
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 11 November 2022
Sec. Chemical Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.1004177
This article is part of the Research TopicMethods in Chemical Ecology: 2022/23View all 8 articles
Morphologically cryptic taxa must be accounted for when quantifying biodiversity and implementing effective conservation measures. Some orchids pollinated by sexual deception of male insects contain morphologically cryptic ecotypes, such as the warty hammer orchid Drakaea livida (Orchidaceae). This species is comprised of three cryptic pollination ecotypes, which can be distinguished based on differences in pollinator species and floral volatiles. The present study aims were: (a) to investigate the geographic range of the three D. livida ecotypes, enabling assessment of their conservation status; and (b) to test the efficacy of different methods of identifying the D. livida ecotypes. Three methods of ecotype identification were assessed: morphometric analysis, genome size comparison, and analysis of chemical volatile composition of labellum extracts from pollinated flowers. MaxEnt species distribution models revealed that each ecotype has a different predicted geographic range, with small areas of overlap at the range margins. One ecotype is known from just ten populations over a limited geographic area, the majority of which has been cleared for agriculture, and urban development. While there was broad overlap between the ecotypes in individual morphological traits, multivariate analysis of morphological traits provided correct assignment to ecotype in 87% of individuals. Using the labellum of pollinated flowers, screening for volatile chemical compounds associated with particular ecotypes returned an even higher correct assignment rate, of 96.5%. As such, we advocate that the use of volatiles from the labellum of recently pollinated flowers is an effective way to determine the ecotype of unknown individuals of D. livida, with minimal impact on the flowering plant.
Morphologically cryptic taxa, being two or more taxa that are difficult to distinguish based on morphology, must be accounted for in order to recognize, and understand the full extent of Earth’s biodiversity (Bickford et al., 2007). Cryptic taxa can pose a major challenge for conservation efforts (Hebert et al., 2004; Bickford et al., 2007; Angulo and Icochea, 2010) through the difficulty of identification in the field and because newly recognized taxa may have a smaller population size and geographic range than the original species complex (Bickford et al., 2007; Niemiller et al., 2013). Following the recognition of cryptic taxa, conservation efforts must adapt accordingly – populations previously thought to be of low conservation value may become an immediate priority if they represent a rare, newly defined taxon. The strategies employed to conserve populations with reciprocal gene flow will also differ to those required to conserve populations between which there is little or no gene flow (Hufford and Mazer, 2003; Brown et al., 2014). Further, the ecological requirements of the cryptic species may differ from each other (Schönrogge et al., 2002). Therefore, in taxa where there is evidence of potential crypsis, this possibility must be fully investigated before appropriate conservation measures can be implemented.
Speciation without obvious morphological divergence can occur in animals that employ non-visual mating signals, such as acoustic (Narins, 1983; Henry, 1994) or chemical (Byers and Struble, 1990; Kozlov et al., 1996) signals. Some plants may also exhibit cryptic variation, including sexually deceptive plants that exploit animal mating signals to achieve pollination. Pollination via sexual deception is achieved when male insect pollinators are sexually attracted to a flower through chemical and/or visual mimicry of conspecific females (Coleman, 1928; Kullenberg, 1961; Stoutamire, 1974; Schiestl et al., 1999). Sexual deception has been recorded in the Asteraceae (Ellis and Johnson, 2010) and Iridaceae (Vereecken et al., 2012), but is most prevalent among the Orchidaceae, with several 100 species of orchid employing this pollination strategy (Schiestl, 2005; Jersáková et al., 2006; Gaskett, 2011; Bohman et al., 2016). As a by-product of mimicking female sex pheromones, which are targeted at conspecific males, sexually deceptive orchids often have just a single pollinator species (Paulus and Gack, 1990; Blanco and Barboza, 2005; Bower and Brown, 2009; Peakall et al., 2010; Phillips R. et al., 2017). Due to the pivotal role of floral chemistry in pollinator attraction, novel pollinators can be attracted via a change in floral odor, which is not necessarily accompanied by morphological divergence (Bower and Brown, 2009; Breitkopf et al., 2013; Peakall and Whitehead, 2014). As such, the attraction of novel pollinators via a shift in floral odor could initiate pollinator-mediated speciation and thereby the formation of cryptic taxa.
Research on the pollination of sexually deceptive orchids has uncovered a growing number of species containing morphologically cryptic variants, some of which may be worthy of taxonomic recognition (Bower, 2006; Bower and Brown, 2009; Breitkopf et al., 2013; Peakall and Whitehead, 2014; Menz et al., 2015; Phillips et al., 2015). In these species, the potential for cryptic ecotypes is initially recognized by the attraction of different pollinator species in different populations of orchids (Bower, 2006; Bower and Brown, 2009; Peakall et al., 2010; Menz et al., 2015; Phillips et al., 2015). Following such an observation, pollinator choice trials, where flowers are presented sequentially to particular pollinator species (Bower, 1996), can be used to test for differences in pollinator response between orchid populations. Further support for the distinctiveness of variants can come from genetic analysis (e.g., patterns of sharing of chloroplast DNA haplotypes; (Peakall and Whitehead, 2014)) or by studying floral volatiles - either through the identification of the exact compounds involved in pollinator attraction (Peakall and Whitehead, 2014) or by comparing overall chemical composition of the floral scent (Véla et al., 2007; Joffard et al., 2016). In some cases, cryptic orchid taxa may also differ in ploidy level, which is reflected in differences in genome size (Trávníček et al., 2010; Gale et al., 2015). In this situation, determining genome size using flow cytometry can provide a cost-efficient and reliable tool for distinguishing taxa (Trávníček et al., 2010).
A recent study on Drakaea livida (Orchidaceae), a species pollinated by sexual deception of thynnine wasps (Hopper and Brown, 2007; Phillips et al., 2013), used pollinator choice trials and chemical analysis of floral volatiles to demonstrate that the species is comprised of a minimum of three ecotypes, each attracting a different pollinator species (Weinstein et al., 2022). These ecotypes were not detected in a recent taxonomic revision of the genus (Hopper and Brown, 2007), and appear to lack obvious differences in morphology. Analysis of the floral volatiles contained in the labellum, a modified petal that releases the sexual attractant (Phillips et al., 2013), revealed that the ecotypes also differed in floral volatile composition (Weinstein et al., 2022). Two of the ecotypes are characterized by containing different sets of pyrazine compounds, but both also contain homovanillyl alcohol. The third ecotype contains neither pyrazines nor homovanillyl alcohol, and is instead characterized by (methylthio)phenol compounds. In addition to the pyrazines, (methylthio)phenols, and homovanillyl alcohol, each ecotype also contained different unidentified compounds, which can be recognized based on their characteristic mass spectra and gas chromatographic retention data (Weinstein et al., 2022). Pyrazines are important for pollinator attraction in some Drakaea, with a blend of pyrazines attracting pollinators in Drakaea glyptodon (Bohman et al., 2014; Bohman and Peakall, 2014), and two pyrazines together with a drakolide attracting pollinators in Drakaea micrantha (Bohman et al., 2019). Further, in some populations of D. livida the attraction of Catocheilus sp. is achieved by a blend of an alkylpyrazine and hydroxymethylpyrazines (Bohman et al., 2012a; Bohman and Peakall, 2014). In other populations, a different hydroxymethylpyrazine is found in flowers attracting male Zaspilothynnus nigripes (Bohman et al., 2012b). This compound is emitted by calling female Z. nigripes and is electrophysiologically active to male Z. nigripes (Bohman et al., 2012b). While attraction to (methylthio)phenols has not been confirmed in pollinators of Drakaea, they are known to play a role in the sexual attraction of thynnine wasps in some Caladenia species that are pollinated by sexual deception (Bohman et al., 2017a,b).
One of the three D. livida ecotypes identified in Weinstein et al. (2022) appears to have a small geographic distribution and is known from a relatively small number of plants, raising the possibility that it may be threatened with extinction. A key impediment to the effective conservation of the D. livida ecotypes is the difficulty of identifying them and establishing their full geographic range. The ecotypes were initially identified based on the attraction of different pollinator species in field experiments, while chemical analyses were also useful in distinguishing the ecotypes. However, both of these methods entail the picking of fresh flowers. Thus, an ideal identification method for the ecotypes would not impact their reproductive success. Further, the ability to conduct pollinator experiments is constrained by the weather conditions, with male thynnine wasps being most active on sunny days ≥20°C (Stoutamire, 1974).
The present study aims were: (a) to investigate the geographic range of the three D. livida ecotypes identified in Weinstein et al. (2022), enabling assessment of their conservation status; and (b) to test the efficacy of different methods in identifying the D. livida ecotypes. In addressing (a), the chemical compounds identified in Weinstein et al. (2022) were used to determine the ecotype of additional populations across the geographic range of D. livida. This larger distribution dataset was then used to generate species distribution models to identify the predicted geographic ranges of the ecotypes. To address (b), three methods of identifying ecotypes were tested. Firstly, we conducted a morphometric analyses on flowers of wild plants, using the traits from the most recent taxonomic revision of the genus (Hopper and Brown, 2007). Secondly, we tested whether the method of identifying ecotypes described in Weinstein et al. (2022), which involved chemical analysis of compounds extracted from the labellum of unpollinated flowers, could be successfully applied to sampling of the labella from pollinated flowers, thereby not affecting reproductive success. Lastly, it was investigated whether potential differences in pollinia genome size between species could be used to distinguish the ecotypes. Variation in genome size has been found in other orchid species (Trávníček et al., 2010; Gale et al., 2015), and intraspecific variation in chromosome number occurs in some members of the Drakaeinae subtribe (Peakall and James, 1989).
Drakaea plants are dormant in summer, develop a single leaf during the late autumn-winter growth period, and flower in spring. Individual plants do not flower every flowering season, and when they do they produce only a single scape bearing a single flower (Hopper and Brown, 2007). The proportion of flowers setting fruit is typically high (>30%) for a deceptive orchid, with greater per-plant reproduction at small population sizes (Phillips et al., 2014). Drakaea flowers achieve pollination by sexually luring a male thynnine wasp into attempting copulation with their labellum, which mimics a female wasp (Peakall, 1990). A hinge located mid-way along the labellum is critical for pollination - the momentum of a male wasp that has grasped the labellum causes them to be swung upside down, with the dorsal side of the thorax contacting the floral reproductive structures (Peakall, 1990). The pollinators of the three pollination ecotypes in D. livida are Z. nigripes (Ecotype 1 sensu Weinstein et al., 2022), Catocheilus sp. (Ecotype 2), and Zaspilothynnus dilatatus (Ecotype 3). In addition to different pollinators, Ecotypes One and Two can be distinguished by different pyrazine compounds in their floral scent, and Ecotype Three by the presence of (methylthio)phenol compounds (Weinstein et al., 2022).
Drakaea livida is endemic to South-West Western Australia, where it spans an approximately 500 km distribution encompassing a variety of different vegetation communities (Hopper and Brown, 2007). The three ecotypes are all almost entirely restricted to well-drained gray sandy soils. All Drakaea species are reliant on the mycorrhizal fungus Tuslasnella secunda for germination and annual growth (Phillips et al., 2014; Linde et al., 2017).
Previous studies have successfully used pollinator choice experiments to determine the ecotype of individual Drakaea flowers (Menz et al., 2015; Phillips et al., 2015; Weinstein et al., 2022). Here we used the methodology of Weinstein et al. (2022) to assign pollinated flowers to ecotypes based on the presence–absence of 20 compounds (Table 1) in extracts of the labellum. Repeating this methodology for additional populations will increase the number of populations for which the ecotype is known, providing data that can be used in species distribution modeling.

Table 1. Characteristic ions and retention indices (RI) of compounds from Weinstein et al. (2022) that were used to assign ecotypes.
Drakaea livida flowers were collected from 22 previously un-sampled populations of unknown ecotype between 2011 and 2018, broadly following the methodology of Weinstein et al. (2022). In brief, labella were extracted in the field in 100 μL of dichloromethane for 24 h. Extracts were kept at –20°C before being analyzed by gas chromatography-mass spectrometry (GC-MS). GC–MS analyses of the floral extracts were conducted using an Agilent 5973 Network Mass Selective Detector connected to an Agilent 6890N Network GC system equipped with an HP5MS-UI column (30 m × 0.25 mm × 0.25 μm film thickness, Agilent), using helium as a carrier gas at 1 mL/min. Peak detection and deconvolution were conducted using the EasyGC python pipeline [based on PyMS python library (O’Callaghan et al., 2012)]1 with the default parameters. The mass spectra of the 20 detected compounds from Weinstein et al. (2022) were added to an AMDIS target library, which was used to individually screen each floral extract for the presence of compounds in the target library, with all extracts being manually checked when a hit occurred. The default AMDIS search settings were applied with the exception of “Sensitivity” which was set to “High.” Screening data was collated in a binary presence–absence matrix. For floral extracts that contained one or more or the 20 ecotype-informative compounds from Weinstein et al. (2022), ecotypes were predicted using a Partial Least Squares Discriminant Analysis (PLS-DA) with the R package “mixOmics” using the default settings with maximum distance as the distance measure (Rohart et al., 2017). As a training dataset (dataset used for model learning), a matrix of presence–absences for ecotype–informative compounds was generated, using the 345 extracts with pollinator data from Weinstein et al. (2022). Populations assigned to ecotypes were checked on a map to see if newly assigned ecotypes fell within or nearby the pre-established ranges of the ecotypes.
To predict the geographic range of the ecotypes, species distribution modeling using MaxEnt was conducted (Phillips S. J. et al., 2017). The analysis was undertaken in R v 3.5.1 (R Core Team, 2018) using the package “dismo” (Hijmans et al., 2017), with bioclimatic variables calculated to a 1 × 1 km scale in ANUCLIM v 6.1 (Xu and Hutchinson, 2011). Default model settings were used: a betamultiplier of one, maximum background points of 10,000, convergence threshold of 1.0E-5, and a default prevalence of 0.5. In addition to abiotic factors, pollinator presence is potentially an important predictor of distributions in plant species with specialist pollination strategies (Duffy and Johnson, 2017). For Ecotypes One and Three, where pollinator distribution data were available, bioclimatic suitability for the pollinator was generated in a separate MaxEnt model, which was included as an explanatory variable in addition to bioclimatic variables. For Ecotype Two, where the pollinator species is not represented in museum collections and shows an infrequent response to orchids, no suitable data were available to model pollinator distribution.
Distribution records for the pollinators of Ecotype One (Z. nigripes) and Three (Z. dilatatus) were obtained from the Western Australian Museum records and from field records from other publications (Phillips et al., 2009, 2013; Menz et al., 2013; Tomlinson and Phillips, 2015; Phillips R. et al., 2017; Phillips and Peakall, 2018; Weinstein et al., 2022). To model pollinator distributions, bioclimatic layers likely to influence pollinator habitat suitability were used. Following the finding of Feng et al. (2019) that MaxEnt is robust to predictor collinearity in model training and can account for redundant variables, variables for the present study were selected based on those that had predictive power in an initial run. Based on the initial run, the following variables had the greatest predictive power: Bio01 annual mean temperature, Bio05 maximum temperature of warmest week, Bio06 minimum temperature of coldest week, Bio10 mean temperature of warmest quarter, Bio11 mean temperature of coldest quarter, Bio12 annual precipitation, bio18 precipitation of warmest quarter, Bio19 precipitation of coldest quarter, Bio20 annual mean radiation, and Bio28 annual mean moisture index.
The resultant bioclimatic pollinator suitability layers were included as an explanatory variable in the models for Ecotypes One (Z. nigripes suitability) and Three (Z. dilatatus suitability). Presence records for each ecotype were based on the pollinator species responding to the flower (Weinstein et al., 2022), and the assignment to ecotypes based on floral chemistry in the present study. Bioclimatic layers were selected for modeling orchid ecotype distributions that influenced the habitat generally, and that were specific to the winter growth months (critical for spring-flowering Drakaea). Following an initial run, layers that had shown predictive power in the initial run were selected: Bio01 annual mean temperature, Bio08 mean temperature of wettest quarter, Bio11 mean temperature of coldest quarter, Bio12 annual precipitation, Bio16 precipitation of wettest quarter, Bio18 precipitation of warmest quarter, Bio24 radiation of wettest quarter, Bio28 annual mean moisture index, and Bio32 mean moisture index of wettest quarter.
Model training regions (the geographic area that model learning is restricted to) for pollinators and orchids were set as the Interim Biogeographic Regionalisation for Australia (IBRA) (Thackway and Cresswell, 1995) bioregions where the species occurred, and any bioregions directly adjoining to bioregions where the species occurred. For species/ecotypes that occurred on the Swan Coastal Plain only, Warren was also included as an adjoining bioregion due to its close proximity to the Swan Coastal Plain bioregion. For Z. nigripes (N = 126 unique locations), 20% of the presence data were withheld for subsequent use in model testing. Data were not withheld for model testing for the other taxa due to their lower number of presence records (Z. dilatatus N = 19, Ecotype One N = 25, Ecotype Two N = 15, Ecotype Three N = 10).
To determine whether the ecotypes differed in their floral morphology, a minimum of 13 flowers from each ecotype were measured (Ecotype One, N = 13 flowers, five populations; Ecotype Two, N = 19, four populations, Ecotype Three, N = 14, six populations) with digital calipers for 17 traits, including those used in the most recent taxonomic revision of Drakaea (Supplementary Table 1; Hopper and Brown, 2007). Significant differences among ecotype floral trait means were tested for using pairwise Holm-corrected t-tests in R v 3.5.1 (R Core Team, 2018). To test for overall differences in morphology, which may not be evident through a trait-by-trait analysis, a Principal Component Analysis was generated from the trait data in R v 3.5.1 (R Core Team, 2018). The ecotypes of flowers were predicted using Linear Discriminant Analysis (LDA) with leave-one-out cross validation, where the model is run N times, with N-1 as the training set and each sample point being predicted individually in a single iteration of the model. LDA was undertaken using the R package “MASS” (Ripley et al., 2013).
Screening for ecotype-informative compounds in extracts of unpollinated D. livida labella can be used to determine their ecotype (Weinstein et al., 2022). To test whether this methodology based on GC-MS could also be successfully applied to labella of pollinated flowers, extracts were made from populations of known ecotype [either identified in this study or in Weinstein et al. (2022)] at various extents of post-pollination withering. The removal of the labellum naturally occurs in rare instances when wasps vigorously attempt to copulate, break the hinge mechanism, and fly off with the labellum. The artificial removal of the labellum after pollination does not adversely affect fruit set–fruit set has been observed on Drakaea with missing labella (A. Weinstein, pers. obvs.). We aimed to determine the extent of post-pollination withering where ecotypes can still be reliably identified. Two metrics were calculated for each flower: the extent of post-pollination withering, and the proportion of the known ecotype-informative compounds detected for the expected ecotype (see Table 2).
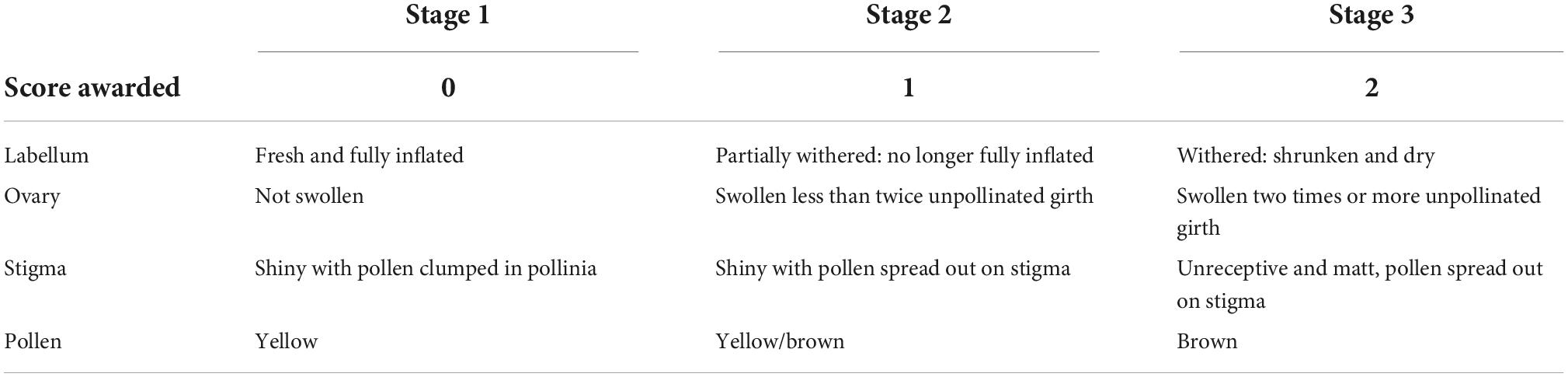
Table 2. Description of the pollination stages of the labellum, ovary, stigma, and pollen that were used to assess degree of post-pollination withering, and the corresponding post-pollination scores awarded.
After being pollinated, in Drakaea the withering of the flower is visible in the deflation of the labellum, and changes in the stigmatic surface, which loses its shiny and sticky appearance and becomes opaque. On initial deposition, the pollen mass remains largely intact and retains its bright yellow coloration. However, as time progresses the original mass loses shape and takes on a brown appearance. At the same time as these changes appear to the labellum and stigma, the ovary begins to swell as the plant nears fruit set. In the present study we investigated at which stage during this post-pollination withering process ecotype-informative chemical compounds were still present. The results of this analysis would provide guidance on which plants could be sampled if using labella from pollinated flowers to identify ecotypes in populations of D. livida.
Floral extracts were made in the field from labella of 85 pollinated flowers at populations of known ecotype (Ecotype One N = 21 flowers, seven populations: Ecotype Two N = 41, eight populations: Ecotype Three N = 23, six populations). Before extracts were made, photos were taken of the labellum, ovary, and stigmatic surface of the flower. To ensure consistency across the dataset, all the photos were assessed in one batch at the end of the flowering season. Four traits were assessed on a scale of three sequential, mutually exclusive stages, and a post-pollination score from 0 to 2 awarded (Figure 1 and Table 2). The total post-pollination withering score was calculated by summing the scores from each trait to give a total score out of eight, where zero represents freshly pollinated and eight represents the most advanced stage of withering.

Figure 1. Flowers displaying different post-pollination stages (stage one being the first stage post-pollination, and stage three being the last) of the labellum, ovary, stigma, and pollen at which chemical sampling was conducted.
Chemical extracts of volatiles from labella of pollinated flowers of each ecotype were made in the field immediately after the plants were photographed, following the methodology of Weinstein et al. (2022). GC-MS and screening for ecotype-informative compounds was conducted as outlined earlier for the assignment of unknown populations to ecotypes. To test for an association between the post-pollination withering score and the proportion of relevant ecotype-informative compounds detected, a generalized linear mixed effects model with the binomial family was conducted using the R package “lme4” (Bates et al., 2015). To account for overdispersion, an observation level random effect was included in the model, where each data point was allocated a unique level of a random effect (Harrison, 2014). The ecotype of the plant was included in the model as a fixed effect. PLS-DA was then implemented to predict the ecotype of the pollinated labella using the data from Weinstein et al. (2022) as a training set, as was done in the assignment of ecotypes to unknown populations.
To investigate whether the ecotypes differed in genome size, flow cytometry was conducted on pollinia from 45 D. livida plants collected from 20 populations, including representatives of all three ecotypes. Pollen was used as this tissue is a reliable standard that is not prone to progressive partial endoreplication, a major problem in orchid flow cytometric analyses (Trávníček et al., 2015). Data were acquired using an Attune NxT acoustic focusing flow cytometer as per Doležel and Bartoš (2005) with a Tris-MgCl2 buffer. For all samples, one of either Pisum sativum (2C = 9.09 pg) or Triodia longiceps (2C = 2.928 pg) was analyzed as a standard with the orchid samples, depending on availability. Data were analyzed in Flowing Software v2.5.1 (freely accessible from),2 and genome sizes calculated using standards as per Doležel and Bartoš (2005). Both distinct 1C and 2C peaks were returned in our analyses (from haploid vegetative nuclei and 2C generative nuclei), as is common in orchid species (Trávníček et al., 2015). Genome sizes were calculated using the 2C values, as these peaks had a lower coefficient of variation error value (CV, calculated as the standard deviation of the peak divided by the mean channel position of the peak, multiplied by 100). Differences in genome size between ecotypes were tested for with an ANOVA in R v 3.5.1.
Analysis of chemical composition enabled the assignment of ecotypes to populations for which the ecotype (and pollinator species) was previously unknown. Of the 74 floral extracts, 95% (70) contained one or more of the ecotype-informative compounds identified in Weinstein et al. (2022). Ecotype-informative compounds were detected in each population. Eleven of the analyzed populations contained compounds indicative of the attraction of Z. nigripes only (assigned Ecotype One in PLS-DA), eight populations contained compounds indicative of the attraction of Catocheilus sp. only (assigned Ecotype Two in PLS-DA), and two populations contained compounds indicative of the attraction of Z. dilatatus only (assigned Ecotype Three in PLS-DA). The locations of these newly identified populations were all congruent with the previously identified ecotype geographic ranges, in that no newly identified populations were disjunct from the known ecotype ranges.
One population situated at the southernmost extent of the range of Ecotype Three, and near the range margins of Ecotypes One and Two, contained compounds indicative of more than one ecotype. Of the 10 samples from this population, eight single-flower extracts contained one or more ecotype-informative compounds identified in Weinstein et al. (2022) (Table 1), while two extracts did not contain any of the compounds from Table 1. Four of these eight extracts contained 2-(methylthio) benzene-1,4-diol, one of which also contained 4-hydroxy-3-(methylthio)benzaldehyde (both indicative of Ecotype Three). All eight extracts also contained homovanillyl alcohol, indicative of Ecotype One or Two (not Three).
MaxEnt modeling supported the results of Weinstein et al. (2022), with the three ecotypes being found to occupy different geographic ranges. All MaxEnt models returned area under the curve (AUC, a common indicator of model performance) values greater than 0.95 within the training area. The Z. nigripes model testing with withheld data returned an AUC value of 0.96. AUC values greater than 0.9 indicate a good discrimination ability of the model (Pearce and Ferrier, 2000), and thus that the predicted geographic ranges are plausible.
When considering the model for Ecotype One (Figure 2), the bioclimatic suitability for Z. nigripes was the explanatory variable with the highest percentage contribution, followed by Bio32 – mean moisture index of driest quarter. The predicted distribution of Ecotype One was primarily near-coastal, comprising most of the Warren IBRA bioregion, and extending west from Albany along the coast into the Jarrah Forest bioregion. The northern limit of the predicted distribution extended into the southernmost part of the Swan Coastal Plain bioregion at Geographe Bay (see IBRA regions in Figure 3).
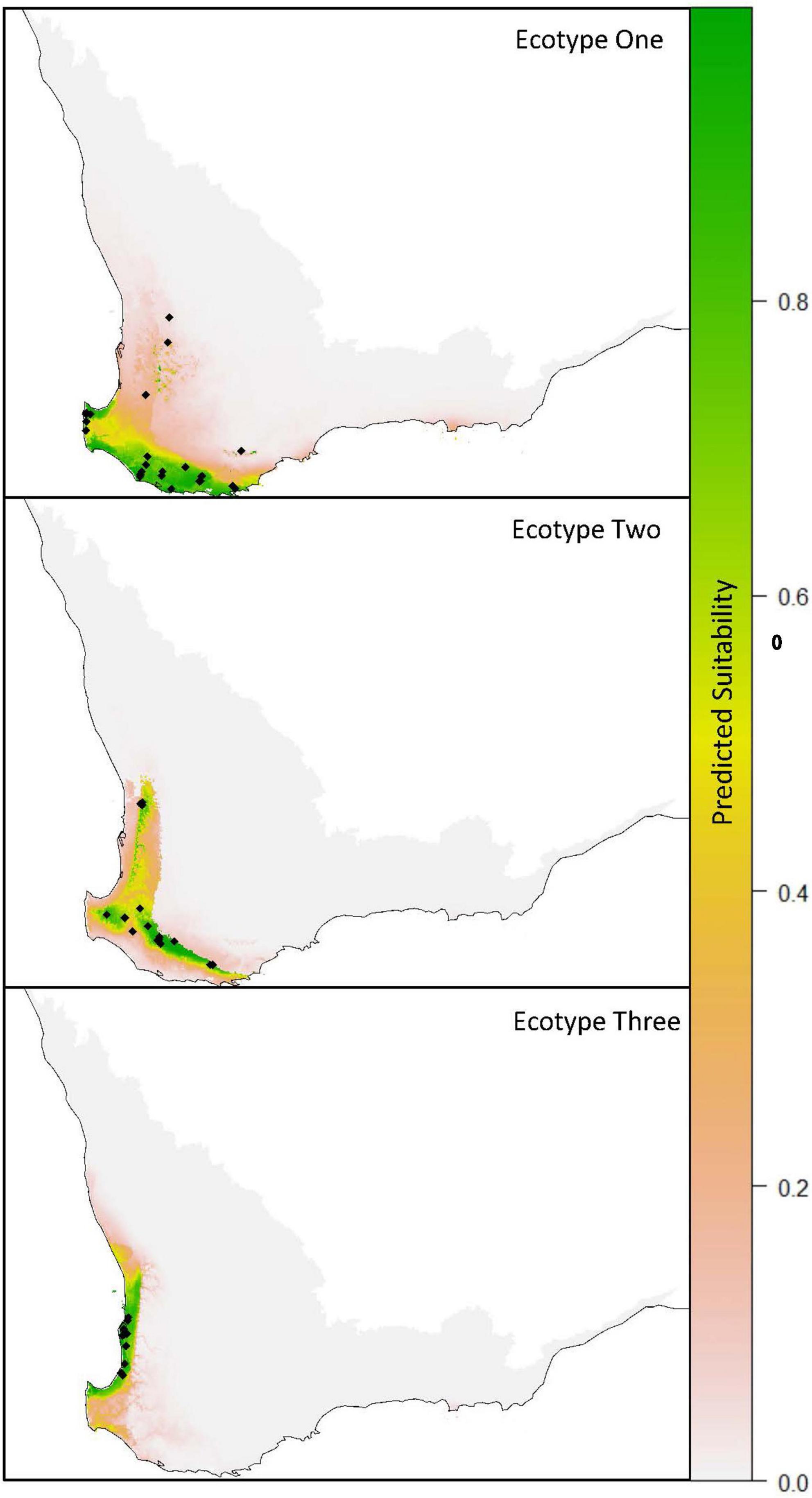
Figure 2. MaxEnt species distribution models for each of the ecotypes of Drakaea livida. Presence points are represented by black dots. Gray denotes the extent of the model prediction area.
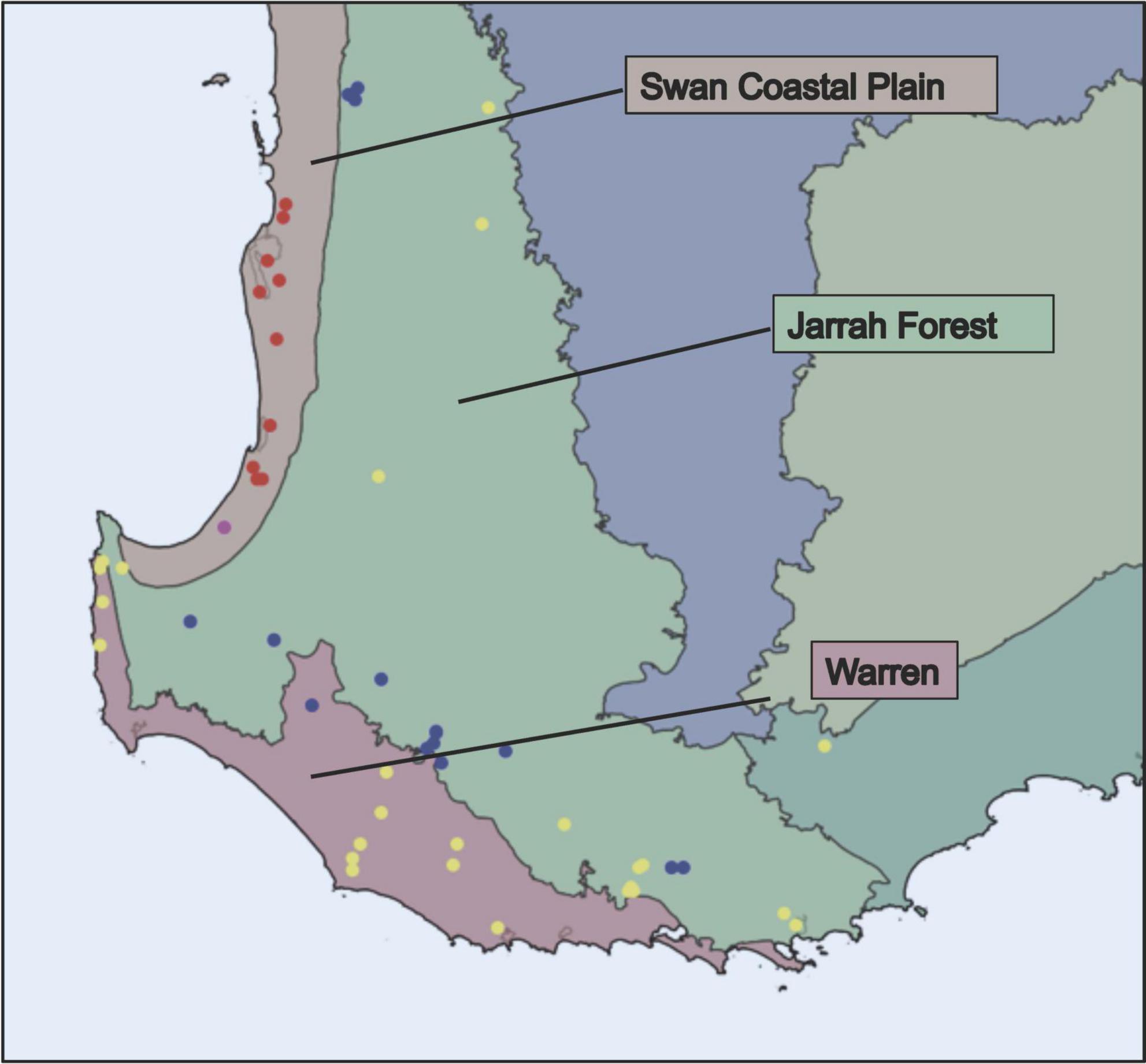
Figure 3. Interim Biogeographic Regionalisation for Australia (IBRA) regions referred to in MaxEnt modeling, and locations of Ecotype One populations (yellow), Ecotype Two populations (blue), Ecotype Three populations (red), and the population that contained flowers with compounds indicative of more than one ecotype (pink).
The habitat suitability for Z. dilatatus and Bio32 were the two variables with the highest percentage contribution in the Ecotype Three model, which predicted Ecotype Three to occur exclusively on the Swan Coastal Plain (Figure 2).
Bio 32 was the variable that explained the most variation in the Ecotype two model, followed by Bio08 mean temperature of wettest quarter, and Bio28 annual mean moisture index (no pollinator data were available for this ecotype). The predicted distribution for Ecotype Two was restricted to the western side of the Jarrah Forest bioregion (Figure 2). While there were some areas of predicted overlap between the ecotypes, each ecotype occupied a distinct core geographic range – Ecotype One on the South Coast, Ecotype Three on the Swan Coastal Plain, and Ecotype Two on the western side of the inland Jarrah forest (Figures 2, 3).
While no single trait could differentiate the ecotypes, they appear to exhibit some divergence in floral morphology. Significant differences were observed between ecotypes in the majority of traits measured, with Ecotype One typically having larger trait values than Ecotypes Two and Three (Supplementary Table 1). For three traits (labellum length, proximal hinge length, and column wing length), the three ecotypes differed significantly from one another (P < 0.05; Figure 4). However, for each trait there was a degree of overlap in the size ranges between ecotypes (Figure 4 and Supplementary Table 1). The ecotypes did not form discrete clusters in a PCA based on all morphological floral traits, but there was some separation on Principal Component 2 of Ecotypes Two and Three, and on Principal Component 3 of Ecotype One from Ecotypes Two and Three (Figure 5). In the LDA with leave one out cross validation, all but six samples (87%) were correctly assigned (i.e., the ecotype assigned matched that of the population). Of the six samples that were not assigned to their chemically defined ecotype, there was one Ecotype One flower, three Ecotype Two, and two Ecotype Three.
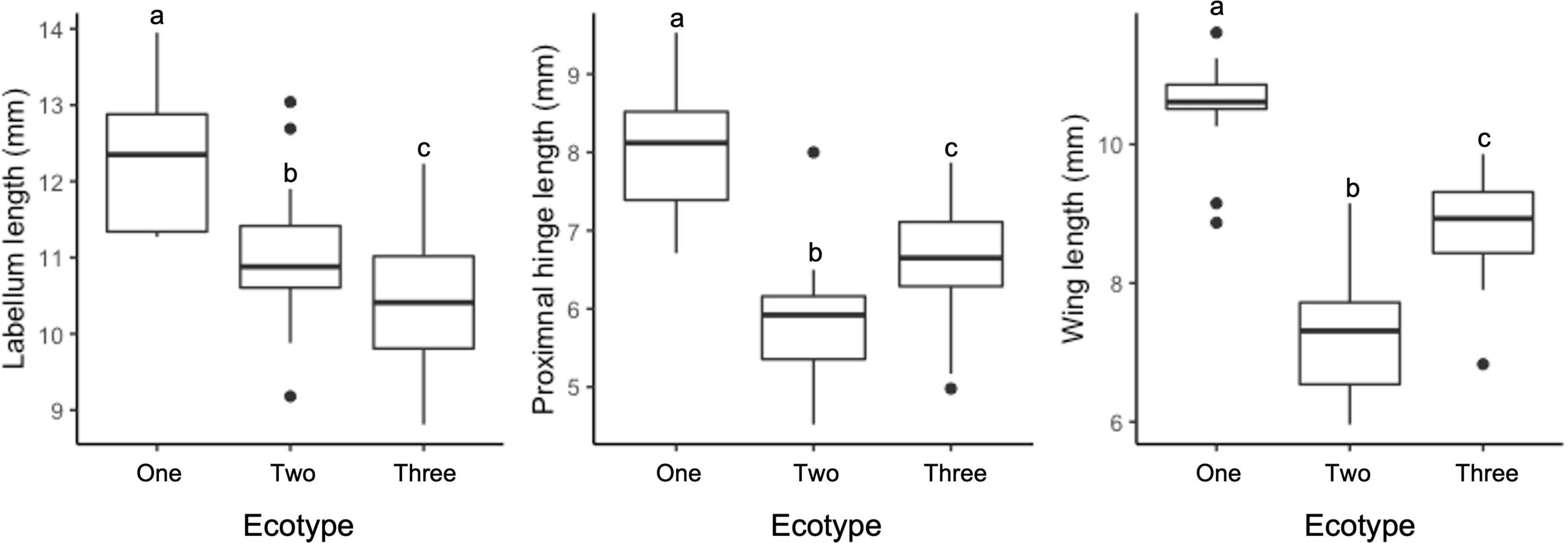
Figure 4. Ecotype means for traits that displayed significant differences between all three ecotypes (P < 0.05); labellum length, proximal hinge length, and column wing length (all in mm). Boxes indicate interquartile ranges with the inner line denoting the median value.
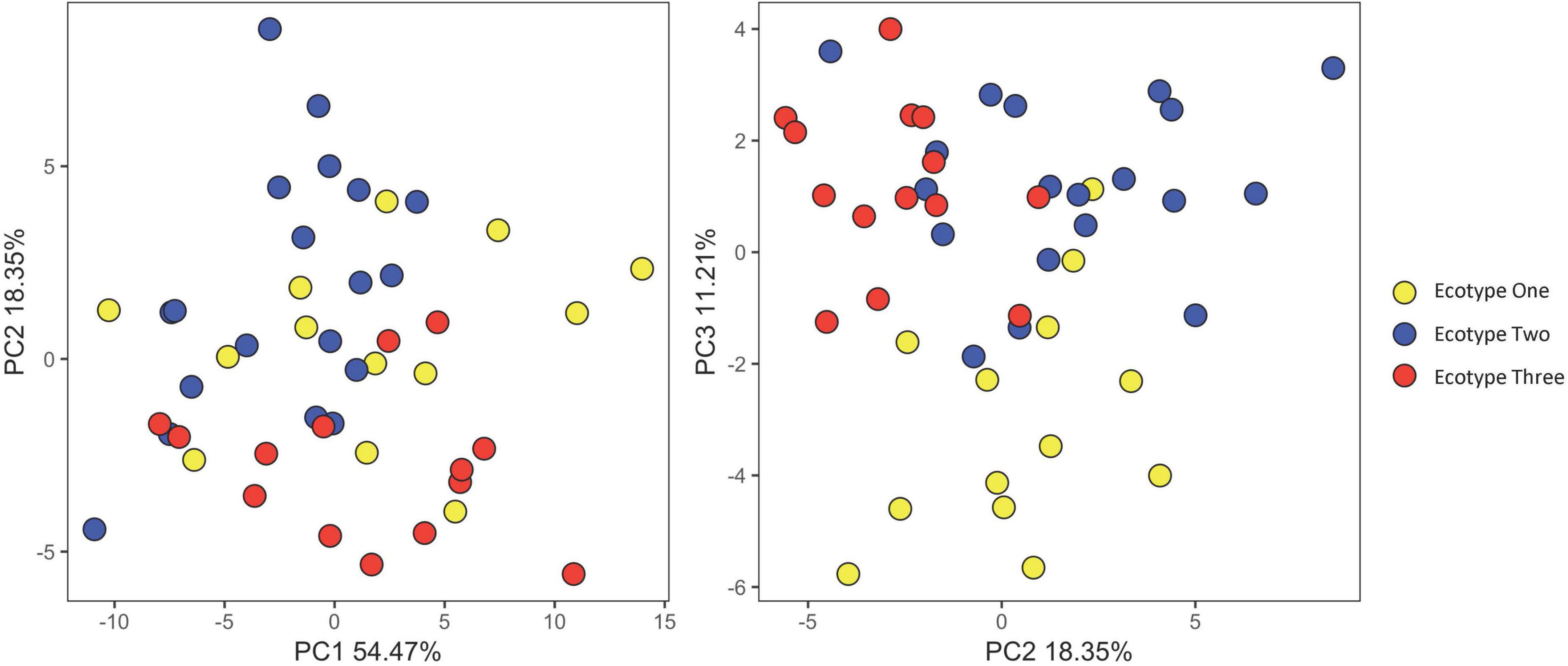
Figure 5. Principal component analysis based off the 17 morphological traits measured for individuals of each ecotype of Drakaea livida.
The sampling of pollinated flowers detected ecotype-informative compounds and allowed the assignment of ecotypes. The average post-pollination withering score was 3.38 ± 0.27 SE out of a possible total of eight (Ecotype One 2.62 ± 0.52 SE, Ecotype Two 3.54 ± 0.41 SE, Ecotype Three 3.83 ± 0.49 SE). The average percentage of ecotype-informative compounds detected per flower was 46.89% ± 2.23 SE (Ecotype One 45.67% ± 5.53 SE, Ecotype Two 45.15% ± 2.73 SE, Ecotype Three 50.00% ± 4.45 SE). Of the 85 flowers analyzed, 82 (96%) were correctly assigned their expected ecotype in the PLS-DA. Extracts from two flowers contained zero ecotype-informative compounds (2.35%, one Ecotype Two, withering score eight; one Ecotype Three, withering score seven). Of the remaining 83 flowers that contained ecotype-informative compounds, 82 (98%) were correctly assigned. The miss-assigned sample (Ecotype Two) only contained one ecotype-informative compound and had the highest possible withering score of eight. There was a significant effect of the extent of post-pollination withering score on the proportion of detected ecotype-informative compounds (P < 0.05) in the generalized linear mixed effects model, with a model estimate of –0.15 and R2 of 0.05 indicating a weak negative correlation. All flowers with a withering score of six or less were correctly assigned (Figure 6).
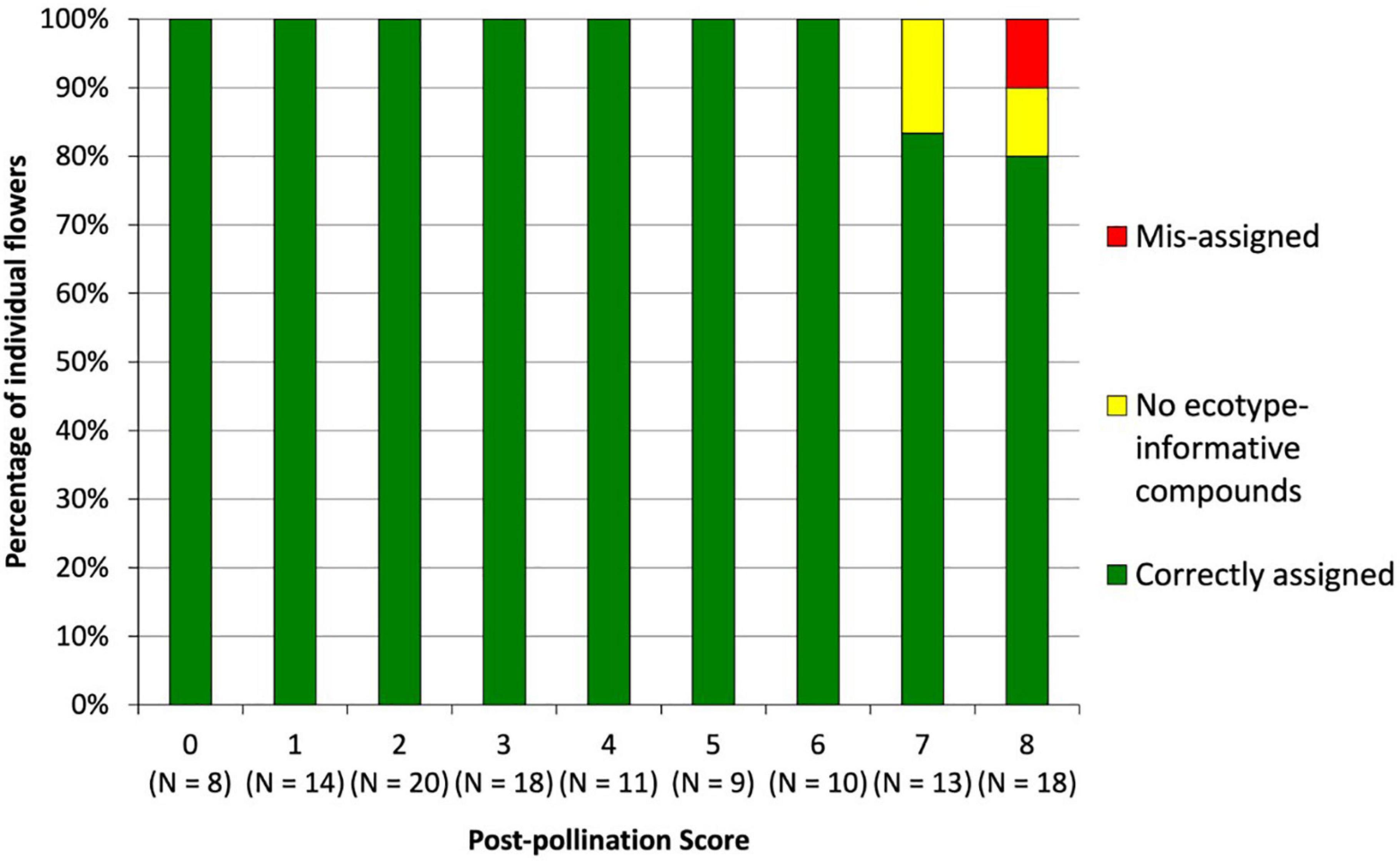
Figure 6. Percentage of individual extracts correctly assigned based on a match with pollination data (green), containing no ecotype-informative compounds (yellow), and miss-assigned (red) by post-pollination score.
There was extensive overlap in the genome sizes of each of the ecotypes (Figure 7). No significant difference in genome size was detected between the ecotypes (Ecotype One 5.17 pg ± 0.006 SE, Ecotype Two 5.24 pg ± 0.009 SE, Ecotype Three 5.26 pg ± 0.011 SE, P = 0.08, Supplementary Table 2).
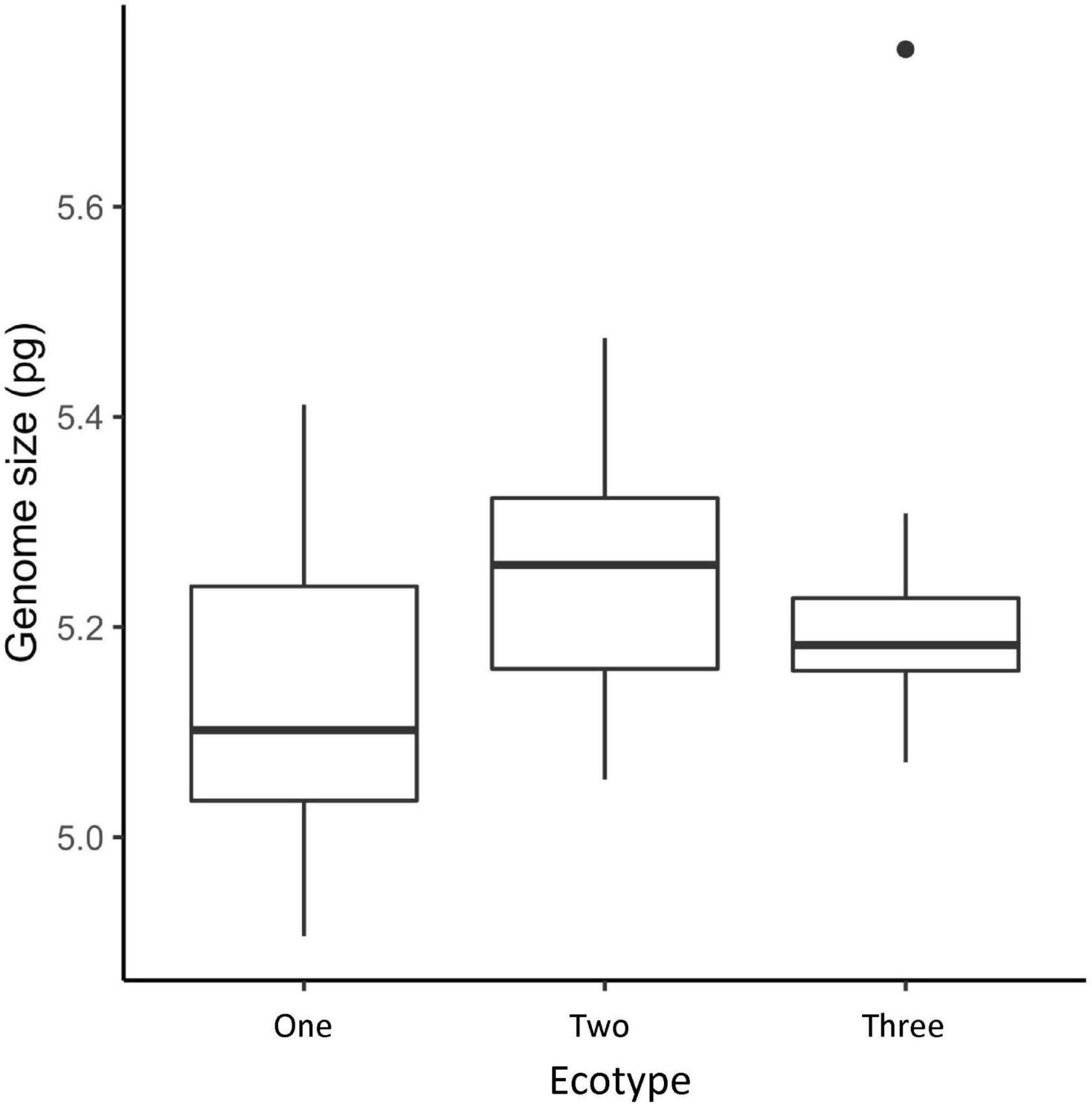
Figure 7. Genome sizes of the Drakaea livida ecotypes in picograms. Boxes indicate interquartile ranges with the inner line denoting the median value.
Species distribution modeling predicted each ecotype to have a different core geographic range, with small areas of overlap predicted at the range margins (Figure 1). Of the three ecotypes, Ecotype One has the broadest geographic range, predominantly occurring on the south coast, but also with isolated patches of suitability in the Stirling Ranges and woodlands east of the Darling Scarp. Ecotype Two has an inland distribution, occurring on the western and southern edge of the Jarrah Forest bioregion. Ecotype Three has the smallest geographic range, being entirely restricted to the Swan Coastal Plain. Ecotype Three has been found to occur in only 10 bushland remnants on the Swan Coastal Plain, which is a known hotspot for orchid rarity (Phillips et al., 2011). Here, regional endemics have become rare through extensive habitat clearing for agriculture and urban development (Shepherd et al., 2002; Horwitz et al., 2008; Phillips et al., 2011) raising conservation concerns for this ecotype. Given that there were areas of predicted range overlap, and that multiple Drakaea species frequently co-occur in suitable habitat (Hopper and Brown, 2007), it is unusual that no populations were found to contain more than one ecotype. The extensive clearing of Ecotype Three habitat has removed much of the habitat where sympatry of ecotypes could have potentially occurred, though rare sympatric populations may yet be revealed in more extensive surveys of the ecotypes’ distributions.
Our analysis is based on the current state of knowledge, where three ecotypes of D. livida have been detected. However, the possibility of additional undiscovered ecotypes of D. livida remains, particularly at the range margins of the species. In these areas, D. livida is often known from very small populations, some of which have not been sampled for floral chemistry or had their pollinator identified. For example, studies of the northern most populations at Watheroo and Wongan Hills, which are in a different biogeographic region to the remaining populations (Hopper and Gioia, 2004; Phillips et al., 2011), would be of particular interest.
Of the 50 populations of D. livida thus far analyzed, 49 contained compounds that are associated with only one of the three pollinator species. The exception was one population in the area of predicted geographic range overlap of Ecotype One and Three, which contained (methylthio)phenols (thus far exclusively found in Ecotype Three) and homovanillyl alcohol (thus far only found in Ecotype One and Two). Given the location of the population at the predicted range margin of Ecotypes One and Three, these flowers may potentially indicate a rare case of hybridization between these two ecotypes. This hybridization scenario is more likely than the flowers representing an undiscovered fourth ecotype given the geographic location of the population, and the similar soil and vegetation type to that of other Ecotype One and Three populations. There is some evidence to suggest that hybridization could have arisen via Z. nigripes, the pollinator of Ecotype One. This species is known to display a partial attraction to Ecotype Two and Three flowers when presented outside their core geographic range (Weinstein et al., 2022), which could potentially lead to occasional cases of pollen transfer.
In D. livida, one limitation of using PLS-DA to identify the ecotype of floral extracts is that potential hybrid populations, such as that detected at the boundary of the Ecotype One and Three predicted ranges, will not be identified as atypical. PLS-DA is trained off the three known groups and will thus only classify samples into these three groups without identifying patterns that may suggest a fourth hybrid grouping. To negate this limitation, it must be ensured that chemical extract data is manually checked, as conducted in the present study, to identify if any samples contain multiple compounds that are normally associated with different ecotypes.
Irrespective of taxonomic recognition, to enable identification of unknown populations and thereby effective conservation management, a practical method for determining the ecotype of a population is required. Ecotype-informative compounds were typically found in pollinated flowers in sufficient quantity to allow identification of ecotypes. Of the 85 labella of pollinated flowers that were analyzed from populations of known ecotype, 82 (96.5%) were correctly assigned to their ecotype using PLS-DA. The three miss-assigned samples all had a post-pollination score of seven or eight (indicating a greatly wilted flower). Therefore, if samples with a post-pollination score greater than six are excluded, analysis of the chemical composition of extracts from labella of pollinated D. livida flowers is a highly accurate method of identifying the ecotype. A previous study on the sexually deceptive orchid Ophrys sphegodes showed that pollinated flowers emitted compounds in different ratios to unpollinated flowers, where the relative amount of hydrocarbons decreased and the relative amounts of some aldehydes and α-pinene increased post-pollination (Schiestl et al., 1997). Quantitative differences in compound abundance may also occur in D. livida, which would not have been detected in the presence–absence analysis. As such, the utility of sampling pollinated flowers, and the need for quantitative versus qualitative sampling, should be validated when working on new species or species complexes. Nonetheless, it is likely that the sampling of pollinated flowers will prove a useful method in other systems where there is a desire to sample without reducing the plant’s reproductive success.
There was broad overlap between the ecotypes in the range of individual floral morphological traits, meaning that ecotypes cannot be confidently distinguished based on trait measurements in the field. However, trait means were often different between ecotypes. This result suggests that some morphological differentiation occurs between the ecotypes, reflected in the 87% correct assignment rate in the LDA. Further investigation using additional traits or methodologies may reveal discriminating traits. The most recent taxonomic revision of the genus (Hopper and Brown, 2007) noted that some populations of D. livida (now recognized as within the geographic range of Ecotype Two) displayed darker coloration and more inflated labella than is typical for the species. Analyses of labellum shape, which may require techniques such as 3D geometric morphometrics (van der Niet et al., 2010), may uncover discrete differences in ecotype floral morphology. Genome size did not prove an informative trait in identifying the ecotypes, which appear to be all of the same ploidy level.
Baiting with fresh flowers to determine the pollinator species attracted (Stoutamire, 1974), which was used in the recognition of the ecotypes (Weinstein et al., 2022), remains an effective method of ecotype identification. However, this method entails the picking of fresh flowers, is weather dependent, and can be more time intensive than the chemical analysis of labella from pollinated flowers. For the D. livida system, of the methods we trialed that did not involve picking whole flowers, GC-MS analysis of volatiles from pollinated labella proved more effective for the identification of ecotypes (96.5% correct assignment) than morphometrics (87%) or estimates of genome size (unable to assign). Given that pollinator switching effected through shifts in floral chemistry is typically associated with speciation in sexually deceptive orchids (Cozzolino and Scopece, 2008; Xu et al., 2012; Peakall and Whitehead, 2014), GC-MS analyses could potentially be implemented to aid in the identification of other morphologically challenging sexually deceptive orchid taxa where differences in floral scent are known to occur.
Thus far, only 10 populations of Ecotype Three have been located. These populations occupy a large proportion of the bushland remnants with suitable habitat on the Swan Coastal Plain. Ecotype Three habitat is readily identifiable - consisting of Banksia woodland with Kunzea ericifolia thickets and an open understory, growing on well-drained gray sandy soils that are typically at lower elevation in the landscape. Each known population of Ecotype Three comprises no more than 150 individual plants (Weinstein and Phillips, unpublished data). At five of the 10 known populations, D. livida was found to co-occur with Drakaea elastica (listed as endangered under the Australian Environment Protection and Biodiversity Conservation Act). However, at four of these populations D. livida was the less numerous of the two species. Using the IUCN Red List Categories assessment, Ecotype Three could be classed as Endangered under Criterion C2 – Population size estimated to number fewer than 2,500 mature individuals and a continuing decline, observed, [estimated], projected, or inferred, in numbers of mature individuals, condition (a) (i) no subpopulation estimated to contain more than 250 mature individuals.
A history of land clearing for agriculture and development of Drakaea habitat on the Swan Coastal Plain (as recent as 2009) has reduced the habitat range and thus population size of both Ecotype Three and the co-occurring D. elastica (Shepherd et al., 2002; Horwitz et al., 2008). Based on known threats to co-occurring species, some of the remaining populations of Ecotype Three are currently threatened by grazing, weeds, and rubbish dumping due to their semi-rural location (Department of Environment and Conservation [DEC], 2009). Many of these threats are exacerbated by edge effects due to the small size of the remnant bushland (Harrison and Bruna, 1999) and by reduced winter rainfall under climate change (McFarlane et al., 2012). Considering the much larger areas of bushland within the predicted ranges of Ecotypes One and Two, where suitable unexplored habitat occurs, it is unlikely that these two ecotypes would qualify for listing as threatened. For effective conservation management to be implemented, such as a formalized recognition as endangered for Ecotype Three, assessment of the taxonomic status of the ecotypes is pivotal.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AW, BB, and RP: conceptualization, methodology, and investigation. AW: formal analysis, writing – original draft preparation, and funding acquisition. AW, BB, CL, and RP: writing – review and editing. CL and RP: supervision. All authors have read and agreed to the published version of the manuscript.
The Holsworth Wildlife Research Endowment and the Australian Systematic Botany Society are thanked for their provision of research funding. AW was supported by an Australian Government Research Training Program (RTP), and BB and RP were supported by the Australian Research Council (ARC) Discovery Early Career Researcher Awards (DE160101313 and DE150101720).
Matt Barrett is thanked for his guidance in conducting the flow cytometric analyses. Tingbao Xu is thanked for his assistance with the MaxEnt analyses. Teresa Neeman and Timothée Bonnet are thanked for their assistance with statistical analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1004177/full#supplementary-material
Angulo, A., and Icochea, J. (2010). Cryptic species complexes, widespread species and conservation: Lessons from Amazonian frogs of the Leptodactylus marmoratus group (Anura: Leptodactylidae). Syst. Biodivers. 8, 357–370. doi: 10.1080/14772000.2010.507264
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bickford, D., Lohman, D. J., Sodhi, N. S., Ng, P. K., Meier, R., Winker, K., et al. (2007). Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22, 148–155. doi: 10.1016/j.tree.2006.11.004
Blanco, M. A., and Barboza, G. (2005). Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Ann. Bot. 95, 763–772. doi: 10.1093/aob/mci090
Bohman, B., and Peakall, R. (2014). Pyrazines attract Catocheilus thynnine wasps. Insects 5, 474–487. doi: 10.3390/insects5020474
Bohman, B., Flematti, G. R., Barrow, R. A., Pichersky, E., and Peakall, R. (2016). Pollination by sexual deception—it takes chemistry to work. Curr. Opin. Plant Biol. 32, 37–46. doi: 10.1016/j.pbi.2016.06.004
Bohman, B., Jeffares, L., Flematti, G. R., Byrne, L. T., Skelton, B. W., Phillips, R. D., et al. (2012a). Discovery of tetrasubstituted pyrazines as semiochemicals in a sexually deceptive orchid. J. Nat. Prod. 75, 1589–1594. doi: 10.1021/np300388y
Bohman, B., Jeffares, L., Flematti, G. R., Phillips, R. D., Dixon, K. W., Peakall, R., et al. (2012b). The discovery of 2-hydroxymethyl-3-(3-methylbutyl)-5-methylpyrazine: A semiochemical in orchid pollination. Org. Lett. 14, 2576–2578. doi: 10.1021/ol300864u
Bohman, B., Phillips, R. D., Flematti, G. R., Barrow, R. A., and Peakall, R. (2017a). The spider orchid Caladenia crebra produces sulfurous pheromone mimics to attract its male wasp pollinator. Angew. Chem. Int. Ed. Engl. 56, 8455–8458. doi: 10.1002/anie.201702864
Bohman, B., Phillips, R. D., Flematti, G. R., and Peakall, R. (2017b). (Methylthio) phenol semiochemicals are exploited by deceptive orchids as sexual attractants for thynnine wasps. Fitoterapia 126, 78–82. doi: 10.1016/j.fitote.2017.09.022
Bohman, B., Phillips, R. D., Menz, M. H. M., Berntsson, B. W., Flematti, G. R., Barrow, R. A., et al. (2014). Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: Implications for the evolution of sexual deception. New Phytol. 203, 939–952. doi: 10.1111/nph.12800
Bohman, B., Tan, M., Phillips, R., Scaffidi, A., Sobolev, A., Moggach, S., et al. (2019). A specific blend of drakolide and hydroxymethylpyrazines–an unusual pollinator sexual attractant used by the endangered orchid Drakaea micrantha. Angew. Chem. Int. Ed. Engl. 59, 1124–1128. doi: 10.1002/anie.201911636
Bower, C. C. (1996). Demonstration of pollinator-mediated reproductive isolation in sexually deceptive species of Chiloglottis (Orchidaceae: Caladeniinae). Aust. J. 44, 15–33. doi: 10.1071/BT9960015
Bower, C. C. (2006). Specific pollinators reveal a cryptic taxon in the bird orchid, Chiloglottis valida sensu lato (Orchidaceae) in south-eastern Australia. Aust. J. Bot. 54, 53–64. doi: 10.1071/BT05043
Bower, C. C., and Brown, G. R. (2009). Pollinator specificity, cryptic species and geographical patterns in pollinator responses to sexually deceptive orchids in the genus Chiloglottis: The Chiloglottis gunnii complex. Aust. J. Bot. 57, 37–55. doi: 10.1071/BT08164
Breitkopf, H., Schlüter, P. M., Xu, S., Schiestl, F. P., Cozzolino, S., and Scopece, G. (2013). Pollinator shifts between Ophrys sphegodes populations: Might adaptation to different pollinators drive population divergence? J. Evol. Biol. 26, 2197–2208. doi: 10.1111/jeb.12216
Brown, R. M., Weghorst, J. A., Olson, K. V., Duya, M. R., Barley, A. J., Duya, M. V., et al. (2014). Conservation genetics of the Philippine tarsier: Cryptic genetic variation restructures conservation priorities for an island archipelago primate. PLoS One 9:e104340. doi: 10.1371/journal.pone.0104340
Byers, J., and Struble, D. (1990). Identification of sex pheromones of two sibling species in dingy cutworm complex, Feltia jaculifera (Gn.)(Lepidoptera: Noctuidae). J. Chem. Ecol. 16, 2981–2992. doi: 10.1007/BF00979489
Coleman, E. (1928). Pollination of an australian orchid by the male ichneumonid Lissopimpla semipunctata, kirby. Trans. Entomol. Soc. London 76, 533–539. doi: 10.1111/j.1365-2311.1929.tb01419.x
Cozzolino, S., and Scopece, G. (2008). Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3037–3046. doi: 10.1098/rstb.2008.0079
Department of Environment and Conservation [DEC] (2009). Glossy-leafed hammer orchid (Drakaea elastica) recovery plan. Western Australia: Department of Environment and Conservation.
Doležel, J., and Bartoš, J. (2005). Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 95, 99–110. doi: 10.1093/aob/mci005
Duffy, K. J., and Johnson, S. D. (2017). Specialized mutualisms may constrain the geographical distribution of flowering plants. Proc. R. Soc. B Biol. Sci. 284:20171841. doi: 10.1098/rspb.2017.1841
Ellis, A. G., and Johnson, S. D. (2010). Floral mimicry enhances pollen export: The evolution of pollination by sexual deceit outside of the Orchidaceae. Am. Nat. 176, E143–E151. doi: 10.1086/656487
Feng, X., Park, D. S., Liang, Y., Pandey, R., and Papeş, M. (2019). Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 9, 10365–10376. doi: 10.1002/ece3.5555
Gale, S. W., Li, J., Kinoshita, A., and Yukawa, T. (2015). Studies in Asian Nervilia (Orchidaceae) V: Nervilia futago, a cryptic new species from southwest Japan confirmed by morphological, cytological, and molecular analyses. Syst. Bot. 40, 413–425. doi: 10.1600/036364415X688772
Gaskett, A. C. (2011). Orchid pollination by sexual deception: Pollinator perspectives. Biol. Rev. 86, 33–75. doi: 10.1111/j.1469-185X.2010.00134.x
Harrison, S., and Bruna, E. (1999). Habitat fragmentation and large-scale conservation: What do we know for sure? Ecography 22, 225–232. doi: 10.1111/j.1600-0587.1999.tb00496.x
Harrison, X. A. (2014). Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2:e616. doi: 10.7717/peerj.616
Hebert, P. D., Penton, E. H., Burns, J. M., Janzen, D. H., and Hallwachs, W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Nat. Acad. Sci. U.S.A. 101, 14812–14817. doi: 10.1073/pnas.0406166101
Henry, C. S. (1994). Singing and cryptic speciation insects. Trends Ecol. Evol. 9, 388–392. doi: 10.1016/0169-5347(94)90061-2
Hopper, S. D., and Gioia, P. (2004). The Southwest Australian floristic region: Evolution and conservation of a global hot spot of biodiversity. Ann. Rev. Ecol. Evol. Syst. 35, 623–50. doi: 10.1146/annurev.ecolsys.35.112202.130201
Hijmans, R. J., Phillips, S., Leathwick, J., Elith, J., and Hijmans, M. R. J. (2017). Package ‘dismo’. Circles 9, 1–68.
Hopper, S. D., and Brown, A. P. (2007). A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators. Aust. Syst. Bot. 20, 252–285. doi: 10.1071/SB06033
Horwitz, P., Bradshaw, D., Hopper, S., Davies, P., Froend, R., and Bradshaw, F. (2008). Hydrological change escalates risk of ecosystem stress in Australia’s threatened biodiversity hotspot. J. R. Soc. West. Aust. 91, 1–11.
Hufford, K. M., and Mazer, S. J. (2003). Plant ecotypes: Genetic differentiation in the age of ecological restoration. Trends Ecol. Evol. 18, 147–155. doi: 10.1016/S0169-5347(03)00002-8
Jersáková, J., Johnson, S. D., and Kindlmann, P. (2006). Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 81, 219–235. doi: 10.1017/S1464793105006986
Joffard, N., Buatois, B., and Schatz, B. (2016). Integrative taxonomy of the fly orchid group: Insights from chemical ecology. Sci. Nat. 103:77. doi: 10.1007/s00114-016-1403-y
Kozlov, M. V., Zhu, J., Philipp, P., Francke, W., Zvereva, E. L., Hansson, B. S., et al. (1996). Pheromone specificity in Eriocrania semipurpurella (Stephens) and E. sangii (Wood)(Lepidoptera: Eriocraniidae) based on chirality of semiochemicals. J. Chem. Ecol. 22, 431–454. doi: 10.1007/BF02033647
Linde, C. C., May, T. W., Phillips, R. D., Ruibal, M., Smith, L. M., and Peakall, R. (2017). New species of Tulasnella associated with terrestrial orchids in Australia. IMA Fungus 8, 28–48. doi: 10.5598/imafungus.2017.08.01.03
McFarlane, D., Stone, R., Martens, S., Thomas, J., Silberstein, R., Ali, R., et al. (2012). Climate change impacts on water yields and demands in south-western Australia. J. Hydrol. 475, 488–498. doi: 10.1016/j.jhydrol.2012.05.038
Menz, M. H. M., Phillips, R. D., Dixon, K. W., Peakall, R., and Didham, R. K. (2013). Mate-searching behaviour of common and rare wasps and the implications for pollen movement of the sexually deceptive orchids they pollinate. PLoS One 8:e59111. doi: 10.1371/journal.pone.0059111
Menz, M. H., Phillips, R. D., Anthony, J. M., Bohman, B., Dixon, K. W., and Peakall, R. (2015). Ecological and genetic evidence for cryptic ecotypes in a rare sexually deceptive orchid, Drakaea elastica. Bot. J. Linn. Soc. 177, 124–140. doi: 10.1111/boj.12230
Narins, P. M. (1983). Divergence of acoustic communication systems of two sibling species of eleutherodactylid frogs. Copeia 4, 1089–1090. doi: 10.2307/1445115
Niemiller, M. L., Graening, G. O., Fenolio, D. B., Godwin, J. C., Cooley, J. R., Pearson, W. D., et al. (2013). Doomed before they are described? The need for conservation assessments of cryptic species complexes using an amblyopsid cavefish (Amblyopsidae: Typhlichthys) as a case study. Biodivers. Conserv. 22, 1799–1820. doi: 10.1007/s10531-013-0514-4
O’Callaghan, S., De Souza, D. P., Isaac, A., Wang, Q., Hodkinson, L., Olshansky, M., et al. (2012). PyMS: A Python toolkit for processing of gas chromatography-mass spectrometry (GC-MS) data. Application and comparative study of selected tools. BMC Bioinformatics 13:115. doi: 10.1186/1471-2105-13-115
Paulus, H. F., and Gack, C. (1990). Pollination of Ophrys (Orchidaceae) in Cyprus. Plant Syst. Evol. 169, 177–207. doi: 10.1007/BF00937674
Peakall, R. (1990). Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Funct. Ecol. 4, 159–167. doi: 10.2307/2389335
Peakall, R., and James, S. (1989). Chromosome numbers of some Australian terrestrial orchids. Lindleyana 4, 85–88.
Peakall, R., and Whitehead, M. (2014). Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Ann. Bot. 113, 341–355. doi: 10.1093/aob/mct199
Peakall, R., Ebert, D., Poldy, J., Barrow, R. A., Francke, W., Bower, C. C., et al. (2010). Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: Implications for pollinator-driven speciation. New Phytol. 188, 437–450. doi: 10.1111/j.1469-8137.2010.03308.x
Pearce, J., and Ferrier, S. (2000). Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Modell. 133, 225–245. doi: 10.1016/S0304-3800(00)00322-7
Phillips, R. D., and Peakall, R. (2018). An experimental evaluation of traits that influence the sexual behaviour of pollinators in sexually deceptive orchids. J. Evol. Biol. 31, 1732–1742. doi: 10.1111/jeb.13370
Phillips, R. D., Brown, A. P., Dixon, K. W., and Hopper, S. D. (2011). Orchid biogeography and factors associated with rarity in a biodiversity hotspot, the Southwest Australian Floristic region. J. Biogeogr. 38, 487–501. doi: 10.1111/j.1365-2699.2010.02413.x
Phillips, R. D., Faast, R., Bower, C. C., Brown, G. R., and Peakall, R. (2009). Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae). Aust. J. Bot. 57, 287–306. doi: 10.1071/BT08154
Phillips, R. D., Peakall, R., Hutchinson, M. F., Linde, C. C., Xu, T., Dixon, K. W., et al. (2014). Specialized ecological interactions and plant species rarity: The role of pollinators and mycorrhizal fungi across multiple spatial scales. Biol. Conserv. 169, 285–295. doi: 10.1016/j.biocon.2013.11.027
Phillips, R. D., Xu, T., Hutchinson, M. F., Dixon, K. W., and Peakall, R. (2013). Convergent specialization–the sharing of pollinators by sympatric genera of sexually deceptive orchids. J. Ecol. 101, 826–835. doi: 10.1111/1365-2745.12068
Phillips, R., Bohman, B., Anthony, J., Krauss, S., Dixon, K., and Peakall, R. (2015). Mismatch in the distribution of floral ecotypes and pollinators: Insights into the evolution of sexually deceptive orchids. J. Evol. Biol. 28, 601–612. doi: 10.1111/jeb.12593
Phillips, R., Brown, G., Dixon, K., Hayes, C., Linde, C., and Peakall, R. (2017). Evolutionary relationships among pollinators and repeated pollinator sharing in sexually deceptive orchids. J. Evol. Biol. 30, 1674–1691. doi: 10.1111/jeb.13125
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E., and Blair, M. E. (2017). Opening the black box: An open-source release of Maxent. Ecography 40, 887–893. doi: 10.1111/ecog.03049
R Core Team (2018). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ripley, B., Venables, B., Bates, D. M., Hornik, K., Gebhardt, A., Firth, D., et al. (2013). Package ‘mass’, Cran R.
Rohart, F., Gautier, B., Singh, A., and Lê Cao, K.-A. (2017). mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13:e1005752. doi: 10.1371/journal.pcbi.1005752
Schiestl, F. P. (2005). On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften 92, 255–264. doi: 10.1007/s00114-005-0636-y
Schiestl, F. P., Ayasse, M., Paulus, H. F., Erdmann, D., and Francke, W. (1997). Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J. Chem. Ecol. 23, 2881–2895. doi: 10.1023/A:1022527430163
Schiestl, F. P., Ayasse, M., Paulus, H. F., Lofstedt, C., Hansson, B. S., Ibarra, F., et al. (1999). Orchid pollination by sexual swindle. Nature 399, 421–421. doi: 10.1038/20829
Schönrogge, K., Barr, B., Wardlaw, J. C., Napper, E., Gardner, M. G., Breen, J., et al. (2002). When rare species become endangered: Cryptic speciation in myrmecophilous hoverflies. Biol. J. Linn. Soc. 75, 291–300. doi: 10.1111/j.1095-8312.2002.tb02070.x
Shepherd, D. P., Beeston, G., and Hopkins, A. (2002). Native vegetation in Western Australia: Extent, type and status. South Perth: Dept. of Agriculture.
Stoutamire, W. P. (1974). Australian terrestrial orchids, thynnid wasps, and pseudocopulation. Am. Orchid Soc. Bull. 43, 13–18.
Thackway, R., and Cresswell, I. (1995). An interim biogeographic regionalisation for Australia: A framework for establishing the national system of reserves, Version 4.0. Canberra: Australian Nature Conservation Agency.
Tomlinson, S., and Phillips, R. D. (2015). Differences in metabolic rate and evaporative water loss associated with sexual dimorphism in thynnine wasps. J. Insect Physiol. 78, 62–68. doi: 10.1016/j.jinsphys.2015.04.011
Trávníček, P., Kubátová, B., Čurn, V., Rauchová, J., Krajníková, E., Jersáková, J., et al. (2010). Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): Evidence from flow cytometry. Ann. Bot. 107, 77–87. doi: 10.1093/aob/mcq217
Trávníček, P., Ponert, J., Urfus, T., Jersáková, J., Vrána, J., Høibová, E., et al. (2015). Challenges of flow-cytometric estimation of nuclear genome size in orchids, a plant group with both whole-genome and progressively partial endoreplication. Cytometry A 87, 958–966. doi: 10.1002/cyto.a.22681
van der Niet, T., Zollikofer, C. P., León, M. S., Johnson, S. D., and Linder, H. P. (2010). Three-dimensional geometric morphometrics for studying floral shape variation. Trends Plant Sci. 15, 423–426. doi: 10.1016/j.tplants.2010.05.005
Véla, E., Tirard, A., Renucci, M., Suehs, C. M., and Provost, E. (2007). Floral chemical signatures in the genus Ophrys L.(Orchidaceae): A preliminary test of a new tool for taxonomy and evolution. Plant Mol. Biol. Report. 25, 83–97. doi: 10.1007/s11105-007-0009-0
Vereecken, N. J., Wilson, C. A., Hötling, S., Schulz, S., Banketov, S. A., and Mardulyn, P. (2012). Pre-adaptations and the evolution of pollination by sexual deception: Cope’s rule of specialization revisited. Proc. R. Soc. B Biol. Sci. 279, 4786–4794. doi: 10.1098/rspb.2012.1804
Weinstein, A. M., Bohman, B., Flematti, G. R., and Phillips, R. D. (2022). Three chemically distinct floral Ecotypes in Drakaea livida, an orchid pollinated by sexual deception of thynnine wasps. Plants 11:260. doi: 10.3390/plants11030260
Xu, S., Schlüter, P. M., and Schiestl, F. P. (2012). Pollinator-driven speciation in sexually deceptive orchids. Int. J. Ecol. 2012, 1–9. doi: 10.1155/2012/285081
Keywords: ecotypes, sexual deception, floral volatiles, cryptic taxa, pollinated flowers, species distribution modeling, genome size
Citation: Weinstein AM, Bohman B, Linde CC and Phillips RD (2022) Conservation assessment of the Drakaea livida (Orchidaceae) ecotypes and an evaluation of methods for their identification. Front. Ecol. Evol. 10:1004177. doi: 10.3389/fevo.2022.1004177
Received: 27 July 2022; Accepted: 07 October 2022;
Published: 11 November 2022.
Edited by:
Ping Wen, Xishuangbanna Tropical Botanical Garden (CAS), ChinaReviewed by:
Tamara Pokorny, University of Regensburg, GermanyCopyright © 2022 Weinstein, Bohman, Linde and Phillips. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan D. Phillips, Ui5QaGlsbGlwc0BsYXRyb2JlLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.