
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 12 October 2022
Sec. Ecophysiology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1017691
This article is part of the Research TopicBehavioral and Physiological Adaptations of Mammals and Birds to Anthropogenic DisturbancesView all 11 articles
A correction has been applied to this article in:
Corrigendum: Maize monoculture causes niacin deficiency in free-living European brown hares and impairs local population development
Maize (Zea mays) is the most produced crop worldwide and the second most important bio-energy plant. Huge maize monoculture is considered a threat to biodiversity in agricultural landscapes and may also contribute to the decline of European brown hares (Lepus europaeus, Pallas 1778). Indeed, the intensification of agriculture has been identified as one of the main factors responsible for the decline of brown hare populations. A reason why large maize cultures can be particularly detrimental to animals consuming this plant is its poor nutritional value with respect to niacin. In this study, we investigated the effects of the proportion of area under maize crops on liver concentrations of niacin in free-living hares, on the reproductive output of does (females), and on the development of local populations, at nine study sites in Lower Austria. Hare numbers were estimated from spotlight counts in spring and autumn. Liver samples and uteri were obtained from hares shot in the same areas during regular autumn hunts. Number of offspring born to an individual female during the preceding reproductive period was determined by counting placental scars. Our results show a significant negative effect of the area under maize crops on liver concentrations of niacin of does and on their reproductive output. Further, we found a significant negative effect of the area under maize on the development of a population. Altogether, our findings indicate that high proportions of the area under maize crops contribute to the decline of brown hares by reduced fecundity of does and impaired development of local populations.
Maize (Zea mays) is the most produced crop on a global scale (Nuss and Tanumihardjo, 2010), and is, with rapeseed, one of the two main energy crops cultivated in Europe (Klenke et al., 2017). Maize is considered as having the highest yield potential of energy crops grown in central Europe (Amon et al., 2007), and will therefore probably become increasingly important for energy/biogas production (Nielsen and Oleskowicz-Popiel, 2008). However, large-scale cultivation of maize is increasingly threatening biodiversity (Fargione et al., 2009), and affects most farmland animals negatively (Klenke et al., 2017). Low habitat quality, e.g., loss of rotational set-aside or loss of habitat heterogeneity due to maize cultivation, with a landscape structure no longer meeting the ecological requirements of many species, seems to be responsible (Gevers et al., 2011).
Population trends of a typical farmland species, the European brown hare (Lepus europaeus, Pallas 1778), show a strong decline in many European countries over the last decades (Mary and Trouvilliez, 1995). As this species also represents an important game species (Pielowski, 1976), long-term trends can be monitored by hunting bag data (Tapper and Parsons, 1984; Langbein et al., 1999). These records show a dramatic population decline throughout Europe [Poland: Pielowski and Raczynski (1976), Denmark: Madsen et al. (1996), Wincentz Jensen (2009), parts of Croatia: Pintur et al. (2006); Popović et al. (2008), Serbia: Ristić et al. (2021), Germany, Austria, Bulgaria, Luxembourg, Netherlands, Slovakia, Switzerland: Mary and Trouvilliez (1995), United Kingdom: Smith et al. (2005)].
A large number of studies tried to identify causes for this decline (reviewed in Smith et al., 2005). Negative effects of predators like a red fox (Vulpes vulpes, Linnaeus 1758) on brown hare abundance were reported from Poland, Germany, and the United Kingdom (Reynolds and Tapper, 1995; Panek et al., 2006; Kalchreuter, 2015). However, Weber et al. (2019) showed that populations could grow despite high predator abundance when sufficient habitat structures offering cover to leverets were present. There was no evidence for reduced fertility of brown hare does as a potential cause of the decline (Hackländer et al., 2001; Schai-Braun et al., 2020), but low temperatures and high precipitation during spring increase leveret mortality (Hackländer et al., 2002). Farming practice, particularly the intensification of agriculture, was identified as a significant factor negatively affecting the abundance and population dynamics of European brown hares (Smith et al., 2005), presumably due to poor nutrition caused by low crop diversity and large fields (Tapper, 1987). However, Smith et al. (2005) found no effect of field size per se on population densities, but report a negative effect of extensive monocultures on hare abundance. Accordingly, population decline in Bulgaria, for instance, began simultaneously with the increase of monocultures (Petrov, 1976). Increase in maize monoculture was also identified as a potential cause of a population decline in Germany (Sliwinski et al., 2019). A decline in hare densities of up to 90% after a change to maize monoculture was reported by Bertóti (1975).
Altogether, loss of cover and malnutrition are considered important causes of low reproduction, and especially high postnatal mortality of brown hares in Europe (Hansen, 1992; Edwards et al., 2000). The diet of hares is twice as diversified in mixed compared to monocultural farmed landscapes (Frylestam, 1986). There is, however, local variation according to the type of cultivated crops, field size, and alternative field habitats (Reichlin et al., 2006; Petrovan et al., 2012). The brown hare is highly selective in its food consumption and is, to some extent, actively searching for plants rich in fat (Schai-Braun et al., 2015). To meet their nutritional requirements, hares rely strongly on weeds (Reichlin et al., 2006; Schai-Braun et al., 2015), and specific crops such as beets (Beta vulgaris), soybean (Glycine max), and fodder crops like alfalfa (Medicago sativa) or red clover (Trifolium pratense). However, most weeds have vanished from farmed landscapes because of the high utilization of herbicides in conventional farming (Wilson et al., 1999; Stoate et al., 2001; Gaba et al., 2016), and fodder crops are nowadays far less cultivated at the expense of energy crops or cereals (Klenke et al., 2017).
An alternative, but not mutually exclusive hypothesis for the negative effects of maize on animals involves its low nutritional value for species consuming this crop. Indeed, although maize is particularly valued for its fatty acid composition (especially adapted for livestock diets), and its high sugar level (for ethanol production), it contains low proportions of many essential micronutrients including calcium, manganese, copper, and iron (Nuss and Tanumihardjo, 2010; INRA et al., 2011). Moreover, maize seeds and leaves are highly deficient in niacin and its precursor tryptophan (Hogan et al., 1955; Henderson et al., 1959; Goss, 1968; Mawson and Jacobs, 1978). Tryptophan (trp) is an essential amino acid for all eukaryotes and even some prokaryotes (de Groot, 1953; Meisinger, 1978; Kantak et al., 1980; Walz et al., 2013). It is the precursor of 5-hydroxytryptophan, an important monoamine neurotransmitter, and of niacin (Kohlmeier, 2003). Niacin can be decomposed into two molecules, nicotinic acid and nicotinamide, essential for the in vivo synthesis of nicotinamide adenine dinucleotide (NAD) (Wan et al., 2010). NAD is indispensable for the effective functioning of the Krebs cycle and therefore for cell respiration and ATP synthesis. Animals must obtain trp and most of their niacin from food on a daily basis. Only plants and microorganisms can synthesize trp, and niacin synthesis from trp is very inefficient in many animal species (Baker, 2008). However, in addition to the deficiency of trp in maize, up to 90% of niacin is present as niacytin in mature maize grains, i.e., bound up in a complex with hemicellulose which renders it unavailable to vertebrates (Ammerman et al., 1995; Ball, 2005; Baker, 2008). Deficiencies in trp and its derivatives, especially nicotinamide, lead to dementia, diarrhea, and dermatitis (i.e., skin rashes) in humans and hamsters (Hegyi et al., 2004; Wan et al., 2010; Tissier et al., 2017), the black-tongue syndrome in dogs (Baker, 2008), and aggressiveness and growth retardation in rats (Krehl et al., 1945; Kantak et al., 1980; Walz et al., 2013). Tissier et al. (2017) recently highlighted that niacin deficiency causes high rates of maternal infanticides in European hamsters fed a diet dominated by maize, reducing reproductive success by up to 70%.
Considering the increase in field size and the amount of maize cultivation, as well as the fact that brown hares select the maize plant as a food source in certain periods of the year (Schai-Braun et al., 2015), and the negative effects of maize dominated diets, we expected with increasing proportions of maize crops in the agricultural landscape
1. Negative effects on niacin concentrations in the liver of brown hares where “de novo biosynthesis” of nicotinic acid solely takes place (Yang and Sauve, 2016), and reserves of this vitamin are stored (Podlogar and Smollich, 2019).
2. Negative effects on hare densities due to detrimental effects of niacin and trp malnutrition on survival and reproductive output of does.
We gathered data at nine study sites in Lower Austria during 2017 and at one site also in 2018 (for more detailed information about each study site, see Supplementary Table 1). From these sites, we selected 226 does for tissue sampling from a total of 1,054 hares shot during regular autumn hunts. Selected animals were shot during our presence. The selection was made to guarantee high-quality, and fresh samples. Of these 226 carcasses, only 117 had intact uteri. We assumed random shooting and hence considered local hunting bags as representative samples of hare populations. Carcasses were sexed by optical inspection of secondary sexual characteristics by experienced biologists. After the first examinations in the field, carcasses were cooled to 2°C. Liver samples (1 g of tissue) and uteri were stored at −18°C until further analyses.
In the laboratory, we determined in liver samples three forms of niacin, free nicotinic acid, free nicotinamide, and nicotinamide moiety of NAD+/NADP+, by gas chromatography with flame ionization detection (Hämmerle et al., 2020). Total niacin concentrations were expressed as the sum of these three forms in μg per g of liver tissue. Reproductive output of does during the preceding season of reproduction was determined by counting placental scars (Hackländer et al., 2001). In situ age classification was verified by the weight of dried eye lenses (Suchentrunk et al., 1991).
Population sizes (individuals per 100 ha) were estimated by spotlight counts (Langbein et al., 1999; Schai-Braun et al., 2013) in spring (late March/early April) and autumn (October) at the nine study sites in Lower Austria during years 2002–2015.
The proportion of arable land cultivated with maize crops was obtained for each study site and year from Statistik Austria (2018).
All statistical analyses were performed using R v4.2.1 (R Core Team, 2021). If necessary for obtaining the normal distribution of residuals, we log-transformed variables. We investigated the effects of the area under maize crops, and of liver vitamin B3 concentrations, on the difference between spring and autumn counts of hares with linear mixed effects models [lme, package “nlme”; Pinheiro et al. (2021)]. The study area was included in these models as a random effect to account for repeated measurements at one site. To identify a potential effect of maize monoculture on the reproductive output of does, independent of the effect of niacin concentration in the liver, we calculated a generalized linear mixed effects model with family “poisson” (glmer, package “lme4; Bates et al. (2015). The response variable was in this model the number of uterine scars. The concentration of niacin in the liver and proportion of area under maize crop were entered as fixed effects, study area as random effect. Plots were generated using the “ggplot2” package (Wickham, 2016) with a back transformation of logarithmized values.
The proportion of area under maize crops of total arable land at our study sites had a significant negative effect on the development of the local hare populations (Figure 1, p < 0.005, t-value = −2.872, R2 = 0.063). For every 10% increase of the area under maize crops, the difference between spring and autumn counts of hares decreased on average by 2.2 hares per 100 ha. To some degree, this negative influence was caused by an increasingly lower fecundity of does. The more maize was cultivated at a study site, the lower the number of uterine scars found in does shot there (Figure 2, glmer: p = 0.002, z-value = −3.097, R2 = 0.083).
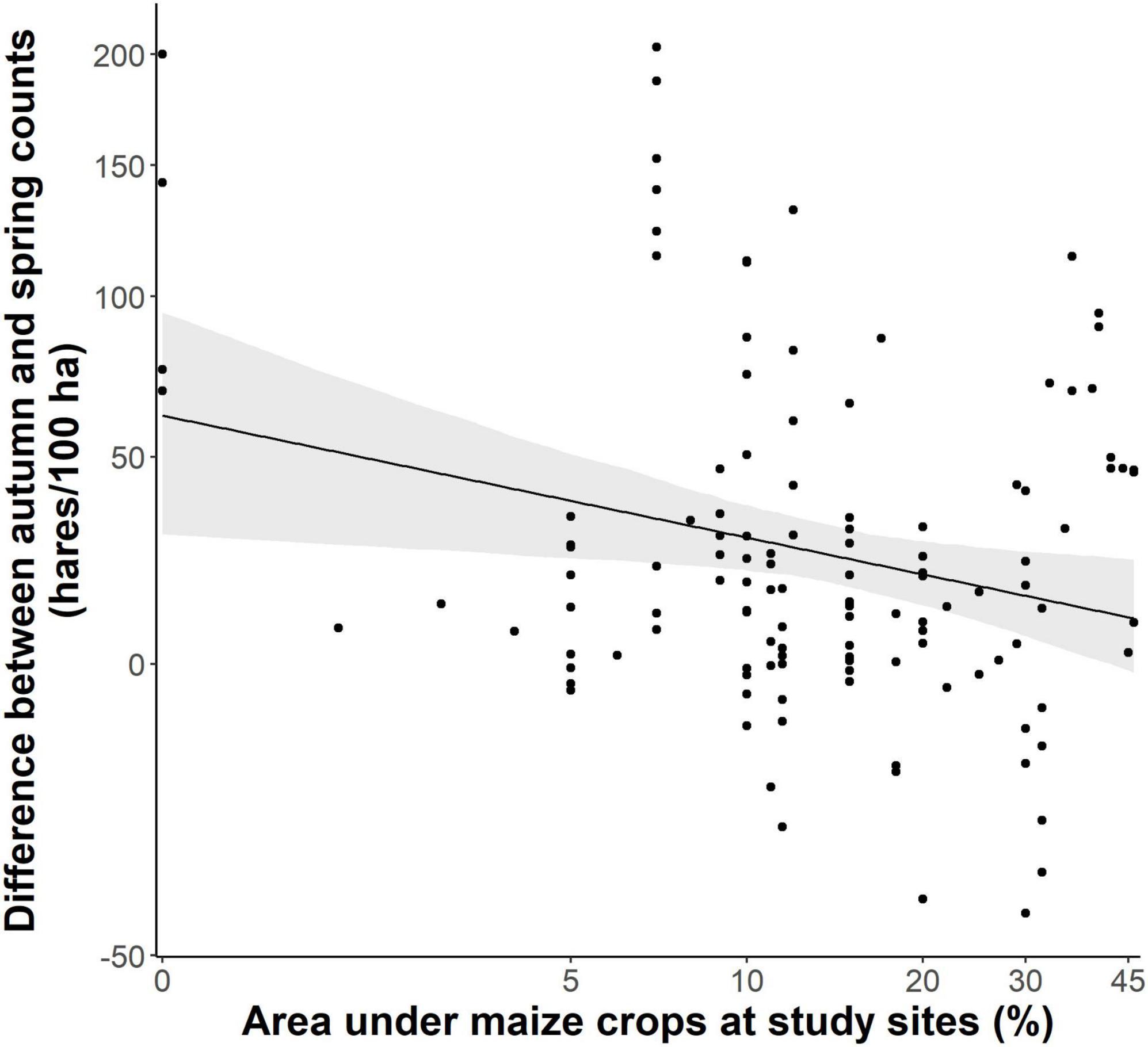
Figure 1. Proportion of maize of total arable land and differences between autumn and spring population counts of hares per 100 ha (light shaded: 95% confidence interval). Both variables were transformed with natural logarithm for analysis and back-transformed for the plot.
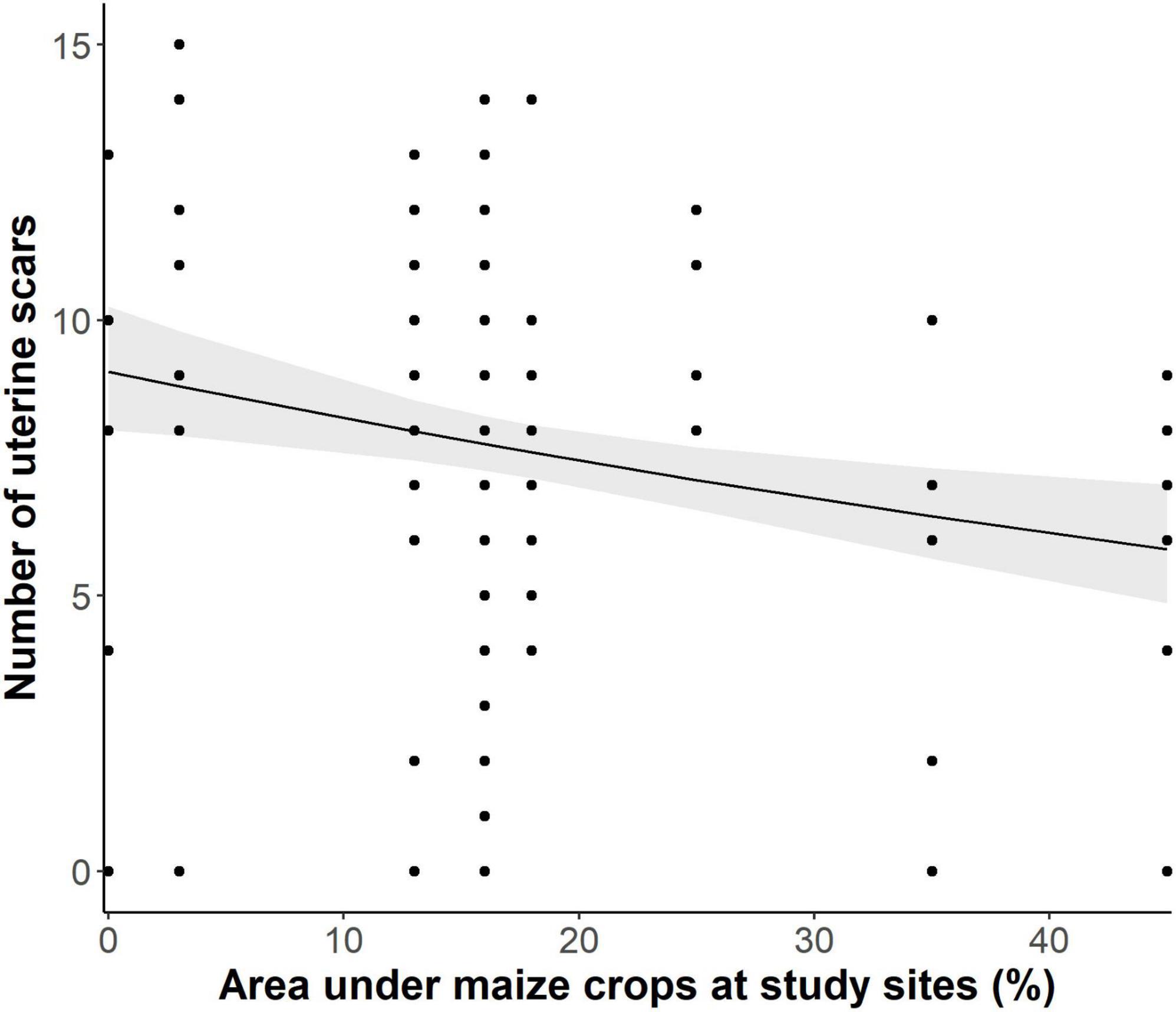
Figure 2. Proportion of maize of total arable land and numbers of uterine scars as a measure of fecundity, i.e., total reproductive output of a female during the preceding period of reproduction (light shaded: 95% confidence interval).
We further found that the proportions of arable land used for growing maize had a significant negative relation with niacin concentrations in liver samples of does living there (Figure 3, p = 0.013, t-value = −2.497, R2 = 0.182), with a decrease of on average 0.97 μg/g with every 10% increase of the area under maize crops. In line with these results, we also found a negative association between niacin concentrations in livers and the population development of local hare populations (Figure 4, p = 0.018, t-value = 2.627, R2 = 0.257). However, a direct effect of niacin deficiency on the number of uterine scars, independent of other potential effects of maize abundance, was statistically not detectable (partial regression coefficient −0.008, z-value = −0.934, p = 0.346). Conversely, the negative effect of the proportion of arable land used for maize production on the reproductive output of does remained significant when adjusting for a direct effect of niacin (partial regression coefficient −0.01, z-value = −3.186, p = 0.001), suggesting that other aspects of maize cultivation were also detrimental, on top of an undersupply of niacin.
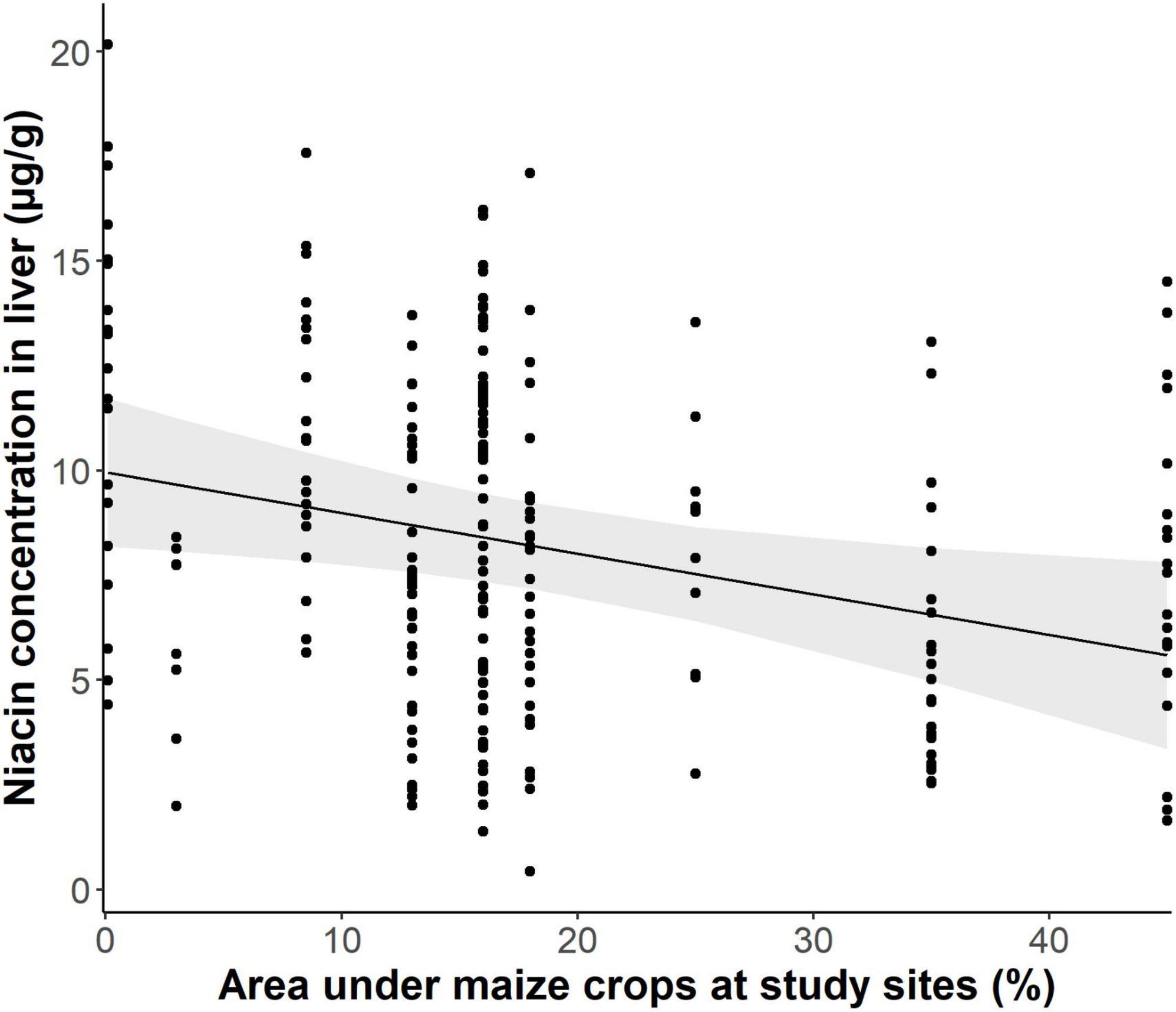
Figure 3. Proportion of maize of total arable land and concentrations of niacin in liver samples (light shaded: 95% confidence interval).
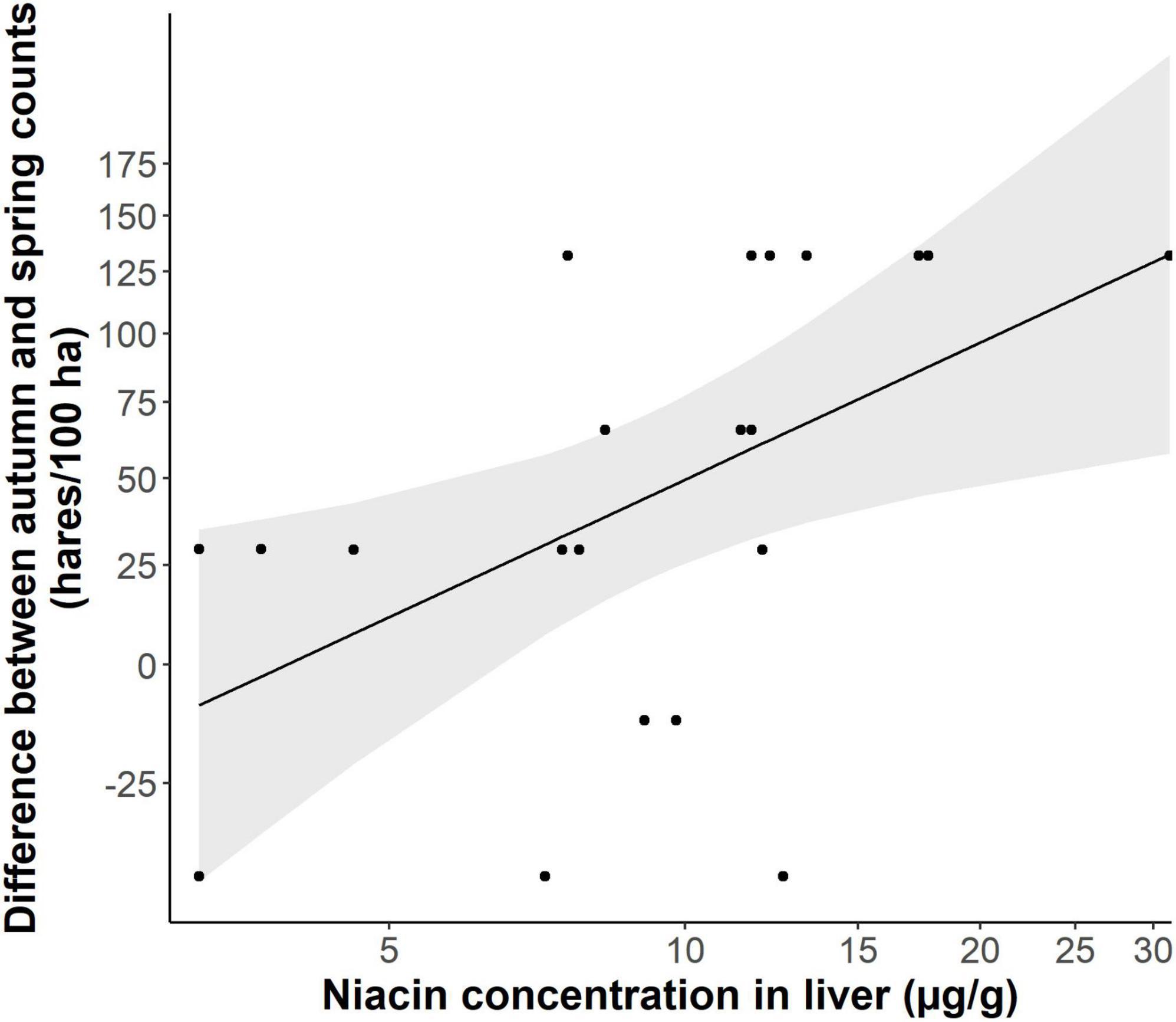
Figure 4. Differences between autumn and spring population counts of hares and its relation to the liver concentrations of niacin found at a study site (light shaded: 95% confidence interval). Both variables were transformed with natural logarithms for analysis and back-transformed for the plot.
In this study, we discovered several important effects of maize monoculture on local brown hare populations. Our results show that populations rather declined from spring to autumn, or grew less, the higher the proportion of maize crops on total arable land was. A similar negative effect of maize cultures on brown hare abundance has been reported by Mayer and Sunde (2020). Potential causes could be temporal migration of hares and a shift or increase in their home ranges after harvest events, as described by Ullmann et al. (2018) and Schai-Braun and Hackländer (2014). However, a recent study by Ullmann et al. (2020) showed, based on telemetry data, that hares specifically shift their home ranges toward maize fields after the harvest of maize. These areas are selected because of potential cover and forage (Mayer et al., 2018). It therefore seems unlikely that dispersal contributes significantly to the change of hare abundance in a given area from spring to autumn. Instead, our findings suggest that the detrimental effects of maize monoculture on survival and reproduction are responsible.
Brown hares select plants rich in fat and protein and strongly rely on weeds (Reichlin et al., 2006). Similar feeding behavior was also observed in the Mountain hare (Lepus timidus, Linnaeus 1758) (Dingerkus and Montgomery, 2001), in the Arctic hare (Lepus arcticus, Ross 1819) (Klein and Bay, 1994), in the Italian hare (Lepus corsicanus, de Winton 1898) (Rizzardini et al., 2019), and the Granada hare (Lepus granatensis, Rosenhauer 1856) (Paupério and Alves, 2008). Therefore, brown hares likely suffer malnutrition when living in areas with monocultures poor in plant biodiversity (Schai-Braun et al., 2015). It is well known that malnutrition negatively impacts body condition and hence the survival of brown hares (Hansen, 1992; Edwards et al., 2000). Further, malnutrition impairs reproductive success, because lactating does experience high energetic costs. Particularly for nursing litters in spring, does rely to a certain degree on energy reserves stored in body fat depots (Parkes, 1989; Valencak et al., 2009). Female hares nurse their offspring only once a day (Broekhuizen and Maaskamp, 1980). Further, leverets lack shelters like burrows and are therefore exposed to unfavorable weather conditions, particularly in spring (Andersen, 1952; Broekhuizen and Maaskamp, 1980; Hackländer et al., 2002; Karp and Gehr, 2020). It is therefore pivotal for leverets to obtain milk with a high energy content. To meet this requirement, does produce milk with a high-fat content, which in spring is even fatter than average (30% vs. 20%, Valencak et al., 2009). Sufficient plant biodiversity is absent in areas of maize monoculture even after harvest in autumn and winter (Fuchs et al., 2021), presumably resulting in malnutrition of does during the period when they accumulate body fat for subsequent reproduction. This reduces the survival chances of leverets, particularly in spring litters, which are most important for population development (Olesen and Asferg, 2006).
We also found a decrease in reproductive output of does with the proportion of maize in arable land. Our measure of reproductive output is the number of scars found in the uteri of does shot during the autumn hunting season. These numbers reflect the total number of juveniles born by a doe during the preceding season of reproduction (Hackländer et al., 2001), and are therefore a measure of female fecundity. Numbers of uterine scars could be affected either by direct negative effects of the area of maize monoculture on brown hare nutrition, and therefore body condition, or by general detrimental effects of monoculture. However, a more likely explanation for the effects on uterine scars is changes in the age structure of a population, because we did not find a direct effect of niacin stored in the liver on the number of uterine scars. Schai-Braun et al. (2021) showed that heavier does have larger litters. The same study reported that primiparous does, which are lighter, had smaller litters than an adult does. Unfortunately, we have no reliable information about the age structure of females at our study sites to confirm that a higher number of juvenile does in the bag was responsible for lower numbers of uterine scars. Alternatively, the direct effects of niacin stores on the reproductive output of does could have remained undetected due to the small sample size. From 223 uteri gathered, only 117 were suitable for analysis, and few were obtained from areas with no maize monoculture or with high proportions of maize fields (n = 5 and n = 10 respectively).
For the first time, we identified here that maize monoculture is detrimental to brown hare populations because it leads to niacin deficiency. The concentration of this vitamin in liver tissue of does decline with the area under maize crops. The concentration of niacin is very low in maize. Further, niacin in maize is mainly present in its bound form, rendering it inaccessible to vertebrates (Ammerman et al., 1995; Ball, 2005; Baker, 2008). In addition to the low content of niacin, maize is also poor in its precursor tryptophan (Carpenter, 1983). Consumption of maize therefore also impairs the potential, for an inefficient pathway of biosynthesis of niacin from tryptophan. Fragments of maize plants were found in the stomach contents of brown hares even at low proportions of areas under maize crops, showing that brown hares readily consume maize plants as well as corn (Steineck, 1978; Reichlin et al., 2006).
Diets with maize as a major component can lead to severe health problems. In humans, for instance, such a diet causes the pathology of “pellagra” (WHO [World Health Organization], 2000). In European hamsters (Cricetus, Linnaeus 1758), less than 12% of pups born to mothers fed a niacin deficient diet survived a 30-day period (Tissier et al., 2017). As a reason for infanticide and insufficient maternal care, Tissier et al. (2017) suggested low levels of oxytocin concentrations in the blood of the mothers, caused by niacin deficiency, as this peptide, which acts as a neurotransmitter, plays an important role in maternal and sexual behavior. Stronger effects of maize diets on niacin deficiency on the reproductive output of hamsters could be a result of less pronounced “caecotrophy” compared to hares (National Research Council [NRC], 1995; Weinhold and Kayser, 2006; Miśta et al., 2015). If niacin deficiency has similar effects in hares, does could show abnormal maternal behavior and give up nursing. As a result, offspring survival would be jeopardized because leverets strongly rely on milk supply during the first three weeks of life (Hackländer et al., 2002). Keeping in mind that hare offspring require daily nursing with milk rich in fat, as described above, additional negative effects of niacin deficiency on the fat content of milk, as reported by Havlin et al. (2017), could have further impaired offspring survival.
Our study clearly identified the negative impacts of maize monoculture on population dynamics and reproductive output of European brown hares. Most importantly, we discovered that niacin deficiency, resulting from the consumption of maize, is a likely, so far unknown mediator. However, whether a maize-dominated diet results in hares in similar severe health issues and impairment of reproduction like in other mammals remains an open question. In contrast to humans and mice, brown hares regularly consume soft feces with caecum content, a nutritional pathway making nutrients and vitamins produced by caecal microorganisms available to the host. Therefore, brown hares may be less affected than other species by consuming a maize-dominated diet, a hypothesis that needs to be investigated experimentally.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because samples analysed in this study were taken from legally shot animals during seasonal hunts according to the hunting laws in Austria.
WA and MT conceived the study. AS performed field work. WA and AS analyzed the data. AS, WA, and MT wrote the manuscript. All authors approved the final version and are accountable for all aspects of the work.
We thank the Austrian Federal Ministry of Education, Science and Culture, the Austrian hunting associations, the City of Vienna, the province of Lower Austria, and the Academy for the Protection of Zoo Animals and Wildlife for financial support.
We thank our colleagues Anna Kübber-Heiss and Annika Posautz for help with counting uterine scars, Michael Hämmerle, Minh Hien Le for help with analyzing niacin in liver samples, and Renate Hengsberger for help with manuscript formatting. We also thank the hunting association of Lower Austria and all hunters that helped us with gathering of samples and counting brown hares.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1017691/full#supplementary-material
Ammerman, C. B., Baker, D. H., and Lewis, A. J. (1995). Bioavailability of nutrients for animals. Amino acids, minerals, and vitamins. San Diego, CA: Academic Press, 441.
Amon, T., Amon, B., Kryvoruchko, V., Zollitsch, W., Mayer, K., and Gruber, L. (2007). Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 118, 173–182. doi: 10.1016/j.agee.2006.05.007
Andersen, J. (1952). Fluctuations in the field hare population in Denmark compared with certain climatic factors. Pap. Game Res. 8, 41–43.
Baker, D. H. (2008). Animal models in nutrition research. J. Nutr. 138, 391–396. doi: 10.1177/011542659200700137
Ball, G. F. M. (2005). Vitamins in foods. Analysis, bioavailability, and stability. Boca Raton, FL: CRC Press, 824.
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bertóti, I. (1975). The effect of modern corn production system on hare production [in Hungarian]. A Vadgazdalkodas Fejlesztese 15, 33–41.
Broekhuizen, S., and Maaskamp, F. (1980). Behaviour of does and leverets of the European hare (Lepus europaeus) whilst nursing. J. Zool. 191, 487–501. doi: 10.1111/j.1469-7998.1980.tb01480.x
Carpenter, K. J. (1983). “The relationship of pellagra to corn and the low availability of niacin in cereals,” in Nutritional adequacy, nutrient availability and needs: Nestlé nutrition research symposium, Vevey, September 14–15, 1982, ed. J. Mauron (Basel: Birkhäuser), 197–222. doi: 10.1007/978-3-0348-6540-1_12
de Groot, A. P. (1953). “Protein and amino acid requirements of the honeybee (Apis mellifica L.),” in Proceedings of the 67th communication of the research institute for animal husbandry, T.N.O, (The Hague: W. Junk), 199–285.
Dingerkus, S. K., and Montgomery, W. I. (2001). The diet and landclass affinities of the Irish hare Lepus timidus hibernicus. J. Zool. 253, 233–240. doi: 10.1017/S0952836901000206
Edwards, P. J., Fletcher, M. R., and Berny, P. (2000). Review of the factors affecting the decline of the European brown hare, Lepus europaeus (Pallas, 1778) and the use of wildlife incident data to evaluate the significance of paraquat. Agric. Ecosyst. Environ. 79, 95–103. doi: 10.1016/S0167-8809(99)00153-X
Fargione, J. E., Cooper, T. R., Flaspohler, D. J., Hill, J., Lehman, C., McCoy, T., et al. (2009). Bioenergy and Wildlife: Threats and opportunities for grassland conservation. BioScience 59, 767–777. doi: 10.1525/bio.2009.59.9.8
Frylestam, B. (1986). Agricultural land use effect on the winter diet of Brown hare (Lepus europaeus Pallas) in southern Sweden. Mamm. Rev. 16, 157–161. doi: 10.1111/j.1365-2907.1986.tb00037.x
Fuchs, A., Berger, V., Steinbauer, K., Köstl, T., Wuttej, D., and Jungmeier, M. (2021). The long-term effects of monoculture maize cultivation on plant diversity. Phytocoenologia 50, 397–408. doi: 10.1127/phyto/2021/0382
Gaba, S., Gabriel, E., Chadœuf, J., Bonneu, F., and Bretagnolle, V. (2016). Herbicides do not ensure for higher wheat yield, but eliminate rare plant species. Sci. Rep. 6:30112. doi: 10.1038/srep30112
Gevers, J., Høye, T. T., Topping, C. J., Glemnitz, M., and Schröder, B. (2011). Biodiversity and the mitigation of climate change through bioenergy: Impacts of increased maize cultivation on farmland wildlife. Glob. Change Biol. Bioenergy 3, 472–482. doi: 10.1111/j.1757-1707.2011.01104.x
Goss, J. A. (1968). Development, physiology, and biochemistry of corn and wheat pollen. Bot. Rev. 34, 333–358.
Hackländer, K., Arnold, W., and Ruf, T. (2002). Postnatal development and thermoregulation in the precocial European hare (Lepus europaeus). J. Comp. Physiol. B 172, 183–190. doi: 10.1007/s00360-001-0243-y
Hackländer, K., Frisch, C., Klansek, E., Steineck, T., and Ruf, T. (2001). Die Fruchtbarkeit weiblicher Feldhasen (Lepus europaeus) aus Revieren mit unterschiedlicher Populationsdichte. Z. Jagdwiss. 47, 100–110. doi: 10.1007/BF02239822
Hämmerle, M., Le, M. H., and Hekmat, O. (2020). GC-FID-based quantification of the sum of the three forms of vitamin B3 from animal liver. Anal. Biochem. 601:113778. doi: 10.1016/j.ab.2020.113778
Havlin, J. M., Robinson, P. H., and Garrett, J. E. (2017). Niacin feeding to fresh dairy cows: Immediate effects on health and milk production. Anim. Prod. Sci. 57, 1069–1078. doi: 10.1071/AN15419
Hegyi, J., Schwartz, R. A., and Hegyi, V. (2004). Pellagra: Dermatitis, dementia, and diarrhea. Int. J. Dermatol. 43, 1–5. doi: 10.1111/j.1365-4632.2004.01959.x
Henderson, L. M., Someroski, J. F., Rao, D. R., Wu, P. H. L., Griffith, T., and Byerrum, R. U. (1959). Lack of a tryptophan-niacin relationship in corn and tobacco. J. Biol. Chem. 234, 93–95.
Hogan, A. G., Gillespie, G. T., Koçtürk, O., O’Dell, B. L., and Flynn, L. M. (1955). The percentage of protein in corn and its nutritional properties. J. Nutr. 57, 225–239. doi: 10.1093/jn/57.2.225
INRA, CIRAD, AFZ, and FAO (2011). Feedipedia. Animal feed resources information system. Available Online at: http://www.feedipedia.org/ (accessed August 1, 2022).
Kalchreuter, H. (2015). Die Sache mit der Jagd. Perspektiven für die Zukunft des Waidwerks, Vol. 6. Nashville, TN: Kosmos, 560.
Kantak, K. M., Hegstrand, L. R., and Eichelman, B. (1980). Dietary tryptophan modulation and aggressive-behavior in mice. Pharmacol. Biochem. Behav. 12, 675–679. doi: 10.1016/0091-3057(80)90147-1
Karp, D., and Gehr, B. (2020). Bad hare day: Very low survival rate in brown hare leverets. Wildlife Biol. 2020:00645. doi: 10.2981/wlb.00645
Klein, D. R., and Bay, C. (1994). Resource partitioning by mammalian herbivores in the high Arctic. Oecologia 97, 439–450. doi: 10.1007/BF00325880
Klenke, R., Frey, B., and Zarzycka, A. (2017). “Case study 5: The effects of increased rape and maize cropping on agricultural biodiversity,” in Service contract to support follow-up actions to the mid-term review of the EU biodiversity strategy to 2020 in relation to target 3A – agriculture. Report to the European commission, eds G. Siriwardena and G. Tucker (London: Institute for European Environmental Policy), 147–183.
Kohlmeier, M. (2003). Nutrient metabolism. A volume in food science and technology. Cambridge, MA: Academic Press, 840.
Krehl, W. A., Teply, L. J., Sarma, P. S., and Elvehjem, C. A. (1945). Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophane. Science 101, 489–490. doi: 10.1126/science.101.2628.489
Langbein, J., Hutchings, M. R., Harris, S., Stoate, C., Tapper, S. C., and Wray, S. (1999). Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mamm. Rev. 29, 93–116. doi: 10.1046/j.1365-2907.1999.00040.x
Madsen, J., Asferg, T., Clausager, I., and Noer, H. (1996). Status og jagttider for danske vildtarter, TEMA-rapport fra DMU. Aarhus: Aarhus Universitet.
Mary, C., and Trouvilliez, J. (1995). Special lièvre d’Europe. Bull. Mens. Off. Natl. Chasse 204:96.
Mawson, A. R., and Jacobs, K. W. (1978). Corn consumption, tryptophan, and cross-national homicide rates. J. Orthomol. Psychiatry 7, 227–230.
Mayer, M., and Sunde, P. (2020). The role of maize cultivation on European hare abundance. Agric. Ecosyst. Environ. 295:106909. doi: 10.1016/j.agee.2020.106909
Mayer, M., Ullmann, W., Sunde, P., Fischer, C., and Blaum, N. (2018). Habitat selection by the European hare in arable landscapes: The importance of small-scale habitat structure for conservation. Ecol. Evol. 8, 11619–11633. doi: 10.1002/ece3.4613
Meisinger, D. J. (1978). The tryptophan requirement for reproduction in swine. Dr. phil Dissertation. Ames, IA: Iowa State University.
Miśta, D., Króliczewska, B., Marounek, M., Pecka, E., Zawadzki, W., and Nicpoń, J. (2015). In Vitro study and comparison of caecal methanogenesis and fermentation pattern in the brown hare (Lepus europaeus) and Domestic Rabbit (Oryctolagus cuniculus). PLoS One 10:e0117117. doi: 10.1371/journal.pone.0117117
National Research Council [NRC] (1995). “Nutrient requirements of the hamster,” in Proceedings of the nutrient requirements of laboratory animals: Fourth revised edition, 1995, (Washington, DC: National Academies Press), 125–139.
Nielsen, J. B. H., and Oleskowicz-Popiel, P. (2008). “Biogas – a promising renewable energy source for Europe,” in Proceedings of the AEBIOM workshop - European parliament, Brussels.
Nuss, E. T., and Tanumihardjo, S. A. (2010). Maize: A paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 9, 417–436. doi: 10.1111/j.1541-4337.2010.00117.x
Olesen, C. R., and Asferg, T. (2006). Assessing potential causes for the population decline of European brown hare in the agricultural landscape of Europe - review of the current knowledge”, in: NERI Technical Report. Denmark: National Environmental Research Institute.
Panek, M., Kamieniarz, R., and Bresiński, W. (2006). The effect of experimental removal of red foxes Vulpes vulpes on spring density of brown hares Lepus europaeus in western Poland. Acta Theriol. 51, 187–193. doi: 10.1007/BF03192670
Parkes, J. P. (1989). Annual patterns in reproduction and perirenal fat of hares (Lepus europaeus) in sub-alpine Canterbury, New Zealand. J. Zool. 217, 9–21. doi: 10.1111/j.1469-7998.1989.tb02471.x
Paupério, J., and Alves, P. C. (2008). Diet of the Iberian hare (Lepus granatensis) in a mountain ecosystem. Eur. J. Wildl. Res. 54, 571–579. doi: 10.1007/s10344-008-0181-z
Petrov, P. (1976). “Über die Faktoren die den realen Zuwachs des Hasen bestimmen,” in Ecology and management of European hare populations, eds Z. Pielowski and Z. Pucek (Warszawa: Polish Hunting Association), 119–126.
Petrovan, S. O., Ward, A. I., and Wheeler, P. M. (2012). Habitat selection guiding agri-environment schemes for a farmland specialist, the brown hare. Anim. Conserv. 16, 344–352. doi: 10.1111/acv.12002
Pielowski, Z. (1976). “On the present state and perspectives of the European hare breeding in Poland,” in Ecology and management of European hare populations, eds Z. Pielowski and Z. Pucek (Warszawa: Polish Hunting Association), 25–27.
Pielowski, Z., and Raczynski, J. (1976). “Ecological conditions and rational management of hare populations,” in Ecology and management of European hare populations, eds Z. Pielowski and Z. Pucek (Warszawa: Polish Hunting Association), 269–286.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Eispack, Heisterkamp, S., et al. (2021). nlme: Linear and nonlinear mixed effects models (R Package Version 3.1-158). Available Online at: https://CRAN.R-project.org/package=nlme (accessed August 1, 2022).
Pintur, K., Popović, N., Alegro, A., Severin, K., Slavica, A., and Kolić, E. (2006). Selected indicators of brown hare (Lepus europaeus Pallas, 1778) population dynamics in Northwestern Croatia. Vet. Arh. 76(Suppl.), S199–S209.
Podlogar, J., and Smollich, M. (2019). Vitamine-mineralstoffe–spurenelemente. beratungswissen für die apothekenpraxis. Gerlingen: Deutscher Apotheker Verlag, 94.
Popović, N., Pintur, K., Alegro, A., Slavica, A., Lacković, M., and Sertić, D. (2008). Temporal changes in the status of the European hare (Lepus europaeus Pallas, 1778) population of Međimurje, Croatia. Nat. Croat. 17, 247–257.
R Core Team (2021). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Reichlin, T., Klansek, E., and Hackländer, K. (2006). Diet selection by hares (Lepus europaeus) in arable land and its implications for habitat management. Eur. J. Wildl. Res. 52, 109–118. doi: 10.1007/s10344-005-0013-3
Reynolds, J. C., and Tapper, S. C. (1995). Predation by foxes Vulpes vulpes on brown hares Lepus europaeus in central southern England, and its potential impact on annual population growth. Wildlife Biol. 1, 145–158. doi: 10.2981/wlb.1995.019
Ristić, Z., Ponjiger, I., Matejević, M., Kovačević, M., Ristić, N., and Marković, V. (2021). Effects of factors associated with the decline of brown hare abundance in the Vojvodina region (Serbia). Hystrix 32, 67–71. doi: 10.4404/hystrix-00334-2020
Rizzardini, G., Fascetti, S., Pietri, C., Riga, F., Cosentino, C., and Freschi, P. (2019). Feeding preferences in dry season of the Italian hare (Lepus corsicanus) in two sites of Corsica. Eur. J. Wildl. Res. 65:43. doi: 10.1007/s10344-019-1284-4
Schai-Braun, S. C., and Hackländer, K. (2014). Home range use by the European hare (Lepus europaeus) in a structurally diverse agricultural landscape analysed at a fine temporal scale. Acta Theriol. 59, 277–287. doi: 10.1007/s13364-013-0162-9
Schai-Braun, S. C., Reichlin, T. S., Ruf, T., Klansek, E., Tataruch, F., Arnold, W., et al. (2015). The European hare (Lepus europaeus): A picky herbivore searching for plant parts rich in fat. PLoS One 10:e0134278. doi: 10.1371/journal.pone.0134278
Schai-Braun, S. C., Ruf, T., Klansek, E., Arnold, W., and Hackländer, K. (2020). Positive effects of set-asides on European hare (Lepus europaeus) populations: Leverets benefit from an enhanced survival rate. Biol. Conserv. 244:108518. doi: 10.1016/j.biocon.2020.108518
Schai-Braun, S. C., Steiger, P., Ruf, T., Arnold, W., and Hackländer, K. (2021). Maternal effects on reproduction in the precocial European hare (Lepus europaeus). PLoS One 16:e0247174. doi: 10.1371/journal.pone.0247174
Schai-Braun, S. C., Weber, D., and Hackländer, K. (2013). Spring and autumn habitat preferences of active European hares (Lepus europaeus) in an agricultural area with low hare density. Eur. J. Wildl. Res. 59, 387–397. doi: 10.1007/s10344-012-0684-5
Sliwinski, K., Ronnenberg, K., Jung, K., Strauß, E., and Siebert, U. (2019). Habitat requirements of the European brown hare (Lepus europaeus Pallas 1778) in an intensively used agriculture region (Lower Saxony, Germany). BMC Ecol. 19:31. doi: 10.1186/s12898-019-0247-7
Smith, R. K., Jennings, N. V., and Harris, S. (2005). A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mamm. Rev. 35, 1–24. doi: 10.1111/j.1365-2907.2005.00057.x
Steineck, T. (1978). Die botanische Zusammensetzung des Mageninhaltes bei Feldhasen (Lepus europaeus P.). Dr. med. vet. Dissertation. Budapest: University of Veterinary Medicine.
Stoate, C., Boatman, N. D., Borralho, R. J., Carvalho, C. R., Snoo, G. R. D., and Eden, P. (2001). Ecological impacts of arable intensification in Europe. J. Environ. Manag. 63, 337–365. doi: 10.1006/jema.2001.0473
Suchentrunk, F., Willing, R., and Hartl, G. B. (1991). On eye lens weights and other age criteria of the Brown hare (Lepus europaeus Pallas, 1778). Z. Säugetierkd. 56, 365–374.
Tapper, S., and Parsons, N. (1984). The changing status of the Brown hare (Lepus capensis L.) in Britain. Mamm. Rev. 14, 57–70. doi: 10.1111/j.1365-2907.1984.tb00339.x
Tissier, M. L., Handrich, Y., Dallongeville, O., Robin, J.-P., and Habold, C. (2017). Diets derived from maize monoculture cause maternal infanticides in the endangered European hamster due to a vitamin B3 deficiency. Proc. R. Soc. B Biol. Sci. 284:20162168. doi: 10.1098/rspb.2016.2168
Ullmann, W., Fischer, C., Kramer-Schadt, S., Pirhofer-Walzl, K., Glemnitz, M., and Blaum, N. (2020). How do agricultural practices affect the movement behaviour of European brown hares (Lepus europaeus)? Agric. Ecosyst. Environ. 292:106819. doi: 10.1016/j.agee.2020.106819
Ullmann, W., Fischer, C., Pirhofer-Walzl, K., Kramer-Schadt, S., and Blaum, N. (2018). Spatiotemporal variability in resources affects herbivore home range formation in structurally contrasting and unpredictable agricultural landscapes. Landsc. Ecol. 33, 1505–1517. doi: 10.1007/s10980-018-0676-2
Valencak, T. G., Tataruch, F., and Ruf, T. (2009). Peak energy turnover in lactating European hares: The role of fat reserves. J. Exp. Biol. 212(Pt 2), 231–237. doi: 10.1242/jeb.022640
Walz, J. C., Stertz, L., Fijtman, A., dos Santos, B. T. M. Q., and de Almeida, R. M. M. (2013). Tryptophan diet reduces aggressive behavior in male mice. Psychol. Neurosci. 6, 397–401. doi: 10.3922/j.psns.2013.3.18
Wan, P., Moat, S., and Anstey, A. (2010). Pellagra: A review with emphasis on photosensitivity. Br. J. Dermatol. 164, 1188–1200. doi: 10.1111/j.1365-2133.2010.10163.x
Weber, D., Roth, T., and Kohli, L. (2019). Increasing brown hare (Lepus europaeus) densities in farmland without predator culling: Results of a field experiment in Switzerland. Eur. J. Wildl. Res. 65:75. doi: 10.1007/s10344-019-1306-2
Weinhold, U., and Kayser, A. (2006). Der Feldhamster. Cricetus cricetus. Hohenwarsleben: Westarp Wissenschaften, 130.
WHO [World Health Organization], Nutrition and Food Safety, and United Nations High Commissions for Refugees (2000). Pellagra and its prevention and control in major emergencies world health organization. Available online at: https://www.who.int/publications/i/item/WHO-NHD-00.10
Wickham, H. (2016). ggplot2. Elegant graphics for data analysis. Available Online at: https://ggplot2.tidyverse.org (accessed August 1, 2022).
Wilson, J. D., Morris, A. J., Arroyo, B. E., Clark, S. C., and Bradbury, R. B. (1999). A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric. Ecosyst. Environ. 75, 13–30. doi: 10.1016/S0167-8809(99)00064-X
Wincentz Jensen, T.-L. (2009). Identifying causes for population decline of the brown hare (Lepus europaeus) in agricultural landscapes in Denmark. Aarhus: Aarhus University.
Keywords: niacin, placental scars, maize crops, Lepus europaeus, monoculture, fecundity, population development
Citation: Selimovic A, Tissier ML and Arnold W (2022) Maize monoculture causes niacin deficiency in free-living European brown hares and impairs local population development. Front. Ecol. Evol. 10:1017691. doi: 10.3389/fevo.2022.1017691
Received: 12 August 2022; Accepted: 21 September 2022;
Published: 12 October 2022.
Edited by:
Maria K. Oosthuizen, University of Pretoria, South AfricaReviewed by:
Pierangelo Freschi, University of Basilicata, ItalyCopyright © 2022 Selimovic, Tissier and Arnold. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aldin Selimovic, QWxkaW4uU2VsaW1vdmljQHZldG1lZHVuaS5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.