
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 16 December 2021
Sec. Ecophysiology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.759726
This article is part of the Research TopicAvian Behavioral and Physiological Responses to Challenging Thermal Environments and Extreme Weather EventsView all 12 articles
Torpor is a controlled reduction of metabolism and body temperature, and its appropriate use allows small birds to adapt to and survive challenging conditions. However, despite its great energy conservation potential, torpor use by passerine birds is understudied although they are small and comprise over half of extant bird species. Here, we first determined whether a free-living, small ∼20 g Australian passerine, the eastern yellow robin (Eopsaltria australis), expresses torpor by measuring skin temperature (Ts) as a proxy for body temperature. Second, we tested if skin temperature fluctuated in relation to ambient temperature (Ta). We found that the Ts of eastern yellow robins fluctuated during winter by 9.1 ± 3.9°C on average (average minimum Ts 30.1 ± 2.3°C), providing the first evidence of torpor expression in this species. Daily minimum Ts decreased with Ta, reducing the estimated metabolic rate by as much as 32%. We hope that our results will encourage further studies to expand our knowledge on the use of torpor in wild passerines. The implications of such studies are important because species with highly flexible energy requirements may have an advantage over strict homeotherms during the current increasing frequency of extreme and unpredictable weather events, driven by changing climate.
Endotherms can maintain a high body temperature (Tb) across a range of ambient temperatures (Ta) via appropriate adjustment of internal heat production. However, the energetic costs to thermoregulate outside of the thermal neutral zone can be expensive (Mckechnie and Lovegrove, 2002; Angilletta et al., 2010). To deal with these energetic costs, many endothermic species use torpor, a controlled reduction in metabolism and typically Tb (Namekata and Geiser, 2009; Ruf and Geiser, 2015). Torpor is used as a strategy to overcome energetically challenging periods such as cold (Maddocks and Geiser, 2007; Romano et al., 2019; Wolf et al., 2020), reduced food/water or foraging opportunities (Nicol and Andersen, 1996; Nord et al., 2009; Smit et al., 2011) or even hot conditions (Reher and Dausmann, 2021), and its use varies considerably among avian taxa (Geiser, 2021). Some evidence of intra-specific variation in torpor patterns along latitudinal or elevation gradients (Geiser and Ferguson, 2001; Dunbar and Brigham, 2010; Zervanos et al., 2010; Stawski and Geiser, 2011) suggests that Ta may play a major role in torpor expression in passerines, but only little information on geographical variation is available for birds (Chaplin, 1976; Sharbaugh, 2001). Although some potential costs may occur at low Tbs on a cellular level (Nowack et al., 2019), the use of torpor may reduce predation risk by increasing antipredator behavior (but see Amo et al., 2011; Turbill and Stojanovski, 2018), and in small diurnal birds can reduce metabolic demands by as much as 50% (Cooper and Gessaman, 2005) and may increase survival by 58% (Brodin et al., 2017).
The use of torpor for energy conservation is common in a range of small mammals (e.g., Ruf and Geiser, 2015; Nowack et al., 2020) and in several non-passerine bird species such as hummingbirds and nightjar relatives (Hiebert, 1992; Körtner et al., 2000; Shankar et al., 2020). In contrast, the extent of the use of torpor by passerines, a group which includes over half of extant birds species, is understudied (Mckechnie and Lovegrove, 2002). Despite evidence for large nocturnal Tb reduction dating back to the late 1950’s [e.g., in Redpoll Carduelis flammea and tree sparrow Passer montanus, Steen (1958)], and further anecdotal evidence of nocturnal torpor in multiple species in the 1970’s [Crimson chat (Epthianura tricolor), red-capped robin (Petroica goodenovii), white-fronted honeyeater (Phylidonyris albifrons) and banded whiteface (Aphelocephala nigricincta); Ives (1973), white-backed swallow (Cheramoeca leucosternum); Serventy (1970)], little progress has been made in the field. Some small passerine species have been reported to use torpor under controlled conditions: Golden-collared manakins [Manacus vitellinus; Bucher and Worthington (1982)], dusky woodswallows [Artamus cyanopterus; Maddocks and Geiser (2007)], rifleman [Acanthisitta chloris; Mcnab and Weston (2018)], and malachite sunbirds [Nectarina famosa; Downs and Brown (2002)] dropped their Tb to 27–30°C at TaS of 19.5, 6, 11, and 5°C, respectively. The rifleman has also been reported to decrease metabolic rate to as low as 21% of its expected basal metabolic rate (Mcnab and Weston, 2018). Torpor under controlled environment has also been reported in species adapted to very cold conditions. The black-capped chickadees (Poecile atricapilla) from Alaska reduced Tb by about 8°C in both winter and summer when exposed to Ta −30°C, and actively rewarmed (Sharbaugh, 2001). Metabolic rate reduction has also been observed in scarlet-backed Flowerpecker (Dicaeum cruentatum), which reduced metabolic rates by over 70% during torpor vs. non-torpor state (Bushuev et al., 2021). While this evidence supports torpor use in passerine species, it is important to evaluate these functions in the wild. Captive conditions do not represent the complexity of the thermal conditions and ecological complexity (e.g., food abundance, predation risk) experienced in the wild, and laboratory studies may therefore underestimate the use of torpor by wild animals (Geiser et al., 2000; O’connor et al., 2017).
To date, only two passerine species studied in the wild have been shown to express torpor. The Tb of noisy miners (Manorina melanocephala) fell by 7°C on average, to a minimum of 33°C during winter in eastern Australia, where night time temperatures frequently drop to near 0°C (Geiser, 2019), and fairy wrens (Malurus cyaneus) dropped their skin temperature (Ts) by over 14.5°C, with minimum Ts of 27.4°C recorded at average minimum Ta of about 3°C (Romano et al., 2019). Other passerines species which were studied in the wild remained euthermic, even during challenging conditions [e.g., red-headed finch Amadina erythrocephala (Mckechnie and Lovegrove, 2003), five species of tropical montane passerines (Burnett et al., 2019), willow tit Parus montanus (Reinertsen and Haftorn, 1984), bronze mannikins Spermestes cucullatus (Lovegrove and Smith, 2003), great tits Parus major (Nilsson et al., 2020), blue tits Cyanistes caeruleus (Nord et al., 2009)]. The lack of extensive studies on torpor use in wild passerines is somewhat surprising, because although evidence is limited, data in the wild and under controlled environment do suggests that some passerine species are capable of large Tb reduction. Moreover, many passerine species are insectivorous, small and diurnal and cannot feed at night when Tas are low. The abundance of insects and other food also typically decreases in winter, therefore, it is likely that many passerine species could benefit greatly from the use of torpor.
The aim of our study was to examine the thermal energetics of a small passerine species, the eastern yellow robin (Eopsaltria australis; hereafter “eastern robin”), during winter at a cool temperate climate site in the eastern Australian Northern Tablelands (elevation range 980–1050 m). We determined whether individuals express torpor by measuring the magnitude of Ts reduction and whether this reduction is related with ambient temperature (Ta). Additionally, we discuss our results in comparison with the thermal energetics of a closely related species, the western yellow robin (Eopsaltria griseogularis) (hereafter “western robin”), which is the only other member of the genus Eopsaltria in Australia (Loynes et al., 2009). The western robin was studied in a Mediterranean climate in WA (Douglas, 2017).
We captured four adult wild-living eastern robins (2 male, 2 female; mean body mass 19.4 g) during the southern hemispheric winter (July–August) in Imbota Nature Reserve, NSW, Australia (30.58°S, 151.72°E), a 218 ha open Eucalyptus and Acacia woodland. The population of eastern yellow robins in Imbota included on average nine pairs between 2000 and 2003, but the population likely declined (Debus, 2006) as a result of low reproduction in this region, mainly due to nest predation by Pied Currawongs (Strepera graculina) (Debus and Ford, 2012), and eastern robins produce too few independent young to replace adult mortality (Zanette, 2000; Debus, 2006). Additionally, eastern robins appear to be most sensitive to loss, fragmentation and degradation of habitat in rural landscapes (Watson et al., 2001, 2002; Lambeck, 2002), and an intense drought from mid-2017 to 2020 and major fire events in summer 2019 in NSW may deteriorated the quality of the site and caused further population decline. Indeed, during our fieldwork we could not find more than five individual eastern robins on the site.
Average daily minimum and maximum Tas for Imbota in midwinter (July) are 1.3 and 12.2°C, and average summer Ta reach up to 26.3°C (Bureau of Meteorology, 2021). After capture, birds were held in a cotton bag until processed. The birds were weighed to the nearest g with a digital scale (HCB-1002, Adam highland HCB). Each bird was banded with an individual numbered metal band and a unique combination of color bands. We then attached a temperature-sensitive radio transmitter (LB-2XT, 0.33 g, Holohil Systems Ltd., Canada) directly to the skin, between the shoulder blades using a latex-based adhesive (12% resin, Manfred Sauter GmbH). Transmitters were calibrated in a water bath to the nearest 0.1°C between 25.0 and 40.0°C in ∼5.0°C increments with a precision digital thermometer (Model 15-077-8, Fisherbrand, United States) before attachment. Ts of each individual bird was calculated from the interval between two pulses following the calibration curve (R2 > 0.98), and was recorded automatically in 10-min intervals with receiver/loggers fitted with an H-frame antenna (Titley Electronics or Telonics Inc., ARI, United States) placed near the roost site. Transmitters remained attached to the birds for 7–15 days, and we recorded Ts over a total of 30 days. Birds were active during the day and mostly remained within range of the logger. We excluded from the analysis daytime data for one bird, which often was beyond range during the day. We also excluded nights where signal was lost (as a result of bird moving or signal interrupted), which resulted in a total of 7 measurement nights for 3 individuals and 6 measurement nights for 1 individual and a total of 2678 Ts data points.
Air temperature (Ta) was recorded every 10 min using four temperature loggers (iButton DS1922L, 0.06°C resolution, Maxim Integrated Products, Inc., Sunnyvale, CA, United States) placed on the southern (shady) side of trees near the roosting locations. We fitted two separate linear mixed effect models with bird identity as a random effect to explain (1) minimum Ts as a function of body mass and mean Ta and (2) minimum Ts as a function of body mass and minimum Ta as explanatory variables. Second, we also ran a linear mixed effect model with average Ts range (maximum Ts–minimum Ts), again as a function of body mass and mean and minimum Ta in two separate models. The torpor threshold was defined as a reduction by >5°C below the resting Ts (Schleucher, 2004; Ruf and Geiser, 2015). The resting Ts was calculated as the average minimum Ts during daytime, between 10:00 and 15:00 of two of our studied individuals, which had sufficient daytime measurements to calculate average minimum Ts.
Statistical analysis was conducted using R version 3.6.0 (R Development Core Team).1 The R-function lme in R package nlme was used for the mixed effect models (Pinheiro et al., 2020). Data are presented as the mean ± s.d. of individual mean daily values.
The average daytime Ta (between sunrise and sunset) at our study site for the eastern robin was 9.5 ± 4.3°C (range −3.5 to 19.5°C) and average night-time Ta was 4.0 ± 3.1°C (range −3.5 to 10°C). The average maximum and minimum Ta recorded were 14.3 ± 2.2°C and 1.3 ± 3.1°C, respectively. Ta dropped below 0°C on three days (Figure 1). Rain (21 and 7 mm) occurred only on 2 days (26 and 27 July), and coincide with Ts measurements of only one bird. We therefore did not analyze the effect of rain on torpor use in this study.
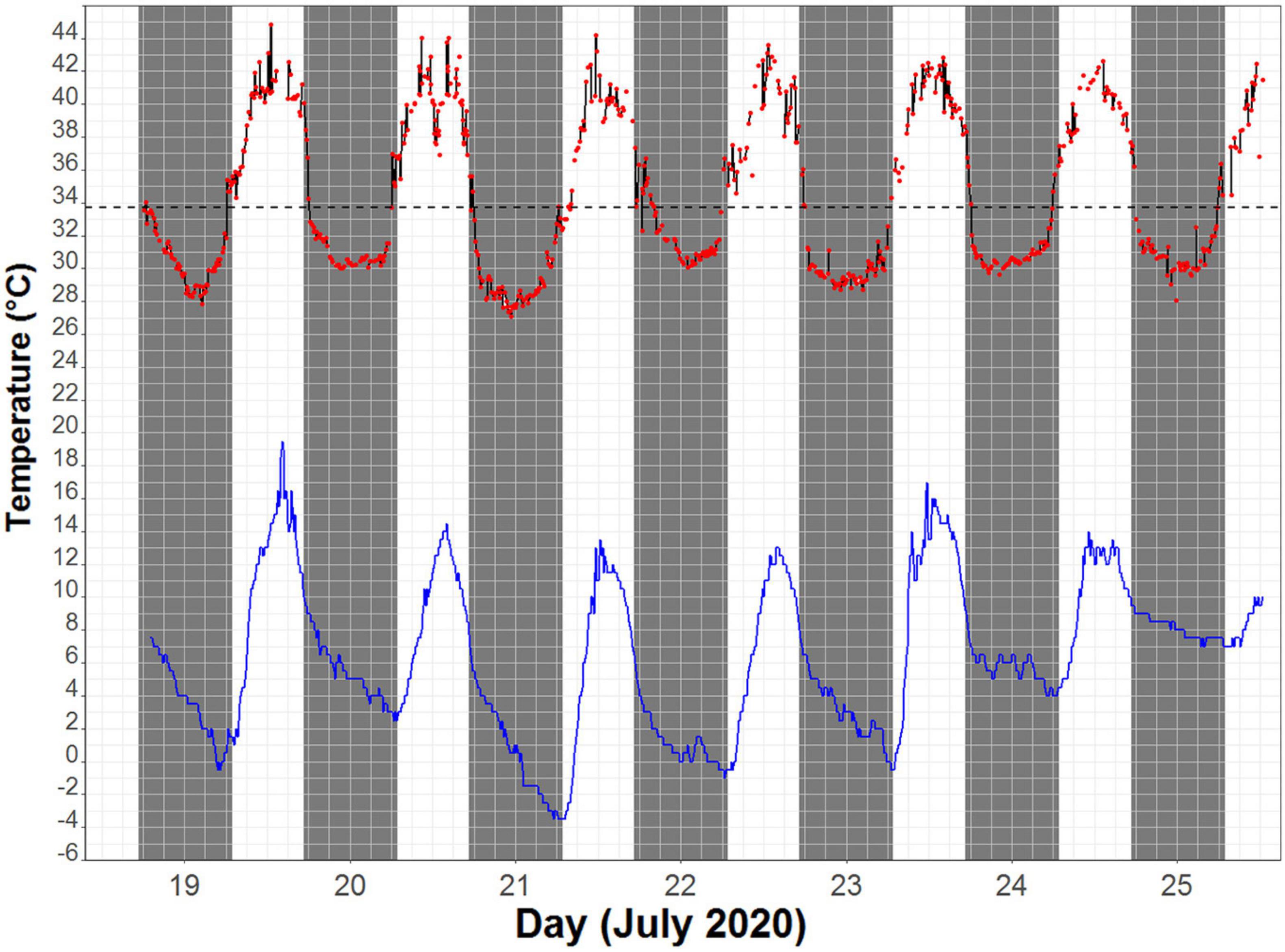
Figure 1. An example of skin temperature (Ts) fluctuation of one individual (red line) and the corresponding ambient temperature (Ta) measured near the roosting site (blue line) over a 7-day period. Black dashed line depicts the torpor threshold (33.7°C), calculated as 5°C below the resting Ts.
The Ts of eastern robins decreased from an average maximum Ts of 39.6 ± 3.1°C (range 44.8–34.0°C), 0.3 ± 0.3 h after sunset (19:10 h), to average minimum Ts of 30.1 ± 2.3°C (range 26.0–34.6°C). The torpor threshold was set at 33.7°C based on average resting Tb of 38.7°C, which is similar to a rest phase Tb of 38.9°C found previously in birds from the order Passeriformes (Prinzinger et al., 1991). Torpor bout duration (TBD) during which the Ts remained below the torpor threshold lasted for 10.3 h on average, with a maximum individual TBD of 14 h. Rewarming of Ts (the time in the morning where Ts was only followed by higher Ts for at least six consecutive measurements) started −2.6 ± 0.9 h relative to sunrise (06:45 h), when the average Ta was 2.1 ± 2.9°C. Minimum Ts was reached at 00:36 h on average (range between 21:21 and 02:41; Figure 2). Average individual daily fluctuations of Ts were 9.1 ± 3.9°C (range: 3.4–17.1°C), with maximum daily Ts range of 17.1°C recorded on 20 July (11:21 h), when the Ts of one individual dropped to 27.1°C (at a Ta of 0.5°C), the absolute minimum recorded (Figure 1).
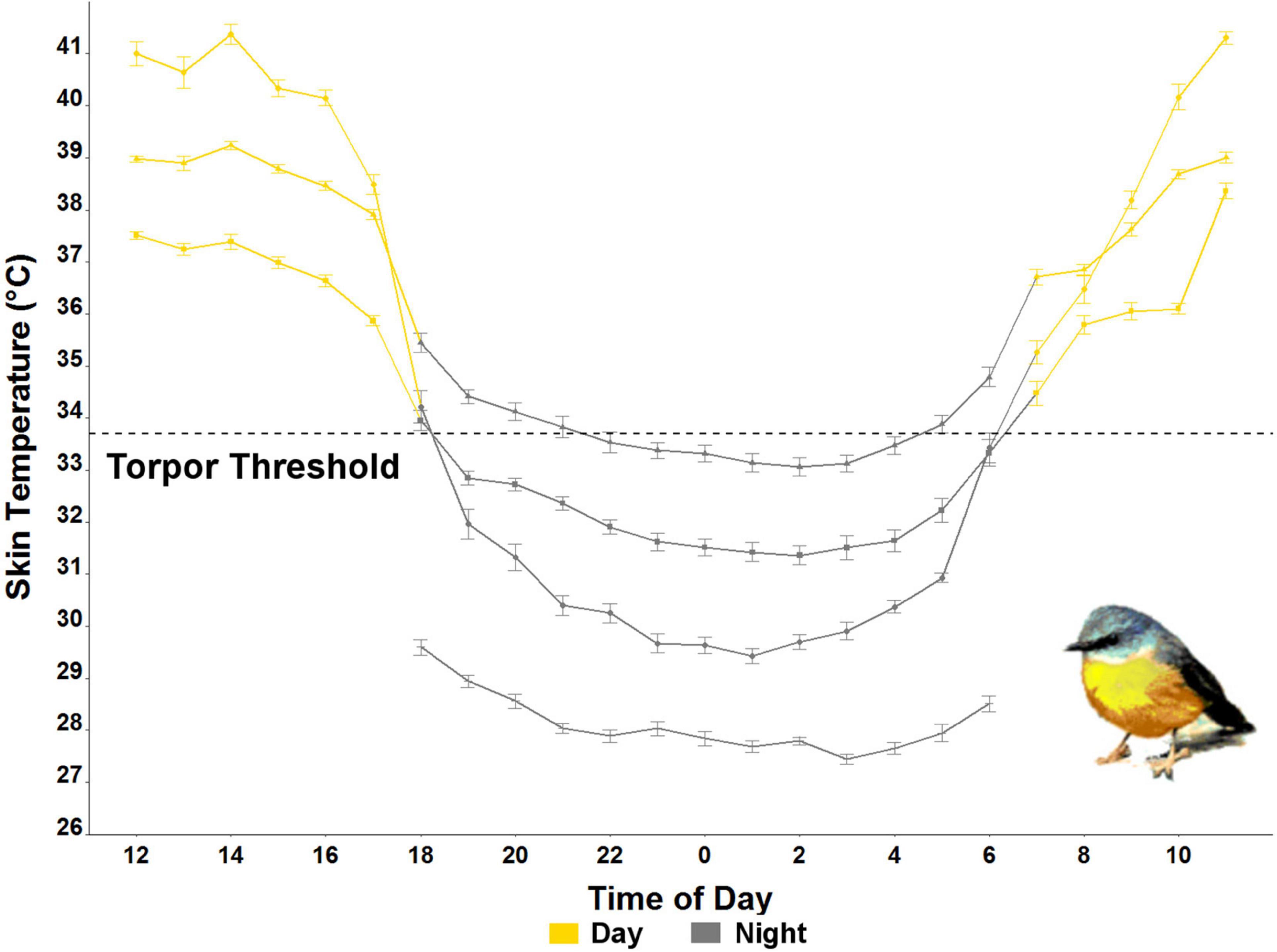
Figure 2. Average hourly skin temperature (Ts) ± standard error of the four individual eastern yellow-robins measured in July in the wild in NSW, Australia (2020). The torpor threshold (black dashed line at 33.7°C) was calculated as 5°C below the resting Ts.
The average minimum Ts, and average daily range of Ts of the eastern robin were significantly affected by average Ta (Ts = 28.01 + 0.34Ta, df = 22, t = 3.18, P < 0.01; Ts range = 2.37–0.05Ta, df = 19, t = −2.25, p < 0.05, respectively), and by minimum Ta (Ts = 29.82 + 0.18Ta, df = 22, t = 2.21, P < 0.05; Tsrange = 2.13–0.03Ta, df = 19, t = −2.30, p < 0.05, respectively), but were independent of body mass at capture (p > 0.05).
We provide new evidence that the eastern robin, a small ∼20 g Australian passerine, uses nocturnal torpor and reduces its night-time Ts during winter to as low as 27.1°C, with TBD lasting over 10 h on average. At the time rewarming began, Tas were still low, on some days below 0°C, and rewarming commenced almost 3 h before sunrise (Figure 1), suggesting endogenously controlled rewarming to prepare for the active phase of morning foraging (Hiebert, 1992).
Although our measurements are based on only four individuals and Ts was used to quantify their thermal biology, we contend that our data are meaningful. All individuals showed a similar, highly predictable daily Ts change and while there is some gradient between core Tb and Ts (Lovegrove and Smith, 2003), Ts is very close to core Tb in small birds, variation being less than 2°C in the 50 g common poorwill (Brigham, 1992), and not exceeding 4°C even in an 80 g owl (Smit and Mckechnie, 2010). In comparison to a rest phase Tb of 38.9°C in birds from the order Passeriformes (Prinzinger et al., 1991), Ts reduction in our study is on average 8.7°C, with maximum reduction of 12.9°C. Moreover, if Ta was influencing the measurement Ts significantly, we would expect to see Ts fluctuate closely with Ta. Instead, Ts started to increase when Ta was still low (about 2°C on average) and even falling (Figure 1). Therefore, even with a small error from the Ts measurements, the data clearly show that the birds used nocturnal torpor.
The magnitude of nocturnal Ts drop during torpor increased with decreasing mean and minimum Ta. This relationship was previously found in other passerine species (Reinertsen and Haftorn, 1983; Nord et al., 2009, 2011; Romano et al., 2019) and supports the assumption that the controlled reduction of Ts aims to conserving energy during energetically expensive periods, such as high energy expenditure required for thermoregulation. A reduction of energy expenditure by 10–20% was demonstrated in several captive avian species when exposed to Ta 0–10°C, with Tb reduction of 3–11°C [mountain chickadees Poecile atricapillus, and juniper titmice Baeolophus ridgwayi (Cooper and Gessaman, 2005); willow tit Parus montanus (Reinertsen and Haftorn, 1984)]. Captive noisy miners Manorina melanocephala reduced metabolic rate by ∼40% when they were measured under Tas of 0–15°C, with a Tb drop of only 4°C (Geiser, 2019). To calculate the predicted metabolic reduction in our studied eastern robins, we followed the equation for metabolic rate M = C′ (Tb-Ta) (Snyder and Nestler, 1990), where M = metabolic rate and C′ = thermal conductance. C was set as 0.186 following Douglas (2017) for western robin, a closely related species of similar size as the eastern robin, because our birds were in steady-state torpor and thermal conductance under such conditions is strongly related to body mass (Schleucher and Withers, 2001; Geiser, 2004) and is often steady below TNZ over a wide range of Ta [but may deviate under extreme conditions; Fristoe et al. (2015)]. Based on these calculations, we estimated that the metabolic rate of a 20 g eastern robin during torpor was about 4.9 ml O2 g–1h–1 at Ta = 0.5°C compared to predicted metabolic rate of over 7 ml O2 g–1h–1 at the same Ta if the bird remained euthermic. Our calculated MR of torpid birds is still above BMR of eastern robin (3.28 ml O2 g–1 h–1; Bech et al., 2016), but this is often the case during nocturnal torpor in small passerines (e.g., Maddocks and Geiser, 2000). Importantly, the calculation predicts that the eastern robin reduced MR during torpor by about 24% derived from the average minimum Ts reduction, compared to its rest-phase Ts of 38.7°C. This prediction is appropriate here because eastern robins maintained a Tb-Ta differential of >25°C via thermoregulation during torpor, unlike many torpid mammals, which when thermoconforming, can have Tb-Ta differentials of often <1°C (Geiser, 2021). When the minimum recorded Ts of 27.1°C was used for the calculations, the MR was reduced by about 32%.
Our data suggest torpor use by eastern robins as an energy saving strategy. Although our study only report data from four individuals, it clearly demonstrates that eastern robins are capable of such energy savings through thermal flexibility. However, we do not argue that our results apply to the whole species. Over its entire range, local conditions are key elements in driving physiological strategies, and we therefore expect some variance in thermal energetics among geographically separated species and/or populations. Indeed, the western robin, a closely related species to the eastern robin (mean body mass 18.5 g), and the only other member of the genus Eopsaltria in Australia (Loynes et al., 2009), remained euthermic in Dryandra woodland National Park (mean Ts 35°C), with a Mediterranean climate, lower elevation site (228 m) in WA during winter (Douglas, 2017). The minimum Ts recorded of the western robins were about 5°C higher on average than that of the eastern robins. The variation in the thermal energetics between the western and eastern robins cannot be explained by the acute exposure to different Ta because night-time Ta in Dryandra dropped similarly to our study site, to a minimum Ta of −3.95°C (Figure 3). The two robin species are also very similar in external appearance and size, excluding body mass as a potential explanation for the observed differences in thermal biology. The climate in Dryandra is mild relative to Imbota (i.e., winter Ta rarely drop below 0°C; Bureau of Meteorology, 2021). Therefore, a potential explanation for these differences may be that long-term thermal adaptation to the conditions in the occupied habitat may drive geographical variation in physiological traits, likely to minimize the potential costs accompanying the use of torpor. Similar differences have been observed between captive bred and wild-caught feathertail gliders (Geiser and Ferguson, 2001) and between captive woodchucks originate from different populations (Fenn et al., 2009).
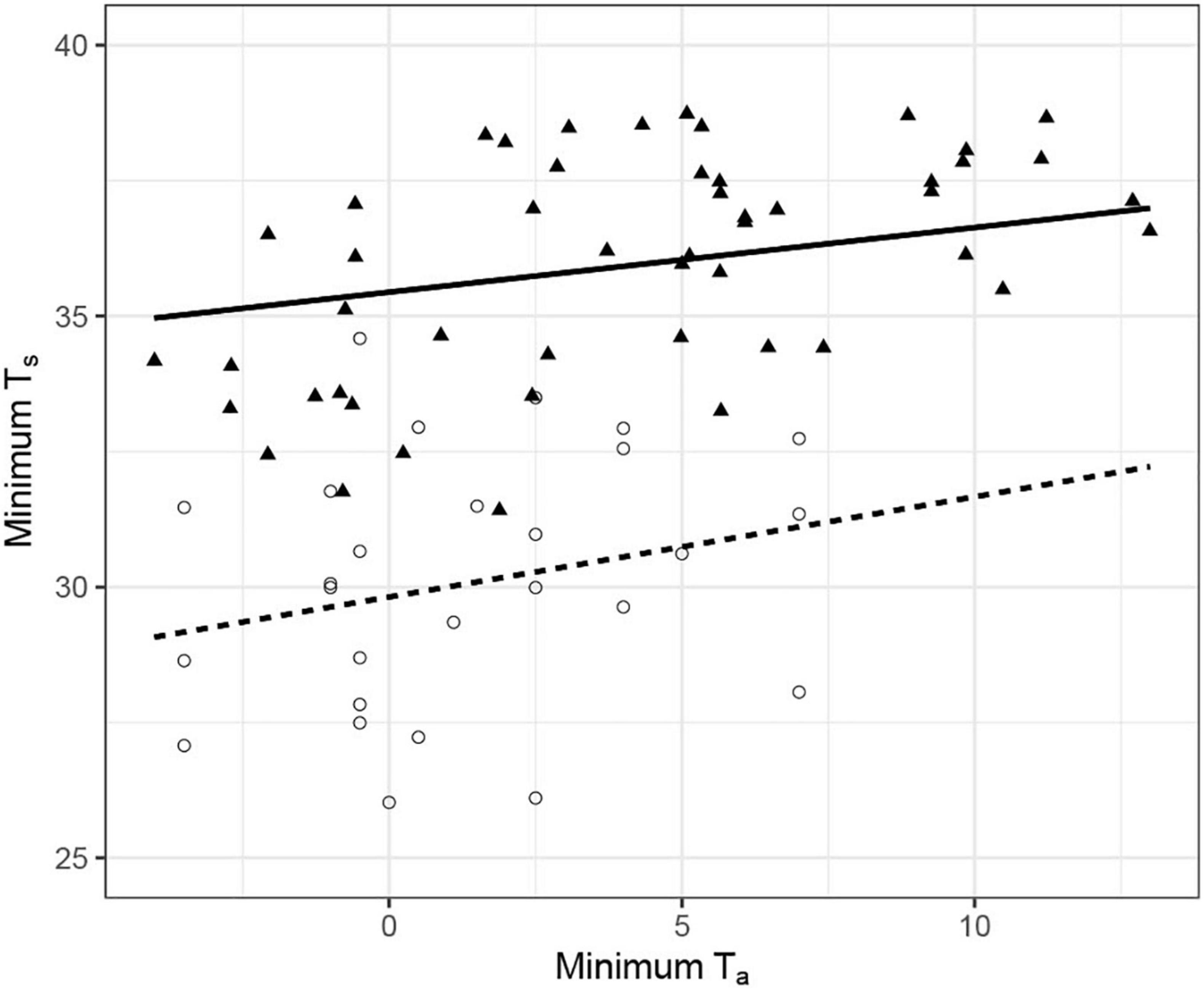
Figure 3. The relationship between minimum skin temperature (Ts°C) and ambient temperature (Ta°C) in the eastern (N = 4; open circles, dashed line) and western (N = 7; filled triangles, solid line) yellow robin. Lines were fitted with a linear mixed effect model with minimum Ts of robins from east and west population separately, in relation to minimum Ta and bird identity as a random effect. Data for the western yellow robin was extracted from Douglas (2017). Like eastern robins (details of statistics in the text), the minimum Ts of the wester robins were significantly affected by minimum Ta (Ts = 35.44 + 0.11Ta, df = 40, t = 2.86, P < 0.01).
Although the western robins did not express torpor like the eastern robins in our study, we do not argue that western robins, or any other passerine species which were studied in the wild and did not express torpor, are not capable of some form of heterothermy to conserve energy. Scarlet-backed flowerpeckers Dicaeum cruentatum, for example, which were studied in captivity for 3 years, appear to express torpor only in years with poor food quality (Bushuev et al., 2021). Other species have been considered homeothermic for decades until they were studied at the relevant conditions that trigger the use of torpor [e.g., mouse lemurs Microcebus murinus and M. myoxinus; (Ortmann et al., 1997)] and there are many other examples. Therefore, western robins, although they may not use torpor routinely as a means to balance their daily energy budget, still may express torpor under challenging conditions, such as drought or extreme cold, to increase the probability of survival.
Our study provides evidence for torpor expression in a small ∼20 g passerine, and suggests plasticity in the expression of torpor, in response to local environment. The resulting flexibility in energy requirements allows animals to optimize energy conservation to increase fitness and has the advantage over strict homeotherms during the current increasing frequency of extreme and unpredictable weather events, driven by changing climate. Indeed, despite range reduction and extreme climatic events, eastern yellow robins manage to survive in a marginal reserve and perhaps torpor expression permits them to overcome these energetically challenging periods and maintain fitness. The question that remains to be answered is whether individuals that previously did not encounter extreme climatic events, or local climatic changes, are able to apply thermal strategies to adjust to new environmental conditions. Resolving these questions is important in light of climatic changes that are expected to change local conditions for populations, but also for translocation conservation programs (Cooper et al., 2018), which need to consider the physiological limits of introduced species.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the University of New England Animal Ethics Committee (Authority No. AEC20-009).
YA-R and FG formulated the idea and designed the study. YA-R, CB, and JM performed the fieldwork. YA-R performed the data analysis with advice from JM. YA-R wrote the manuscript with advice from all other authors. All authors contributed to the article and approved the submitted version.
The study was funded by the University of New England-PDF program awarded to YA-R.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Kathrin Dausmann for valuable comments on previous version of the manuscript, Gerhard Körtner for programming the loggers and advice throughout the study, Sharon McGavin and Chris Wacker for help in the field and Christine Cooper and Tegan Douglas for their permission to use the data on western yellow robin.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.759726/full#supplementary-material
Amo, L., Caro, S. P., and Visser, M. E. (2011). Sleeping birds do not respond to predator odour. PLoS One 6:e27576. doi: 10.1371/journal.pone.0027576
Angilletta, M., Cooper, B. S., Schuler, M. S., and Boyles, J. G. (2010). The evolution of thermal physiology in endotherms. Front. Biosci. 2, 861–881.
Bech, C., Chappell, M. A., Astheimer, L. B., Londoño, G. A., and Buttemer, W. A. (2016). A ‘slow pace of life’in Australian old-endemic passerine birds is not accompanied by low basal metabolic rates. J. Comp. Physiol. 186, 503–512. doi: 10.1007/s00360-016-0964-6
Brigham, R. M. (1992). Daily torpor in a free-ranging goatsucker, the common poorwill (Phalaenoptilus nuttallii). Physiol. Zool. 65, 457–472. doi: 10.1086/physzool.65.2.30158263
Brodin, A., Nilsson, J.-A., and Nord, A. (2017). Adaptive temperature regulation in the little bird in winter: predictions from a stochastic dynamic programming model. Oecologia 185, 43–54. doi: 10.1007/s00442-017-3923-3
Bucher, T. L., and Worthington, A. (1982). Nocturnal hypothermia and oxygen consumption in manakins. Condor 84, 327–331.
Bureau of Meteorology (2021). Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/averages/tables/cw_056238.shtml and http://www.bom.gov.au/climate/averages/tables/cw_010614.shtml. [Online]. [Accessed 30/04/2021 2021]
Burnett, K., Zipple, M. N., Phillips, L. T., Panwar, P., Mcguire, L. P., and Boyle, W. A. (2019). Nocturnal reductions in body temperature in high-elevation Neotropical birds. Trop. Ecol. 60, 581–586.
Bushuev, A., Zubkova, E., and Kerimov, A. (2021). Evidence of torpor in a tropical passerine, the Scarlet-backed Flowerpecker Dicaeum cruentatum. Ornitholog. Sci. 20, 213–222.
Chaplin, S. B. (1976). The physiology of hypothermia in the black-capped chickadee, Parus atricapillus. J. Comp. Physiol. 112, 335–344. doi: 10.1007/bf00692303
Cooper, C. E., Withers, P. C., Munns, S. L., Geiser, F., and Buttemer, W. A. (2018). Geographical variation in the standard physiology of brushtail possums (Trichosurus): implications for conservation translocations. Conservat. Physiol. 6:coy042. doi: 10.1093/conphys/coy042
Cooper, S. J., and Gessaman, J. A. (2005). Nocturnal hypothermia in seasonally acclimatized mountain chickadees and juniper titmice. Condor 107, 151–155. doi: 10.1086/342256
Debus, S. J. S. (2006). Breeding and population parameters of robins in a woodland remnant in northern New South Wales. Australia. Emu-Austral Ornithol. 106, 147–156. doi: 10.1071/mu04013
Debus, S. J. S., and Ford, H. A. (2012). Responses of Eastern Yellow Robins Eopsaltria australis to translocation into vegetation remnants in a fragmented landscape. Pacific Conserv. Biol. 18, 194–202. doi: 10.1071/pc130194
Douglas, T. K. (2017). Thermoregulatory responses of Australian birds to environmental challenges (Doctoral dissertation). Doctoral dissertation. Australia: Curtin University.
Downs, C. T., and Brown, M. (2002). Nocturnal heterothermy and torpor in the malachite sunbird (Nectarinia famosa). Auk 119, 251–260. doi: 10.1093/auk/119.1.251
Dunbar, M. B., and Brigham, R. M. (2010). Thermoregulatory variation among populations of bats along a latitudinal gradient. J. Comp. Physiol. 180, 885–893. doi: 10.1007/s00360-010-0457-y
Fenn, A. M., Zervanos, S. M., and Florant, G. L. (2009). Energetic relationships between field and laboratory woodchucks (Marmota monax) along a latitudinal gradient. Ethol. Ecol. Evol. 21, 299–315. doi: 10.1080/08927014.2009.9522485
Fristoe, T. S., Burger, J. R., Balk, M. A., Khaliq, I., Hof, C., and Brown, J. H. (2015). Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals. Proc. Natl. Acad. Sci. 112, 15934–15939. doi: 10.1073/pnas.1521662112
Geiser, F. (2004). Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. doi: 10.1146/annurev.physiol.66.032102.115105
Geiser, F. (2019). Frequent nocturnal torpor in a free-ranging Australian honeyeater, the noisy miner. Sci. Nat. 106:28. doi: 10.1007/s00114-019-1626-9
Geiser, F. (2021). Ecological Physiology of Daily Torpor and Hibernation. Switzerland: Springer Nature.
Geiser, F., and Ferguson, C. (2001). Intraspecific differences in behaviour and physiology: effects of captive breeding on patterns of torpor in feathertail gliders. J. Comp. Physiol. 171, 569–576. doi: 10.1007/s003600100207
Geiser, F., Holloway, J. C., Körtner, G., Maddocks, T. A., Turbill, C., and Brigham, R. M. (2000). “Do patterns of torpor differ between free-ranging and captive mammals and birds?,” in Life in the cold, eds G. Heldmaier and M. Klingenspor (Berlin: Springer), 95–102.
Hiebert, S. M. (1992). Time-dependent thresholds for torpor initiation in the rufous hummingbird (Selasphorus rufus). J. Comp. Physiol. 162, 249–255. doi: 10.1007/BF00357531
Ives, N. (1973). Overnight torpidity in Australian arid-country birds. Emu-Austral Ornithol. 1973:73.
Körtner, G., Brigham, R. M., and Geiser, F. (2000). Winter torpor in a large bird. Nature 407:318. doi: 10.1038/35030297
Lambeck, R. J. (2002). Focal species and restoration ecology: response to Lindenmayer et al. Conserv. Biol. 16, 549–551. doi: 10.1046/j.1523-1739.2002.02007.x
Lovegrove, B. G., and Smith, G. A. (2003). Is ‘nocturnal hypothermia’a valid physiological concept in small birds?: a study on Bronze Mannikins Spermestes cucullatus. Ibis 145, 547–557. doi: 10.1046/j.1474-919x.2003.00166.x
Loynes, K., Joseph, L., and Keogh, J. S. (2009). Multi-locus phylogeny clarifies the systematics of the Australo-Papuan robins (Family Petroicidae, Passeriformes). Mole. Phylogenet. Evol. 53, 212–219. doi: 10.1016/j.ympev.2009.05.012
Maddocks, T. A., and Geiser, F. (2000). Seasonal variations in thermal energetics of Australian silvereyes (Zosterops lateralis). J. Zool. 252, 327–333. doi: 10.1111/j.1469-7998.2000.tb00627.x
Maddocks, T. A., and Geiser, F. (2007). Heterothermy in an Australian passerine, the dusky woodswallow (Artamus cyanopterus). J. Ornithol. 148, 571–577.
Mckechnie, A. E., and Lovegrove, B. G. (2002). Avian facultative hypothermic responses: a review. Condor 104, 705–724. doi: 10.1093/condor/104.4.705
Mckechnie, A. E., and Lovegrove, B. G. (2003). Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala). J. Comp. Physiol. 173, 339–346. doi: 10.1007/s00360-003-0341-0
Mcnab, B. K., and Weston, K. A. (2018). The energetics of torpor in a temperate passerine endemic to New Zealand, the Rifleman (Acanthisitta chloris). J. Comp. Physiol. 188, 855–862. doi: 10.1007/s00360-018-1175-0
Namekata, S., and Geiser, F. (2009). Effects of nest use, huddling, and torpor on thermal energetics of eastern pygmy-possums. Austral. Mammal. 31, 31–34.
Nicol, S. G., and Andersen, N. (1996). “Hibernation in the echidna: not an adaptation to the cold?,” in Adaptations to the Cold: Tenth International Hibernation Symposium, eds F. Geiser, A. J. Hubert, and S. C. Nicol (Armidale: University of New England Press), 7–12.
Nilsson, J. F., Nilsson, J.-A., Broggi, J., and Watson, H. (2020). Predictability of food supply modulates nocturnal hypothermia in a small passerine. Biol. Lett. 16:20200133. doi: 10.1098/rsbl.2020.0133
Nord, A., Nilsson, J. F., and Nilsson, J.-A. (2011). Nocturnal body temperature in wintering blue tits is affected by roost-site temperature and body reserves. Oecologia 167, 21–25. doi: 10.1007/s00442-011-1972-6
Nord, A., Nilsson, J. F., Sandell, M. I, and Nilsson, J.-A. (2009). Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J. Comp. Physiol. 179, 737–745. doi: 10.1007/s00360-009-0357-1
Nowack, J., Levesque, D. L., Reher, S., and Dausmann, K. H. (2020). Variable climates lead to varying phenotypes:‘weird’mammalian torpor and lessons from non-Holarctic species. Front. Ecol. Evol. 8:60.
Nowack, J., Tarmann, I., Hoelzl, F., Smith, S., Giroud, S., and Ruf, T. (2019). Always a price to pay: hibernation at low temperatures comes with a trade-off between energy savings and telomere damage. Biol. Lett. 15:20190466. doi: 10.1098/rsbl.2019.0466
O’connor, R. S., Brigham, R. M., and Mckechnie, A. E. (2017). Diurnal body temperature patterns in free-ranging populations of two southern African arid-zone nightjars. J. Avian Biol. 48, 1195–1204. doi: 10.1111/jav.01341
Ortmann, S., Heldmaier, G., Schmid, J., and Ganzhorn, J. (1997). Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften 84, 28–32. doi: 10.1007/s001140050344
Pinheiro, J., Bates, D., Debroy, S., and Sarkar, D. and R-Core-Team. (2020). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-139.
Prinzinger, R., Preßmar, A., and Schleucher, E. (1991). Body temperature in birds. Comp. Biochem. Physiol. Part A: Physiol. 99, 499–506.
Reher, S., and Dausmann, K. H. (2021). Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc. R. Soc. B 288:20202059. doi: 10.1098/rspb.2020.2059
Reinertsen, R. E., and Haftorn, S. (1983). Nocturnal hypothermia and metabolism in the Willow Tit Parus montanus at 63 N. J. Comp. Physiol. 151, 109–118. doi: 10.1007/bf00689908
Reinertsen, R. E., and Haftorn, S. (1984). The effect of short-time fasting on metabolism and nocturnal hypothermia in the Willow Tit Parus montanus. J. Comp. Physiol. 154, 23–28. doi: 10.1007/bf00683212
Romano, A. B., Hunt, A., Welbergen, J. A., and Turbill, C. (2019). Nocturnal torpor by superb fairy-wrens: a key mechanism for reducing winter daily energy expenditure. Biol. Lett. 15:20190211. doi: 10.1098/rsbl.2019.0211
Ruf, T., and Geiser, F. (2015). Daily torpor and hibernation in birds and mammals. Biol. Rev. 90, 891–926. doi: 10.1111/brv.12137
Schleucher, E. (2004). Torpor in birds: taxonomy, energetics, and ecology. Physiol. Biochem. Zool. 77, 942–949. doi: 10.1086/423744
Schleucher, E., and Withers, P. C. (2001). Re-evaluation of the allometry of wet thermal conductance for birds. Comp. Biochem. Physiol. Part A: Mole. Integr. Physiol. 129, 821–827. doi: 10.1016/s1095-6433(01)00356-7
Serventy, D. L. (1970). Torpidity in the white-backed swallow. emu-Austral Ornithol. 70, 27–28. doi: 10.1071/mu970027a
Shankar, A., Schroeder, R. J., Wethington, S. M., Graham, C. H., and Powers, D. R. (2020). Hummingbird torpor in context: duration, more than temperature, is the key to nighttime energy savings. J. Avian Biol. 2020:51.
Sharbaugh, S. M. (2001). Seasonal acclimatization to extreme climatic conditions by black-capped chickadees (Poecile atricapilla) in interior Alaska (64 °N). Physiol. Biochem. Zool. 74, 568–575. doi: 10.1086/322170
Smit, B., and Mckechnie, A. E. (2010). Do owls use torpor? Winter thermoregulation in free-ranging pearl-spotted owlets and African scops-owls. Physiol. Biochem. Zool. 83, 149–156. doi: 10.1086/605457
Smit, B., Boyles, J. G., Brigham, R. M., and Mckechnie, A. E. (2011). Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J. Biol. Rhythms. 26, 241–248. doi: 10.1177/0748730411402632
Snyder, G. K., and Nestler, J. R. (1990). Relationships between body temperature, thermal conductance, Q 10 and energy metabolism during daily torpor and hibernation in rodents. J. Comp. Physiol. B 159, 667–675. doi: 10.1007/BF00691712
Stawski, C., and Geiser, F. (2011). Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? Am. J. Physiol. Regulat. Integr. Comparat. Physiol. 301, R542–R547. doi: 10.1152/ajpregu.00792.2010
Steen, J. (1958). Climatic adaptation in some small northern birds. Ecology 39, 625–629. doi: 10.2307/1931602
Turbill, C., and Stojanovski, L. (2018). Torpor reduces predation risk by compensating for the energetic cost of antipredator foraging behaviours. Proc. R. Soc. B 285:20182370. doi: 10.1098/rspb.2018.2370
Watson, J., Freudenberger, D., and Paull, D. (2001). An assessment of the focal-species approach for conserving birds in variegated landscapes in southeastern Australia. Conserv. Biol. 15, 1364–1373. doi: 10.1046/j.1523-1739.2001.00166.x
Watson, J., Watson, A., Paull, D., and Freudenberger, D. (2002). Woodland fragmentation is causing the decline of species and functional groups of birds in southeastern Australia. Pacific Conserv. B iol. 8, 261–270. doi: 10.1071/pc030261
Wolf, B. O., Mckechnie, A. E., Schmitt, C. J., Czenze, Z. J., Johnson, A. B., and Witt, C. C. (2020). Extreme and variable torpor among high-elevation Andean hummingbird species. Biol. Lett. 16:20200428. doi: 10.1098/rsbl.2020.0428
Zanette, L. (2000). Fragment size and the demography of an area-sensitive songbird. J. Anim. Ecol. 69, 458–470. doi: 10.1098/rspb.2003.2620
Keywords: torpor, thermoregulation, passerines, metabolism, climate change, heterothermy, geographical variation, yellow robin
Citation: Aharon-Rotman Y, McEvoy JF, Beckmann C and Geiser F (2021) Heterothermy in a Small Passerine: Eastern Yellow Robins Use Nocturnal Torpor in Winter. Front. Ecol. Evol. 9:759726. doi: 10.3389/fevo.2021.759726
Received: 16 August 2021; Accepted: 19 November 2021;
Published: 16 December 2021.
Edited by:
Andreas Nord, Lund University, SwedenReviewed by:
J. F. Staples, Western University, CanadaCopyright © 2021 Aharon-Rotman, McEvoy, Beckmann and Geiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaara Aharon-Rotman, eWFhcmEuYWhhcm9uLXJvdG1hbkB1bmUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.