
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 21 October 2021
Sec. Behavioral and Evolutionary Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.759438
Animals often exhibit conspicuous, and sometimes curious, courtship traits, such as nestling-like courtship display in birds, though modern studies of nestling-like courtship display (and calls) are virtually lacking. An exception is previous experiments on the barn swallow Hirundo rustica, demonstrating that females are equally attracted to playback of two structurally similar calls, nestling-like male courtship calls and nestling food-begging calls. The experiments support the sensory trap hypothesis, i.e., that male signals mimic nestling stimuli to exploit female parental care for nestlings. However, female attraction might not be the sole function of nestling-like traits, and males might also have a sensory bias toward nestling-like traits, in which males would be less aggressive toward characteristics typical of immature individuals. Here, I conducted playback experiments to study the function of nestling-like male courtship calls in the context of male–male interactions. Playback of male courtship songs induced frequent approaches by neighbouring males, while nestling-like male courtship calls or nestling food-begging calls induced fewer approaches, though male responses to the latter two vocalisations increased when approaching the nestling period. The observed pattern indicates that, by mimicking immature individuals, males attract intended signal receivers (i.e., females) while avoiding interference from eavesdroppers (i.e., neighbouring males). This unique function can explain why species with parental care exhibit immature-like behaviour.
Animals often perform conspicuous, and sometimes curious, courtship behaviours, which attract the attention of evolutionary ecologists to study the origin and maintenance of these behaviours (Andersson, 1994). Nestling-like courtship displays in birds are one such behaviour: courting males (and females) often perform nestling-like behaviours, including wing fluttering and/or nestling-like vocalisation in many bird species (Bradbury and Vehrencamp, 1998). The resemblance to young may have evolved as a sensory trap to exploit the parental care of potential mates (Christy, 1995; Stålhandske, 2002), although there is only one series of experimental tests of the sensory trap hypothesis in birds (see below).
Hasegawa and colleagues experimentally demonstrated that, during the courtship period, female barn swallows Hirundo rustica were, similarly, attracted to playback of two structurally similar calls: male enticement calls (i.e., nestling-like courtship calls that are emitted from nest sites to attract females) and nestling food-begging calls, supporting the sensory trap hypothesis [e.g., Hasegawa et al., 2013; Hasegawa and Arai, 2016; reviewed in Hasegawa (2018); also see Hasegawa and Arai, 2018 for close adult-young resemblance of appearance in parental, but not non-parental, birds]. This explains why male barn swallows emit enticement calls rather than courtship songs when attracting females to their nest sites (e.g., see Supplementary Figure 1 for sonograms; see Hasegawa et al., 2016a for videos). In other words, as cuckoos exploit the parental care behaviour of the host species by mimicking the begging behaviour of their nestlings, males might exploit conspecific female parental care for nestlings (Hasegawa et al., 2013). Males utter the enticement calls only when attracting females to their nest sites, which seems necessary for males to pair (and use complex songs in other timings; Hasegawa et al., 2013).
Female attraction, however, might not be the sole function of courtship signals. It is now well-known that male courtship traits such as songs can be dual-function signals, by attracting females and stimulating rival males [e.g., see Berglund et al., 1996 for a general pattern; see Galeotti et al., 1997; Wilkins et al., 2015 for the song of the barn swallow; reviewed in Turner (2006)]. Even when courtship displays are solely directed at females, these displays can be exploited by eavesdroppers, including neighbouring males, which can affect the design of courtship signals (e.g., see Reichard and Anderson, 2015 for the use of low-amplitude song to avoid eavesdroppers). Because nestling-like courtship displays attract females as a sensory trap to exploit female parental care for nestlings (i.e., approaching to the vocalisation; see above), males might also have a sensory bias toward nestling-like behaviours, behaving less aggressively toward characteristics typical of immature birds (sensu Foster, 1987; see Turner, 2006 for tolerance of adult swallows toward food-begging young). In other words, male agonistic response to neighbouring males would be cancelled out by their tolerant response to immature birds. Nestling-like traits might therefore function to avoid interference from neighbouring males.
Here, I experimentally examined male responses to the playback of male enticement calls, male courtship songs, and nestling food-begging calls in the barn swallow. I predicted that, if nestling-like traits function to avoid interference from neighbouring males, there would be fewer male responses to male enticement calls and nestling food-begging calls than to male courtship songs. Although males would have limited intrinsic responsiveness to nestling calls compared with females due to the uncertain paternity [e.g., Møller, 1988; reviewed in Turner (2006)], I also predicted that male responses to male enticement calls and nestling food-begging calls would increase when approaching the nestling period, during which parents respond to nestling food-begging calls (e.g., Saino et al., 2000).
This study was conducted in a residential area in Tsurugi-machi (ca. 35 km2), Ishikawa Prefecture, Japan (36°26N, 136°37E) from late-March to May 2019–2021. In this area, barn swallows nest under the eaves of a covered sidewalk along the street (Hasegawa and Arai, 2016). This is in sharp contrast to the dense colonies often found in European subspecies [e.g., a similar outdoor population has 20.39 ± 20.02 m (mean ± SD, N = 52) distance between the nearest males, whereas European colonies have much dense populations (ca. 3–5 m between the nearest males); Hasegawa et al., 2010; reviewed in Turner (2006)]. Due to their low population density, extrapair paternity is virtually absent in Japanese outdoor populations, possibly including this population (<3%; Hasegawa et al., 2010), and thus extrapair paternity would not confound the study design. Male enticement calls are, in fact, not used for extrapair mating (Møller, 1994; Turner, 2006). I inspected nests every third day to determine laying dates and clutch sizes (Hasegawa et al., 2016b). The permits for the current study including capturing were provided by Ishikawa Prefecture in Japan (#18148, #19109, and #20113) and Ishikawa Prefectural University (#31-14-1, #R2-14-1, and #R3-14-3), following the Wildlife Protection and Hunting Management Law.
After the start of incubation (i.e., after females completed their clutches), adult males were captured in sweep nets while roosting at night. The birds were fitted with a numbered aluminium ring and an individual combination of two coloured rings (Arai et al., 2009), and thus I could track the behaviour of the focal male. The sex of each individual was determined based on the presence or absence of a brood patch (Turner, 2006).
After capturing males, I conducted playback experiments between 0500 and 0800 h during the incubation period in 2019 and 2020 (1–13 days after the start of incubation, when all eggs remained unhatched) and during the pairing period in 2021, in which I used males with previously attached rings. I conducted three 10-min trials using 17 different males during the incubation period (14 males were tested in 2019 and the remaining 3 individuals were tested in 2020). Males were identified by their coloured rings to prevent confusion with other birds. In 2021, I conducted supplementary experiments using eight males that remained unpaired in the preceding day (i.e., eight different adult males were tested) to examine whether male responses observed in preceding years were unique to the incubation period or were applicable to the paring (and thus fertilization) period. Because three of them accompanied females at the start of the experiment, I also tested the effects of the treatment after excluding these seemingly “paired” males; see Supplementary Table 1). In each trial, I used a Sony SRS-M30-S speaker on a tripod to broadcast recordings of male enticement calls, male songs, and food-begging calls of nestlings with short intervals (1–10 min) between trials (i.e., after an interval of at least 1 min, although I had to wait a maximum of 10 min until the focal swallows returned after flying away).
I used three male enticement calls, three male songs, and three nestling food-begging calls for the playback experiments (see Supplementary Figure 1 for some examples). Male enticement calls and songs were obtained from courting males. All of these vocalisations were obtained during previous years from the Joetsu or Yokosuka populations (see Hasegawa et al., 2010; Hasegawa et al., 2016b for detailed information on these populations), and thus no males had ever heard these calls before. Each vocalisation (recorded as a .wav file) was obtained from a different nest; in the first ten trials, I randomly chose one of the two male enticement calls, one of the two male songs, and one of the two nestling food-begging calls; from the eleventh trial on, I started to use the third vocalisations (in addition to the other vocalisations) to minimise pseudoreplication (with no nests received the exact same call order with call ID). I broadcast each recording (again using a .wav file) approximately 3 m from a territory boundary (the sound level was about 70 dB(A) from a 1-m distance, which is audible from the focal males), as in the preceding study (Hasegawa and Arai, 2016; see Hasegawa et al., 2012 for detailed information of territory; note that Hirundo rustica gutturalis defend “visually isolated area” from the outsides and other territories, which corresponds to ca. 10–15 m2 in our study populations; Fujita and Higuchi, 2007). The exact broadcasting point was not visible from breeding nests of focal swallows and was not defended by any swallows (note that I did not change the exact broadcasting point between trials). In these populations, males sometimes emit enticement calls to attract nearby females in such empty sites before establishing their territories, and thus the current study design would not be unnatural. In other words, the current study focussed on the behaviour of breeding males to (perceived) late-arriving males. During the 10 min of playback, I recorded the number of male responses (i.e., the number of times males came within 1 m of the speaker, which is an appropriate measure in this highly aerial species: see Supplementary Videos 1a–c; also see Hasegawa et al., 2013; Hasegawa and Arai, 2016 for female responses; note that I can follow only one bird during the experiment).
A generalised linear mixed-effects model (GLMM) with a Poisson error distribution was used to test the effect of treatment (vocalisation type) on the number of male responses. Each playback trial (i.e., observation number) was included as a random factor, as was male identity, since the data were overdispersed (Bates et al., 2015). The order of presentation was first included as a fixed factor but was far from significant and hence removed from the model (pairing period; df = 2, χ2 = 0.54, P = 0.76; incubation period: df = 2, χ2 = 0.52, P = 0.77; also see Hasegawa and Arai, 2016 for the lack of a detectable effect of presentation order on the number of female responses to the playback of vocalisations). I also investigated the correlation among the numbers of male responses to each kind of call to study whether males that frequently responded to certain vocalizations (e.g., nestling food-begging calls), similarly responded to another (e.g., male enticement calls). The relationship between the number of male responses to each treatment and the progress of the incubation period (i.e., number of days after the start of incubation) was analysed using a GLM with a Poisson error distribution (using family = “quasipoisson”). For this analysis, I controlled for the potential confounding factors of observation date and male body condition, which would affect male responsiveness, to detect subtle patterns of male responses toward each vocalisation. I used body condition as residual body mass against tarsus length as in previous studies (Hasegawa et al., 2017). All data analyses were performed using R version 3.5.0 (R Core Team, 2018).
During the incubation period, vocalisation type had a significant effect on the number of male responses (GLMM with a Poisson error distribution: nmales = 17, ntotal = 51, χ2 = 15.30, P < 0.001). The number of male responses to male enticement calls was significantly lower than that to male songs (Figure 1 and Table 1; the number of male responses to nestling food-begging calls was also significantly lower than that to male songs: difference ±SE = −1.01 ± 0.38, z = 2.69, P < 0.01). Similar pattern was found during the pairing period (Supplementary Figure 2 and Supplementary Table 1), though sample size was limited (nmales = 8, ntotal = 24).
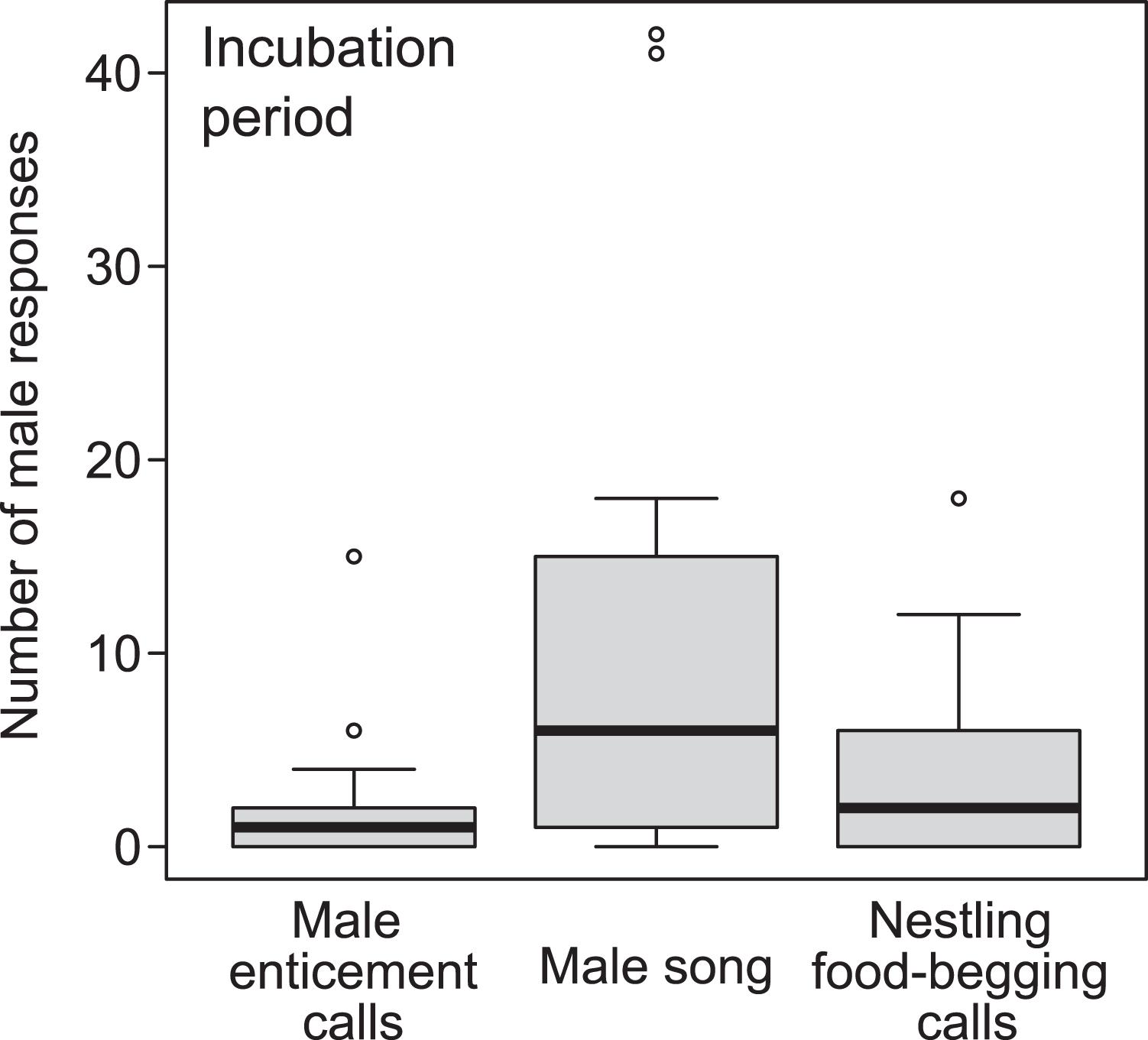
Figure 1. Boxplot of the number of male responses to each type of vocalisation during the playback experiment during the incubation period. The bar in each boxplot indicates the median value, and the box shows the first and third quartiles of data. The whiskers range from the lowest to the highest data points within 1.5× the interquartile range of the lower and upper quartiles, respectively. Data points beyond the range of the whiskers are outliers and are depicted by circles. Each call was broadcast in a random order.
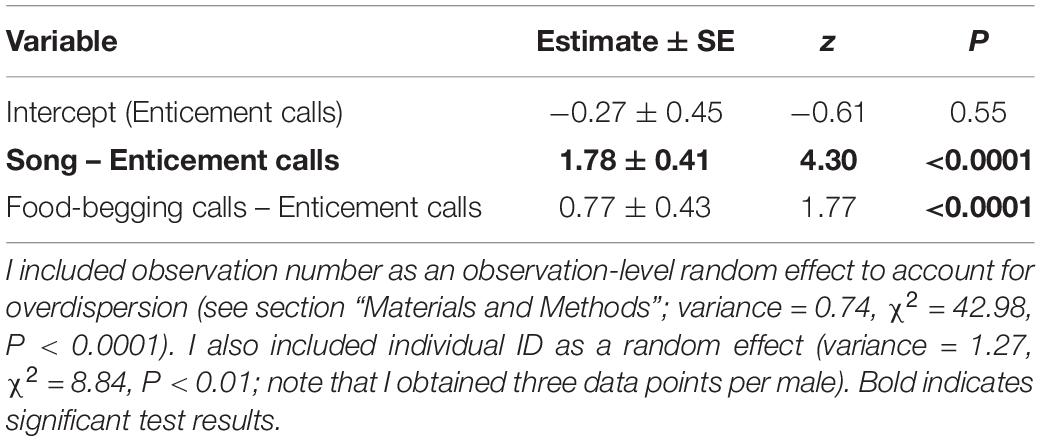
Table 1. Multivariable generalised linear mixed-effects model (GLMM) with a Poisson error distribution predicting the number of male responses to the experimental playback of male enticement calls, male songs, and nestling food-begging calls during the incubation period (nmale = 17, nobservation = 51).
No significant correlations were found between the numbers of male responses to each treatment (Spearman’s rank correlation coefficient, |rs| < 0.44, n = 17, P > 0.07), although the sample size was small. When the male responses to each vocalisation were analysed separately, the number of male responses to the playback of each treatment increased with the progress of the incubation period, although this relationship was not significant in the analysis of male responses to male songs (Figure 2 and Table 2). Rather, male responses to male songs marginally increased with body condition (Table 2 and Supplementary Figure 3).
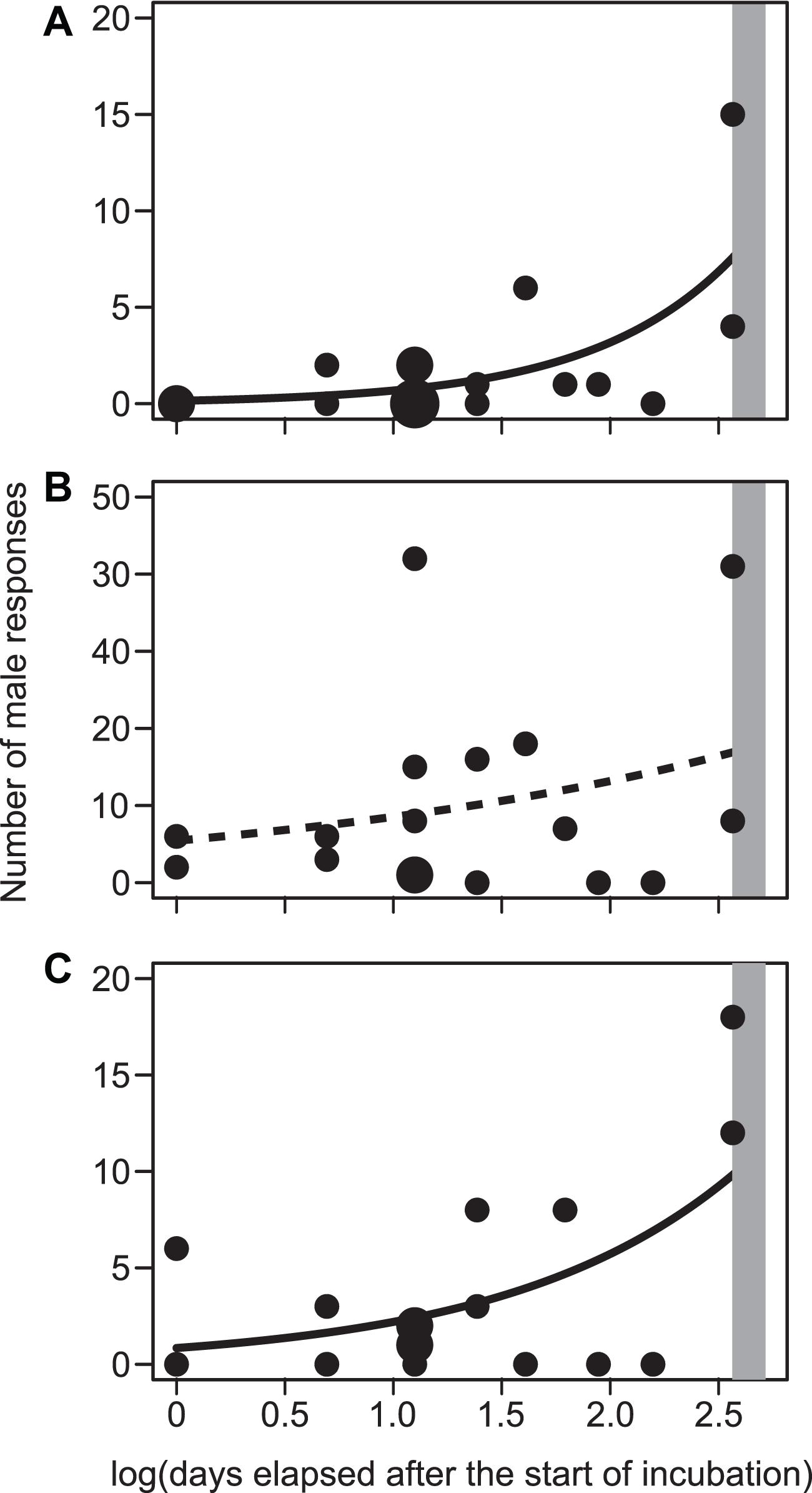
Figure 2. Relationship between the number of male responses to the playback of male enticement calls (A), male songs (B), and nestling food-begging calls (C) in relation to the progress of incubation period, measured as log(days elapsed after the start of incubation). Lines indicate simple Poisson regressions [using the glm function with family = “quasipoisson”; y = slope (±SE)x + intercept (±SE); a: y = 1.54 (±0.47)x –1.92 (±0.99); b: y = 0.44 (±0.42)x + 1.69 (±0.71); c: y = 0.96 (±0.41)x –0.17 (±0.78)]. Circle size indicates the number of overlapping points. Dashed line indicates non-significant regression lines. Shaded bars indicate mean hatching dates (13–15 days; Turner, 2006). I confirmed that all eggs remained unhatched during our experiment.
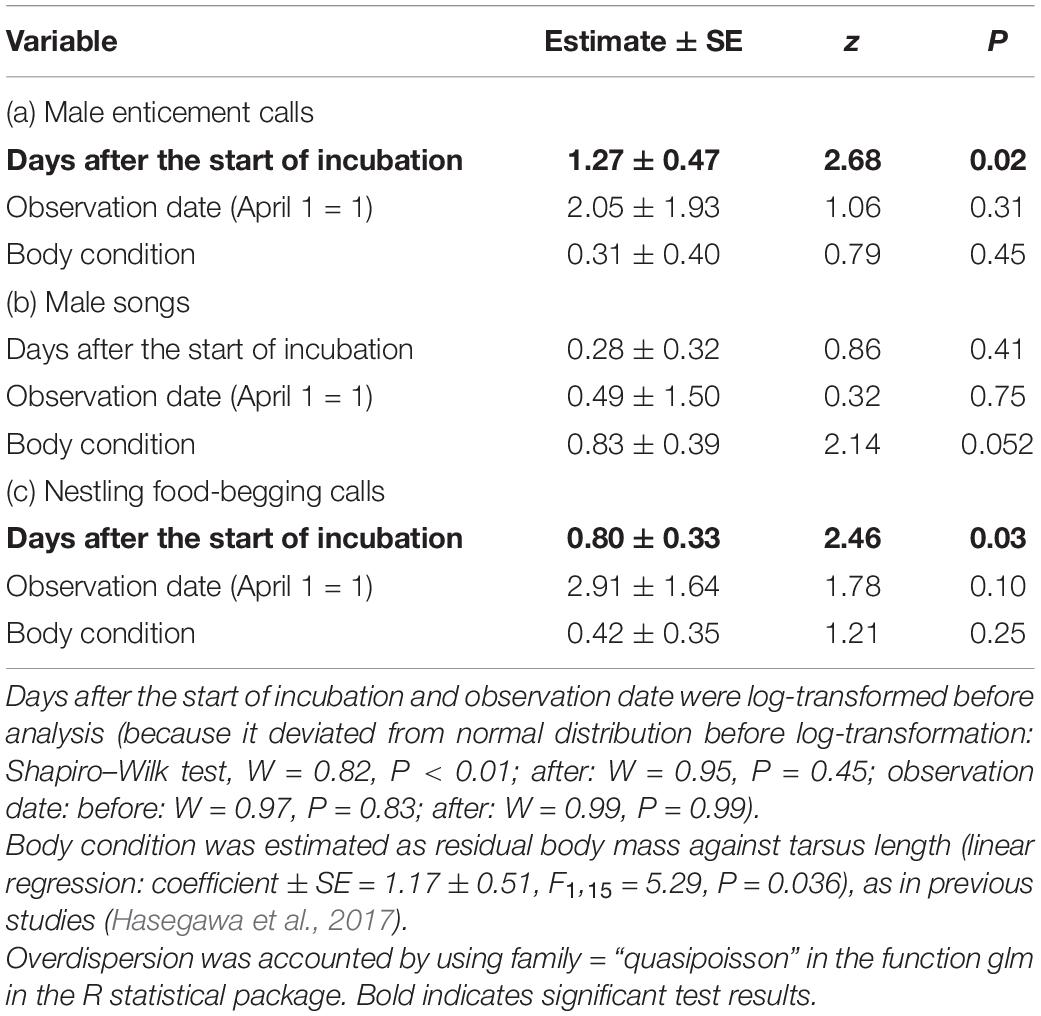
Table 2. Separate multivariable GLM with a Poisson error distribution predicting the number of male responses to the experimental playback of male enticement calls (a), male songs (b) and nestling food-begging calls (c), respectively (nmale = 17 each).
The main finding of the current study is that, although both male enticement calls and songs function as courtship signals, male songs elicited frequent male responses, while male enticement calls (and nestling food-begging calls) elicited fewer responses. This indicates that males emitting enticement calls rather than songs would be selected for by avoiding interference from early-breeding, neighbouring males. Because males ignored male enticement calls near their territory boundaries (see section “Materials and Methods”), male enticement calls might allow for high density breeding in the barn swallow, which often breeds in nests that are close to (though visually hidden from) other nests (Fujita and Higuchi, 2007).
Previous studies on courtship signals have often focussed on the positive responses of potential signal receivers, in which signals that enhance the receiver’s attention should be effective. An exception is the “soft song,” a low-amplitude, short-range song, which confers a mating/reproductive advantage to males by not attracting the attention of conspecifics simply because it is inaudible from a distance (e.g., Vargas-Castro et al., 2017; reviewed in Reichard and Anderson (2015)]. Male enticement calls might have similar functions but, unlike the soft song, male enticement calls are loud, long-range vocalisations, with which males attract females from nest sites even when the females cannot see them (Hasegawa et al., 2013). By using vocalisations that are effective at drawing a response from potential mates, but not neighbouring males, males can attract females from a distance without the cost of reduced communication with the signal receivers.
Because the enticement call is emitted during the courtship sequence for pair formation (Hasegawa et al., 2013), the tolerance of male enticement calls increases the possibility that other males will breed nearby. Thus, as is the case for the male courtship song, neighbouring males should keep signallers away, even outside of their own mating periods, from their territories to reduce nest competition for current and future clutches (Turner, 2006; also see Arai et al., 2009 for frequent re-clutches due to nest predation in Japanese barn swallows). An explanation for why neighbouring males rarely responded to male enticement calls is that males would be less aggressive toward characteristics typical of immature birds [sensu Foster, 1987; reviewed in Hawkins et al. (2012); see Turner, 2006 for tolerance of adult swallows toward food-begging young]. As females confuse male enticement calls with nestling food-begging calls (Hasegawa et al., 2013; Hasegawa and Arai, 2016), males might have similar sensory or perceptive bias toward nestling calls (i.e., non-hostile behaviour toward nestlings). Such male behaviour would be maladaptive but can have persisted through evolutionary time scale as was the case with other sensory trap systems even when mimics and models were temporally separated: for example, in some insects, male penis mimics egg ovulation to elicit females consuming the other males’ sperm stored [reviewed in Christy (1995) and Arnqvist (2006)]. In consistent with this perspective, male responses toward enticement calls increased with progress of the incubation period, i.e., when approaching the nestling period (Figure 2 and Table 2), supporting this sensory trap explanation, or more specifically, nestling-mimicry hypothesis. A previous study showed that less-competitive males with drab throat colouration mimic nestlings more than do colourful males (Hasegawa and Arai, 2016). This finding further supports the nestling-mimicry hypothesis, given that competitively inferior males should avoid, rather than compete with, dominant and colourful males that breed early in high-quality territories when they attempt pair formation among these competitive males (Hasegawa et al., 2014; Wilkins et al., 2015).
Clearly, this is not the sole explanation for the observed pattern, and males might tolerate nestling-like calls for other reasons. For example, males might ignore nestling-like calls and concentrate on songs to simplify their sensory pathways, which can effectively prevent most neighbours’ breeding attempts nearby, because courting males often, but not always, use songs before/after emitting enticement calls (see section “Introduction”). However, this possibility alone cannot explain the reason why male responsiveness increased when approaching the nestling period. The actual mechanism remains to be shown in future studies.
In conclusion, I demonstrated here that the male enticement call, a nestling-like courtship behaviour of barn swallows, elicited few responses from neighbouring males. Together with a previous study showing female attraction to nestling-like calls, the current study indicates that male enticement calls have a dual function. However, unlike male song, the nestling-like courtship call is unique in attracting females while being tolerated by neighbouring males. Males as well as females would have sensory bias (but have contrasting behaviour) toward signals with nestling characteristics, simultaneously selecting for nestling-like courtship signals. This could explain why species with parental care exhibit immature-like behaviour.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Ishikawa Prefectural University.
MH conducted all field work and wrote the manuscript.
I was supported by the Research Fellowship of the Japan Society for the Promotion of Science (19K06850).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
I am grateful to the residents of Ishikawa Prefecture for their kind support and assistance. I thank Emi Arai, Shumpei Kitamura, and his lab members at Ishikawa Prefectural University for their kindest advices. I also thank reviewers for their helpful comments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.759438/full#supplementary-material
Arai, E., Hasegawa, M., and Nakamura, M. (2009). Divorce and asynchronous arrival in Barn Swallows Hirundo rustica. Bird Study 56, 411–413. doi: 10.1080/00063650902968342
Arnqvist, G. (2006). Sensory exploitation and sexual conflict. Phil. Trans. R. Soc. B 361, 375–386. doi: 10.1098/rstb.2005.1790
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Berglund, A., Bisazza, A., and Pilastro, A. (1996). Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399. doi: 10.1006/bijl.1996.0043
Bradbury, J. W., and Vehrencamp, S. L. (1998). The Principles of Animal Communication. Sunderland, MA: Sinauer.
Christy, J. H. (1995). Mimicry, mate choice, and the sensory trap hypothesis. Am. Nat. 146, 171–181. doi: 10.1086/285793
Foster, M. S. (1987). Delayed maturation, neoteny, and social system differences in two manakins of the genus Chiroxiphia. Evolution 41, 547–558. doi: 10.1111/j.1558-5646.1987.tb05825.x
Fujita, G., and Higuchi, H. (2007). Barn swallows prefer to nest at sites hidden from neighboring nests within a loose colony. J. Ethol. 25, 117–123. doi: 10.1007/s10164-006-0005-0
Galeotti, P., Saino, N., Sacchi, R., and Møller, A. P. (1997). Song correlates with social context, testosterone and body condition in male barn swallows. Anim. Behav. 53, 687–700. doi: 10.1006/anbe.1996.0304
Hasegawa, M. (2018). Beauty alone is insufficient: female mate choice in the barn swallow. Ecol. Res. 33, 3–16. doi: 10.1007/s11284-017-1527-3
Hasegawa, M., and Arai, E. (2016). Female attraction to higher pitched male enticement calls in barn swallows. Ethology 122, 430–441. doi: 10.1111/eth.12492
Hasegawa, M., and Arai, E. (2018). Differential visual ornamentation between brood parasitic and parental cuckoos. J. Evol. Biol. 31, 446–456. doi: 10.1111/jeb.13240
Hasegawa, M., Watanabe, M., and Nakamura, M. (2016a). Promiscuous copulation attempts and discriminate pairing displays in male barn swallows as revealed by model presentation. Ethol. Ecol. Evol. 28, 163–174. doi: 10.1080/03949370.2015.1026404
Hasegawa, M., Arai, E., Ito, S., and Wakamatsu, K. (2016b). High brood patch temperature of less colourful, less pheomelanic female Barn Swallows. IBIS 158, 808–820. doi: 10.1111/ibi.12405
Hasegawa, M., Arai, E., Watanabe, M., and Nakamura, M. (2012). Female mate choice based on territory quality in barn swallows. J. Ethol. 30, 143–150. doi: 10.2326/osj.17.125
Hasegawa, M., Arai, E., Kojima, W., Kitamura, W., Fujita, G., Higuchi, H., et al. (2010). Low level of extra-pair paternity in a population of the Barn Swallow Hirundo rustica gutturalis. Ornithol. Sci. 9, 161–164. doi: 10.2326/osj.9.161
Hasegawa, M., Arai, E., Sato, M., and Sakai, H. (2017). Plasma testosterone levels increase with expression of male ornaments during mating, but not incubation, in Japanese barn swallows. Zool. Sci. 34, 261–266. doi: 10.2108/zs160187
Hasegawa, M., Arai, E., Watanabe, M., and Nakamura, M. (2013). Male nestling-like courtship calls attract female barn swallows Hirundo rustica gutturalis. Anim. Behav. 86, 949–953. doi: 10.1016/j.anbehav.2013.08.012
Hasegawa, M., Arai, E., Watanabe, M., and Nakamura, M. (2014). Colourful males hold high quality territories but exhibit reduced paternal care in barn swallows. Behaviour 151, 591–612. doi: 10.1163/1568539X-00003150
Hawkins, G. L., Hill, G. E., and Mercadante, A. (2012). Delayed plumage maturation and delayed reproductive investment in birds. Biol. Rev. 87, 257–274. doi: 10.1111/j.1469-185X.2011.00193.x
Møller, A. P. (1988). Paternity and paternal care in the swallow, Hirundo rustica. Anim. Behav. 36, 996–1005. doi: 10.1016/S0003-3472(88)80059-9
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Reichard, D. G., and Anderson, R. C. (2015). Why signal softly? The structure, function, and evolutionary significance of low-amplitude signals. Anim. Behav. 105, 253–265. doi: 10.1016/j.anbehav.2015.04.017
Saino, N., Ninni, P., Incagli, M., Calza, S., Sachi, R., and Møller, A. P. (2000). Begging and parental care in relation to offspring need and condition in the barn swallow (Hirundo rustica). Am. Nat. 156, 637–649. doi: 10.1086/316996
Stålhandske, P. (2002). Nuptial gifts of male spiders function as sensory trap. Proc. R. Soc. Lond. B 269, 905–908. doi: 10.1098/rspb.2001.1917
Vargas-Castro, L. E., Sandoval, L., and Searcy, W. A. (2017). Eavesdropping avoidance and sound propagation: the acoustic structure of soft song. Anim. Behav. 134, 113–121. doi: 10.1016/j.anbehav2017.10.0008
Keywords: courtship behaviour, Hirundo rustica, nestling-mimicry, signal design, dual function
Citation: Hasegawa M (2021) Male Barn Swallows Tolerate Nestling-Like Courtship Calls of Rival Males. Front. Ecol. Evol. 9:759438. doi: 10.3389/fevo.2021.759438
Received: 16 August 2021; Accepted: 30 September 2021;
Published: 21 October 2021.
Edited by:
Hope Klug, University of Tennessee at Chattanooga, United StatesReviewed by:
Mark Stanback, Davidson College, United StatesCopyright © 2021 Hasegawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masaru Hasegawa, cGVyb3JvYm9tdXNhZGlvYmVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.