
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 01 February 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.816557
This article is part of the Research Topic Research Advances on Drosophila suzukii View all 7 articles
Among insects, female oviposition preferences are critical to understanding the evolutionary dynamics between herbivores and hosts. Previous studies have shown Drosophila resource use has a strong genetic basis, although there is evidence that preferences are adaptable given isolation from ancestral hosts. Given the high degree of adaptability and behavioral plasticity of invasive species, we were interested in the mechanisms affecting host preferences of the invasive fruit fly, Drosophila suzukii, which in recent years has developed a nearly global range infesting small fruit crops. We studied the diet hierarchies of D. suzukii using a combination of laboratory and field assays designed to assess how female oviposition host choice differs given the availability of, and experience with, different fruit and non-fruit hosts. We found that host preferences did not shift over time and flies reared on two differential isolated diets up to F5 behaved and performed similarly regardless of diet lineage. Rather, female host choice appeared guided by a fixed hierarchical system of host preferences. Raspberry was more preferred to mushroom, which was more preferred to goose manure. However, if preferred resources were absent, the use of less-preferred resources was compensatory. We suggest that among niche specialists, such as D. suzukii, these hierarchies may support a bet-hedging strategy, rather than multiple-niche polymorphism, allowing for niche separation during periods of increased competition, while maintaining more diverse, ancestral feeding behaviors when preferred resources are scarce.
Proximate insect herbivore host preferences are guided by a suite of complex sensory interactions with the environment as a means for detecting suitable host plants for foraging and reproduction (Dethier, 1954). Female insects are capable of discerning hosts even within heterogeneous landscapes and rely on a combination of visual and olfactory kairomones to ensure that her offspring develop in favorable conditions. In addition to nutritional differences among different plants, female host preferences are often driven by factors such as interspecific competition and predator avoidance (Futuyma and Moreno, 1988; Thompson, 1988). For that reason, in some cases, nutritionally inferior plants are used because the benefits of niche separation outweigh the costs of polyphagy (Björkman et al., 1997). In some species, female host choice depends on fixed hierarchies that direct resource use toward those that produce beneficial developmental or reproductive outcomes. There is a clear ranked order to the preference of females for certain oviposition hosts and less-preferred hosts will only be used if more preferred hosts are absent (Jaenike, 1990). For example, the cactus feeding species, Drosophila mojavensis, feeds on decaying cactus in the Sonoran Desert of Southern California and Arizona, down into Mexico and Baja California. As early as the 1970s, ecologists noted that the female host preferences of this species appeared to follow a hierarchical pattern. Agria cactus is heavily preferred over organ pipe, senita, saguaro, or cina, even among populations in which agria is absent, indicating that larval conditioning does not drive host specificity (Fellows and Heed, 1972). However, while these hierarchical preferences are fixed, they are subject to adaptive shifts. Indeed, later research found that in the Sonoran D. mojavensis population, where agria is absent for more than 120 km, females responses to agria are significantly more muted, compared to females from areas where agria is present (Newby and Etges, 1998). Similarly, a study of 13 different strains of Drosophila tripunctata found that flies from different geographic locations have distinct preference patterns reflecting genetic differences among populations that likely occurred over time due to sympatric isolation and differences in selection pressure for differential host use (Jaenike and Grimaldi, 1983).
Among Drosophila, the diversity of host preference mechanisms and host specializations are still being described, as species are found in both arid deserts and lush rainforests, tropical and temperate climates alike. Because Drosophila larvae are restricted to the substrate in which they develop, maternal host selection is critical to offspring survival, performance, and fitness (Whiteman and Pierce, 2008). Although most Drosophila, such as the model species Drosophila melanogaster, are considered saprotrophic generalists, opportunistically feeding and reproducing nearly indiscriminately in rotten and decaying plant material, some are highly specialized, such as Drosophila sechellia, which feeds and reproduces exclusively on the fruit of a single shrub species native to the Seychelles islands (Jones, 2005). Spotted-wing drosophila (SWD), Drosophila suzukii Matsumura, is a niche specialist species closely related to D. melanogaster in the subgenus Sophophora, that uses a large, serrated ovipositor to access ripening, not rotten, soft-skinned fruit, such as raspberries and cherries (Kanzawa, 1939). Since its accidental introduction to the United States and Europe in 2008, D. suzukii has quickly become established throughout fruit producing regions, resulting in devastating losses to berry crops (Walsh et al., 2011; Asplen et al., 2015). Previous research on the behavioral ecology and host use of this species has indicated that it is highly polyphagous and can feed and reproduce on a wide array of crop and non-crop host fruits (Lee et al., 2015). D. suzukii likely evolved its niche separation as a means of escaping intense interspecific competition from nearby and often overlapping temperate Drosophila (Shrader et al., 2020). While its sister species are highly competitive on overripe and rotting fruit, and indeed, D. suzukii is easily outperformed on those resources by D. melanogaster (Dancau et al., 2017), a move to intact fruit likely allowed D. suzukii to access resources otherwise avoided. In addition to developing a specialized ovipositor capable of puncturing through intact fruit skin, D. suzukii also appears to have developed behavioral differences in gravid females favoring the plant canopy and oviposition into intact and attached ripening fruit, as opposed to fruit that has fallen to the ground (Poyet et al., 2015). Further, D. suzukii appears more sensitive than its sister species to leaf volatiles indicative of host plant phenology that allow it to detect suitable hosts in the field (Keesey et al., 2015). Comparative analysis of Drosophila olfaction has shown that at least two odorant receptors responsible for detecting volatiles associated with fermentation in D. melanogaster, Or85a and Or22a, are modified and more responsive to leaf volatiles in D. suzukii (Ramasamy et al., 2016). Meanwhile, microbiological studies have revealed species-specific differences between D. suzukii and D. melanogaster in their attraction to certain yeasts (Jones et al., 2021), which suggests functional differences in the nutritional needs of the larvae and reflects the niche separation these species have developed over time.
Although D. suzukii has moved away from saprotrophic host use, the ability to reproduce and use those ancestral resources remains (Stemberger, 2016). Indeed, D. suzukii may use compost (Bal et al., 2017), tree sap (Kanzawa, 1939), and extra floral nectaries (Tochen et al., 2016) as food sources, and possibly reproductive sites, when preferred resources are scarce, such as the spring or early summer before fruit are available in the landscape. We additionally reported that female D. suzukii fully reproduce in non-fruit resources such as mushroom and even bird manure (Stockton et al., 2019). However, the mechanisms underlying D. suzukii oviposition preferences and the stability of those preferences remains unclear. Do female D. suzukii host preferences function within a fixed diet hierarchy or are they adaptively learned? Are they subject to adaptive shifts over time given repeated, generational exposure to certain hosts types? How rapidly could shifts occur toward a host resource given previous experience? There are a number of reports of artificially breeding polyphagous insects and mites to prefer a certain host plant which was originally less-preferred, indicating that these shifts can occur rather quickly and within just a few generations (Dethier, 1954; Agrawal, 2000). This is particularly relevant to invasive species, such as D. suzukii, given that population radiation is occurring at an unprecedented pace due to human movement and the transport of produce containing D. suzukii eggs (dos Santos et al., 2017). In fact, D. suzukii moved from the West Coast of the United states, where it was first detected in 2008, to the East Coast within just a few years, by 2013 (Asplen et al., 2015). Since that time, D. suzukii is now established in at least seven different climate zones in the United States (Hauser, 2011), Europe (Calabria et al., 2012; Cini et al., 2014), and South America (Deprá et al., 2014; Andreazza et al., 2017) with marked differences in resource availability, both geographically and temporally. The highly invasive nature of this pest is an arguably strong indicator of the degree of physiological and behavioral trait plasticity associated with host range and acceptance (Stockton et al., 2018, 2020; Little et al., 2020).
Factors governing host preference in D. suzukii are of practical importance for several reasons. The first is assessing host risk. Do crops have different levels of risk relative to the abundance of the most preferred oviposition sites? In Central Florida, D. suzukii is not a major pest, despite it’s prevalence in the surrounding area where it is consistently detected in monitoring traps (Dean et al., 2012; Renkema et al., 2018). Meanwhile, in Northern Spain, strawberry is one the primary reproductive hosts reported for D. suzukii (Arnó et al., 2016). We are interested in the extent to which regional differences in crop infestation are associated with differences in rank order availability of preferred hosts. Second, there are implications for pest management and predictive infestation risk. In Northern growing regions of the United States, such as New York, we have been particularly interested in understanding seasonal host use, as preferred resources are scarce or entirely absent for nearly 6 months of the year during the winter/early spring seasons. Do D. suzukii that develop on certain resources at one time of the year respond differently to host cues than those that develop on other hosts? From a management standpoint, could seasonality produce experiential effects on female olfactory responses to monitoring lures? Finally, the ability and propensity to use non-crop hosts is directly relevant to management decisions related to high-risk resources. While fungal fruiting bodies may not be a host during the summer fruiting season, it is unclear whether mushrooms and other non-crop resources could host early season generations derived from overwintered females. If this is the case, those resources could be potentially managed to reduce pest pressure later in the season.
In the present study we aimed to address the most fundamental of these questions: do female D. suzukii host preferences shift based on experiential effects or are preferences fixed and based on an innate hierarchy of host suitability? To test these hypotheses regarding female D. suzukii oviposition preferences, we set up two experiments. In the first (the generations assay), we reared flies on either the standard colony cornmeal diet, or a modified diet using raspberry puree, for five generations. At each generation we assessed adult female oviposition preferences and we conducted morphometric assessments of male and female offspring to determine if host preferences were affected by prior exposure both acutely and over generations. This also allowed us to rule out random fluctuations in host preference (Jaenike, 1983). In the second experiment (diet hierarchy assay), we tested hierarchical effects on female oviposition preferences. We established a ranked host preference order using a single comparative assessment of seven probable host substrates, then used three of those substrates and presented them singly or in combination with each other substrate. Using that approach we were able to compare female host acceptance given situations when preferred hosts are more or less available. We concluded our study by comparing differential host use in the field using the same three substrates. We deployed sentinel traps just before and during the fruiting period in New York during late summer 2020 and compared the number of offspring that developed from each sample. In this way, we were able to observe host use in the field in real time as the phenology of the preferred host crop shifted into availability.
The D. suzukii flies in this study were maintained in a colony at Cornell AgriTech in Geneva, NY. The original colony was found in 2014 using wild flies collected at nearby raspberry farms, although the colony stock was refreshed yearly using introduced females. The colony was maintained indoors in a walk-in growth chamber set at 25 ± 1°C, 16L: 8D light cycle, and 55 ± 5% relative humidity (RH). The colony was fed the standard D. suzukii cornmeal-agar diet (Markow and O’Grady, 2006). We added 50 ml of diet to the bottom of colony bottles (236.5 ml polypropylene drosophila stock bottles; VWR International, Radnor, PA, United States), each of which housed approximately 100 mixed sex flies. The colony bottles were replaced once per week to refresh the diet and promote oviposition.
We reared flies in isolation on two different diets in order to test the hypothesis that diet preferences are affected by previous exposure. Over subsequent generations, we tested the female diet preference of flies for each diet type (oviposition preference) and after the offspring matured (5 days post-eclosion), we collected morphometric data to determine whether body size changed over time in each dietary lineage (Figure 1A). We developed two separate colonies reared on either the standard cornmeal diet (described above) or an alternative diet that substituted 25 g of cornmeal for 200 g of fresh raspberry puree (organic berries purchased at Wegmans Grocery Store, Geneva, NY, United States) per liter. To compensate for differences in carbohydrate content, the amount of white cane sugar used in the diet prep was reduced to 30 g/L in the raspberry diet (compared to 40 g/L in the cornmeal diet). This standardized the carbohydrates to approximately 55 g/L in both diets. Differences in water volume were adjusted by adding water to the raspberry diet minus the volume of the raspberry puree, up to 1 L.
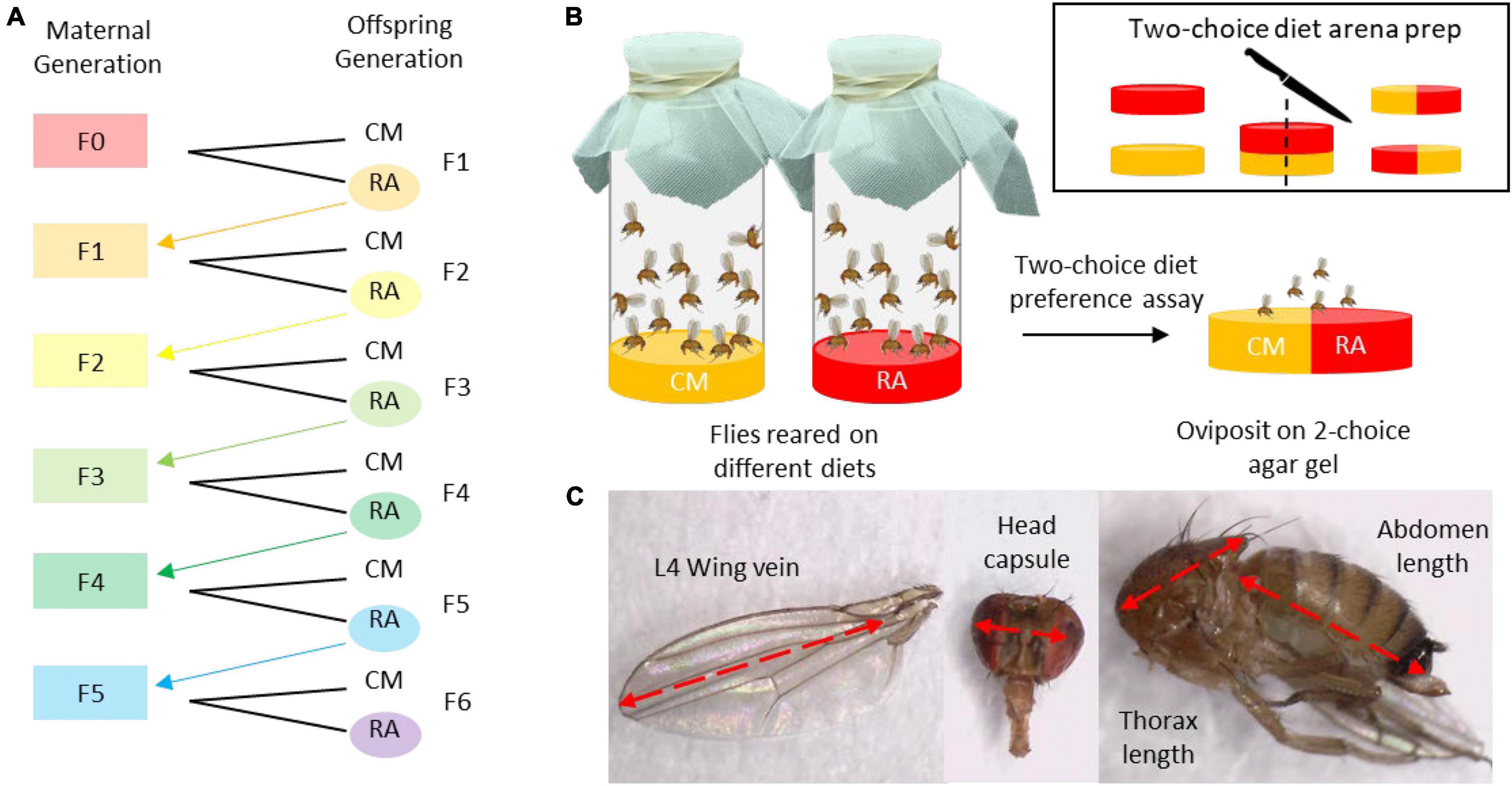
Figure 1. The generations assay was conducted by rearing flies on cornmeal (CM) or raspberry (RA) for successive generations up to F5 (A). Once offspring were sexually mature (5 day post-eclosion), females were presented with a two-choice oviposition assay to quantify differences in oviposition preference among females reared on different diets and at each generation (B). A subsample of males and females from each generation were selected randomly for morphometric assessments in which wing length (L4 wing vein), head capsule width, thorax length, and abdominal length were measured (C).
During the first round of the assay, flies from the cornmeal colony diet were placed on either cornmeal or raspberry diet and allowed to oviposit for 24 h. After 24 h the adult flies were removed and the bottles were stored in the colony room until offspring eclosed. Once the offspring reached sexual maturity 5 days post-eclosion, a subsample of females from each diet were used for the oviposition preference assay (Figure 1B). Oviposition frequency was evaluated using a two-choice diet assay in which five females were placed on an agar diet arena containing two equal diet halves for 24 h (Jaenike and Grimaldi, 1983). The two diets were first prepared separately by pouring 40 ml diet into 4-ounce PLA plastic condiment containers (Fabri-Kal, Kalamazoo, MI, United States) until they set. Then the diets disks were sliced in half and recombined to create two-choice arenas. A total of 30 replicates were run per diet lineage during each generation.
In addition to the subsample used for the behavioral assessment, another subsample including 20–30, 5-day-old males and females were stored in 95% ethanol in a −4°C freezer and used for morphometric assessments (Figure 1C). Using a stereomicroscope, the samples were measured with an ocular micrometer. We recorded left L4 wing vein length (Stockton et al., 2020), head capsule length, thorax length, and abdomen length. The remaining offspring, not used for the behavioral or morphological assessments, were used to start the next generation of flies.
To understand host ranking and develop a crop/non-crop host use profile, we ran a preliminary host range assessment to pre-determine rank host order. We conducted a screening assay that compared survival and reproduction on seven probable D. suzukii host substrates including: blueberry, raspberry, grape, tomato, mushroom, goose manure, and cow manure. Organic blueberries (Vaccinium corymbosum), red raspberries (Rubus idaeus), grapes (Vitis labrusca var. ‘Condord’), tomatoes (Solanum lycopersicum var. cerasiforme), and white button mushrooms (Agaricus bisporus) were purchased at Wegman’s grocery store in Geneva, NY. Goose manure was collected near Geneva, NY near various ponds where wild Canada Geese (Branta canadensis) tended to gather. Domestic cow manure was sourced from a dairy farm in Stanley, NY. To avoid the risk of non-target infestation in our manure samples, we froze the manure samples for at least 3 days at −4°C prior to use. For each diet, we prepared five replicate arenas (16-ounce (473 ml) deli containers; Fabri-Kal, Kalamazoo, MI, United States) containing 10 g of whole host substrate material placed on 20 ml agar gel (9 g agar per L water). We released 20 adult females (5–7 days old) into each arena for 24 h. Afterward, we assessed female survival. The samples were stored in the colony room until offspring eclosion, approximately 10 days later, when the number of offspring on each substrate was counted.
We directly tested the hypothesis of female host preference hierarchies using an ABC assay. This type of assessment presented three diet alternatives in varying combinations. Using this method, we were able to determine whether female host preferences were fixed or determined by resource availability. In total there were seven different arena presentations: 1 choice (raspberry, mushroom, or goose manure alone), 2-choice (raspberry + mushroom, raspberry + goose manure, or mushroom + goose manure), or 3-choice (raspberry + mushroom + goose manure). The substrates we selected for this assay were based on the host rank screening described above. A fruit, a fungal host, and manure were chosen to better understand how host use may shift from fruit to non-fruit given availability. Each diet was prepared as previously described for the raspberry agar diet, with raspberry substituted by mushroom or goose manure. We released 20 females (aged 5–7 days post-eclosion) into each arena for 24 h and counted the number of eggs laid in each diet target. In total, we conducted 15 replicates per treatment.
We placed sentinel traps in the field for 24 h containing different oviposition substrate targets (raspberry, mushroom, or goose manure) and counted the number of D. suzukii to emerge. Each trap contained approximately 10 g of substrate material. We placed three replicate traps per substrate at four different field sites in Geneva, NY. The traps were deployed on three dates just before and during the early stages of the raspberry fruiting period in that area of New York (July 8, July 22, and August 10, 2020). Two of the field sites (Darrow Farm and Research North) contained a raspberry field (R. idaeus var. ‘Caroline’), while the other two sites (Robbins Farm and the Vignoles site) contained trellised grape vines (Vitis vinifera, V. labrusca, V. vinifera interspecific hybrids interplanted at Robbins Farm; V. vinifera-V. rupestris interspecific hybrid at the Vignoles site).
Statistical analyses were conducted using R (The R Foundation for Statistical Computing) version 4.0.5. For the generations assay we compared diet choice in the 2-choice oviposition experiment using linear regression. The percentage of eggs laid in raspberry was compared between raspberry reared and cornmeal reared flies at each successive generation beginning with generation F1. Model assumptions were verified using diagnostic plots of the residuals to assess normality and heteroscedasticity. Next, we compared the effects of diet lineage, rearing diet, fly sex, and maternal generation (Fn) on body size measures. Interaction terms were included for each factor except fly sex. Because the data were not normally distributed, the data were log10 transformed due to positive skew. Levene’s was used to validate homogeneity of variances following transformation. Because only L4 wing vein length and abdominal length showed equal variances following transformation, only these measures were used for further analysis. We conducted a multivariate analysis using generalized linear models with gaussian distribution. Post hoc pairwise comparisons (Fisher LSD) were conducted using the package emmeans. The results are reported as significant differences among groups between each generation.
For the host rank screening we compared 24 h survival of female D. suzukii on seven host substrates using logistic regression. Post hoc analysis of different between groups was conducted using Tukey LSD using the package emmeans. For the diet hierarchy assay we compared the oviposition responses among females given different substrate combinations, we used Poisson regression to model oviposition to each diet separately. We then used post hoc pairwise comparisons to determine ranked differences in oviposition among groups using the package emmeans.
The mean number of eggs laid by cornmeal reared flies was greater ( = 43.22 ± 2.22) than the number of eggs laid by flies reared on raspberry ( = 22.39 ± 1.65) (Figure 2A). Raspberry reared flies laid 51.62% of eggs in raspberry compared to cornmeal, whereas cornmeal reared flies laid only 42.80% of eggs in raspberry diet. However, although there were differences in the proportion of eggs laid on raspberry diet among successive generations (Table 1 and Supplementary Table 1), there was no discernable pattern indicating that reproductive isolation on a single diet led to an increase in oviposition on that diet. Rather, there was a decrease in the proportion of eggs laid on raspberry by raspberry reared flies over time.
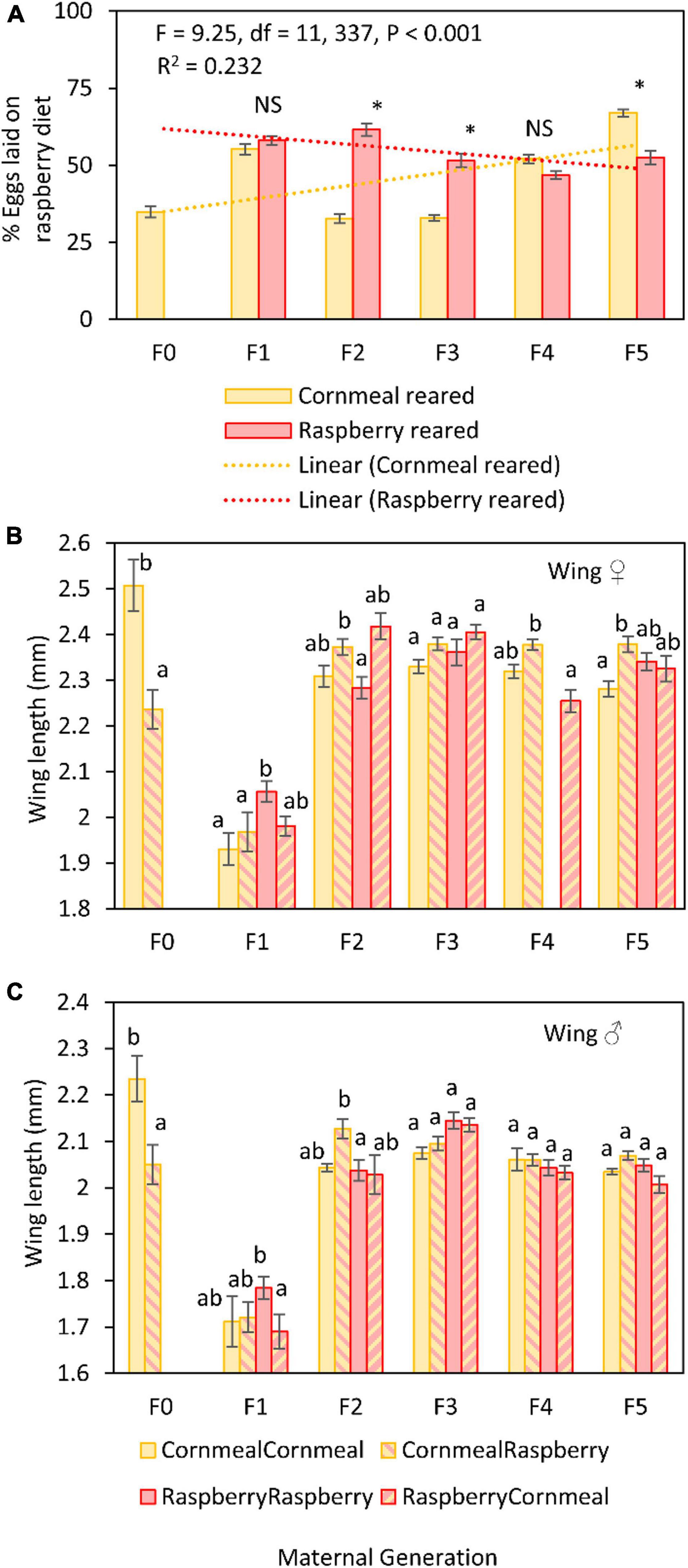
Figure 2. Comparative differences in the percent eggs laid on raspberry diet in a 24 h two-choice oviposition assay between D. suzukii reared on raspberry or cornmeal diet at each successive generation (A). Asterisks indicate statistical differences between groups in the proportion of eggs laid on raspberry, *≥0.05. Morphometric assessments of L4 wing vein length in females (B) and males (C) reared on different diet treatments: Cornmeal lineage, reared on cornmeal (CornmealCornmeal); Cornmeal lineage, reared on raspberry (CornmealRaspberry); Raspberry lineage, reared on raspberry (RaspberryRaspberry); raspberry lineage, reared on cornmeal (RaspberryCornmeal). Different letters indicate statistically significant differences among treatment groups.
Table 1. Modeling the effect of rearing diet (cornmeal, raspberry) and maternal generation (F1–F5) on subsequent D. suzukii diet preference in a two-choice oviposition assay using linear regression.
Fly sex, diet lineage, and maternal generation were the most significant factors affecting D. suzukii body size (Table 2 and Supplementary Table 2). Rearing diet was also a significant factor, although the effect size of this factor was less robust. The effect of sex on body size was expected given known sexual dimorphism in this species; females were approximately 8–12% larger than males (Figures 2B,C). To discern whether diet lineage produced a shift in size over time, we looked at the difference between two groups in particular, cornmeal lineage flies reared on raspberry and raspberry lineage flies reared on raspberry. When we looked at the differences between these two key groups, we did not find significant differences in favor of selection for favorable development on raspberry at any point except at F1, where “RaspberryRaspberry” flies were larger than “CornmealRaspberry” flies. For this reason, maternal generation effects were likely derived from the large dip in size that occurred uniformly during the F1 generation, rather than an increase in fitness associated with diet lineage. All other generations showed similar size patterns.
Table 2. Generalized linear model summary of the effects of diet lineage, rearing diet, maternal generation, and fly sex on D. suzukii offspring wing length (log10 transformed) measures.
Female survival on the seven crop and non-crop hosts followed a different pattern than offspring eclosion frequency (Figure 3A). Survival was similar among females placed on blueberry (99.1%), mushroom (99.1%), raspberry (94.4%), cow manure (93%), and grape (86.9%). The lowest survival was on tomato (79.6%), followed by a large decrease on goose manure (7.3%) (Logistic regression: X2 = 354.36, df = 6, 28; P < 0.001; Supplementary Table 3). In contrast, the number of offspring that were produced on each host favored fruit (Figure 3B). The greatest reproduction occurred on blueberry (Mean = 71.4 ± 9.8 offspring per sample replicate), while the least occurred on cow manure (Mean = 1.6 ± 0.7 offspring) (Poisson regression: X2 = 852.75, df = 6, 28; P < 0.001; Supplementary Table 4).
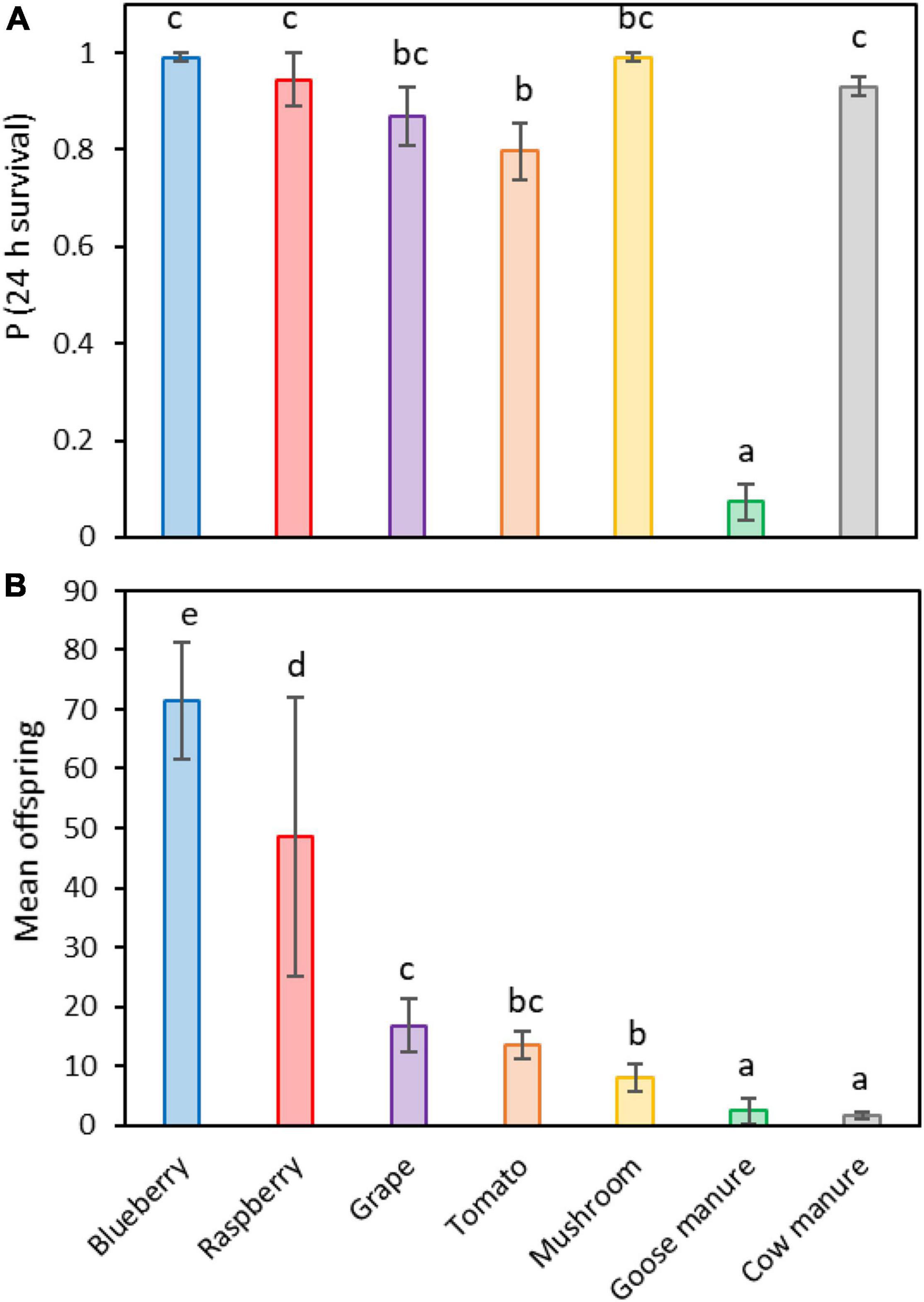
Figure 3. Differences in maternal survival (A) and the mean number of offspring that were reared on each of seven different potential host substrates (B). Different letters indicate statistically significant differences among groups, Tukey contrasts (α ≤ 0.05).
Presentation of three diets with known preference levels altered the response to each diet depending on the availability of preferred substrates (Tables 3, 4). For the most preferred diet substrate, raspberry (RA), oviposition on raspberry was greatest on raspberry when no other choices were available (Figures 4A,B). Similarly, oviposition was greatest on mushroom and manure when they were presented as the single diet choice. However, when a second diet was introduced, preference for each diet shifted depending on whether the second choice was lesser or more preferred relative to the first diet. For example, mean oviposition on mushroom increased (Mean = 18.67 ± 4.18 eggs) when goose manure was introduced (a less preferred diet), while mean oviposition decreased (Mean = 6.4 ± 1.81 eggs) if raspberry was introduced (a more preferred diet). Oviposition on goose manure was lowest (Mean = 0.46 ± 0.16 eggs) when both raspberry and mushroom substrates were available (RA-MU-GO).
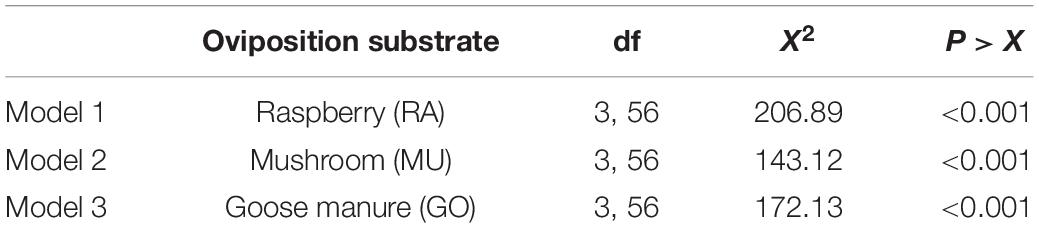
Table 3. The significance of three individual Poisson models evaluating the effect of differential substate availability on mean oviposition frequencies on each diet.
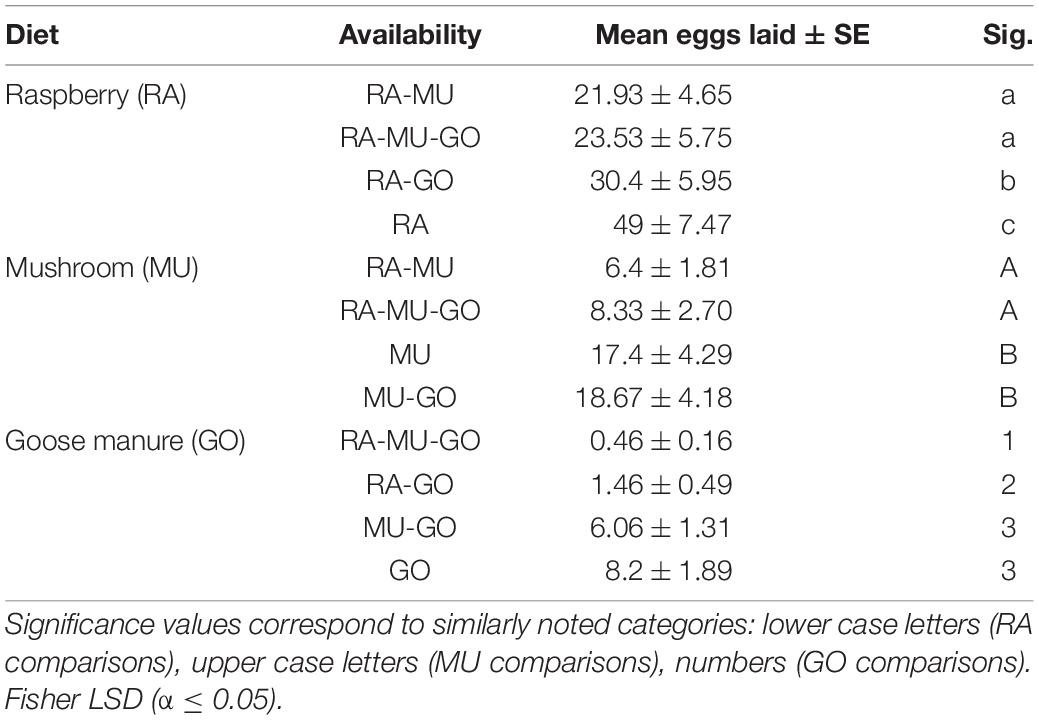
Table 4. Diet hierarchy analysis of the ranked mean oviposition on each diet substrate depending on availability.
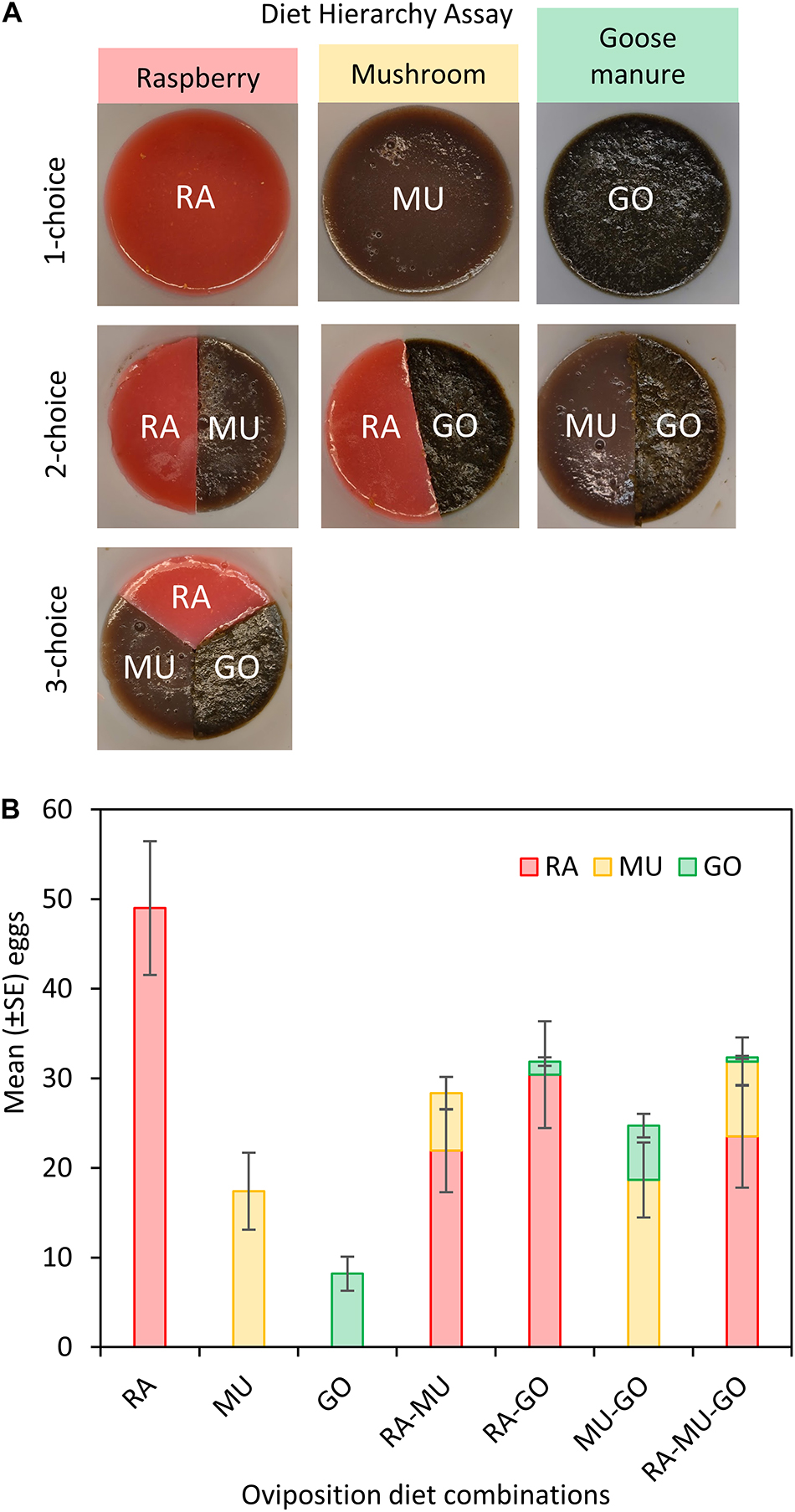
Figure 4. The diet hierarchy assay compared oviposition frequency on three diet substrates: raspberry (RA), white button mushroom (MU), and goose manure (GO) (A). Female D. suzukii were presented with either single diet, two-choice diet arenas, or three-choice diet arenas. Comparative differences in mean oviposition on each diet substrate after 24 h (B).
Trap disturbance, probably by foraging animals in the area, affected a large number of our samples and many of the substrates were absent from the traps when they were recollected after 24 h. Of the samples we successfully recovered, D. suzukii were reared from samples at two sites, Darrow and Research North, both raspberry sites (Table 5). A total of three D. suzukii were reared from manure samples at Darrow farm on July 8, 2020. No mushroom samples contained D. suzukii. No D. suzukii were reared from samples at Robbins farm or the Vignoles site. The majority of flies were recovered from raspberry samples at the Research North farm on July 22, 2020, and from Darrow farm on August 10, 2020. The staggered dates appeared to correspond to differences in fruit ripening phenology at each site, with the fruit at Research North ripening a few weeks earlier than at Darrow farm.
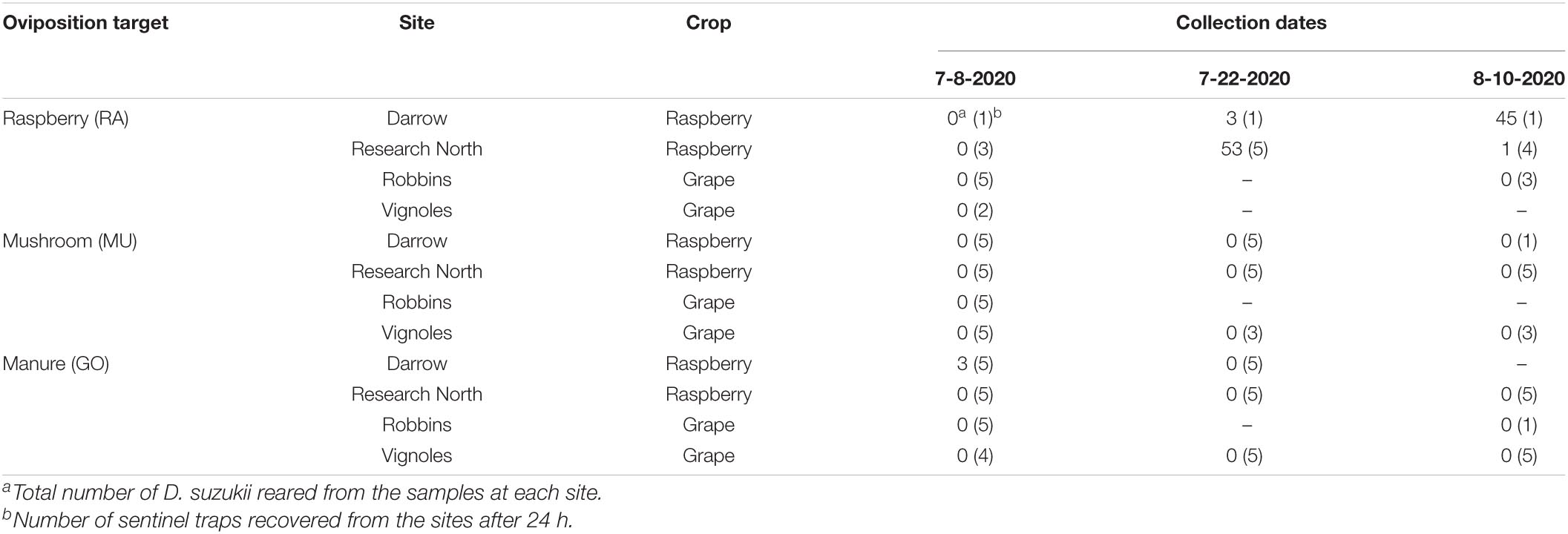
Table 5. Number of D. suzukii reared from three sentinel oviposition substrate targets (raspberry, mushroom, goose manure) placed in the field for 24 h during summer 2020 near Geneva, NY.
Our results showed that D. suzukii oviposition preferences are guided by a fixed diet hierarchy. Further, isolation on a particular diet was not associated with a consistent shift in preference or performance toward that diet across generations. This suggests that the experiential effects of larval diet or early adult oviposition attempts, do not significantly alter host preferences. The hierarchy we observed placed fresh fruit ahead of tomatoes and mushrooms, followed by manure. Our initial host range assessment showed that raspberries were preferred 20:1 over goose manure. This was true in our 2-choice tests as well. However, when raspberries were not available, oviposition on goose manure increased more than fourfold. In a 2-choice arena with mushroom, eggs laid in goose manure accounted for about a third of all eggs, and this was comparable to the total number of eggs laid in 2 and 3-choice arenas featuring raspberry. This shows that host acceptance is directly related to resource availability relative to the innate fixed hierarchy, meaning less preferred resources may be utilized, but only if more preferred resources are absent or scarce. Previous reports of D. suzukii host preference hierarchies showed different ranks. One study conducted in California reported a rank order of strawberry, raspberry, blackberry, blueberry, and grape at the bottom of the list (Bellamy et al., 2013). Another laboratory assessment conducted in North Carolina reported the oviposition host preference order to be raspberry, strawberry, blackberry, and blueberry (Burrack et al., 2013). In contrast, a more recent study from France demonstrated a very different rank ordering of oviposition hosts, with blackberry leading in preference, followed by raspberry, grape, and strawberry at the very bottom out of a complete list of 12 host fruits (Olazcuaga et al., 2019). These examples demonstrate variable reordering of the hierarchy depending on the study, which our data would suggest was likely attributable to differences in cultivar selection for the trials, or differences in skin firmness or sugar content among sample fruits (Entling et al., 2018), rather than an experiential difference in host preference among different D. suzukii populations, as is the case with other insects that display a high degree of behavioral plasticity in oviposition site selection (Jaenike, 1983; Rausher, 1983; Papaj and Prokopy, 1988).
Although previous work suggested that experiential effects were greater than indicated presently (Stockton et al., 2019), our present study took this further and reared the lines out to the F5. This is because multigenerational assessments reduce type I error by correcting for random fluctuations in intergenerational fitness, sometimes leading to inaccurate conclusions (Jaenike, 1983). Our present approach captured a more complete view and suggests that when viewed longitudinally, experiential effects on D. suzukii host preference and offspring performance were minimal and no discernable pattern emerged indicative of learning or physiological adaptations, at least not on the scale we employed. Indeed, given a broader scope, our results are more in line with that reported by Diepenbrock et al. (2016), which found little effect of host origin on subsequent preference in a comparison of cultivated blackberry (Rubus L. subgenus rubus Watson) and a wild non-cultivated host, American pokeweed berry (Phytolacca americana). Rather, females reared on pokeweed preferred to oviposit on blackberry and blackberry reared females showed no preference. Further blackberry reared flies were always more fit than those reared from pokeweed, indicating a performance benefit for the hierarchical structure of host selection. We previously reported similar performance data for apple, mushroom, and chicken manure (Stockton et al., 2019) indicating maternal oviposition preferences are positively correlated with hierarchical performance benefits during larval development. One area that our study did not address was the distinction between female host use for reproduction versus feeding. Although it seems likely that if female D. suzukii were biologically prepared to learn (Jermy, 1987; Papaj and Prokopy, 1989) about familiar or acceptable host substrates we would have noted this in our study, it is possible that these effects occur in other contexts, such as resource selection for adult feeding or habitat use. Among many species, resource use varies considerably between the sites of feeding and reproduction (McQuate and Vargas, 2007) and successful location of both resources is a fitness requirement (Jaenike, 1978). This is also the case with D. suzukii—adults feed on carbohydrate sources apart from fruit like extrafloral nectaries (Tochen et al., 2016; Plantamp et al., 2017) and leaf guttation droplets for sustenance (Urbaneja-Bernat et al., 2020). Future studies may benefit from a separation in the role of these different resources on behavior and functional role of plasticity in each context.
The hierarchical preference structure we observed are certainly not limited to D. suzukii. Hierarchical host use has been reported in both generalist species such as D. tripunctata (Jaenike and Grimaldi, 1983) and Drosophila busckii (Courtney and Hard, 1990), and specialists such as Drosophila suboccidentalis (Courtney and Chen, 1988) and D. mojavensis (Fellows and Heed, 1972). It was among other Drosophila that the hierarchy-threshold model was proposed by Courtney et al. (1989), wherein insects are predicted to rank host plants in order of decreasing acceptability or preference. In this way, when high-ranking hosts are scarce, low-ranking hosts become acceptable. The model suggests pleiotropy associated with variable host use, particularly the ability to use and access low-ranking hosts, should be maintained in populations because there is rarely selection against these traits (Courtney and Hard, 1990). However, there is frequent selection for high-ranking hosts, leading to less variability within populations. In the case of D. suzukii this is supported by low variability in preference for fruit in the genus Rubus and consistently high ranking for raspberry in particular. In contrast, preference for lower-order hosts including grape, blueberry, and wild hosts are more variable. In some cases, however, use of less-preferred hosts occurs even in the presence of higher ranked hosts. Among swallowtail butterflies, Papilio machaon L., while oviposition behavior occurs on three primary host plants, the use of alternative secondary hosts does occur when primary hosts are present (Wiklund, 1981). In our own trials, we observed a similar pattern in our two and three-way choice tests, where female D. suzukii laid eggs on less-preferred mushroom or manure substrates even in the presence of raspberry, although in low numbers. While it is unclear how common non-fruit host use is among wild flies in nature, it is possible that use of non-fruit hosts occurs in low levels throughout the year in an opportunistic manner.
Among many insects, the use of some hosts is considered conditional, meaning that although the insect can reproduce and develop on that host, it is not known to do so in nature (Aluja and Mangan, 2008; Follett et al., 2021). Following our controlled laboratory-based preference assays, we sampled host use in the field using sentinel hosts just before and during raspberry fruiting season. In this way, we hoped to monitor the use of our experimental three host substrates (raspberry, mushroom, and goose manure) over time under natural conditions. Our data were limited by low oviposition, trap disturbance by wild animals, and substrate consumption by sap beetles. Of the traps we recovered each week, only one trap baited with manure was found to contain D. suzukii and none were found in those baited with mushroom. The flies in the manure sample were found prior to the fruit period. Once fruit were available, D. suzukii were only detected in a total of four raspberry-baited traps located at our two raspberry field sites. Unfortunately, due to the low numbers we observed, interpretation of these data is difficult, and it remains unclear whether mushroom fruiting bodies and avian manure are natural hosts or are only conditional. Gut contents analysis of wild caught flies would allow for real-time study of these effects. If mushroom, other fungi, and manure sources are used in the natural environment, removal of these resources or mitigation at these sites, particularly during the early spring months when fruit are scarce, may be beneficial (Tait et al., 2021).
Due to the highly invasive nature of D. suzukii (dos Santos et al., 2017), the high degree of behavioral and physiological plasticity it displays (Little et al., 2020; Stockton et al., 2020), and its ability to establish in extreme climates with greatly differing natural resources, it is interesting to consider what host use will look like over time. On the one hand, the hierarchy-threshold model predicts that the presence of genetic variance in novel host use may proceed rapid host shifts (Courtney et al., 1989). Because D. suzukii appears well-equipped to utilize a wide range of wild and cultivated hosts (Lee et al., 2011), and presumably countless more novel hosts, we would expect it to fit into this category easily. However, feeding preference alone is not the only factor likely driving divergence and host shifts (Dethier, 1954). Rather, if D. suzukii population movement is regular enough, either through natural yearly migration events or human directed movement (i.e., interstate commerce and pest hitchhiking), then host race formation is unlikely. A multi-locus microsatellite approach revealed at least two distinct invasion events into North America on the east and western sites of the continent, although the data indicated at least five admixture events producing heterogeneous populations, particularly in the eastern United States (Fraimout et al., 2017). Similarly, single nucleotide polymorphism (SNP) analysis was used to estimate population movement of D. suzukii through the United States and found that although there were two distinct groups of insects largely isolated to the eastern and western sides of the continental divide, within these regions there was a great degree of genetic transmission, indicating continual admixture (Lewald et al., 2021). These invasive populations have since showed signs of bottlenecking, displaying losses of up to 23.3% of heterozygosity and up to 54.2% of allelic diversity compared to native populations in southeast China (Fraimout et al., 2017). Further, differences in phenotype expression regionally has produced distinct changes in non-genetic traits like fecundity, rates of parasitization, and Wolbachia frequencies (Rota-Stabelli et al., 2020). From an evolutionary perspective, it will be interesting to see whether divergence in host preference occurs in the new invaded ranges this pest now inhabits based on local resource availability and genetic differences among allopatric populations, two factors, along with experiential effects and positive assortative mating, thought to promote host race formation in herbivorous insects (Prokopy et al., 1988). Over time, we may expect the hierarchical ordering or maternal host preferences to shift in favor of locally available hosts.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DS and GL conceived the project and acquired the funding. DS collected and analyzed the data and prepared the manuscript. GL edited and critiqued the manuscript. All authors contributed to the article and approved the submitted version.
This funding was provided by the United States Department of Agriculture (USDA); National Institute for Food and Agriculture (NIFA) SCRI award, #2015-51181-24252; and the USDA-NIFA Postdoctoral Fellowship program award, #2020-67034-31785.
Opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA. USDA is an equal opportunity provider and employer.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Stephen Hesler, Karen Wentworth, Gabrielle Brind’Amore, Mason Clark, Molly Cappiello, Rowan Collins, Kayli Harding, and Rachel Brown for their assistance with this project.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.816557/full#supplementary-material
Agrawal, A. A. (2000). Host range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81, 500–508. doi: 10.1890/0012-9658
Aluja, M., and Mangan, R. L. (2008). Fruit fly (Diptera: tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 53, 473–502. doi: 10.1146/ANNUREV.ENTO.53.103106.093350
Andreazza, F., Bernardi, D., dos Santos, R. S. S., Garcia, F. R. M., Oliveira, E. E., Botton, M., et al. (2017). Drosophila suzukii in southern neotropical region: current status and future perspectives. Neotrop. Entomol. 46, 591–605. doi: 10.1007/S13744-017-0554-7
Arnó, J., Solà, M., Riudavets, J., and Gabarra, R. (2016). Population dynamics, non-crop hosts, and fruit susceptibility of Drosophila suzukii in Northeast Spain. J. Pest Sci. 89, 713–723. doi: 10.1007/S10340-016-0774-3
Asplen, M. K., Anfora, G., Biondi, A., Choi, D.-S., Chu, D., Daane, K. M., et al. (2015). Invasion biology of spotted-wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J. Pest Sci. 88, 469–494. doi: 10.1007/s10340-015-0681-z
Bal, H. K., Adams, C., and Grieshop, M. (2017). Evaluation of off-season potential breeding sources for spotted-wing Drosophila (Drosophila suzukii Matsumura) in Michigan. J. Econ. Entomol. 110, 2466–2470. doi: 10.1093/jee/tox252
Bellamy, D. E., Sisterson, M. S., and Walse, S. S. (2013). Quantifying host potentials: indexing postharvest fresh fruits for spotted-wing Drosophila, Drosophila suzukii. PLoS One 8:e61227. doi: 10.1371/journal.pone.0061227
Björkman, C., Larsson, S., Bommarco, R., and Bjorkman, C. (1997). Oviposition preferences in pine sawflies: a trade-off between larval growth and defence against natural enemies. Oikos 79:45. doi: 10.2307/3546088
Burrack, H. J., Fernandez, G. E., Spivey, T., and Kraus, D. A. (2013). Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: drosophilidae), an invasive frugivore. Pest Manag. Sci. 69, 1173–1180. doi: 10.1002/ps.3489
Calabria, G., Máca, J., Bächli, G., Serra, L., and Pascual, M. (2012). First records of the potential pest species Drosophila suzukii (Diptera: drosophilidae) in Europe. J. Appl. Entomol. 136, 139–147. doi: 10.1111/j.1439-0418.2010.01583.x
Cini, A., Anfora, G., Escudero-Colomar, L. A., Grassi, A., Santosuosso, U., Seljak, G., et al. (2014). Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 87, 559–566. doi: 10.1007/S10340-014-0617-Z
Courtney, S. P., and Chen, G. K. (1988). Genetic and environmental variation in oviposition behaviour in the mycophagous Drosophila suboccidentalis. Funct. Ecol. 2:521. doi: 10.2307/2389395
Courtney, S. P., Chen, G. K., and Gardner, A. (1989). A general model for individual host selection. Oikos 55:55. doi: 10.2307/3565872
Courtney, S. P., and Hard, J. J. (1990). Host acceptance and life-history traits in Drosophila busckii: tests of the hierarchy-threshold model. Heredity. 64, 371–375. doi: 10.1038/hdy.1990.46
Dancau, T., Stemberger, T. L. M., Clarke, P., and Gillespie, D. R. (2017). Can competition be superior to parasitism for biological control? The case of spotted wing Drosophila (Drosophila suzukii), Drosophila melanogaster and Pachycrepoideus vindemmiae. Biocontrol Sci. Technol. 27, 3–16. doi: 10.1080/09583157.2016.1241982
Dean, D., Price, J. F., Steck, G., and Nagle, C. A. (2012). Development and impact of the spotted-wing Drosophila, Drosophila suzukii, in Florida strawberries. Int. J. Fruit Sci. 13, 67–75. doi: 10.1080/15538362.2012.696992
Deprá, M., Poppe, J. L., Schmitz, H. J., De Toni, D. C., and Valente, V. L. S. (2014). The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 87, 379–383. doi: 10.1007/s10340-014-0591-5
Dethier, V. G. (1954). Evolution of feeding preferences in phytophagous insects. Evolution 8, 33–54. doi: 10.1111/j.1558-5646.1954.tb00107.x
Diepenbrock, L. M., Swoboda-Bhattarai, K. A., and Burrack, H. J. (2016). Ovipositional preference, fidelity, and fitness of Drosophila suzukii in a co-occurring crop and non-crop host system. J. Pest Sci. 89, 761–769. doi: 10.1007/s10340-016-0764-5
dos Santos, L. A., Mendes, M. F., Krüger, A. P., Blauth, M. L., Gottschalk, M. S., Garcia, F. R. M., et al. (2017). Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS One 12:e0174318. doi: 10.1371/journal.pone.0174318
Entling, W., Anslinger, S., Jarausch, B., Michl, G., and Hoffmann, C. (2018). Berry skin resistance explains oviposition preferences of Drosophila suzukii at the level of grape cultivars and single berries. J. Pest Sci. 92, 477–484. doi: 10.1007/S10340-018-1040-7
Fellows, D. P., and Heed, W. B. (1972). Factors affecting host plant selection in desert-adapted cactiphilic Drosophila. Ecology 53, 850–858. doi: 10.2307/1934300
Follett, P. A., Haynes, F. E. M., and Dominiak, B. C. (2021). Host suitability index for polyphagous tephritid fruit flies. J. Econ. Entomol. 114, 1021–1034. doi: 10.1093/JEE/TOAB035
Fraimout, A., Debat, V., Fellous, S., Hufbauer, R. A., Foucaud, J., Pudlo, P., et al. (2017). Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol. Biol. Evol. 34, 980–996. doi: 10.1093/molbev/msx050
Futuyma, D. J., and Moreno, G. (1988). The evolution of ecological specialization. Ann. Rev. Ecol. Syst. 19, 207–240.
Hauser, M. (2011). A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 67, 1352–1357.
Jaenike, J. (1978). On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 14, 350–356. doi: 10.1016/0040-5809(78)90012-6
Jaenike, J. (1983). Induction of host preference in Drosophila melanogaster. Oecologia 58, 320–325. doi: 10.1007/BF00385230
Jaenike, J. (1990). Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273.
Jaenike, J., and Grimaldi, D. (1983). Genetic variation for host preference within and among populations of Drosophila tripunctata. Evolution 37, 1023–1033. doi: 10.1111/j.1558-5646.1983.tb05630.x
Jermy, T. (1987). “The role of experience in the host selection of phytophagous insects,” in Perspectives in Chemoreception and Behavior, eds R. F. Chapman, E. A. Bernays, and J. G. Stoffolano (New York, NY: Springer-Verlag), 143–159.
Jones, C. D. (2005). “The genetics of adaptation in Drosophila sechellia,” in Genetics of Adaptation, ed. R. Mauricio (Dordrecht: Springer), 137–145.
Jones, R., Fountain, M. T., Günther, C. S., Eady, P. E., and Goddard, M. R. (2021). Separate and combined Hanseniaspora uvarum and Metschnikowia pulcherrima metabolic volatiles are attractive to Drosophila suzukii in the laboratory and field. Sci. Rep. 11:1201. doi: 10.1038/s41598-020-79691-3
Keesey, I. W., Knaden, M., and Hansson, B. S. (2015). Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 41, 121–128. doi: 10.1007/s10886-015-0544-3
Lee, J. C., Bruck, D. J., Curry, H., Edwards, D., Haviland, D. R., Van Steenwyk, R. A., et al. (2011). The susceptibility of small fruits and cherries to the spotted-wing Drosophila, Drosophila suzukii. Pest Manag. Sci. 67, 1358–1367. doi: 10.1002/ps.2225
Lee, J. C., Dreves, A. J., Cave, A. M., Kawai, S., Isaacs, R., Miller, J. C., et al. (2015). Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: drosophilidae). Ann. Entomol. Soc. Am. 108, 117–129. doi: 10.1093/aesa/sau014
Lewald, K. M., Abrieux, A., Wilson, D. A., Lee, Y., Conner, W. R., Andreazza, F., et al. (2021). Population genomics of Drosophila suzukii reveal longitudinal population structure and signals of migrations in and out of the continental United States. G3 Genes Genomes Genet. 11:jkab343. doi: 10.1093/G3JOURNAL/JKAB343
Little, C. M., Little, C. M., Chapman, T. W., and Hillier, N. K. (2020). Plasticity is key to success of Drosophila suzukii (Diptera: drosophilidae) invasion. J. Insect Sci. 20:5. doi: 10.1093/jisesa/ieaa034
Markow, T. A., and O’Grady, P. M. (2006). Drosophila: A Guide to Species Identification and Use. Amsterdam: Elsevier.
McQuate, G. T., and Vargas, R. I. (2007). Assessment of attractiveness of plants as roosting sites for the melon fly, Bactrocera cucurbitae, and oriental fruit fly, Bactrocera dorsalis. J. Insect Sci. 7:57. doi: 10.1673/031.007.5701/873523
Newby, B. D., and Etges, W. J. (1998). Host preference among populations of Drosophila mojavensis (Diptera: drosophilidae) that use different host cacti. J. Insect Behav. 11, 691–712.
Olazcuaga, L., Rode, N. O., Foucaud, J., Facon, B., Ravigné, V., Ausset, A., et al. (2019). Oviposition preference and larval performance of Drosophila suzukii (Diptera: drosophilidae), spotted-wing Drosophila: effects of fruit identity and composition. Environ. Entomol. 48, 867–881. doi: 10.1093/EE/NVZ062
Papaj, D. R., and Prokopy, R. J. (1988). The effect of prior adult experience on components of habitat preference in the apple maggot fly (Rhagoletis pomonella). Oecologia 76, 538–543. doi: 10.1007/BF00397866
Papaj, D. R., and Prokopy, R. J. (1989). Ecological and evolutionary aspects of learning in phytophagous insects. Annu. Rev. Entomol. 34, 315–350.
Plantamp, C., Estragnat, V., Fellous, S., Desouhant, E., and Gibert, P. (2017). Where and what to feed? Differential effects on fecundity and longevity in the invasive Drosophila suzukii. Basic Appl. Ecol. 19, 56–66. doi: 10.1016/J.BAAE.2016.10.005
Poyet, M., Le Roux, V., Gibert, P., Meirland, A., Prévost, G., Eslin, P., et al. (2015). The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: the key of its invasion success in temperate Europe? PLoS One 10:e0142785. doi: 10.1371/journal.pone.0142785
Prokopy, R. J., Diehl, S. R., and Cooley, S. S. (1988). Behavioral evidence for host races in Rhagoletis pomonella flies. Oecologia 76, 138–147.
Ramasamy, S., Ometto, L., Crava, C. M., Revadi, S., Kaur, R., Horner, D. S., et al. (2016). The evolution of olfactory gene families in Drosophila and the genomic basis of chemical-ecological adaptation in Drosophila suzukii. Genome Biol. Evol. 8, 2297–2311. doi: 10.1093/GBE/EVW160
Rausher, M. D. (1983). Conditioning and genetic variation as causes of individual variation in the oviposition behaviour of the tortoise beetle, Deloyala guttata. Anim. Behav. 31, 743–747. doi: 10.1016/S0003-3472(83)80231-0
Renkema, J. M., Iglesias, L. E., Bonneau, P., and Liburd, O. E. (2018). Trapping system comparisons for and factors affecting populations of Drosophila suzukii and Zaprionus indianus in winter-grown strawberry. Pest Manag. Sci. 74, 2076–2088. doi: 10.1002/PS.4904
Rota-Stabelli, O., Ometto, L., Tait, G., Ghirotto, S., Kaur, R., Drago, F., et al. (2020). Distinct genotypes and phenotypes in European and American strains of Drosophila suzukii: implications for biology and management of an invasive organism. J. Pest Sci. 93, 77–89. doi: 10.1007/s10340-019-01172-y
Shrader, M. E., Burrack, H. J., and Pfeiffer, D. G. (2020). Effects of interspecific larval competition on developmental parameters in nutrient sources between Drosophila suzukii (Diptera: drosophilidae) and Zaprionus indianus. J. Econ. Entomol. 113, 230–238. doi: 10.1093/JEE/TOZ297
Stemberger, T. L. M. (2016). Survey of hanging and fallen cherry fruit use by spotted wing drosophila, Drosophila suzukii (Matsumura, 1931) (Diptera: drosophilidae), and other Drosophilidae species. Pan-Pac. Entomol. 91, 347–431. doi: 10.3956/2015-91.4.347
Stockton, D. G., Brown, R., and Loeb, G. (2019). Not berry hungry? Discovering the hidden food sources of a small fruit specialist, Drosophila suzukii. Ecol. Entomol. 44, 810–822. doi: 10.1111/een.12766
Stockton, D. G., Wallingford, A., and Loeb, G. (2018). Phenotypic plasticity promotes overwintering survival in a globally invasive crop pest, Drosophila suzukii. Insects 9:105. doi: 10.3390/INSECTS9030105
Stockton, D. G., Wallingford, A. K., Brind’amore, G., Diepenbrock, L., Burrack, H., Leach, H., et al. (2020). Seasonal polyphenism of spotted-wing Drosophila is affected by variation in local abiotic conditions within its invaded range, likely influencing survival and regional population dynamics. Ecol. Evol. 10, 7669–7685. doi: 10.1002/ece3.6491
Tait, G., Mermer, S., Stockton, D., Lee, J., Avosani, S., Abrieux, A., et al. (2021). Drosophila suzukii (Diptera: drosophilidae): a decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 114, 1950–1974. doi: 10.1093/JEE/TOAB158
Thompson, J. N. (1988). Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 47, 3–14. doi: 10.1111/j.1570-7458.1988.tb02275.x
Tochen, S., Walton, V. M., and Lee, J. C. (2016). Impact of floral feeding on adult Drosophila suzukii survival and nutrient status. J. Pest Sci. 89, 793–802. doi: 10.1007/s10340-016-0762-7
Urbaneja-Bernat, P., Tena, A., González-Cabrera, J., and Rodriguez-Saona, C. (2020). Plant guttation provides nutrient-rich food for insects. Proc. R. Soc. B 287:20201080. doi: 10.1098/RSPB.2020.1080
Walsh, D. B., Bolda, M. P., Goodhue, R. E., Dreves, A. J., Lee, J., Bruck, D. J., et al. (2011). Drosophila suzukii (Diptera: drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2, G1–G7. doi: 10.1603/IPM10010
Whiteman, N. K., and Pierce, N. E. (2008). Delicious poison: genetics of Drosophila host plant preference. Trends Ecol. Evol. 23, 473–478. doi: 10.1016/J.TREE.2008.05.010
Keywords: Drosophila suzukii (Diptera: Drosophilidae), SWD, preference and performance, host choice, plasticity, insects, invasive, entomology
Citation: Stockton DG and Loeb GM (2022) Diet Hierarchies Guide Temporal-Spatial Variation in Drosophila suzukii Resource Use. Front. Ecol. Evol. 9:816557. doi: 10.3389/fevo.2021.816557
Received: 16 November 2021; Accepted: 20 December 2021;
Published: 01 February 2022.
Edited by:
Josephine Antwi, University of Mary Washington, United StatesReviewed by:
Martín Aluja, Instituto de Ecología (INECOL), MexicoCopyright © 2022 Stockton and Loeb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dara G. Stockton, ZGFyYS5zdG9ja3RvbkB1c2RhLmdvdg==
†Present address: Dara G. Stockton, USDA-ARS, Daniel K. Inouye U.S. Pacific Basin Agricultural Research Center, Hilo, HI, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.