
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Ecol. Evol. , 04 October 2021
Sec. Urban Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.765950
This article is part of the Research Topic Effects of Artificial Light at Night on Organisms: From Mechanisms to Function View all 13 articles
Parts of this article's content have been modified or rectified in:
Erratum: A systematic review of research investigating the combined ecological impact of anthropogenic noise and artificial light at night
Levels of anthropogenic noise and artificial light at night (ALAN) are rapidly rising on a global scale. Both sensory pollutants are well known to affect animal behavior and physiology, which can lead to substantial ecological impacts. Most studies on noise or light pollution to date have focused on single stressor impacts, studying both pollutants in isolation despite their high spatial and temporal co-occurrence. However, few studies have addressed their combined impact, known as multisensory pollution, with the specific aim to assess whether the interaction between noise and light pollution leads to predictable, additive effects, or less predictable, synergistic or antagonistic effects. We carried out a systematic review of research investigating multisensory pollution and found 28 studies that simultaneously assessed the impact of anthropogenic noise and ALAN on animal function (e.g., behavior, morphology or life-history), physiology (e.g., stress, oxidative, or immune status), or population demography (e.g., abundance or species richness). Only fifteen of these studies specifically tested for possible interactive effects when both sensory pollutants were combined. Four out of eight experimental studies revealed a significant interaction effect, in contrast to only three out seven observational studies. We discuss the benefits and limitations of experimental vs. observational studies addressing multisensory pollution and call for more specific testing of the diverse ways in which noise and light pollution can interact to affect wildlife.
The natural world is under threat due to a multitude of anthropogenic disturbances, including habitat destruction, climate change, pollution, and urbanization (Vitousek et al., 1997). Many of these human-induced environmental stressors covary in space and time making their combined impact difficult to predict (Crain et al., 2008; Piggott et al., 2015; Orr et al., 2020; Tekin et al., 2020). Artificial light at night (ALAN) and anthropogenic noise are two environmental stressors associated with urbanization, transport and industry, and are well known to influence biological processes ranging from individual physiology, reproduction and survival, to large scale processes occurring across whole ecosystems (Halfwerk and Slabbekoorn, 2015; Swaddle et al., 2015; Dominoni D.M. et al., 2020; Svechkina et al., 2020; Jerem and Mathews, 2021). High levels of anthropogenic noise and ALAN often co-occur at the same location and at the same time of day, depending on the latitude and time of year (Halfwerk and Slabbekoorn, 2015; Buxton et al., 2020; Dominoni D.M. et al., 2020). Despite this frequent covariance, the majority of studies have addressed their biological impact in isolation, ignoring potential interactive mechanisms (Piggott et al., 2015; Orr et al., 2020). Most reports therefore over- or underestimate effect sizes when these sensory pollutants are combined (Dominoni D. et al., 2020).
Our understanding of the combined impact of anthropogenic noise and ALAN can benefit from concepts and theory applied to situations involving other multiple stressors (Crain et al., 2008; Darling and Côté, 2008; Orr et al., 2020). Multi-stressor research has a long history in various fields of biology, ranging from eco-toxicological lab studies to global conservation modeling, and from aquatic to terrestrial study systems (Orr et al., 2020). In general, most of these studies acknowledge combined exposure to multiple stimuli or stressors can have functional, physiological or demographic impacts that cannot be understood when studying these stressors in isolation. The different research fields share little overlap in theory or nomenclature, however, which is why we adhere to the terms and definitions outlined by Piggott et al. (2015) and Orr et al. (2020) to study multi-stressor impacts. According to Orr et al. (2020), the simplest (and easiest to predict) effect of multisensory pollution is an additive effect, which occurs when the combined impact of noise and light pollution is similar to the sum of their parts (Orr et al., 2020). When the effect of anthropogenic noise is affected or modulated by exposure to light pollution and/or vice versa, we consider their combined impact to be interactive (Figure 1). The interactive impact can either result in less than the expected additive effect (antagonistic), or more than the expected effect (synergistic) (Orr et al., 2020). The most extreme form of interaction occurs where individual exposure to noise and light has no impact, but their combined exposure does—known as an emergent effect (Halfwerk and Slabbekoorn, 2015).
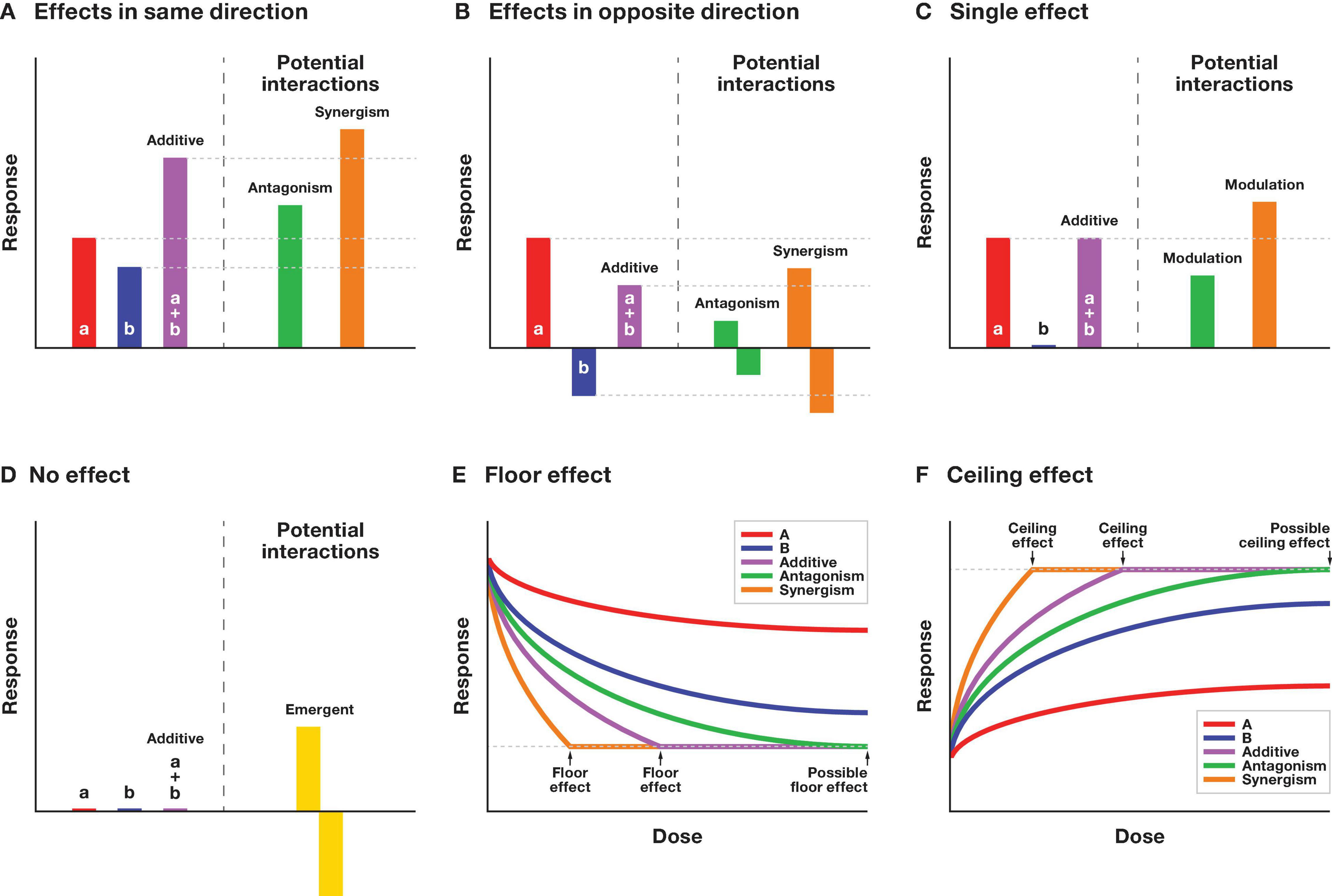
Figure 1. Classification of different types of interactive effects of noise and light pollution. Various scenarios where noise and light pollution have either the same (A), opposite (B), no effect (C,D) or a dose-dependent effect (E,F) when exposed in isolation. The effect of noise and light can either add up (a + b) or interact and lead to a lower (antagonistic) or higher (synergistic) than expected effect when compared to the additive effect. (A) Scenario when the effect of noise and light pollution is in the same direction. (B) Scenario when noise and light have opposite effects. Antagonistic effects can be in either direction, as long as they are closer to zero compared to either one of the single effects or the additive effect. Synergistic effects should be either further away from zero compared to additive effects, or single effects, but not necessarily both. (C) Modulation scenario where one sensory pollutant has no effect on its own. Interactive effects are considered to be a modulation of the dominant pollutant by the other pollutant. The modulatory effect can either be lower (antagonistic) or higher (synergistic) than the additive effect, which is the same as the effect of the dominant pollutant. (D) Emergent scenario where both sensory pollutants have no effect on their own, whereas their combined exposure either has a positive or negative effect. (E) Illustration of the floor effect. Depending on the dose of pollutant (A,B), their additive effect may reach a physical or energetic limit. Synergistic effects can only be assessed at low dosage levels, whereas at high dosages, antagonistic and additive effects may not be discernable. (F) Illustration of the ceiling effect.
Researchers examining impacts of sensory pollutants have recently started paying greater attention to the combined and possible interactive effects of ALAN and anthropogenic noise (Casasole et al., 2017; McMahon et al., 2017; Raap et al., 2017; Dominoni D. et al., 2020; Wilson et al., 2021). To provide an overview of this work, we performed a systematic review with two specific aims. Firstly, to determine whether combined impacts deviate from additive expectations. And secondly, to evaluate which study systems and trait types are most commonly affected where any such deviations occur. We discuss whether our findings help identify conditions under which sensory pollutants should be studied in conjunction, or can be considered in isolation. Additionally, we highlight the most common pitfalls relating to observational and experimental methods and provide some thoughts on the design of future studies in this developing research field.
We used an “All Database” Web of Science search (Clarivate Analytics, 2021) to create our candidate study list. We first performed a search using the following terms (within which speech marks define phrases, and asterisks indicate truncation wildcards).
TS = (“anthropogenic noise*” OR “noise* pollution” OR “sound* pollution” OR “anthropogenic sound*”) AND TS = (“light*” OR “light* pollution” OR “artificial light*” OR “artificial light at night” OR “ALAN”).
Preliminary searches showed that including simple “noise*” and “sound*” terms returned an unworkably large number of articles for screening (∼30,000 when “noise*” was added, and ∼80,000 when both terms were included). Given the multiple potential meanings of both words, most of these articles would be beyond the scope of this review. Therefore, we only incorporated “noise” in our search term when included in phrases alongside the filtering words “anthropogenic” and “pollution.” After screening all papers returned by our initial search (see below), we augmented our candidate study list with papers identified on Web of Science as citing articles from the initial search which met our screening criteria (and so likely focused on relevant topics). Our initial search and the subsequent identification of citing articles were carried out between 26 and 28th July, 2021.
We initially applied the inclusion criteria to all titles and abstracts. Full-texts were gathered when the title and abstract appeared to meet all criteria, or when there was insufficient information to form a judgment. The inclusion criteria were then re-applied to the full-texts to confirm eligibility. Our inclusion criteria were defined using a PICO framework (Frampton et al., 2017). Articles were included in our analyses if they presented data from primary research addressing the question “What are the combined effects of anthropogenic noise and ALAN on non-human animals?” We specified the PICO components for this question as: Population = non-human animals; Interventions = anthropogenic noise and ALAN; Comparators = absence and/or differing types/levels of anthropogenic noise and ALAN; Outcomes = functional, demographic or physiological effects. Accordingly, articles were considered eligible for inclusion if the research presented met each of the following criteria:
• Investigated populations of non-human animals
• Examined effects of both anthropogenic noise and ALAN
• Compared anthropogenic noise and ALAN with non-noise and -light controls, or different types/levels of anthropogenic noise and ALAN
• Assessed functional, demographic or physiological outcomes.
For each included full-text, we first characterized experimental design as either observational or experimental. We then noted subject taxon, effect(s) and response traits assessed (categorized as physiological—e.g., effects on endocrine or immune systems, functional—e.g., behavior, morphology or life history, or demographic—e.g., abundance, population density, or spatial distribution). Additionally, we recorded whether statistically significant individual effects of ALAN and anthropogenic noise were observed, and whether any relationship was positive or negative. Finally, we assessed whether possible interactions between ALAN and noise were explicitly tested for, and if so, whether the interaction was statistically significant. We considered interaction testing to be explicit either when an interaction term was specified in statistical models, or where light, noise and combined light and noise groups were statistically compared with each other and a control group. We categorized interaction effects as significant when estimate 95% confidence intervals did not include zero. And, we classified significant interactive effects as either additive, antagonistic, synergistic or emergent according to the definitions set out in the Introduction and Figure 1, by comparing effect sizes in text, tables, or figures.
We identified 839 unique articles through our literature search, of which 28 met the inclusion criteria, and so were incorporated into our analyses (see Supplementary Figure 1 for numbers of articles identified and screened at each stage).
Our analysis revealed that most multisensory pollution studies (21 out of 28) addressed effects of both noise and light pollution on birds (Table 1, Figure 2A, and Supplementary Data Sheet). In particular, on the timing of dawn singing (eight out of 21 studies), but also on a range of other functional, physiological and demographic traits, including breeding success, stress, immune and oxidative status, abundance, and species richness. Functional traits were studied almost twice as often as physiological and demographic traits combined (Figure 2B). Seven out of eight studies on dawn song were observational in nature and only one study explicitly tested whether noise and light exposure interacted. Across all study systems, more research reported observational data (20 out of 28 studies), than experimental (10 out of 28 studies). Only eight observational studies statistically tested for possible interactive effects of noise and light pollution (Figure 2C). Of these studies, four reported significant interactions. In contrast, eight out of ten experimental studies tested for interaction effects, with interactions detected in four instances. Two studies reported statistical interactions between noise and light without performing follow-up analyses to identify interaction effect type. For these studies we scored effect type from figures or effect sizes reported in tables or text (listed as “possible” effects in Table 1). The eight studies reporting interaction effects of noise and light (Table 1, also see Discussion for details) covered a range of characteristics, including physiological (e.g., haptoglobin and body temperature), functional (life history and behavior), and demographic (abundance) traits. Interactions detected included antagonistic, synergistic and emergent types, and one example of a floor effect. Of three experimental studies testing for but not reporting an interactive effect, two were carried out with groups of fish in the lab (Table 1).
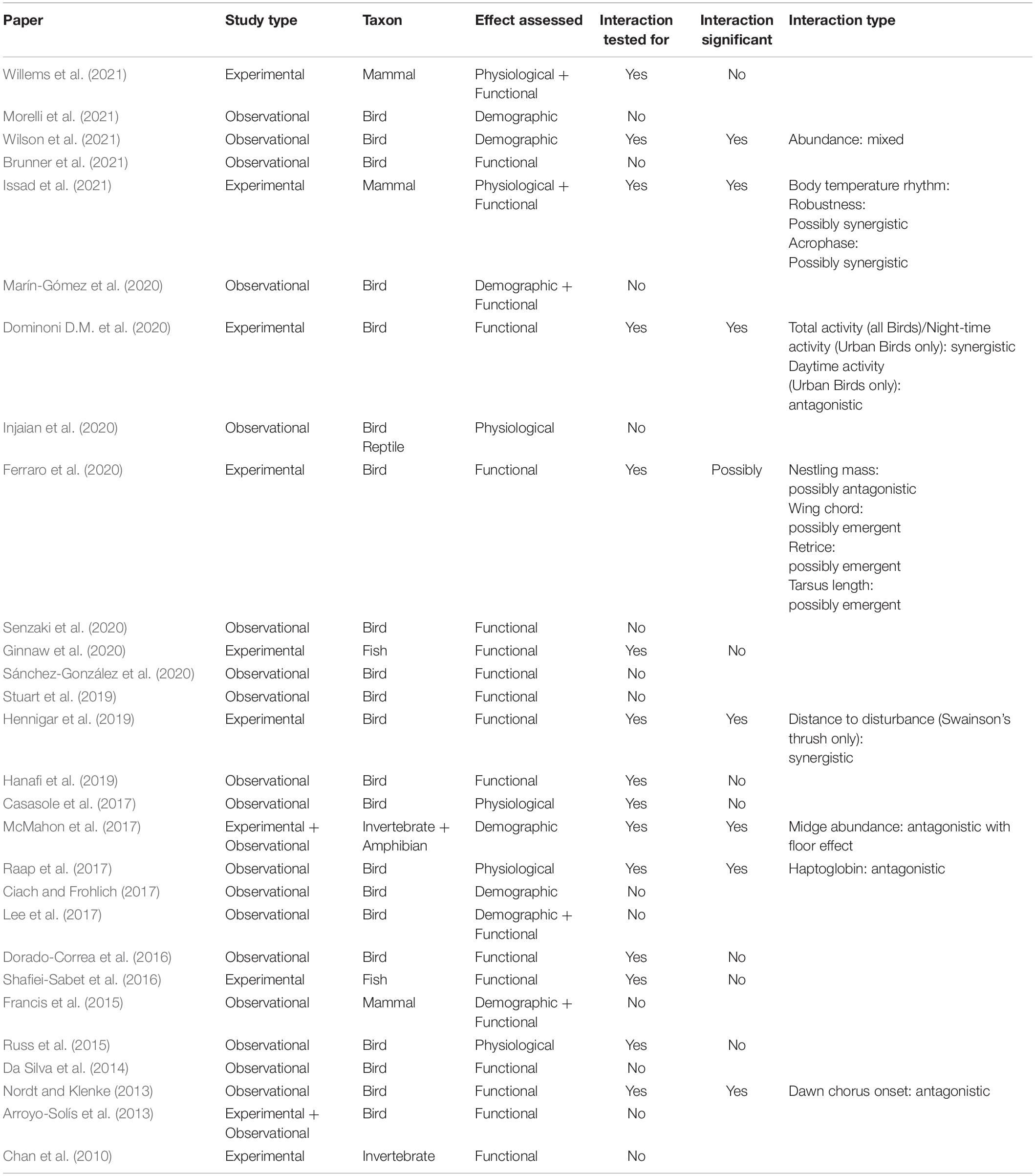
Table 1. Studies included in the systematic review of research investigating combined ecological impact of anthropogenic noise and artificial light at night.
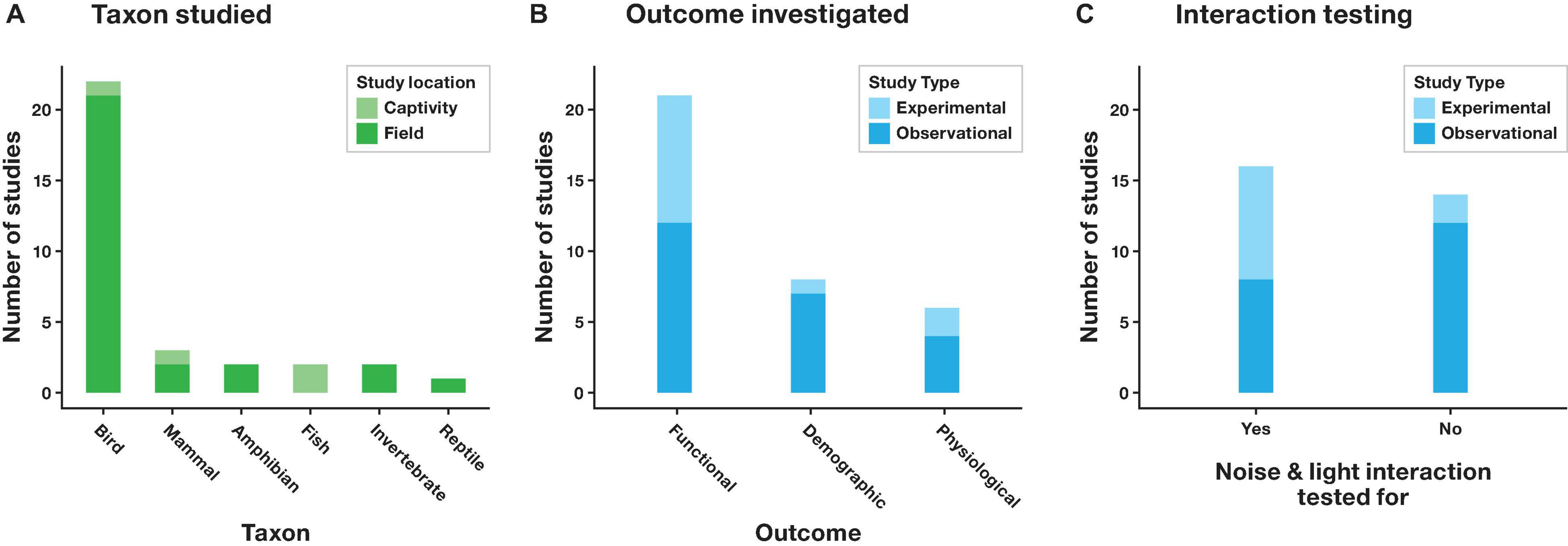
Figure 2. (A) Taxa and (B) outcomes investigated in included studies, and (C) numbers of included studies where interaction effects were explicitly tested for.
We screened the literature for research that simultaneously assessed ecological impacts of noise and light pollution, to determine how frequently possible interactive impacts of both sensory pollutants were tested for. We found 15 of 28 studies that statistically tested for interactive effects of noise and light. Within this group, four out of eight observational studies and four out of eight experimental studies detected an interactive effect, which was either antagonistic, synergistic, or emergent.
Six studies reported an antagonistic, or less-than expected effect of combined noise and light exposure, whereas four studies reported a synergistic, or more-than expected effect of noise and light pollution. Two studies found emergent effects, one observational, the other experimental. Ferraro et al. (2020) exposed free-living Western blue birds (Sialia Mexicana) to noise and light at their nests and tracked chick development. Chick fledging success, mass and size were influenced by noise and light exposure in different ways, suggesting emergent effects in some cases (Supplementary Data Sheet). For example, chicks grew shorter wing chords during combined exposure, but not during single exposure of noise and light treatments. However, emergent effects were not specifically tested for (Ferraro et al., 2020).
Some studies reported multiple types of interactive effects, depending on the trait that was measured, or the species that was studied (Table 1). Dominoni D. et al. (2020) scored overall activity of captive great tits and found light on its own increased activity at night, while noise on its own reduced activity during the day. When combined, the nighttime effect was enhanced, demonstrating a synergistic impact, whereas the daytime effect was reduced, revealing an antagonistic effect. Interestingly, these patterns were stronger in urban than forest birds. Wilson et al. (2021) related data from bird feeders throughout continental United States to modeled data on ALAN and anthropogenic noise. Using more than a million sightings from thousands of feeder stations they found an interactive effect of both pollutants on abundances for 50 out of 140 species. Noise and light had either an effect on abundances in the same or opposite direction (see also Figure 1), depending on bird species, and their combined effect was either antagonistic, synergistic, or emergent.
Finally, two experimental fish behavior studies suggested noise and light operate independently from each other (Shafiei-Sabet et al., 2016; Ginnaw et al., 2020). For example, fish actively avoided the loudspeaker and showed more freezing behavior during sound playback. Whereas, they spent more time in the upper water column in response to light treatment, but showed no avoidance or preference for the dark part of the tank (Shafiei-Sabet et al., 2016). Noise and light did not therefore interact. Although, any effect on individual behavior may have been overruled by group-level processes, as both studies focused on social fish during schooling formation.
Studies reporting antagonistic effects may suffer from ceiling or floor effects (Figures 1E,F), especially when working with numbers or percentages (Tekin et al., 2020). McMahon et al. (2017) for example, compared isolated effects of noise and light exposure to a control treatment and found a strong reduction in the number of parasitic midges approaching calling male frogs. The single effects were already so severe that their combined estimated additive effect should have resulted in a negative value for abundance. The reported significant interaction effect of noise and light might therefore result from statistical limitations, rather than a genuine underlying mechanism driving an antagonistic response. Likewise, an observational study on dawn song found birds started singing later than expected based on single effects of noise and light exposure (Nordt and Klenke, 2013), which could relate to energetic or physiological limits. In both cases, accounting for the dosages of noise and light might have provided insight into possible floor or ceiling effects.
Observational studies have the benefit of testing hypotheses under realistic field scenarios where sensory pollutants often vary in space and time in their intensity and composition, and covary with other important environmental variables, such as temperature, habitat structure and dietary composition. Potential pitfalls related to observational studies include: (i) insufficient statistical power to test for interactive effects; (ii) non-linear responses; (iii) biases toward single effect models; and (iv) unstandardized variables. Studies in which noise and light levels are correlated to other factors, such as food availability or temperature may require higher sample sizes to test for interactive effects, especially when these effects follow a non-linear function (e.g., when effects only start to occur after a certain threshold level is reached; Figures 1F,G). Furthermore, observational studies often use an information theoretic approach based on Aikaike values, as did all but one of the included observational studies that tested for interaction effects. Such methods penalize the number of terms added to candidate models, thus biasing single effect models over full models (especially for low sample sizes). And, environmental factors are not always standardized and scaled. For example, only three of the eight included observational studies testing for interaction effects standardized/scaled environmental variables. Failure to standardize and scale potentially biases one factor over the other, which makes statistical testing for interaction terms difficult (Stuart et al., 2019).
Future observational studies should thus take environmental covariance into account, ideally carrying out some a priori modeling or power analyses using standardized variables to inform on the best sampling design and sample sizes required to test for the different type of interactive effects. Also, while information theoretic approaches can be informative, penalization of interactive terms should be taken into account in some way.
Experimental studies have the benefit of enabling randomized and balanced full-factorial designs, which control for any of the possible confounding effects that occur in observational studies. Experimental studies also allow testing for dose-dependent effects of single vs. combined pollutants, commonly used in eco-toxicological studies, but not in other disciplines. Notably, of all included experimental studies only one (McMahon et al., 2017) included more than a single dosage level. While experimental studies are often easier to carry out in the laboratory, most lab-based studies are limited in terms of how generalizable their findings are to field conditions, as any interactive impact can be context-dependent. For example, under optimal conditions such as ad libitum food, provided in many captive bird and rodent studies (including the two experimental captive avian/rodent studies identified in this review), mechanisms necessary for interactive multi-stressor effects may not be in place. Therefore, although experimental studies might be better suited to demonstrate interactive effects of multisensory pollutants when compared to observational studies, care must be taken with their interpretation. In that sense, field experiments—ideally carried out across multiple (breeding) seasons—seem to provide the best design for studying impacts of noise and light pollution under realistic ecological conditions, while controlling for confounding effects.
The newly emerged field of multisensory pollution is in dire need of theories regarding the underlying mechanisms to improve our predictions of combined effects of anthropogenic noise and ALAN. Such mechanisms appear likely to differ across levels of biological organization. At an organismal level, sensory pollutants can alter an individual’s physiology and behavior through multiple perceptual mechanisms. Both noise and light can mask important signals and cues, distract animals from challenging cognitive tasks, or lead to misidentification of sensory pollutants as relevant natural signals or cues (Dominoni D.M. et al., 2020). Theory on multisensory pollution at the individual level could therefore usefully concentrate on situations where both pollutants influence the same perceptual mechanism and/or whether the same type of response is observed (e.g., both pollutants influencing hormone concentrations, or specific behavior such as dawn song).
At the community-level, biotic interactions and associated positive or negative feedback loops will strongly determine the outcome of single as well as combined effects of sensory pollutants. Species experiencing a direct negative impact of either high levels of noise or light (or both) on their behavior and/or physiology may simultaneously indirectly profit from impacts on their predators, prey or competitors. Blue tits (Cyanistes caeruleus) for example, benefit by occupying noisy nest boxes that are abandoned by their larger great tit (Parus major) competitor (Halfwerk et al., 2016). Theories on population-level or community-level impacts of multisensory pollution may therefore benefit from approaches developed for other multi-stressor impacts, which aim to predict when combined impacts are either synergistic or antagonistic (Bulleri et al., 2018; Griffith et al., 2019; Orr et al., 2020).
In conclusion, our bibliographic analysis revealed few studies have specifically addressed combined impact of anthropogenic noise and ALAN, despite the fact that these sensory pollutants often co-occur, especially in urbanized areas. This small number of studies limits conclusions that can be drawn with respect to whether noise and light pollution should be considered in conjunction, or can be studied on their own, for a given context and/or study system. Consequently, we call for more dedicated observational and experimental work on multisensory pollution, necessarily based on theoretical understanding of the underlying mechanisms through which interactive impacts may arise, and using appropriate experimental designs. Such approaches will greatly improve our understanding of the risks presented by sensory pollution, and provide greater predictive power to identify the most urgent conservation issues, and design the most cost-effective mitigation measures.
WH and PJ conceived the idea and wrote the manuscript. PJ carried out the literature search.
This study was supported by the ERC-Starting Grant—CITISENSE (802460).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.765950/full#supplementary-material
Supplementary Figure 1 | Flow chart of the systematic review process detailing numbers of studies identified at each stage.
Supplementary Data Sheet | Data for each stage of the systematic review process, comprising separate spreadsheets for the initial Web of Science search, included studies from the initial Web of Science search, articles citing the included studies (with and without duplicates removed), and the final list of all included studies.
Arroyo-Solís, A., Castillo, J. M., Figueroa, E., López-Sánchez, J. L., and Slabbekoorn, H. (2013). Experimental evidence for an impact of anthropogenic noise on dawn chorus timing in Urban Birds. J. Avian Biol. 44, 1–9. doi: 10.1111/j.1600-048X.2012.05796.x
Brunner, N., Kühleitner, M., and Renner-Martin, K. (2021). Bertalanffy-Pütter models for avian growth. PLoS One 16:e0250515. doi: 10.1371/journal.pone.0250515
Bulleri, F., Eriksson, B. K., Queirós, A., Airoldi, L., Arenas, F., Arvanitidis, C., et al. (2018). Harnessing positive species interactions as a tool against climate-driven loss of coastal biodiversity. PLoS Biol. 16:e2006852. doi: 10.1371/journal.pbio.2006852
Buxton, R. T., Seymoure, B. M., White, J., Angeloni, L. M., Crooks, K. R., Fristrup, K., et al. (2020). The relationship between anthropogenic light and noise in US national parks. Landsc. Ecol. 35, 1371–1384.
Casasole, G., Raap, T., Costantini, D., AbdElgawad, H., Asard, H., Pinxten, R., et al. (2017). Neither artificial light at night, anthropogenic noise nor distance from roads are associated with oxidative status of nestlings in an urban population of songbirds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 210, 14–21. doi: 10.1016/j.cbpa.2017.05.003
Chan, A. A. Y.-H., Giraldo-Perez, P., Smith, S., and Blumstein, D. T. (2010). Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461. doi: 10.1098/rsbl.2009.1081
Ciach, M., and Fröhlich, A. (2017). Habitat type, food resources, noise and light explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst. 20, 547–559. doi: 10.1007/s11252-016-0613-6
Crain, C. M., Kroeker, K., and Halpern, B. S. (2008). Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x
Da Silva, A., Samplonius, J. M., Schlicht, E., Valcu, M., and Kempenaers, B. (2014). Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 25, 1037–1047. doi: 10.1093/beheco/aru103
Darling, E. S., and Côté, I. M. (2008). Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x
Dominoni, D., Smit, J. A., Visser, M. E., and Halfwerk, W. (2020). Multisensory pollution: artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environ. Pollut. 256:113314. doi: 10.1016/j.envpol.2019.113314
Dominoni, D. M., Halfwerk, W., Baird, E., Buxton, R. T., Fernández-Juricic, E., Fristrup, K. M., et al. (2020). Why conservation biology can benefit from sensory ecology. Nat. Ecol. Evol. 4, 502–511. doi: 10.1038/s41559-020-1135-4
Dorado-Correa, A. M., Rodríguez-Rocha, M., and Brumm, H. (2016). Anthropogenic noise, but not artificial light levels predicts song behaviour in an equatorial bird. R. Soc. Open Sci. 3:160231. doi: 10.1098/rsos.160231
Ferraro, D. M., Le, M.-L. T., and Francis, C. D. (2020). Combined effect of anthropogenic noise and artificial night lighting negatively affect Western Bluebird chick development. Condor 122:duaa037.
Frampton, G. K., Livoreil, B., and Petrokofsky, G. (2017). Eligibility screening in evidence synthesis of environmental management topics. Environ. Evid. 6, 1–13. doi: 10.1097/00587875-200609000-00001
Francis, M. J., Spooner, P. G., and Matthews, A. (2015). The influence of urban encroachment on squirrel gliders (Petaurus norfolcensis): effects of road density, light and noise. Wildlife Res. 42, 324–333. doi: 10.1071/WR14182
Ginnaw, G. M., Davidson, I. K., Harding, H. R., Simpson, S. D., Roberts, N. W., Radford, A. N., et al. (2020). Effects of multiple stressors on fish shoal collective motion are independent and vary with shoaling metric. Anim. Behav. 168, 7–17. doi: 10.1016/j.anbehav.2020.07.024
Griffith, G. P., Strutton, P. G., Semmens, J. M., and Fulton, E. A. (2019). Identifying important species that amplify or mitigate the interactive effects of human impacts on marine food webs. Conserv. Biol. 33, 403–412. doi: 10.1111/cobi.13202
Halfwerk, W., Both, C., and Slabbekoorn, H. (2016). Long-term nestbox noise experiments reveal an impact on nest-site selection but not on reproduction. Behav. Ecol. 27, 1592–1600.
Halfwerk, W., and Slabbekoorn, H. (2015). Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol. Lett. 11:e20141051. doi: 10.1098/rsbl.2014.1051
Hanafi, S., Chong, L. P., Maruthaveeran, S., and Yeong, K. L. (2019). Vocal response of oriental magpie robin (Copsychus saularis) to urban environmental factors in peninsular Malaysia. Sains Malaysiana. 48, 2061–2069. doi: 10.17576/jsm-2019-4810-01
Hennigar, B., Ethier, J. P., and Wilson, D. R. (2019). Experimental traffic noise attracts birds during the breeding season. Behav. Ecol. 30, 1591–1601. doi: 10.1093/beheco/arz123
Injaian, A. S., Francis, C. D., Ouyang, J. Q., Dominoni, D. M., Donald, J. W., Fuxjager, M. J., et al. (2020). Baseline and stress-induced corticosterone levels across birds and reptiles do not reflect urbanization levels. Conserv. Physiol. 8:coz110. doi: 10.1093/conphys/coz110
Issad, S. M., Benhafri, N., El Allali, K., Farsi, H., Ouali-Hassenaoui, S., and Dekar-Madoui, A. (2021). Effects of prolonged night-time light exposure and traffic noise on the behavior and body temperature rhythmicity of the wild desert rodent, Gerbillus tarabuli. Chronobiol. Int. 38, 415–425. doi: 10.1080/07420528.2020.1862858
Jerem, P., and Mathews, F. (2021). Trends and knowledge gaps in field research investigating effects of anthropogenic noise. Conserv. Biol. 35, 115–129.
Lee, J. G. H., Macgregor-Fors, I., and Yeh, P. J. (2017). Sunrise in the city: disentangling drivers of the avian dawn chorus onset in urban greenspaces. J. Avian Biol. 48, 955–964. doi: 10.1111/jav.01042
Marín-Gómez, O. H., García-Arroyo, M., Sánchez-Sarria, C. E., Sosa-López, J. R., Santiago-Alarcon, D., and Macgregor-Fors, I. (2020). Nightlife in the city: drivers of the occurrence and vocal activity of a tropical owl. Avian Res. 11:9. doi: 10.1186/s40657-020-00197-7
McMahon, T. A., Rohr, J. R., and Bernal, X. E. (2017). Light and noise pollution interact to disrupt interspecific interactions. Ecology 98, 1290–1299. doi: 10.1002/ecy.1770
Morelli, F., Benedetti, Y., Ibáñez-Álamo, J. D., Tryjanowski, P., Jokimäki, J., Kaisanlahti-Jokimäki, M.-L., et al. (2021). Effects of Urbanization on taxonomic, functional and phylogenetic avian diversity in Europe. Sci. Total Environ. 795:148874. doi: 10.1016/j.scitotenv.2021.148874
Nordt, A., and Klenke, R. (2013). Sleepless in town–drivers of the temporal shift in dawn song in urban European blackbirds. PLoS One 8:e71476. doi: 10.1371/journal.pone.0071476
Orr, J. A., Vinebrooke, R. D., Jackson, M. C., Kroeker, K. J., Kordas, R. L., Mantyka-Pringle, C., et al. (2020). Towards a unified study of multiple stressors: divisions and common goals across research disciplines. Proc. R. Soc. B 287:20200421. doi: 10.1098/rspb.2020.0421
Piggott, J. J., Townsend, C. R., and Matthaei, C. D. (2015). Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547. doi: 10.1002/ece3.1465
Raap, T., Pinxten, R., Casasole, G., Dehnhard, N., and Eens, M. (2017). Ambient anthropogenic noise but not light is associated with the ecophysiology of free-living songbird nestlings. Sci. Rep. 7:2754.
Russ, A., Reitemeier, S., Weissmann, A., Gottschalk, J., Einspanier, A., and Klenke, R. (2015). Seasonal and urban effects on the endocrinology of a wild passerine. Ecol. Evol. 5, 5698–5710. doi: 10.1002/ece3.1820
Sánchez-González, K., Aguirre-Obando, O. A., and Ríos-Chelén, A. A. (2020). Urbanization levels are associated with the start of the dawn chorus in vermilion flycatchers in Colombia. Ethol. Ecol. Evol. 33, 377–393. doi: 10.1080/03949370.2020.1837963
Senzaki, M., Barber, J. R., Phillips, J. N., Carter, N. H., Cooper, C. B., Ditmer, M. A., et al. (2020). Sensory pollutants alter bird phenology and fitness across a continent. Naure 587, 605–609. doi: 10.1038/s41586-020-2903-7
Shafiei-Sabet, S., Van Dooren, D., and Slabbekoorn, H. (2016). Son et lumiere: sound and light effects on spatial distribution and swimming behavior in captive zebrafish. Environ. Pollut. 212, 480–488. doi: 10.1016/j.envpol.2016.02.046
Stuart, C. J., Grabarczyk, E. E., Vonhof, M. J., and Gill, S. A. (2019). Social factors, not anthropogenic noise or artificial light, influence onset of dawn singing in a common songbird. Auk 136:ukz045. doi: 10.1093/auk/ukz045
Svechkina, A., Portnov, B. A., and Trop, T. (2020). The impact of artificial light at night on human and ecosystem health: a systematic literature review. Landsc. Ecol. 35, 1725–1742. doi: 10.1007/s10980-020-01053-1
Swaddle, J. P., Francis, C. D., Barber, J. R., Cooper, C. B., Kyba, C. C., Dominoni, D. M., et al. (2015). A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. doi: 10.1016/j.tree.2015.06.009
Tekin, E., Diamant, E. S., Cruz-Loya, M., Enriquez, V., Singh, N., Savage, V. M., et al. (2020). Using a newly introduced framework to measure ecological stressor interactions. Ecol. Lett. 23, 1391–1403. doi: 10.1111/ele.13533
Vitousek, P. M., Mooney, H. A., Lubchenco, J., and Melillo, J. M. (1997). Human domination of Earth’s ecosystems. Science 277, 494–499. doi: 10.1126/science.277.5325.494
Willems, J. S., Phillips, J. N., Vosbigian, R. A., Villablanca, F. X., and Francis, C. D. (2021). Night lighting and anthropogenic noise alter the activity and body condition of pinyon mice (Peromyscus truei). Ecosphere 12:e03388. doi: 10.1002/ecs2.3388
Keywords: multisensory pollution, anthropogenic noise, emergent properties, synergism, antagonism, artificial light at night (ALAN)
Citation: Halfwerk W and Jerem P (2021) A Systematic Review of Research Investigating the Combined Ecological Impact of Anthropogenic Noise and Artificial Light at Night. Front. Ecol. Evol. 9:765950. doi: 10.3389/fevo.2021.765950
Received: 27 August 2021; Accepted: 15 October 2021;
Published: 30 November 2021.
Edited by:
Jennifer N. Phillips, Texas A&M University San Antonio, United StatesReviewed by:
Valentina J. Alaasam, University of Nevada, Reno, United StatesCopyright © 2021 Halfwerk and Jerem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Jerem, cGF1bEBwYXVsamVyZW0uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.