- 1School of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding, China
- 2College of Plant Protection, Northwest A&F University, Xianyang, China
- 3State Key Laboratory of Crop Stress Biology for Arid Areas, Northwest A&F University, Xianyang, China
- 4Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Science (CAS), Kunming, China
Chemoreceptive sensilla are abundantly distributed on antennal lamellae of scarab beetles. Olfactory reception by these sensory lamellae plays a major role in feeding behaviors and sexual communication of these beetles. A new electroantennogram (EAG) recording technique is here described for evaluation of electrophysiological responses of antennal lamellae of Pseudosymmachia flavescens to sex pheromones and host plant-related compounds. EAG responses were recorded simultaneously from each lamella and the closed antennal club. All test stimuli elicited similar EAG depolarization profiles in all the three lamellae and the closed club although EAG amplitudes from the same lamella or the club varied widely among different chemical stimuli. The mid lamella tended to produce significantly greater EAG responses. EAG responses evoked by a sex pheromone component, anisole, showed a significant correlation with the density of sensilla placodea subtype 1 (SP1). However, no general patterns were obtained for correlations between the density of any test sensilla type and EAG amplitudes evoked by all the six plant volatiles. Single sensillum recordings are needed to elucidate the specific roles of these sensilla in intraspecific sexual communication and perception of host plant volatiles.
Introduction
As an important feature of living organisms, olfaction plays a key role in regulating essential behaviors. In insects, the location of mates, food sources, and oviposition sites, as well as avoidance of predators and other threats, primarily relies on olfactory perception of environmental chemical signals. The main olfactory organs in insects include antennae, maxillary palps, labial palps, ovipositor, and feet. Among them, the antennae are the most important sensory organ and their function in olfaction in insects has been commonly recognized. A variety of sensilla are distributed on the surface of the antennae, and mainly tuned to detect odorants in the environment (Altner and Prillinger, 1980; Zacharuk, 1985; Steinbrecht, 1997).
The antennae of scarab beetles (Coleoptera: Scarabaeidae) are usually 10-segmented with the last 3–7 segments forming a lamellate club (Meinecke, 1975). The lamellar segments are always folded together to protect their inner surfaces when resting or crawling in soil, but wide-open during olfaction in mate finding and host plant location. As most olfactory sensilla are present on the surfaces of lamellae, it is crucial to allow lamellae to stay open when recording EAG response to odorants. However, such antennal structures and the closing feature of the antennal club in the resting position have made the antennal preparation for an electrophysiological study difficult. Several different techniques have been used to manually maneuver the antennal club for EAG recordings from scarab beetles [reviewed by Chen et al. (2019)]. With these techniques, electrical conductivity between antenna and the recording electrode may be insufficient, resulting in non-negligible background noises. Recently, we have developed a practical technique for recording EAG responses from one of the three lamellae of a scarab beetle, Pseudosymmachia flavescens (Brenske) (Coleoptera: Scarabaeidae: Melolonthinae). A key step of this antennal preparation technique is to make glass electrodes with appropriate size at opening to rightly fit the base or the tip of an excised antenna. One lamella was held apart from the other two on the antenna with a minuten pin and a disposable syringe needle, and directly connected to the recording electrode. The Beadle-Ephrussi Ringer solution modified with Tween® 80 (0.05%, W/V) was used to improve the signal-to-noise ratio. This technique is very useful even when testing EAG responses from very tiny antennae of small-sized scarab beetles (Chen et al., 2019).
In the present study, we developed a new technique to record EAG responses from three lamellae of one antenna and the closed club of the other one at same time to sex and host plant-related compounds. This approach allows to test whether the three lamellae on an antenna respond the same or differently to the same compounds, and whether the amplitudes of the EAG responses are comparable among the three lamellae and the closed club. In a previous study, four different types of sensilla on the antennal club of P. flavescens were observed, including sensilla basiconica, coeloconica, placodea, and trichodea (Li et al., under review). Porous sensilla placodea are predominant on lamellar surfaces (Bohacz et al., 2020), which have been demonstrated to detect sex pheromones and host plant-related volatiles in melolonthids (subfamilies Melolonthinae, Rutelinae, and Dynastinae) (Hansson et al., 1999; Nikonov et al., 2001; Ochieng et al., 2002). The presence of cuticular wall pores on sensilla basiconica intermingled with sensilla placodea all over the lamellar surface suggests an olfactory role for them. A comparison of the electrophysiological data with previous morphological data is aimed at demonstrating likely correlations between the number of any specific olfactory sensilla and the strength of EAG responses to certain compounds.
Materials and Methods
Insects
Adult beetles were collected from clove [Syzygium aromaticum (L.) Merr. and L. M. Perry] tree leaves on the campus of Institute of Zoology, Chinese Academy of Sciences, Beijing, China (116.39° E, 40.01° N). The beetles were sexed and the two sexes were held separately in clear plastic boxes (30 cm × 12 cm × 22 cm) containing moisturized soil at 25°C, 80% RH, and under a 16L:8D photoperiod. Fresh clove leaves were supplied daily to the beetles. Both female and male beetles were used for electrophysiological recordings.
Chemicals
HPLC grade hexane was purchased from CNW Technologies GmbH (Düsseldorf, Germany). Anisole (99%), Z-3-hexenal (50% in triacetin, stabilized), eucalyptol (99%), 6-methyl-5-hepten-2-one (99%), 1-hexanol (98%), (-)-E-pinocarveol (96%), and 4-ethyl-phenol (99%) were all purchased from Sigma-Aldrich (St. Louis, MO, United States). Anisole is a major component identified from the sex gland of female P. flavescens (unpublished data). The other compounds are electrophysiologically active, derived from leaves of walnut (Juglans regia L.), a preferred host plant of P. flavescens (Chen et al., 2019). Anisole was prepared in hexane and diluted to 0.1 and 0.01 μg/μL while all other synthetic plant volatiles were diluted with hexane to 10 and 1 μg/μL.
Electroantennographic Recordings
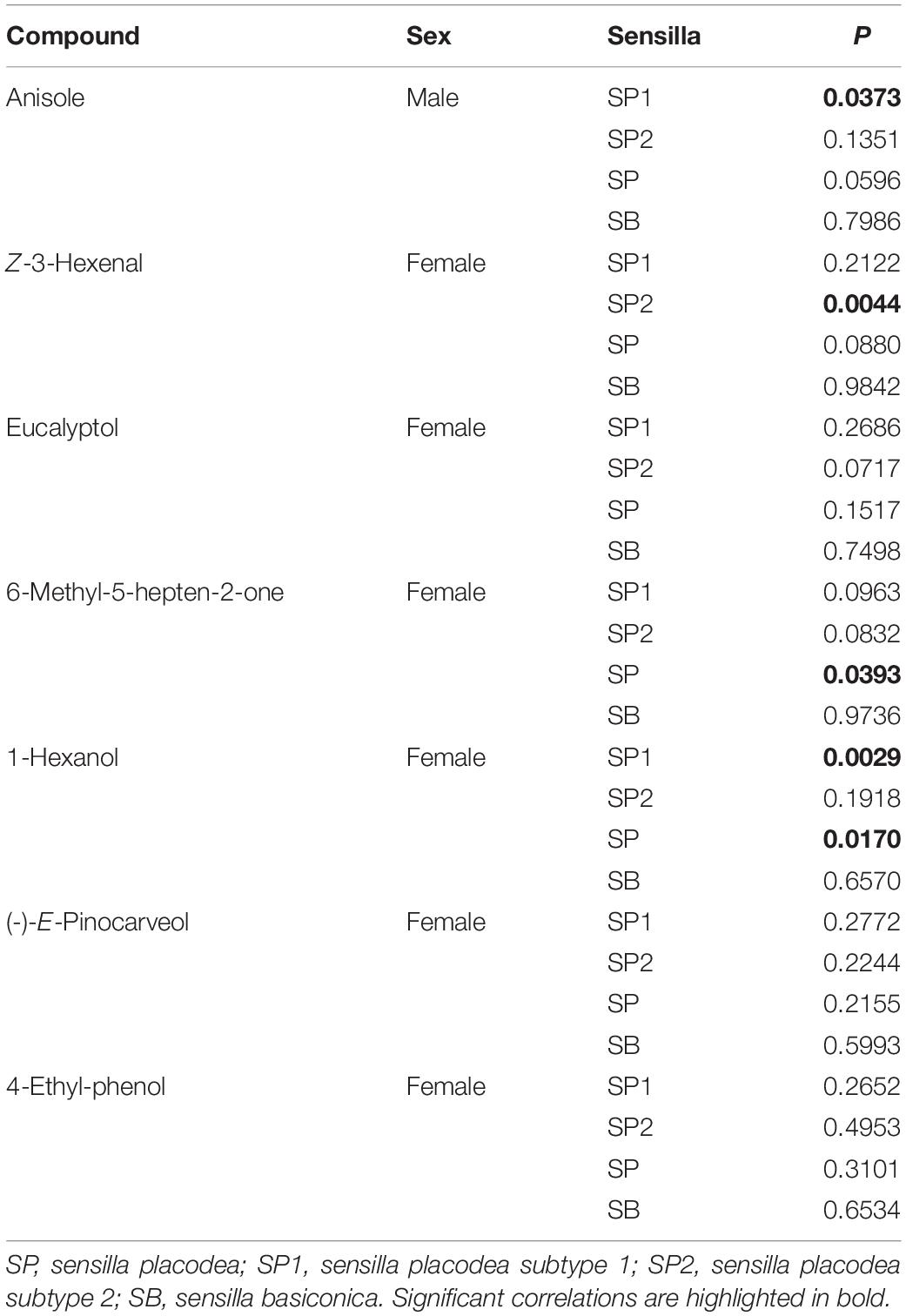
Olfactory responses of antennal lamellae of P. flavescens adults to volatile organic compounds were measured by the conventional EAG technique. The protocol for antennal preparation was similar to Chen et al. (2019). Glass capillary tubes (O.D. 1.5 mm, I.D. 0.84 mm, VitalSense Scientific Instruments Co., Ltd., Chengdu, China) were manually pulled over the flame of an alcohol lamp, and then cut with a column cutter, having tip openings with an internal diameter of 20 μm. Some heated capillary tubes were bent to 120–150° by further heating the point about 1 cm away from their tips (Figure 1A). All these capillary tubes were filled with the Tween® 80-modified Beadle-Ephrussi Ringer solution (Chen et al., 2019), serving as reference and recording electrodes. The head of a beetle was excised with a microknife, and fixed on a holding stage with dental wax in a downright position (Figure 1B). The reference electrode was connected to the neck of the isolated head. The three lamellae of one antennal club were carefully separated with a minuten pin and connected to three recording electrodes. The mid-lamella (L2 in Figure 1B) was allowed to connect to the second recording electrode (2 in Figure 1A) followed by the proximal (L1 in Figure 1B) and distal lamellae (L3 in Figure 1B) connecting to the first (1 in Figure 1A) and third recording electrodes (3 in Figure 1A), respectively. The other antennal club (Figure 1B) was directly connected to the fourth recording electrode (4 in Figure 1A). This connecting procedure ensured a high success rate of antennal preparation. Platinum wire (D. 0.4 mm) was used to establish an electrical connection between the electrode and the customized EAG probe (Wen et al., 2017).
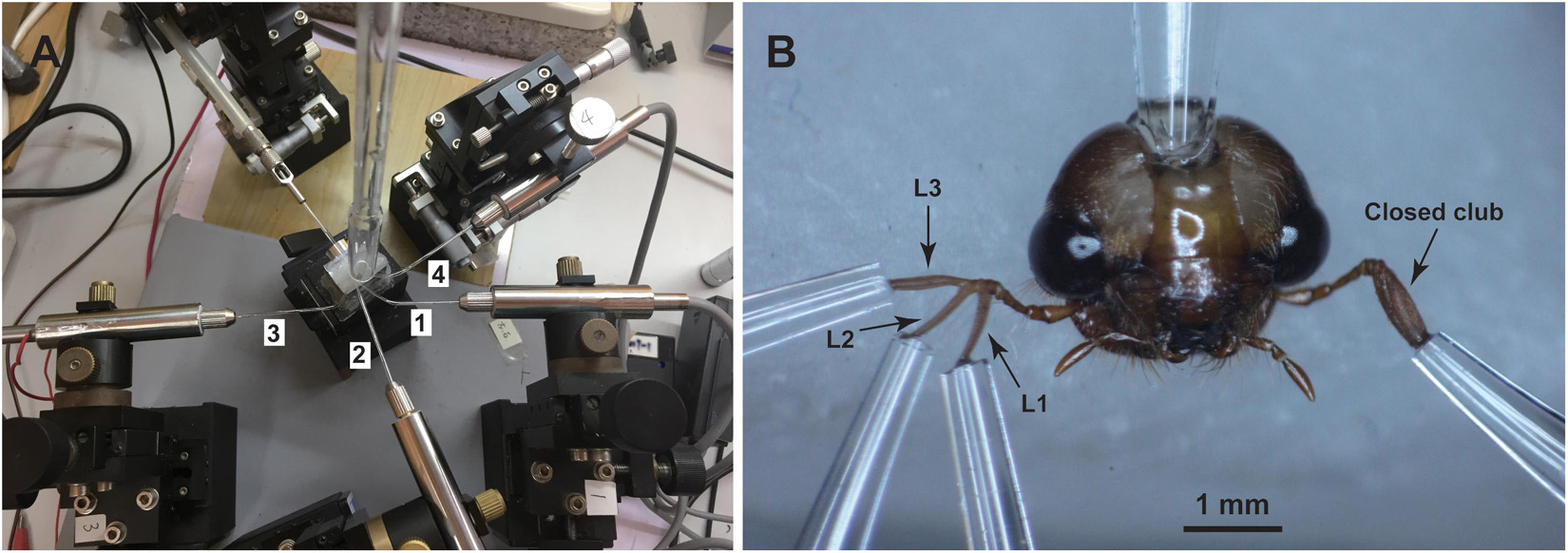
Figure 1. Experimental set-up (A) and antennal preparation (B) for EAG recordings. The isolated head of a beetle was mounted on a dental wax stage. The reference electrode was connected to the neck while the recording electrodes were connected to the three separated lamellae, L1, L2, and L3, of one antennal club and the other closed club. The numbers 1, 2, 3, and 4 of the recording electrodes correspond to the lamellae L1, L2, L3, and one antennal club, respectively.
A 10-μL aliquot of each solution was loaded on a filter paper strip (4 mm × 40 mm), which was then inserted into a glass Pasteur pipette to constitute an odor cartridge. The odor cartridge was connected to an air stimulus controller CS-55 (Syntech, Kirchzarten, Germany) to deliver odor stimuli through a small hole in the wall of a PTFE tube (I.D. 6 mm) as 0.2-s puffs. The antennae were flushed continuously by a charcoal-filtered and humidified air stream at 800 mL/min in the PTFE tube that ended directly at the antennal preparation. A single antennal preparation was used for a series of measurements of various compounds at the same concentration. A blank stimulus (solvent control) was presented before testing the compounds, and the testing sequence of these compounds was randomized. Intervals of 2 min between successive stimulations were used to ensure antennal recovery. For strict signal separation, signals captured by the four individual battery powered EAG probes were output to four independent HP-34465A digital multimeters (Keysight, United States) controlled by a BenchVue software (Keysight, United States) running on a PC (Wen et al., 2017). There were two dose groups, designated as low (0.1 μg anisole and 10 μg plant volatile compounds) and high (1 μg anisole and 100 μg plant volatile compounds). EAG recordings were obtained from 10 antennal preparations for each dose group. To minimize any variation among antennae, EAG responses in a lamella or the closed antennal club were corrected by deducting the EAG amplitude to the solvent control from the EAG amplitude elicited by the test compound.
Statistical Analyses
All values reported were mean ± standard error. One-way analysis of variance with Tukey’s HSD multiple comparisons was used to investigate significant differences in the EAG responses of different lamellae and the closed club to the same odor. The effects of sex, compound, dose, and lamella, as well as two-way, three-way, and four-way interactions (a total of 11 possible interactions) among these four variables on the EAG amplitudes were analyzed using a four-way mixed ANOVA. Data on the abundance of sensilla placodea (SP) and sensilla basiconica (SB) published in a previous study (Li et al., under review) were used for correlational analyses. The correlation between sensillar abundance and EAG responses of male beetles to high-dose sex pheromone or EAG responses of female beetles to high-dose host plant-related compounds was analyzed using the PROC REG procedure. Statistical significance was set at the α level of 0.05. All analyses were performed using the SAS statistical software (SAS Institute, 2004).
Results
All the tested stimuli evoked EAG responses of a fairly constant and reproducible time course and a characteristic shape (Figure 2). These responses were similar in the shape across all individual lamellae and the closed antennal club on antennae of P. flavescens.
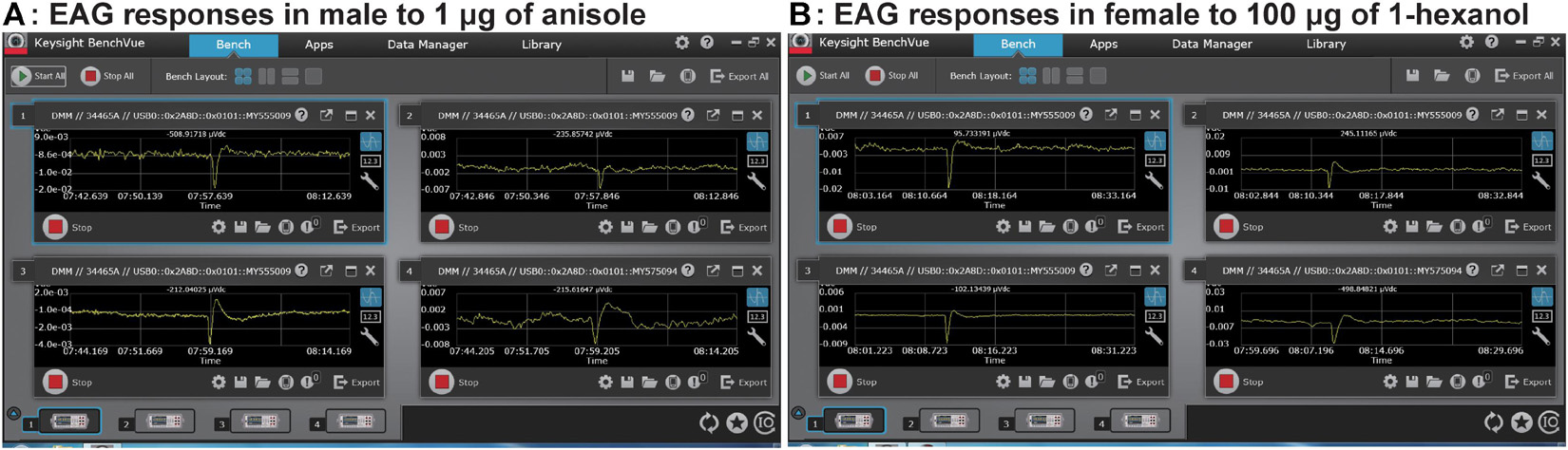
Figure 2. Simultaneous EAG recordings from three lamellae of one antennal club and the closed club of the second antenna in response to anisole (A) and 1-hexanol (B). The amplitudes in each small window were instantaneous reads recorded by a customized probe. The windows 1, 2, 3, and 4 display EAG traces captured by the recording electrodes 1, 2, 3, and 4 in Figure 1, respectively.
Multifactorial ANOVA revealed significant effects of sex, compound, dose, and lamella on EAG responses of P. flavescens. Various interactions among these factors were also significant (Table 1). For each sex and dose, all tested compounds triggered apparent antennal responses in all lamellae and the closed club (Figure 3). In both sexes, the mid-lamella L2 appeared to show higher EAG response than the other two lamellae and the closed club, but the differences were not always significant. Anisole elicited significantly stronger responses in males, with 1.77–2.83 mV from 0.1 μg and 3.21–4.27 mV from 1.0 μg, showing a sex-specific response pattern. Generally, male lamellae tended to show significantly higher responses to most plant-related compounds (except 4-ethyphenol), especially at high doses, than female counterparts (Supplementary Table 1). The EAG responses to all compounds except for 6-methyl-5-hepten-2-one and eucalyptol significantly increased with dose in females (significant dose effect, Figure 3 and Supplementary Table 2). Among the six plant-related compounds, 1-hexanol generally elicited the highest EAG responses in females at low dose and in males at both doses, followed by 6-methyl-5-hepten-2-one, (-)-E-pinocarveol, and eucalyptol. Z-3-Hexenal and 4-ethylphenol were the least active in most cases (Supplementary Figure 1). The EAG amplitude patterns to these volatiles across all lamellae were similar. In the case of low dose, however, there were no significant differences in EAG responses of female lamellae to 1-hexanol and 6-methyl-5-hepten-2-one.
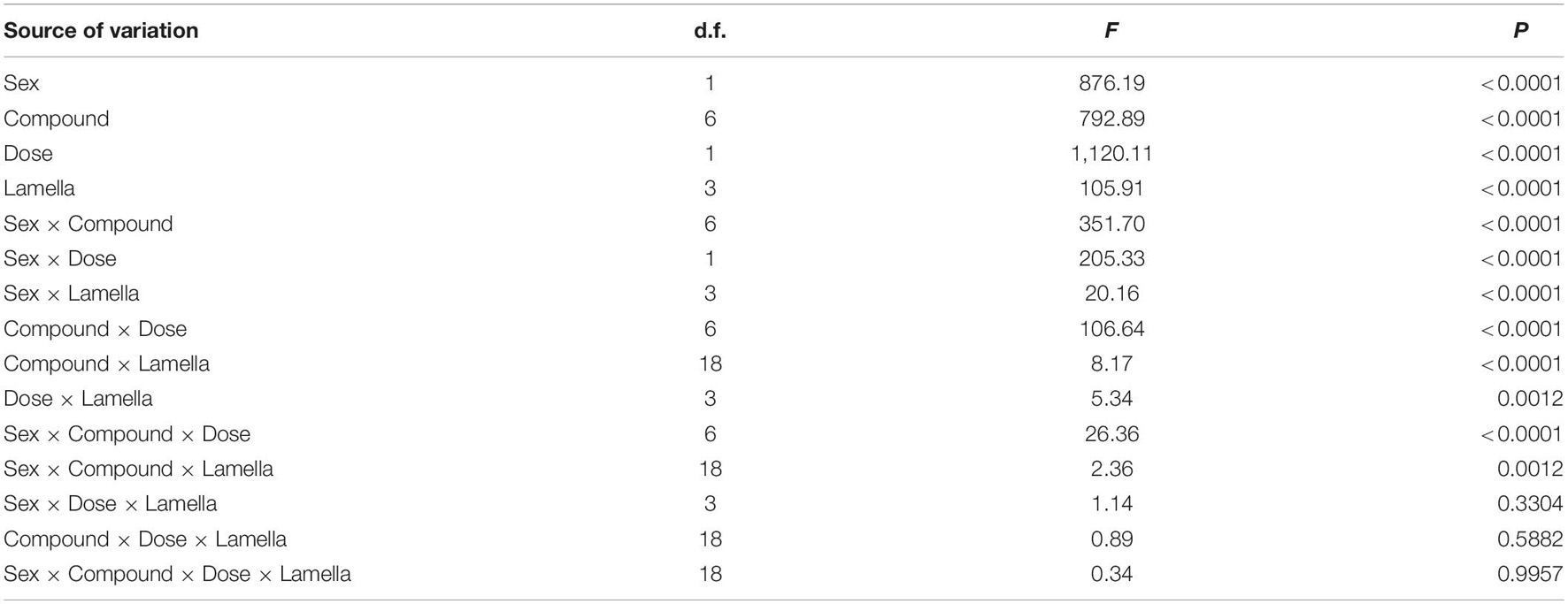
Table 1. The effects of sex, compound, dose, lamella, and interactions of these variables on absolute EAG responses.
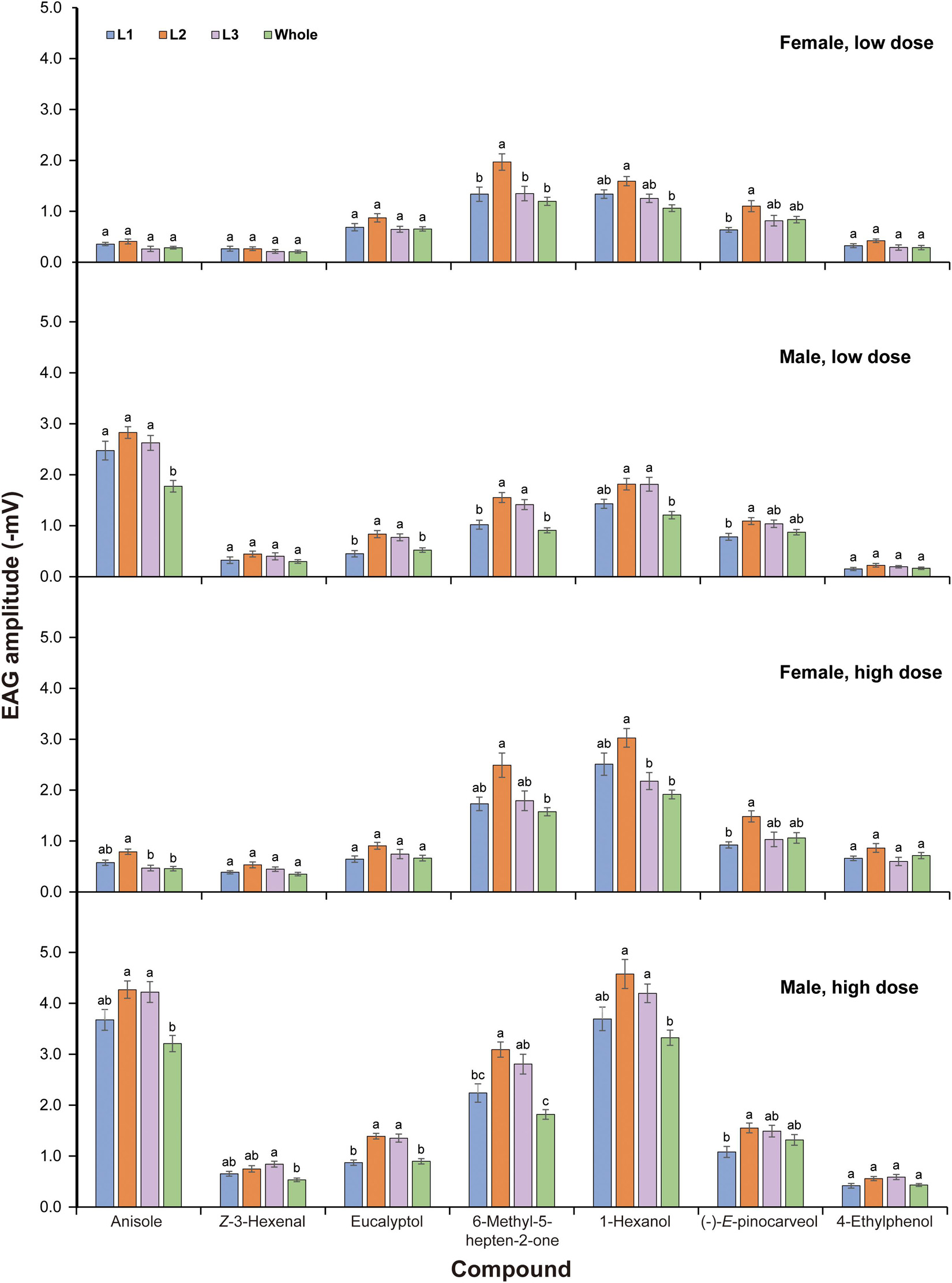
Figure 3. EAG responses of antennae of Pseudosymmachia flavescens to synthetic volatile compounds. L1: proximal lamella; L2: mid lamella; L3: distal lamella; Whole: the closed club. Low dose: 0.1 μg for anisole and 10 μg for all plant volatile compounds; High dose: 1 μg for anisole and 100 μg for all plant volatile compounds. Columns with different lowercase letters are significantly different at P < 0.05 (N = 10, Tukey tests after significant ANOVA).
By means of the linear regression analysis, a significant correlation between the sensillar abundance and EAG responses of males to high dose of anisole was obtained for sensilla placodea subtype 1 (SP1) (P = 0.0373) (Table 2). This thus suggests that olfactory neurons in SP1 in males is tuned to anisole. EAG values for Z-3-hexenal, 6-methyl-5-hepten-2-one, and 1-hexanol were correlated with the population density values of SP1 or SP2 (sensilla placodea subtype 2). The EAG responses to eucalyptol was likely to correlate with the population density of SP2 (P = 0.0717). However, there was no correlational relationship between the sensilla abundance and EAG responses to (-)-E-pinocarveol and 4-ethyl-phenol. The EAG values of all stimuli were not correlated with the density of SB at all (Table 2).
Discussion
The antennae of scarab beetles show a characteristic lamellicorn shape, on which olfactory sensilla are concentrated (Meinecke, 1975). All lamellar segments of the antennal club are involved in sexual chemical communication and perception of plant volatiles (Leal, 1998). For the first time, we presented differences in EAG amplitudes among the three lamellae of a scarab beetle. The mid lamella of P. flavescens tended to show greater EAG responses as compared to the first and third ones and the whole club to the host plant-related compounds, eucalyptol, 6-methyl-5-hepten-2-one, 1-hexanol, and (-)-E-pinocarveol. This tendency correlated well to a greater number of sensilla placodea on the mid lamella (Li et al., under review). It is therefore reasonable to estimate the electrical contribution provided to the EAG by such sensilla distributed on the lamellar surface. If the sensitivities of same-type sensilla on different lamellae to the same odor stimulation are quite similar, the contributions by the receptors in each sensilla would be directly related to its density on the individual lamella. Therefore, the distribution of specialized sensilla tuned to sex pheromones or host plant-related compounds determines the EAG amplitudes. The position of the recording electrode on an insect antenna affects conclusions on the detection of odorants (Biasazin et al., 2014; Jacob et al., 2017; Jacob, 2018). Our data showed that the three lamellae detected the same odorants, however, the sensitivities to different compounds varied with lamella position although the difference were not always significant (Figure 3). The receptors housed in sensilla placodea have proven to respond to sex pheromones and host plant-related volatiles in melolonthids (Hansson et al., 1999; Nikonov et al., 2001; Ochieng et al., 2002). Significant correlations found between population density of sensilla placodea (SP1 and/or SP2) and EAG responses evoked by anisole, Z-3-hexenal, 6-methyl-5-hepten-2-one, and 1-hexanol (Table 2) indicate that sensilla placodea are responsible for perception to the above test stimuli. However, no satisfactory correlations were obtained for (-)-E-pinocarveol and 4-ethyl-phenol (Table 2). These results suggested that morphological features could be somewhat insufficient to distinguish between sensillar types responsible for the EAG responses to odorant stimuli.
In Rutelinae, sensilla placodea without pits in the smooth area of the lamella surface respond to sex pheromones (Leal and Mochizuki, 1993; Larsson et al., 1999, 2001; Kim and Leal, 2000), whilst sensilla placodea with pits in the longitudinal heterogeneous area respond to host plant-related compounds (Hansson et al., 1999; Larsson et al., 2001; Bengtsson et al., 2011). Similar spatial division of the lamellar surface occurs in Cetoniinae (Stensmyr et al., 2001). Sensilla placodea in smooth areas in both grooved and smooth areas are capable of detecting host plant-related compounds (Stensmyr et al., 2001; Bengtsson et al., 2011). In Melolonthinae, there is no spatial separation of the lamellar surface, and sensilla involved in the reception of sex pheromones and plant-associated volatiles are uniformly distributed (Ochieng et al., 2002; Romero-López et al., 2004). As EAG responses to anisole significantly correlated with the density of SP1, it is very likely that SP1 in male P. flavescens generates responses to sex pheromones. Provided that SP1 is pheromone-sensitive, the percentage of SP1 in total SP is 69% in males (Li et al., under review) is consistent with the percentage of the pheromone-sensitive sensilla placodea (68%) in male Japanese beetle (Kim and Leal, 2000; Nikonov and Leal, 2002). In P. flavescens, sensilla placodea are significantly more abundant in males than in females (Li et al., under review). It has been previously demonstrated that both female and male scarabs can detect their own pheromones, and the response properties of pheromone-sensitive receptor neurons are similar in female and male scarabs, but with a lower sensitivity in females (Larsson et al., 1999; Nikonov et al., 2001, 2002). The sexual differences in the number of pheromone-sensitive sensilla (SP1, in the case of P. flavescens) and pheromone sensitivity may account for significantly greater EAG responses to anisole in males than in females. Because there is no general pattern of correlation between abundance of a specific sensilla placodea subtype with EAG responses, it is unlikely to determine which sensilla subtype responds to plant volatiles.
In comparison with sensilla placodea, sensilla basiconica on each lamella was at least 10 times less abundant (Li et al., under review). A significant correlation has been previously found between EAG amplitude and population density of long sensilla basiconica in Bactrocera oleae, which specifically responds to host plant-related compounds (Crnjar et al., 1989). However, no satisfactory correlations between EAG amplitude and population density of sensilla basiconica in P. flavescens were found for any test stimuli (Table 2). Although the multiporous feature of sensilla basiconica suggests an olfactory role for them (Zacharuk, 1980; Keil and Steinbrecht, 1984), it might be difficult to obtain EAG responses due to the very low-density distribution. Their contributions to EAG responses cannot be determined in the present study. Further electrophysiological recordings from single sensilla are desperately needed to better differentiate sensilla types, and assess the specific sensitivity spectra of a specific sensillar type in P. flavescens.
Scarab beetles usually fold their lamellae forming a compact club at rest to protect sensorial areas located in the inner lamella surfaces. The closed club showed evident EAG responses to sex pheromone and plant volatiles, suggesting that scarabs are responsive to chemical stimuli at rest with functioning sensilla placodea on the outer surfaces of L1 and L3. However, we cannot rule out the possibility that air may be able to enter in between the lamellae even when the club is closed. The capability of detecting environmental and chemical cues at rest appears to be related to the synchronized behavior in both female and male P. flavescens emerging simultaneously from belowground in search for food and mate. The significant olfactory responses to the six host plant-related compounds are consistent with findings in Chen et al. (2019) (Figure 3 and Supplementary Figure 1). In general, lamellae in males showed significantly greater EAG responses to these plant volatiles than those in females. Significantly longer lamellae and higher number of sensilla placodea on males than females (Li et al., under review) may account for the higher EAG responses in males. The higher sensitivity in males is likely related to the male’s ability to find a conspecific mate on a host plant, which may be linked to strong competition for mates among males. The biological significance of higher electrophysiological sensitivity in males requires further investigation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LC and PW conceived and designed the experiments. Y-YL, LC, and PW carried out the experiments. Y-YL, LC, PW, and DL analyzed the data and wrote the manuscript. All authors agreed to publish this manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 31171847 and 31460474) and the High-level Talents Research Start-up Project of Hebei University (Grant No. 521000981387).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.759778/full#supplementary-material
References
Altner, H., and Prillinger, L. (1980). Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 67, 69–139. doi: 10.1016/S0074-7696(08)62427-4
Bengtsson, J. M., Khbaish, H., Reinecke, A., Wolde-Hawariat, Y., Negash, M., Seyoum, E., et al. (2011). Conserved, highly specialized olfactory receptor neurons for food compounds in 2 congeneric scarab beetles, Pachnoda interrupta and Pachnoda marginata. Chem Senses 36, 499–513. doi: 10.1093/chemse/bjr002
Biasazin, T. D., Karlsson, M. F., Hillbur, Y., Seyoum, E., and Dekker, T. (2014). Identification of host blends that attract the african invasive fruit fly, Bactrocera invadens. J. Chem. Ecol. 40, 966–976. doi: 10.1007/s10886-014-0501-6
Bohacz, C., du G. Harrison, J., and Ahrens, D. (2020). Comparative morphology of antennal surface structures in pleurostict scarab beetles (Coleoptera). Zoomorphology 139, 327–346. doi: 10.1007/s00435-020-00495-0
Chen, L., Li, Y.-Y., and Shao, K.-M. (2019). A practical technique for electrophysiologically recording from lamellated antenna of scarab beetle. J. Chem. Ecol. 45, 392–401. doi: 10.1007/s10886-019-01059-3
Crnjar, R., Scalera, G., Liscia, A., Angioy, A. M., Bigiani, A., Pietra, P., et al. (1989). Morphology and EAG mapping of the antennal olfactory receptors in Dacus oleae. Entomol. Exp. Appl. 51, 77–85. doi: 10.1111/j.1570-7458.1989.tb01216.x
Hansson, B. S., Larsson, M. C., and Leal, W. S. (1999). Green leaf volatile-detecting olfactory receptor neurones display very high sensitivity and specificity in a scarab beetle. Physiol. Entomol. 24, 121–126. doi: 10.1046/j.1365-3032.1999.00121.x
Jacob, V., Scolari, F., Delatte, H., Gasperi, G., Jacquin-Joly, E., Malacrida, A. R., et al. (2017). Current source density mapping of antennal sensory selectivity reveals conserved olfactory systems between tephritids and Drosophila. Sci. Rep. 7:15304. doi: 10.1038/s41598-017-15431-4
Jacob, V. E. J. M. (2018). Current source density analysis of electroantennogram recordings: a tool for mapping the olfactory response in an insect antenna. Front. Cell. Neurosci. 12:287. doi: 10.3389/fncel.2018.00287
Keil, T. A., and Steinbrecht, R. A. (1984). “Mechanosensitive and olfactory sensilla of insects,” in Insect Ultrastructure, eds R. C. King and H. Akai (Boston: Springer).
Kim, J. Y., and Leal, W. S. (2000). Ultrastructure of pheromone-detecting sensillum placodeum of the Japanese beetle, Popillia japonica newmann (Coleoptera: Scarabaeidae). Arthropod Struct. Dev. 29, 121–128. doi: 10.1016/s1467-8039(00)00022-0
Larsson, M. C., Leal, W. S., and Hansson, B. S. (1999). Olfactory receptor neurons specific to chiral sex pheromone components in male and female Anomala cuprea beetles (Coleoptera: Scarabaeidae). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 184, 353–359. doi: 10.1007/s003590050334
Larsson, M. C., Leal, W. S., and Hansson, B. S. (2001). Olfactory receptor neurons detecting plant odours and male volatiles in Anomala cuprea beetles (Coleoptera: Scarabaeidae). J. Insect Physiol. 47, 1065–1076. doi: 10.1016/S0022-1910(01)00087-7
Leal, W. S. (1998). Chemical ecology of phytophagous scarab beetles. Annu. Rev. Entomol. 43, 39–61. doi: 10.1146/annurev.ento.43.1.39
Leal, W. S., and Mochizuki, F. (1993). Sex pheromone reception in the scarab beetle Anomala cuprea: enantiomeric discrimination by sensilla placodea. Naturwissenschaften 80, 278–281. doi: 10.1007/bf01135914
Meinecke, C.-C. (1975). Riechsensillen und systematik der lamellicornia (insecta, Coleoptera). Zoomorphologie 82, 1–42. doi: 10.1007/bf00995905
Nikonov, A. A., and Leal, W. S. (2002). Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle, Popillia japonica. J. Chem. Ecol. 28, 1075–1089.
Nikonov, A. A., Peng, G., Tsurupa, G., and Leal, W. S. (2002). Unisex pheromone detectors and pheromone-binding proteins in scarab beetles. Chem. Senses 27, 495–504. doi: 10.1093/chemse/27.6.495
Nikonov, A. A., Valiyaveettil, J. T., and Leal, W. S. (2001). A photoaffinity-labeled green leaf volatile compound ‘tricks’ highly selective and sensitive insect olfactory receptor neurons. Chem. Senses 26, 49–54. doi: 10.1093/chemse/26.1.49
Ochieng, S. A., Robbins, P. S., Roelofs, W. L., and Baker, T. C. (2002). Sex pheromone reception in the scarab beetle Phyllophaga anxia (Coleoptera: Scarabaeidae). Ann. Entomol. Soc. Am. 95, 97–102. doi: 10.1603/0013-8746(2002)095[0097:sprits]2.0.co;2
Romero-López, A. A., Arzuffi, R., Valdez, J., Morón, M. A., Castrejón-Gómez, V., and Villalobos, F. J. (2004). Sensory organs in the antennae of Phyllophaga obsoleta (Coleoptera: Melolonthidae). Ann. Entomol. Soc. Am. 97, 1306–1312. doi: 10.1603/0013-8746(2004)097[1306:soitao]2.0.co;2
Steinbrecht, R. A. (1997). Pore structures in insect olfactory sensilla: A review of data and concepts. Int. J. Insect Morphol. Embryol. 26, 229–245. doi: 10.1016/S0020-7322(97)00024-X
Stensmyr, M. C., Larsson, M. C., Bice, S., and Hansson, B. S. (2001). Detection of fruit-and flower-emitted volatiles by olfactory receptor neurons in the polyphagous fruit chafer Pachnoda marginata (coleoptera: Cetoniinae). J. Comp. Physiol. A Sens. Neural Behav. Physiol. 187, 509–519. doi: 10.1007/s003590100222
Wen, P., Cheng, Y., Qu, Y., Zhang, H., Li, J., Bell, H., et al. (2017). Foragers of sympatric asian honey bee species intercept competitor signals by avoiding benzyl acetate from Apis cerana alarm pheromone. Sci. Rep. 7:6721. doi: 10.1038/s41598-017-03806-6
Zacharuk, R. Y. (1980). Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 25, 27–47. doi: 10.1146/annurev.en.25.010180.000331
Keywords: Scarabaeidae, electroantennogram, Pseudosymmachia flavescens, sex pheromone, host plant volatiles
Citation: Li Y-Y, Liu D, Wen P and Chen L (2021) Detection of Volatile Organic Compounds by Antennal Lamellae of a Scarab Beetle. Front. Ecol. Evol. 9:759778. doi: 10.3389/fevo.2021.759778
Received: 17 August 2021; Accepted: 28 September 2021;
Published: 25 October 2021.
Edited by:
Xin-Cheng Zhao, Henan Agricultural University, ChinaReviewed by:
Qi Yan, Nanjing Agricultural University, ChinaStefan Dötterl, University of Salzburg, Austria
Copyright © 2021 Li, Liu, Wen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Wen, d2VucGluZ0B4dGJnLmFjLmNu; Li Chen, Y2hlbmxpMUBoYnUuZWR1LmNu; orcid.org/0000-0002-0394-4387
 Ya-Ya Li1,2,3
Ya-Ya Li1,2,3 Deguang Liu
Deguang Liu Ping Wen
Ping Wen Li Chen
Li Chen