
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol. , 09 December 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.794708
The modification of male pedipalps into secondary sexual intromittent organs is one of the hallmark characteristics of spiders, yet understanding the development and evolution of male genitalia across the order remains a challenging prospect. The embolus – the sclerite bearing the efferent spermatic duct or spermophor, and used to deliver sperm directly to the female genitalia during copulation – has always been considered the single unambiguously homologous palpal sclerite shared by all spider species, fundamental to the bauplan of the order and to the evolution and functional morphology of spider reproductive systems. Indeed, after two centuries of comparative research on spider reproduction, the presence of a single spermophor and embolus on each of a male spider’s two pedipalps remains a central tenet of evolutionary arachnology. Our findings challenge this premise, and reveal a remarkable twin intromittent organ sperm transfer system in a lineage of Australian palpimanoid spiders, characterized by a bifurcate spermophor and the presence of two efferent ducts leading to a pair of embolic sclerites on each pedipalp. This is the first time such a remarkable conformation has been observed in any group of arachnids with direct sperm transfer, complicating our understanding of palpal sclerite homologies, and challenging ideas about the evolution of spider genitalia.
Sperm transfer in Arachnida is achieved either indirectly with spermatophores (e.g., in Amblypygi, Scorpiones, and Pseudoscorpiones), or directly using a penis (e.g., in Opiliones), the chelicerae (e.g., in Solifugae) or modified appendages (e.g., in Araneae and Ricinulei). Most prominent are the male intromittent organs of spiders, the so-called palpal organs or genital bulbs situated on the pedipalps, which directly transfer sperm to the female gonopore during copulation (Coddington and Levi, 1991; Coddington et al., 2004). To do so, the male spider must first release sperm from his gonopore onto a sperm web, after which the sperm is taken up into the palpal organ. The palpal organs of adult male spiders vary enormously in their morphology, complexity, and ontogeny (e.g., Coddington, 1990; Griswold et al., 2005; Dederichs et al., 2019), thought to be originally derived from a tarsal claw (Coddington, 1990; Quade et al., 2019) and consisting at their simplest of an undivided piriform sclerite (the “bulb”) with a terminal embolus (Magalhães and Ramírez, 2017; Michalik et al., 2019), the latter enclosing an efferent duct (the spermatic duct or “spermophor”) which is gradually enlarged proximally (Foelix, 2011). In most spider taxa the palpal organ is more complex – spectacularly so in some lineages, e.g., Linyphiidae (Hormiga, 1994, 2000) – with divided bulb (i.e., tegular and subtegular) sclerites, inflatable membranous elements (haematodochae) that rely on hydraulic pressure, and multiple tegular apophyses in addition to the ubiquitous internal spermophor and sclerotized embolus bearing a terminal sperm pore (Griswold et al., 2005). These palpal sclerites function in concert during copulation, engaging and inter-locking with the female genitalia (e.g., Uhl et al., 2007; Mouginot et al., 2015; Poy et al., 2020), which may explain the diversity of species-specific morphologies that occur in these structures. Given this morphological complexity, and the importance of male palpal morphology to spider reproduction and systematics, ontological and comparative morphological research has long focused on trying to determine sclerite homologies across and within spider taxa (Comstock, 1910; Shear, 1967; Kraus, 1978; Raven, 1985; Coddington, 1990; Ramírez, 2014). In all such studies it has consistently been shown that the embolus is the only primary homologous palpal sclerite shared by all known spider species, with other putatively symplesiomorphic sclerites (e.g., the conductor and median apophysis) prone to fusion or loss. By extension, the internal spermophor is also homologous across all spiders, a proposition borne out by ontogeny (Coddington, 1990) and over two centuries of taxonomic research.
Spiders of the superfamily Palpimanoidea have long been of interest to arachnologists, due to their phylogenetic antiquity (Rix and Harvey, 2012c; Wood et al., 2012, 2013, 2014), highly modified somatic morphology (Wood et al., 2012; Wood and Parkinson, 2019) and phylogenetic position as sister clade to the Entelegynae (Ramírez et al., 2021) – the latter a highly diverse lineage containing the bulk of spider diversity, with a modified “flow-through” female genitalic system bearing distinct copulatory and fertilization ducts (Griswold et al., 2005; Wheeler et al., 2017; Michalik et al., 2019). With five unambiguously monophyletic families, a remarkably rich fossil record, and an evolutionary origin on the Pangaean supercontinent (Wood et al., 2013, 2018), the Palpimanoidea are a rare example of an arthropod lineage of putatively ancient origin to have a traditionally vicariant biogeographical hypothesis now strongly supported by dated phylogenetics (Wood et al., 2013). “Assassin” or “pelican” spiders of the family Archaeidae are a lineage of Palpimanoidea with a particularly fragmented modern distribution in Australia and the Old World Afrotropics (southern Africa and Madagascar), temporally and geographically consistent with the breakup of East and West Gondwana (Wood et al., 2013). However, perhaps unsurprisingly for a lineage with an extant crown group of Jurassic age (Wood et al., 2013, 2014), comparative morphological work on the five extant genera of Archaeidae is challenging given certain divergent character systems that exist within and between lineages. Unlike in their diverse sister-group, the Entelegynae, the female genitalia of palpimanoids are haplogyne, exhibiting a cul-de-sac system with only one duct connecting to the spermathecae (Michalik et al., 2019). Indeed, despite having rather simple haplogyne female genitalia, the male genitalic morphology of Archaeidae is surprisingly complex, sometimes with a functional tegular and subtegular division of the male palpal bulb, and always with expandable basal and/or distal haematodochae, and highly flexible tegular sclerites in various taxa (Rix and Harvey, 2011, 2012a; Figure 1). In life, most of these complex structures are sunken and partially enclosed within the distal rim of the bulb (except during copulation, when the bulb greatly expands), making comparative morphological assessment especially difficult in the unexpanded state. Thus, despite the highly derived yet nonetheless geologically conserved somatic morphology and ecology of both Afrotropical and Australian Archaeidae, a consensus on palpal sclerite homologies within the family is yet to be achieved (Wood, 2008; Rix and Harvey, 2011, 2012a,b; Wood and Scharff, 2018).
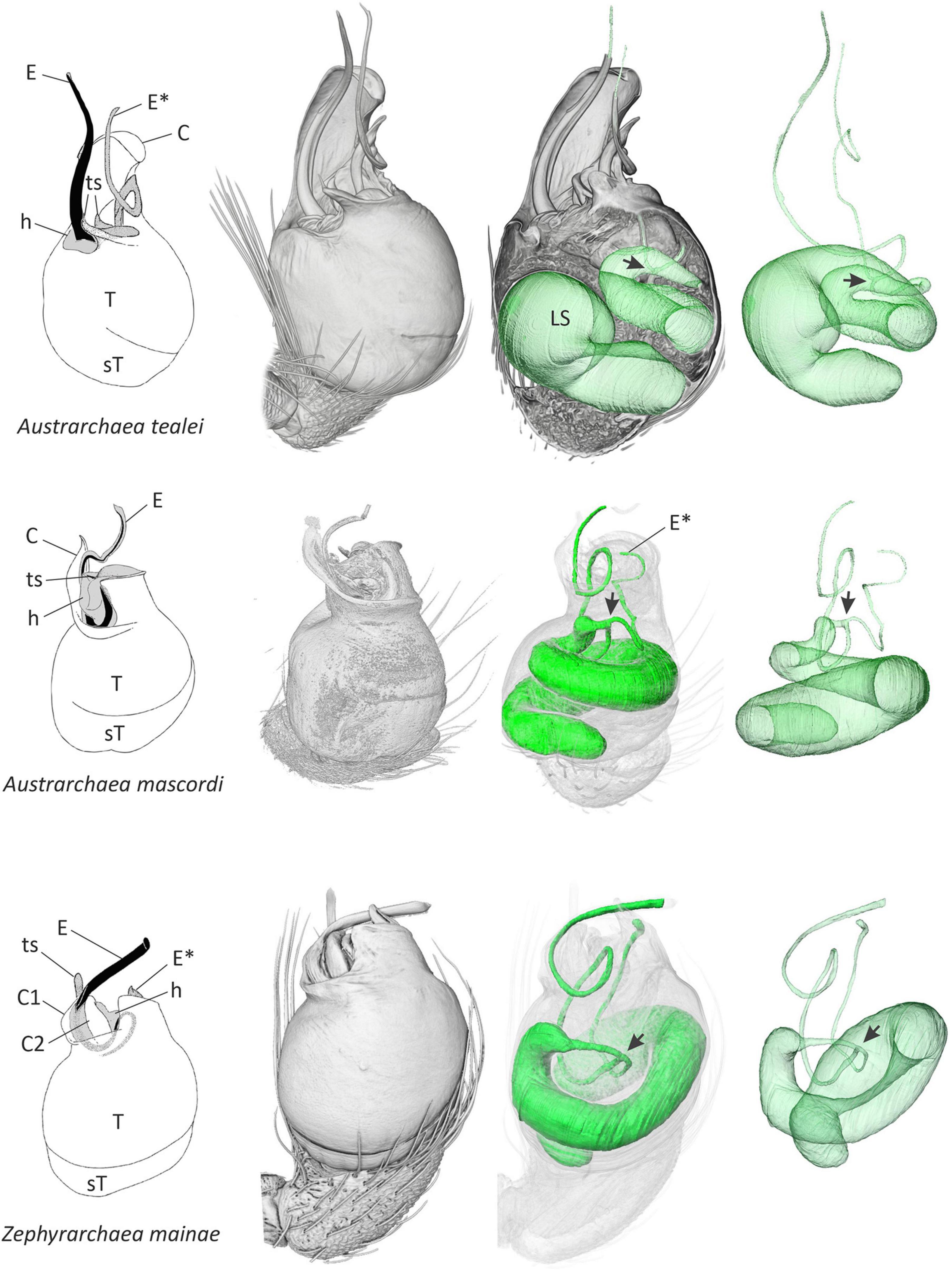
Figure 1. Structural morphology of the male pedipalp of three species of Australian Archaeidae. Line drawings at left (modified from Rix and Harvey, 2011, 2012a,b) depict the external morphology of the unexpanded left palpal bulb in retrolateral view, showing variation in the shape, size, and relative position of primary sclerites. Other images in each series show three-dimensional volume-rendered micro-CT visualizations of the left pedipalp, trajectory of the internal spermophor and efferent ducts in situ, the latter rendered in isolation at right. Note the point of bifurcation (arrow) of each spermophor, and the separate ducts leading to each embolic sclerite. C, conductor; E, primary embolic sclerite; E*, secondary embolic sclerite; h, haematodochal membrane; LS, lumen of spermophor; sT, subtegulum; T, tegulum; ts, tegular sclerite.
In this study, our initial aim was to use the trajectory of the spermophor to identify the embolus in lineages of Palpimanoidea, especially in Archaeidae from the Wet Tropics of north-eastern Queensland (Australia), for which the identification of the embolus was unexpectedly problematic (Rix and Harvey, 2012b). In doing so, we revealed a remarkable bifurcate conformation of the spermophor and a twin intromittent organ in the archaeid genera from Australia. Our secondary aims, therefore, were to understand the morphology of the palpal organ with a focus on the sperm transferring parts in the Afrotropical archaeid genera and the remaining palpimanoid families, and to pose a number of hypotheses that could be used to understand possible evolutionary or ontogenetic drivers in the context of correlated female genitalic morphology and sexual selection.
Ten exemplar male specimens from each of the five palpimanoid families, and from all five genera of Archaeidae, were sourced from collections held at the California Academy of Sciences (San Francisco, CA, United States), the Western Australian Museum (Perth, WA, Australia) and the Zoologisches Institut und Museum (Greifswald, Germany) (Table 1). Animals were preserved in either 70 or 95% ethanol, prior to stereo and compound microscopy, and micro-computed tomography (micro-CT) analysis.
Preserved male pedipalps were dehydrated in graded ethanol and stained with a 1% iodine solution overnight. Subsequently, the samples were washed in absolute ethanol and critical-point dried using a Leica CPD300 automated dryer (Leica Microsystems GmbH, Wetzlar, Germany). After mounting on a plastic rod, each pedipalp was scanned using an XRadia MicroXCT-200 X-Ray imaging system (Carl Zeiss Microscopy GmbH, Pleasanton, CA, United States). The tomography projections were reconstructed using the XMReconstructor software (Carl Zeiss Microscopy GmbH, Pleasanton, CA, United States). The volume rendering of the image stacks and the segmentation of the spermophor was performed by Amira 6.4 (Thermo Fisher Scientific Inc., Waltham, CA, United States). Post-processing of images was performed in Corel PaintShop Pro 2020 and Corel Draw 2020.
The phylogenetic topology used for character-state mapping against all 10 exemplar palpimanoid taxa (Figure 2) was modified from previously published phylogenetic studies. For inter-familial relationships, the latest phylogenomic topology of Ramírez et al. (2021) was applied, and for inter-generic relationships within Archaeidae, the topology followed the results of Rix and Harvey (2012c) and Wood et al. (2012, 2013, 2014, 2018).
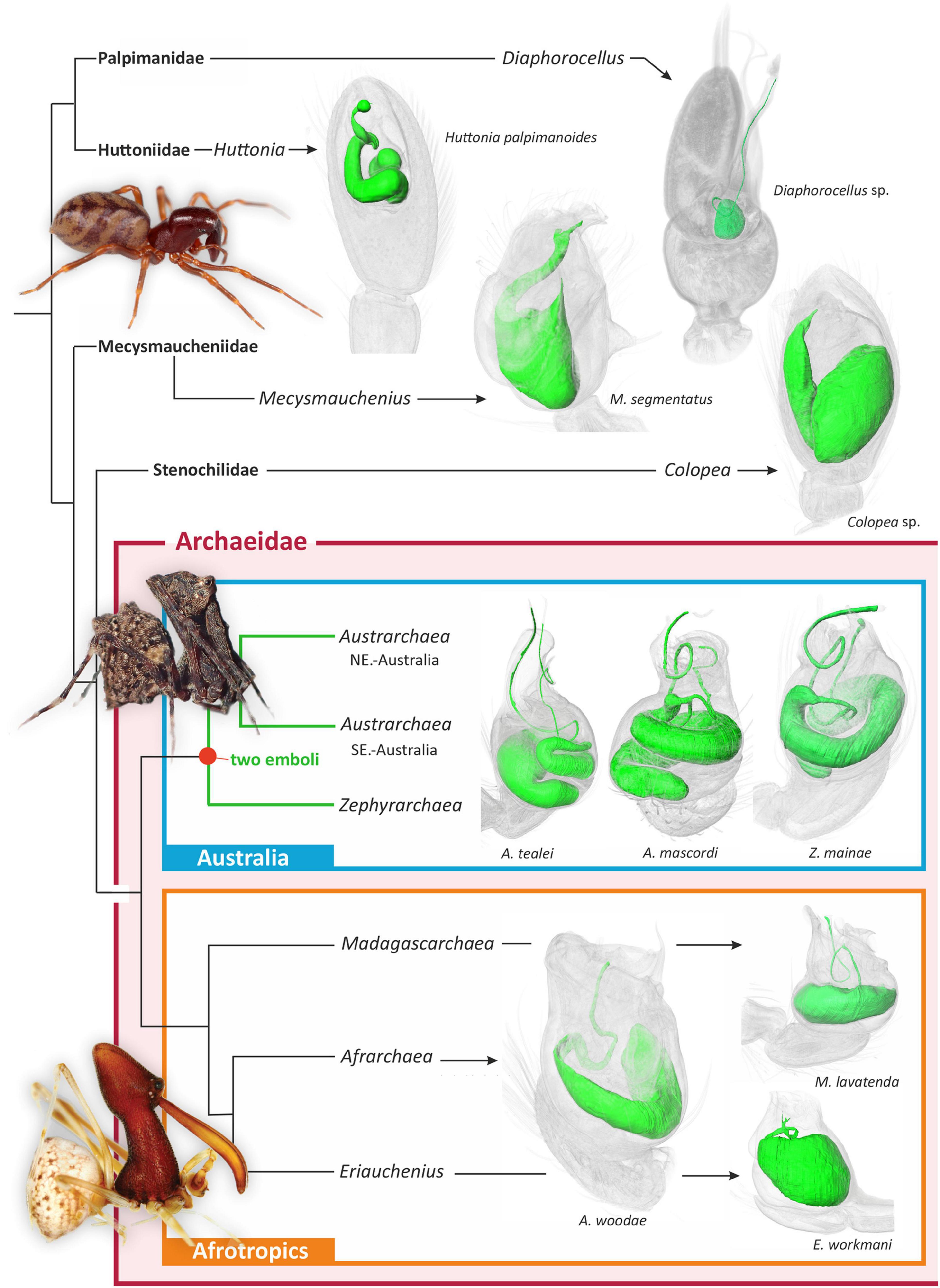
Figure 2. Summary higher-level phylogeny of Archaeidae and other palpimanoid spiders, modified from previously published phylogenetic studies (Rix and Harvey, 2012c; Wood et al., 2012, 2013, 2014, 2018; Ramírez et al., 2021). Shown to the right of each clade are three-dimensional micro-CT visualizations of the spermophors of exemplar taxa. Note the presence of a bifurcating spermophor and two efferent ducts in all three major lineages of Australian Archaeidae.
Comparative micro-CT analysis of the palpal organ across palpimanoid spider families revealed a medio-distal bifurcation of the spermophor and two efferent distal ducts in all three major monophyletic lineages of Archaeidae from Australia, i.e., in exemplar species belonging to the Austrarchaea nodosa species-group from south-eastern Australia (exemplar Austrarchaea mascordi), the Austrarchaea daviesae species-group from north-eastern Australia (exemplar Austrarchaea tealei), and the genus Zephyrarchaea from southern Australia (exemplar Zephyrarchaea mainae) (Rix and Harvey, 2012c; Figures 1, 2). In the three specimens scanned the spermophor was highly sinuous, bifurcating deep within the tegular division of the bulb and splitting into two relatively long, filiform efferent ducts (Figures 1, 2). These ducts had convoluted, looped trajectories each leading to separate embolic sclerites representing a twin intromittent organ, one of which (the primary embolic sclerite; E) was enlarged and supported by a tegular conductor or conductor-like analog (Figure 1). The secondary embolic sclerite (E*) differed markedly in size between taxa, from relatively small and partially concealed in Z. mainae and A. mascordi, to extremely large, whip-like and exposed in A. tealei (Figure 1).
The morphology of the spermophor in all other examined palpimanoid exemplars was unmodified and symplesiomorphic in structure relative to Austrarchaea and Zephyrarchaea, including in those representatives of the three extant Afrotropical archaeid genera (Figure 2). Exemplar species of Afrarchaea, Eriauchenius, and Madagascarchaea all had spermophors which were shorter and less sinuous than those of Australian archaeid species, and there was also no evidence in Afrotropical taxa for a division of the palpal organ into a tegulum and subtegulum (cf. Figure 1). Similarly, in Huttonia (Huttoniidae), Mecysmauchenius (Mecysmaucheniidae), Diaphorocellus (Palpimanidae), and Colopea (Stenochilidae), there was no evidence of a bifurcate spermophor, despite the presence of an unusual divided embolic sclerite in Diaphorocellus. Mapping of the bifurcate state onto the phylogeny of Archaeidae and other Palpimanoidea thus revealed it to be an unambiguous synapomorphy of the Australian archaeid clade [i.e., (Austrarchaea + Zephyrarchaea)] (Figure 2).
The embolus – enclosing the efferent spermatic duct or spermophor, and used to deliver sperm to the female genitalia during copulation – has always been considered the single unambiguously homologous sclerite on a male spider’s pedipalp, shared by all known species and fundamental to the bauplan of the order and to the evolution, architecture and functional morphology of spider reproductive systems. Indeed, despite extraordinary variation in the structure and complexity of spider palpal sclerites (Hormiga, 1994, 2000; Griswold et al., 2005), the presence of a single embolus on each of a male spider’s two pedipalps remains a central tenet of evolutionary arachnology. Even after two centuries of taxonomic, ontological and comparative morphological research, no species has ever been found with multiple “emboli,” and by extension, no spermophor has ever been recorded as having more than one efferent duct. Our findings challenge this premise, as in this study we report a lineage of palpimanoid spiders with a remarkable twin intromittent organ, whereby males possess a uniquely bifurcate spermophor with two efferent distal ducts leading to a pair of prominent embolic sclerites on each palpal bulb (Figures 1, 2). This “double-embolic” morphology (Figures 1, 2) was found to occur in all studied archaeid species from Australia, and optimizes as a unique and unambiguous synapomorphy of the “Australian clade” (Figure 2).
Understanding how, and especially why, stem-group archaeids of the Australian clade evolved such an extraordinary deviation from the normal spider bauplan is difficult to explain, both evolutionarily and ontogenetically. Post-copulatory sexual selection (Eberhard, 1985, 2004; Simmons, 2001, 2013) may be invoked as a possible evolutionary driver, as the female genitalia of Australian assassin spiders are unique among Archaeidae in possessing multiple aciniform clusters of defined spermathecae (Rix and Harvey, 2011, 2012a,b). Indeed, with so many potential sperm reservoirs (>20 in some taxa) in each female, it would reasonably be expected that sperm competition would favor males with functional access to the most spermathecae. Other Afrotropical Archaeidae, in contrast, share a relatively simple female genital system, consisting of a haplogyne bursa bearing clusters of secretory pores on modified “poreplates” (Wood and Scharff, 2018). The “double-embolic” state is documented here to have evolved one time in spiders, and as such, we are restricted to the simple observation that female genitalia differ markedly in Australian taxa relative to their Afrotropical relatives. However, within the Australian clade, significant inter-specific variation in the morphology of the secondary embolus (E*; see Figure 1) does lend itself to evolutionary analysis. For example, future studies could examine whether the size, length or shape of E* across multiple species in both genera is phylogenetically correlated to the number of spermathecae in females, the length of the female bursa, or the length of the spermathecal stalks. Similarly, intra-specific studies could examine whether paternity success is related to embolic morphometrics (with implications for post-copulatory sexual selection), as has been examined in other terrestrial arthropods with secondary sexual intromittent organs (e.g., Wojcieszek and Simmons, 2011).
Ontogenetically, a morphological shift of this magnitude seems harder to explain. The spider spermophor forms from an invagination of cells, with the duct anlage developing from the distal tip of the pedipalp and later spiraling proximally (Coddington, 1990; Quade et al., 2019). The embolus itself is one of the last sclerites to differentiate in the apical division, surrounding the distal spermophor and sperm pore. At present, the developmental genetic architecture required for such a fundamental transition of this conserved phenotypic system – and the complexity or otherwise of this transition – are unknown, although the trait has seemingly evolved just once in the long evolutionary history of spiders, sometime between the mid-late Mesozoic and early-mid Cenozoic (during which time Australian stem-group archaeids diverged from Old World relatives; Wood et al., 2013, 2014). Understanding the ontogenetic architecture underlying this transition would therefore be a particularly valuable avenue for future research, not least because it may provide insight into the sort of evolutionary genetic shift required for bauplan modification more generally. Our results highlight the need for ongoing functional genomic, ontogenetic, morphometric, and sexual selection studies of Archaeidae and related Palpimanoidea, and highlight the importance of understanding the internal morphology of the palpal organ as it relates to the external functional morphology of spider pedipalps.
Data are available from the Morphobank Digital Repository: http://morphobank.org/permalink/?P4100.
Fieldwork was conducted under the following permits in: Queensland, Australia (No. WITK03859806); New South Wales, Australia (Nos. S13035 and XX48918); Western Australia, Australia (Nos. SF005357, SF005814, SF006247, and SF006821); Chile (No. 01/2008); and New Zealand (No. CA-30389-FAU). Specimens from Brunei Darussalam, Madagascar, and South Africa were sourced from existing museum material.
MR, HW, MH, and PM performed the study and wrote the manuscript. PM conducted micro-CT analysis. All authors agreed to be held accountable for the content therein and approved the final version of the manuscript.
Funding for MR and MH was made possible with an Australian Biological Resources Study (ABRS) Taxonomy Research Grant (Grant No. 209-09), with additional funding from Grant Nos. RF215-06 and RG18-03. The micro-computed tomography was funded by the state of Mecklenburg-Vorpommern and the German Research Foundation (DFG INST 292/119–1 FUGG and DFG INST 292/120–1 FUGG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the various collectors who assisted with field work, and colleagues for discussion, especially Charles Griswold (California Academy of Sciences, United States). We further thank Shou-Wang Lin (University of Greifswald, Germany) for his technical assistance. The image of Mecysmauchenius segmentatus in Figure 2 was provided courtesy of Martín J. Ramírez (Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina).
Coddington, J. A. (1990). Ontogeny and homology in the male palpus of orb-weaving spiders and their relatives, with comments on phylogeny (Araneoclada: Araneoidea, Deinopoidea). Smithson. Contrib. Zool. 496, 1–52. doi: 10.5479/si.00810282.496
Coddington, J. A., Giribet, G., Harvey, M. S., Prendini, L., and Walter, D. E. (2004). “Arachnida,” in Assembling the Tree of Life, eds J. Cracraft and M. J. Donoghue (New York, NY: Oxford University Press), 296–318.
Coddington, J. A., and Levi, H. W. (1991). Systematics and evolution of spiders (Araneae). Annu. Rev. Ecol. Syst. 22, 565–592.
Comstock, J. H. (1910). The palpi of male spiders. Anna. Entomol. Soc. Am. 3, 161–185. doi: 10.1093/aesa/3.3.161
Dederichs, T. M., Müller, C. H. G., Sentenská, L., Lipke, E., Uhl, G., and Michalik, P. (2019). The innervation of the male copulatory organ of spiders (Araneae) – a comparative analysis. Front. Zool. 16:39. doi: 10.1186/s12983-019-0337-6
Eberhard, W. G. (1985). Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard University Press.
Eberhard, W. G. (2004). Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. J. Arachnol. 32, 545–556. doi: 10.1636/0161-8202(2004)032[0545:wsssst]2.0.co;2
Griswold, C. E., Ramírez, M. J., Coddington, J. A., and Platnick, N. I. (2005). Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proc. Calif. Acad. Sci. 56, 1–324.
Hormiga, G. (1994). Cladistics and the comparative morphology of linyphiid spiders and their relatives (Araneae, Araneoidea, Linyphiidae). Zool. J. Linn. Soc. 111, 1–71. doi: 10.1111/j.1096-3642.1994.tb01491.x
Hormiga, G. (2000). Higher level phylogenetics of erigonine spiders (Araneae, Linyphiidae, Erigoninae). Smithson. Contrib. Zool. 609, 1–160.
Kraus, O. (1978). Liphistius and the evolution of spider genitalia. Sympos. Zool. Soc. Lond. 42, 235–254.
Magalhães, I. L. F., and Ramírez, M. J. (2017). Relationships and phylogenetic revision of Filistatinella spiders (Araneae: Filistatidae). Invertebr. Systemat. 31, 665–712. doi: 10.1071/is16083
Michalik, P., Kallal, R., Dederichs, T. M., Labarque, F. M., Hormiga, G., Giribet, G., et al. (2019). Phylogenomics and genital morphology of cave raptor spiders (Araneae, Trogloraptoridae) reveal an independent origin of a flow-through female genital system. J. Zool. Systemat. Evol. Res. 57, 737–747.
Mouginot, P., Prügel, J., Thom, U., Steinhoff, P. O. M., Kupryjanowicz, J., and Uhl, G. (2015). Securing paternity by mutilating female genitalia in spiders. Curr. Biol. 25, 2980–2984. doi: 10.1016/j.cub.2015.09.074
Poy, D., Ramírez, M. J., Michalik, P., and Piacentini, L. N. (2020). Copulatory mechanics in the wolf spider Agalenocosa pirity reveals a hidden diversity of locking systems in Lycosidae (Araneae). J. Morphol. 281, 250–257. doi: 10.1002/jmor.21095
Quade, F. S. C., Holtzheimer, J., Frohn, J., Töpperwien, M., Salditt, T., and Prpic, N.-M. (2019). Formation and development of the male copulatory organ in the spider Parasteatoda tepidariorum involves a metamorphosis-like process. Scientific Reports 9, 6945. doi: 10.1038/s41598-019-43192-9
Ramírez, M. J. (2014). The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bull. Am. Mus. Nat. His. 390, 1–374. doi: 10.1206/821.1
Ramírez, M. J., Magalhaes, I. L. F., Derkarabetian, S., Ledford, J., Griswold, C. E., Wood, H. M., et al. (2021). Sequence capture phylogenomics of true spiders reveals convergent evolution of respiratory systems. Syst. Biol. 70, 14–20. doi: 10.1093/sysbio/syaa043
Raven, R. J. (1985). The spider infraorder Mygalomorphae (Araneae): cladistics and systematics. Bull. Am. Mus. Nat. His. 182, 1–180.
Rix, M. G., and Harvey, M. S. (2011). Australian assassins, part I: a review of the assassin spiders (Araneae, Archaeidae) of mid-eastern Australia. ZooKeys 123, 1–100. doi: 10.3897/zookeys.123.1448
Rix, M. G., and Harvey, M. S. (2012a). Australian assassins, part II: a review of the new assassin spider genus Zephyrarchaea (Araneae, Archaeidae) from southern Australia. ZooKeys 191, 1–62. doi: 10.3897/zookeys.191.3070
Rix, M. G., and Harvey, M. S. (2012b). Australian assassins, part III: a review of the assassin spiders (Araneae, Archaeidae) of tropical north-eastern Queensland. ZooKeys 218, 1–55. doi: 10.3897/zookeys.218.3662
Rix, M. G., and Harvey, M. S. (2012c). Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within tertiary refugia. Mol. Phylogenet. Evol. 62, 375–396. doi: 10.1016/j.ympev.2011.10.009
Simmons, L. W. (2001). Sperm Competition and its Evolutionary Consequences in the Insects. Princeton, NJ: Princeton University Press.
Uhl, G., Nessler, S. H., and Schneider, J. (2007). Copulatory mechanism in a sexually cannibalistic spider with genital mutilation (Araneae: Araneidae: Argiope bruennichi). Zoology 110, 398–408. doi: 10.1016/j.zool.2007.07.003
Wheeler, W. C., Coddington, J. A., Crowley, L. M., Dimitrov, D., Goloboff, P. A., Griswold, C. E., et al. (2017). The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616. doi: 10.1111/cla.12182
Wood, H. (2008). A revision of the assassin spiders of the Eriauchenius gracilicollis group, a clade of spiders endemic to Madagascar (Araneae: Archaeidae). Zool. J. Linn. Soc. 152, 255–296. doi: 10.1111/j.1096-3642.2007.00359.x
Wood, H. M., Gillespie, R. G., Griswold, C. E., and Wainwright, P. C. (2014). Why is Madagascar special? The extraordinarily slow evolution of pelican spiders (Araneae, Archaeidae). Evolution 69, 462–481. doi: 10.1111/evo.12578
Wood, H. M., González, V. L., Lloyd, M., Coddington, J., and Scharff, N. (2018). Next-generation museum genomics: phylogenetic relationships among palpimanoid spiders using sequence capture techniques (Araneae: Palpimanoidea). Mol. Phylogenet. Evol. 127, 907–918. doi: 10.1016/j.ympev.2018.06.038
Wood, H. M., Griswold, C. E., and Gillespie, R. G. (2012). Phylogenetic placement of pelican spiders (Archaeidae, Araneae), with insight into evolution of the “neck” and predatory behaviours of the superfamily Palpimanoidea. Cladistics 28, 598–626.
Wood, H. M., Matzke, N. J., Gillespie, R. G., and Griswold, C. E. (2013). Treating fossils as terminal taxa in divergence time estimation reveals ancient vicariance patterns in the palpimanoid spiders. Syst. Biol. 62, 264–284. doi: 10.1093/sysbio/sys092
Wood, H. M., and Parkinson, D. Y. (2019). Comparative morphology of cheliceral muscles using high-resolution X-ray microcomputed-tomography in palpimanoid spiders (Araneae, Palpimanoidea). J. Morphol. 280, 232–243. doi: 10.1002/jmor.20939
Wood, H. M., and Scharff, N. (2018). A review of the Madagascan pelican spiders of the genera Eriauchenius O. Pickard-Cambridge, 1881 and Madagascarchaea gen. n. (Araneae, Archaeidae). ZooKeys 727, 1–96. doi: 10.3897/zookeys.727.20222
Keywords: Archaeidae, haplogyne, morphology, palpal organ, Palpimanoidea, phylogeny
Citation: Rix MG, Wood HM, Harvey MS and Michalik P (2021) Micro-Computed Tomography Reveals a Remarkable Twin Intromittent Organ in Spiders – A Novelty for Arachnids With Direct Sperm Transfer. Front. Ecol. Evol. 9:794708. doi: 10.3389/fevo.2021.794708
Received: 14 October 2021; Accepted: 18 November 2021;
Published: 09 December 2021.
Edited by:
Stano Pekar, Masaryk University, CzechiaReviewed by:
Matthias Foellmer, Adelphi University, United StatesCopyright © 2021 Rix, Wood, Harvey and Michalik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael G. Rix, bWljaGFlbC5yaXhAcW0ucWxkLmdvdi5hdQ==
†ORCID: Michael G. Rix, orcid.org/0000-0001-5086-3638; Hannah M. Wood, orcid.org/0000-0003-0453-2699; Mark S. Harvey, orcid.org/0000-0003-1482-0109; Peter Michalik, orcid.org/0000-0003-2459-9153
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.