- 1Department of Zoology, Stockholm University, Stockholm, Sweden
- 2Department of Biology, Lund University, Lund, Sweden
- 3Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland
- 4Institute for Biomedical Engineering, University and ETH Zūrich, Zurich, Switzerland
Heatwaves are increasingly common globally and are known to have detrimental impacts on animal morphology and behaviour. These impacts can be severe, especially if heatwaves occur during development, even on animals that can regulate the temperature of their developing young. The onset and duration of heatwaves are stochastic and therefore may affect all or only part of development. In the heterothermic bumblebee Bombus terrestris, elevated temperatures over the entirety of development cause morphological changes in adults, despite their capability to regulate brood temperature. However, the effects of heatwaves that occur during a short period of development are unclear. We test the impact of elevated developmental temperature during the latter fraction of development on the behaviour and morphology of adult worker B. terrestris. We show that exposure to elevated temperature over a portion of late development is sufficient to impair the initial behavioural responses of workers to various sensory stimuli. Despite this, exposure to elevated temperatures during a period of development did not have any significant impact on body or organ size. The negative effect of elevated developmental temperatures was independent of the exposure time, which lasted from 11–20 days at the end of the workers’ developmental period. Thus, heat stress in bumblebees can manifest without morphological indicators and impair critical behavioural responses to relevant sensory stimuli, even if only present for a short period of time at the end of development. This has important implications for our understanding of deleterious climactic events and how we measure indicators of stress in pollinators.
Introduction
For many animals, abnormal temperatures during development, either elevated or depressed, are known to have profound impacts on adult phenotype. This sometimes takes the form of phenotypic plasticity, such as the reptile temperature-dependent sex determination systems (Singh et al., 2020) or the spring/summer morphs of certain butterfly species (Gilbert, 2001). Other times, deviation from an optimum developmental temperature is negative, causing adult morphological deformation (Groh et al., 2004; Gerard et al., 2018), behavioural impairment (Van Damme et al., 1992; Tautz et al., 2003), deficient neurological development (Groh et al., 2004) and physiological disruption (Watkins, 2000; Singh et al., 2020).
Across exothermic vertebrates, non-adaptive deviations in adult phenotypes due to abnormal developmental temperatures have been well investigated when temperature stress occurs during the entirety of development (Burger, 1991; Van Damme et al., 1992; Watkins, 2000; Micheli-Campbell et al., 2011; Sfakianakis et al., 2011; Singh et al., 2020). Zebra fish embryos reared in warmer temperatures swim better as adults than their cooler compatriots, but show no discernible morphological differences (Sfakianakis et al., 2011). In contrast, hatchlings of the turtle Elusor macrurus from cooler incubation temperatures are the better swimmers (Micheli-Campbell et al., 2011; Singh et al., 2020). Even in insects, such as damselflies, exposure to elevated temperature throughout development has been shown to affect both wing shape and flight performance (Arambourou et al., 2017). The solitary bee Osmia bicornis suffers increased adult mortality, as well as decreases in prepupal weight, under hotter developmental temperatures (Radmacher and Strohm, 2011).
Exposure to elevated ambient temperatures has also been shown to affect bee colonies, albeit in a species-dependant manner. Honeybees that experience heat stress during development do not show any morphological indicators (Jones et al., 2005) (though see Groh et al., 2004 for reports of morphological disfigurement) but display learning and memory impairment (Tautz et al., 2003). Furthermore, adult honeybees reared under naturally occurring but undesirable temperatures had fewer mushroom body microglomeruli in the region dedicated to olfactory learning (Groh et al., 2004), suggesting that their capacity to learn olfactory stimuli would be impaired. Bumblebees subjected to heat stress over the duration of development have modified wing shapes as adults (Gerard et al., 2018), although it remains unknown if these or any other potential morphological variations have any behavioural consequences.
While we now have a good general understanding of how exposure to abnormal temperatures during the entirety of development affect a range of organisms, we still know little about the effects of abnormal temperature exposure during only a part of development. Understanding such effects, however, is becoming increasingly important if we are to understand how animals will be impacted by global warming. Global warming is increasing the frequency of heatwaves (Perkins-Kirkpatrick and Lewis, 2020) – prolonged periods of excessive heat (Perkins and Alexander, 2013; Perkins-Kirkpatrick and Lewis, 2020) – a weather phenomenon that may only impact an animal over a portion of their development. Animals like bumblebees that grow their colonies over spring and summer, when extreme heat events are most common, are particularly vulnerable to excessive temperature events. While the influence of elevated temperatures over a fraction of development is relatively unknown, investigations into the impacts of heatwaves specifically indicate they may have a profound effect on all organisms (Stillman, 2019).
Short-term heat-shock in insects often causes reproductive disruptions (Sales et al., 2018; Chen et al., 2019; Martinet et al., 2020), particularly impacting male fertility by altering spermatozoa (Sales et al., 2018; Martinet et al., 2020). In O. bicornis, exposure to elevated temperatures during pupation only disrupted male mate attraction behaviour (Conrad et al., 2017). In eusocial insects, capable of intranidal temperature regulation, the effect of partial developmental heatwaves has only been investigated in Africanized honeybees (Medina et al., 2018; Poot-Báez et al., 2020). A naturally occurring heatwave prior to pupation caused smaller adults (Poot-Báez et al., 2020) whereas a simulated heatwave during pupation affected wing shape, altered the strength of fluctuating asymmetry and lowered the age at onset of foraging (Medina et al., 2018).
The stochasticity of heatwaves means that eusocial insect larvae will be at different developmental stages at the onset of a heatwave and will therefore experience differential exposure to elevated temperatures. Having uneven exposure to elevated temperatures may still affect colony performance. Here, we begin to address the functional consequences of elevated temperatures during varied periods of mid and late development in the bumblebee Bombus terrestris. Bumblebees are an important model for this work because they tightly regulate both their colony (Schultze-Motel, 1991; Jones and Oldroyd, 2006; Gardner et al., 2007) and brood temperatures (Vogt, 1986; Weidenmüller et al., 2002). The proper development and function of sensory systems in bumblebees is essential for their pollination activities as they rely heavily on visual, olfactory, gustatory and mechanosensory information to navigate between flower patches and their colony (Dyer and Chittka, 2004; Orbán and Plowright, 2014; Wilmsen et al., 2017; Sprayberry, 2018) and to optimise their foraging (Spaethe et al., 2001; Chittka et al., 2003; Kulahci et al., 2008). Furthermore, bumblebees are already experiencing range shrinkage and population declines in both Europe (Kerr et al., 2015) and North America (Cameron et al., 2011; Kerr et al., 2015) as a result of a warming climate. The capacity for this genus to adapt to hotter temperatures may be physiologically constrained; Bombus vosnesenkii workers show no geographical variability regarding their critical thermal maximum (Pimsler et al., 2020), indicating a lack of local adaptation. We predicted that elevated temperatures over a portion of development, akin to a partial developmental heatwave, would impair behavioural responses to relevant sensory stimuli and cause morphological changes in adult worker bumblebees. Our results indicate that, despite having no discernible impact on morphology, partially elevated temperatures impair bumblebee responses to important sensory information, likely leading to decreased colony fitness.
Materials and Methods
Animals
Eight Bombus terrestris (L., 1758) colonies from Koppert (Berkel en Rodenrijs, Netherlands) were reared in the dark in climate control cabinets (Panasonic MIR 123L, Tokyo, Japan). The colonies (consisting of 1 queen, 19–40 workers – the variation was due to the initial colony state – and all brood cells) were kept in plastic boxes (Supplementary Figure 1) filled with unscented cat litter to absorb humidity. Individuals from each colony were reared and experimented upon concurrently and the colonies were randomly assigned to treatments on arrival to the laboratory. Four colonies were kept in one incubator maintained at an air temperature of 26 ± 0.07°C and another four colonies were kept in another incubator at an air temperature of 33 ± 0.5°C. Climate loggers (Lascar Electronics, EasyLog EL-USB-2, Whiteparish, United Kingdom) recording temperature and humidity every 10 s were placed in each incubator (outside of the colonies). Two additional loggers recorded the air temperature and humidity inside two of the four colonies by means of small wire probes (attached to data loggers placed inside the cabinets but outside the colonies) that were placed beside the brood cells. At the end of the experiment, the data was downloaded from the loggers and average temperature and humidity calculated. All data loggers indicated that experimental temperatures and humidity were maintained in both the incubators and in the colonies and that they were stable throughout the experiment (indicating that bees were not able to significantly modify the ambient colony temperature from that set by the incubator). The relative humidity in the 26°C incubator was 53 ± 5.5% and 56.5 ± 8.8% in the 33°C incubator. Bees were fed ad libitum with 50% w/v refined white sugar water solution and fresh-frozen, organic pollen every 2–3 days (Naturprodukter Raspowder Bipollen, Stockholm, Sweden). After 7 days in the incubator, all bees in each colony were marked with non-toxic paint (Färgpenna Lackstift, Biltema, Helsingborg, Sweden) to identify bees that had already completed, or were close to, adult development.
Between 11 and 20 days after the colonies were placed in the incubators, individual bees without prior colour markings were tagged with individual colour/number plate combinations that were glued to their thorax and subjected to testing within 4 days. The time each bee was exposed to the temperature treatment at the end of its development (Table 1) was explicitly included in our statistical models (see below) to control for the effects of exposure length. Note that each individual only experienced the temperature treatment for a maximum of 20 days at the end of their development, rather than for their entire developmental period – typically 25 days (Cnaani et al., 2000) – as such, they experienced simulated heatwave conditions rather than long periods of sustained high temperature. Based on an estimated 25 days of development time from egg to adult, bees were first exposed to their treatment temperatures from day 5 to day 14 (corresponding to approximately when bees hatch through to pre-pupation and the cessation of feeding) (Cnaani et al., 2000). A total of 60 workers from eight colonies were tested (with only a single worker being excluded from the behavioural analysis due to insufficient data) – 30 workers from four colonies at 33°C and 29 workers from four colonies at 26°C. Sample sizes for the morphological analysis vary because some specimens were damaged during the preparation process.
Table 1. The number of days each bee spent at treatment temperatures before being marked and subjected to behavioural tests.
Selecting Treatment Temperatures
Considerable increases in bumblebee thermoregulatory behaviour have been observed when colonies were exposed to ambient temperatures of 26°C (Weidenmüller et al., 2002) and 27°C (Nasir et al., 2019). Other investigations have found that colonies function optimally below 30°C but temperatures in excess of this are detrimental to the number of workers produced in the first brood and to the lifespan of the colony (Nasir et al., 2019). Vogt (1986) considered colonies that experience temperatures of 32°C as heat stressed – ambient temperatures at and above 32°C elicit increased wing fanning, to cool the colony, with an associated increase in metabolic costs (Vogt, 1986). While 32°C has been reported as the preferred temperature for brood to be kept at Weidenmüller et al. (2002), brood temperature are typically 2°C warmer than the ambient air temperature inside the colony (Vogt, 1986; Weidenmüller et al., 2002). Therefore, an air temperature of 33°C inside the colony is likely to be beyond the optimal temperature for developing B. terrestris, with evidence of this being found in the modified wing shapes of adults which developed at 32°C (Gerard et al., 2018). Due to the above-described effects, 33°C was selected as the elevated temperature treatment.
Behavioural Experiments
Experimental Set-Up
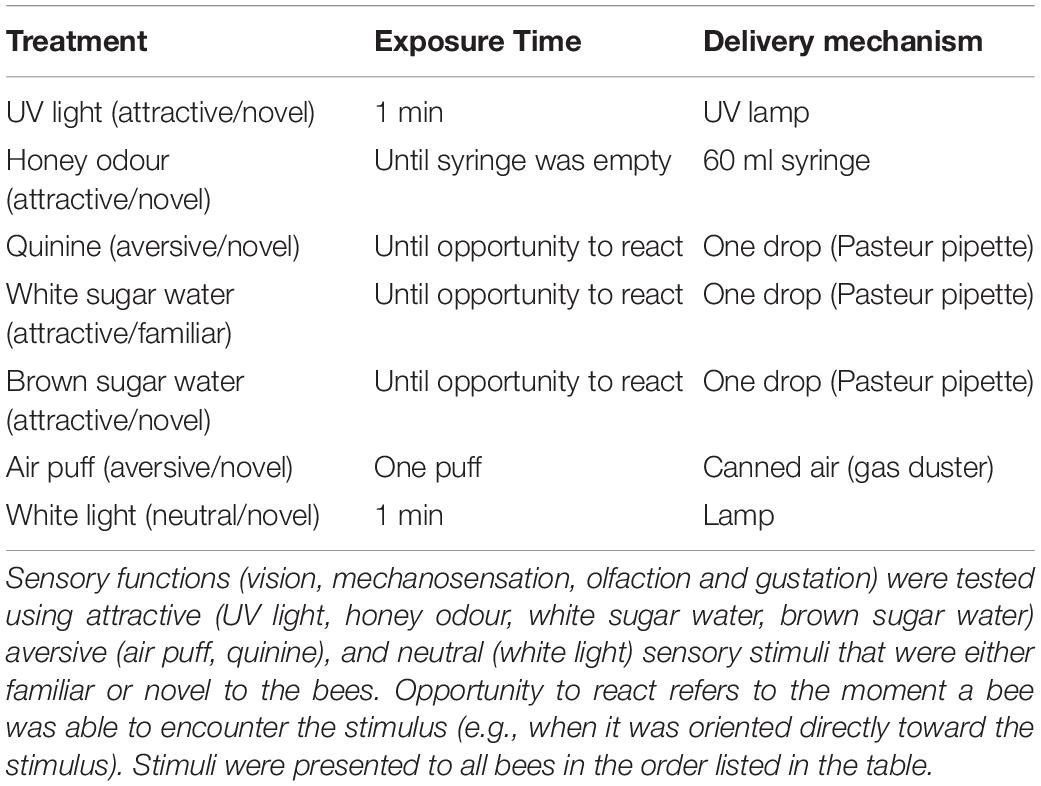
Adult bees were fed ad libitum before the experiment and kept in darkness for the duration of the experiment. The experimental setup consisted of a circular transparent plastic arena with a plexiglass lid and metal mesh bottom (Figure 1) 9 cm in diameter and 14 cm in height. Individual bees were caught in the colony and placed in the experimental setup in the dark for 2 min acclimation before being sequentially exposed to seven stimuli with 2 min dark rest periods in between stimulus presentations. The behavioural tests were performed at room temperature, 23 ± 2°C. Bees were recorded under red light using a video camera (Sony FDR-AX33, Tokyo, Japan) positioned above the arena. The first response upon exposure to each stimulus was examined via frame-by-frame playback of the video recording using VLC Media Player (VideoLan, 2006) and classified as either expected or unexpected (for details, see section Table 2) by an investigator blind to the treatment group of each bee.
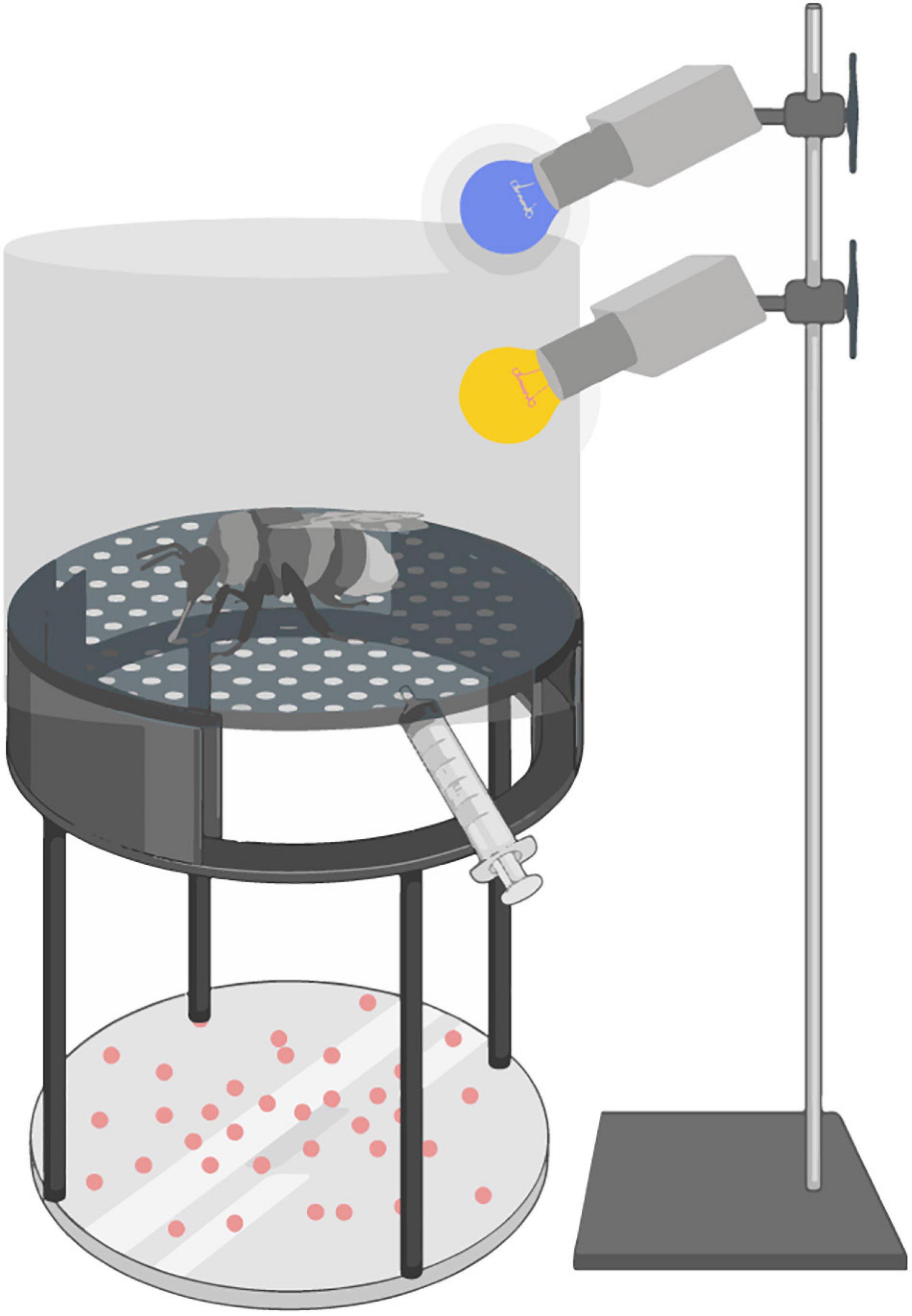
Figure 1. Behavioural arena set-up. A schematic drawing of the experimental set-up used to investigate the effect of rearing temperature on the behavioural responses of bumblebees to different sensory stimuli. Bees were placed in the centre of the arena and presented with a light stimulus from above (UV or white light), an olfactory or mechanosensory stimulus delivered through a syringe from below (honey water odour or an air puff) or a drop of liquid (white sugar water or quinine) placed on the floor of the arena. Responses to these stimuli were recorded using a camera that filmed the set-up from above.
Stimuli
The stimuli were chosen to test the function of different sensory systems – vision, mechanosensation, olfaction and gustation – and were expected to elicit attraction, aversion or neutral responses from the bees (Table 3). The stimuli were always presented to the bees in the same sequence, the order of which can be found in Table 3. The attractive stimuli consisted of honey odour, white sugar water, brown sugar water and UV light, the aversive stimuli consisted of an air puff or quinine and the neutral stimulus consisted of white light. The brown sugar water was the solution that the supplier, Koppert, uses as a standard nutritional food source in commercial colonies. The response of the bees to the stimulus was recorded upon their exposure to it. In the case of the UV and white lights (Supplementary Figure 2), the bees were given 1 min to respond, in the case of the odour and air puff stimuli, the response of the bees was recorded in the duration of the delivery and in the case of the sugar solutions and quinine, a droplet of the solutions were placed on the floor of the experimental setup and a response was recorded once the bees had encountered (walked over) the solution (for details see Table 3).
List of Behaviours
Based on preliminary experiments and whether the stimulus was aversive, attractive or neutral, a list of expected and unexpected responses (an ethogram) was generated for each sensory stimulus (Table 2). These expected responses were considered to be reasonable initial reflex reactions from the bees on exposure to these stimuli. The unexpected responses were reactions considered functionally inappropriate on exposure to that stimulus.
Morphological Measurements
Antennal length, forewing size and eye areas were selected as features to measure, because the functioning of these organs will affect how the bees interact with the different presented stimuli. Body size was measured as fresh mass to the nearest μg using a balance (Sartorius BP 310S, Göttingen, Germany). Bumblebees were euthanised using ethyl acetate and their mass was documented within 5 min of death followed by subsequent dissections. Antennae and proboscides were dissected from bumblebee workers, laid flat on 1 mm grid paper and photographed. The length of the right antenna from each individual was measured in ImageJ (Schneider et al., 2012). Right forewings were removed from each individual, photographed and their length and width was measured in ImageJ (Schneider et al., 2012). 3D volumetric scans of the head were acquired through X-ray microtomographic (micro-CT) scans (Taylor et al., 2019, 2020) at the TOMCAT beamline of the Swiss Light Source (beamtime number: 20191425). The left eyes were cropped using Drishti 2.6.4 (Limaye, 2012) and imported into Amira 6.2.0 (Thermo Fisher Scientific, Whaltham, United States). Eye surface area was determined by creating a patch of the left eye using the “Patchify Surface” function in Amira 6.2.0.
Statistics
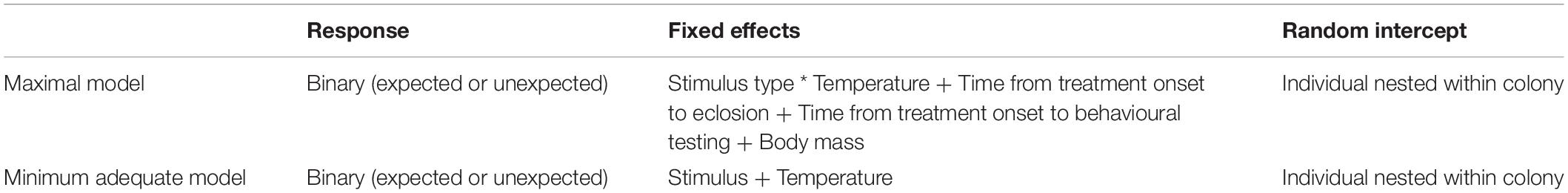
All statistics were conducted using R v.4.0.2 (R Core Team, 2016). Binary response data were analysed using generalized mixed effects models with a binomial family from the lme4 package (Bates et al., 2015). A maximal model was constructed and simplified using likelihood ratio tests (LRT) to arrive at the minimum adequate model. The initial maximal model was fitted with main effects of temperature, time from treatment onset to being tagged, time from treatment onset to behavioural testing (equal to the total time spent in the treatment), stimulus type and body mass (Table 4). An interaction between temperature and stimulus type was also fitted along with a random intercept of bee ID nested in colony (Table 4). Significance of explanatory variables in the final model were analysed using type II ANOVAs from the car package (Fox and Weisberg, 2019). Subsequent pairwise comparisons were Tukey adjusted and estimated using the multcomp package (Hothorn et al., 2008). Morphological differences were assessed using a two-sample t-test.
Results
There was no significant interaction between temperature and stimulus type (LRT, X6 = 4.04, p = 0.62) nor was there a significant effect of fresh mass (LRT, X1 = 0.02, p = 0.89). There was no significant effect of days taken to eclose or days to onset of behavioural testing (LRT, X1 = 3.27, p = 0.07) There was a significant effect of both temperature (type II ANOVA, X59,1 = 5.22, p < 0.03) and stimulus type on the likelihood of bees responding in accordance with the expected criteria (type II ANOVA, X59,6 = 0.03, p < 0.001).
Post-hoc pairwise comparisons revealed that bees reared at 26°C made a greater number of expected responses compared with bees reared at 33°C (Figure 2, Z = 2.29, p = 0.02). Irrespective of temperature treatment, bees were more likely to respond in accordance with the expected criteria to UV light, honey water odour, quinine, an air puff and brown sugar water than they were to white sugar water and white light (Figure 2, Z > 3.73, p < 0.01).
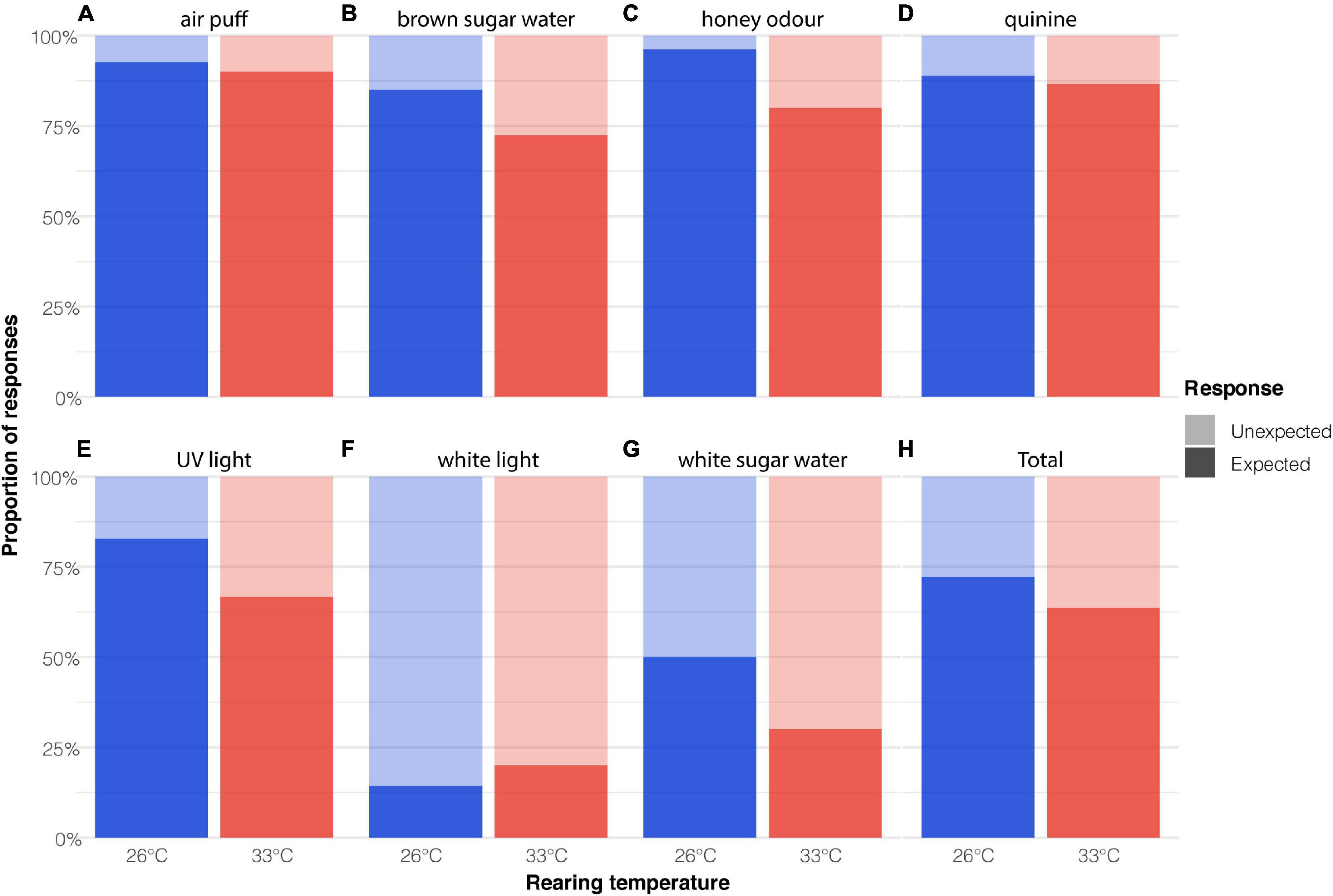
Figure 2. The effect of developmental heatwaves on the behavioural response to sensory stimuli. The proportional of expected and unexpected behavioural responses (Table 2) of bees exposed to (A) air puff, (B) brown sugar water, (C) honey odour, (D) quinine, (E) UV light, (F) white light (G) white sugar water. (H) Total proportion of expected and unexpected behavioural responses for all presented stimuli. Sample size = 59.
There was no significant difference in the mean size of any of the measured morphological features (antennae length, eye area, forewing length, forewing width, proboscis length, body mass) between temperature treatments (Figure 3, T-test, t22–60,17–58, < 1.85, P > 0.17).
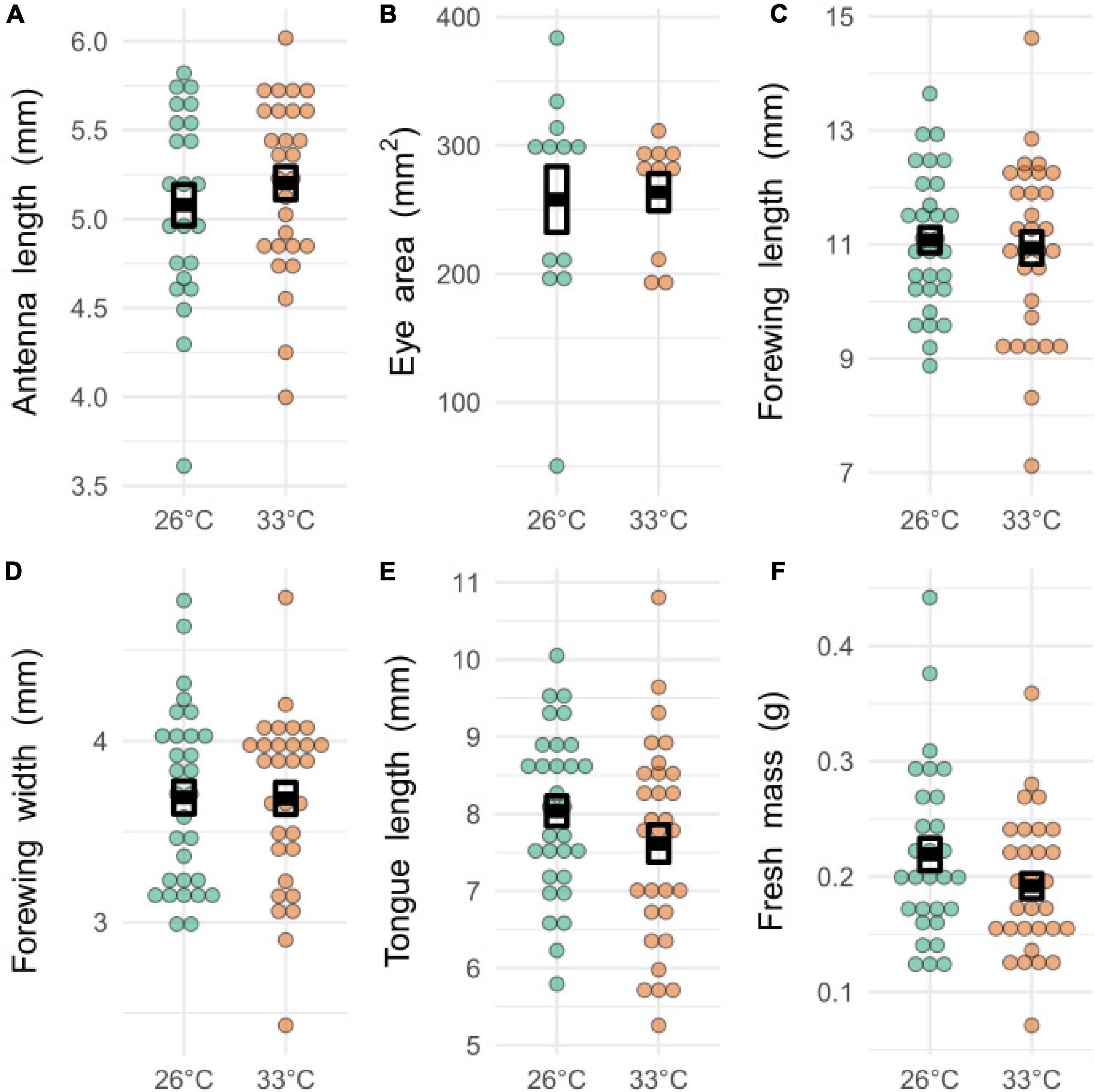
Figure 3. Morphological measurements from bees exposed to developmental heatwaves of different temperatures. Each dot represents a single measurement from an individual bee. Dark boxes represent means ± S.E. There was no significant difference between the mean values of any of the morphological measurements. (A) Antenna length: t51,44.4 = 0.83, p = 0.41, size range = 3.61–6.02 mm. (B) Eye area: t22,16.96 = 0.19, p = 0.85, size range = 0.005–0.0384 mm2. (C) Forewing length: t60,54.64 = 0.38, p = 0.70, size range = 7.12–14.6 mm. (D) Forewing width: t60,57.96 = 0.04, p = 0.96, size range = 2.43–4.79 mm. (E) Tongue length: t60,55.51 = 1.35, p = 0.18, size range = 5.26–10.8 mm. (F) Fresh mass: t59,53.16 = 1.53, p = 0.13, size range = 0.071–0.442g.
Discussion
We explored the functional consequences of elevated rearing temperatures during a period of development – such as those likely to be induced by heatwaves arising from global warming – on bumblebee behaviour by testing their initial responses to various novel and familiar sensory stimuli (Table 3). Bees that spent a portion of their development time at 33°C made 8% more unexpected responses than those reared at 26°C, a difference that would represent a significant negative effect on behaviour. Though these bees were naïve and tested in laboratory conditions, it is not implausible to posit that this could lead to many incorrect choices when extrapolated across the lifetime of a colony. Although maladaptive responses to relevant sensory stimuli could potentially be rectified by experience, previous work showing that honeybees raised at elevated temperatures have impaired memory (Tautz et al., 2003; Jones et al., 2005), suggests that this process may also be affected. The effect of elevated temperatures on the response to sensory stimuli was independent of the time the bees spent at that temperature. This suggests that elevated developmental temperatures can disrupt adult behaviour, independent of their duration – a shorter period of exposure to elevated temperatures is as detrimental as a longer period. This effect of elevated temperature on behavioural responses to sensory stimuli did not appear to be related to morphology; we found no differences between body masses or the size of important sensory structures such as antennae, eyes, wings or proboscides between the two treatment conditions (Figure 2).
Morphological changes, such as organ shape (Gerard et al., 2018), body size (Gerard et al., 2018) or levels of fluctuating asymmetry (Klingenberg, 2015) are often employed as indicators of environmental stress. We found that behavioural impairment occurred without a morphological correlate, indicating that the approach of using morphological differences to indicate environmental stress alone is not necessarily sufficient and other factors, such as behaviour, should be considered during such investigations. The elevated temperatures presented during the mid- and late stages of development in this study did not induce any morphological changes, unlike in Gerard et al. (2018), where a constantly elevated temperature throughout development caused changes to wing morphology. Since we exposed bumblebees in later stages of development to heatwave conditions, it is likely that body size and relative organ sizes were already developmentally fixed (Tian and Hines, 2018). A useful extension of this study would be to examine the effect of elevated temperatures during early development and assess the extent of behavioural and morphological alteration.
The impairments in behaviour observed here, as a result of a heatwave-like event, likely have a neurological, rather than morphological basis. It is unclear if the impairment lies in sensory structures, ascending or descending neurons or in areas of the brain. Tautz et al. (2003) and Jones et al. (2005) concluded that decreased temperature during development caused subtle neurological changes that impaired short-term memory in honeybees. Further evidence for this can be found when examining mushroom bodies of honeybees reared outside of their optimum temperature (Groh et al., 2004). The number of microglomeruli (distinct synaptic complexes) fell as rearing temperature deviated away from the naturally held optimum. This effect was most pronounced within the olfactory input region, while the visual region of the mushroom body was less affected (Groh et al., 2004). Our data are consistent with these findings – a subtle neurological impairment could explain the poorer responses observed in bumblebees reared partly at 33°C. Further, short-term memory in honeybees (Tautz et al., 2003; Jones et al., 2005) was examined via the proboscis extension reflex, a common method for establishing learning and memory in bees (Giurfa and Sandoz, 2012). One of the responses of the bumblebees in this study was the extension of their proboscides (Table 2), indicating that the short-term memory issues reported in honeybees reared at abnormal temperatures might be mechanistically or neurologically similar to the unexpected behavioural responses we observe.
Although we generally observed an effect of elevated developmental temperature on the behavioural responses of bees, there was variation in the extent of the impairment depending on the sensory stimulus. For example, the proportion of expected responses to brown sugar water was similar in both temperature groups (85% at 33°C, 75% at 26°C), while the response differed more markedly in response to white sugar water (70% at 33°C, 50% at 26°C). It is unclear why there was a difference in the response to these two stimuli but it possibly relates to the fact that the brown sugar water had a “honey-like” odour while the white sugar water did not. There was also a difference in how the two temperature groups responded to the two light stimuli that were presented. The bees in this study were reared in the dark and had thus never seen light before, which may explain the higher proportion of unexpected responses to white light. Though, this does not explain the higher proportion of expected responses to UV light, which was equally novel to them. As the stimuli were always presented in the same order, it is also possible that the differences in the proportion of expected responses are related to the order of stimulus presentation. Nonetheless, this does not explain differences between the proportion of responses observed in the two temperature groups to each stimulus as both groups experienced the same order of presentation. Further experimentation and analysis would be required to ascertain why bees reared under elevated temperatures were better able to respond to some stimuli than others.
It is unclear at what temperature B. terrestris colonies begin to experience heat stress. The value appears to be somewhere between 26°C (Weidenmüller et al., 2002) and 32°C (Vogt, 1986). Whether the bees or the colonies were indeed under heat stress in this study is somewhat moot concerning what our results indicate – the elevated developmental temperatures were sufficient to induce an effect on adult behaviour. Heat stressed brood are an obvious and likely explanation behind our results, however, even if colonies were not under heat stress, the indication that sub-stressful elevated temperatures can cause behavioural impairment should be troubling to anyone paying attention to current trends in global climate.
Our data also do not indicate whether the elevated temperature experienced by the colony was the direct cause of the behavioural impairment we observed. Bumblebees can regulate the air and the brood temperatures independently (Vogt, 1986). We measured only the ambient temperature inside the colonies and did not directly measure the brood temperature; therefore it is possible that the brood were kept above or below the temperature set by the incubator, despite the air temperature within the colonies being consistently 33°C or 26°C. The temperature of the brood would need to be directly measured to assess the capacity of the bees to combat the overwhelming air temperature. If the brood are reared at temperatures different from ambient, it would indicate that there are indirect temperature effects on developing bees, likely a result of modified action by nurses. It is known that increased thermoregulatory behaviour lessens the attention that nurses provide to brood (Vogt, 1986). The observed behavioural impairments may therefore be caused directly via the temperature of the brood or indirectly via temperature causing disruption across the whole colony. Either proximate mechanism indicates that elevated temperatures over portions of development create behavioural impairments in adult bumblebees.
Our finding that bees experiencing periods of elevated temperature during development make sub-optimal responses to relevant sensory stimuli is more evidence of the dangers of climate change to heterothermic animals, which are susceptible despite their capacity to independently regulate their temperature. Bees in our experiment experienced elevated temperatures during their mid- and late developmental stages, mimicking heatwave periods, the risk of which are increasing across the northern hemisphere (Qiu and Yan, 2020) and increasing in frequency and magnitude across the globe (Perkins-Kirkpatrick and Lewis, 2020). Therefore, our data provide an indicator of how current and near-future climate change may affect bumblebees. Responding poorly to stimuli, relative to bees reared in more optimal temperatures, could come at a greater energetic cost and result in early worker death, which is a source of stress for a colony (Bryden et al., 2013). Furthermore, a reduced capacity to make appropriate responses to sensory stimuli will affect the speed at which correct decisions are made, impacting foraging efficiency and therefore colony fitness (Spaethe et al., 2001; Chittka et al., 2003; Kulahci et al., 2008).
Our findings add to an existing body of work that indicates that global warming will have a significant effect on pollinators (Shrestha et al., 2018), though few experiments have explicitly examined the effects of elevated rearing temperature on adult behaviour. The impairments presented here show that elevated rearing temperatures affect innate behavioural responses to sensory stimuli. Our experiment tested basic behavioural responses but the results indicate that elevated rearing temperatures are likely to affect more complex behaviours, as well as learning and memory (Tautz et al., 2003; Jones et al., 2005). Further investigations are necessary to understand if these behavioural impairments are mitigated by age, the effects of increased learning and to uncover the mechanisms by which impairments occur.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MG, ZJ, CR, and EB designed the study. CP, ZJ, VW, ZM, MG, and CR collected the data. AM acquired data and provided technical micro-CT expertise. CP performed the analysis and drafted the manuscript with EB. All authors critically revised the manuscript and gave approval for publication.
Funding
This research was supported by grants to EB from Vetenskaprådet (2018-06238), and the Human Frontiers Science Program (RGP0002/2017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to Julia Meneghello and Fatih Aksoy for their hard work measuring compound eyes with AMIRA and Andrea Gonsek who created excellent custom wing measuring software. Special thanks to Maxence Gérard and Inga Tuminaite for providing impeccable comments on the manuscript. Thanks to Tunhe Zhou and Jenny Romell for their invaluable assistance with the micro-CT. We would also like to acknowledge the Paul Scherrer Institut, Villigen, Switzerland for provision of synchrotron radiation beamtime at the TOMCAT beamline X02DA of the SLS.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.776830/full#supplementary-material
References
Arambourou, H., Sanmartín-Villar, I., and Stoks, R. (2017). Wing shape-mediated carry-over effects of a heat wave during the larval stage on post-metamorphic locomotor ability. Oecologia 184, 279–291. doi: 10.1007/s00442-017-3846-z
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Bryden, J., Gill, R. J., Mitton, R. A. A., Raine, N. E., and Jansen, V. A. A. (2013). Chronic sublethal stress causes bee colony failure. Ecol. Lett. 16, 1463–1469. doi: 10.1111/ele.12188
Burger, J. (1991). Effects of incubation temperature on behavior of hatchling pine snakes: implications for reptilian distribution. Behav. Ecol. Sociobiol. 28, 297–303.
Cameron, S. A., Lozier, J. D., Strange, J. P., Koch, J. B., Cordes, N., Solter, L. F., et al. (2011). Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S.A. 108, 662–667. doi: 10.1073/pnas.1014743108
Chen, C., Donner, S. H., Biere, A., Gols, R., and Harvey, J. A. (2019). Simulated heatwave conditions associated with global warming affect development and competition between hyperparasitoids. Oikos 128, 1783–1792.
Chittka, L., Dyer, A. G., Bock, F., and Dornhaus, A. (2003). Bees trade off foraging speed for accuracy. Nature 424:388. doi: 10.1038/424388a
Cnaani, J., Robinson, G. E., and Hefetz, A. (2000). The critical period for caste determination in Bombus terrestris and its juvenile hormone correlates. J. Comp. Physiol. A 186, 1089–1094. doi: 10.1007/s003590000163
Conrad, T., Stöcker, C., and Ayasse, M. (2017). The effect of temperature on male mating signals and female choice in the red mason bee, Osmia bicornis (L.). Ecol. Evol. 7, 8966–8975. doi: 10.1002/ece3.3331
Dyer, A. G., and Chittka, L. (2004). Bumblebee search time without ultraviolet light. J. Exp. Biol. 207, 1683–1688. doi: 10.1242/jeb.00941
Fox, J., and Weisberg, S. (2019). An R Companion To Applied Regression, 3rd Edn. Thousand Oaks, CA: Sage.
Gardner, K. E., Foster, R. L., and O’Donnell, S. (2007). Experimental analysis of worker division of labor in bumblebee nest thermoregulation (Bombus huntii, Hymenoptera: Apidae). Behav. Ecol. Sociobiol. 61, 783–792.
Gerard, M., Michez, D., Debat, V., Fullgrabe, L., Meeus, I., Piot, N., et al. (2018). Stressful conditions reveal decrease in size, modification of shape but relatively stable asymmetry in bumblebee wings. Sci. Rep. 8:15169. doi: 10.1038/s41598-018-33429-4
Gilbert, S. F. (2001). Ecological developmental biology: developmental biology meets the real world. Dev. Biol. 233, 1–12.
Giurfa, M., and Sandoz, J. C. (2012). Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54–66. doi: 10.1101/lm.024711.111
Groh, C., Tautz, J., and Rössler, W. (2004). Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. U.S.A. 101, 4268–4273. doi: 10.1073/pnas.0400773101
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. doi: 10.1002/bimj.200810425
Jones, J. C., and Oldroyd, B. P. (2006). Nest thermoregulation in social insects. Adv. Insect Phys. 33, 153–191.
Jones, J. C., Helliwell, P., Beekman, M., Maleszka, R., and Oldroyd, B. P. (2005). The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A 191, 1121–1129. doi: 10.1007/s00359-005-0035-z
Kerr, J. T., Pindar, A., Galpern, P., Packer, L., Potts, S. G., Roberts, S. M., et al. (2015). Climate change impacts on bumblebees converge across continents. Science 349, 177–180. doi: 10.1126/science.aaa7031
Klingenberg, C. (2015). Analyzing fluctuating asymmetry with geometric morphometrics: concepts, methods, and applications. Symmetry (Basel) 7, 843–934.
Kulahci, I. G., Dornhaus, A., and Papaj, D. R. (2008). Multimodal signals enhance decision making in foraging bumble-bees. Proc. R. Soc. B 275, 797–802. doi: 10.1098/rspb.2007.1176
Limaye, A. (2012). “Drishti: a volume exploration and presentation tool,” in Developments in X-Ray Tomography VIII, ed. S. R. Stock (Bellingham, WAS: SPIE), 85060X.
Martinet, B., Zambra, E., Przybyla, K., Lecocq, T., Anselmo, A., Nonclercq, D., et al. (2020). Mating under climate change: impact of simulated heatwaves on the reproduction of model pollinators. Funct. Ecol. 35, 739–752.
Medina, R. G., Paxton, R. J., De Luna, E., Fleites-Ayil, F. A., Medina Medina, L. A., and Quezada-Euán, J. J. G. (2018). Developmental stability, age at onset of foraging and longevity of Africanized honey bees (Apis mellifera L.) under heat stress (Hymenoptera: Apidae). J. Therm. Biol. 74, 214–225. doi: 10.1016/j.jtherbio.2018.04.003
Micheli-Campbell, M. A., Campbell, H. A., Cramp, R. L., Booth, D. T., and Franklin, C. E. (2011). Staying cool, keeping strong: incubation temperature affects performance in a freshwater turtle. J. Zool. 285, 266–273.
Nasir, M., Mohsan, A., Ahmad, M., Saeed, S., Aziz, M. A., Imran, M., et al. (2019). Effect of different temperatures on colony characteristics of Bombus terrestris (Hymenoptera: Apidae). Pak. J. Zool. 51, 1315–1322.
Orbán, L. L., and Plowright, C. M. S. (2014). Getting to the start line: how bumblebees and honeybees are visually guided towards their first floral contact. Insectes Soc. 61, 325–336. doi: 10.1007/s00040-014-0366-2
Perkins, S. E., and Alexander, L. V. (2013). On the measurement of heat waves. J. Clim. 26, 4500–4517.
Perkins-Kirkpatrick, S. E., and Lewis, S. C. (2020). Increasing trends in regional heatwaves. Nat. Commun. 11:3357. doi: 10.1038/s41467-020-16970-7
Pimsler, M. L., Oyen, K. J., Herndon, J. D., Jackson, J. M., Strange, J. P., Dillon, M. E., et al. (2020). Biogeographic parallels in thermal tolerance and gene expression variation under temperature stress in a widespread bumble bee. Sci. Rep. 10:17063. doi: 10.1038/s41598-020-73391-8
Poot-Báez, V., Medina-Hernández, R., Medina-Peralta, S., and Quezada-Euán, J. J. G. (2020). Intranidal temperature and body size of Africanized honey bees under heatwaves (Hymenoptera: Apidae). Apidologie 51, 382–390.
Qiu, W., and Yan, X. (2020). The trend of heatwave events in the Northern Hemisphere. Phys. Chem. Earth, Parts A B C 116:102855. doi: 10.1038/s41467-017-02699-3
Radmacher, S., and Strohm, E. (2011). Effects of constant and fluctuating temperatures on the development of the solitary bee Osmia bicornis (Hymenoptera: Megachilidae). Apidologie 42, 711–720.
Sales, K., Vasudeva, R., Dickinson, M. E., Godwin, J. L., Lumley, A. J., Michalczyk, Ł, et al. (2018). Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 9:4771. doi: 10.1038/s41467-018-07273-z
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schultze-Motel, P. (1991). Heat loss and thermoregulation in a nest of the bumblebee Bombus lapidarius (Hymenoptera, Apidae). Thermochim. Acta 193, 57–66.
Sfakianakis, D. G., Leris, I., and Kentouri, M. (2011). Effect of developmental temperature on swimming performance of zebrafish (Danio rerio) juveniles. Environ. Biol. Fishes 90, 421–427.
Shrestha, M., Garcia, J. E., Bukovac, Z., Dorin, A., and Dyer, A. G. (2018). Pollination in a new climate: assessing the potential influence of flower temperature variation on insect pollinator behaviour. PLoS One 13:e0200549. doi: 10.1371/journal.pone.0200549
Singh, S. K., Das, D., and Rhen, T. (2020). Embryonic temperature programs phenotype in reptiles. Front. Physiol. 11:31. doi: 10.3389/fphys.2020.00035
Spaethe, J., Tautz, J., and Chittka, L. (2001). Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl. Acad. Sci. U.S.A. 98, 3898–3903. doi: 10.1073/pnas.071053098
Sprayberry, J. D. H. (2018). The prevalence of olfactory- versus visual-signal encounter by searching bumblebees. Sci. Rep. 8:14590. doi: 10.1038/s41598-018-32897-y
Stillman, J. H. (2019). Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86–100. doi: 10.1152/physiol.00040.2018
Tautz, J., Maier, S., Groh, C., Rössler, W., and Brockmann, A. (2003). Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. U.S.A. 100, 7343–7347. doi: 10.1073/pnas.1232346100
Taylor, G. J., Hall, S. A., Gren, J. A., and Baird, E. (2020). Exploring the visual world of fossilized and modern fungus gnat eyes (Diptera: Keroplatidae) with X-ray microtomography. J. R. Soc. Interface 17:20190750. doi: 10.1098/rsif.2019.0750
Taylor, G. J., Tichit, P., Schmidt, M. D., Bodey, A. J., Rau, C., and Baird, E. (2019). Bumblebee visual allometry results in locally improved resolution and globally improved sensitivity. Elife 8:e40613. doi: 10.7554/eLife.40613
Tian, L., and Hines, H. M. (2018). Morphological characterization and staging of bumble bee pupae. PeerJ 6:e6089. doi: 10.7717/peerj.6089
Van Damme, R., Bauwens, D., Braña, F., and Verheyen, R. F. (1992). Incubation temperature differentially affects hatching time, egg survival, and hatchling performance in the lizard Podarcis muralis. Herpetologica 48, 220–228.
VideoLan (2006). VLC Media Player. Available online at: https://www.videolan.org/vlc/index.html
Vogt, F. D. (1986). Thermoregulation in bumblebee colonies. I. Thermoregulatory versus brood-maintenance behaviors during acute changes in ambient temperature. Physiol. Zool. 59, 55–59.
Watkins, T. B. (2000). The effects of acute and developmental temperature on burst swimming speed and myofibrillar ATPase activity in tadpoles of the Pacific tree frog, Hyla regilla. Physiol. Biochem. Zool. 73, 356–364. doi: 10.1086/316744
Weidenmüller, A., Kleineidam, C., and Tautz, J. (2002). Collective control of nest climate parameters in bumblebee colonies. Anim. Behav. 63, 1065–1071.
Keywords: bumblebee, heatwave, behaviour, sensory system, reflex, Bombus terrestris
Citation: Perl CD, Johansen ZB, Moradinour Z, Guiraud M, Restrepo CE, Wen Jie V, Miettinen A and Baird E (2022) Heatwave-Like Events During Development Are Sufficient to Impair Bumblebee Worker Responses to Sensory Stimuli. Front. Ecol. Evol. 9:776830. doi: 10.3389/fevo.2021.776830
Received: 14 September 2021; Accepted: 13 December 2021;
Published: 31 January 2022.
Edited by:
Michael Hrncir, University of São Paulo, BrazilReviewed by:
Natalie Hempel de Ibarra, University of Exeter, United KingdomCarita Lindstedt, University of Jyväskylä, Finland
Copyright © 2022 Perl, Johansen, Moradinour, Guiraud, Restrepo, Wen Jie, Miettinen and Baird. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily Baird, ZW1pbHkuYmFpcmRAem9vbG9naS5zdS5zZQ==
†Present address: Craig D. Perl, School of Life Sciences, Arizona State University, Tempe, AZ, United States; A. Miettinen, Department of Physics, University of Jyväskylä, Jyväskylä, Finland
 Craig D. Perl
Craig D. Perl Zanna B. Johansen1
Zanna B. Johansen1 Marie Guiraud
Marie Guiraud Emily Baird
Emily Baird