- 1State Key Laboratory of Grassland Agro-Ecosystems, School of Life Sciences, Lanzhou University, Lanzhou, China
- 2Desert Animal Adaptations and Husbandry, Wyler Department of Dryland Agriculture, Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Beersheba, Israel
- 3Qinghai Provincial Key Laboratory of Restoration Ecology of Cold Areas, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, China
- 4Qinghai Provincial Key Laboratory of Adaptive Management on Alpine Grassland, Qinghai Academy of Animal and Veterinary Sciences, Qinghai University, Xining, China
Asymbiotic nitrogen-fixing (ANF) bacteria contribute a substantial amount of nitrogen in ecosystems, especially in those with low symbiotic nitrogen fixation (SNF) capability. Degradation of alpine grassland is widespread on the Tibetan Plateau and sown grassland has become one of the main strategies for grassland restoration. However, the diversity and community structure of ANF bacteria in different grassland types remain unknown. The aim of this study was to fill this gap. Soil samples were obtained from 39 grassland plots selected from three counties in the eastern Tibetan Plateau. The plots were classified as natural grassland (NG), sown grassland (SG), lightly degraded grassland (LDG), and severely degraded grassland (SDG). ANF microbial communities of the four grassland types were compared at the level of community and species diversity by 16S rRNA high-throughput sequencing technology. The phylum Proteobacteria accounted for >72% of the ANF bacteria. The community structures of soil ANF bacteria differed significantly (p < 0.01) among grassland types. We concluded that: (1) planting gramineous forage could possibly mitigate the decrease in diversity of soil ANF bacteria caused by grassland degradation; and (2) the diversity of soil ANF bacteria in alpine grassland of the Tibetan Plateau is closely related to grassland degradation and restoration.
Introduction
Biological nitrogen fixation, a process in which some prokaryotic microorganisms reduce atmospheric nitrogen molecules to ammonia through the catalytic action of nitrogenase (Dart and Wani, 1982; Cleveland et al., 1999), is the main natural input form of nitrogen in ecosystems (Barron et al., 2009; Reed et al., 2011; Zheng et al., 2018). Approximately 120 million tons per year of active nitrogen are converted from inert N2 by biological nitrogen fixation (Galloway and Cowling, 2002). Microbial nitrogen fixation in soil compensates for the nitrogen loss from soil to the atmosphere (Kennedy and Islam, 2001), and plays an important role in improving soil fertility and enhancing the soil nitrogen cycle (Reed et al., 2007; Gupta et al., 2014).
Based on the interaction between microorganisms and plants, biological nitrogen-fixation can be divided into symbiotic nitrogen fixation (SNF), free-living nitrogen fixation and associative nitrogen fixation. The latter two are collectively referred to as asymbiotic nitrogen fixation (ANF) (Bottomley and Myrold, 2015). Symbiotic nitrogen-fixing bacteria fix nitrogen effectively only when they are symbiotic with plants and generally have high host specificity. In contrast, ANF bacteria do not require symbiosis with plants. Taylor et al. (2019) reported that with an increase in forest age, the main biological nitrogen fixation input is transformed gradually from symbiotic to ANF. Studies indicated that there are many factors affecting the community structure of nitrogen-fixing bacteria, including altitude and soil organic carbon, pH, and total nitrogen content (Limmer and Drake, 1996; Pérez et al., 2004; Zhang et al., 2006; Wang et al., 2017; Zheng et al., 2018; Bomfim et al., 2019).
Mainly nitrogenase genes (nifH) are associated with nitrogen fixation (Poly et al., 2001; Zehr et al., 2003; Wakelin et al., 2010). Zhang et al. (2005) amplified the nifH genes of alpine meadow soil microorganisms in the Three-Rivers Headwater Region by PCR to examine the structural characteristics and diversity of microbial communities. They concluded that nifH sequences of soil microorganisms in alpine meadow are highly diverse, and that nitrogen-fixing microbial communities are substantially different among plots.
The Tibetan Plateau is the highest and youngest plateau in the world, and is one of the regions with the lowest disturbance by human activities, not only in China but in the whole world (Zhang et al., 2006). However, under the impact of climate change, overgrazing by livestock and human activities, degradation of the alpine grassland has become widespread on the Tibetan Plateau (Cao et al., 2019; Miehe et al., 2019; Li et al., 2020). In efforts to restore degraded grassland, fencing and sown grassland (SG) are used widely (Zhen et al., 2018; Chen et al., 2021). Biological nitrogen fixation is an important nitrogen source for plant growth in alpine grassland ecosystem (Wang et al., 2017). Microthermal and anoxic environmental conditions limit the SNF to a great extent; nevertheless, soil nitrogen content is still at a high level on the Tibetan Plateau (Ma et al., 2020). We reasoned that ANF plays an important role in nitrogen input to the alpine grassland ecosystem. Soil nitrogen content has been reported to be related to grassland degradation and restoration, but little is known about ANF during these processes. To fill this gap, we examined: 1) the community structure of ANF microorganisms in the process of grassland degradation; and 2) the community structure and diversity of ANF bacteria in sown grassland.
Materials and Methods
Study Site Description
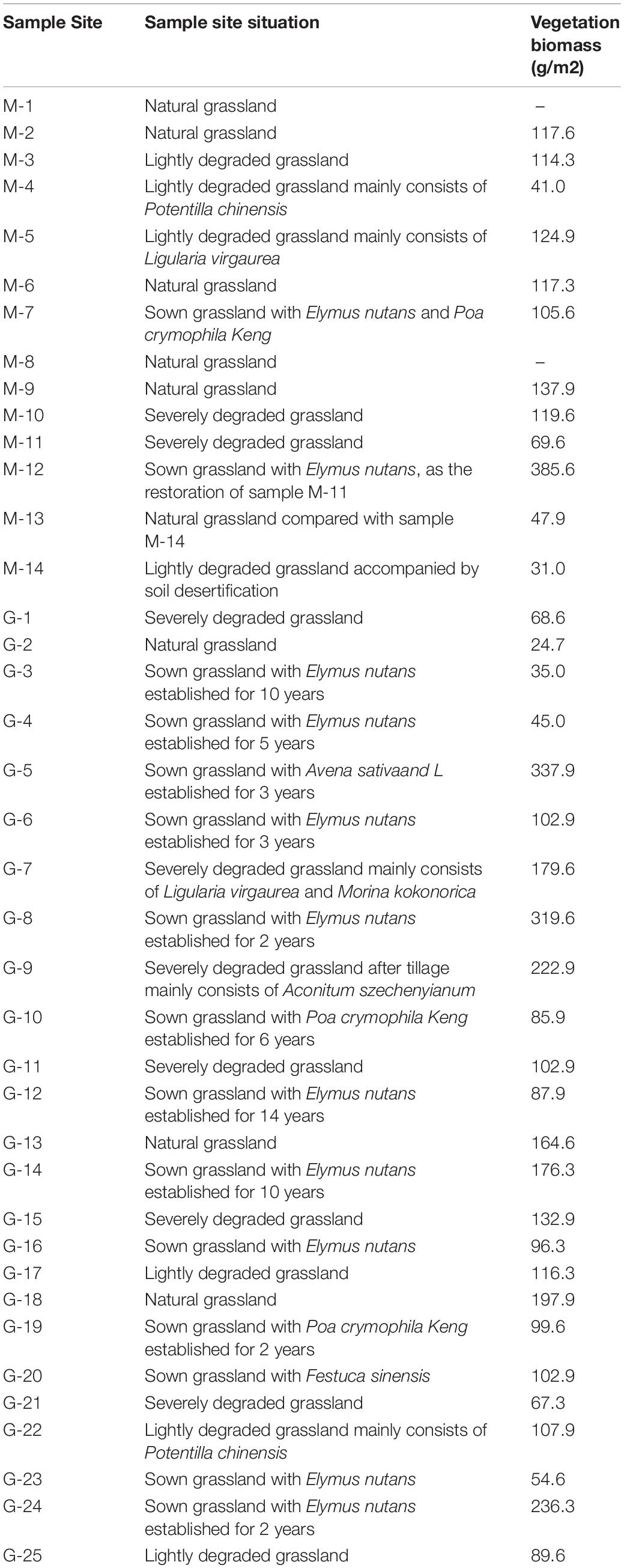
In total, 39 samples sites were selected in three counties: Maqu County (33°41′15″N∼33°52′58″N, 101°43′14″E∼102°12′44″E), Gannan Autonomous Prefecture, Gansu Province and Maqin and Gande counties (34°00′48″N∼34°28′21″N, 99°48′52″E∼100°32′45″E), Guoluo Autonomous Prefecture, Qinghai Province, located on the eastern Tibetan Plateau (Figure 1 and Table 1). The three counties are situated in the headwater area of Yellow river. Altitude ranges between 3,300 and 4,800 m, average annual temperature ranges between −3.8 and 3.5°C and annual precipitation ranges between 421 and 612 mm at these sites. Kobresia meadow is the main vegetation type. Due to the cold and dry climate, the area is characterized by low soil temperature, low plant metabolism, mainly single vegetation types and relatively poor biological yield (Zhang et al., 2005; Zhen et al., 2018). The grasslands are being used mainly for raising livestock and are being degraded by overgrazing.
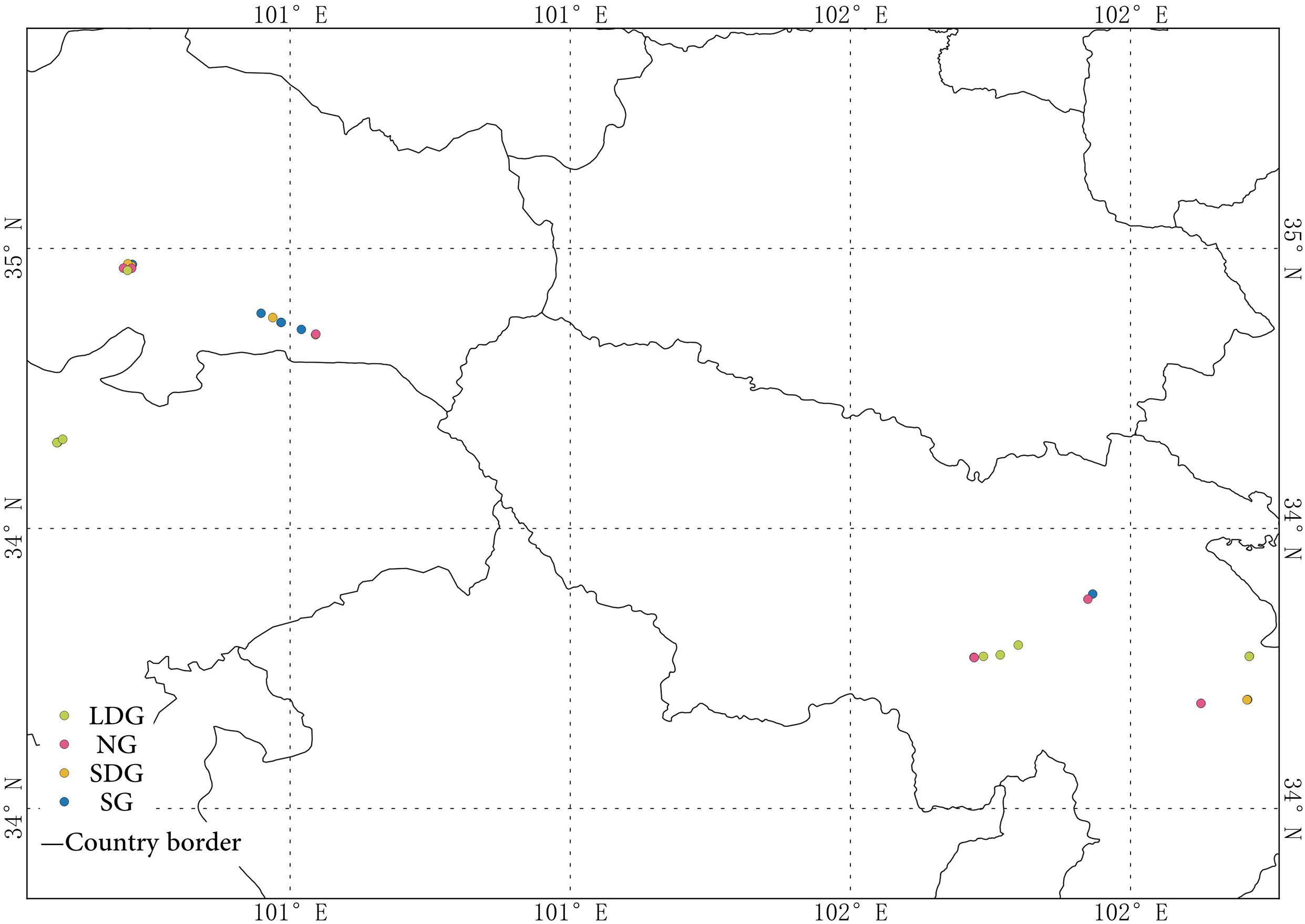
Figure 1. Distribution of the sample sites. NG (red), natural grassland; SG (blue), sown grassland; LDG (green), lightly degraded grassland; SDG (yellow), severely degraded grassland.
Soil Sampling and Preservation
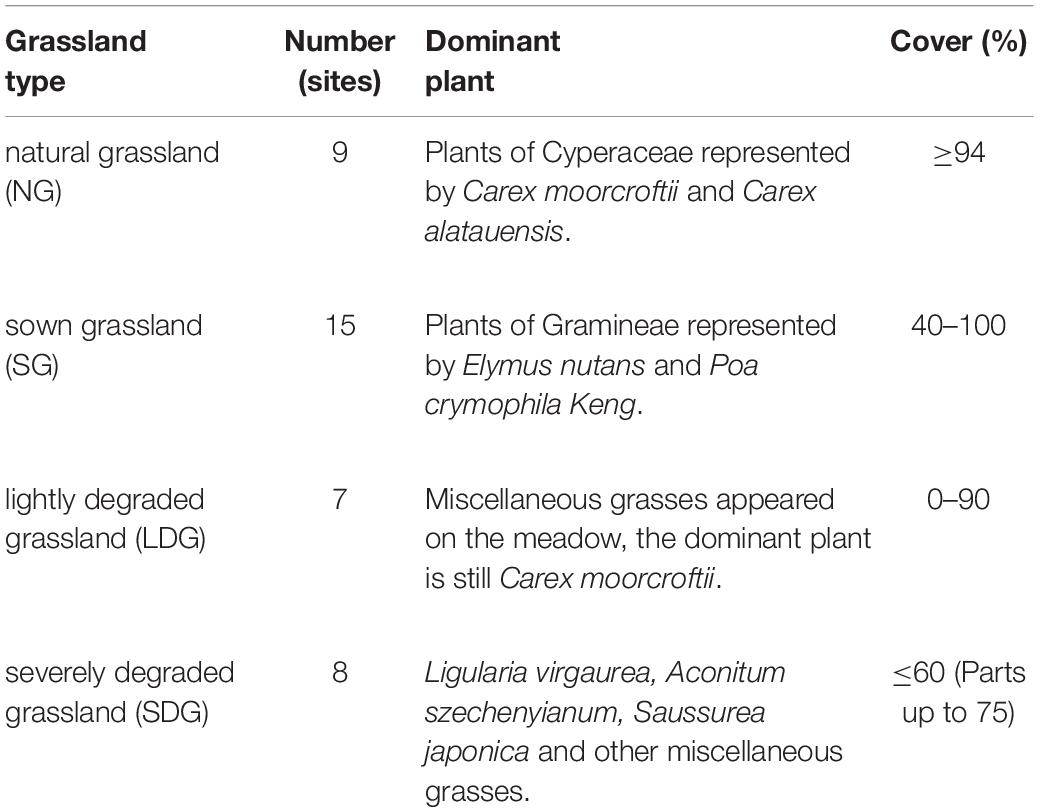
In August, 2014, we collected soil samples at 39 random sites, including 14 in Maqu County and 25 in Maqin and Gande counties (Figure 1). By asking local people about land use and by a vegetation survey (Table 1), the sites were classified into four types of grasslands: natural grassland (NG; n = 9), sown grassland with gramineous pastures (SG; n = 15), lightly degraded grassland (LDG; n = 7), and severely degraded grassland (SDG; n = 8) (Table 2). The 3-point square sampling method was employed to collect bulk soil samples of 0–10 cm in depth. The 39 soil samples were each mixed thoroughly, placed into aseptic self-sealing bags and kept in styrofoam boxes with ice packs to maintain the soil microbes alive. The boxes were brought back to the laboratory and stored at −80°C for later analyses.
DNA Extraction, PCR Amplification and Hiseq Sequencing
Total genomic DNA of soil microorganisms was extracted by TIANamp Stool DNA Kit (TIANGEN, China) following instructions with the kits. The V3 + V4 region of the 16S rRNA gene was amplified with a universal primer 341F-805R (341F:5′-CCTACGGGNGGCWGCAG-3′,805R:5′-GACTACHVGGGTATCTAATCC-3′). The EXtaq enzyme of TaKaRa was used to ensure the amplification efficiency and accuracy. Hiseq was applied to sequence the purified PCR products with a sequencing strategy of PE250 and the sequencing was completed by Annoroad Gene Technology (Beijing) Co., Ltd.
Sequence Processing and Statistical Analyses
Sequence data were filtered by removing low-quality bases, Ns, joint contamination sequences and other processes to obtain Clean Reads, that is, the trusted target sequences for subsequent analysis. The corresponding Read1 and Read2 of double-end sequencing, obtained from 5′ and 3′ terminals, respectively, were spliced with the sequence splicing method PEAR. Then, the spliced sequences were analyzed by QIIME version 1.8.0 (Caporaso et al., 2010; Vasileiadis et al., 2012).
A cumulative curve was drawn to measure and predict the increase of species richness in the community with the increase in sample size, to assess whether the sample size was sufficient for analyses and to estimate community richness (Figure 2). OriginPro 2021 was employed to draw the Venn diagram of operational taxonomic units (OTUs) distribution. Software R (version 4.1.0) analyzed the relative abundance and alpha diversity of ANF microorganism species in different grassland types (Johnsen et al., 2001), including Shannon, Simpson, Chao1 and Ace. In addition, cluster analysis and non-metric multidimensional scaling analysis (NMDS) were performed on soil ANF microbial species. Then the diversity and community structures of ANF bacteria in different grassland types were analyzed.
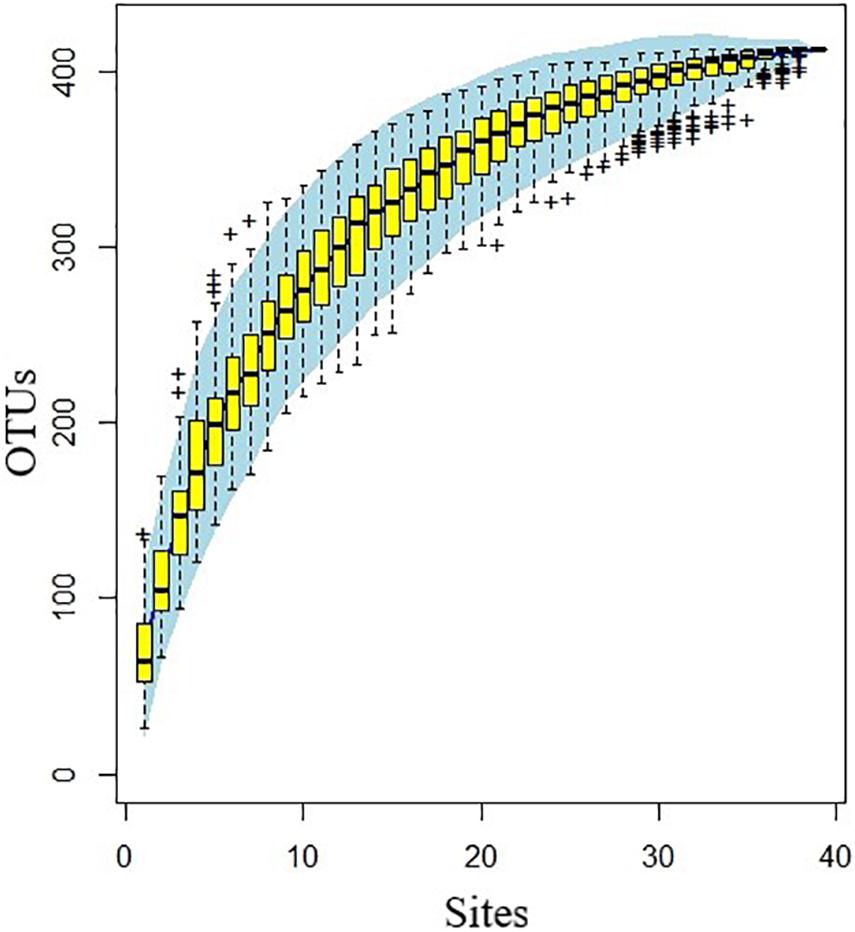
Figure 2. Operational taxonomic units (OTUs) accumulation curve of asymbiotic nitrogen-fixing microorganisms at 39 sampling sites.
Results
A total of 392,684 bacterial sequences and 46,474 actinomycetes sequences were compared to a reference database in all soil samples, which were clustered to 68,215 and 9,179 OTUs, respectively. Known ANF microorganisms were identified from the sequencing results, and the data were analyzed.
Composition of Asymbiotic Nitrogen-Fixing Microbial Communities
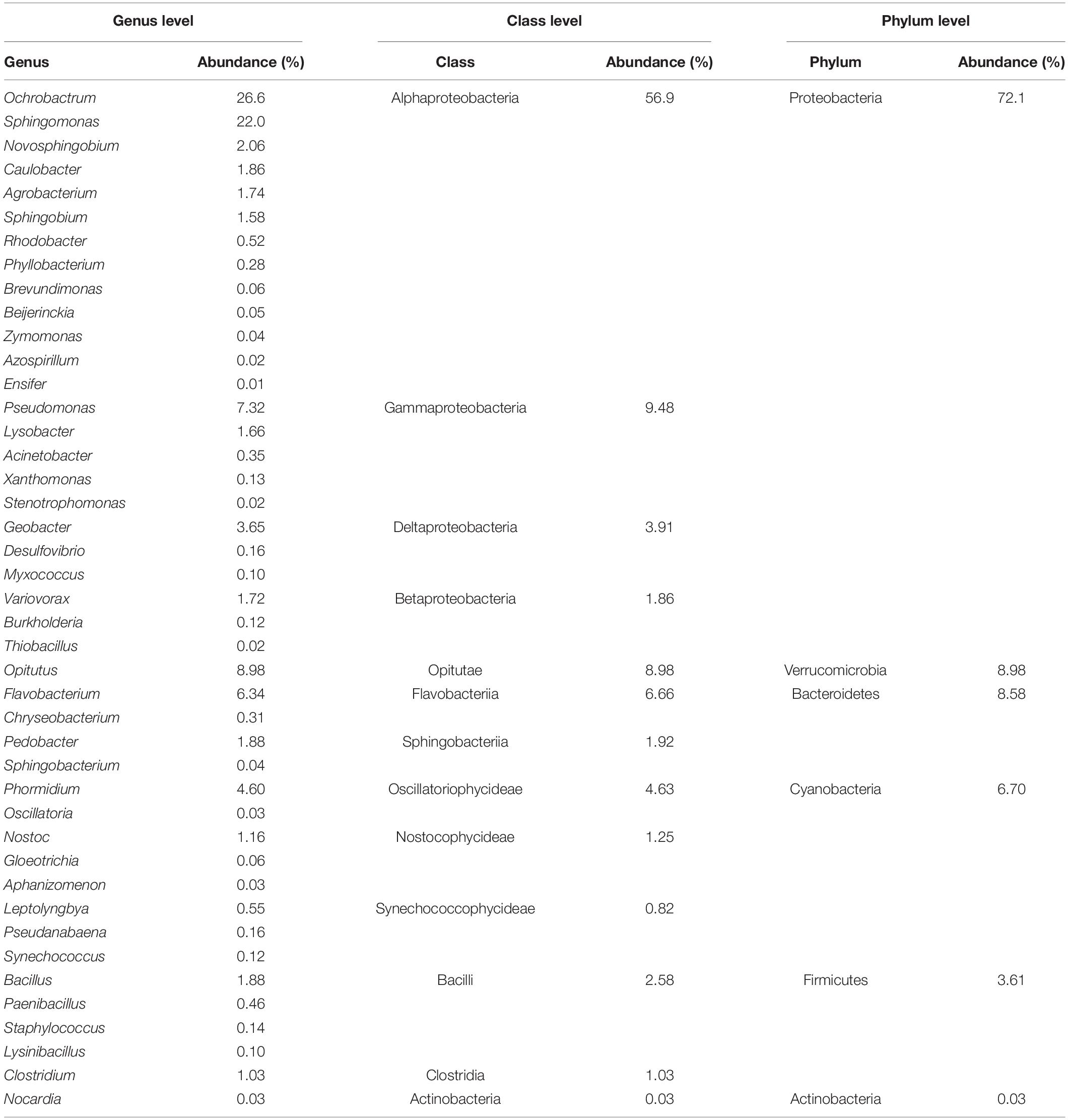
After screening, all the ANF bacteria contained six phyla, 13 classes and 43 genera (Table 3). Proteobacteria was the dominant phylum, with an abundance of 72.1%. At the level of class, Alphaproteobacteria and Gammaproteobacteria were dominant, with abundances of 56.9 and 9.48%, respectively, whereas the abundance of Actinobacteria was only 0.03% (Table 3). At the genera level, Ochrobactrum and Sphingomonas were most abundant, followed by Opitutus and Pseudomonas (Table 3 and Figure 3). The ANF bacteria species richness of NG and SG were the highest of the grassland types (Figure 3). The Venn diagram (Figure 4) displayed that SG had the most OTUs species of ANF bacteria (299), while LDG had the least (198) of the four grassland types. There were 109 OTUs species shared by the four grassland types, while 51 and 23 OTUs species were endemic to NG and SG, respectively.
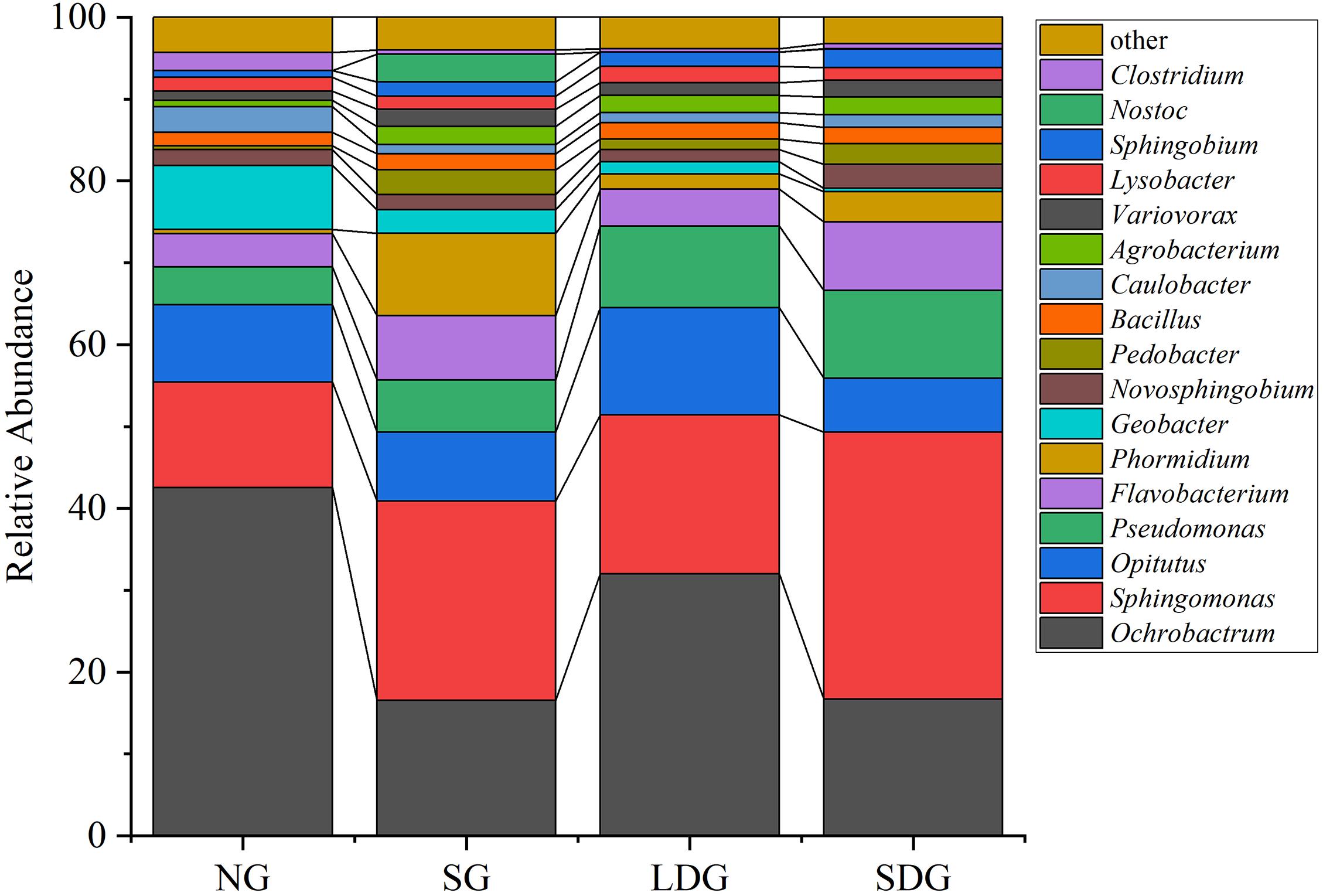
Figure 3. Relative abundance of asymbiotic nitrogen-fixing bacteria at the genus level in different grassland types. Each color represents the corresponding genus. NG, natural grassland; SG, sown grassland; LDG, lightly degraded grassland; SDG, severely degraded grassland.
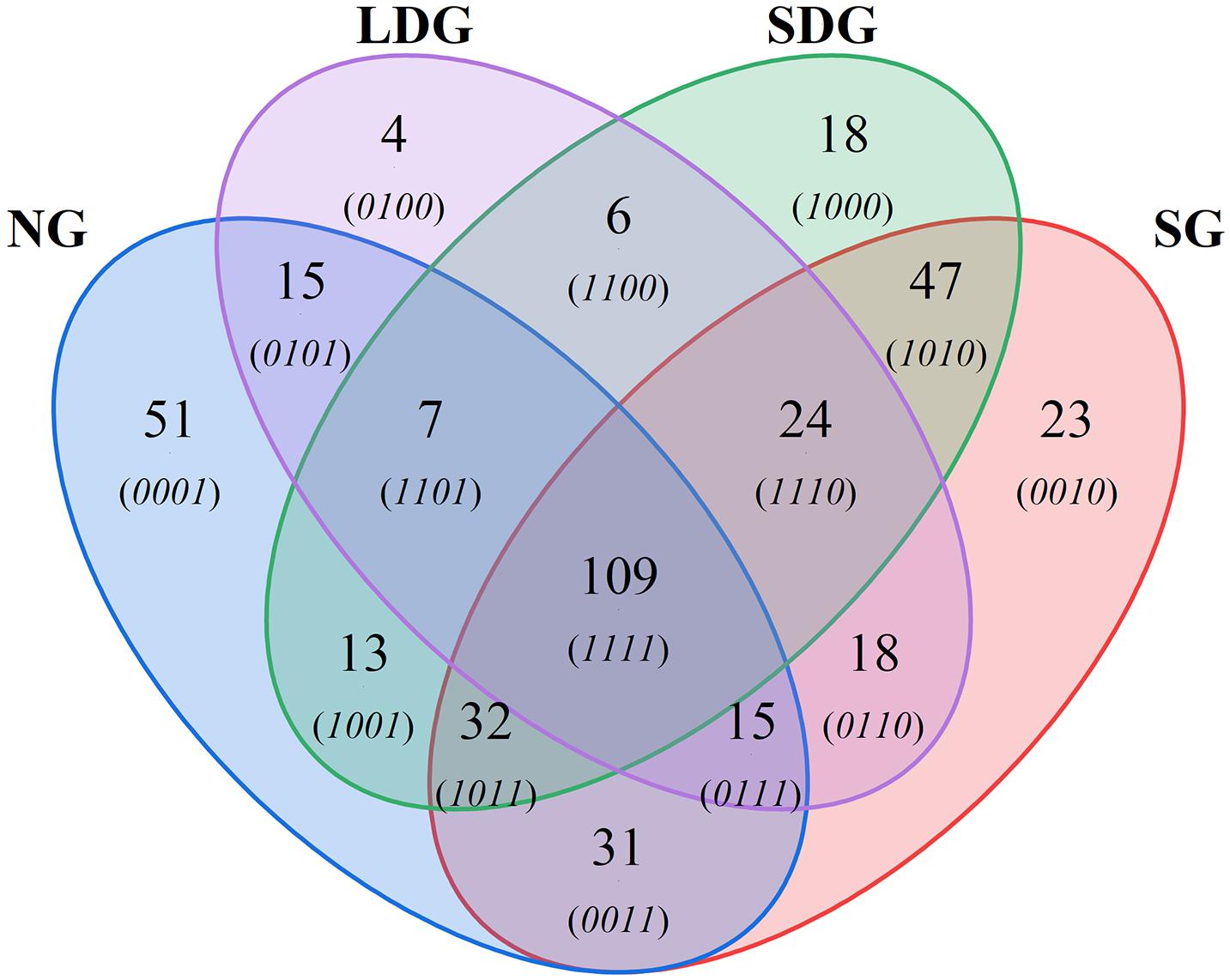
Figure 4. Quantitative analysis of operational taxonomic units (OTUs) species in different grassland types. NG (blue), natural grassland; SG (red), sown grassland; LDG (purple), lightly degraded grassland; SDG (green), severely degraded grassland.
Alpha Diversity Index of Asymbiotic Nitrogen-Fixing Bacteria
Alpha diversity index reflects the richness, evenness and diversity of the biological community in numerical form. Chao1 ranged between 107 and 131 and Ace index ranged between 118 and 132 in all grasslands (Table 4). SDG had the highest values in both indices, while LDG had the lowest Chao1 (107 ± 21.1) and SG had the lowest Ace (118 ± 27.9). Shannon index ranged between 4.31 and 5.02 and Simpson index ranged between 0.83 and 0.93 in the four grassland types. SDG had the highest Shannon index (5.02 ± 0.75), followed by SG (4.90 ± 0.84), while NG had the lowest Simpson index (0.83 ± 0.16), followed by SG (0.90 ± 0.10). Therefore, we speculate that SG may alleviate the decrease of Alpha diversity of asymbiotic nitrogen-fixing microorganisms during grassland degradation. This is based only on rank, however, we are aware that the sampling methods, the processes of DNA extraction, and the sequencing of DNA may have high variability.

Table 4. Alpha diversity of asymbiotic nitrogen-fixing microbial communities in different grassland types.
The Similarities of Asymbiotic Nitrogen-Fixing Bacteria Communities in the Four Grasslands
Based on alpha diversity, the cluster analysis of ANF bacteria (Figure 5) displayed that, except for LDG, Sphingomonas accounted for a large proportion of all grassland bacteria. Ochrobactrum was common in NG and LDG, whereas Pseudomonas and Flavobacterium, while not as widely distributed as those of the dominant bacteria, were most abundant in SG and SDG (Figures 3, 5). The distribution of Opitutus in alpine grassland was most uniform (Figure 5). Figure 6 is a scatter plot on NMDS based OTUs to analyze the soil ANF microbial community. Most points representing SG and SDG were close to each other and some points of LDG approached SDG. The points of LDG were scattered far apart, while those of NG were most concentrated. The ANF bacteria communities in SG and SDG were similar to each other (Figure 6). The community structures differed significantly among the four grassland types (p < 0.01) (Figures 5, 6).
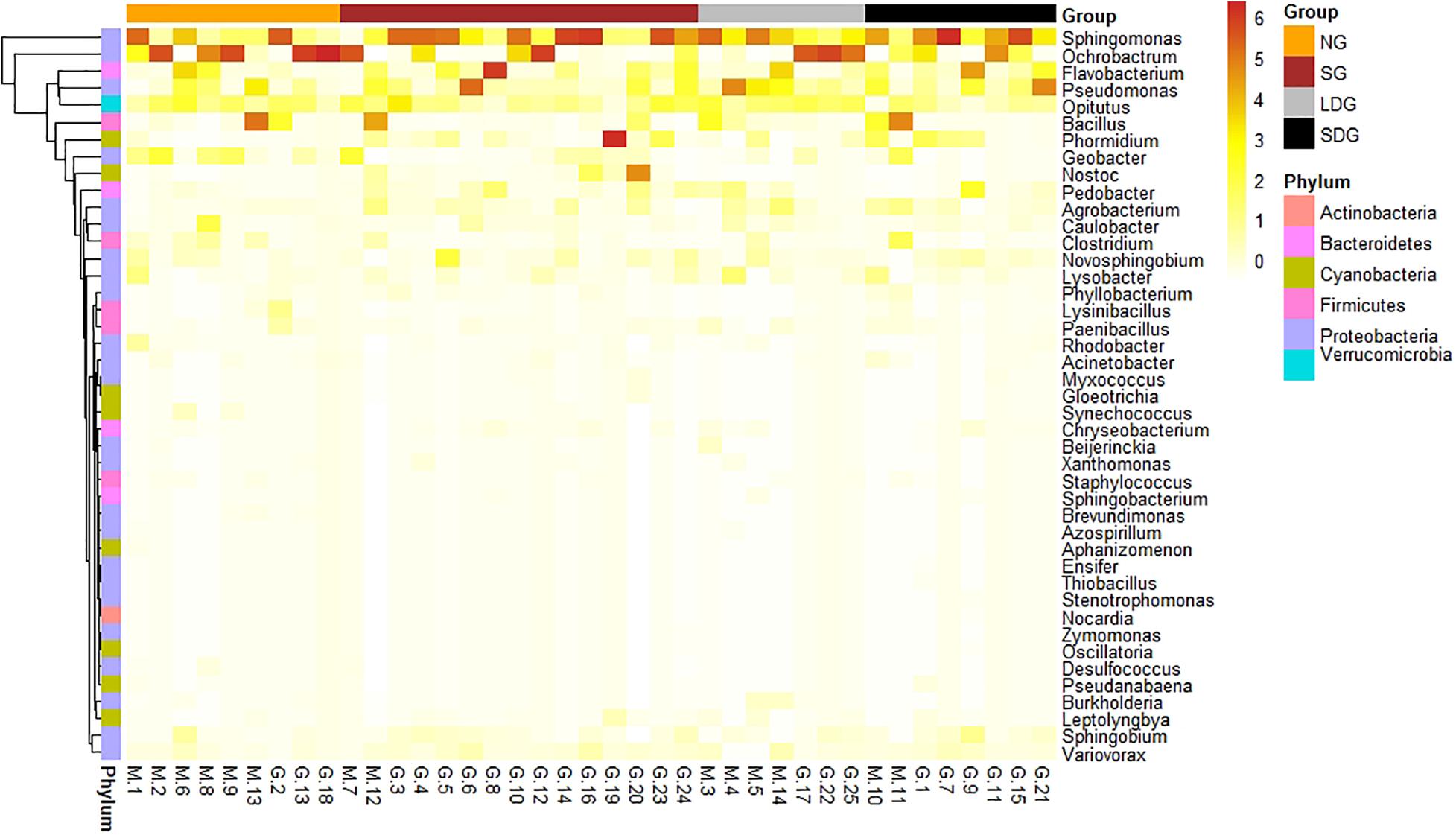
Figure 5. Relative abundance clustering heat map of asymbiotic nitrogen-fixing bacteria. Horizontal axis: Sample names, Group refers to different types of grassland, vertical axis: asymbiotic nitrogen-fixing genus, each belonging to a different Phylum. NG (orange), natural grassland; SG (brown), sown grassland; LDG (gray), lightly degraded grassland; SDG (blank), severely degraded grassland.
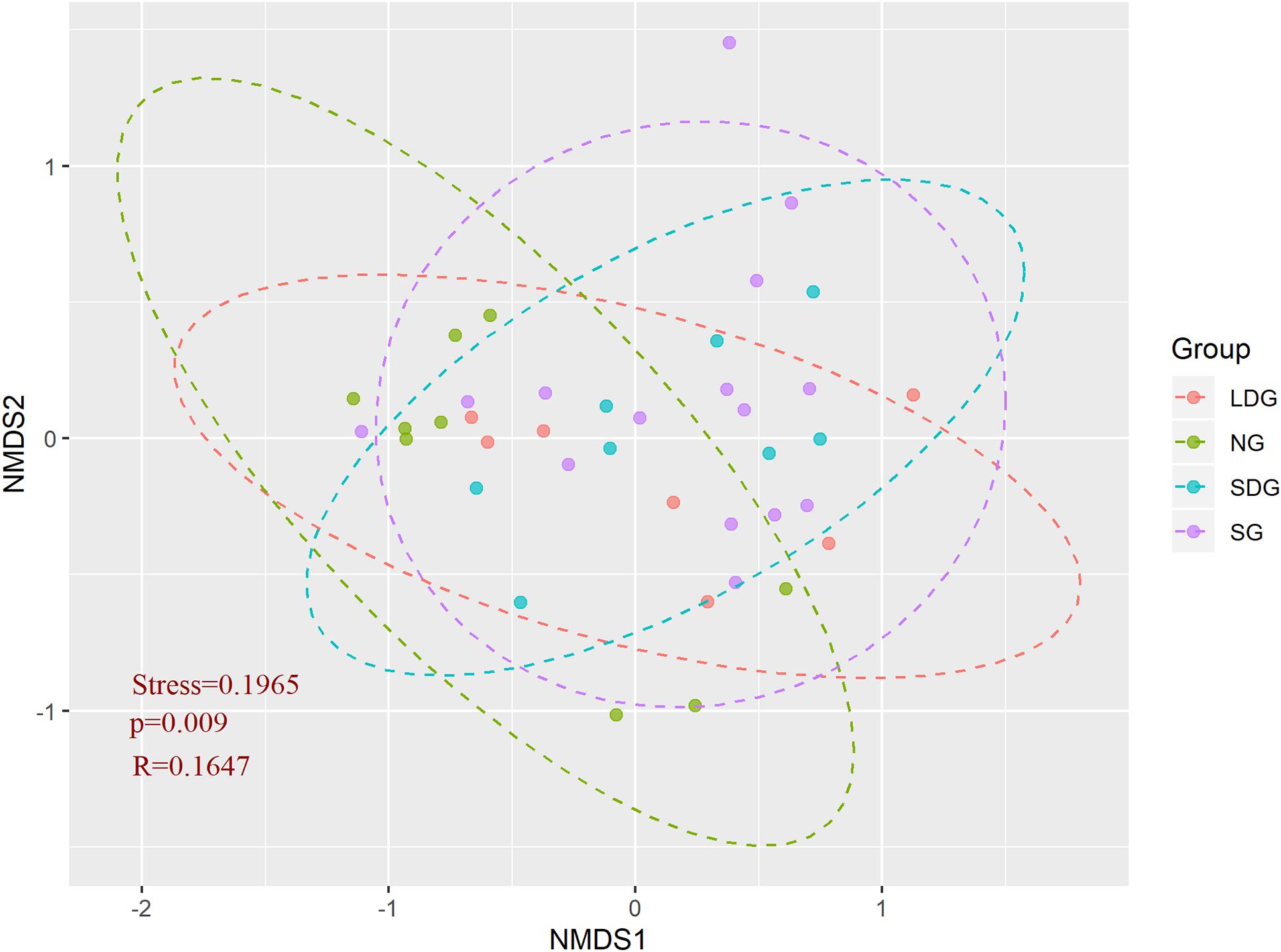
Figure 6. Non-metric multidimensional scale (NMDS) analysis of soil asymbiotic nitrogen-fixing microorganisms in 39 study sites. Group refers to different types of grassland, NG (green dots and dotted lines), natural grassland; SG (purple), sown grassland; LDG (red), lightly degraded grassland; SDG (blue), severely degraded grassland.
Discussion
The input of biological nitrogen is the main driving force of soil nutrient enrichment in the alpine grassland ecosystem of the Tibetan Plateau (Keuter et al., 2014; Wang et al., 2017); consequently, microbial nitrogen fixation processes in soil are crucial for maintaining nitrogen balance (Lannetta et al., 2016; Landriscini et al., 2019). In the present study, the ANF microorganisms were mainly bacteria and actinomycetes. Alphaproteobacteria and Gammaproteobacteria were the dominant classes, which was consistent with the results of previous studies (Zhan and Sun, 2012; Fernández-Méndez et al., 2016); whereas the genera Sphingomonas, Ochrobactrum, Opitutus and Pseudomonas were the most widely distributed.
It was reported that, except for extremely degraded grassland, soil nitrogen content in the grassland of the Tibetan Plateau is at a high level (Ma et al., 2020). With increasing degradation of the grasslands, there are alterations in the ANF microbial community in the soil, which also affects the development of the surrounding environment (Saravanan et al., 2008). In the present study, the diversity of the ANF bacteria community decreased with grassland degradation, but increased with the sowing of gramineous pastures. In addition, miscellaneous grasses increased the number of OTU species (Figure 4). In agreement with these results, Valkó et al. (2020) reported that the planting of gramineous grasses was beneficial to the growth of perennials. This could have occurred by effectively changing the community structure of soil microorganisms (Zhu et al., 2020) and by promoting various species of rhizosphere ANF microbial populations (Minamisawa et al., 2004; Miyamoto et al., 2004; Zhang et al., 2008). The dominant bacteria genera in the present study existed mainly in the soil of NG and SG, indicating that the restoration with sown gramineous grasses had a positive effect. In addition, the ANF microbial communities in SG and SDG had high similarity, which suggests that many bacteria in the sown grassland were still present from the time before restoration.
Asymbiotic nitrogen fixation contributes a large proportion of the nitrogen input (Wang et al., 2017). In the present study, Proteobacteria was identified as the dominant ANF phylum in all grasslands, which was consistent with the findings of Zhang et al. (2006). In addition to Proteobacteria, it has been reported that Cyanobacteria and some Firmicutes are widespread ANF bacteria in farmland ecosystems where land use of arable soil could affect the communities of ANF bacteria and nitrogen fixation efficiency (Wolińska et al., 2017). However, the addition of nitrogen in agricultural systems inhibited biological nitrogen fixation (Orr et al., 2011; Fan et al., 2019). These changes could occur over small scales, as was noted by Li et al. (2019) who examined free-living nitrogen fixation in three kinds of karst shrubs in southwest China and reported large differences in nitrogen fixation efficiency among them. In conclusion, SG increased species richness, evenness and stability of ANF bacteria community in degraded grassland, but not to the level of non-degraded NG.
There are some limitations to our study. First of all, the V3 + V4 region of the 16S rRNA gene was sequenced and the microbial species were identified by sequence alignment with basic database. The later data selection was based on the NCBI nitrogenase-related gene bank and the recently identified microbial genera. The phylogenetic analysis of the nitrogenase gene could not be done without sequence alignment. Secondly, we lacked experimental data for measuring nitrogenase activity or nitrogen fixation efficiency to determine whether sown grassland can change nitrogen fixation during grassland degradation. In addition, the result of redundancy analysis (RDA) showed that the above-ground biomass was insufficient to explain the difference in the structure of asymbiotic nitrogen-fixing bacteria community in the soil. No correlation was found between succession stages of the sown grassland and the alpha diversity of the asymbiotic nitrogen-fixing bacteria community in the soil due to the lack of effective repetition.
The ANF microorganisms play an important role in restoring degraded grassland in the Tibetan Plateau alpine ecosystem. Planting gramineous grass is beneficial as it increases the diversity of ANF microorganisms in soil, accelerates nitrogen fixation, and thus improves the low nitrogen content in degraded grassland. Future research should focus on the development of bacterial strains with high-efficiency of nitrogen fixation, which can promote nitrogen input into alpine grassland ecosystems, thereby, reducing fertilizer application and facilitating the restoration of degraded grassland.
Conclusion
The main ANF microorganisms in the Tibetan Plateau alpine grassland were bacteria and actinomycetes, among which the genera Sphingomonas, Ochrobactrum, Opitutus and Pseudomonas were widely distributed. The community structures of ANF microorganisms differed among the four grassland types. The decrease of richness and diversity caused by degraded grassland was improved by sown grassland, which could be beneficial in the restoration of degraded grassland.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WL: data curation, formal analysis, methodology, and writing—original draft. FL: field sampling, methodology, laboratory assays, and data curation. HZ: data curation and picture plots. LM: data curation. LQ, XW, WW, and ZP: formal analysis. AD: writing—review and editing. YB and TZ: field sampling. MH: field sampling and data curation. JH: field sampling and laboratory assays. ZS: funding acquisition, conceptualization, field sampling, methodology, formal analysis, and writing—review and editing. All authors read and approved the final manuscript.
Funding
This study was supported by the Second Tibetan Plateau Expedition (2019QZKK0302), the National Natural Science Foundation of China (31870433 and 31961143012), the National Key Research and Development Project (2016YFC0501906), and the “111” Program 2.0 (BP0719040).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Central Laboratory of the School of Life Science, Lanzhou University, for providing various instruments and equipment.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.702848/full#supplementary-material
References
Barron, A. R., Wurzburger, N., Bellenger, J. P., Wright, S. J., Kraepiel, A. M. L., and Hedin, L. O. (2009). Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nat. Geosci. 2, 42–45. doi: 10.1038/ngeo366
Bomfim, B., Silva, L. C. R., Doane, T. A., and Horwath, W. R. (2019). Interactive effects of land-use change and topography on asymbiotic nitrogen fixation in the Brazilian Atlantic Forest. Biogeochemistry 142, 137–153. doi: 10.1007/s10533-018-0525-z
Bottomley, P. J., and Myrold, D. D. (2015). Biological N inputs. Soil Microbiol. Ecol. Biochem. 3, 365–388. doi: 10.1016/b978-0-08-047514-1.50018-4
Cao, J., Adamowski, J. F., Deo, R. C., Xu, X., Gong, Y., and Feng, Q. (2019). Grassland degradation on the qinghai-tibetan plateau: reevaluation of causative factors. Rangeland Ecol. Manag. 72, 988–995. doi: 10.1016/j.rama.2019.06.001
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336.
Chen, X., Zhang, T., Guo, R., Li, H., Zhang, R., Degen, A. A., et al. (2021). Fencing enclosure alters nitrogen distribution patterns and tradeoff strategies in an alpine meadow on the Qinghai-Tibetan Plateau. Catena 197:104948. doi: 10.1016/j.catena.2020.104948
Cleveland, C. C., Townsend, A. R., Schimel, D. S., Fisher, H., Howarth, R. W., Hedin, L. O., et al. (1999). Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 13, 623–645. doi: 10.1029/1999gb900014
Dart, P. J., and Wani, S. P. (1982). “Non-symbiotic nitrogen fixation and soil fertility,” in Proceedingsof the 12th International Congress of Soil Science: Managing Soil Resources to Meet the Challenges of Mankind, I. (New Delhi: Indian Society of Soil Science), 3–27.
Fan, K., Delgado-Baquerizo, M., Guo, X., Wang, D., Wu, Y., Zhu, M., et al. (2019). Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 7:143.
Fernández-Méndez, M., Turk-Kubo, K. A., Buttigieg, P. L., Rapp, J. Z., Krumpen, T., Zehr, J. P., et al. (2016). Diazotroph diversity in the Sea Ice, Melt Ponds, and surface waters of the Eurasian Basin of the Central Arctic Ocean. Front. Microbiol. 7:1884. doi: 10.3389/fmicb.2016.01884
Galloway, J. N., and Cowling, E. B. (2002). Reactive nitrogen and the world: 200 years of change. AMBIO J. Hum. Environ. 31, 64–71. doi: 10.1579/0044-7447-31.2.64
Gupta, V. V. S. R., Kroker, S. J., Hicks, M., Davoren, C. W., Descheemaeker, K., and Llewellyn, R. (2014). Nitrogen cycling in summer active perennial grass systems in South Australia: non-symbiotic nitrogen fixation. Crop Pasture Sci. 65, 1044–1056. doi: 10.1071/cp14109
Johnsen, K., Jacobsen, C. S., Torsvik, V., and Sørensen, J. (2001). Pesticide effects on bacterial diversity in agricultural soils - a review. Biol. Fertil. Soils 33, 443–453. doi: 10.1007/s003740100351
Kennedy, I. R., and Islam, N. (2001). The current and potential contribution of asymbiotic nitrogen fixation to nitrogen requirements on farms: a review. Austral. J. Exp. Agric. 41, 447–457. doi: 10.1071/ea00081
Keuter, A., Veldkamp, E., and Corre, M. D. (2014). Asymbiotic biological nitrogen fixation in a temperate grassland as affected by management practices. Soil Biol. Biochem. 70, 38–46. doi: 10.1016/j.soilbio.2013.12.009
Landriscini, M. R., Galantini, J. A., Duval, M. E., and Capurro, J. E. (2019). Nitrogen balance in a plant-soil system under different cover crop-soybean cropping in Argentina. Appl. Soil Ecol. 133, 124–131. doi: 10.1016/j.apsoil.2018.10.005
Lannetta, P. P. M., Young, M., Bachinger, J., Bergkvist, G., Doltra, J., Lopez-Bellido, R. J., et al. (2016). A comparative nitrogen balance and productivity analysis of legume and non-legume supported cropping systems: the potential role of biological nitrogen fixation. Front. Plant Sci. 7:1700. doi: 10.3389/fpls.2016.01700
Li, C., Jong, R. D., Schmid, B., Wulf, H., and Schaepman, M. E. (2020). Changes in grassland cover and in its spatial heterogeneity indicate degradation on the Qinghai-Tibetan Plateau. Ecol. Indic. 119:106641. doi: 10.1016/j.ecolind.2020.106641
Li, D., Zhang, Q., and Wang, Z. (2019). Free-living N2 Fixation in Three Karst Shrublands, Southwest China. Ecosystems 22, 818–826. doi: 10.1007/s10021-018-0305-6
Limmer, C., and Drake, H. L. (1996). Non-Symbiotic N2-fixation in acidic and pH-neutral forest soils: aerobic and anaerobic differentials. Soil Biol. Biochem. 28, 177–183. doi: 10.1016/0038-0717(95)00118-2
Ma, X., Asano, M., Tamura, K., Zhao, R., Nakatsuka, H., Wu, Y., et al. (2020). Physicochemical properties and micromorphology of degraded alpine meadow soils in the Eastern Qinghai-Tibet Plateau. Catena 194:104649. doi: 10.1016/j.catena.2020.104649
Miehe, G., Schleuss, P., Seeber, E., Babel, W., Biermann, T., Braendle, M., et al. (2019). The Kobresia pygmaea ecosystem of the Tibetan highlands - Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem Kobresia pastures of Tibet. Sci. Total Environ. 648, 754–771. doi: 10.1016/j.scitotenv.2018.08.164
Minamisawa, K., Nishioka, K., and Miyaki, T. (2004). Anaerobic nitrogen-fixing consortia consisting of clostridia isolated from gramineous Plants. Appl. Environ. Microbiol. 70, 3096–3102. doi: 10.1128/aem.70.5.3096-3102.2004
Miyamoto, T., Kawahara, M., and Minamisawa, K. (2004). Novel endophytic nitrogen-fixing clostridia from the grass Miscanthus sinensis as revealed by terminal restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 70, 6580–6586. doi: 10.1128/aem.70.11.6580-6586.2004
Orr, C. H., James, A., Leifert, C., Cooper, J. M., and Cummings, S. P. (2011). Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Appl. Environ. Microbiol. 77, 911–919. doi: 10.1128/aem.01250-10
Pérez, C. A., Carmona, M. R., Aravena, J. C., and Armesto, J. J. (2004). Successional changes in soil nitrogen availability, non-symbiotic nitrogen fixation and carbon/nitrogen ratios in southern Chilean forest ecosystems. Oecologia 140, 617–625. doi: 10.1007/s00442-004-1627-y
Poly, F., Ranjard, L., Nazaret, S., Gourbière, F., and Monrozier, L. J. (2001). Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl. Environ. Microbiol. 67, 2255–2262. doi: 10.1128/aem.67.5.2255-2262.2001
Reed, S. C., Cleveland, C. C., and Townsend, A. R. (2011). Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 42, 489–512. doi: 10.1146/annurev-ecolsys-102710-145034
Reed, S. C., Seastedt, T. R., Mann, C. M., Suding, K. N., Townsend, A. R., and Cherwin, K. L. (2007). Phosphorus fertilization stimulates nitrogen fixation and increases inorganic nitrogen concentrations in a restored prairie. Appl. Soil Ecol. 36, 238–242. doi: 10.1016/j.apsoil.2007.02.002
Saravanan, V. S., Madhaiyan, M., Osborne, J., Thangaraju, M., and Sa, T. M. (2008). Ecological occurrence of gluconacetobacter diazotrophicus and nitrogen-fixing acetobacteraceae members: their possible role in plant growth promotion. Microb. Ecol. 55, 130–140. doi: 10.1007/s00248-007-9258-6
Taylor, B. N., Chazdon, R. L., and Menge, D. D. L. (2019). Successional dynamics of nitrogen fixation and forest growth in regenerating Costa Rican rainforests. Ecology 100:e02637.
Valkó, O., Deák, B., Török, P., Tóth, K., Kiss, R., Kelemen, A., et al. (2020). Vegetation and seed bank dynamics highlight the importance of post-restoration management in sown grasslands. bioRxiv [Preprint]. doi: 10.1101/2020.01.20.913426
Vasileiadis, S., Puglisi, E., Arena, M., Cappa, F., Cocconcelli, P. S., and Trevisan, M. (2012). Soil bacterial diversity screening using single 16S rRNA gene v regions coupled with Multi-Million read generating sequencing technologies. PLoS One 7:e42671. doi: 10.1371/journal.pone.0042671
Wakelin, S. A., Gupta, V. V. S. R., and Forrester, S. T. (2010). Regional and local factors affecting diversity, abundance and activity of free-living, N2-fixing bacteria in Australian agricultural soils. Pedobiologia 53, 391–399. doi: 10.1016/j.pedobi.2010.08.001
Wang, Y., Li, C., Kou, Y., Wang, J., Tu, B., Li, H., et al. (2017). Soil pH is a major driver of soil diazotrophic community assembly in Qinghai-Tibet alpine meadows. Soil Biol. Biochem. 115, 547–555. doi: 10.1016/j.soilbio.2017.09.024
Wolińska, A., Kuźniar, A., Zielenkiewicz, U., Banach, A., Izak, D., Stępniewska, Z., et al. (2017). Metagenomic analysis of some potential nitrogen-fixing bacteria in arable soils at different formation processes. Microb. Ecol. 73, 162–176. doi: 10.1007/s00248-016-0837-2
Zehr, J. P., Jenkins, B. D., Short, S. M., and Steward, G. F. (2003). Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5, 539–554. doi: 10.1046/j.1462-2920.2003.00451.x
Zhan, J., and Sun, Q. (2012). Diversity of free-living nitrogen-fixing microorganisms in the rhizosphere and non-rhizosphere of pioneer plants growing on wastelands of copper mine tailings. Microbiol. Res. 167, 157–165. doi: 10.1016/j.micres.2011.05.006
Zhang, G. X., Peng, G. X., Wang, E. T., Yan, H., Yuan, Q. H., Zhang, W., et al. (2008). Diverse endophytic nitrogen-fixing bacteria isolated from wild rice Oryza rufipogon and description of Phytobacter diazotrophicus gen. nov. sp. nov. Arch. Microbiol. 189, 431–439. doi: 10.1007/s00203-007-0333-7
Zhang, Y., Li, D., Wang, H., Xiao, Q., and Liu, X. (2006). Molecular diversity of nitrogen-fixing bacteria from the Tibetan Plateau, China. FEMS Microbiol. Lett. 260, 134–142. doi: 10.1111/j.1574-6968.2006.00317.x
Zhang, Y., Wang, H., Li, D., Xiao, Q., and Liu, X. (2005). Molecular diversity and phylogenetic analysis of nitrogen-fixing (nifH) genes in alp prairie soil of Sanjiangyuan natural reserve. Acta Microbiol. Sin. 260, 134–142.
Zhen, L., Du, B., Wei, Y., Xiao, Y., and Sheng, W. (2018). Assessing the effects of ecological restoration approaches in the alpine rangelands of the Qinghai-Tibetan Plateau. Environ. Res. Lett. 13:095005. doi: 10.1088/1748-9326/aada51
Zheng, M., Zhang, W., Luo, Y., Li, D., Wang, S., Huang, J., et al. (2018). Stoichiometry controls asymbiotic nitrogen fixation and its response to nitrogen inputs in a nitrogen-saturated forest. Ecology 99, 2037–2046. doi: 10.1002/ecy.2416
Keywords: asymbiotic nitrogen-fixing bacteria, alpine grassland, Tibetan Plateau, diversity, community structure
Citation: Li W, Li F, Zeng H, Ma L, Qi L, Wang X, Wang W, Peng Z, Degen AA, Bai Y, Zhang T, Huang M, Han J and Shang Z (2021) Diversity and Variation of Asymbiotic Nitrogen-Fixing Microorganisms in Alpine Grasslands on the Tibetan Plateau. Front. Ecol. Evol. 9:702848. doi: 10.3389/fevo.2021.702848
Received: 30 April 2021; Accepted: 16 July 2021;
Published: 13 August 2021.
Edited by:
Jian Sun, Institute of Tibetan Plateau Research (CAS), ChinaReviewed by:
Xiaoyong Cui, University of Chinese Academy of Sciences, ChinaFei Peng, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences (CAS), China
Copyright © 2021 Li, Li, Zeng, Ma, Qi, Wang, Wang, Peng, Degen, Bai, Zhang, Huang, Han and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanhuan Shang, shangzhh@lzu.edu.cn
 Wenyan Li1
Wenyan Li1