- 1Hunan Changsha Municipal Tobacco Company, Changsha, China
- 2Hunan Province Tobacco Company, Changsha, China
- 3College of Plant Protection, Hunan Agricultural University, Changsha, China
- 4Hunan Forestry Academy, Changsha, China
Background: Egg cannibalism is common in nature. In China, Arma custos (Hemiptera: Asopinae) has been widely used as a natural enemy to control agricultural and forestry pests. A previous study showed that adult A. custos devour their eggs. However, no research has investigated the interaction between A. custos cannibalism and egg development. Clarifying the mechanisms involved in egg cannibalism by A. custos improves our understanding of the evolutionary relationships to enable more efficient mass rearing and biological control systems.
Results: Virgin females showed a lower egg cannibalism inclination than gravid females. Both virgin and mated females showed a higher egg cannibalism inclination than virgin and mated males. The first and second instar nymphs did not devour eggs. The third, fourth, and fifth instar nymphs devoured eggs. Younger eggs were more readily eaten than older eggs. Neither A. custos nymphs nor female adults consumed all the available eggs, allowing an emergence ratio of >70%.
Conclusion: Arma custos females exhibit a higher tendency for egg cannibalism than males. Egg cannibalism varies not only with the developmental stage of the eggs and nymphs but also with sex and reproductive status of A. custos females. These findings help us to better understand the evolutionary relationships in egg cannibalism by A. custos and contribute to the efficient mass rearing and realization of A. custos in biological control systems.
Introduction
In China, Arma custos Fallou (Hemiptera: Asopinae) (Zhao et al., 2018) is a well-known predaceous pentatomid that prefers to prey on insects of coleopterans, hymenopterans, hemipterans, and lepidopterans (Zou et al., 2012; Wu et al., 2020). Since the 1970s, it has been used to control many agricultural and forestry pests in China (Chai et al., 2000; David and Zheng, 2002; Gao et al., 2011; Zou et al., 2012). Target species include Leptinotarsa decemlineata (Say), Spodoptera litura (fabricius), Beet armyworm (Hübner), and Hyphantria cunea (Drury) (Chai et al., 2000; David and Zheng, 2002; Gao et al., 2011; Zou et al., 2012), with an efficiency of over 60% (Zheng and Chen, 1992; Gao et al., 2011). In recent years, the application area of A. custos has been increasing annually, exceeding 100,000 ha in China in 2018 (Zhang and Jia, 2018).
Egg cannibalism is a very common behavior in nature (Fox, 1975; Polis, 1981), but it is unfavorable in large-scale breeding as it increases breeding costs and reduces biocontrol efficiency (Kuriwada et al., 2013). However, egg cannibalism occurs because the acquisition of nutrients directly benefits the consumer through increased growth and development rate, and the elimination of conspecific competition is also an indirect benefit (Block and Stoks, 2004; Richardson et al., 2010). Some insects cannibalize eggs to avoid starvation (Pizzatto and Shine, 2008; Dobler and Koelliker, 2010), such as flour beetles (Tribolium confusum Duval) (Parsons et al., 2013), European earwigs (Forficula Auricularia Linnaeus) (Dobler and Koelliker, 2010, 2011), and Adalia bipunctata Linnaeus (Agarwala and Dixon, 1992). In addition, many factors can influence egg cannibalism behavior, including predator gender (Revynthi et al., 2018a, b), predator or prey density (Kakimoto et al., 2003; Erica et al., 2011), the kinship between predator and prey (Samu et al., 1999; Hoffman, 2012; Parsons et al., 2013), and the reproductive status of the predator (Momen and AbdelKhalek, 2009; Bayoumy and Michaud, 2015; Maleknia et al., 2016).
Many Hemiptera insects are known for their cannibalistic behavior, such as Arizona backswimmers Frank (Zalom, 1978), Triatoma infestans Klug and Triatoma sordida Stal (Ryckman, 1951), Arctocorisa carinata and Callicorixa producta (Pajunen and Pajunen, 1991), Xylocoris flavipes Reuter (Richard, 1979), Notonecta hoffmanni (Orr et al., 1990), Dicyphus cerastii Wagner, and Macrolophus pygmaeus Ranmbur (Goncalo et al., 2020) which would prey on its immature offspring. Our previous research showed that female A. custos were more active predators (ate more eggs than males) (Wu et al., 2020), while males did not participate in egg cannibalism. However, it remains unclear whether reproductive status affects egg cannibalism by A. custos. There may be differences in both male and female A. custos between mated and virgin stages. For example, Bayoumy and Michaud (2015) found that virgin female Hippodamia convergens Guerin-Meneville (Coleoptera) are less cannibalistic than mated adults, while males are more cannibalistic than mated adults. Meanwhile, we identified the cannibalistic behavior by adults mainly occurred 48 h after initiating the experiments. We also found that neither A. custos males nor females consumed all the available eggs, regardless of the predator-to-prey ratio. This may be due to egg age or because adults did not want to consume all the eggs. In addition, although egg cannibalism by larvae has been widely studied, few studies have investigated egg cannibalism by A. custos larvae. Pajunen and Pajunen (1991) found that female rock-pool corixids cannibalized new eggs more frequently than 1-day-old eggs. It is difficult to construct experiments in the laboratory that interact nymphs with eggs, but in the natural environment where nymphs may change and encounter other A. custos eggs. Significant egg cannibalism has been found in many insects, such as Formica aquilonia Yarrow (Hymenoptera) (Schultner et al., 2013) and Triboliunm castaneum Herbst (Coleoptera) (Frank and Peter, 1966). Moreover, older nymphs disperse further, requiring more energy than earlier-stage nymphs; thus, older nymphs may consume more eggs in natural environments. Therefore, it is necessary to investigate egg cannibalism by A. custos nymphs. This research not only improves our understanding of cannibalism evolutionary selectivity but also increases the efficiency of larger-scale breeding and release biological programs.
To identify the relationship between egg cannibalism and reproductive status, egg age, sex, and life stage of A. custos, the following three hypotheses were tested in the laboratory. (1) Mated female A. custos devour more eggs than virgin females, and reproductive status has no effect on male egg cannibalism. (2) Younger eggs are more likely to be eaten than older ones. (3) Older nymphs consume more eggs than younger nymphs.
Materials and Methods
Experimental Insects and Conditions
Arma custos adults were collected in Langfang, Hebei Province, China, in 2018 and taken to the laboratory. Specimens were fed Chinese oak silk moth pupae (Antherea pernyi Guer.) purchased from a supermarket in Liaoning, China. The insects were reared in artificial climate boxes as previously described by Pan et al. (2019). Briefly, the first instar nymphs of A. custos from a single egg mass were placed in transparent plastic dishes and fed only water via a piece of moist absorbent cotton. Chinese oak silk moth pupae were provided food from the second instar nymph stage, with the supply replenished every 4 to 5 days (Pan et al., 2019).
Recently emerged adults (<6 h old) were paired and placed in a 6 × 10 cm Petri dish, and they would mate after 5–6 days. The Petri dish contained one Chinese oak silk moth pupae lined with a piece of paper and formed into a tube for food (diameter: 1 cm, height: 6 cm). The female had laid eggs after 4–5 d and the insects (and the remains of the moth pupa) were removed. Eggs laid on the paper were used in the experiment within 24 h.
We assessed the cannibalistic behavior of adult A. custos under laboratory conditions by placing recently laid eggs (<24 h old) in small plastic dishes (10 × 1.5 cm) covered with an insect-proof screen (80-μm mesh) for ventilation. All adults were 8 days old and had been starved for 24 h before beginning the experiment. The specimens used included females that had previously laid eggs and males that had previously mated. All treatments were performed at 25°C ± 2°C, 60% ± 10% relative humidity, and a photoperiod of 16:8 (L:D).
Egg Cannibalism in Different Female and Male A. custos Developmental Stages
We performed four treatments to verify whether the female and male A. custos cannibalistic behavior was affected by the age of the female. The treatments were the following: virgin and mated. The adults were placed in plastic cups containing 30 eggs (<24 h) that were not their own offspring. Each treatment had 30 replicates with 30 different adults, and each cup was considered an experimental unit.
Egg Cannibalism by Female and Male A. custos of Different Aged Eggs
We performed two tests to verify whether female and male A. custos cannibalistic behavior was related to egg developmental stage: (1) with different aged eggs and (2) supplying new eggs (<24 h) after 48 h.
(1) Different aged egg tests: We collected and dated eggs of a female A. custos and stored them in different boxes according to date so that we could distinguish the eggs’ age. Five treatments were compared to verify whether the female A. custos cannibalistic behavior was affected by the age of the eggs in free-choice and no-choice conditions. Each treatment involved 30 replicates with 30 different adults, and each cup was considered an experimental unit.
No-choice test: one female or male A. custos was placed in a plastic cup containing 24, 48, 72, 96, and 120-h-old eggs. Every plastic cup contained 30 eggs that were not offspring of the female.
Free-choice test: at the beginning of the free-choice experiments, we drew five flabellate grids on the Petri dishes, which divided the dishes into five equal parts. Then six eggs of 24, 48, 72, 96, and 120 h old were put into the five equal parts of Petri dishes at random. Then, one female or male A. custos was placed in a plastic cup containing 30 eggs.
(2) Supplying new eggs test: This experiment involved two treatments, as follows: supplying new eggs (<24 h) after 48 h and not supplying new eggs (<24 h) after 48 h. The adults were placed in plastic cups containing 30 eggs that were not their offspring. Each treatment had 30 replicates with 30 different adults, and each cup was considered an experimental unit.
Egg Cannibalism in Different Nymph Developmental Stages
We performed five treatments to verify whether the A. custos cannibalistic behavior was affected by the age of the nymph. Treatments included: first, second, third, fourth, and fifth instar nymphs. All first, second, third, fourth and fifth instar nymphs were 1 day old and had been starved for 24 h before beginning the experiment. The nymphs were placed in plastic cups containing 30 non-related eggs (<24 h). Each treatment had 30 replicates with 30 different nymphs, and each cup was considered an experimental unit.
Data Collection
In all treatments, A. custos adults were monitored for 5 min post-release, and further observations were carried out every 24 h until the death of all individuals. The number of unconsumed eggs and consumed eggs (broken eggshells) was counted. We determined (1) the cannibalistic behavior of A. custos adults and (2) the number of consumed and unconsumed eggs. From previous research, eggs with broken and diaphanous eggshells were considered cannibalized eggs. We did not provide additional food to males and females after initiating the cannibalism experiments.
Statistical Analysis
A chi-squared test was used to estimate whether the development progress of the eggs, nymphs, and females was related to their cannibalism. An analysis of variance was performed to verify the differences between female A. custos egg consumption and egg emergence ratio. Bartlett’s test was used to test the homogeneity of variances, and sqrt was used to analyze datasets with p < 0.05. Multiple comparisons were performed using Tukey’s HSD test. All analyses was performed using R v.3.3.3 (R Development Core Team, 2017).
Results
Egg Cannibalism by Female and Male A. custos at Different Developmental Stages
Virgin females showed a lower egg cannibalism inclination than gravid females. Both virgin and mated females showed a higher egg cannibalism inclination than virgin and mated males (Figure 1). The number of virgin females showing egg cannibalism behavior was significantly lower than that of mated females (Figure 1A: chi-squared test, χ2 = 6.87, df = 1, p < 0.05). The number of virgin females showing egg cannibalism behavior was four times higher than virgin males (Figure 1A: chi-squared test, χ2 = 5.69, df = 1, p < 0.05). The number of mated females showing egg cannibalism behavior was eight times higher than mated males (Figure 1A: chi-squared test, χ2 = 24.50, df = 1, p < 0.05). Meanwhile, the number of eggs consumed by virgin female A. custos was lower than that of mated females (Figure 1B: Tukey HSD test, p < 0.05). Both virgin and mated females consumed more eggs than virgin and mated males (Figure 1B: All, Tukey HSD test, p < 0.05). The egg emergence ratio when cannibalized by virgin females was significantly higher than that of mated females. The egg emergence ratio when cannibalized by both virgin and mated females was significantly lower than that of virgin and mated males (Figure 1C: Tukey HSD test, p < 0.05).
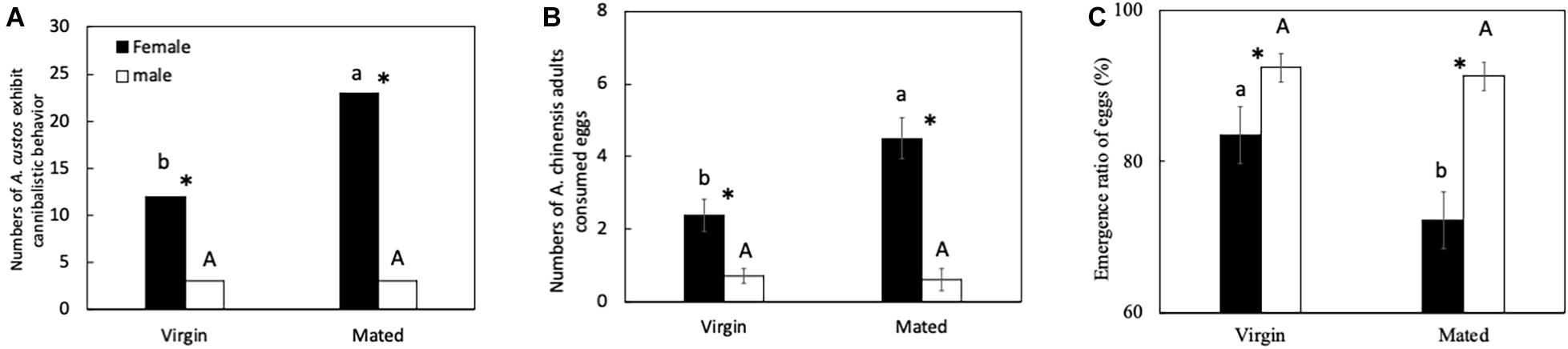
Figure 1. (A–C) Predatory behavior by A. custos on different-aged eggs. Different lowercase letters indicate significant differences among females at p < 0.05 and different uppercase letters indicate significant differences among males at p < 0.05. The investigation was repeated 30 times. * indicates a significant difference between the black bar and white bar at p < 0.05 (female and male virgins or female and male mated individuals).
Egg Cannibalism of A. custos to Different Ages of Eggs
The predatory behavior by female A. custos is affected by the egg development stage. After supplying new eggs (at 48 h), female A. custos exhibited a more active predatory behavior than female A. custos who were not supplied with new eggs after 48 h (Figure 2A: before supplying new eggs, chi-squared test, χ2 = 0, df = 1, p = 1; after supplying new eggs, chi-squared test, χ2 = 29.93, df = 1, p < 0.05). Female A. custos who were supplied with new eggs consumed more eggs than the female A. custos that were not supplied with new eggs after 48 h (Figure 2B: before supplying new eggs, Tukey HSD test, p = 0.78; after supplying new eggs, Tukey HSD test, p < 0.05). However, supplying new eggs did not affect the emergence ratio of the eggs (Figure 2C: Tukey HSD test, p < 0.05) and more than 70% of eggs emerged (Figure 2C).
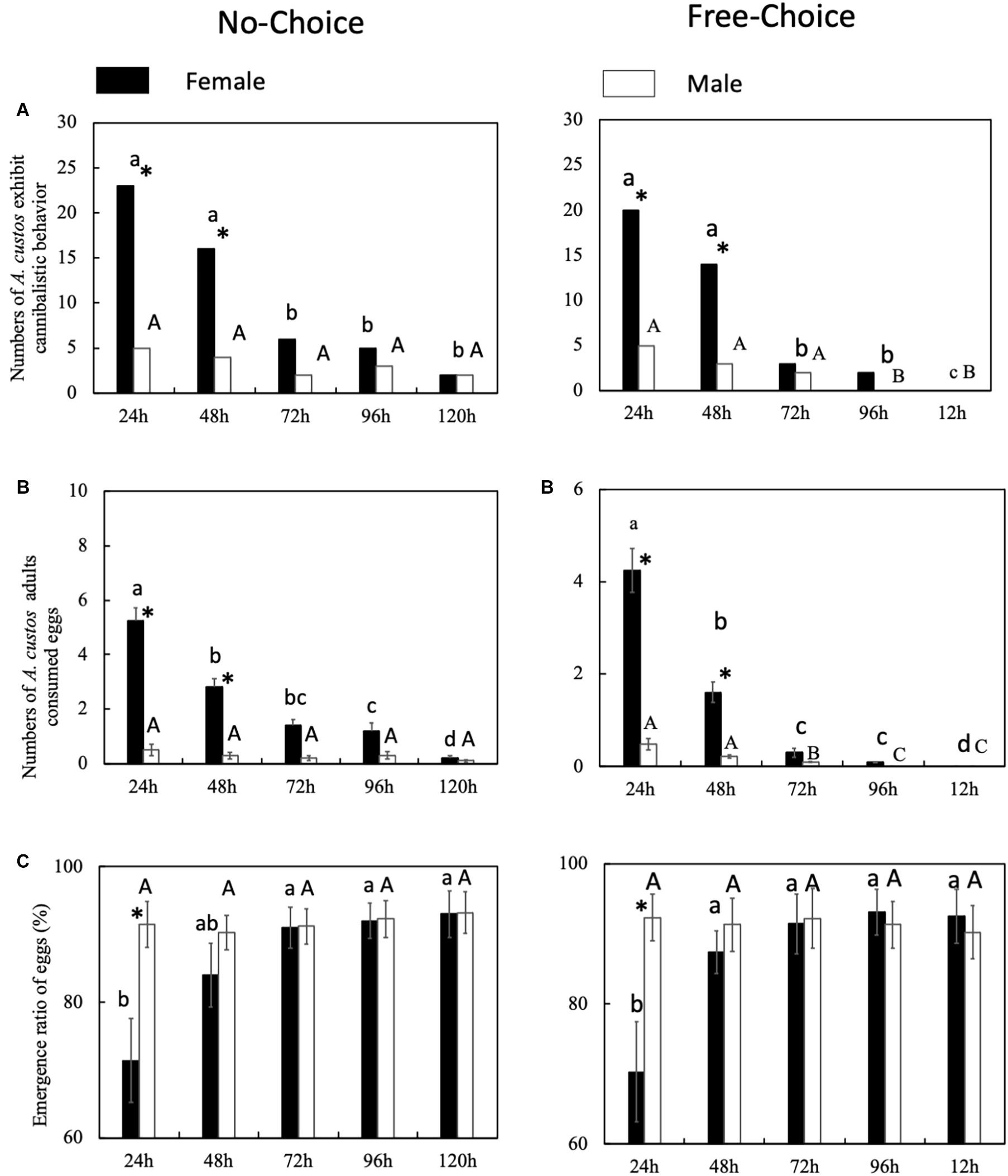
Figure 2. (A–C) Predatory behavior by A. custos with different aged eggs under no choice and free choice conditions. Different lowercase letters indicate significant differences among females at P < 0.05 and different uppercase letters indicate significant differences among males at p < 0.05. The investigation was repeated 30 times. * indicates a significant difference between the black bar (females) and white bar (males) at P < 0.05.
Female A. custos cannibalized old eggs less than young eggs (Figure 3). The cannibalism on eggs that had been laid over 72 h earlier was significantly lower than the cannibalism on younger eggs (Figure 3A: 48–72 h, No choice, chi-squared test, χ2 = 5.81, df = 1, p < 0.05; Free choice, χ2 = 8.21, df = 1, p < 0.05). However, eggs that had been laid over 48 h before were also consumed significantly less often than younger eggs (Figure 3B: 24–48 h, No choice, Tukey HSD test, p < 0.05; Free choice, Tukey HSD test, p < 0.05). In addition, in the no-choice conditions, the number of eggs consumed by female A. custos on 72-h-old eggs was not significantly lower than the consumption of eggs that were 48 h old (Figure 3B: 48–72 h, No choice, Tukey HSD test, p > 0.05). The number of 72-h-old eggs consumed by female A. custos was significantly lower than the consumption of 48-h-old eggs in the free choice conditions (Figure 3B: 48–72 h, Free choice, Tukey HSD test, p < 0.05). Meanwhile, the number of 24-h-old eggs consumed by female A. custos was significantly higher than the 48 h eggs regardless of the conditions (choice or free choice) (Figure 3B: 48–72 h, No choice, Tukey HSD test, p < 0.05; Free choice, Tukey HSD test, p < 0.05). In addition, the emergence ratio of the 24-h-old eggs was significantly lower than the other aged eggs in both the no-choice and free-choice conditions (Figure 3C: No choice, Tukey HSD test, p < 0.05; Free choice, Tukey HSD test, p < 0.05).
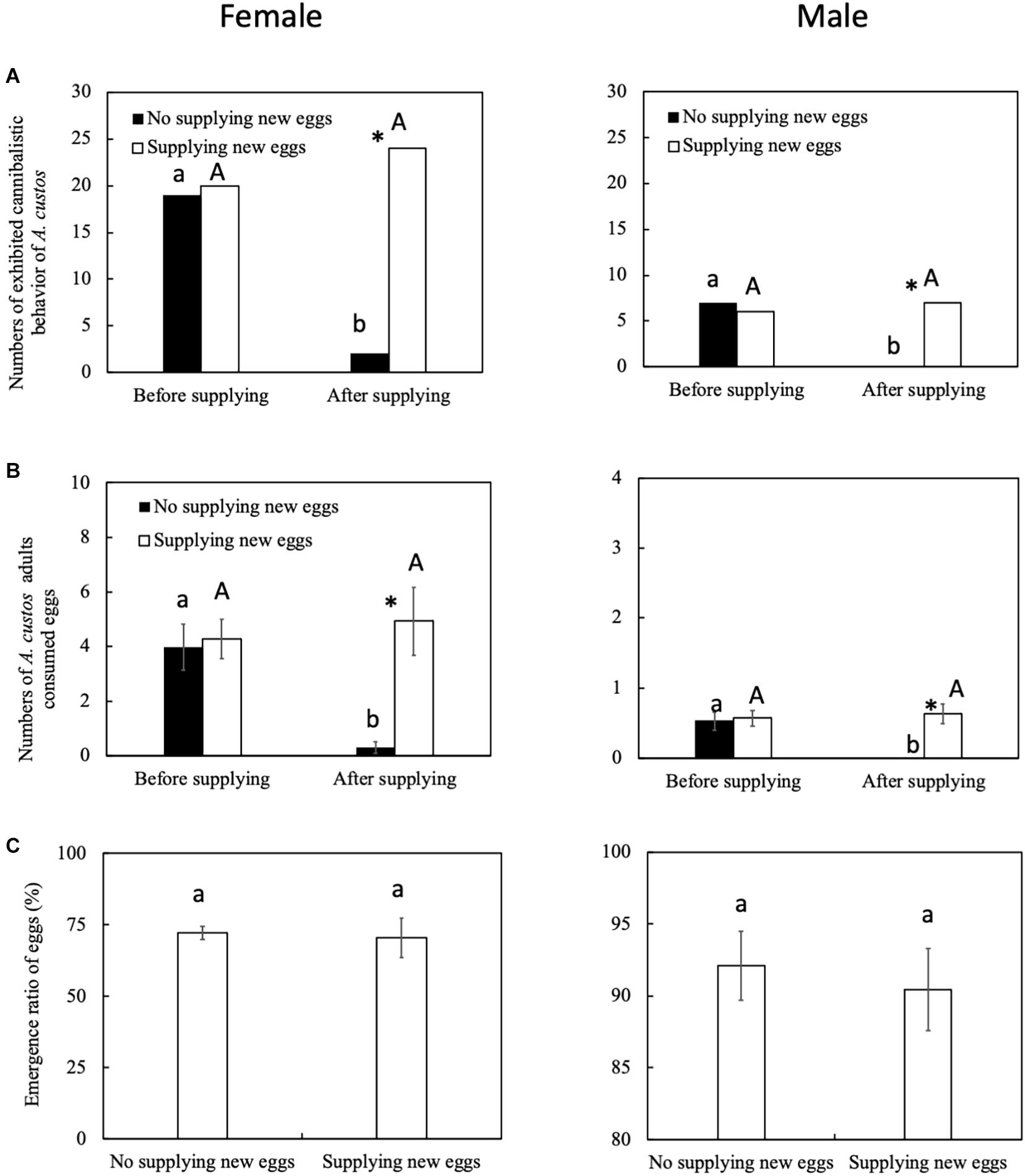
Figure 3. (A–C) Predatory behavior by A. custos when supplied new eggs. Different lowercase or uppercase letters indicate a significant difference of different treatments at P < 0.05. The investigation was repeated 30 times. * indicates a significant difference between the black bar (not supplied new eggs) and white bar (supplied new eggs) at p < 0.05.
Egg Cannibalism by Nymphs at Different Developmental Stages
The developmental stage of the nymph significantly influenced the cannibalistic behavior by nymph A. custos (Figure 4). First and second instar nymphs did not cannibalize eggs. The third, fourth, and fifth instar nymphs cannibalized eggs (Figure 4). The number of third instar A. custos nymphs exhibiting cannibalistic behavior was significantly lower than that of fourth instar nymphs (Figure 4A: chi-squared test, χ2 = 8.10, df = 1, p < 0.05) and fifth instar nymphs (Figure 4A: chi-squared test, χ2 = 9.61, df = 1, p < 0.05). In addition, third instar nymph females consumed fewer eggs than fourth or fifth instar nymph females (Figure 4B: Tukey HSD test, p < 0.05). In addition, the egg emergence ratios in the first, second, and third instar nymph experiments were significantly higher than the fourth and fifth instar nymph experiments (Figure 4C: Tukey HSD test, All, p < 0.05).
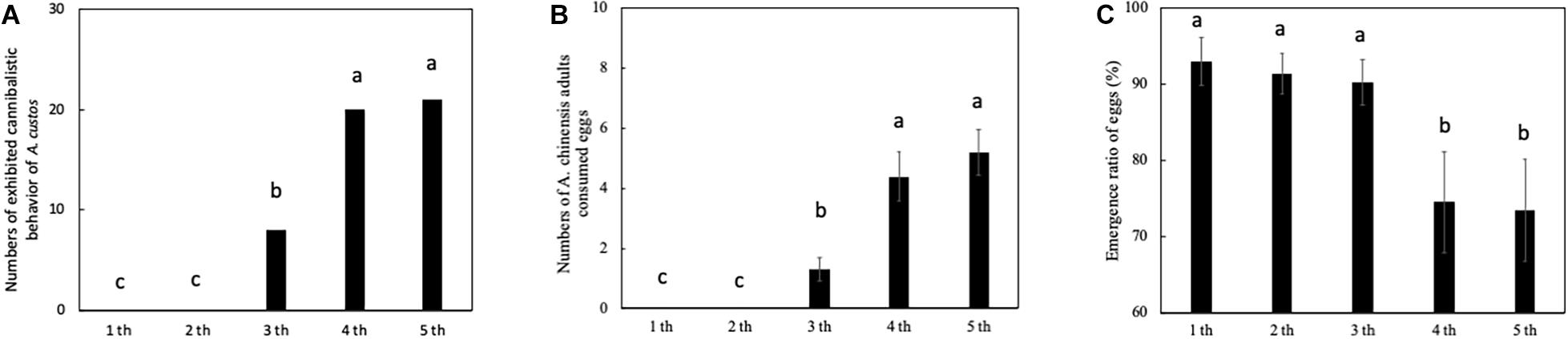
Figure 4. (A–C) Predatory behavior by A. custos in different nymph developmental stages. Different lowercase letters indicate a significant difference in different nymph developmental stages at p < 0.05. The investigation was repeated 30 times.
Discussion
Egg cannibalism is influenced by many factors (Samu et al., 1999; Kakimoto et al., 2003; Momen and AbdelKhalek, 2009; Erica et al., 2011; Hoffman, 2012; Parsons et al., 2013; Maleknia et al., 2016). Sex, reproductive status, and egg age are major factors in egg cannibalism (Momen and AbdelKhalek, 2009; Maleknia et al., 2016).
Our results indicate that virgin females showed a lower egg cannibalism inclination than gravid females, both virgin and mated females showed a higher egg cannibalism inclination than virgin and mated males, the number of eggs consumed by virgin female A. custos was lower than that of mated females, both virgin and mated females consumed more eggs than virgin and mated males, and both virgin and mated females showed a lower egg cannibalism inclination, supporting our first hypothesis: mated female A. custos devour more eggs than virgin females, and reproductive status has no effect on male egg cannibalism. We found that virgin females showed a lower egg cannibalism inclination than gravid females. This result indicates that egg cannibalism behavior by A. custos is influenced by the developmental stage of the female. This may be because gravid females need more energy for breeding (Neff, 2003; Miller and Zink, 2012). Therefore, we believe that oviposition may be one of the reasons for A. custos egg cannibalism behavior. Our results were similar to those of female Hippodamia convergens Guerin-Meneville (Coleoptera) (Bayoumy and Michaud, 2015) and some mite species (Schausberger, 2003). Conversely, our results were different from those of Tribolium confusum Duval (Parsons et al., 2013), Coccinella undecimpunctata L. (Bayoumy et al., 2016), and male Hippodamia convergens Guerin-Meneville (Coleoptera) (Bayoumy and Michaud, 2015), whose virgin adults are more cannibalistic than mated adults. However, whether female spawning behavior is related to predation remains unresolved. Does female spawning behavior stimulate egg cannibalism behavior? In future studies we will attempt to answer this question by inhibiting the expression of genes related with oviposition.
Our results also indicate that eggs aged <48 h were more likely to be cannibalized under no-choice and free-choice conditions. When we supplied new eggs after 48 h, the control group exhibited higher egg cannibalism, consuming more eggs. Therefore, we believe that egg development stage can inhibit egg cannibalism behavior by female A. custos. These results support our second hypothesis: younger eggs are more likely to be eaten than older ones. Furthermore, egg cannibalism behavior may be influenced by egg development as female A. custos no longer preyed on the eggs when the test eggs were older than 48 h. This may be because eggs have start to develop 48 h after being laid. Our results are similar to female rock-pool corixids, who cannibalized new eggs more frequently than 1-d-old eggs (Pajunen and Pajunen, 1991).
Nymphs are an important stage in the life cycle of A. custos. In our experiments, we supplied suitable food for nymphs from the second instar stage in the laboratory. Previous studies have shown that there are many younger cannibalistic insect that prey on eggs, such as Formica aquilonia Yarrow (Schultner et al., 2013) and Triboliunm castaneum Herbst (Frank and Peter, 1966). Our results showed that the developmental stage of the nymphs significantly influences the cannibalistic behavior by nymph A. custos. We found that older nymphs cannibalize more eggs than younger nymphs, supporting our third hypothesis: older nymphs consume more eggs than younger nymphs. The first and second instar nymphs did not cannibalize eggs, which is unlike the third, fourth and fifth instar nymphs who cannibalized eggs. This result is similar to Hippodamia convergens Guerin-Meneville (Coleoptera) (Bayoumy and Michaud, 2015). However, the reason why both adults and nymphs avoid eating +48-h old eggs remains unresolved. It may be that adults and nymphs can distinguish between developed and undeveloped eggs? Alternatively, there are other potential explanations. For example, eggs may exude a repellent that restricts cannibalistic behavior (Narasimha et al., 2019) or it may be because adults refrain from eating more eggs (Polis, 1981; Smith and Reay, 1991; Manica, 2002). Further research is required to understand the causes of this behavior.
Previous studies have also demonstrated that selective cannibalism provides a food source for adult insects while also ensuring the survival of most of their offspring, thus maintaining the population levels (Polis, 1981; Smith and Reay, 1991; Manica, 2002). In this instance, A. custos females would not consume all available eggs, regardless of the predator-to-prey ratio. Similar results have also been obtained for Anisolabis maritima Bon. (Miller and Zink, 2012) and Euborellia annulipes Lucas (Jacobs and Stigall, 2019). Our results also demonstrate that neither A. custos nymphs nor female adults consumed all of the available eggs, and the emergence ratio was > 70%. This result indicates that most eggs were left alive. Obviously, there was a balance and a significant decision by female A. custos to consume less than one-third of the offspring, like Anisolabis maritima Bon. (Miller and Zink, 2012), and allowed the majority of the offspring to survive. This selective cannibalism would not only extend the lifespan of the adult insects but also ensure the survival of most offspring, which is more helpful in preventing populations from dwindling (Polis, 1981; Smith and Reay, 1991; Manica, 2002). Our results were similar to those for Euborellia annulipes Lucas (Dermaptera: Anisolabididae) (Jacobs and Stigall, 2019). This process is not only beneficial for the health of offspring, but also beneficial for females (Okada et al., 2015).
The evolution of cannibalism is driven by the balance between its benefits and costs (Hamilton, 1964). One evident benefit of egg cannibalism is starvation avoidance (Pizzatto and Shine, 2008; Dobler and Koelliker, 2010). Moreover, selective cannibalism provides an alternative food source for adult insects, while ensuring the survival of most of their offspring and maintaining the population size (Polis, 1981; Smith and Reay, 1991; Manica, 2002). Our study revealed that hungry A. custos do not prey on all the available eggs (>70% of the eggs were unconsumed, and males exhibit minimal egg cannibalism). Moreover, virgin females showed a lower egg cannibalism inclination than gravid females; first and second instar nymphs did not cannibalize the eggs. Egg cannibalism offers insect species a means to avoid starvation and prolong lifespan by providing an alternative source of nutrition and energy (Polis, 1981; Smith and Reay, 1991; Pizzatto and Shine, 2008; Okada et al., 2015). However, under field conditions, whether A. custos would exhibit the same behavior, possibly avoiding egg cannibalism by protecting at least some of its eggs and instead searching for other prey, is still unknown (Revynthi et al., 2018b). Furthermore, egg cannibalistic behavior has also been reported in the nymphs of F. aquilonia Yarrow (Schultner et al., 2013) and T. castaneum Herbst (Frank and Peter, 1966). Given that, compared with laboratory conditions, nymphs generally have little difficulty in locating eggs in the wild, it will be instructive to investigate egg cannibalism among A. custos nymphs, as well as egg cannibalism as a whole, under natural conditions. However, our observations were conducted under confined conditions, and it remains to be determined whether A. custos adult would exhibit the same behavior at more extensive spatial scales.
Conclusion
Overall, A. custos females exhibit a higher tendency for egg cannibalism than males. Egg cannibalism varies not only with the developmental stage of the eggs and nymphs but also with the sex and reproductive status of A. custos females. Virgin females showed a lower egg cannibalism inclination than gravid females. Both virgin and mated females showed a higher egg cannibalism inclination than virgin and mated males. First and second instar nymphs did not cannibalize the eggs. The third, fourth, and fifth instar nymphs cannibalized eggs, and younger eggs were more often eaten than older eggs. However, neither A. custos nymphs nor female adults consumed all of the available eggs, and the emergence ratio of the remaining eggs was >70%. Our findings help us to better understand the evolutionary relationships in egg cannibalism by A. custos and contribute to the efficient mass rearing and realization of A. custos in biological control systems.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
SW, WZ, ML, ZZ, and WD performed the experiments. SW, WZ, WH, ZZ, and WD conceived and designed the experiments, analyzed the data, and wrote the manuscript. All authors have read and approved the final manuscript.
Funding
Funding for this study was provided by the Major Program for Science and Technology of Hunan Province (No. 2020NK2034), Natural Science Foundation of Hunan Province (No. 2020JJ5290), and Double First-Class Construction Project of Hunan Agricultural University (No. SYL 2019029). The Natural Enemies Breeding project was supported by the Science Foundation of Hunan (No. 18-21 Aa06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the following individuals: Changhua Zhang and Fangzhao Jia for supplying the insects; Anfang Zhu for feeding insects.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.705318/full#supplementary-material
References
Agarwala, B. K., and Dixon, A. F. G. (1992). Laboratory study of cannibalism and interspecific predation in ladybirds. Ecol. Entomol. 17, 303–309. doi: 10.1111/j.1365-2311.1992.tb01062.x
Bayoumy, M. H., and Michaud, J. P. (2015). Egg cannibalism and its life history consequences vary with life stage, sex, and reproductive status in Hippodamia convergens (Coleoptera: Coccinellidae). J. Econ. Entomol. 108, 1665–1674. doi: 10.1093/jee/tov148
Bayoumy, M. H., Abou-Elnaga, A. M., Ghanim, A. A., and Mashhoot, G. A. (2016). Egg cannibalism potential benefits for adult reproductive performance and offspring fitness of coccinella undecimpunctata L. (Coleoptera: Coccinellidae). Egypt. J. Biol. Pest. Control. 26, 35–42.
Block, M. D., and Stoks, R. (2004). Cannibalism-mediated life history plasticity to combined time and food stress. Oikos 106, 587–597. doi: 10.2307/3548381
Chai, X. M., He, Z. H., Jiang, P., Wu, Z., Pan, C. R., and Hu, R. D. (2000). Studies on natural enemies of dendrolimus punctatus in zhejiang province. Forestry Technol. Zhejiang. 61, 1–56.
David, A., and Zheng, L. Y. (2002). Checklist and nomenclatural notes on the Chinese Pentatomidae (Heteroptera) I. asopinae. Entomotaxonomia 24, 107–115. doi: 10.1520/STP11985S
Dobler, R., and Koelliker, M. (2010). Kin-selected siblicide and cannibalism in the European earwig. Behav. Ecol. 21, 257–263. doi: 10.1093/beheco/arp184
Dobler, R., and Koelliker, M. (2011). Influence of weight asymmetry and kinship on siblicidal and cannibalistic behaviour in earwigs. Anim Behav. 82, 667–672. doi: 10.1016/j.anbehav.2011.06.017
Erica, L. W., Douglas, P. C., Joseph, M. K., and Andrew, R. B. (2011). The effects of food level and conspecific density on biting and cannibalism in larval long-toed salamanders, Ambystoma macrodactylum. Oecol 128, 202–209. doi: 10.1007/s004420100641
Fox, L. R. (1975). Factors influencing cannibalism, a mechanism of population limitation in the predator Notonecta hoffmanni. Ecol. 56, 933–941. doi: 10.2307/1936303
Frank, K. H., and Peter, S. D. (1966). Egg cannibalism by tribolium larvae. Ecol. Soc. Am. 47, 318–322. doi: 10.2307/1933784
Gao, Z., Wang, X., Zhang, L., Sun, Y., Fan, J., and Wang, G. (2011). Biological characteristic of Arma chinensis. J. Eng. Heilongjiang Univ. 2, 72–77.
Goncalo, A. D., Filipa, C., Ariadna, P., Elsa, B. S., and Elisabete, F. (2020). Intraguild predation and cannibalism among dicyphini: dicyphus cerastii vs. two commercialized species. Entomol. Exp. Appl. 169, 90–96. doi: 10.1111/eea.12943
Hoffman, C. R. (2012). Kinship and Familiarity Affect Recognition and Foraging in the Wolf Spider, Pardosa Milvina (Araneae: Lycosidae). Ph. D, Thesis. Miyami University.
Jacobs, A. C., and Stigall, T. (2019). Paternity and egg cannibalism in the ringlegged earwig Euborellia annulipes (Dermaptera: Anisolabididae). Entomol. Sci. 22, 250–257. doi: 10.1111/ens.12363
Kakimoto, T., Fujisaki, K., and Miyatake, T. (2003). Egg laying preference, larval dispersion, and cannibalism in Helicoverpa armigera (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 96, 793–798.
Kuriwada, T., Kumano, N., and Haraguchi, S. D. (2013). High population density and egg cannibalism reduces the efficiency of mass-rearing in euscepes postfasciatus (Coleoptera: Curculionidae). Fla Entomol. 92, 221–228. doi: 10.1653/024.092.0205
Maleknia, B., Fathipour, Y., and Soufbaf, M. (2016). Intraguild predation among three phytoseiid species, Neoseiulus barkeri, Phytoseiulus persimilis and Amblyseius swirskii. Syst. Appl. Acarol. 21, 417–426. doi: 10.11158/saa.21.4.4
Manica, A. (2002). Alternative strategies for a father with a small brood: mate, cannibalize or care. Behav. Ecol. Sociobiol. 51, 319–323. doi: 10.1007/s00265-001-0444-0
Miller, J. S., and Zink, A. G. (2012). Parental care trade-offs and the role of filial cannibalism in the maritime earwig. Anisolabis Maritima. Anim. Behav. 83, 1387–1394. doi: 10.1016/j.anbehav
Momen, F. M., and AbdelKhalek, A. (2009). Cannibalism and intraguild predation in the phytoseiid mites Typhlodromips swirskii, Euseius scutalis and Typhlodromus athiasae (Acari: Phytoseiidae). Acari 17, 223–229.
Narasimha, S., Nagornov, K. O., Menin, L., Mucciolo, A., Rohwedder, A., Humbel, B. M., et al. (2019). Drosophila melanogaster cloak their eggs with pheromones, which prevents cannibalism. PLoS Biol. 17:e2006012. doi: 10.1371/journal.pbio.2006012
Neff, B. D. (2003). Paternity and condition affect cannibalistic behavior in nest-tending bluegill sunfish. Behav. Ecol. Sociobiol. 54, 377–384. doi: 10.1016/j.yhbeh.2009.05.002
Okada, S., Fukuda, Y., and Takahashi, M. K. (2015). Paternal care behaviors of Japanese giant salamander Andrias japonicus in natural populations. J. Ethol. 33, 1–7. doi: 10.1007/s10164-014-0413-5
Orr, B. K., Murdoch, W. W., and Bence, J. R. (1990). Population regulation, convergence, and cannibalism in notonecta (Hemiptera). Ecology 71, 68–82. doi: 10.2307/1940248
Pajunen, V. I., and Pajunen, I. (1991). Oviposition and egg cannibalism in rock-pool corixids (Hemiptera: Corixidae). Oikos 60, 83–90.
Pan, M. Z., Zhang, H., Zhan, L., and Chen, H. (2019). Effects of starvation and prey availability on predation and dispersal of an omnivorous predator Arma chinensis Fallou. J. Insect. Behav. 32, 134–144. doi: 10.1007/s10905-019-09718-9
Parsons, W., Zhong, W., and Rudolf, V. H. W. (2013). Mating status and kin recognition influence the strength of cannibalism. Anim. Behav. 85, 365–369. doi: 10.1016/j.anbehav.2012.11.006
Pizzatto, L., and Shine, R. (2008). The behavioral ecology of cannibalism in cane toads (Bufo marinus). Behav. Ecol. Sociobiol. 63, 123–133. doi: 10.1007/s00265-008-0642-0
Polis, G. (1981). The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 12, 251–255. doi: 10.1146/annurev.es.12.110181.001301
R Development Core Team. (2017). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available at: https://www.R-project.org/
Revynthi, A. M., Egas, M., Janssen, A., and Sabelis, M. W. (2018a). Prey exploitation and dispersal strategies vary among natural populations of a predatory mite. Ecol. Evol. 8, 10384–10394. doi: 10.1002/ece3.4446
Revynthi, A. M., Janssen, A., Egas, M. E., and Acarology, A. (2018b). Gender-specific differences in cannibalism between a laboratory strain and a field strain of a predatory mite. Exp. Appl. Acarol. 74, 239–247. doi: 10.1007/s10493-018-0232-4
Richard, T. A. (1979). Cannibalism in Xylocoris flavipes (Hemiptera: anthocoridae), a predator of stored-product insects. Ent. Exp. Appl. 25, 128–135.
Richardson, M. L., Mitchell, R. F., Reagel, P. F., and Hanks, L. M. (2010). Causes and consequences of cannibalism in non-carnivorous insects. Annu. Rev. Entomol. 55, 39–53. doi: 10.1146/annurev-ento-112408-085314
Ryckman, R. E. (1951). Section 1 | | recent observations of cannibalism in triatoma (Hemiptera: Reduviidae). J. Parasitol. 37, 433–434.
Samu, F., Toft, S., and Kiss, B. (1999). Factors influencing cannibalism in the wolf spider Pardosa agrestis (Araneae: Lycosidae). Behav. Ecol. Sociobiol. 45, 349–354. doi: 10.1007/s002650050570
Schausberger, P. (2003). Cannibalism among phytoseiid mites: a review. Exp. Appl. Acarol. 29, 173–191. doi: 10.1023/A:1025839206394
Schultner, E., d’Ettorre, P., and Helantera, H. (2013). Social conflict in ant larvae: egg cannibalism occurs mainly in males and larvae prefer alien eggs. Behav. Ecol. 24, 1306–1311. doi: 10.1093/beheco/art067
Smith, C., and Reay, P. (1991). Cannibalism in teleost fish. Rev. Fish. Biol. Fish. 1, 41–64. doi: 10.1007/BF00042661
Wu, S. L., Deng, W., Cai, H. L., Yang, J. S., Zeng, W. A., Zhou, Z. C., et al. (2020). The occurrence period and effect of intraspecific cannibalism behavior of arma chinensis under starvation. Chin. J. Biol. 36, 169–174. doi: 10.16409/j.cnki.2095-039x.2020.03.001
Zalom, F. G. (1978). Backswimmer prey selection with observations on cannibalism (Hemiptera: Notonectidae). Southwestern Naturalist 23, 617–622.
Zhao, Q., Wei, J., Wenjun, B. U., Liu, G., and Zhang, H. (2018). Synonymize Arma chinensis as Arma custos based on morphological, molecular and geographical data. Zootaxa 4455:161.
Zheng, Z. Y., and Chen, Y. W. (1992). Experiments on the use of Arma Custos (Fabricius) to control forist pesrs. Chin. J. Biol. Control 8, 155–156.
Keywords: Arma custos, egg cannibalism, gravid female, natural enemy, biocontrol
Citation: Wu S, Zeng W, Deng W, Li M, Hu W, Cai H, Li Y, Xie P, Tan L and Zhou Z (2021) Egg Cannibalism Varies With Sex, Reproductive Status, and Egg and Nymph Ages in Arma custos (Hemiptera: Asopinae). Front. Ecol. Evol. 9:705318. doi: 10.3389/fevo.2021.705318
Received: 05 May 2021; Accepted: 06 August 2021;
Published: 08 September 2021.
Edited by:
Cleber Galvão, Oswaldo Cruz Institute, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Talita Roell, University of São Paulo, BrazilLuiz Campos, Federal University of Rio Grande do Sul, Brazil
Copyright © 2021 Wu, Zeng, Deng, Li, Hu, Cai, Li, Xie, Tan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Tan, aHFsdGFubGluQDE2My5jb20=; Zhicheng Zhou, emhvdXpjaG55Y0AxMjYuY29t
†These authors have contributed equally to this work
 Shaolong Wu1,2,3†
Shaolong Wu1,2,3† Wan Deng
Wan Deng Youzhi Li
Youzhi Li