
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Ecol. Evol., 30 July 2021
Sec. Conservation and Restoration Ecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.648495
This article is part of the Research TopicConservation of European Freshwater CrayfishView all 16 articles
Native European crayfish conservation was triggered by invasion of crayfish plague disease agent, Aphanomyces astaci, starting 1860s in Northern Italy. Resulting crayfish plague epidemics quickly spread over Continental Europe, then to Finland, Sweden and finally, after running amok around Europe, A. astaci was discovered also in Iberian Peninsula, Norway, Ireland, and United Kingdom in 1970s and 1980s. By that time significant proportion of native crayfish stocks had been lost, and while crayfish plague epidemics were still recorded, also industrialization and waterways construction were causing damage to remaining native crayfish stocks. While alien crayfish introductions, at least Faxonius limosus, already gave rise to first wave of crayfish plague epidemics in late 19th century, later in 1960s it was decided that introductions of alien Pacifastacus leniusculus should be initiated to replace native European crayfish populations. Decisions were based on presumed advantages for fishery, suitable habitat requirements and supposed immunity against A. astaci. Furthermore, conservation of native European crayfish species was sidelined and focus shifted toward alien crayfish stocking routine and consumption. Alien crayfish species introductions resulted in repeated waves of crayfish plague epidemics among remaining native crayfish stocks. It was soon discovered that alien crayfish of North American origin were, as suspected, permanent reservoirs for A. astaci, that some of those alien species were losing their resistance against selected strains of A. astaci and struggled in European aquatic ecosystems. In this article, we introduce numerous motives behind grand mistake of introducing alien crayfish species to Europe and then promoting their stocks instead of focusing on conservation of native crayfish species. We outline how false economical, biological and ecologic assumptions were used to justify a hasty introduction of alien crayfish, which has further devastated native crayfish and also permanently changed European aquatic ecosystems, both with disastrous consequences. Lesson to be learnt is that science-based warnings about alien species damage to native ecosystems and native crayfish must be taken with utmost caution. Protection of native European crayfish should be core issue, not commercial activities. Finally, we summarize main threats and actions needed to protect remaining native freshwater crayfish fauna in Europe.
Of all ecosystems in the world, freshwaters are among the most diverse and vulnerable, while constituting only 0.8% of the Earth’s surface (Strayer and Dudgeon, 2010). Inland waters exhibit a high degree of endemism and extinction rates (Dudgeon et al., 2006) due to their insular nature, frequently small size, and the limited dispersal ability of many freshwater species which commonly results in adaptation to narrow habitat conditions. These characteristics make freshwaters vulnerable to extensive and growing human pressures (Naiman and Turner, 2000; Jackson et al., 2001; Strayer and Dudgeon, 2010). In the past century, the rapid increase of human populations accompanied by economic development resulted in an equally rapid increase in the demand for freshwater provisioning services, such as water for consumptive use, irrigation, power generation or transport as indicated in the Millennium Ecosystem Assessment from 2005. Among many pressures to freshwaters and their rich biodiversity, land-use change, pollution, physical alteration and damming, water abstraction, climate change and introduction of alien species have caused the most severe degradations (Dudgeon et al., 2006; Domisch et al., 2011).
Freshwater biodiversity is significantly influenced by freshwater crayfish, which are keystone species and ecosystem engineers, important components of freshwater food webs, due their relatively large body size, long life span and omnivorous feeding habits (Holdich, 2002; Usio and Townsend, 2008; Weinländer and Füreder, 2016). Owing to these characteristics, crayfish can directly affect ecosystem processes, species abundance and diversity (Pyšek and Richardson, 2010). Thus, crayfish disappearance from an ecosystem can significantly alter freshwater ecosystem processes and services, species abundance and diversity, leading to changes in habitat structure and functioning as well as to watercourse succession and changes in the dynamics of sediment transport (Moorhouse and Macdonald, 2011). Since native freshwater crayfish have a key role in the ecosystem by ensuring its normal functioning, and frequently being economically important, they need to be conserved. In a modern approach to species conservation, apart from habitat conservation, the focus is also on the preservation of species genetic diversity. High genetic diversity enables survival of species through time due to higher adaptive potential and fast evolutionary response to environmental changes (Bickford et al., 2007; Eizaguirre and Baltazar-Soares, 2014).
On the other hand, crayfish are among the most widely translocated aquatic invertebrates, introduced both within and between continents mainly through extensive harvesting for food, aquaculture and aquarium trade (Kouba et al., 2014; Loureiro, 2020) (Table 1). Once brought into a new habitat some alien crayfish species frequently become established and invasive. Invasive crayfish species are characterized by advantageous life history traits such as fast growth rate, high fecundity and early maturation (Souty-Grosset et al., 2006), which contribute to their invasive success. Their aggressiveness, e.g., Pacifastacus leniusculus (Dana, 1852) (Söderbäck, 1991), Procambarus clarkii (Girard, 1852) (Souty-Grosset et al., 2006; Arce and Dieguez-Uribeondo, 2015), enable them to exclude native species in competition for space and food sources (e.g., Söderbäck, 1994a, 1995; Hudina et al., 2014; Pacioglu et al., 2020). Additionally, they may outcompete native species by reproductive interference (Söderbäck, 1994b). Further, their tolerance to a broad range of conditions including pollution or organic enrichment, e.g., Faxonius limosus (Rafinesque, 1817) (Souty-Grosset et al., 2006), and transmission of diseases such as crayfish plague, caused by pathogen Aphanomyces astaci Schikora (Persson and Söderhäll, 1983; Vey et al., 1983; Diéguez-Uribeondo and Söderhäll, 1993), enhances their potential to drastically affect native crayfish populations. Thus, invasive species are recognized as the second most important factor affecting biodiversity loss worldwide (Lodge et al., 2000; Fahrig, 2003) and determining the factors of their invasive success is a key issue in invasive species management (Capinha et al., 2013) in order to conserve native crayfish populations as well as local biodiversity and ecosystem functioning.
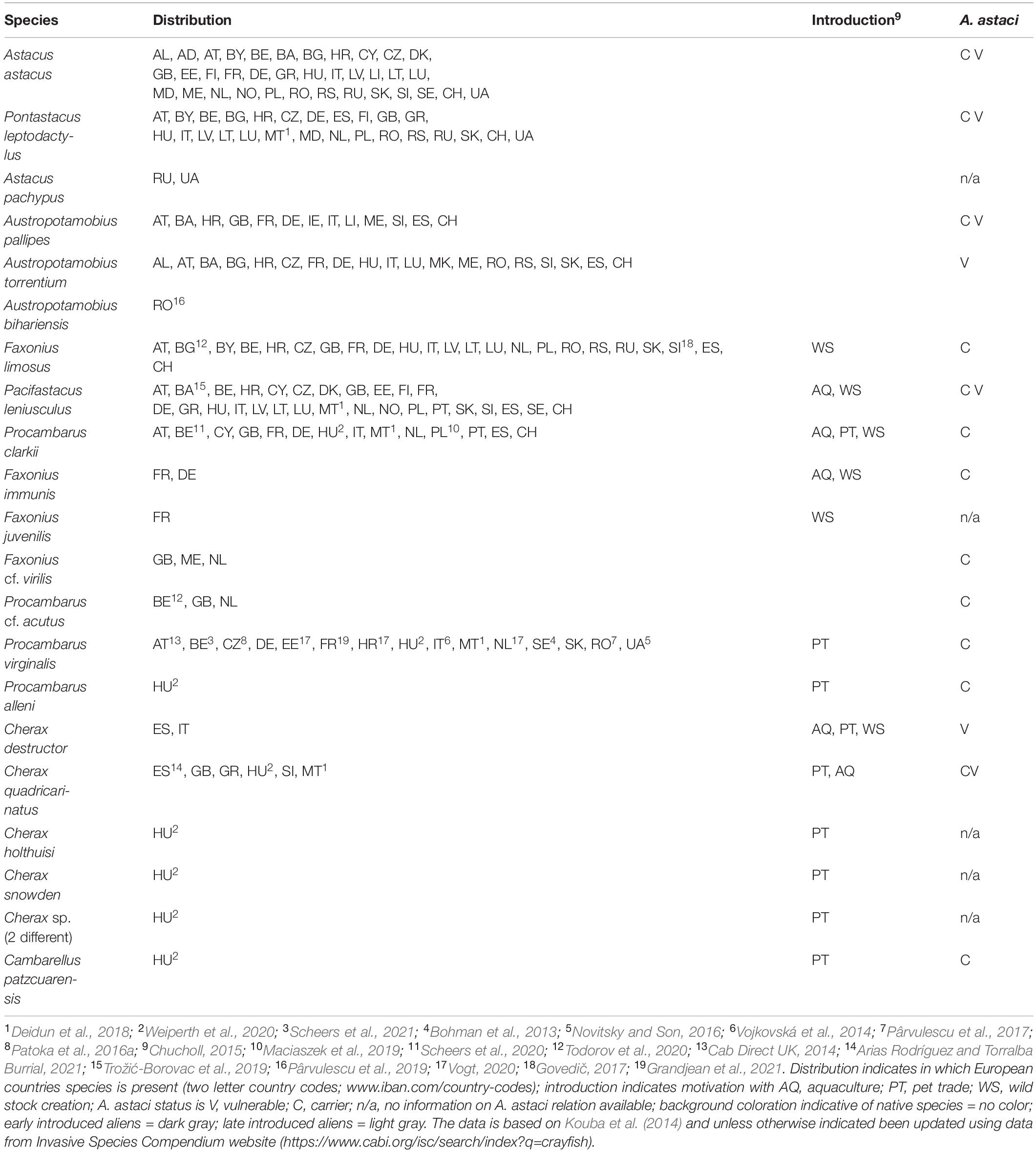
Table 1. Native and alien crayfish in Europe: distribution, introduction motivation and A. astaci relationship. Distribution indicates in which European countries species is present (two letter country codes; www.iban.com/country-codes); introduction indicates motivation with AQ, aquaculture; PT, pet trade; WS, wild stock creation; A. astaci status is V, vulnerable; C, carrier; n/a, no information on A. astaci relation available; background coloration indicative of native species = no color; early introduced aliens = dark gray; late introduced aliens = light gray. The data is based on Kouba et al. (2014) and unless otherwise indicated been updated using data from Invasive Species Compendium website (https://www.cabi.org/isc/search/index?q=crayfish).
Commercial activity seems to have had a key role in the introduction of alien crayfish and their associated diseases to Europe, and thus to the devastation of the native European crayfish. Crayfish have been traded since the discovery of their worth as food item, either for nutritional purposes as a valuable source of proteins in remote and sometimes poor parts of Europe (Holdich, 2002; Maguire and Gottstein-Matočec, 2004; Gherardi, 2011) and other regions (Andriantsoa et al., 2019), or as a focal point of cultural events (Jussila, 1995; Edsman, 2004; Alonso et al., 2000). They have even been used as food during fasting within religious communities, in order to bypass regulations forbidding the consumption of animals during fasting (e.g., Swahn, 2004; Ackefors, 2005; Patoka et al., 2016b) and also as a source of additional income for people catching and marketing them to bourgeois and religious communities (e.g., Lehtonen, 1975; Bohman et al., 2006; Gherardi, 2011). Trading frequently included crayfish transport over long distances (Anon, 1899; Edsman and Schröder, 2009; Jussila et al., 2013b) resulting in a mix of the natural genetic composition of native species (e.g., Edsman et al., 2002; Makkonen et al., 2015). Moreover, by trading live alien crayfish species within Europe, the spreading of the crayfish plague pathogen A. astaci was facilitated around the continent, as crayfish were often placed in water bodies along the way to markets (e.g., Alderman, 1996).
After struggling for around 100 years with A. astaci epidemics, some countries of Northern and Southern Europe, losing more native crayfish stocks and failing with previous introductions of F. limosus and Faxonius virilis (Hagen, 1870) decided, largely for commercial purposes, that mass introduction of other alien crayfish should start from North America, such as P. leniusculus in Sweden 1960s (Svärdson, 1965, 1995; Abrahamsson, 1973), P. leniusculus in Finland 1960s (e.g., Westman, 1973), F. limosus and P. leniusculus in Austria 1960s and 1970s (Spitzy, 1973) and P. leniusculus and P. clarkii in Spain 1970s (e.g., Diéguez-Uribeondo et al., 1997a). Previous attempts to also introduce other North American species had happened, but not on such a massive scale (Alonso et al., 2000; Souty-Grosset et al., 2006). As mentioned above, the introduction of the alien P. leniusculus, together with organized hatchery and stockling production, resulted amongst other matters in further waves of A. astaci epidemics among native European crayfish stocks. The commercial value of the native crayfish such as Astacus astacus (Linnaeus, 1758), Pontastacus leptodactylus (Eschscholtz, 1823) and Austropotamobius pallipes (Lereboullet, 1858), has partially contributed to protecting their wild stocks via a possibility for their profitable exploitation. When facing extinction or severe decline in Continental Europe and Fennoscandia (A. astacus), the Mediterranean countries (A. pallipes), Eastern Europe and Turkey (P. leptodactylus), their commercial value could be seen as directed against these European native crayfish. This resulted in the potential income from crayfish being used several times, one could say every time, when introductions of alien crayfish were discussed and justified (e.g., Westman, 1973; Söderbäck and Edsman, 1998; Alonso et al., 2000; Sahlin et al., 2010).
Monetary benefits have played a crucial role in the alien crayfish introductions, as there have also been at least temporary economic gains from alien species through aquaculture (e.g., Bohman et al., 2006) and exploiting wild stocks (Jussila and Mannonen, 2004; Bohman et al., 2006). In Fennoscandia, the economic benefits may have initially been obvious (e.g., Kirjavainen and Sipponen, 2004), Spain appears to be the same (e.g., Gutiérrez-Yurrita et al., 2017), while in Central Europe and Balkans the economics gains have been considerably smaller (e.g., Maguire and Gottstein-Matočec, 2004; Souty-Grosset et al., 2006). Even when discussing the initial cumulative benefits, the long term direct and indirect economic gains have so far been negligible or even negative when the whole aquatic ecosystem and society is taken into account (e.g., Gren et al., 2009). In Spain, the Scientific Committee of Spanish Ministry has finally declared that alien P. leniusculus and alien P. clarkii are not naturalized but invasive and detrimental to local aquatic ecosystems (Díaz, 2021). The entire ecosystem should always be considered when alien species benefits are discussed, even though short-term thinking favors money and ignores intangible benefits.
One crucial issue in the native crayfish conservation has been, and also will be in the future, several contrasting interests promoting either native crayfish conservation or alien species introductions. This issue has been discussed in detail by Biasetti et al. (2021), highlighting the complex network of intangible ideas and values relevant to different interest groups with also strong economic interest involved. Conservation obstinacy has been mentioned as an example of wasting resources to fight lost causes, as conservation outcome could, in some cases, be hard or even impossible to predict (Lehmann et al., 2002; Gontier et al., 2006) while we argue that lost causes can only be species extinctions. Animal welfare issues can also be relevant, since native species might have to be raised under artificial conditions and alien species eradication could be cruel (e.g., Cowan and Warburton, 2011). Finally, social factors of conservation are important and actions would require community acceptance and support with intensive awareness raising campaigns taking place. Ecosystem health or biodiversity as such are complex entities and adding diverse individual attitudes and expectations to considerations when native species conservation acts are planned, the result can easily be counterproductive for native species. The conservation of native crayfish should thus be seen as an attempt to conserve native ecosystem health, as the native crayfish are true keystone species and ecosystem managers in the most positive sense of the phrase.
We have used cases from Fennoscandian policies and practices as an overwhelming example of official management strategies, based often on economical justifications rather than ecological, to highlight the detrimental effects of alien species to native species and ecosystems. In addition, P. leniusculus alone is the most widespread alien crayfish species in Europe and is present in at least 28 countries (Kouba et al., 2014; Table 1). The magnitude of alien crayfish promotion by governmental institutions has been of such fundamental proportions in Fennoscandia, that several cases have been discussed from a Fennoscandian view point, however, with cases from Continental Europe being introduced. The regions might differ, the species might differ, but the outcome seems to be repeated. Money talks and native European crayfish walks, this time, out. This review on policy and practice is about the dramatic chain of events affecting the fate of European freshwater crayfish.
Over the past 150 years, freshwater crayfish in Europe have faced a severe challenge caused by the pathogen A. astaci, probably introduced with alien crayfish species of North American origin, with mass mortalities first reported by Cornalia (1860). Today, the European native crayfish populations are in decline nearing extinction both regionally and species-wise (e.g., Souty-Grosset et al., 2006). Due to its devastating effects on the native crayfish populations of Europe, A. astaci is today considered among the world’s 100 worst invasive species (Lowe et al., 2004). A. astaci belongs to the class of Oomycetes a diverse group of fungus-like organisms, including not only a wide variety of plant and animal pathogens, but also saprophytic species (Diéguez-Uribeondo et al., 2009). A. astaci itself is originally a very specific parasite (Unestam, 1969a; Unestam, 1972; Diéguez-Uribeondo et al., 2009) of freshwater crayfish species from North America that have developed some tolerance and resistance whilst also alternative hosts have been reported and speculated (e.g., Unestam and Weiss, 1970; Svoboda et al., 2014; Martín-Torrijos et al., 2021b) (Table 2). However, in the European crayfish, the parasite causes a lethal disease known as crayfish plague. The pathogen spreads from host to host by producing free-swimming zoospores; should a suitable host be found, the zoospores then encyst, germinate, and start to grow hyphae into the host tissues, typically resulting in death of the host (Söderhäll and Cerenius, 1999; Cerenius et al., 2009; Rezinciuc et al., 2016). By contrast, in A. astaci infected North American crayfish species, there is a continual but low level of sporulation (Strand et al., 2012; Svoboda et al., 2013).
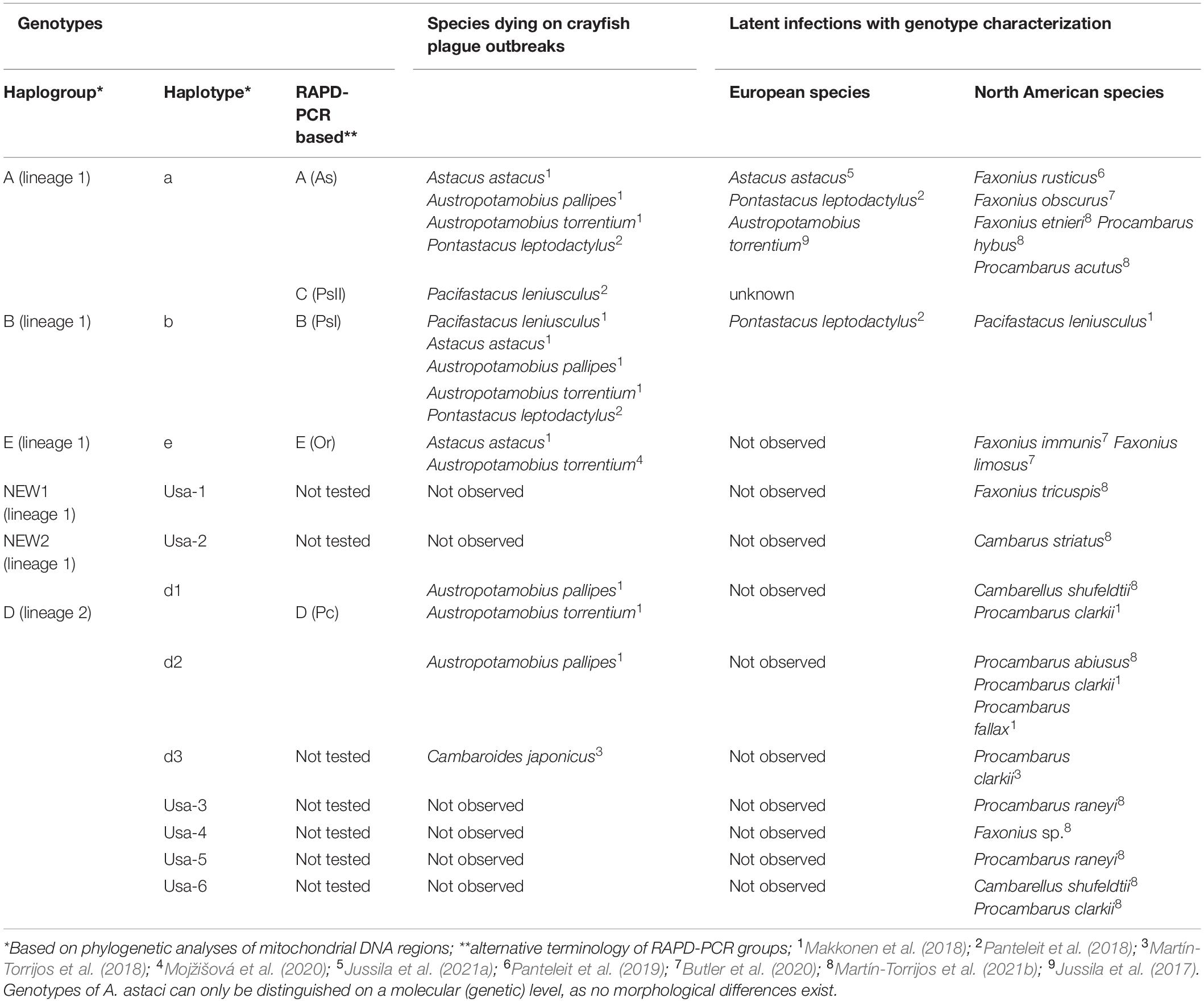
Table 2. Aphanomyces astaci haplogroups (genotypes) and crayfish carrying these haplotypes as latent infections. Genotypes of A. astaci can only be distinguished on a molecular (genetic) level, as no morphological differences exist.
The presence of North American crayfish, and thus most likely the pathogen A. astaci, has caused high mortalities and numerous population collapses among all native European crayfish species (e.g., Souty-Grosset et al., 2006). The invasive North American crayfish species appears to tolerate and resist the crayfish plague infection across their original distribution in North America (e.g., Martín-Torrijos et al., 2021b). This seems to be the result of a balanced relationship arising from coevolution (Unestam, 1969b; Cerenius et al., 2009). Thus, the pathogen was usually unable to freely infect its North American host because their immune system can encase the pathogen within the cuticle (Söderhäll and Cerenius, 1999; Cerenius et al., 2009). However, susceptible crayfish and A. astaci seemed to have created a novel and complex relationship, with evidence for a rapid co-evolution of native crayfish and A. astaci (Jussila et al., 2015, 2021a). Moreover, the alien crayfish in their newly invaded biogeographic regions in Europe show increased susceptibility with even population collapses reported (e.g., Jussila et al., 2014a; Sandström et al., 2014; Thomas et al., 2020). As a result, among crayfish stocks in Europe, the resistance of both native European and alien crayfish against the crayfish plague has changed, as has the virulence of the disease agent A. astaci (e.g., Jussila et al., 2015).
Currently, four distinct haplogroups (i.e., group of strains evolutionarily related as judged by mitochondrial concatenated sequences, sensu Makkonen et al., 2018) of A. astaci are known to infect native and alien crayfish in Europe and Asia (Table 2), named as A, B, D, and E haplogroups (e.g., Kozubíková et al., 2011; Kozubíková-Balcarová et al., 2013; Makkonen et al., 2018; Martín-Torrijos et al., 2018), with haplogroup D consisting of d1, d2, and d3 haplotypes (Makkonen et al., 2018; Martín-Torrijos et al., 2018). These haplogroups and haplotypes can only be distinguished on a genetic level, as no morphological differences exist. Recent studies on A. astaci in the south-eastern United States indicate that this region seems to be a center of diversity of the pathogen. In this region, 19 additional North American crayfish species were found to carry A. astaci and six new haplotypes of the pathogen were identified (Martín-Torrijos et al., 2021b). Laboratory infection trials have shown extensive variation in the virulence among different strains of some haplogroups (e.g., Makkonen et al., 2012, 2014; Francesconi et al., 2021). In general, strains of haplogroups B, D and E seem to possess higher virulence (e.g., Makkonen et al., 2012; Jussila et al., 2013a; Francesconi et al., 2021) than those of haplogroup A, which, on the other hand, appear to be more variable in their virulence (Makkonen et al., 2012, 2014). Furthermore, latent crayfish plague infections with A. astaci from the haplogroup A, and in some cases also haplogroup B, without mass mortalities have been reported in the native European A. astacus (Jussila et al., 2011; Viljamaa-Dirks et al., 2011; Maguire et al., 2016), P. leptodactylus (Kokko et al., 2012; Svoboda et al., 2012; Ungureanu et al., 2020), A. torrentium (Van Paula Schrank, 1803) (Kušar et al., 2013; Maguire et al., 2016; Jussila et al., 2017) and A. pallipes (Manfrin and Pretto, 2014; Maguire et al., 2016) (Tables 1, 2).
Aphanomyces astaci has been under high selective pressure to adapt to the European crayfish hosts and its new environmental conditions since its original introduction to Europe 150 years ago (Jussila et al., 2015, 2016a). After its presumed arrival in Europe in the 1850s (Alderman, 1996), it had access to a variety of host habitats across the European native crayfish spectrum (Souty-Grosset et al., 2006) (Table 1). All European crayfish species were susceptible to A. astaci of haplogroup A, the first intruder, and the outcome of the crayfish plague epidemic during the first decades was massive mortality among European crayfish populations. If it had not had assistance from humans, the disease might have had a short history in Europe. But the spread of the disease agent was unintentionally aided by transferring it to new water bodies and populations through commercial marketing chains and through natural water ways also along with alien invasive crayfish species (Alderman, 1996). The rapid and efficient spread allowed for both the constant presence of epidemics and opportunities for A. astaci to jump from one European crayfish species host to the next and, apparently, to also jump back and forward among crayfish species in close proximity (e.g., Diéguez-Uribeondo, 2006; Jussila et al., 2015; James et al., 2017).
It has long been presumed that A. astaci strains of haplogroup A may have been naturally selected by lowered virulence toward a balanced host-pathogen relationship, which has been demonstrated experimentally only during the last decade (e.g., Makkonen et al., 2014; Jussila et al., 2014b; Francesconi et al., 2021). Some wild native European and Turkish crayfish stocks, which are viable and producing commercial catches, have been shown to be latent carriers of A. astaci (e.g., Svoboda et al., 2012; Kokko et al., 2018; Jussila et al., 2021a). Laboratory scale infection studies have revealed significant virulence differences among and within A. astaci haplogroups and even the existence of very low virulent strains (e.g., Makkonen et al., 2012; Jussila et al., 2017; Francesconi et al., 2021). The invasive crayfish, especially P. leniusculus, have lately been shown to be susceptible to A. astaci, which points to the high virulence of the infecting A. astaci haplogroup B and possibly a lowered resistance of P. leniusculus toward A. astaci (Aydin et al., 2014; Jussila et al., 2014a; Thomas et al., 2020). Laboratory experiments have demonstrated that the A. astaci of haplogroup B, although highly virulent, is also capable of exhibiting a significant but narrow range of virulence variation (Jussila et al., 2013a). This indicates that even A. astaci of haplogroup B could be adapting in Europe (Ungureanu et al., 2020), while the presence of a permanent host habitat for the A. astaci of haplogroup B allows for the maintenance of high virulence without the immediate threat of the parasite’s evolutionary suicide due to the outbreak of a devastating crayfish plague epidemic (Jussila et al., 2015). A similar trend has been observed in A. astaci of haplogroup D currently infecting some Procambarus species (e.g., Martín-Torrijos et al., 2017; Makkonen et al., 2018). Furthermore, some strains of this haplogroup have shown adaptation to warmer temperatures than other strains from other haplogroups (Diéguez-Uribeondo et al., 1995; Rezinciuc et al., 2014) and tolerance to brackish waters (Martín-Torrijos et al., 2021b). These evolutionary adaptations might be regional between specific populations of crayfish and A. astaci, as a consequence of host-parasite co-evolution (e.g., Svoboda et al., 2012; Makkonen et al., 2014; Jussila et al., 2017). It has recently been shown that the A. astaci strain carried by Faxonius rusticus (Girard, 1852) is genetically different from all other A. astaci strains described to date (Panteleit et al., 2019), leading the authors to hypothesize that each North American crayfish species might carry its own A. astaci haplogroup, or haplotype, to which it evolved resistance against. In other words, there might be as many strains of A. astaci as there are different species of crayfish in North America; over 300 (Mathews et al., 2008). If the mode of action and virulence of A. astaci depends on the different host species or populations, this could have a high impact on the invasion success of the invasive species. However, recent studies in North America by Martín-Torrijos et al. (2021b) do not seem to support this hypothesis since no clear species-specific or distributional patterns of the haplotypes and crayfish species were found. Further investigations are needed to enhance our understanding on the phylogeography of A. astaci.
The introduction of different A. astaci haplogroups into Europe and the repeated introductions of its chronically infected hosts are a classic example of a man-made ecological disaster, stemming from the naive belief that the manipulation of an ecosystem would be straightforward. Currently, the native European crayfish species are on the brink of extinction (Richman et al., 2015). A. astaci itself has apparently adapted rather well to European conditions (Jussila et al., 2016a), and seems to be currently co-evolving while maintaining contact with its relatively resistant hosts as new crayfish stocks were imported from North American into Europe (e.g., Jussila et al., 2016a). One could predict that this will inevitably lead to possible total eradication of the remaining native European crayfish stocks. The original introduction of A. astaci to Europe, though it was most probably purely accidental, has not only seriously devastated native crayfish populations throughout Europe, but also resulted in further damage due to misguided management attempts such as further introductions of alien crayfish species from North America to rectify the situation (Souty-Grosset et al., 2006; Jussila et al., 2015, 2016a).
The high financial and cultural values of the freshwater crayfish in Europe, namely A. pallipes, A. astacus, and P. leptodactylus, and the devastation of the native crayfish stocks during 19th and 20th century (Alderman, 1996; Alonso et al., 2000; Jussila et al., 2016a) encouraged fisheries officers and researchers in several European countries to grasp the opportunity to introduce alien freshwater crayfish into Europe (Lodge et al., 2000; Holdich et al., 2009). During the first wave of the crayfish plague epidemics in Europe from the 1860s onward, the cause of mass mortalities among European crayfish populations was not known to the scientific community, not to mention administrators or the common public. It took until the 1930s to discover that A. astaci was the organism causing crayfish plague epidemics (Schikora, 1903, 1906; Schäperclaus, 1935; Nybelin, 1936), while it took much longer to fully understand how to halt its spread. Within 50 years from arrival, the crayfish plague epidemic’s first wave had permanently changed European aquatic ecosystems by almost wiping out native crayfish from Continental Europe (Jussila et al., 2015) and collapsing native crayfish stocks in Fennoscandia (Jussila and Mannonen, 2004; Bohman et al., 2006; Jussila et al., 2015). Within one century, crayfish plague epidemics were even reported from the Iberian Peninsula to the United Kingdom and Ireland, which were all but the last safe havens for the native European crayfish (Reynolds, 1988; Holdich and Reeve, 1991; Diéguez-Uribeondo et al., 1997a, respectively). Even though in some parts of south-eastern Europe alien crayfish were introduced quite early, e.g., in Greece (Perdikaris et al., 2017), other parts of south-eastern Europe resisted alien crayfish introductions for a long time compared to the rest of Europe (Holdich, 2002). The first record of F. limosus in freshwaters of Croatia was in 2003 (Maguire and Gottstein-Matočec, 2004), while P. leniusculus was first recorded in 2008 (Maguire et al., 2008). In both cases, alien crayfish species spread naturally through big rivers (Danube and Mura, respectively) from neighboring countries (Hungary and Slovenia, respectively) and continued their expansion toward east (i.e., F. limosus to Serbia and Romania) causing irreversible negative impact onto the native astacofauna in the region, i.e., in the south-eastern Europe. It was only in 2011 that P. leniusculus was illegally introduced to the Korana River (Hudina et al., 2013) situated in the continental part of Croatia in the karstic region that is known as a hotspot of the A. torrentium and A. astacus diversity (Klobučar et al., 2013; Lovrenčić et al., 2020; Gross et al., 2021), inflicting great damage to the populations of these vulnerable species distributed in this area.
A common mistake made whenever a large-scale epidemic is happening is the open spreading of the disease agent and organisms that are carrying the disease (Alonso et al., 2000; Bohman et al., 2006; Diéguez-Uribeondo, 2006). In most cases, it can be claimed that due to the lack of prior knowledge, it was not possible to apply strategies or tactics that would have prevented the disease spreading and resulting wide epidemic. When the pandemic started in the late 19th century, the cause of the mass mortalities among native European crayfish populations was not known, while several alternative theories were discussed (Alderman, 1996). Some of those were based on the actual disease agent being responsible, but even then there did not seem to be rational strategies implemented to save the valuable natural resource of European freshwater crayfish (e.g., Fiskeriverket, 1993; Alderman, 1996; Jussila et al., 2016a). The Europeans were caught by surprise regarding A. astaci, and proper means of attempting to stop the spreading of A. astaci happened only after the whole Continental Europe and large parts Fennoscandia were hit by crayfish plague epidemics.
Repeated introductions of the disease agent carriers, i.e., alien crayfish of North American origin, but also pathogens spreading through natural waterways, made matters even more complicated, as eradication of the permanent disease carriers became impossible and prevention of spreading very hard (e.g., Alonso et al., 2000; Pârvulescu et al., 2012; Peay et al., 2019). Europeans have imported alien crayfish for various reasons from North America since the late 19th century (Henttonen and Huner, 1999; Alonso et al., 2000; Souty-Grosset et al., 2006), but the prime mistake was made during the mid-20th century when decisions to start mass introductions of alien P. leniusculus to fill the now mostly empty freshwater courses in Europe were made. The possibility of introducing novel diseases, e.g., Psorospermium haeckeli, Saprolegnia spp. (Diéguez-Uribeondo and Söderhäll, 1993; Diéguez-Uribeondo et al., 1994), even the possibility of introducing novel strains of A. astaci, were played down (e.g., Westman, 1973), while the Swedes had their concerns (e.g., Svärdson, 1995). The decision to introduce crayfish from the region where the crayfish plague disease originated, the disease that had already eradicated most European crayfish stocks, was made (e.g., Kilpinen, 2003). Several North America species have been introduced, but the main focus was on P. leniusculus (Abrahamsson, 1973; Souty-Grosset et al., 2006; Holdich et al., 2009). The deliberate large-scale introduction of P. leniusculus in Sweden starting in the 1960s resulted in a fivefold increase in the spread of crayfish plague epidemics in the country (Bohman et al., 2006; Bohman, 2020).
It is difficult to appreciate how this decision was made and justified from an ecologically perspective, mainly because it was obvious that the introduction of P. leniusculus would result in further spreading of A. astaci in Europe (e.g., Kilpinen, 2003). Field introductions during the period of 1960–1967, thus before the massive importation of P. leniusculus to Fennoscandia, indicated that P. leniusculus was a carrier of A. astaci and thus eradicated coexisting A. astacus populations (Unestam, 1969b). The import license application of P. leniusculus to Finland was first rejected in 1967 by the veterinary administration due to risks of introducing diseases, namely crayfish plague (Kilpinen, 2003). Later the same year, the veterinary administration was bypassed and an import license granted using political maneuvers and ignoring the risks. Unestam (1969a) already discussed P. leniusculus resistance against A. astaci, clearly indicating that the possibility of P. leniusculus acting as a permanent reservoir existed, yet permits were given to stock 58,100 adult P. leniusculus from Lake Tahoe, California, into 64 natural waters in Sweden in 1969 (Svärdson, 1968; Abrahamsson, 1973). Also, alien P. leniusculus was introduced as resistant to A. astaci, even immune, while the obvious outcome from this resistance, i.e., P. leniusculus as a spreading vector for A. astaci, was largely ignored.
Soon after the introduction of P. leniusculus to Fennoscandia, it was discovered that even the most sophisticated stockling production systems might produce P. leniusculus that were carriers of A. astaci (e.g., Makkonen et al., 2010), while some P. leniusculus stocks remained in disease-free status for a while once established from farm raised stocklings. This did not halt the stocking of the disease carrying P. leniusculus into numerous water bodies, even though there were strict national regulations banning introductions of diseased organisms into the wild (e.g., Fiskeriverket, 1993; Ruokonen et al., 2018; Jussila and Edsman, 2020): a case of fisheries administration favoring the distribution of an alien species even if it was suspected or known to carry and spread A. astaci (e.g., Kilpinen, 2003). The relaxed attitude among fisheries administrators, even an attitude that favors alien species over native ones (e.g., Ruokonen et al., 2018; Jussila and Edsman, 2020), is bound to cause devastation of the native crayfish, even when it is already claimed to be vulnerable and threatened, as has been the case in Sweden (Bohman and Edsman, 2011).
Initial optimistic assumptions, based sometimes on biased analyses and even wishful thinking, can be hard to correct when new scientific information surfaces. It was discovered some 20 years after the initiation of major alien crayfish stocking campaigns that the P. leniusculus wild stocks were declining, even collapsing in some cases (Jussila et al., 2014a, 2016b; Sandström et al., 2014). Sometimes this information was ignored and those managing the wild P. leniusculus stocks were kept in the dark, which was evident during meetings with stake holders in Finland and Sweden. The initial inflated information regarding the resistance of P. leniusculus against A. astaci infection was not corrected when the first stock collapses were observed and reported (e.g., Jussila et al., 2014a; Sandström et al., 2014) and local fisheries managers were wondering how the collapses were even possible. The original assumption of disease resistance was used to justify further spreading of the alien crayfish, even after it was discovered that it was spreading very virulent A. astaci of haplogroup B and it was quite obvious that it too was suffering from the infection itself (e.g., Aydin et al., 2014; Jussila et al., 2014a).
It is common to deprecate novel findings indicating that an alien harmful species could have developed new diseases under the new environmental conditions. In Fennoscandia, it was discovered some 20 years after the intensive P. leniusculus stocking program that established P. leniusculus stocks were showing gross symptoms that were then studied and described as eroded swimmeret syndrome, i.e., ESS (Sandström et al., 2014; Edsman et al., 2015). The suspected disease causes total or partial erosion of the female swimmerets and thus prevents the female from hatching eggs, resulting in reproductive failures. Later it was been discovered that male P. leniusculus also show similar gross symptoms including gonopod trauma (Jussila et al., 2016b, 2021b). It took a while before the existence of ESS was admitted to affect populations of P. leniusculus in Finland. Even then, the response was based on undermining the possible population level effects of ESS. This was motivated by trying to maintain the suspected good reputation of P. leniusculus in Fennoscandia (e.g., Jussila and Edsman, 2020). Despite struggling in some parts of Europe, alien P. leniusculus is still the second worst alien crayfish in Europe and still spreading (Table 1).
Short-term monetary benefits are sometimes regarded as more valuable than long-term ecological sustainability, and thus the promotion of the alien species could be justified by economic reasons. The designated area for the introductions of the alien P. leniusculus in Sweden was originally limited to the south-eastern part of the country (Bohman et al., 2006). The designated area in Finland was originally only the great lakes in southern Finland, too (Ruokonen et al., 2018). Due to intensive promotion of P. leniusculus as a commercially lucrative species and initial good development of the introduced stocks (e.g., Ackefors, 1999; Kirjavainen and Sipponen, 2004), the illegal introductions of P. leniusculus northwards were commonplace (Bohman et al., 2006; Bohman and Edsman, 2011; Ruokonen et al., 2018). In Finland, fisheries administration, instead of taking a firm stand against illegal introductions, drafted several crayfishery strategies, which all included the regions of illegal introductions within the newly designated region for P. leniusculus (Ruokonen et al., 2018). This only encouraged the spread of the alien crayfish and A. astaci it is carrying, resulting in further devastation of the remaining native A. astacus stocks.
Emphasizing the alien species’ economic benefits could sideline conservation attempts of the native species. The start of the crayfish season is one of the widely publicized events in Fennoscandian late summer (Taugbøl et al., 2004; Jussila et al., 2015; Jussila and Edsman, 2020). The premium price for the crayfish is paid during the first few days of the crayfishing season due to high demand, while the prices stay considerably high throughout the season (Jussila, 1995). To boost the start of the crayfish season trade, the ministry in charge of fisheries arranged an importation of alien P. leniusculus from England to Finland, an exception to the EU Alien Species Regulation 1143/2014, using an economic justification. This is again an example of trying to boost the economic reputation of the alien species and thus undermine the fundamentals of the EU Alien Species Regulation 1143/2014.
The strategy for alien species eradication can be deliberately and erroneously implemented to actually give an upper hand to the alien harmful species over the native species. Eradication of the harmful alien P. leniusculus is one of the EU Alien Species Regulation No 1143/2014 aims and there have been national strategies drafted and also implemented to test different strategies and techniques (Edsman and Schröder, 2009; Bohman and Edsman, 2013; Huusela-Veistola et al., 2019). In Finland, the National Research Institute for Natural Resources, LUKE, has been asked by Ministry of Agriculture and Forestry to assess the possible gains on relaxing P. leniusculus trapping regulations, such as open or early season, no bag limits, no specific trapping licenses, encouragement to removal trapping, etc. (Anon, 2019). The official justification is that the relaxed trapping regulations would encourage recreational trappers to increase their trapping pressure, which would then result in halting the spread, or even cause eradication, of the wild P. leniusculus stocks. While in theory this might seem achievable, the reality is different. Anyone understanding motivational aspects of the crayfish trapping would claim that recreational trappers would stop trapping when the catch per unit effort (CPUE) falls below a certain limit, for example 0.5, which is well above any rational CPUE that would result in eradication of the population. The planned relaxation of P. leniusculus trapping regulations would make the alien harmful P. leniusculus a more tempting target than A. astacus for a recreational crayfish trapper as the alien species’ stocks would then be easier to access. This would only encourage the general public to spread the alien harmful species even more and it would also give a hidden message of the alien harmful species actually being more desirable than the native species. At four international freshwater crayfish scientific conferences, conclusions and resolutions have been issued: IAA17 in Kuopio, Finland (2008), Crayfish conservation meeting in Olot, Spain (2015), IAA21 in Madrid, Spain (2016) and IAA Gotland, Sweden (2019). At all four meetings it was clearly stated that “the control of invasive crayfish species by intensive recreational and commercial fisheries does not represent a feasible method for this purpose. Instead, it favors the further spread and increase of these alien populations” (Furse, 2008; Edsman et al., 2019).
The ornamental aquatic pet trade is another important but frequently overlooked pathway for the introduction of alien crayfish species and their diseases into Europe (Hänfling et al., 2011; Chucholl and Wendler, 2017). While there are numerous parasitic organisms and viral diseases in crustaceans worldwide (Bojko et al., 2020), some of which might become a serious threat for crayfish in the future, the main disease threatening crayfish in Europe to date is the crayfish plague. Crayfish imported from North America to be sold as pets or kept in private aquaria are often vectors of the crayfish plague pathogen A. astaci. In a study by Mrugała et al. (2014) six crayfish species were identified as vectors for the first time, with horizontal transmission of A. astaci, i.e., the transmission of the pathogen between crayfish individuals kept in close proximity. These results were confirmed in a study by Panteleit et al. (2017), where a further nine crayfish species were identified as vectors for A. astaci for the first time. One of the most problematic crayfish in the pet trade is probably the marbled crayfish, P. virginalis Lyko, 2017, due to its parthenogenetic reproduction and its high popularity as a pet (Patoka et al., 2014). It probably evolved from Procambarus fallax Hagen, 1870, an American species native to Florida and South Georgia, after triploidization in the German pet trade in the mid 1990s (Vogt et al., 2018). The species was first recorded in the wild in Germany in 2003 (Marten et al., 2004) and has since established numerous populations in at least 17 countries worldwide, mainly in Europe1 (Vogt, 2020; Scheers et al., 2021) (Table 1). However, European mean water temperatures are widely below the optimum for reproduction of P. virginalis (Seitz et al., 2005). Consequently it has been suggested that when new P. virginalis populations become established, it is to some extent due to the invasive potential of this species, but the location of the occurrences is rather dependent on human-mediated releases (Martin et al., 2010). The pet trade presumably led to the introduction of P. virginalis into Sweden, Romania, Ukraine, and many other countries (Marten et al., 2004; Chucholl and Pfeiffer, 2010; Bohman et al., 2013; Novitsky and Son, 2016; Weiperth et al., 2020), and the number of different alien crayfish species could be expected to increase as the pet trade through different channels, e.g., on-line trade, will develop (Kotovska et al., 2016; Vodovsky et al., 2017; Weiperth et al., 2020).
Some countries in Europe have stricter rules for pet trade. Examples are Ireland and Scotland, where keeping alien crayfish is illegal and the crayfish pet trade is strictly regulated (Peay, 2009), yet P. virginalis is still for sale on the pet market in Ireland (Faulkes, 2015a). Another example is Sweden where all importation, transport and keeping of any live crayfish species from abroad is banned (Edsman, 2004). Laws and regulations can only be effective if they are also enforced (Faulkes, 2015b). When this is not possible, other methods (e.g., education of pet traders and pet owners) to reduce the negative effects of the pet trade need to be implemented. Recognizing threats that alien species and pet trade pose to native European biodiversity, the EU recently adopted regulations dealing with alien invasive species in Europe, including crayfish (EU Pet Trade Regulation No 2016/1141). However, the list of species of Union concern includes only five alien crayfish species which already have established viable populations in Europe, namely F. limosus, F. virilis, P. leniusculus, and P. virginalis. This list does not include species that are imported through international pet trade, but are not yet invasive or established in the European aquatic ecosystems. It is very important, that species which are known to have a high invasive potential or species which are known carriers of A. astaci, are added to the invasive species list or, alternatively, to prepare a white list of crayfish that would not present concern to European freshwaters and native astacofauna, supported by scientific evidence.
One common way to cause confusion among the general public and thus also those pondering conservation issues, is a deliberate erroneous usage of alien species arguments when discussing native species (e.g., Courtine and Willett, 1986; Clavero et al., 2016). The concept of an alien species, in the case of Finland, is that the species has spread to Finland later than 1850 and the spreading has been assisted by man (Niemivuo-Lahti, 2012). If arrived later than 1850 by natural spreading to Finland, the species is considered a newcomer, but not an alien species. In the EU Alien Species Regulation No 1143/2014, for matters to be simpler and easier to define, whole nations or geographical regions, such as peninsulas, are considered one entity with regard to alien species spreading. Thus, A. astacus in Finland, even though originally considered as a southern species in its spreading (Lehtonen, 1975), is regarded a native species in all regions within Finland. Regardless, to improve the status of alien P. leniusculus and to weaken the status of native A. astacus, there have been claims that native A. astacus is actually an alien species above Jyväskylä (latitude 62°14′), according to information regarding its distribution during early days (e.g., Lehtonen, 1975). In the same way, claims have been made that A. astacus was introduced into Sweden during the 1500s by the kings. Later genetic studies have shown that A. astacus has inhabited Swedish aquatic ecosystems since the last ice age, thus being native to Sweden (Edsman et al., 2002; Gross et al., 2013; Dannewitz et al., 2021). Similarly, A. pallipes has been claimed to have been introduced to Spain from Italy only in the 1500s (Clavero et al., 2016), despite genetic evidence dating the species origin on the Iberian Peninsula from the last glaciation (Matallanas et al., 2011, 2016; Jelić et al., 2016; Martín-Torrijos et al., 2021a). Such scenarios, of course, create confusion and employ everyone to waste resources in order to correct the deliberate misinterpretation in any given case.
Another twisted argument in favor of alien invasive species is the claim that introduced North American crayfish species P. leniusculus, P. clarkii, or F. limosus are immune against A. astaci infection, a definition that is commonly used when justifying the introduction and spreading for example alien P. leniusculus in Europe (e.g., Bohman et al., 2006; Jussila et al., 2015). However, it has been shown directly in laboratory tests (Unestam and Weiss, 1970; Persson and Söderhäll, 1983; Vey et al., 1983) and indirectly from wild stock observations, that these alien species, particularly P. leniusculus, can be quite often susceptible to A. astaci infection and stock collapses due the crayfish plague epidemics have been reported (Jussila et al., 2014a; Sandström et al., 2014; Martín-Torrijos et al., 2019; Thomas et al., 2020). This, again, is one example on how decision makers aim to justify their considerations of the first alien species introductions, and how difficult it is to get the novel message of state of the art facts recognized, even though being supported by reliable data. The debates regarding alien P. leniusculus spreading A. astaci and thus devastating native crayfish populations, quite often bring up claims that not all alien signal stocks or individuals are chronically infected with A. astaci, which also neglects the long term competitive displacement of native species by alien P. leniusculus. This argument of exceptions to the rule, i.e., alien P. leniusculus being most often infected with A. astaci, should bring up the magnitude of the risks when attempting to spread alien P. leniusculus, but in most cases a principle of cautious approach is ignored, leading to actions detrimental to native crayfish. The possibility of a favored outcome, a false positive expectation, seems to be a strong motivator sidelining serious ecological considerations and cautious approach.
Alien species eradication is a very complex task, especially for aquatic species, and thus bound to cause problematic situations. Risks are often unknown both because little data is available on the magnitude of the introduction of alien species and also uncertainty about positive and negative effects of potential measures and actions to be undertaken. Under severe uncertainty about knowledge and value ambiguity in management objectives, the best initial step would be to perform a robust decision analyses (Sahlin et al., 2021).
The eradication of P. leniusculus from limited water bodies, such as golf courses or irrigation ponds, has been tried and shown to be successful (Peay et al., 2006; Sandodden, 2019). Successful attempts have also been reported for the eradication of P. clarkii and Australian Cherax destructor (Clark, 1936) in Spain (Alcorlo and Diéguez Uribeondo, 2014). Biocides, even though discussed controversially, have been used efficiently in the United Kingdom, Sweden, Norway, and Spain to tackle alien crayfish introductions (Peay et al., 2006, 2019; our personal observation). In the Italian project RARITY different approaches (e.g., removal by trapping, pheromones, sterilization of males) have been applied simultaneously, resulting in temporary significant reduction in the P. clarkii population size in Italy (RARITY, 2020). Other methods that were applied with more or less success include electrofishing, manual removal of crayfish and introduction of predators or specific diseases into the system, building physical barriers, water body drainage and shock liming (Gherardi et al., 2011; Stebbing et al., 2014; Bohman and Edsman, 2013). A recent study (Krieg et al., 2020) showed that implementation of different drastic measures, e.g., drainage of water body in combination with chemicals or barriers, could reduce alien crayfish population size or even eradicate a whole population. On the other hand, controlling invasive crayfish in big rivers (e.g., Danube and Drava) is almost impossible, and mechanical removal from the water body could only slow down their dispersal (Hudina et al., 2017; Krieg and Zenker, 2020). Still, achievable strategies for alien crayfish management in such systems include a combination of methods that would increase ecosystem resilience and continuous crayfish trapping (both fishermen and authorities) as well as involvement of well-informed citizens, as shown in the study of Lemmers et al. (2021). Also, the application of biological and ecological data on invasive crayfish to develop new tailored approaches to the management of specific invasive populations may improve invasive crayfish control (Hudina et al., 2016).
When eradication attempts are planned in habitats common especially in Fennoscandia, but also elsewhere in Europe, where lakes are interconnected by rivers to form watercourses stretching hundreds of kilometers, it is soon realized that effective eradication is impossible, or the vanishing of the alien harmful P. leniusculus could be an indication of drastic changes in the aquatic ecosystem. In this case, the alien species could be of least concern. Even though roughly 10% of Finnish surface area is freshwater (MMM, 2020), there have been plans for eradication or limiting the spreading of the harmful alien species (Erkamo et al., 2019). However, once P. leniusculus is released into the aquatic ecosystem there are very limited possibilities for its practical and effective eradication. In addition to the large size of the watercourses in Finland, most of them are shallow, lacelike structures, allowing basically their whole benthic area for crayfish settling.
The release of diseases targeting alien species has been widely suggested and even used in some cases (e.g., McColl et al., 2018; Wells et al., 2018). They have been shown to function as planned initially in some cases (e.g., Saunders et al., 2010), while some have faced problems right from the start (e.g., Holden, 1995). Pathogens are known to have evolved into diseases targeting different species, as is the case with A. astaci (e.g., Jussila et al., 2015; Simmonds et al., 2019). In Finland, the authority responsible of veterinary issues was planning to eradicate a sparse A. astacus population, a protected species, suspected to be carriers of A. astaci, by using another virulent haplogroup B strain of A. astaci. Once the suspected remaining A. astacus would have been eradicated, it would have allowed reintroduction of healthy stock into those water bodies. The scheme was introduced in a research proposal and later discussed in a conference in Olot (Girona, Spain, 2015), with a lively interaction between audience and presenter. Luckily, the project was not funded. The idea of fixing an obvious mistake, in this case the introduction of A. astaci to Europe several times, by making another, known to be a potential mistake from experience and reports, is a strange human urge.
One of the main driving forces behind the spreading of the alien crayfish species and devastation of the native European species has been ongoing exploitation of those alien species stocks that have been illegally introduced to regions which have specifically been allocated for the native species. This has been a common phenomenon in Finland, Sweden, and Spain (Alonso et al., 2000; Sahlin et al., 2017; Ruokonen et al., 2018). Thus, even though there have been attempts to halt the spreading of the alien crayfish species, the actions of the fisheries administrations in all three countries have actually encouraged the spreading of the alien crayfish (e.g., Alonso et al., 2000; Bohman et al., 2006; Ruokonen et al., 2018), despite national laws banning these introductions for various reasons, mostly motivated by conservation (e.g., Edsman and Schröder, 2009; Caffrey et al., 2014; Erkamo et al., 2019) and legislation (e.g., Jussila and Edsman, 2020). In Finland, a partial motivation must have been the alien P. leniusculus population crashes (e.g., Jussila et al., 2014a), resulting in the urge to push up the alien P. leniusculus catch figures, all in promotion of the alien P. leniusculus and at the cost of native A. astacus. It should clearly be mentioned here that although population density can be thinned and their spreading slowed down through intense trapping (e.g., Hein et al., 2007), there are no examples of successful eradication of crayfish populations by strong trapping pressure. Intensive fishing with baited traps as well as hand searching and removal is highly unsuccessful since only a minimal fraction of the total population is removed (Chadwick et al., 2020; Krieg et al., 2020). On the contrary, intensive trapping as a control measure may rather be a potential damaging activity by limiting cannibalistic predation pressure on the remaining population (Houghton et al., 2017), increasing fitness in remaining individuals (Moorhouse and Macdonald, 2011), inducing early onset of sexual maturity (Holdich et al., 2014), increasing intentional anthropogenic spread (Edsman, 2004) and by increasing bycatch of non-target species (De Palma-Dow et al., 2020).
The promotion of the alien P. leniusculus in Finland has been taken to the level of selecting a Crayfish King annually, namely a person who has done the most to promote alien P. leniusculus in Pirkanmaa county in southern Finland and thus causing the most damage to native A. astacus in that region, the latter normally not mentioned in this context. The nomination gets wide media coverage, not least because quite often the award is handed over by a minister in charge of fisheries and thus also crayfisheries. This minister should also be in charge of protecting native aquatic resources, such as fish and crayfish, but quite ironically does not see any conflict here.
Our attempts to solve ecological problems tend to be based on short-term thinking. While timeframes of decades or even centuries are required to remedy some matters, the lack of immediate personal or corporate benefit (e.g., Wu et al., 2017) and higher levels of uncertainty are seen as difficult to justify. Looking for the quick fix might be practical when trying to show benefits to the general public, but from the natural ecosystem’s viewpoint this time frame is negligible. The idea to compensate for the declining native natural resources by introducing alien species while there are still native specimens left is bizarre. On the other hand, it is quite understandable that the time scale of human thinking is rather short and quite often does not stretch over generations, not to mention over decades or millennia. From nature’s viewpoint, thousand years is not a long time frame and is in many cases not even long enough for any kind of drastic evolutionary changes. Animal species are normally spreading with variable pace (e.g., Messager and Olden, 2018; Melotto et al., 2020), while introductions of alien crayfish species have happened via quick and violent moments, in the false belief that human actions do not result in negative changes within ecosystems.
How does the obvious human selfishness affect decision making? Are we bound to only look for solutions which allow us to reap the glory for ourselves? Are the decisions actually based on selfish gains (like, e.g., political votes) instead of trying to actually solve the problems in the long run and maintain rational balance for the foreseeable future? Quite often the best solution would have been to do nothing and let solutions be based on natural progression of matters, even though, in the case of European crayfish, that would have taken a very long time for them to possibly bounce back. At least in Finland and Spain, there are now obvious indications that the native crayfish could be recovering in some water bodies previously considered void of native species (personal observation from Finland and Spain; Diéguez-Uribeondo et al., 1997a; Jussila et al., 2016b; Martín-Torrijos et al., 2017). This often happened in Sweden before the introduction of P. leniusculus, and later more frequently, as a result of liming in acidified waters (Bohman, 2020). It is also rather common that after alien P. leniusculus introductions, there is a short period when also A. astacus turns up in trap catches (e.g., Jussila et al., 2016b; Bohman, 2020), even for several years (e.g., Westman and Savolainen, 2001; Westman et al., 2002), while even in these reported Finnish cases of co-existence A. astacus have since disappeared (Erkamo, 2020). Thus, A. astacus has been in these water bodies, though at such low densities that trapping them has not been worth the effort. However, A. astacus must have been waiting for the moment to bounce back and take a stronger position in the aquatic ecosystem. After the hasty introduction of the alien P. leniusculus, A. astacus faces little or no chance to recover in the long run. It would have been wiser to wait.
Predicting the outcome of an alien species introduction is quite often made too soon, before it has taken its niche properly, leading to false promotion of the alien species role in the aquatic ecosystem (e.g., Kirjavainen and Westman, 1999; Kirjavainen and Sipponen, 2004; Jussila et al., 2016b). In Fennoscandia and Spain, the promotion of the alien P. leniusculus (and also P. clarkii in Spain) was based on the period when it was only settling down to aquatic ecosystem and was not properly established yet: populations were growing, there was a lot of free habitat, plenty of resources and as a result stress levels were low. This resulted in rapid spreading of these alien crayfish and the virulent A. astaci strains they have been carrying (Diéguez-Uribeondo et al., 1997b; Bohman et al., 2006; Ruokonen et al., 2018; Martín-Torrijos et al., 2019). In Fennoscandia and Spain, only 20 years after introduction, the alien P. leniusculus stocks were showing signs of maladaptation (Jussila et al., 2014a, 2016b; Sandström et al., 2014; Larumbe, 2020) and warning signs of not being quite suitable to conditions in their novel habitat. From the native European crayfish perspective this was too late, while this still was only early stages when the spreading of the alien P. leniusculus is considered. It is hard to change the positive message later, even though it is obvious that alien P. leniusculus stocks are not performing as originally told, especially since the alien P. leniusculus promoters do not change their story but largely ignore the bad news.
The urge to plan and introduce alien species to new regions has long been a temptation. The fundamental question is why alien species introductions are more tempting than conservation of native species. The already known to be flawed justifications are repeated in order to hide the quite obvious indications of introduction failures and severe negative impact on native species (e.g., Lodge et al., 2000; Westman, 2002; Jussila and Edsman, 2020). In Finland, the catch of the P. leniusculus was predicted to double every year due to an increasing number of introduced alien P. leniusculus populations being established and starting to produce commercial size crayfish after the early 1990s (e.g., Kirjavainen and Sipponen, 2004; Jussila and Edsman, 2020). This prediction was made early into the introduction scheme, mainly to encourage those managing wild crayfish stocks to introduce alien P. leniusculus instead of native A. astacus. An annual doubling of catches would have easily resulted in some 30,000,000,000 alien P. leniusculus been caught by 2010, which is not exactly what happened, since annual P. leniusculus catch briefly peaked at 7 million in mid 2010s and then leveled at around 3 million (Erkamo, 2019). Instead, alien P. leniusculus was discovered to be suffering similar population collapses during 1990s and 2000s as A. astacus in the past (Jussila et al., 2014a; Sandström et al., 2014) and being affected by A. astaci and novel diseases (Jussila et al., 2013b; Edsman et al., 2015). Both consequences were largely ignored and vigorously debated against in public. It took the government research institute LUKE until the mid-2010s to admit that their statistics showed the introductions of the alien P. leniusculus actually having only a small, even negligible, impact on the total catch of crayfish in Finland (Erkamo, 2019).
One of the unexpected dangers A. astacus is facing is possible restrictions on trapping wild A. astacus stocks, as has been suggested in Sweden, due to a fundamentalist view on conservation practices (Edsman, 2020a). Crayfish have been traditionally trapped because of their market value, the beach price for A. astacus, a minimum 10 cm long, being between one and two euro each (Jussila, 1995; Jussila and Mannonen, 2004). The income for a crayfish trapper could easily be several thousand euros during the crayfish season, which amongst other matters is a valuable lesson to a young crayfish trapper of the value of the natural resource (Jussila, 1995). If trapping of the A. astacus is restricted, one can always illegally introduce P. leniusculus in the water body, because trapping of P. leniusculus will not be restricted in the foreseeable future and it would thus enable trapping incomes. Even though the beach price of P. leniusculus is less than half compared to A. astacus, this scheme would work against conservation of the native A. astacus. Sometimes it would definitely be worth catching and eating a few endangered crayfish, for the benefit of the rest of the population. Even without the economic argument a carefully managed fishery by many local fishing right owners will be favorable for conservation by increasing the will for local people to protect the native crayfish (Edsman and Śmietana, 2004).
The fast buck ideology might have something to do with the alien species introduction and bluntly ignoring the necessity to conserve native species. It might be easy to predict a bright future in the case of unknown factors affecting the outcome of the alien species introduction. The North American crayfish species considered for introduction to Europe were thriving in their original distribution area despite A. astaci being present there in its most virulent haplotypes (e.g., Makkonen et al., 2019). It must have been tempting to claim that these species would be doing similarly in Europe, despite the fact that only the general climate features would be the same, while there are differences between North American and European aquatic ecosystems in terms of potential pathogens and parasites (e.g., Martiny et al., 2006; Litchman, 2010). Most of the aquatic and geological features would be different from, for example, conditions in Lake Tahoe, which was one of the main sources of P. leniusculus being introduced to Europe (Westman, 1973; Henttonen and Huner, 1999). Ignoring the fact that Lake Tahoe is very deep and rather constant in water temperature compared to rather shallow and low volume water courses in Europe is a grave mistake, which was verified by a Swedish research group (e.g., Sandström et al., 2014), showing that one of the main variables explaining alien P. leniusculus population crashes was warmer water temperature. In this case, climate change would make matters even worse for alien P. leniusculus, while it might not help native A. astacus either (e.g., Capinha et al., 2013; Préau et al., 2020).
One cannot ignore recent political changes across Europe and at the national level, with the more populist and nationalistic tendencies gaining support (Aalberg et al., 2016; Scoones et al., 2018; Borras, 2020). Quite often these populist movements tend toward conservative and rightwing policies, which tend to ignore the importance of conservation values (Cortes-Vazquez, 2020). There has been an increase in ideologies and movements characterizing nature conservation and an ecologically sound lifestyle as being detrimental to the well-being of individuals, and even a threat to the western life style as such (Apostolopoulou and Adams, 2015). In this political atmosphere, short-term economic benefits tend to gain the upper hand and indirect or intangible long-term benefits, such as ecosystems with biodiversity and strength, are not considered valid priorities (e.g., McCarthy, 2019). These political tendencies, of course, threaten the existence of vulnerable native species and whole native ecosystems, including native European crayfish struggling with detrimental diseases, such as A. astaci infections, and pollutants from industrial activities. The well-being of society, in this context, does not include the well-being of natural, native resources (Cortes-Vazquez, 2020) but rather short-term economic benefits.
Lively and productive native ecosystems, as they can be taken when considering native European crayfish stocks in their prime, offer both intangible benefits in the form of recreation and economic benefits in the form of trapping income and sales of trapping related gear and licenses (Jussila, 1995; Jussila and Mannonen, 2004; Bohman and Edsman, 2011). In Fennoscandia, productive native crayfish stocks have been used in the tourism industry as sites for trapping crayfish and then having crayfish parties on the lakesides (e.g., Jussila et al., 2016a), as have lately also the alien P. leniusculus stocks been utilized. In the context of conservation, the general public could be educated during the recreational trapping and the following crayfish parties on the importance of native crayfish stocks from both recreational and economic viewpoints. As most, if not all, of the native A. astacus populations are not open access, the hospitality enterprises are important in terms of widening possibilities for positive experiences been offered by the productive native crayfish stocks.
We would like to summarize the threats and necessary actions to ensure the maximum conservation outcome for the native European crayfish, with several aspects presented in the Table 3 and outlined in the following paragraphs (cf. Caffrey et al., 2014).
First of all, if ever again attempting to introduce alien species to substitute declining native species, one should be aware that such actions will cause more damage than benefit to the ecosystem or society (Kouba et al., 2021). In Sweden, from a purely national economic perspective, disregarding the disastrous effects on biodiversity, the massive introduction of P. leniusculus resulted in a cost rather than a benefit in the end (Gren et al., 2009). The loss of local native species populations, especially if it is limited to a certain region as opposed to a species extinction, even though a serious issue as such, is not a reason to correct the mistake by making another one. The spreading of alien crayfish in Europe is a classic and sad example of how matters can easily be made worse for the native species by introducing alien species to compensate local or regional losses of native species stocks (Gherardi and Holdich, 1999). The causes for their decline have multiple origins, and some of those must be corrected, such as pollution of water, acid rain and waterways construction (Edsman and Schröder, 2009), which the EU has taken a firm stand against (e.g., Paloniitty, 2016). Then, when habitats and environment have been restored to an ecologically maintainable level, one has to wait and see how ecosystem resilience will do its job. Sometimes less is more. If alien species have been introduced, on purpose or by accident, conclusions regarding the establishment and success of these populations should be reached only after a considerable time period, in the case of P. leniusculus in Finland more than 20 years after its introductions. In most cases this is too late and the progress of matters cannot be reversed. Maybe being more cautious and suspicious in the first place and eschewing the introduction of alien crayfish into Europe would have been best.
Alien crayfish should at least be restricted to limited designated regions, as is the general aim of the all European national crayfisheries strategies, and those alien crayfish populations which have been stocked, without permission and in most cases illegally, should be banned from all exploitation (e.g., Edsman et al., 2019). It thus would be strictly pointless to spread alien crayfish, which so far has been common practice and partially encouraged by the fisheries administration at least in Spain, Finland and Sweden (e.g., Alonso et al., 2000; Ruokonen et al., 2018; Jussila and Edsman, 2020). If broadly adopted, by banning introductions and trapping of the illegally established populations of alien crayfish at least some social pressure would be created, potentially resulting in hesitation when planning the next illegal introduction. The role of information campaigns should be more focused in this context, as it has been clear in the past that one of the main reasons for the irresponsible spreading of alien crayfish species has been messages which do not clearly state the risks related to alien species. One of the main reasons for this misleading information has been the reluctance of those in charge to admit that expectations have not been met and thus the instructions should be revised in order to avoid repeating mistakes.
While competitive exclusion by alien over native crayfish is a major risk to the long-term persistence of native crayfish in Europe, the hitchhiking disease pathogen that comes along with the alien crayfish possesses a much more immediate threat (e.g., Unestam, 1969a; Jussila et al., 2014b). Now that various A. astaci strains from different genotypes are present in various European water bodies (Table 2), its spread not only via alien crayfish, but also via fish (Oidtmann et al., 2002) or other species transporting A. astaci zoospores, i.e., birds and mammals preying on crayfish (Anastácio et al., 2014) appears inexorable, and we might have to start to think about how to make co-existence of the pathogen and native crayfish possible. It has been suggested that the main mechanism underlying the increased resistance of the North American crayfish species against the crayfish plague is the constant overexpression of prophenoloxidase-related genes, inhibiting pathogen growth and hence infection development. In European crayfish species, the enzymatic activation of the prophenoloxidase-cascade is often too inefficient and slow to successfully combat the disease (Cerenius et al., 2003). Therefore, in European native crayfish the infection leads to death usually within a few days or weeks, depending on the pathogen strain and virulence (Makkonen et al., 2014; Becking et al., 2015). However, recent reports indicate that European native crayfish wild populations exposed to A. astaci of haplogroup A, in some cases even haplogroup B and D, can sometimes resist the deadly acute crayfish plague infection (Svoboda et al., 2012; Jussila et al., 2017; Martín-Torrijos et al., 2017). Significant differences in disease resistance have also been observed in controlled infection experiments (Makkonen et al., 2012; Martín-Torrijos et al., 2017; Francesconi et al., 2021). It is thus a major future challenge to identify target genes and molecular pathways, which underlie the defense mechanisms of the crayfish immune system under an A. astaci challenge that might be responsible for an increased resistance toward crayfish plague infection. In perspective, such results might become the basis of selective breeding programs focusing on resistance-genes. Subsequently, reintroduction programs could make use of crayfish plague resistant crayfish to be released into their original habitats. That being said, there would then be a risk of promoting yet another reservoir for A. astaci among native crayfish populations. Thus, speeding up the positive selection process by genetic enhancement of resistance against pathogens has to be carefully considered in the context of the conservation of the native crayfish species.
International pet trade polices like EU Pet Trade Regulation 2016/1141 need to be extended to cover more species which have high invasive potential and are known A. astaci vectors. More conservatively, instead of a blacklist of forbidden species, which takes too much time on EU level to be extended by additional species, it could be suggested to have a white list of species allowed for trade within the EU. Such a list seems to be more in line with a precautionary principle regarding the prevention of introducing invasive species unintentionally. Additionally, a frequent eDNA test of ballast water and the water used during animal cargo for presence of A. astaci spores and other emerging diseases using molecular methods would be highly advisable and definitely compulsory in cases where animal cargo could be entering the EU market (Brunner, 2020). Without effective implementation of national and international biosecurity measures, the occurrence, transboundary spread and serious economic and ecological impact of aquatic animal diseases will continue. In this regard, globally agreed standards for sanitary measures to apply to international trade in live aquatic animals are laid out in the OIE Aquatic Animal Health Code2 and in the OIE Manual of Diagnostic Tests for Aquatic Animals3. Finally, public education is probably the key factor to reduce the risk of alien crayfish to be released into the wild. Education of retailers and pet crayfish owners is an important aspect to alleviate the threat posed by the pet trade.
People’s awareness can contribute to public engagement benefiting nature conservation, which would be initiated by environmental education as part of the school curriculum. Recent successful conservation campaigns in Finland and Sweden (e.g., LIFE+ CrayMate and other regional campaigns; Jussila, 2016) have resulted in the common public being more aware of the possible benefits of native A. astacus stocks and the dangers of the alien P. leniusculus (Jussila, 2016). During the 3-year LIFE+ CrayMate awareness campaign, 2013 – 2016, the targeted fishing rights owners, mostly private persons responsible of the management wild fish and crayfish stock, initiated A. astacus restocking programs within carefully selected waters and at least in Southern Karelia region the success rate of the introductions was above 50% (Tiitinen, 2020) similarly to what has been observed in Northern Savo during the 2020s (Kosunen, 2020). At the same time, people became more aware of the role of alien P. leniusculus as the main reason for the spreading of A. astaci and the devastation of the native A. astacus stocks. This came as a surprise since the Finns have a strong tradition of trapping and eating crayfish, while the knowledge regarding the basics of crayfish biology and ecology seemed thin. In Fennoscandia an information campaign called “The Crayfish Myth Buster”4 (in Swedish, Finnish, and English) was launched on the web in 2006 and people were directed to the website by advertisements in commercial radio jingles, TV, newspapers, flyers, special hats, and information on milk cartons. The campaign dealt with the 21 most common myths, exaggerations and misunderstandings of freshwater crayfish. In a very recent project (“MaNaKa,” 2017–2020) German authorities funded an awareness campaign to encourage and instruct fishing clubs, water leaseholders and nature conservation authorities to safely stock suitable water bodies with the endangered native A. astacus. The colonization of waters previously free from crayfish plague, if carefully selected, should make an important contribution to the preservation of A. astacus in Germany. As an example from the south-east Europe, there have been campaigns in Croatia dedicated to raise awareness of the problems that invasive crayfish cause to freshwater diversity (Pavić et al., 2021). Unfortunately, those activities rarely attained the expected result. Even though there were workshops organized for local inhabitants focusing on the problems that P. leniusculus could cause to karstic habitats, P. leniusculus was illegally introduced into another karstic river (Una River) bordering Croatia and Bosnia and Herzegovina (Trožić-Borovac et al., 2019). Local education campaigns and workshops are needed in the regions where alien crayfish are present and also where they are not yet present but highly likely to spread. Currently, an action plan for alien crayfish in Croatia is being developed, involving local stake holders (fisherpersons, policy makers, protected areas employees, local inhabitants, school teachers, NGOs, etc.) as well as astacologists and the wider scientific community (Faller, 2020). In Croatia, a mobile application for invasive species alert has recently been developed and is now available for citizens (MGOR, 2020).
Finally, natural resources, such as reproductive native crayfish populations, can be taken as exploitable resources, while this approach should not be applied to all native crayfish populations or their distribution regions. The exploitation, and thus the commercial value of the wild crayfish stock, might be a means to protect and conserve native crayfish populations or even a whole species (e.g., Taugbøl, 2004), providing that exploitation is sustainable and spreading of diseases is prevented. When exploitation is discussed, one should introduce ecology into the debate and bear in mind that exploitation should not result in biodiversity decline or drastic ecosystem changes. Exploitation should thus be based on wide ecosystem sustainability, which would then allow both ecosystem health and thriving variety of species, in this case aquatic species, while also ensuring income and benefits to those attempting to exploit natural resources. Discussions regarding exploitation of natural resources quite often, if not always, focus on maximum economical gain, ignoring the long-term health of the exploited natural resource or the ecosystem where this resource belongs to. It never ceases to surprise how those interested in economic gain tend to forget that conservation is actually a rather selfish activity, in most cases, if successful, allowing the existence of human beings and the cultural frame that we rely on. Thus, conserving native European crayfish, protecting them against alien species and their diseases, works for us, too, allowing us to enjoy natural resources and, in the case of the Swedes and maybe the Finns, also crayfish parties, while having nicely prepared A. astacus (e.g., Fürst and Törngren, 2003; Edsman, 2004; Jussila et al., 2016a), but not in excess.
Quite a few of the suggested management and conservations actions have been implemented at least partially and with some success, for example LIFE+ CrayMate awareness campaign in Finland. As more radical actions we suggest that a fundamental principle of polluter pays should be enforced in the cases where the alien species are spreading and resulting in damage to the native ecosystems (e.g., Gaines, 1991). In Finland, and many other European countries, spreading of alien species is illegal, while so far the legal system fails to find culprits or ignores the cases as meaningless in their impacts (our observation). If an alien species cannot be eradicated, a functional eradication could be limiting or even eliminating ecological damage, as has been observed in the case of P. rusticus in North America (Green and Grosholz, 2021). Recent advances in bioengineering would also allow genetic biocontrol, which is based on modifications of the organism’s genome in a heritable way that would for example disrupt the reproduction of the alien species (e.g., Teem et al., 2020). However, such methods are to date only applicable for some insect and vertebrate model species. In the case of freshwater crayfish, the most basic genomic knowledge required for genetic bioengineering, i.e., a fully annotated reference genome, is still lacking.
In this paper we clearly articulate and showcase that human perception and knowledge, or rather the lack of them, together with plain greed, are the most crucial components of the sad predicament of native freshwater crayfish species in Europe. The alien species threat has to be dealt with resolutely. However, to solve the problem of the invasive freshwater crayfish spread today, the most important thing is to manage people rather than the crayfish themselves (e.g., Robbins, 2011; Edsman, 2020b).
JJ, LE, IM, JD-U, and KT contributed equally in fact finding and writing process. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank our two reviewers for their very constructive comments that significantly improved this manuscript. We would like express our deepest respect to all those who have been fighting an uphill battle to support the native ecosystems below and above water level, especially the inspiring atmosphere of the International Association of Astacology (IAA). Remember, it is never too late and, please, never give up. In loving memory of Vesa Tiitinen’s life: he was a good boy.
Aalberg, T., Esser, F., Reinemann, C., Strömbäck, J., and de Vreese, C. H. (2016). Populist Political Communication in Europe. London: Routledge.
Abrahamsson, S. (1973). The crayfish, Astacus astacus, in Sweden and the introduction of the American crayfish, Pacifastacus leniusculus. Freshw. Crayfish 1, 27–40. doi: 10.5869/fc.1973.v1.027
Ackefors, H. (1999). “The positive effects of established crayfish introductions in Europe,” in Crayfish as Alien Species. How to Make the Best of a Bad Situation. Crustacean Issues, 11, eds F. Gherardi and D. M. Holdich (Rotterdam: A.A. Balkema), 49–62. doi: 10.1201/9781315140469-5
Alcorlo, P., and Diéguez Uribeondo, J. (2014). El cangrejo señal y el declive de las poblaciones de cangrejo autóctono. Ambienta 109, 52–61.
Alderman, D. J. (1996). Geographical spread of bacterial and fungal diseases of crustaceans. Rev. Sci. Tech. 15, 603–632. doi: 10.20506/rst.15.2.943
Alonso, F., Temiño, C., and Diéguez-Uribeondo, J. (2000). Status of the white clawed crayfish, Austropotamobius pallipes (Lereboullet, 1858) in Spain: distribution and legislation. Bull. Fr. Pêche Piscic. 356, 31–54. doi: 10.1051/kmae:2000003
Anastácio, P. M., Ferreira, M. P., Banha, F., and Capinha, C. (2014). Waterbird-mediated passive dispersal is a viable process for crayfish (Procambarus clarkii). Aquat. Ecol. 48, 1–10. doi: 10.1007/s10452-013-9461-0
Andriantsoa, R., Tönges, S., Panteleit, J., Theissinger, K., Carneiro, V. C., Rasamy, J., et al. (2019). Ecological plasticity and commercial impact of invasive marbled crayfish populations in Madagascar. BMC Ecol. 19:8. doi: 10.1186/s12898-019-0224-1
Anon (2019). Maa – Ja Metsätalousministeriön Ja Luonnonvarakeskuksen Tulossopimus 2020-2024. Helsinki: Maa- ja metsätalousministeriö.
Apostolopoulou, E., and Adams, W. M. (2015). Neoliberal capitalism and conservation in the post-crisis era: the dialectics of “green” and “un−green” grabbing in Greece and the UK. Antipode 47, 15–35. doi: 10.1111/anti.12102
Arce, J., and Dieguez-Uribeondo, J. (2015). Structural damage caused by the invasive crayfish Procambarus clarkii (Girard, 1852) in rice fields of the Iberian Peninsula: a study case. J. Fundam. Appl. Limnol. 186, 259–269. doi: 10.1127/fal/2015/0715
Arias Rodríguez, A., and Torralba Burrial, A. (2021). First record of the redclaw crayfish Cherax quadricarinatus (Von Martens, 1868) on the Iberian Peninsula. Limnetica 40, 33–42. doi: 10.23818/limn.40.03
Aydin, H., Kokko, H., Makkonen, J., Kortet, R., Kukkonen, H., and Jussila, J. (2014). The signal crayfish is vulnerable to both the As and the PsI-isolates of the crayfish plague. Knowl. Manag. Aquat. Ecosyst. 413:3. doi: 10.1051/kmae/2014004
Becking, T., Mrugała, A., Delaunay, C., Svoboda, J., Raimond, M., Viljamaa-Driks, S., et al. (2015). Effect of experimental exposure to differently virulent Aphanomyces astaci strains on the immune response of the noble crayfish Astacus astacus. J. Invertebr. Pathol. 132, 115–124. doi: 10.1016/j.jip.2015.08.007
Biasetti, P., Ferrante, L., Bonelli, M., Manenti, R., Scaccini, D., and de Mori, B. (2021). Value-conflicts in the conservation of a native species: a case study based on the endangered white-clawed crayfish in Europe. Rend. Fish. Acc. Lincei 32, 389–406. doi: 10.1007/s12210-021-00987-1
Bickford, D., Lohman, D. J., Sodhi, N. S., Ng, P. K., Meier, R., Winker, K., et al. (2007). Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 22, 148–155. doi: 10.1016/j.tree.2006.11.004
Bohman, P. (ed) (2020). Swedish Crayfish Database (In Swedish: Nationella Kräftdatabasen). Slu, Department Of Aquatic Resources. Available online at: http://www.slu.se/kraftdatabasen (accessed December 22, 2020).
Bohman, P., and Edsman, L. (2011). Status, management and conservation of crayfish in Sweden: results and the way forward. Freshw. Crayfish 18, 19–26. doi: 10.5869/fc.2011.v18.19
Bohman, P., and Edsman, L. (2013). Marmorkräftan i Märstaån. Riskanalys och åtgärdsförslag. (English title: Marmorkrebs in River Märstaån. Risk Assessment And Action Proposals.) Aqua Reports 2013:17. Drottningholm: Sveriges lantbruksuniversitet.
Bohman, P., Edsman, L., Martin, P., and Scholtz, G. (2013). The first marmorkrebs (Decapoda: Astacida: Cambaridae) in Scandinavia. BioInv. Rec. 2, 227–232. doi: 10.3391/bir.2013.2.3.09
Bohman, P., Nordwall, F., and Edsman, L. (2006). The effect of the large-scale introduction of signal crayfish on the spread of crayfish plague in Sweden. Bull. Fr. Pêche Piscic. 380-381, 1291–1302. doi: 10.1051/kmae:2006026
Bojko, J., Jennings, L. A., and Behringer, D. C. (2020). A novel positive single-stranded RNA virus from the crustacean parasite, Probopyrinella latreuticola (Peracarida: Isopoda: Bopyridae). J. Invertebr. Pathol. 177:107494. doi: 10.1016/j.jip.2020.107494
Borras, S. M. Jr. (2020). Agrarian social movements: The absurdly difficult but not impossible agenda of defeating right-wing populism and exploring a socialist future. J. Agrar. Change 20, 3–36. doi: 10.1111/joac.12311
Brunner, J. L. (2020). Pooled samples and eDNA-based detection can facilitate the “clean trade” of aquatic animals. Sci. Rep. 10:10280. doi: 10.1038/s41598-020-66280-7
Butler, E., Crigler, P., Robbins, G., and Blair, J. E. (2020). Preliminary survey of Aphanomyces sp. associated with native and invasive crayfish in the Lower Susquehanna watershed of South Central Pennsylvania. J. Freshw. Ecol. 35, 223–233. doi: 10.1080/02705060.2020.1779141
Cab Direct UK (2014). Procambarus fallax f. virginalis (Martin et al., 2010), Marmorkrebs. [invasive species]. Procambarus fallax f. virginalis (Martin et al., 2010), Marmorkrebs. [invasive species]., (AQB ISC record). Available online at: www.cabdirect.org/cabdirect/abstract/20107601509 [accessed 10 March 2021]
Caffrey, J. M., Baars, J. R., Barbour, J. H., Boets, P., Boon, P., Davenport, K., et al. (2014). Tackling invasive alien species in Europe: The top 20 issues. Manag. Biol. Inv. 5, 1–20. doi: 10.3391/mbi.2014.5.1.01
Capinha, C., Larson, E. R., Tricarico, E., Olden, J. D., and Gherardi, F. (2013). Effects of climate change, invasive1021 species, and disease on the distribution of native European crayfishes. Conserv. Biol. 27, 731–1022. doi: 10.1111/cobi.12043
Cerenius, L., Andersson, M. G., and Söderhäll, K. (2009). “Aphanomyces astaci and crustaceans,” in Oomycete Genetics and Genomics: Diversity, Interactions and Research Tools, eds K. Lamour and D. Kamoun (Hoboken, NJ: John Wiley & Sons Inc), 425–433. doi: 10.1002/9780470475898.ch21
Cerenius, L., Bangyeekhun, E., Keyser, P., Söderhäll, I., and Söderhäll, K. (2003). Host prophenoloxidase expression in freshwater crayfish is linked to increased resistance to the crayfish plague fungus, Aphanomyces astaci. Cell. Microbiol. 5, 353–357. doi: 10.1046/j.1462-5822.2003.00282.x
Chadwick, D. D. A., Pritchard, E. G., Bradley, P., Sayer, C. D., Chadwick, M. A., Eagle, L. J. B., et al. (2020). A novel ‘triple drawdown’ method highlights deficiencies in invasive alien crayfish survey and control techniques. J. Appl. Ecol 29, 316–326. doi: 10.1111/1365-2664.13758
Chucholl, C. (2015). “Marbled crayfish gaining ground in Europe: the role of the pet trade as invasion pathway,” in Freshwater Crayfish – A Global Overview, eds T. Kawai, Z. Faulkes, and G. Scholtz (London: Taylor and Francis Group), 83–114. doi: 10.1201/b18723-8
Chucholl, C., and Pfeiffer, M. (2010). First evidence for an established Marmorkrebs (Decapoda, Astacida, Cambaridae) population in Southwestern Germany, in syntopic occurrence with Orconectes limosus (Rafinesque, 1817). Aquat. Inv. 5, 405–412. doi: 10.3391/ai.2010.5.4.10
Chucholl, C., and Wendler, F. (2017). Positive selection of beautiful invaders: long-term persistence and bioinvasion risk of freshwater crayfish in the pet trade. Biol. Inv. 19, 197–208. doi: 10.1007/s10530-0161272-5
Clavero, M., Nores, C., Kubersky-Piredda, S., and Centeno-Cuadros, A. (2016). Interdisciplinarity to reconstruct historical introductions: solving the status of cryptogenic crayfish. Biol. Rev. 91:10361049. doi: 10.1111/brv.12205
Cornalia, E. (1860). “Sulla malattia dei gamberi,” in Atti Della Societa Italiana Di Scienze Naturali E Del Museo Civili Di Storia Naturale, Vol. 2, Milano, 334–336.
Cortes-Vazquez, J. A. (2020). In the name of the people: The populist redefinition of nature conservation in post-crisis Spain. Geoforum 108, 110–118. doi: 10.1016/j.geoforum.2019.12.004
Courtine, J. J., and Willett, L. (1986). A brave new language: Orwell’s invention of ‘newspeak’ in 1984. SubStance 15, 69–74. doi: 10.2307/3684756
Cowan, P., and Warburton, B. (2011). “Animal welfare and ethical issues in island pest eradication,” in Island Invasives: Eradication And Management, eds C. R. Veitch, M. N. Clout, and D. R. Towns (Gland: IUCN), 418–421.
Dannewitz, J., Palm, S., and Edsman, L. (2021). Colonization history and human translocations explain the population genetic structure of noble crayfish (Astacus astacus) in Fennoscandia – implications for management of a critically endangered species. Aquat. Conserv. doi: 10.1002/aqc.3632 [Epub ahead of print].
De Palma-Dow, A., \urti, J., and Fergus, E. (2020). It’s a Trap! An evaluation of different passive trap types to effectively catch and control the invasive red swamp crayfish (Procambarus clarkii) in streams of the Santa Monica Mountains. Manag. Biol. Inv. 11, 44–62. doi: 10.3391/mbi.2020.11.1.04
Deidun, A., Sciberras, A., Formosa, J., Zava, B., Insacco, G., Corsini-Foka, M., et al. (2018). Invasion by non-indigenous freshwater decapods of Malta and Sicily, central Mediterranean Sea. J. Crust. Biol. 38, 748–753. doi: 10.1093/jcbiol/ruy076
Díaz, M. (2021). Solicitud de dictamen sobre efectos negativos de Procambarus clarkii Y Pacifastacus leniusculus sobre el medio natural. Madrid: MITECO.
Diéguez-Uribeondo, J. (2006). Dispersion of the Aphanomyces astaci-carrier, Pacifastacus leniusculus, by humans represents the main cause of disappearance of native populations of Austropotamobius pallipes in Navarra. Bull. Fr. Pêche Piscic 4, 1303–1312. doi: 10.1051/kmae:2006036
Diéguez-Uribeondo, J., and Söderhäll, K. (1993). Procambarus clarkii as a vector for the crayfish plague fungus Aphanomyces astaci Schikora. Aquacult. Res. 24, 761–765. doi: 10.1111/j.13652109.1993.tb00655.x
Diéguez-Uribeondo, J., Cerenius, L., and Söderhäll, K. (1994). Saprolegnia parasitica and its virulence on three different species of freshwater crayfish. Aquaculture 120, 219–228. doi: 10.1016/0044-8486(94)90080-9
Diéguez-Uribeondo, J., García, M. A., Cerenius, L., Kozubíková, E., Ballesteros, I., Windels, C., et al. (2009). Phylogenetic relationships among plant and animal parasites, and saprobionts in Aphanomyces (Oomycetes). Fungal Genet. Biol. 46, 365–376. doi: 10.1016/j.fgb.2009.02.004
Diéguez-Uribeondo, J., Huang, T.-S., Cerenius, L., and Söderhäll, K. (1995). Physiological adaptation of an Aphanomyces astaci strain isolated from the freshwater crayfish Procambarus clarkii. Mycol. Res. 99, 571–578. doi: 10.1016/S0953-7562(09)80716-8
Diéguez-Uribeondo, J., Rueda, A., Castien, E., and Bascones, J. C. (1997a). A plan of restoration for the native freshwater crayfish species, Austropotamobius pallipes, in Navarra. Bull. Fr. Pêche Piscic. 347, 625–637. doi: 10.1051/kmae/1997056
Diéguez-Uribeondo, J., Temiño, C., and Muzquiz, J. L. (1997b). The crayfish plague fungus, Aphanomyces astaci in Spain. B. Fr. Pêche Piscic. 347, 753–763. doi: 10.1051/kmae/1997051
Domisch, S., Jaehnig, S. C., and Haase, P. (2011). Climate−change winners and losers: Stream macroinvertebrates of a submontane region in Central Europe. Freshw. Biol. 56, 2009–2020. doi: 10.1111/j.1365-2427.2011.02631.x
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Leveque, C., et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. doi: 10.1017/S1464793105006950
Edsman, L. (2004). The Swedish story about import of live crayfish. Bull. Franc. Pêche Piscic. 372-373, 281288. doi: 10.1051/kmae:2004003
Edsman, L. (2020a). “Flodkräfta,” in Fisk- och skaldjursbestånd i hav och sötvatten 2019: Resursöversikt. Rapport 2020:3, eds A. Bryhn, A. Sundelöf, A. Lingman, A. B. Florin, E. Petersson, F. Vitale, et al. (Göteborg: Havs- och vattenmyndigheten), 55–57.
Edsman, L. (2020b). “The rise and fall of the introduced invasive signal crayfish in Sweden – sixty years with an alien,” in Proceedings of the Abstract of oral presentation, Ecology in the Anthropocene, The fourth conference of the Nordic Society Oikos, Reykjavik.
Edsman, L., and Schröder, S. (2009). Åtgärdsprogram För Flodkräfta 2008–2013 (Astacus Astacus). Report nr. 5955. Drottningholm: Fiskeriverket och Naturvårdsverket.
Edsman, L., and Śmietana, P. (2004). Exploitation, conservation and legislation. Bull. Franc. Pêche Piscic. 372-373, 457–464. doi: 10.1051/kmae:2004019
Edsman, L., Farris, J. S., Källersjö, M., and Prestegaard, T. (2002). Genetic differentiation between noble crayfish, Astacus astacus (L.), populations detected by microsatellite length variation in the rDNA ITS1 region. Bull. Franç. Pêche Piscic. 367, 691–706. doi: 10.1051/kmae:2002082
Edsman, L., Nyström, P., Sandström, A., Stenberg, M., Kokko, H., Tiitinen, V., et al. (2015). Eroded swimmeret syndrome in female crayfish Pacifastacus leniusculus associated with Aphanomyces astaci and Fusarium spp. infections. Dis. Aquat. Org. 112, 219–228. doi: 10.3354/dao02811
Eizaguirre, C., and Baltazar-Soares, M. (2014). Evolutionary conservation – evaluating the adaptive potential of species. Evol. Appl. 7, 963–967. doi: 10.1111/eva.12227
Erkamo, E. (2019). Conference Presentation. Jyväskylä: Kansalliset kalantutkimuspäivät, (In Finnish).
Erkamo, E., Tulonen, J., and Kirjavainen, J. (2019). Kansallinen Rapustrategia 2019–2022. Helsinki: MMM.
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst. 34, 487–515.
Faulkes, Z. (2015a). The global trade in crayfish as pets. Crust. Res. 44, 75–92. doi: 10.18353/crustacea.44.0_75
Faulkes, Z. (2015b). A bomb set to drop: parthenogenetic Marmorkrebs for sale in Ireland, a European location without non-indigenous crayfish. Manag. Biol. Inv. 6:111. doi: 10.3391/mbi.2015.6.1.09
Fiskeriverket (1993). Ways Of Increasing Populations Of Noble Crayfish Astacus astacus In Swedish Freshwaters. Information From The Institute Of Freshwater Research. Drottningholm: Fiskeriverket.
Francesconi, C., Makkonen, J., Schrimpf, A., Jussila, J., Kokko, H., and Theissinger, K. (2021). Elevated resistance and latent infections caused by reduced virulence: marbled crayfish and noble crayfish challenged with the crayfish plague disease agent. Front. Ecol. Evol. (in press)
Fürst, M., and Törngren, K. (2003). Våra Älskade Kräftor. Från Bur Till Bord. Stockholm: Bäckströms förlag.
Gaines, S. E. (1991). The polluter-pays principle: from economic equity to environmental ethos. Tex. Int. Law J. 26, 463–496.
Gherardi, F. (2011). Towards a sustainable human use of freshwater crayfish (Crustacea, Decapoda, Astacidea). Knowl. Manag. Aquat. Ecosyst. 401:2. doi: 10.1051/kmae/2011038
Gherardi, F. and Holdich, D. M. (eds.) (1999). Crayfish in Europe as Alien Species. How to Make Best of a Bad Situation. Rotterdam: A. A. Balkema.
Gherardi, F., Aquiloni, L., Diéguez-Uribeondo, J., and Tricarico, E. (2011). Managing invasive crayfish: is there a hope? Aquat. Sci. 73, 185–200. doi: 10.1007/s00027-011-0181-z
Gontier, M., Balfors, B., and Mörtberg, U. (2006). Biodiversity in environmental assessment—current practice and tools for prediction. Environ. Imp. Assessm. Rev. 26, 268–286. doi: 10.1016/j.eiar.2005.09.001
Govedič, M. (2017). First record of the spiny-cheek crayfish (Orconectes limosus) in Slovenia–300 km upstream from its known distribution in the Drava River. Knowl. Manag. Aquatic Ecosyst. 418, 7. doi: 10.1051/kmae/2016039
Grandjean, F., Collas, M., Uriarte, M., and Rousset, M. (2021). First record of a marbled crayfish Procambarus virginalis (Lyko, 2017) population in France. Bioinv. Rec. 10, 341–347. doi: 10.3391/bir.2021.10.2.12
Green, S. J., and Grosholz, E. D. (2021). Functional eradication as a framework for invasive species control. Front. Ecol. Environ. 19:98–107. doi: 10.1002/fee.2277
Gren, M., Isacs, L., and Carlsson, M. (2009). Costs of alien invasive species in Sweden. AMBIO 38:135140. doi: 10.1579/0044-7447-38.3.135
Gross, R., Jelić, M., Lovrenčić, L., Grandjean, F., Ðuretanović, S., Simić, V., et al. (2021). Genetic diversity and structure of the noble crayfish populations in the Balkan Peninsula revealed by mitochondrial and microsatellite DNA markers. PeerJ (in press)
Gross, R., Palm, S., Koiv, K., Prestegaard, T., Jussila, J., Paaver, T., et al. (2013). Microsatellite markers reveal clear geographic structuring among threatened noble crayfish (Astacus astacus) populations in Northern and Central Europe. Conserv. Genet. 14, 809–882. doi: 10.1007/s10592-013-0476-9
Gutiérrez-Yurrita, P. J., Marténez, J. M., Bravo-Utrera, M. Á, Montes, C., Ilhéu, M., and Bernardo, J. M. (2017). “The status of crayfish populations in Spain and Portugal,” in Crayfish as Alien Species. How to Make the Best of a Bad Situation. Crustacean Issues, 11, eds F. Gherardi and D. M. Holdich (Rotterdam: A.A. Balkema), 161–192. doi: 10.1201/9781315140469-12
Hänfling, B., Edwards, F., and Gherardi, F. (2011). Invasive alien Crustacea: dispersal, establishment, impact and control. Biocontrol 56, 573–595. doi: 10.3391/ai.2017.12.1.07
Hein, C. L., Vander Zanden, M. J., and Magnuson, J. J. (2007). Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshw. Biol. 52, 1134–1146. doi: 10.1111/j.1365-2427.2007.01741.x
Henttonen, P., and Huner, J. V. (1999). “The introduction of alien species of crayfish in Europe: A historical introduction,” in Crayfish as Alien Species. How to Make the Best of a Bad Situation. Crustacean Issues, 11, eds F. Gherardi and D. M. Holdich (Rotterdam: A.A. Balkema), 13–22. doi: 10.1201/9781315140469-2
Holdich, D. M. (2002). Distribution of crayfish in Europe and some adjoining countries. Bull. Fr. Pêche Piscic. 367, 611–650. doi: 10.1051/kmae:2002055
Holdich, D. M., and Reeve, I. D. (1991). Distribution of freshwater crayfish in the British Isles, with particular reference to crayfish plague, alien introductions and water quality. Aquat. Conserv. 1, 139–158. doi: 10.1002/aqc.3270010204
Holdich, D. M., James, J., Jackson, C., and Peay, S. (2014). The North American signal crayfish, with particular reference to its success as an invasive species in Great Britain. Ethol. Ecol. Evol. 26, 232–262. doi: 10.1080/03949370.2014.903380
Holdich, D. M., Reynolds, J. D., Souty-Grosset, C., and Sibley, P. J. (2009). A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 394395, 11. doi: 10.1051/kmae/2009025
Houghton, R., Wood, C., and Lambin, X. (2017). Size-mediated, density dependent cannibalism in the signal crayfish Pacifastacus leniusculus (Dana, 1852) (Decapoda, Astacidea), an invasive crayfish in Britain. Crustaceana 90, 417–435. doi: 10.1163/15685403-00003653
Hudina, S., Galić, N., Kutleša, P., Duplić, A., and Maguire, I. (2016). ““Range expansion of the signal crayfish (Pacifastacus leniusculus) in a recently invaded region in Croatia and potential for its control”,” in 21st Symposium of the International Association of Astacology – Program and Book of Abstracts, ed. J. Diéguez-Uribeondo (Madrid: Real Jardín Botánico), 29.
Hudina, S., Hock, K., and Žganec, K. (2014). The role of aggression in range expansion and biological invasions. Curr. Zool. 60, 401–409. doi: 10.1093/czoolo/60.3.401
Hudina, S., Kutleša, P., Trgovčević, K., and Duplić, A. (2017). Dynamics of range expansion of the signal crayfish (Pacifastacus leniusculus) in a recently invaded region in Croatia. Aquat. Invasions 12, 67–75.
Hudina, S., Žganec, K., Lucić, A., Trgovčić, K., and Maguire, I. (2013). Recent invasion of the karstic river systems in Croatia through illegal introductions of the signal crayfish. Freshw. Crayfish 19, 21–27. doi: 10.5869/fc.2013.v19.021
Huusela-Veistola, E., Holmala, K., Hyvönen, T., Hyvönen, T., Kauhala, K., Ryttäri, T., et al. (2019). Ehdotus Haitallisten Vieraslajien Hallintasuunnitelmaksi Ja Leviämisväyliä Koskevaksi Toimintasuunnitelmaksi. Helsinki: Valtioneuvoston kanslia.
Jackson, D. A., Peres-Neto, P. R., and Olden, J. D. (2001). What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aqua. Sci. 58, 157–170. doi: 10.1139/cjfas-58-1-157
James, J., Mrugała, A., Oidtmann, B., Petrusek, A., and Cable, J. (2017). Apparent interspecific transmission of Aphanomyces astaci from invasive signal to virile crayfish in a sympatric wild population. J. Invertebr. Pathol. 145, 68–71. doi: 10.1016/j.jip.2017.02.003
Jelić, M., Klobučar, G. I. V., Grandjean, F., Puillandre, N., Franjević, D., Futo, M., et al. (2016). Insights into the molecular phylogeny and historical biogeography of the white-clawed crayfish (Decapoda, Astacidae). Mol. Phylogenet. Evol. 103, 26–40. doi: 10.1016/j.ympev.2016.07.009
Jussila, J. (1995). On the cost of crayfish trapping in central Finland. Freshw. Crayfish 8, 215–227. doi: 10.5869/fc.1995.v8.215
Jussila, J., and Edsman, L. (2020). Relaxed attitude towards spreading of alien crayfish species affects protection of native crayfish species: case studies and lessons learnt from a Fennoscandian viewpoint. Freshw. Crayfish 25, 39–46. doi: 10.5869/fc.2020.v25-1.039
Jussila, J., and Mannonen, A. (2004). Crayfisheries in Finland, a short overview. Bull. Fr. Pêche Piscic. 372-373, 263–273. doi: 10.1051/kmae:2004001
Jussila, J., Francesconi, C., Theissinger, K., Kokko, H., and Makkonen, J. (2021a). Is Aphanomyces astaci loosing its stamina: a latent crayfish plague disease agent from lake Venesjärvi, Finland. Freshw. Crayfish. (Submitted).
Jussila, J., Kokko, H., Kortet, R., and Makkonen, J. (2013a). Aphanomyces astaci PsI-genotype isolates from different Finnish signal crayfish stocks show variation in their virulence but still kill fast. Knowl. Manag. Aquat. Ecosyst. 411:10. doi: 10.1051/kmae/2013077
Jussila, J., Maguire, I., Kokko, H., and Makkonen, J. (2016a). “Chaos and adaptation in the pathogen-host relationship in relation to the conservation. The case of the crayfish plague and the noble crayfish,” in Freshwater Crayfish – A Global Overview, eds T. Kawai, Z. Faulkes, and G. Scholtz (London: Taylor and Francis Group), 246–274. doi: 10.1201/b18723-15
Jussila, J., Makkonen, J., Kokko, H., and Mäkinen, P. (2014a). Numerous population crashes of wild signal crayfish (Pacifastacus leniusculus) in Southern Finland. Freshw. Crayfish 20, 73–79. doi: 10.5869/fc.2014.v20-1.73
Jussila, J., Makkonen, J., Vainikka, A., Kortet, R., and Kokko, H. (2011). Latent crayfish plague (Aphanomyces astaci) infection in a robust wild noble crayfish (Astacus astacus) population. Aquaculture 321, 17–20. doi: 10.1016/j.aquaculture.2011.08.026
Jussila, J., Makkonen, J., Vainikka, A., Kortet, R., and Kokko, H. (2014b). Crayfish plague dilemma: how to be a courteous killer? Boreal Env. Res. 19, 235–244.
Jussila, J., Tiitinen, V., Edsman, L., Kokko, H., and Fotedar, R. (2016b). Signal crayfish in Lake Saimaa could be maladapted to the local conditions due to Aphanomyces astaci infection: a seven-year study. Freshw. Crayfish 22, 53–60. doi: 10.5869/fc.2016.v22-1.53
Jussila, J., Tiitinen, V., Fotedar, R., and Kokko, H. (2013b). A simple and efficient cooling method for postharvest transport of the commercial crayfish catch. Freshw. Crayfish 19, 15–19. doi: 10.5869/fc.2013.v19.015
Jussila, J., Tiitinen, V., Makkonen, J., Kokko, H., Bohman, P., and Edsman, L. (2021b). Eroded swimmeret syndrome: update of the current knowledge. Freshw. Crayfish 26, 63–68. doi: 10.5869/fc.2021.v26-1.63
Jussila, J., Vrezec, A., Jaklič, T., Kukkonen, H., Makkonen, J., and Kokko, H. (2017). Aphanomyces astaci isolate from latently infected stone crayfish (Austropotamobius torrentium) population is virulent. J. Invertebr. Pathol. 149, 15–20. doi: 10.1016/j.jip.2017.07.003
Jussila, J., Vrezec, A., Makkonen, J., Kortet, R., and Kokko, H. (2015). “8. Invasive crayfish and their invasive diseases in Europe with the focus on the virulence evolution of the crayfish plague,” in Biological Invasions in Changing Ecosystems. Vectors, Ecological Impacts, Management and Predictions, ed. J. Canning-Clode (Warsaw: De Gryuter), 183–211. doi: 10.1515/9783110438666-013
Kirjavainen, J., and Sipponen, M. (2004). Environmental benefit of different crayfish management strategies in Finland. Fish. Manag. Ecol. 11, 213–218. doi: 10.1111/j.1365-2400.2004.00388.x
Kirjavainen, J., and Westman, K. (1999). Natural history and development of the introduced signal crayfish, Pacifastacus leniusculus, in a small, isolated Finnish lake, from 1968 to 1993. Aquat. Living Res. 12, 387–401. doi: 10.1016/S0990-7440(99)00110-2
Klobučar, G., Podnar, M., Jelić, M., Franjević, D., Faller, M., Štambuk, A., et al. (2013). Role of the Dinaric Karst (western Balkans) in shaping the phylogeographic structure of the threatened crayfish Austropotamobius torrentium. Freshw. Biol. 58, 1089–1105. doi: 10.1111/fwb.12110
Kokko, H., Harlioglu, M. M., Aydin, H., Makkonen, J., Gökmen, G., and Aksu, Ö, et al. (2018). Observations of crayfish plague infections in commercially important narrow-clawed crayfish populations in Turkey. Knowl. Manag. Aquatic Ecosyst. 419:10. doi: 10.1051/kmae/2018001
Kokko, H., Koistinen, L., Harlioğlu, M. M., Makkonen, J., AydIn, H., and Jussila, J. (2012). Recovering Turkish narrow clawed crayfish (Astacus leptodactylus) populations carry Aphanomyces astaci. Knowl. Manag. Aquat. Ecosyst. 404:12. doi: 10.1051/kmae/2012006
Kotovska, G., Khrystenko, D., Patoka, J., and Kouba, A. (2016). East European crayfish stocks at risk: arrival of non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 417:37. doi: 10.1051/kmae/2016024
Kouba, A., Oficialdegui, F. J., Cuthbert, R. N., Kourantidou, M., South, J., Tricarico, E., et al. (2021). SI on economic costs of invasions – feeling the pinch: global economic costs of crayfish invasions and comparison with other aquatic crustaceans. Res. Sq. 13, 1488–1530. doi: 10.21203/rs.3.rs-381161/v1
Kouba, A., Petrusek, A., and Kozák, P. (2014). Continental-wide distribution of crayfish species in Europe: update and maps. Knowl. Manag. Aquat. Ecosyst. 413:5. doi: 10.1051/kmae/2014007
Kozubíková, E., Viljamaa-Driks, S., Heinikainen, S., and Petrusek, A. (2011). Spiny-cheek crayfish Orconectes limosus carry a novel genotype of the crayfish plague pathogen Aphanomyces astaci. J. Invertebr. Pathol. 108, 214–216. doi: 10.1016/j.jip.2011.08.002
Kozubíková-Balcarová, E., Koukol, A., Martín, M. P., Svoboda, J., Petrusek, A., and Diéguez-Uribeondo, J. (2013). The diversity of Oomycetes on crayfish: Morphological vs molecular identification of cultures obtained while isolating the crayfish plague pathogen. Fungal Biol. 117, 682–691. doi: 10.1016/j.funbio.2013.07.005
Krieg, R., and Zenker, A. (2020). A review of the use of physical barriers to stop the spread of nonindigenous crayfish species. Rev. Fish Biol. Fish. 30, 423–435. doi: 10.1007/s11160-020-09606-y
Krieg, R., King, A., and Zenker, A. (2020). Measures to control invasive crayfish species in Switzerland: A success story? Front. Env. Sci. 8:609129. doi: 10.3389/fenvs.2020.609129
Kušar, D., Vrezec, A., Ocepek, M., and Jenčič, V. (2013). Aphanomyces astaci in wild crayfish populations in Slovenia: first report of persistent infection in a stone crayfish Austropotamobius torrentium population. Dis. Aquat. Organ. 103, 157–169. doi: 10.3354/dao02567
Lehmann, A., Overton, J. M., and Austin, M. P. (2002). Regression models for spatial prediction: their role for biodiversity and conservation. Biodiv. Conserv. 11, 2085–2092. doi: 10.1023/A:1021354914494
Lehtonen, J. U. E. (1975). Kansanomainen Ravustus Ja Rapujen Hyväksikäyttö Suomessa. Tapiola: SuomiFinland Oy Weilin+Göös Ab.
Lemmers, P., Collas, F. P. L., Gylstra, R., Crombaghs, B. H. J. M., van der Velde, G., and Leuven, R. S. E. W. (2021). Risks and management of alien freshwater crayfish species in the Rhine-Meuse river district. Manag. Biol. Inv. 12, 193–220. doi: 10.3391/mbi.2021.12.1.13
Litchman, E. (2010). Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial 1298 ecosystems. Ecol. Lett. 13, 1560–1572. doi: 10.1111/j.1461-0248.2010.01544.x
Lodge, D. M., Taylor, C. A., Holdich, D. M., and Skurdal, J. (2000). Nonidigenous crayfish threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25, 7–20. doi: 10.1577/15488446
Loureiro, T. G. (2020). “Non-indigenous crayfish species: a global assessment and future perspectives,” in Crayfish: Evolution, Habitat and Conservation Strategies, ed. F. Bezerra Ribeiro (Hauppauge, NY: Nova Science Publishers), 91–119.
Lovrenčić, L., Bonassin, L., Boštjančić, L. J. L., Podnar, M., Jelić, M., Klobučar, G., et al. (2020). New insights into the genetic diversity of the stone crayfish: taxonomic and conservation implications. BMC Evol. Biol. 20:146. doi: 10.1186/s12862-020-01709-1
Lowe, S., Browne, M., Boudjelas, S., and De Poorter, M. (2004). 100 of the world’s worst invasive alien species: A selection from the Global Invasive Species Database. Auckland: University of Auckland.
Maciaszek, R., Bonk, M., and Strużyñski, W. (2019). New records of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852)(Decapoda: Cambaridae) from Poland. Knowl. Manag. Aquat. Ecosyst. 420, 39. doi: 10.1051/kmae/2019033
Maguire, I., and Gottstein-Matočec, S. (2004). The distribution pattern of freshwater crayfish in Croatia. Crustaceana 77, 25–49. doi: 10.1163/156854004323037874
Maguire, I., Jelić, M., Klobučar, G., Delpy, M., Delaunay, C., and Grandjean, F. (2016). Prevalence of the pathogen Aphanomyces astaci in freshwater crayfish populations in Croatia. Dis. Aquat. Org. 118, 45–53. doi: 10.3354/dao02955
Maguire, I., Klobučar, G., Marčić, Z., and Zanella, D. (2008). The first record of Pacifastacus leniusculus in Croatia. Crayfish News 30:4.
Makkonen, J., Jussila, J., Kortet, R., Vainikka, A., and Kokko, H. (2012). Differing virulence of Aphanomyces astaci isolates and elevated resistance of noble crayfish Astacus astacus against crayfish plague. Dis. Aquat. Org. 102, 129–136. doi: 10.3354/dao02547
Makkonen, J., Jussila, J., Panteleit, J., Keller, N. S., Schrimpf, A., Theissinger, K., et al. (2018). MtDNA allows the sensitive detection and haplotyping of the crayfish plague disease agent Aphanomyces astaci showing clues about its origin and migration. Parasitology 145, 1210–1218. doi: 10.1017/s0031182018000227
Makkonen, J., Kokko, H., and Jussila, J. (2015). Mitochondrial cytochrome oxidase I gene analysis indicates a restricted genetic background in Finnish noble crayfish (Astacus astacus) stocks. Knowl. Manag. Aquat. Ecosyst. 416:21. doi: 10.1051/kmae/2015017
Makkonen, J., Kokko, H., Gökmen, G., Ward, J., Umek, J., Kortet, R., et al. (2019). The signal crayfish (Pacifastacus leniusculus) in Lake Tahoe (USA) hosts multiple Aphanomyces species. J. Invertebr. Pathol. 166:107218. doi: 10.1016/j.jip.2019.107218
Makkonen, J., Kokko, H., Henttonen, P., and Jussila, J. (2010). Crayfish plague (Aphanomyces astaci) can be vertically transferred during artificial incubation of crayfish eggs: preliminary results. Freshw. Crayfish 17, 151–153. doi: 10.5869/fc.2010.v17.151
Makkonen, J., Kokko, H., Vainikka, A., Kortet, R., and Jussila, J. (2014). Dose-dependent mortality of the noble crayfish (Astacus astacus) to different strains of the crayfish plague (Aphanomyces astaci). J. Invertebr. Pathol. 115, 86–91. doi: 10.1016/j.jip.2013.10.009
Manfrin, A., and Pretto, T. (2014). Aspects of health and disease prevention. RARITY Report, 123-125. RARITY project LIFE10 NAT/IT/000239. Trieste.
Marten, M., Werth, C., and Marten, D. (2004). Der Marmorkrebs (Cambaridae, Decapoda) in Deutsch- land – ein weiteres Neozoon im Einzugsgebiet des Rheins. Lauterbornia 50, 17–23.
Martin, P., Shen, H., Füllne, G., and Scholtz, G. (2010). The first record of the parthenogenetic Marmorkrebs (Decapoda, Astacida, Cambaridae) in the wild in Saxony (Germany) raises the question of its actual threat to European freshwater ecosystems. Aquat. Inv. 5, 397–403. doi: 10.3391/ai.2010.5.4.09
Martín-Torrijos, L., Campos Llach, M., Pou-Rovira, Q., and Diéguez-Uribeondo, J. (2017). Resistance to the crayfish plague, Aphanomyces astaci (Oomycota) in the endangered freshwater crayfish species, Austropotamobius pallipes. PLoS One 12:e01812. doi: 10.1371/journal.pone.0181226
Martín-Torrijos, L., Correa-Villalona, A., Pradillo, A., and Dieìguez-Uribeondo, J. (2021a). Coexistence of two invasive species, Procambarus clarkii and Aphanomyces astaci, in brackish waters of a Mediterranean coastal lagoon. Front. Ecol. Evol 8:622434. doi: 10.3389/fevo.2020.622434
Martín-Torrijos, L., Kawai, T., Makkonen, J., Jussila, J., Kokko, H., and Diéguez-Uribeondo, J. (2018). Crayfish plague in Japan: A real threat to the endemic Cambaroides japonicus. PLoS One 13:e0195353. doi: 10.1371/journal.pone.0195353
Martín-Torrijos, L., Kokko, H., Makkonen, J., Jussila, J., and Diéguez-Uribeondo, J. (2019). Mapping 15 years of crayfish plague in the Iberian Peninsula: the impact of two invasive species on the endangered native crayfish. PLoS One 14:e0219223. doi: 10.1371/journal.pone.0219223
Martín-Torrijos, L., Martínez-Ríos, M., Casabella-Herrero, G., Collins, J., Adams, S., and Dieìguez-Uribeondo, J. (2021b). Tracing the origin of the crayfish plague pathogen, Aphanomyces astaci, to the Southeastern United States. Sci. Rep. 11:9332. doi: 10.1038/s41598-021-88704-8
Martiny, J. B. H., Bohannan, B. J., Brown, J. H., Colwell, R. K., Fuhrman, J. A., Green, J. L., et al. (2006). Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112. doi: 10.1038/nrmicro1341
Matallanas, B., Ochando, M. D., Alonso, F., and Callejas, C. (2016). Update of genetic information for the white-clawed crayfish in Spain, with new insights into its population genetics and origin. Org. Divers. Evol. 16, 533–547. doi: 10.1007/s13127-016-0268-4
Matallanas, B., Ochando, M. D., Vivero, A., Beroiz, B., Alonso, F., and Callejas, C. (2011). Mitochondrial DNA variability in Spanish populations of A. italicus inferred from the analysis of a COI region. Knowl. Manag. Aquat. Ecosyst. 401:30. doi: 10.1051/kmae/2011052
Mathews, L. M., Adams, L., Anderson, E., Basile, M., Gottardi, E., and Buckholt, M. A. (2008). Genetic and morphological evidence for substantial hidden biodiversity in a freshwater crayfish species complex. Mol. Phylogen. Evol. 48, 126–135. doi: 10.1016/j.ympev.2008.02.006
McCarthy, J. (2019). Authoritarianism, populism, and the environment: comparative experiences, insights, and perspectives. Ann. Am. Assoc. Geograph. 109, 301–313. doi: 10.1080/24694452.2018.1554393
McColl, K. A., Sunarto, A., and Neave, M. J. (2018). Biocontrol of carp: more than just a herpesvirus. Front. Microbiol. 9:2288. doi: 10.3389/fmicb.2018.02288
Melotto, A., Manenti, R., and Ficetola, G. F. (2020). Rapid adaptation to invasive predators overwhelms natural gradients of intraspecific variation. Nat. Commun. 11, 1–10. doi: 10.1038/s41467-020-17406-y
Messager, M. L., and Olden, J. D. (2018). Individual-based models forecast the spread and inform the management of an emerging riverine invader. Diver. Distrib. 24, 1816–1829. doi: 10.1111/ddi.12829
MGOR (2020). Invazivne Strane Vrste. Available online at: https://gospodarstvo.gov.hr/aktualno/ministarstvo-pokrenulomobilnu-aplikaciju-za-prijavu-invazivnih-vrsta/12796. (Accessed December 30, 2020).
MMM (2020). Vesistöt (Water bodies). Available online at: https://mmm.fi/vesistot (Accessed December 18, 2020)
Mojžišová, M., Mrugała, A., Kozubíková-Balcarová, E., Vlach, P., Svobodová, J., Kouba, A., et al. (2020). Crayfish plague in Czechia: Outbreaks from novel sources and testing for chronic infections. J. Invertebr. Pathol. 173:107390. doi: 10.1016/j.jip.2020.107390
Moorhouse, T. P., and Macdonald, D. W. (2011). The effect of removal by trapping on body condition in populations of signal crayfish. Biol. Cons. 144, 1826–1831. doi: 10.1016/j.biocon.2011.03.017
Mrugała, A., Kozubíková-Balcarová, E., Chucholl, C., Cabanillas Resino, S., Viljamaa-Drinks, S., Vukiæ, J., et al. (2014). Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: crayfish plague and white spot syndrome. Biol. Inv. 17, 1313–1326. doi: 10.1007/s10530-014-0795-x
Naiman, R. J., and Turner, M. G. (2000). A future perspective on North America’s freshwater ecosystems. Ecol. Appl. 10, 958–970.
Novitsky, R. A., and Son, M. O. (2016). The first records of Marmorkrebs [Procambarus fallax (Hagen, 1870) f. virginalis] (Crustacea, Decapoda, Cambaridae) in Ukraine. Ecol.onten. 5, 44–46. doi: 10.37828/em.2016.5.8
Nybelin, O. (1936). Untersuchungen Uber Die Ursache Der In Schweden Gegenvartig Vorkommenden Krebspest. Report of the Institute of Freshwater Research, Drottningholm. Drottningholm: Carl Bloms Boktr Lund Sweden, 3–29.
Oidtmann, B., Heitz, E., Rogers, D., and Hoffmann, R. W. (2002). Transmission of crayfish plague. Dis. Aquat. Organ. 52, 159–167. doi: 10.3354/dao052159
Pacioglu, O., Theissinger, K., Alexa, A., Samoilã, C., Sîrbu, O., Schrimpf, A., et al. (2020). Multifaceted implications of the competition between native and invasive crayfish: a glimmer of hope for the native’s long-term survival. Biol. Invasive 22, 827–842. doi: 10.1007/s10530-019-02136-0
Paloniitty, T. (2016). The Weser case: Case C-461/13 Bund v Germany. J. Environ. Law 28, 151–158. doi: 10.1093/jel/eqv032
Panteleit, J., Horvath, T., Jussila, J., Makkonen, J., Perry, W., Schulz, R., et al. (2019). Invasive rusty crayfish (Faxonius rusticus) populations in North America are infected with the crayfish plague disease agent (Aphanomyces astaci). Freshw. Sci. 38, 425–433. doi: 10.1086/703417
Panteleit, J., Keller, N. S., Diéguez-Uribeondo, J., Makkonen, J., Martín-Torrijos, L., Patrulea, V., et al. (2018). Hidden sites in the distribution of the crayfish plague pathogen Aphanomyces astaci in Eastern Europe: relicts of genetic groups from older outbreaks? J. Invertebr. Pathol. 157, 117–124. doi: 10.1016/j.jip.2018.05.006
Panteleit, J., Keller, N. S., Kokko, H., Jussila, J., Makkonen, J., Theissinger, K., et al. (2017). Investigation of ornamental crayfish reveals new carrier species of the crayfish plague pathogen (Aphanomyces astaci). Aquat. Inv. 12, 77–83. doi: 10.3391/ai.2017.12.1.08
Pârvulescu, L., Pérez-Moreno, J. L., Panaiotu, C., Schrimpf, L., Popovici, A., Zaharia, I.-A., et al. (2019). A journey on plate tectonics sheds light on European crayfish phylogeography. Ecol. Evol. 9, 1957–1971. doi: 10.1002/ece3.4888
Pârvulescu, L., Schrimpf, A., Kozubíková, E., Cabanillas Resino, S., Vrålstad, T., Petrusek, A., et al. (2012). Invasive crayfish and crayfish plague on the move: first detection of the plague agent Aphanomyces astaci in the Romanian Danube. Dis. Aquat. Organ. 98, 85–94. doi: 10.3354/dao02432
Pârvulescu, L., Togor, A., Lele, S. F., Scheu, S., Şinca, D., and Panteleit, J. (2017). First established population of marbled crayfish Procambarus fallax (Hagen, 1870) f. virginalis (Decapoda, Cambaridae) in Romania. BioInv. Rec. 6, 357–362. doi: 10.3391/bir.2017.6.4.09
Patoka, J., Buřič, M., Kolář, V., Bláha, M., Petrtýl, M., Franta, P., et al. (2016a). Predictions of marbled crayfish establishment in conurbations fulfilled: evidences from the Czech Republic. Biologia 71, 1380–1385. doi: 10.1515/biolog-2016-0164
Patoka, J., Kocánová, B., and Kalous, L. (2016b). Crayfish in Czech cultural space: the longest documented relationship between humans and crayfish in Europe. Knowl. Manag. Aquat. Ecosyst. 417:5. doi: 10.1051/kmae/2015038
Patoka, J., Petrtýl, M., and Kalous, L. (2014). Garden ponds as potential introduction pathway of ornamental crayfish. Knowl. Manag. Aquat. Ecosyst. 414:8. doi: 10.1051/kmae/2014019
Pavić, D., Bielen, A., Hudina, S., Špoljarić, I., Grandjean, F., Jussila, J., et al. (2021). Monitoring of Aphanomyces astaci, causative agent of crayfish plague, in the Plitvice Lakes National Park. Bioinv. Rec. 10. (in press).
Peay, S. (2009). Invasive non-indigenous crayfish species in Europe: recommendations on managing them. Knowl. Manag. Aquat. Ecosyst. 394-395:3. doi: 10.1051/kmae/2010009
Peay, S., Hiley, P. D., Collen, P., and Martin, I. (2006). Biocide treatment of ponds in Scotland to eradicate signal crayfish. Bull. Fr. Pêche Piscic. 380-381, 1363–1379. doi: 10.1051/kmae:2006041
Peay, S., Johnsen, S. I., Bean, C. W., Dunn, A. M., Sandodden, R., and Edsman, L. (2019). Biocide treatment of invasive signal crayfish: successes, failures and lessons learned. Diversity 11:29. doi: 10.3390/d11030029
Perdikaris, C., Konstantinidis, E., Georgiadis, C., and Kouba, A. (2017). Freshwater crayfish distribution update and maps for Greece: combining literature and citizen-science data. Knowl. Manag. Aquat. Ecosyst. 418:51. doi: 10.1051/kmae/2017042
Persson, M., and Söderhäll, K. (1983). Pacifastacus leniusculus Dana and its resistance to the parasitic fungus Aphanomyces astaci Schikora. Freshw. Crayfish 5, 292–298. doi: 10.5869/fc.1983.v5.292
Préau, C., Nadeau, I., Sellier, Y., Isselin-Nondedeu, F., Bertrand, R., Collas, M., et al. (2020). Niche modelling to guide conservation actions in France for the endangered crayfish Austropotamobius pallipes in relation to the invasive Pacifastacus leniusculus. Freshw. Biol. 65, 304–315. doi: 10.1111/fwb.13422
Pyšek, P., and Richardson, D. M. (2010). Invasive species, environmental change and management, and health. Ann. Rev. Env. Res. 35, 25–55. doi: 10.1146/ANNUREV-ENVIRON-033009-095548
RARITY (2020). LIFE – Rarity Project. Available online at: http://www.ismar.cnr.it/projects/internationalprojects/copy14_of_project-001/rarity-project?set_language=en&cl=en (accessed December 28, 2020)
Reynolds, J. D. (1988). Crayfish extinctions and crayfish plague in central Ireland. Biol. Cons. 45, 279–285. doi: 10.1016/0006-3207(88)90059-6
Rezinciuc, S., Galindo, J., Montserrat, J., and Diéguez-Uribeondo, J. (2014). AFLP-PCR and RAPD-PCR evidences of the transmission of the pathogen Aphanomyces astaci (Oomycetes) to wild population of European crayfish from the invasive crayfish species, Procambarus clarkii. Fungal Biol. 118, 612–620. doi: 10.1016/j.funbio.2013.10.007
Rezinciuc, S., Sandoval-Sierra, J. V., Oidtmann, B., and Diéguez-Uribeondo, J. (2016). “The biology of crayfish plague pathogen Aphanomyces astaci: current answers to most frequent questions,” in Freshwater Crayfish – A Global Overview, eds T. Kawai, Z. Faulkes, and G. Scholtz (London: Taylor and Francis Group), 182–204. doi: 10.1201/b18723-12
Richman, N. I., Böhm, M., Adams, S. B., Alvarez, F., Bergey, E. A., Bunn, J. J., et al. (2015). Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philos. Tr. R. Soc. B 370:20140060. doi: 10.1098/rstb.2014.0060
Ruokonen, T. J., Sjövik, R., Erkamo, E., Tulonen, J., Ercoli, F., Kokko, H., et al. (2018). Introduced alien signal crayfish (Pacifastacus leniusculus) in Finland- uncontrollable expansion despite numerous crayfisheries strategies. Knowl. Manag. Aquatic Ecosyst. 419:27. doi: 10.1051/kmae/2018016
Sahlin, U., Edsman, L., and Bohman, P. (2017). Riskanalys av Signalkräfta i Sverige. CEC Rapport Nr 04. Lund: Centre for Environmental and Climate Research (CEC), Lund University.
Sahlin, U., Smith, H. G., Edsman, L., and Bengtsson, G. (2010). Time to establishment success for introduced signal crayfish in Sweden–a statistical evaluation when success is partially known. J. Appl. Ecol 47, 1044–1052. doi: 10.1111/j.1365-2664.2010.01849.x
Sahlin, U., Troffaes, M. C., and Edsman, L. (2021). Robust decision analysis under severe uncertainty and ambiguous tradeoffs: an invasive species case study. Risk Anal. doi: 10.1111/risa.13722
Sandodden, R. (2019). “Eradication of invasive alien crayfish: past experiences and further possibilities,” in Island invasives: Scaling up to Meet the Challenge, Occasional Paper SSC no. 62, eds C. R. Veitch, M. N. Clout, A. R. Martin, J. C. Russell, and C. J. West (Gland: IUCN), 405–409.
Sandström, A., Andersson, M., Asp, A., Bohman, P., Edsman, L., Engdahl, F., et al. (2014). Population collapses in introduced non-indigenous crayfish. Biol. Invasions 16, 1961–1977. doi: 10.1007/s10530-014-0641-1
Saunders, G., Cooke, B., McColl, K., Shine, R., and Peacock, T. (2010). Modern approaches for the biological control of vertebrate pests: an Australian perspective. Biol. Control 52, 288–295. doi: 10.1016/j.biocontrol.2009.06.014
Scheers, K., Boets, P., Abeel, T., and Van den Neucker, T. (2020). First records of alien crayfish of the Procambarus acutus species complex in Belgium. BioInv. Rec. 9, 562–569. doi: 10.3391/bir.2020.9.3.11
Scheers, K., Brys, R., Abeel, T., Halfmaerten, D., Neyrinck, S., and Adriaens, T. (2021). The invasive parthenogenetic marbled crayfish Procambarus virginalis Lyko, 2017 gets foothold in Belgium. BioInv. Rec. 10, 326–340. doi: 10.3391/BIR.2021.10.2.11
Scoones, I., Edelman, M., Borras, S. M. Jr., Hall, R., Wolford, W., and White, B. (2018). Emancipatory rural politics: confronting authoritarian populism. J. Peasant Stud. 45, 1–20. doi: 10.1080/03066150.2017.1339693
Seitz, R., Vilpoux, K., Hopp, U., Harzsch, S., and Maier, G. (2005). Ontogeny of the Marmorkrebs (marbled crayfish): a parthenogenetic crayfish with unknown origin and phylogenetic position. J. Exp. Zool. A Comp. Exp. Biol. 303, 393–405. doi: 10.1002/jez.a.143
Simmonds, P., Aiewsakun, P., and Katzourakis, A. (2019). Prisoners of war—host adaptation and its constraints on virus evolution. Nat. Rev. Microbiol. 17, 321–328. doi: 10.1038/s41579-018-0120-2
Söderbäck, B. (1991). Interspecific dominance and aggressive interactions in the freshwater crayfishes Astacus astacus (L.) and Pacifastacus leniusculus (Dana). Can. J. Zool. 69, 1321–1325. doi: 10.1139/z91186
Söderbäck, B. (1994a). Interactions among juveniles of two freshwater crayfish species and a predatory fish. Oecologia 100, 229–235. doi: 10.1007/BF00316949
Söderbäck, B. (1994b). Reproductive interference between two co-occurring crayfish species, Astacus astacus L. and Pacifastacus leniusculus Dana. Nord. J. Freshw. Res. 69, 137–143.
Söderbäck, B. (1995). Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a Swedish lake: possible causes and mechanisms. Freshw. Biol. 33, 291–304. doi: 10.1111/j.1365-2427.1995.tb01168.x
Söderbäck, B., and Edsman, L. (1998). Action Plan For The Restoration Of Noble Crayfish. Stockholm: Fiskeriverket and Naturvårdsverket.
Söderhäll, K., and Cerenius, L. (1999). The crayfish plague fungus: history and recent advances. Freshw. Crayfish 12, 11–35. doi: 10.1016/0044-8486(92)90133-6
Souty-Grosset, C., Holdich, D., Noel, P., Reynolds, J. D., and Haffner, P. (2006). Atlas of Crayfish in Europe. Paris: Museìum national d’Historie naturelle.
Spitzy, R. (1973). Crayfish in Austria, history and actual situation. Freshw. Crayfish 1, 9–14. doi: 10.5869/fc.1973.v1.009
Stebbing, P., Longshaw, M., and Scott, A. (2014). Review of methods for the management of non-indigenous crayfish, with particular reference to Great Britain. Ethol. Ecol. Evol. 26, 204–231. doi: 10.1080/03949370.2014.908326
Strand, D. A., Jussila, J., Viljamaa-Drinks, S., Kokko, H., Makkonen, J., Holst-Jensen, A., et al. (2012). Monitoring the spore dynamics of Aphanomyces astaci in the ambient water of latent carrier crayfish. Vet. Mic. 160, 99–107. doi: 10.1016/j.vetmic.2012.05.008
Strayer, D. L., and Dudgeon, D. (2010). Freshwater biodiversity conservation: recent progress and future challenges. J. North Am. Benthol. Soc. 29, 344–358. doi: 10.1899/08-171.1
Svärdson, G. (1965). The American crayfish Pacifastacus leniusculus (DANA) introduced into Sweden. Rep. Inst. Freshw. Res. Drottningholm 46, 90–94.
Svärdson, G. (1995). The early history of signal crayfish introduction into Europe. Freshw. Crayfish 8, 68–77. doi: 10.5869/fc.1995.v8.068
Svoboda, J., Kozubíková, E., Kozák, P., Kouba, A., Koca, S. B., and Diler, Ö, et al. (2012). PCR detection of the crayfish plague pathogen in narrow-clawed crayfish inhabiting Lake Eğirdir in Turkey. Dis. Aquatic Org 98, 255–259. doi: 10.3354/dao02445
Svoboda, J., Kozubíková-Balcarová, E., Kouba, A., Buřič, M., Kozák, P., Dieìguez-Uribeondo, J., et al. (2013). Temporal dynamics of spore release of the crayfish plague pathogen from its natural host, American spiny-cheek crayfish (Orconectes limosus), evaluated by transmission experiments. Parasitology 140, 792–801. doi: 10.1017/S0031182012002223
Svoboda, J., Strand, D. A., Vrålstad, T., Grandjean, F., Edsman, L., Kozák, P., et al. (2014). The crayfish plague pathogen can infect freshwater−inhabiting crabs. Freshw. Biol. 59, 918–929. doi: 10.1111/fwb.12315
Taugbøl, T. (2004). Exploitation is a prerequisite for conservation of Astacus astacus. Bull. Fr. Pêche Piscic. 372-373, 275–279. doi: 10.1051/kmae:2004002
Taugbøl, T., Edsman, L., and Souty-Grosset, C. (2004). Preface second thematic meeting of CRAYNET, Halden Norway, 1-4 September 2003. Bull. Fr. Pêche Piscic. 372-373, 233–239. doi: 10.1051/kmae:2004022
Teem, J. L., Alphey, L., Descamps, S., Edgington, M. P., Edwards, O., Gemmell, N., et al. (2020). Genetic biocontrol for invasive species. Front. Bioeng. Biotech. 8:452. doi: 10.3389/fbioe.2020.00452
Thomas, J. R., Robinson, C., Mrugaa, A., Ellison, A., Matthews, E., Griffiths, S., et al. (2020). Crayfish plague affects juvenile survival and adult behaviour of invasive signal crayfish. Parasitol. 147, 706–714. doi: 10.1017/s0031182020000165
Todorov, M., Trichkova, T., Hubenov, Z., and Jurajda, P. (2020). Faxonius limosus (Rafinesque, 1817) (Decapoda: Cambaridae), a new invasive alien species of European Union concern in Bulgaria. Acta Zool. Bulgar. 72, 113–121.
Trožić-Borovac, S., Škrijelj, R., Vesnić, A., Ðug, S., Mušović, A., Šljuka, S., et al. (2019). ““Negative effects of introducing allochthonous species Pacifastacus leniusculus (Dana, 1852) into aquatic ecosystems of Bosnia and Herzegovina”,” in Book of Abstracts – 3rd Symposium of Freshwater Biology, eds M. Ivković, I. Stanković, R. Matoničkin Kepčija, and R. Gračan (Zagreb: Croatian Association of Freshwater Ecologists), 33–33.
Unestam, T. (1969a). On the adaptation of Aphanomyces astaci as a parasite. Physiol. Plant. 22, 221–235. doi: 10.1111/j.1399-3054.1969.tb07371.x
Unestam, T. (1969b). Resistance to the crayfish plague in some American, Japanese and European crayfishes. Rep. Inst. Freshw. Res., Drott. 49, 202–209.
Unestam, T. (1972). On the host range and origin of the crayfish plague fungus. Rep. Inst. Freshw. Res. Drott. 52, 192–198.
Unestam, T., and Weiss, D. W. (1970). Host parasite relationship between freshwater crayfish and crayfish disease fungus, Aphanomyces astaci. Responses to injection by a susceptible and a resistant species. J. Gen. Microbiol. 60, 77–90. doi: 10.1099/00221287-60-1-77
Ungureanu, E., Mojžišová, M., Tangerman, M., Ion, M. C., Pârvulescu, L., and Petrusek, A. (2020). The spatial distribution of Aphanomyces astaci genotypes across Europe: introducing the first data from Ukraine. Freshw. Crayfish 25, 77–87. doi: 10.5869/fc.2020.v25-1.077
Usio, N., and Townsend, C. R. (2008). Functional significance of crayfish in stream food webs: roles of omnivory, substrate heterogeneity and sex. Oikos 98, 512–522. doi: 10.1034/j.1600-0706.2002.980316.x
Vey, A., Söderhäll, K., and Ajaxon, A. (1983). Susceptibility of the Orconectes limosus Raff. to the crayfish plague, Aphanomyces astaci Schikora. Freshw. Crayfish 5, 284–291. doi: 10.5869/fc.1983.v5.284
Viljamaa-Dirks, S., Heinikainen, S., Nieminen, M., Vennerström, P., and Pelkonen, S. (2011). Persistent infection by crayfish plague Aphanomyces astaci in a noble crayfish population–a case report. B. Eur. Ass. Fish Pathologists 31, 182–188.
Vodovsky, N., Patoka, J., and Kouba, A. (2017). Ecosystem of Caspian Sea threatened by pet-traded nonindigenous crayfish. Biol. Invasions 19, 2207–2217. doi: 10.1007/s10530-017-1433-1
Vogt, G. (2020). “Biology, ecology, evolution, systematics and utilization of the parthenogenetic marbled crayfish, Procambarus virginalis,” in Crayfish: Evolution, Habitat and Conservation Strategies, ed. F. Bezerra Riveiro (Hauppauge, NY: Nova Science Publishers), 137–227.
Vogt, G., Lukhaup, C., Williams, B. W., Pfeiffer, M., Dorn, N. J., Schulz, R., et al. (2018). Morphological characterization and genotyping of the marbled crayfish and new evidence on its origin. Zootaxa 4524, 329–350. doi: 10.11646/zootaxa.4524.3.3
Vojkovská, R., Horká, I., Tricarico, E., and Ďuriš, Z. (2014). New record of the parthenogenetic marbled crayfish Procambarus fallax f. virginalis from Italy. Crustaceana 87, 1386–1392. doi: 10.1163/15685403-00003365
Weinländer, M., and Füreder, L. (2016). Native and alien crayfish species: do their trophic roles differ? Freshw. Sci. 35, 1340–1353. doi: 10.1086/689031
Weiperth, A., Bláha, M., Szajbert, B., Seprõs, R., Bányai, Z., Patoka, J., et al. (2020). Hungary: a European hotspot of non-native crayfish biodiversity. Know. Manag. Aquat. Ecosyst. 421, 43. doi: 10.1051/kmae/2020035
Wells, K., Fordham, D. A., Brook, B. W., Cassey, P., Cox, T., O’Hara, R. B., et al. (2018). Disentangling synergistic disease dynamics: Implications for the viral biocontrol of rabbits. J. Anim. Ecol. 87, 1418–1428. doi: 10.1111/1365-2656.12871
Westman, K. (1973). The population of the crayfish, Astacus astacus L. in Finland and the introduction of the American crayfish Pacifastacus leniusculus Dana. Freshw. Crayfish 1, 41–55. doi: 10.5869/fc.1973.v1.041
Westman, K. (2002). “Alien crayfish in Europe: negative and positive impacts and interactions with native crayfish,” in Invasive Aquatic Species of Europe. Distribution, Impacts and Management, eds E. Leppäkoski, S. Gollasch, and S. Olenin (Dordrecht: Springer), 76–95. doi: 10.1007/978-94-015-9956-6_9
Westman, K., and Savolainen, R. (2001). Long term study of competition between two co-occurring crayfish species, the native Astacus astacus L. and the introduced Pacifastacus leniusculus Dana, in a Finnish lake. Bull. Franç. Pêche Piscic. 361, 613–627. doi: 10.1051/kmae:2001008
Westman, K., Savolainen, R., and Julkunen, M. (2002). Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a small, enclosed Finnish lake: a 30−year study. Ecography 25, 53–73. doi: 10.1034/j.1600-0587.2002.250107.x
Keywords: conservation, biased decision making, environmental economics, political ecology, fisheries administration, native and alien crayfish, shortsightedness
Citation: Jussila J, Edsman L, Maguire I, Diéguez-Uribeondo J and Theissinger K (2021) Money Kills Native Ecosystems: European Crayfish as an Example. Front. Ecol. Evol. 9:648495. doi: 10.3389/fevo.2021.648495
Received: 06 January 2021; Accepted: 29 June 2021;
Published: 30 July 2021.
Edited by:
Orsolya Valkó, Centre for Ecological Research, Hungarian Academy of Sciences, HungaryReviewed by:
Christoph Chucholl, Fischereiforschungsstelle Baden-Württemberg, GermanyCopyright © 2021 Jussila, Edsman, Maguire, Diéguez-Uribeondo and Theissinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Japo Jussila, amFwby5qdXNzaWxhQHVlZi5maQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.