinity of Sunnyside Harbor, Pineland, and Dollar Point from April 2019 through September 2020 (Figure 1; Table 1) as part of an evaluation of nuisance algal growth in this area of the lake, conducted for California’s Lahonton Regional Water Quality Control Board. Both the eulittoral zone, a high energy wave zone 0-2 m depth, and the sublittoral zone (>2 m) were sampled because previous work has shown distinctions in algal assemblages (Loeb and Reuter, 1984; Aloi et al., 1988; Atkins et al., 2021). A subset of 19 samples were then enumerated to determine relative species abundance. The enumerated samples include 8 samples from rock scrapes in the eulittoral zone in Spring (4-23-19), early Summer (6-1-20), and Fall (9-29-20) seasons spanning the 3 sites, 8 samples from rock scrapings in the sublittoral zone, and 3 samples from surface sediment scrapings in the sublittoral zone. All sublittoral zone samples are from outside of Sunnyside Harbor at approximately 8 m depth from Fall (November 12 and 13, 2019) and Spring (July 21, 2020) seasons. Enumerated data were used as a means of evaluating community composition and seasonal differences in epilithic eulittoral and sublittoral zones, and the epipsammic community.
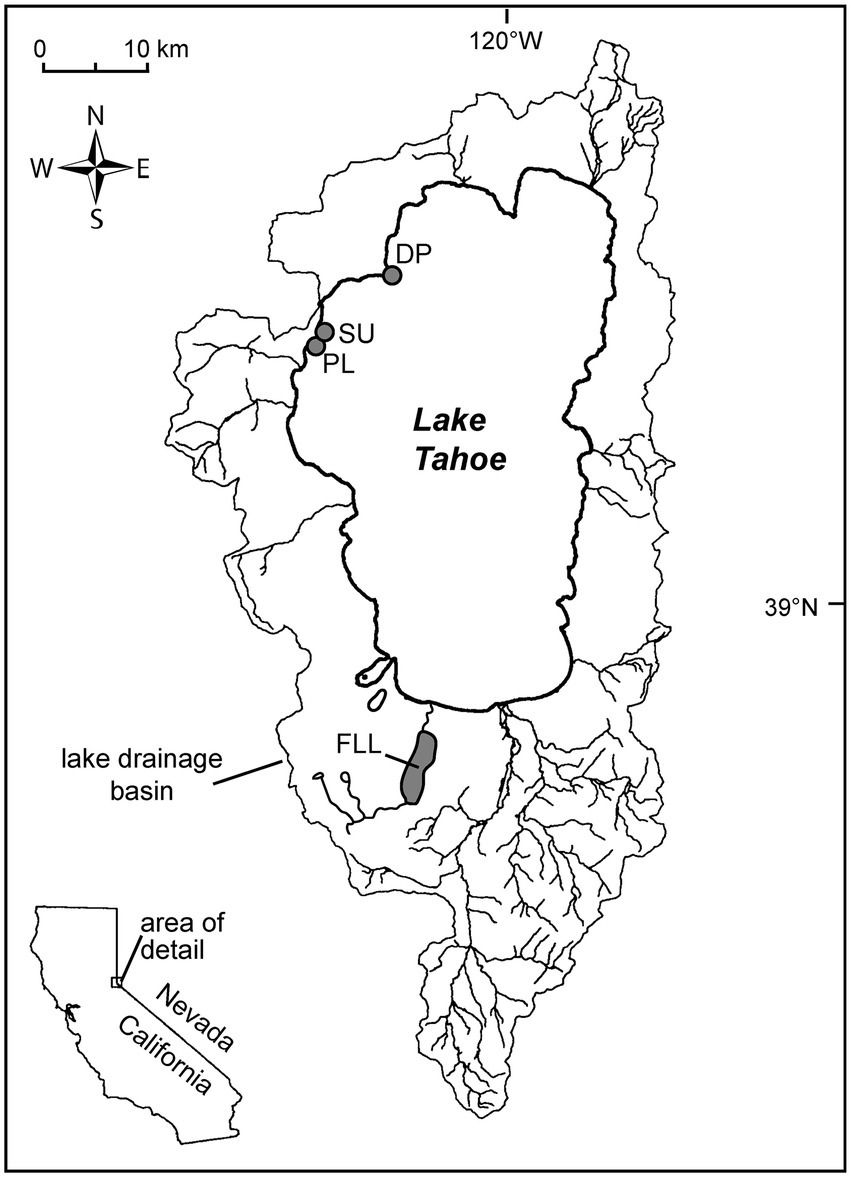
Figure 1. Locality map showing the extent of the watershed for Lake Tahoe, major drainages, and the 3 sampling localities on the west shore. SU: Sunnyside Harbor; PL: Pineland, and DP: Dollar Point, FLL: Fallen Leaf Lake.
The 29 diatom samples were processed using a hydrogen peroxide digestion method, where samples were heated for ~1 h in a 15% solution of peroxide. Samples were decanted then acidified using 20% HCl solution, heated for an additional hour, then decanted multiple times over the course of a week to remove acid. Cleaned siliceous slurries were then pipetted onto glass coverslips and permanent mounts of strewn slides were made using Zrax mounting medium. Slides were scanned and species documented with transmitted light microscopy, using DIC, and a 100x oil immersion lens on an Olympus BX51 microscope. Photomicrographs were taken using a Leica DMLB II microscope and Leica DMC6200 camera (Leica Microsystems, Wetzlar, Germany) and then sorted into operational taxonomic units (OTU) that were each assigned a provisional species code. In cases where the genus assignment of an OTU appeared clear, the species code used was a combination of the abbreviated genus name and a numerical designation (e.g., NAV01 for a species of Navicula). In some cases, taxa were later assigned to a different genus after the OTU had been in use (e.g., ENC04 = Cymbella neoleptoceros; Krammer, 2002). In cases where genus assignment would require investigation into the literature, the species code was abbreviated from a broader morphologic group (e.g., FRAG01 for a species of fragilarioid). Following the initial designation of OTUs, identification of diatom OTUs to the species level was undertaken using a variety of resources available, including the Diatoms of North America website (Spaulding et al., 2021), and many other resources from both North America and Europe (see Supplementary material). Initial identifications underwent a reconciliation process, to cross-check identifications and split out additional OTUs from morphologically similar arrays. Additionally, the voucher flora was harmonized with an unpublished flora constructed as part of a MS thesis by Stratton (2013) on Fallen Leaf Lake, a smaller subalpine lake in the Tahoe watershed (Figure 1). The Fallen Leaf Lake flora has served as an internal reference in author Noble’s laboratory, that is used for enumeration of Holocene Lake cores in the Tahoe basin (Ball et al., 2018; Johnson et al., 2018) and shares many taxa with the present sample set. All slides examined in this study have been accessioned in the Natural History Museum at the University of Nevada Reno and thus are available to the public and other scientists for examination.
Samples were then enumerated by counting n = 200 valves across multiple transects of the strewn slides. For broken valves, those greater than half were counted. All data are reported as percentage valve counts and not converted to biovolume data, as is typical in many of the Tahoe enumeration work done by Utermöhl chambers and inverted light microscopy (i.e., Goldman, 1974). The enumerated data were analyzed and plotted using the software PAST v.3.24 (Hammer et al., 2001) to look for differences between epipsammic versus epilithic habitats and seasonal variations, using Principal Components Analysis (PCA) and indices of species richness and species dominance. The PCA analysis was used to determine the number of statistically significant axes (Birks, 2012).
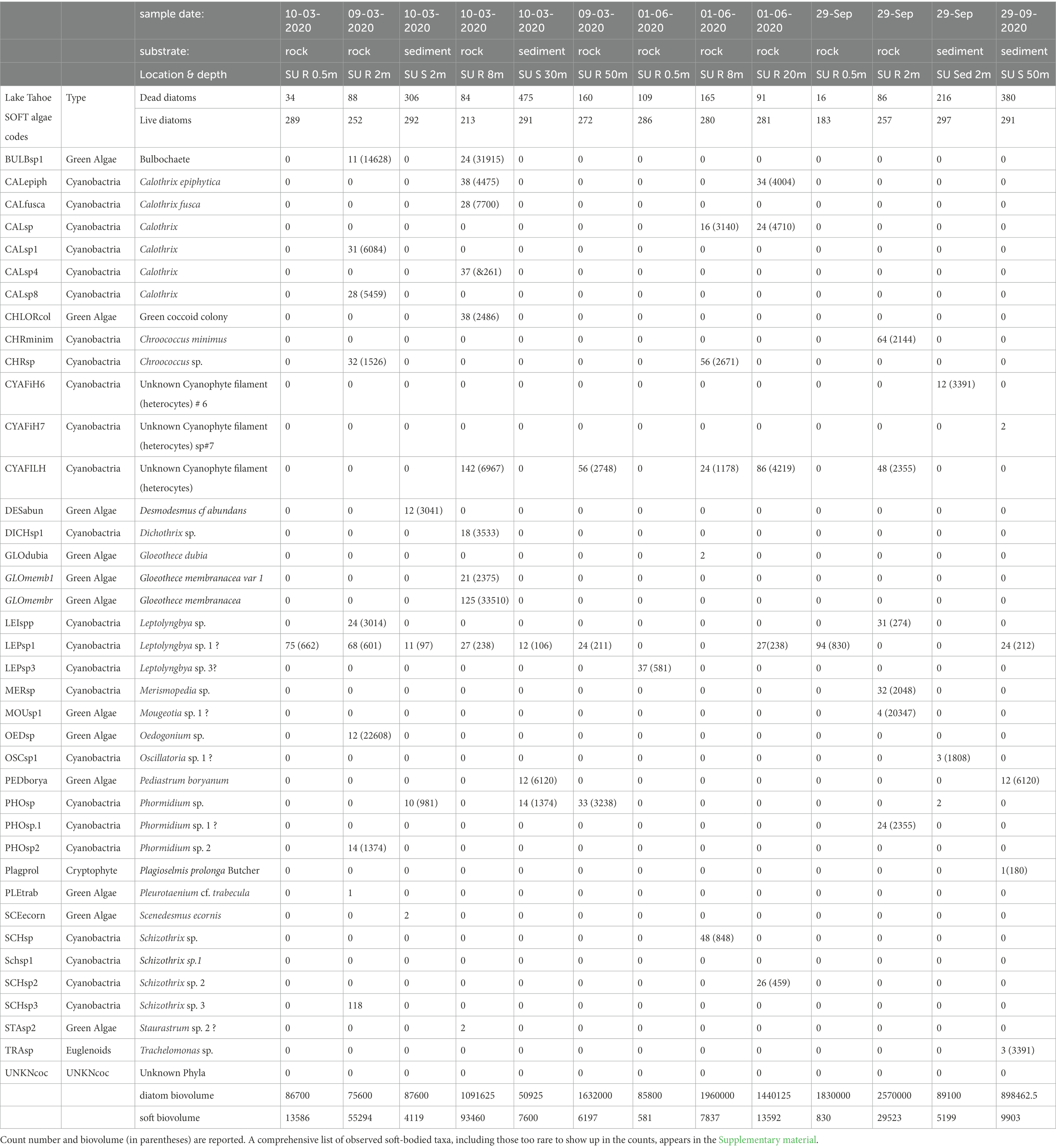
In addition to diatom enumeration, a suite of 13 samples was separately collected from transects at Sunnyside Harbor from 0.5 to 50 m water depth for algal identification and enumeration to better characterize the entire periphyton community, especially the soft-bodied algae. Samples included 7 sediment and rock scrapes from the eulittoral zone from 3 seasons (3/9/20, 6/1/20, and 9/29/20), 2 rock scrapes at 8 m, and an additional 4 rock and sediment scrapes going down to 50 m (Table 2). Samples were collected and preserved in Lugol’s solution (APHA, 2012). Lugol’s preserved samples can be stored for several years. With Lugol’s preservation, shape, size, colony formation, structures, etc. are useful for identification, yet it prevents any taxonomic characteristics of color since starch in a sample is turned black due to the iodine. These samples were then examined at 400x magnification, and a minimum of 300 algal natural units were enumerated including live diatoms using a Palmer-Maloney cell (Palmer and Maloney, 1954) with ceramic chamber with a depth of 0.4 mm holding a volume of 0.1 ml. Counts were performed on a Leica DM2500 microscope with a DFC295 Camera (Leica Microsystems, Wetzlar, Germany). A minimum of 300 units was chosen, compared to the 200 valve counts for the diatom enumerations on the permanent mounts, in order to capture more of the soft-bodied assemblage.
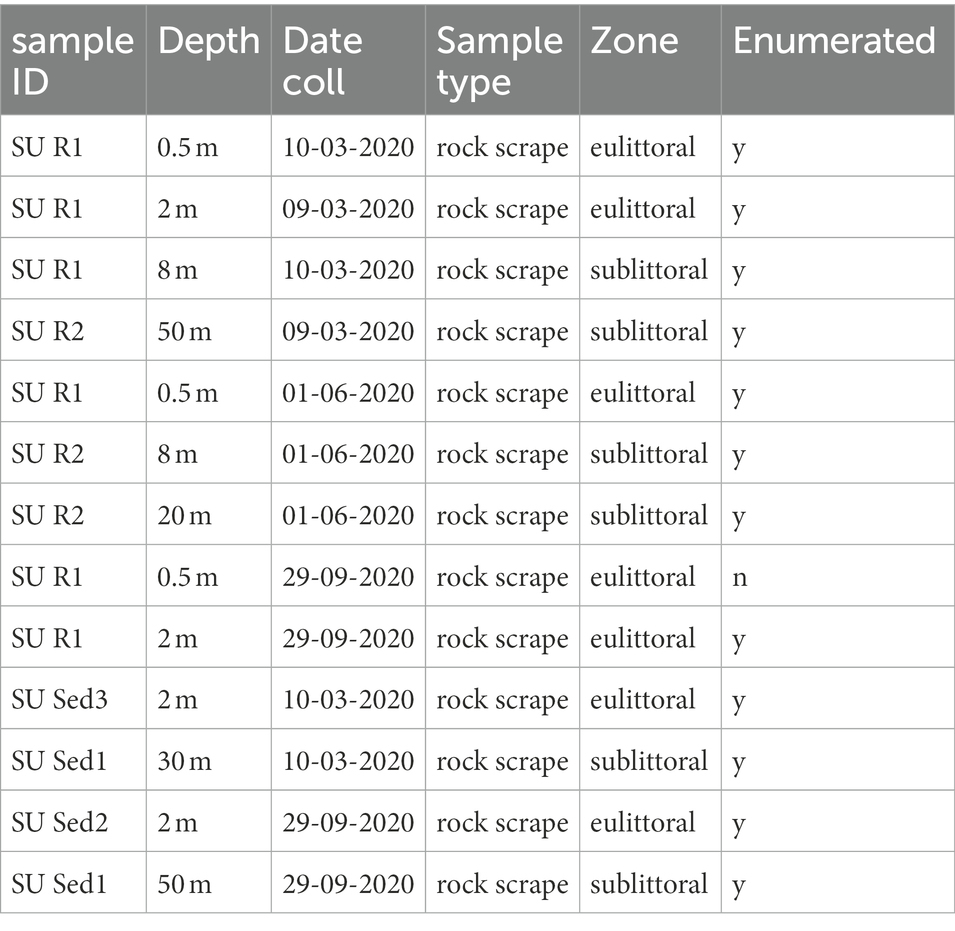
Table 2. List of samples examined for soft-bodied algal taxonomy and enumerated for algal biovolume (soft-bodied and diatoms combined).
Lugol’s samples were shaken to homogenize, and checked for unit density not to exceed 25 units per field of view of the counting chamber. Number of cells within each colony unit were estimated. For diatoms only frustules with a visible, healthy looking chloroplast were enumerated. Dead diatoms were enumerated additionally and allowed for the determination of the proportion of live to dead diatoms. A natural algal unit is defined as a natural grouping of algae (e.g., a unicellular flagellate or isolated cell; an individual filament; or a connected cluster or colony). Unlike the soft-bodied algal counting units, each diatom frustule is always a natural counting unit, even if attached to other frustules in a chain. The main purpose of using ‘natural counting units’ is to prevent cells from large colonial or filamentous forms from dominating a count. That also allows documentation of higher diversity with just 300 units enumerated. After counting, a minimum of 10 fields of view were scanned for each sample, or until there was no new taxa observed in five fields of view. In samples where coccoid colonies were in high abundance, and Lugol’s preservation (which can obscure pigmentation color) did not allow recognition of cyanobacteria versus chlorophyte colonies, the algal material was emptied in a small (2.5 cm diameter) Petri dish and diluted with distilled water to check for color and finalize the identification. The soft-bodied taxa were assigned OTUs and names using a variety of references, including Stancheva et al. (2014), Komárek and Hauer (2015), and Wehr et al. (2015). Taxa were harmonized to the names used in the SWAMP (Surface Water Ambient Monitoring Program) database used by the California Water Boards (Table 3). Taxon specific biomass was calculated based on cell number times cell biovolume. Biovolume estimations were based on measurements of the dimensions of a minimum of 10 cells, including their length, width, height, and diameter (depending on the shape, Hillebrand et al., 1999) of common taxa (i.e., wih a relative abundance higher than 10 units). Taxon biomass was determined by multiplying cell density and cell biovolume (μm3).
3. Results
3.1. Community composition
Counts of the total assemblage from Lugol’s preservation show most samples were dominated by diatoms, followed by filamentous and coccoid forms of cyanobacteria, and lesser amounts of green algae (Table 3). A few representatives of Dinoflagellata and Euglenophyta were also documented. Many filamentous and colonial algae were fragmented from collection processes and differentiation of genera like Calothrix, Dichothrix, Dasygloea etc. was inconclusive. Diatoms comprised over 90% of the biovolume and cell counts, regardless of depth, habitat or season. In terms of biomass, algae were relatively sparse in the 0.5 m epilithic samples, comprising a thin biofilm, but increased in samples greater than 2 m depth. Dead diatom frustule accumulations were highest in the sediment samples regardless of season, and increased in the deeper samples in the transect. Live Epithemia and Staurosirella chains were observed in all sublittoral samples, including the deepest samples, and comprised nearly 100% of the live algal biovolume at 30 and 50 m depth.
The observed OTU diversity consisted of 115 diatom species spanning 44 genera, as identified in the cleaned strewn slides, and 78 soft bodied taxa observed in the Lugol’s preparations. Of the diatom taxa, 87 are formally described species or varieties of species known in the published diatom literature, an additional 28 were left in open nomenclature with only a provisional species code designated for this project. Given that this was a benthic survey, many of the diatoms documented were benthic stalked and prostrate forms. A small percentage of phytoplankton were included, and their presence was attributed to their entrainment in algal mats, or adjacent sediment. Also present were a number of attached lotic forms which may represent washed in species entering from Ward Creek, and smaller inflows nearby.
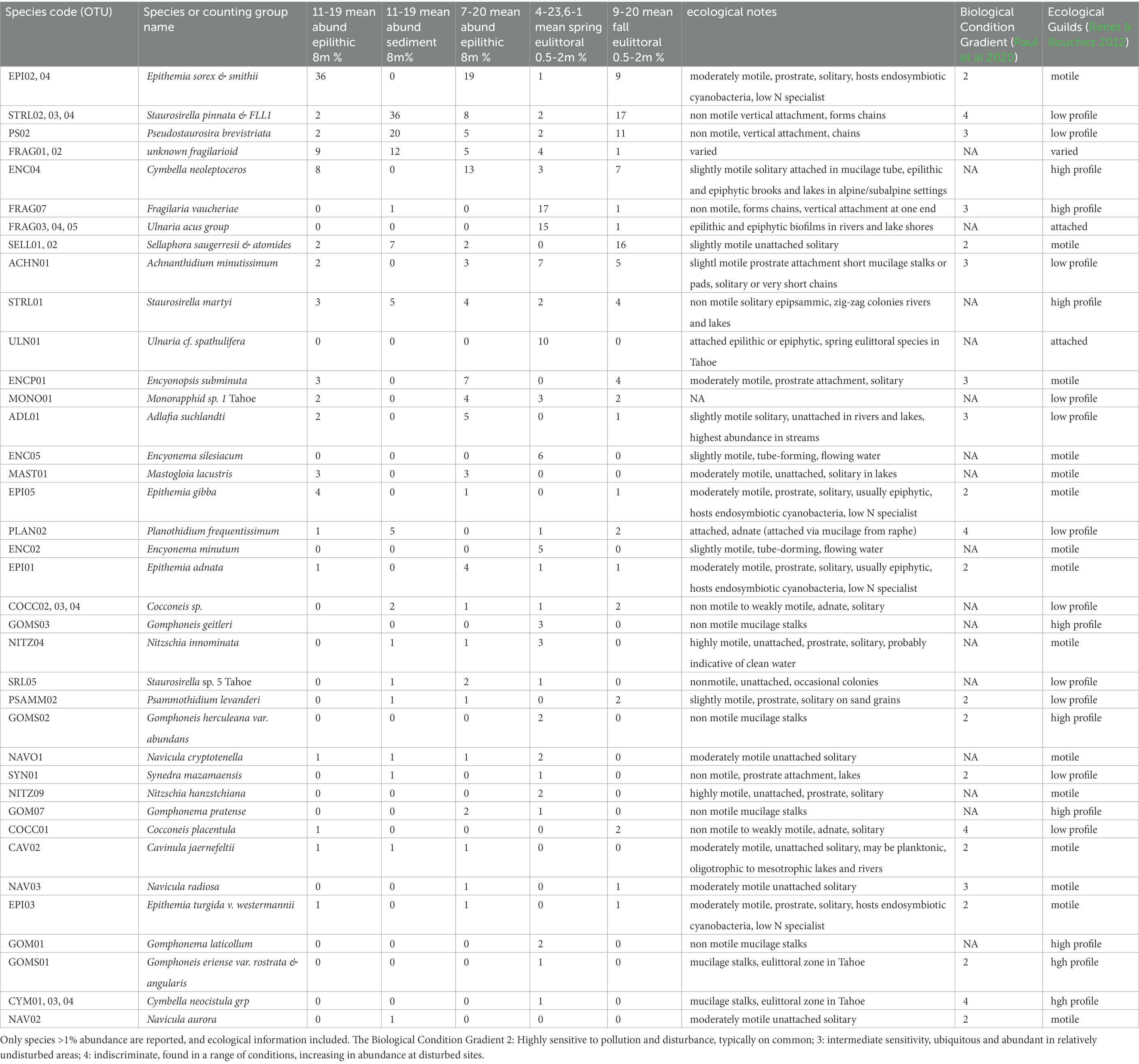
The most common members of the diatom assemblage in the samples included fragilarioids and stalked or entubed asymmetrical biraphids. The fragilarioids were dominated by species assigned to Fragilaria, Synedra, Staurosirella, Staurosira, Pseudostaurosira, and Ulnaria. The stalked or entubed biraphids were abundant and dominated by species of Epithemia, Encyonema, Gomphonema, and Gomphoneis, and to a lesser extent by species of Amphora, Cymbopleura, Gomphosphenia, and Reimeria. Large solitary biraphid forms were a consistent component of the flora and include several species of Navicula and Nitzschia. Smaller biraphids also made up a notable component, the common species being assigned to Adlafia, Mastogloia, Encyonopsis, Sellaphora, Geissleria, Cavinula, and Brachysira. A smaller component of the flora consisted of monoraphids, the most common of which belong to the genera Psammothidium, Cocconeis, Gliwiczia, and Planothidium. The 37 most common taxa from the enumerations appear in Table 4, along with abundance and ecological notes, and images of the most common taxa appear in Figures 2, 3.
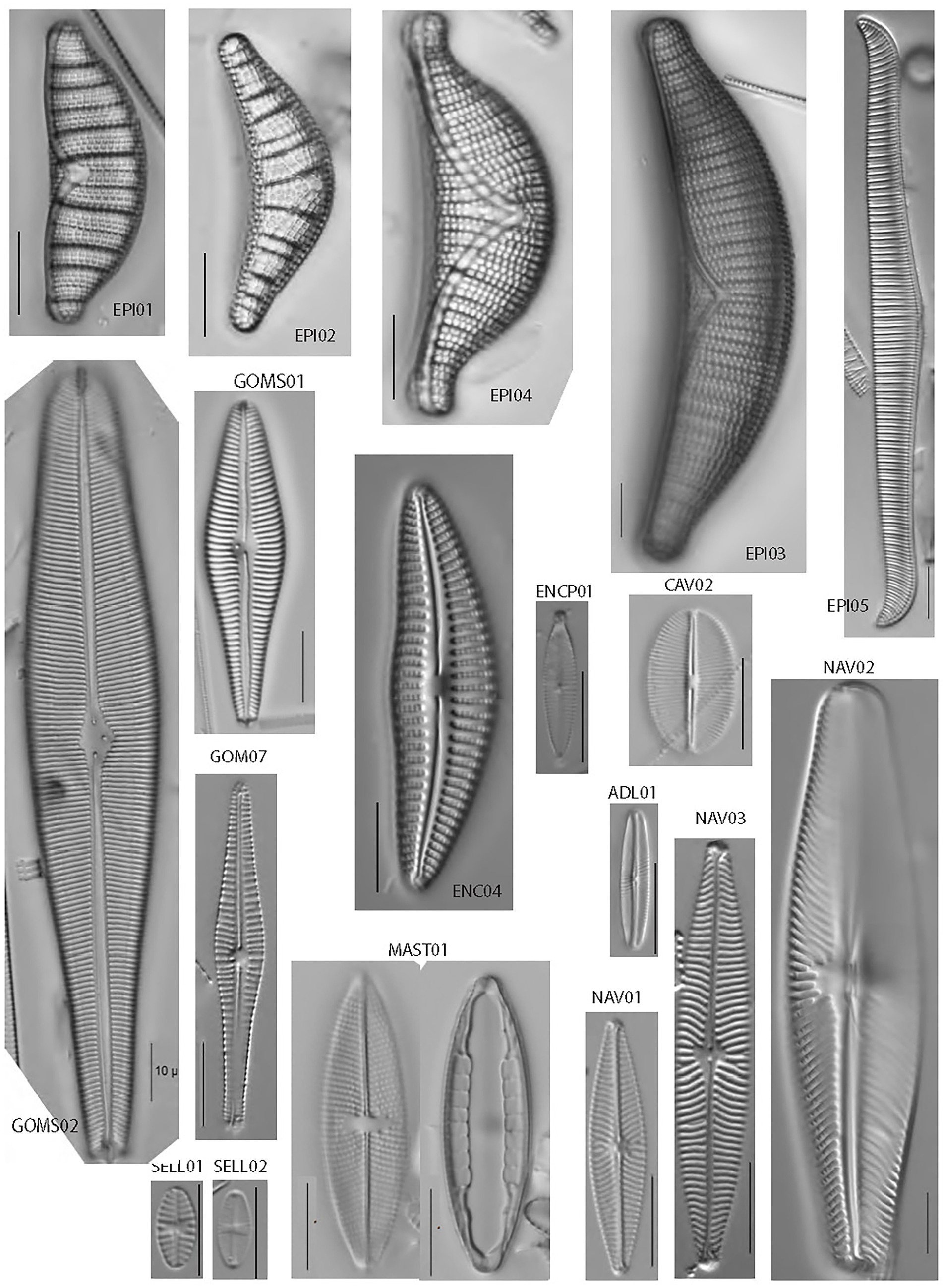
Figure 2. Light micrographs of common epithemioid and biraphid diatoms identified in this study. All scale bars are 10 microns in length.
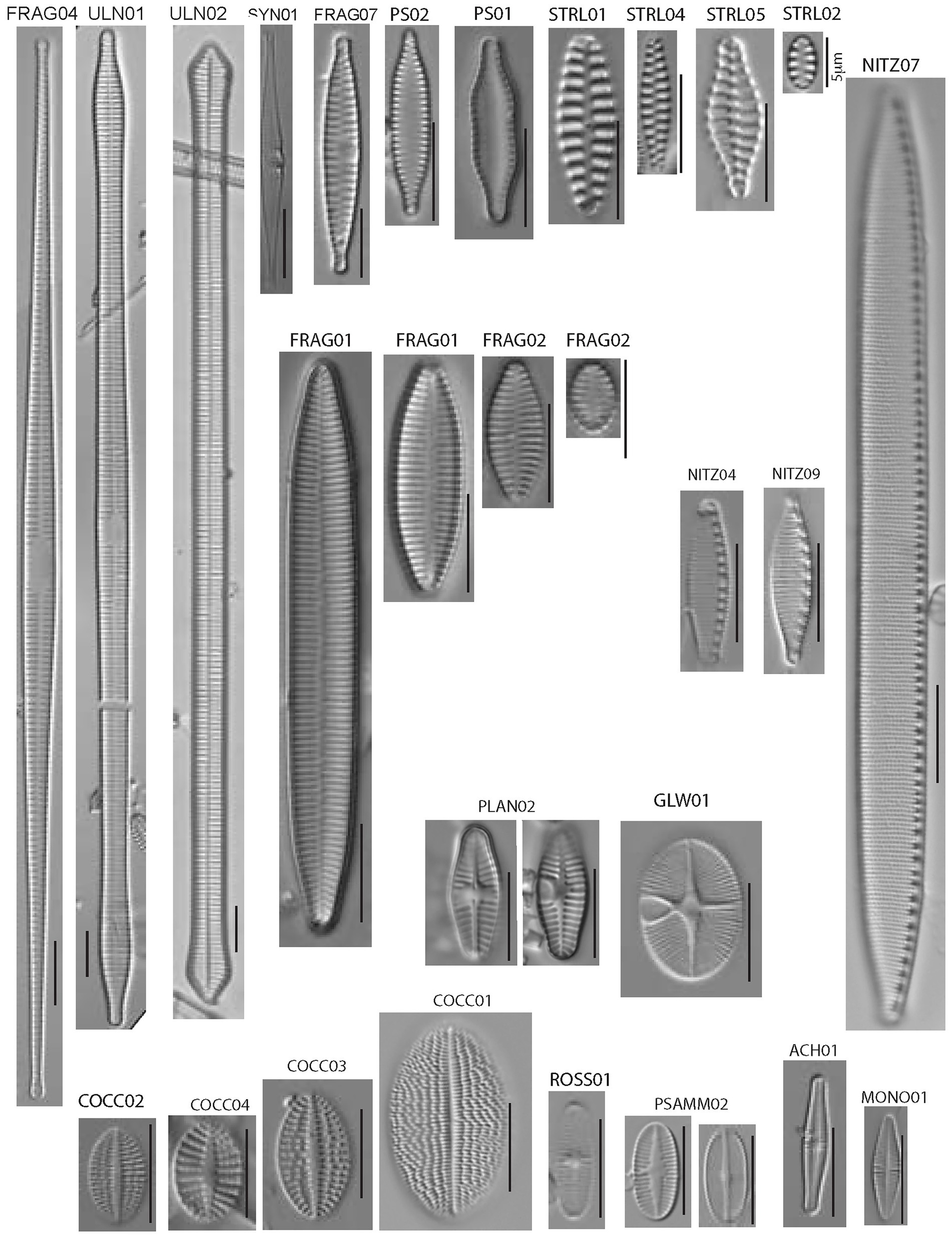
Figure 3. Transmitted light photomicrographs of common araphid, nitzschioid, and monoraphid diatoms identified in this study. All scale bars are 10 microns in length unless otherwise noted.
Additional reference material for the taxonomic identifications and enumerations is provided as Supplementary material. Supplementary Table 1 is the diatom voucher file; a complete list of the taxa, their basionym, and references used in making taxonomic identifications for each species. Also in Supplementary Table 1 are cross-referencing to identifications in the Fallen Leaf Lake voucher flora of Stratton (2013), and the taxonomic list of periphyton from Goldman (1974). Diatom species counts are found in Supplementary Table 2, and the whole floral counts from Lugol’s preserved samples appear in Supplementary Table 3. Supplementary Data Sheet 1 contains photomicrographs of the common soft-bodied algae observed. Supplementary Data Sheet 2 contains photomicrographs of all diatom taxa using transmitted light microscopy, with plate numbers and species codes referenced in Supplementary Table 1. The full reference list for species identifications for both diatoms and soft-bodied algae appears in Supplementary Data Sheet 3.
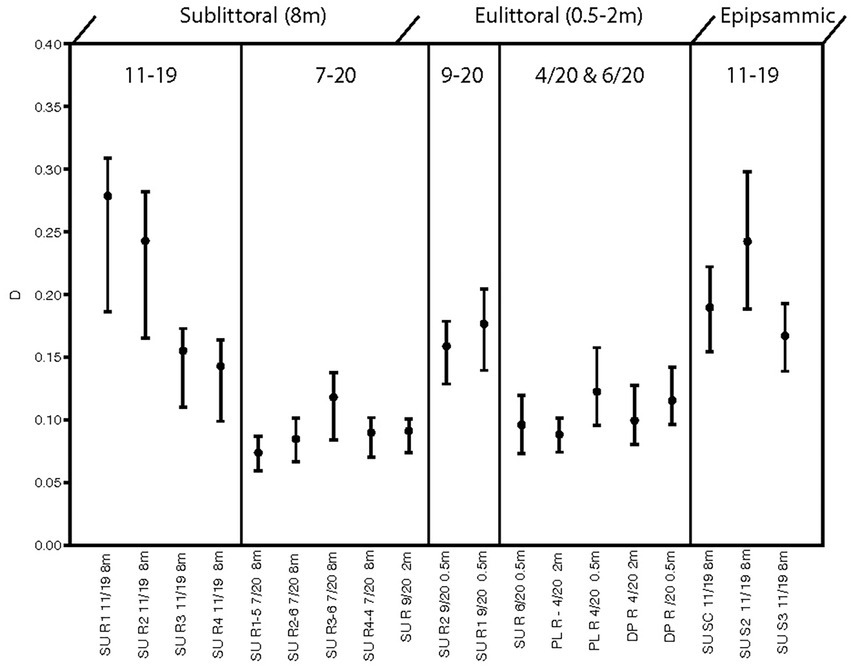
Results of the PCA performed on the cleaned diatom sample data are illustrated in a biplot (Figure 4). The first 2 axes of this PCA were found to be significant accounting for 43 and 31% of the variance, respectively. Diatom species composition showed distinct groupings between epilithic and epipsammic communities, as well as between the Spring eulittoral and the sublittoral assemblages. The species which accounted for the largest differences are plotted as a vector overlay. Alpha diversity indices were also calculated in PAST, showing a range in species richness, S, from 22 to 36, and species dominance, D, from 0.07 to 2.7 (Figure 5). These values can be found in Supplementary Table 2. In PAST, D is calculated as 1-Simpson (Hammer et al., 2001), with higher numbers indicating a more uneven sample dominated by a particular species. These communities are discussed in further detail below.
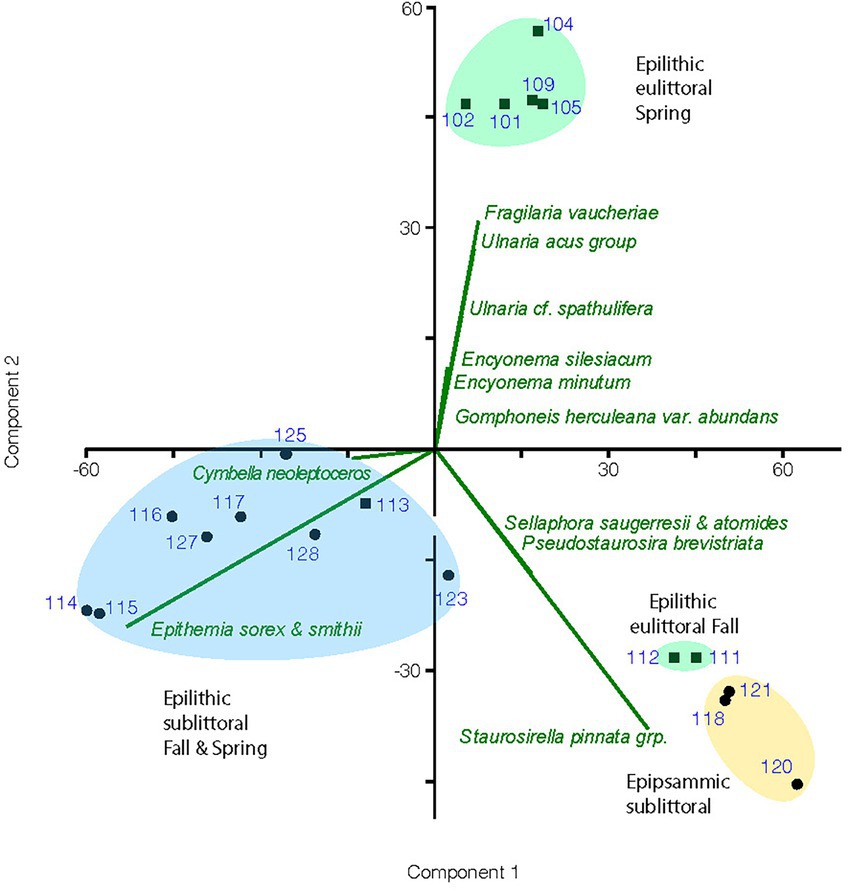
Figure 4. Biplot of principal component analysis for enumerated diatom samples showing groupings by habitat and season of samples and a vector overlay of the diatom species most responsible for the variation in assemblage composition between groupings. Sample numbers are the UNRMNH catalog # found in Table 1. Eulittoral samples are marked with a square symbol and sublittoral samples with a circle.
3.1.1. Eulittoral zone flora
For the epilithic samples, there was a sharp distinction in the composition of the eulittoral samples (0.5–2 m depth) versus the sublittoral samples, as described in previous investigations. The eulittoral zone contained a mix of diatoms and soft-bodied algae. Diatoms represented between 42 and 93% of the enumerated cells from Spring samples, and 56–66% of the September samples, with the remainder of the flora composed of filamentous and coccoidal cyanobacteria, and green algae. Common taxa observed were Calothrix, Leptolyngbya, Phormidium, Schizothrix, Gloeothece and Chroococcus (Table 3). Common diatoms that typified the eulittoral epilithic habitats were species of fragilarioids, gomphonemoids, cymbelloids and epithemioids, including Gomphoneis herculeana var. abundans Kociolek and Stoermer, Gomphoneis eriense var. rostrata Kociolek and Stoermer, Gomphoneis eriense var. angularis Kociolek and Stoermer, Encyonema minutum (Hilse) D.G. Mann and E. siliacum (Beisch) D.G. Mann, Cymbella neocistula group, Ulnaria cf. ulna var. spathulifera (Grunow) Aboal, Fragilaria vaucheriae (Kützing) Peterson, Synedra mazamaensis Sovereign, Ulnaria acus group, and FRAG01-02 species complex. These taxa were particularly prominent in the Spring samples. In the September samples, chains of Pseudostaurosira and Staurosirella became more prominent. Epithemioids were near absent in the Spring samples, but became prominent in the eulittoral zone in the Fall.
3.1.2. Sublittoral zone flora
Epilithic samples from the November 2019 and July 2020 samplings at Sunnyside that were taken in the sublittoral zone, were characterized in previous studies as a flora dominated by nitrogen-fixing cyanobacteria (Calothryx and Tolypothrix), with diatoms as the second most important group (Loeb and Reuter, 1984; Atkins et al., 2021). Our data show that diatoms represented the bulk of the biovolume relative to the soft-bodied algae, ranging from 58 to 100% of the biovolume. This high abundance persisted throughout the transects from 8 m to 50 m, regardless of substrate or season. Using valve counts, diatoms represent a smaller percentage, but were still the dominant algal group in most samples, ranging from 30 to 93%. The discrepancy between the valve and biovolume percentages is attributed to the difference in cell size where the diatom cells have an overall larger biovolume. The soft-bodied community was predominantly cyanobacteria, including the filamentous and coccoidal forms observed in the eulittoral zone (Table 3). No seasonal difference was observed in the composition of the soft-bodied taxa. However, differences by substrate and depth were observed in that the filamentous branching cyanobacterial filaments that were epilithic were found in lesser abundance with depth. The diatom fraction was dominated by species of Epithemia (largely Epithemia sorex Kützing, but also E. adnata (Kützing) Brébisson, E. gibba (Ehrenberg) Kützing, and E. turgida var. westermannii (Ehrenberg) Grunow), ranging from 18 to 44% of the valves counted in a given sample. Epithemia spp. were more abundant in the November, 2019 samples than the July, 2020 samples (mean abundance of 27% valves counted in November 2019, and 20% in July, 2020). Another abundant species amongst the epilithic samples was Cymbella neoleptoceros, ranging from 2 to 13% of the valves counted in a given epilithic sample.
3.1.3. Epipsammic habitat
The epipsammic samples taken from surface sediment represent a different habitat for diatoms and have a markedly different proportion of taxa in the assemblage, despite its proximity to the rock samples in the same plots. In addition, sediment samples may represent a mixed assemblage of diatoms living in-situ in the sediment surface, plus washed in diatoms from nearby habitats. The % dead diatoms was higher in epipsammic samples (ranging from 42 to 62%) than epilithic samples (ranging from 11 to 37%). The epipsammic samples counted were dominated by colonial fragilarioid chains, mostly belonging to Staurosirella pinnata group. S. confusa Morales, S. martyi (Héribaud) E. Morales and Manoylov, and Pseudostaurosira brevistriata (Grunow) D.M. Williams and Round, and FRAG01, a taxon morphologically close to Fragilariforma virescens (Ralfs) Williams and Round. Interestingly, Epithemia and Encyonema were rare in these samples, despite the proximity to rocks covered in these taxa, and indicates a strong habitat partitioning in the diatoms by substrate. Soft-bodied algae also showed strong habitat distinctions. The attached filamentous branched cyanobacteria, typical in the epilithic samples, like Calothrix, were generally absent in the epipsammic samples.
4. Discussion
4.1. Comparison with 1960’s–80’s surveys
In the early surveys, Goldman (1974) identified 60 species of diatoms in his periphyton samples. Of the 115 taxa observed in the Voucher flora, 23 can be harmonized with taxa in Goldman’s list, and another 3 tentatively harmonized. Of the remaining taxa another 27 have sufficiently enduring and stable species concepts to confidently conclude that they were not reported by Goldman during his surveys. The remaining taxa, roughly 1/3 of those we observed, could not be reconciled with Goldman’s species lists, and may in fact have been observed in the 1970’s. Our survey, while encompassing fewer years and fewer samples, was able to recognize roughly 2-fold the number of species than was observed by Goldman (1974). This does not necessarily indicate that biodiversity today is that much greater than it was in the early 1970’s, but may rather reflect methodological differences. In our study, we used permanent mounts of cleaned siliceous material, whereas Goldman (1974) used inverted light microscopy on settled live material. Our method allows for more extensive scanning of strewn slides at higher magnification, that may allow for the identification of more species than with live material using the Utermöhl method used by Goldman’s group. Many of the species we documented were rare and did not show up in the enumerations, save for 1 or 2 valves.
As is typical of diatom communities, there is a strong seasonal component as well as high species dominance during times of rapid growth, such as during Spring bloom. This was observed in Goldman’s 1971 growth experiments on glass cylinders set up in the sublittoral zone. Goldman (1974) found that in both the Summer and Winter, the majority of the taxa were represented by ~8 species each season, but the species composition differed. Summer samples showed roughly equal dominance between Mougeotia green algae and diatoms Epithemia argus (Ehrenberg) Kützing, E. gibba, Synedra ulna (Nitsch) Ehrenberg, Cymbella ventricosa Agardh, Navicula aurora Sovereign, and Fragilaria capucina Desmazières. In contrast, the Winter samples were dominated by Fragilaria actinastroides Lemmermann, Fragilaria crotonensis Kitton, Gomphonema parvulum Kützing, Lindavia bodanica (Eulenstein ex Grunow) Nakov, Guillory, Julius, Theriot and Alverson, Synedra ulna, Gomphoneis herculeana, and Melosira crenulata [possibly = Aulacoseira pusilla (Meister) Tuji and Houki]. These observations differ substantially from our observations of the most dominant species. Notably less green algae was present, and there was a greater abundance of the Epithemia-cyanobacteria assemblage components, especially in November samples. Community analysis from studies in the 1980’s provides less species-level data, but still indicates there was a significant green algae component during that time. The eulittoral habitat in the 1980’s was dominated by Gomphoneis-Synedra diatoms and green algae, whereas the sublittoral zone (>2 m) was dominated by nitrogen fixing cyanobacteria (Loeb and Reuter, 1984; Aloi et al., 1988; Atkins et al., 2021). By comparison, our data show cyanobacteria to be more prominent in both eulittoral and sublittoral zones, and green algae much less abundant. The 1980’s community observations showing distinctions between the eulittoral and sublittoral zones still persist today with stalked diatoms abundant in the eulittoral zone. Dominant seasonal species observed in the Goldman 1970–71 experiments differ from the 2019–20 data, yet the explanation for these differences cannot be attributed to a specific factor. The data are merely 2 annual snapshots spaced 50 years apart, and may in part reflect interannual variation, seasonal differences, and differences in natural versus artificial substrate. None-the-less, the differences are noted. Of the dominant species observed in the sublittoral zone by Goldman (1974), nearly all have been surpassed by other species.
4.2. Community analysis
4.2.1. Assemblage composition and seasonality
4.2.1.1. Soft algal community
Algal community ecology information, including potential toxicity, and genus/species function in habitats, can be inferred from the observed morphological traits (filamentous, heterocystous, motile, stalked, monoraphid, biraphid) and growth forms (colonial, unicellular, planktonic, benthic; Stevenson et al., 2010). The abundance of heterocytes of cyanobacteria are indicators of nitrogen fixation (Porter et al., 2008). Algal groups summarized in proportions could be used as a food web indicator, because invertebrate grazers have strong preferences for diatoms (unicellular and stalked growth forms) versus other algal groups (Wellnitz and Poff, 2012; Stevenson, 2014). Cell and biovolume counts have been found to be complementary in the information they produce. Biovolume is a good tool for understanding community structure and biomass changes. Meanwhile, cell relative abundance was found to be better than biovolume in its correlation environmental factors, such as nutrient concentrations (Lavoie et al., 2006).
4.2.1.2. Fragilarioid-gomphonemoid assemblage
Eulittoral samples were largely inhabited by an assemblage of stalked gomphonemoid diatoms and large elongate solitary fragilarioids that showed a strong seasonal variation. The highest numbers of gomphonemoids, Ulnaria spp. and Fragilaria vaucheriae occurred in the Spring, decreasing in the Fall. Spring samples were also favored by the smaller Encyonema species, E. minutum and E. silesiacum. Fall samples showed increased numbers of small biraphids such as Adlafia and Sellaphora, monoraphids, and colonial fragilarioid chains (Table 3). As a result, the Sunnyside Fall eulittoral samples inhabited by this flora plot separately from the Spring eulittoral samples on the PCA biplot, and were grouped closer to the epipsammic samples (Figure 4). One possible explanation for this result is that the colonial fragilarioid chains form chains or ribbons that passively tangle amongst periphyton, but can also be entrained in the water column and move between epilithic and epipsammic habitats. Likewise, small biraphid species are solitary motile species that prefer moving water, and can move between the epipsammic and epilithic communities (Table 4).
4.2.1.3. Epithemioid-dominated assemblage
The epithemioid-dominated assemblage occurs largely in the sublittoral zone epilithic habitats. At Sunnyside, it occurred only in the 0.5 m samples, as is illustrated through enumeration of a 2 m September 2020 sample, which plotted with the sublittoral samples in the PCA biplot (Figure 4). Seasonal variance in the epithemioid-dominated community was less apparent than with the Ulnaria-gomphonemoid dominated community in the eulittoral zone. Spring and Fall sublittoral rock scrape samples grouped together in the PCA biplot, represented by greater abundances of E. sorex, E. smithii Carruthers and Cymbella neoleptoceros (Figure 4). Smaller variations in Spring and Fall composition can be seen in the abundance count summaries (Table 4). The strong seasonal preference in gomphonemoids seen in the eulittoral zone was also manifested in sublittoral counts to a lesser extent. They were present in minor amounts in the sublittoral zone (1–6% of valve counts) in the July 2020 enumerations (i.e., Gomphonema pratense Lange-Bertalot and Reichardt, Gomphosinica geitleri (Kociolek and Stoermer) Kociolek, You, Wang, and Liu, Gomphoneis eriense var. rostrata), but were near absent (<1%) in the Fall 2019 samples.
All the Epithemia species are benthic, low nitrogen (N) specialists, and indicative of N-limited environments, as they host endosymbiotic N-fixing spheroid bodies derived from cyanobacteria (DeYoe et al., 1992; Nakayama et al., 2011). These species are also considered to be highly sensitive to human disturbance and ecologic degradation, associated with good water quality (score of 2 on Biological Condition Gradient in Paul et al., 2020, Table 4). Epithemia species are all moderately motile, solitary forms attaching prostrate to the substrate via mucilage. The high abundance of Epithemia supports the soft-bodied algal data, showing that cyanobacteria was a large component of the algal population. Cymbella neoleptoceros is also a slightly motile solitary benthic form. In boil mounts it was found in close association with Epithemia and filamentous cyanobacteria. Encyonema species are slightly motile, forming mucilage sheaths or tubes and may be solitary or extend in long chains (Spaulding et al., 2021). Tube forming diatoms like Encyonema and C. neoleptoceros are in a high profile guild with taller stature (Rimet and Bouchez, 2012) and can add to the thickness of the biomass attached to the rock surface.
4.2.2. Species diversity
Alpha diversity indices calculated from the cleaned diatom samples and run in PAST show variation in species richness (i.e., number of taxa/sample), ranging from 22 to 36 taxa. This number is not terribly informative nor accurate because most of the species are rare, with a low chance of appearing in the enumerations. Scanning of these slides showed much larger numbers of the entire 115 OTUs are in fact present in a given sample, and the many rare taxa were overshadowed by a few dominant species. Different methods are necessary to approach true species richness through enumeration (Tyree et al., 2020b), and were beyond the scope of this present work. A more informative metric is species dominance, D, which ranges from 0 to 1. A D = 0 indicates all taxa are equally present, whereas 1 indicates a species dominates the sample completely. For the sublittoral zone, there was higher species dominance in the Fall epilithic samples at 0.5 m compared to Spring (Figure 5). At Sunnyside, the Epithemia-cyanobacteria dominated assemblage shoaled into the base of the eulittoral zone, as is shown in the PCA biplot mentioned in the previous section (Figure 4), as well as with the diversity index. At Sunnyside, the September 2020 2 m Sunnyside sample has lower D value then the 0.5 m samples, similar to the 8 m sublittoral samples from this location (Figure 5). While Fall and Spring samples plotted similarly in the PCA multivariate space (Figure 4) based on composition, they separated based on dominance of epithemioids, especially E. sorex and E. smithii (Table 4). There was also a high dominance in the Fall epipsammic samples, reflective of high numbers of small Sellaphora and fragilarioid chains. Diatoms are known to have strongest growth during the Spring. It was therefore anticipated that species dominance would be greater during the July sampling versus the November sampling. However, this was not the case; there was a lower species dominance in July that may reflect the high level of adaption of the sublittoral epithemioid-cyanobacteria assemblage of low nutrient N-fixing specialists as the season progressed. The Fall eulittoral and epipsammic communities showed higher dominance (Figure 5), but dominant species were Sellaphora and colonial fragilarioid chains. No explanation can presently be given for this increased dominance, and it will require further investigation.
4.2.3. Implications for management
The degradation of nearshoe habitats in relatively pristine lake waters has been of increasing concern in the last decade (Vadeboncoeur et al., 2021) with increased obsevations from many of the world’s iconic lakes. A major challenge in understanding nearshore water quality degradation resulting from benthic biofilms and metaphytic growth is that rarely do we have an understanding of the taxonomy of the community, and often no understanding of the growth phases of dominant taxa. If we are going to understand and manage nearshore eutrophication in the 21st century, then we need to promote and enhance taxonomic level identifications in lake monitoring programs and rapid response studies during algal blooms. Another challenge in long-term monitoring programs of critical water bodies is maintaining taxonomic consistency amongst the datasets (e.g., Kovalenko and Reavie, 2022; Riato et al., 2022). In the Lake Tahoe basin, as more agencies and research groups become involved, these challenges will grow and are met best through the analytical methods that result in taxonomically consistent practices. Our use of a diatom voucher flora for the most speciose part of the algal assemblages provides a means of consistency between projects and research groups, providing it is adopted. Adopting a voucher flora approach earlier in assessment programs can prevent the need for resource intensive post-hoc harmonization, which may require re-analysis of slides and loss of species information when taxa cannot be reconciled (Potapova et al., 2022). Our voucher flora shows a 2-fold level of species diversity than was previously documented, most likely because of the methods involved in examining the diatom fraction through cleaned permanent mounts. As additional samples are analyzed from other parts of the nearshore, we expect the biodiversity to grow. As such the voucher flora can become a living document which is expanded and updated periodically and provides an opportunity for open-source based collaborations among scientists to discuss the importance of taxa in driving changes to ecosystem level properties.
5. Conclusion
Herein algal survey and enumeration of diatoms, the most speciose group in the Lake Tahoe nearshore assemblages, is documented and enumerated by the construction of a voucher flora. These data were then combined with taxonomy and enumeration of algal samples preserved in Lugol’s solution collected from 0.5 to 50 m depth, to provide data on the whole algal community. This method captures detailed snapshots of the biodiversity and community structure of nearshore algae epilithic and epipsammic habitats. Additionally, this method provides a means of reconciling taxonomic assignments in any future diatom-based work in the basin, a necessary function in long-term monitoring of ecosystems.
Despite challenges in harmonizing these data with past species lists, it is clear that several prominent taxa have continued to dominate the nearshore since cultural eutrophication and algal blooms became problematic. These include a fragilarioid-gomphonemoid dominated assemblage in the eulittoral zone, and an epithemioid dominated assemblage in the sublittoral zone. Seasonal changes were noted, and the epithemioid assemblage at Sunnyside Harbor impinged into the eulittoral zone seasonally in the Fall with the expansion of cyanobacterial blooms. Significant differences were also seen between epilithic and epipsammic sublittoral assemblages, despite their being spatially adjacent, and underscores the importance of substrate in establishing the algal communities. Comparison between the 1970–71 and 2019–20 taxonomic data, while representing only limited snapshots that are 50 years apart, show substantial changes in the nearshore sublittoral community. Such changes include a decrease in the green alga Mougeotia, increases in Epithemia, and shoaling of the Epithemia-cyanobacteria assemblage to 2 m at Sunnyside. Of the 13 most common taxa in 1970–71, only 1 remains relatively common in the sublittoral zone, Epithemia gibba. These data will play a role in continued assessment of algal biodiversity and the overall health in the nearshore environment, providing a means of gauging future biodiversity change from the present conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
The voucher flora was constructed by PN, SL, and CS, with additional taxonomic verification by KM. Soft-body algal analysis was conducted by KM. The project was designed by PN and SC. Manuscript preparation was lead by PN with contributions by all other authors in data analysis, interpretation, and writing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Lahontan Regional Water Quality Control Board area 6 to Noble and Chandra, and via agreement number 14-068-160 to R. Naranjo and R. Niswonger, and then to Chandra. Additional funding was provided by the Georgia Power Endowed Professorship, Georgia College & State University Foundation, Inc. provided to Manoylov.
Acknowledgments
We acknowledge the Lakeside Laboratory, University of Iowa, and Iowa State University for providing access to literature and photomicroscopy, to Dr. Ed Krynak and Marine Taxonomic Service Divers (Seth Jones, and Monique Rydel-Fortner) who collected the periphyton samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or the policies of the US Environmental Protection Agency. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the US Environmental Protection Agency.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1053499/full#supplementary-material.
References
Alers-García, J., Lee, S. S., and Spaulding, S. A. (2021). Resources and practices to improve diatom data quality. Limnol. Oceanogr. Bull. 30, 48–53. doi: 10.1002/lob.10433
Aloi, J. E., Loeb, S. L., and Goldman, C. R. (1988). Temporal and spatial variability of the Eulittoral Epilithic Periphyton, Lake Tahoe, California-Nevada. J. Freshw. Ecol. 4, 401–410. doi: 10.1080/02705060.1988.9665189
APHA. (2012). Standard methods for examination of water and wastewater. Washington: American Public Health Association.
Atkins, K. S., Hackley, S. H., Allen, B. C., Watanabe, S., Reuter, J. E., and Schladow, S. G. (2021). Variability in periphyton community and biomass over 37 years in Lake Tahoe (CA-NV). Hydrobiologia 848, 1755–1772. doi: 10.1007/s10750-021-04533-w
Ball, G. I., Noble, P. J., Stephens, B. M., Higgins, A., Mensing, S. A., Aluwihare, L. I., et al. (2018). “The Pleistocene–Holocene transition at fallen leaf Lake, Sierra Nevada, California, USA” in From saline to freshwater: The diversity of Western Lakes in space and time: Geological Society of America special paper 536. eds. S. W. Starratt and M. Rosen (United States: The Geological Society of America), 1–18.
Birks, H. J. B. (2012). “Overview of numerical methods in Palaeolimnology” in Tracking environmental change using Lake sediments: Data handling and numerical techniques. eds. H. J. B. Birks, A. F. Lotter, S. Juggins, and J. P. Smol (Dordrecht: Springer Netherlands), 19–92.
Bishop, I. W., Esposito, R. M., Tyree, M., and Spaulding, S. A. (2017). A diatom voucher flora from selected southeast rivers (USA). Phytotaxa 332, 101–140. doi: 10.11646/phytotaxa.332.2.1
Chandra, S., Vander Zanden, M. J., Heyvaert, A. C., Allen, B. C., and Goldman, C. R. (2005). The effects of cultural eutrophication on the coupling between pelagic primary producers and benthic consumers. Limnol. Oceanogr. 50, 1368–1376. doi: 10.4319/lo.2005.50.5.1368
DeYoe, H., Lowe, R., and Marks, J. (1992). Effects of nitrogen and phosphorus on the endosymbiont load of Rhopalodia gibba and Epithemia turgida (Bacillariophyceae). J. Phycol. 28, 773–777. doi: 10.1111/j.0022-3646.1992.00773.x
Goldman, C. R. (1974). Eutrophication of Lake Tahoe emphasizing water quality. United States: Ecol Res Ser EPA.
Goldman, C. R., and De Amezaga, E. (1975). Spatial and temporal changes in the primary productivity of Lake Tahoe, California-Nevada between 1959 and 1971. Verh. Internat. Verein. Limnolo 19, 812–825. doi: 10.1080/03680770.1974.11896127
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). Past: Paleontological Statistics Software Package for Education and Data Analysis Palaeontol, Electron. 4, 1–9. Available at: http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hillebrand, H., Dürselen, C.-D., Kirschtel, D., Pollingher, U., and Zohary, T. (1999). Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 35, 403–424. doi: 10.1046/j.1529-8817.1999.3520403.x
Johnson, B. E., Noble, P. J., Heyvaert, A. C., Chandra, S., and Karlin, R. (2018). Anthropogenic and climatic influences on the diatom flora within the fallen leaf Lake watershed, Lake Tahoe Basin, California over the last millennium. J. Paleolimnol. 59, 159–173. doi: 10.1007/s10933-017-9961-3
Kociolek, J. P., and Stoermer, E. F. (1988). Taxonomy, ultrastructure and distribution of Gomphoneis herculeana, G. eriense and closely related species (Naviculales: Gomphonemataceae). Proc. Acad. Nat. Sci. Philadelphia 140, 24–97.
Komárek, J., and Hauer, T. (2015). Cyano DB.Cz—on-line database of cyanobacterial genera. Wordwide Electron. Publ. Univ. South Bohemia Inst. Bot. Available at: http://www.cyanodb.cz
Kovalenko, K. E., and Reavie, E. D. (2022). Taxonomic bias in freshwater phytoplankton communities and its effect on environmental assessment metrics. Phycologia 61, 436–443. doi: 10.1080/00318884.2022.2073077
Krammer, K. (2002). The genus Cymbella. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats 3, 1–584.
Lavoie, I., Campeau, S., Fallu, M.-A., and Dillon, P. J. (2006). Diatoms and biomonitoring: should cell size be accounted for? Hydrobiologia 573, 1–16. doi: 10.1007/s10750-006-0223-z
Loeb, S. L., and Reuter, J. E. (1984). Littoral Zone Investigations, Lake Tahoe 1982: Periphyton. Institute of Ecology, UC Davis, 66p.Loeb, S.L. 1983. Near-shore (Littoral Zone) Monitoring Program. July 1981-July 1982. Tahoe Research Group Technical Report, UC Davis, 193 p.
Nakayama, T., Ikegami, Y., Nakayama, T., Ishida, K. I., Inagaki, Y., and Inouye, I. (2011). Spheroid bodies in rhopalodiacean diatoms were derived from a single endosymbiotic cyanobacterium. J. Plant Res. 124, 93–97. doi: 10.1007/s10265-010-0355-0
Naranjo, R. C., Niswonger, R. G., Smith, D., Rosenberry, D., and Chandra, S. (2019). Linkages between hydrology and seasonal variations of nutrients and periphyton in a large oligotrophic subalpine lake. J. Hydrol. 568, 877–890. doi: 10.1016/j.jhydrol.2018.11.033
Palmer, C. M., and Maloney, T. E. (1954). A new counting slide for nannoplankton. Am. Soc. Limnol. Oceanogr. 21:6.
Paul, M. J., Jessup, B., Brown, L. R., Carter, J. L., Cantonati, M., Charles, D. F., et al. (2020). Characterizing benthic macroinvertebrate and algal biological condition gradient models for California wadeable streams, USA. Ecol. Indic. 117:106618. doi: 10.1016/j.ecolind.2020.106618
Porter, S. D., Mueller, D. K., Spahr, N. E., Munn, M. D., and Dubrovsky, N. M. (2008). Efficacy of algal metrics for assessing nutrient and organic enrichment in flowing waters. Freshw. Biol. 53, 1036–1054. doi: 10.1111/j.1365-2427.2007.01951.x
Potapova, M. G., Lee, S. S., Spaulding, S. A., and Schulte, N. O. (2022). A harmonized dataset of sediment diatoms from hundreds of lakes in the northeastern United States. Sci. Data 9:540. doi: 10.1038/s41597-022-01661-3
Riato, L., Hill, R. A., Herlihy, A. T., Peck, D. V., Kaufmann, P. R., Stoddard, L., et al. (2022). Genus-level, trait-based multimetric diatom indices for assessing the ecological condition of rivers and streams across the conterminous United States. Ecol. Indic. 141:109131. doi: 10.1016/j.ecolind.2022.109131
Rimet, F., and Bouchez, A. (2012). Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manag. Aquat. Ecosyst. 406:1. doi: 10.1051/kmae/2012018
Spaulding, S. A., Potapova, M. G., Bishop, I. W., Lee, S. S., Gasperak, T. S., Jovanoska, E., et al. (2021). Diatoms.org: supporting taxonomists, connecting communities. Diatom Res. 36, 291–304. doi: 10.1080/0269249X.2021.2006790
Stancheva, R., Fuller, C., and Sheath, R. G. (2014). Soft-bodied stream algae of California. Available at: http://dbmuseblade.colorado.edu/DiatomTwo/sbsac_site/index.php.
Stevenson, J. (2014). Ecological assessments with algae: a review and synthesis. J. Phycol. 50, 437–461. doi: 10.1111/jpy.12189
Stevenson, R. J., Pan, Y., and van Dam, H. (2010). Assessing environmental conditions in rivers and streams with diatoms. The Diatoms 57–85:005. doi: 10.1017/CBO9780511763175.005
Stratton, L. E. (2013). Feasibility study for using diatom assemblages from a small dilute subalpine lake as an indicator of past megadroughts in the Sierra Nevada.
TERC (2022). Tahoe State of the Lake Report :2022. Electronic report found at: https://tahoe.ucdavis.edu/stateofthelake
Tyree, M. A., Bishop, I. W., Hawkins, C. P., Mitchell, R., and Spaulding, S. A. (2020a). Reduction of taxonomic bias in diatom species data. Limnol. Oceanogr. Methods 18, 271–279. doi: 10.1002/lom3.10350
Tyree, M. A., Carlisle, D. M., and Spaulding, S. A. (2020b). Diatom enumeration method influences biological assessments of southeastern Usa streams. Freshw. Sci. 39, 183–195. doi: 10.1086/707725
Vadeboncoeur, Y., Moore, M. V., Stewart, S. D., Chandra, S., Atkins, K. S., Baron, J. S., et al. (2021). Blue Waters, green bottoms: benthic filamentous algal blooms are an emerging threat to Clear Lakes worldwide. Bioscience 71, 1011–1027. doi: 10.1093/biosci/biab049
Van Landingham, S. L. (1987). Observations on the ecology and trophic status of Lake Tahoe (Nevada and California, USA) based on the algae from three independent surveys (1965-1985). Gt. Basin Nat. 47, 562–582.
Wehr, J. D., Sheath, R. G., and Kociolek, J. P. (2015). “Copyright” in Freshwater algae of North America (second edition) aquatic ecology (Boston: Academic Press), 4.
Keywords: diatom, voucher flora, lake Tahoe, periphyton, cyanobacteria
Citation: Noble PJ, Seitz C, Lee SS, Manoylov KM and Chandra S (2023) Characterization of algal community composition and structure from the nearshore environment, Lake Tahoe (United States). Front. Ecol. Evol. 10:1053499. doi: 10.3389/fevo.2022.1053499
Edited by:
David R. L. Burge, Science Museum of Minnesota, United StatesReviewed by:
Edna Pedraza Garzón, Rhithro Associates, United StatesXu Chen, China University of Geosciences Wuhan, China
Copyright © 2023 Noble, Seitz, Lee, Manoylov and Chandra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula J. Noble, ✉ bm9ibGVwakB1bnIuZWR1
 Paula J. Noble
Paula J. Noble Carina Seitz
Carina Seitz Sylvia S. Lee3
Sylvia S. Lee3 Kalina M. Manoylov
Kalina M. Manoylov