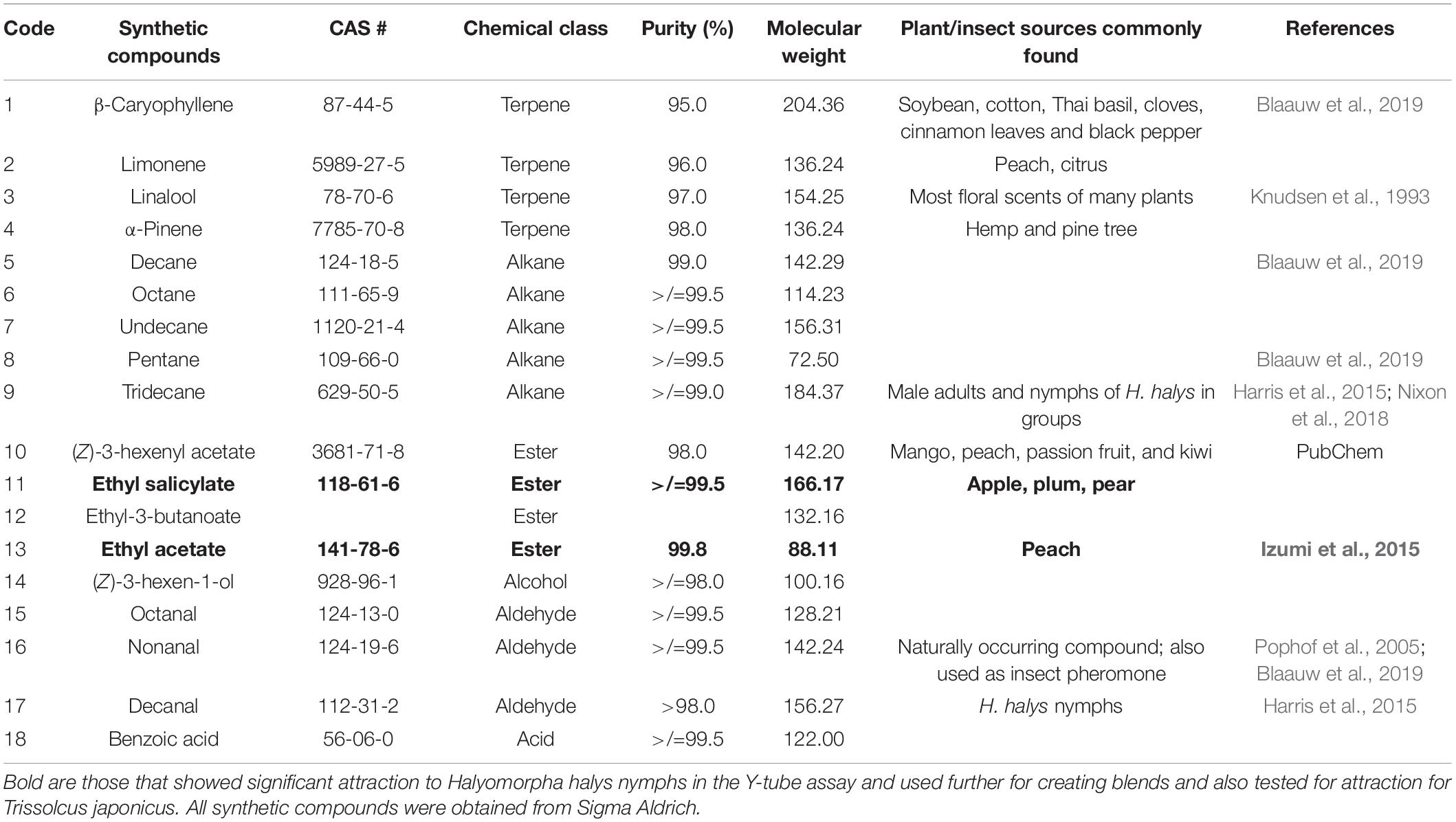
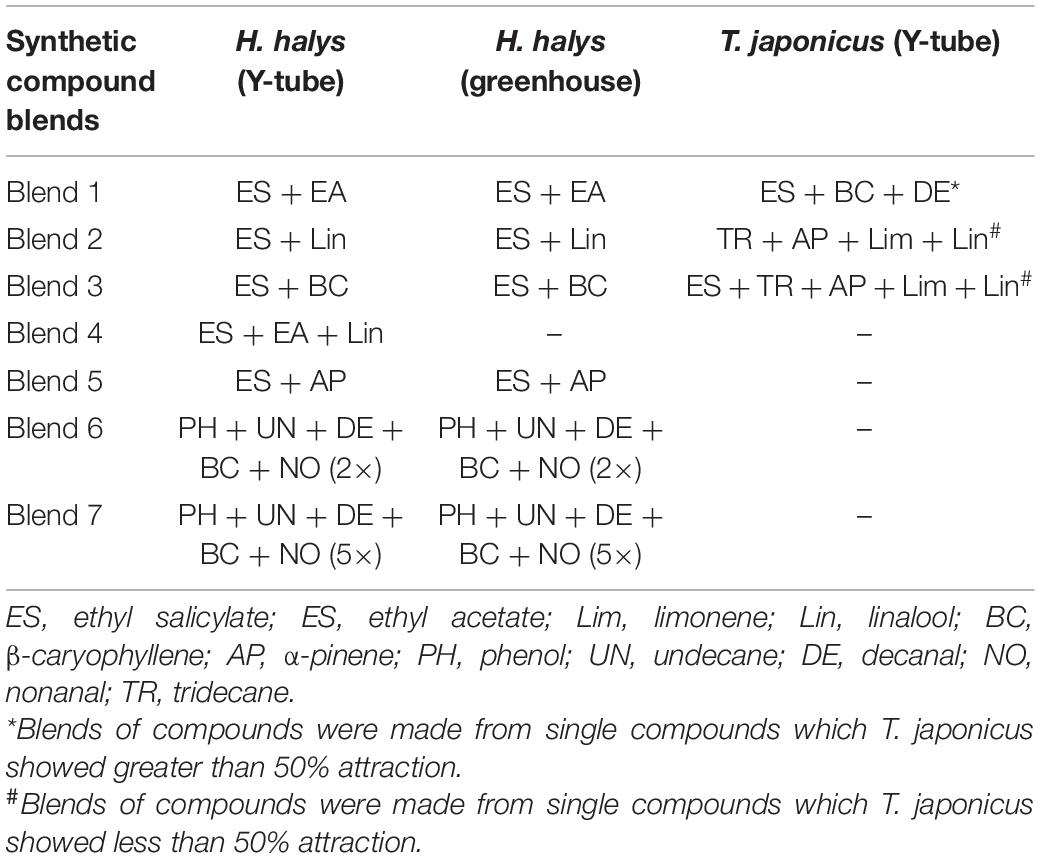
- 1Lincoln University Cooperative Extension, Jefferson City, MO, United States
- 2Department of Entomology, University of Georgia, Athens, GA, United States
- 3Department of Entomology, University of California, Riverside, Riverside, CA, United States
- 4Department of Entomology, Rutgers University, New Brunswick, NJ, United States
Insects use a range of cues to help them interact with each other and their host plants. Among these cues, olfaction plays a major role in host selection. The present study investigated the behavioral response of the brown marmorated stink bug, Halyomorpha halys (Stål), and its egg parasitoid, Trissolcus japonicus (Ashmead), to host plant-related odors. We used H. halys nymphs since their response to host odors is relatively unknown. In a Y-tube, we first evaluated the behavioral response of H. halys nymphs to whole-fruit odors of apple [Malus domestica (Borkh.)] and peach [Prunus persica (L.) Batsch)]. Subsequently, we tested the behavioral response of H. halys and T. japonicus to 18 selected synthetic volatiles previously identified from H. halys and its common host plants. In the greenhouse, we further tested H. halys attraction to the most promising of these volatiles individually and as blends. In single-choice tests, H. halys nymphs preferred odors from apple and peach over the control (no odor). In dual-choice tests, H. halys did not show any preference between apple and peach odors. Among the 18 volatiles tested, H. halys nymphs were attracted to ethyl salicylate (ES), undecane (UN), and ethyl acetate (EA) compared to the control. In the greenhouse, H. halys nymphs were similarly attracted to blends of 1:1 ratio of ES and EA but not to single compounds. Also in the Y-tube, female T. japonicus preferred the arm that had ES, β-caryophyllene, and decanal and a blend of these three compounds at a 1:1:1 ratio. Trissolcus japonicus was more attracted to the control arm than to the arm containing tridecane or α-pinene. These results indicate the potential of developing H. halys and T. japonicus attractants or/and repellents based on host plant volatiles and suggest possible adaptive responses of this pest and its egg parasitoid to similar host plant odors.
Introduction
Research in insect chemical ecology has led to the discovery of effective attractants for use in insect pest monitoring. Most insects, particularly herbivores, have evolved to depend on plants for survival (Ehrlich and Raven, 1964). Insects use plants to obtain resources, like food and oviposition sites, which they locate through chemical cues commonly referred to as volatile organic compounds (VOCs) produced by intact [host plant volatiles (HPVs)] or mechanically and/or herbivore-damaged plants [herbivore-induced plant volatiles (HIPVs)] (Choh et al., 2013; Xu and Turlings, 2018). Although plants are ubiquitous, their availability and quality changes because of selection pressure (Bruce et al., 2005), and production of volatiles varies in space and time (Holopainen and Blande, 2013; Conchou et al., 2017, 2019), which means insects have to develop efficient and effective sensory organs to discriminate between host and non-host odors.
Several VOCs have been identified from different plant parts (Knudsen et al., 1993), with each of the compounds or their combinations reported to perform different biological roles during an insect’s trophic interactions with plants (Paré and Tumlinson, 2002; Reddy and Guerrero, 2004). In many situations, phytophagous insects in particular respond to individual VOCs described as signature compounds or blends (Szendrei and Rodriguez-Saona, 2010; Rodriguez-Saona et al., 2012) that assist in locating plant species on which they can feed and reproduce. Moreover, insects respond to VOCs when they synergize with visual and olfactory cues, such as color, shape, and pheromones, which are used in monitoring traps particularly in moths (Yang et al., 2004; Varela et al., 2011; Sans et al., 2016; Rodriguez-Saona et al., 2020). This has provided opportunities where VOCs are used as stand-alone compounds or synergized with other cues to monitor many destructive agricultural and forest pests and improve pest management by increasing accuracy of detection (Knight and Light, 2001; Light and Knight, 2005).
The brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), is a key invasive pest responsible for causing severe feeding damage to major fruit, vegetable, and field crops in North America and Europe (Leskey et al., 2012; Rice et al., 2014; Leskey and Nielsen, 2018). Halyomorpha halys is a polyphagous insect, and its behavioral ecology, feeding habits, and movement within the landscape are driven by a number of factors. Notably among them are host switching due to decreasing host quality, strong requirement for diet mixing to satisfy its developmental requirements (Nielsen and Hamilton, 2009; Acebes-Doria et al., 2016; Dingha and Jackai, 2017), and VOCs produced by host plant species during attractive stages (Blaauw et al., 2019). Among these factors, HPVs inform host selection when the insect is in the active accessible space for host choice. Yet, little is known on the behavioral response of H. halys nymphs to host plant odors.
Like most insects, H. halys’ selection of host plants is dependent on the chemical profile released by the plants. However, polyphagous insects accept a wide range of host plants with distinct odor profiles. Thus, selection of candidate compounds is more challenging with generalist than specialist insects (Braasch et al., 2012; Kaplan, 2012).
Additionally, there is strong evidence that entomophagous insects including parasitoids also use VOCs associated with herbivory and egg deposition to locate their host and thus may respond to synthetic VOCs associated with these behaviors (Turlings and Tumlinson, 1992; Van Loon et al., 2000; Dudareva et al., 2006; Tentelier and Fauvergue, 2007; Ngumbi et al., 2009; Pashalidou et al., 2015). This has been demonstrated in some Trissolcus species (Colazza et al., 2004). However, limited studies have been done on the behavioral responses of H. halys’ coevolved egg parasitoid, Trissolcus japonicus Ashmead (Hymenoptera: Scelionidae), although T. japonicus does positively respond to host kairomones on exposed leaf surfaces (Boyle et al., 2020).
Our primary aim was to investigate the behavioral response of H. halys nymphs and female T. japonicus parasitoids to HPVs that may serve as attractants for food and oviposition sites, respectively, and for improving the monitoring of both species. Specifically, as adult and nymphs share the same host plants, we tried to isolate host plant cues that nymphs might use during host searching behaviors. As a non-reproductive stage, nymphs are only seeking nutritional resources, which may be signaled by VOCs. This study follows up on Blaauw et al. (2019) findings that H. halys nymphs chose host plants depending on host phenology and characterized VOCs that are present during attraction. Apple and peach fruits were chosen because they are common host plants of H. halys (Akotsen-Mensah et al., 2018), and H. halys survive well and damage peach and apple based on laboratory feeding studies (Acebes-Doria et al., 2016; Dingha and Jackai, 2017). Whole fruits were used because H. halys attack mainly fruiting parts, and plant parts are commonly used as experimental methods to identify novel plant compounds for herbivore insects (Knolhoff and Heckel, 2014).
Further, we hypothesized that T. japonicus, during its own host searching behaviors, will respond positively to similar plant volatiles as its primary host, H. halys. Our specific objectives were to: (1) evaluate the behavioral response of H. halys nymphs to whole-fruit odors of apple [Malus domestica (Borkh.)] and peach [Prunus persica (L.) Batsch] in laboratory (Y-tube) assays; (2) test the behavioral response of H. halys and T. japonicus to 18 selected, previously identified synthetic fruit volatiles in laboratory (Y-tube) assays; and (3) test H. halys attraction to the most promising of these volatiles individually and as blends in a greenhouse.
Materials and Methods
Test Insects
Halyomorpha halys second instars used for the study came from eggs sourced from the New Jersey Department of Agriculture (NJDA, Trenton, NJ, United States), and a colony at Rutgers Agricultural Research and Extension Center (RAREC; Bridgeton, NJ, United States). Newly hatched nymphs were fed ad libitum with pesticide-free raw sunflower seeds, green beans, carrot, and water provided in a cotton wick and were kept in Petri dishes until ready for use.
Trissolcus japonicus females came from the laboratory colony maintained at RAREC using protocols developed by Talamas et al. (2015) and Zhang et al. (2017). Briefly, fresh H. halys eggs obtained from the NJDA were exposed to the parasitoids for 24 h. Upon emergence, the parasitoids were sexed, and females were transferred to plastic containers with a feeding station (honey solution). Females aged between 2 and 7 days were used in the experiments.
Insect colonies were maintained in an incubator at 25 ± 1°C, 65–70% relative humidity, and 16:8 h (L:D) photoperiod.
Y-Tube Olfactometer Bioassays
A Y-tube olfactometer (Sigma Scientific LLC, Micanopy, FL, United States) was used to determine the behavioral response of second instar H. halys starved for 3 h and adult (female) T. japonicus. The Y-tube was placed in a particle board box in a darkroom illuminated with a 20-W red LED light and maintained at approximately 25°C. The olfactometer was similar to that described by Blackmer et al. (2004). Briefly, the apparatus consisted of a Y-tube glass (40-mm diameter × 36-cm length) with the two arms angled at 50° through which laboratory-grade air (Airgas Company, Vineland, NJ, United States) was drawn into each arm to create a single or two potential odor sources, and a central junction where mixing of the airflow from the arms occurred. The airflow was controlled by an inline flowmeter (Gilmont Instr., Barnant Co., Barrington, IL, United States) set at 5 L/min to deliver 2.5 L/min to the olfactometer arm. The arm in which the treatments were placed was randomized in all experiments, and the Y-tube was rinsed in acetone and air dried between replicates.
Whole undamaged apple (M. domestica) and peach (P. persica) fruit were tested in two customized 4.5-L stainless steel stock pots (Figure 1; Experiment 1 below). Each pot had two openings through which air could flow in and out of a glass chamber (odor source). Volatile sources (apple vs. peach) were placed in their designated olfactometer arm for 2 min prior to release of test insects to ensure the stabilization of the diffusion of the test material. Synthetic compounds (2- and 0.5-μl aliquots of synthetic VOCs for H. halys and T. japonicus, respectively) were pipetted onto 5-cm2 filter paper (Whatman®, Thermo Fisher Scientific, Waltham, MA, United States), and the filter paper was replaced after testing 10 insects per compound. In each experiment, a single insect was introduced into the base of the Y-tube and allowed to walk to either arm at the Y-junction, and the time taken to make a choice was recorded. The insects were continuously observed until they made a choice. Individuals that did not make a choice after 12 min were recorded as not responding and removed from the analysis. Thirty (30) insects were tested for each compound, with each insect representing a replicate. Pots and other apparatus were cleaned with 5% bleach and 95% ethanol between replicates.
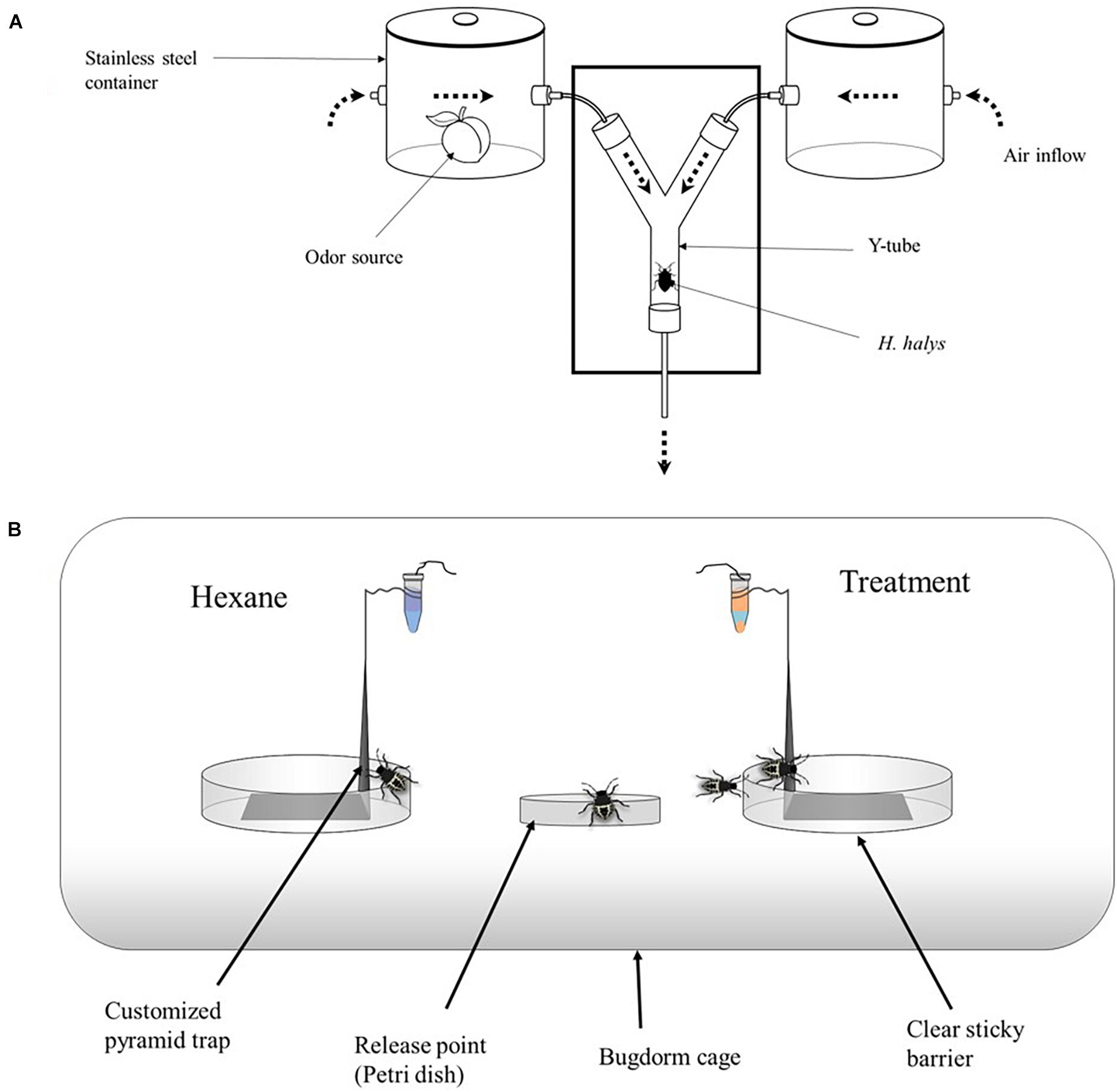
Figure 1. (A) Customized olfactometer, showing the source of stimuli via airflow through each of the two arms. Air was pumped from a gas cylinder through the stainless container to the two-way part of the olfactometer. The flow rate of the air was checked regularly, ensuring that it was 100 ml/min through each arm (200 ml/min into the central portion of the olfactometer). (B) Greenhouse setup consisted of a customized black pyramid trap placed in a bugdorm and nymphs released from the center of the two treatments. The pyramid trap is 15 cm tall and 10 cm wide at the base. These were placed in a 90 × 45 × 45 BugDorm tent. The release mechanism consisted of a 1.5-ml microcentrifuge vial with a cotton string (∼2.5 cm long) threaded through a hole drilled (0.125 mm) through the lid and placed on top of the pyramid trap.
Halyomorpha halys Response to Apple and Peach Fruit Volatiles
Experiment 1
In experiment 1, we tested the response of H. halys to volatiles from apple, M. domestica var. “Golden Delicious” and “Ginger Gold,” and matured peach, P. persica var. “Encore” and “Harrow Beauty”; peach and apple fruits that were not treated with insecticides were harvested in late June and late August, respectively, at RAREC. These time points were chosen because fruit were nearing maturity and harvest which are vulnerable to H. halys attack. The responses of H. halys nymphs to three choices (apple vs. control, peach vs. control, and peach vs. apple) were assessed by placing two similar-sized fruit into one of the pots and connected to the one arm of the Y-tube olfactometer.
Halyomorpha halys Response to Synthetic HPVs
Experiment 2
In experiment 2, second instar H. halys behavioral responses to volatiles identified in headspace collections from pepper (Capsicum frutescens Mill.), soybean (Glycine max L.), sweet corn (Zea mays L. saccharata Sturt.), Swiss chard (Beta vulgaris L.) (Blaauw et al., 2019), peach (P. persica), and apple (M. domestica) (Akotsen-Mensah et al., unpublished), and those reported in the literature (Horvat and Chapman, 1990; Horvat et al., 1990; Knudsen et al., 1993; Casado et al., 2006; Rout et al., 2012; Izumi et al., 2015) were tested. In all, 18 synthetic compounds consisting of five alkanes, four terpenes, four esters, four aldehydes, an alcohol, and an acid (Table 1) and their blends (Table 2) were purchased from commercial sources (Sigma Aldrich, St. Louis, MO, United States) and tested in the Y-tube olfactometer. Compounds that significantly attracted nymphs in the olfactometer were tested as binary and ternary blends of equal ratios to investigate the existence of possible synergisms. Blends consisted of two (binary) compounds in a ratio of 1:1 and three (ternary) compounds in ratios of 1:1:1. Because of potential of compounds reacting when mixed, blends used in the Y-tube were released from separate filter papers.
Experiment 3
In experiment 3, nymphal response to promising compounds from experiment 2 were tested in a mesh cage under greenhouse conditions. Six mesh cages were used for each trial (93.0 cm × 47.5 cm × 47.5 cm, BugDorm-44590DH, Megaview Science Co., Ltd., Taiwan) and separated by at least 0.5 m. The cages were placed in a greenhouse aligned lengthwise from north to south, and the test compounds and control (hexane) were randomly assigned to either end of the cage. The test compounds (treatments) consisted of four single compounds, namely, (1) ethyl salicylate (ES), (2) ethyl acetate (EA), (3) nonanal (NO), and (4) tridecane (TR) (Table 1), and seven blends, namely, (5) blend 1, (6) blend 2, (7) blend 3, (8) blend 4, (9) blend 5, (10) blend 6, and (11) blend 7 (Table 2). Each blend was prepared by putting 2 μl each in 2 ml of hexane and tested individually. Individual compounds or blends were dispensed by transferring 1.0-ml aliquots into release devices consisting of a 1.5-ml microcentrifuge vial (VWR International, Radnor, PA, United States) with a cotton string (∼2.5 cm long) threaded through a hole drilled (0.125 mm) in the lid. The release device was suspended on top of a customized trap (Figure 1B) to collect insects attracted to the compounds. For each compound, 10 late second instar H. halys, starved for 3 h, were released from Petri dishes (100 mm × 15 mm; VWR International, Radnor, PA, United States) placed in the center of each cage. After 24 h, the location of the nymphs was recorded. A nymph was recorded as making a “choice” if it was on a baited trap or on the cage directly above it (e.g., a circle of a given diameter directly aligned above the treatments; defined as a “choice-zone”). Cages were soaked in 10% bleach for 2 h and washed thoroughly with tap water before each trial.
Trissolcus japonicus Response to Synthetic HPVs
Experiment 4
To measure the behavioral response of T. japonicus to volatiles identified as attractive to H. halys (based on experiments 2 and 3 above), mated 4- to 7-day-old female T. japonicus adults were evaluated individually in the Y-tube olfactometer, as described previously (experiment 2). Twenty (20) adults were used for each treatment. Responses of adults to individual synthetic VOCs and blends were tested in separate experiments. The treatments tested individually were (1) ES, (2) linalool (Lin), (3) limonene (lim), (4) α-pinene (AP), (5) β-caryophyllene (BC), (6) decanal (DE), and (7) TR. Compounds in which T. japonicus showed greater than 50% response were made into a blend (blend 1) and tested in a Y-tube. Also, those which showed less than 50% response were made into a blend (blend 2) and also tested. The blends (Table 2) used were (1) blend 1 (ES + BC + DE), (2) blend 2 (AP + TR + lim + Lin), and (3) blend 3 (blend 2 + ES). To maintain the integrity of the compounds and avoid potential interaction of compounds, blends were not mixed, but each was put separately on a filter paper. The loading rate tested for each compound was 0.5 μl.
Statistical Analyses
For all tests involving choice between two treatments, data were analyzed using Chi-square contingency analyses. A Chi-square goodness-of-fit test based on the assumption that there would be 50:50 chance of insect responding to both treatments was used to test for significance at p = 0.05. For the greenhouse experiment, a Student’s t-test was used to determine difference between the treatments. All analyses were done using JMP®, Version 15 (SAS Institute Inc., Cary, NC, United States).
Results
Halyomorpha halys Response to Apple and Peach Fruit Volatiles
Halyomorpha halys nymphs were significantly attracted to apples of the variety Golden Delicious (20.0%) (χ2 = 16.28, p < 0.001), but not to those of the variety Ginger Gold (χ2 = 1.68, p = 0.302). Peaches of the Encore variety attracted significantly more H. halys nymphs, while those of the variety Harrow Beauty did not exhibit significant attraction between the odors of apples (var. Golden Delicious: 65.4%, χ2 = 0.28, p = 0.599) and peach (var. Encore: 34.6%, χ2 = 3.28, p = 0.0673), although nymphs tended to orient more to the arm containing apples.
Halyomorpha halys Response to Synthetic HPVs
Among the 18 synthetic compounds evaluated in the Y-tube, ES (χ2 = 8.53, p = 0.004), undecane (UN) (χ2 = 8.53, p = 0.004), and EA (χ2 = 6.08, p = 0.014) were significantly attractive to the nymphs (blank) (Figure 2). In contrast, nymphs demonstrated repellant behavior to BC (χ2 = 8.53, p = 0.004), octanal (χ2 = 5.83, p = 0.016), DE (χ2 = 4.67, p = 0.031), and octane (χ2 = 4.67, p = 0.031) (Figure 2). When ES and EA were blended (blend 1) and compared to other blends including those modeled from volatiles emitted from pepper, soybean, sweet corn, and Swiss chard at 2× (blend 6) and 5× (blend 7) concentrations (Blaauw et al., 2019), blend 1 (χ2 = 4.52, p = 0.0336) and blend 2 (ES and Lin) (χ2 = 9.5, p = 0.002) significantly attracted the nymphs (Figure 3), while blends 6 and 7 showed no attraction compared with control. The combined blends of ES and EA were not more attractive than individual compounds, which suggests there was no synergistic effect between ES and EA and other blends too.
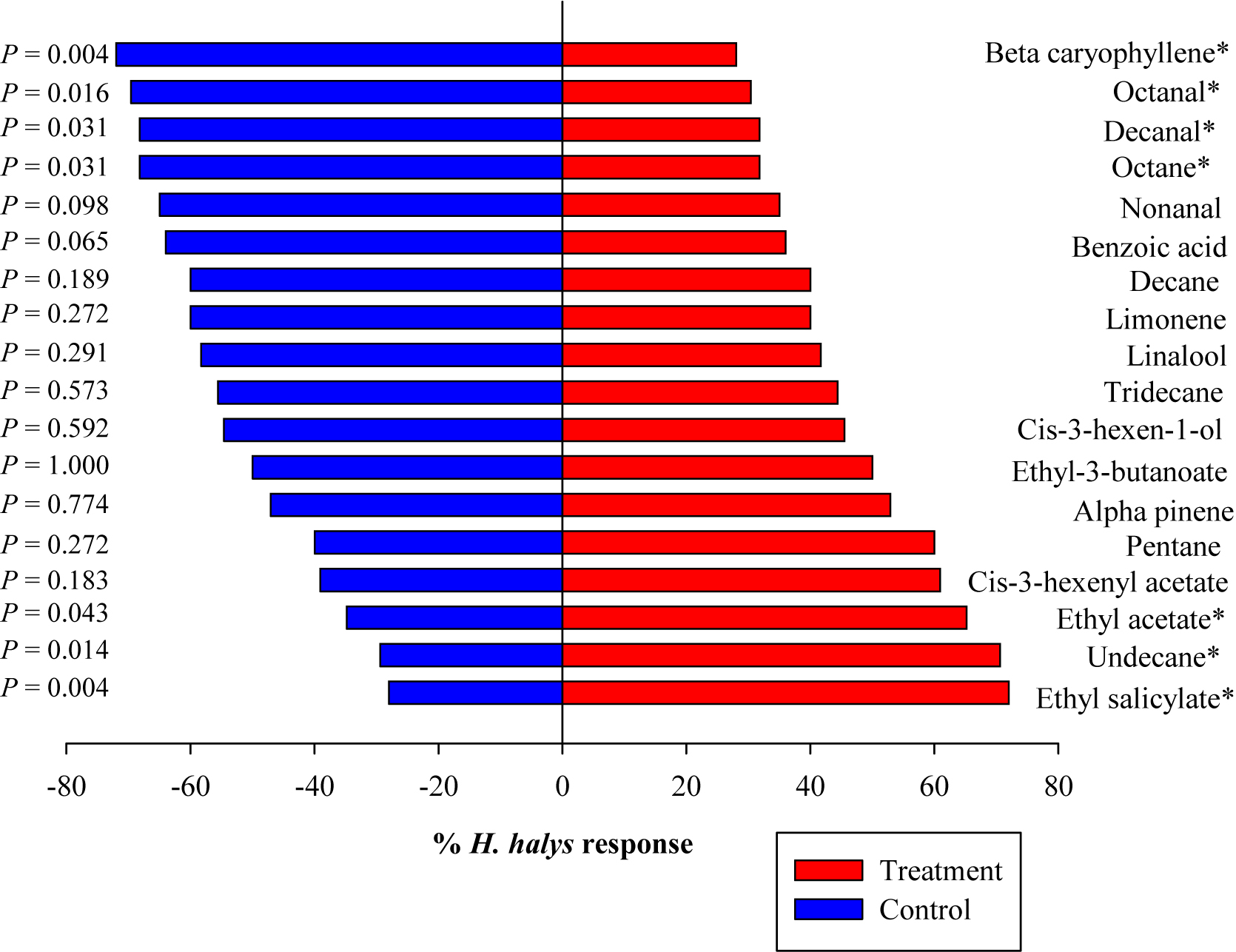
Figure 2. Responses of Halyomorpha halys nymphs to synthetic compounds in a Y-tube olfactometer. Percentage of H. halys (n = 30) responding to the single compounds. Asterisks indicate significant difference between the treatments at p = 0.05 using likelihood ratio test.
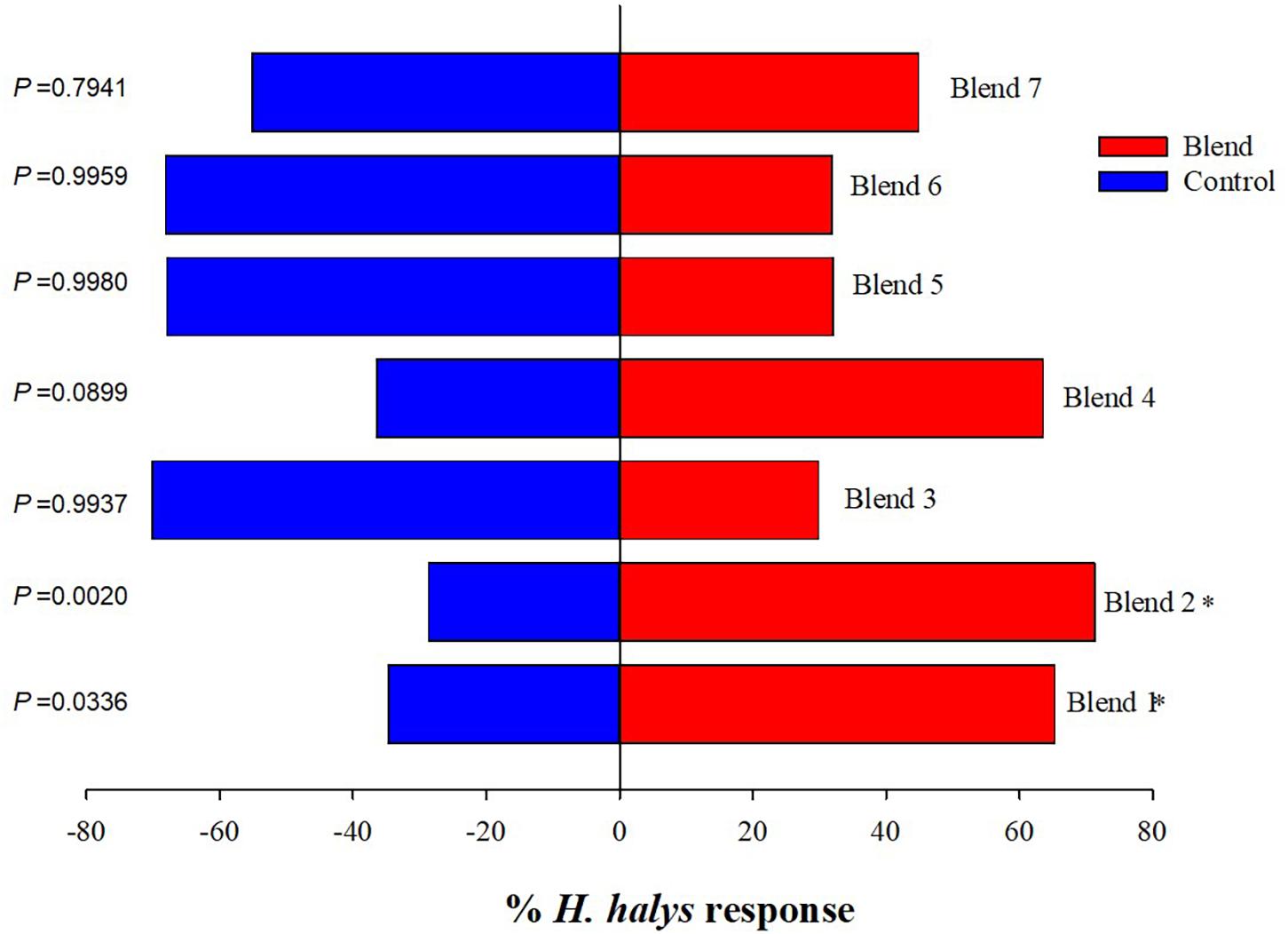
Figure 3. Responses of Halyomorpha halys nymphs to different blends of synthetic compounds. Blend 1 (ES and EA), blend 2 (ES and Lin), blend 3 (ES and BC), blend 4 [ES, EA, and (±)-Lin], blend 5 [ES and (±)-AP], blend 6 (phenol, UN, decane, BC, and NO at 2×), and blend 7 (phenol, UN, decane, BC, and NO at 5×). Asterisks indicate significant difference between the treatments at p = 0.05 using likelihood ratio test.
In the greenhouse, response of H. halys nymphs to the four individual synthetic VOCs tested (ES, EA, NO, and TR) was not significant compared to the control (Figure 4). However, of the six blends tested, H. halys showed significant positive response to blend 1 (ES + EA) (t = 8.93, p = 0.014) but were repelled by blend 6 (t = 7.27, p = 0.022) (Figure 4).
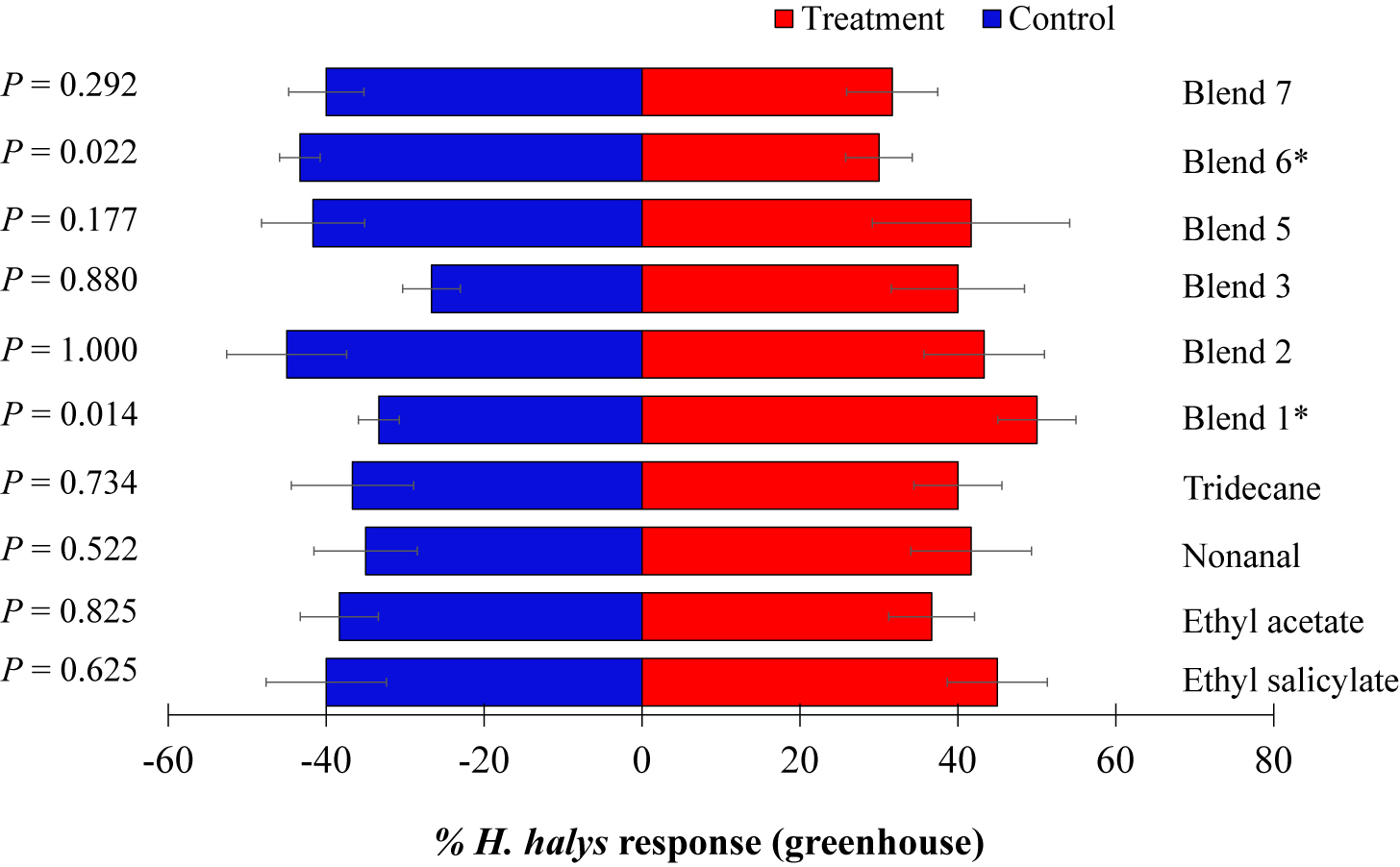
Figure 4. Responses of Halyomorpha halys nymphs to synthetic compounds in greenhouse. Blend 5 [ES and (±)-AP], blend 6 phenol, UN, decane, BC, and NO at 2×), and blend 7 (phenol, UN, decane, BC, and NO at 5×). Asterisks indicate significant difference between the treatments at p = 0.05 using likelihood ratio test.
Trissolcus japonicus Response to Synthetic HPVs
Trissolcus japonicus females were attracted to ES, BC, and DE, but not to AP and TR (Figure 5). To test whether there could be a synergistic effect of VOCs that showed significant attraction and those that did not, only blend 1 (ES + BC + DE) showed significant attraction (χ2 = 4.9, p = 0.026); however, the attraction was not greater than the individual compounds. Also, when ES was added to compounds that did not show attraction (blend 2), ES did not improve the attraction of this blend. This indicates that ES alone may be attractive, but its effect may be suppressed when combined with other non-attractive compounds.
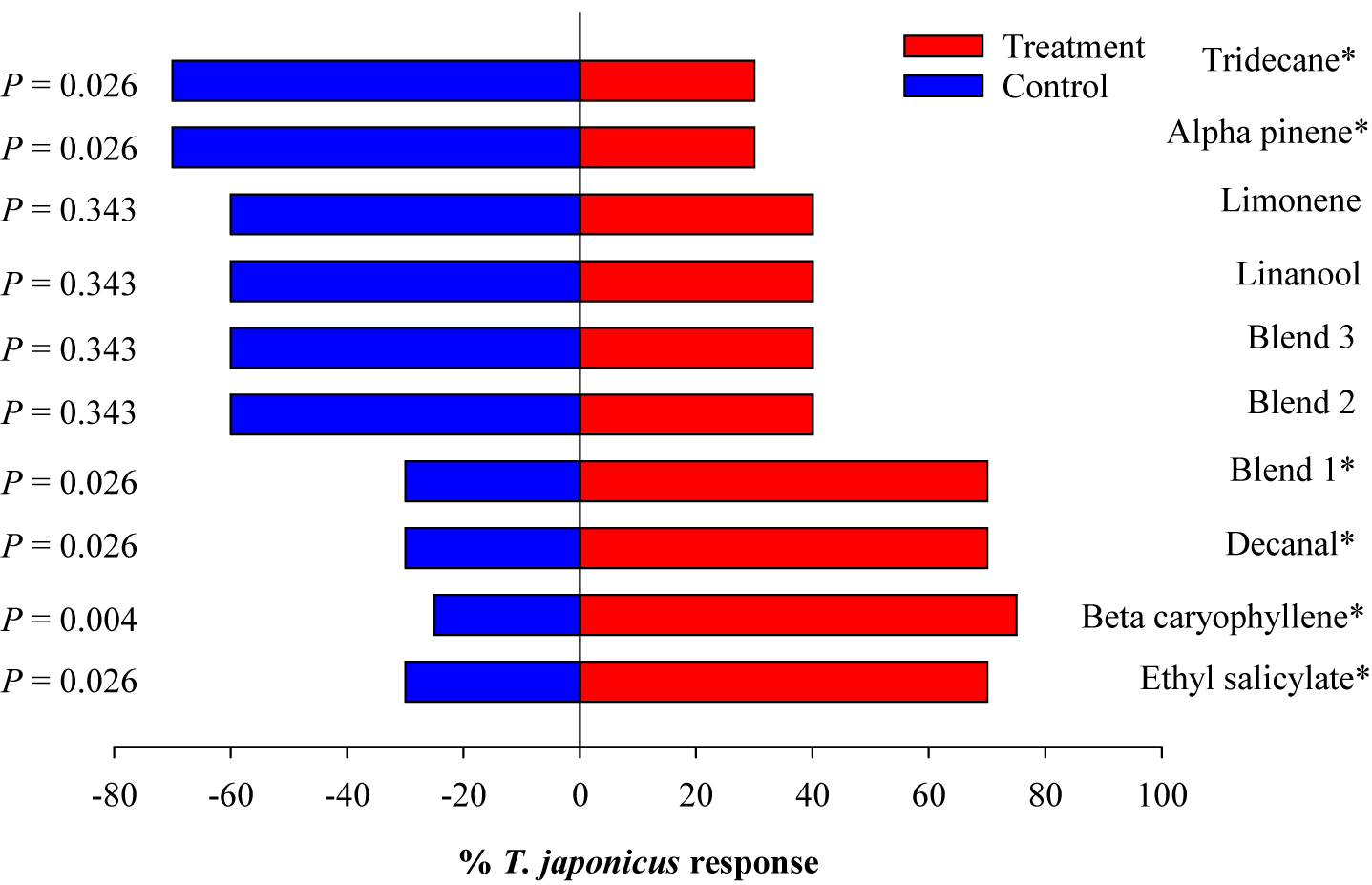
Figure 5. Responses of Trissolcus japonicus to synthetic compounds in Y-tube. Blend 1 (ES, BC, and decanal), blend 2 (TR, BC, lim, and Lin), and blend 3 (ES, TR, BC, lim, and Lin). Asterisks indicate significant difference between the treatments at p = 0.05 using likelihood ratio test.
Discussion
The primary aim of this study was to investigate the behavioral responses of H. halys nymphs and females of the H. halys parasitoid T. japonicus to HPVs, with the goal of identifying attractants for improving the monitoring of both species. The results showed that H. halys nymphs are attracted to volatiles from whole apple and peach fruit and to some synthetic VOCs and their blends. When offered matured Golden Delicious apple or matured Encore peach, nymphs showed attraction to volatiles emitted by both fruit compared to control (only air). However, when given a choice between these fruits, H. halys nymphs responded similarly to both odors. This is in broad agreement with host discrimination by insects using plant volatiles (Bruce et al., 2005; Webster et al., 2010; Bruce and Pickett, 2011; Pickett et al., 2012) and shows a clear preference of H. halys nymphs for fruit volatiles in the absence of other choices but a lack of discrimination when two attractive volatile blends are present simultaneously. Although H. halys survival on peaches is higher than on apples (Acebes-Doria et al., 2016), this may be due to host plant quality or nutrition during fruit development, whereas we evaluated mature fruits. As a generalist insect, H. halys specializes on maturing fruits in landscapes with highly heterogeneous and ephemeral resources (Martinson et al., 2015; Blaauw et al., 2019); thus, we expected mature fruits to be more attractive. Peach is a common early season host for H. halys (Leskey and Nielsen, 2018), and further studies including fruits across different phenological growth stages (i.e., early maturation to mature) are needed to correlate emissions of fruit volatiles with H. halys nymphal arrival to hosts. Although H. halys nymphs did not seem to discriminate between the odors of mature apple and peach VOCs, differences in the fruit quality, which is a combination of total number, identity, and concentration of VOCs emitted by different cultivars of apples (Dixon and Hewett, 2000) and peaches (Akotsen-Mensah et al., unpublished), could explain the observed differences in attractiveness between peach and apple varieties.
With respect to the synthetic VOCs, the results revealed that H. halys nymphs showed attraction to ES, UN, and EA. These compounds are commonly found in some host plants of H. halys, and thus, it is not surprising to observe this positive response of the nymphs toward them. The strongest attraction was found for ES, which is emitted in high amounts from several fruits including apples (e.g., var. “Granny Smith” and “Jonagold”), blueberries, plum, passion fruit, feijoa fruit, raspberry, tomato, among others (Knudsen et al., 1993), many of which are preferred host of H. halys. Also, EA is a volatile found in nature and emitted by many plants (Knudsen et al., 2006).
Although, H. halys did not respond to the majority of VOCs, individually and as blends, tested in this study, we cannot conclusively establish that they were not attractive for several reasons. First, it is possible that the dose and blend ratio (Bruce et al., 2005; Tasin et al., 2006a, b; Najar-Rodriguez et al., 2010; Bruce and Pickett, 2011) and release rates of some of the tested compounds and blends were not optimized to trigger a behavioral response. Another reason may be because most Pentatomid receptor neurons are not strongly tuned toward specific plant odor compounds (Dickens et al., 1993) and, thus, the variations in the response of H. halys to these compounds. The combined blends of ES and EA were not more attractive than the individual compounds, which suggests that there was no synergistic effect between ES and EA and other blends too. Morrison et al. (2018) tested blends of ubiquitous compounds found in apple and peach headspace volatiles and green leaf volatile compounds commonly produced by most plants, but found little evidence for synthetic compounds retaining H. halys in apple and peach fields. They also found no evidence for synthetic host plant blends synergizing with the H. halys aggregation pheromone. They, however, noted that the compounds used were not optimized for H. halys in either emission rate, concentration, or deployment strategy, which provides further opportunity to investigate the responses of H. halys to other potential HPVs.
For some of the VOCs tested, the nymphs selected the clean air. This indicates that some HPVs may repel H. halys and that no single compound may fully account for the response to fruit volatiles. Thus, host location in H. halys is likely not mediated by a single compound but instead by a combination of attractive (and possibly also repellent) compounds in the blend, which makes the polyphagous H. halys attracted to multiple hosts. This also means some of these VOCs could be potential repellents and could have a place in a push–pull strategy in managing H. halys.
Our results support our hypothesis that the coevolved parasitoid T. japonicus is attracted to similar VOCs as its primary host H. halys. The HPV ES elicited positive behavioral responses by both species. This response to the same VOCs across trophic levels has likely been selected to increase the likelihood of T. japonicus locating host resources (i.e., H. halys eggs) across a diverse landscape (Aldrich et al., 1995; Tillman et al., 2010). Further studies should evaluate if there is an increase in host finding (i.e., H. halys eggs) by T. japonicus in the presence of attractive VOCs as well as their potential use as a monitoring lure for both species.
In conclusion, we have demonstrated convergence across trophic levels by a polyphagous species and its coevolved natural enemy to similar HPVs. We found that H. halys nymphs are attracted to odors from peach and apple fruits and that one common host-associated ester, ES, was attractive to H. halys nymphs and T. japonicus females. While we evaluated nymphs, adult and nymphal stages of H. halys share host plants and are expected to respond to the same cues. Cues shared between H. halys and its parasitoid suggest that T. japonicus utilizes HPVs to locate egg masses for oviposition. Given that plants emit a complex mixture of VOCs, belonging to the same or to other chemical groups, other VOCs not tested here could also be involved. However, the VOCs tested in our study are all found in host plants of H. halys, and thus, its response to them is highly plausible. If ES and EA, and their blends, are further identified in the field to be attractive, then they can be used in conjunction with the aggregation pheromone to improve H. halys trapping.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
BB, CA-M, AN, and CR-S conceived the idea, planned the experiments, and analyzed and interpreted the data. CA-M performed both the greenhouse and Y-tube experiments. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the USDA NIFA-SCRI grant “Management of Brown Marmorated Stink Bug in US Specialty Crops” (no. 2016-51181-25409).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank and recognize the contributions from Ann Rucker, Meghin Rollins, and Nicolas Avila for their assistance in the laboratory and greenhouse studies.
References
Acebes-Doria, A. L., Leskey, T. C., and Bergh, J. C. (2016). Injury to apples and peaches at harvest from feeding by Halyomorpha halys (Stål) (Hemiptera: pentatomidae) nymphs early and late in the season. Crop Prot. 89, 58–65. doi: 10.1016/j.cropro.2016.06.022
Akotsen-Mensah, C., Kaser, J. M., Leskey, T. C., and Nielsen, A. L. (2018). Halyomorpha halys (Hemiptera: pentatomidae) responses to traps baited with pheromones in peach and apple orchards. J. Econ. Entomol. 111, 2153–2162. doi: 10.1093/jee/toy200
Aldrich, J. R., Rosi, M. C., and Bin, F. (1995). Behavioral correlates for minor volatile compounds from stink bugs (Heteroptera: pentatomidae). J. Chem. Ecol. 21, 1907–1920. doi: 10.1007/BF02033851
Blaauw, B. R., Hamilton, G., Rodriguez-Saona, C., and Nielsen, A. L. (2019). Plant Stimuli and Their Impact on Brown Marmorated Stink Bug Dispersal and Host Selection. Front. Ecol. Evol. 7:414. doi: 10.3389/fevo.2019.00414
Blackmer, J. L., Rodriguez-Saona, C., Byers, J. A., Shope, K. L., and Smith, J. P. (2004). Behavioral response of Lygus hesperus to conspecifics and headspace volatiles of alfalfa in a Y-tube olfactometer. J. Chem. Ecol. 30, 1547–1564. doi: 10.1023/B:JOEC.0000042067.27698.30
Boyle, S. M., Weber, D. C., Hough-Goldstein, J., and Hoelmer, K. A. (2020). Host kairomones influence searching behavior of Trissolcus japonicus (Hymenoptera: Scelionidae), a parasitoid of Halyomorpha halys (Heteroptera: Pentatomidae). Environ. Entomol. 49, 15–20. doi: 10.1093/ee/nvz155
Braasch, J. E., Wimp, G., and Kaplan, I. (2012). Testing for phytochemical synergism: arthropod community responses to induced plant volatile blends across crops. J. Chem. Ecol. 38, 1264–1275. doi: 10.1007/s10886-012-0202-y
Bruce, T. J. A., and Pickett, J. A. (2011). Perception of plant volatile blends by herbivorous insects – finding the right mix. Phytochemistry 72, 1605–1611. doi: 10.1016/j.phytochem.2011.04.011
Bruce, T. J. A., Wadhams, L. J., and Woodcock, C. M. (2005). Insect host location: a volatile situation. Trends Plant Sci. 10, 269–274. doi: 10.1016/j.tplants.2005.04.003
Casado, D., Gemeno, C., Avilla, J., and Riba, M. (2006). Day-night and phenological variation of apple tree volatiles and electroantennogram responses in Cydia pomonella (Lepidoptera: Tortricidae). Environ. Entomol. 35, 258–267. doi: 10.1603/0046-225X-35.2.258
Choh, Y., Ozawa, R., and Takabayashi, J. (2013). Do plants use airborne cues to recognize herbivores on their neighbours? Exp. Appl. Acarol. 59, 263–273. doi: 10.1007/s10493-012-9616-z
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., Bin, F. (2004). Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207, 47–53. doi: 10.1242/jeb.00732
Conchou, L., Anderson, P., and Birgersson, G. (2017). Host plant species differentiation in a polyphagous moth: olfaction is enough. J. Chem. Ecol. 43, 794–805. doi: 10.1007/s10886-017-0876-2
Conchou, L., Lucas, P., Meslin, C., Proffit, M., Staudt, M., and Renou, M. (2019). Insect odorscapes: from plant volatiles to natural olfactory scenes. Front. Physiol. 10:972. doi: 10.3389/fphys.2019.00972
Dickens, J. C., Visser, J. H., and Van Der Pers, J. N. C. (1993). Detection and deactivation of pheromone and plant odor components by the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: noctuidae). J. Insect Physiol. 39, 503–516. doi: 10.1016/0022-1910(93)90083-4
Dingha, B. N., and Jackai, L. E. N. (2017). Laboratory rearing of the brown marmorated stink bug (Hemiptera: pentatomidae) and the impact of single and combination of food substrates on development and survival. Can. Entomol. 149, 1–14. doi: 10.4039/tce.2016.39
Dixon, J., and Hewett, E. W. (2000). Factors affecting apple aroma/flavour volatile concentration: a review. New Zeal. J. Crop Hortic. Sci. 28, 155–173. doi: 10.1080/01140671.2000.9514136
Dudareva, N., Negre, F., Nagegowda, D. A., and Orlova, I. (2006). Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–470. doi: 10.1080/07352680600899973
Ehrlich, P. R., and Raven, P. H. (1964). Butterflies and Plants: a Study in Coevolution. Evolution (N. Y). 18, 586–608. doi: 10.2307/2406212
Harris, C., Abubeker, S., Yu, M., Leskey, T., and Zhang, A. (2015). Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha halys. PLoS One 10:e0140876. doi: 10.1371/journal.pone.0140876
Holopainen, J. K., and Blande, J. D. (2013). Where do herbivore-induced plant volatiles go? Front. Plant Sci. 4:185. doi: 10.3389/fpls.2013.00185
Horvat, R. J., and Chapman, G. W. (1990). Comparison of Volatile Compounds from Peach Fruit and Leaves (cv. Monroe) during Maturation. J. Agric. Food Chem. 38, 1442–1444. doi: 10.1021/jf00097a002
Horvat, R. J., Chapman, G. W., Robertson, J. A., Meredith, F. I., Scorza, R., Callahan, A. M., et al. (1990). Comparison of the Volatile Compounds from Several Commercial Peach Cultivars. J. Agric. Food Chem. 38, 234–237. doi: 10.1021/jf00091a051
Izumi, Y., Tian, R., Sonoda, S., Imayoshi, Y., Iwabuchi, H., Miyashita, Y., et al. (2015). Analysis of peach fruit headspace volatiles and response by the fruit-piercing moth Oraesia excavata (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 50, 231–238. doi: 10.1007/s13355-015-0330-2
Kaplan, I. (2012). Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire? Biol. Cont. 60, 77–89. doi: 10.1016/j.biocontrol.2011.10.017
Knight, A. L., and Light, D. M. (2001). Attractants from Bartlett pear for codling moth, Cydia pomonella (L.), larvae. Naturwissenschaften 88, 339–342. doi: 10.1007/s001140100244
Knolhoff, L. M., and Heckel, D. G. (2014). Behavioral Assays for Studies of Host Plant Choice and Adaptation in Herbivorous Insects. Annu. Rev. Entomol. 59, 263–278. doi: 10.1146/annurev-ento-011613-161945
Knudsen, J. T., Eriksson, R., Gershenzon, J., and Stahl, B. (2006). Diversity and distribution of floral scent. Bot. Rev. 72:1.
Knudsen, J. T., Tollsten, L., and Bergström, L. G. (1993). Floral scents – a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33, 253–280. doi: 10.1016/0031-9422(93)85502-I
Leskey, T. C., and Nielsen, A. L. (2018). Impact of the invasive brown marmorated stink bug in North America and europe: history, biology, ecology, and managemenT. Ann. Rev. Entomol. 63, 599–618. doi: 10.1146/annurev-ento-020117-043226
Leskey, T. C., Hamilton, G. C., Nielsen, A. L., Polk, D. F., Rodriguez-Saona, C., Christopher Bergh, J., et al. (2012). Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag. 23, 218–226. doi: 10.1564/23oct07
Light, D. M., and Knight, A. (2005). Specificity of codling moth (lepidoptera: tortricidae) for the host plant kairomone, ethyl (2E,4Z)-2,4-decadienoate: field bioassays with pome fruit volatiles, analogue, and isomeric compounds. J. Agric. Food Chem. 53, 4046–4053. doi: 10.1021/jf040431r
Martinson, H. M., Venugopal, P. D., Bergmann, E. J., Shrewsbury, P. M., and Raupp, M. J. (2015). Fruit availability influences the seasonal abundance of invasive stink bugs in ornamental tree nurseries. J. Pest Sci. 88, 461–468. doi: 10.1007/s10340-015-0677-8
Morrison, W. R., Allen, M., and Leskey, T. C. (2018). Behavioral response of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to host plant stimuli augmented with semiochemicals in the field. Agric. Forest Entomol. 20, 62–72. doi: 10.1111/afe.12229
Najar-Rodriguez, A. J., Galizia, C. G., Stierle, J., and Dorn, S. (2010). Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exper. Biol. 213, 3388–3397. doi: 10.1242/jeb.046284
Ngumbi, E., Chen, L., and Fadamiro, H. Y. (2009). Comparative GC-ead responses of a specialist (microplitis croceipes) and a generalist (cotesia marginiventris) parasitoid to cotton volatiles induced by two caterpillar species. J. Chem. Ecol. 35, 1009–1020. doi: 10.1007/s10886-009-9700-y
Nielsen, A. L., and Hamilton, G. C. (2009). Seasonal occurrence and impact of halyomorpha halys (Hemiptera: pentatomidae) in tree fruit. J. Econ. Entomol. 102, 1133–1140. doi: 10.1603/029.102.0335
Nixon, L. J., Morrison, W. R., Rice, K. B., Brockerhoff, E. G., Leskey, T. C., Guzman, F., et al. (2018). Identification of volatiles released by diapausing brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). PLoS One 13:e0191223. doi: 10.1371/journal.pone.0191223
Paré, P. W., and Tumlinson, J. H. (2002). Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 121, 325–332. doi: 10.1104/pp.121.2.325
Pashalidou, F. G., Gols, R., Berkhout, B. W., Weldegergis, B. T., van Loon, J. J. A., Dicke, M., et al. (2015). To be in time: egg deposition enhances plant-mediated detection of young caterpillars by parasitoids. Oecologia 177, 477–486. doi: 10.1007/s00442-014-3098-0
Pickett, J. A., Midega, C. A. O., Khan, Z. R., Woodcock, C. M., Smart, L. E., Birkett, M. A., et al. (2012). Aspects of insect chemical ecology: exploitation of reception and detection as tools for deception of pests and beneficial insects [electronic resource]. Physiol. Entomol. 37, 2–9.
Pophof, B., Stange, G., and Abrell, L. (2005). Volatile organic compounds as signals in a plant-herbivore system: electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 30, 51–68. doi: 10.1093/chemse/bji001
Reddy, G. V. P., and Guerrero, A. (2004). Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 9, 253–261. doi: 10.1016/j.tplants.2004.03.009
Rice, K. B., Bergh, C. J., Bergmann, E. J., Biddinger, D. J., Dieckhoff, C., Dively, G., et al. (2014). Biology, ecology, and management of brown marmorated stink bug (Hemiptera: pentatomidae). J. Integr. Pest Manag. 5, A1–A13. doi: 10.1603/IPM14002
Rodriguez-Saona, C., Blaauw, B. R., and Isaacs, R. (2012). “Manipulation of Natural Enemies in Agroecosystems: habitat and Semiochemicals for Sustainable Insect Pest Control,” in Integrated Pest Management and Pest Control - Current and Future Tactics, eds M. L. Larramendy and S. Soloneski (US: Intech).
Rodriguez-Saona, C., Urbaneja-Bernat, P., Salamanca, J., and Garzón-Tovar, V. (2020). Interactive effects of an herbivore-induced plant volatile and color on an insect community in cranberry. Insects 11:524. doi: 10.3390/insects11080524
Rout, P. K., Rao, Y. R., and Naik, S. (2012). Analysis of floral volatiles by using headspace-solid phase microextraction: a review. Asian J. Chem. 24, 945–956.
Sans, A., Morán, M., Riba, M., Guerrero, Á, Roig, J., and Gemeno, C. (2016). Plant volatiles challenge inhibition by structural analogs of the sex pheromone in Lobesia botrana (Lepidoptera: tortricidae). Eur. J. Entomol. 113, 579–586. doi: 10.14411/eje.2016.078
Szendrei, Z., and Rodriguez-Saona, C. (2010). A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 134, 201–210. doi: 10.1111/j.1570-7458.2009.00954.x
Talamas, E. J., Herlihy, M. V., Dieckhoff, C., Hoelmer, K. A., Buffington, M. L., Bon, M.-C., et al. (2015). Trissolcus japonicus (Ashmead) emerges in North America. J. Hymenop. Res. 43, 119–128. doi: 10.3897/JHR.43.4661
Tasin, M., Bäckman, A. C., Bengtsson, M., Ioriatti, C., and Witzgall, P. (2006a). Essential host plant cues in the grapevine moth. Naturwissenschaften 93, 141–144. doi: 10.1007/s00114-005-0077-7
Tasin, M., Bäckman, A. C., Bengtsson, M., Varela, N., Ioriatti, C., and Witzgall, P. (2006b). Wind tunnel attraction of grapevine moth females, Lobesia botrana, to natural and artificial grape odour. Chemoecology 16, 87–92. doi: 10.1007/s00049-005-0332-6
Tentelier, C., and Fauvergue, X. (2007). Herbivore-induced plant volatiles as cues for habitat assessment by a foraging parasitoid. J. Anim. Ecol. 76, 1–8. doi: 10.1111/j.1365-2656.2006.01171.x
Tillman, P. G., Aldrich, J. R., Khrimian, A., and Cottrell, T. E. (2010). Pheromone attraction and cross-attraction of nezara, acrosternum, and euschistus spp. Stink Bugs (Heteroptera: pentatomidae) in the field. Environ. Entomol. 39, 610–617. doi: 10.1603/EN09114
Turlings, T. C., and Tumlinson, J. H. (1992). Systemic release of chemical signals by herbivore-injured corn. Proc. Natl. Acad. Sci. U. S. A. 89, 8399–8402. doi: 10.1073/pnas.89.17.8399
Van Loon, J. J. A., De Boer, J. G., and Dicke, M. (2000). Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol. Exp. Appl. 97, 219–227. doi: 10.1023/A:1004032225239
Varela, N., Avilla, J., Anton, S., and Gemeno, C. (2011). Synergism of pheromone and host-plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 141, 114–122. doi: 10.1111/j.1570-7458.2011.01171.x
Webster, B., Bruce, T., Pickett, J., and Hardie, J. (2010). Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 79, 451–457. doi: 10.1016/j.anbehav.2009.11.028
Xu, H., and Turlings, T. C. J. (2018). Plant Volatiles as Mate-Finding Cues for Insects. Trends Plant Sci. 23, 100–111. doi: 10.1016/j.tplants.2017.11.004
Yang, Z., Bengtsson, M., and Witzgall, P. (2004). Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J. Chem. Ecol. 30, 619–629. doi: 10.1023/B:JOEC.0000018633.94002.af
Keywords: brown marmorated stink bug, Y-tube olfactometer, invasive species, host plant volatiles, tritrophic interaction
Citation: Akotsen-Mensah C, Blaauw BR, Rivera MJ, Rodriguez-Saona C and Nielsen AL (2021) Behavioral Response of Halyomorpha halys (Hemiptera: Pentatomidae) and Its Egg Parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to Host Plant Odors. Front. Ecol. Evol. 9:696814. doi: 10.3389/fevo.2021.696814
Received: 17 April 2021; Accepted: 28 July 2021;
Published: 08 September 2021.
Edited by:
Ahmed M. Saveer, North Carolina State University, United StatesReviewed by:
Ayako Wada-Katsumata, North Carolina State University, United StatesKevin Farnier, Agriculture Victoria Research, Australia
Copyright © 2021 Akotsen-Mensah, Blaauw, Rivera, Rodriguez-Saona and Nielsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clement Akotsen-Mensah, QWtvdHNlbi1tZW5zYWhjQGxpbmNvbG51LmVkdQ==
 Clement Akotsen-Mensah
Clement Akotsen-Mensah Brett R. Blaauw
Brett R. Blaauw Monique J. Rivera
Monique J. Rivera Cesar Rodriguez-Saona
Cesar Rodriguez-Saona Anne L. Nielsen
Anne L. Nielsen