
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 October 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1028686
This article is part of the Research Topic Recent Advances in Restoration, Preservation and Eco-Morphophysiology of Plants Under Integrated Management Approaches and Current Climate Change View all 11 articles
The diversity of the rhizosphere arbuscular mycorrhizal fungi (AMF) community is a crucial factor affecting root-soil interaction. They can absorb carbohydrates from the host body and return the nutrient elements from the soil to the host. Using 15 Chinese fir (Cunninghamia lanceolata Lamb. Hook.) clones, the AMF richness, abundance and community structure in “Root system-Rhizosphere soil-Bulk soil” were obtained by Real-time quantitative PCR (qPCR) and Illumina Miseq sequencing techniques. The results showed that under the same Chinese fir clone, the total amount of AMF was in the order of rhizosphere soil > root system > bulk soil. The species diversity and uniqueness of AMF were in the order of root system > rhizosphere soil > bulk soil. There was a significant correlation between soil-available phosphorus and AMF diversity and its dominant genera and species. Regarding AMF abundance, Chinese fir clone S18 is the highest, followed by clones Y061 and P17. There was a significant difference in AMF richness among different clones, and Glomus was the dominant genus of AMF. The AMF species diversity of P17 and S2 in roots and rhizosphere soil was high, indicating a good symbiosis between roots and the AMF community. However, the AMF diversity of clones P11 and P41 was low, and the variation of AMF community composition in the group was small. The root-soil interaction caused the AMF community to gather in the rhizosphere but had less symbiosis present with roots. Still, the AMF diversity of the rhizosphere soil of both clones was high. There was a significant correlation between the soil-available phosphorus content and the species diversity of AMF and its dominant genera and species. In conclusion, Clone P17 has high AMF richness and abundance and forms a good symbiosis with AMF, which could be a nutrient-efficient clone of Chinese fir.
Soil fauna is central to key ecological functions of soil ecosystems (Farooq et al., 2021a; Yan et al., 2021). They break down soil organic matter and release carbon (C), oxygen (O), nitrogen (N), and phosphorus (P) into the soil (Farooq et al., 2022). Arbuscular mycorrhizal fungi (AMF) are soil’s most widely distributed microbial components. They can absorb carbohydrates from the host body and nutrient elements from the soil to supply the host. Improving the nutrient use efficiency of the host is conducive to its growth and biomass accumulation to enhance its stress resistance (Smith and Read, 2008; Smith and Smith, 2011; Tufail et al., 2021). Therefore, the symbiosis between plants and AMF is key to improving forest productivity.
Chinese fir (Cunninghamia lanceolata Lamb. Hook.) is the most common fast-growing timber species in subtropical China, with good material and high yield per unit area (Li Y. et al., 2021); however, the management mode of continuous planting of Chinese fir plantations for multiple generations has significantly reduced the availability of soil nutrients, and the productivity of stands was difficult to maintain for a long time (Farooq et al., 2019a). However, with the maturing of Chinese fir breeding technology, excellent breeding clones have become the main means of afforestation of Chinese fir plantation. In recent years, various studies have been carried out on using mycorrhizal symbionts to maintain the high productivity of Chinese fir plantations. Some studies have shown that AMF significantly improves the aboveground biomass of Chinese fir seedlings by promoting the absorption of nutrients (Jing et al., 2020), and AMF has made great progress in improving nutrient efficiency and its mechanism (Martin et al., 2017).
Since plant productivity in a specific ecosystem depends on the diversity of fungal symbionts, AMF diversity is an important determinant of plantation ecosystems (Heijden et al., 1998). For an individual plant, its productivity increases with the increase of AMF community diversity (Vogelsang et al., 2006). At the same time, there was a preferential association between some AMF and plant genotypes (clones or varieties) (Pivato et al., 2007), and significant differences are noticed in the impact of different kinds of AMF on plants (Heijden et al., 1998). From the perspective of the plant-soil system, the rhizosphere interaction process plays a key role in the nutrient use efficiency of the system. The diversity of the AMF community is of certain importance in maintaining the sustainable production of the system (Manoharan et al., 2017). The “host preference” of AMF was considered an important factor in determining plant community structure and ecosystem function (Heijden et al., 1998; Vandenkoornhuyse et al., 2002; Sanders, 2003). Different species of AMF have different effects on host plants’ competitiveness and relative abundance (Scheublin et al., 2007). Studies have shown that even if AMF of different species appear in the same area at the same time, there are differences in root colonization ability, impact on plant performance and compatibility with existing plants (Van der Heijden et al., 1998; Helgason et al., 2002).
The special behavior of AMF involves an external interface with soil and an internal interface with the root cortex to exchange nutrients with plants. Therefore, AMF diversity may be affected differently at different interfaces. From the perspective of the “root-rhizosphere-soil” system, revealing the interaction process of the rhizosphere has become the focus of research. This also raises the question about the influence of the spatial heterogeneity of the “Root system- Rhizosphere soil-Bulk soil” system on the diversity of AMF.
Currently, most of the research mainly focuses on a single rhizosphere process; thus, in-depth research from the “Root system-Rhizosphere soil-Bulk soil” system multi-interface interaction is scarce. Moreover, the research on AMF in Chinese fir plantations mainly focuses on the influence of external factors such as nitrogen deposition, continuous planting, and afforestation density on the interface rate and community structure of AMF. Still, it does not involve the response of AMF to different Chinese fir clones, genotypes or varieties. Therefore, this paper focuses on (1) the diversity, richness and community structure of AMF in the root system, rhizosphere, and bulk soil under 15 Chinese fir clones and (2) the difference in the impact of the spatial heterogeneity of the root-rhizosphere- bulk soil on AMF diversity. We hypothesized that due to the “host preference” of AMF, there were significant differences in AMF community diversity among roots of different clones of Chinese fir.
The research was carried out on the Shanghang Baisha state-owned forest farm in Longyan City, Fujian Province (Figure 1). The test site has an altitude of 780 m. It belongs to the subtropical monsoon climate, with an average annual precipitation of 1780 mm, an average annual temperature of 18.5°C, and a frost-free period of about 270 days. The mountain soil is mainly red. The test plots present a long strip with different Chinese fir clones planted adjacent to each other. The afforestation was carried out in the spring of 2019 using 1-year-old cuttings of Chinese fir clones collected from the Chinese Fir Engineering Technology Research Center of the State Forestry and Grassland Administration. Afforestation density was 278 plants/hm2 for all the Chinese clones.
Figure 1. The map of the study area (Longyan City, Fujian Province, 116°37′53′′E—116°37′59′′E, 25°09′46′′N—25°09′51′′N).
According to the research objectives, fifteen Chinese fir clones (L27, P2, P11, P17, P18, P41, S22, S2, S4, S18, S23, Y020, Y061, W2, and 302) were selected for the research. An entire plot (from uphill to downhill) was used for the study without establishing the smaller sample plot. In October 2020, all the trees in the sample plots were tested for height and diameter. Then, three healthy standard trees per clone were selected. After carefully removing the litter layer, fine roots (≤2 mm) from the standard trees were collected. The shaking soil method sampled rhizosphere soil (0–5 mm from the root’s surface). To collect the bulk soil sample, the soil profiles were excavated from the upper, middle and lower slopes, and undisturbed soil samples were taken from 0 to 20 and 20 to 40 cm depth using a 100 cm3 ring knife. Soil and root samples were put into sterile plastic bags, placed in a 4°C incubator, and returned to the laboratory. Bulk soil was divided into two parts. Chinese fir roots, rhizosphere soil and one part of bulk soil were placed in an ultra-low temperature refrigerator (−80°C) to detect the abundance and diversity of AMF. The remaining bulk soil samples were used to determine the soil’s physical and chemical properties. The repetitions were used for each parameter and each clone.
Soil pH was measured using the Potentiometric method (1:2.5 soil:water) (Farooq et al., 2019b). Soil water content was measured by the drying method. Whereas soil bulk density (BD), total porosity (TPOR), and capillary porosity (CPOR) were measured by following Core method of the Nanjing Institute of Soil, Chinese Academy of Science (1978). Total phosphorus (TP) was measured by molybdenum antimony anti colorimetry, whereas available phosphorus (AP) by using the method of Bray and Kurtz (1945). Rapidly available potassium (AK) was measured by Flame atomic spectrophotometry. The total potassium (TK) content was measured by an inductively coupled plasma emission spectrometer. Total carbon (TC) and total N (TN) was measured using a C-N elemental analyzer (Farooq et al., 2021b). An organic carbon analyzer measured the total organic C (TOC). Table 1 shows the physical and chemical properties of the bulk soil at the test site (Table 1).
Nested PCR was used to quantitatively analyze the fluorescence of AMF in roots, rhizosphere soil, and bulk soil. ABI7500 fluorescent quantitative PCR instrument was used for the determination. The process included four parts: primer design of AMF (Table 2; Tavasolee et al., 2011), genomic DNA extraction of samples (omega soil DNA Extraction Kit), plasmid cloning (PCR amplification, TA cloning, colony PCR identification positive cloning, Plasmid Extraction) and RealTime PCR sample detection.
Arbuscular mycorrhizal fungi richness was measured in 96 samples of bulk soil, mixed roots, and rhizosphere soil of 15 Chinese fir clones. For microbial diversity detection, fungal AMF was selected. The DNA samples were sent to Beijing ovison Gene Technology Co., Ltd. Paired-end sequencing was used through Illumina miseq PE300 high throughput sequencing platform. AMF first round amplification was primer FLR1 (5′-GCATATCAATAAG CGGAGGA-3′) and FLR2 (5′ GTCGTTTAAAGCCAT TACGTC-3′). Whereas second round amplification was primer FLR3-F (5′-TTGAAAGGGAAACGATTGAAGT-3′) and FLR4-R (5′-TACGTCAACATCCTTAACGAA-3′).
SPSS Statistical Package (SPSS 25.0, Chicago, IL, USA) was used for variance analysis, and QIIME1 (V1.8.0) software was used for α Diversity index analysis (including Shannon and Chao1 indexes). Based on species annotation and relative abundance results, R (V3.6.0) software was used to analyze the histogram of species composition and PCoA. Mothur (V.1.34.4) software for meta stats group difference analysis, Phython (V2.7) software for lefse analysis, Canoco 4.5 software for RDA analysis, and Origin Pro 2021 was used for mapping.
Apart from the S18 clone, the total amount of AMF for all the clones was in the order of rhizosphere soil > root > bulk soil. For the S18 clone, the total AMF amount was higher in roots than in the rhizosphere soil. Overall, the total amount of AMF in root and bulk soil was relatively close (Figure 2). However, due to the large variation within the group, no significant difference was observed in the total amount of AMF between 96 samples of the root system, rhizosphere soil, and bulk soil of all the clones (P > 0.05).
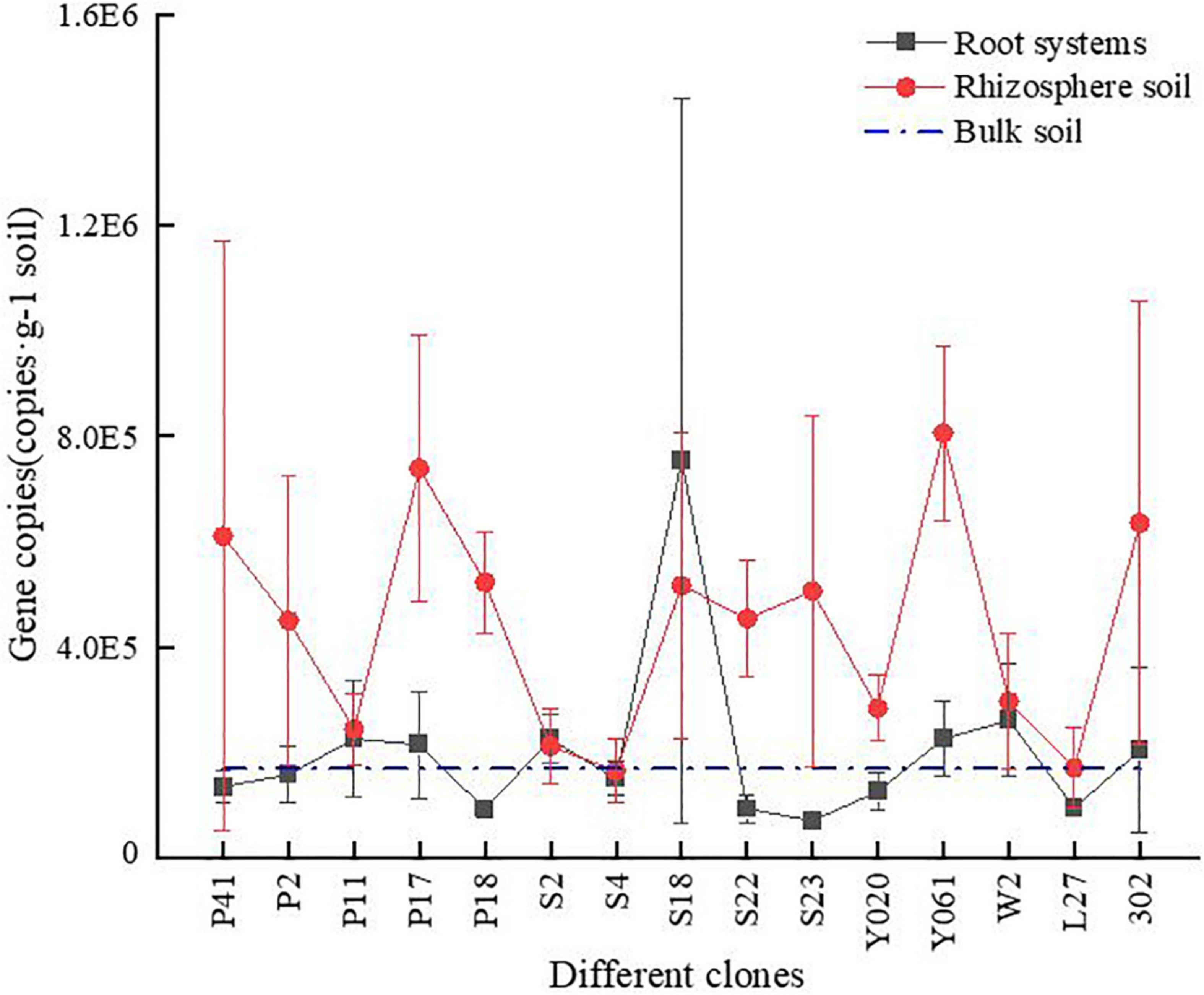
Figure 2. The gene copies of AMF in different clones of Chinese fir plantations at different sampling positions. On the horizontal axis, S-NR represents bulk soil, R-represents root system, S-represents rhizosphere soil, and the subsequent number indicates clones.
A total of 3,206 OTUs were generated from bulk soil, rhizosphere soil, and root system samples. There were 2,930 OTUs left after leveling. The diversity and uniqueness of AMF species were in the order of root > rhizosphere soil > bulk soil. The AMF composition of rhizosphere soil was similar to that of root and contains most of the AMF composition of bulk soil (Figure 3).
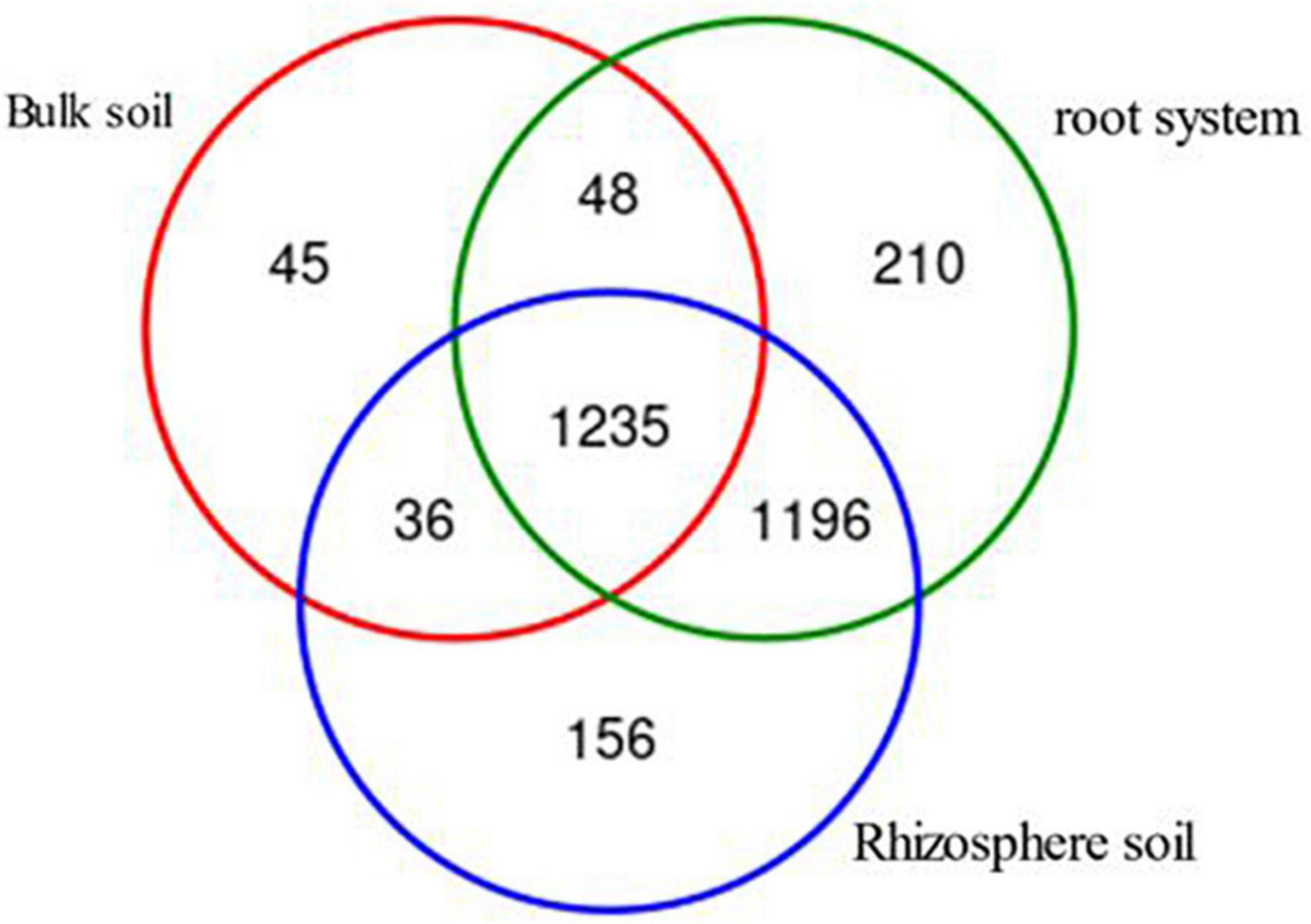
Figure 3. Venn diagram of the arbuscular mycorrhizal fungi (AMF) community based on the operational taxonomic units (OTUs) level in different clones of Chinese fir plantations at different sampling positions.
The total OTU number and the unique number of roots of Chinese Fir Clone S2 were the highest, 1641 (Figure 4A) and 532 (Figure 4B), respectively. The maximum number of OTU shared by rhizosphere soil and root system was in clone P17 (923) (Figure 4C), and the maximum number of OTU unique to rhizosphere soil was observed in clone P11 (837) (Figure 4D). It showed that there were significant differences in AMF flora in different clones. AMF species diversity in Chinese fir clones S2, S23, and P17 were high, and the similarity of AMF composition between rhizosphere soil and root system of clone P17 was the largest.
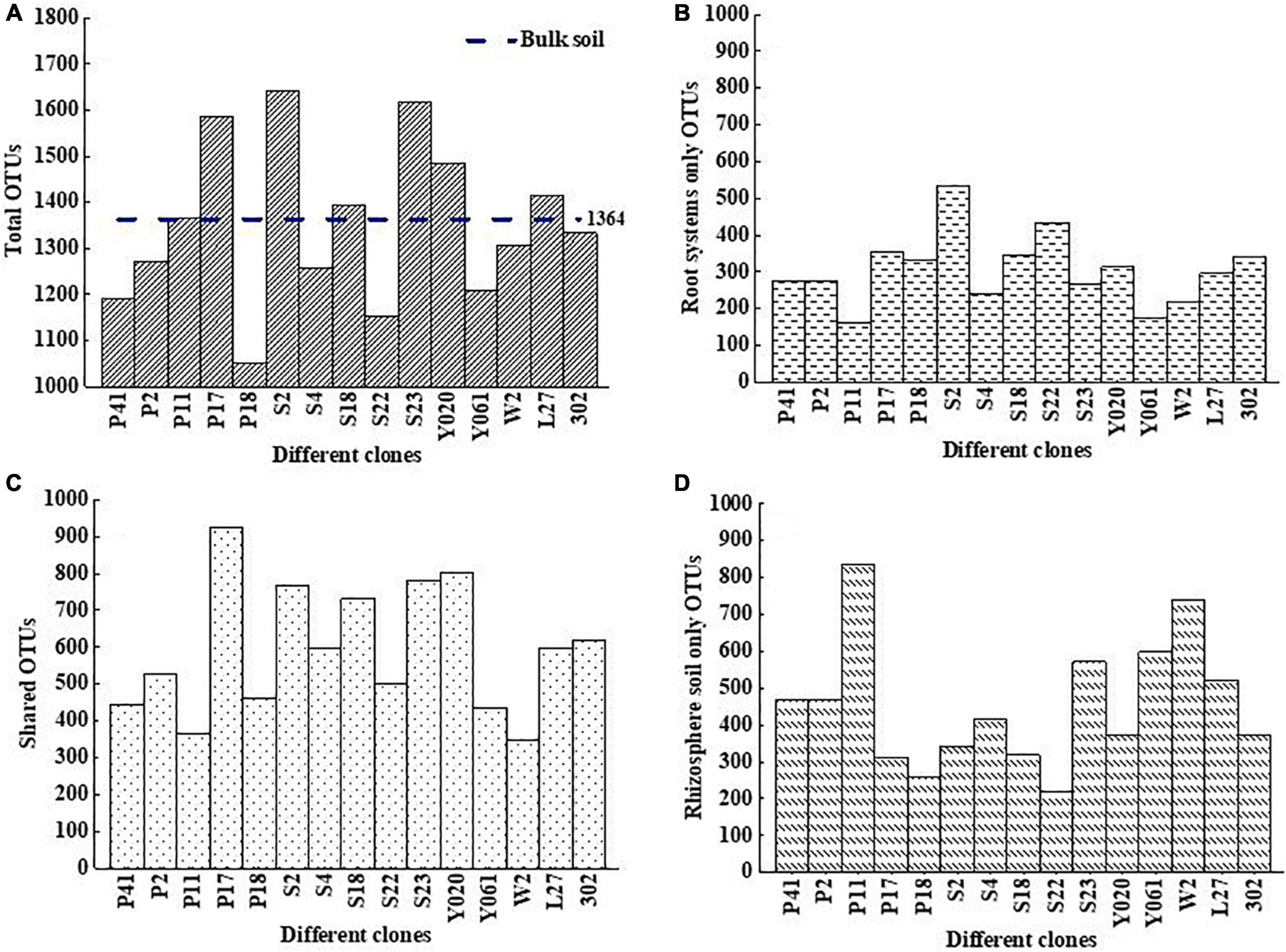
Figure 4. Venn diagram of AMF OTUs number in different clones of Chinese fir plantations, the total OTUs (A), root system only OTUs (B), shared OTUs (C), and rhizosphere soil only OTUs (D), respectively. On the horizontal axis, S-NR represents bulk soil, R-represents root system, S-represents rhizosphere soil, and the subsequent number indicates clones.
The change of the α diversity index showed that AMF richness in 15 clone systems was significantly different (Figure 5). The Chao1 index of roots of Chinese fir clones S2 and P17 were considerably higher while clone P11 was the least, while the Chao1 index of rhizosphere soil of clones S23, P17, Y020, W2, and P11 was significantly higher, whereas S22 clone was the least (Figure 5A). The Shannon index of the roots of Chinese Fir Clone P17 was significantly higher, whereas clones P41 and P11 were the least (Figure 5B), and the Shannon index of its rhizosphere soil was considerably higher than that of clone P18.
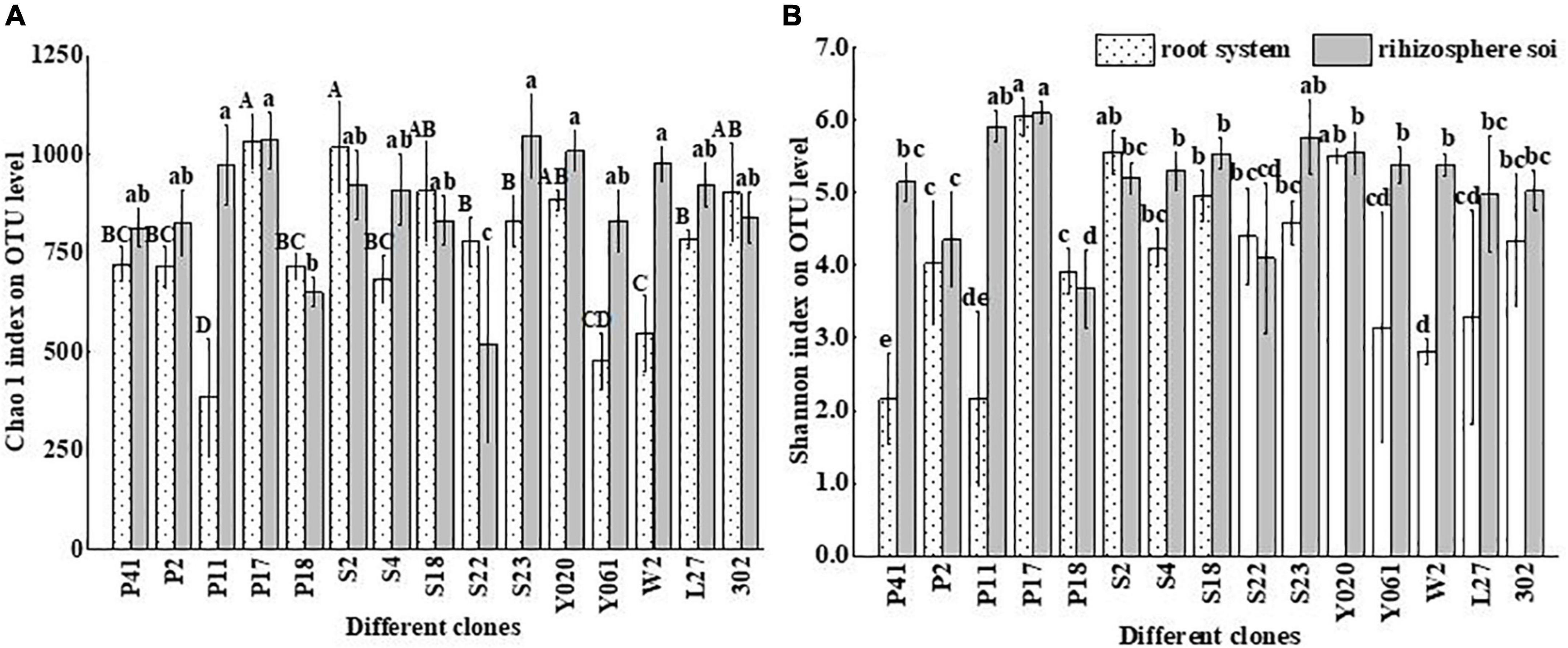
Figure 5. The alpha diversity index of AMF in different clones of Chinese fir plantations (A,B). Bars (means ± standard error) accompanied by the same lowercase letter(s) are not significantly different at the 5% probability level. The capital letters in the panel indicate that the difference is very significant (P < 0.01), lowercase letters indicate significant differences (P < 0.05), the same below. On the horizontal axis, S-NR represents bulk soil, R-represents root system, S-represents rhizosphere soil, and the subsequent number indicates clones.
After 96 samples were identified and classified, 2,930 AMF OTUs were obtained, covering 65 species of AMF in 1 phylum, 1 class, 4 orders, 8 families, and 11 genera. Glomerales had the highest relative abundance of AMF among all the samples, accounting for more than 99.7%. In addition, there were a certain number of unclassified-Fungi. In terms of the genus, Glomus accounted for 90.86%, followed by Rhizophagus accounted for 7.71%, Rhizoglomus accounted for 1.37%, and Scutellospora, 0.03%. Specifically talking about bulk soil, Glomus accounts for 89.88%, followed by Rhizophagus accounts for 9.67%. The AMF genus diversity of clone S2 included up to 7 identified and unidentified genera. Other genera and unidentified genera account for very little, and the proportions of different clones were quite different (Figure 6A).
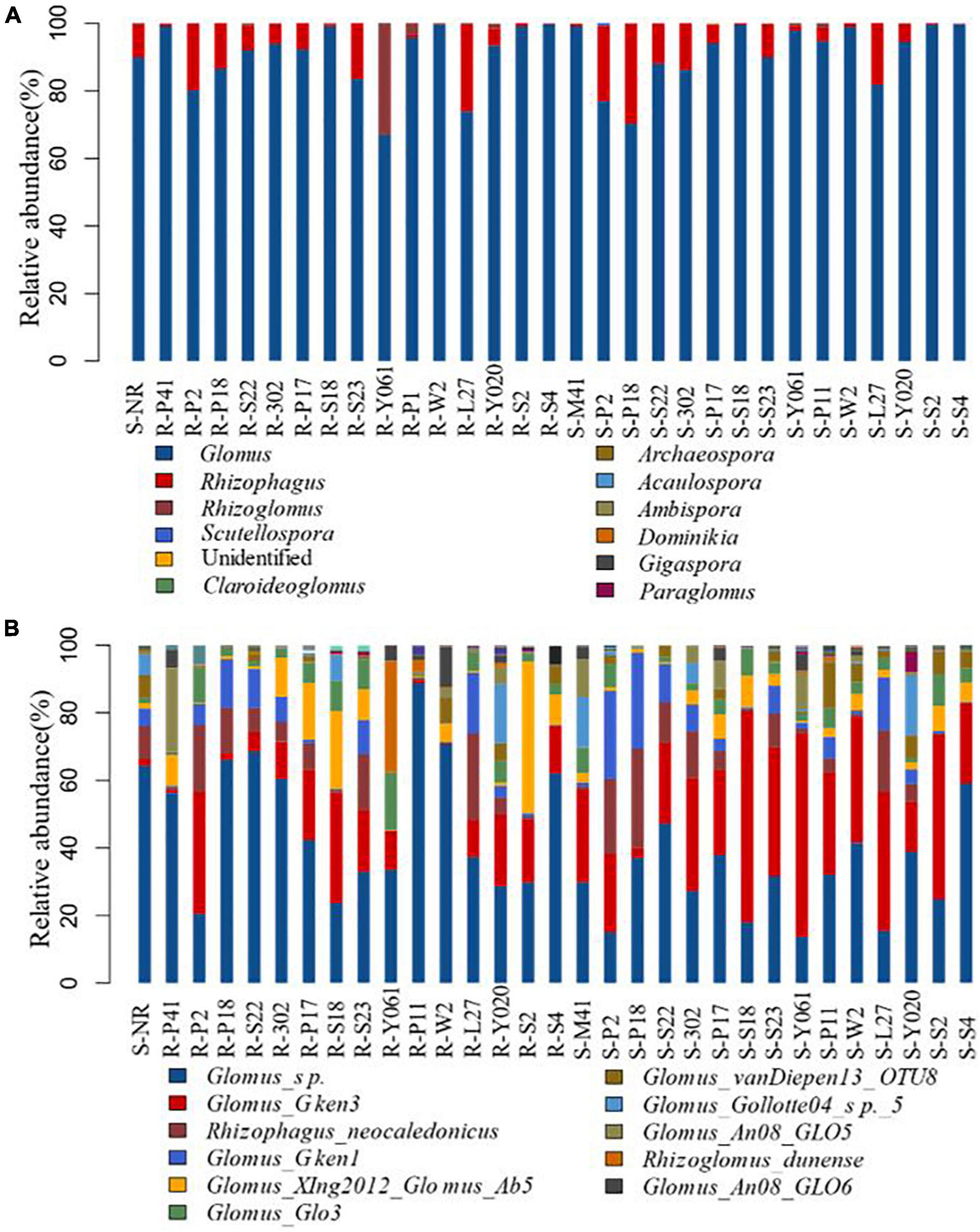
Figure 6. The relative abundance at AMF genus level (A) and species level (B) in different clones of Chinese fir plantations. On the horizontal axis, S-NR represents bulk soil, R-represents root system, S-represents rhizosphere soil, and the subsequent number indicates clones.
Among the 15 clones, Glomus and Rhizophagus existed in all samples. Glomus accounted for more than 99.7% of the root system of clone S4 and the rhizosphere soil of clone S2. Rhizophagus accounted for 29.6% of the rhizosphere soil of clone P18 (the highest). Rhizoglomus did not exist in the roots of clone S4 or P17 rhizosphere soil. The 11 AMFs identified at the species level with relative abundances greater than 1% were included in the NCBI nucleoside database (Figure 6B).
Figure 7 showed that Rhizoglomus and Rhizophagus displayed the most significant contributions to the difference between groups at the genus level for all the Chinese fir-tested clones. AMF dominant genera and species contributed more to the difference in AMF community structure of different Chinese fir clones than other genera and species.
PCoA map showed that in the root AMF community of different clones, the β diversity difference was highly significant (P = 0.002; Figure 8A). The variation within the clone P11 group was the smallest. The AMF community of the two roots was less affected by external factors, and the degree of overlap between the two was high. The composition and diversity of the two root communities were similar (Figure 8A). There was no significant difference in the β diversity of AMF communities in rhizosphere soil of different clones (P = 0.057; Figure 8B).
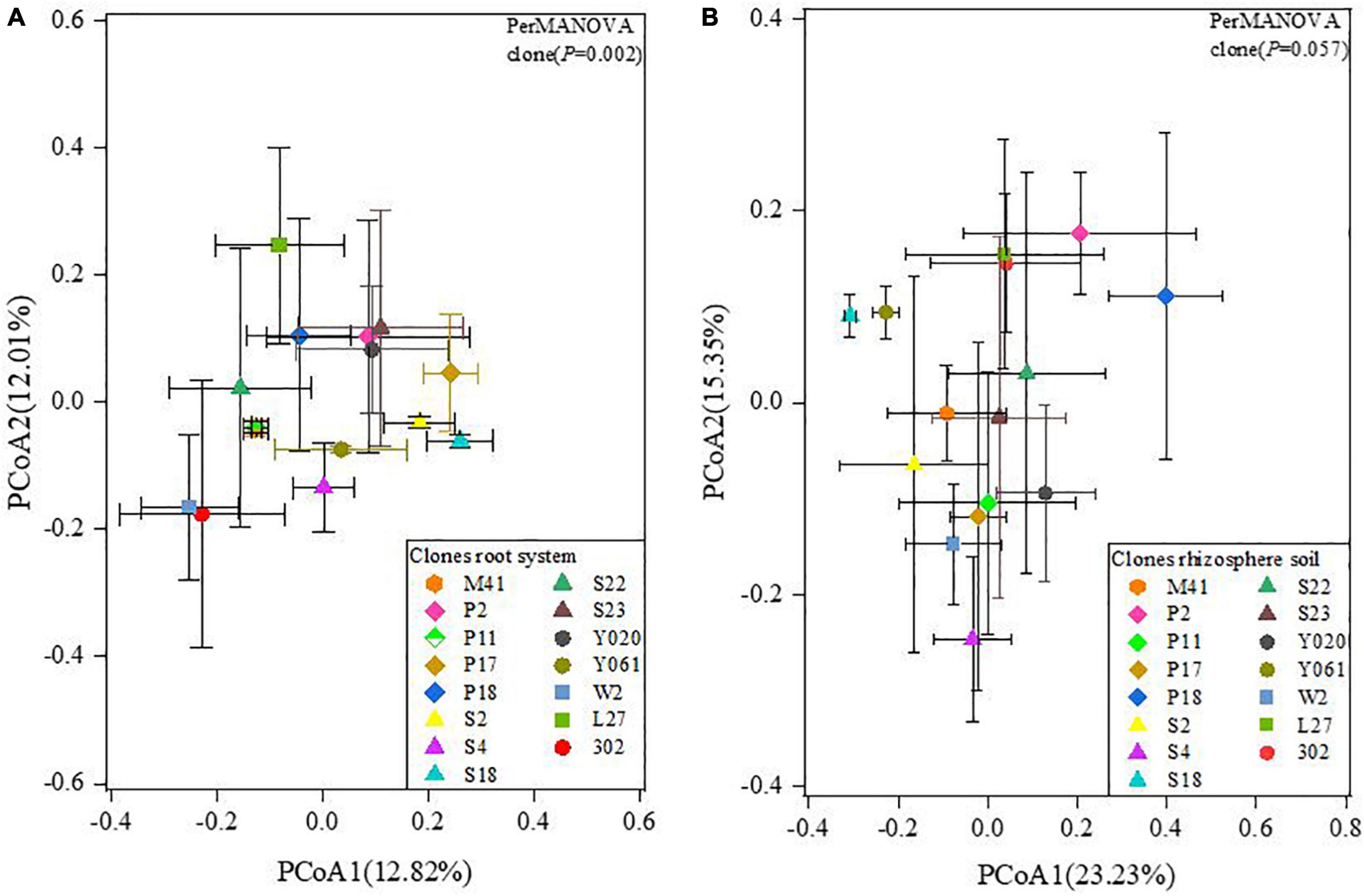
Figure 8. PCoA analysis of AMF community in root (A) and rhizosphere soil (B) of different Chinese fir clones. The numbers in the lower right corner represent the different clones.
Available phosphorus content was significantly positively correlated with the Shannon index, and the TK content was negatively correlated with the Chao1 index. The TP content was negatively correlated with both Shannon index and Chao1 index. AP was significantly negatively correlated with Glomus, and significantly positively correlated with Rhizophagus, pH, Scutellospora, TPOR and Rhizoglomus. There was a significant negative correlation between Glomus and Rhizophagus (Figures 9A,B).
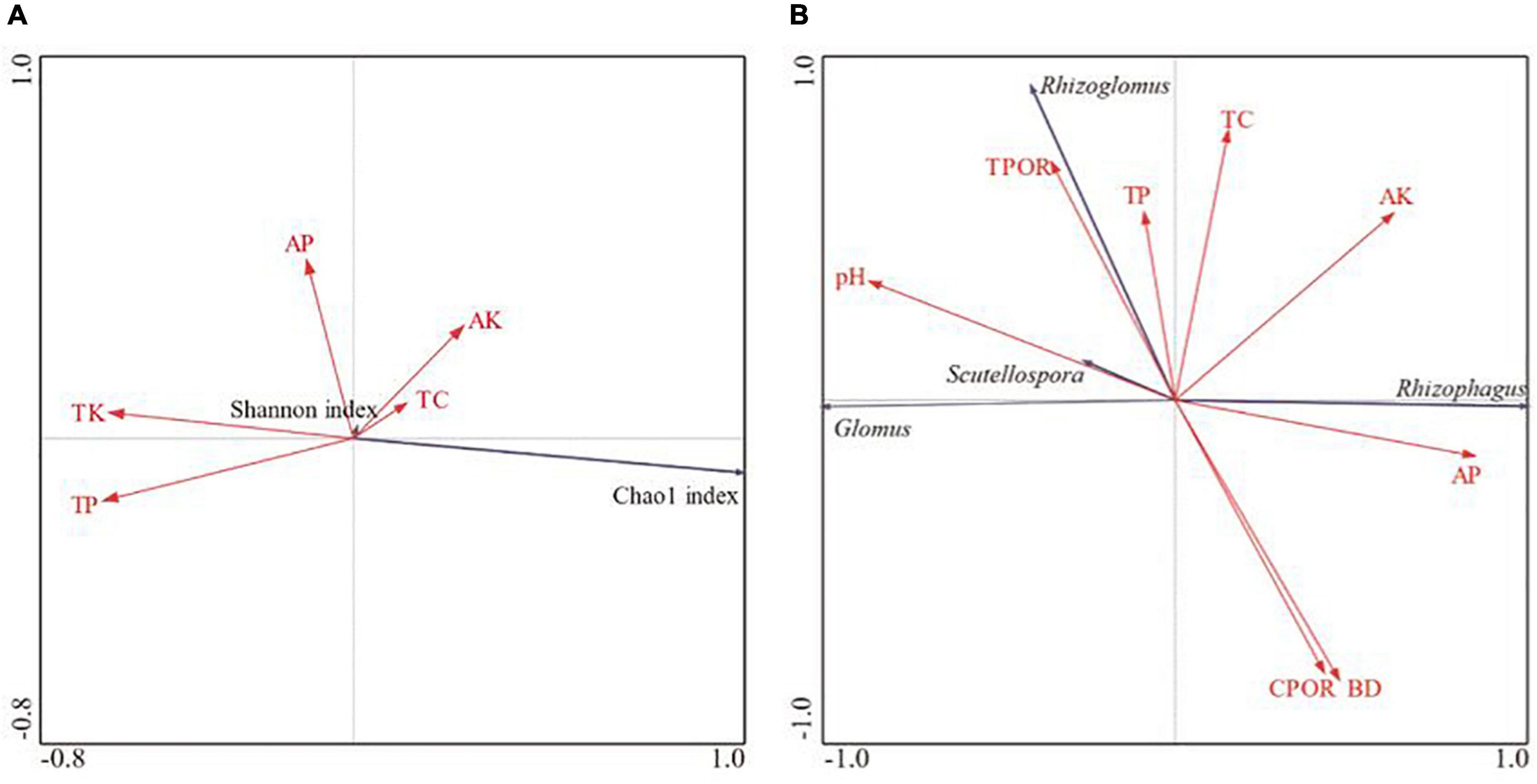
Figure 9. Correlation analysis of soil physical and chemical properties with AMF diversity index (A) and relative abundance (B) in bulk soils.
In general, the diversity of AMF determines plant growth, ecosystem productivity and variability (Van der Heijden et al., 1998; Vogelsang et al., 2006). On the contrary, AMF diversity is also regulated by different plant genotypes (Hart and Reader, 2002). In this study, overall, strong ecological adaptability and rich species diversity were present for AMF; moreover, there was a considerable difference in the physiological characteristics of AMF in different species and genera.
The AMF richness and root β diversity of different clones were significantly different. The AMF species diversity in roots and rhizosphere soil was high for clones P17 and S2. It is probably because they may have more root exudates, attracting different kinds of AMF. On the other hand, they may have better compatibility with multiple species of AMF and benefit from the functional complementarity of multiple AMF species (Koide and Kabir, 2000). The AMF diversity of clones P11 and P41 was low, with a relatively stable AMF β diversity; however, the AMF diversity of rhizosphere soil was high. The reason is that plants will use the supply of C sources to exert different selection pressures on different kinds of AMF and prioritize AMF that is more beneficial to themselves (Bever et al., 2009). Different plant genotypes will affect the coexistence of different AMF species in the same root system, and specific plant species will preferentially choose specific AMF species (Alkan et al., 2006; Pivato et al., 2007). This “host preference” makes different AMF C sources or other benefits and affects the AMF community composition of different clones. In the process of plant growth, when the early colonized AMF occasionally replaces the dominant species, the species diversity of AMF will significantly decline (Husband et al., 2002).
Arbuscular mycorrhizal fungi abundance quantifies the impact of Chinese fir clones on it, and it is more sensitive to individual characteristics and genetic diversity of tree species (De Deyn et al., 2011). The AMF abundances of Chinese fir clones S18, Y061 and P17, are high, indicating that these three clones are highly dependent on mycorrhiza and can provide sufficient space and resources for AMF by releasing root exudates to slow down the interspecific competition of AMF, which is conducive to the diffusion and reproduction of AMF. According to the correlation analysis, the relative abundance of Glomus and Rhizophagus is negatively correlated, indicating that the two AMF species may compete for common resources and space due to their similar niche. As the dominant genus of AMF, Glomus will restrict the propagation and diffusion of Rhizophagus and reduce its abundance.
Arbuscular mycorrhizal fungi diversity is not only regulated by plant genotypes (clones or varieties) but also affected by the soil environment. Under the long-term influence, AMF will evolve into different community compositions (Mirzaei and Moradi, 2017). Some studies have compared natural forests and Chinese fir plantations and concluded that the decrease in soil physical and chemical properties of Chinese fir plantations had caused the diversity changes in the fungal community (Guo et al., 2022). This study shows a significant correlation between AP content and AMF species diversity and its dominant genera. Studies have found that with the increase of AMF species richness, the improvement of plant species diversity and productivity is determined by individual AMF and depends on soil P content (Vogelsang et al., 2006), which further shows that soil P nutrition level is one of the leading factors of AMF diversity. The AMF diversity of rhizosphere soil is higher than that of non-rhizosphere soil. It may be that soil diffusion is less restricted, and AMF taxa with special ecological requirements are also less restricted in the region (Victorino et al., 2021; Zheng et al., 2021). The other reason could be that the rhizosphere is affected by the root exudates of Chinese fir; and the enhanced availability of insoluble nutrients in the rhizosphere changes the interaction between the rhizosphere to facilitate the recruitment and supply of AMF (Zhang et al., 2016).
The diversity of AMF in roots is higher than that in the rhizosphere, which may be because the biomass density of AMF extracellular hyphae in the soil is usually lower than that in the root system, and the distribution of nuclei in extracellular hyphae during spore formation has high genetic variability (Hart and Reader, 2002; Olsson et al., 2010; Deepika and Kothamasi, 2015; Herrmann et al., 2016). It may also be the low saprophytic ability of AMF, which can obtain more C sources in symbiosis with Chinese fir roots, which is conducive to its own growth (Smith and Read, 2008). In addition to the influence of the external environment, different AMFs have different life strategies, resulting in different AMFs appearing in different habitats and obtaining nutrients at different distances/areas from plant roots (Opik et al., 2006; Thonar et al., 2011). Fungal spores may effectively use favorable conditions to form a pool of propagules so that the richness of AMF community in a single plant root or vegetation sample varies with spatial distance and environmental gradient (Li X. L. et al., 2021; Muneer et al., 2022). Therefore, the spatial heterogeneity of the “root-rhizosphere-bulk soil system” has significantly different effects on AMF diversity.
Arbuscular mycorrhizal fungi abundance also has spatial differences, and in the “root-rhizosphere-soil” system, the total amount of AMF is in the order of rhizosphere soil > root > non-rhizosphere soil. It might be because the colonization of AMF in the root system of Chinese fir may be limited by the root space and resources, resulting in a lower abundance in the root system than in the rhizosphere soil (Zhang et al., 2016). On the other hand, the time of AMF infection of Chinese fir seedlings may not be long enough. At the same time, the poor soil environment can limit the survival of AMF, promote AMF to seek nutrients and hosts by using extrarhizosphere hyphae, and thus reduce the abundance of AMF in non-rhizosphere soil (Davison et al., 2015).
By comparing the differences in AMF richness and abundance in the “Root system-Rhizosphere soil-Bulk soil” system of different Chinese fir clones, it was found that the AMF richness and abundance of bulk soil within the same Chinese fir clone plantations were significantly lower than that of root and rhizosphere soil. It indicates that root exudates might activate AMF in the root system and rhizosphere soil, gather in the rhizosphere, and mutually coexist with roots. This study shows Glomus is the dominant AMF genus in the infection process among Chinese fir clones. The screening of different Chinese fir clones depicts that clone P17 has high richness and abundance, which may be a nutrient-efficient clone of Chinese fir. Based on the impact of AMF diversity and the difference in symbiosis with different clones, we suggest that AMF diversity can be artificially increased. The screened nutrient-efficient clone (P17) can be planted to coordinate with productivity, resource efficiency and environmental safety of Chinese fir. It will reduce forest management costs and provide a new path for sustainable development of Chinese fir plantation. This laid a foundation for revealing the host genetic regulation mechanism of AMF infecting roots and also provides a reference for the coordinated regulation research and sustainable development of nutrient utilization of Chinese fir in the future.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
PW and YC conceived the idea and designed the experiment. YC, NL, YZ, and JL conducted the study. NL and YC wrote the manuscript. YZ and XM gave suggestions to improve the manuscript. PW reviewed the manuscript and contributed to the discussion. All authors contributed to the article and approved the submitted version.
This study was funded by the Key Program of Natural Science of Fujian Province, China (grant number: 2020J02029) and the Science and Technology Project of Fuzhou Science and Technology Bureau, Fujian Province, China (grant number: 2021-P-035).
We thank the Baisha state-owned forest farm in Shanghang, Longyan City, Fujian Province, for providing experimental sites.
Author JL was employed by Fujian Shanghang Baisha Forestry Farm.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BD, soil bulk density; TCPOR, total porosity; CPOR, capillary porosity; AP, available phosphorus; TP, total phosphorus; TK, total potassium; AK, rapidly available potassium; TC, total carbon.
Alkan, N., Gadkar, V., Yarden, O., and Kapulnik, Y. (2006). Analysis of quantitative interactions between two species of arbuscular mycorrhizal fungi, Glomus mosseae and G. intraradices, by real-time PCR. AEM 72, 4192–4199. doi: 10.1128/AEM.02889-05
Bever, J. D., Richardson, S. C., Lawrence, B. M., Holmes, J., and Watson, M. (2009). Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 12, 13–21. doi: 10.1111/j.1461-0248.2008.01254.x
Bray, R. H., and Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46.
Davison, J., Moora, M., Opik, M., Adholeya, A., Ainsaar, L., Ba, A., et al. (2015). Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349, 970–973. doi: 10.1126/science.aab1161
De Deyn, G. B., Quirk, H., and Bardgett, R. D. (2011). Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility. Biol. Lett. 7, 75–78. doi: 10.1098/rsbl.2010.0575
Deepika, S., and Kothamasi, D. (2015). Soil moisture-a regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza 25, 67–75. doi: 10.1007/s00572-014-0596-1
Farooq, T. H., Kumar, U., Shakoor, A., Albasher, G., Alkahtani, S., Rizwana, H., et al. (2021a). Influence of intraspecific competition stress on soil fungal diversity and composition in relation to tree growth and soil fertility in sub-tropical soils under Chinese fir monoculture. Sustainability 13:10688.
Farooq, T. H., Chen, X., Shakoor, A., Li, Y., Wang, J., Rashid, M. H. U., et al. (2021b). Unraveling the influence of land-use change on δ13C, δ15N, and soil nutritional status in coniferous, broadleaved, and mixed forests in southern China: A field investigation. Plants 10:1499. doi: 10.3390/plants10081499
Farooq, T. H., Kumar, U., Yan, Y., Arif, M. S., Shakoor, A., Tayyab, M., et al. (2022). Receptiveness of soil bacterial diversity in relation to soil nutrient transformation and canopy growth in Chinese fir monoculture influenced by varying stand density. Trees 36, 1149–1160.
Farooq, T. H., Yan, W., Rashid, M., Tigabu, M., Gilani, M. M., Zou, X. H., et al. (2019a). Chinese fir (Cunninghamia lanceolata) a green gold of China with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 17, 11055–11067. doi: 10.15666/aeer/1705_1105511067
Farooq, T. H., Ma, X., Rashid, M. H. U., Wu, W., Xu, J., Tarin, M. W. K., et al. (2019b). Impact of stand density on soil quality in Chinese fir (Cunninghamia lanceolata) monoculture. Appl. Ecol. Environ. Res. 17, 3553–3566.
Guo, J. H., Feng, H. L., Roberge, G., Feng, L., Pan, C., McNie, P., et al. (2022). The negative effect of Chinese fir (Cunninghamia lanceolata) monoculture plantations on soil physicochemical properties, microbial biomass, fungal communities, and enzymatic activities. For. Ecol. Manage. 519:120297. doi: 10.2139/ssrn.4075914
Hart, M. M., and Reader, R. J. (2002). Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 153, 335–344. doi: 10.1046/j.0028-646X.2001.00312.x
Heijden, M. G. A. V., Boller, T., and Sanders, W. I. R. (1998). Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79, 2082–2091. doi: 10.2307/176711
Helgason, T., Merryweather, J. W., Denison, J., Wilson, P., Young, J., and Fitter, A. H. (2002). Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J. Ecol. 90, 371–384. doi: 10.1046/j.1365-2745.2001.00674.x
Herrmann, L., Lesueur, D., Brau, L., Davison, J., Jairus, T., Robain, H., et al. (2016). Diversity of root-associated arbuscular mycorrhizal fungal communities in a rubber tree plantation chronosequence in Northeast Thailand. Mycorrhiza 26, 863–877. doi: 10.1007/s00572-016-0720-5
Husband, R., Herre, E. A., and Young, J. (2002). Temporal variation in the arbuscular mycorrhizal communities colonising seedlings in a tropical forest. FEMS Microbiol. Ecol. 42, 131–136. doi: 10.1016/S0168-6496(02)00323-9
Jing, Y. B., Mao, J. H., Li, R. B., Chen, L. M., Li, Y. P., Cao, J. X., et al. (2020). Diversity of arbuscular mycorrhizal fungi in Cunninghamia lanceolata plantation and their effects on growth of seedlings. J. West Chin. For. Sci. 49, 100–106. doi: 10.16473/j.cnki.xblykx1972.2020.06.014
Koide, R. T., and Kabir, Z. (2000). Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 148, 511–517. doi: 10.1046/j.1469-8137.2000.00776.x
Li, X. L., Qi, Z. Q., Yu, X. L., Xu, M., Liu, Z. H., Du, G. F., et al. (2021). Soil pH drives the phylogenetic clustering of the arbuscular mycorrhizal fungal community across subtropical and tropical pepper fields of China. Appl. Soil Ecol. 165:103978. doi: 10.1016/j.apsoil.2021.103978
Li, Y., Heal, K., Wang, S. Z., Cao, S., and Zhou, C. F. (2021). Chemodiversity of soil dissolved organic matter and its association with soil microbial communities along a chronosequence of Chinese fir monoculture plantations. Front. Microbiol. 12:729344. doi: 10.3389/fmicb.2021.729344
Manoharan, L., Rosenstock, N. P., Williams, A., and Hedlund, K. (2017). Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Appl. Soil Ecol. 115, 53–59. doi: 10.1016/j.apsoil.2017.03.012
Martin, F. M., Uroz, S., and Barker, D. G. (2017). Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 356:eaad4501. doi: 10.1126/science.aad4501
Mirzaei, J., and Moradi, M. (2017). Relationships between flora biodiversity, soil physiochemical properties, and arbuscular mycorrhizal fungi (AMF) diversity in a semi-arid forest. Plant Ecol. Evol. 150, 151–159. doi: 10.5091/plecevo.2017.1249
Muneer, M. A., Tarin, M., Chen, X. H., Afridi, M. S., Iqbal, A., Munir, M. Z., et al. (2022). Differential response of mycorrhizal fungi linked with two dominant plant species of temperate grassland under varying levels of N-addition. Appl. Soil Ecol. 170:104272. doi: 10.1016/j.apsoil.2021.104272
Nanjing Institute of Soil Science, Chinese Academy of Sciences (1978). Soil physical and chemical analysis. Shanghai: Shanghai Science and Technology Press, 481.
Olsson, P. A., Rahm, J., and Aliasgharzad, N. (2010). Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 72, 123–131. doi: 10.1111/j.1574-6941.2009.00833.x
Opik, M., Moora, M., Liira, J., and Zobel, M. (2006). Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 94, 778–790. doi: 10.1111/j.1365-2745.2006.01136.x
Pivato, B., Mazurier, S., Lemanceau, P., Siblot, S., Berta, G., Mougel, C., et al. (2007). Medicago species affect the community composition of arbuscular mycorrhizal fungi associated with roots. New Phytol. 176, 197–210. doi: 10.1111/j.1469-8137.2007.02151.x
Sanders, I. R. (2003). Preference, specificity and cheating in the arbuscular mycorrhizal symbiosis. Trends Plant Sci. 8, 143–145. doi: 10.1016/S1360-1385(03)00012-8
Scheublin, T. R., Van Logtestijn, R., and Van der Heijden, M. (2007). Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 95, 631–638. doi: 10.1111/j.1365-2745.2007.01244.x
Smith, S. E., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250. doi: 10.1146/annurev-arplant-042110-103846
Tavasolee, A., Aliasgharzad, N., Salehi, G. R., Mardi, M., Asgharzadeh, A., and Akbarivala, S. (2011). Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on fungal occupancy in chickpea root and nodule determined by real-time PCR. Curr. Microbiol. 63, 107–114. doi: 10.1007/s00284-011-9951-z
Thonar, C., Schnepf, A., Frossard, E., Roose, T., and Jansa, J. (2011). Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant Soil 339, 231–245. doi: 10.1007/s11104-010-0571-3
Tufail, M. A., Bejarano, A., Shakoor, A., Naeem, A., Arif, M. S., Dar, A. A., et al. (2021). Can bacterial endophytes be used as a promising bio-inoculant for the mitigation of salinity stress in crop plants?—A global meta-analysis of the last decade (2011–2020). Microorganisms 9:1861. doi: 10.3390/microorganisms9091861
Van der Heijden, M., Klironomos, J. N., Ursic, M., Moutoglis, P., Streitwolf-Engel, R., Boller, T., et al. (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69–72. doi: 10.1038/23932
Vandenkoornhuyse, P., Husband, R., Daniell, T. J., Watson, I. J., Duck, J. M., Fitter, A. H., et al. (2002). Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol. Ecol. 11, 1555–1564. doi: 10.1046/j.1365-294X.2002.01538.x
Victorino, G., Santos, E. S., Abreu, M. M., Viegas, W., and Nogales, A. (2021). Detrimental effects of copper and EDTA co-application on grapevine root growth and nutrient balance. Rhizosphere 19:100392. doi: 10.1016/j.rhisph.2021.100392
Vogelsang, K. M., Reynolds, H. L., and Bever, J. D. (2006). Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol. 172, 554–562. doi: 10.1111/j.1469-8137.2006.01854.x
Yan, Y., Li, B., Huang, Z., Zhang, H., Wu, X., Farooq, T. H., et al. (2021). Characteristics and driving factors of rhizosphere bacterial communities of Chinese fir provenances. Forests 12:1362.
Zhang, D., Zhang, C., Tang, X., Li, H., Zhang, F., Rengel, Z., et al. (2016). Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol. 209, 823–831. doi: 10.1111/nph.13613
Keywords: arbuscular mycorrhizal fungi, diversity and abundance, root structure, rhizosphere soil, Cunninghamia lanceolata
Citation: Cao Y, Li N, Lin J, Zhang Y, Ma X and Wu P (2022) Root system-rhizosphere soil-bulk soil interactions in different Chinese fir clones based on fungi community diversity change. Front. Ecol. Evol. 10:1028686. doi: 10.3389/fevo.2022.1028686
Received: 26 August 2022; Accepted: 20 September 2022;
Published: 04 October 2022.
Edited by:
Wen Xing Long, Hainan University, ChinaReviewed by:
Babar Iqbal, Jiangsu University, ChinaCopyright © 2022 Cao, Li, Lin, Zhang, Ma and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengfei Wu, Zmp3dXBlbmdmZWlAMTI2LmNvbQ==, Zmp3dXBlbmdmZWlAZmFmdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.