
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 15 September 2022
Sec. Interdisciplinary Climate Studies
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1015468
 Wei Shui1*†
Wei Shui1*† Yuanmeng Liu1†
Yuanmeng Liu1† Cong Jiang2*†
Cong Jiang2*† Xiang Sun1†
Xiang Sun1† Xiaomei Jian3
Xiaomei Jian3 Pingping Guo4
Pingping Guo4 Hui Li1
Hui Li1 Sufeng Zhu5
Sufeng Zhu5 Sili Zong1
Sili Zong1 Meiqi Ma1
Meiqi Ma1Karst tiankengs, as one of the most magnificent negative topographies, are capable of forming a bank for species diversity conservation easily. More than 300 karst tiankengs have been discovered and identified worldwide. Given its treacherous terrain, although original karst tiankeng were identified as species refuges, the broader distribution of degraded karst tiankeng has not been systematically studied. Our study area comprised the degraded karst tiankeng cluster immersed in the fragmented karst forests of Yunnan, China. Fifty-eight plant samples were selected from karst tiankengs and surface. We compared species composition, and analyzed diversity indices and similarity coefficients to verify the isolation effect of karst tiankengs on floras. The results indicated that: (1) In the degraded karst tiankeng, there were 24 families, 37 genera and 48 species in the tree layer and 27 families, 43 genera and 49 species in the shrub layer. Outside the degraded karst tiankengs, 20 families, 31 genera and 39 species were in the tree layer, and the shrub layer included 26 families, 44 genera and 55 species. (2) The species composition reached significant differences within and outside degraded karst tiankeng (p < 0.05) based on the analysis of variance (ANOVA). (3) In the degraded karst tiankeng, species richness/diversity in trees were higher than those in the shrub layer, while at the surface, shrubs had higher richness and lower diversity than trees by Alpha-diversity index. And for Beta-diversity index, species similarity among degraded karst tiankengs (0.215) was extremely dissimilar, which was even lower than the contrast within and outside the degraded karst tiankengs (0.272). (4) Shared species ranged from 1 to 5 among the four habitats, with high variability in plant species across the habitat matrices. Through a comparative analysis of systematic biodiversity methods, we found that the degraded karst tiankengs, an independent type of karst tiankeng, are the unreported refugia. Species records in degraded karst tiankeng cluster will contribute to plant diversity conservation and resource management, and to the linkage with broader China’s karst floras. Karst tiankeng botanical habitats possess not only biodiversity value for in situ conservation, but will further support the ecological recovery of surface flora. While its mechanism needs to be further revealed.
Karst tiankengs are extra-large negative karst terrains, with spatial and morphological features such as large volume, steep and circled rock walls, and barrel-shaped contours, with width and depth greater than 100 m, and the bottom is usually attached to an underground river (Zhu et al., 2003). Most of them appear in tropical or subtropical karst areas with abundant precipitations. Karst region in China is distributed over an area of 3.44 million km2, more than 1/3 of the total terrestrial area (Song et al., 2017). More than 300 karst tiankeng have been discovered and identified worldwide. Discussions on karst tiankengs’ value, research on conservation and development have attracted an increasing amount of attention from scholars at home and abroad. Previous studies have pointed out that karst tiankeng can be distinguished from funnels and waterfalls according to their size, form, formation environment, development process and formation mechanism (Shui et al., 2015). Owing to the unique hydrogeological properties of karst environments, fragile non-zonal ecological zones are characterized by limited environmental capacity, weak disturbance tolerance, low stability and poor self-regulation. Thus, its forests tend to be highly vulnerable to destruction (Zhang et al., 2015). In the internal environment of karst tiankeng, the forests matrices different from the surface landscape, and plants usually grow on more fertile soils. Such an environment can be considered a terrestrial island-like system with multiple ecological characteristics (Shui et al., 2015; Jiang et al., 2021). By providing favorable growing conditions for a variety of organisms, karst tiankeng’s unique microclimate potentially serves as an important habitat for certain species under global warming (Redžić et al., 2011; Vilisics et al., 2011; Su et al., 2017). Some findings from the Zhanyi karst tiankeng groups of China’s Yunnan suggest that the flora of karst tiankeng-inside environment underwent stages of pioneering, settlement, competition and adaptation, and eventually evolving into underground forests (Jiang et al., 2022a,b; Shui et al., 2022), presenting unique community characteristics with significant edge effects and trapping effects (Su et al., 2014; Harper Karen et al., 2015). Considerable work has been performed on a wide variety of terrestrial island-like systems grounded in island biogeography theory. Species composition and abundance patterns mechanism on island-like systems (e.g., inselbergs) have been extensively discussed (Mendez-Castro et al., 2021). While karst tiankeng are the magnificent natural landscapes formed along with violent geological movements and are characterized by the attributes of rare, typical, systematic, non-renewable and world-class geological heritages. Research on karst tiankeng will help deepen the understanding of the basic nature of karst action and further realize karst tiankeng’s biodiversity and its mechanisms of community assembly. Hence, karst tiankeng, with unique microclimate features, are also ideal places to explore plant distribution and diversity (Jian et al., 2018).
Driven by human activities and environmental changes, sharp declines in biodiversity have become a global environmental threat (Yang et al., 2003; Kreyling et al., 2010; Thomas, 2013; Kidane Yohannes et al., 2019). Plant diversity, as an important component of biodiversity, remains a core issue for ecologists worldwide (Gao et al., 2008). Contraction of species habitats results in decline of numerous populations, which contributes to a severe decline in plant diversity (Zhang and Han, 2018). Eco-degradation has various adverse effects on the stable development of human society, and the extinction of plant species involves not only their genetic, cultural and scientific value, but also the associated extinctions of 10–30 other species. As an important ingredient of ecological resources, plants potentially contain great value when applied in the future (Sun et al., 2013; Li et al., 2018), which makes the conservation of plant diversity an indispensable effort. Investigations on plant diversity have been continuously pursued at home and abroad. In a Mediterranean study, plant interactions from semiarid to subalpine habitats shaped the structure and composition of plant diversity, and species nesting and regional dispersal of shrubs created a diverse islands (Arroyo et al., 2021). Study of an Mexican coastal ecosystems showed that the invasive species did not affect spatial patterns of species diversity, but rather strongly influence the dominance patterns of species (Parra Tabla et al., 2018). Along the Himalayan gradient in India, elevation is an essential influence on species diversity, emphasizing the importance of species selection and forestation with native species (Sharma and Kala, 2022). Relevant studies have contributed to decision-making for the conservation of plant diversity, and examining the characteristics and diversity of flora in landscapes and habitats is important (Ochoa et al., 2016).
Most of the rare and endangered species assessed in the Red List of Chinese Biodiversity are scattered in various nature reserves, but the value of karst areas, which occupy over 30% of the national territory, is often overlooked (Shui and Xu, 2015). Karst tiankeng are represent the magnificent negative terrain created by the collapse of the surface, usually located in secluded environments with little disturbance and insufficient accessibility. Therefore, field trips in and out of one degraded karst tiankeng can typically be up to 4–6 h/day, and we found that one of the more maturely developed karst tiankengs was covered over 70,000 m2 in size and contained many species with better growth and species richness than the surface area. However, to date, they still have not been fully recognized and valued (Zhu and Waltham, 2006).
The selected study area is the Zhanyi karst tiankeng groups located in the “Kingdom of Plants,” Yunnan, China. Currently, study have revealed that original karst tiankeng are considered as suitable species pool and refuges (Shui et al., 2022). However, there are no systematic studies and reports on negative terrain represented by degraded karst tiankeng, which are more widely distributed and commonly visited. Research on the biodiversity characteristics of a specific karst tiankeng also lacks representation. Furthermore, little association between degraded karst tiankeng and species composition has been made in independent studies of species diversity (Jian et al., 2018; Zhang et al., 2019). Relevant studies on environment–plant relationships have shown that cryptic habitats in karst landscapes have high species diversity, and new taxa have been identified (Parmentier and Hardy, 2009; Li et al., 2020; Peng et al., 2020; Liu et al., 2021; Ren et al., 2021). However, little is known about how the flora composition and diversity varies and what effects that degraded karst tiankengs inserted in a karst forest matrix may exert on these plant communities. In this research, we quantified the species composition and diversity of woody layers and verified whether degraded karst tiankengs serve as a species diversity conservation reservoir.
Is it possible that the existence of karst tiankeng makes biodiversity more higher in the region than elsewhere? Considering the close association between plants and the surrounding matrix in karst areas, and the fact that species are shared within and outside degraded karst tiankeng, we hypothesized that, due to the location of degraded karst tiankeng, which allows the forests to perform differently in terms of species composition and diversity characteristics within and outside degraded karst tiankengs. Our findings will provide useful references and a scientific basis for the ecological restoration of species in nature reserves and other regions with similar habitats.
The Zhanyi Karst Tiankeng Group (25°35′∼ 25°57′ N, 103°29′∼ 103°39′ E) is located in Haifeng Nature Reserve, Yunnan Province (Figure 1). Their geological environments are characterized by varied rocks and stratigraphy, showing the plurality of tectonic systems. The strata are all Paleozoic series except for the Quaternary river, lake and cave accumulations, where carbonates are the main rocks exposed, followed by clastic sandy shales, with a small amount of igneous rocks and modern deposits (Li et al., 2001). Within karst tiankeng group area, soils are in the laterite zone, including red soil, yellow–brown soil, purple soil and limestone soil (Fu et al., 2007). This area has a typical subtropical plateau monsoon climate, an annual average temperature of approximately 14°C and an annual rainfall of 1073.5–1089.7 mm (Shui et al., 2018), The average temperature in karst tiankeng-inside was 1.93°C lower than the surface temperature, which provide an important foundation for a diversity of plant species in regard to hydrothermal conditions.
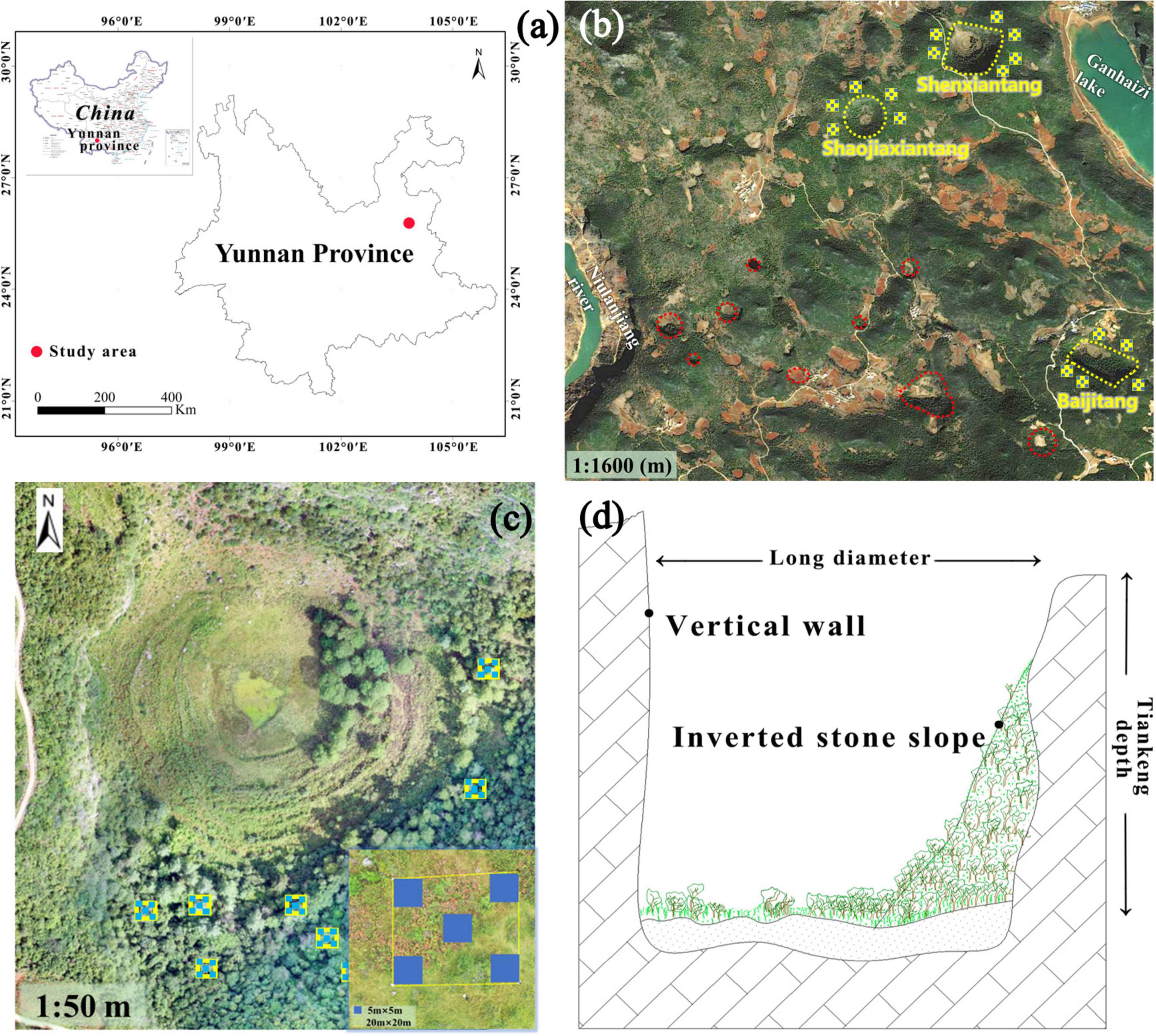
Figure 1. Study region (a,b), top view of karst tiankengs with sample design (c), and longitudinal profile of karst tiankeng (d). (a) Map source: Natural Resources Standard Map Service Review Number: GS(2019)1686. Sampled area are highlighted in yellow (b), among them, circle: karst tiankeng groups, yellow circle: karst tiankeng occurring in the study area, squares: schematic of surface sample.
Our selected degraded karst tiankeng are located in the northeastern part of the karst group. After fieldwork, the area is dotted with dozens of concentrated clusters of karst tiankeng. Not only is it a unique landscape resource in China, but it also provides scientific research sites for ecological and environmental disciplines. With an elevation of 2,013–2,070 m, a measured long diameter of degraded karst tiankeng ranging from 277 to 422 m, a short diameter from 187 to 349 m, and a depth from 105 to 149 m. The bottoms of karst tiankeng, formerly used for farming activities, has been retired and its flora has been restored gradually. The collapsed inverted stone slope, due to the massive pile-up collapse and runoff scouring from the outer slopes of the karst tiankeng, became the “entrance” to karst tiankeng, the community features are mainly characterized by interspersed trees and shrubs. The inverted stone slope, as an ecological staggered zone, represents a hub connecting the interior with external areas (Chen et al., 2018).
Previous visits revealed that obvious stratification of flora in the karst tiankeng group is existed, thus, information on the growth-form definition was cited for plant identification to avoid statistical bias. We examined woody plants in the community, with the tree layer being taller than at least 5 m and having a diameter at breast height (DBH) greater than 5 cm (partially less than 5 cm). The shrub layer was less than 5 m in height and had a DBH below 5 cm (partly above 5 cm). To avoid analytical bias caused by higher number of missing data, herbaceous samples were excluded.
Fifty-eight samples were collected from the study area (Figure 1 and Table 1). Specifically, samples of karst tiankeng were collected from the locally named “Shaojia Xiantang tiankeng” (code: SJXT, 10 samples), “Shenxiantang tiankeng” (code: SXT, 10 samples), and “Baijitang tiankeng” (code: BJT, 10 samples). A total of 20 m × 20 m tree samples were set in the forest area of the inverted stone slopes, and 5 m × 5 m shrub samples were set in each tree sample according to the “plum blossom type.” Based on the survey, 29 samples were selected, with an area of 1.16 ha. Sampling of the surface was based on the distribution of karst tiankeng. We placed tree and shrub samples around karst tiankeng, 29 samples are effective on the surface with an area of 1.16 ha. and detailed identification of plant species were performed in karst tiankengs and surface samples, species categories and number of plants were noted, and geographic information such as latitude, longitude, elevation, slope position, and slope aspects were recorded by handheld Global Positioning System (GPS). To assess the isolation of karst degraded tiankengs, we measured the closest distance between environmental gradients (both within and outside degraded karst tiankengs and between karst degraded tiankeng groups), as measured by the Euclidean distance metric.
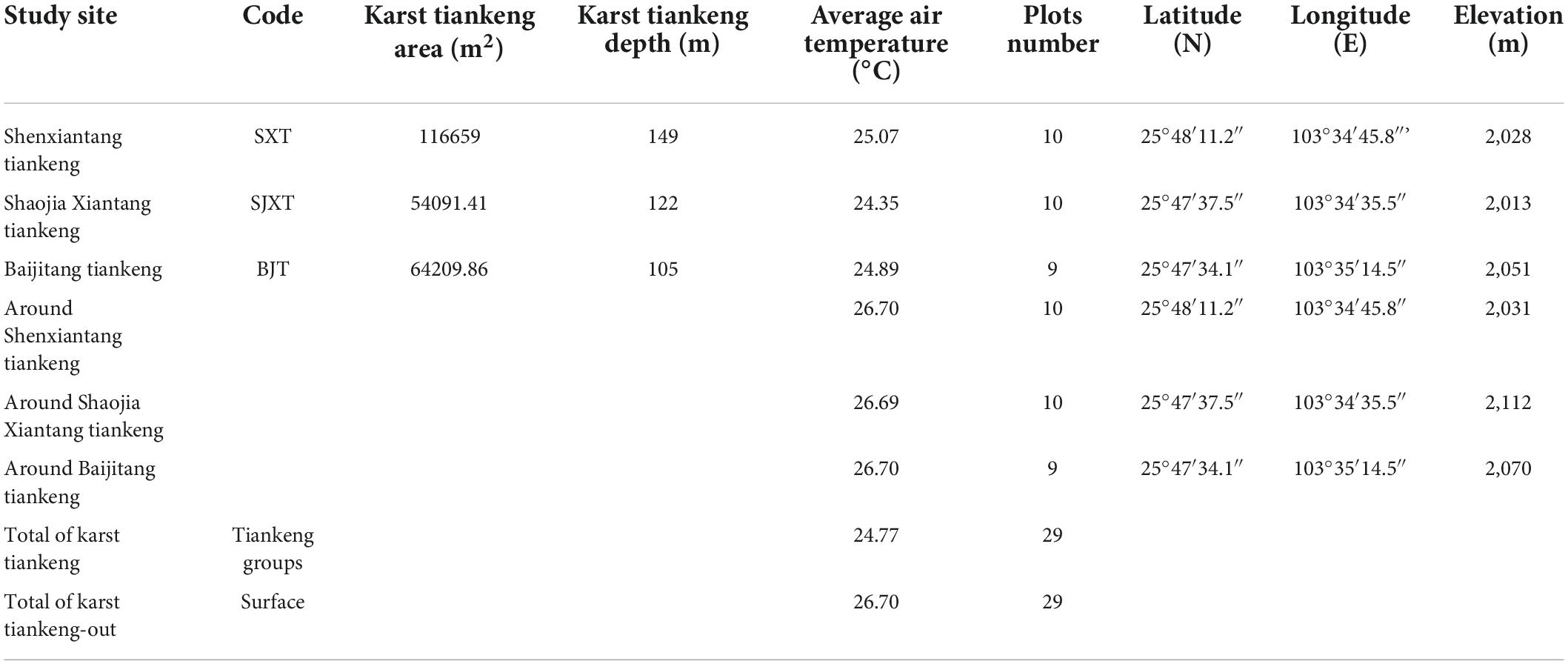
Table 1. List of study sites within and outside the karst tiankeng forests region of southwest China.
Conducting field plant diversity measurements requires the identification of plant species as a decisive matter. Through a preliminary survey, we found that the blooming period of vegetation occurs mostly from April to May, when plants were growing more luxuriantly and leaf buds basically unfolded; hence, we decided to investigate in mid-April.
To compare species composition between habitats and distinguish the variation in trees and shrubs within and outside degraded karst tiankeng, all woody plant taxonomic information recorded from fieldwork was analyzed by the OmicStudio platform based on the UpSet package in R. Subsequently, the species recorded at the four sites were numbered, and taking the analysis of variance (ANOVA) with the species (tiankeng vs. surface) as a treatment and site (four habitats) as a random blocking variable. However, the tree data did not meet the ANOVA requirements. Specifically, the tree data did not passed the normality test and the chi-square test; the shrub data passed the normality test (by Shapiro test, p = 0.001) and the chi-square test (by Bartlett test, p = 0.28). Therefore, we used the permutation test to obtain p-values. Then, difference in species composition between groups was visualized using boxplert.R (R Core Team, 2018).
The alpha-diversity index refers to the variety in a community or habitat characterized within a spatial unit, including the species diversity index, species richness index, species evenness index, and species dominance index (Chen et al., 2018). We used the Shannon–Wiener index to indicate species diversity. Species richness was calculated by the Margalef index, and evenness and dominance were calculated by the Pielou index and Simpson index, respectively. For clarifying the differences of species abundance (Romanuk and Kolasa, 2002), we further compared the species distributions within and outside degraded karst tiankengs by taking the cumulative distribution function of experience (ECDF). And comparing the variation in species abundance by the Wilcoxon rank sum test.
The beta-diversity index represents the simplest way to determine species differences among communities and is generally combined with the similarity coefficient (Jian et al., 2018). The Whittaker index, Jaccard index, and Sørenson index are the most widely used, suggesting differences in species composition among communities under environmental gradients. The value of the similarity coefficient ranges from 0 to 1. If it is equal to 0, then the two community species are completely different; if it is 1, then the two community species are identical.
To evaluate the relationship between species distribution and spatial distance, standard linear models were used to test the correlation between species similarity and distance between environmental gradients that passed the Mantel test (p < 0.05) (Liu et al., 2020). Standard linear models were used, as the variables were not integer data, to compare the correlation between spatial distance and flora distance (beta-diversity), and significance was assessed using Pearson correlation coefficients at the 5% significance level.
In the karst tiankeng groups, we recorded 24 families, 37 genera and 48 species in the tree layer. Among them, SJXT had 16 families, 22 genera and 26 species (Supplementary Table 1); SXT had 11 families, 15 genera and 20 species (Supplementary Table 2); and BJT had 11 families, 16 genera and 19 species (Supplementary Table 3). The dominant tree including Anacardiaceae, Ericaceae, Adoxaceae, Rosaceae, Fagaceae, and Pinaceae (Table 2). There were 49 species of 43 genera in 27 families in the shrub layer. Among them, 21 families, 28 genera and 31 species were in SJXT; 15 families, 22 genera and 24 species were in SXT, and 14 families, 21 genera and 24 species were in BJT. Classified by “family,” the dominant shrubs were mainly Rosaceae, Cornaceae, Oleaceae, Fagaceae, Fabaceae, Adoxaceae, and Anacardiaceae (Table 2). Several species, such as Xylosma congesta, Toxicodendron succedaneum, Rhus chinensis, and Carallia brachiate in the karst tiankeng groups can be found in the species protection list of Haifeng Nature Reserve.
At the surface (karst degraded tiankeng-outside), the taxonomic numbers of species “families” were relatively less than those in the karst tiankengs, whereas “species” of shrubs had more taxonomic numbers (Supplementary Table 4). Specifically, the tree layer had 39 species in 31 genera and 20 families, dominant tree including Fabaceae, Ericaceae, Cupressaceae, Rosaceae, Fagaceae, Pinaceae, and Anacardiaceae (Table 3). The shrub layer contained approximately 26 families, 44 genera and 55 species; the dominant shrub comprising Oleaceae, Fabaceae, Ericaceae, Rosaceae, Berberidaceae, Hypericaceae, Fagaceae, and Rhamnaceae (Table 3). Overall, trees were less abundant than shrubs.

Table 3. Shared species within and outside the karst tiankeng groups. SJXT, SXT, BJT, and Surface indicates shared species in four habitats, SXT and BJT; SJXT and BJT; and SJXT and SXT means shared species in two habitats.
Through the survey of woody plants in karst tiankeng and on the surface, we found that there were nine tree species more in the tiankeng than on the surface, while six shrub species less in the tiankeng than on the surface. In terms of individual karst tiankeng, SJXT had a higher number of species at 26. There are a relatively small number of species in each karst tiankeng, and the ascending scale reaches 48 species in the cluster of degraded karst tiankeng, suggesting that the distribution of species varies across karst tiankengs.
Significant divergence in forests existed between within and outside the karst tiankengs, and between karst tiankeng groups, together with some commonality. Among the surveyed species, more species categories are found on the surface, and a fewer are found in karst degraded tiankeng (Figure 2). At the tree level, the surface reached a significant difference with SJXT (p < 0.001), while no significant difference was found between BJT, SXT, and the surface. The species composition in SJXT were significantly different from those in BJT, SXT, and the surface (p < 0.001). The species components shared between sites were shown specifically in Table 3. We found that only Oleaceae, Fagaceae, and Rosaceae were shared within and outside degraded karst tiankengs. Significant differences between karst tiankeng groups were also different. For instance, SJXT and BJT shared one family, two genera and two species, with the specific species Rhus chinensis and Carallia brachiata.
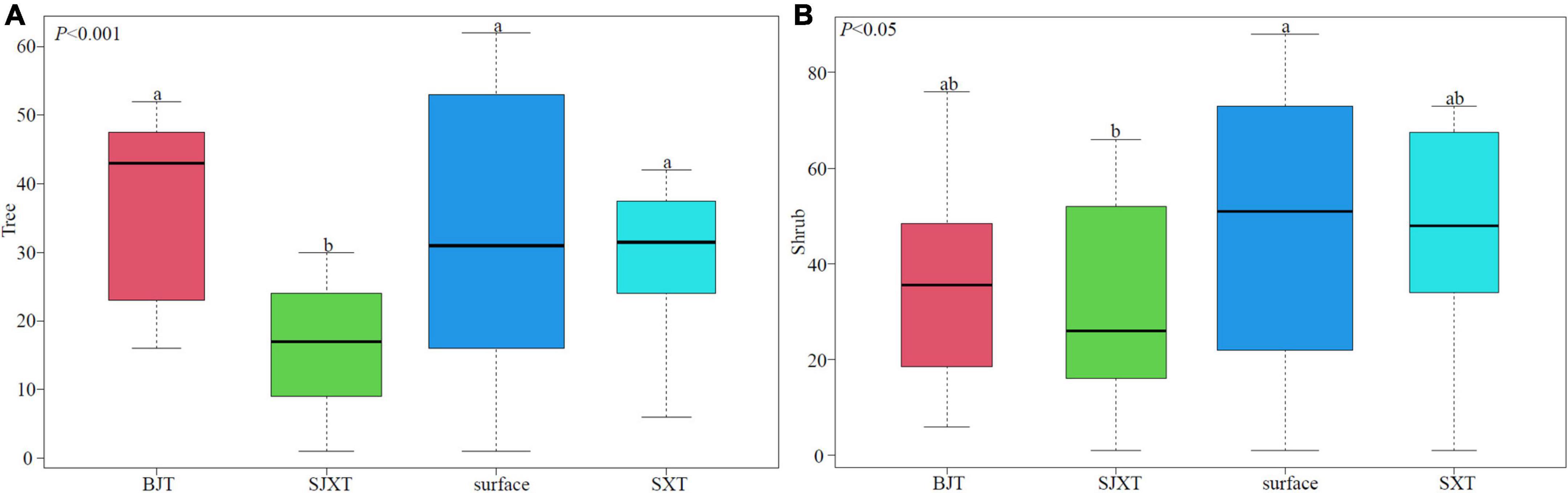
Figure 2. Differences in species composition of forests between sites, with different lowercase letters indicating significant differences. (A) Tree, (B) shrub.
Among shrub species, species composition of both surface and tiankeng groups were significantly different (p < 0.05). Similar to the tree species composition pattern, the shrub composition in SJXT was significantly different from that of BJT, SXT, and the surface (p < 0.05). Similarly, in Table 3, the karst tiankeng complex (SJXT, SXT, and BJT) and the surface shared five species, including Photinia glomerata, Pyracantha fortuneana, Coriaria nepalensis, Pistacia weinmanniifolia, and Viburnum propinquum. Among the tiankeng complex, only one shrub could be found in common. For instance, the shared species found in SJXT and BJT was Xylosma congesta. Karst tiankeng environment allows for more inclusiveness to accommodate the growth and reproduction of species with a broader range of habits.
At the tree level, a certain change was occurred in four indices (Margalef, Shannon–Wiener, Pielou, and Simpson) for each karst degraded tiankeng, with the characteristics ranked as “SJXT > SXT > BJT” and variation coefficients reaching 20.7, 18.9, 15.2, and 12.6%, respectively (Table 4). The Margalef, Shannon–Wiener, Pielou, and Simpson indices of shrubs in each karst degraded tiankeng were differed slightly from those of trees, ranked as “SJXT > BJT > SXT.” Moreover, a high coefficient of variation of 18.9% was observed for the Shannon–Wiener index, whereas the other three were 10.6, 15.3, and 15.7%, respectively.
Diversity differentiation between trees and shrubs was also demonstrated. For trees, we observed a higher Margalef index than that of shrubs, where the Shannon–Wiener index, Pielou index, and Simpson index were lower than those of shrubs. For species diversity differences in specific karst tiankengs, we noticed that both trees and shrubs in SJXT possessed higher species richness (Tree = 3.72, Shrub = 3.63) and species diversity (Tree = 2.30, Shrub = 2.48) than those in SXT and BJT.
The Margalef and Shannon–Wiener indices in the tree layer of all karst degraded tiankengs were greater than those of the surface, and the greatest differences were found in the species richness index. In contrast, in the shrub layer, the species richness, species diversity and species evenness were higher at the surface. Overall, the species diversity of the tree layer was high, while the dominance of the shrub species was low in the karst tiankeng.
Two species growth of the community within and outside the karst tiankengs presented a fixed S-shaped empirical cumulative distribution function (ECDF), with a high percentage of rare species in different topographies (Figure 3). A higher proportion of trees and shrubs in karst tiankengs than that of the surface, and the overall proportion of shrubs is higher than that of trees. Furthermore, according to the Wilcoxon rank sum test, in the tree and shrub layer, there were significant variations (p < 0.01) in the ECDFs within and outside degraded karst tiankeng.

Figure 3. Empirical cumulative distribution function (ECDF) of species within and outside the degraded karst tiankeng forest communities.
The Whittaker index decreased gradually from the degraded tiankeng groups to that within and outside the degraded karst tiankeng, and the Jaccard and Sørenson coefficients gradually increased. Moreover, decreasing Jaccard coefficients showed that the communities within and outside degraded karst tiankengs mainly belonged to the medium dissimilarity level (only the tree communities within and outside BJT fell into extreme dissimilarity) (Figure 4). Conversely, lower community similarity occurred between the karst tiankeng groups. For example, the Whittaker index (tree level: 0.795, shrub level: 0.667) was higher in the gradients of SXT and BJT compared with other two tiankeng groups, showing extremely habitat heterogeneity.
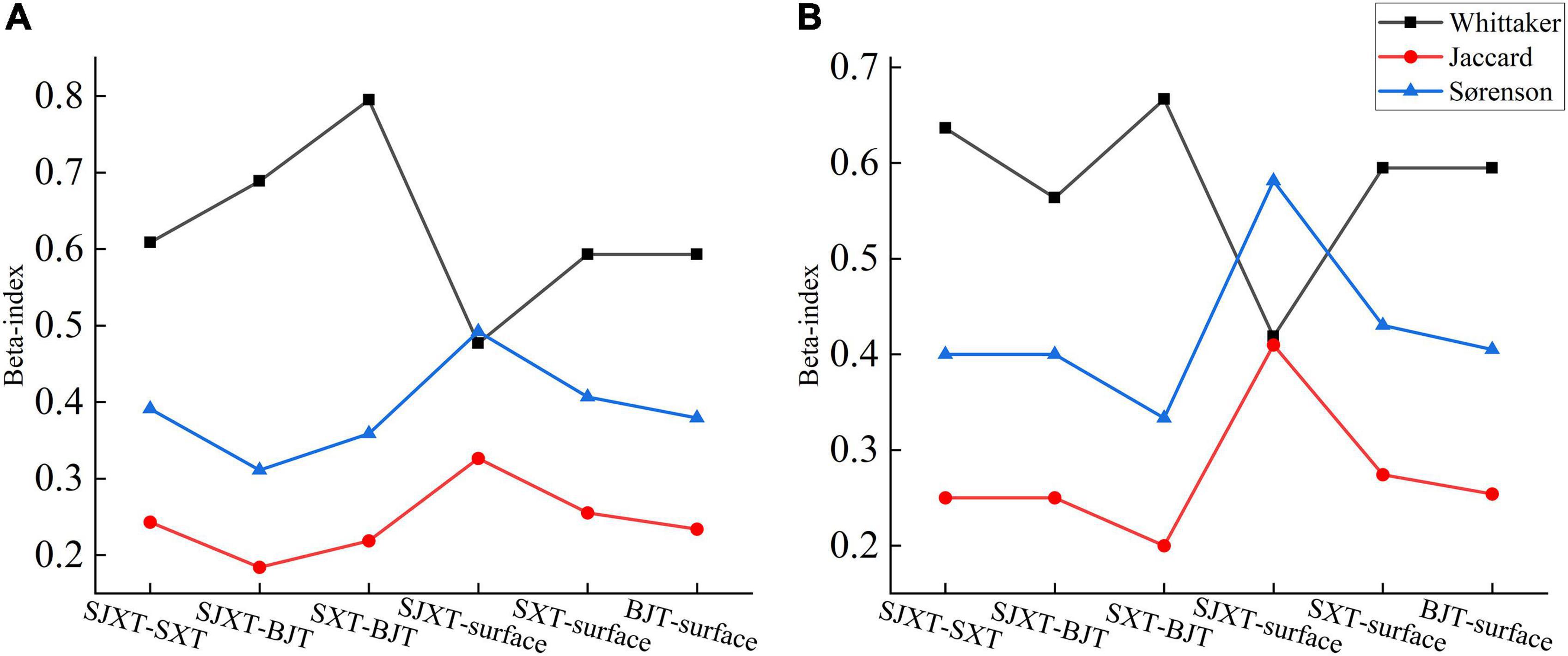
Figure 4. Similarity coefficient comparison within and outside the degraded karst tiankeng. (A) Tree, (B) shrub.
From the Jaccard factor, the similarity intervals with SJXT and SXT, and SXT and BJT were equivalently ranked as very dissimilar, showing the high-level of species segregation and habitat heterogeneity, where SJXT and BJT were the lowest in community similarities (SJXT: tree level: 0.184, shrub level: 0.250. BJT: tree level: 0.311, shrub level: 0.400). The similarity degree of species between karst tiankeng groups was even lower than the environmental gradient that within and outside degraded karst tiankeng. Additionally, the highest value of Jaccard locates in that within and outside SJXT (tree level: 0.327, shrub level: 0.410), similar to the gradients that within and outside SXT, the communities were moderately dissimilar. Although the variability of communities within and outside degraded karst tiankeng outweighs the similarity, the differences between karst tiankeng groups are greater.
The model and Mantel test (p < 0.05) indicated that flora distribution exhibited notable spatial dependence. Spatial distance between degraded karst tiankeng groups shaped the similarity of the flora, as the distance between habitats decreased the community similarity (Figure 5). Habitats that within and outside degraded karst tiankengs derived less separation from species than among the karst tiankeng groups, with certain similarities in communities.
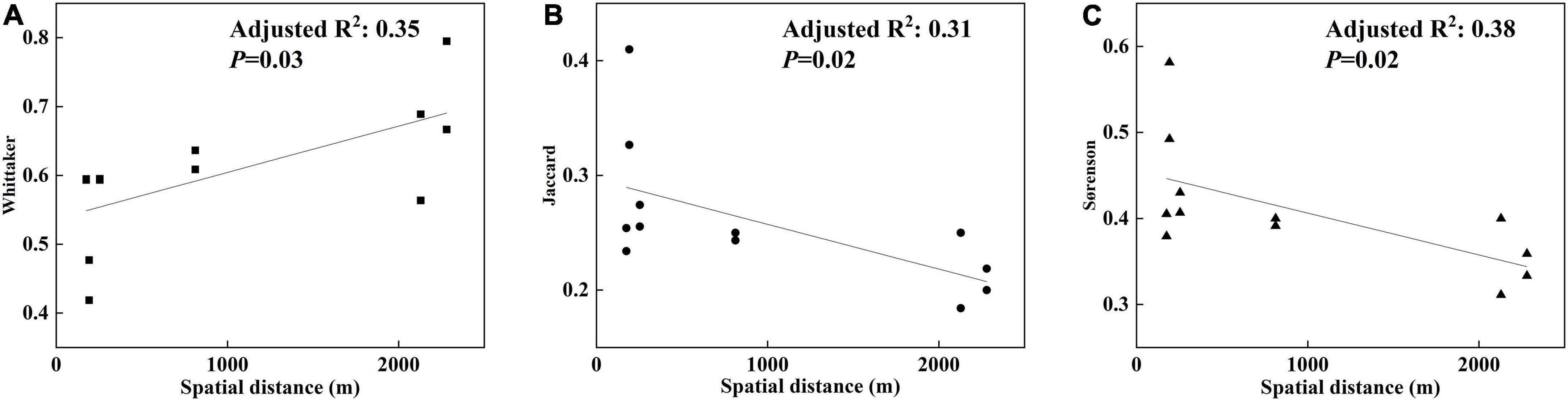
Figure 5. Relationship between flora beta-diversity and spatial distance of environmental gradients, within and outside the degraded karst tiankengs and between karst tiankeng groups in Yunnan, China. (A–C) Refer to the relationships between Jaccard, Sørenson, Whittaker and spatial distances, respectively. Black line is the best-fit linear regression model.
Karst tiankengs constitute an essential habitat for species and contribute to the ecological conditions for communities of plants with various growth strategies. Our species investigation revealed trees in 26 species, 16 families and 21 genera and shrubs in 32 species, 21 families and 29 genera among the shared species within and outside degraded karst tiankengs. This indicates their floristic proximity. The plant communities were slightly less similar between karst tiankeng groups, especially the plant composition between SXT, BJT, and SJXT was significantly different. It suggests that different karst tiankeng have different species distribution, i.e., there are differences in the species conservation targets (Shui et al., 2015). According to previous species investigation, species distributions in extremely karst areas have been well-documented (Wen et al., 2015; Zhang et al., 2020). Species in karst tiankeng are dominated by shade-tolerant plants, accompanied by some heliophytic plants. On the surface, the species are largely distributed as heliophytic plants. Previous studies concluded that the effect was due to the influence of karst tiankeng wall height on the distribution of light resources (Chen et al., 2018). In particular, the shade-loving plants were located more intensively on the higher wall side of karst tiankeng. The presence of degraded pit walls in karst tiankeng explains the association of sun-loving species within karst tiankeng. Thus, it affects the distribution of different life-type species. Similar to the studies of Bátori et al. (2014), Raschmanová et al. (2017), negative topography is an important species conservation bank for regional species. Karst tiankeng, on the other hand, are more magnificent in scale and have better entrapment. It is also a natural active open top chamber for studying forest ecosystems (Yang et al., 2019). In this article, Species gradually increase from the surface to degraded karst tiankengs, although their growth habits differed between the main dominant species within and outside degraded karst tiankengs, which was probably correlated with the habitat environment. Strong light and heat radiation combine with drought and water scarcity in the surface karst environment, screening for drought-tolerant and light-adapted species. The degraded karst tiankengs create a semi-enclosed secluded habitat based on volume and depth, with thermal radiation greatly weakened and high humidity maintained by negative topographic factors that intercept evaporation and rainfall; thus, plant characteristics are mainly shade-tolerant and hygrophilous. These unique microclimates contribute to specific cyclic ecosystems composed of different organisms (Bátori et al., 2014). Moreover, contrary to mine pits, degraded karst tiankeng have a richer species diversity, and new species could be found in this place, as Bartgis (1992) observed that rare or endangered plant species survived in seepage ponds, furthermore, an previous study (Dang et al., 2016) discovered one new genus and five new species in the Dashiwei series of karst tiankengs in Guangxi.
The higher tree diversity that occurred in degraded karst tiankeng habitat vs. karst tiankeng-outside forests supports a basic prediction in island biogeography—the isolate effect in species distribution. Research on terrestrial island-like systems based on island biogeography offers an important theoretical basis for biodiversity conservation in various habitats (Su et al., 2014; Song et al., 2018). Loss, acquisition, and replacement of community species by habitat fragmentation, leading to alteration of community structure, composition, and diversity (Gonzalez et al., 2010), is considered one of the causes of biodiversity and ecosystem degradation (Haddad et al., 2015). The special ecological environment of karst areas forms numerous fragmented habitats under natural disturbance, with high spatial heterogeneity (Zhang et al., 2019). Generally, the vertical steep cliff walls and enclosed form of degraded karst tiankengs created by the negative topography do not render it an isolated island. Conversely, forest cover is highly diversified. Habitat characteristics are similar to rock outcrops, springs, fens and inselbergs, which differ from ordinary island isolation (Mendez-Castro et al., 2021). As previously mentioned, the habitats within and outside degraded karst tiankeng are not completely independent, and biodiversity is often richer on the inverted stone slopes (Chen et al., 2018), as evidenced by the flora findings. It shows differently from native karst tiankeng due to environmental differences in soil, light, and wind speed, etc.
Furthermore, relatively modest shrub diversity in karst degraded tiankeng is also attributed to the negative topography (Shui et al., 2015; Chen et al., 2018). Flora form the products of plant–environment interactions (Yuan et al., 2014; Li et al., 2019); the more diverse the niche in the community is, the richer the clusters within it, indicating the distribution structure and diversity patterns of communities and their relationship with environmental factors for maintaining community stability and biodiversity conservation (Kidane Yohannes et al., 2019). Although habitats in the degraded karst tiankeng are limited and environmental resources (e.g., light, temperature, and wind speed) are weaker than karst tiankeng-outside, differences in slope collapse and wall degradation leading to local environments are more suitable for species survival among different degraded karst tiankengs. For instance, due to competition for light and heat resources caused by topography, a study found that tree height in a karst tiankeng averaged approximately 5 m taller than the karst tiankeng-outside (Zhang et al., 2022), with more trees and fewer shrubs; thus, species in karst tiankeng were characterized by higher tree diversity and lower shrub diversity.
Forest similarity between degraded karst tiankengs was lower than that between within and outside degraded karst tiankengs, and we found a higher limitation in spatial distance, although species dispersal within degraded karst tiankeng was limited by large depths and microclimatic factors. Our results showed that both spatial distance and environmental gradients contribute to explaining the community structure of distance decay in species similarity. Studies from Brazilian forests demonstrated that spatial distance highly correlated with plant similarity (Leitman et al., 2015), and comparable conclusions emerged for African rainforest regions—island-like systems (Parmentier and Hardy, 2009). Species similarity among regions can be better explained by spatial autocorrelation. In addition, species similarity between habitats decreases with increasing geospatial distance, also reflecting the view of species dispersal limitation proposed by Macarthur and Wilson (1967). However, the pattern of spatial variation was strongly scale-dependent. In other words, no significant changes in species diversity characteristics may be related to too small gradient changes. Furthermore, species similarity could also be potentially impacted by other nutrient gradients or environmental substrate heterogeneity.
Overall, a lower plant similarity among degraded karst tiankengs in part revealed that karst tiankengs provide shelters for not only specific plants but also more diverse species. Perhaps protected by the natural barrier of karst tiankeng walls, a more complete type of native vegetation remains in karst tiankengs, enabling its flora to survive against climate change and human activities (Chen et al., 2018). Different karst tiankeng had similar alpha biodiversity indices, but greater differences in similarity indices. Consequently, degraded karst tiankeng are not only valuable for species conservation, but also have different conservation targets for different species.
Certainly, specific mechanisms of environmental factors influencing the flora in degraded karst tiankengs will be further revealed. For the karst region of Yunnan, China, degraded karst tiankengs represent the important shelter for rare species with important world heritage value (Li et al., 2013), it is worth prioritizing as the bank for biodiversity conservation in the widely distributed karst region.
This research provides reliable information for future biodiversity conservation efforts in karst areas under climate change. Our findings of flora differences, species diversity and species similarity within and outside degraded karst tiankengs contribute to the species survey and habitat diversity. It may help to highlight differentiated conservation strategies for karst tiankeng systems, which are important habitats for threatened species under extreme environmental change. The conclusions are as follows:
(1) Based on the differences of species abundance and alpha-diversity index, our study shows that despite the large flora similarity that within and outside degraded karst tiankengs, their species composition appears to be highly variable. According to the calculation of intersection by species and ANOVA for species composition, there were significant differences in tree and shrub stratification within and outside degraded karst tiankeng. Furthermore, no more than five species are shared among degraded karst tiankengs, with the possibility of different microhabitat features among degraded karst tiankeng groups.
(2) Species similarity between degraded karst tiankeng groups and that within and outside degraded karst tiankeng are both at dissimilar levels, but to a higher degree in the former. According to the standard linear model and Pearson correlation coefficient, similarity under environmental gradients was correlated significantly with spatial distance, showing a distance decay pattern of species similarity, while species increased through spatial autocorrelation, demonstrating species dispersal limitations driven by spatial distance and topographic factors.
(3) Despite the limited area and resources, degraded karst tiankengs possess high species diversity values with differentiated species conservation targets, which should serve as a priority concern for in situ species conservation and ecological restoration of the surface flora. Additionally, microclimate monitoring of long time series and community assembling mechanisms of underground forests in karst tiankengs are also needed in the future. And whether the negative terrain represented by karst tiankeng is similar to island system still needs further work.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
WS, YL, and CJ: conceptualization and methodology. YL: software, data curation, and visualization. WS and YL: validation, formal analysis, and writing—original draft preparation. WS, YL, CJ, XS, XJ, PG, SuZ, and HL: investigation. WS, YL, XS, XJ, PG, and SuZ: resources. WS, YL, CJ, XS, HL, SuZ, SZ, and MM: writing—review and editing. WS: supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Natural Science Foundation of China (Grant No. 41871198).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1015468/full#supplementary-material
Arroyo, A. I., Pueyo, Y., Saiz, H., and Alados, C. L. (2021). Plant-plant interactions and local patterns of diversity from semi-arid to subalpine Mediterranean plant communities. Biodiv. Conserv. 30, 3481–3508. doi: 10.1007/s10531-021-02257-w
Bartgis, R. L. (1992). The endangered sedge Scirpus ancistrochaetus and the flora of sinkhole ponds in Maryland and West Virginia. Castanea 57, 46–51.
Bátori, Z., Farkas, T., Erdös, L., Tölgyesi, C., Körmöczi, L., and Vojtkó, A. (2014). A comparison of the vegetation of forested and non-forested solution dolines in Hungary: a preliminary study. Biologia 69, 1339–1348.
Chen, Y., Jiang, C., Jian, X., Shui, W., Hu, Y., Ma, T., et al. (2018). Spatial distribution characteristics of grassland plant communities in a moderately degraded tiankeng in Zhanyi, Yunnan. Acta Ecol. Sin. 38, 8008–8021.
Dang, G., Feng, H., Tang, Q., Mo, F., and Xue, Y. (2016). New recorded plant species in Guangxi China. Nat. Sci. Edit. 34, 147–150.
Fu, Q., Li, X., Zhou, W., Cui, G., and Li, F. (2007). Protection order and exploitation of tourism resources on Haifeng Natural Reserve in Yunnan. J. Cent. South Univ. Forest. Technol. 27, 60–65.
Gao, J., Ma, K., Qi, J., Feng, Y., and Feng, Z. (2008). Effects of anthropogenic disturbance and environmental pattern on plant diversity in Dongling mountain, Beijing, China. Acta Bot. Boreal Occid. Sin 28, 2506–2513.
Gonzalez, M., Ladet, S., Deconchat, M., Cabanettes, A., Alard, D., and Balent, G. (2010). Relative contribution of edge and interior zones to patch size effect on species richness: An example for woody plants. Forest Ecol. Manag. 259, 266–274. doi: 10.1016/j.foreco.2009.10.010
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., Gonzalez, A., Holt, R. D., et al. (2015). Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 1:e1500052. doi: 10.1126/sciadv.1500052
Harper Karen, A., Macdonald, S., Ellen, Mayerhofer Michael, S., Biswas Shekhar, R., Esseen, P., et al. (2015). Edge influence on vegetation at natural and anthropogenic edges of boreal forests in Canada and Fennoscandia. J. Ecol. 103, 550–562. doi: 10.1111/1365-2745.12398
Jian, X., Shui, W., Chen, Y., Jiang, C., Hu, Y., Wang, Q., et al. (2018). Interspecific relationships of dominant species in the grassland community of moderately degraded tiankeng of Yunnan, China. Chin. J. Appl. Ecol. 29, 492–500.
Jiang, C., Feng, J., Zhu, S., and Shui, W. (2021). Characteristics of the Soil Microbial Communities in Different Slope Positions along an Inverted Stone Slope in a Degraded Karst Tiankeng. Biology 10:474. doi: 10.3390/biology10060474
Jiang, C., Sun, X., Feng, J., Zhu, S., and Shui, W. (2022a). Metagenomic analysis reveals the different characteristics of microbial communities inside and outside the karst tiankeng. BMC Microbiol. 22:115. doi: 10.1186/s12866-022-02513-1
Jiang, C., Zhu, S., Feng, J., and Shui, W. (2022b). Slope aspect affects the soil microbial communities in karst tiankeng negative landforms. BMC Ecol. Evol. 22:54. doi: 10.1186/s12862-022-01986-y
Kidane Yohannes, O., Steinbauer Manuel, J., and Beierkuhnlein, C. (2019). Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob. Ecol. Conserv. 19:e00670. doi: 10.1016/j.gecco.2019.e00670
Kreyling, J., Wana, D., and Beierkuhnlein, C. (2010). Potential consequences of climate warming for tropical plant species in high mountains of southern Ethiopia. Div. Distrib. 16, 593–605. doi: 10.1111/j.1472-4642.2010.00675.x
Leitman, P., Amorim, A. M., Sansevero, J. B. B., and Forzza, R. C. (2015). Floristic patterns of epiphytes in the Brazilian Atlantic Forest, a biodiversity hotspot. Bot. J. Linn. Soc. 179, 587–601. doi: 10.1111/boj.12342
Li, A., Sun, S., and Guo, L. (2018). Ecological roles of bryophytes in carbon cycling in subalpine ecosystems-A case study in Gongga Mountain, China. Mount. Res. 36, 693–698.
Li, G., Xiong, K., Xiao, S., and Zhou, M. (2013). Research on World Heritage Geomorphologic Value of the Shibing Karst. Trop. Geogr. 33, 562–569.
Li, K., Zhang, M., Li, Y., Xing, X., Fan, S., Cao, Y., et al. (2020). Karren Habitat as the Key in Influencing Plant Distribution and Species Diversity in Shilin Geopark, Southwest China. Sustainability 12:5808. doi: 10.3390/su12145808
Li, T., Ji, L., Yu, D., Zhou, L., Zhou, W., Mao, Y., et al. (2019). Forest community classification, ordination, and comparison of species diversity in broadleaved-Korean pine mixed forests of Northeast China. Acta Ecol. Sin. 39, 620–628. doi: 10.5846/stxb201802080339
Li, W., Xiang, Y., and Du, Y. (2001). An analysis on characteristics of underground forest communities in Haifeng Natural Protection area Zhanyi Yunnan Province. J. South. Forest. Coll. 3, 8–13.
Liu, J., Zhong, Y., Zhong, L., Wei, B., Zheng, S., Xie, Y., et al. (2020). The asymmetric relationships of the distribution of conspecific saplings and adults in forest fragments. J. Plant Ecol. 13, 398–404. doi: 10.1093/jpe/rtaa026
Liu, Y., Qi, W., He, D., Xiang, Y., Liu, J., Huang, H., et al. (2021). Soil resource availability is much more important than soil resource heterogeneity in determining the species diversity and abundance of karst plant communities. Ecol. Evol. 11, 16680–16692. doi: 10.1002/ece3.8285
Macarthur, R. H., and Wilson, E. O. (1967). The Theory of Island Biogeography. Princeton, NJ: Princeton University Press.
Mendez-Castro, F. E., Conti, L., Chytry, M., Jimenez-Alfaro, B., Hajek, M., Horsak, M., et al. (2021). What defines insularity for plants in edaphic islands? Ecography 44, 1249–1258. doi: 10.1111/ecog.05650
Ochoa, D., Zavada Michael, S., Liu, Y., and Farlow James, O. (2016). Floristic implications of two contemporaneous inland upper Neogene sites in the eastern US: Pipe Creek Sinkhole, Indiana, and the Gray Fossil Site, Tennessee (USA). Palaeobiodiv. Palaeoenviron. 96, 239–254. doi: 10.1007/s12549-016-0233-4
Parmentier, I., and Hardy, O. J. (2009). The impact of ecological differentiation and dispersal limitation on species turnover and phylogenetic structure of inselberg’s plant communities. Ecography 32, 613–622. doi: 10.1111/j.1600-0587.2008.05697.x
Parra Tabla, V., Albor Pinto, C., Tun Garrido, J., Angulo Perez, D., Barajas, C., Silveira, R., et al. (2018). Spatial patterns of species diversity in sand dune plant communities in Yucatan, Mexico: importance of invasive species for species dominance patterns. Plant Ecol. Div. 11, 157–172. doi: 10.1080/17550874.2018.1455232
Peng, W., Song, T., Du, H., Chen, H., Zeng, F., Liu, Y., et al. (2020). Inconsistent diversity patterns of soil fungi and woody plants among habitat types in a karst broadleaf forest. Forest Ecol. Manag. 474:118367. doi: 10.1016/j.foreco.2020.118367
Raschmanová, N., Žurovcová, M., Kováč, L., Paučulová, Ľ., Šustr, V., Jarošová, A., et al. (2017). The cold-adapted population of Folsomia manolachei (Hexapoda, Collembola) from a glaciated karst doline of Central Europe: evidence for a cryptic species? J. Zool. Syst. Evolut. Res. 55, 19–28. doi: 10.1111/jzs.12150
Redžić, S., Barudanovic, S., Trakić, S., and Kulijer, D. (2011). Vascular plant biodiversity richness and endemo-relictness of the karst mountains Prenj-Čvrsnica-Čabulja in Bosnia and Herzegovina (W. Balkan). Acta Carsol. 40, 527–555. doi: 10.3986/ac.v40i3.64
Ren, H., Wang, F., Ye, W., Zhang, Q., Han, T., Huang, Y., et al. (2021). Bryophyte diversity is related to vascular plant diversity and microhabitat under disturbance in karst caves. Ecol. Indic. 120:106947. doi: 10.1016/j.ecolind.2020.106947
Romanuk, T., and Kolasa, J. (2002). Abundance and species richness in natural aquatic microcosms: A test and refinement of the Niche-Limitation Hypothesis. Commun. Ecol. 3, 87–94. doi: 10.1556/ComEc.3.2002.1.10
Sharma, N., and Kala, C. P. (2022). Patterns in plant species diversity along the altitudinal gradient in Dhauladhar mountain range of the North-West Himalaya in India. Trees Forest. People 7:100196. doi: 10.1016/j.tfp.2022.100196
Shui, W., Chen, Y., Jian, X., Jiang, C., Wang, Q., and Guo, P. (2018). Spatial pattern of plant community in original karst tiankeng: A case study of Zhanyi tiankeng in Yunnan, China. Chin. J. Appl. Ecol. 29, 1725–1735.
Shui, W., Chen, Y., Jian, X., Jiang, C., Wang, Q., Zeng, Y., et al. (2022). Original karst tiankeng with underground virgin forest as an inaccessible refugia originated from a degraded surface flora in Yunnan, China. Sci. Rep. 12:9408. doi: 10.1038/s41598-022-13678-0
Shui, W., Chen, Y., Wang, Y., Su, Z., and Zhang, S. (2015). Origination, study progress and prospect of karst tiankeng research in China. Acta Geogr. Sin. 70, 431–446.
Shui, W., and Xu, G. (2015). Analysis of the influential factors for changes to land use in China’s Xingwen Global Geopark against a tourism development background. Geoc. Int. 31, 1–44. doi: 10.1080/10106049.2015.1041558
Song, N., Wang, X., Chen, L., Xue, Y., Chen, J., Sui, J., et al. (2018). Co-existence mechanisms of plant species within “soil islands” habitat of desert steppe. Biodiv. Sci. 26, 667–677. doi: 10.17520/biods.2018045
Song, X., Gao, Y., Wen, X., Guo, D., Yu, G., He, N., et al. (2017). Carbon sequestration potential and its eco-service function in the karst area, China. J. Geogr. Sci. 27, 967–980. doi: 10.1007/s11442-017-1415-3
Su, X., Yuan, J., Hu, G., Xu, G., and Yu, M. (2014). Edge effect of the plant community structure on land-bridge islands in the Thousand Islan Lake. Chin. J. Appl. Ecol. 25, 77–84.
Su, Y., Tang, Q., Mo, F., and Xue, Y. (2017). Karst tiankengs as refugia for indigenous tree flora amidst a degraded landscape in southwestern China. Sci. Rep. 7:4249. doi: 10.1038/s41598-017-04592-x
Sun, S., Wu, Y., Wang, G., Zhou, J., Yu, D., Bing, H., et al. (2013). Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain, China. PLoS One 8:e58131. doi: 10.1371/journal.pone.0058131
R Core Team (2018). R: A language and environment for statistical computing. Vienna Austr. 1, 12–21.
Thomas, C. D. (2013). Local diversity stays about the same, regional diversity increases, and global diversity declines. Proc. Natl. Acad. Sci. U.S.A. 110, 19187–19188. doi: 10.1073/pnas.1319304110
Vilisics, F., Solymos, P., Nagy, A., Farkas, R., Zita, K., and Elisabeth, H. (2011). Small scale gradient effects on isopods (Crustacea: Oniscidea) in karstic sinkholes. Biologia 66, 499–505. doi: 10.2478/s11756-011-0042-1
Wen, L., Song, T., Du, H., Wang, K., Peng, W., Zeng, F., et al. (2015). The succession characteristics and its driving mechanism of plant community in karst region, Southwest China. Acta Ecol. Sin. 35, 5822–5833. doi: 10.5846/stxb201310192524
Yang, G., Peng, C., Liu, Y., and Dong, F. (2019). Tiankeng: an ideal place for climate warming research on forest ecosystems. Environ. Earth Sci. 78:46.
Yang, L., Zhou, G., Wang, G., and Wang, Y. (2003). Effect of human activities on soil environment and plant species diversity of elm sparse woods. Chin. J. Appl. Ecol. 14, 321–325.
Yuan, T., Zhang, H., Ou, Z., and Tan, Y. (2014). Effects of topography on the diversity and distribution pattern of ground plants in karst montane forests in Southwest Guangxi, China. Chin. J. Appl. Ecol. 25, 2803–2810.
Zhang, F., Du, H., Zeng, F., Peng, W., and Song, T. (2019). Regeneration dynamics of primary forest in the karst peak-cluster depression. Acta Ecol. Sin. 39, 8516–8525. doi: 10.5846/stxb201808251813
Zhang, H., Yang, Y., and Li, Y. (2015). Discussion on ecosystem degradation in karst rock desertification areas of southwest China. Ecol. Sci. 34, 169–174.
Zhang, L., and Han, G. (2018). A review on the relationships between plant genetic diversity and ecosystem functioning. Chin. J. Appl. Ecol. 42, 977–989. doi: 10.17521/cjpe.2018.0013
Zhang, Y., Shui, W., Feng, J., Sun, X., Sun, X., Liu, Y., et al. (2022). Tree-hight characterization of karst degraded tiankeng underground forests using unmanned aerial vehicles. Remote Sens. Technol. Appl. 37, 1–11.
Zhang, Z., Huang, X., and Liu, Y. (2020). Species composition and diversity of plants at different successional stages in small catchments of karst areas. Pakist. J. Bot. 52, 551–556. doi: 10.30848/PJB2020-2(33)
Zhu, X., and Waltham, T. (2006). Tiankeng: Definition and description. Speleogen. Evolut. Karst Aquif. 4:8.
Keywords: karst tiankeng, species records, plant diversity, refugia, Yunnan
Citation: Shui W, Liu Y, Jiang C, Sun X, Jian X, Guo P, Li H, Zhu S, Zong S and Ma M (2022) Are degraded karst tiankengs coupled with microclimatic underground forests the refugia of surface flora? Evidence from China’s Yunnan. Front. Ecol. Evol. 10:1015468. doi: 10.3389/fevo.2022.1015468
Received: 09 August 2022; Accepted: 26 August 2022;
Published: 15 September 2022.
Edited by:
Dingde Xu, Sichuan Agricultural University, ChinaCopyright © 2022 Shui, Liu, Jiang, Sun, Jian, Guo, Li, Zhu, Zong and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Shui, c2h1aXdlaUBmenUuZWR1LmNu; Cong Jiang, amNvbmdAcGt1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.