- 1Center of Natural History, University of Hamburg, Hamburg, Germany
- 2Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
- 3Department of Cariology, Endodontics and Periodontology, University of Leipzig, Leipzig, Germany
- 4Institute for Terrestrial and Aquatic Wildlife Research (ITAW), University of Veterinary Medicine Hannover, Foundation, Büsum, Germany
Marine mammals are increasingly threatened in their habitat by various anthropogenic impacts. This is particularly evident in prey abundance. Understanding the dietary strategies of marine mammal populations can help predict implications for their future health status and is essential for their conservation. In this study we provide a striking example of a new dietary proxy in pinnipeds to document marine mammal diets using a dental record. In this novel approach, we used a combination of 49 parameters to establish a dental microwear texture (DMTA) as a dietary proxy of feeding behaviour in harbour seals. This method is an established approach to assess diets in terrestrial mammals, but has not yet been applied to pinnipeds. Our aim was to establish a protocol, opening DMTA to pinnipeds by investigating inter- and intra-individual variations. We analysed the 244 upper teeth of 78 Atlantic harbour seals (Phoca vitulina vitulina). The specimens were collected in 1988 along the North Sea coast (Wadden Sea, Germany) and are curated by the Zoological Institute of Kiel University, Germany. An increasing surface texture roughness from frontal to distal teeth was found and related to different prey processing biomechanics. Ten and five year old individuals were similar in their texture roughness, whereas males and females were similar to each other with the exception of their frontal dentition. Fall and summer specimens also featured no difference in texture roughness. We established the second to fourth postcanine teeth as reference tooth positions, as those were unaffected by age, sex, season, or intra-individual variation. In summary, applying indirect dietary proxies, such as DMTA, will allow reconstructing dietary traits of pinnipeds using existing skeletal collection material. Combining DMTA with time series analyses is a very promising approach to track health status in pinniped populations over the last decades. This approach opens new research avenues and could help detect dietary shifts in marine environments in the past and the future.
Introduction
Marine mammals are increasingly threatened by cumulative anthropogenic activities, such as chemical pollution, marine litter, noise pollution, climate change, shipping, and offshore-construction (Lotze et al., 2005; Das et al., 2008; Gilles et al., 2009; Unger et al., 2017; Lehnert et al., 2018; Mikkelsen et al., 2019; Baltzer et al., 2020). Given their long life spans, their predatory feeding at high trophic levels as well as their proximity to coastal regions, marine mammals can be considered sentinels for ocean and human health (Bossart, 2011). The understanding of their population viability (aquatic food web, prey availability, distribution pattern) and the assessment of possible threats is an important basis for an informed management and conservation measures (Parrish et al., 2002; Kovacs et al., 2012).
The harbour seal (Phoca vitulina Linnaeus, 1758) is the most widespread pinniped confined to the Northern Hemisphere and a successful apex-predator in the Wadden Sea (Jensen et al., 2017). However, the status of the species is highly affected by human impact. In the first half of the 20th century, the harbour seal population in the Wadden Sea has been severely decimated by a heavy hunting pressure (Reijnders, 1981). After the hunting prohibition in the mid-1970s and the establishment of national parks in German waters, the harbour seal population recovered gradually (Brasseur et al., 2018). Since then, the growing seal population became increasingly threatened by a shift of prey availability as a result of fisheries interaction and climate change (Kovacs et al., 2012). Moreover, reoccurring virus epizootics have led to increased harbour seal mortality. In 2014, the Influenza A virus subtype H10N7 has caused the death of several thousand European harbour seals, with approximately 1,400 dead seals in the coastal waters of Schleswig Holstein, Germany, alone (Bodewes et al., 2015). In 1988 and 2002, the north-European population was struck by large scale epizootics caused by the phocine distemper virus (PDV), resulting in the largest mass mortality events in marine mammals recorded, with 23,000 and 30,000 dead harbour seals respectively (Härkönen et al., 2006). The PDV epidemics were associated with chronic exposure to contaminant loads that accumulate through aquatic food chains and weaken the immune function in harbour seals (Hall et al., 1992; De Swart et al., 1996; Ross et al., 1996). Many of the PDV infected harbour seal carcasses have been studied in an effort to better understand the risks and dangers to the population and to improve the conservation management (Härkönen and Heide-Jørgensen, 1990; Abt, 2002; Müller et al., 2004). As a side effect, the skeletal material has been accumulated in scientific collections. These large series of the harbour seal population are an exceptional well-documented treasure with the potential to reconstruct harbour seal ecology and to engineer new tools to assess the health status of populations (Kahle et al., 2018; Ludolphy et al., 2018; Kierdorf et al., 2019).
The dental wear in curated tooth material can be a powerful tool for dietary discrimination. This dietary proxy has been studied on skeletal material from scientific collections for more than 40 years (Walker et al., 1978; Ungar, 2004). Dental wear occurs whenever a tooth comes into contact with another tooth (attrition) or ingesta (abrasion) (Kaiser et al., 2015). During the last decade, these microscopic scars have increasingly been interpreted by three-dimensional non-contact automated methods (for review see Schulz-Kornas et al., 2021) called dental microwear texture analysis (DMTA). These scars are continuously recorded and erased with each subsequent feeding event (Müller et al., 2014; Winkler et al., 2019a), thus the enamel surface merely contains a record of an individual’s last few meals (Teaford and Oyen, 1989). Winkler et al. (2020a) showed in feeding experiments with rats (Rattus norvegicus forma domestica), that the overwriting of wear features on a micrometer scale takes at least two weeks.
Regarding terrestrial carnivorans, most DMTA measurements were taken on shearing and crushing facets of the maxillary fourth premolar and the mandibular first molar (Schubert et al., 2010; Ungar et al., 2010; De Santis et al., 2013; De Santis, 2016), which are differentiated into a pair of carnassial (Van Valen, 1969). These teeth are most probably used to efficiently process prey by shearing flesh and breaking bones. However, the dentition of marine mammals at large is specialised for capturing, rather than processing (Werth, 2000; Armfield et al., 2013; Loch and Simoes-Lopes, 2013). In contrast to terrestrial carnivorans, marine carnivores are not able to pin down struggling prey in their aqueous environment (Kienle and Berta, 2016). Furthermore, the mastication is reduced in marine mammal species, since oral processing of prey would be difficult and most probably inefficient, as prey items may drift away during consumption (Werth, 2000; Churchill and Clementz, 2015). To overcome those difficulties, the majority of marine mammals use a piercing bite to capture rapidly moving prey items, then swallow them whole (Adam and Berta, 2002; Armfield et al., 2013; Jones et al., 2013; Kienle and Berta, 2016). Regarding its skeletal and dental anatomy, the harbour seal is categorised as a pierce feeder (Adam and Berta, 2002; Churchill and Clementz, 2015), using its frontal teeth to seize agile prey, then swallowing it whole (Adam and Berta, 2002; Armfield et al., 2013; Jones et al., 2013; Kienle and Berta, 2016). The harbour seal uses raptorial biting to capture large prey, whereas smaller prey is captured using suction feeding (Kienle, 2014; Marshall et al., 2014; Ydesen et al., 2014). Similar to other phocids, the harbour seal displays an increased homodonty, simple cusp morphology, reduced carnassials (typical of carnivoran dentition) and a serial eruption sequence of the postcanine teeth (Loughlin, 1982; Adam and Berta, 2002; Churchill and Clementz, 2015). The deciduous teeth shed already in utero or shortly after birth, in order for the permanent dentition to be fully functional at the end of a short suckling period (4 – 6 weeks) (Meyer and Matzke, 2004).
In the North Sea, the diet composition of the harbour seal consists mostly of gadoid and flat fishes or clupeids and sand eels (Pierce et al., 1991; Gilles et al., 2008; de la Vega et al., 2016). Stomach content analyses conducted in the 70s and 80s revealed benthic fish, such as flounders, plaice, and gobies to be the most important prey species in the Wadden Sea (Behrends, 1985; Sievers, 1989), reflecting that harbour seals are opportunistic hunters. Additionally, harbour seals’ food composition changed with increasing age, depending on size of both hunter and prey and availability of prey species (Behrends, 1985). Between 1981 and 1984 juvenile harbour seal stomach contents consisted of flatfish, gobies, and shrimp, while a shift towards more plaice, flounders and gadoids was observed with increasing age in sub-adults and adults (Behrends, 1985). Behavioural differences in harbour seals exist not only between age classes, but also between the sexes (Härkönen et al., 1999). Temporal and spatial behavioural differences between male and female harbour seals have been reported for water depth use, dive duration, dive shapes as well as movement, side fidelity and haul-out behaviour and are most likely linked to the annual reproduction cycle (Thompson et al., 1989; Baechler et al., 2002; Dietz et al., 2012; Wilson et al., 2015). Seasonal changes in diet composition indicated by gut content, spew/scat samples and stable isotopes have been linked to periodic shifts in prey availability and in movement patterns of harbour seals (Pierce et al., 1991; Hall et al., 1998; Dietz et al., 2012; de la Vega et al., 2016).
Considering dietary analysis in predatory taxa, DMTA has been successfully applied to terrestrial carnivorans (Schubert et al., 2010; De Santis, 2016), microbats (Purnell et al., 2013), fishes (Purnell et al., 2012; Purnell and Darras, 2015), odontocete whales (Purnell et al., 2017), and reptiles (Bestwick et al., 2021, 2019; Winkler et al., 2019b), but not yet to pinnipeds. Therefore, our goal is to establish a workflow for applying DMTA in pinnipeds and determine suitable tooth positions for measuring the dental microwear texture in harbour seals. We aim to develop a standardised sampling protocol for targeting tooth sampling positions in order to reduce variation in texture roughness due to differences in feeding biomechanics, which could possibly obscure dietary signals (Gordon, 1982). For this study, archived material from museum collections was used to establish DMTA as sensible tool to assess long term dietary and ecosystem changes in vulnerable marine top predators. We tested the following hypotheses.
(a) The dental microwear texture in harbour seals should differ between frontal and distal tooth positions because of their dissimilar demands.
(b) The dental microwear texture should differ between age classes, sexes, and seasons according to reported observed differences in foraging behaviour.
Materials and Methods
Specimen Selection
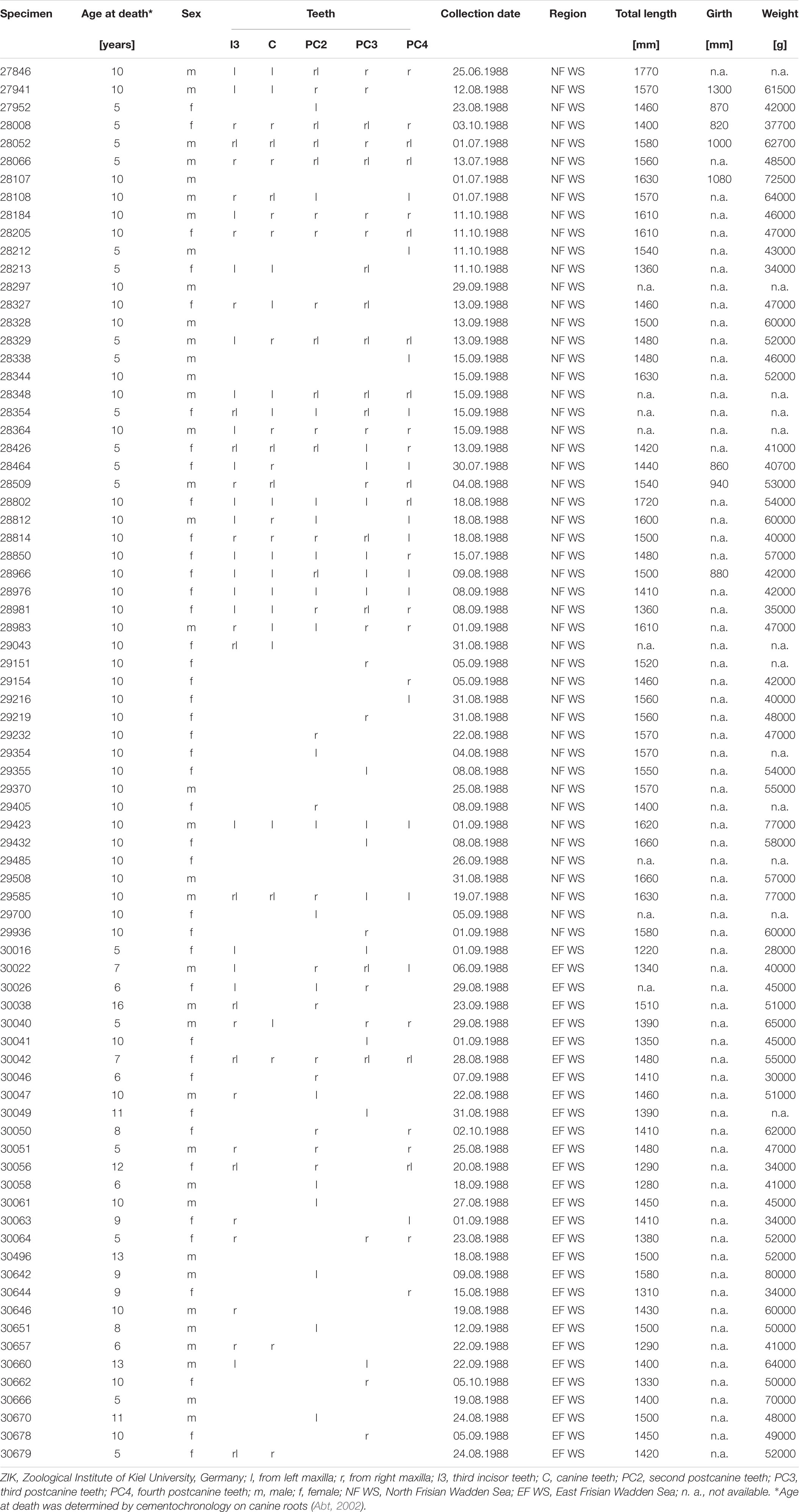
This study focused on the upper dentition of skeletal material from adult harbour seal specimens (Figure 1) (41 females, 37 males), curated at the osteological collection of the Zoological Institute of Kiel University, Germany. We concentrated on the dental record of harbour seals, which were collected in the year 1988 between June and October along the German Wadden Sea coast (Figure 1). Since the North European harbour seal population of 1988/89 was highly decimated by the phocine distemper virus (PDV), we were provided with a large number of individuals from both sexes across all age groups, which had died within a short period of time (4 month). The period of time reflects two seasons, summer = June, July, August; fall = September, October. This gave us a snapshot of the population and minimised the effect of other seasonal and annual prey availability. Due to the fragile preservation of the dental enamel of harbour seals, not all age groups were available for sampling and only two adult age groups (≥ 5 years and ≥ 10 years were available in sample sizes with n ≥ 10). Further, due to damage of single teeth (Supplementary Figure 1), we selected one side of the jaw only and chose the tooth of the better-preserved jaw side (left or right). Information on sex, age at death, date, and location of collection as well as data on body length and weight was available for each animal. Age at death was determined by using cement-layer-analysis of the upper canine teeth (Abt, 2002). The 49 examined specimens from the North Friesian Wadden Sea (Figure 1) were 5 years (n = 12) and 10 years old at death (n = 37) (Table 1). An additional 30 examined specimens stem from the East Frisian Wadden Sea, ranging from 5- to 16-year old individuals (Table 1). We focused on the upper dentition (Figure 2) since during the epizootic the skulls were more often retrieved from the carcasses and curated than mandibles. The upper permanent dentition of harbour seals consists of tooth classes according to the dental formula (I 3/2, C 1/1, P 4/4, M 1/1 = 34) (Jefferson et al., 1993; Figures 2A,B). The premolar and molar teeth in pinnipeds are not clearly distinguishable. Therefore, those teeth are often referred to as postcanine teeth (Hillson, 1986). In harbour seals, these postcanines are small, uniform, of simple shape and have a main cusp and smaller cusps located mesial and distal (Hillson, 1986; Figure 2). Along the upper dentition, the following tooth positions (Figure 2) were measured: third incisor (I3), canine tooth (C), second (PC2), third (PC3) and fourth postcanine teeth (PC4). The first and second incisor as well as the first and the fifth postcanine teeth were excluded from this study. The first two incisors (I1, I2) of the upper dentition were too small to take adequate moulds. In most cases, those teeth were glued into the maxillary bone with an incorrect orientation. The first postcanine (PC1) exhibited no wear as it possibly experienced no tooth-tooth contact and/or limited contact to ingesta due to its small size and its situation between much larger teeth. The fifth postcanine (PC5) was often calcified and/or exhibited sections were the thin enamel was flaked off, potentially due to excessive heating and drying during skull preparation (Aalderink et al., 2015). Specimens with dental anomalies (f. e. supernumerary teeth, abnormal tooth morphologies) and lesions as well as strong pathological changes of the temporomandibular joint (Kahle et al., 2018; Ludolphy et al., 2018) were excluded from this study.
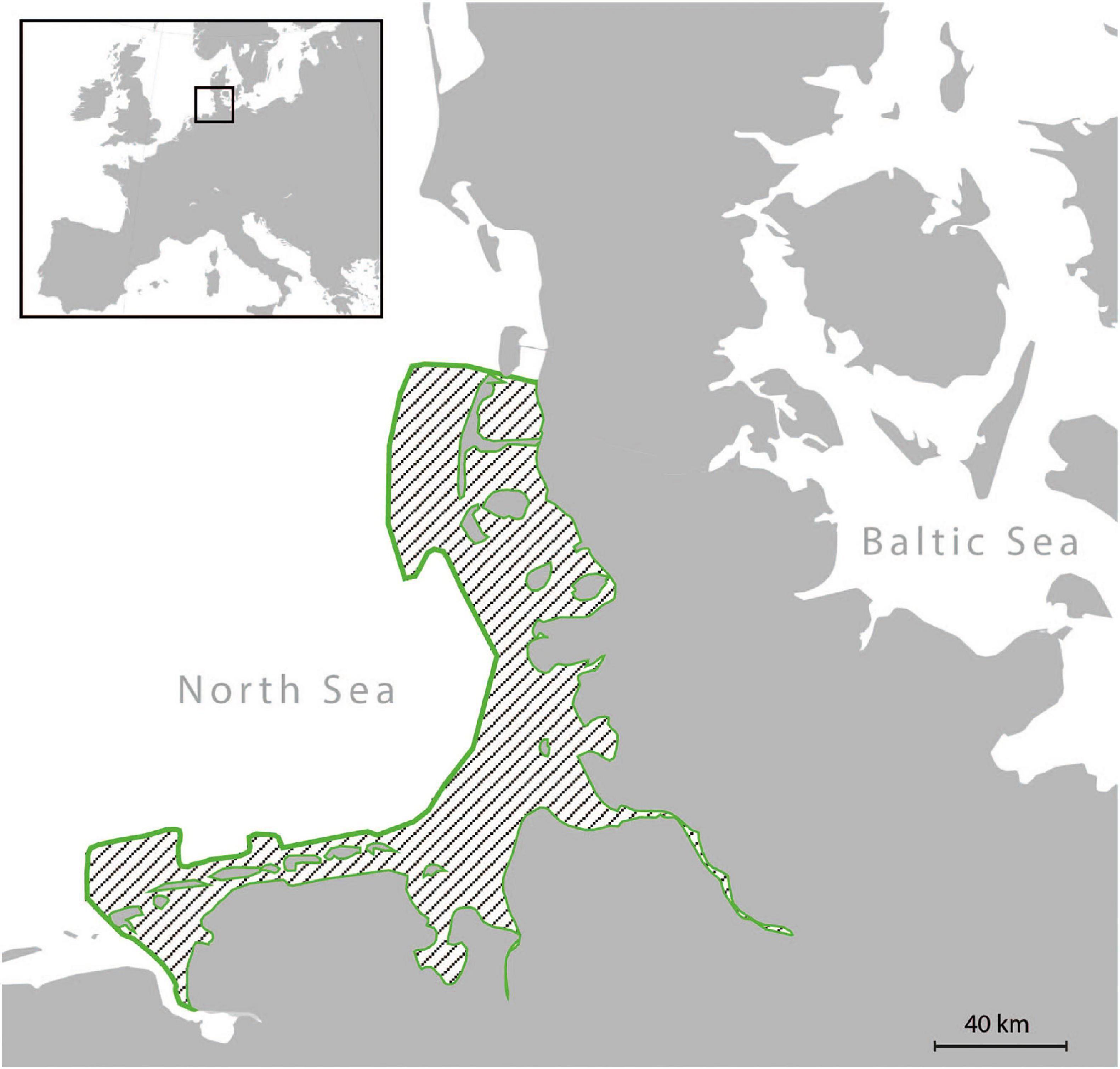
Figure 1. Map of the German Wadden Sea. During the summer/fall of 1988, all examined specimens were collected along the coast of the German Wadden Sea (area outlined in green).
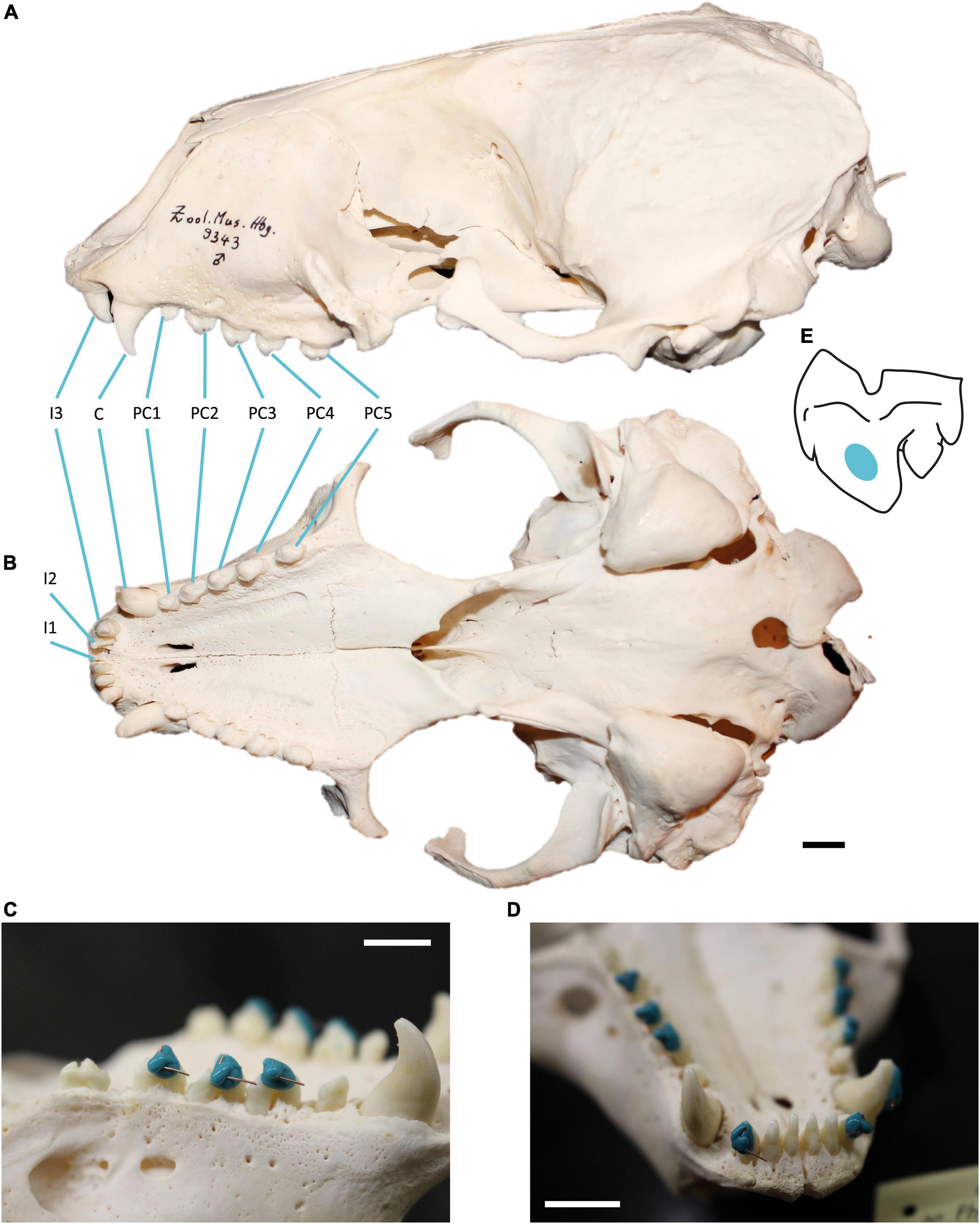
Figure 2. Harbour seal (Phoca vitulina) dentition and moulding process. (A) Skull in ventral and (B) lateral view showing the upper dentition. Tooth positions from mesial to distal: first incisor (I1), second incisor (I2), third incisor (I3), canine (C), first postcanine (PC1), second postcanine (PC2), third postcanine (PC3), fourth postcanine (PC4), fifth postcanine (PC5). Specimen 9343, curated at Center of Natural History, Hamburg, Germany. (C) Moulding process on the buccal side of PC2, PC3 and PC4 and (D) on C and I3. The short wire is oriented towards cusp tip, the long wire towards mesial. Specimen 30670, curated at the skeletal collection of the Zoological Institute, University of Kiel, Germany. Scale = 1 cm. (E) Buccal view of measurement area (coloured) on the third upper postcanine tooth of Phoca vitulina.
Moulding
After cleaning the dentition with acetone-drenched soft cotton, moulds of upper teeth surfaces were made, using a high resolution silicone (Provil novo Light C.D.2 regular set; Type 3; Heraeus Kulzer, Dormagen, Germany) following established procedures (Schulz et al., 2010). To ensure measurements on intact enamel regardless of the wear stage, the silicone was applied in all specimens on an enamel surface not prone to chipping. On the second to fourth postcanine teeth of older animals, the thin enamel was occasionally abraded mesially along the narrow ridge of the main cusp. It was also worn down distal-lingually on the smaller cusps exposing areas of dentine (Supplementary Figure 1). Therefore, the silicone was applied not on the occluding dental facets between upper and lower dentition, but instead buccally on the main cusp of the postcanines on the straight surface between the maximum convexity at the cervical third and the tip (Figures 2C–E). The third incisors were moulded on the labial side, level with the postcanine moulds (Figure 2D). The canines of older individuals showed signs of lateral wear, most likely caused by attritional contacts between upper and lower canine teeth. Therefore, all moulds of the canines were taken on the labial side (Figure 2D).
Data Acquisition
Surface scans of enamel facets were conducted by the high-resolution disk scanning confocal microscope μsurf Custom with a blue LED (470 nm) and a high-speed progressive-scan digital camera (984 × 984 pixels) (NanoFocus AG, Oberhausen, Germany) following the established protocol (Schulz et al., 2010). Pre-processing and filtering following the established protocol (Schulz et al., 2013, 2010); the template used is given as Supplementary Material 1. Where possible, four non-overlapping measurement fields per facet with a square area of 160 μm2 were collected. We used a 100 × long distance objective (numerical aperture of 0.8, a resolution in x, y = 0.16 μm and z = 0.06 μm). Measurements with < 95% surface points or a vertical displacement range of δz > 40 μm were rejected as well as surface areas with defects or adherent dirt. The dental microwear texture (DMT) was quantified by employing two methods: (1) scale-sensitive fractal analysis (SSFA, four parameters, Table 2) using length-scale and area-scale fractal analyses (Ungar et al., 2003; Scott et al., 2006) and (2) three-dimensional surface texture analysis (3DST, 46 parameters, Table 2) using standardised roughness (ISO 25178) and flatness (ISO 12781) parameters combined with non-standardised further motif, furrows, direction and isotropy parameters (Schulz et al., 2010, 2013; Calandra et al., 2012). A full description of the 49 microwear texture parameters used is given in Table 2. The analysis was conducted using the μsoft analysis premium v.7.0.6672 software (NanoFocus AG, Oberhausen, Germany; a derivative of Mountains® Analysis software by Digital Surf, Besançon, France).
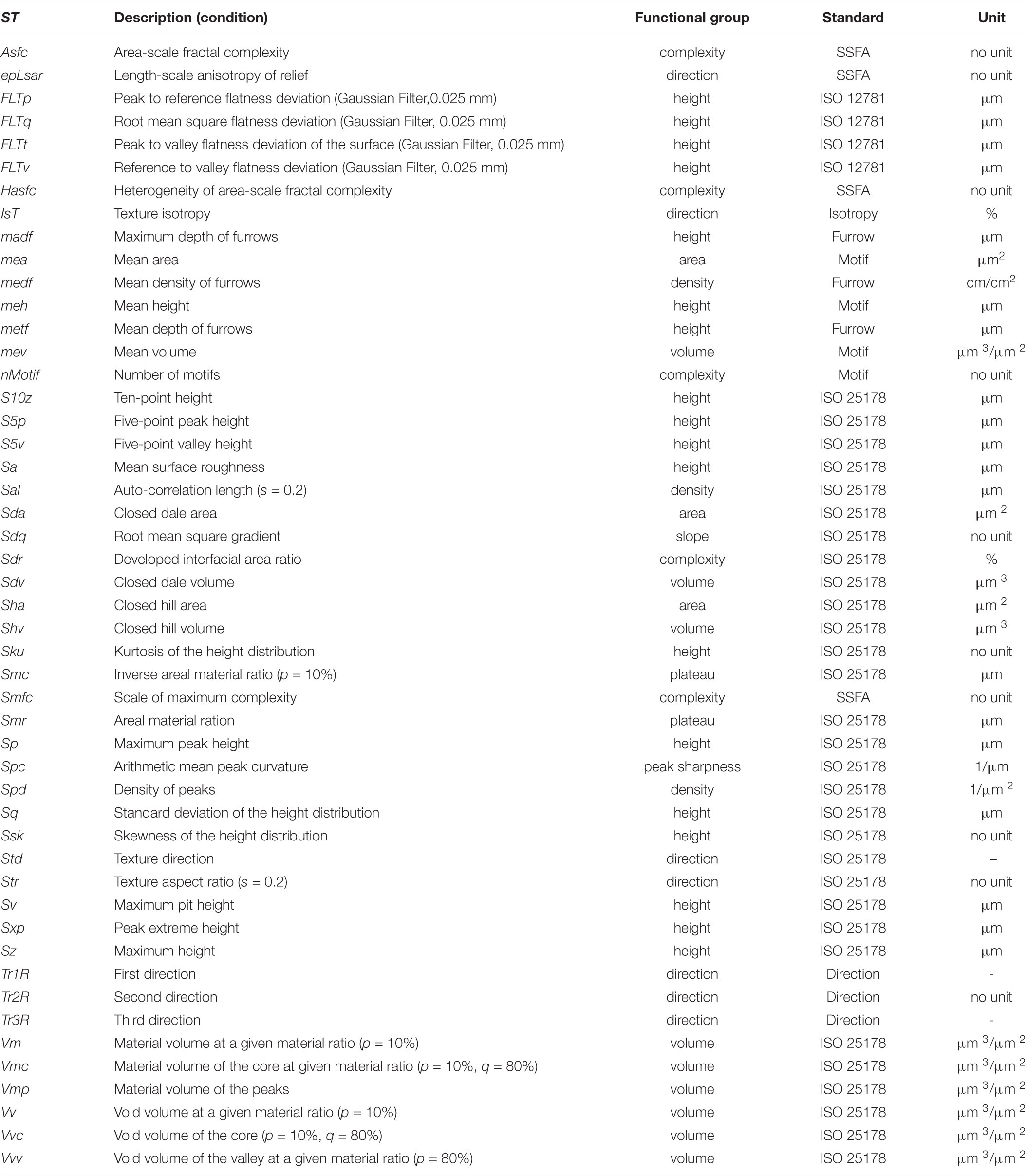
Table 2. Description of applied microwear texture parameters according to ISO 25178, ISO 12781, motif, furrow, texture direction, isotropy and SSFA.
Statistics
The statistical analyses were carried out using the open-source software R versions 2.15.1 (R Development Core Team, 2011) following an established statistical procedure (Calandra et al., 2012). The R packages xlsx version 0.3.0 (Dragulescu, 2011), rJava (Urbanek, 2016), doBy version 4.5.9 (Højsgaard and Halekoh, 2013), grDevices version 2.15.1 (R Development Core Team, 2011) and R.utils version 1.9.11 (R Development Core Team, 2011) were used. All statistical tests were carried out using functions (Wilcox et al., 2005) which are included in the package WRS (Wilcox and Schönbrodt, 2010, v. 0.12.1). The groups tested were defined in four datasets according to the hypotheses (Table 3): sex (females versus males), age (5 years old versus 10 year old), season (summer = June/July versus fall = September/October); in each group sorted according to tooth position (I3/C/PC2/PC3/PC4); and additionally a pooled dataset with all specimens sorted according to tooth position (I3/C/PC2/PC3/PC4). All data were trimmed 15% in each tail to compensate for non-normality and heteroscedastic tests were applied due to heterogeneity of variances. The robust Welch-Yuen omnibus test (Welch, 1938; Yuen, 1974) was performed to test for significant differences (Supplementary Table 3) between the tested groups (Wilcox, 2003; Wilcox et al., 2005). Subsequently, the source of significant differences within the groups (arithmetical means) was determined using a heteroscedastic pairwise comparison test analogue to Dunnett’s T3 (“Dunnett test” hereafter) (Dunnett, 1980). The results were accepted if Cliff’s ordinal method (“Cliff test” hereafter) (Cliff, 1996), and the combination of the Welch-Yuen test and the Dunnett’s test showed a significant output (p ≤ 0.05). We applied the combination of the three tests using robust means to control over type I error (probability of detecting a false difference). This combination is similar to the F-test to test for group differences. Whenever a significant difference was found, the heteroscedastic pair-wise Dunnett’s test was subsequently employed to reveal the source of difference. In order to control for type II error (probability of not detecting a genuine difference, related to the power of the test) we applied a heteroscedastic rank-based test that performs Cliff’s test for all pairs, in which the family-wise-error is controlled via Hochberg’s method (Hochberg, 1988).
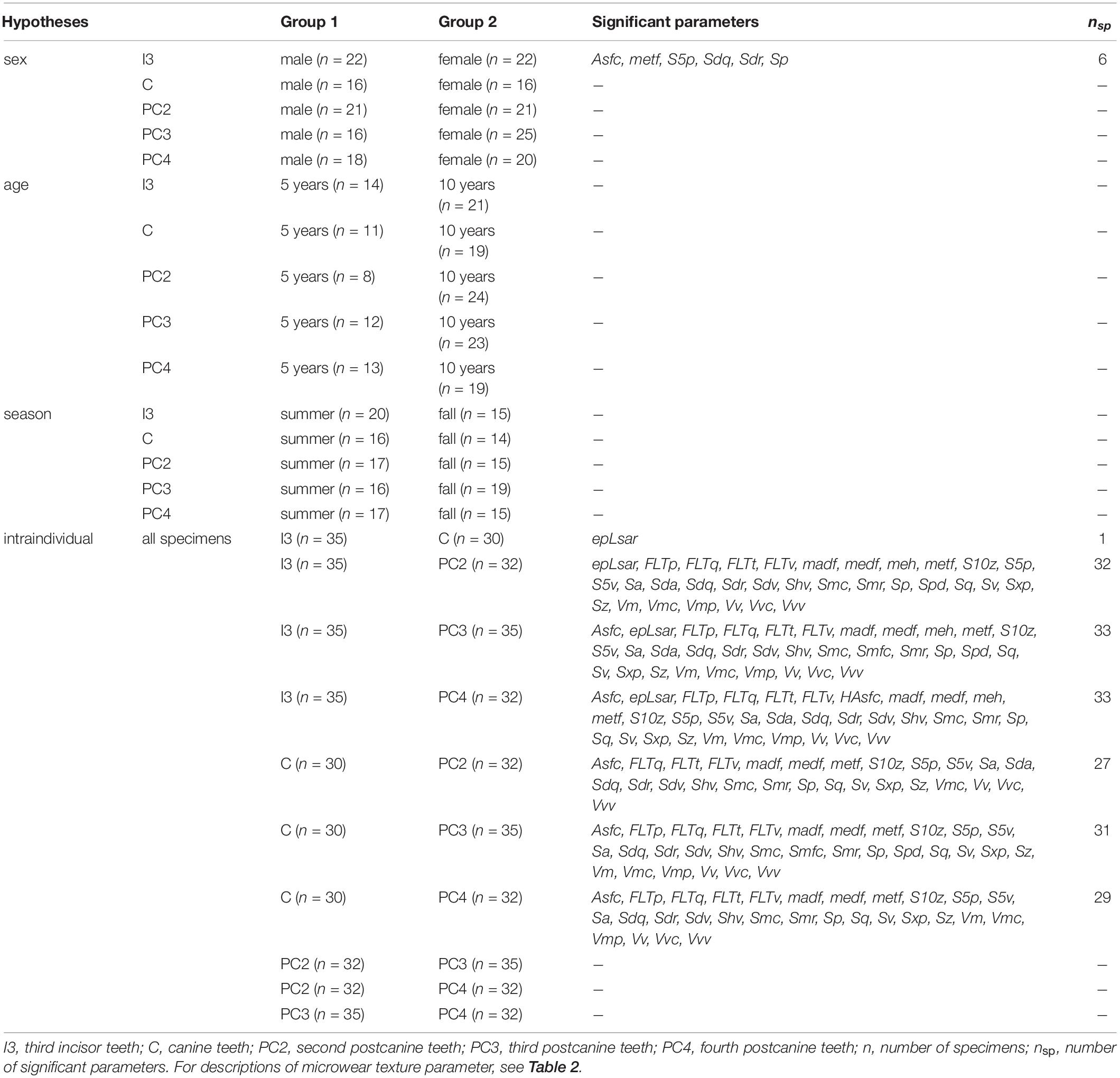
Table 3. Overview table of the tested hypotheses and the given statistical significant microwear texture parameters.
Results
Descriptive statistical values, including the mean and standard derivation (SD) for the surface texture parameters are given in Supplementary Tables 1–3. The parameters are described in Table 2.
Intra-Individual Variation
The microwear texture along the tooth rows of individuals differentiated into two groups: Firstly, the mesial teeth (I3, C) with a less rough surface texture (only difference in one out of 49 parameters), and, secondly, the more rough distal teeth (PC2, PC3, PC4) (no difference in 49 parameters), while I3 and PC3/4 differ in 33 out of 49 parameters. The distal teeth displayed a complex, anisotropic texture (Asfc, Smfc, Sdr) with a higher surface profile (meh, FLTp, FLTq, FLTt, FLTv, S10z, S5p, S5v, Sa, Sp, Sq, Sv, Sxp, Sz, Smc, Smr) featuring voluminous, more steep, pointed peaks (Sdv, Sdq, Shv, Spc) and deeper, denser furrows (medf, metf, madf) of greater volume (Vm, Vmc, Vmp, Vv, Vvc, Sha) (Table 3 and Figure 3). The frontal tooth positions I3 and C were similar in their texture roughness. The only significant difference between I3 and C was the higher anisotropy (epLsar) in the texture of the canine teeth, rather resembling the postcanine dentition. A trend of increasing surface roughness along the tooth row from mesial to distal was found, with most voluminous furrows and highest peaks in the postcanine teeth (Table 3, Figure 2).
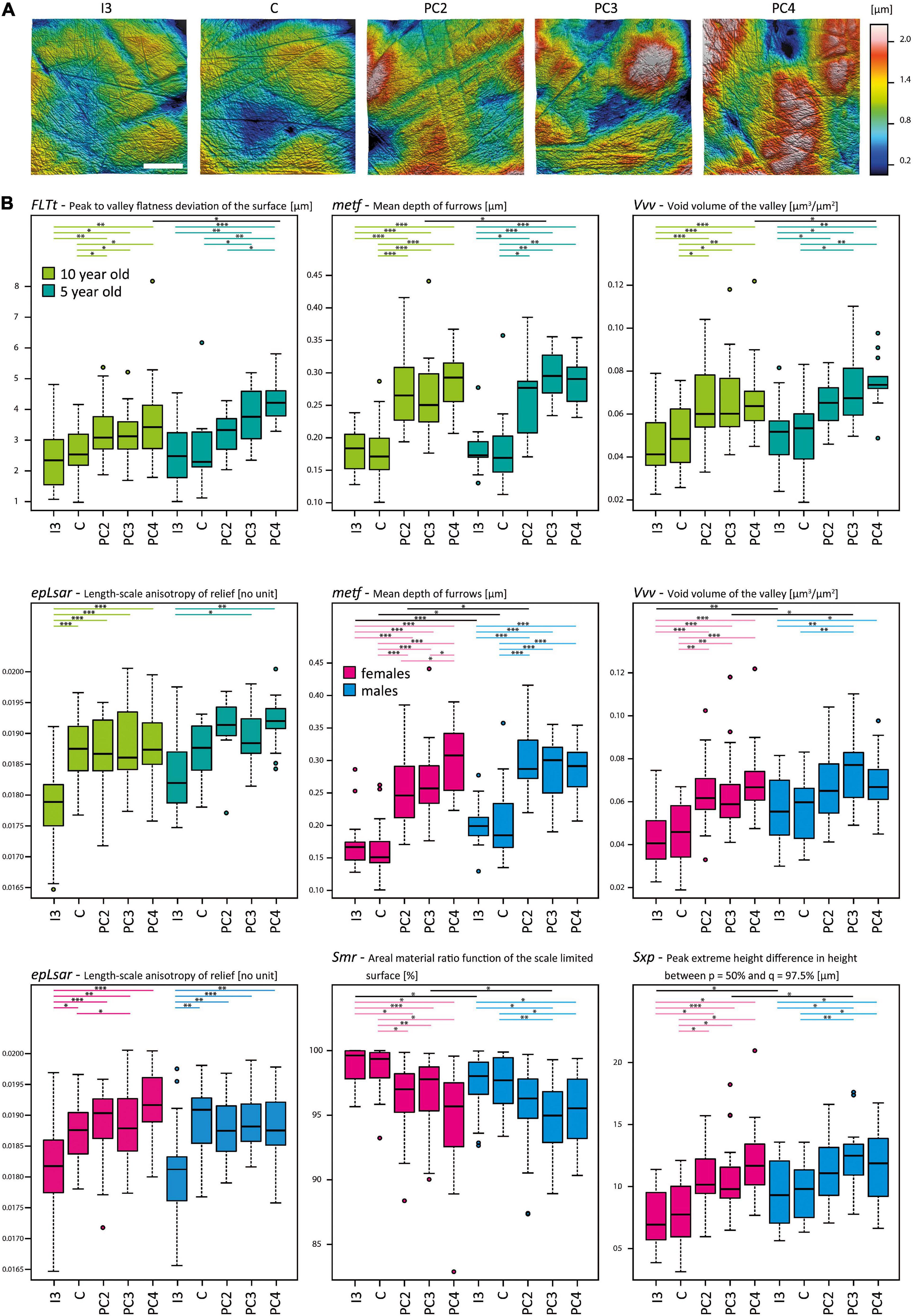
Figure 3. Dental microwear texture in the upper dentition of Phoca vitulina. (A) Three-dimensional (3D) microwear textures in Phoca vitulina (ZIK-28348) for all examined tooth positions. The textures are sorted from mesial to distal along the upper dentition. Scale = 40 μm. (B) Boxplots of selected surface texture parameters measured along the upper dentition of Phoca vitulina specimens at the age of 10 years (light green) and 5 years (dark green) as well as female (pink) and male (blue) specimens. The boxplots are sorted from mesial to distal teeth. Microwear texture parameters: FLTt = Peak to valley flatness deviation of the surface [μm] (ISO 12781), metf = Mean depth of furrows [μm] (Motif), epLsar = Length-scale anisotropy of the relief [no unit] (SSFA), Vvv = Void volume of the valley at a given material ratio (p = 80%) [μm3/μm2] (ISO 25178), Smr = Areal material ration [μm] (ISO 25178), Sxp = Peak extreme height [μm] (ISO 25178). Test statistics following the established protocol (Calandra et al., 2012) combining the Welch-Yuen-Test, the equivalent of Dunnett’s T3-test, and Cliff’s method; significance levels: ∗ = p ≤ 0.05, ∗∗ = p ≤ 0.01, ∗∗∗ = p ≤ 0.005. Abbreviations for tooth positions from mesial to distal teeth: I3, third upper incisor; C, canine; PC2, second postcanine; PC3, third postcanine; PC4, fourth postcanine.
Individual Age
The microwear texture of the frontal dentition in harbour seals of different age classes appeared to be similar (Table 3 and Figure 3). The variability within the age classes is larger than the difference between 5- and 10-year old specimens (Supplementary Table 3). Regarding the intra-individual variation in the 10-year old group, it is notable that microwear textures were undistinguishable between PC2, PC3 and PC4. In contrast, in 5-year old individuals the microwear texture of the second postcanine was significantly less complex (Asfc) than in the third postcanine (Supplementary Table 3). In contrast to the I3 in 10 year olds, the second postcanines exhibited a higher surface profile (FLTq, FLTv, S10z, S5v, Sa, Smc, Smr, Sq, Sv, Sxp, Sz) with pointed peaks (Sdv, Shv) and deeper, denser furrows of greater volume (madf, metf, Vmc, Vv, Vvv) (differences in 19 out of 49 parameters), whereas in 5 year olds, no differences between PC2 and I3 were detected (Supplementary Table 3).
Sexual Segregation
Sexual differences were most prominent in the I3 with 6 out of 49 microwear texture parameters (Table 3 and Figure 3). Regarding this tooth position, males (n = 22) displayed a microwear texture with higher complexity (Asfc, Sdr), higher pointy peaks (S5p, Sdq, Sp) as well as deeper furrows (metf) compared to females (n = 21) (Table 3 and Figure 3). In both sexes, no intra-individual differences between I3 and C or between the postcanines were detected (Supplementary Table 3). Both within females and males, the frontal tooth positions were clearly distinguishable from the postcanines in their texture roughness. In females, the postcanines (PC3/PC4) were rougher than I3 (difference in 24/24 out of 49 parameters) (Supplementary Table 3). In contrast, the differences in males between I3 and PC3/PC4 were not as pronounced (difference in 9/1 out of 49 parameters).
Seasonality of Feeding Behaviour
We were unable to fully examine seasonal variations dependent on sex and age classes because of the sample size. When pooled, the results revealed no differences in texture roughness between different seasons (summer = June, July, August; fall = September, October) (Table 3). In comparison to the summer specimens (n = 28), the fall specimens (n = 24) displayed a tendency of a more exaggerated gradient from smoother surface texture in frontal teeth (I3 lower values for FLTt, FLTv, Smr, Sv) to rougher texture in distal teeth (PC2 higher values for FLTp, FLTt, Vm, Vmp) (Supplementary Table 3). Within the fall specimens, the most differences in microwear texture were between I3 and PC4 (23 parameters out of 49), with PC4 displaying a more complex texture (Asfc, epLsar, Sdr), a larger texture profile (FLTq, FLTt, FLTv, Smr, madf, metf, S5v, Sa, Sdq, Sq, Sv, Sxp) and more voluminous peaks and furrows (Sdv Shv, Vm, Vmc, Vmp, Vv, Vvc, Vvv) (Supplementary Table 3). Within summer specimens, the differences between I3 and the postcanines were not as pronounced (9 parameters out of 49 between I3 and PC3).
Discussion
Intra-Individual Variation
Distal teeth exhibited larger texture depths and feature volumes than frontal teeth. We interpret the gradual increase of enamel surface roughness along the dentition from mesial to distal as a biomechanical trend along the tooth row, due to the functional shifts. Our results are in line with former results in marine odontocete whales (Purnell et al., 2017), which found that dental microwear texture (especially Sa, Sq and Sku values) varies with tooth location in the jaw in marine odontocete whales. Harbour seals use their frontal teeth to capture prey, whereas the postcanines are only engaged, if prey items are too large to swallow in whole and need to be reoriented or fractured (Adam and Berta, 2002; Fahlke et al., 2013). The posterior teeth in mammals transmit higher bite forces due to their proximity to the mandibular condyle (Greaves, 2002, 2000; Santana and Dumont, 2009). This might result in the rougher microwear texture of distal teeth. In contrast, short capturing bites with the frontal dentition could have caused less roughness. Soft ingesta (with less ingesta abrasives) tend to smoothen the tooth surface of soft-food feeders (Lucas et al., 2008). A similar effect for the surface topography but with a different cause was proposed (Kaiser et al., 2015) relating lower height and volume enamel surface texture parameters to a higher matrix resistance of the ingesta (independent of ingesta abrasives). The last case could be the situation we observed in harbour seals. Therefore, we consider it possible, that the piercing of soft fish might have led to more pronounced polishing in frontal teeth. We consider sediment, fish scales and exoskeletons to be the main abrasive agents in harbour seal ingesta. Previous studies on ungulates, rodents and primates attributed the main ingesta abrasiveness to airborne mineral dust and soil particles adherent to vegetation (Kaiser et al., 2018; Schulz-Kornas et al., 2019), which should play a minor role in a marine environment of the pelagic prey species. However, when hunting and capturing benthic prey species like sand eels (Ammodytidae), flatfish (Pleuronectiformes), and gobies (Gobiidae), the ingestion of additional sediment seems to be inevitable. The occasionally observed regurgitation behaviour in harbour seals has in fact been interpreted as a mechanism to expel swallowed sediment after feeding events targeting benthic prey (Bowen et al., 2002; Heithaus et al., 2009). It is known that quartz sand dominated most sediment sections of the German Wadden Sea (Volkman et al., 2000), but the indentation hardness of underwater quartz sand is unknown to us. According to nanoindentation studies, quartz dust (12.8 GPa) must be considered harder than dental enamel of several vertebrates, including humans (4.88 GPa), wild boars (Sus scrofa, 6.5 GPa) and dolphins (Steno bredanensis, 3.86 GPa, Pontoporia blainvillei, 2.36 GPa) (Loch and Simoes-Lopes, 2013; Lucas et al., 2013; Kaiser et al., 2018). However, the actual influence of sediment on the enamel microwear texture would be dependent on grain size, shape and quantity, as fine external abrasive have been reported to cause a polishing effect (Ackermans et al., 2020; Winkler et al., 2020b).
Other than incidentally ingested sediment, we propose the scales of modern teleost fish to be a potential abrasive agent in both pelagic and benthic prey species. Teleost fish scales consist of tough collagen reinforced with hydroxyapatite crystallites (Ikoma et al., 2003; Vernerey and Barthelat, 2010; Zhu et al., 2013; Naleway et al., 2016). Pleuronectiformes have ctenoid scales exhibiting complex external rugosities and surface appendices that entrap additional abrasive particles (Spinner et al., 2016; Minicozzi et al., 2019). Those surface appendices are found to be the hardest part of the ctenoid scale in the common sole (Solea solea, 0.40 GPa, Spinner et al., 2019). The contact between accumulated abrasives (ctenoid scales, overlying sediment) and harbour seal teeth would be amplified by repeated contact, which would be necessary when handling larger, unwieldy fish (Bowen et al., 2002). Therefore, we consider cryptic flatfish to be a more abrasive prey item than demersal fish with smooth cycloid scales, f. e. gadoids and gobies. Pelagic fish with cycloid scales, like smelt, we regard as less abrasive prey.
The anisotropy (epLsar) in the third incisor (I3) was significantly lower than all other (more distal) tooth positions. The higher anisotropy values in the canine and postcanine dentition are likely due to numerous parallel scratches (Figure 3A). This finding is in accordance with results from odontocete whales (Fahlke et al., 2013), who investigated 2D dental microwear of extinct whales and modern marine mammals. According to their results, the enamel surfaces on cheek teeth of harbour seals and other pinnipeds featured many parallel to subparallel scratches compared to other marine mammals, indicating a straight ortho-retractional occlusal movement.
Individual Age
No differences in dental microwear texture between 5- and 10- year old animals were detectable This could not be related to a slight shift in prey spectrum, driven by increased body mass, resulting in a preferences for larger prey (Sievers, 1989; de la Vega et al., 2016). We assume that during the (relatively short) study period both age groups used similar available dietary sources. Therefore, a pooling of adult samples in further analyses of our study was justified. It is important to note that both groups can be considered adult individuals (Sievers, 1989) and diet items are likely similar in composition and might differ only in size (larger prey size in older animals (Behrends, 1985).
Apart from the general trend toward more texture roughness in younger adults, the microwear texture between both adult age groups can be considered similar and could be explained by similar body size. For future studies we recommend to test larger age ranges that differ more widely in body size; for example juvenile animals below 2 years in comparison to 10-year old adult individuals. It would be promising to test if similar like in Californian sea lions four to seven years old individuals have highest frequency of excessive tooth wear in the species age profile, which was interpreted as an indication of deficient feeding and/or chronic malnutrition (Labrada-Martagon et al., 2007). This would allow to test if hunting success between juvenile individuals (< 2 years) and older individuals (> 5 years) that differ more clearly in body size, is more reflected in microwear texture signals.
Sexual Segregation
We found differences in microwear texture roughness between males and females in the frontal I3 position (Asfc, metf, S5p, Sdq, Sdr, Sp); within females, there were more pronounced differences between frontal and distal teeth (I3 – PC3: 24 differences) than within males (I3 – PC3: 9 differences).
As the texture in frontal teeth reflects the initial contact with prey and/or sediment and as males showed more roughness, this might indicate a sex-specific foraging style or aggressive behaviour during male-male competitions and mating in the breeding season. In previous studies, DMTA was successfully used to detect significant sex-related differences in the diet of terrestrial mammals (Merceron et al., 2010). For harbour seals, sexual segregation has been documented in foraging area, traveling distances and diving patterns (Baechler et al., 2002). Sexual segregation of habitat use has been reported as mechanism to minimise the effect of consumptive competition in harbour seals and other pinnipeds (Herreman et al., 2009; Wilson et al., 2015). Dietary differences between sexes in the Norwegian population of the harbour seals were found (Herreman et al., 2009), which the authors attributed to increased competition and predation-risk. This resulted in longer travel distances for males and in nearshore hunting behaviour in females during pupping and weaning. Since the Norwegian harbour seal population belongs to the same subspecies (Phoca vitulina vitulina) as our study material (Bjørge et al., 2010), we infer a similar foraging behaviour for the individuals from the German Wadden Sea. Further sex-related dietary resource partitioning was reported for two fur seal species (Arctocephalus forsteri and Arctocephalus pusillus) as a potential for reducing foraging competition in nearby waters (Page et al., 2006). We conclude that the variation in dental microwear texture between harbour seal males and females is caused by a sexual segregation to reduce forage competition.
Seasonality of Feeding Behaviour
The enamel surfaces of summer and fall specimens were indistinguishable in their microwear texture. Within fall specimens there is a microwear texture gradient along the tooth row that might reflect a seasonal shift in prey availability (prey distribution and abundance, feeding/spawning activity, prey size), which has been reported in several dietary studies (Pierce et al., 1991; Hall et al., 1998; de la Vega, 2016). During the summer, pelagic fish are the most abundant in the Wadden Sea, however it is the benthic species, which are mostly consumed by harbour seals (de la Vega et al., 2016). During fall, a higher use of North Sea dietary resources has been reported (de la Vega et al., 2016). A large moving distance and increased range size has been reported for harbour seals from the Danish Kattegat region (Dietz et al., 2012). As a result, minor seasonal variations in enamel surface roughness could reflect either no shift in prey composition or a switch in high abrasive foraging habitat from coastal to less abrasive offshore foraging grounds that are too small to be detected by microwear texture.
Microwear Texture as a Tool to Access Ecosystem Changes
Our data showed the importance of standard measuring protocols since the tooth position has a significant effect on variability of the dietary signal. The postcanine dentition, preferably PC4 - PC2, proved most suitable to assess dietary signals; it was the least affected by age and sex. For future studies, we recommend to test frontal C tooth position to discern a wider range of foraging behaviour. Additionally, seal populations from different geographic regions should be investigated to infer interspecific dietary preferences related to prey species abundance in different habitats. This would allow tracking the feeding ecology in various seal populations and ecosystems. Following this direction by sorting harbour seal museum material from different periods and under various anthropogenic endangerment, long term dietary monitoring of harbour seals would be possible. We propose that in harbour seals microwear texture has a very interesting potential to be an indicator of a common dietary niche or specific example of the available food in addition to gut content analyses that allow a very particular short-term snapshot of diet. This could lead to a better understanding of critical extinction events and especially shed some light on the feeding ecology’s role and habitat use.
Conclusion
This study successfully established a combination of 49 microwear texture parameters to quantify the enamel surface texture as an indicator of dietary behaviour in harbour seals. The buccal side of the upper teeth were selected for analysis and the second to fourth postcanine tooth positions were established as a reference. Increasing enamel texture roughness from frontal to distal was found along the tooth row. Compared to the abundant intra-individual differences, only small intra-specific differences dependent on age, sex and season were detected. Variations in microwear texture between ages were limited to the posterior teeth with a tendency toward more roughness in younger adults. The variations between sexes were more pronounced, with a decreasing trend from the most frontal tooth to the middle tooth positions and with slightly rougher microwear textures in males than in females. Minor seasonal variations were reflected in a stronger texture roughness gradient along the tooth row of fall specimens. Marine mammal collections contain useful records in detecting changes in food spectrum and prey availability. A better understanding of shifts in marine environments of the past might help employ conservation measurements for marine mammals in a changing environment. Future microwear texture studies on pinnipeds should adopt defined homogeneous parameters regarding tooth position and orientation to avoid obscuring dietary patterns with high variability linked to biomechanical intra-individual variation and to ensure comparability.
Data Availability Statement
The original contributions presented in the study are included in the article and the Supplementary Material (Supplementary Tables 1–4). Additionally, the Supplementary Template 1 and the surface measurements are accessible through the open source data repository of the University of Hamburg (template via doi: 10.25592/uhhfdm.8981, surface measurements (*.nms files) via doi: 10.25592/uhhfdm.8979).
Ethics Statement
Ethical review and approval was not required for the animal study because the dental specimen were parts of skeletal material in a bone collection in a Natural History Museum. No animal testing of living specimens was conducted.
Author Contributions
TK and ES-K designed the study. EB collected and analysed the data and wrote the manuscript with input from all co-authors (ES-K, KL, US, TK). All authors contributed to the article and approved the submitted version
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Volkswagen Foundation (AZ 89911) for funding this study within the framework of the program “Research in Museums,” as well as the Center of Natural History (CeNak), Hamburg, Germany, for their financial support. We would like to express our gratitude to Günther B. Hartl and Renate Lücht (Zoological Institute of Kiel University, Germany) for granting access to the osteological collection of the Zoological Institute of Kiel University. We would like to extend our thanks to Uwe Kierdorf, Patricia Kahle and Catharina Ludolphy (Department of Biology, University of Hildesheim, Germany) for their feedback and for access to publications and additional data. We would like to thank Sasha Viquerat (Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany) for helpful advice in statistics and Holger Krohn (CeNak) for assisting with measuring the DMTs.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.644019/full#supplementary-material
Supplementary Figure 1 | Lateral view of the upper postcanine dentition of Phoca vitulina displaying abraded enamel and calcification. Exposed dentine mesially along the narrow ridge of the main cusp and distal-lingually on the smaller cusps marked by white arrows on PC2, PC3 and PC4 (from left to right). Calcified area on PC5 marked by black arrow. Scale = 1 cm.
Supplementary Table 1 | Test statistics given for microwear texture data sorted. I3, third incisor teeth; C, canine teeth; PC2, second postcanine teeth; PC3, third postcanine teeth; PC4, fourth postcanine teeth; n, number of specimens; ST, surface texture parameter; Ft/ph, test statistics; p, level of significance (bold values indicate significant levels < 0.05); nu1/nu2/df, degree of freedom; pl, lower 95% confidence interval; pu, upper 95% confidence interval; pcrit, critical significance level α = 0.05; pcomp, 1 (p < pcrit) or 0 (p > pcrit). For descriptions of microwear texture parameter, see Table 2.
Supplementary Table 2 | Descriptive statistics (mean ± SD) for all DMTA parameters for the upper dentition of Phoca vitulina. For descriptions of microwear texture parameter, see Table 2.
Supplementary Table 3 | Overview table of the inter-individual variation within the tested groups and the given statistical significant microwear texture parameters. I3, third incisor teeth; C, canine teeth; PC2, second postcanine teeth; PC3, third postcanine teeth; PC4, fourth postcanine teeth; n, number of specimens; nsp, number of significant parameters. For descriptions of microwear texture parameter, see Table 2.
Supplementary Table 4 | Raw dental microwear data for all 242 measured teeth. phvit, Phoca vitulina; ZIK, Zoological Institute of Kiel University, Germany; I3, third incisor teeth; C, canine teeth; PC2, second postcanine teeth; PC3, third postcanine teeth; PC4, fourth postcanine teeth; n Scans, number of valid scans included in analysis; Total length [mm], Weight [g]; For descriptions of parameters, see Table 2. Surface texture files are stored at the database of the public natural history museum (University of Hamburg, Center of Natural History Hamburg, mammal collection) and can be accessed via the open source data repository of the University of Hamburg (doi: 10.25592/uhhfdm.8979).
Supplementary Template 1 | Template to analyse the raw files indicating a detailed batch analyses to run pre-and post-processing filtering of the surface texture files as conducted in established protocols (Schulz et al., 2013, 2010). The template is accessible through the open-source data repository Zenodo: doi: 10.25592/uhhfdm.8981.
References
Aalderink, M. T., Nguyen, H. P., Kass, P. H., Arzi, B., and Verstraete, F. J. M. (2015). Dental and temporomandibular joint pathology of the eastern pacific harbour seal (Phoca vitulina richardii). J. Comp. Pathol. 152, 335–344. doi: 10.1016/j.jcpa.2015.02.003
Abt, K. F. (2002). Phänologie und Populationsdynamik des Seehundes (Phoca Vitulina) im Wattenmeer: Grundlagen zur Messung von Statusparametern. Ph.D. thesis, Christian-Albrechts-University Kiel, Kiel.
Ackermans, N. L., Winkler, D. E., Martin, L. F., Kaiser, T. M., Clauss, M., and Hatt, J. M. (2020). Dust and grit matter: abrasives of different size lead to opposing dental microwear textures in experimentally fed sheep (Ovis aries). J. Exp. Biol. 223, 1–9. doi: 10.1242/jeb.220442
Adam, P. J., and Berta, A. (2002). Evolution of prey capture strategies and diet in the Pinnipedimorpha (Mammalia. Carnivora). Oryctos 4, 83–107.
Armfield, B. A., Zheng, Z., Bajpai, S., Vinyard, C. J., and Thewissen, J. G. M. (2013). Development and evolution of the unique cetacean dentition. PeerJ 1:e24. doi: 10.7717/peerj.24
Baechler, J., Beck, C. A., and Bowen, W. D. (2002). Dive shapes reveal temporal changes in the foraging behaviour of different age and sex classes of harbour seals (Phoca vitulina). Can. J. Zool. 80, 1569–1577. doi: 10.1139/z02-150
Baltzer, J., Maurer, N., Schaffeld, T., Ruser, A., Schnitzler, J. G., and Siebert, U. (2020). Effect ranges of underwater noise from anchor vibration operations in the Wadden Sea. J. Sea Res. 162:101912. doi: 10.1016/j.seares.2020.101912
Behrends, G. (1985). Zur nahrungswahl von seehunden (Phoca vitulina L.) im wattenmeer schleswig-holsteins. Zeitschrift Jagdwissenschaft 31, 3–14.
Bestwick, J., Unwin, D. M., Henderson, D. M., and Purnell, M. A. (2021). Dental microwear texture analysis along reptile tooth rows: complex variation with non-dietary variablese. R. Soc. Open Sci. 7:201754. doi: 10.1098/rsos.201754
Bestwick, J., Unwin, D. M., and Purnell, M. A. (2019). Dietary differences in archosaur and lepidosaur reptiles revealed by dental microwear textural analysis. Sci. Rep. 9, 1–11. doi: 10.1038/s41598-019-48154-9
Bjørge, A., Desportes, G., Waring, G. T., and Rosing-Asvid, A. (2010). Introduction: the harbour seal (Phoca vitulina) - a global perspective. NAMMCO Sci. Publ. 8:7. doi: 10.7557/3.2668
Bodewes, R., Bestebroer, T. M., Van Der Vries, E., Verhagen, J. H., Herfst, S., Koopmans, M. P., et al. (2015). Avian influenza a(H10n7) virus–associated mass deaths among harbor seals. Emerg. Infect. Dis. 21, 720–722. doi: 10.3201/eid2104.141675
Bossart, G. D. (2011). Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 48, 676–690. doi: 10.1177/0300985810388525
Bowen, W. D., Tully, D., Boness, D. J., Bulheier, B. M., and Marshall, G. J. (2002). Prey-dependent foraging tactics and prey profitability in a marine mammal. Mar. Ecol. Prog. Ser. 244, 235–245. doi: 10.3354/meps244235
Brasseur, S. M. J. M., Reijnders, P. J. H., Cremer, J., Meesters, E. H. W. G., Kirkwood, R., Jensen, L. F., et al. (2018). Echoes from the past: regional variations in recovery within a harbour seal population. PLoS One 13:e0189674. doi: 10.1371/journal.pone.0189674
Calandra, I., Schulz, E., Pinnow, M., Krohn, S., and Kaiser, T. M. (2012). Teasing apart the contributions of hard dietary items on 3D dental microtextures in primates. J. Hum. Evol. 63, 85–98. doi: 10.1016/j.jhevol.2012.05.001
Churchill, M., and Clementz, M. T. (2015). Functional implications of variation in tooth spacing and crown size in pinnipedimorpha (Mammalia: carnivora). Anat. Rec. 298, 878–902. doi: 10.1002/ar.23082
Das, K., Siebert, U., Gillet, A., Dupont, A., Di-Poï, C., Fonfara, S., et al. (2008). Mercury immune toxicity in harbour seals: links to in vitro toxicity. Environ. Heal. A Glob. Access Sci. Source 7, 1–17. doi: 10.1186/1476-069X-7-52
de la Vega, C. (2016). Influence of Top Predators on the Wadden Sea Food Web. Ph.D. thesis, Christian-Albrecht University, Kiel.
de la Vega, C., Lebreton, B., Siebert, U., Guillou, G., Das, K., Asmus, R., et al. (2016). Seasonal variation of harbor seal’s diet from the wadden sea in relation to prey availability. PLoS One 11:e0155727. doi: 10.1371/journal.pone.0155727
De Santis, L. R. G. (2016). Dental microwear textures: reconstructing diets of fossil mammals. Surf. Topogr. Metrol. Prop. 4:023002. doi: 10.1088/2051-672X/4/2/023002
De Santis, L. R. G., Scott, J. R., Schubert, B. W., Donohue, S. L., McCray, B. M., Van Stolk, C. A., et al. (2013). Direct comparisons of 2D and 3D dental microwear proxies in extant herbivorous and carnivorous mammals. PLoS One 8:e071428. doi: 10.1371/journal.pone.0071428
De Swart, R. L., Ross, P. S., Vos, J. G., and Osterhaus, A. D. M. E. (1996). “Impaired immunity in harbour seals (Phoca vitulina) exposed to bioaccumulated environmental contaminants: Review of a long-term feeding study,” in Environmental Health Perspectives, ed. J. D. Kaufman, (Durham, NC: National Institute of Environmental Health Sciences), 823–828.
Dietz, R., Teilmann, J., Andersen, S. M., Rigét, F. F., and Olsen, M. T. (2012). Movements and site fidelity of harbour seals (Phoca vitulina)in Kattegat, Denmark, with implications for the epidemiology of the phocine. ICES J. Mar. Sci. 69, 1–10. doi: 10.1093/icesjms/fss144.Received
Dragulescu, A. A. (2011). Read, Write, Format Excel 2007 and Excel 97/2000/XP/2003 Files. R Packag. version 0.3.0.
Dunnett, C. W. (1980). Pairwise multiple comparisons in the unequal variance case. J. Am. Stat. Assoc. 75, 796–800.
Fahlke, J. M., Bastl, K. A., Semprebon, G., and Gingerich, P. D. (2013). Paleoecology of archaeocete whales throughout the eocene: dietary adaptations revealed by microwear analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 386, 690–701. doi: 10.1016/j.palaeo.2013.06.032
Gilles, A., Andreasen, H., Müller, S., and Siebert, U. (2008). Nahrungsökologie von marinen Säugetieren und Seevögeln für das Management von NATURA 2000 Gebieten. Teilvorhaben: Marine Säugetiere. Endbericht für das Bundesamt für Naturschutz F+E Vorhaben FKZ: 805 85018. 1–82.
Gilles, A., Scheidat, M., and Siebert, U. (2009). Seasonal distribution of harbour porpoises and possible interference of offshore wind farms in the German North Sea. Mar. Ecol. Prog. Ser. 383, 295–307. doi: 10.3354/meps08020
Gordon, K. D. (1982). A study of microwear on chimpanzee molars: implications for dental microwear analysis. Am. J. Phys. Anthropol. 59, 195–215. doi: 10.1002/ajpa.1330590208
Greaves, W. S. (2000). Location of the vector of jaw muscle force mammals. J. Morphol. 243, 293–299. doi: 10.1002/(SICI)1097-4687(200003)243:3<293::AID-JMOR6<3.0.CO;2-5
Greaves, W. S. (2002). Modeling the distance between the molar tooth rows in mammals. Can. J. Zool. 80, 388–393.
Hall, A. J., Law, R. J., Wells, D. E., Harwood, J., Ross, H. M., Kennedy, S., et al. (1992). Organochlorine levels in common seals (Phoca vitulina) which were victims and survivors of the 1988 phocine distemper epizootic. Sci. Total Environ. 115, 145–162. doi: 10.1016/0048-9697(92)90039-U
Hall, A. J., Watkins, J., and Hammond, P. S. (1998). Seasonal variation in the diet of harbour seals in the south-western North Sea. Mar. Ecol. Prog. Ser. 170, 269–281. doi: 10.3354/meps170269
Härkönen, T. J., Dietz, R., and Reijnders, P. J. H. (2006). A review of the 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Dis. Aquat. Organ. 68, 115–130.
Härkönen, T. J., Hårding, K. C., and Lunneryd, S. G. (1999). Age- and sex-specific behaviour in harbour seals Phoca vitulina leads to biased estimates of vital population parameters. J. Appl. Ecol. 36, 825–841. doi: 10.1046/j.1365-2664.1999.00434.x
Härkönen, T. J., and Heide-Jørgensen, M. P. (1990). Comparative life histories of east atlantic and other harbour seal populations. Ophelia 32, 211–235. doi: 10.1080/00785236.1990.10422032
Heithaus, M. R., Dill, L. M., and Kiszka, J. J. (2009). “Feeding Strategies and Tactics,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Wursig, and J. G. M. Thewissen, (Amsterdam: Elsevier), 354–363.
Herreman, J. K., Blundell, G. M., and Ben-David, M. (2009). Evidence of bottom-up control of diet driven by top-down processes in a declining harbor seal Phoca vitulina richardsi population. Mar. Ecol. Prog. Ser. 374, 287–300. doi: 10.3354/meps07719
Hillson, S. (1986). Teeth, 2nd Edn. Cambridge: Cambridge University Presse: Cambridge University Press.
Hochberg, Y. (1988). A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802.
Højsgaard, S., and Halekoh, U. (2013). doBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. R Packag. version 4.5.9.
Ikoma, T., Kobayashi, H., Tanaka, J., Walsh, D., and Mann, S. (2003). Microstructure, mechanical, and biomimetic properties of fish scales from Pagrus major. J. Struct. Biol. 142, 327–333. doi: 10.1016/S1047-8477(03)00053-4
Jefferson, T. A., Leatherwood, S., and Webber, M. A. (1993). FAO Species Identification Guide - Marine Mammals of the World. Rome: Food and Agriculture Organization.
Jensen, L. F., Teilmann, J., Galatius, A., Pund, R., Czeck, R., Jess, A., et al. (2017). “Marine mammals,” in Wadden Sea Quality Status Report 2017, eds S. Kloepper, J. M. Baptist, A. Bostelmann, and J. A. Busch, (Wilhelmshaven: Common Wadden Sea Secretariat).
Jones, K. E., Ruff, C. B., and Goswami, A. (2013). Morphology and biomechanics of the pinniped jaw: mandibular evolution without mastication. Anat. Rec. 296, 1049–1063. doi: 10.1002/ar.22710
Kahle, P., Ludolphy, C., Kierdorf, H., and Kierdorf, U. (2018). Dental anomalies and lesions in Eastern Atlantic harbor seals, Phoca vitulina vitulina (Carnivora, Phocidae), from the German North Sea. PLoS One 13:e0204079. doi: 10.1371/journal.pone.0204079
Kaiser, T. M., Braune, C., Kalinka, G., and Schulz-Kornas, E. (2018). Nano-indentation of native phytoliths and dental tissues: implications for herbivore-plant combat and dental wear proxies. Evol. Syst. 2, 55–63. doi: 10.3897/evolsyst.2.22678
Kaiser, T. M., Clauss, M., and Schulz-Kornas, E. (2015). A set of hypotheses on tribology of mammalian herbivore teeth. Surf. Topogr. Metrol. Prop. 4, 014003. doi: 10.1088/2051-672X/4/1/014003
Kienle, S. E. S. (2014). Eat You With: The Comparative Feeding Morphology and Evolution of Feeding Strategies in Phocid Seals (Pinnipedia, Phocidae). San Diego, CA: San Diego State University.
Kienle, S. E. S., and Berta, A. (2016). The better to eat you with: the comparative feeding morphology of phocid seals (Pinnipedia. Phocidae). J. Anat. 228, 396–413. doi: 10.1111/joa.12410
Kierdorf, U., Olsen, M. T., Kahle, P., Ludolphy, C., and Kierdorf, H. (2019). Dental pulp exposure, periapical inflammation and suppurative osteomyelitis of the jaws in juvenile Baltic grey seals (Halichoerus grypus grypus) from the late 19th century. PLoS One 14:e0215401. doi: 10.1371/journal.pone.021540
Kovacs, K. M., Aguilar, A., Aurioles, D., Burkanov, V., Campagna, C., Gales, N., et al. (2012). Global threats to pinnipeds. Mar. Mammal Sci. 28, 414–436. doi: 10.1111/j.1748-7692.2011.00479.x
Labrada-Martagon, V., Aurioles-Gamboa, D., and Castro-Gonzalez, M. I. (2007). Relation of dental wear to the concentrations of essential minerals in teeth of the California sea lion Zalophus californianus californianus. Biol. Trace Elem. Res. 115, 107–126. doi: 10.1007/s12011-007-0002-3
Lehnert, K., Desforges, J.-P., Das, K., Siebert, U. (2018). “Ecotoxicological biomarkers and accumulation of contaminants in pinnipeds,” in Marine Mammal Ecotoxicology, eds M. C., Fossi, and C., Panti, (San Diego: Academic Press), 261–289.
Loch, C., and Simoes-Lopes, P. C. (2013). Dental wear in dolphins (Cetacea: delphinidae) from southern Brazil. Arch. Oral Biol. 58, 134–141. doi: 10.1016/j.archoralbio.2012.08.002
Lotze, H. K., Reise, K., Worm, B., van Beusekom, J., Busch, M., Ehlers, A., et al. (2005). Human transformations of the Wadden Sea ecosystem through time: a synthesis. Helgol. Mar. Res. 59, 84–95. doi: 10.1007/s10152-004-0209-z
Loughlin, T. R. (1982). Functional adaptation of eruption sequence of pinniped postcanine teeth. J. Mammal. 63:3.
Lucas, P. W., Constantino, P., Wood, B., and Lawn, B. (2008). Dental enamel as a dietary indicator in mammals. BioEssays 30, 374–385. doi: 10.1002/bies.20729
Lucas, P. W., Omar, R., Al-Fadhalah, K., Almusallam, A. S., Henry, A. G., Michael, S., et al. (2013). Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J. R. Soc. Interface 10:20120923. doi: 10.1098/rsif.2012.0923
Ludolphy, C., Kahle, P., Kierdorf, H., and Kierdorf, U. (2018). Osteoarthritis of the temporomandibular joint in the Eastern Atlantic harbour seal (Phoca vitulina vitulina) from the German North Sea?: a study of the lesions seen in dry bone. BMC Vet. Res. 14:150. doi: 10.1186/s12917-018-1473-5
Marshall, C. D., Wieskotten, S., Hanke, W., Hanke, F. D., Marsh, A., Kot, B., et al. (2014). Feeding kinematics, suction, and hydraulic jetting performance of harbor seals (Phoca vitulina). PLoS One 9:e86710. doi: 10.1371/journal.pone.0086710
Merceron, G., Escarguel, G., Angibault, J. M., and Verheyden-Tixier, H. (2010). Can dental microwear textures record inter-individual dietary variations? PLoS One 5:e9542. doi: 10.1371/journal.pone.0009542
Meyer, W., and Matzke, T. (2004). On the development of the deciduous teeth in the common seal (Phoca vitulina). Mamm. Biol. 69, 401–409. doi: 10.1078/1616-5047-00162
Mikkelsen, L., Johnson, M., Wisniewska, D. M., van Neer, A., Siebert, U., Madsen, P. T., et al. (2019). Long-term sound and movement recording tags to study natural behavior and reaction to ship noise of seals. Ecol. Evol. 9, 2588–2601. doi: 10.1002/ece3.4923
Minicozzi, M. R., Perez, J., Kimball, D. S., and Gibb, A. C. (2019). Scale thickness predicts skin puncture-force resistance in three pleuronectiform fishes. Integr. Org. Biol. 1:obz005. doi: 10.1093/iob/obz005
Müller, G., Wohlsein, P., Beineke, A., Haas, L., Greiser-Wilke, I., Siebert, U., et al. (2004). Phocine distemper in German Seals, 2002. Emerg. Infect. Dis. 10, 723–725. doi: 10.3201/eid1004.030591
Müller, J., Clauss, M., Codron, D., Schulz, E., Hummel, J., Fortelius, M., et al. (2014). Growth and wear of incisor and cheek teeth in domestic rabbits (Oryctolagus cuniculus) fed diets of different abrasiveness. J. Exp. Zool. Part A-Ecological Genet. Physiol. 321, 283–298. doi: 10.1002/jez.1864
Naleway, S. E., Taylor, J. R. A., Porter, M. M., Meyers, M. A., and McKittrick, J. (2016). Structure and mechanical properties of selected protective systems in marine organisms. Mater. Sci. Eng. C 59, 1143–1167. doi: 10.1016/j.msec.2015.10.033
Page, B., McKenzie, J., Sumner, M. D., Coyne, M., and Goldsworthy, S. D. (2006). Spatial separation of foraging habitats among New Zealand fur seals. Mar. Ecol. Prog. Ser. Ecol. Prog. Ser. 323, 263–279.
Parrish, F. A., Abernathy, K., Marshall, G. J., and Buhleier, B. M. (2002). Hawaiian monk seals (Monachus schauinslandi) foraging in deep-water coral beds. Mar. Mammal Sci. 18, 244–258. doi: 10.1111/j.1748-7692.2002.tb01031.x
Pierce, G. J., Thompson, P. M., Miller, A., Diack, J. S. W., Miller, D., and Boyle, P. R. (1991). Seasonal variation in the diet of common seals (Phoca vitulina) in the Moray Firtg area of Scotland. J. Zool. London 223, 641–652. doi: 10.1111/j.1469-7998.1991.tb04393.x
Purnell, M. A., Crumpton, N., Gill, P. G., Jones, G., and Rayfield, E. J. (2013). Within-guild dietary discrimination from 3-D textural analysis of tooth microwear in insectivorous mammals. J. Zool. 291, 249–257. doi: 10.1111/jzo.12068
Purnell, M. A., and Darras, L. P. G. (2015). 3D tooth microwear texture analysis in fishes as a test of dietary hypotheses of durophagy. Surf. Topogr. Metrol. Prop. 4:014006. doi: 10.1088/2051-672X/4/1/014006
Purnell, M. A., Goodall, R. H., Thomson, S., and Matthews, C. J. D. (2017). Tooth microwear texture in odontocete whales?: variation with tooth characteristics and implications for dietary analysis. Biosurface and Biotribol. 3, 184–195. doi: 10.1016/j.bsbt.2017.11.004
Purnell, M. A., Seehausen, O., and Galis, F. (2012). Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. J. R. Soc. Interface 9, 2225–2233. doi: 10.1098/rsif.2012.0140
R Development Core Team. (2011). R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. R Foundation for Statistical Computing. Vienna: R Core Team.
Reijnders, P. J. H. (1981). Management and conservation of the harbour seal, Phoca vitulina, population in the international wadden sea area. Biol. Conserv. 19, 213–221. doi: 10.1016/0006-3207(81)90036-7
Ross, P. S., De Swart, R. L., Addison, R., Van Loveren, H., Vos, J., and Osterhaus, A. D. M. E. (1996). Contaminant-induced immunotoxicity in harbour seals: wildlife at risk? Toxicology 112, 157–169. doi: 10.1016/0300-483X(96)03396-3
Santana, S. E., and Dumont, E. R. (2009). Connecting behaviour and performance: the evolution of biting behaviour and bite performance in bats. J. Evol. Biol. 22, 2131–2145. doi: 10.1111/j.1420-9101.2009.01827.x
Schubert, B. W., Ungar, P. S., and De Santis, L. R. G. (2010). Carnassial microwear and dietary behaviour in large carnivorans. J. Zool. 280, 257–263. doi: 10.1111/j.1469-7998.2009.00656.x
Schulz, E., Calandra, I., and Kaiser, T. M. (2010). Applying tribology to teeth of hoofed mammals. Scanning 32, 162–182. doi: 10.1002/sca.20181
Schulz, E., Calandra, I., and Kaiser, T. M. (2013). Feeding ecology and chewing mechanics in hoofed mammals: 3D tribology of enamel wear. Wear 300, 169–179. doi: 10.1016/j.wear.2013.01.115
Schulz-Kornas, E., Kaiser, T. M., Calandra, I., and Winkler, D. E. (2021). “A brief history of quantitative wear analyses with an appeal for a holistic view on dental wear processes,” in Mammalian Teeth – Form and Function, eds T. Martin and W. von Koenigswald (Munich: Verlag Dr. Friedrich Pfeil), 44–53.
Schulz-Kornas, E., Stuhlträger, J., Clauss, M., Wittig, R. M., and Kupczik, K. (2019). Dust affects chewing efficiency and tooth wear in forest dwelling Western chimpanzees (Pan troglodytes verus). Am. J. Phys. Anthropol. 169, 1–12. doi: 10.1002/ajpa.23808
Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Childs, B. E., Teaford, M. F., et al. (2006). Dental microwear texture analysis: technical considerations. J. Hum. Evol. 51, 339–349. doi: 10.1016/j.jhevol.2006.04.006
Sievers, U. (1989). Nahrungsökologische Untersuchungen an Seehunden (Phoca vitulina, Linne 1758) aus dem schleswig-holsteinischen Wattenmeer. Zool. Anz. 222, 249–260.
Spinner, M., Kortmann, M., Traini, C., and Gorb, S. N. (2016). Key role of scale morphology in flatfishes (Pleuronectiformes) in the ability to keep sand. Sci. Rep. 6:26308. doi: 10.1038/srep26308
Spinner, M., Schaber, C. F., Chen, S. M., Geiger, M., Gorb, S. N., and Rajabi, H. (2019). Mechanical behavior of ctenoid scales: joint-like structures control the deformability of the scales in the flatfish Solea solea (Pleuronectiformes). Acta Biomater. 92, 305–314. doi: 10.1016/j.actbio.2019.05.011
Teaford, M. F., and Oyen, O. J. (1989). Differences in the rate of molar wear between monkeys raised on different diets. J. Dent. Res. 68, 1513–1518. doi: 10.1177/00220345890680110901
Thompson, P. M., Fedak, M. A., McConnell, B. J., and Nicholas, K. S. (1989). Seasonal and sex-related variation in the activity patterns of common Seals (Phoca vitulina). J. Appl. Ecol. 26, 521–535.
Ungar, P. S. (2004). Dental topography and diets of Australopithecus afarensis and early Homo. J. Hum. Evol. 46, 605–622. doi: 10.1016/j.jhevol.2004.03.004
Ungar, P. S., Brown, C. A., Bergstrom, T. S., and Walker, A. (2003). Quantification of dental microwear by tandem scanning confocal microscopy and scale-sensitive fractal analyses. Scanning 25, 185–193. doi: 10.1002/sca.4950250405
Ungar, P. S., Scott, J. R., Schubert, B. W., and Stynder, D. D. (2010). Carnivoran dental microwear textures: comparability of carnassial facets and functional differentiation of postcanine teeth. Mammalia 74, 219–224. doi: 10.1515/MAMM.2010.015
Unger, B., Herr, H., Benke, H., Böhmert, M., Burkhardt-Holm, P., Dähne, M., et al. (2017). Marine debris in harbour porpoises and seals from German waters. Mar. Environ. Res. 130, 77–84. doi: 10.1016/j.marenvres.2017.07.009
Van Valen, L. (1969). Evolution of dental growth and adaptation in mammalian carnivores. Evolution (N. Y). 23, 96–117.
Vernerey, F. J., and Barthelat, F. (2010). On the mechanics of fishscale structures. Int. J. Solids Struct. 47, 2268–2275. doi: 10.1016/j.ijsolstr.2010.04.018
Volkman, J. K., Rohjans, D., Rullkötter, J., Scholz-Böttcher, B. M., and Liebezeit, G. (2000). Sources and diagenesis of organic matter in tidal flat sediments from the German Wadden Sea. Cont. Shelf Res. 20, 1139–1158. doi: 10.1016/S0278-4343(00)00016-9
Walker, A., Hoeck, H. N., and Perez, L. (1978). Microwear of mammalian teeth as an indicator of diet. Science 201, 908–910. doi: 10.1126/science.684415
Welch, B. L. (1938). The significance of the difference between two means when the population variances are unequal. Biometrika 29, 350–362.
Werth, A. (2000). “Feeding in marine mammals,” in Feeding, ed. K. Schwenk (San Diego: Academic Press), 487–526.
Wilcox, R. R., Holland, B. A., and Dooley, A. G. (2005). Introduction to robust estimation and hypothesis testing. Technometrics 47, 525–526. doi: 10.1198/tech.2005.s334
Wilson, R. P., Liebsch, N., Gómez-Laich, A., Kay, W. P., Bone, A., Hobson, V. J., et al. (2015). Options for modulating intra-specific competition in colonial pinnipeds: the case of harbour seals (Phoca vitulina) in the Wadden Sea. PeerJ 3:e957. doi: 10.7717/peerj.957
Winkler, D. E., Schulz-Kornas, E., Kaiser, T. M., Codron, D., Leichliter, J., Hummel, J., et al. (2020a). The turnover of dental microwear texture: testing the” last supper” effect in small mammals in a controlled feeding experiment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 557:109930. doi: 10.1016/j.palaeo.2020.109930
Winkler, D. E., Tütken, T., Schulz-Kornas, E., Kaiser, T. M., Müller, J., Leichliter, J., et al. (2020b). Shape, size, and quantity of ingested external abrasives influence dental microwear texture formation in guinea pigs. Proc. Natl. Acad. Sci. U.S.A. 117, 22264–22273. doi: 10.1073/pnas.2008149117
Winkler, D. E., Schulz-Kornas, E., Kaiser, T. M., De Cuyper, A., Clauss, M., and Tütken, T. (2019a). Forage silica and water content control dental surface texture in guinea pigs and provide implications for dietary reconstruction. Proc. Natl. Acad. Sci. 116, 1325–1330. doi: 10.1073/pnas.1814081116
Winkler, D. E., Schulz-Kornas, E., Kaiser, T. M., and Tütken, T. (2019b). Dental microwear texture reflects dietary tendencies in extant Lepidosauria despite their limited use of oral food processing. Proc. R. Soc. B Biol. Sci. 286:20190544. doi: 10.1098/rspb.2019.0544
Ydesen, K. S., Wisniewska, D. M., Hansen, J. D., Beedholm, K., Johnson, M., and Madsen, P. T. (2014). What a jerk: prey engulfment revealed by high-]rate, super-]cranial accelerometry on a harbour seal (Phoca vitulina). J. Exp. Biol. 217, 2239–2243. doi: 10.1242/jeb.100016
Yuen, K. K. (1974). The two-sample trimmed t for unequal population variances. Biometrika 61, 165–170.
Keywords: dental microwear texture analysis (DMTA), marine mammals, Phoca vitulina (Harbour seal), German Wadden Sea, pinnipeds, diet
Citation: Bethune E, Schulz-Kornas E, Lehnert K, Siebert U and Kaiser TM (2021) Tooth Microwear Texture in the Eastern Atlantic Harbour Seals (Phoca vitulina vitulina) of the German Wadden Sea and Its Implications for Long Term Dietary and Ecosystem Changes. Front. Ecol. Evol. 9:644019. doi: 10.3389/fevo.2021.644019
Received: 19 December 2020; Accepted: 17 March 2021;
Published: 13 April 2021.
Edited by:
Javier Perez-Barberia, University of Castilla-La Mancha, SpainReviewed by:
Jacqueline Potts, Biomathematics and Statistics Scotland, United KingdomGildas Merceron, UMR 7262 Institut de Paléoprimatologie, Paléontologie Humaine Évolution et Paléoenvironnements (IPHEP), France
Laura McLennan, University of Derby, United Kingdom
Copyright © 2021 Bethune, Schulz-Kornas, Lehnert, Siebert and Kaiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen Schulz-Kornas, ZWxsZW5fc2NodWx6QGV2YS5tcGcuZGU=
 Elehna Bethune
Elehna Bethune Ellen Schulz-Kornas
Ellen Schulz-Kornas Kristina Lehnert
Kristina Lehnert Ursula Siebert
Ursula Siebert Thomas M. Kaiser1
Thomas M. Kaiser1