- 1Horticultural Crops Research Unit, USDA Agricultural Research Service, Corvallis, OR, United States
- 2Hermiston Agricultural Research and Extension Center, Oregon State University, Hermiston, OR, United States
Methyl salicylate (MeSA) is an herbivore-induced plant volatile widely tested for attracting natural enemies for pest control. MeSA is commercially sold as slow-release lures or as a spray. While MeSA application has increased the abundance of natural enemies in numerous food crops, its ability to reduce pests for crop protection is not as frequently demonstrated. Our first objective was to test MeSA lures in ornamental fields where few studies have been done, and monitor natural enemies, pests, and crop protection. A 2-year study in spruce container yards revealed more aphid parasitoids (Pseudopraon sp.), fewer aphids (Mindarus obliquus) on shoot tips, and less shoot tip damage in MeSA plots during the first year. A 2-year study in red maple fields revealed more predatory lady beetles and rove beetles, and parasitic Ceraphronidae, Diapriidae, and Chalcidoidea in one or both years with MeSA. Fewer pest thrips were also captured in MeSA plots, though it is not clear whether this was due to enhanced predation or reduced colonization. Maple growth as measured by stem diameter change did not differ with MeSA use. A 2-year study examining predation on sentinel Halyomorpha halys eggs in various mature ornamental stock blocks found no increase in predation except for 1 month, though green lacewings, lady beetles, and predatory thrips occurred more in MeSA plots in the first year. While MeSA is expected to enhance biological control by herding in natural enemies, the impacts that applied volatiles have on predator efficiency is mostly unknown. Thus, our second objective examined how volatiles would impact feeding rates at close-range. Adult carabid Pterostichus melanarius, adult coccinellids Coccinella septempunctata and Harmonia axyridis, and larval lacewing Chrysoperla rufilabris consumed their prey at similar rates in the presence/absence of MeSA when food was presented directly in a 28 cm2 or 30 ml arena, or when foraging in a 520 cm2 outdoor soil arena or 946 ml arena with aphids on leaves.
Introduction
When herbivores feed on plants, herbivore-induced plant volatiles (HIPVs) are released that attract natural enemies and are considered as an indirect defensive response. While many HIPVs have been identified, methyl salicylate (MeSA) is often studied for biological control (rev. by Khan et al., 2008; Rodriguez-Saona et al., 2011) and is commercially available as slow-release lures or tank mix for sprays. Insects are attracted to MeSA in the laboratory, including anthocorids (Drukker et al., 2000), green lacewings, lady beetles (Salamanca et al., 2017), parasitic wasps (Williams et al., 2008), predatory mites (De Boer and Dicke, 2004), predatory thrips and rove beetles (Shimoda et al., 2002). MeSA application in the field has attracted natural enemies and subsequently decreased pest abundance in soybean (Mallinger et al., 2011), potted cucumbers in an open field (Dong and Hwang, 2017), hop yards (Woods et al., 2011), and beans (Salamanca et al., 2018). On the other hand, other studies have shown no/limited benefits with the MeSA-only treatment on ash trees (McPike and Evenden, 2021), sweet sorghum (Mercer et al., 2020), and cotton (Naranjo et al., 2021). Or, MeSA only elevated natural enemy abundance without consistently decreasing pest levels in vineyards and strawberry fields (Lee, 2010; Gadino et al., 2012).
Fewer studies have examined the impacts on plants. In one study, MeSA treatments did not reduce associated earworm damage in corn (Simpson et al., 2011b). In another study, MeSA treatment lowered spider mite damage on bean plants, but eventual seed yield was not improved (Salamanca et al., 2018). In studies showing promise, MeSA treatment resulted in similar cucumber yield as insecticide-treated plants (Dong and Hwang, 2017) and higher wheat yield than control plots (Wang et al., 2011). MeSA has also stimulated plant defenses making them more resistant to an herbivore and fungal pathogen (Rowen et al., 2017). Lastly, most MeSA studies have been conducted in food crops with variable results (Rodriguez-Saona et al., 2011); variability is expected since the crop background will affect attraction to MeSA (Braasch et al., 2012). Therefore, our first objective was to evaluate MeSA lures in ornamental fields, where few studies have been done, during three 2-year studies on natural enemy and pest abundance, and plant damage or growth.
The first field study was conducted in Alberta spruce, Picea glauca ‘Conica’ (Moench) Voss (Pinales: Pinaceae), which is grown in containers. Large growers have acres of gravel lots filled with potted spruce. The spruce woolly aphid, Mindarus obliquus (Chol.) (Hemiptera: Aphididae), feeds on the newly formed shoot tips causing it to twist and accumulate wax (Supplementary Material 1). Such aesthetic damage is costly, as growers prune off current-year damage and spray with insecticides multiple times a year. Augmentative releases of green lacewings have reduced a related aphid, Mindarus abietnus Koch densities in Christmas tree farms (Fondren et al., 2004). Thereby, using MeSA lures to attract lacewings may be a cheaper alternative to enhance biological control. The second study was conducted in red maples, Acer rubrum L. (Sapindales: Sapindaceae), grown in the soil. Growers spray the maples multiple times a season to control a variety of pests such as aphids, leafhoppers, lygus, spider mites, and thrips (J. Lee, personal communication). These seedlings are planted early spring about ∼12 cm tall, and grow rapidly through summer. The third field study was conducted in a mix of mature ornamental plants grown for scion cuttings, and infrequently sprayed with insecticides. During the study in 2015-16, the invasive brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae), was a pest throughout the United States (Rice et al., 2014). Although it has a wide host range, it prefers some ornamental hosts especially plants with fruit (Martinson et al., 2015; Bergmann et al., 2016). MeSA was tested for its ability to increase predation and parasitism of sentinel H. halys egg masses.
After establishing that MeSA attracts natural enemies, some studies have followed up to demonstrate that this leads to predation on aphids in the soybean field (Mallinger et al., 2011), sentinel aphids in a bean plot (Salamanca et al., 2018), and sentinel eggs in a cranberry bog (Salamanca et al., 2019). The expectation is that once natural enemies are drawn in, numerically more predators/parasitoids will suppress the pest. Yet, the per capita efficiency of a natural enemy is of interest. Puente et al.’s (2008) model predicts that once a natural enemy is in the vicinity, there is a possibility that synthetic HIPVs will saturate the system and interfere with its search for pests. This is supported by a field cage study where synthetic MeSA lures near tomatoes reduced per capita predation by a stink bug (Vidal-Gomez et al., 2018). Yet, other HIPVs have suggested the opposite effect. Treating cabbage with a cocktail of four HIPVs enhanced parasitism on caterpillars (Uefune et al., 2012), and treating corn with jasmonic acid did not interfere with a parasitoid’s ability to orient toward caterpillar-infested plants over uninfested plants (Ozawa et al., 2004). Does MeSA then reduce, increase or have no impact on an individual’s attack rate? Our second objective was to evaluate how concurrent exposure to MeSA volatiles affects predator consumption and foraging within a small-scale arena in outdoor settings near a MeSA lure. This was tested with a predatory carabid beetle, Pterostichus melanarius (Illiger) (Coleoptera: Carabidae), lady beetles, Coccinella septempunctata L. and Harmonia axyridis Pallas (Coleoptera: Coccinellidae), and green lacewing, Chrysoperla rufilabris Burmeister (Neuroptera: Chrysopidae). The lacewing was tested at the larval stage since the adults are not predaceous and leave after egg laying, and their offspring larvae remain near lures.
Materials and Methods
Spruce Field Trial
MeSA was evaluated in 0.8–1.2 m tall potted Alberta spruce, P. glauca ‘Conica’ in commercial fields (Supplementary Material 1A). MeSA-treated and control plots were 5.5 × 30.5 m (18 × 100 ft) in size, and separated 100+ m apart. In each plot, three 30-d Predalure® sachets (AgBio, Westminster, Colorado) or three white cards were placed equidistantly near the top of a spruce plant. MeSA was applied at 180 lures/ha (73 lures/acre). In year 1, four MeSA-treated and four control plots were set up in a randomized complete block design on 1-May 2009 and sampled for 6 weeks. See below for sampling details. In year 2, the experiment was conducted in the same nursery but in different sites. Five MeSA and five control plots were set up in a completely randomized design on 23-April 2010 and sampled for 10 weeks; lures were replaced after 5 weeks. Sampling continued past the 30-d lure expiration point to compare efficacy over time and monitor for any decline.
Each week, five trees within 1 m of a lure/blank and seven trees about 4–7 m away were visually inspected for aphid abundance, natural enemies, and rated for damage. Aphids were counted on three shoot tips per tree. Damage ratings were: (1) none, (2) trace – mild twisting, (3) some – twisting and wax accumulation visible upon opening a shoot tip, (4) visible – twisting and wax accumulation noticeable from 1 m distance, and (5) high – shoot tip stunted in growth and multiple tips noticeably infested (Supplementary Material 1B–D). In year 1, one clear sticky trap and one yellow pan trap were placed within 1 m of the nearest lure/blank, and another set of traps was placed 4–7 m away. In year 2, sticky traps but no pan traps were used with one sticky trap placed within 1 m, and two traps placed 4–7 m away from a lure/blank. Clear sticky traps passively caught insects and were 10 × 16 cm double-sided made from a folded transparency with Tanglefoot® (Scotts Miracle Gro, Grand Rapids, MI, United States). Sticky traps were attached 1 m aboveground to a metal post with a cement base that could be moved each week. Yellow pan traps were 5.7 ml plastic containers painted bright yellow on the exterior and replaced with soapy water each week. All traps were moved around weekly to sample the 168 m2 plot.
Analyses were conducted using R v. 4.0.3 (R Core Team, 2020). Insect counts were compared separately each year since different plots were used. Treatment, position (‘near’ or ‘away’ from MeSA/blank lure), time, and relevant interaction terms were tested as fixed effects using a negative binomial GLMM (‘glmmTMB’ package, Brooks et al., 2017). Each block/plot/position was a unique physical observation included as a random factor and in a repeated measures model. In 2009, block was a random factor and in 2010, blocking was not in the design because the fields available for testing were scattered. The number of days between collections was an offset for trap counts; the number of trees was an offset for visual counts. GLMM models were validated by visually examining a scaled residual plot (‘DHARMa’ package, Florian, 2020). If overdispersion was >1.2, the model was re-run with a Poisson distribution. If the variance of the data was bigger than the variance given by Poisson or negative binomial distributions, then an observation-level random effect to account for observation-level noise or an optimizer (bobyqa) to extend the maximum number of iterations was added to the negative binomial model. Data were tested using Bayesian analysis with penalized regression via diffuse priors where complete separation existed (‘blme’ Chung et al., 2013). Fixed factors and their interaction were checked for significance with likelihood ratio tests fitting a full and reduced model. If an interaction was not significant, it was removed from the model. For plant assessments, damage ratings (none, trace, some, visible, high) were pooled by treatment per date and tested by permutation test (‘predictmeans’ Luo et al., 2018).
Red Maple Field Trial
Methyl salicylate was evaluated in red maple A. rubrum cultivars in commercial fields. In year 1, four MeSA and control plots 56 m2 (600 ft2) were set up among two ‘Redpointe,’ and one ‘Red Sunset’ and ‘Autumn Blaze’ maple blocks. Plots were 200+ m apart. One 30-d Predalure® or white tag was hung on a maple tree per plot with 180 lure/ha rate. Plots were set up on 22-23-July 2009 and sampled for 6 weeks. Each week, 20 leaves were inspected for spider mites and other insects, and one white sticky trap and one yellow pan trap were collected within 1 m of the lure/blank from each plot, and another set of samples were taken 4–7 m away. White sticky cards (Great Lakes IPM, Vestaburg, MI, United States) considered moderately attractive were used, they were hung ∼1 m high beneath the canopy. Sticky and pan traps were moved weekly within the plot.
In year 2, five different MeSA and five control plots were set up among two ‘Autumn Blaze’ blocks, two ‘Red Sunset’ blocks, and one ‘October Glory’ block. Plots were 200+ m apart and different from the prior year. Each 167 m2 (1800 ft2) plot contained three lures or blanks placed equidistantly, with 180 lures/ha rate. Plots were set up on 7–8-July 2010 and sampled for 7 weeks. Each week, 20 leaves, one white sticky trap and one yellow pan trap were sampled within 1 m of a lure/blank from each plot, and another 20 leaves, two sticky traps, and one pan trap were sampled 4–7 m away. Traps were moved weekly. To determine if MeSA affected plant growth, the diameter of the stem 15 cm (6 in) aboveground was monitored for growth. The same ten trees next to each MeSA or blank, 30 trees per plot, were marked and measured at the beginning and end of the experiment.
As described earlier, insect counts were compared separately each year using a GLMM with treatment, position, time, and relevant interaction terms as fixed effects, incorporating random effects, overdispersion adjustment, offsets and use of penalized regression for complete separation as in the Spruce analyses. A negative binomial or Poisson distribution was used.
Halyomorpha halys Egg Predation Field Trial
Methyl salicylate was evaluated in various nursery crops for enhancing predation and parasitism on sentinel H. halys eggs. Established stock block plantings for scion collection were used since they were infrequently sprayed with insecticides. Two blocks were set up in maple Acer platanoides L., A. ginnala Maxim., A. miyabei Maxim. (Sapindaceae), and two blocks in crabapple Malus spp. (Rosaceae), and one block in Tilia cordata Mill. and T. x euchlora (Malvaceae). Plots were 56 m2 (600 ft2) and 50+ m apart. In each plot, a 90-day MeSA Predalure® sachet or white card was hung in the center canopy on 16-June 2015 and 7-June 2016, with the same plots used each year. Trials ended in September.
Samples were taken once a month for 4 months. First, three sentinel H. halys egg clusters collected from the lab colony on filter paper (McIntosh et al., 2019) were placed randomly in plots and hung on the underside of leaves with a paperclip. Egg clusters were previously stored at –80°C and were frozen within 0–3 days of being laid. One egg cluster was placed in a 5 × 5 cm cage with 1 mm mesh size to prevent removal by larger predators but allow parasitism. The other two egg clusters were uncaged and accessible to a variety of predators. A white sticky card was hung in the canopy within 2 m of a H. halys cluster. Sentinel eggs and white sticky cards were collected after a week in the field and the eggs examined for predation upon return to the lab and reared for parasitism. In year 1, vacuum samples were taken on collection day; the foliage was vacuumed for 1 min at each site using an inverted leaf blower with a mesh collection bag (Black & Decker, Towson, MD, United States). In 2016, a second white sticky card was added on a tree near an egg cluster to increase sampling effort because vacuum samples collected very few insects in year 1 and were not analyzed.
The percent egg predation in caged or uncaged clusters and sticky card insect counts were compared separately each year since some methodology changed in the second year. A GLMM tested treatment, time, and interactions as fixed effects; block and the subject that was repeatedly sampled (site) were random effects. A binomial was used for percent egg predation and a negative binomial or Poisson for sticky card counts. Overdispersion adjustment, correlation of errors, and use of penalized regression for complete separation were conducted as described in the Spruce trial.
Methyl Salicylate Effect on Feeding
To determine if MeSA affects predation rate, several predator species were tested for consumption of prey in the presence of MeSA. Carabid adults, P. melanarius, were collected from pitfall traps in a strawberry field and maintained on dog food in the lab. Coccinellid adults, C. septempunctata and H. axyridis, were collected from weedy fields and maintained with aphids. Green lacewing 2nd instar larvae, C. rufilabris, were purchased in hexcel units (Evergreen Growers, Clackamas, OR, United States) right before trials. Prey were raspberry aphid, Amphorophora agathonica Hottes, pea aphid, Acyrthosiphon pisum Harris (Hemiptera: Aphidae), and black vine weevil larvae, Otiorhynchus sulcatus Fabricius (Coleoptera: Curculionidae), from laboratory colonies.
Table 1 summarizes the setup for each experiment. Multiple replicates were set up on five or more trial dates. Before testing, carabid and coccinellid adults were starved 20 h with water, and lacewing larvae were held at 10°C as recommended until augmentative releases.
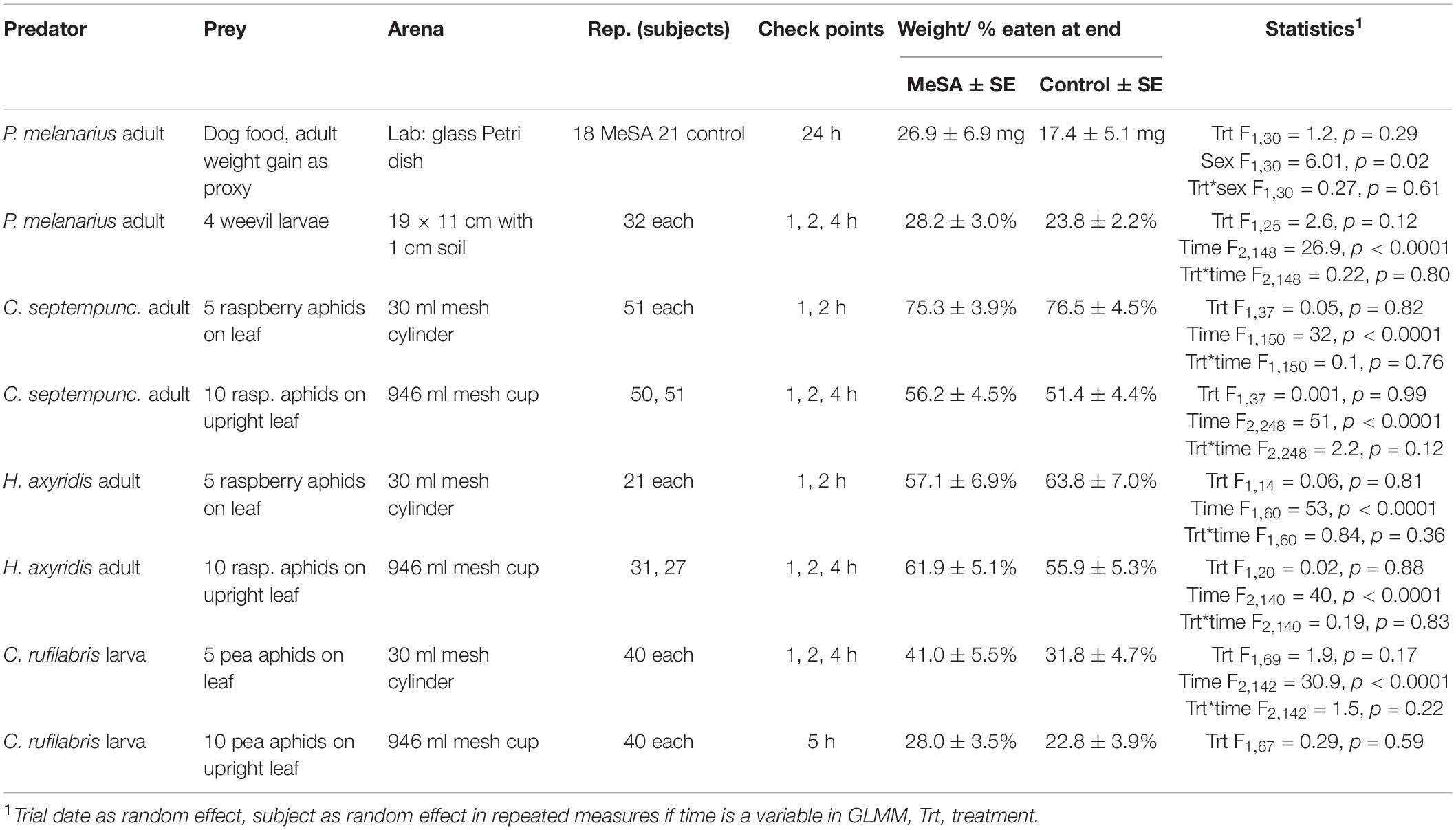
Table 1. Experimental design, mean predation, and statistical outcomes of predation trials in the presence/absence of MeSA volatiles.
The first laboratory trial weighed each carabid and placed it in a 9 cm diameter glass Petri dish with 1 cm layer of sterilized moistened loam dirt. Filter paper with 0.1 μl of water or MeSA (Sigma Aldrich, St. Louis, MO, United States) was affixed to the lid while dog food (Alpo®, Neenah, WI, United States) was placed on the dirt. After 24 h, carabids were weighed again to calculate weight lost or gained as a sign of predation.
Outdoor trials tested carabid adults, coccinellid adults, and lacewing larvae by placing them in open/mesh arenas in a shaded station with a Predalure® 30-day sachet or blank card hung 40 cm aboveground. Stations were 50+ m apart and separated by buildings. Individual carabids were given 5–6th instar weevil larvae in 1 cm of soil in a 19 × 11 × 7 cm (l × w × h) metal pan. Individual coccinellids were given 4th instar raspberry aphids on a raspberry leaf, Rubus idaeus L. (Rosaceae), and lacewing larvae were given 2nd-3rd instar pea aphids on a fava bean leaf, Vicia faba L. (Fabaceae). To test direct consumption, prey on a leaf were presented in a 30 ml cylindrical container with mesh ends to limit predator movement. To test small-scale foraging, prey were on an upright leaflet in a floral water wick in a 946 ml cup with mesh sides and a predator was placed at the bottom. Predation included missing or eaten prey at various time intervals (Table 1). For lacewings placed in a cup, predation was only checked at 5 h to minimize disturbance.
For statistical analysis, weight gain between MeSA-exposed and unexposed carabids in the laboratory was compared with treatment, sex and their interaction as fixed effects, and trial as a random effect and normal distribution. Most outdoor experiments tracked predation over time and tested the proportion of prey consumed with treatment, time and the interaction as fixed effects, trial as random, and treatment*replicate as the random subject effect with an autoregressive correlation for repeated measures with a binomial or normal distribution. To simplify, the analysis combining both sexes is shown for coccinellids since outcomes were similar among both sexes. For lacewings placed in a cup, predation at 5 h was compared with treatment as a fixed effect, and trial as a random effect using a binomial distribution. Analyses of predation studies were done in Proc Glimmix SAS 9.3 (SAS Institute, 2016).
Results
All stated differences are significant with p < 0.05, unless otherwise stated. Statistical results are in Tables 1–4. Only p-values are shown in Tables 2–4 to reduce size, and full statistics are shown for significant treatment effects in Supplementary Material 2.
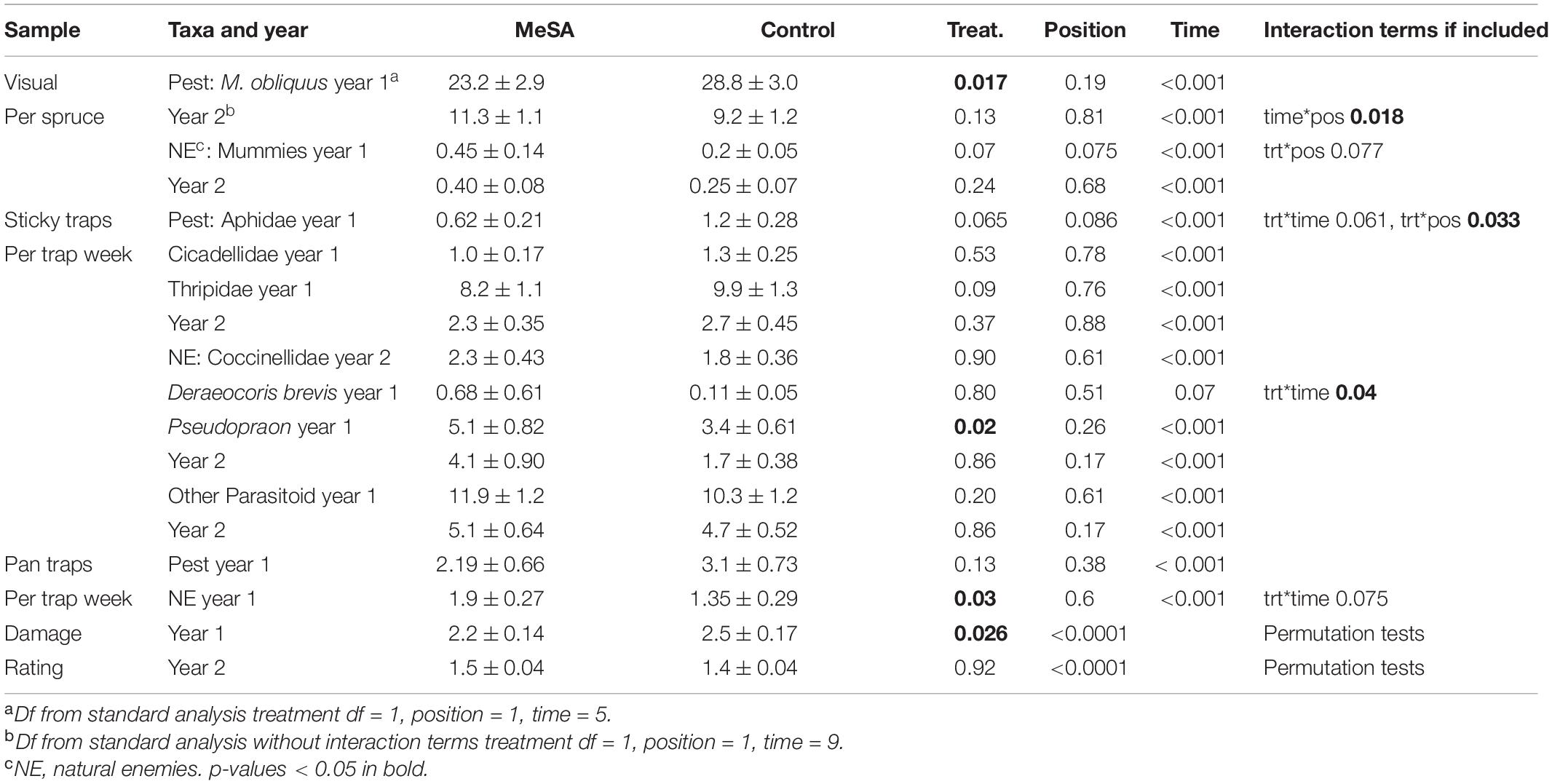
Table 2. Mean counts (±SE) of insects in spruce container yards with and without MeSA lures, and p-values from GLMM.
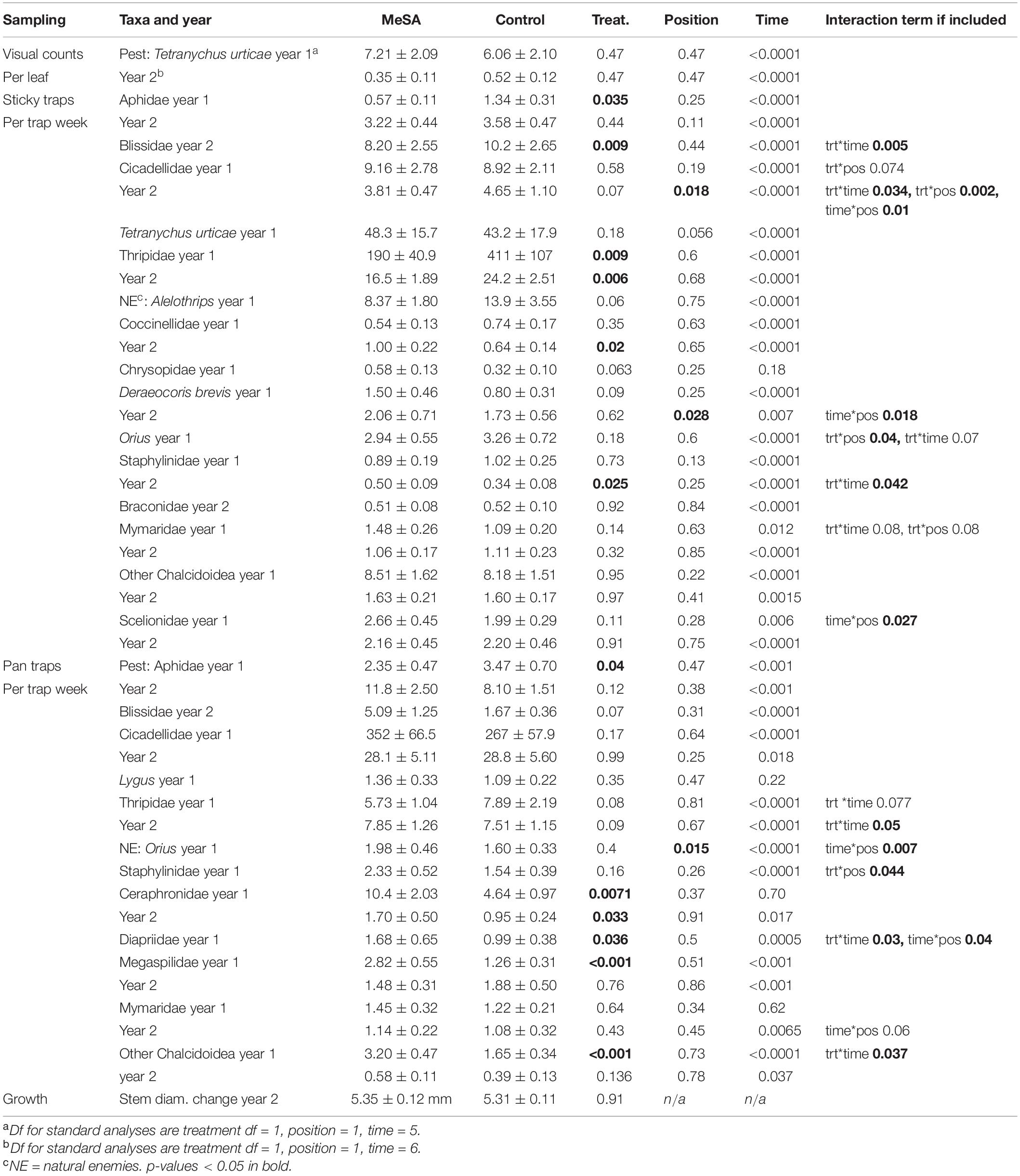
Table 3. Mean (±SE) counts of insects in red maple plots with and without MeSA lures, and p-values from GLMM.

Table 4. Mean (+SE) predation of sentinel H. halys eggs and counts of insects in nursery plots with and without MeSA lures, and p-values from GLMM.
Spruce Field Trial
In year 1, few aphids were observed inside spruce shoot tips on 1-May when lures were set up. By 21-May, aphid counts were visibly lower in MeSA than control plots (Figure 1A). Aphids decreased by 19% over the season with an overall treatment effect (Table 2 and Supplementary Material 2). Pseudopraon sp. (Hymenoptera: Braconidae) wasps were identified from spruce aphid mummies, and collected as adults on sticky cards. Adult wasps were visibly abundant in MeSA plots by 7-May (Figure 1B), with a 50% overall increase. Aphid mummies observed on spruce tips were 125% higher in MeSA plots (marginal p = 0.07, Figure 1B). Also, 41% more natural enemies were found in pan traps in MeSA plots. Subsequently, shoot tip damage caused by aphids differed (Figure 1C). Spruce with ‘some,’ ‘visible,’ and ‘heavy’ damage was 27% less frequent in MeSA than control plots with 75 versus 104 damaged trees, respectively. Year 1 trends consistently showed the benefit of MeSA. More aphid parasitoids, fewer aphids, and fewer aphid-induced plant damage occurred in MeSA than control spruce plots.
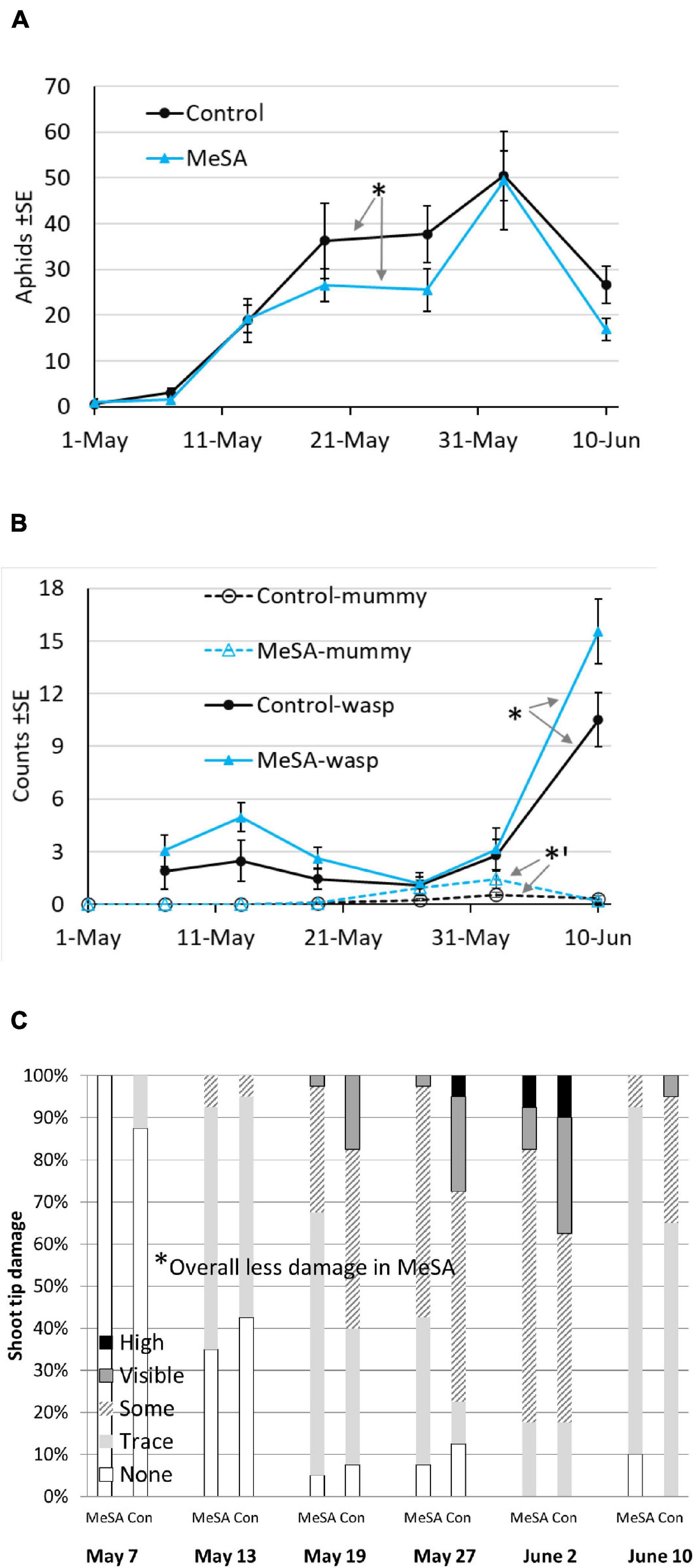
Figure 1. Mean number of aphids on shoot tips (A), Pseudopraon wasps on sticky cards and aphid mummies on shoot tips (B), and percent of trees by damage ranking (C) in a container-grown spruce field study during year 1. Asterisk indicates a difference between treatments over season, GLMM *p < 0.05, *’p = 0.07.
Despite the promising trends in year 1, there were no significant differences in year 2 when plots were set up in different sites (Table 2). For most comparisons and in the two field studies to follow, time was significant which was expected since populations increase with plant growth and fluctuate with weather. For the most part, sampling position did not affect insect counts suggesting that most impacts were spread over 4–7 m. Only in year 1, aphid counts on shoot tips varied by treatment*position. Aphid abundance increased 34% in MeSA plots as sampling moved away from lures (MeSA near 18.5 ± 2.4, MeSA away 28.0 ± 5.2). Aphid reduction was stronger closer to the MeSA lure.
Maple Field Trial
In both years with plots set up in different fields, thrips were the most common pest found on sticky cards, with fewer thrips in MeSA than control maple plots (Figure 2). A 54% decrease occurred when thrips were caught by the hundreds per trap per week in year 1, and 32% decrease when thrips were caught by the tens in year 2 (Table 3). Aphids were 57 and 32% less abundant in MeSA than control plots based on sticky cards and pan traps in year 1, respectively. Aphids did not differ in year 2. Cinch bugs (blissids) were 30% less abundant in MeSA plots in year 1. The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetrachynidae), was found at similar rates during visual leaf inspection, and on sticky cards.
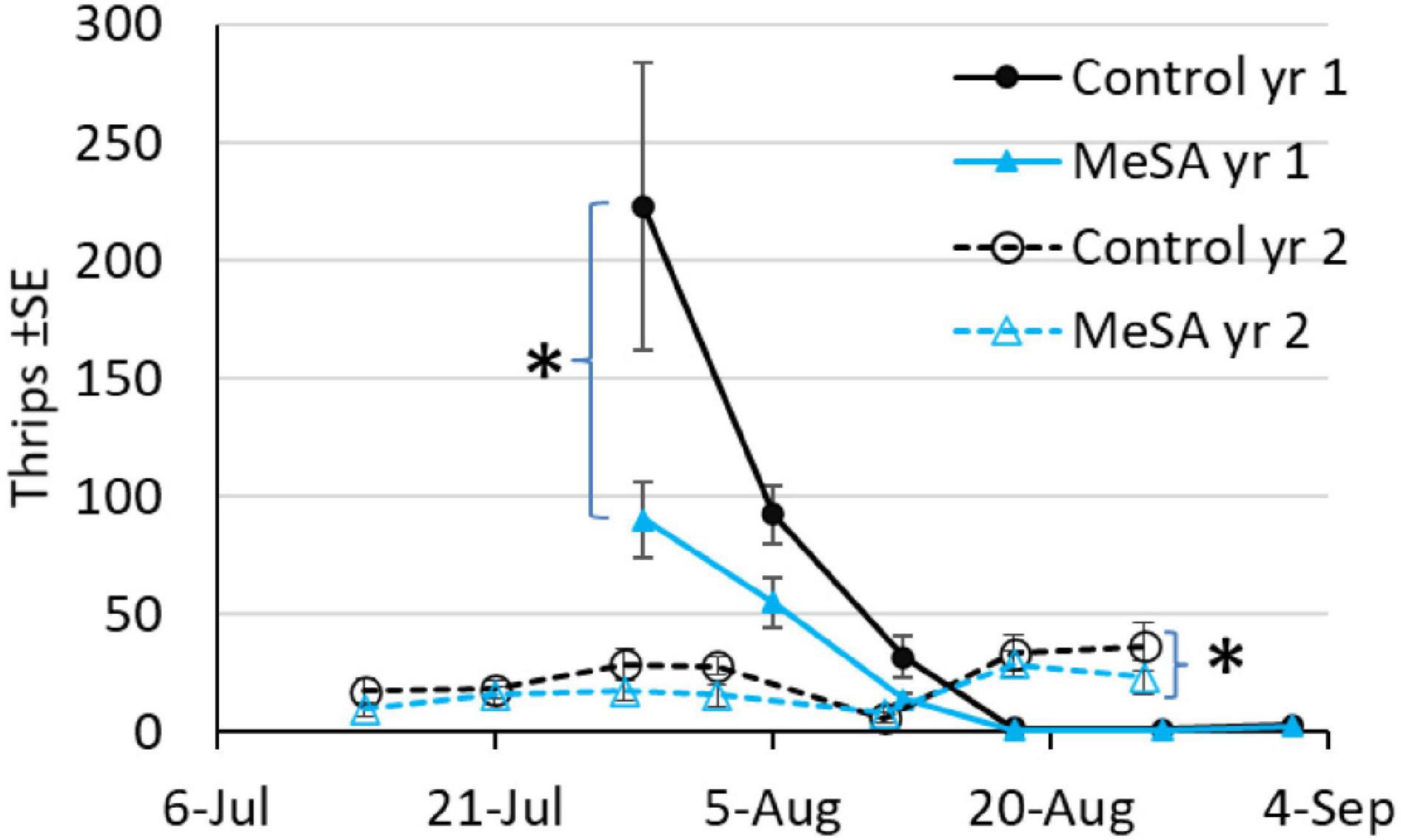
Figure 2. Mean number of thrips on sticky cards during a 2 year red maple field study. Asterisk indicates a difference between treatments over the season, GLMM p < 0.05.
In both years, natural enemies were more abundant in MeSA than control maple plots. Unlike the spruce trials, it is not clear that ‘attracted’ natural enemies were controlling the thrips or aphids. For instance, coccinellids which consume aphids were captured 56% more on sticky cards in MeSA plots in year 2, whereas aphids were captured less in year 1 (Table 3). Other natural enemies such as staphylinids, and parasitic wasps such as ceraphronids, diapriids, megaspilids, and chalcidoids were caught 47, 79–124, 124, and 94% more, respectively, in MeSA than control plots.
Out of 45 comparisons over 2 years, leafhoppers (cicadellids), Orius, mymarids, and rove beetles (staphylinids) responded to treatment*position interactions (Table 3). However, no differences in counts were significant when near and away positions were compared within MeSA or control plots. Lastly, stem diameter at the base of maple trees were measured at the beginning and end of the trial, and no difference was observed between maples grown with or without MeSA lures.
Halyomorpha halys Egg Predation Field Trial
During both years, caged and uncaged sentinel H. halys eggs experienced similar predation rates between MeSA and control ornamental plots (Table 4), and egg parasitism was not observed. Predation among caged eggs was 7–8%, and uncaged eggs was 32–39%. Caged egg masses could only be accessed by predators less than 1.1 mm. A difference occurred among uncaged eggs in August of year 1 with a treatment*time effect, with 49 ± 14% of eggs predated in MeSA plots and 9 ± 8% in control plots. No pests were enhanced with MeSA lures except for leafhoppers (cicadellids) collected from sticky traps in year 1, with a 110% increase in MeSA plots. Predatory thrips (Aeolothrips), green lacewing adults (chrysopids), and coccinellid adults were captured 563, 325, and 95% more from sticky cards placed in MeSA than control plots in year 1.
Methyl Salicylate Effect on Feeding
Overall, there was no effect of being in proximity to MeSA on predation. Direct feeding on prey by the carabid adult P. melanarius, coccinellid adult H. axyridis and C. septempunctata, and lacewing larva C. rufilabris (Table 1) was similar among MeSA-exposed and unexposed treatments overall or during the 1–4 h observational period. Likewise, when the predators were placed in small arenas where they needed to walk on a leaf or search the soil for prey, predation rates were also similar. This suggests that exposure to MeSA neither enhances nor interferes with close-range predation activity.
Discussion
Natural Enemy Attraction and Efficiency
Our paper demonstrates that MeSA attracts natural enemies in potted spruce container yards, red maple seedling fields, and mature maple-crabapple-Tilia stock blocks. This trend occurs with varied management, the spruce and red maple production fields were sprayed with insecticides, and the stock block was generally unmanaged. While drawing in natural enemies is desired, Kaplan (2012) cautioned about interpreting trap data where natural enemies can merely come and go, versus in-plant sampling which better reflect natural enemy activity. While we visually inspected shoot tips, leaves, or vacuumed the foliage in our three studies, few natural enemies were collected for statistical analysis. Only mummified M. obliquus aphids which are not mobile could be counted on spruce trees, and they were 125% higher in MeSA than control plots. Though this increase was marginal (p = 0.07), it matched the significant increase of Pseudopraon wasps on sticky cards from MeSA plots. Pseudopraon wasps emerged from mummified aphids.
In red maple fields, sticky cards and pan traps in MeSA plots had more lady beetles, predatory mirid D. brevis, and rove beetles. In the mature stock blocks, sticky cards in MeSA plots had more predatory thrips Aeolothrips, green lacewings, and lady beetles. Our results are consistent with prior studies, D. brevis responded to MeSA in vineyards (James and Price, 2004). Green lacewings responded in cranberry (Rodriguez-Saona et al., 2011), hops (James, 2003), soybean (Mallinger et al., 2011), strawberry (Lee, 2010), and vineyards (James and Price, 2004). Lady beetles grouped at the family level responded to MeSA in cranberry bogs (Rodriguez-Saona et al., 2011) and vineyards (Gadino et al., 2012), and specific aphidophagous species responded in cotton (Zhu and Park, 2005) and potted cucumbers (Dong and Hwang, 2017). Rove beetles responded to MeSA in laboratory settings (Shimoda et al., 2002). Predatory thrips responded to MeSA in corn fields (Braasch et al., 2012).
Once natural enemies are attracted, whether their foraging is similar, diminished or enhanced with externally applied MeSA is of interest. Our studies revealed that adult carabid P. melanarius, adult lady beetle C. septempunctata and H. axyridis, and larval green lacewing C. rufilabris feed similarly when given food directly, or when foraging in a small outdoor arena regardless of MeSA exposure. This outcome favors incorporating MeSA for pest control. However, another study that examined per capita predator efficiency found a negative impact. The number of Podisus maculiventris (Say) (Hemiptera: Pentatomidae) nymphs observed on plants was 26% lower and predation on caterpillars was 22% lower on tomatoes caged with MeSA than untreated tomatoes (Vidal-Gomez et al., 2018). The constitutive emission of MeSA was suggested to camouflage necessary odors and reduce predator efficiency.
Different outcomes from Vidal-Gomez et al.’s (2018) and our study might be influenced by the predator’s inherent response to MeSA, prior experience, and rearing/origins. The latter has affected a predatory mite’s response to MeSA (Krips et al., 1999; Dicke et al., 2000). However, no clear trend appeared among the one negatively responding species and four neutrally responding species in terms of having a known attraction to MeSA, being reared/wild, or experience with HIPVs. Pterostichus melanarius was not attracted to MeSA in strawberry fields (Lee, 2010), whereas the other four predators have responded to MeSA in various systems (Rodriguez-Saona et al., 2011; Kelly et al., 2014). Podisus maculiventris and C. rufilabris were lab-reared while the others were field-collected. Both naïve and experienced P. maculiventris fed less in the presence of MeSA (Vidal-Gomez et al., 2018). The lady beetles would be considered experienced since they were maintained on aphid-infested plants that may have produced various HIPVs. In contrast, P. melanarius was fed dog food, and C. rufilabris was fed moth eggs and would not have been recently exposed to MeSA.
The scale of the arenas and plants used in Vidal-Gomez et al.’s (2018) and our study differed affecting the context of outcomes. Podisus maculiventris foraged in a larger 1 m3 field cage for 12 h on tomato plants, and MeSA can elevate defensive proteins in tomatoes (Rowen et al., 2017). Thus, in a more complex environment and larger foraging range, MeSA interfered with nymphal P. maculiventris efficiency. Our four predators were either given prey within a few centimeters, or foraged in 520 cm2 or 946 ml arenas for up to 4–5 h. We used food/prey with no plant material or prey on leaf cuttings which might not be affected by MeSA lures. While insect herbivores are known to feed less on plants treated and subsequently induced by MeSA (Rowen et al., 2017; Kalaivani et al., 2018), and MeSA is a common topical analgesic for human and veterinary uses (PubChem, 2021), how MeSA volatiles affect predator feeding rates was unknown. The direct feeding assays suggested that MeSA itself (without crop influence) did not change short-term consumption rates among the four predators. The outdoor foraging assays suggested that per capita consumption remains unchanged in the presence of MeSA, but foraging beyond 5 h at a larger scale with induced plants is unknown.
Pest and Crop Protection
From three 2-year field studies, MeSA lures applied at 180 lures/ha showed promise in commercial Alberta spruce can yards. This led to more Pseudopraon parasitoids and marginally more mummified aphids, and subsequently fewer aphids and aphid-associated damage occurred in shoot tips. Pseudopraon was identified from mummified M. obliquus aphids, making it clear that MeSA enhanced biological control, and reduced plant damage which would require labor to prune. However, these trends did not appear in the second year when plots were set up in different spruce blocks to minimize carry-over effects. The yearly differences could be due to dimethoate sprays to minimize economic losses. The first year was conducted with spruce ∼80 cm tall, and the second year with spruce 100+ cm tall.
The red maple study showed some benefit as MeSA plots had fewer pest thrips and aphids. However, it is not clear that this was mediated through biological control. Aphidophagous predators were enhanced in year 2 of the study while aphids were reduced in year 1. Also, no obvious predators of thrips were enhanced during both years. Various parasitoids were also enhanced, but none could be classified as targeting aphids or thrips. MeSA may have directly impacted maple pests; semiochemicals can affect pests directly via attraction or repellency (Szendrei and Rodriguez-Saona, 2010). MeSA has slowed colonization of aphids in barley (Ninkovic et al., 2003) and repelled aphids in hops (Losel et al., 1996). In another case, MeSA had no impact on alate aphids in soybean (Zhu and Park, 2005). Lastly, MeSA had no clear impacts on plant growth as measured by stem diameter change. This would be a concern for growers since MeSA can induce allelopathic effects as shown in rice (Bi et al., 2007).
The study with sentinel H. halys egg masses revealed no consistent benefit from MeSA. Only in 1 month of the first year did predation increase in MeSA plots. This may have been mediated by lady beetles and green lacewings as their abundance increased. No parasitism was observed, and the second year was conducted just as the exotic parasitoid Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) of H. halys was detected in Oregon (Hedstrom et al., 2017). In the East Coast, sentinel H. halys eggs placed next to MeSA or other host cues did not increase egg mortality (Morrison et al., 2018). Thus, externally applied MeSA does not appear beneficial for controlling H. halys eggs.
Future Directions
To integrate MeSA and HIPVs into management programs, continued documentation of crop protection is critical to change grower practices. While MeSA is attractive, further elucidation on what happens to natural enemy foraging efficiency is necessary. Interestingly, MeSA has been coupled with augmentative releases to retain predators (Kelly et al., 2014). Kaplan (2012) warns that natural enemies herded into regions with no food may associate the odor with no reward, and thereby reduce the effectiveness of MeSA. For example, anthocorids were exposed to MeSA in the lab during prey deprivation and were later not attracted to MeSA (Drukker et al., 2000). Thus, coupling MeSA with floral plants or food rewards is warranted (Simpson et al., 2011a), and have shown promise in apples (Jaworski et al., 2019), beans (Salamanca et al., 2018), and vegetables grown in tunnels (Ingwell et al., 2018). As HIPV research continues, more studies are combining MeSA with plant volatile blends for synergy (Jones et al., 2016; Lucchi et al., 2017; Pålsson et al., 2019), including attracting natural enemies for ornamental pests (Velasco Graham et al., 2020). Moreover, adding tactile stimuli can enhance egg laying by adult lacewings, which is necessary for biological control since many green lacewings are predaceous at the larval but not adult stage (Koczor et al., 2017).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SF, JL, and VS conceived the study and carried out the work. JL and KVG analyzed the data. JL wrote the remaining first draft. All the authors drafted methods.
Funding
Funding was provided by base funds USDA CRIS 5358-22000-032-00D and 2072-22000-044-00D, Northwest Nursery Crop Grant, and USDA Specialty Crop Research Initiative grant on brown marmorated stink bug, 2011-51181-39037, and 2016-51181-25409.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dave Edwards, Christina Fieland, Nikko Fujita, Kathleen Knight, Danielle Lightle, Emily Parent, Danielle Selleck, Thomas Whitney, and Jeff Wong for assistance. We also thank the nurseries for use of their fields.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.788187/full#supplementary-material
References
Bergmann, E. J., Venugopal, P. D., Martinson, H. M., Raupp, M. J., and Shrewsbury, P. M. (2016). Host plant use by the invasive Halyomorpha halys (Stal) on woody ornamental trees and shrubs. PLoS One 11:30149975. doi: 10.1371/journal.pone.0149975
Bi, H. H., Zeng, R. S., Su, L. M., An, M., and Luo, S. M. (2007). Rice allelopathy induced by methyl jasmonate and methyl salicylate. J. Chem. Ecol. 33, 1089–1103. doi: 10.1007/s10886-007-9286-1
Braasch, J., Wimp, G. M., and Kaplan, I. (2012). Testing for phytochemical synergism: arthropod community responses to induced plant volatile blends across crops. J. Chem. Ecol. 38, 1264–1275. doi: 10.1007/s10886-012-0202-y
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/rj-2017-066
Chung, Y., Rabe-Hesketh, S., Dorie, V., Gelman, A., and Liu, J. (2013). A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika 78, 685–709. doi: 10.1007/s11336-013-9328-2
De Boer, J. G., and Dicke, M. (2004). The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 30, 255–271. doi: 10.1023/b:joec.0000017976.60630.8c
Dicke, M., Schutte, C., and Dijkman, H. (2000). Change in behavioral response to herbivore-induced plant volatiles in a predatory mite population. J. Chem. Ecol. 26, 1497–1514.
Dong, Y. J., and Hwang, S. Y. (2017). Cucumber plants baited with methyl salicylate accelerates Scymnus (Pullus) sodalis (Coleoptera: Coccinellidae) visiting to reduce cotton aphid (Hemiptera: Aphididae) infestation. J. Econ. Entomol. 110, 2092–2099. doi: 10.1093/jee/tox240
Drukker, B., Bruin, J., and Sabelis, M. W. (2000). Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol. Entomol. 25, 260–265.
Florian, H. (2020). DHARMa: Residual Diagnostics For Hierarchical (Multi-Level/Mixed). Regression Models. R Package Version 0.2.7. Available online at: https://CRAN.Rproject.org/package=DHARMa.
Fondren, K. M., McCullough, D. G., and Walter, A. J. (2004). Insect predators and augmentative biological control of balsam twig aphid (Mindarus abietinus Koch) (Homoptera : Aphididae) on Christmas tree plantations. Environ. Entomol. 33, 1652–1661.
Gadino, A. N., Walton, V. M., and Lee, J. C. (2012). Evaluation of methyl salicylate lures on populations of Typhlodromus pyri (Acari: Phytoseiidae) and other natural enemies in western Oregon vineyards. Biol. Control 63, 48–55.
Hedstrom, C., Lowenstein, D., Andrews, H., Bai, B., and Wiman, N. (2017). Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 90, 1169–1179.
Ingwell, L. L., Avila-Ruiz, D. A., Foster, R., and Kaplan, I. (2018). Tailoring insect biocontrol for high tunnels. Biol. Control 123, 76–86.
James, D. G. (2003). Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 29, 1601–1609.
James, D. G., and Price, T. S. (2004). Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 30, 1613–1627. doi: 10.1023/b:joec.0000042072.18151.6f
Jaworski, C. C., Xiao, D., Xu, Q., Ramirez−Romero, R., Guo, X., Wang, S., et al. (2019). Varying the spatial arrangement of synthetic herbivore−induced plant volatiles and companion plants to improve conservation biological control. J. Appl. Ecol. 56, 1176–1188. doi: 10.1111/1365-2664.13353
Jones, V. P., Horton, D. R., Mills, N. J., Unruh, T. R., Baker, C. C., Melton, T. D., et al. (2016). Evaluating plant volatiles for monitoring natural enemies in apple, pear, and walnut orchards. Biol. Control 102, 53–65.
Kalaivani, K., Kalaiselvi, M. M., and Senthil-Nathan, S. (2018). Effect of methyl salicylate (MeSA) induced changes in rice plant (Oryza sativa) that affect growth and development of the rice leaffolder, Cnaphalocrocis medinalis. Physiol. Mol. Plant Pathol. 101, 116–126. doi: 10.1016/j.pmpp.2017.07.001
Kaplan, I. (2012). Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire? Biol. Control 60, 77–89.
Kelly, J. L., Hagler, J. R., and Kaplan, I. (2014). Semiochemical lures reduce emigration and enhance pest control services in open-field predator augmentation. Biol. Control 71, 70–77. doi: 10.1016/j.biocontrol.2014.01.010
Khan, Z. R., James, D. A., Midega, C. A. O., and Pickett, J. A. (2008). Chemical ecology and conservation biological control. Biol. Control 45, 210–224. doi: 10.1016/j.biocontrol.2007.11.009
Koczor, S., Szentkirályi, F., Fekete, Z., and Tóth, M. (2017). Smells good, feels good: oviposition of Chrysoperla carnea-complex lacewings can be concentrated locally in the field with a combination of appropriate olfactory and tactile stimuli. J. Pest Sci. 90, 311–317. doi: 10.1007/s10340-016-0785-0
Krips, O. E., Willems, P. E. L., Gols, R., Posthumus, M. A., and Dicke, M. (1999). The response of Phytoseiulus persimilis to spider mite-induced volatiles from gerbera: influence of starvation and experience. J. Chem. Ecol. 25, 2623–2641.
Lee, J. C. (2010). Effect of methyl salicylate-based lures on beneficial and pest arthropods in strawberry. Environ. Entomol. 39, 653–660. doi: 10.1603/EN09279
Losel, P. M., Lindemann, M., Scherkenbeck, J., Maier, J., Engelhard, B., Campbell, C. A. M., et al. (1996). The potential of semiochemicals for control of Phorodon humuli (Homoptera: Aphididae). Pest Sci. 48, 293–303. doi: 10.1002/(sici)1096-9063(199612)48:4<293::aid-ps479>3.0.co;2-5
Lucchi, A., Loni, A., Gandini, L. M., Scaramozzino, P., Ioriatti, C., Ricciardi, R., et al. (2017). Using herbivore-induced plant volatiles to attract lacewings, hoverflies, and parasitoid wasps in vineyards: achievements and constraints. Bull. Insectol. 70, 273–282.
Luo, D., Ganesh, S., and Koolaard, J. (2018). Available online at: https://www.rdocumentation.org/packages/predictmeans/versions/1.0.6
Mallinger, R. E., Hogg, D. B., and Gratton, C. (2011). Methyl salicylate attracts natural enemies and reduces populations of soybean aphids (Hemiptera: Aphididae) in soybean agroecosystems. J. Econ. Entomol. 104, 115–124. doi: 10.1603/ec10253
Martinson, H. M., Venugopal, P. D., Bergmann, E. J., Shrewsbury, P. M., and Raupp, M. J. (2015). Fruit availability influences the seasonal abundance of invasive stink bugs in ornamental tree nurseries. J. Pest Sci. 88, 461–468.
McIntosh, H., Lowenstein, D. M., Wiman, N. G., Wong, J. S., and Lee, J. C. (2019). Parasitism of frozen Halyomorpha halys eggs by Trissolcus japonicus: applications for rearing and experimentation. Biocontrol Sci. Tech. 29, 478–493. doi: 10.1080/09583157.2019.1566439
McPike, S. M., and Evenden, M. L. (2021). Host plant volatile lures attract Apanteles polychrosidis (Hymenoptera: Braconidae) to ash trees infested with Caloptilia fraxinella (Lepidoptera: Gracillariidae). Front. Ecol. Evol. 9:701954. doi: 10.3389/fevo.2021.701954
Mercer, N. H., Bessin, R. T., and Obrycki, J. J. (2020). Impact of buckwheat and methyl salicylate lures on natural enemy abundance for early season management of Melanaphis sacchari (Hemiptera: Aphididae) in sweet sorghum. Crop Protect. 137:105279. doi: 10.1016/j.cropro.2020.105279
Morrison, W. R., Blaauw, B. R., Nielsen, A. L., Talamas, E., and Leskey, T. C. (2018). Predation and parasitism by native and exotic natural enemies of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) eggs augmented with semiochemicals and differing host stimuli. Biol. Control 121, 140–150.
Naranjo, S. E., Hagler, J. R., and Byers, J. A. (2021). Methyl salicylate fails to enhance arthropod predator abundance or predator to pest ratios in cotton. Environ. Entomol. 50, 293–305. doi: 10.1093/ee/nvaa175
Ninkovic, V., Ahmed, E., Glinwood, R., and Pettersson, J. (2003). Effects of two types of semiochemical on population development of the bird cherry oat aphid Rhopalosiphum padi in a barley crop. Agric. For. Entomol. 5, 27–33. doi: 10.1046/j.1461-9563.2003.00159.x
Ozawa, R., Shiojiri, K., Sabelis, M. W., Arimura, G. I., Nishioka, T., and Takabayashi, J. (2004). Corn plants treated with jasmonic acid attract more specialist parasitoids, thereby increasing parasitization of the common armyworm. J. Chem. Ecol. 30, 1797–1808. doi: 10.1023/b:joec.0000042402.04012.c7
Pålsson, J., Thöming, G., Silva, R., Porcel, M., Dekker, T., and Tasin, M. (2019). Recruiting on the spot: a biodegradable formulation for lacewings to trigger biological control of aphids. Insects 10:6. doi: 10.3390/insects10010006
PubChem (2021). Compound Summary: Methyl Salicylate. National Library of Medicine: National Center for Biotechnology Information. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-salicylate
Puente, M. E., Kennedy, G. G., and Gould, F. (2008). The impact of herbivore-induced plant volatiles on parasitoid foraging success: a general deterministic model. J. Chem. Ecol. 34, 945–958. doi: 10.1007/s10886-008-9471-x
R Core Team (2020). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rice, K. B., Bergh, C. J., Bergmann, E. J., Biddinger, D. J., Dieckhoff, C., Dively, G., et al. (2014). Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 5:3. doi: 10.1603/IPM14002
Rodriguez-Saona, C., Kaplan, I., Braasch, J., Chinnasamy, D., and Williams, L. (2011). Field responses of predaceous arthropods to methyl salicylate: a meta-analysis and case study in cranberries. Biol. Control 59, 294–303. doi: 10.1016/j.biocontrol.2011.06.017
Rowen, E., Gutensohn, M., Dudareva, N., and Kaplan, I. (2017). Carnivore attractant or plant elicitor? Multifunctional roles of methyl salicylate lures in tomato defense. J. Chem. Ecol. 43, 573–585. doi: 10.1007/s10886-017-0856-6
Salamanca, J., Souza, B., and Rodriguez-Saona, C. (2018). Cascading effects of combining synthetic herbivore-induced plant volatiles with companion plants to manipulate natural enemies in an agro-ecosystem. Pest Manag. Sci. 74, 2133–2145. doi: 10.1002/ps.4910
Salamanca, J., Souza, B., Kyryczenko-Roth, V., and Rodriguez-Saona, C. (2019). Methyl salicylate increases attraction and function of beneficial arthropods in cranberries. Insects 10:423. doi: 10.3390/insects10120423
Salamanca, J., Souza, B., Lundgren, J. G., and Rodriguez-Saona, C. (2017). From laboratory to field: electro-antennographic and behavioral responsiveness of two insect predators to methyl salicylate. Chemoecology 27, 51–63.
Shimoda, T., Ozawa, R., Arimura, G., Takabayashi, J., and Nishioka, T. (2002). Olfactory responses of two specialist insect predators of spider mites toward plant volatiles from lima bean leaves induced by jasmonic acid and/or methyl salicylate. Appl. Entomol. Zool. 37, 535–541.
Simpson, M., Gurr, G. M., Simmons, A. T., Wratten, S. D., James, D. G., Leeson, G., et al. (2011b). Field evaluation of the ‘attract and reward’ biological control approach in vineyards. Ann. Appl. Biol. 159, 69–78. doi: 10.1111/j.1744-7348.2011.00477.x
Simpson, M., Gurr, G. M., Simmons, A. T., Wratten, S. D., James, D. G., Leeson, G., et al. (2011a). Insect attraction to synthetic herbivore-induced plant volatile-treated field crops. Agric. For. Entomol. 13, 45–57. doi: 10.1111/j.1461-9563.2010.00496.x
Szendrei, Z., and Rodriguez-Saona, C. (2010). A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 134, 201–210. doi: 10.1111/j.1570-7458.2009.00954.x
Uefune, M., Kugimiya, S., Sano, K., and Takabayashi, J. (2012). Herbivore-induced plant volatiles enhance the ability of parasitic wasps to find hosts on a plant. J. Appl. Entomol. 136, 133–138.
Velasco Graham, K., Choi, M. Y., and Lee, J. C. (2020). Attracting Chrysopidae with plant volatiles for lace bug (Hemiptera: Tingidae) control in rhododendrons and azaleas. J. Insect Sci. 20:5. doi: 10.1093/jisesa/ieaa078
Vidal−Gomez, U., Rodriguez-Saona, C., and Kaplan, I. (2018). Constitutive exposure to the volatile methyl salicylate reduces per-capita foraging efficiency of a generalist predator to learned prey associations. Entomol. Exp. Appl. 166, 661–672. doi: 10.1111/eea.12713
Wang, G., Cui, L.-L., Dong, J., Francis, F., Liu, Y., and Tooker, J. (2011). Combining intercropping with semiochemical releases: optimization of alternative control of Sitobion avenae in wheat crops in China. Entomol. Exp. Appl. 140, 189–195.
Williams, L., Rodriguez-Saona, C., Castle, S. C., and Zhu, S. (2008). EAG-active herbivore-induced plant volatiles modify behavioral responses and host attack by an egg parasitoid. J. Chem. Ecol. 34, 1190–1201. doi: 10.1007/s10886-008-9520-5
Woods, J. L., James, D. G., Lee, J. C., and Gent, D. H. (2011). Evaluation of airborne methyl salicylate for improved conservation biological control of two-spotted spider mite and hop aphid in Oregon hop yards. Exp. Appl. Acarol. 55, 401–416. doi: 10.1007/s10493-011-9495-8
Keywords: aphid, biological control, foraging, Halyomorpha halys, herbivore induced plant volatile (HIPV), maple, spruce, thrips
Citation: Lee JC, Flores SM, Velasco Graham K and Skillman VP (2022) Methyl Salicylate Can Benefit Ornamental Pest Control, and Does Not Alter Per Capita Predator Consumption at Close-Range. Front. Ecol. Evol. 9:788187. doi: 10.3389/fevo.2021.788187
Received: 01 October 2021; Accepted: 22 December 2021;
Published: 27 January 2022.
Edited by:
Maria L. Pappas, Democritus University of Thrace, GreeceReviewed by:
Cesar Rodriguez-Saona, Rutgers, The State University of New Jersey, United StatesAntonino Cusumano, University of Palermo, Italy
Copyright © 2022 Lee, Flores, Velasco Graham and Skillman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jana C. Lee, amFuYS5sZWVAdXNkYS5nb3Y=
 Jana C. Lee
Jana C. Lee Salvador M. Flores1
Salvador M. Flores1 Katerina Velasco Graham
Katerina Velasco Graham