- 1Theoretical Research in Evolutionary Life Sciences, TRÊS, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, Netherlands
- 2The Faculty of Life Sciences, Bar-Ilan University, Ramat Gan, Israel
- 3School of Zoology, Tel Aviv University, Tel Aviv, Israel
Competition in group-living animals often results in a dominance hierarchy. The sex that is larger (usually the males) generally dominates the one that is smaller (the females). In certain species, however, despite being smaller, the females dominate several males. Female dominance over males may here arise from the self-reinforcing effects of winning and losing fights, the so-called winner-loser effect, as demonstrated in the model DomWorld. In the model, females may become dominant over more males when the percentage of males in the group is higher due to the higher intensity of aggression of males than females combined with the higher frequency of male–male fights. This association between female dominance and the percentage of adult males in the group has been confirmed in several primate species. Since in the model DomWorld this association requires few assumptions, it should be tested beyond primates. In the present study, we investigated it in the group-living rock hyrax (Procavia capensis), because it fulfilled most requirements. We used data on adults from six groups, collected over 20 years in natural colonies in Israel. We confirmed that body weight and intensity of aggression was greater in males than females. Three measurements indicated that females dominated ca. 70% of the males. Unexpectedly, only in the data where groups comprised several males, female dominance over males was shown to increase with male percentage, but not when including (the many) years in which groups comprised a single male. We attribute this non significance to the limited male–male interactions. One of the requirements of DomWorld is that individuals live in permanent groups, but in rock hyrax there were also bachelor males, that were not permanently associated with a group. Thus, we expected and confirmed that there was no association between the percentage of males and female dominance over males when including them. In conclusion, our results support the hypothesis that the winner-loser effect contributes to the dominance of females over males, and the association between the percentage of males in a group and female dominance over males requires an extra criterion: that most groups contain multiple males.
Introduction
Competitive interactions among individuals in a group often result in a dominance hierarchy (Drews, 1993). When the hierarchy is steep, meaning that differences in rank among individuals are large, the society is despotic. Here, the dominant individuals have more access to resources than the subordinates. In an egalitarian society, in contrast, individuals are similar in rank and have comparable access to resources (Vehrencamp, 1983). Higher rank has been linked to individual attributes, such as larger body size (Beacham, 1988). However, females sometimes dominate several males despite the smaller body size of females, such as in macaques (Macaca spp.; Hemelrijk et al., 2008), vervet monkeys (Chlorocebus pygerythrus; Hemelrijk et al., 2020a,b), and capuchin monkeys (Sapajus libidinosus; S. nigritus, and S. xanthosternos; Izar et al., 2021). Causes of dominance rank are, thus, more complex and do not depend merely on body size. For instance, dominance rank may depend on other processes such as coalitional support (Vullioud et al., 2019), or the self-reinforcing effect of winning and losing competitive interactions (Franz et al., 2015), the so-called “winner-loser effect,” which is prevalent in the animal kingdom (Hsu et al., 2006). The winner-loser effect causes winners to be more likely to win subsequent fights and losers to be more likely to lose them. In the present paper we examine how the winner-loser effect influences dominance between sexes.
The consequences of these self-reinforcing effects regarding both dominance style and intersexual dominance have been demonstrated in the computational model DomWorld (Hemelrijk, 1999). In this model, individuals aggregate and may attack when they are near others. They are more likely to attack when their own dominance value (representing their fighting capacity) is relatively high compared to that of their opponent. After a fight is decided, the dominance value of the winner increases, enhancing the likelihood that in the next fight it will win again and that of the victim decreases, making it more likely that the loser will be beaten in the next fight. The model demonstrates that a dominance hierarchy will develop even if all individuals start with the same initial dominance value (Hogeweg, 1988; Hemelrijk, 1999).
Regarding dominance style, the model demonstrates that when aggression is fierce (such as biting) the hierarchy becomes steeper, resembling that of a despotic society. This arises because fierce aggression has greater impact than mild aggression (such as staring or threatening) on the subsequent winning tendency of the opponents. When aggression is mild, the impact of conflicts is small and the hierarchy differentiates little, resembling that of an egalitarian society (Vehrencamp, 1983; Hemelrijk, 1999).
Regarding intersexual dominance, the model DomWorld demonstrates that even though the fighting capacity of females is initially lower than that of males (reflecting the females’ smaller body size and lower intensity of aggression), some females may still become dominant over some males (Hemelrijk et al., 2003). This occurs, however, only when aggression intensity is high, because the hierarchy differentiates strongly due to the high impact of fights and this causes overlap between the dominance of males and females. If aggression intensity is weak, fight outcomes have little impact on the hierarchy and the initially more powerful males remain dominant over all the females. Thus, DomWorld demonstrates that female dominance is stronger in species with more intense aggression. This has been confirmed in macaques (Hemelrijk et al., 2008). The model also reveals that female dominance over males increases with the percentage of males in the group (Hemelrijk et al., 2008). This we refer to as the self-organisation hypothesis. It is explained by a higher percentage of males resulting in a relatively higher percentage of male–male fights. Through the higher intensity of aggression by males than females, this higher percentage of male–male fights leads to stronger female dominance over males because more males are defeated and sink to a lower rank than some females. This association between the percentage of males and female dominance in the group has been confirmed in empirical studies of macaques (Hemelrijk et al., 2008), vervet monkeys (Hemelrijk et al., 2020b), and capuchin monkeys (Izar et al., 2021).
The assumptions in DomWorld underlying the self-organisation hypothesis, namely the association between female dominance and percentage of males, are that: (1) individuals live permanently in a group; (2) the agonistic interactions result in the winner-loser effect; (3) the initially greater fighting capacity of males than females (e.g., in real animals body weight of males is greater than that of females); (4) the intensity of aggression is high; (5) and higher in males than females; and (6) the range of adult sex ratios across groups is sufficiently large.
Although these assumptions are expected to be met in many group-living species, this association has not been tested to date beyond primates. Therefore, in the present study we investigate natural groups of rock hyrax (Procavia capensis) in Ein Gedi, Israel. The rock hyrax fulfils many of the requirements of the DomWorld model: individuals live in permanent groups with both sexes, aggression is sometimes intense, males are slightly larger than females on average (Koren, 2006), and these groups show a large range of sex ratios. Although the winner-loser effect has not been studied in this species, it has been shown in all taxa where it has been tested, namely, insects, crustacean, amphibia, reptilia, fish, birds and mammals, including humans (Hsu et al., 2006). Besides, in the present study, we confirmed that the body size and intensity of aggression in males of rock hyrax is greater than in females. We quantified female dominance over males and studied whether with a greater percentage of males in the group there was an increase in the dominance of females over males and the percentage of male–male fights of all fights of males with adults. As an alternative, we also investigated whether the dominance of females over males was greater when the percentage of young males or “late dispersers” in the group was higher, because these are males over which females could dominate easily.
The groups of rock hyrax comprise not only resident males (that reside in a group for a few years), but also so-called “bachelor” males. Bachelor males often reside alone, occasionally in all-male groups and sometimes interact with groups, but are not permanently associated with a specific group (Koren, 2000). Herewith, bachelor males do not fulfil the requirement from DomWorld of permanent group living (requirement 1). Thus, we expected no association between the percentage of males and female dominance over males when including bachelor males.
Materials and methods
Study animals, field procedures and behavioural observations at the Ein Gedi Nature Reserve in Israel
The rock hyrax belongs to the order hyracoidea (Afrotheria; Murata et al., 2003; Springer et al., 1997). This species is widely distributed across Africa and the Middle East, where it inhabits mostly rocky areas. Males and females reach sexual maturity at the age of 17 to 24 months (Hoeck et al., 1982) and can live up to 12 years (Mendelssohn, 1965; Glover and Sale, 1968). Most adolescent males disperse upon reaching sexual maturity (Hoeck et al., 1982), with those males that remain in their natal group past sexual maturity being referred to as “late-dispersers” (Koren, 2006). When the males disperse, they either join a new group as residents or remain on the periphery of groups as “bachelors,” mostly sleeping alone or on rare occasions in all-male bachelor groups (Koren, 2000). Resident males reside in a group of females for an average of 3 years (maximum 5) before leaving the group and being replaced by another male. In both sexes aggressive behaviour is sometimes intense, such as biting, fighting and chasing, even killing has been observed (Supplementary Table S1). Both males and females have long incisors (i.e., tusks), that can inflict fatal wounds (LK pers. observation). Rock hyraxes breed seasonally (Mendelssohn, 1965; Millar, 1971; Frey and Miller, 1972; Neaves, 1973), with synchronised parturition (Mendelssohn, 1965; Sale, 1965). Although resident males guard their mates and bachelor males also sire offspring (Bar Ziv et al., 2016).
Rock hyraxes were studied at the Ein Gedi Nature Reserve, (31°28′N, 35°24′E), near the Dead Sea (Supplementary Figure S1A; Supplementary material). The reserve comprises two deep gorges, David and Arugot. Field seasons lasted for 5–6 months each year, from March to September. Data were collected yearly between 2000 and 2019 on 1,213 days on about 4 h a day. Data from 2006 and 2019 are missing due to insufficient observations. The total population size was between 500 and 1,000 individuals (Barocas et al., 2011). Six groups were studied (7 groups when including bachelors, Table 1; Supplementary Table S3; Supplementary Figure S1B). Since we studied each group over several years, we refer to these data-points as group-years. Data were collected using binoculars, a telescope, and paper and pencil (Supplementary Table S2). All individuals were recognisable by marking them with a subcutaneous tag, earrings, and a collar (weighing 5 g). To mark the individuals (including females, resident males and bachelor males) they were caught yearly using live box traps and anaesthetized with ketamine hydrochloride. Following the protocols established by Koren et al. (2006, 2009), groups were observed mostly in the morning, from first light to noon; and, after a period of when hyrax were resting because of the heat, they were observed for ~2 h in the later afternoon until dusk. Observers sat at fixed points and scanned the area for rock hyrax. Once a group was detected, it was followed until it retreated underground because of high temperatures. Observation time was distributed approximately equally over all groups.

Table 1. Summary results of agonistic interactions among adults in rock hyrax groups in Ein Gedi, Israel.
We sampled agonistic interactions by all occurrences, because the activity level was low and the group sizes were small, and we continuously could see all individuals of a group. We recorded the behaviour of resident and bachelor males at a similar frequency.
We defined individuals to be adult when they were older than 2 years and focused on their interactions within the same group. Because we recorded behaviours with several observers, at the beginning of each season we practised observing the same interactions to train all people to note behaviour in the same way. For all agonistic interactions we recorded the initiator of the interaction (namely the one that approached the other), the receiver, the outcome of the fight (the loser being the one who retreated or fled and the other one being the winner) and the agonistic behaviour of both opponents. An interaction ends with one individual walking away or running away. Agonistic acts involved elements of the ethogram, namely attack, fight, chase, flee, displace, retreat, threat, bite and kill (see Supplementary Table S1). Agonistic interactions were subdivided into fierce and mild, with attacking, chasing, biting, fighting, and killing being counted as fierce and threatening, and displacing as mild. If several agonistic elements were observed in an interaction, we categorised the interaction by the element of the highest intensity. The order of the elements, from lowest to highest intensity was: displace, threat, attack, chase, bite, fight, and kill. When comparing the intensity of aggression between the sexes we used the proportion of fights per individual that were of high intensity of all fights that an individual initiated.
Rank order and female dominance over males
We determined the rank order in the dominance hierarchy of adults of both sexes in each group in each year we studied it (group-year), using the Average Dominance Index, ADI, namely the average percentage of conflicts with which each adult was victorious over all its adult opponents (Hemelrijk et al., 2005). It is similar to David’s Score (Gammell et al., 2003), but has a better treatment of missing values as is shown in studies of hierarchical steepness (Saccà et al., 2022) and its computation is simpler and easier to interpret. The degree of dominance of females over males was measured using the Female Dominance Index, FDI, which gives the percentage of males that rank below females on average (Hemelrijk et al., 2008, 2020a). As a robustness measure of the Female Dominance Index, FDI, we calculated two additional measures of female dominance over males: (a) the average percentage per group-year that each female wins fights from each of her male opponents; and (b) the percentage of intersexual dyads (with interactions) in which females won more than half of their fights.
Statistical analysis
To derive a dominance hierarchy, we considered interactions within groups among adult individuals (older than 2 years) that were resident in a group, ignoring bachelor males because they are not integrated in the group. Note that we have only included groups if they contained both sexes, and if at least three individuals were involved in at least one competitive interaction with an opponent.
Data were tested for normality by conducting Shapiro–Wilk tests and examining qq-plots. Where data were normally distributed, parametric tests were used. Otherwise non-parametric tests were used. Data analysis were done in R version 4.1.2 (R Core Team, 2021) and we used packages glmmTMB (Brooks et al., 2017) to conduct GLMMs and LMMs and tested their goodness-of-fit by comparing residuals with simulated residuals using the package DHARMa (Hartig, 2019). Likelihood ratio tests were performed, comparing full models to null models (containing only the intercept and random effects; package lmtest Zeileis and Hothorn, 2002).
We tested the difference in weight and intensity of aggression between females and males using linear mixed models, with ID as a random effect. The relationship between the percentage of males, the Female Dominance Index and percentage of male–male fights (number of fights initiated by males against other males divided by the total number of fights initiated by males towards either male or female adults) were tested using a GLMM with a beta-binomial family to account for possible over-dispersion and with group as a random effect. We investigated whether the presence of late-dispersers in group-years influenced our results by testing in group-years with multiple males, whether the percentage of late-dispersing males in the group was associated with the degree of female dominance (FDI) or the percentage of males. We did this by performing a binomial GLMM with group as a random effect. For this model we included an observation level random effect (OLRE) to reduce over-dispersion (Harrison, 2015). For all linear or general models, we report the estimate and standard error. For significance, we report the likelihood ratio test between the full and null models.
Results
Resident group members
Partial dominance of females over males became clear in several ways. The position of females in the dominance hierarchy among adults (Figure 1), reveals that in 18 of the 27 group-years one or more females had occupied the alpha position exclusively; in 7 cases females shared the alpha position with one or more males; and only in a single case did a male hold the alpha position alone. On average, females dominated 69% of the resident males (Female Dominance Index; Tables 1, 2), meaning that they were subordinate to only 31% of the resident males. The other two measures, based on the percentage of intersexual fights won, provided similar results: females dominated 72% and 67% of the males (Table 2). In our remaining analyses we used the Female Dominance Index, FDI, because this measure was also used in setting up the predictions of DomWorld (Hemelrijk et al., 2008) and testing them in primates (Hemelrijk et al., 2008, 2020b; Izar et al., 2021) and the FDI realistically incorporates both intra- and intersexual interactions when determining female dominance over males. The dominance hierarchy among resident group members shows numerous shared ranks (Figure 1), probably due to the low number of interactions. The frequency of agonistic interactions among adults is low as is typical of this species, per group-year it ranges from 2 interactions in small groups of 3 individuals to 28 in a group of 7 (Table 1).
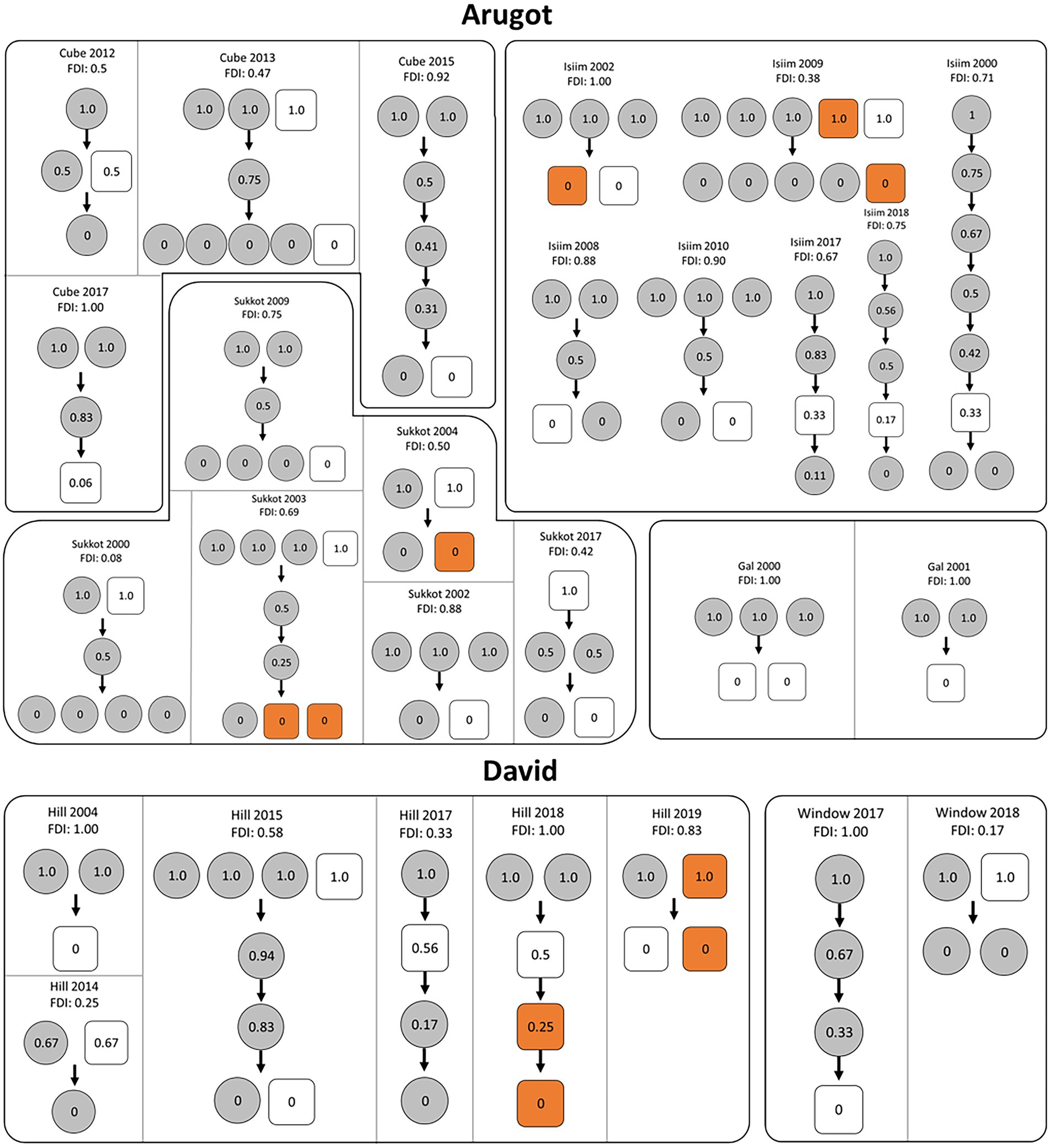
Figure 1. Dominance hierarchies in groups of rock hyrax among adults of both sexes per group-year at two sites, Arugot and David in Ein Gedi, Israel. Partitions indicate name of the group and year of study. FDI represents the Female Dominance Index per group-year. Circles represent females and squares represent males. The average dominance index of each individual is shown in the circles and squares. Late dispersers (males) are indicated in orange.

Table 2. Partial female dominance over males according to three different measurements for interactions between adult group members only and group members with bachelor males.
The requirements of DomWorld regarding sexual dimorphism held true: namely, compared to females, resident males weighed more (average weight of males 2.76 ± 0.07 and of females 2.23 ± 0.03 kg, LMM, ID as random effect, nMales = 25, nFemales = 85, estimate (SE) = 0.52 (0.08), z = 6.12, LRT: χ2 = 29.91, p < 0.001, Supplementary Figure S2A) and the percentage of fights of high intensity initiated by males was greater than that by females (binomial GLMM with ID as random effect nMales = 20, nFemales = 80, estimate (SE) = 1.20 (0.46), LRT: χ2 = 6.43, p = 0.01, Supplementary Figure S2B).
Unexpectedly, the Female Dominance Index, FDI, did not increase significantly with the percentage of males in the group (test 1 in Table 3; Figure 2A), but the percentage of male–male fights did (test 2 in Table 3; Figure 2B). The relationship between the percentage of males in the group and the Female Dominance Index may have been nonsignificant due to the low absolute number of male–male interactions. This was a consequence of the high number of group-years (17 of the 27 group-years) comprising a single male only and the low number of group-years (10) comprising more than a single male (namely 2 or 3 males; Table 1; Figure 2B).

Table 3. Statistical results (GLMM) for the relationship between the percentage of males (predictor) and either the Female Dominance Index (FDI) or the percentage of male–male fights out of all fights involving males with other adult hyraxes of either sex (dependent variable).
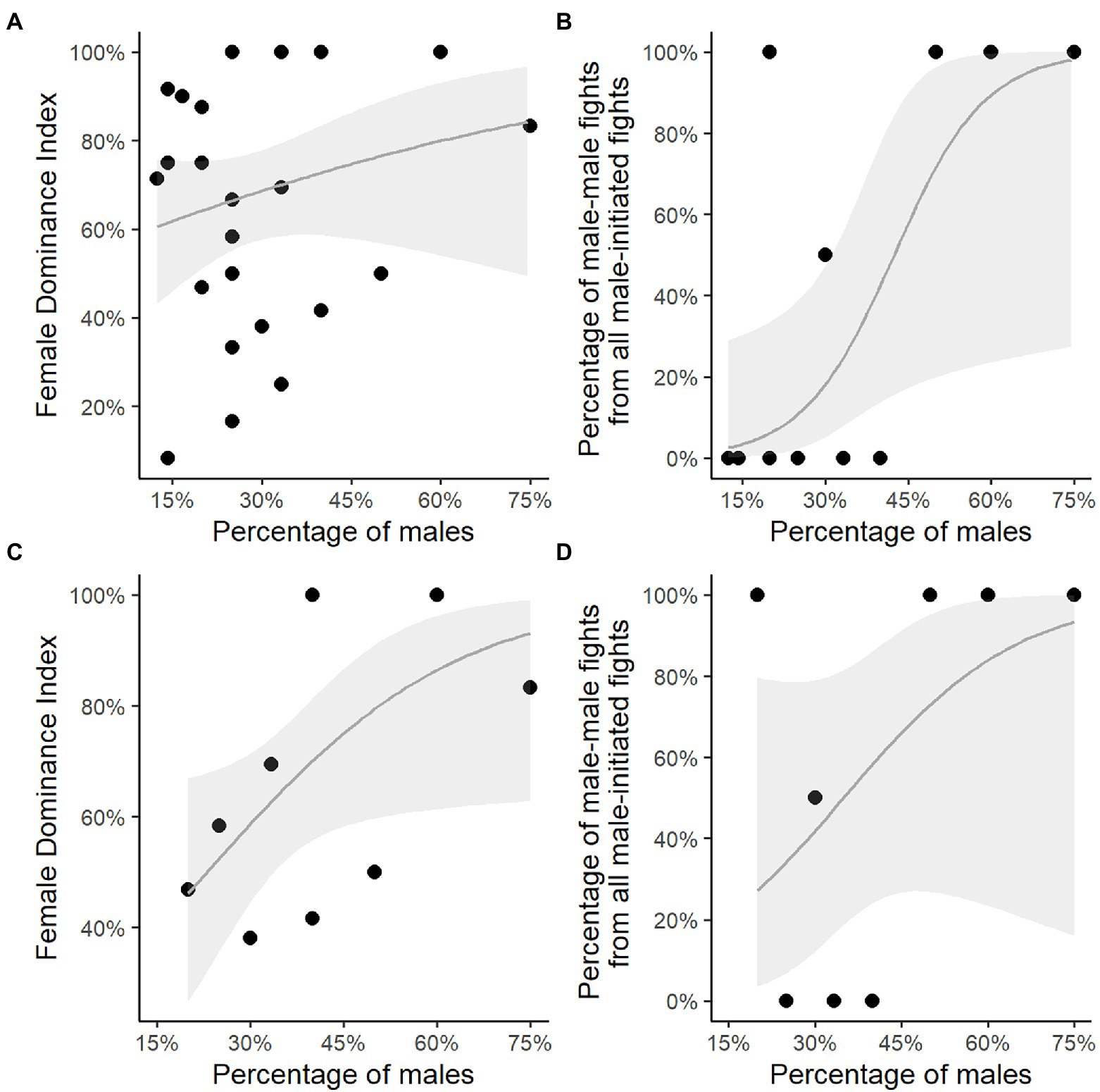
Figure 2. Percentage of resident males in rock hyrax groups versus the Female Dominance Index and the percentage of male–male fights. Percentage of resident males in groups with a single male or multiple males versus (A) the Female Dominance Index; (B) the percentage of male–male fights of male-all fights. Percentage of resident males in multi-male groups only versus (C) the Female Dominance Index; (D) the percentage of male–male fights of male-all fights. The grey line represents the fitted regression line, grey polygons represent the 95% confidence intervals.
When limiting our analyses to group-years with multiple males, by excluding single male group-years (Figures 2C,D), the Female Dominance Index significantly increased with the percentage of males (test 3 in Table 3; Figure 2C). However, the percentage of male–male fights did not increase with the percentage of males in the group (test 4 in Table 3; Figure 2D).
Alternatively, female dominance may increase with a higher percentage of males in the group because in groups with multiple males, some could be young males that have not yet dispersed (late-dispersers) and females may be dominant over these males. We did not find evidence for this type of dominance since in group-years containing multiple males (which we will refer to as multi-male group-years), late dispersers were neither lower in rank than residents (t-test, nMaleResidents = 13, nLate-disperser Males = 10, t = −0.74, df = 18.99, p = 0.47) nor did the degree of female dominance over males increase with the percentage of late dispersers (binomial GLMM with group as random effect, multi-male group-years n = 10, estimate (SE) = 1.9 (1.46), LRT: χ2 = 1.62, p = 0.20).
Including interactions with bachelor males
Because bachelor males did not live permanently in groups (requirement 1 of DomWorld) but interacted now and then with a few groups (Supplementary Figure S3), we did not expect a significant correlation between the Female Dominance Index and proportion of males when including bachelor males.
We confirm that when adding the interactions with bachelor males, the correlation between the Female Dominance Index and proportion of males was not significant (test 5 in Table 3; Figure 3A), also not when only group-years with several males were used (40 group-years, test 7 in Table 3; Figure 3C); nor was the percentage of males related to the percentage of male–male fights (test 6, 8 in Table 3; Figures 3B, 3D).
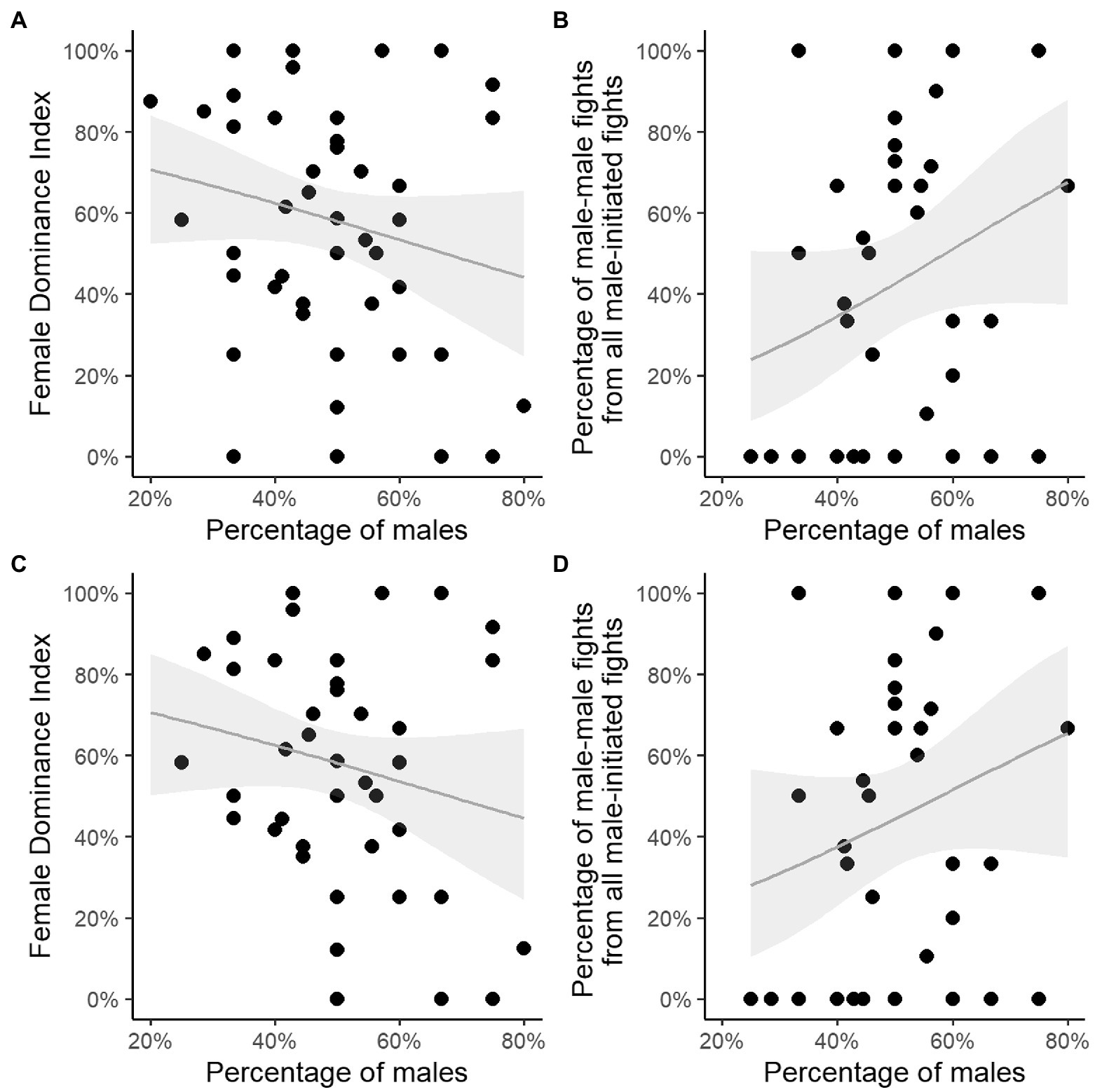
Figure 3. Percentage of resident plus bachelor males in groups with a single male or multiple males versus (A) Female Dominance Index; (B) percentage of male–male fights of male-all fights. Percentage of resident plus bachelor males in multi-male groups only versus (C) Female Dominance Index; (D) percentage of male–male fights of male-all fights. Grey line represents the fitted regression line, grey polygons represent the 95% confidence intervals. Note that some data-points overlap, see T able S3 for all values.
Note that including interactions with bachelor males reduced the degree to which females were dominant over males in all three measurements (Table 2; Supplementary Table S3).
Discussion
The results of the present study support the earlier findings that in the rock hyrax the females dominate most of the males (Koren, 2000; Koren et al., 2006; Koren and Geffen, 2009). Here we show that this dominance exists despite the females weighing less and displaying milder aggression than the males do. Females dominated on average 69% of the males [according to the Female Dominance Index (Hemelrijk et al., 2008, 2020b; Izar et al., 2021)]. This value is consistent with that of our other two measurements, which only included intersexual fights. Such consistency among different measures of female dominance over males has recently been found in a theoretical study and an empirical study on several species of primates, rock hyrax and hyenas (Seex et al., Accepted/In press; Kappeler et al., 2022, this issue). Note that despite the similar values of the different types of measurement, the Female Dominance Index is the most appropriate tool because it was used in the predictions of DomWorld and it is based on the dominance hierarchy including both sexes. Since interactions among individuals of the same sex as well as the opposite sex are likely to lead to the winner-loser effect, both will impact each individual’s ability to win in subsequent fights and therefore the position of each individual (of either sex) in the dominance hierarchy.
In the subset of group-years of rock hyrax that included multiple males, female dominance over males increased with the percentage of males in the group. In line with the self-organisation hypothesis from the DomWorld model (Hemelrijk et al., 2008), this association may arise in rock hyrax from the dynamics of the self-reinforcing effects of winning and losing fights. The self-organisation hypothesis argues that when the percentage of males in the group is higher, females become dominant over more males because of the relatively more frequent male–male fights. This is due to the higher intensity of aggression of males than females. When male–male fights are more numerous, males will be beaten by other males more often, resulting in more males dropping in rank, even below some females (Hemelrijk et al., 2020a; Izar et al., 2021). Yet, the relationship between the percentage of male–male fights and the percentage of males in groups with more than one male was not significant in rock hyrax. This lack of significance may be due to the small sample size of only eight group-years, and the number of males per group-year being small (two or three males). Note that this relationship was significant in the 17 group-years when including groups with a single male.
The relationship between the percentage of males in the group and the Female Dominance Index was significant when considering only multi-male groups. We must note, however, that multi-male groups are rare in the rock hyrax and single-male groups are the norm (Koren, 2006). Thus, in our study, the range in sex ratios among group-years was due to the large range in the number of females rather than males. Our study indicates that an additional, new, seventh requirement is necessary to establish the self-organisation hypothesis of female dominance over males (Hemelrijk et al., 2008). Not only should: (1) individuals live in permanent groups; (2) the agonistic interactions result in the winner-loser effect; (3) body size be larger in males than females; (4) the intensity of aggression be high; (5) and be higher in males than females; and (6) the range of adult sex ratios across groups be sufficiently large; but also, (7) most groups should include multiple males. This is important because the presence of more males increases the average aggression intensity and thus the hierarchical differentiation, which causes stronger overlap in dominance between the sexes. Thus, logically, we do not expect any relation between female dominance and sex-ratio in species that live in one-male groups, such as hamadryas baboons.
Alternatively, female dominance over males may increase with a higher percentage of males due to a higher percentage of young “late-disperser” males. We rejected this alternative explanation because a higher percentage of late dispersers in group-years was not associated with stronger dominance of females over males, and late dispersers were not significantly lower in rank than resident males.
We confirmed that when we violated requirement 1 of permanent group-living, of the self-organisation hypothesis of DomWorld, by including interactions with bachelor males (that were not permanently associated with the group), the relationship between the percentage of males and female dominance was not significant (also not significant when only group-years with multiple males were considered). Thus, in general, when including individuals that do not live permanently in a group, the correlation between percentage of males and female dominance over males is less likely. Thus, this correlation is less likely when dealing with groups in societies that are very loose, such as fission fusion societies where subgroups split up and merge continuously as in chimpanzees unless subgroups are larger, such as in bonobos (Furuichi, 2009).
Whether and why bachelor males in our study of rock hyrax are more dominant over females than resident males requires further investigation. According to the self-organisation hypothesis this may be because bachelor males were less often defeated by resident males, thus their dominance relative to females depended more on their body size (which is larger than that of females) than in resident males. This issue should be further explored in future studies. The self-organisation hypothesis from DomWorld was designed to predict within-group interactions and does not work with the inclusion of outsiders such as bachelor males. To gain more knowledge on dominance in bachelor males, detailed empirical data should be collected by focussing particularly on them. Regarding why bachelor males interact with groups, we hypothesise that they do so in particular to gain access to females. Indeed, bachelor males have been observed to copulate with females at the same rate as resident males (Bar Ziv et al., 2016).
We conclude that female dominance over males is a dynamic trait rather than a static feature (Chase, 1985; Lindquist and Chase, 2009), and may partially rely on the winner-loser effect, because it depends on the adult sex ratio in a group. We have shown that the positive relationship between the percentage of males in the group and the degree of dominance of females over males occurs in the rock hyrax and thus is not limited to primates. Because the general requirements for such a relationship, as presented in the DomWorld model, are met in many species, we expect it to be found also in other animals that are living in permanent groups.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Our study was conducted under annual permits from the Israeli Nature and Parks Authority (NPA) for capturing, handling, and tagging the hyraxes at the Ein Gedi Nature Reserve (2002/14674, 2003/14674, 2004/17687, 2007/27210, 2008/31138, 2009/32871, 2010/37520, 2011/38061, 2012/38400, 2013/38803, 2017/41507, 2018/41880). All procedures performed in this study involving animals were in accordance with the ethical standards of the NPA and the state of Israel.
Author contributions
The scientific conceptualization came from CH and LK. Data were collected and provided by LK, AI, and EG. Data analyses were done by MP and LS. Figures were created by LS. The writing was done by CH with help of LS, LK, AI, and EG. All authors contributed to the article and approved the submitted version.
Funding
The empirical study was supported by grants from and the U.S.-Israel Binational Science Foundation (2015088, 2019156) and the Israel Science Foundation (577/99, 488/05, 461/09, 550/14, 767/16, 244/19, 245/19). LS was supported by the Leverhulme Trust, SAS-2020-026/2. This cooperation came out of the Workshop of the Lorentz Center in Netherlands awarded to CH, “Dynamics of dominance of females relative to males in a group.”
Acknowledgments
The authors are grateful for the logistic support provided by the staff of the Ein Gedi Nature Reserve and the Ein Gedi Field School, and to the Nature and Park Authority for permission to work at the site. The authors thank all the former students, field assistants, and guests for their valuable help in the field, and Naomi Paz for the linguistic editing. We are grateful to the statistician Gerrit Gort for his statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1004919/full#supplementary-material
References
Bar Ziv, E., Ilany, A., Demartsev, V., Barocas, A., Geffen, E., and Koren, L. (2016). Individual, social, and sexual niche traits affect copulation success in a polygynandrous mating system. Behav. Ecol. Sociobiol. 70, 901–912. doi: 10.1007/s00265-016-2112-4
Barocas, A., Ilany, A., Koren, L., Kam, M., and Geffen, E. (2011). Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS One 6:e22375. doi: 10.1371/journal.pone.0022375
Beacham, J. L. (1988). The relative importance of body size and aggressive experience as determinants of dominance in pumpkinseed sunfish, Lepomis gibbosus. Anim. Behav. 36, 621–623. doi: 10.1016/S0003-3472(88)80042-3
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/RJ-2017-066
Chase, I. D. (1985). The sequential analysis of aggressive acts during hierarchy formation: an application of the “jigsaw puzzle” approach. Anim. Behav. 33, 86–100. doi: 10.1016/S0003-3472(85)80122-6
Drews, C. R. (1993). The concept and definition of dominance in animal behaviour. Behaviour 125, 283–313. doi: 10.1163/156853993X00290
Franz, M., McLean, E., Tung, J., Altmann, J., and Alberts, S. C. (2015). Self-organizing dominance hierarchies in a wild primate population. Proc. R. Soc. Lond. B Biol. Sci. 282:20151512. doi: 10.1098/rspb.2015.1512
Frey, D. F., and Miller, R. J. (1972). The establishment of dominance relationships in the blue gourami Trichogaster trichopterus (Pallas). Behaviour 42, 8–60. doi: 10.1163/156853972X00103
Furuichi, T. (2009). Factors underlying party size differences between chimpanzees and bonobos: a review and hypotheses for future study. Primates 50, 197–209. doi: 10.1007/s10329-009-0141-6
Gammell, M. P., de Vries, H., Jennings, D. J., Carlin, C. M., and Hayden, T. J. (2003). David’s score: a more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 66, 601–605. doi: 10.1006/anbe.2003.2226
Glover, T. D., and Sale, J. B. (1968). Reproductive system of male rock hyrax (Procavia and Heterohyrax). J. Zool. 156, 351–362. doi: 10.1111/j.1469-7998.1968.tb04358.x
Harrison, X. A. (2015). A comparison of observation-level random effect and Beta-binomial models for modelling overdispersion in binomial data in ecology & evolution. PeerJ 3:e1114. doi: 10.7717/peerj.1114
Hartig, F. (2019). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.6. Available at: http://florianhartig.github.io/DHARMa
Hemelrijk, C. K. (1999). An individual-orientated model of the emergence of despotic and egalitarian societies. Proc. R. Soc. B Biol. Sci. 266, 361–369. doi: 10.1098/rspb.1999.0646
Hemelrijk, C. K., Wantia, J., and Dätwyler, M. (2003). Female co-dominance in a virtual world: ecological, cognitive, social and sexual causes. Behaviour 140, 1247–1273. doi: 10.1163/156853903771980585
Hemelrijk, C. K., Wantia, J., and Gygax, L. (2005). The construction of dominance order: comparing performance of five methods using an individual-based model. Behaviour 142, 1037–1058. doi: 10.1163/156853905774405290
Hemelrijk, C. K., Wantia, J., and Isler, K. (2008). Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS One 3:e2678. doi: 10.1371/journal.pone.0002678
Hemelrijk, C. K., Wubs, M., Gort, G., Botting, J., and van de Waal, E. (2020a). Dynamics of intersexual dominance and adult sex-ratio in wild Vervet monkeys. Front. Psychol. 11, 10–11. doi: 10.3389/fpsyg.2020.00839
Hemelrijk, C. K., Wubs, M., Gort, G., Botting, J., and van de Waal, E. (2020b). Corrigendum: Dynamics of inter-sexual dominance and adult sex-ratio in wild vervet monkeys. Front. Psychol. 11:2734. doi: 10.3389/fpsyg.2020.598699
Hoeck, H. N., Klein, H., and Hoeck, P. (1982). Flexible social organization in Hyrax. 2. Ticrpsychol 59, 265–298. doi: 10.1111/j.1439-0310.1982.tb00343.x
Hogeweg, P. (1988). “MIRROR beyond MIRROR, puddles of LIFE” in Artificial Life, SFI Studies in the Sciences of Complexity. ed. C. Langton (Redwood City, CA: Adisson-Wesley Publishing Company), 297–316.
Hsu, Y., Earley, R. L., and Wolf, L. L. (2006). Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. Camb. Philos. Soc. 81, 33–74. doi: 10.1017/S146479310500686X
Izar, P., Fernández-Bolaños, M., Seex, L., Gort, G., Suscke, P., Tokuda, M., et al. (2021). Female emancipation in a male dominant, sexually dimorphic primate under natural conditions. PLoS One 16, e0249039–e0249016. doi: 10.1371/journal.pone.0249039
Kappeler, P. M., Huchard, E., Baniel, A., Canteloup, C., Charpentier, M. J. E., Cheng, L., et al. (2022). Sex and dominance: how to assess and interpret intersexual dominance relationships in mammalian societies. Front. Ecol. Evol. 10:918773. doi: 10.3389/fevo.2022.918773
Koren, L. (2000). Hyrax socialization: first evidence for a matriarchal society. Tel Aviv: Tel Aviv University.
Koren, L. (2006). Vocalization as an indicator of individual quality in the rock hyrax. Tel-Aviv University.
Koren, W. B., and Geffen, E. (2009). Androgens and social status in female rock hyraxes. Anim. Behav. 77, 233–238. doi: 10.1016/j.anbehav.2008.09.031
Koren, L., Mokady, O., and Geffen, E. (2006). Elevated testosterone levels and social ranks in female rock hyrax. Hormones and Behavior. 49, 470–477. doi: 10.1016/j.yhbeh.2005.10.004
Lindquist, W. B., and Chase, I. D. (2009). Data-based analysis of winner-loser models of hierarchy formation in animals. Bull. Math. Biol. 71, 556–584. doi: 10.1007/s11538-008-9371-9
Millar, R. P. (1971). Reproduction in the rock hyrax (Procavia Capensis). Zool. Afr. 6, 243–261. doi: 10.1080/00445096.1971.11447418
Neaves, W. B. (1973). Changes in testicular Leydig cells and in plasma testosterone levels among seasonally breeding rock hyrax. Biol. Reprod. 8, 451–466. doi: 10.1093/biolreprod/8.4.451
R Core Team (2021). “R: A language and environment for statistical computing,” R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org
Saccà, T., Gort, G., van de Waal, E., and Hemelrijk, C. K. (2022). Reducing the bias due to unknown relationships in measuring the steepness of a dominance hierarchy. Anim. Behav. 193:131. doi: 10.1016/j.anbehav.2022.09.002
Sale, J. B. (1965). Gestation period and neonatal weight of hyrax. Nature 205, 1240–1241. doi: 10.1038/2051240a0
Seex, L., Saccà, T., and Hemelrijk, C. K. (Accepted/In press). How to measure intersexual dominance? Front. Ecol. Evol.
Vehrencamp, S. L. (1983). A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667–682. doi: 10.1016/S0003-3472(83)80222-X
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. Evol. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Keywords: female dominance, Procavia capensis, self-organisation, sex ratio, intersexual dominance, winner-loser effect, computational model
Citation: Hemelrijk CK, Seex L, Pederboni M, Ilany A, Geffen E and Koren L (2022) Adult sex ratios and partial dominance of females over males in the rock hyrax. Front. Ecol. Evol. 10:1004919. doi: 10.3389/fevo.2022.1004919
Edited by:
Michael Griesser, University of Konstanz, GermanyReviewed by:
Ignacio A. Lazagabaster, University of Liverpool, United KingdomChristian Deschodt, University of Pretoria, South Africa
Copyright © 2022 Hemelrijk, Seex, Pederboni, Ilany, Geffen and Koren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte K. Hemelrijk, Yy5rLmhlbWVscmlqa0BydWcubmw=
 Charlotte K. Hemelrijk
Charlotte K. Hemelrijk Lauren Seex
Lauren Seex Matteo Pederboni1
Matteo Pederboni1 Amiyaal Ilany
Amiyaal Ilany Eli Geffen
Eli Geffen Lee Koren
Lee Koren