- 1The Albert Katz International School for Desert Studies, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beer-Sheva, Israel
- 2Southern District, Israeli Nature and Parks Authority, Beer Sheva, Israel
- 3Mitrani Department of Desert Ecology, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beer-Sheva, Israel
The increasing pressure of ecotourism on wildlife in their natural habitats leads many wild animals to alter their behaviors. The restrictions issued in many places due to COVID-19 provide a rare opportunity to examine wildlife behavior in nature reserves with reduced human presence, and to reveal the impact of human visitation on the behaviors and fitness of local wildlife species. In 2019 and 2020 we placed trail cameras next to two natural springs in the Israeli Negev Desert, Ein-Avdat and Ein-Shaviv, located 9 km apart. Both sites serve as the main water source for local Nubian ibex (Capra nubiana) populations, but Ein-Avdat is situated within a popular national park into which visitors’ entrance was restricted due to COVID-19 regulations in 2020, while Ein-Shaviv is more remote and thus attracts only few visitors regardless of COVID-19 regulations. Our study revealed that during 2020, ibex in Ein-Avdat arrived to drink earlier in the day and the population’s Female:Kids ratio more than doubled. These changes were not observed in Ein-Shaviv. We found that the daily number of visitors in Ein-Avdat affected the arrival time of ibex to the water pool. We conclude that the reduced number of visitors to Ein-Avdat in 2020 compared to 2019 may have allowed ibex to arrive in preferred hours, and may have contributed to the increased kid-to-females ratio. Our study shows that behavioral adaptions to human visitation in nature reserves might carry a high fitness cost.
Introduction
Human’s attraction to nature probably offers one of the most effective tools for education and for raising awareness to nature conservation (Fennell, 2015; Soga and Gaston, 2016). From this point of view, ecotourism plays an important role in the conservation of species and ecosystems in a world where most regions are utilized and dominated by humans (Fulton, 2002; Foley, 2005). Nevertheless, as the global ecotourism market grows and the number of humans visiting national parks and protected areas around the globe keeps increasing, wildlife in protected areas are increasingly exposed to human disturbances. With the global ecotourism industry generating over eight billion ecotourists a year (Balmford et al., 2015), wildlife are being pressured either to displace into less favorable locations (Griffiths and Schaik, 1993; George and Crooks, 2006; Blumstein et al., 2017) or to develop behavioral adjustments (Griffiths and Schaik, 1993; Naylor et al., 2009; Marchand et al., 2014; Geffroy et al., 2015; Blumstein et al., 2017; Reilly et al., 2017).
Behavioral adjustments manifest themselves in various ways. For example, animals can shift their activity periods to reduce interactions with visitors (Griffiths and Schaik, 1993; Olson et al., 1998; Marchand et al., 2014; Reilly et al., 2017), with a recent meta-analysis showing that all over the world mammals are becoming more nocturnal to avoid encounters with humans (Gaynor et al., 2018). Other behavioral adjustments include trading-off the times devoted to certain activities over others (Naylor et al., 2009; Geffroy et al., 2017), which may have fitness consequences. For instant, investing more time in vigilance behavior, or spending more time in refuges, will negatively affect the time available for foraging (i.e., the well-studied predation-starvation tradeoff; Hochman and Kotler, 2007; Cañadas Santiago et al., 2020; Montero-Quintana et al., 2020). Another example occurs in the Tatra National Park in Poland, where Tatra chamois (Rupicapra rupicapra tatrica) moved more when they were close to hiking trails at the expense of foraging and resting (Pȩksa and Ciach, 2018). Alternatively, some animals may habituate to humans, decreasing their behavioral response to visitors due to repeated exposure (Stankowich, 2008; Mccleery, 2009; Blumstein, 2016; Saltz et al., 2018; Uchida et al., 2019), and creating the impression that the presence of humans in natural areas has no effect on the animals’ fitness.
However, although behavioral adaptations may seem to provide animals with the tools to deal with human disturbance, they may come at a cost of decreased fitness and destabilization of community interactions (Bejder et al., 2006; Berger-Tal et al., 2011; Higham and Shelton, 2011; Bateman and Fleming, 2017). The negative effects of behavioral adaptations can be hard to discern, especially when human disturbance is constant and we have limited knowledge on how the disturbed individuals would choose to behave in the absence of that disturbance. Thus, it is difficult to assess the effects ecotourism have on the behavior and fitness of seemingly adapted wildlife populations. Nevertheless, understanding these effects, especially in nature reserves, is essential for proper management of protected areas (Haysmith and Hunt, 1995; Roe et al., 1997; Higginbottom et al., 2001; Wolf et al., 2019).
In this respect, the restrictions that followed the COVID-19 outbreak provide us with a rare opportunity to examine the impacts of ecotourism in natural areas, particularly in protected areas that have restricted visitors’ entry due to the pandemic. We monitored the activity times and population composition of two adjacent but separated populations of Nubian ibex, Capra nubiana, in the spring and summer of 2019 and 2020. The fact that one of these populations inhabited a National Park that restricted visitor access during the spring and summer of 2020 allowed us to explore the potential effects of human visitation to the park on the behavior of the ibex.
The Nubian ibex is a desert ungulate whose conservation status is Vulnerable according to the IUCN red-list (Ross et al., 2020) and is protected by the Israel Nature and Parks Authority (INPA). Ibex are social animals living in herds, usually divided into groups of females, kids and young males (1–3 years old) and groups of males, although males can be found roaming alone as well (Gross et al., 1995; Tadesse and Kotler, 2010, 2012). Ibex can live up to 12 years in the wild (Kingdon and Hoffmann, 2013) and females start breeding at the age of two. The rutting season takes place during the autumn months (September–November) and births occur during the spring (March–April). Every female usually give birth to 1–2 kids (Kingdon and Hoffmann, 2013) and the birth sex ratio is even (Habibi, 1997; Kingdon and Hoffmann, 2013). Ibex are diurnal; however, there is evidence of ibex activity during the night, mostly males during the rutting season (Tichon, personal communication). The main natural predators of ibex in Israel used to be leopards (Panthera pardus), but since their local extinction (Tadesse and Kotler, 2010; Kingdon and Hoffmann, 2013), wolves (Canis lupus) and feral dogs are the main predators, mostly threatening females and kids (Tadesse and Kotler, 2010). Despite its global risk status, the species is common in the Israeli desert in proximity to cliffs and desert oases. Ibex need to drink almost daily and therefore in the absence of hunting and their main natural predators (i.e., leopards); water is the main factor limiting their distribution (Habibi, 1994; Gross et al., 1995).
Materials and Methods
Study Sites
We examined the activity patterns of two Nubian ibex populations at two natural oases in the Negev Desert of Israel; Ein-Avdat National Park and Ein-Shaviv in the Zin Valley Nature Reserve (Figure 1).
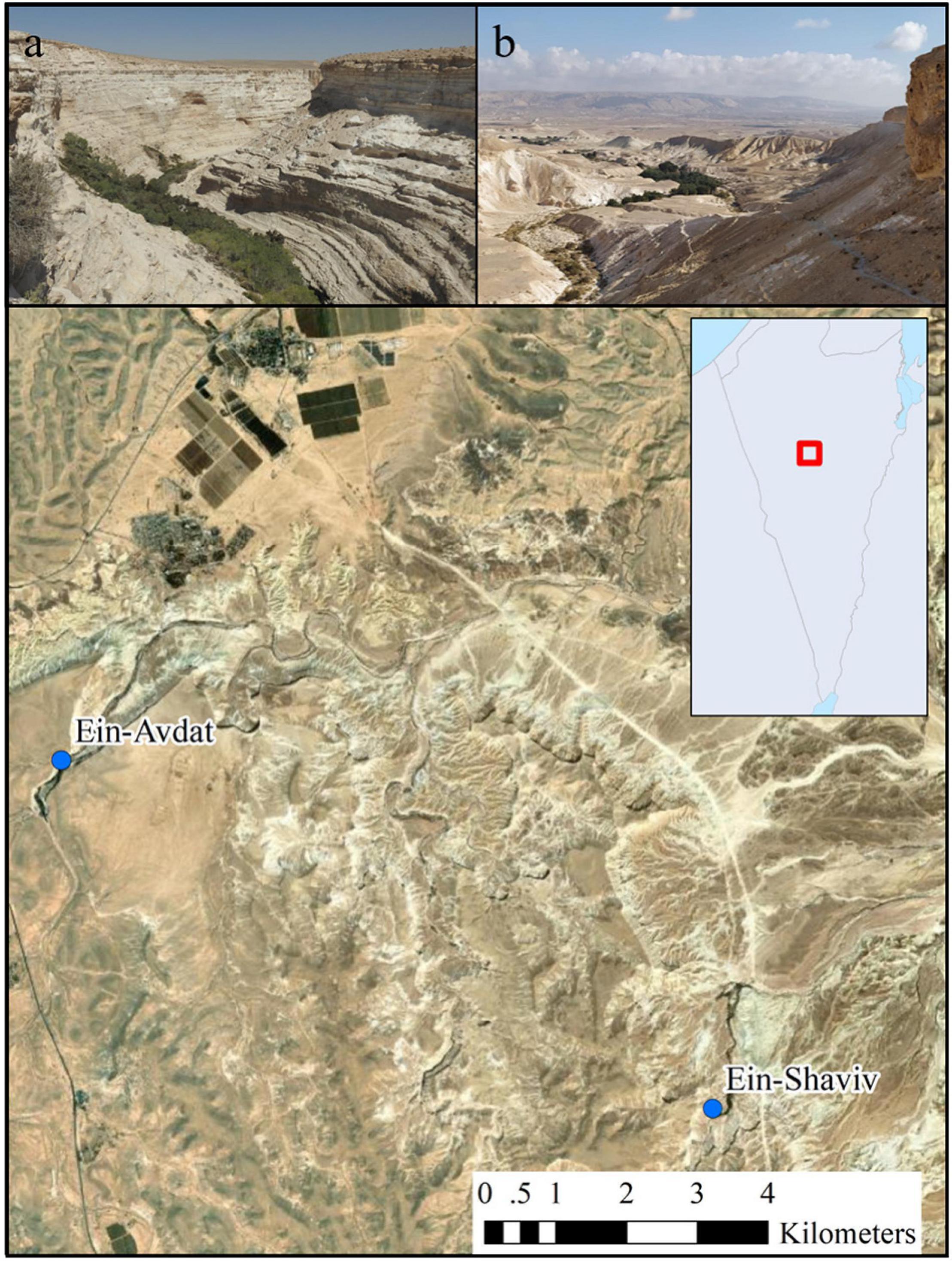
Figure 1. A map of the study area that includes the two desert oases: (a) Ein-Avdat (photography by Yuval Zukerman) and (b) Ein-Shaviv (photography by Tomer Mizrachi).
Ein Avdat National Park is a steep desert canyon, with three springs and relatively a high richness of plant species. The park is easily accessible by road and attracts an unrestricted large number of visitors year-round. In 2020 visitors’ entry was restricted according to COVID-19 guidelines (to a maximum of 130 visitors every hour) which reduced the daily visitors’ number from 315 ± 193 (mean ± SE) visitors per day in 2019 to 104 ± 107 visitors per day in 2020 (paired t-test, t23 = 2.96, p < 0.01). Nevertheless, the proportion of visitors per hour (i.e., the daily activity pattern of the visitors) didn’t change between the years [chi-square, χ2(1, 23) = 0.05, p = 1.00]. Ein Shaviv is a spring located in the Zin Valley Nature Reserve, about 9 km east of Ein Avdat National Park. It is located in a remote desert area with access by 4 × 4 vehicles only. Due to the difficult access to the site, it is one of the only water sources in the region that is not crowded with visitors on a daily basis and is therefore less affected by human presence. Since there is no official tracking of the visitor number to Ein-Shaviv, we counted the number of visitors during two morning hours (10:00–12:00) during the end of the fall, 3 days in the middle of the week, and 1 day in the weekend in 2020 (November 16th, November 19th, November 29th, and December 2nd). According to this sample, there are only 6 ± 9 visitors per hour. Both Ein Avdat and Ein Shaviv serve as main water sources for local ibex populations. The maximum number of ibex individuals seen at the same time in the local populations of Ein Avdat and Ein Shaviv were approximately 50 and 40 individuals, respectively. According to data collected over the years by the INPA that includes observations of tagged individuals and information on individuals with GPS or VHF collars, females tend to have small home ranges centered on one water source, while males can sometimes travel between water sources (Tichon, 2020). Despite their proximity, no movement of individuals between our two study sites was recorded.
Trail Cameras
We used images from three motion-detecting trail cameras to examine the arrival time of the ibex to the water at the study sites. A single camera was placed on a thin iron rod 30 cm above the ground, hidden from sight in front of the main water pool in Ein Avdat National Park, known as the ibex’s main source of drinking in the reserve (Figure 2a). This area is off-limits to visitors of the National Park. The camera operated in the summers of 2019 and 2020. In 2019 the camera type was a BolyMedia SG968K with a 2-min lapse after a picture was taken, and in 2020 the camera type was a Uovision UV785 with a 5-min lapse after a picture was taken. Importantly, the difference in the time lapses between years did not affect our results since we did not use the cameras to estimate the numbers of ibex visiting the sites, but to note their time of arrival to the site and the ratio of Female:Kids within each year. However, it did result in a smaller sample size in 2020. All other settings were identical in both cameras, including their exact location. A second camera (Bushnell 119875C 24MP Trophy Cam HD Low Glow) was placed on the ground secured by rocks and hidden in front of the only Pistacia atlantica tree in the National Park, located where the ibex usually descends from the cliffs to forage around it. The Pistacia atlantica is also located next to the only hiking trail in the National Park and all visitors must pass through it, therefore the camera captures both ibex and visitors (Figures 2b,c). The camera operated in the summer of 2020. A third camera (ATC 128x) was placed hidden on a tree trunk 1 m high with a downward viewing angle, in front of the main water pool in Ein Shaviv with a 5-min lapse after a picture was taken (Figure 2d). The camera operated in the summers of 2019 and 2020. Overall, we captured 567 pictures of ibex coming to drink in 2019 (May 27th–July 26th), and 217 in 2020 during the same period (May 27st–July 26th) in Ein-Avdat National Park. In Ein Shaviv, we captured 250 pictures of ibex coming to drink in 2019 and 174 in 2020 (May 23th–July 4th in 2019 and May 23st–July 4th in 2020). Around the Pistacia atlantica tree in Ein Avdat National Park we got 590 captures of ibex and 571 captures of visitors between July 14th and October 31th in 2020. We analyzed the images using Timelapse2 software.
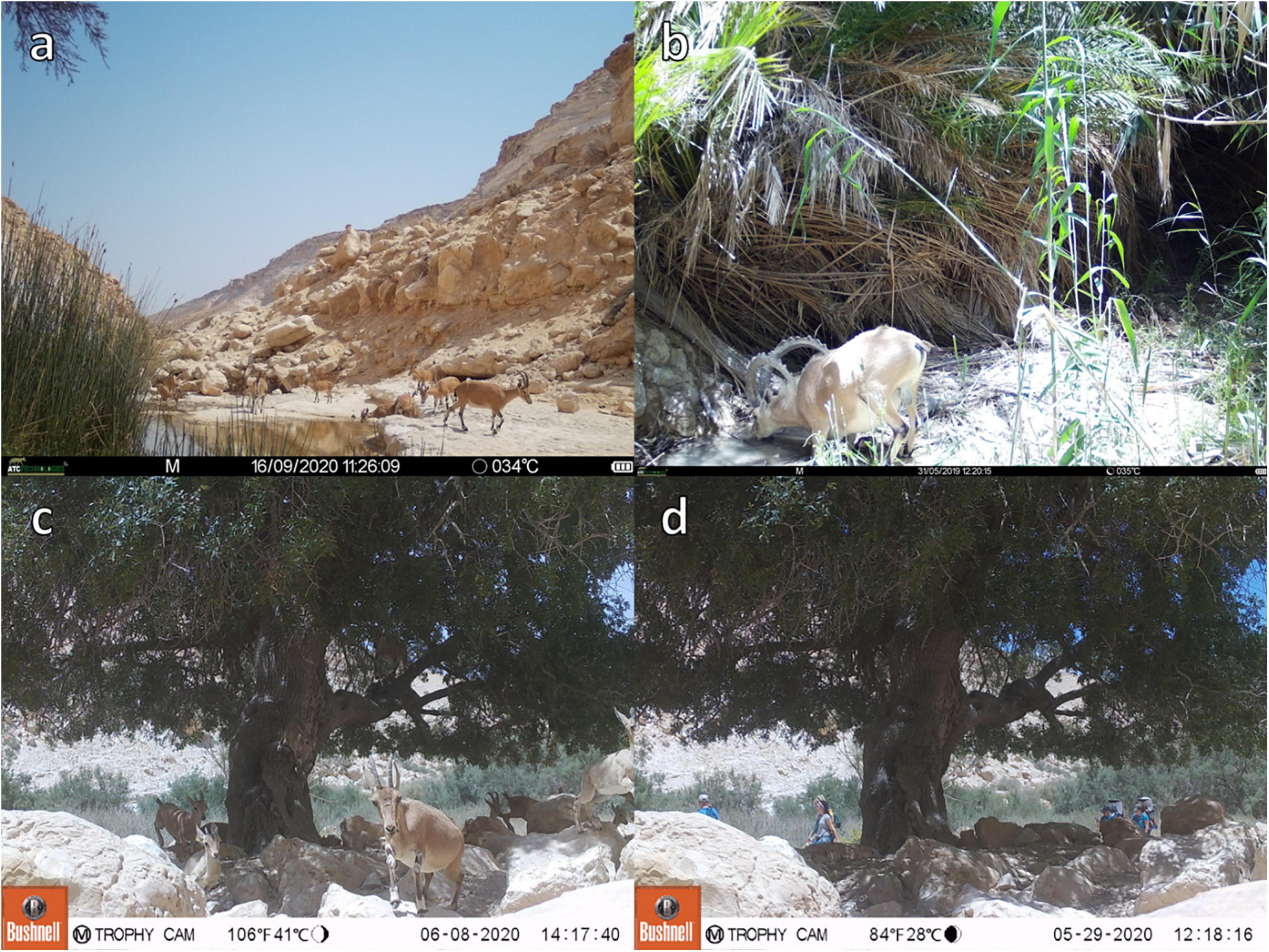
Figure 2. Camera footage from the camera traps used in the study: (a) in front of the water pool in Ein-Avdat. (b) In front of the water pool in Ein-Shaviv, (c,d) in front of the Pistacia atlantica tree in Ein Avdat.
Statistical Analyses
For each site we fitted circular kernel density models of the time in which the ibex arrived in 2019 and in 2020, and used the Wald test (Wald and Wolfowitz, 1940) for pairwise comparisons between activity levels at different times of day (Rovero and Zimmermann, 2016). We used the cameras near the water sources to test for any changes to the Female:Kids ratio in 2019 and 2020 using a chi-square test. Using data from the camera near the Pistacia atlantica, we tested for a correlation between the times that visitors and ibex were captured by the camera. Since we did not expect a linear correlation, we used Spearman’s correlation. We also calculated the coefficient of overlap (Δ) between the two kernel densities of two periods of time: lockdown period, when humans were not allowed in the park (September 18th–October 19th) and COVID-19 restriction period, during which up to 130 visitors were allowed to enter the site every hour (July 14th–September 17th, October 19th–October 31th). Since there were no visitors during the lockdown, we used the kernel density of the visitors before the lockdown to represent a regular visitors’ daily activity (Ridout and Linkie, 2009; Rovero and Zimmermann, 2016). Finally, we used visitor data from the Israel Nature and Parks Authority (Qlik sense software and Checkfront software) to test the effect of the daily number of visitors on the arrival time of ibex to the water pool in Ein Avdat in 2019 and in 2020 using a general linear model (GLM). To avoid biases caused by the COVID-19 regulations that limited the daily number of visitors in 2020, we included the year in the model as well. For that purpose, we counted the minutes from 00:00 for each arrival time, and used it as our response variable, with a quasipoisson distribution (since our model was over-dispersed). It is important to note that all of our analyses are aimed at comparing the behaviors of ibex between years within each site. We make no attempt to statistically compare between the two sites. Statistical tests were made using RStudio Version 1.3.1073 with the packages “stats,” “activity,” “overlap,” and “performance” (Ridout and Linkie, 2009; R Core Team, 2020; Lüdecke et al., 2021; Rowcliffe, 2021).
Results
In Ein-Avdat, ibex in 2020 arrived to the main spring approximately 80 min earlier than in 2019 (Wald test, W = 24.64, p < 0.01; Figure 3A). However, we didn’t find any significant differences between 2019 and 2020 in Ein Shaviv (Wald test, W = 0.93, p = 0.33; Figure 3B). In Ein-Avdat, we found that ibex delayed their arrival time to the water pool as the daily number of visitors increased (t777 = 2.78, p = 0.01; Figure 4), regardless of the year (t777 = 1.09, p = 0.27; Figure 4). Additionally, we found that the overlap between the times that the ibex were near the Pistacia atlantica tree and tourists’ visiting hours almost doubled during the lockdown when no visitors were present (Δ of 0.46 and 0.81, respectively). We also found a negative correlation between times ibex and visitors were present near the Pistacia atlantica, indicating that ibex avoided the area when visitors were present (rs = −0.75, p < 0.01, Figure 5). In Ein-Avdat the ratio of Female:Kids more than doubled in 2020 compared to 2019, while in Ein Shaviv the proportion did not change [χ2(1, 784) = 27.52, p < 0.01 and χ2(1, 424) = 0.31, p = 0.58, respectively; Figure 6].
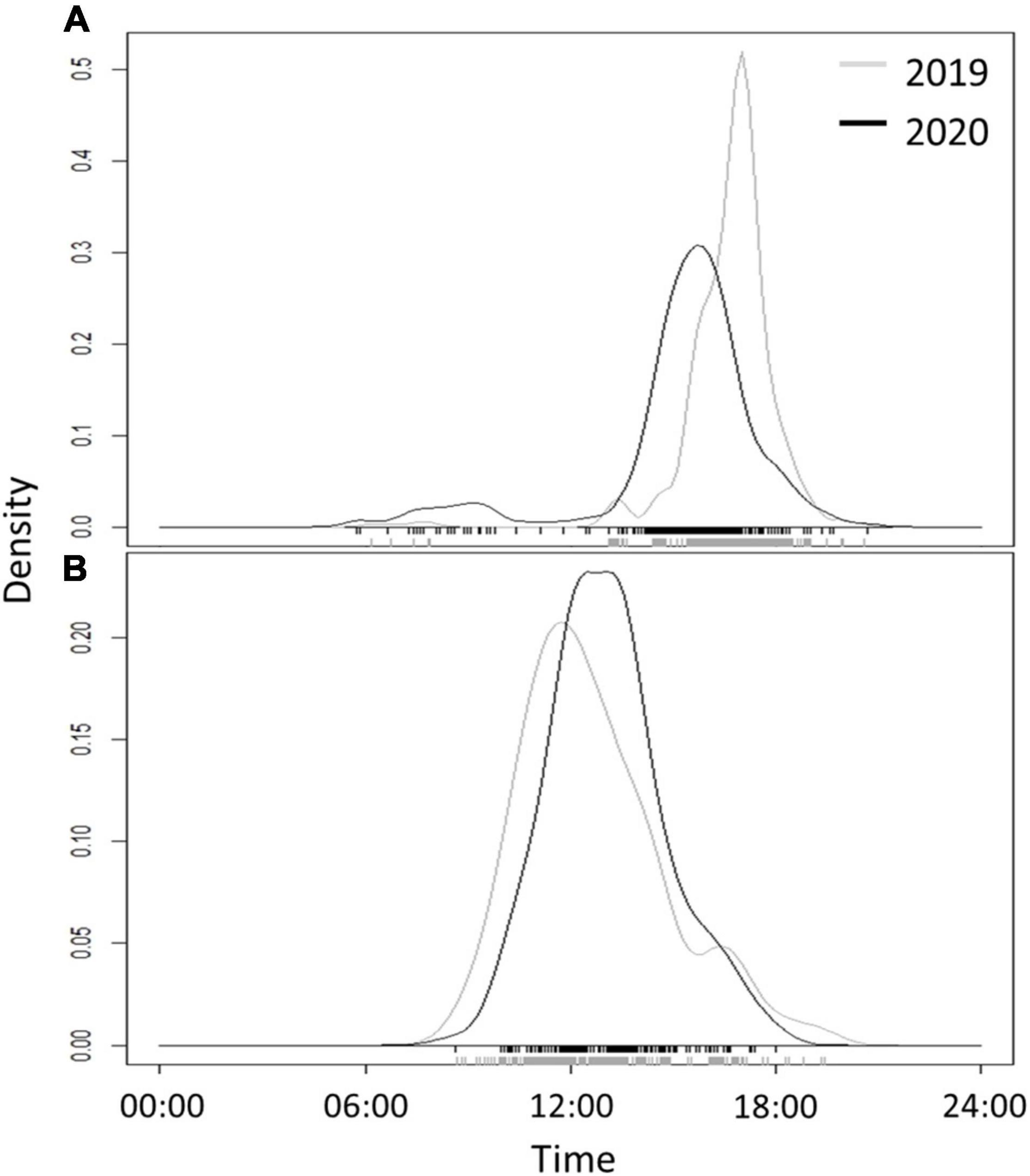
Figure 3. The daily time curve of arrival of ibex to the water pools in Ein-Avdat (A) and Ein-Shaviv (B) during the beginning of the summer in 2019 (gray line) and 2020 (black line). The original records of the data are shown at the foot of the charts as rugs.
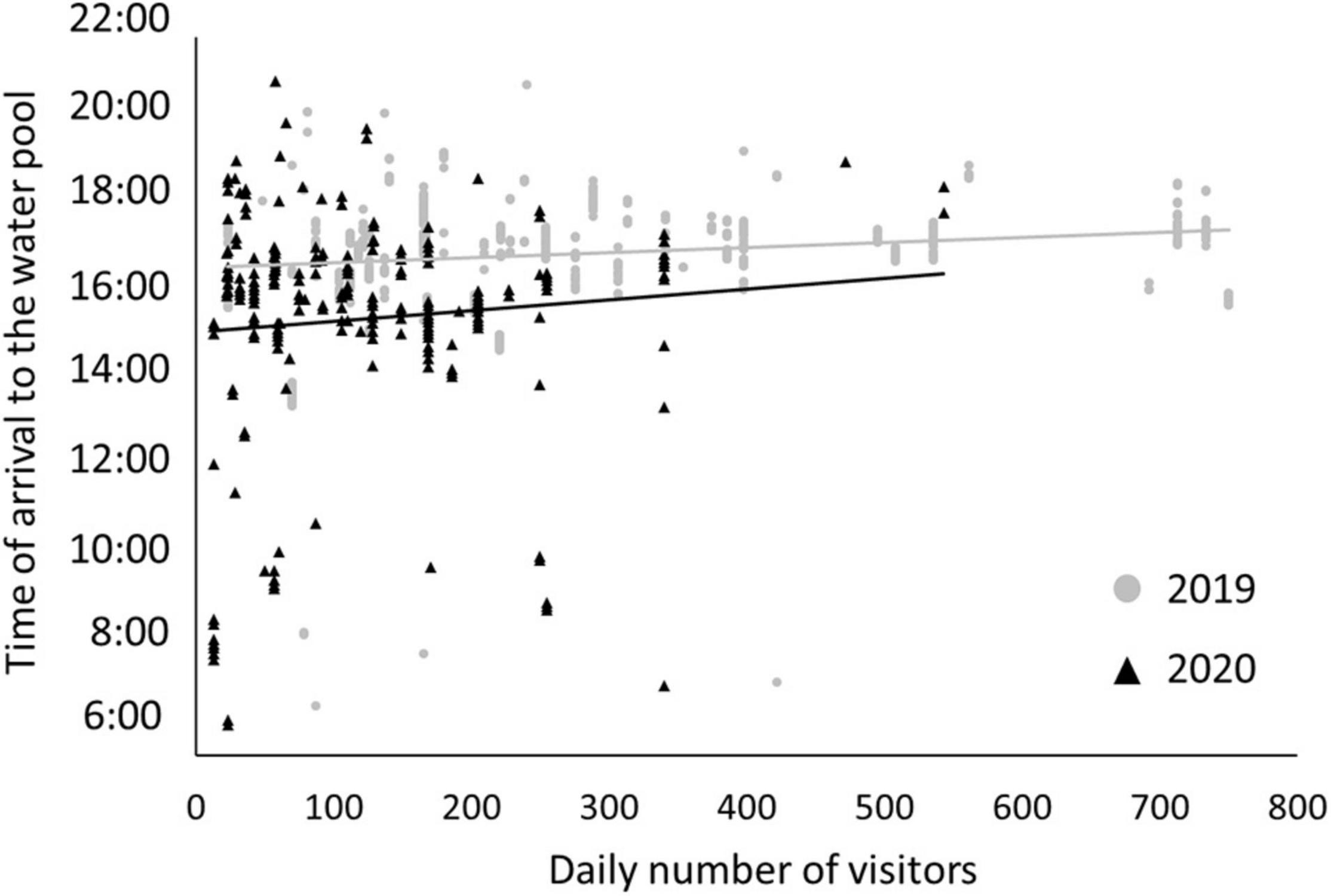
Figure 4. The effect of the daily number of visitors on the arrival time of ibex to the water pool in Ein-Avdat during 2019 (gray circles) and 2020 (black triangles).
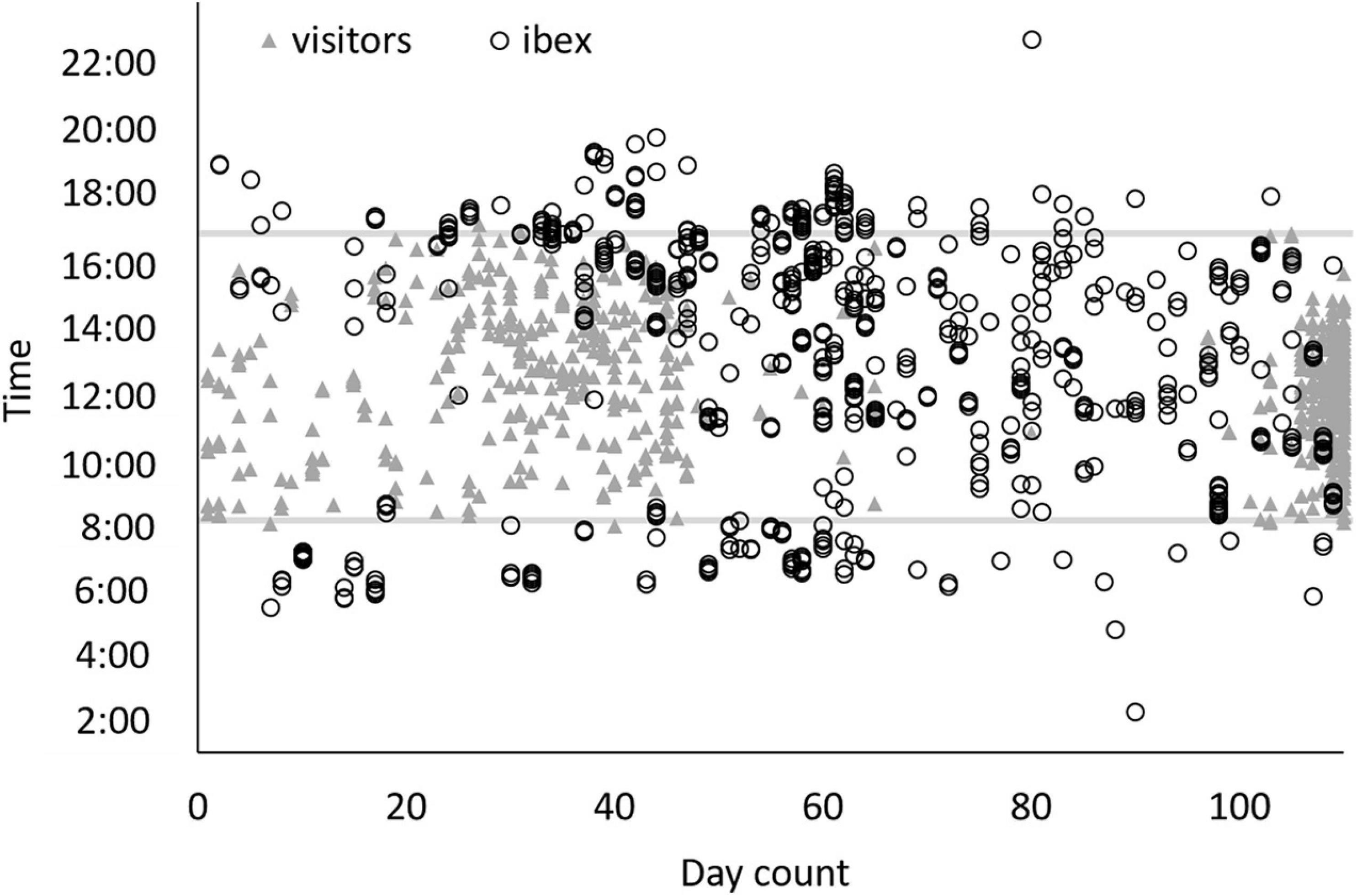
Figure 5. Times that ibex (hollow black circles) and visitors (gray triangles) were spotted around the Pistacia atlantica (a popular location in the park for both humans and ibex). X-axis is the count of the days starting from July 14 to October 31, 2020. Gray lines represent the daily opening (bottom line) and closing (upper line) hours of Ein Avdat National Park. The site was under lockdown between days 67 and 97.
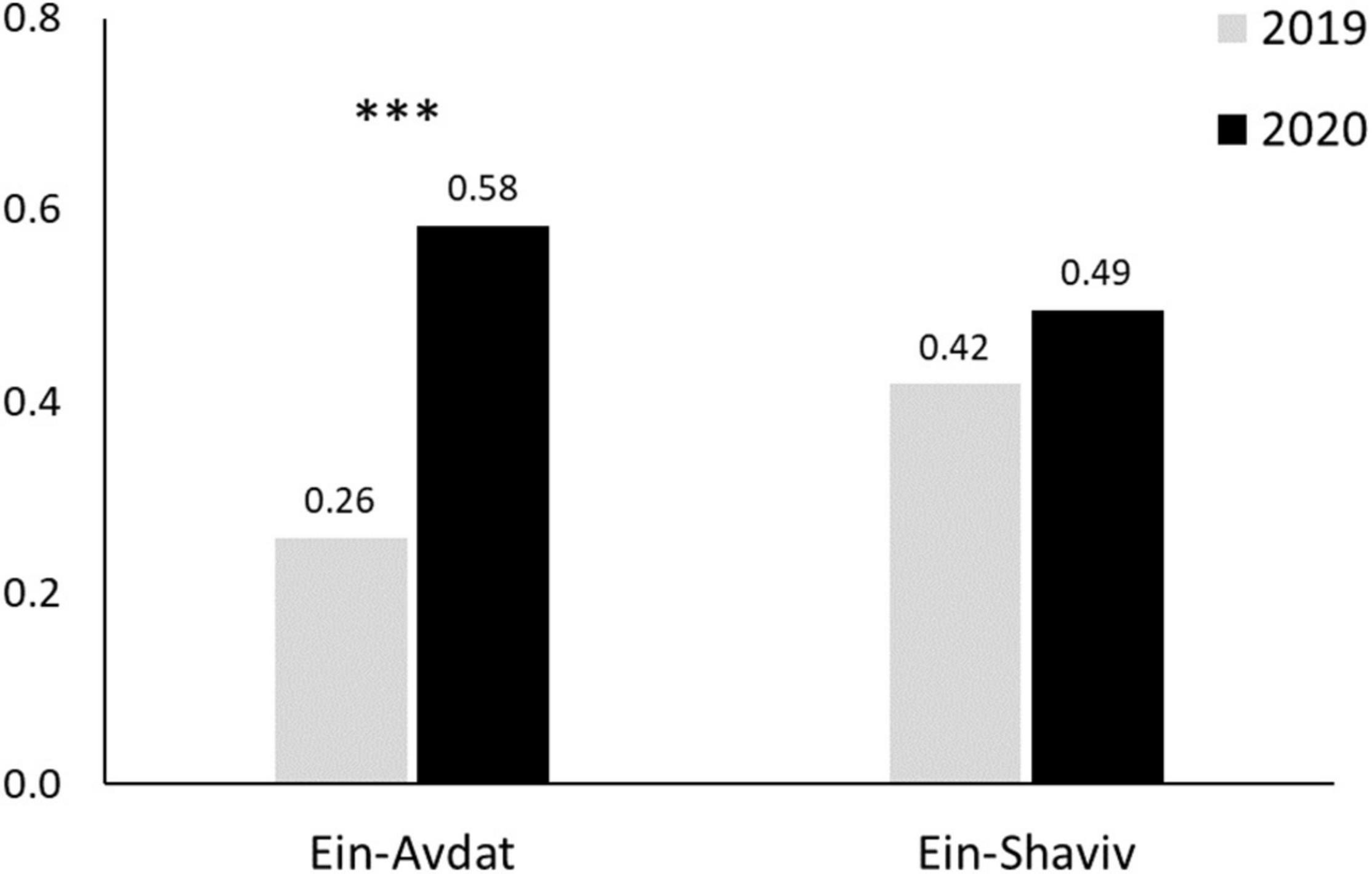
Figure 6. Female:Kids Ratio in Ein Avdat and Ein Shaviv in 2019 and 2020. Three stars represent significance of p < 0.001.
Discussion
The results of our study indicate that the presence of human visitors in a protected area may elicit a behavioral change in local species that could have a negative effect on their fitness. While the limitations of having a single treatment site over a short period of time prevents us from ruling out additional factors that may have led to the observed change in behavior and in Female:Kids ratio in Ein-Avdat (such as changes in the micro-climatic conditions or in predator abundance, or even a random anomaly), the proximity of our two study sites makes human presence the most likely driver of behavioral change.
Ein Avdat National Park closes to visitors at 17:00 each day, and in past years the local population of Nubian ibex usually arrived to drink around that time (average arrival time was 16:37 ± 1:22 for 2019). When the number of visitors to Ein-Avdat was dramatically reduced in 2020 due to COVID-19 restrictions, the local population of ibex significantly changed their behavior, arriving to drink much earlier in the day (average arrival time was 15:19 ± 2:26 in 2020, Figure 3A). We did not observe such a change in the adjacent and more remote Ein-Shaviv population (Figure 3B). These results support our hypothesis that the presence of human visitors in Ein-Avdat forced the ibex to delay their drinking hours. This delay in drinking hours allowed the Ibex to avoid interactions with visitors, but also prevented them from resting and foraging in preferred areas, such as next to the Pistacia atlantica tree that is usually a preferred location for ibex when visitors are not around (Figure 5).
Avoidance of areas highly visited by humans and changes to wildlife behavior in response to ecotourism pressure has been documented in many species. Some examples include, Tatra chamois (Rupicapra rupicapra tatrica) that were found to avoid areas near cable cars in the Tatra National Park in Poland as the number of the visitors using them increased (Pȩksa and Ciach, 2015); North American Elk (Cervus elaphus) feeding and resting less when visitors were present at the Starkey Experimental Forest and Range in northern Oregon (Naylor et al., 2009); and in the Sumatran Rain Forest barking deer (Muntiacus muntjak) and sambar deer (Rusa unicolor) avoided using areas with high human visitation, while the tigers (Panthera tigris) and sun bears (Helarctos malayanus) altered their activity time to be less diurnal rather than avoiding these areas (Griffiths and Schaik, 1993). Human recreational activity negatively affected patch use behavior of Capercaillie (Tetrao urogallus) in the Bohemian Forest, as they avoided high-quality areas with high recreation intensity (Rösner et al., 2014). These seemingly small alterations to wildlife behaviors bring up the question of whether these behavioral shifts carry with them fitness costs. While our findings are strictly correlational, they do suggest a steep fitness cost of ecotourism.
In 2020, when Ein Avdat was under COVID-19 regulations that restricted visitors’ access, the ratio of Female:Kids in the local ibex population more than doubled itself compared to 2019, when the site was open for an unlimited number of visitors (0.58 and 0.26 respectably; Figure 6). At Ein Shaviv we did not find a significant difference in the Female:Kids ratio between 2019 and 2020 (0.42 and 0.49 respectably; Figure 6).
Of course, other environmental factors may have contributed to the change in Female:Kids ratio that we have observed. According to the Israel Meteorological Service the annual rainfall in Avdat station (2.5 Km south-east to Ein Avdat) was higher in 2020 (165.5 mm) than in 2019 (81.5 mm), while the temperatures were approximately the same (maximum average of 25.9°C in 2019 and 25.4°C in 2020, minimum average of 13.9°C in 2019 and 13.2°C in 2020). Since water is crucial for ibex survival, the increase in the Female:Kids ratio in Ein Avdat in 2020 could be related to the higher amount of precipitation in 2020. However, if that was the case we would also expect to see a more positive change in the kid-female ratio in Ein Shaviv, which is just 9 km away, although we cannot rule out the possibility that microclimatic conditions have led to differences in rainfall between these two adjacent sites. Moreover, although Ein Shaviv and Ein Avdat are very similar to each other in many aspects, they are not identical and small differences in their characteristics may lead to differences in the distribution of plants in these sites.
Another important environmental factor that needs to be considered is predators’ dynamics. Predation not only lowers the survival of ibex, in particular kids, predation risk can also influence the behavior and movement patterns of ibex by creating a “landscape of fear” (Laundré et al., 2001; Rösner et al., 2014; Berger-Tal and Saltz, 2019). In Israel, some predators such as jackals can be attracted to human-disturbed areas, leading to increased predation risk within these areas, and forcing prey species such as gazelles to shift their activity time or spend less time in these areas (Shamoon et al., 2018). Alternatively, the presence of humans can generate a “human shield” caused by reduced predation risk in close proximity to humans (Geffroy et al., 2015; Blumstein, 2016; Schakner and Blumstein, 2016). In our study, we observed a similar pattern to that found in Shamoon et al. (2018). The number of local predator species (jackals, wolf, hyaena, and fox) that we observed with the camera near the water pool in Ein Avdat declined by 35% (37 observations in 2019 and 13 observations in 2020) when there were fewer visitors. Such a reduction in predators’ presence near the water source can promote the arrival of kids (that are most vulnerable to predation) and decrease predation risk. As such, the restricted visitors’ access to Ein Avdat National Park may have benefited the local ibex population both directly (by reducing human presence) and indirectly (by reducing predation pressure). In Ein Shaviv, we observed too few predators each year (7 observations in 2019 and 4 observations in 2020), to make any conclusions regarding the change in predation pressure.
Human presence is known to often increase stress hormones (Ellenberg et al., 2007; Shutt et al., 2014; Blumstein et al., 2017), which may have a negative effect on fitness (Brown et al., 2005; Meylan and Clobert, 2005; Saino et al., 2005; Cabezas et al., 2007). For example, a study made on mountain goats (Oreamnos americanus; also of the Caprinae subfamily) found that the proportion of reproductive females in the population decreased with the increase of glucocorticoid stress hormones (Dulude-de Broin et al., 2020). Additionally, female ibex are known to have a more restrictive diet than males. Their smaller body size (compared to males) and higher risk of predation requires them to be more efficient when foraging and more selective in their diet choice (Tadesse and Kotler, 2010, 2012). Given our results and the data available, we hypothesize the intense visitors’ pressure in Ein-Avdat is delaying the drinking hours of the ibex, restricting their access to high-quality food that is found close to the water, and increasing their stress level. While we cannot rule out alternative explanations, we hypothesize that this combined effect contributed to an increase in abortion rate or a decrease in mating success for the local ibex population and consequently significantly lowered the ibex annual recruitment.
Many desert species depend on scarce water sources in the arid desert environment. However, this scarcity of water is what makes many of these water sources attractive to humans (Malo et al., 2011; Santarém et al., 2020). Thus, such species must become tolerant to human disturbance, since individuals that do not tolerate the presence of humans near desert water sources will less likely survive. Our study suggests that any such tolerance may be misleading, because it may lead to the false belief that these species are unaffected by human presence. Our study shows that high tolerance does not mean that the population is not negatively affected by disturbance, which emphasizes the necessity of conducting ecological and behavioral research and communicating with the bodies responsible for ecotourism policy and management within protected areas for nature and wildlife conservation.
In Israel, the COVID-19 regulations led to the implementation new tools for managing visitors in nature reserves by using a web platform for pre-registration, and limiting the number of visitors allowed in a site each hour. This approach can improve the conservation of local wildlife species (as shown in this study) and also the traveler’s experience. Thus, we highly recommend continued use of such a platform even after the COVID-19 restrictions are removed. Furthermore, we think that in the case of the ibex, there might be a threshold to the number of visitors that allowed the ibex to drink, even during the site’s opening hours. We suggest that with further research, such a threshold can be quantified and perhaps be used to design daily number of visitors to the park.
Despite the possible negative effects caused by adaptation to human disturbance, behavioral adaptations are essential for fostering coexistence between wildlife and humans in a world where the human population is constantly growing. It is our responsibility to understand the consequences of these behavioral adaptations and to adjust our management schemes accordingly, so both wildlife and humans can enjoy the resources nature has to offer.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was not obtained from the individual(s), nor the minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
OB-T and YZ conceived the study and drafted the manuscript. ZS collected field data and critically revised the manuscript. YZ collected field data and carried out the statistical analyses. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Funding
This work was supported by the Israeli Nature and Park Authority and the German-Israeli Foundation for Scientific Research and Development (Grant I-2504-413.13/2018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staff of the Zin Valley Nature Reserve for their assistance in maintaining the cameras and for being our eyes in the field, Nadav Taube for providing the park visitation data, Gopal Murali for programming and statistics help, Yael Lehnardt for reporting observations during the COVID-19 closure that led to the study idea, and to Uri Roll for encouragement to initiate this study.
References
Balmford, A., Green, J. M. H., Anderson, M., Beresford, J., Huang, C., Naidoo, R., et al. (2015). Walk on the wild side: estimating the global magnitude of visits to protected areas. PLoS Biol. 13:e1002074. doi: 10.1371/journal.pbio.1002074
Bateman, P. W., and Fleming, P. A. (2017). Are negative effects of tourist activities on wildlife over-reported? A review of assessment methods and empirical results. Biol. Conserv. 211, 10–19. doi: 10.1016/j.biocon.2017.05.003
Bejder, L., Samuels, A., Whitehead, H., Gales, N., Mann, J., Connor, R., et al. (2006). Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 20, 1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x
Berger-Tal, O., and Saltz, D. (2019). Invisible barriers: Anthropogenic impacts on inter- and intra-specific interactions as drivers of landscape-independent fragmentation. Philos. Trans. R. Soc. B Biol. Sci. 374:20180049. doi: 10.1098/rstb.2018.0049
Berger-Tal, O., Polak, T., Oron, A., Lubin, Y., Kotler, B. P., and Saltz, D. (2011). Integrating animal behavior and conservation biology: a conceptual framework. Behav. Ecol. 22, 236–239. doi: 10.1093/beheco/arq224
Blumstein, D. T. (2016). Habituation and sensitization: new thoughts about old ideas. Anim. Behav. 120, 255–262. doi: 10.1016/j.anbehav.2016.05.012
Blumstein, D. T., Geffroy, B., Samia, D. S. M., and Bessa, E. (2017). Ecotourism’s Promise and Peril: A Biological Evaluation, eds D. T. Blumstein, B. Geffroy, D. S. M. Samia, and E. Bessa (Cham: Springer International Publishing), doi: 10.1007/978-3-319-58331-0
Brown, C. R., Brown, M. B., Raouf, S. A., Smith, L. C., and Wingfield, J. C. (2005). Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecology 86, 1034–1046. doi: 10.1890/04-0740
Cabezas, S., Blas, J., Marchant, T. A., and Moreno, S. (2007). Physiological stress levels predict survival probabilities in wild rabbits. Horm. Behav. 51, 313–320. doi: 10.1016/j.yhbeh.2006.11.004
Cañadas Santiago, S., Dias, P. A. D., Garau, S., Coyohua Fuentes, A., Chavira Ramírez, D. R., Canales Espinosa, D., et al. (2020). Behavioral and physiological stress responses to local spatial disturbance and human activities by howler monkeys at Los Tuxtlas, Mexico. Anim. Conserv. 23, 297–306. doi: 10.1111/acv.12541
Dulude-de Broin, F., Hamel, S., Mastromonaco, G. F., and Côté, S. D. (2020). Predation risk and mountain goat reproduction: evidence for stress-induced breeding suppression in a wild ungulate. Funct. Ecol. 34, 1003–1014. doi: 10.1111/1365-2435.13514
Ellenberg, U., Setiawan, A. N., Cree, A., Houston, D. M., and Seddon, P. J. (2007). Elevated hormonal stress response and reduced reproductive output in yellow-eyed penguins exposed to unregulated tourism. Gen. Comp. Endocrinol. 152, 54–63. doi: 10.1016/j.ygcen.2007.02.022
Foley, J. A. (2005). Global consequences of land use. Science 309, 570–574. doi: 10.1126/science.1111772
Fulton, G. R. (2002). Positive effects of wildlife tourism on wildlife. Pacific Conserv. Biol. 8:141. doi: 10.1071/pc020141
Gaynor, K. M., Hojnowski, C. E., Carter, N. H., and Brashares, J. S. (2018). The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235. doi: 10.1126/science.aar7121
Geffroy, B., Sadoul, B., and Ellenberg, U. (2017). “Physiological and behavioral consequences of human visitation,” in Ecotourism’s Promise and Peril, eds D. T. Blumstein, B. Geffroy, D. S. M. Samia, and E. Bessa (Cham: Springer International Publishing), 9–28.
Geffroy, B., Samia, D. S. M., Bessa, E., and Blumstein, D. T. (2015). How nature-based tourism might increase prey vulnerability to predators. Trends Ecol. Evol. 30, 755–765. doi: 10.1016/j.tree.2015.09.010
George, S. L., and Crooks, K. R. (2006). Recreation and large mammal activity in an urban nature reserve. Biol. Conserv. 133, 107–117. doi: 10.1016/j.biocon.2006.05.024
Griffiths, M., and Schaik, C. P. (1993). The impact of human traffic on the abundance and activity periods of sumatran rain forest wildlife. Conserv. Biol. 7, 623–626. doi: 10.1046/j.1523-1739.1993.07030623.x
Gross, J. E., Alko, P. U., and Demment, M. W. (1995). Grouping patterns and spatial segregation by nubian ibex. J. Arid Environ. 30, 423–439. doi: 10.1006/jare.1995.0037
Habibi, K. (1994). The Desert Ibex: Life History, Ecology, And Behavior Of The Nubian Ibex In Saudi Arabia. London: Immel Publishing.
Habibi, K. (1997). Group dynamics of the nubian ibex (Capra ibex nubiana) in the Twwayiq Canyons, Saudi Arabia. K. J. Zool. 241, 791–801. doi: 10.1111/j.1469-7998.1997.tb05748.x
Haysmith, L., and Hunt, J. D. (1995). “Nature tourism: impacts and management,” in Wildlife and Recreationists: Coexistence Through Management And Research, eds R. L. Knight and K. Gutzwiller (Washington, DC: Island Press), 203–219.
Higginbottom, K., Rann, K., Moscardo, G., Davis, D., and Muloin, S. (2001). Status Assessment Of Wildlife Tourism In Austrialia: An Overview. Part I: Descriptive Overview Of Wildlife Tourism. Gold Coast, QLD: CRC for Sustainable Tourism.
Higham, J. E. S., and Shelton, E. J. (2011). Tourism and wildlife habituation: Reduced population fitness or cessation of impact? Tour. Manag. 32, 1290–1298. doi: 10.1016/j.tourman.2010.12.006
Hochman, V., and Kotler, B. P. (2007). Patch use, apprehension, and vigilance behavior of nubian ibex under perceived risk of predation. Behav. Ecol. 18, 368–374. doi: 10.1093/beheco/arl087
Kingdon, J., and Hoffmann, M. (2013). Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids. London: Bloomsbury Publishing, 600–603.
Laundré, J. W., Hernández, L., and Altendorf, K. B. (2001). Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 79, 1401–1409. doi: 10.1139/cjz-79-8-1401
Lüdecke, D., Ben-Shachar, M., Patil, I., Waggoner, P., and Makowski, D. (2021). Performance: an r package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6:3139. doi: 10.21105/joss.03139
Malo, J. E., Acebes, P., and Traba, J. (2011). Measuring ungulate tolerance to human with flight distance: a reliable visitor management tool? Biodivers. Conserv. 20, 3477–3488. doi: 10.1007/s10531-011-0136-7
Marchand, P., Garel, M., Bourgoin, G., Dubray, D., Maillard, D., and Loison, A. (2014). Impacts of tourism and hunting on a large herbivore’s spatio-temporal behavior in and around a French protected area. Biol. Conserv. 177, 1–11. doi: 10.1016/j.biocon.2014.05.022
Mccleery, R. A. (2009). Changes in fox squirrel anti-predator behaviors across the urban-rural gradient. Landsc. Ecol. 24, 483–493. doi: 10.1007/s10980-009-9323-2
Meylan, S., and Clobert, J. (2005). Is corticosterone-mediated phenotype development adaptive? Maternal corticosterone treatment enhances survival in male lizards. Horm. Behav. 48, 44–52. doi: 10.1016/j.yhbeh.2004.11.022
Montero-Quintana, A. N., Vázquez-Haikin, J. A., Merkling, T., Blanchard, P., and Osorio-Beristain, M. (2020). Ecotourism impacts on the behaviour of whale sharks: an experimental approach. Oryx 54, 270–275. doi: 10.1017/S0030605318000017
Naylor, L. M., Wisdom, M. J., and Anthony, R. G. (2009). Behavioral responses of north american elk to recreational activity. J. Wildl. Manage. 73, 328–338. doi: 10.2193/2008-102
Olson, T. L., Squibb, R. C., and Gilbert, B. K. (1998). Brown bear diurnal activity and human use: a comparison of two salmon streams. Ursus 10, 547–555. doi: 10.2307/3873167
Pȩksa, Ł, and Ciach, M. (2015). Negative effects of mass tourism on high mountain fauna: the case of the Tatra chamois Rupicapra rupicapra tatrica. Oryx 49, 500–505. doi: 10.1017/S0030605313001269
Pȩksa, Ł, and Ciach, M. (2018). Daytime activity budget of an alpine ungulate (Tatra chamois Rupicapra rupicapra tatrica): influence of herd size, sex, weather and human disturbance. Mammal. Res. 63, 443–453. doi: 10.1007/s13364-018-0376-y
R Core Team (2020). R: A Language And Environment For Statistical Computing. Vienna: R Foundation Statistical Computing.
Reilly, M. L., Tobler, M. W., Sonderegger, D. L., and Beier, P. (2017). Spatial and temporal response of wildlife to recreational activities in the San Francisco Bay ecoregion. Biol. Conserv. 207, 117–126. doi: 10.1016/j.biocon.2016.11.003
Ridout, M. S., and Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 14, 322–337. doi: 10.1198/jabes.2009.08038
Roe, D., Leader-williams, N., and Dalal-clayton, B. (1997). Take Only Photographs, Leave Only Footprints. London: International Institute for Environment and Development (IIED).
Rösner, S., Mussard-Forster, E., Lorenc, T., and Müller, J. (2014). Recreation shapes a “landscape of fear” for a threatened forest bird species in Central Europe. Landsc. Ecol. 29, 55–66. doi: 10.1007/s10980-013-9964-z
Ross, S., El Alqamy, H., and Alsaid, T. (2020). Capra Nubiana, Nubian Ibex. The IUCN Red List of Threatened Species 2020. Gland: IUCN.
Rovero, F., and Zimmermann, F. (2016). Camera Trapping For Wildlife Research. Exeter: Pelagic Publishing.
Rowcliffe, M. (2021). Activity: Animal Activity Statistics. R Packag. version 1.3.1. Available online at: https://cran.r-project.org/package=activity (accessed May 7, 2021).
Saino, N., Romano, M., Ferrari, R. P., Martinelli, R., and Møller, A. P. (2005). Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. Part A Comp. Exp. Biol. 303, 998–1006. doi: 10.1002/jez.a.224
Saltz, D., Berger-Tal, O., Motro, U., Shkedy, Y., and Raanan, N. (2018). Conservation implications of habituation in nubian ibex in response to ecotourism. Anim. Conserv. 22, 220–227. doi: 10.1111/acv.12456
Santarém, F., Saarinen, J., and Brito, J. C. (2020). Mapping and analysing cultural ecosystem services in conflict areas. Ecol. Indic. 110:105943. doi: 10.1016/j.ecolind.2019.105943
Schakner, Z., and Blumstein, D. T. (2016). “Learning and conservation behavior: an introduction and overview,” in Conservation Behavior: Applying Behavioral Ecology to Wildlife Conservation and Management, eds B.-T. Oded and S. David (Cambridge: Cambridge University Press), 66–92. doi: 10.1017/cbo9781139627078.005
Shamoon, H., Maor, R., Saltz, D., and Dayan, T. (2018). Increased mammal nocturnality in agricultural landscapes results in fragmentation due to cascading effects. Biol. Conserv. 226, 32–41. doi: 10.1016/j.biocon.2018.07.028
Shutt, K., Heistermann, M., Kasim, A., Todd, A., Kalousova, B., Profosouva, I., et al. (2014). Effects of habituation, research and ecotourism on faecal glucocorticoid metabolites in wild western lowland gorillas: Implications for conservation management. Biol. Conserv. 172, 72–79. doi: 10.1016/j.biocon.2014.02.014
Soga, M., and Gaston, K. J. (2016). Extinction of experience: the loss of human-nature interactions. Front. Ecol. Environ. 14:94–101. doi: 10.1002/fee.1225
Stankowich, T. (2008). Ungulate flight responses to human disturbance: a review and meta-analysis. Biol. Conserv. 141, 2159–2173. doi: 10.1016/j.biocon.2008.06.026
Tadesse, S. A., and Kotler, B. P. (2010). Habitat choices of nubian ibex (Capra Nubiana) evaluated with A habitat suitability modeling and isodar analysis. Isr. J. Ecol. Evol. 56, 55–74. doi: 10.1560/ijee.56.1.55
Tadesse, S. A., and Kotler, B. P. (2012). Impact of tourism on nubian ibex (Capra nubiana) revealed through assessment of behavioral indicators. Behav. Ecol. 23, 1257–1262. doi: 10.1093/beheco/ars110
Tichon, J. (2020). Demography, Movement, and Social Structure of the Nubian ibex (Capra nubiana) Population of the Judean Desert, and Conservation Implications. Ph.D. thesis. Beer-Sheva: Ben-Gurion University of the Negev.
Uchida, K., Suzuki, K. K., Shimamoto, T., Yanagawa, H., and Koizumi, I. (2019). Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behav. Ecol. 30, 1583–1590. doi: 10.1093/beheco/arz117
Wald, A., and Wolfowitz, J. (1940). On a test whether two samples are from the same population. Ann. Math. Stat. 11, 147–162. doi: 10.1214/aoms/1177731909
Keywords: ecotourism, COVID-19, behavioral flexibility, animal behavior, wildlife management
Citation: Zukerman Y, Sigal Z and Berger-Tal O (2021) COVID-19 Restrictions in a Nature Reserve Reveal the Costs of Human Presence for the Threatened Nubian Ibex (Capra nubiana). Front. Ecol. Evol. 9:751515. doi: 10.3389/fevo.2021.751515
Received: 01 August 2021; Accepted: 03 November 2021;
Published: 02 December 2021.
Edited by:
Alvaro Soutullo, Universidad de la República, UruguayReviewed by:
Francesca Brivio, University of Sassari, ItalyUrsula Ellenberg, La Trobe University, Australia
Richard Patrick Reading, Butterfly Pavilion, United States
Copyright © 2021 Zukerman, Sigal and Berger-Tal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuval Zukerman, eXV2YWx6dWtAcG9zdC5iZ3UuYWMuaWw=
 Yuval Zukerman
Yuval Zukerman Zehava Sigal2
Zehava Sigal2 Oded Berger-Tal
Oded Berger-Tal