- 1Departamento de Biología, Facultad Experimental de Ciencias y Tecnología, Universidad de Carabobo, Valencia, Venezuela
- 2Centro de Ecología, Instituto Venezolano de Investigaciones Científicas (IVIC), Caracas, Venezuela
Islands are well-known as particular and vulnerable ecosystems with evolutionary histories, environmental characteristics, and original communities different from those of continents. On the contrary, urban environments are recent, artificial, and structurally similar among distant regions. To assess the relative importance of regional and local processes on urban biota, we chose two urban environments, i.e., one on the mainland and another on an island in the same ecoregion. We asked whether the urbanization process affects the avian biodiversity of the ISLAND in the same way as in the continent. We defined an urban gradient with three levels of building density, namely, patches of native vegetation (remnant woodlands in the urban matrix), medium density urbanized areas that maintain vegetation along the streets and gardens, and residential areas with less vegetation cover and higher building density. In each geographical locality, we selected three sites (replicates) for each level of the urban gradient and did bird surveys. We found two times as many species in the urban landscape of the continent (69) as on the island (35), with the analogous richness decrease along the gradient in both regions. Species similarity was higher between urbanized sites of both regions compared with the similarity between woodlands and urbanized sites, showing that urban matrix filters similar species of each pool regionally. Individual species responded to urban structure in different ways. We found 32% of bird species were urban exploiters, 48% urban tolerant, and 20% urban avoiders in both regions. However, some species showed different frequencies of occurrence on the island and the continent. Species turnover contributed more than richness differences to species dissimilarity along the urban gradient on the continent. Contrarily, the nestedness component (i.e., species being a strict subset of the species at a richer site) was higher on the island. We concluded that the negative impact of highly urbanized areas on birds was stronger on the island than on the continent. Our results may help to assess the implications of beta-diversity loss, especially on islands.
Introduction
Urban development is associated with the loss of natural ecosystems and a decrease in biodiversity. Latin America has one of the highest urbanization rates in the world, with 79.5% of its total population living in cities, and this figure is expected to increase up to 86.2% by the year 2050 (United Nations, 2017). This fact imposes great challenges for nature conservation, as the region also harbors some of the most biodiversity-rich ecosystems in the world (Myers et al., 2000). These characteristics, and the extensive natural areas that remain, make this region an interesting place for research on urban ecology and for exploring the impacts that cities have on biodiversity.
Islands are well-known as particular and vulnerable ecosystems. The geographical isolation of island ecosystems favors the appearance of original communities with patterns, evolutionary histories, and environmental characteristics different from those observed in nearby mainland communities (for review, refer to Brown and Lomolino, 1998; Whittaker et al., 2017). On the contrary, urban environments are recent, artificial, and structurally similar among distant regions. That is, cities homogenize the physical environment (McKinney, 2006; Groffman et al., 2014). Therefore, it can be expected that similarity will be high in urban species composition even in different regions, including islands. But, urbanized areas are also an environmental filter that leaves many species outside their borders (Croci et al., 2008). General observed trends worldwide are as follows: (1) biotic richness is lower in cities than in surrounding areas and (2) specialist species (urban avoiders) are replaced by generalists (i.e., urban adapters and urban exploiters), which are able to use urban resources such as food and shelter (McKinney, 2002).
In a previous study on Margarita Island (Venezuela), we found that, as expected, avian richness decreased as urbanization increased, being very low in the recent suburbs with scarce vegetation cover. The urban assemblage consisted mainly of generalist species. Omnivorous birds were common along the gradient, and granivores were also tolerant of urban development. On the contrary, specialized insectivores and frugivores were the most negatively affected groups (Sanz and Caula, 2015). Regarding these observed general patterns, we asked whether the urbanization process affects islands, with their reduced geographical extension, isolation, lower richness, and higher endemism, in the same way as to continental cities in the same region, usually richer, with more connectivity, and greater extension. Would species turnover and/or loss be the same under these contrasting conditions?
In recent years, novel methods have been developed to separate the components underlying the differences in species composition between different places or beta diversity: (1) spatial turnover, the substitution of species from one site to another as a consequence of environment selection and dispersal processes and (2) species richness differences (nested or not nested) related to extinction-colonization dynamics in areas where there have been environmental alterations by natural or induced-man factors (Baselga, 2010, 2012, 2017). Quantifying changes in species composition may help to reveal the ecological processes in the human-modified ecosystems and to plan conservation strategies (Gutiérrez-Cánovas et al., 2013; Socolar et al., 2016). If nestedness component is dominant, species being a strict subset of the species at a richer site, it is suggested the need to prioritize sites with richer species for protection, while, if the turnover component is dominant, a regional approach focusing on multiple sites is required (Wright and Reeves, 1992; Angeler, 2013).
To assess the relative importance of the regional and local processes on urban biota establishment, we chose two urban environments, namely, one on the mainland and another on an island in the same neotropical ecoregion but with different geographical isolation levels. We hypothesized that (1) due to the simplification and loss of vegetation cover as urbanization progress, species richness will decrease as built surface increases through the gradient, in both island and continent, (2) as urbanized areas are structurally similar, we would expect that species of highly urbanized sites in both geographical localities will be more similar than those from less urbanized ones, i.e., the effects for the more urbanized sectors should be mainly regional more than local, and (3) due to true isolation and limited size, islands would have lower species richness than the continent, therefore, is less likely to replace species after a perturbation. Then, the effect of urbanization should be higher on islands, producing a loss of specialists and higher levels of nestedness on the beta-diversity component compared with the continent.
Materials and Methods
Study Areas
We worked at the only insular Venezuelan location with an important urban development (i.e., Margarita Island), and we compared our study with another one made in a location in the mainland (i.e., Valencia city). We focused on medium-sized urban developments as they are less studied but can also have the highest impact on biodiversity because they have a greater perimeter/area ratio and usually are surrounded by natural ecosystems (Pauchard and Barbosa, 2013).
Margarita Island (951 km2, 491, 610 inhab.) is located in the Caribbean Sea 25 km far from the Venezuelan coast (10°51′ to 11°11′N, 63°46′ to 63°25′W). Most of the residents live in the oriental sector of the island, where the main urban development has occurred, especially in the city of Porlamar and adjoining towns of Pampatar, El Valle, and Los Robles. This urban conurbation is 26 km2 (Sanz et al., 2011). The population is growing very fast, and the annual geometric growth rate from 2001 to 2014 was 2.8%. The data from the last census in 2011, when compared to 1961, showed a 5-fold increase in the population (from 89,492 to 491,610 inhab.) (Instituto Nacional de Estadística-INE, 2014).
Valencia city (245 km2, 1,396,322 inhab.) is the capital of Carabobo State and the third-largest city of Venezuela, and it is ~180 km away to the west of Caracas (10°06′ to 10°17′N, 68°03′ to 67°57′W). The city is located in a central valley (479–520 m.a.s.l) and is surrounded by a mountain range called the Cordillera de la Costa. The city is an economic hub that hosts top industries and manufacturing companies in Venezuela.
Both localities are in the same time zone (i.e., GMT-4:30), and both have a tropical savanna climate. The weather is hot all year around and with a seasonal rainfall pattern. On the island, there are two rainy seasons, namely, the main one from November to February and a second one from June to August, alternating with two dry seasons. The average total rainfall in wooded lowlands in the island is 743 mm, and the annual mean temperature is 26.8°C. On the continent, there are two seasons, namely, the rainy (May to October) and the dry season (November to May). The average total rainfall is 1295.4 mm, and the annual mean temperature is 24.1°C (http://www.weatherbase.com/). In both regions, the selected urban areas are located at the base of mountain ranges covered with scrubs and deciduous dry forests in the lower slopes.
In both studies, an urban gradient was defined with three levels of building density, starting from the patches of native vegetation [remnant woodlands (W)], following with medium density (MD) urbanized areas that maintain vegetation along the streets and gardens, up to the residential zones with less vegetation cover and higher building density. In each geographical locality, we selected three sites (replicates) for each level of the urban gradient, for a total of nine sampling units. The W were the patches of 5–10 ha of original native vegetation or secondary forests within the urban matrix. In Margarita Island, native dry deciduous or semi-deciduous forest has trees of 5–20 m tall of species as Lonchocarpus atropurpureus, Inga spp., Platymiscium diadelphum, Caesalpinia mollis, or Machaerium latialatum. In the understory shrubs, 2–3 m high of Bourreria cumanensis and Erythroxylum cumanense are common and also lianas (González, 1999). In Valencia city, although the vegetation presents a very high degree of human intervention due to the urbanization process, the urban W maintain the native vegetation of dry deciduous and semi-deciduous forests. The main arboreal species are Bourreria cumanensis, Acacia glomerosa, Guapira pacurero, Erythroxylon havanense, Bursera simaruba, Lonchocarpus violaceus, Guaiacum officinale, Chlorophora tinctoria, and Vitex compressa. Woody lianas of Bignoniaceae and Asclepiadaceae families are relatively abundant (Fajardo et al., 2005).
The MD areas were the residential places with small buildings and houses with ornamental gardens and a mix of vegetation of dry woodland, scrubs, or grassland in small allotments of lands not urbanized or recreational parks, as well as native and exotic trees along the streets. The high building density level [high density (HD)] was the recent residential development-type “townhouse,” consisting mostly of two-storied small houses attached, with a low proportion of green spaces, with small ornamental gardens with grasses and shrubs, in the houses.
On Margarita Island (i.e., Island), we chose three traditional towns adjacent to Cerro El Copey National Park (oriental sector): La Asunción (23,616 inhab.), El Valle del Espíritu Santo (49,967 inhab.), and San Juan Bautista (39,490 inhab.). In each village, we chose residential areas with MD and the W. Additionally, near the largest city of the island, Porlamar (98,000 inhab.), we selected HD areas in three recent urbanizations (Table 1 and Supplementary Material 1).
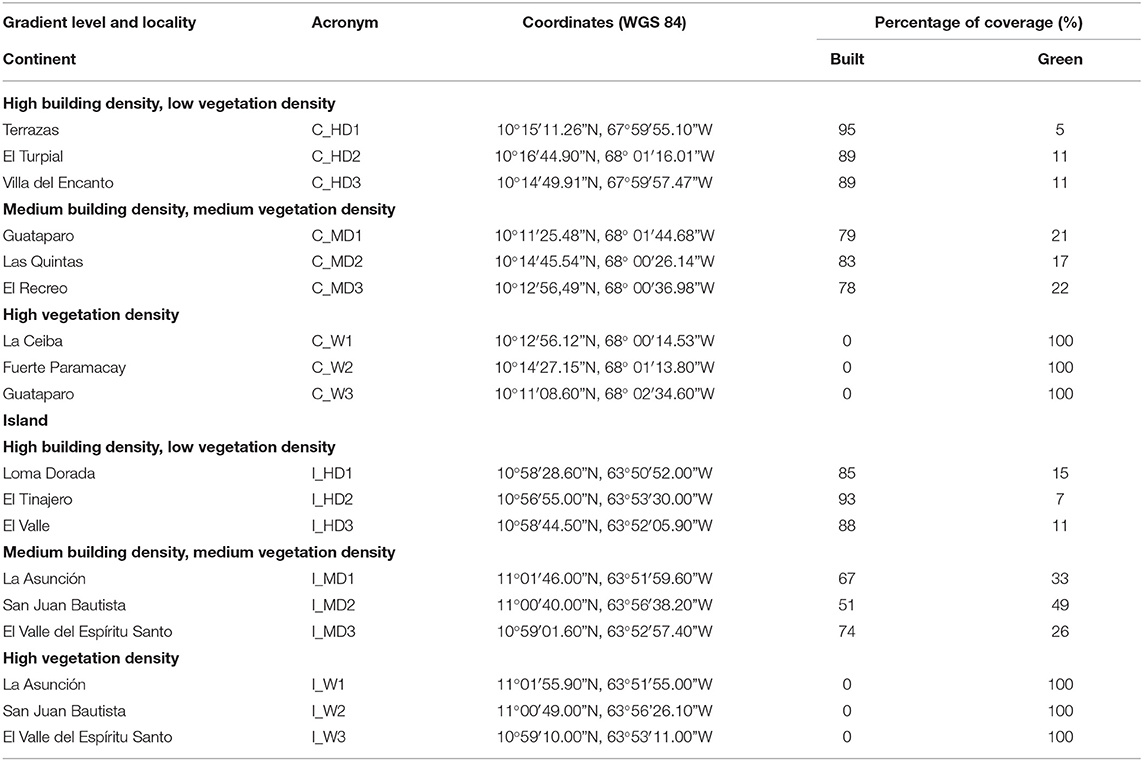
Table 1. Location, acronyms, and relative coverage of built and paved, as well as vegetation surface of the 18 sampling units selected along the urban gradient of Continent and Island.
The continental study (i.e., continent) was carried out in the north part of Valencia city at two sectors, namely, Naguanagua municipality (157,000 inhab.) and San José parish (132,534 inhab.). Despite the differences in size and population between the two localities, urban development in north Valencia is similar to that in Margarita Island. Originally, these places were the small towns adjacent to Valencia, which were incorporated as part of an urban agglomeration or “Greater Valencia” due to the progressive growth of the city. In this locality, we selected the nine sampling units close to the mountains (Supplementary Material 1). At Naguanagua, we chose three recent residential developments with HD-type “townhouse,” i.e., one residential place with small buildings and houses with ornamental gardens and vegetated street, Las Quintas (MD), and remnant dry woodland (W) inside Fuerte Paramacay, a military base. At San José, we chose two residential places with similar characteristics to Las Quintas, namely, El Recreo and Guataparo, and two remnant dry woodlands near the residential places.
Using images from Google Earth, which is a free application, as the geographical source, we digitized the patches of vegetation (i.e., forests, trees, and gardens) and built-up area (i.e., houses, buildings, and streets) with QGIS version 2.14 software (QGIS Development Team, 2016) each cover in relation to the area evaluated (Table 1).
Avian Survey
In both localities, the urban bird communities were sampled with the same method. In the residential sectors (HD and MD), systematic and standardized walks were performed in each sampling unit during each visit. These walks lasted 20 min and covered an area of ~200 × 200 m. In the woodlands (W), we selected two points with the distance of 150 m and counted every bird observed, or heard for 10 min.
The bird assemblage was surveyed four times in the dry season and four times in the rainy season. On the island, the sampling months were October 2010 and May 2011 for the dry season, as well as February and July 2011 for the rainy season. On the continent, February and April 2011 were chosen for the dry season, as well as June and September 2011 for the rainy season. The Fuerte Paramacay, one of the woodland sampling units in Valencia, is a military area and was not sampled during the rainy season because the entry was denied.
The surveys were carried out in the morning, starting 15 min after sunrise and ending ~3 h later on days with no wind, rain, or heavy cloud cover. The records were limited to terrestrial and diurnal birds using specifically the evaluated urban sector. Therefore, aquatic, aerial feeders (swallows and swifts) and crepuscular species were excluded from the analysis because the methods used were not appropriate to assess their presence. The identification of the species was based on the studies by Hilty (2003) and Restall et al. (2007), and the taxonomy follows the South American Classification Committee (SACC), version June 2020 (Remsen et al., 2020).
Data Analysis
We did species accumulative curves for each urban sector in each geographical area to verify if sampling effort was adequate, and we calculated corrected species richness with Chao 2 estimator.
To conduct the rest of the statistical analysis, we excluded the species observed only once and retained the most constant species (65 species), i.e., those recorded at least two times in each locality. We conducted a correspondence analysis (CA) to test for seasonal differences (dry and rainy) of each locality, but we did not find any significant effects on the richness and bird species composition [refer to the “Results” section on Margarita Island in the study by Sanz and Caula (2015)]; therefore, the eight surveys were used as replicas.
In this study, we used the frequency as a surrogate for relative abundance (i.e., the proportion of subsamples that contain the species). We defined the frequency of detection of each species as the number of times a species was detected divided by the total number of surveys performed in each sampling unit. We have taken into account that for some species of birds living in pairs or groups, the frequency is not equivalent to their abundance.
To evaluate compositional similarity in bird assemblages through the urban gradient in both regions, we conducted an abundance-based Bray–Curtis cluster analysis using Past4 project 1.0 software (Hammer et al., 2001). Also, we analyzed the distribution of species of both urban landscapes with the CA with 65 species, using the frequency data as a quantitative measure to visualize whether the gradient levels segregate as a result of the composition of different bird species.
To characterize the sensitivity of each species to the urbanization along the gradient, we ranked the species based on their frequency in each sampling unit in the three levels of the gradient. We categorized the species into urban exploiters, e.g., species well-adapted to human-dominated landscapes (i.e., HD) and become dependent on urban resources (Shochat et al., 2006), urban-tolerant species, with the ability to persist, despite the disturbance caused by the human population (MD), and urban avoiders, e.g., species that do not adapt to the changes to their natural habitat caused by intense urbanization and occur almost exclusively in areas with a high percentage of native vegetation (W) (Kark et al., 2007).
Each species was assigned to a feeding guild following the information from the literature (Poulin et al., 1994; Pérez et al., 2001; Quilarque et al., 2010; Sainz-Borgo, 2016). To simplify the analysis, we included a species in a particular feeding guild based on the main items of the reported diet, although it could include others. We discriminated granivorous birds (feeding mainly seeds of grasses or herbs) from seedeaters (using seeds from fruits of trees or shrubs) because, even though they use the same item (seeds), their sources imply different structural vegetations (i.e., grasslands or open habitats in the first case and forest-woodland in the latter).
To evaluate the taxonomic differences along the urban gradient in each region and between regions, we compared dissimilarity associated with nested dissimilarity and species turnover using presence-absence data (Baselga, 2010) and abundance data (Baselga, 2017). Both phenomena produce dissimilarity, but they are opposite: Spatial turnover is the replacement of some species by others, and nestedness is the loss of species where the poorest site is a strict subset of the richest site (Baselga, 2010). In terms of species abundances partition, the two components of dissimilarity are as follows: (i) balanced variation in abundance, where the individuals of some species in one site are substituted by the same number of individuals of different species in another site and (ii) abundance gradients, whereby some individuals are lost from one site to the other. The indices were computed, using the betapart package 1.4 (Baselga and Orme, 2012) in R 3.2 (RStudioTeam, 2020).
Results
We found 78 species using the whole urban gradient in both the continent and the island. On the continent, we recorded a total of 69 species, on the island, almost half, 35 (Supplementary Material 2). Accumulation species curves show that the sampling effort worked well to catch most species on the island, but on the continent, especially in the woodland patches, and MD zones, several species were not detected.
The pattern of avian richness was the same for both localities, following a decrease along the urban gradient, being highest in the woodland and lowest in the high building density areas (Figure 1). On the continent, however, the major consecutive loss of species occurred between the remnant forests and the MD zones (18 spp.). On the island, the highest difference was between the MD and the HD zones (11 spp.). The proportion of the double number of estimated species on the continent over island remains constant at every level of the gradient (Continent_W = 65.65 ± 4.70/Island_W = 33.15 ± 1.78, Continent_MD = 48.18 ± 6.1/Island_MD = 26.87 ± 3.30), except for HD areas, where the continent has three times more species than the island (Continent_HD = 35.02 ± 4.17/Island_HD = 13.00 ± 0.46), which reinforces the low capacity of the high building density areas on islands to maintain bird species. This occurs though the proportion of built surface in the HD sector on the island is similar ( = 89%) to the continent ( = 91%).
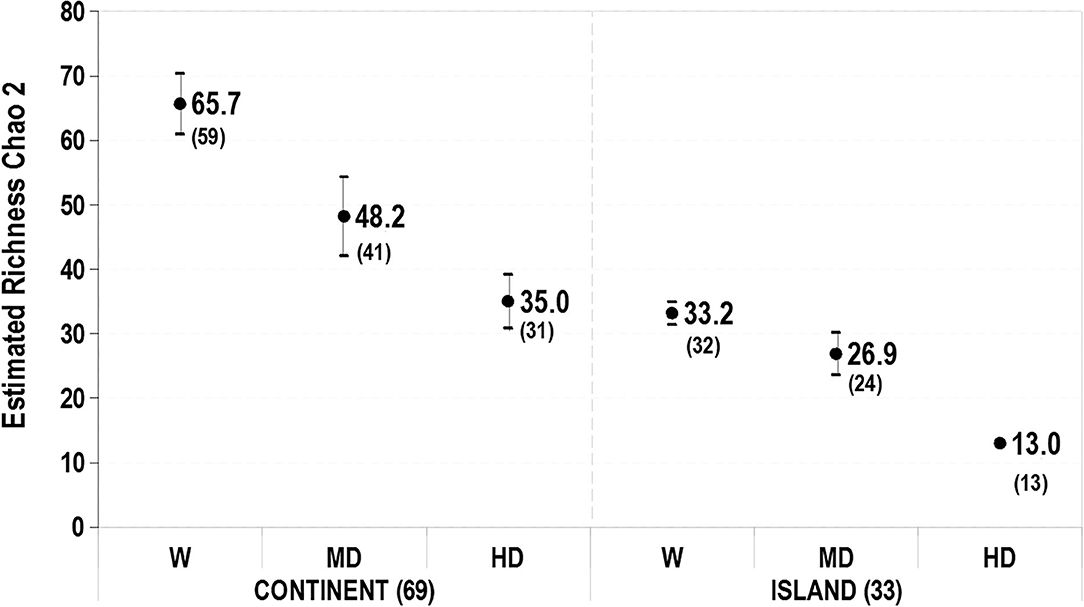
Figure 1. The estimated richness of bird species (Chao 2 index), in the three types of urban habitats in Continent and Island. Total species observed in parenthesis. Vertical bars represent SD. W, remnant woodland; MD, medium density; HD, high density.
The Bray–Curtis cluster analysis showed three distinct groups (Figure 2A), i.e., one consisting of most urbanized sites from the island (I_HD–I_MD) and another consisting of most urbanized sites from the continent (C_HD–C_MD) with 40% of similarity between them. These two are linked with the third one, consisting of the W in both regions that share 40% similarity among them but <30% the similarity with urbanized sites in both regions. Although the similarity was low in general, it was higher between urbanized sites in both regions than between woodlands and urbanized sites.
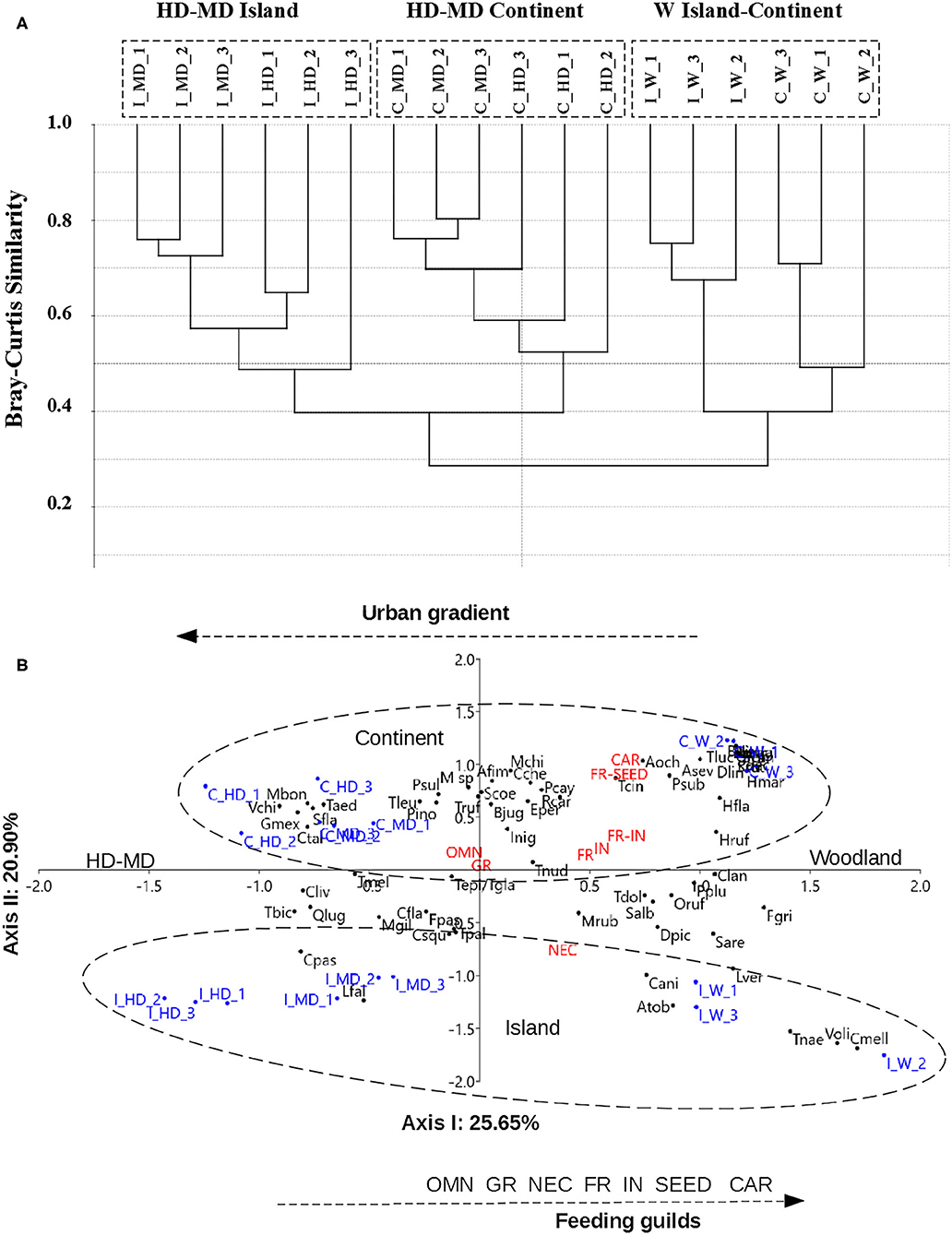
Figure 2. (A) Hierarchical clustering dendrogram from the Bray–Curtis similarity analysis in the three levels of urban gradient (i.e., HD, MD, and W) in Continent and Island. (B) Correspondence analysis based on the frequency of bird species along the urban gradient in Continent and Island with 65 widespread species recorded at least two times in one region. For the acronyms of bird species, refer to Bird List in Supplementary Material 2. For the acronyms of localities, refer to Table 1. C, Continent; I, Island; W, remnant woodland; MD, medium density; HD, high density. Feeding guilds: OMN, omnivores; GR, granivores; IN, insectivores; FR, frugivores; NEC, nectarivores; SEED, seedeaters; and CAR, carnivores.
The cluster results are confirmed by the CA, which clearly separates all levels and replicates of the continent and the island (Figure 2B). In the CA with 65 species, the first two axes accounted for about 46.6% of the total variance. The first axis (horizontal) shows the urban gradient, separating a group of species associated with urban woodland (positive scores) from species associated with higher building density (HD and MD negative scores) in both regions. The second axis (vertical) segregated the bird species associated with the sampling units of the island (negative scores) from bird assemblage associated with the sampling units of the continent (positive scores). In the middle zone, there are species common to both regions. On the island, the three levels of the gradient are more clearly segregated, but on the continent, the sampling units of high and medium built areas are very close together. This could be due to the difference in coverage of vegetation between high and MD areas are lower on the continent ( = 11%) than on the island ( = 25%).
Regarding the feeding guilds in the CA, omnivores and granivores are observed in the center of the scatter plot, meaning that they are present throughout the whole gradient in both regions. As the building density decreases (horizontal axis, positive scores), insectivore and frugivore groups increase. On the island, nectarivores and frugivores are predominant in the areas of intermediate vegetation density and woodland patches, and carnivores were not recorded in the urban landscape. On the continent, seedeaters and carnivores are mainly associated with woodland.
There were marked contrasts in the way individual species responded to urban structure. This can be illustrated by the differences in the response to urbanization observed in the 25 shared species recorded in our samples (Figure 4).
The first nine species are urban exploiters: Columba livia, Quiscalus lugubris, Tiaris bicolor, and Columbina passerina all have a higher frequency at MD and HD sectors, and the following five species are present in the whole urban sectors: Thraupis episcopus/glaucocolpa, Icterus nigrogularis, Mimus gilvus, Coereba flaveola, and Tyrannus melancholicus all have a similar frequency of occurrence in the three levels of the gradient. Notably, T. episcopus (only continent) and T. glaucocolpa (only island) are the only two congeneric species recorded in this study, which are present in one locality and absent in the other. Therefore, we can refer to them as “replacements.” These species belong to a species complex, closely related due to their recent evolutionary formation (Cueva, 2018). They have morphological and ecological similarities, but T. glaucocolpa replaces T. episcopus in the driest zones (Hilty, 2003), and thus, we have treated them as equivalent species to each region. Among these urban exploiter species, the only exotic species registered was C. livia. The second group, conformed by 12 species, are the urban tolerant and urban avoiders, and They were quite common in woodland and their occurrence decreased through MD and they were absent from HD sectors. The 9 exploiters and 12 urban-tolerant species have similar behavior in both regions. Conversely, the third group, conformed by four species, i.e., three Columbiformes (Columbina talpacoti, Columbina squammata, and Leptotila verreauxi) and one Passeriforme (Turdus nudigenis) have different distributions on the urban landscape of island and continent. It is interesting to highlight the different behaviors shown by T. nudigenis and C. talpacoti in the regions. Both species are common urban dwellers in high and medium build density areas of the continent, but they are rare in the whole island, except in the woodland, where T. nudigenis is frequent. L. verreauxi and C. squammata, which are very common on the island, are infrequent in woodland habitats in both regions.
The mean values of dissimilarity along the gradient on continent and island are analogous. Slightly less than half of the assemblage remains similar, in the species presence (C 55.1%; I 52.6%) and the number of individuals (C 52.6%; I 58.9%) throughout from woodland to HD. However, the species nestedness component (Jned) along the gradient is higher on the island (38.3%) than the continent (22.5%) (Figure 3). This means that on the island, dissimilarity across the gradient was mainly caused by nestedness, e.g., the poorest areas (HD and MD) are a subset of the richest areas in species (W). On the contrary, on the continent, species replacement (Jturn 32.6%) along the gradient is more important compared with the island (14.3%), i.e., new species appear at each level while others disappear. There are seven species present in MD and HD in the continent, namely, C. livia, C. passerina, Gymnomystax mexicanus, Molothrus bonariensis, Q. lugubris, T. bicolor, and Vanellus chilensis, not recorded in woodlands, while, on the island, only C. livia is present in MD and HD and not in woodland (Figure 4).
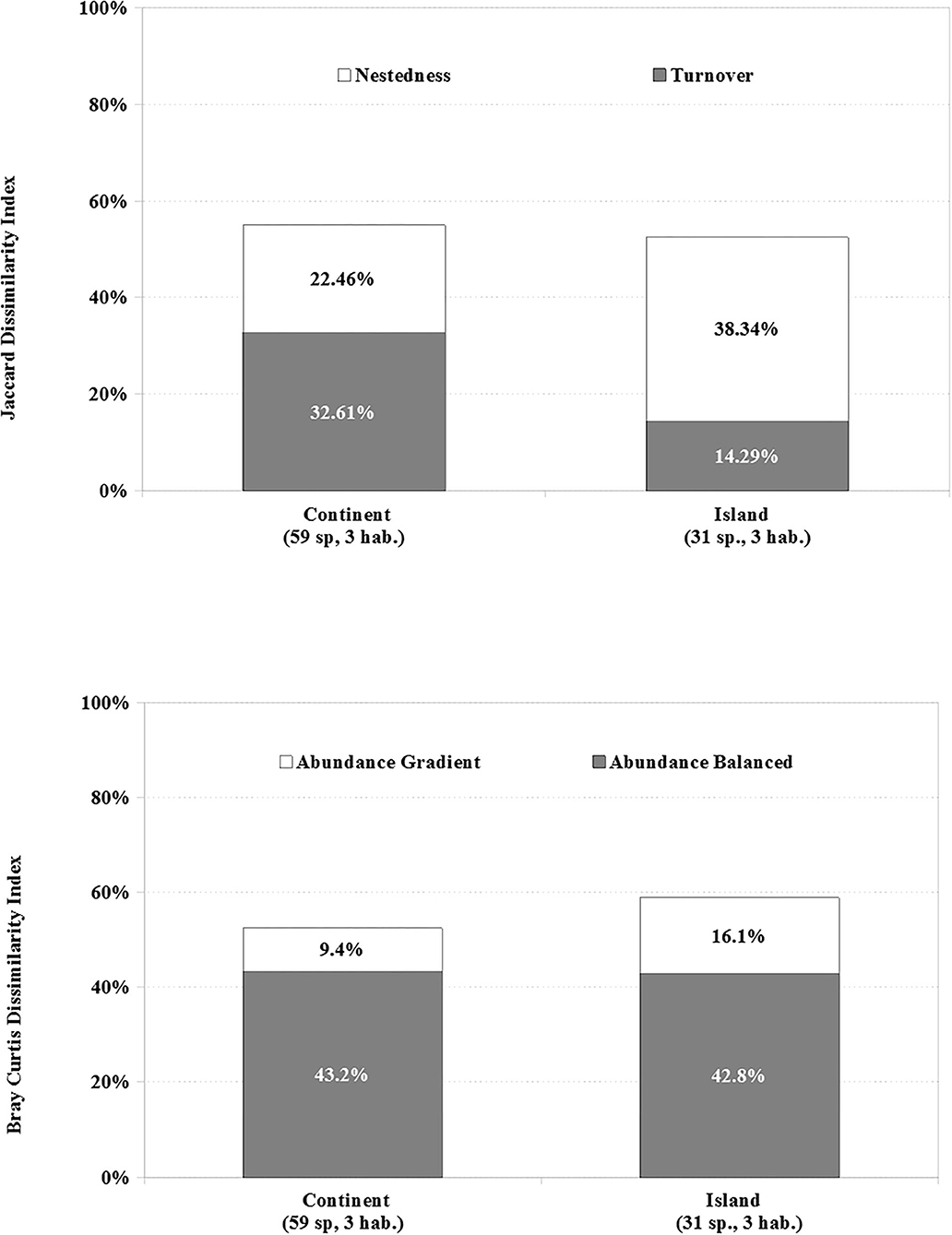
Figure 3. Mean values of the index of incidence-based dissimilarity (Jaccard) and abundance-based dissimilarity (Bray–Curtis) among W, MD, and HD sectors, in each region with 31 and 59 widespread species recorded at Island and Continent, respectively.
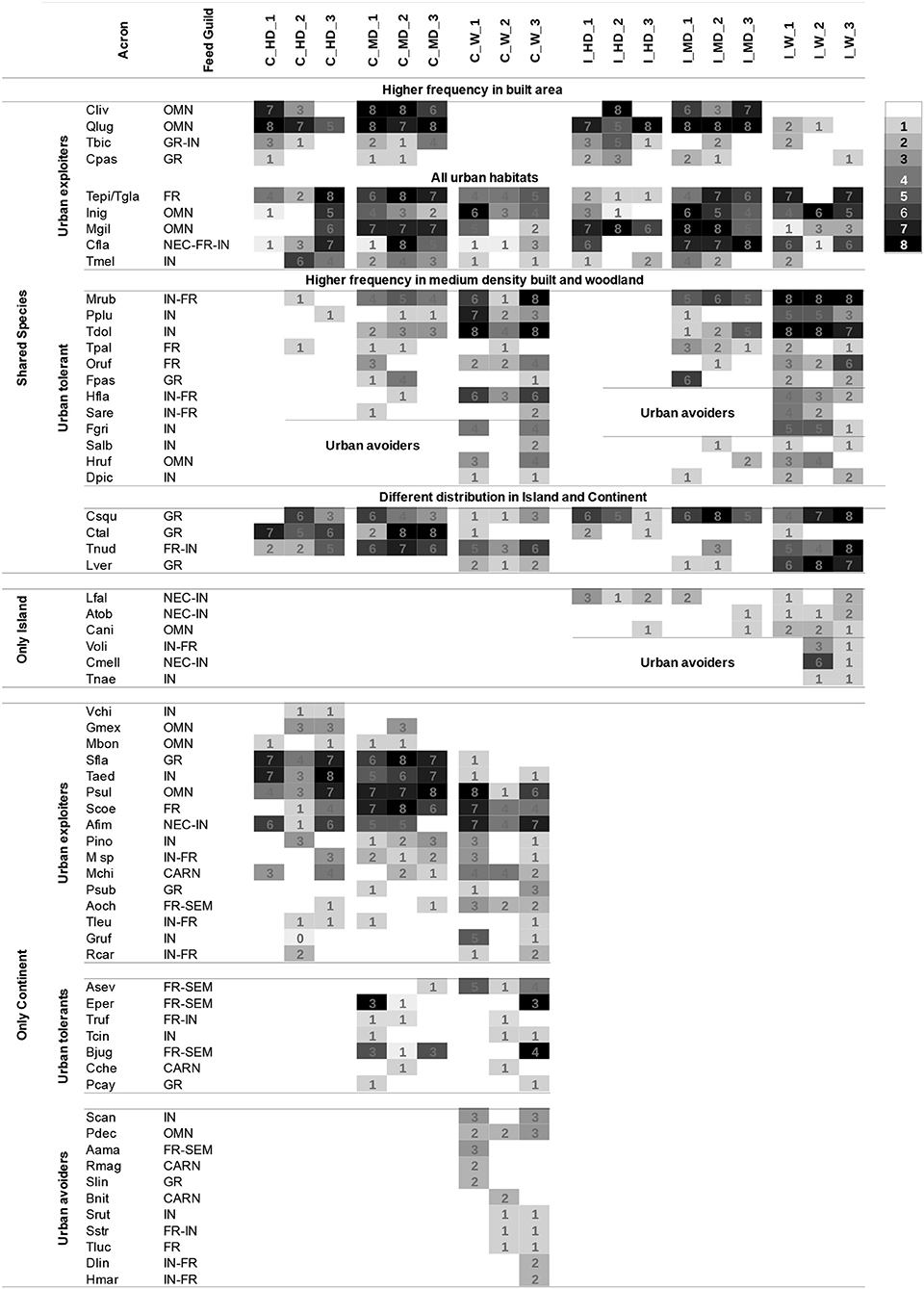
Figure 4. Record of species at each habitat and region, ranked by the uses of the urban landscape and frequency. Grayscale: Frequency of detection of each species at each habitat type during 8 sampling days (refer to legends 1–8). The Paramacay woodland (C-W-2) was visited only in the dry season, i.e., four times out of the total of eight; therefore, the maximum detection frequencies were 4 in this unit. For the acronyms of localities, refer to Table 1; for the acronyms of bird species, refer to Supplementary Material 2.
The abundance balanced variation and gradient components of Bray–Curtis dissimilarity were 43.2 and 9.4% for the continent and 42.8 and 16.1% for an island, respectively. Thus, dissimilarity in continent and island was mostly related to balanced variation in abundance, e.g., the increases of abundance in some species are the same as the decreases of abundance in other species through the gradient. However, the abundance gradient was two times as relevant in the island as in continent, where species decrease their abundance following the urban gradient.
Discussion
As far as we are aware, this is the first study comparing the impact of urban development on bird assemblages on an island and an equivalent locality on the continent. We found similar patterns in several variables but also relevant differences that suggest that the bird communities of the island are more vulnerable to urbanization than their continental counterparts.
Considering all bird species present in the urban samples (78), richness was two times higher on the continent (69) than on the island (35). This remarkable difference is attributable, rather than to limitations posed by urban habitats, to the larger regional pool of species on the continent. The number of species able to reach an island depends on the ability of dispersion of the species in the continent and the availability of ecological resources, usually more limited in the islands (Brown and Lomolino, 1998; Whitaker, 1998).
As predicted, the pattern of decreasing species richness with increasing building density and structure simplification was observed in both island and continent and was consistent with results from earlier studies (e.g., Chace and Walsh, 2006; Clergeau et al., 2006; Faeth et al., 2011; Mikami and Mikami, 2014; Sanz and Caula, 2015), suggesting that fewer species are able to exploit increasingly urbanized habitats and that the vegetation cover is an important component of bird species conservation in cities. However, the island lost more species in the HD units than the continent, even though the proportion of impervious surface was similar in both conditions. Therefore, other variables are acting on the higher susceptibility of the island birds to high urbanized sites. Several authors have found a negative relationship between several human activities as city noise (Francis et al., 2009; Proppe et al., 2013), vehicle traffic (Verma and Murmu, 2015), and pedestrians (MacGregor-Fors and Schondube, 2011; Verma and Murmu, 2015) with bird richness. This is not our case because, although we did not measure these variables, MD sectors in the villages have regular traffic of vehicles and people since they are crossed by roads that connect different towns of the island. Contrarily, the HD building areas chosen in this study are private and closed residential sites, which usually have very few people walking and with cars circulating infrequently. Undoubtedly, the MD zones were the ones with the higher levels of the above urban disturbances, but the number of species was higher in these zones than in high built density places.
On a global scale, it has been found that urban avian assemblages strongly reflect their regional species pools, indicating a weaker role for homogenization than what had been previously recognized (Aronson et al., 2014). However, we found a higher species similarity between urbanized sites of the island and continent (40%) than between urbanized sectors and woodlands remnants inside the same region (<30%). This was so, even though there were differences in the species pool of each region, as was shown by merely 40% similarity between the species present on island and continent woodland remnants. The homogenization is partially a consequence of the presence of non-native birds with worldwide distribution (McKinney, 2006). The number of exotic species, however, differs broadly among cities worldwide with a median of 3.5 exotic birds (range: 0–23) (Aronson et al., 2014). In Latin America, only two cosmopolitan species occur in most cities, C. livia and Passer domesticus (Aronson et al., 2014). In our study, the only exotic species registered was C. livia. This result shows that similar species of each regional pool were successful in overcoming the urban matrix filter.
Most shared species of both regions are distributed similarly on urban areas on the island and the continent, i.e., urban exploiters, urban tolerant, and urban avoiders have a frequency of occurrence similar in each sector, in both regions. However, there were also important local effects that influence the constitution of a particular assemblage of bird species in urban areas of each region (e.g., T. nudigenis, an urban exploiter on the continent, which changes to urban avoider behavior on the island). An ecologically equivalent species, M. gilvus, is very abundant on the island and perhaps is replacing T. nudigenis on island ecosystems. C. talpacoti is a very common dove in the continental city but very rare on the island. In this study, a similar species seems to replace it, C. passerina, which in turn is scarce on the continent. Thus, as pointed out by Sorace and Gustin (2008), urbanization can promote taxonomic homogenization, but local factors still play a role in the composition of urban bird communities.
Although there are studies that have attempted to make generalizations about how species with similar traits respond to urbanization, the results differed across cities and seasons. However, the prevalence of omnivores and granivores, with decreased prevalence of insectivores, has often been associated with the success of these sinantropics species, which agree to the general consensus reached by prior research (Hensley et al., 2019). In the continental urban landscape, 11 feeding guilds were observed and were present along the gradient, while, on the island, only 9 were registered, and they decreased to 7 in the HD construction area. Carnivores and frugivorous seedeaters were absent from the island urban area. Therefore, there is also a reduction in the number of feeding guilds on the island in areas with high urbanization, which means a loss of ecological functionality. We found omnivorous, gregarious, and granivorous ground feeders to be most abundant in more urbanized areas (HD and MD). Omnivorous species would benefit from the variety of foods offered directly or indirectly by humans in the more urbanized areas. Gregarious behavior aids in finding food and avoiding predators (Kark et al., 2007; Sol, 2007; Croci et al., 2008). The carnivores, nectar feeders, and frugivores were dominant at the intermediate levels of urbanization. The vegetation in this study is characterized by several native and exotic flowering and fruiting trees, available in gardens, parks, and streets that benefit nectarivorous and frugivorous birds offering a more constant source of food (Blair, 1996). The high richness of birds in these areas may favor those carnivorous species that feed on eggs and nestlings such as Milvago chimachima (Leveau and Leveau, 2004).
We carried out two beta-diversity analyzes, i.e., one based on presence/absence and another one on the abundance data following the suggestion of Anderson et al. (2011). They proposed the use of a combination of beta-diversity analyses that emphasize the presence of species and those that emphasize the differences in relative abundances because they offer different and complementary results that can help to understand the nature of the changes in the composition of the assemblage. However, if conservation is the main concern, it is better to give more relevance to the incidence index, with the strong relevance for rare species, than measures based on abundance, where common and numerically dominant species play a stronger role (Socolar et al., 2016). In the urban landscape, this is important because, as we have noted, the urban exploiters or tolerants are also the more generalist species, therefore, with less value for conservation.
Species-based beta diversity had a much higher nesting component on the island than on the mainland, where the turnover component was dominant. This was expected, given the isolation of a true island, which limits the ability of many species to colonize. However, in the case of birds, turnover could also be expected to be an important component on the island due to the high dispersal capacity of many species present in the region, such as Columbidae, Tyrannidae, or Icteridae. In studies comparing the diversity of birds and other terrestrial vertebrates, birds had higher levels of turnover or lower levels of nesting (Calderón-Patrón et al., 2013; Si et al., 2016). On the island, the result is clear, as the only new species in the more urbanized space is the non-native C. livia. In contrast, on the continent, six species were able to establish in more urbanized areas. Probably the greater geographical extension, greater connectivity, and the potential presence of more vegetation types close to cities in the continent favored the colonization of new species to the urban landscape. Environmental heterogeneity is a very relevant variable when measuring the impact on beta diversity in other regions (Melo et al., 2009; Calderón-Patrón et al., 2016). The nested pattern on the island would explain the low species richness in HD areas, as there are no new native species colonizing those areas.
Differences in species dissimilarity between the island and the continent are dampened when analyzed considering the abundance. Both regions share a similar and relatively high abundance-balanced variation component. However, in this case, also, the island has two times the value of the abundance-gradient component, which implies that a greater number of species also lose individuals without being compensated by others.
Although the greater sensitivity of islands to human disturbance and species extinction has been well-established for years (Beissinger, 2000; Hughes, 2004; Pimm et al., 2006), the impact of urbanization has not been equally analyzed. In this study, we found that the islands also appear to be more vulnerable to urbanization. Urbanization is a contemporary process that causes bird species displacement and extinction where they occur, despite some species are able to remain or recolonize the urbanized area. On the island, species present in the urbanized area are the result of two environmental filters, i.e., the first one related to the isolation of the region and the second one related to the impact of urbanization. The group of birds that manages to remain, especially in sites with an HD of the built-up area, is a subgroup of generalist and tolerant species that constitute a taxonomically, numerically, and functionally simplified assemblage of the original pool. Margarita Island has certain conditions that promote high biodiversity: medium size (950 km2), proximity to the mainland (23 km), topographic variety (up to 950 m.a.s.l.), and variety of vegetation types (19 vegetation units, Sanz et al., 2011). If under these conditions we find a strong negative impact of highly urbanized areas, the effect could be even greater on islands with more simplified or isolated characteristics. More comparative studies in the future are needed to support whether the patterns we found in this study are common to island ecosystems and to elucidate the underlying process that causes them.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because we do not capture or handle any animals to conduct this research. We have only observed them through binoculars.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was financially supported by the Council for Scientific and Technological Development of the University of Carabobo (CDCH-UC) No 0432–10 and the Venezuelan Institute for Scientific Research (IVIC) project 657.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the improvements in English usage made by Bruce Peterson through the Editorial Assistance Program of the Association of Field Ornithologists. We are grateful to Ian MacGregor-Fors and referees whose comments helped to improve the manuscript. We are also grateful to Elysa Silva for the GIS support in the digitalization of the urban gradient and Rodrigo Díaz for his assistance in the field.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.727879/full#supplementary-material
References
Anderson, M. J., Crist, T. O., Chase, J. M., Vellend, M., Inouye, B. D., Freestone, A. L., et al. (2011). Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14, 19–28. doi: 10.1111/j.1461-0248.2010.01552.x
Angeler, D. G. (2013). Revealing a conservation challenge through partitioned long-term beta diversity: increasing turnover and decreasing nestedness of boreal lake metacommunities. Divers. Distrib. 19, 772–781. doi: 10.1111/ddi.12029
Aronson, M. F., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. Biol. Sci. 281:20133330. doi: 10.1098/rspb.2013.3330
Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 1, 134–143. doi: 10.1111/j.1466-8238.2009.00490.x
Baselga, A. (2012). The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecol. Biogeogr. 21, 1223–1232. doi: 10.1111/j.1466-8238.2011.00756.x
Baselga, A. (2017). Partitioning abundance-based multiple-site dissimilarity into components: balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 8, 799–808. doi: 10.1111/2041-210X.12693
Baselga, A., and Orme, C. D. L. (2012). Betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812. doi: 10.1111/j.2041-210X.2012.00224.x
Beissinger, S. R. (2000). Ecological mechanisms of extinction. PNAS 97, 11688–11689 doi: 10.1073/pnas.97.22.11688
Blair, R. B. (1996). Land use and avian species diversity along an urban gradient. Ecol. Appl. 6, 506–519. doi: 10.2307/2269387
Brown, J. H., and Lomolino, M. V. (1998). Biogeography. 2nd Ed. Sunderland, MA: Sinauer Associates, Inc. Publishers.
Calderón-Patrón, J. M., Goyenechea, I., Ortiz-Pulido, R., Castillo-Cerón, J., Manriquez, N., Ramírez-Bautista, A., et al. (2016). Beta diversity in a highly heterogeneous area: Disentangling species and taxonomic dissimilarity for terrestrial vertebrates. PLoS ONE 11:e0160438. doi: 10.1371/journal.pone.0160438
Calderón-Patrón, J. M., Moreno, C., Pineda-López, R., Sánchez-Rojas, G., and Zuria, I. (2013). Vertebrate dissimilarity due to turnover and richness differences in a highly beta diverse region: the role of spatial grain size, dispersal ability and distance. PLoS ONE. 8:e82905. doi: 10.1371/journal.pone.0082905
Chace, J., and Walsh, J. J. (2006). Urban effects on native avifauna: a review. Landscape Urban Plann. 74, 46–69. doi: 10.1016/j.landurbplan.2004.08.007
Clergeau, P., Croci, S., Jokimäki, J., Kaisanlahti-Jokimäki, M. L., and Dinetti, M. (2006). Avifauna homogenisation by urbanisation: Analysis at different European latitudes. Biol. Conserv. 127, 336–344. doi: 10.1016/j.biocon.2005.06.035
Croci, S., Butet, A., and Clergeau, P. (2008). Does urbanization filter birds on the basis of their biological traits. Condor 110, 223–240. doi: 10.1525/cond.2008.8409
Cueva, D. A. (2018). Molecular Phylogeny of Thraupis, Boie, 1826 (Aves: Passeriformes) and Taxonomic Review of the Thraupis episcopus (Linnaeus, 1766) - Thraupis sayaca (Linnaeus, 1766) species complex. [dissertation/master's thesis]. [Säo Paulo(Br)]: Museu de Zoologia da Universidade de Säo Paulo.
Faeth, S. H., Bang, C., and Saari, S. (2011). Urban Biodiversity: Patterns and Mechanisms. New York, NY: Annals New York Academic Sciences.
Fajardo, L., González, V., Nassar, J. M., Lacabana, P., Portillo, C. A., Carrasquel, F., et al. (2005). Tropical dry forests of Venezuela: Characterization and current conservation status. Biotropica 37, 531–546. doi: 10.1111/j.1744-7429.2005.00071.x
Francis, C. D., Ortega, C. P., and Cruz, A. (2009). Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. doi: 10.1016/j.cub.2009.06.052
González, V. (1999). Vegetación Estado Nueva Esparta. Programa DAO-Palmaven. Caracas, Venezuela: Palmaven S.A and Universidad Central de Venezuela.
Groffman, P. M., Cavender-Bares, J., Bettez, N. D., Grove, J. M., Hall, S. J., Heffernan, J. B., et al. (2014). Ecological homogenization of urban USA. Front. Ecol. Environ. 12:120374. doi: 10.1890/120374
Gutiérrez-Cánovas, C., Millán, A., Velasco, J., Vaughan, I. P., and Ormerod, S. J. (2013). Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Global Ecol. Biogeogr. 22, 796–805. doi: 10.1111/geb.12060
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electr. 4, 1–99.
Hensley, C. B., Trisos, C. H., Warren, P. S., MacFarland, J., Blumenshine, S., Reece, J., et al. (2019). Effects of urbanization on native bird species in three southwestern US Cities. Front Ecol. Evol. 7:71. doi: 10.3389/fevo.2019.00071
Hughes, A. L. (2004). A statistical analysis of factors associated with historical extinction and current endangerment of non-passerine birds. Wilson Bull. 116, 330–336. doi: 10.1676/03-088
Instituto Nacional de Estadística-INE (2014). Censo Nacional de Población y Vivienda. Resultados por entidad federal y municipios del Estado Nueva Esparta. Available online at: http://www.ine.gov.ve/demografica/censopoblacionvivienda.asp (accessed May 10, 2016).
Kark, S., Iwaniuk, A., Schalimtzek, A., and Banker, E. (2007). Living in the city: can anyone become an 'urban exploiter'?. J. Biogeogr. 34, 638–651. doi: 10.1111/j.1365-2699.2006.01638.x
Leveau, L. M., and Leveau, C. M. (2004). Comunidades de aves en un gradiente urbano de la ciudad de Mar del Plata, Argentina. Hornero 19, 13–21.
MacGregor-Fors, I., and Schondube, J. E. (2011). Gray vs. green urbanization: Relative importance of urban features for urban bird communities. Basic Appl. Ecol. 12, 372–381. doi: 10.1016/j.baae.2011.04.003
McKinney, M. L. (2002). Urbanization, biodiversity, and conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52, 883–890. doi: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260.
Melo, A. S., Rangel, T. F. L. V., and Diniz-Filho, J. A. F. (2009). Environmental drivers of betadiversity patterns in New-World birds and mammals. Ecography 32, 226–236. doi: 10.1111/j.1600-0587.2008.05502.x
Mikami, O., and Mikami, K. (2014). Structure of the Japanese avian community from city centers to natural habitats exhibits a globally observed pattern. Landscape Ecol. Eng. 10, 355–360 doi: 10.1007/s11355-012-0201-8
Myers, N., Mittermeier, R., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Pauchard, A., and Barbosa, O. (2013). “Regional assessment of Latin America: Rapid urban development and social economic inequity threaten biodiversity hotspots,” in Urbanization, Biodiversity, and Ecosystem Services: Challenges and Opportunities: A Global Assessment Dordrecht; Heidelberg; New York, NY: Springer. doi: 10.1007/978-94-007-7088-1_28
Pérez, E. M., Bulla, L., and Santiago, E. (2001). Similitudes dietarias entre ocho aves granívoras en la estación experimental “La Iguana”, estado Guárico, Venezuela. Ecotropicos 14, 49–56.
Pimm, S., Raven, P., Peterson, A., Sekercioglu, Ç. H., and Ehrlich, P. R. (2006). Human impacts on the rates of recent, present, and future bird extinctions. PNAS 103, 10941–10946. doi: 10.1073/pnas.0604181103
Poulin, B., Lefebvre, G., and McNeil, R. (1994). Diets of land birds from northeastern Venezuela. Condor 96, 354–367. doi: 10.2307/1369320
Proppe, D. S., Sturdy, C. B., and St. Clair, C. C. (2013). Anthropogenic noise decreases urban songbird diversity and may contribute to homogenization. Glob. Change Biol. 19, 1075–1084. doi: 10.1111/gcb.12098
QGIS Development Team (2016). QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.org
Quilarque, E., Marín, G., Carvajal, M., and Ferrer, H. (2010). Componentes de la dieta de Sporophila minuta, S. Intermedia (Emberizidae), Myiozetetes similis y Elaenia flavogaster (Tyrannidae), en un ecotono bosque palustre-basi-montano de Venezuela. Bol. Ctro. Inv. Biol. 44, 161–172.
Remsen, J. V., Areta, J. I., Cadena, C. D., Claramunt, S., Jaramillo, A., Pacheco, J. F., et al (2020). A Classification of the Bird Species of South America. Available online at: http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm (accessed November 20, 2020).
Restall, R., Rodner, C., and Lentino, M. (2007). Birds of Northern South America: An Identification Guide. New Haven, CT: Yale University Press.
RStudioTeam (2020). RStudio: Integrated Development for R. Rstudio. http://www.rstudio.com/
Sainz-Borgo, C. (2016). Diet composition of birds associated to an urban forest patch in Northern Venezuela. Interciencia 41, 119–126.
Sanz, V., and Caula, S. (2015). Assessing bird assemblages along an urban gradient in a Caribbean Island (Margarita, Venezuela). Urban Ecosyst. 18, 729–746. doi: 10.1007/s11252-014-0426-4
Sanz, V., Riveros, M., Gutiérrez, M., and Moncada, R. (2011). Vegetación y uso de la tierra en el estado Nueva Esparta, Venezuela: un análisis desde la ecología del paisaje. Interciencia 36, 881–887.
Shochat, E., Warren, P. S., Faeth, S. H., McIntyre, N. E., and Hope, D. (2006). From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 4, 186–191. doi: 10.1016/j.tree.2005.11.019
Si, X., Baselga, A., Leprieur, F., Song, X., and Ding, P. (2016). Selective extinction drives taxonomic and functional alpha and beta diversities in island bird assemblages. J. Anim. Ecol. 85, 409–418. doi: 10.1111/1365-2656.12478
Socolar, J. B., Gilroy, J. J., Kunin, W. E., and Edwards, D. P. (2016). How should beta-diversity inform biodiversity conservation. Trends Ecol. Evol. 31, 67–80. doi: 10.1016/j.tree.2015.11.005
Sol, D. (2007). “Do successful invaders exist? Pre-adaptations to novel environments in terrestrial vertebrates,” in Biological Invasions, ed Nentwig, W., (Berlin: Springer). doi: 10.1007/978-3-540-36920-2_8
Sorace, A., and Gustin, M. (2008). Homogenisation processes and local effects on avifaunal composition in Italian towns. Acta Oecol. Int. J. Ecol. 33, 15–26. doi: 10.1016/j.actao.2007.07.003
United Nations (2017). Habitat III Regional Report. Latin America and the Caribbean. Sustainable Cities With Equality. New York, NY: United Nations Publication.
Verma, S. K., and Murmu, T. D. (2015). Impact of environmental and disturbance variables on avian community structure along a gradient of urbanization in Jamshedpur, India. PLoS ONE 10:e0133383. doi: 10.1371/journal.pone.0133383
Whitaker, R. J. (1998). Island Biogeography: Ecology, Evolution and Conservation. Oxford: Oxford University Press.
Whittaker, R. J., Fernández-Palacios, J. M., Matthews, T. J., Borregaard, M. K., and Triantis, K. A. (2017). Island biogeography: Taking the long view of nature's laboratories. Science 357:eaam8326. doi: 10.1126/science.aam8326
Keywords: beta diversity, biodiversity conservation, urban bird, Margarita Island, Caribbean, Island-Continent, Neotropical Cities, Latin American
Citation: Caula SA and Sanz D'Angelo V (2021) Impact of Urbanization to an Island and the Continent: Species Turnover and Nestedness in Neotropical Bird Assemblages. Front. Ecol. Evol. 9:727879. doi: 10.3389/fevo.2021.727879
Received: 20 June 2021; Accepted: 07 September 2021;
Published: 11 October 2021.
Edited by:
Álvaro Garitano-Zavala, Universidad Mayor de San Andrés, BoliviaReviewed by:
Wayne C. Zipperer, United States Forest Service (USDA), United StatesWenjie Wang, Northeast Forestry University, China
Copyright © 2021 Caula and Sanz D'Angelo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina A. Caula, c2FiaW5hY2F1bGFAZ21haWwuY29t
†Present address: Sabina A. Caula, Unión Venezolana de Ornitólogos, Caracas, Venezuela
‡These authors have contributed equally to this work
 Sabina A. Caula
Sabina A. Caula Virginia Sanz D'Angelo2‡
Virginia Sanz D'Angelo2‡