- 1School of Biological and Environmental Sciences, Liverpool John Moores University, Liverpool, United Kingdom
- 2Department of Recovery Ecology, Conservation Science and Wildlife Health, San Diego Zoo Wildlife Alliance, Escondido, CA, United States
Shifts in resource availability due to environmental change are increasingly confronting animals with unfamiliar food types. Species that can rapidly accept new food types may be better adapted to ecological change. Intuitively, dietary generalists are expected to accept new food types when resources change, while dietary specialists would be more averse to adopting novel food. However, most studies investigating changes in dietary breadth focus on generalist species and do not delve into potential individual predictors of dietary wariness and the social factors modulating these responses. We investigated dietary wariness in the Gouldian finch, a dietary specialist, that is expected to avoid novel food. This species occurs in two main head colors (red, black), which signal personality in other contexts. We measured their initial neophobic responses (approach attempts before first feed and latency to first feed) and willingness to incorporate novel food into their diet (frequency of feeding on novel food after first feed). Birds were tested in same-sex pairs in same and different head color pairings balanced across experiments 1 and 2. Familiar and novel food (familiar food dyed) were presented simultaneously across 5 days for 3 h, each. Gouldian finches fed on the familiar food first demonstrating food neophobia, and these latencies were repeatable. Birds made more approach attempts before feeding on novel than familiar food, particularly red-headed birds in experiment 1 and when partnered with a black-headed bird. Individuals consistently differed in their rate of incorporation of novel food, with clear differences between head colors; red-headed birds increased their feeding visits to novel food across experimentation equaling their familiar food intake by day five, while black-headed birds continually favored familiar food. Results suggest consistent among individual differences in response to novel food with red-headed birds being adventurous consumers and black-headed birds dietary conservatives. The differences in food acceptance aligned with responses to novel environments on the individual level (found in an earlier study) providing individuals with an adaptive combination of novelty responses across contexts in line with potential differences in movement patterns. Taken together, these novelty responses could aid in population persistence when faced with environmental changes.
Introduction
Human activities are increasingly confronting animal species with environmental challenges such as changes in habitat, which affects resource availability. New food resources may appear, such as the emergence of invasive species or incidental food provisioning by humans, meanwhile preferred food may disappear as native habitats dwindle. The ability to adapt to changes in food resources, has far-reaching consequences on distribution and population development (McKinney, 1997). However, a species’ response to novelty is rarely uniform, but harbors considerable among individual variation (personality) in response to environmental challenges (White et al., 2013; Boulton et al., 2014; Edwards et al., 2016). Additionally, seemingly independent behaviors can be correlated, forming behavioral syndromes that define an individual’s response across contexts (e.g., Sih et al., 2015). Such among individual variation reflects different strategies to cope with environmental stressors, giving certain individuals advantages depending on environmental conditions (e.g., Dingemanse et al., 2004). Therefore, it has been proposed that populations with individual differences improve a species’ ability to respond to environmental change (Delarue et al., 2015). Accordingly, understanding the overall level and individual differences within a species’ response to novel resources may be of conservation value (Kelleher et al., 2018).
Responses to novel food consist of two different and independent processes. First animals must overcome neophobic responses toward the sight or smell of novel food. Then they must incorporate the novel food consistently into their diet, i.e., dietary conservatism (Marples and Mappes, 2011). Both processes together are termed dietary wariness (Marples and Kelly, 1999; Kelly and Marples, 2004; Marples et al., 2007; McMahon et al., 2014). Food neophobia is an adaptive mechanism to avoid potentially harmful substances and has been shown to be a widespread behavioral strategy demonstrated in humans (Cooke et al., 2007; Knaapila et al., 2007), non-human primates (Johnson, 2000; Visalberghi and Addessi, 2000; Visalberghi et al., 2003; Gustafsson et al., 2014; Forss et al., 2019), rodents (Hall et al., 1997; Lin et al., 2009; Modlinska et al., 2015), carnivores (Malmkvist et al., 2003), and birds (Marples et al., 1998; Camín et al., 2016). It has a genetic component (Jones, 1986; Marples and Brakefield, 1995; Turro-Vincent et al., 1995; Boliver and Flaherty, 2004; Cooke et al., 2007; Knaapila et al., 2007), though social and asocial environmental factors and experience modulate food neophobia (Marples et al., 2007; Doktorovová et al., 2019). For example, naïve individuals are more likely to try novel food that they have seen others consume (Dally et al., 2008; Chiarati et al., 2012; Greggor et al., 2016b). Moreover, group composition (Oostindjer et al., 2011) and an individual’s own position in a group (Amici et al., 2020) can affect food neophobic responses. Finally, juveniles have been found less food neophobic than adults in some primates (Visalberghi et al., 2003; Ueno and Matsuzawa, 2005; Addessi and Visalberghi, 2006; but see Gustafsson et al., 2014; Arnaud et al., 2017). While studies generally find considerable differences in food neophobia between individuals (e.g., Marples et al., 1998; Exnerova et al., 2010; Liebl and Martin, 2014), few studies have investigated whether or not individuals are consistent in these differences. Coleman and Wilson (1998) demonstrated consistent individual differences in food neophobia in fish, as did Prasher et al. (2019) in birds, indicating that food neophobia forms part of personality traits.
Fewer studies have looked into the neophilic or exploratory component of sampling novel food, which prompts an individual to approach and collect information about the unfamiliar food source. Chimpanzees (Pan troglodytes) – who are food neophobic – have been found to extensively explore novel food items before first tasting, and also rely heavily on social information (Gustafsson et al., 2014). Likewise, mink (Mustela vision) sniffed more often and for longer on novel than familiar food (Malmkvist et al., 2003). Forss et al. (2019) found that ape species with a more solitary lifestyle relied more on individual exploration of novel food than more social ape species who used social observation. While exploration of novel objects or environments has been shown to be consistent individual traits (Williams et al., 2012; Boulton et al., 2014), there are no studies that have tested this for novel food.
Once novel food has been tasted, food neophobia terminates and dietary conservatism begins. Dietary conservatism describes the process of incorporating novel food into the diet over time (Marples et al., 1998; Marples and Kelly, 1999; Kelly and Marples, 2004), and can be divided into two stages; an assessment stage where novel food is occasionally sampled, but not preferred, and a full acceptance stage in which novel food is consumed at equal or higher rates than familiar food (Marples and Kelly, 1999). Dietary conservatism is often assessed by comparing the amount of novel versus familiar food consumed, with novel food often ingested at a lower rate than familiar food (Malmkvist et al., 2003; Visalberghi et al., 2003; Gustafsson et al., 2014). Individuals fall into two genetically different types – adventurous consumers and dietary conservatives (Marples and Brakefield, 1995; Marples et al., 1998, 2007; Thomas et al., 2003, 2004). Adventurous consumers accept the novel food as soon as neophobia has ceased, whereas dietary conservatives demonstrate a prolonged aversion (sometimes months or years) to accept novel food into the diet (Marples and Brakefield, 1995). Both types are found on the species level in quails and a wide range of passerines, with dietary conservative individuals comprising between 30–50% of many populations (Thomas et al., 2010). The two foraging strategies may reflect different risk-reward trade-offs (Toscano et al., 2016), with adventurous consumers maximizing food intake through a generalist foraging approach at the risk of occasional food poisoning, while dietary conservatives (e.g., specialist foragers) may have high efficiency in exploiting a few familiar resources without risk (Thomas et al., 2010). Whether adventurous consumers and dietary conservatives also reflect consistent individual strategies is unclear as some studies have found consistency (Thomas et al., 2010), whereas others did not (Marples et al., 1998; Prasher et al., 2019).
Most studies on dietary wariness focus on the phenomenon itself and its mechanisms. Few studies have investigated how environmental conditions affect dietary wariness or how dietary wariness is linked to other traits. Two general hypotheses should be mentioned. The Neophobia Threshold hypothesis predicts that neophobia preserves ecological specialization (Greenberg, 1983) and limits ecological plasticity, having been confirmed in closely related diet and habitat specialist and generalist species, with specialists showing more (spatial) neophobia when encountering novel micro-habitats (Greenberg, 1983, 1984). The highly specialized snail kite (Rostrhamus sociabilis) serves as a supporting example of a species with both high food neophobia and diet specialization, since they reject even similar but unfamiliar snails (Beissinger et al., 1994). The Dangerous Niche hypothesis, in contrast, addresses the general risk inherent to the environment rather than ecological plasticity (Greenberg, 2003). In support of this hypothesis, higher neophobia has been demonstrated in species that are more likely to encounter dangerous situations, such as poisonous prey items (Greenberg, 2003; Mettke-Hofmann et al., 2013). Camín et al. (2016) compared dietary wariness between the rufous collared sparrow (Zonotrichia capensis), a dietary specialist, and the many colored chaco finch (Saltatricula multicolour), a dietary generalist. While both species had similar latencies to feed on the novel food, the generalist took significantly longer to taste the novel than the familiar food showing clear food neophobia. Interestingly, the proportion of dietary conservative individuals in the generalist species was 37%, whereas it was only 12% in the specialist species. The authors concluded that the generalist encountered more food containing toxic secondary compounds causing food neophobia, a result which was consistent with the Dangerous Niche hypothesis (Greenberg, 2003) but in contrast to the Neophobia Threshold hypothesis (Greenberg, 1983). This is one of very few studies involving specialists as most studies focus on generalist foragers (Turro-Vincent et al., 1995; Marples et al., 1998; Kelly and Marples, 2004).
Responses toward novel food may have ramifications beyond diet. A newly emerging area of research connects movement and dietary wariness since animals moving into unfamiliar environments are also more likely to encounter novel food. For example, in invasive species lower food neophobia was found in populations at the invasion front as compared to native or more established populations in two bird species (Martin and Fitzgerald, 2005; Cohen et al., 2020). Lower food neophobia helps to adapt to unfamiliar environments (Martin and Fitzgerald, 2005; Cohen et al., 2020). Martin and Fitzgerald (2005) also measured the amount of novel food consumed, which did not differ between the established and invading populations of house sparrows (Passer domesticus). While this study showed house sparrows from native and invasive populations consumed similar amounts of novel food, they found the invader was less neophobic. In other scenarios, lower food neophobia could theoretically give invading species a competitive edge over native ones, thereby compromising the persistence of native species. With changes in climate and species distribution shifts documented globally (Perry et al., 2005; Boisvert-Marsh et al., 2014; Pacifici et al., 2017; Cohen et al., 2019), species that are likely most at risk of population declines are those that occupy a specialist lifestyle. Therefore, investigating dietary wariness in specialized species could help predict certain vulnerability to changes in resources or competitors.
Due to the potential importance of dietary wariness for long-term population persistence, and the lack of knowledge how specialist species deal with novel food, we studied dietary wariness in the Gouldian finch (Erythrura gouldiae), a diet and habitat specialist. The Gouldian finch predominantly forages on grass seeds, particularly annual Sorghum species (Brazill-Boast et al., 2011). Changes in food availability caused by grazing and changed fire regimes have resulted in steep population declines (Dostine et al., 2001; Legge et al., 2015; Weier et al., 2016, 2017, 2018), with the species now listed as endangered by the Australia Government (Environment Protection and Biodiversity Conservation, EPBC, 2018). Higher stress levels and lower physiological condition scores in response to food shortages, particularly during the wet season, have been reported in this food specialized species compared to other sympatric but more generalist finches (Maute et al., 2013).
Gouldian finches are a unique example of a non-melanin-based color-polymorphism, where head colors co-exist in sympatry in the wild with 70% black-headed, 30% red-headed and <1% yellow-headed birds (Kim et al., 2019). In situ research has shown head color signals personality; black-headed birds are consistently less aggressive yet readily investigate changes in their familiar environment (novel objects) and take greater risk in potentially dangerous situations than red-headed birds (Williams et al., 2012). However, black-headed birds hesitate longer to enter unsuitable novel habitats (Mettke-Hofmann et al., 2020). The combination of high interest in changes in the familiar environment and less interest in entering unfamiliar environments in the black-headed birds is consistent with a resident cognitive strategy (Mettke-Hofmann et al., 2020). This allows tracking of changes in the familiar environment facilitating persistence at a site (Mettke-Hofmann et al., 2005, 2012). In contrast, the higher willingness of red-headed birds to enter unfamiliar environments but refraining from investigating changes in the familiar environment is consistent with a migratory/nomadic cognitive strategy (Mettke-Hofmann et al., 2020). Moreover, morph composition has been shown to affect novelty responses in Gouldian finch social groups. The presence of black-headed birds increased cautious behavior toward novel environments in other Gouldian finches, whereas red-headed birds did not (Mettke-Hofmann et al., 2020).
Besides their extreme food specialization, little is currently known about Gouldian finches’ responses (a) to unfamiliar food and their willingness to incorporate novel food into the diet, (b) whether head colors and/or personality types respond differently to novel food and (c) how morph group composition may affect responses. However, such responses could have far-reaching consequences for population recovery in light of further habitat change. Moreover, few food neophobia studies have been conducted on food specialists and color polymorphism has only been considered from a predator perspective, i.e., how rare color morphs can induce neophobic reactions and dietary conservatism in predators (Thomas et al., 2003, 2010). The current study aimed to investigate the entire process of dietary wariness in the food specialized Gouldian finch, considering potential differences in responses of color morphs reflecting underlying differences in personality, and in relation to group composition. We combined two experimental approaches for studying dietary wariness: (1) we investigated food neophobia by considering both, food neophobia and food neophilia as separate processes consistent with other studies (e.g., Gustafsson et al., 2014; Forss et al., 2019). (2) We investigated the process of dietary conservatism following the approach by Marples et al. (1998) which distinguishes food neophobia from dietary conservatism. To avoid confusion between the umbrella term of dietary conservatism describing the process of accepting novel food and one of its outcomes (i.e., being dietarily conservative) we will refer to the process as the rate of incorporation of novel food into the diet.
Based on the existing literature and the dietary specialism of Gouldian finches, the following predictions were made.
Prediction 1 – Individual consistency: In line with other studies, we expected consistent among individual variation in food neophobia (Coleman and Wilson, 1998; Prasher et al., 2019). This may extend to the willingness to incorporate novel food into the diet.
Prediction 2 – Effects of color morphs/personalities: Morphs have been found to reflect different personalities (Mafli et al., 2011; Mateos-Gonzalez and Senar, 2012; Williams et al., 2012). In the Gouldian finch, black-headed birds’ personalities combine into a resident cognitive strategy, whereas in red-headed birds they align with a migratory/nomadic cognitive strategy (Mettke-Hofmann et al., 2020). We therefore expected black-headed birds to be more food neophobic than red-headed birds. The more spatially novelty-prone red-headed birds are likely to encounter unfamiliar food on a regular basis and sampling novel food may be part of their cognitive adaptation (Martin and Fitzgerald, 2005; Cohen et al., 2020). Whether this extends to dietary conservatism is unclear (Martin and Fitzgerald, 2005) but if so, we expect red-headed birds to incorporate novel food faster than black-headed birds facilitating their higher movement potential.
Prediction 3 – Evidence for a novelty syndrome: As the Mettke-Hofmann et al. (2020) study tested exactly the same birds in the same setting as in the current study, we were able to test for a novelty syndrome. We expected a positive correlation between spatial and food neophobia on the individual level, which would equip birds with a high propensity to enter novel environments with a high willingness to try and accept novel food.
Prediction 4 – Social effects: Group composition can affect foraging efficiency (Paijmans et al., 2020) and responses to novel food (Oostindjer et al., 2011). Different scenarios are possible: (a) If red-headed birds are less food neophobic than black-headed birds (see prediction 2), then pure red-headed pairs may be fastest to sample and incorporate novel food into their diet, whereas pure black-headed pairs may be the most food neophobic. Mixed pairs may fall in between, with either black-headed birds slowing down red-headed birds, similar to their influence on spatial neophobia (Mettke-Hofmann et al., 2020), or red-headed birds reducing neophobia in black-headed birds. (b) Alternatively, mixed morph pairs may be fastest as studies have found higher foraging efficiency and faster approach to novel feeders in mixed personality groups as compared to groups consisting of one type only (Dyer et al., 2009; Paijmans et al., 2020).
Prediction 5 – Dietary wariness: We expected to find species-level dietary wariness, in line with the neophobia threshold hypothesis (Greenberg, 1983). Gouldian finches are food specialists feeding nearly exclusively on Poaceae, e.g., Sorghum spec (Dostine and Franklin, 2002), a plant group that has very low toxicity levels (Diaz, 1996). Therefore, we predicted (a) food neophobia evidenced by hesitating longer before feeding on novel food in comparison to familiar food, and (b) dietary conservatism evidenced by birds continuing to prefer familiar over novel food after any initial neophobia has ceased.
Materials and Methods
Study Group and Housing
Thirty-two Gouldian finches originating from 12 private breeders were used. Birds were acquired at roughly 1 year old and had spent different amounts of time in our Animal Facility, but at least 2 months before the experiments began. All birds were wild type, parent reared, and ages ranged from 1 to 6 years. Sex ratios were equal with 16 males (eight red-head, eight black-head) and 16 females (seven red-head, nine black-head). All birds were housed together within six free-flight cages (1.20 m long × 80 cm deep × 1.00 m high) in groups of 5–6 individuals. All birds were grouped in mixed sexes, ages and head colors with the exception of the 1-year-old individuals (10 birds) who were housed in same sex groups. Birds were fed a 6:3:1 mixture of 6 units Astrilden Spezial, 3 units Amadinen-Zucht Spezial and 1 unit red sibirica millet (referred to as familiar seed hereon), plus grit (all purchased from Blattner-Heimtierfutter, Ermengerst, Germany) and egg shells in separate feeders located at the front of the cage. French red spray millet (Blattner-Heimtierfutter) was located next to the feeders. Water was available ad libitum. Once per week Blattner’s vitamins (Blattner-Hiemtierfutter) were supplemented in the drinking water. Birds were kept at a temperature of 24°C and 51% humidity and provided with a full spectrum light source with a light:dark cycle of 13:11 h. In addition to the two wooden perches located within each cage, natural branches and twigs were available.
Experimental Set Up
Testing took place in four experimental cages (1.20 m long × 0.7 m deep × 1.00 m high) in a separate room from the housing. Experimental cages each comprised of three wooden walls and a wire mesh front and ceiling. Each cage was furnished with a front perch, running parallel to the front wire mesh with two plastic plant pot saucers (14 cm diameter × 2.50 cm depth) as feeders attached side-by-side between the mesh and the perch (Figure 1). Two additional perches at the same height as the first perch were positioned on the left- and right-hand side of the cage running perpendicular to the front perch with a water dispenser each attached to it from the outside. The front perch was marked at 7.5 cm away from each feeder as this is the average body length of a Gouldian finch and was used to determine distance within the data collection sessions. Birds in different cages could not see each other but were in auditory contact. A digital video camera was positioned on a tripod one meter in front of each experimental cage, connected to GeoVision 1480 recording software for later analysis.
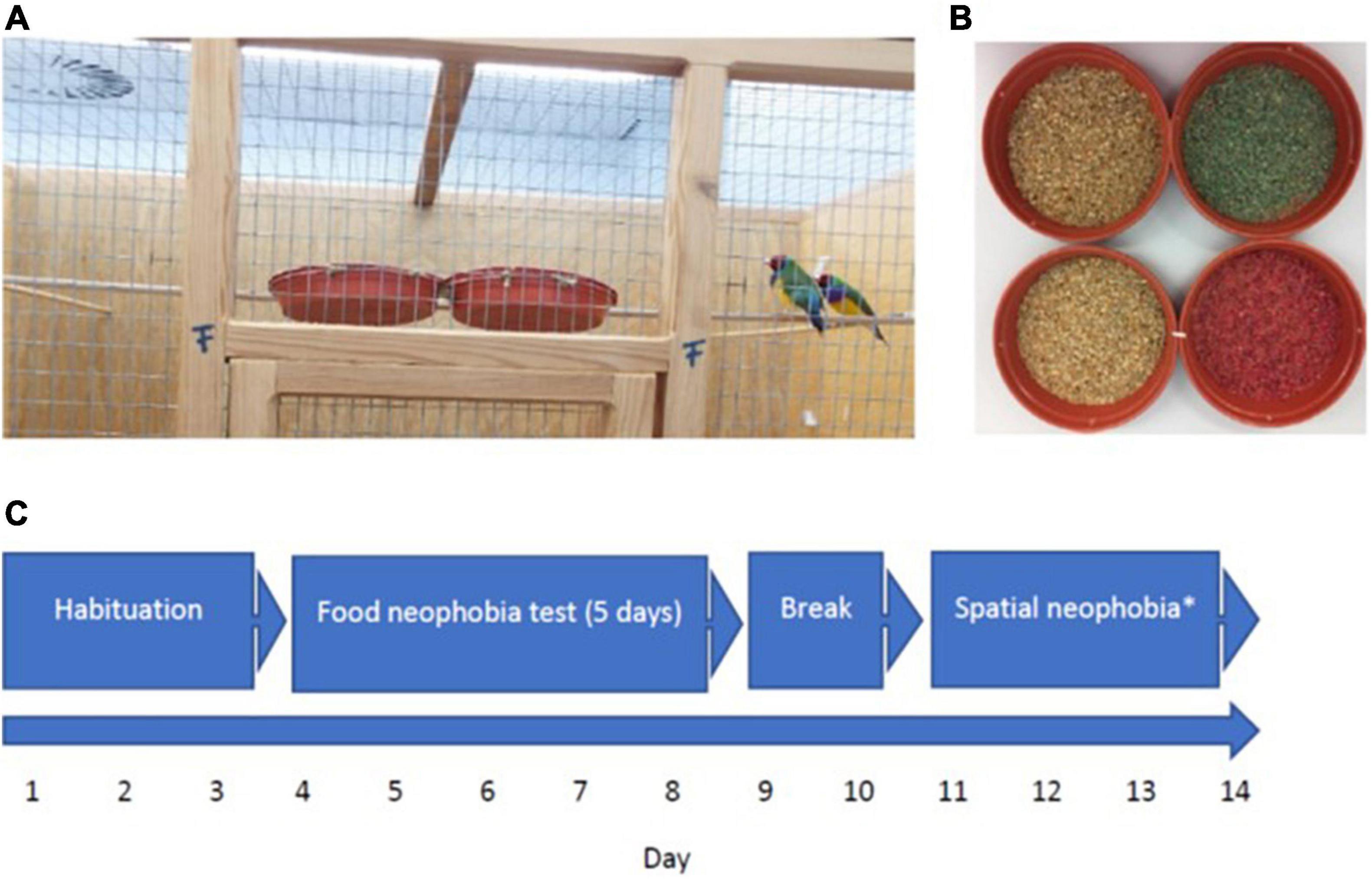
Figure 1. (A) Upper part of experimental cage with the two feeders side-by-side, (B) familiar (left) and novel green (experiment 1) and red (experiment 2) food (right), (C) flow chart demonstrating experimental design (repetition of experiment not shown); *see Mettke-Hofmann et al. (2020).
In the experimental cages, birds were fed two types of food. Familiar food was the seed they were fed in their normal housing. Novel food was produced by dying the familiar food, which is a common procedure to create novelty (e.g., Marples et al., 2007; Greggor et al., 2016b). Novel seed were either green (peppermint green pastel paste gel edible concentrated food coloring) for experiment 1 or red (scarlet red pastel paste gel edible concentrated food coloring; both from Sugarflair Colours Limited, Essex, United Kingdom) for experiment 2 (Figure 1). We selected glycerine-based products over sugar-based ones to minimize effects of taste. The seed was dyed by boiling 50 parts seed (g) in a solution of 1 part dye (ml) with 100 parts water (ml) for 20 min at a low heat. Dyed seeds were dried at room temperature for 24 h then separated into 20 g portions and frozen at a temperature of –21°C until required at which point it was defrosted early morning prior to use. Gouldian finches readily consume undyed boiled seeds as they may be more palatable than dry seeds (CMH, own obs.).
Procedure
Birds were assigned to same sex pairs for testing as Gouldian finches are highly social and testing in isolation would produce unnatural conditions (Brush and Seifried, 1968; Mettke-Hofmann, 2012). It also allowed testing for effects of group composition. Same sex pairs were matched for size and weight. Age was controlled for by pairing birds that were at least 2 years apart as age effects have been shown to influence object neophobia in this species (Mettke-Hofmann, 2012). One bird in each pair was fitted with two white leg bands (one per leg) for identification purposes and all birds were weighted and their tarsus length taken as a measure of body size. To investigate effects of group composition, each bird was tested once in a same head color pair (red-red or black-black) and once in a different head color pair (red-black) in two separate experiments. In experiment 1, half of the birds were paired with a partner of the same head color and half of the birds with a partner of the opposite head color. In experiment 2, which took place after all birds had finished experiment 1, the pairings were reversed so that birds who were paired with a partner of the same head color in experiment 1 were paired with a bird with the opposite head color in experiment 2 and vice versa. As we had more black-headed than red-headed females and uneven numbers within head colors, two black-headed females were tested with partner birds in both experiments that had gone through their own testing already (hereafter named experienced partner bird). In experiment 2, two additional black-headed females and one red-headed female were tested with experienced partner birds. Only the data from the focal birds were included from these pairings. The interval between experiment 1 and 2 for individual birds ranged from 3 to 18 weeks.
Four pairs were tested simultaneously with a total of four batches of four pairs, each. Head color combination and age were balanced across cages and batches. Birds could settle and feed on their standard (familiar) food in both feeders in the experimental cages for 3 days prior to the start of testing. Experiments ran from day 4 to day 8 followed by spatial neophobia testing between day 11 and 14 (Mettke-Hofmann et al., 2020) after which birds were moved back into their home cage (Figure 1).
During experiment 1, each morning for five consecutive days the two feeders containing the familiar food were removed for 1 h (8:00 to 9:00 AM) directly after the lights went on to control for hunger levels at the start of testing. At 9:00 AM each day the feeders were returned, but this time only one contained the familiar seed, whereas the other one contained the novel green seed. The birds’ feeding behavior was video recorded for 3.5 h. At the end of each session the novel food was removed from the experimental cage and replaced with familiar food. The positions of the familiar and novel food were counter-balanced to the left and right locations across days within and across cages. Once all birds had completed experiment 1, they went through experiment 2 following the same procedure as before except birds were paired with a new partner in a different head color combination than in experiment 1 and the novel seed was red to retain novelty. Due to the new pairing, it was not possible to counter-balance novel food colors within experiments.
Data Preparation
Data preparation and statistical analyses were performed in R version 3.6.0. (R Core Team, 2017). Raw data can be found in the Supplementary Materials S1,S2. To assess neophobia we extracted two response variables: approach frequency prior to first feed and latency to first feed. Approach frequency before feeding reflects the conflict between the motivation to approach and feed (food neophilia) and the motivation to avoid the unfamiliar food due to its potential harmfulness (food neophobia) and helps identifying the contribution of each motivation to the latency to feed (Mettke-Hofmann et al., 2009). Meanwhile, latency to feed is a standard measure of neophobia (e.g., Camín et al., 2016; Forss et al., 2019). Response variables were not correlated with body mass (Spearman correlation: n = 31, familiar food: approach frequency, corr coef = 0.05, P = 0.791, first feeding latency, corr coef = 0.306, P = 0.96; novel food: approach frequency, corr coef = 0.135, P = 0.469, first feeding latency, corr coef = 0.159, P = 0.392) or body size (familiar food: approach frequency, corr coef = –0.013, P = 0.946, first feeding latency, corr coef = 0.177, P = 0.341; novel food: approach frequency, corr coef = 0.128, P = 0.491, first feeding latency, corr coef = 0.022, P = 0.907).
To assess dietary conservatism, the rate of incorporation of novel food into the diet was measured as feeding frequency following the first feed on each food type. Feeding frequency was recorded for each of the 5 days of testing in experiment 1 and experiment 2, separately.
All three response variables did not meet the requirement of normality in raw or transformed form. Therefore, untransformed data were used, and appropriate model error structures specified. Sample size was N = 31 birds for the analysis of neophobia, as one bird died between experiment 1 and 2 due to circumstances unrelated to the experiments and their data were therefore removed from the study. Sample size for assessment of dietary conservatism was N = 30 birds, due to a transcription error resulting in missing data for one bird for frequency of feeding after the first feed.
Statistical Analysis
To address prediction 1 about consistent among individual variation, we assessed repeatability (R) of behavior by accounting for the degree of variation attributable to bird identity using the rptR package (Stoffel et al., 2017). Repeatability highlights persistent differences in novelty reactions between individuals (Dingemanse et al., 2003; Nakagawa and Schielzeth, 2010). All birds had also been tested on spatial neophobia directly following the food neophobia testing (see Figure 1; Mettke-Hofmann et al., 2020), allowing us to test for a novelty syndrome (prediction 3). From their familiar test cage, birds gained access on 1 day to a novel open habitat and on another day to a novel dense habitat. Approach frequencies before entering and latencies to enter the novel habitats were measured. Like in the current study, all birds were tested in same and mixed head color combinations across the two experiments. We correlated the variables for spatial neophobia with the dietary wariness measures (approach frequency and latency to first feed for novel and familiar food, frequency of feeding visits after first feed to novel and familiar food on day 1 and day 5) of experiment 1 only using a Spearman rank correlation. Responses in the first experiment were chosen to exclude any habituation effects.
To address predictions 2 (effects of color morphs/personalities), 4 (social effects) and 5 (species-level dietary wariness), we fitted generalized linear mixed models (GLMM) for all three response variables using the R package ‘lme4’ version 1.1-20 (Bates et al., 2015), with a specified Poisson family error distribution with log-link function. We developed four statistical models tailored to our predictions. One model to address predictions 2 and 5 for food neophobia (model A) and a second model to address prediction 4 about social effects of morph composition within pairings on food neophobia (model B). Note that we had two response variables for model A and B. Similarly, we had one model to address prediction 2 and 5 for dietary conservatism (model C) and another one to address prediction 4 for dietary conservatism (model D). The analyses were conducted as separate models to avoid inclusion of too many variables in any single model which would cause over-parametrization issues (Crawley, 2012). Bird identity, partner identity and cage number were entered as crossed random effects in all models (crossed rather than nested because assigning birds to new pairings for experiment 2 precluded birds being tested in the same cage as in experiment 1). To account for using the same data in both models (for food type and head color) we used sequential Bonferroni adjustments were necessary (Rice, 1989; Chandler, 1995).
Generalized Linear Mixed Models for Food Neophobia
Model A: Approach to and Feed on Novel Food
Response variables were approach frequency prior to first feed and latency to first feed. Each GLMM contained two predictor variables: food type (familiar, novel) and head color morph (black, red); with two control variables: age [1 year old (N = 10), older than 1 year (N = 21)]; and experiment (1, 2; to account for the repeated testing). Sex was not included as earlier screening showed no effect of sex which corroborates our previous findings in other contexts that sex did not influence neophobic responses (Eccles, 2018; Mettke-Hofmann et al., 2020). Food type, morph and experiment were entered as a three-way interaction term in the full model as morphs were expected to differ in their response to novel food and these differences may be more prevalent during the first experiment. Sample sizes in the three-way interaction for all comparisons were N = 31 birds (124 rows of data) as all birds were tested in both experiments with both food types. Where the three-way interaction was not significant, we tested the following two-way interactions: food type × morph, food type × experiment and morph × experiment.
Model B: Social Factors Influencing Neophobia
Response variables were approach frequency prior to first feed and latency to first feed. Each GLMM contained three predictor variables: food type (familiar, novel), head color morph (black, red) and partner head color (black, red) and one control variable: relative age within each pairing (younger or older to account for age effects within pairings as found in earlier studies; Mettke-Hofmann, 2012). We included food type, morph and partner head color as a three-way interaction term as the combination of morphs may influence behaviors (e.g., Dyer et al., 2009). Where the three-way interaction was not significant, we tested the following two-way interactions: food type × morph, food type × partner head color and morph × partner head color.
Generalized Linear Mixed Models for Dietary Conservatism
Model C: The Rate of Incorporation of Novel Food Into the Diet
The response variable was the feeding frequency on each food type after first feed. The GLMM contained three predictor variables: food type (familiar, novel), morph (black, red) and day (1 – 5); and three control variables: age (1 year old, older than 1 year), experiment (1, 2) and latency to feed (a continuous variable to control for the variation in time that feed frequency was recorded for each bird and which was scaled to a mean of 0 and SD = 1 to aid model interpretation). Food type, morph, and experiment were entered as a three-way interaction term, as were food type, morph and day. Sample sizes in the three-way interaction for all comparisons were N = 30 birds (120 rows of data) as all birds were tested in both experiments with both food types. Where the three-way interactions were not significant the following two-way interactions were tested: food type × morph, food type × experiment, morph × experiment, food type × day, morph × day and experiment × day.
Model D: Social Factors Influencing Dietary Conservatism
The response variable was the feeding frequency on each food type after first feed. The model contained three predictor variables: food type (familiar, novel), morph (black, red) and partner head color (black, red) and two control variables: relative age within each pairing and latency to first feed. Food type, morph, and partner head color were included as a three-way interaction term, with subsequent two-way interactions: food type × morph, food type × partner head color and morph × partner head color in case of a non-significant outcome.
Model Simplification for Generalized Linear Mixed Models
Interaction terms were retained where P < 0.05, and excluded where they failed to reach this criterion, in a stepwise model simplification, following Crawley (2012). Orthogonal data are robust to stepwise removal of interaction terms as variation attributable to each factor is constant at each stage of the stepwise simplification (Crawley, 2007). All predictor and control variables were retained in all final models. Retaining fixed effects in final models minimized repeated testing and hence concern about the risk of type I errors (e.g., Steel et al., 2013) and increased our ability to interpret model output and effect size calculations in a biologically meaningful way (e.g., Nakagawa and Cuthill, 2007). We adjusted convergence tolerance using the arguments ‘allFit’ and ‘control’ to specify the optimizer to ‘bobyqa’ and increased the number of iterations to 100,000, a practice considered ‘gold standard’ for ensuring stable model fit (Bates et al., 2019). Model fit was assessed by visually inspecting plots of fitted model residuals to ensure an even spread of residuals, which we found in all cases. We assessed each final model by comparing it against the null model (an identical model except for the removal of the predictor and control variables, with an intercept of 1 specified) using the anova command in R. The final model was only accepted where it was a significantly better fit than the null model (Burnham and Anderson, 2002).
We checked for evidence of collinearity within models using the function ‘vif’ (variance inflation factor) in the package ‘car,’ and extracted effect sizes using the r.squaredGLMM command in the package MuMIn (Barton, 2015). To facilitate future meta-analyses, we report both marginal and conditional effect sizes, r2m and r2c respectively, where r2m explains variance due to fixed effects and r2c explains variance due to fixed and random effects (Nakagawa and Cuthill, 2007; Nakagawa and Schielzeth, 2013).
Ethical Note
We conducted all experiments in accordance with published guidelines for the treatment of animals in behavioral research (ASAB/ABS guidelines, Animal Behaviour, 2018; ARRIVE guidelines, Kilkenny et al., 2010). Holding and experimental aviaries conformed to Home Office codes of practice (Home Office, 2013) and were carried out in approved facilities within Liverpool John Moores University. All experiments were non-regulated by the Home Office and complied with the ethical and welfare guidelines for animals and the legal requirements of the University (CMH_GE/2016-5) and the United Kingdom.
Results
Food Neophobia
All birds fed on the familiar food on the first day of presentation in both, experiment 1 and experiment 2. There was variation between birds in day of first feed on the novel food. In experiment 1, 20 birds fed on the novel food (green seed) on the first day, one bird on the second day, four birds on the third day and two birds on the fourth day. Four birds never fed on the novel food in experiment 1. In experiment 2, all birds fed on the novel food (red seed): 20 birds on the first day, six birds on the second day, one bird on the third day, three birds on the fourth day and one bird on the fifth day.
Consistency of Responses and Novelty Syndromes
There was no evidence for significant repeatability in the number of approaches prior to first feed, to familiar seed [R = 0 (0 – 0.20), P = 1], with marginal evidence for novel seed [R = 0.283 (0 – 0.62), P = 0.077]. The cross study analysis for a potential novelty syndrome did not reveal a correlation between approach frequencies before first feed and any measures of spatial novelty reactions (Table 1).
Latency to first feed was moderately repeatable and significant for familiar seed [R = 0.391 (0.024 – 0.673), P = 0.015] but not for novel seed [R = 0.17 (0 – 0.57), P = 0.189]. Cross study Spearman correlations linking latency to feed with spatial novelty reactions were all non-significant, although we found one marginal and positive trend between the latency to first feed on familiar food and approach frequency to dense habitat (Spearman: n = 31, Corr Coef = 0.34, P = 0.060). Birds that were hesitant to enter unsuitable habitats tended to feed later on familiar food (Table 1).
Number of Approaches Before First Feed to Familiar and Novel Food
Results of the GLMMs for number of approaches are shown in Table 2. For model A, testing for effects of head color and seed type (Table 2A), there was a significant three-way interaction between food type, head color and experiment for number of approaches prior to first feed [GLMM: n = 31 birds (124 data points), LRT = 4.56, df = 1, P = 0.033; Figure 2] including a two-way interaction between food type and head color (z = 2.43, P = 0.015). Posthoc tests revealed that red-headed birds made significantly more approaches to novel food in experiment 1 than experiment 2 (z = –4.901, P < 0.001), whereas black-headed birds made significantly fewer approaches to novel food prior to first feed in experiment 1 than they did in experiment 2 (z = 3.673, P < 0.001). Also, red-headed birds tended to make more approaches to novel food before first feed than did black-headed birds in experiment 1 (z = 9.911, P = 0.056), while there was no difference in number of approaches to novel food before first feed between head morphs in experiment 2 (z = –0.063, P = 0.95).
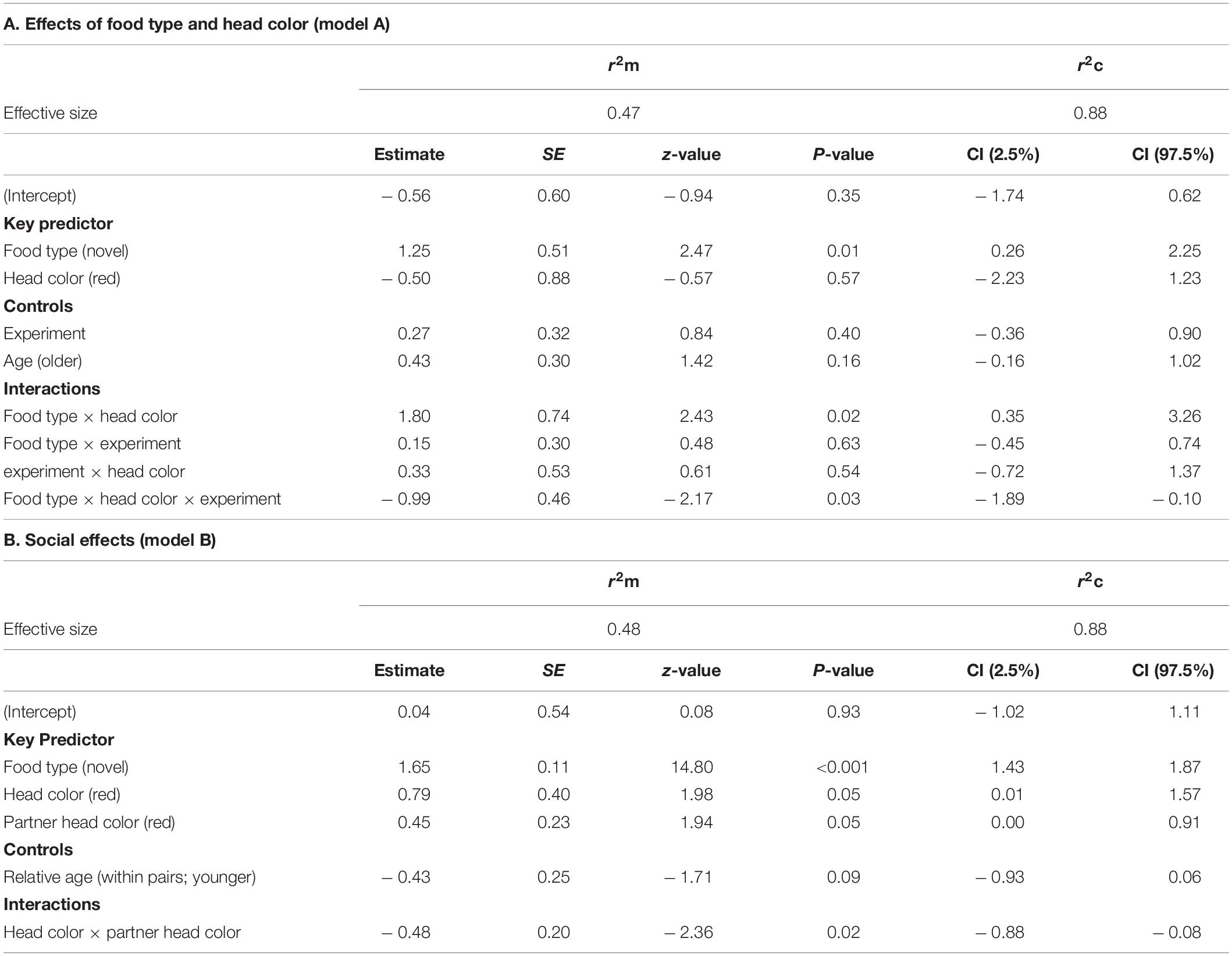
Table 2. Results of the general linear mixed effects model on the number of approaches before first feed on familiar and novel food of Gouldian finches addressing (A) the effect of food type and color morphs (model A) and (B) social effects (model B). Only the final model of each analysis is shown. The reference modality is in parentheses.
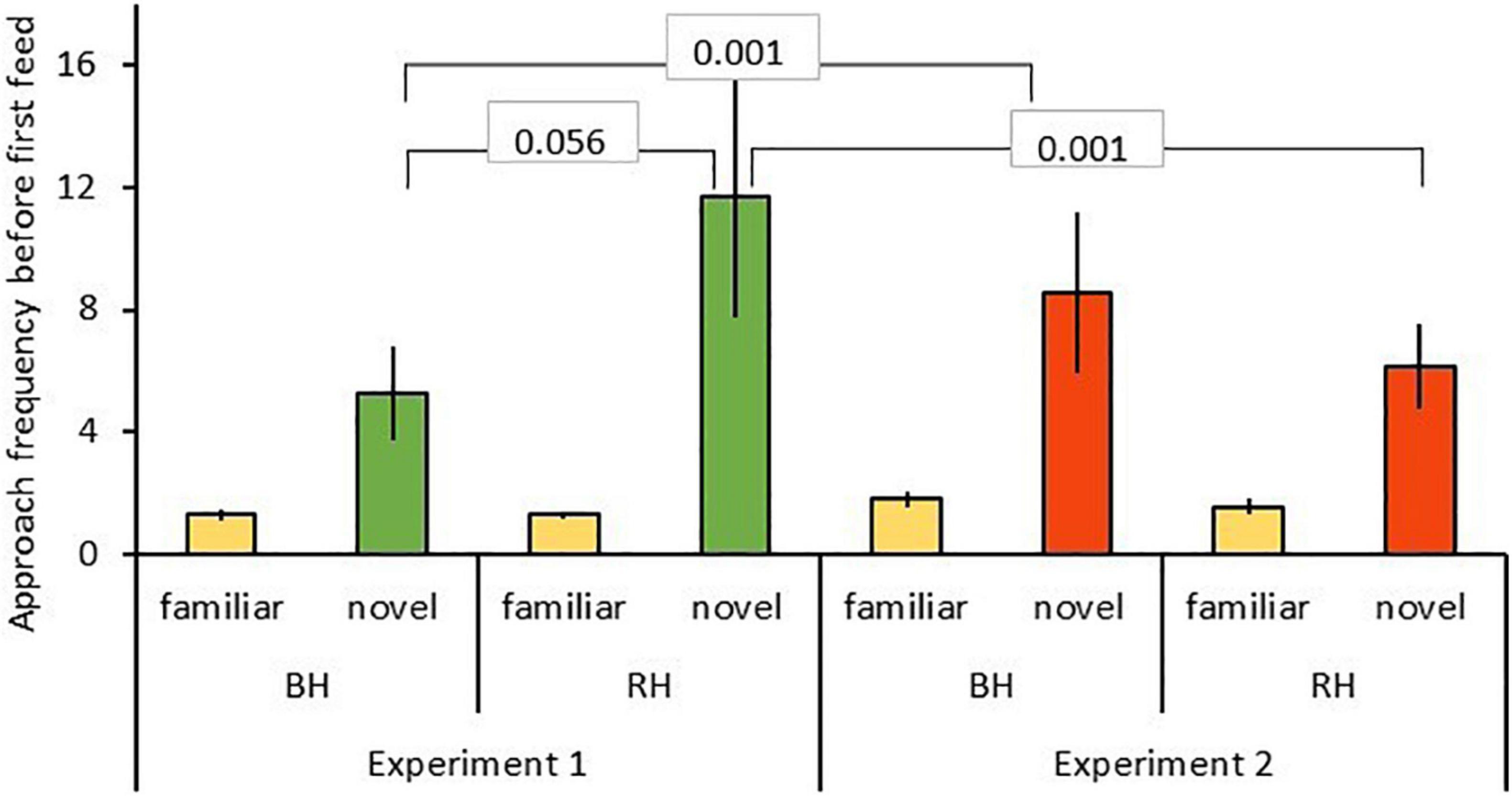
Figure 2. Approach frequencies before first feed (mean ± SE) to familiar (beige) and novel food (green, red) in experiment 1 and 2 for red-headed (RH) and black-headed (BH) Gouldian finches. Numbers in the figure represent p-values.
Irrespective of head color, birds made more approach attempts toward novel than familiar food (z = 2.47, P = 0.014; Figure 2). There were no main effects of either head color, experiment or age.
Results for model B testing for social effects of own and partner head color on food neophobia are shown in Table 2B. There was no significant three-way interaction between food type, head color morph and partner head color. Removal of this term revealed a significant two-way interaction between head color morph and partner head color (GLMM: n = 31 (124 data points), LRT = 5.35, P = 0.021; Figure 3). Post hoc tests revealed that black-headed partners led to significantly more approach attempts in red-headed birds (mean 6.00 ± SE 2.11) than black-headed birds (mean 3.59 ± SE 0.91; z = 10.478, P < 0.001), whereas red-headed partners did not differentially impact number of approach attempts by red and black headed birds (red: mean 4.36 = SE 1.04; black: mean 4.85 = SE 1.39; z = 1.591, P = 0.112). The significant main effect of food type already reported in model 1 was again evident (LRT = 290.26, P = 0.001). There was no main effect of relative age within pair (LRT = 3.04, P = 0.081).
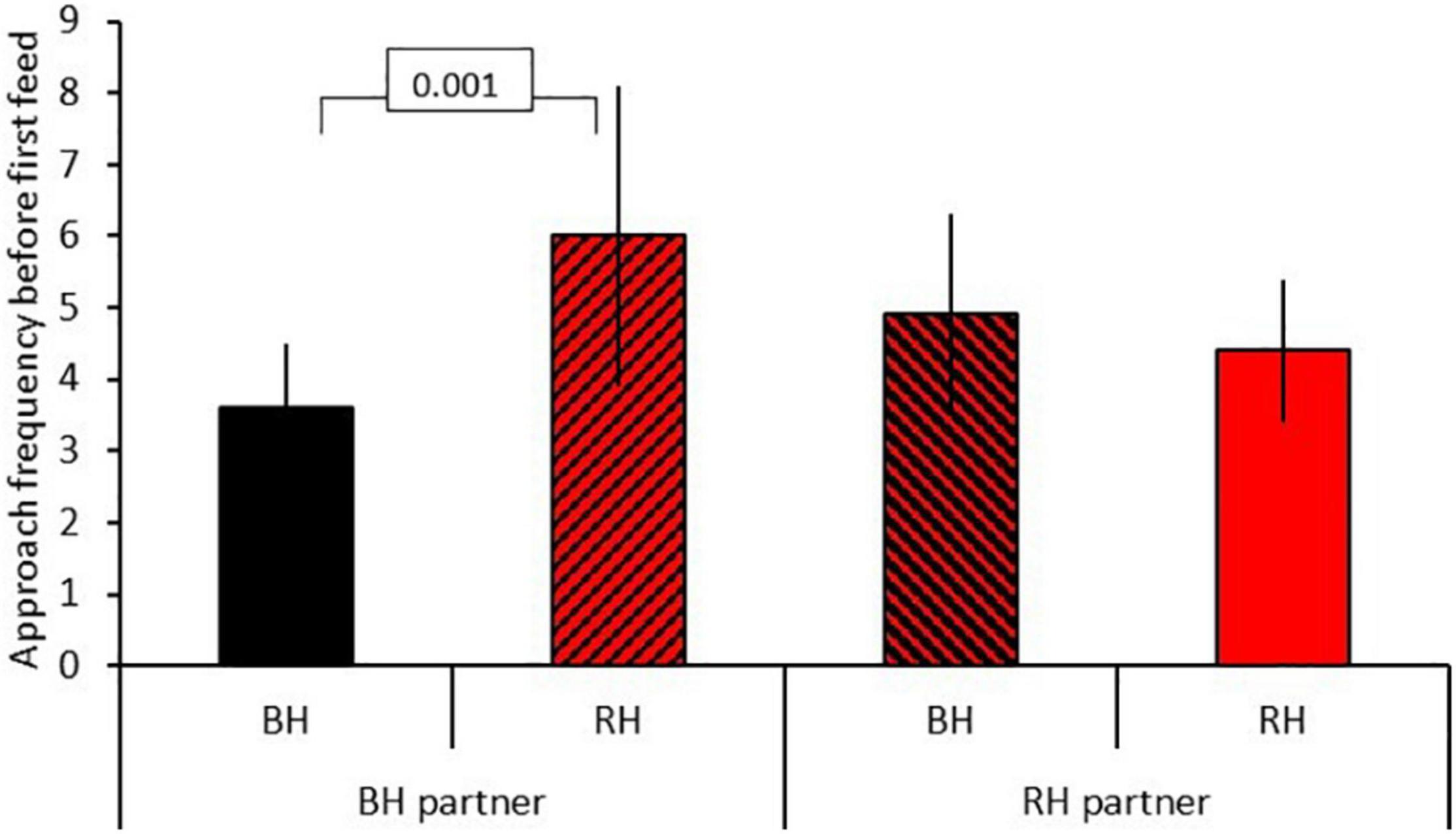
Figure 3. Effect of partner head color on approach frequency before first feed (mean(SE) for red-headed (RH) and black-headed (BH) birds. Numbers in the figure represent p-values; black bars = black-headed pairs, hatched bars = mixed head color pairs, red bars = red-headed pairs.
Latency to First Feed on Familiar and Novel Food
Results of the GLMMs for latency to first feed are shown in Table 3. For model A, testing for effects of head color and seed type (Table 3A), there were no significant interactions. There were significant main effects of food type (LRT = 57.09, df = 1, P = 0.001) and age (LRT = 5.25, df = 1, P = 0.022). Birds were faster to feed on familiar food (mean = 356 s ± 542 s) than on novel food (mean = 7202 s ± 10698 s). One-year old birds were faster to first feed, irrespective of food type (mean = 1272 ± 2949 s) than were older birds (mean = 4973 ± 9659 s).
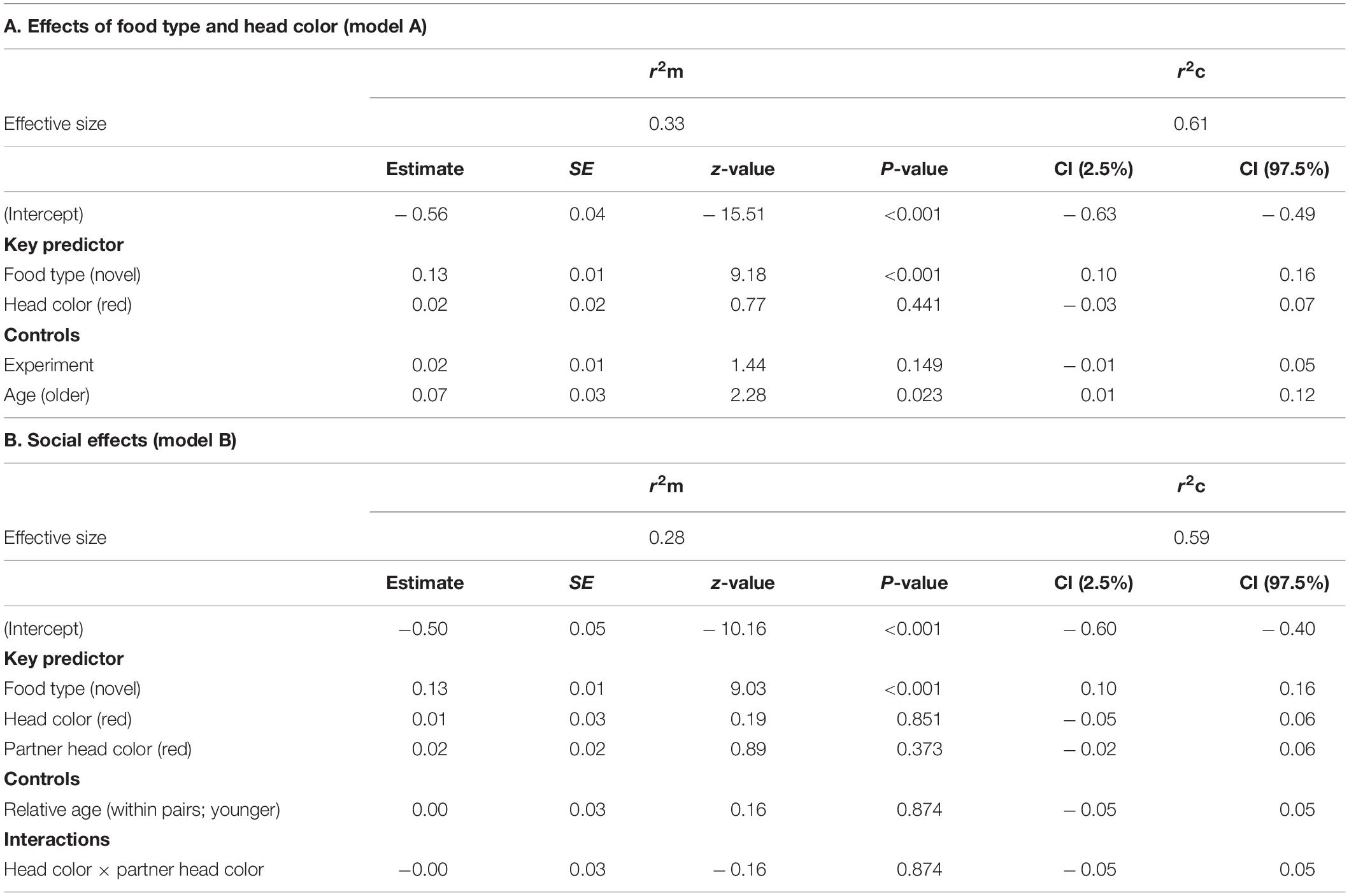
Table 3. Results of the linear mixed effects models on the latencies to first feed on familiar and novel food of Gouldian finches addressing (A) the relationship between food type and color morph (model A) and (B) social effects (model B). Only the final model for each analysis is shown. The reference modality is in parentheses.
Results for model B, testing for social effects, are shown in Table 3B. There were no significant interactions and no main effects of any of the variables associated with social context. The main effect of food type revealed in model A was retained (LRT = 55.78, df = 1, P < 0.001).
Rate of Incorporation of Novel Food Into the Diet
Consistency of Responses and Novelty Syndromes
Repeatability for feed frequency overall was moderate and significant (R = 0.26, P < 0.001), and present for both novel seed [R = 0.384 (0.18 – 0.47), P < 0.001] and familiar seed [R = 0.361 (0.196 – 0.509), P < 0.001]. In the cross species comparison some correlations were found. The frequencies of feeding visits to familiar food on day 1 and 5 were positively correlated with the approach frequency to open (suitable) habitat; birds with many approach attempts before entering the novel environment made more feeding visits to familiar food (Table 4). A similar trend was found for novel food on day 1. Moreover, feeding visits to novel food on day 5 were negatively correlated with the latency to enter open (trend) and dense habitats. Birds that entered novel habitats sooner also made frequent visits to the novel food on day 5 (Table 4).
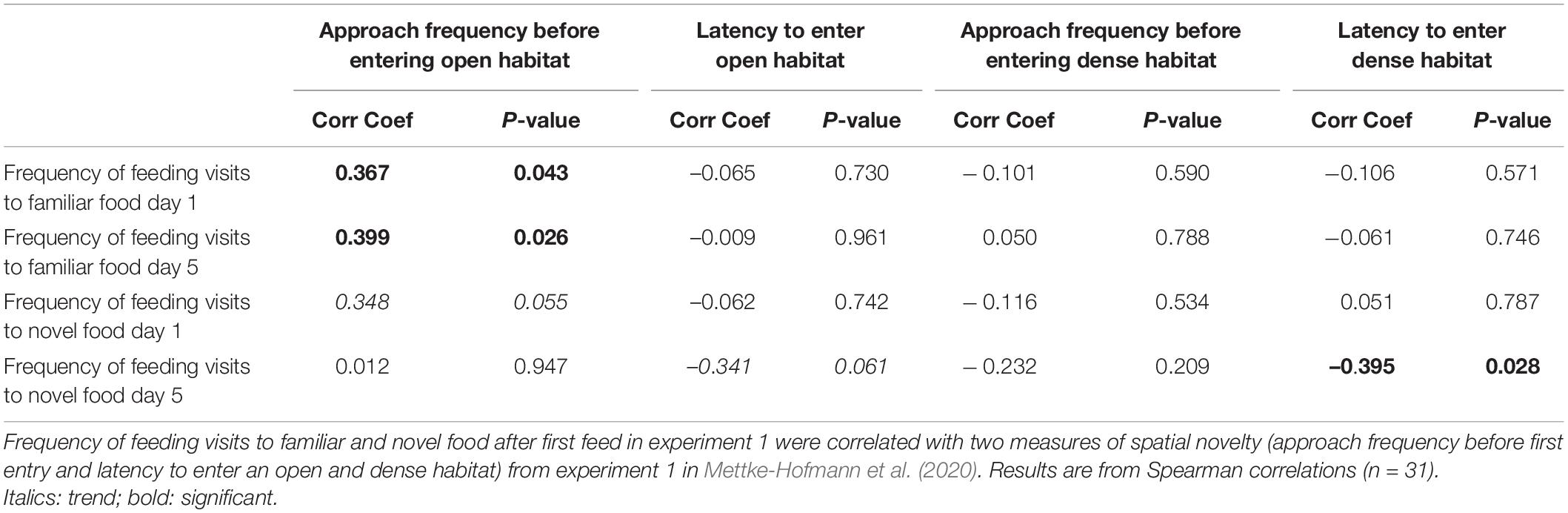
Table 4. Correlation between rate of incorporation of novel food into the diet and spatial novelty reactions.
Rate of Incorporation of Novel Food
Results for rate of incorporation of food into the diet after the first feed are shown in Table 5. For model C, testing for effects of head color and seed type, there were significant three-way interactions between food type, head color and day (LRT = 6.85, df = 1, P = 0.009; Table 5A and Figure 4), and between food type, head color and experiment (LRT = 21.97, df = 1, P < 0.001; Table 5A and Figure 5). As part of the three-way interaction, there were significant two-way interactions between food type and head color (z = 3.82, P < 0.001), between food type and experiment (z = 5.60, P < 0.001) and between food type and day (z = 3.55, P < 0.001). Planned post hoc comparisons were conducted to explore the interaction between food type and day, for each head color separately. For red headed birds these revealed a significant increase in frequency of feeds on novel food between day 1 (mean = 6.46 ± 1.62 feeds) and day 5 (mean = 13.14 ± 2.65 feeds; V = 10, P = 0.025), with no difference in frequency of feeds on novel versus familiar food on day five (familiar mean = 14.93 ± 1.48 feeds; V = 62, P = 0.572). For black headed birds there was no increase in number of feeds on novel food from day 1 to 5 (V = 36.5, P = 0.190), and they continued to feed on familiar food significantly more often than novel food on day 5 (V = 127, P = 0.003; Figure 4).
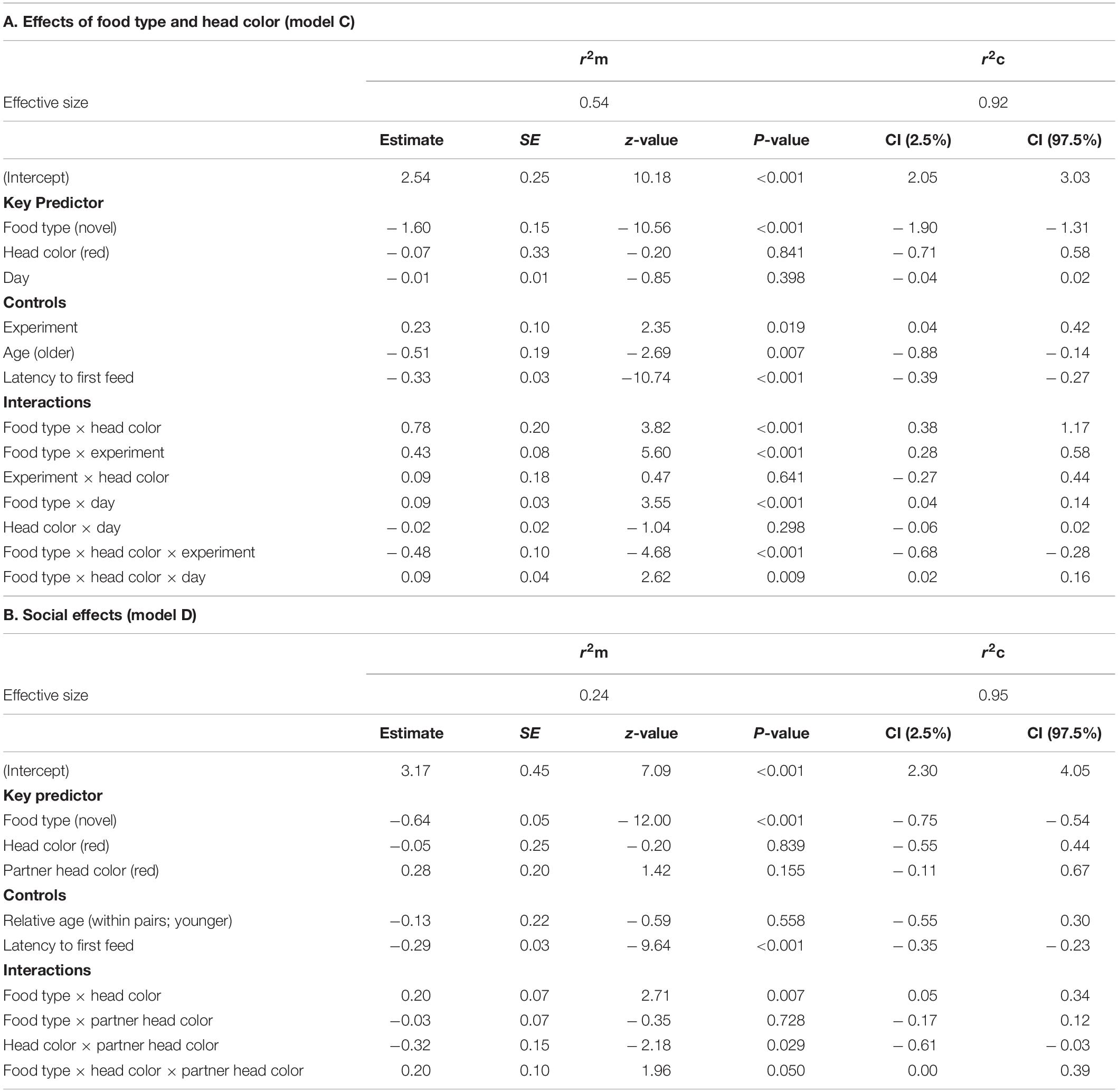
Table 5. Results of the general linear mixed effects model on the frequency of feeding visits after first feed on familiar and novel food (rate of incorporation of novel food into the diet) of Gouldian finches addressing (A) the effect of food type and color morph (model C) and (B) social effects (model D). Only the final model of each analysis is shown. The reference modality is in parentheses.
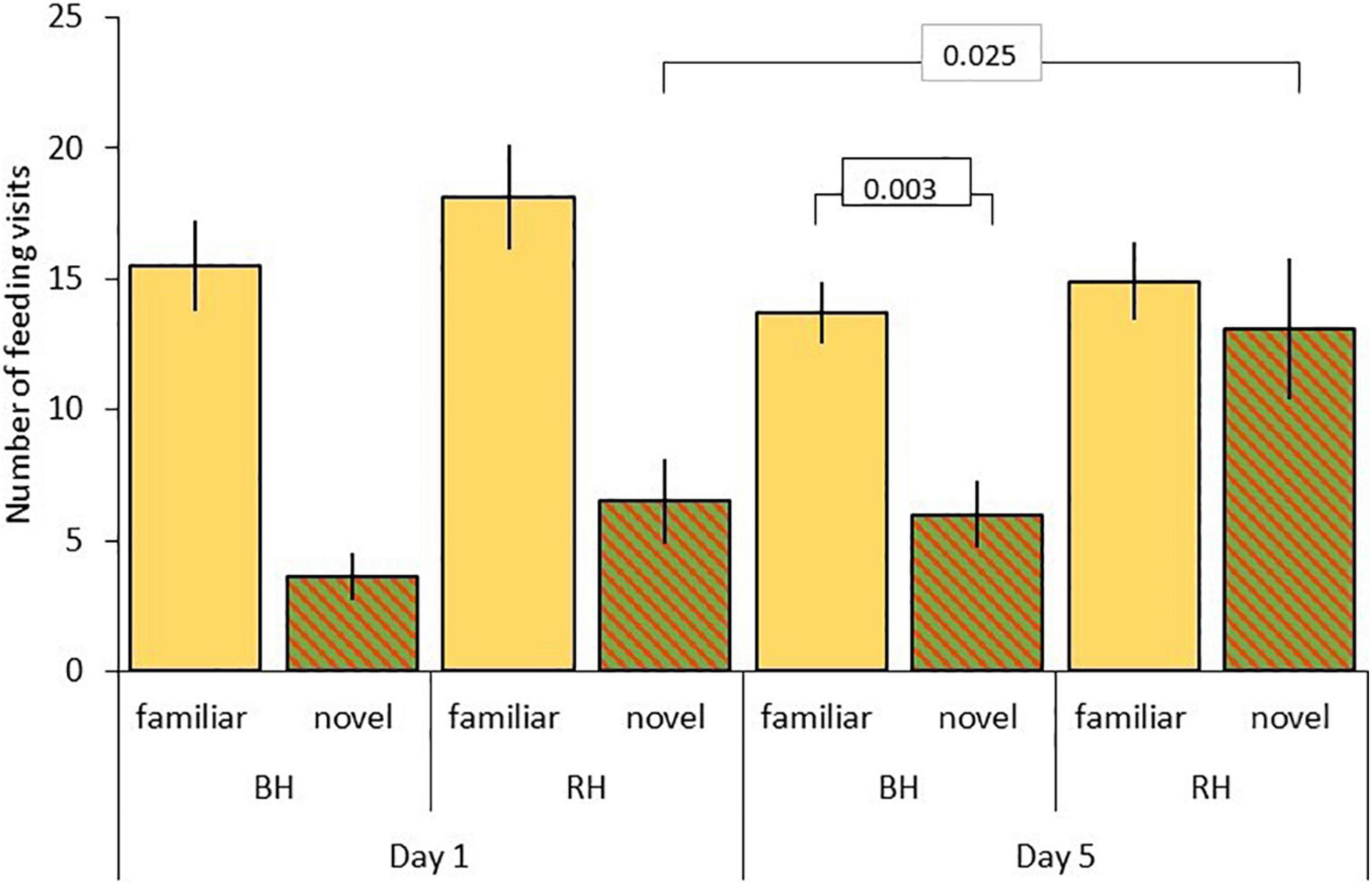
Figure 4. Number of feeding visits after first feed (mean ± SE) to familiar (beige) and novel food (red/green hatched) on day 1 and 5 for red-headed (RH) and black-headed (BH) Gouldian finches. Numbers in the figure represent p-values.
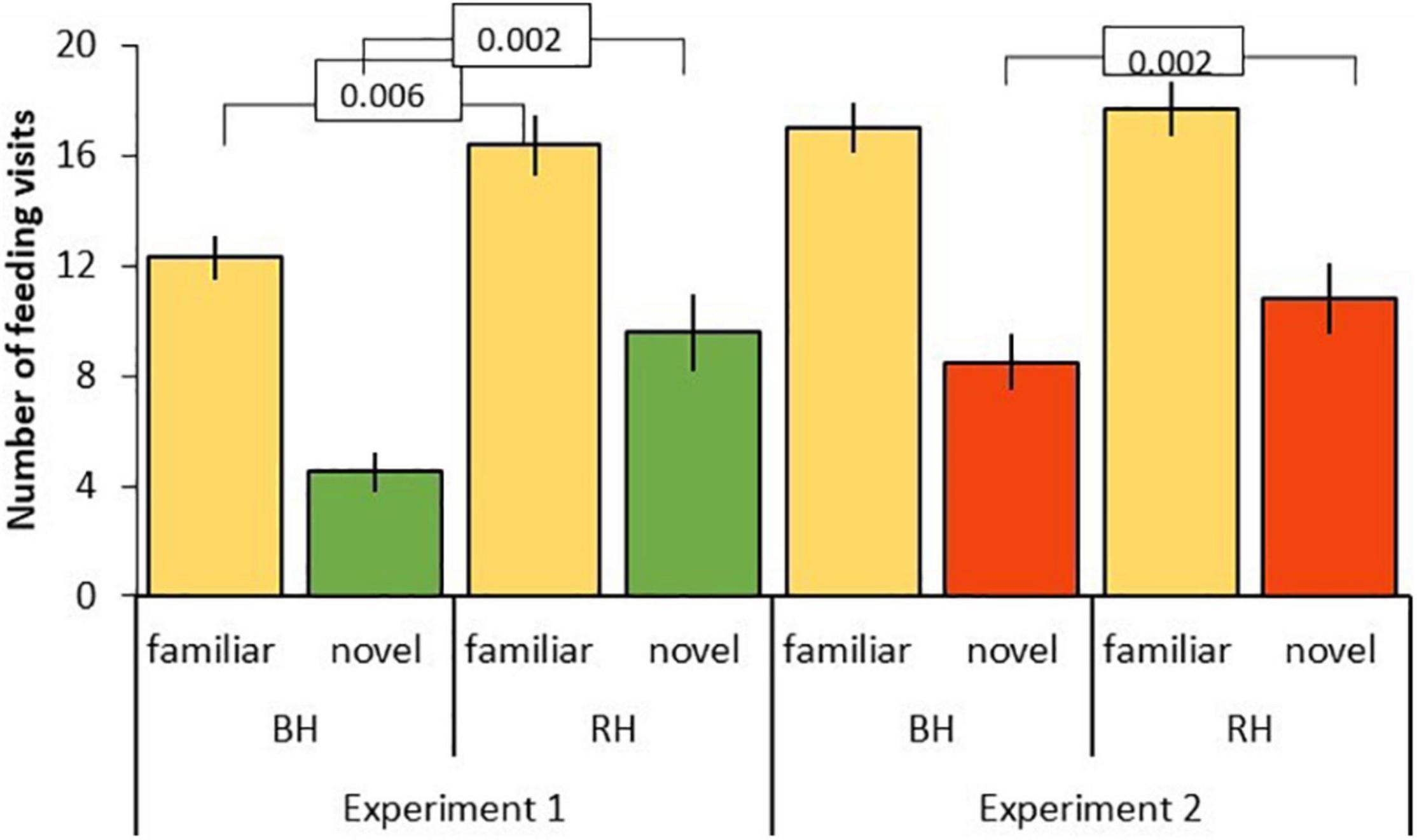
Figure 5. Number of feeding visits after first feed (mean ± SE) to familiar (beige) and novel food (green, red) in experiment 1 and 2 for red-headed (RH) and black-headed (BH) Gouldian finches. Numbers in the figure represent p-values.
The three-way interaction between food type, head color, and experiment was driven by the significantly lower number of feeding visits to familiar food by black-headed birds in experiment 1 as compared to red-headed birds (black: mean 12.3 ± SE 0.8; red: mean 16 ± SE 1.1; df = 153, t = –2.813, P = 0.006). This difference between head colors disappeared in experiment 2 (black: mean 17 ± SE 0.9; red: mean 17.7 ± SE 1.0; df = 153, t = –0.456, P = 0.649). In contrast, feeding visits to novel food by black-headed birds were significantly lower than by red-headed birds in both experiments (exp 1: df = 153, t = –3.200, P = 0.002; exp. 2: df = 168, t = –3.173, P = 0.002; Figure 5).
Furthermore, the main effect of age was significant (LRT = 6.57, df = 1, P = 0.010). One-year old birds generally visited the feeders (irrespective of type) more often (mean 16.34 ± SE 0.67) than older birds (mean 9.78 ± SE 0.47).
Results for model D, testing for social effects, are shown in Table 5B. There was a significant three-way interaction between seed type, head color and partner head color (LRT = 3.84, df = 1, P = 0.050). Red-headed birds made significantly more feeding visits to novel food than black-headed birds when partnered with a red-headed bird (red-headed bird: mean 11.76 ± 1.6; black-headed bird: mean 6.61 = 0.87; df = 1, t = –7.61, P = 0.006) but not when partnered with a black-headed bird (red-headed bird: mean 8.61 ± 1.04; black-headed bird: mean 6.36 ± 0.93; df = 153, t = –1.62, P = 0.107). Partner head color did not affect the number of visits of red-headed or black-headed birds to familiar food (red-headed partner: df = 153, t = –1.57, P = 0.119; black-headed partner: df = 153, t = –1.61, P = 0.110; Figure 6).
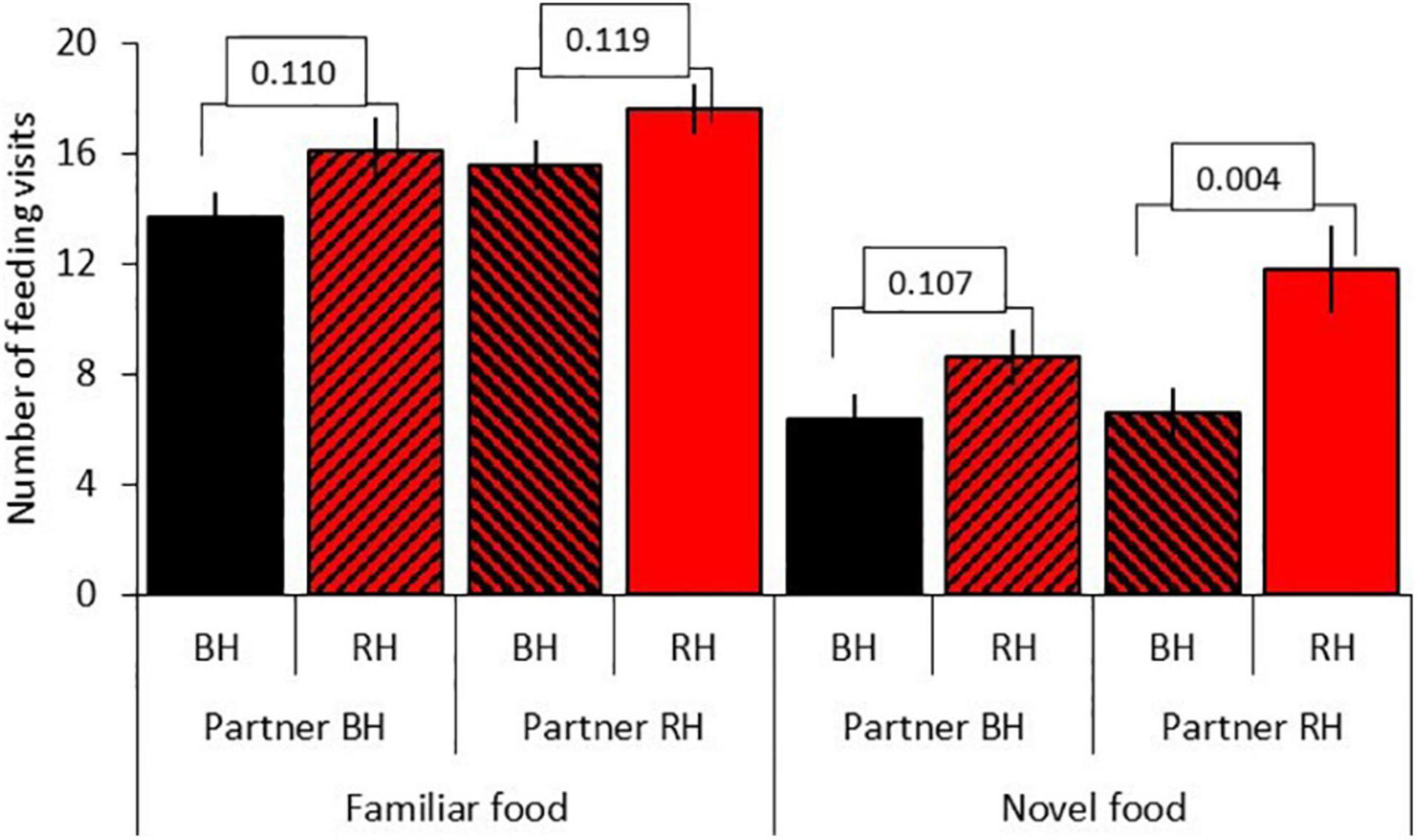
Figure 6. Effect of partner head color on the number of feeding visits after first feed (mean = SE) to familiar and novel food for red-headed (RH) and black-headed (BH) Gouldian finches. Numbers in the figure represent p-values; black bars = black-headed pairs, hatched bars = mixed head color pairs, red bars = red-headed pairs.
Discussion
We investigated whether novelty responses to food and acceptance of novel food into the diet are part of personality traits, whether these traits align with color morph and how group composition affects these responses in the food specialized Gouldian finch. We found that food neophobia and dietary conservatism were differentially expressed and were influenced by head color and group composition. Specifically, we found that birds’ food neophobia was consistent in certain situations, and partially tied to their spatial novelty responses. Meanwhile, they exhibited clear individual consistency and a behavioral syndrome in dietary conservatism. Head color influenced birds’ approach frequencies prior to their first feed and the rate of incorporation of novel food into the diet, further supporting that personalities are linked to head color morphs. Moreover, group composition mattered. The presence of black-headed partners increased the approach frequency before first feed in red-headed but not in black-headed birds. Meanwhile the presence of red-headed partners increased the acceptance of novel food into the diet in other red-headed birds, but not in black-headed birds. On the species level, Gouldian finches were food neophobic and demonstrated dietary conservatism by making more approach attempts to novel food as compared to familiar food before first feed, sampling novel food later than familiar food and continuing to feed on familiar food more often than on novel food. Therefore, we found evidence for species-level dietary wariness.
Food Neophobia
We first predicted that individuals would be consistent in their food neophobic reaction. Individuals’ neophobia reactions showed a weak trend to be consistent for approach frequencies to novel food before first feed and differed consistently in the latency to first feed on familiar food. This confirms our first prediction partly. Approach frequencies before first feed, particularly to novel food, are an indicator of fear as it reflects the conflict between the motivation to approach and sample the novel food and to avoid it (Mettke-Hofmann et al., 2009). Effects of the differently colored food in experiment 1 and 2 and social effects could have masked individual consistency. Birds were tested with partners of different head colors in the two experiments, which affected approach frequencies before first feed in both head colors differently. Testing birds in the same pairing may provide clearer results whether approach frequencies are indeed part of personality traits.
Latency to first feed on familiar food confirmed our prediction 1. This indicates that some individuals consistently approach and consume familiar food fast, whereas others consistently wait longer to consume familiar food when novel food is close by. This hints at a personality trait linked to food neophobia. The reason why we do not find similar consistent latencies to feed on novel food may be that Gouldian finches show conformity in risky and novel situations (King et al., 2015). Sampling novel food involves risk and individuals may align their response with others to reduce risk overwriting individual differences. Moreover, partner effects and the color of the novel food may have masked consistency. Other studies demonstrated consistency in food neophobia between breeding and non-breeding seasons in jackdaws (Corvus monedula; Greggor et al., 2016a). Similarly, Japanese macaques (Macaca fuscata) showed consistent food neophobia reactions (Arnaud et al., 2017).
Approach frequencies toward novel food before first feed differed between head color morphs. Red-headed birds made more approach attempts toward novel food in experiment 1 as compared to experiment 2, whereas this was reversed in black-headed birds. Moreover, red-headed birds tended to have more approach attempts toward novel food than black-headed birds in experiment 1 but not within experiment 2. This indicates that red-headed birds were more drawn to the novel food (food neophilia) but also reluctant to try it out (food neophobia) resulting in more approach attempts in experiment 1 reflecting the conflict between approach and avoidance (Mettke-Hofmann et al., 2009). Black-headed birds, in contrast, had fewer approaches, possibly reflecting less interest in the novel food (neophilia) but also less fear of the novel food (neophobia). This mirrors similar results in a novel spatial context with red-headed birds showing more interest in novel environments than black-headed birds (Mettke-Hofmann et al., 2020). Overall, this contradicts prediction 2, which expected black-headed birds to be more neophobic. As approach attempts to novel food tended to be repeatable, this may be another trait describing differences in personality between head colors. However, more research is needed to fully confirm this.
Despite latency to first feed on familiar food varying consistently among individuals, responses were not linked to head color. Likewise, latencies to first feed on novel food were unrelated to morph. This again contrasts with prediction 2 since individuals do not signal their food neophobia to others or others cannot use head color as a proxy for food neophobia in their peers. This is in line with findings regarding object neophobia in this species, which also showed no relationship to head color (Mettke-Hofmann, 2012; Williams et al., 2012). Signaling personality traits may not always be beneficial (Wolf et al., 2011). While, e.g., signaling aggression in conflict situations reduces agonistic encounters (Pryke, 2009), signaling food neophobia or the lack of it could attract competitors to newly encountered resources in socially foraging birds (Wolf et al., 2011).
There was no indication of a novelty syndrome between approach frequency before first feed and any measures of spatial neophobia. While we did find a positive trend between latency to first feed and latencies to enter unsuitable habitats, these effects were marginal and only slightly support prediction 3. More research is needed into how different novelty reactions are linked with each other.
In mixed head color pairs, black-headed partners induced a higher approach frequency before first feed in red-headed birds, whereas red-headed partners had no effect on black-headed birds. While this confirms that group composition has an effect, as we had predicted (prediction 4), the influence of partner head color did not manifest itself according to any of our predicted scenarios. That red-headed birds become more hesitant to approach food in the presence of black-headed birds is surprising given their higher aggression and ability to displace black-headed birds within competitive scenarios where food is a limited resource (Pryke and Griffith, 2006; Pryke, 2007; Williams et al., 2012). While this result combines approach attempts to both, familiar and novel food, all birds made more approach attempts to novel food (see Figure 2) and differences seem to be primarily down to approach attempts to novel food (see Supplementary Material S3). The following explanation is therefore suggested. The disinterest in novel food by the black-headed partner may have increased fear and cautiousness in red-headed birds. In combination with their interest in the novel food, this increased their approach attempts. Interestingly, black-headed partners had the same effect on red-headed birds in a novel spatial context (Mettke-Hofmann et al., 2020).
On the species level, Gouldian finches showed clear food neophobia by making on average more approach attempts before first feed to novel than familiar food indicating fear of the novel food (Malmkvist et al., 2003; Mettke-Hofmann et al., 2009). They also fed on the familiar food sooner than the novel food. Both findings confirm our fifth prediction (species-level dietary wariness). The results are in line with other studies demonstrating food neophobia in a variety of species (humans, Cooke et al., 2007; Knaapila et al., 2007, non-human primates, Johnson, 2000; Visalberghi and Addessi, 2000; Visalberghi et al., 2003; Gustafsson et al., 2014; Forss et al., 2019, rodents, Hall et al., 1997; Lin et al., 2009; Modlinska et al., 2015, carnivores, Malmkvist et al., 2003 and birds Marples et al., 1998; Kelly and Marples, 2004; Camín et al., 2016). Alternatively, due to the 1-h food deprivation prior to the experiment, birds may have preferred the familiar option over the unfamiliar one to refuel energy reserves before sampling the novel food (Inglis and Ferguson, 1986; Beaulieu and Schaefer, 2014). However, the extended period of avoiding novel food in several individuals in our study indicates a stronger effect of food neophobia than just the desire to refuel energy reserves quickly. In any way, the birds must have perceived the novel food as riskier, otherwise they could have fed from both food types equally fast.
Gouldian finches are specialist granivores, heavily relying upon annual Sorghum species during the breeding season (Brazill-Boast et al., 2011) and on perennial grasses during the wet season (Weier et al., 2016). They have been shown to suffer reduction in body condition and increased stress levels possibly linked to food shortage during the wet season (Maute et al., 2013; Legge et al., 2015), due to intense fire regimes preventing the growth and seeding of perennial grasses (Weier et al., 2016, 2018). Such physiological changes have not been observed in closely related sympatric species that are more diet generalists (Maute et al., 2013). This suggests that Gouldian finches on the species level are reluctant or unable to switch to other seed types. Our study indicates that one factor responsible for this lack of plasticity is food neophobia, since some birds hesitated up to 5 days to sample the novel food. Similar patterns have been found in other food specialized species (Beissinger et al., 1994). High food neophobia may prevent specialized birds from ingesting unsuitable food, which is in line with the Neophobia Threshold hypothesis (Greenberg, 1983). However, future studies should investigate food neophobia in closely related, generalist foragers to support this further. Interestingly, Addessi et al. (2007) found that common marmosets (Callithrix jacchus) who are dietary generalists and occupy small home ranges were less hesitant to sample novel food than Goeldi’s monkeys (Callimico goeldii), who are dietary specialists and utilize a large home range. The marmosets seem to be willing to exploit new resources in their restricted home range, whereas the monkeys were roaming around widely to find familiar food. Gouldian finches are nomadic outside the breeding season tracking suitable food resources (Woinarski and Tidemann, 1992; Dostine et al., 2001) and may follow a similar roaming strategy as the Goeldi’s monkeys.
Incorporation of Novel Food Into the Diet
Individuals showed consistent among individual differences in incorporating novel food into the diet with some individuals being adventurous consumers and others dietary conservatives. Only few other studies have so far shown individual consistency in accepting novel food (Thomas et al., 2010; but see Marples et al., 1998; Prasher et al., 2019). This finding is in support of prediction 1 on top of the consistent differences in food neophobia latencies toward familiar food discussed earlier. Additionally, food acceptance correlated with novelty responses to enter an unfamiliar space (Mettke-Hofmann et al., 2020) in support of a novelty syndrome confirming prediction 3. Individuals that had entered a novel environment quickly, were those that ate less of the familiar food and accepted the novel food by day 5. The combination of a high willingness to enter novel environments and accepting novel food is advantageous as mobile individuals are likely to encounter novel food resources. The novelty syndrome links to the existence of dispersal syndromes. Dispersing individuals in a population are often more spatially explorative, less social and more aggressive than philopatric individuals (Cote et al., 2010; Paulauskaite et al., 2010; Ciani and Capillupi, 2011). They may also be adventurous consumers as it helps individuals to quickly adapt to new resources. The findings are also in support of a resident and nomadic cognitive strategy (Mettke-Hofmann et al., 2020), particularly as red-headed birds turned out to be adventurous consumers and black-headed birds dietary conservatives, which continued to avoid the novel food. In earlier experiments with the same individuals, red-headed birds were more willing to enter unfamiliar environments (Mettke-Hofmann et al., 2020). Therefore, the willingness to accept novel food may be part of the personality syndrome characterizing the two different head colors (Williams et al., 2012) and is in support of prediction 2. Unlike for food neophobia, group members can use head color as a proxy for the willingness to accept novel food. This may help spread acceptance of novel food in a group. Wolf et al. (2011) suggested that signaling traits is beneficial when coordination and collaboration is important. More hesitant individuals could particularly pay attention to more adventurous individuals.
Head color indeed affected the rate of incorporation of novel food into the diet in others, although in the opposite way then laid out above. While black-headed birds’ willingness to incorporate the novel food was unaffected by their partner, red-headed birds became slower in accepting novel food into their diet when paired with a black-headed partner as compared when paired with a red-headed partner. The persistent avoidance of the novel food in black-headed birds likely made the red-headed birds more cautious. This is largely in line with prediction 4 expecting pure red-headed pairings being fastest in accepting novel food and black-headed birds slowing down red-headed ones in mixed pairs. However, in contrast to our prediction, pure black-headed pairings were not slower than mixed pairs. The reason for this result seems to be that black-headed birds do not change their behavior in relation to others. Similar results have been found for object exploration and risk-taking when black-headed birds did not conform when paired with red-headed birds (King et al., 2015). Red-headed birds, in contrast, seem to pay attention to others and become more careful. This is in line with similar effects when entering novel environments; black-headed partners as compared to red-headed partners led to more approach attempts before entering a novel environment in red-headed birds (Mettke-Hofmann et al., 2020). The presence of black-headed birds seemed to act as a cautionary note to red-headed birds, rather them being a motivator for black-headed birds to accept novel food. More research is needed to understand this relationship and the consequences on the species level further.
On the species level, Gouldian finches were not only food neophobic but also hesitated to incorporate novel food into their diet, demonstrating dietary conservatism confirming the second part of prediction 5. This foraging response can be attributed to their food specialism (Brazill-Boast et al., 2011) and does not seem to be the response to interspecific competition (Jessopp et al., 2020). Similar dietary conservatism has been found in the specialized snail kite (Beissinger et al., 1994).
Dietary Wariness in a Changing World
As a dietary specialist with strong dietary wariness Gouldian finches may struggle in a changing world. This is evidenced already in earlier work as Gouldian finches experience reduced body condition and increased stress levels during times of food shortage in the non-breeding season (Maute et al., 2013; Legge et al., 2015). However, we currently do not know whether head colors are differently affected. From our results we would predict that black-headed birds suffer more than red-headed birds. An experimental study manipulating protein content before and during breeding in captive Gouldian finches showed that red-headed birds need a higher protein-diet than black-headed birds to maintain body condition and raise their young successfully (Pryke et al., 2012). Being more open to accept novel food would help red-headed birds to gain access to a high-protein diet during breeding. This may carry over into the non-breeding season and allow red-headed birds to utilize unfamiliar food during times of food shortage.
While the species as a whole is dietary wary, not all Gouldian finches are dietary conservative and consistent among individual variation in their willingness to accept novel food may help to adapt to a changing world. Indeed, Forsman et al. (2008) suggested that polymorphism in traits is advantageous when encountering environmental change as individuals use different resources and strategies. Groups of mixed personalities have been shown to approach novel feeding situations faster and have improved patch exploitation and group cohesion (Dyer et al., 2009; Aplin et al., 2014). While mixed groups in the current study were not the fastest in incorporating novel food, they may maximize use of existing and newly occurring food with black-headed birds preferring the familiar food and red-headed birds diversifying by incorporating novel food. The red-headed birds’ responsiveness to other group members may slow them down enough that black-headed birds can eventually catch up and start exploiting this new food source. Future studies should determine when, if at all, black-headed birds incorporate novel food into their diet and whether mixed groups speed up this process.
In the wild, black-headed birds make up 70% in most populations (Kim et al., 2019). This means that dietary conservatives are by far outnumbering adventurous consumers. However, a few individuals can affect group decisions, e.g., whether and where to move (Couzin et al., 2005). Potentially, a small number of adventurous consumers is enough to slowly spread the use of new food types. More research is needed to better understand how group composition affects individual preferences.
Overall, the results indicate that food neophobia and in particular dietary conservatism contribute to maintaining a specialist’s diet. Moreover, they show that even in a food specialized species consistent among individual variation exists in response to novel food with some individuals being adventurous consumers. This individual variation may help adapting to new resources. In our specific case, individual differences in accepting novel food into the diet were linked to color morph, adding to the increasing evidence that color morphs respond differently to environmental challenges (Mateos-Gonzalez and Senar, 2012; Schweitzer et al., 2015; Mettke-Hofmann et al., 2020). Both, individual and morph-specific differences in response to environmental challenges have rarely been considered in conservation-oriented studies (but see Kelleher et al., 2018). However, they may be an important component of a species’ survival chances.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by LJMU Ethics Committee.
Author Contributions
GE conducted all experiments, transcribed all data, did initial analyses, and co-wrote the sections “Abstract, Introduction, Materials and Methods, and Discussion.” EB analyzed the data for the manuscript, co-wrote the “Materials and Methods” section and wrote the “Results” section. AG contributed to the experimental design and initial analyses and gave important feedback on the manuscript. CM-H came up with the design, advised on data collection and analyses, and co-wrote the sections “Abstract, Introduction, and Discussion.” All the authors contributed to the manuscript revision, read and approved the submitted version.
Funding
GE was self-funded.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Blattner Heimtierfutter for sponsoring the bird food, Peter McGough and other bird breeders for providing some of the birds, the animal facility technicians for assistance and support throughout data collection and Gerhard Hofmann for his valuable assistance with the experimental design. EB would like to thank Julia Schroeder for initial advice on repeatability testing. We would also like to thank Nicola Marples, Hazel Nichols, and Richard Brown for their comments on this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.772812/full#supplementary-material
Supplementary Material S1 | Food Neophobia raw dataset.
Supplementary Material S2 | Dietary Conservatism raw dataset.
Supplementary Material S3 | Approach frequencies (mean ± SE) before first feed to familiar and novel food considering effects of partner head colors on black-headed (BH) and red-headed (RH) birds. Black bars: pure black-headed pairs; hatched red/black bars: mixed head color pairs; red bars: pure red-headed pairs.
References
Addessi, E., Chiarotti, F., Visalberghi, E., and Anzenberger, G. (2007). Response to novel food and the role of social influences in common marmosets (Callithrix jacchus) and Goeldi’s monkeys (Callimico goeldii). Am. J. Primatol. 69, 1210–1222. doi: 10.1002/ajp.20429
Addessi, E., and Visalberghi, E. (2006). “How social influences affect food neophobia in captive chimpanzees: a comparative approach,” in Cognitive Development in Chimpanzees, eds T. Matsuzawa, M. Tomonaga, and M. Tanaka (Tokyo: Springer), 246–264.
Amici, F., Widdig, A., Karimullah, K., Maulany, R. I., Ngakan, P. O., Hamzah, A. S., et al. (2020). Dominance style only partially predicts differences in neophobia and social tolerance over food in four macaque species. Sci. Rep. 10:22069. doi: 10.1038/s41598-020-79246-6
Animal Behaviour (2018). Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 135, 1–10. doi: 10.1016/j.anbehav.2017.10.001
Aplin, L. M., Farine, D. R., Mann, R. P., and Sheldon, B. C. (2014). Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B 281:20141016. doi: 10.1098/rspb.2014.1016
Arnaud, C. M., Suzumura, T., Inoue, E., Adams, M. J., Weiss, A., and Inoue-Murayama, M. (2017). Genes, social transmission, but not maternal effects influence responses of wild Japanese macaques (Macaca fuscata) to novel-object and novel-food tests. Primates 58, 103–113. doi: 10.1007/s10329-016-0572-9
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effect models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.1186/s13071-020-04134-x
Bates, D., Maechler, M., Bolker, B. M., Walker, S. C., Christensen, R. H. B., Singmann, H., et al. (2019). Package ‘lme4’: Linear Mixed-Effects Models using ‘Eigen’ and S4. Available online at: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed March 05, 2019).
Beaulieu, M., and Schaefer, H. M. (2014). The proper time for antioxidant consumption. Physiol. Behav. 128, 54–59.
Beissinger, S. R., Donnay, T. J., and Walton, R. (1994). Experimental analysis of diet specialization in the snail kite: the role of behavioral conservatism. Oecologia 100, 54–65. doi: 10.1007/BF00317130
Boisvert-Marsh, L., Périé, C., and de Blois, S. (2014). Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 5, 1–33.
Boliver, V. J., and Flaherty, L. (2004). Genetic control of novel food preference in mice. Mammal. Genome 15, 193–198.
Boulton, K., Grimmer, A. J., Rosenthal, G. G., Walling, C. A., and Wilson, A. J. (2014). How stable are personalities? A multivariate view of behavioural variation over long and short timescales in the sheepshead swordtail, Xiphophorus birchmanni. Behav. Ecol. Sociobiol. 68, 791–803. doi: 10.1007/s00265-014-1692-0
Brazill-Boast, J., Dessmann, J. K., Davies, G. T. O., Pryke, S. R., and Griffith, S. C. (2011). Selection of breeding habitat by the endangered Gouldian finch (Erythrura gouldiae) at two spatial scales. Emu 111, 304–311. doi: 10.1071/mu10064
Brush, A. H., and Seifried, H. (1968). Pigmentation and feather structure in genetic variants of the Gouldian finch, Poephila gouldiae. Auk 85, 416–430. doi: 10.2307/4083290
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd Edn. New York, NY: Springer.
Camín, S. R., Martín-Albarracin, V., Jefferies, M., and Marone, L. (2016). Do neophobia and dietary wariness explain ecological flexibility. An analysis with two seed-eating birds of contrasting habits. Avian Biol. 47, 245–251. doi: 10.1111/jav.00697
Chandler, C. R. (1995). Practical considerations in the use of simultaneous inference for multiple tests. Anim. Behav. 49, 524–527. doi: 10.1006/anbe.1995.0069
Chiarati, E., Canestrari, D., Vera, R., and Baglione, V. (2012). Subordinates benefit from exploratory dominants: response to novel food in cooperatively breeding carrion crows. Anim. Behav. 83, 103–109. doi: 10.1016/j.anbehav.2011.10.012
Ciani, A. C., and Capillupi, C. (2011). Gene flow by selective emigration as a possible cause for personality differences between small islands and mainland populations. Eur. J. Pers. 25, 53–64.
Cohen, B., Askin, S., Balkcom, G., Benedict, R., Rader, J., James, J., et al. (2019). Survival and distribution of Black-bellied Whistling-duck (Dendrocygna autumnalis) in the Southeastern United States. J. Southeastern Assoc. Fish. Wildl. 6, 123–128.
Cohen, T. M., Kumar, R. S., Nair, M., Hauber, M. E., and Dor, R. (2020). Innovation and decreased neophobia drive invasion success in a widespread avian invader. Anim. Behav. 163, 61–72. doi: 10.1016/j.anbehav.2020.02.012
Coleman, K., and Wilson, D. S. (1998). Shyness and boldness in pumpkinseed sunfish: individualdifferences are context-specific. Anim. Behav. 56, 927–936. doi: 10.1006/anbe.1998.0852
Cooke, L. J., Haworth, C. M. A., and Wardle, J. (2007). Genetic and environmental influences on children’s food neophobia. Am. J. Clin. Nutr. 86, 428–433.
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos. Trans. R. Soc. Lond. B 365, 4065–4076. doi: 10.1098/rstb.2010.0176
Couzin, I. D., Krause, J., Franks, N. R., and Levin, S. A. (2005). Effective leadership and decision makingin animal groups on the move. Nature 433, 513–516.
Dally, J. M., Clayton, N. S., and Emery, N. J. (2008). Social influences on foraging in rooks (Corvus frugilegus). Behaviour 145, 1101–1124.
Delarue, E. M. P., Kerr, S. E., and Rymer, T. L. (2015). Habitat complexity, environmental change and personality: a tropical perspective. Behav. Processes 120, 101–110. doi: 10.1016/j.beproc.2015.09.006
Diaz, M. (1996). Food choice by seed-eating birds in relation to seed chemistry. Comp. Biochem. Physiol. 113A, 239–246. doi: 10.1016/0300-9629(95)02093-4
Dingemanse, N. J., Both, C., Drent, P. J., and Tinbergen, J. M. (2004). Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. Lond. B 271, 847–852. doi: 10.1098/rspb.2004.2680
Dingemanse, N. J., Both, C., van Noordwijk, A. J., Rutten, A. L., and Drent, P. J. (2003). Natal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747. doi: 10.1098/rspb.2002.2300
Doktorovová, L., Exnerová, A., Hotová Svádová, K., Štys, P., Zverev, V., Kozlov, M. V., et al. (2019). Differential bird responses to colour morphs of an aposematic leaf beetle may affect variation in morph frequencies in polymorphic prey populations. Evol. Biol. 46, 34–46.
Dostine, P. L., and Franklin, D. C. (2002). A comparison of the diet of three finch species in the Yinberrie Hills area, Northern Territory. Emu 102, 159–164. doi: 10.1071/mu01034
Dostine, P. L., Johnson, G. C., Franklin, D. C., Zhang, Y., and Hempel, C. (2001). Seasonal use of savanna landscapes by the Gouldian finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern territory. Wildl. Res. 28, 445–458.
Dyer, J. R. G., Croft, D. P., Morrell, L. J., and Krause, J. (2009). Shoal composition determines foraging success in the guppy. Behav. Ecol. 20, 165–171. doi: 10.1093/beheco/arn129
Eccles, G. R. (2018). Phobic Finches: Investigating Neophobia Reactions in a Colour Polymorphic Specialist, The Gouldian Finch, Erythrura gouldiae. Ph.D. thesis. Liverpool: Liverpool John Moores University.
Edwards, H. A., Dugdale, H. L., Richardson, D. S., Komdeur, J., and Burke, T. (2016). Exploration is dependent on reproductive state, not social state, in a cooperatively breeding bird. Behav. Ecol. 27, 1889–1896.
EPBC (2018). Species Profile and Threats Data Base: Erythrura gouldiae, Gouldian finch. Available online at: http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=413 (accessed May 15, 2019).
Exnerova, A., Svadova, K. H., Fucikova, E., Drent, P., and Stys, P. (2010). Personality matters: individual variation in reactions of naïve bird predators to aposematic prey. Proc. R. Soc. Lond. B 277, 723–728. doi: 10.1098/rspb.2009.1673
Forsman, A., Ahnesjoe, J., Caesar, S., and Karlsson, M. (2008). A model of ecological and evolutionary consequences of color polymorphism. Ecology 89, 34–40. doi: 10.1890/07-0572.1
Forss, S. I. F., Motes-Rodrigo, A., Hrubesch, C., and Tennie, C. (2019). Differences in novel food responses between Pongo and Pan. Am. J. Primatol. 81:e22945.
Greenberg, R. (1983). The role of neophobia in determining the degree of foraging-specialisation in some migrant warblers. Am. Nat. 122, 444–453. doi: 10.1086/284148
Greenberg, R. (1984). Differences in feeding neophobia in the tropical migrant wood warblers Dendroica castanea and D. pensylvanica. J. Comp. Psychol. 98, 131–136.
Greenberg, R. (2003). “The role of neophobia and neophilia in the development of innovative behaviour of birds,” in Animal Innovation, eds S. M. Reader, and K. N. Laland (Oxford: Oxford University Press), 175–196. doi: 10.1093/acprof:oso/9780198526223.003.0008
Greggor, A. L., McIvor, G. E., Clayton, N., and Thornton, A. (2016b). Contagious risk taking: social information and context influence wild jackdaws’ responses to novelty and risk. Sci. Rep. 6:27764. doi: 10.1038/srep27764
Greggor, A. L., Jolles, J. W., Thornton, A., and Clayton, N. S. (2016a). Seasonal changes in neophobia and its consistency in rooks: the effect of novelty type and dominance. Anim. Behav. 121, 11–20. doi: 10.1016/j.anbehav.2016.08.010
Gustafsson, E., Jalme, M. S., Bomsel, M. C., and Kreif, S. (2014). Food neophobia and social learning opportunities in great apes. Int. J. Primatol. 35, 1037–1071. doi: 10.1007/s10764-014-9796-y
Hall, F. S., Humby, T., Wilkinson, L. S., and Robbins, T. W. (1997). The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol. Behav. 62, 281–290. doi: 10.1016/s0031-9384(97)00115-7
Home Office (2013). Guidance on the Operation of the UK Legislation on Animals Used in Research and Codes of Practice. Available online at: https://www.gov.uk/guidance/research-and-testing-using-animals (accessed June 06, 2019)
Inglis, I. R., and Ferguson, N. J. (1986). Starlings search for food rather than eat freely-available, identical food. Anim. Behav. 34, 614–617.
Jessopp, M., Arneill, G. E., Nykänen, M., Bennison, A., and Rogan, E. (2020). Central place foraging drives niche partitioning in seabirds. Oikos 129, 1704–1713.
Johnson, E. (2000). Food-neophobia in semi-free ranging rhesus macaques: effects of food limitation and food source. Am. J. Primatol. 50, 25–35. doi: 10.1002/(SICI)1098-2345(200001)50:1<25::AID-AJP3>3.0.CO;2-D
Jones, R. B. (1986). Responses of domestic chicks to novel food as a function of sex, strain and previous experience. Behav. Processes 12, 261–271. doi: 10.1016/0376-6357(86)90040-9
Kelleher, S. R., Silla, A. J., and Byrne, P. G. (2018). Animal personality and behavioral syndromes in amphibians: a review of the evidence, experimental approaches, and implications for conservation. Behav. Ecol. Sociobiol. 72:79. doi: 10.1007/s00265-018-2493-7
Kelly, D. J., and Marples, N. M. (2004). The effects of novel odour and colour cues on food acceptance by the zebra finch, Taeniopygia guttata. Anim. Behav. 68, 1049–1054.
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8:e1000412. doi: 10.1371/journal.pbio.1000412
Kim, K. W., Jackson, B. C., Zhang, H., Toews, D. P. L., Taylor, S. A., Greig, E. I., et al. (2019). Genetics and evidence for balancing selection of a sex-linked colour polymorphism in a songbird. Nat. Commun. 10:1852. doi: 10.1038/s41467-019-09806-6
King, A. J., Williams, L. J., and Mettke-Hofmann, C. (2015). The effects of social conformity on Gouldian finch personality. Anim. Behav. 99, 25–31. doi: 10.1016/j.anbehav.2014.10.016
Knaapila, A., Tuorila, H., Silventoinen, K., Keskitalo, K., Kallela, M., Wessman, M., et al. (2007). Food neophobia shows heritable variation in humans. Physiol. Behav. 91, 573–578.
Legge, S., Garnett, S., Maute, K., Heathcote, J., Murphy, S., Woinarski, J. C. Z., et al. (2015). A landscape-scale, applied fire management experiment promotes recovery of a population of the threatened Gouldian finch, Erythrura goudiae, in Australia’s Tropical Savannas. PLoS One 10:e0137997. doi: 10.1371/journal.pone.0137997
Liebl, A. L., and Martin, L. B. (2014). Living on the edge: range edge birds consume novel foods sooner than established ones. Behav. Ecol. 25, 1089–1096.
Lin, J. Y., Roman, C., St.Andre, J., and Reilly, S. (2009). Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 1251, 195–203. doi: 10.1016/j.brainres.2008.11.040
Mafli, A., Wakamatsu, K., and Roulin, A. (2011). Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Anim. Behav. 81, 859–863. doi: 10.1016/j.anbehav.2011.01.025
Malmkvist, J., Herskin, M. S., and Christensen, J. W. (2003). Behavioural responses of farm mink towards familiar and novel food. Behav. Processes 61, 123–130. doi: 10.1016/s0376-6357(02)00167-5
Marples, N. M., and Brakefield, P. M. (1995). Genetic variation for the rate of recruitment of novel insect prey into the diet of a bird. Biol. J. Linn. Soc. 55, 17–27. doi: 10.1016/0024-4066(95)90026-8
Marples, N. M., and Kelly, D. J. (1999). Neophobia and dietary conservatism: two distinct processes? Evol. Ecol. 13, 641–653. doi: 10.1023/a:1011077731153
Marples, N. M., and Mappes, J. (2011). Can the dietary conservatism of predators compensate for positive frequency dependent selection against rare, conspicuous prey? Evol. Ecol. 25, 737–749.
Marples, N. M., Quinlan, M., Thomas, R. J., and Kelly, D. J. (2007). Deactivation of dietary wariness through exposure of novel food. Behav. Ecol. 18, 803–810.
Marples, N. M., Roper, T. J., and Harper, D. G. C. (1998). Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83, 161–165.
Martin, L. B., and Fitzgerald, S. (2005). A taste for novelty in invading house sparrows, Passer domesticus. Behav. Ecol. 16, 702–707.
Mateos-Gonzalez, F., and Senar, J. C. (2012). Melanin-based trait predicts individual exploratory behaviour in siskins, Carduelis spinus. Anim. Behav. 83, 229–232. doi: 10.1016/j.anbehav.2011.10.030
Maute, K. L., French, K., Legge, S., and Astheimer, L. (2013). Seasonal stress physiology and body condition differ among co-occurring tropical finch species. Comp. Physiol. B 183, 1023–1037. doi: 10.1007/s00360-013-0775-y
McKinney, M. L. (1997). Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516. doi: 10.1146/annurev.ecolsys.28.1.495
McMahon, K., Conboy, A., O’Byrne-White, E., Thomas, R. J., and Marples, N. (2014). Dietary wariness influences the response of foraging birds to competitors. Anim. Behav. 89, 63–69.
Mettke-Hofmann, C. (2012). Head colour and age relate to personality traits in Gouldian finches. Ethology 118, 906–916. doi: 10.1111/j.1439-0310.2012.02079.x
Mettke-Hofmann, C., Eccles, G. R., Greggor, A. L., and Bethell, E. J. (2020). Cognition in a changing world: red-headed Gouldian finches enter spatially unfamiliar habitats more readily than do black-headed birds. Front. Ecol. Evol. 8:498347. doi: 10.3389/fevo.2020.498347
Mettke-Hofmann, C., Lorentzen, S., Schlicht, E., Schneider, J., and Werner, F. (2009). Spatial neophilia and spatial neophobia in resident and migratory warblers (Sylvia). Ethology 115, 482–492. doi: 10.1111/j.1439-0310.2009.01632.x
Mettke-Hofmann, C., Wink, M., Braun, M., and Winkler, H. (2012). Residency and a broad feeding spectrum are related to extensive spatial exploration in parrots. Behav. Ecol. 23, 1365–1371.
Mettke-Hofmann, C., Wink, M., Winkler, H., and Leisler, B. (2005). Exploration of environmental changes relates to lifestyle. Behav. Ecol. 16, 247–254. doi: 10.1093/beheco/arh159
Mettke-Hofmann, C., Winkler, H., Hamel, P. B., and Greenberg, R. (2013). Migratory New World blackbirds (Icterids) are more neophobic than closely related resident Icterids. PLoS One 8:e57565. doi: 10.1371/journal.pone.0057565
Modlinska, K., Stryjek, R., and Pisula, W. (2015). Food neophobia in wild and laboratory rats (multi-strain comparison). Behav. Processes 113, 41–50. doi: 10.1016/j.beproc.2014.12.005
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. doi: 10.1111/j.1469-185X.2007.00027.x
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. doi: 10.1111/j.1469-185X.2010.00141.x
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed−effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1093/sysbio/syy060
Oostindjer, M., Munoz, J. M., van den Brand, H., Kemp, B., and Bolhuis, J. E. (2011). Maternal presence and environmental enrichment affect food neophobia of piglets. Biol. Lett. 7, 19–22. doi: 10.1098/rsbl.2010.0430
Pacifici, M., Visconti, P., Butchart, S. H., Watson, J. E., Cassola, F. M., and Rondinini, C. (2017). Species’ traits influenced their response to recent climate change. Nat. Clim. Chang. 7, 205–208.
Paijmans, K. C., Booth, D. J., and Wong, M. Y. L. (2020). Predation avoidance and foraging efficiency contribute to mixed-species shoaling by tropical and temperate fishes. J. Fish Biol. 96, 806–814. doi: 10.1111/jfb.14277
Paulauskaite, E., Seibokaite, L., and Endriulaitiene, A. (2010). Big five personality traits linked with migratory intentions in Lithuanian student sample. Int. J. Psychol. 7, 41–58.
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915.
Prasher, S., Thompson, M. J., Evans, J. C., El-Nachef, M., Bonier, F., and Morand-Ferron, J. (2019). Innovative consumers: ecological, behavioral, and physiological predictors of responses to novel food. Behav. Ecol. 30, 1216–1225.
Pryke, S. R. (2007). Fiery red heads: female dominance among head colour morphs in the Gouldian finch. Behav. Ecol. 18, 621–627.
Pryke, S. R. (2009). Is red an innate or learned signal of aggression and intimidation? Anim. Behav. 78, 393–398. doi: 10.1016/j.anbehav.2009.05.013
Pryke, S. R., Astheimer, L. B., Griffith, S. C., and Buttemer, W. A. (2012). Covariation in life-history traits: differential effects of diet on condition, hormones, behavior, and reproduction in genetic finch morphs. Am. Nat. 179, 375–390. doi: 10.1086/664078
Pryke, S. R., and Griffith, S. C. (2006). Red dominates black: agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proc. R. Soc. B 273, 949–957. doi: 10.1098/rspb.2005.3362
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution 43, 223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x
Schweitzer, C., Motreuil, S., and Dechaume-Moncharmont, F.-X. (2015). Coloration reflects behavioural types in the convict cichlid, Amatitlania siquia. Anim. Behav. 105, 201–209.
Sih, A., Mathot, K. J., Moiron, M., Montiglio, P.-O., Wolf, M., and Dingemanse, N. J. (2015). Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. doi: 10.1016/j.tree.2014.11.004
Steel, E. A., Kennedy, M. C., Cunningham, P. G., and Stanovick, J. S. (2013). Applied statistics in ecology: common pitfalls and simple solutions. Ecosphere 4:115.
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210x.12797
Thomas, R. J., Bartlett, L. A., Marples, N. M., Kelly, D. J., and Cuthill, I. C. (2004). Prey selection by wild birds can allow novel and conspicuous colour morphs to spread in prey populations. Oikos 106, 285–294. doi: 10.1111/j.0030-1299.2004.13089.x
Thomas, R. J., King, T. A., Forshaw, H. E., Marples, N. M., Speed, M. P., and Cable, J. (2010). The response of fish to novel prey: evidence that dietary conservatism is not restricted to birds. Behav. Ecol. 21, 669–675. doi: 10.1093/beheco/arq037
Thomas, R. J., Marples, N. M., Cuthill, I. C., Takahashi, M., and Gibson, E. A. (2003). Dietary conservatism may facilitate the initial evolution of aposematism. Oikos 101, 458–466.
Toscano, B. J., Gownaris, N. J., Heerhartz, S. M., and Monaco, C. J. (2016). Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia 182, 55–69. doi: 10.1007/s00442-016-3648-8
Turro-Vincent, I., Launay, F., Mills, A. D., Picard, M., and Faure, J. M. (1995). Experiential and genetic influences on learnt food aversions in Japanese quail selected for high or low levels of fearfulness. Behav. Processes 34, 23–41. doi: 10.1016/0376-6357(94)00045-i
Ueno, A., and Matsuzawa, T. (2005). Response to novel food in infant chimpanzees: do infants refer to mothers before ingesting food on their own? Behav. Processes 68, 85–90. doi: 10.1016/j.beproc.2004.09.002
Visalberghi, E., and Addessi, E. (2000). Seeing group members eating a familiar food enhances acceptance of novel food in capuchin monkeys. Anim. Behav. 60, 69–76. doi: 10.1006/anbe.2000.1425
Visalberghi, E., Janson, C. H., and Agostini, I. (2003). Response toward novel foods and novel objects in wild Cebus apella. Int. J. Primatol. 24, 653–675.
Weier, A., Radford, I. J., Manson, A., Durrans, L. J., and Lawes, M. J. (2017). Frequent fires reduce the nutritional quality of Sorghum stipoideum seed, a keystone food resource for the Gouldian finch (Erythrura gouldiae). Rangel. J. 39, 105–112.
Weier, A., Radford, I. J., Oliveira, S. L. J., and Lawes, M. J. (2016). Recently but infrequently burnt breeding sites are favoured by threatened Gouldian finches (Erythrura gouldiae). Int. J. Wildland Fire 25, 1281–1290.
Weier, A., Radford, I. J., Woolley, L. A., and Lawes, M. J. (2018). Fire regime effects on annual grass seeds as food for threatened grass-finch. Fire Ecol. 14, 1–11.
White, J. R., Meekan, M. G., McCormick, M. I., and Ferrari, M. C. O. (2013). A comparison of measures of boldness and their relationships to survival in young fish. PLoS One 8:e68900. doi: 10.1371/journal.pone.0068900
Williams, L. J., King, A. J., and Mettke-Hofmann, C. (2012). Colourful characters: head colour reflects personality in a social bird, the Gouldian finch, Erythrura gouldiae. Anim. Behav. 84, 159–165.
Woinarski, J. C. Z., and Tidemann, S. (1992). Survivorship and some population parameters for the endangered Gouldian Finch Erythrura gouldiae and two other finch species at two sites in tropical northern Australia. Emu 92, 33–38.
Keywords: food neophobia, dietary conservatism, Erythrura gouldiae, color polymorphism, novelty syndrome, specialist, conservation
Citation: Eccles GR, Bethell EJ, Greggor AL and Mettke-Hofmann C (2021) Individual Variation in Dietary Wariness Is Predicted by Head Color in a Specialist Feeder, the Gouldian Finch. Front. Ecol. Evol. 9:772812. doi: 10.3389/fevo.2021.772812
Received: 08 September 2021; Accepted: 20 October 2021;
Published: 11 November 2021.
Edited by:
Edward Narayan, The University of Queensland, AustraliaReviewed by:
Michaël Beaulieu, German Oceanographic Museum, GermanyYang Wang, Hebei Normal University, China
Copyright © 2021 Eccles, Bethell, Greggor and Mettke-Hofmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgina R. Eccles, TWlzc2dyZWNjbGVzQGdtYWlsLmNvbQ==; Claudia Mettke-Hofmann, Qy5DLk1ldHRrZS1Ib2ZtYW5uQGxqbXUuYWMudWs=
 Georgina R. Eccles
Georgina R. Eccles Emily J. Bethell
Emily J. Bethell Alison L. Greggor
Alison L. Greggor Claudia Mettke-Hofmann
Claudia Mettke-Hofmann