
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ecol. Evol., 06 January 2022
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.788764
This article is part of the Research TopicNesting in Reptiles: Natural and Anthropogenic Threats and Evolutionary ResponsesView all 18 articles
 Gerald Kuchling1*
Gerald Kuchling1* Margaretha D. Hofmeyr2†
Margaretha D. Hofmeyr2†In a captive colony of Chersina angulata in Cape Town, South Africa, we observed in 2015/16 retention of the last egg clutch inside the female until the hatching stage was reached, conforming to the generally accepted definition of viviparity. Retrospective climatic analysis indicates egg retention until the hatching stage co-occurred with unusually hot summer weather: the average air temperatures in December 2015 and January and February 2016 were higher than during the preceding five and the following 5 years when facultative viviparity could not be observed. Late December and January appears to be the critical period for females to either deposit their last clutch of the nesting season into a nest, or to retain the last clutch for embryonic development inside the female. Over the 28 December to 24 January period the minimum, average and maximum air temperatures in 2015–16 were about 3°C higher than in the five following years. This association of facultative viviparity with unusual summer heat suggests that hot ambient temperatures at the end of the nesting season may cue females to switch from oviposition to facultative viviparity. Compared to incubation in a nest this phenotypic plasticity of the reproductive mode—to retain during hot summers the season’s last clutch inside the female—may buffer the developing embryos from excessive heat exposure: females can thermo-regulate by moving among microhabitats whereas sun exposed shallow nests cannot escape high ground temperatures. This novel reproductive strategy has the potential to enhance the resilience of species to global warming.
Among living reptilian orders, Testudines, Crocodylia, and Rhynchocephalia are considered strictly oviparous whereas Squamata include both oviparous and viviparous species (Blackburn and Sidor, 2014). Turtles typically lay their eggs in underground nests constructed with their hind legs, although in humid environments some may also deposit eggs on the ground beneath leaf litter or beneath the edge of fallen logs (Kuchling, 1999). No matter how long turtle females may retain eggs, the generally accepted wisdom is that intrauterine development of embryos is arrested at the gastrula stage until oviposition (Ewert, 1985; Kuchling, 1999; Rafferty and Reina, 2012).
In the family Testudinidae, Chersina angulata (Schweigger, 1812) has an unusual reproductive pattern for a tortoise inhabiting climatic zones ranging from winter rainfall with extreme aridity in the northwest (south-western Namibia) to mediterranean in the southwest (Western Cape) to temperate with all-year rainfall in southern South Africa: females produce single-egg clutches nearly year round (March to December) and females lay up to six clutches per year (Hofmeyr, 2004, 2009; Branch, 2008). The nest consists of an about 10 cm wide shallow depression with a small chamber 4 cm wide and deep at its bottom, constructed in sandy soil, in a well-drained, sunny position. After laying, the soil is tamped down by the female with her shell (Branch, 2008). Similar to other tortoises occurring in the winter rainfall region of South Africa, hatching occurs March to April, just before or at the start of the rainy season in autumn (Hofmeyr, 2009). Egg shells crack 6–7 days before hatchlings emerge. Little is known on the ecology of hatchlings and their habitat choice, but high nest and hatchling predation rates from mongooses and jackals have been observed as well as hatchling predation from baboons, rock monitors, secretary birds, sea gulls, and crows (Branch, 2008).
Egg retention time in the oviducts of C. angulata varies substantially: it can last from 23 to 212 days (Hofmeyr, 2004). Observations indicate that C. angulata females can retain eggs until embryonic development has progressed to the hatching stage (Hofmeyr and Kuchling, 2017), conforming to the generally accepted definition of viviparity in reptiles (Shine, 1985; Van Dyke et al., 2014): eggs produced in early summer (December) are occasionally not deposited in nests, but retained by females until embryonic development has progressed to the hatching stage, until the time hatching normally occurs in the species (Hofmeyr and Kuchling, 2017). To our knowledge, no respective observations have been reported for any other chelonian species. However, we observed facultative viviparity in the second author’s long-term captive C. angulata colony only over the summer of 2015/16 (Hofmeyr and Kuchling, 2017) and could not repeat this observation over the five following years.
In the present paper we explore if the switch from oviparity to facultative viviparity in C. angulata in 2015/16 may have coincided with unusual climatic conditions. Cape Town recorded unusually high temperatures in 2015, including its highest temperature of the last 100 years at 42°C (downloaded 01 June 2021).1 A multi-year (2015–2017) drought occurred in the South West of the Western Cape from 2015 to 2017. Total annual rainfall in each of those years was lower than the long-term average, with the strongest anomaly in 2017 (Odoulami et al., 2021). The Western Cape drought continued throughout 2015–2019 and was either the longest or third longest drought on record since 1901 (Kam et al., 2021).
Thus, the climatic condition which differed in 2015/16 from those in the following years was that it was an unusually hot summer period, while severe drought conditions continued for several more years. In the present paper we test the hypothesis that viviparity, resulting from retention of the last egg clutch of the nesting season by female C. angulata, is a phenotypically plastic response to unusually high environmental temperatures or drought at the time they would normally nest. Late December/early January is the critical time period to follow one of the alternative strategies to either nest or to retain the last egg clutch of the nesting season inside the female. We analyze monthly ambient air temperatures and monthly average rainfall from December to March and weekly ambient air temperatures from late December to late January to explore if environmental temperatures and precipitation over the summer of 2015/16, when we observed facultative viviparity, differed from those over the five previous and the five following summers.
The reported observations of facultative viviparity are based on routine observations in the long-term captive research colony of C. angulata established by author 2 in South Africa to investigation the species’ reproductive biology: in 1999 sixteen wild C. angulata females and five males from the West’ Coast National Park (WCNP; 33′13′S; 18′09′E) were transferred to Kuilsrivier (33″56′S; 18″41′E), 90 km from the WCNP. The animals were maintained in a 20 × 10 m outdoor enclosure under generally natural climatic conditions, but in addition it was irrigated with ground water 3 days a week for 20 min. The enclosure contained Kikuyu grass (Pennisetum clandestinum) that provided food and shelter, sandy areas for nesting, dense bushes and artificial shelters; the animals received supplemental food (fresh vegetables, occasional fruits, and chicken eggshell) and drinking water (Hofmeyr, 2004).
At the time of and in the year prior to the reported incidental observation (April 2016) no research interferences occurred with females, nests or eggs in the enclosure. From 2017 until 2021 all 16 females were annually radiographed in late January/early February to establish if any of them retained eggs over the summer, during the period of the year when no new clutches are ovulated (Hofmeyr, 2004). Following the passing of author 2 in February 2020 the captive colony was disbanded in February 2021, with all originally transferred 16 females and five males still alive.
The climate data analysis is based on air temperatures and rainfall collected by the Matroosfontain Meteorological Station at Cape Town International Airport, 10 km from the captive colony, as provided by https://www.wunderground.com/history/monthly/za/matroosfontein/FACT/date. Monthly temperature averages (December–March) from 2011 to 2021 were converted from Degrees Fahrenheit to Degrees Celsius. The daily minimum, average and maximum temperatures for the period 28 December–24 January of the years 2010/11–2020/21 were converted from Degrees Fahrenheit to Degrees Celsius and averaged for 7-day (weekly temperature) and 28-day (four-weekly temperature) periods. The 1979–2000 averages of monthly rainfall are based on a graphic overview provided by https://www.csag.uct.ac.za/current-seasons-rainfall-in-cape-town/ (downloaded 01 June 2021).
Observation of facultative viviparity:
In 2016, the captive females started nesting by mid-March and in the afternoon of 1 April 2016, author 2 found two unburied eggs (ca. 2 m apart) in the captives’ enclosure with no sign of disturbance of the surrounding ground, indicating that one or two females dropped eggs without digging nests. Although laying one egg at a time is the norm for this species, two-egg clutches occasionally occur and we do not know if one female laid both eggs or if two females laid one egg each. Dropping eggs on the surface is not common but neither unusual for C. angulata in the wild or in captivity (author 2, personal observation). The eggs found on 1 April were at most 1-day old, because the tortoises were fed the previous day. Since the eggs have been exposed to full sun, they were left outside to let nature take its course. When the tortoises were fed on the morning of 5 April, each egg had a small opening through which a hatchling and substantial amount of yolk was visible. The eggs were brought indoors, placed in a container, and left so that the hatchlings could internalize the residual yolk. Both hatchlings emerged 4 days later on 9 April 2016. Upon hatching, the hatchlings weighed 19.7 and 16.9 g, respectively, and showed no external abnormalities. Both hatchlings displayed normal activity and growth, and, respectively, weighed 25.9 and 28.5 g 3 months later. Nests from the 2015 nesting season in the enclosure have not been monitored, although some hatchlings emerged in autumn 2016 from nests of the 2015 season we do not know the respective hatching success (percentage of eggs that hatched).
Summer air temperatures:
The monthly average air temperatures for December, January, and February 2015/16 at Matroosfontain Meteorological Station were higher than the monthly averages averaged for the preceding 4 years and the following 5 years. The March 2016 average was only marginally warmer than the average for the five following years, but cooler than the average for the previous 4 years (Figure 1).
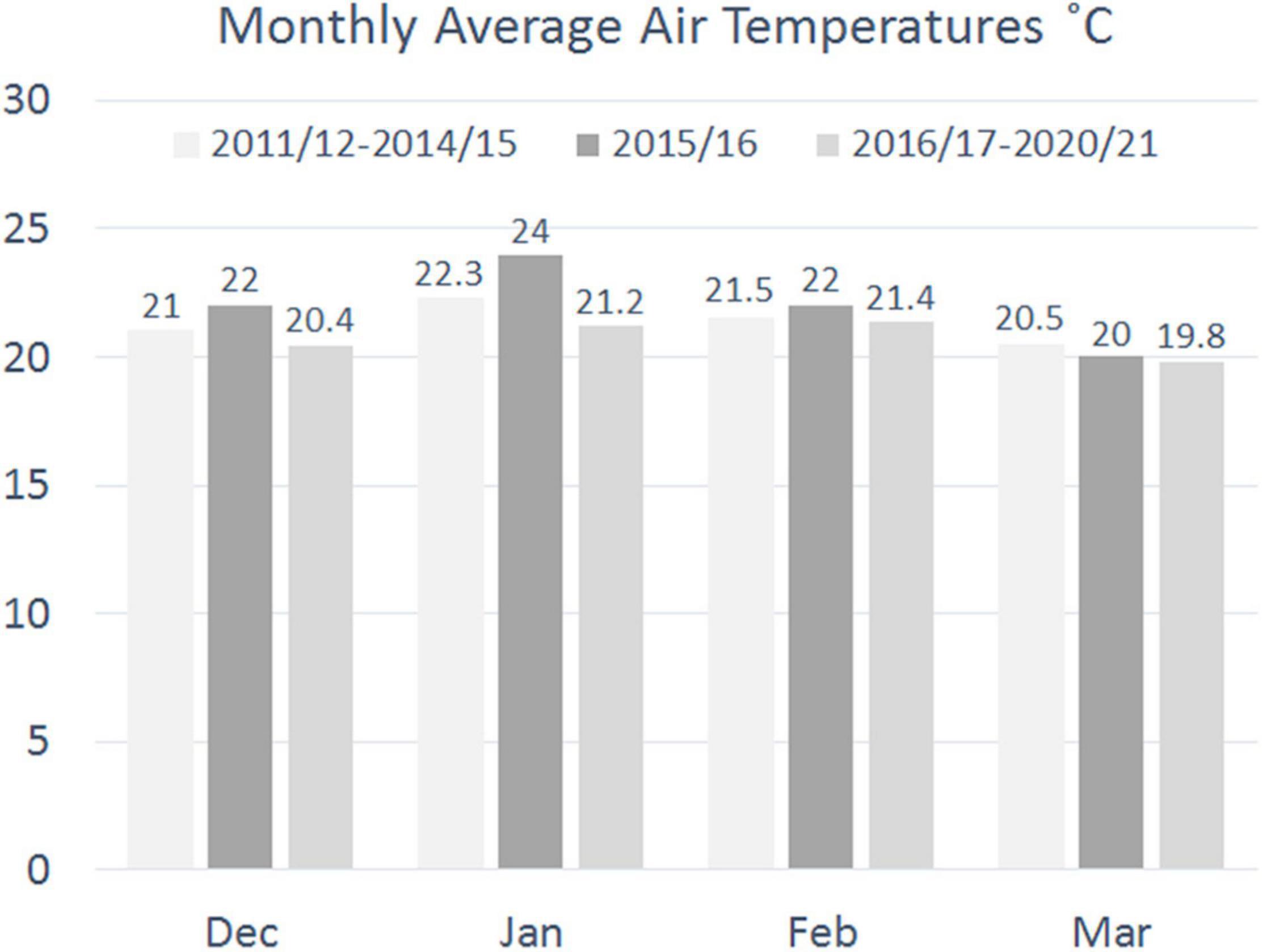
Figure 1. Monthly averages of daily average air temperatures at Matroosfontain Meteorological Station for December to March 2015/16, and the respective averages for the years 2011/12 to 2014/15 and 2016/17 to 2020/21.
Regarding the 4-week (28 days) period 28 December to 24 January, the average of daily minimum, average and maximum air temperatures in 2015/16 was, respectively, 2.8, 3.1, and 3.8°C higher than averaged over the five following years and 2.2, 2.5, and 3.3°C higher than averaged over the four previous years. Considering every year from 2016/17 separately, the average of daily minimum temperatures in 2015/16 was still at least 2.3°C higher than in any year from 2016/17 to 2020/21, the average of daily average temperatures in 2015/16 was at least 2.7°C higher than in any year from 2016/17 to 2020/21 and the average of daily maximum temperatures in 2015/16 was at least 3.3°C higher than in any year from 2016/17 to 2020/21 (Figure 2).
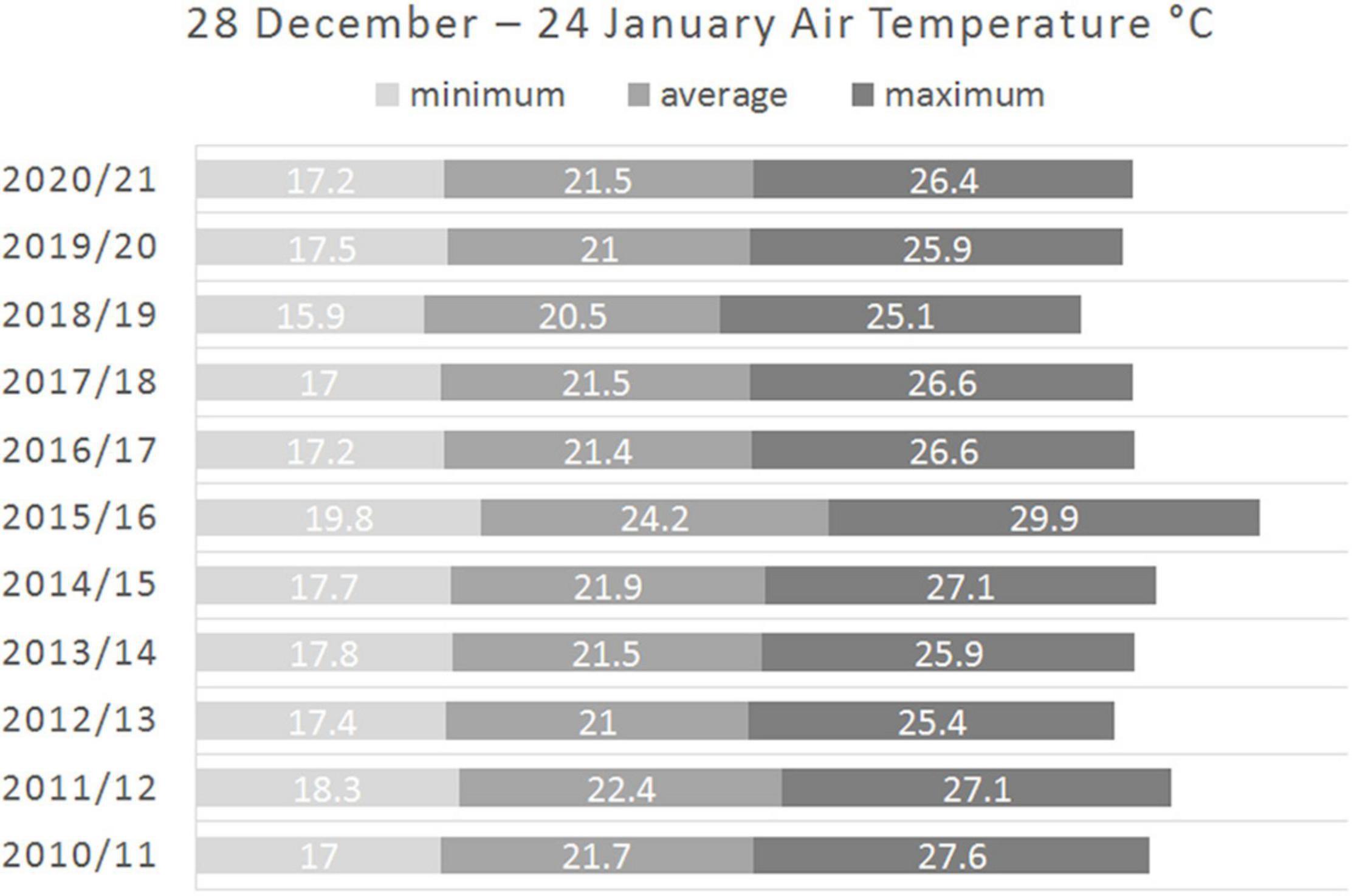
Figure 2. 28 December to 24 January four-weekly averages of daily minimum, average and maximum air temperatures at Matroosfontain Meteorological Station for every year from 2010/11 to 2020/21.
The weekly averages of the daily minimum, average and maximum air temperatures in the 4-week period 28 December to 24 January were also consistently higher in 2015/16 than the respective temperatures averaged for the years 2016/17 to 2020/21. This was particularly pronounced in the 7-day period 28 December to 02 January, when in 2015/16 the average minimum temperature was 3.7°C, the average of the average temperature 3.4°C and the average maximum temperature 4.3°C higher than the respective averages over the following 5-year period (Figure 3).
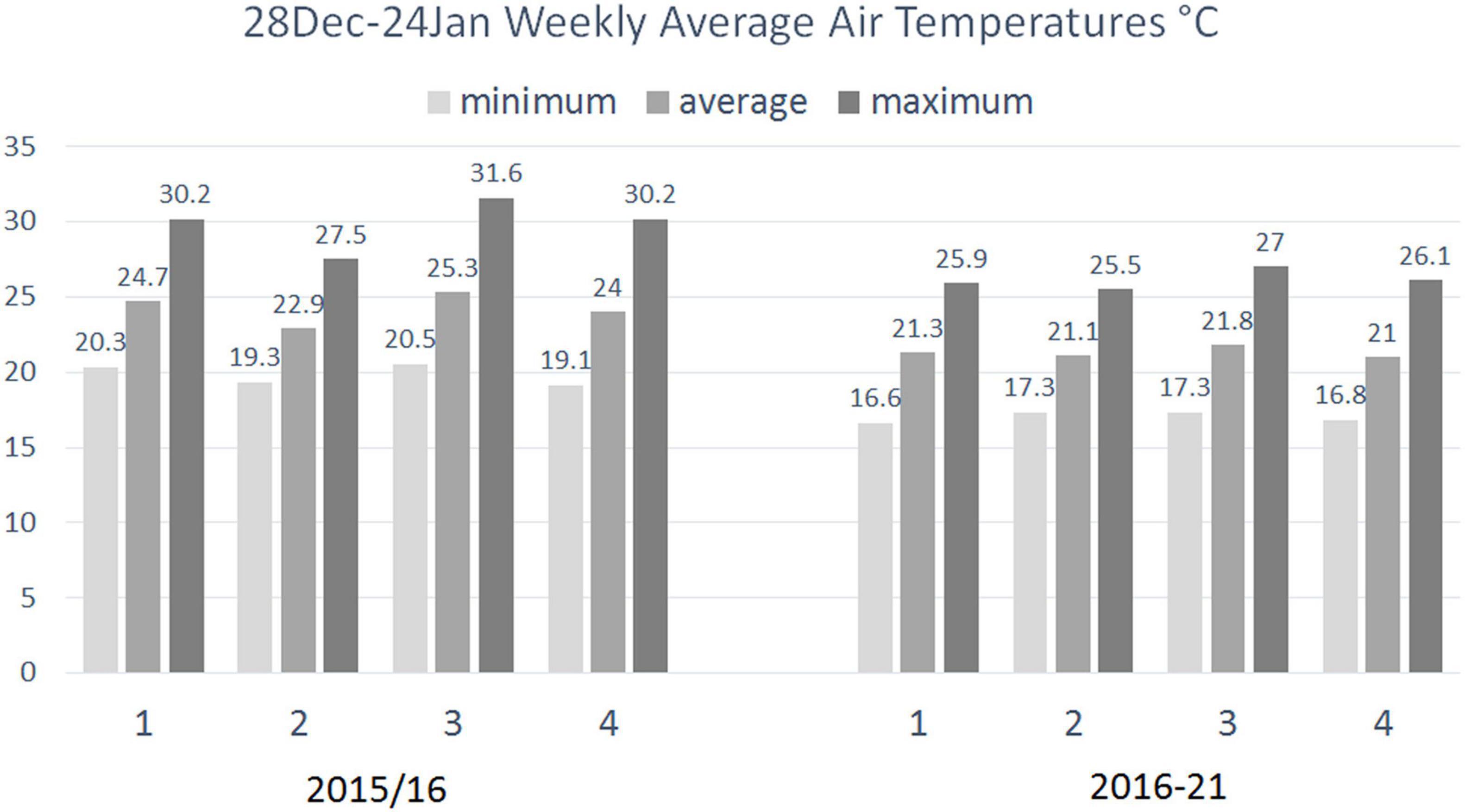
Figure 3. Seven-day averages of daily minimum, average and maximum temperatures (°C) from December 28th to January 24th (1: 28 December–03 January; 2: 04–10 January; 3: 11–17 January; 4: 18–24 January) at Matroosfontain Meteorological Station in the summer of 2015/16 when facultative viviparity was observed and averaged for the five following summers from 2016/17 to 2020/21.
Monthly rainfall at Matroosfontain Meteorological Station from December 2015 to March 2016 (when facultative viviparity was observed) was higher than the historic monthly rainfall averaged from 1979 to 2000 and December rainfall was higher in 2015 than in 2017 and 2020 (Figure 4). December/January rainfall combined was higher in 2015/16 (35.6 mm) than in 2019/20 (34.5 mm) and 2020/21 (18.1 mm).
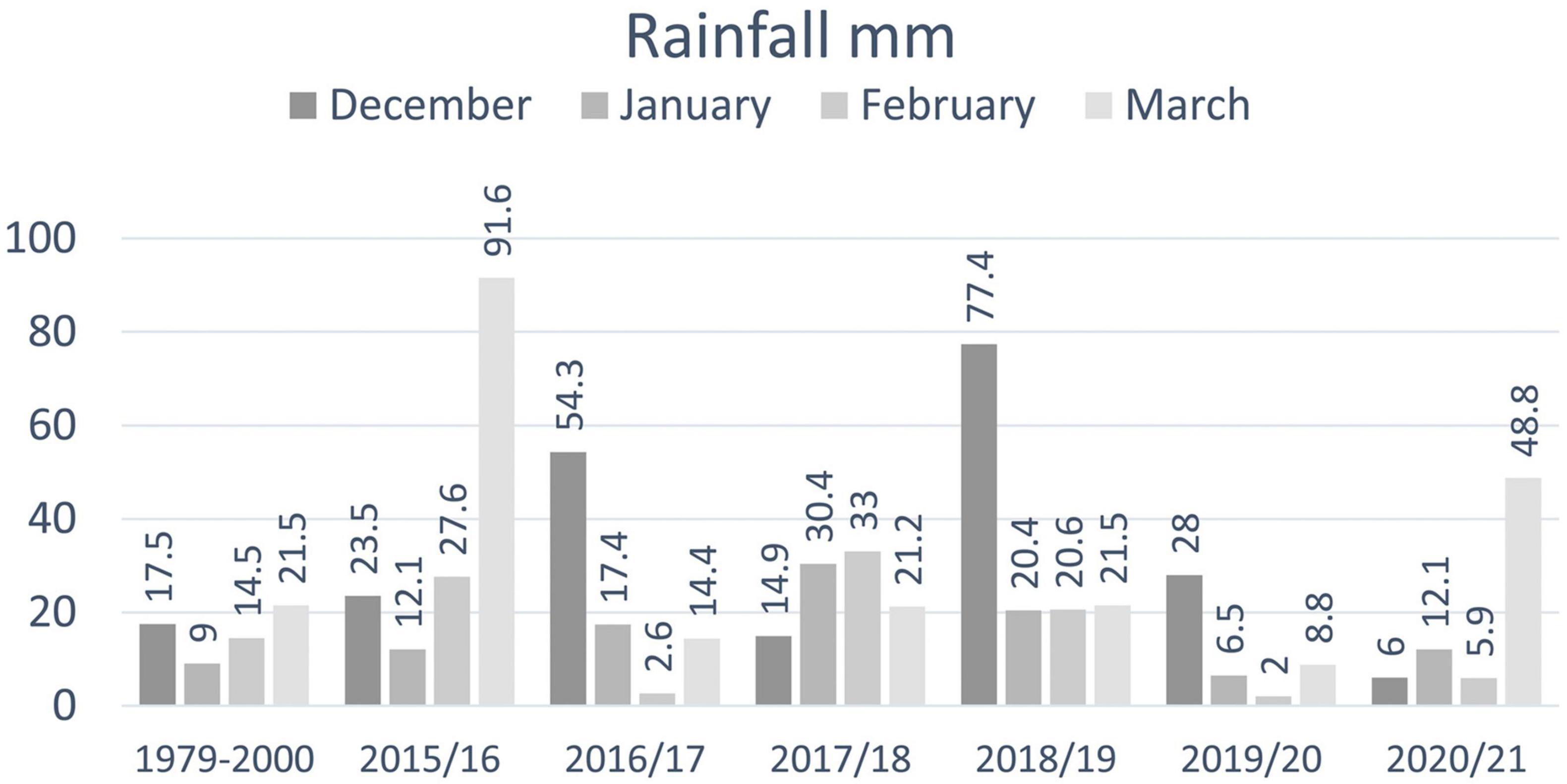
Figure 4. December to March monthly rainfall at Matroosfontain Meteorological Station averaged from 1979 to 2000, monthly rainfall in the summer of 2015/16 when facultative viviparity was observed in the captive colony and in the five following summers when facultative viviparity was not observed.
The discovery of facultative viviparity in the captive C. angulata colony in 2015/16 was unplanned and involved happenstance. Despite increased monitoring we could not repeat this observation over the five following years. The results of the present investigation demonstrate that, locally for the studied captive population, the summer of 2015/16 was the hottest summer of the last decade. We found no indication for rainfall anomaly in the summer of 2015/16, the 2015--2017 drought in Cape Town was primarily the result of reduced winter rainfall (downloaded 01 June 2021).2 Opportunistic studies, like the present one, lack information on the situation earlier on: prior to April 2016 observations were insufficient to indicate if facultative viviparity had already occurred in the captive colony in earlier years. However, the results of this study demonstrate that, over the last 6 years when monitoring was increased, facultative viviparity in the C. angulata captive colony only co-occurred with a single extreme summer heat event. Extreme climatic events happen rarely, thus there are few opportunities to study their effect on an organism’s phenotypically plastic response. However, even observing only one event can tell us what types of responses are possible (Altweg et al., 2017).
Concerning the 2016 case history of the detection of the two fully developed eggs, there was no evidence that the female(s) attempted to dig nests; in fact, the ground surface of the enclosure was completely undisturbed. The two eggs were on open ground close to the enclosure’s entrance where they were instantly visible when nearing the enclosure. Because members of the household visit the enclosure and its surroundings on a regular basis, there is no other explanation than that one or two females laid fully developed eggs on the ground surface shortly before the eggs hatched. It could well be that similar cases have gone undetected in past autumns if females laid fully developed eggs among vegetation. Even though our observations do not indicate viviparity in captivity co-occurred with summer droughts, respective triggers might have been mask by the regular irrigation of the enclosure with bore water. Apart from this possibility, Barrows et al. (2016) stressed the general difficulty of separating temperature and rainfall as driving force in semi-arid to hyper-arid winter rainfall areas, in that case for modeling habitat suitability of the desert tortoise (Gopherus agassizii): since, in these areas, summer temperature (mean July maximum temperature) and rainfall/humidity are negatively auto correlated they used summer temperature alone to identify habitat refuges under climate change. The natural range of C. angulata extends into areas of extreme aridity (south-western Namibia). Low soil humidity may reduce nesting and hatching success and most C. angulata eggs are generally laid within 3 days of rainfall (Hofmeyr, 2004). Therefore, in addition to summer heat, we cannot exclude that in natural populations of C. angulata drought or low humidity may act as a further potential cue for facultative viviparity.
The only earlier observation suggesting the possibility of facultative viviparity in C. angulata was made over two decades ago in a wild population of C. angulata, but we were unable to reconstruct the local climatic conditions of that time to analyze them for inclusion into the results of this paper: while doing fieldwork on Dassen Island, an offshore island in the Atlantic Ocean to the south-west of West Coast National Park, author 1 found a C. angulata egg (length = 41.0 mm, width = 35.2 mm) in the late afternoon of 20 March 1999. The unburied egg was on open, rocky ground, in full sun, relatively close to a laboratory where we assessed the reproductive condition of C. angulata females by ultrasound scanning. No sign of a nest hole could be seen nearby and the eggshell was clean white, without a trace of soil or dirt, as in a freshly laid egg. We did not notice the egg in the morning or in preceding days when field workers frequently passed through this area. Since some females had ovulated by that time, the beginning of a new nesting season, we first assumed that, following the ultrasound examination, a released female had aborted her first shelled egg of the new season and dropped it on the ground without nesting. We assumed it was doomed due to its exposure to the elements, measured the egg and stored it in a glass flask in the laboratory. However, the egg hatched overnight and the following morning we found a fully developed hatchling (mass = 14.5 g, carapace length = 35.6 mm) in the glass flask with all yolk internalized. The hatchling was alert and moved normally, but appeared to have a bilateral microphthalmic condition.
Chersina angulata females occasionally lay eggs on the surface (not into nests underground), but this is not limited to autumn when hatching normally occurs (M. D. Hofmeyr, personal observation). Consequently, egg laying on the surface in itself is not linked to full embryonic development. We detected the fully developed eggs at Dassen Island and in the captives’ enclosure because they were out in the open. Although the incident at Dassen Island over two decades ago perplexed us at the time, its viviparity interpretation conflicted with the (still today) generally accepted wisdom that the order Testudines is strictly oviparous. At the time we were hesitant to interpret and report it correctly as viviparity. Our hesitation disappeared with the second incident in the captive colony, which dispelled any other explanation than that C. angulata mothers can carry embryos to full term. The reason for the rarity of respective reports for reptiles may well be that such cases can easily be overlooked, or shelved away as odd flukes instead of being reported (as we first did in 1999), or that editors reject their publication in scientific journals (as happened during the late life time of the second author, with exception of a conference abstract: Hofmeyr and Kuchling, 2017). Since then facultative viviparity (capacity to deposit either eggs or developed offspring, depending on circumstances) has been reported for one more reptile, the skink Saiphos equalis, with its discovery based on a single female in a captive colony (Laird et al., 2019). Even though this reproductive lability has been rarely described in reptiles, it can be part of a viable reproductive strategy and evolutionary more significant than previously assumed.
The reproductive strategies of oviparity and viviparity both entail advantages and disadvantages, each of which may differ in their applicability to particular species. The universality of internal fertilization in reptiles readily permits the evolution of viviparity which evolved over 100 times in different lineages of the order Squamata, often at subfamilial and subgeneric levels and, in some cases, at the subspecific level (Blackburn, 1999). From the standpoint of life history theory viviparity should evolve only when the benefits of stages that are evolutionarily intermediate outweigh the costs. Selection would only favor uterine retention of eggs if there were a net benefit to female fitness as measured by lifetime reproductive success: the costs of longer egg retention have to be more than counterbalanced by increased egg survivorship through a reduced incubation period (Williams, 1992). Due to the small clutch size of C. angulata, the costs of retaining a clutch for longer periods (impaired mobility, higher risk of predation of the egg carrier, reduced potential for feeding impacting subsequent fecundity: Shine, 1980) appear to be small regarding the residual reproductive value of the female.
One explanation for the, until now, presumed absence of steps toward viviparity in the order Testudines is that, in most species, eggs are laid in an environment removed and quite different from the one in which the turtles live, making the intermediate egg retention stages in oviducts ineffective as a means of later placing eggs in the most optimal environment (Tinkle and Gibbons, 1977). However, this reasoning only applies to aquatic and marine turtles, it does not apply to terrestrial tortoises (Family Testudinidae). Another explanation is that viviparity could be disadvantageous in multi-clutching turtles because longer oviducal egg retention times would reduce annual fecundity (Tinkle and Gibbons, 1977). This constraint also appears not to apply to C. angulata which usually has single-egg clutches and can ovulate a further clutch while the previous clutch is still in the oviduct (Hofmeyr, 2004). Our data suggest that viviparity in C. angulata is facultative and may be restricted to the last clutch of their long nesting season, with gravidity not preventing ovulation of the first clutch of the following nesting season. Facultative viviparity could also occur in other arid-adapted tortoises, particularly in the genus Chersobius, Chersina’s sister genus which diverged from it during the Late Oligocene warming phase (26 Mya). The ranges of all three species of the genus Chersobius, C. boulangeri, C. signatus, and C. solus, are largely embedded in C. angulata’s much larger area of distribution and all three species also produce single egg clutches (Hofmeyr et al., 2016).
The co-occurrence of facultative viviparity in C. angulata with hot summer temperatures is not in agreement with the “cold-climate hypothesis” for the evolution of viviparity in reptiles, but supports the more general “maternal manipulation hypothesis”: that prolonged uterine retention of developing embryos allows a female to provide better incubation conditions for her offspring (Shine, 2014). In the lizard Zootoca vivipara females select lower-than-usual temperatures while carrying eggs and this avoidance of high temperatures early in embryogenesis enhances the viability of offspring (Rodríguez-Díaz and Braña, 2011). Under unusually hot summer temperatures facultative viviparity in C. angulata may provide comparable temperature advantages for embryonic development: we propose that high risks associated with nesting and egg development during hot and dry summer conditions provide selective pressures for the evolution of facultative viviparity in C. angulata. In the captive C. angulata colony the environmental cue to initiate facultative viviparity appears to be unusually high temperatures toward the end of their nesting season when, under normal temperature conditions, they would oviposit their last clutch into a nest. Phenotypic plasticity can be a major mechanism of response to environmental variability, which may allow organisms to cope with rapid environmental changes (Bonamour et al., 2019).
Several aspects of facultative viviparity in C. angulata need to be studied in more detail. Oviparous reptiles, with the exception of crocodiles, arrest embryonic development prior to oviposition. Squamata most often arrest at stage 30 of a 40-stage chronology (Andrews and Mathies, 2000; Van Dyke et al., 2014), whereas in Rhynchocephalia and, as generalization, in Testudines this pre-ovipositional developmental arrest occurs earlier during development at the gastrula stage (Ewert, 1985; Andrews and Mathies, 2000; Rafferty and Reina, 2012). Under hypoxia, in the oviducts as well as experimentally, turtle embryos arrest development at the gastrula stage, until resumption of their development is again triggered by a normoxic environment (e.g., once deposited into the nest). In the marine turtle Chelonia mydas (Cheloniidae) and in the freshwater turtles Chelodina oblonga, Chelodina longicollis, and Emydura macquarii (Chelidae) pre-ovipositional developmental arrest is achieved by a mucus-like substance secreted by the oviducts which retards oxygen diffusion (Rafferty et al., 2013), a mechanism that appears adaptive for these multi-clutching aquatic species. Pre-ovipositional developmental arrest would prevent the transition to viviparity (Andrews and Mathies, 2000), unless it is abolished (as in viviparous squamates) or becomes facultative. Neither details of pre-ovipositional developmental arrest, nor oxygen conditions in the oviducts, nor eggshell morphology and composition have so far been investigated in C. angulata. This basic research will be essential to understand the mechanisms behind this species’ unique suite of reproductive strategies.
Elucidating mechanisms and strategies available to species plays a significant role in assessing and predicting the susceptibility or resilience of species to future, human-induced environmental change (Franklin and Hoppeler, 2021). Our analysis of air temperatures and rainfall for the summer when we observed facultative viviparity in a captive colony of C. angulata, and for the five following summers when we could not observe it, suggests that hot ambient temperatures at the end of this species’ long nesting season can function as environmental cue for females to switch from egg deposition into nests to facultative viviparity. The association of this phenotypic plasticity with inter-annual variations in climate has the potential to buffer the developing embryos of the season’s last clutch from overheating during heat waves or increasing temperatures due to climate change: females can thermo-regulate by moving among different microhabitats whereas, once the nest site is chosen, nests of non-nest-guarding reptiles are passively exposed to hot ambient temperatures. This study provides insight into a, for the order Testudines, so far unrecognized and novel strategy: to change the reproductive mode during extremely hot summer weather from oviparity to facultative viviparity to facilitate successful embryonic development of the last clutch of the season. This novel reproductive strategy has the potential to enhance the resilience of species to global warming.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the University of Western Cape Research Ethics Committee CWE/23 (SANP), 96/10/15.
MH conceived the idea of facultative viviparity in C. angulata, monitored the captive population and wrote an early draft of part of this manuscript with GK providing conceptual input. Following MH’s passing in February 2020, GK conceived the idea that climate variability may influence the occurrence of facultative viviparity in C. angulata, retrieved and analyzed the temperature and rainfall data, and wrote the final draft. Both authors contributed to the article and GK approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Thelma Maans for assistance with the captive colony and Guundie Kuchling for assistance during the field work on Dassen Island. This research was completed under permits CWE/23 (SANP), 96/10/15 (UWC Research Ethics Committee) and permits 224/98, 703/98, 176/2000, and 923/2000 authorized by the Western Cape Nature Conservation Board. We thank Theunis Hofmeyr for maintaining the captive C. angulata colony throughout 2020 and for getting all females radiographed in January 2021. We thank Brian Henen for improving an earlier draft of this manuscript.
Altweg, R., Visser, V., Biley, L. D., and Erni, B. (2017). Learning from single extreme events. Philos. Trans. R. Soc. B 372:2016041. doi: 10.1098/rstb.2016.0141
Andrews, R. M., and Mathies, T. (2000). Natural history of reptilian development: constraints on the evolution of viviparity. BioScience 50, 227–238. doi: 10.1641/0006-3568(2000)050[0227:nhordc]2.3.co;2
Barrows, C. W., Henen, B. T., and Karl, A. E. (2016). Identifying climate refugia: a framework to inform conservation strategies for Agassiz’s desert tortoise in a warmer future. Chelonian Cons. Biol. 15, 2–11. doi: 10.2744/CCB-1157.1
Blackburn, D. G. (1999). “Viviparity and oviparity: evolution and reproductive strategies,” in Encyclopedia of Reproduction, Vol. 4, eds E. Knobil and J. D. Neill (London: Academic Press), 994–1003.
Blackburn, D. G., and Sidor, C. A. (2014). Evolution of viviparous reproduction in paleozoic and mesozoic reptiles. Int. J. Dev. Biol. 58, 935–948. doi: 10.1387/ijdb.150087db
Bonamour, S., Chevin, L.-M., Charmantier, A., and Teplitsky, C. (2019). Phenotypic plasticity in response to climate change: the importance of cue variation. Philos. Trans. R. Soc. B 374:20180178. doi: 10.1098/rstb.2018.0178
Ewert, M. A. (1985). “Embryology of turtles,” in Biology of the Reptilia, Development A, Vol. 14, eds C. Gans, F. Billett, and P. F. A. Maderson (New York, NY: John Wiley & Sons), 75–267.
Franklin, C. E., and Hoppeler, H. H. (2021). Elucidating mechanism is important in forecasting the impact of a changing world on species survival. J. Exp. Biol. 224:jeb242284. doi: 10.1242/jeb.242284
Hofmeyr, M. D. (2004). Egg production in Chersina angulata: an unusual pattern in a Mediterranean climate. J. Herpetol. 38, 172–179. doi: 10.1670/133-03A
Hofmeyr, M. D. (2009). “Chersina angulata (Schweigger 1812) – angulate tortoise, South African bowsprit tortoise,” in Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group (Chelonian Research Monographs No. 5), eds A. G. J. Rhodin, P. C. H. Pritchard, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, J. B. Iverson, et al. (White Lake, NC: Chelonian Research Foundation), 030.1–030.6. doi: 10.3854/crm.5.030.angulata.v1.2009
Hofmeyr, M. D., and Kuchling, G. (2017). “Reproductive strategies of the angulate tortoise Chersina angulata (Testudines: Testudinidae). African Herp News 66,” in Proceedings of the Abstract 13th Herpetological Association of Africa conference, 23-27 January 2017, (Kwazulu Natal), 25–26.
Hofmeyr, M. D., Vamberger, M., Branch, W. R., Schleicher, A., and Daniels, S. R. (2016). Tortoises (Reptilia, Testudinidae) radiations in South Africa from the Eocene to the present. Zool. Scr. 46, 389–400. doi: 10.1111/zsc.12223
Kam, J., Min, S.-K., Wolski, P., and Kug, J.-S. (2021). CMIP6 model-based assessment of anthropogenic influence on the long sustained Western Cape drought over 2015–19. Am. Meteorol. Soc. 102, S45–S50. doi: 10.1175/BAMS-D-20-0159.1
Kuchling, G. (1999). The Reproductive Biology of the Chelonia, Zoophysiology, Vol. 38. Berlin: Springer, 223.
Laird, M. K., Thompson, M. B., and Whittington, C. M. (2019). Facultative oviparity in a viviparous skink (Saiphos equalis). Biol. Lett. 15:20180827. doi: 10.1098/rsbl.2018.0827
Odoulami, R. C., Wolski, P., and New, M. A. (2021). SOM-based analysis of the drivers of the 2015–2017 Western Cape drought in South Africa. Int. J. Climatol. 41, (Suppl. 1) E1518–E1530. doi: 10.1002/joc.6785
Rafferty, A. R., and Reina, R. D. (2012). Arrested embryonic development: a review of strategies to delay hatching in egg-laying reptiles. Proc. R. Soc. Lond. B 279, 2299–2308. doi: 10.1098/rspb.2012.0100
Rafferty, A. R., Evans, R. G., Scheelings, T. F., and Reina, R. D. (2013). Limited oxygen availability in utero may constrain the evolution of live birth in reptiles. Am. Nat. 181, 245–253. doi: 10.1086/668827
Rodríguez-Díaz, T., and Braña, F. (2011). Shift on thermal preferences of female oviparous common lizards during egg retention: insights into the evolution of reptilian viviparity. Evol. Biol. 38, 352–359. doi: 10.1007/s11692-011-9122-y
Schweigger, A. F. (1812). Prodromus monographia Cheloniorum auctore Schweigger. Königsberg. Arch. Naturwiss. Math. 1, 271–368, 406–458.
Shine, R. (1980). “Costs” of reproduction in reptiles. Oecologia 46, 92–100. doi: 10.1007/BF00346972
Shine, R. (1985). “The evolution of viviparity in reptiles: an ecological analysis,” in Biology of the Reptilia, Vol. 14, eds C. Gans, F. Billett, and P. Maderson (New York, NY: Wiley), 605–694.
Shine, R. (2014). Evolution of an evolutionary hypothesis: a history of changing ideas about the adaptive significance of viviparity in reptiles. J. Herpetol. 48, 147–161. doi: 10.1670/13-075
Tinkle, D. W., and Gibbons, J. W. (1977). The Distribution and Evolution of Viviparity in Reptiles, Vol. 154. Ann Arbor: Miscellaneous Publications Museum of Zoology, University of Michigan, 1–55.
Van Dyke, J. U., Brandley, M. C., and Thompson, M. B. (2014). The evolution of viviparity: molecular and genomic data from squamate reptiles advance understanding of live birth in amniotes. Reproduction 147, R15–R26. doi: 10.1530/REP-13-0309
Keywords: heat, rainfall, climate change, reptile, Testudines, oviparity, facultative viviparity, reproductive strategy
Citation: Kuchling G and Hofmeyr MD (2022) Too Hot to Nest? In a Hot Summer the Tortoise Chersina angulata Can Switch From Nesting to Facultative Viviparity. Front. Ecol. Evol. 9:788764. doi: 10.3389/fevo.2021.788764
Received: 03 October 2021; Accepted: 15 December 2021;
Published: 06 January 2022.
Edited by:
J. Sean Doody, University of South Florida, United StatesReviewed by:
Bao-jun Sun, Institute of Zoology, Chinese Academy of Sciences (CAS), ChinaCopyright © 2022 Kuchling and Hofmeyr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerald Kuchling, Z2VyYWxkLmt1Y2hsaW5nQHV3YS5lZHUuYXU=
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.