
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 09 April 2021
Sec. Biogeography and Macroecology
Volume 9 - 2021 | https://doi.org/10.3389/fevo.2021.642309
This article is part of the Research TopicConsequences of Climate Change for Plant Biodiversity in High Mountain EcosystemsView all 18 articles
 Lena Nicklas1*
Lena Nicklas1* Janette Walde2
Janette Walde2 Sonja Wipf3,4
Sonja Wipf3,4 Andrea Lamprecht5
Andrea Lamprecht5 Martin Mallaun1
Martin Mallaun1 Christian Rixen4
Christian Rixen4 Klaus Steinbauer5
Klaus Steinbauer5 Jean-Paul Theurillat6
Jean-Paul Theurillat6 Peter Unterluggauer1
Peter Unterluggauer1 Pascal Vittoz7
Pascal Vittoz7 Dietmar Moser8
Dietmar Moser8 Andreas Gattringer8
Andreas Gattringer8 Johannes Wessely8
Johannes Wessely8 Brigitta Erschbamer1
Brigitta Erschbamer1The alpine life zone is expected to undergo major changes with ongoing climate change. While an increase of plant species richness on mountain summits has generally been found, competitive displacement may result in the long term. Here, we explore how species richness and surface cover types (vascular plants, litter, bare ground, scree and rock) changed over time on different bedrocks on summits of the European Alps. We focus on how species richness and turnover (new and lost species) depended on the density of existing vegetation, namely vascular plant cover. We analyzed permanent plots (1 m × 1 m) in each cardinal direction on 24 summits (24 × 4 × 4), with always four summits distributed along elevation gradients in each of six regions (three siliceous, three calcareous) across the European Alps. Mean summer temperatures derived from downscaled climate data increased synchronously over the past 30 years in all six regions. During the investigated 14 years, vascular plant cover decreased on siliceous bedrock, coupled with an increase in litter, and it marginally increased on higher calcareous summits. Species richness showed a unimodal relationship with vascular plant cover. Richness increased over time on siliceous bedrock but slightly decreased on calcareous bedrock due to losses in plots with high plant cover. Our analyses suggest contrasting and complex processes on siliceous versus calcareous summits in the European Alps. The unimodal richness-cover relationship and species losses at high plant cover suggest competition as a driver for vegetation change on alpine summits.
Alpine plants are highly sensitive to global warming (Kammer et al., 2007; Grytnes et al., 2014; Rixen and Wipf, 2017; Winkler et al., 2019). With rising temperatures, species from lower elevations have been expanding their ranges upward (Rumpf et al., 2018), leading to an accelerated increase of species richness on mountain summits (Steinbauer et al., 2018) and a “thermophilization,” i.e., a directional shift in community composition in favor of warm-affinity species, of mountain plant communities (Vittoz et al., 2008; Erschbamer et al., 2011; Gottfried et al., 2012; Pauli et al., 2012; Wipf et al., 2013; Unterluggauer et al., 2016). Warmer temperatures and a longer growing season have been enhancing plant productivity and causing a greening trend at high elevation during the last decades (Carlson et al., 2017; Rogora et al., 2018; Filippa et al., 2019). Since competitive species with a higher growth rate are particularly benefiting from warming (Farrer et al., 2015; Winkler et al., 2019) it is expected that slow-growing, cold-adapted species are threatened by competitive displacement (Alexander et al., 2015) and/or physiological constraints (Larcher et al., 1998; Marcante et al., 2012, 2014; Lamprecht et al., 2018) over time. Species distribution models predict drastic losses of such cold-adapted species by the end of the 21st century (Dirnböck et al., 2011; Engler et al., 2011; Dullinger et al., 2012). Recent studies in the Central Austrian Alps have already shown losses and decrease in cover of cold-adapted species from permanent plots (Lamprecht et al., 2018; Steinbauer et al., 2020). However, species distribution models might overestimate losses, as extinctions are still rarely encountered in revisitation studies in the European Alps (Kulonen et al., 2018).
The highly heterogeneous relief of alpine terrain (Nagy and Grabherr, 2009) forms a mosaic of closely located distinct climatic “micro-refugia,” which might locally reduce or postpone extinction risks of alpine species (Scherrer and Körner, 2011; Graae et al., 2018). While, on the mesoscale of a whole summit, suitable microhabitats for new and resident species could be available side by side for a long time, especially when taking into account the contrasting temperature regimes between aspects (Winkler et al., 2016), open space for low-competitive alpine plants might get overgrown on the microscale. Here, the availability and distribution of surface cover types such as vascular plants, litter, bare ground, scree or rock might be relevant for species establishment. Open microsites such as rock crevices and scree could function as seed traps, providing new colonization areas (Graae et al., 2011). Kulonen et al. (2018) found that successfully colonizing high-alpine summit plants were most abundant on scree. In contrast, high-alpine species with a prevalence on organo-mineral substrate showed decreasing frequencies over time, suggesting competitive interactions in alpine microhabitats with favorable growing conditions (Kulonen et al., 2018).
The difference in species composition between siliceous versus calcareous bedrock types in the European Alps is an essential driver of the high plant diversity in the alpine region (Grabherr and Mucina, 1993; Wohlgemuth, 2002; Virtanen et al., 2003) and has been the focus of numerous early studies in plant ecology (Gigon, 1971 and references therein). The two bedrocks form contrasting landscape morphologies, typically with large rock walls and extensive scree slopes in calcareous regions, and dense grasslands up to high elevation in siliceous regions (Reisigl and Keller, 1994). Gigon (1971, 1987) and Kinzel (1983) found that most of the competitive species from siliceous grassland communities were inhibited by an excess of calcium ions, and by iron and phosphorus deficiency when they were grown on a calcareous substrate. Calcareous grassland species, on the other hand, were inhibited by an increased solubility of toxic aluminium ions and heavy metals due to a low pH, and – above all – by root competition of calcifuge species when they were grown on a siliceous substrate. These processes point toward a higher level of competition within siliceous plant communities and a higher level of specialization to abiotic conditions in species from calcareous substrates. Additionally, the different erosion potential (i.e., stronger weathering of calcareous bedrock due to carbonate dissolution) and different soil forming processes (Veit, 2002) most likely lead to more open and diverse surface cover types at calcareous sites, thus slowing down vegetation development, preventing the dominance of competitive species, and allowing a higher species richness (Wohlgemuth, 2002; Virtanen et al., 2003) and species turnover.
Whether vegetation in siliceous or calcareous regions will be more strongly affected by climate change is still an open, but highly relevant question in terms of conservation. Dense and competitive communities in siliceous regions might hamper new colonizers from low elevation and thus remain relatively stable, whereas open microsites on calcareous bedrock might facilitate the installation of new species and thus enhance a vegetation change. In the present study, we investigated divergences of ongoing vegetation changes between bedrock types, specifically focusing on the relationship between species richness/species turnover and vascular plant cover on calcareous and siliceous summits. On the microscale, a unimodal relationship of species richness and vascular plant cover might indicate increasing competition in dense vegetation (Grytnes, 2000). We analyzed monitoring data from 1 m × 1 m permanent plots, covering a 14-year period from three siliceous and three calcareous GLORIA (Global Observation Research Initiative in Alpine Environments1) regions across the European Alps (total of 24 summits). We assumed that warming effects on vegetation vary in magnitude (i) between siliceous and calcareous mountains, and (ii) along a gradient from low to high vascular plant cover. Specifically, we address the following three topics and inherent hypotheses:
(1) Surface cover types: (1a) Cover of vascular plants and litter is higher on siliceous bedrock, whereas on calcareous bedrock the cover of bare ground, scree, and rock is higher and, as a consequence, more open and competition free microsites should be present here. (1b) Over time, vascular plant and litter cover increases at the expense of bare ground, scree and rock, however more strongly on siliceous bedrock.
(2) Species richness: (2a) Species richness shows a unimodal relationship with increasing vascular plant cover and highest values at intermediate plant cover. (2b) Species richness increases over time, however at a slower pace on siliceous bedrock.
(3) New species and lost species: (3a) With an increasing vascular plant cover the number of lost species increases more than the number of new species. (3b) The number of lost species exceeds the number of new species at sites with high vascular plant cover, more so, on siliceous than on calcareous bedrock.
Our dataset comprised 24 summits from the GLORIA initiative across the European Alps, in three regions on siliceous and calcareous bedrock, respectively (Table 1 and Supplementary Figure 1). Each region contained permanent plots on four different summits situated along an elevation gradient from the treeline to the subnival ecotone (treeline, lower alpine, upper alpine, subnival). The summits were mostly located in protected areas and were chosen to avoid pressure from human land use (i.e., tourism or intense grazing by livestock or anthropogenically strongly raised numbers of wild ungulates; Pauli et al., 2015; Supplementary Table 1). Species composition was surveyed in each permanent plot at least twice between 2001 and 2017 following the methodology in Pauli et al. (2015). To standardize elevation across different regions, we used the elevation of a summit relative to the position of the regional treeline (defined at the first survey as the highest elevation where groups of trees taller than 3 m occur), instead of absolute elevation (Table 1). On each summit, a quadrat cluster of 3 m × 3 m was established 5 m below the peak in each of the four cardinal directions. In each of the four 1 × 1 m corner plots of each quadrat cluster (in total 345 plots), percent cover was estimated for all occurring vascular plant species and for four different surface types, namely vascular plants (living), litter (dead plant material), bare ground (earthy or sandy surface), scree (unsealed rocks and debris material larger than sand fraction), and solid rock (outcrops and large boulders). As an attempt to minimize phenological variations, the first survey and resurvey were done in peak season. For the analyses, the scree and rock surface cover types were aggregated into one common substrate type “scree & rock,” due to some potential ambiguity in the classification (larger boulders were sometimes classified as rocks and sometimes as scree) and because scree included a larger range of sizes (cm to m), with various potential for seedling establishment. Cover changes of cryptogams were excluded from the analyses due to their higher variability in cover estimation (Vittoz et al., 2010) and ambiguity in the classification. Due to inaccessible terrain on some summits, not all aspects could be equally documented (Table 1). After the baseline surveys from 2001 to 2003, monitoring was repeated approximately every seven years. To estimate long-term trends, we compared the first and the last survey (called resurvey hereafter) to gain the longest possible time interval. Time intervals varied from 12 to 14 years (Table 1).
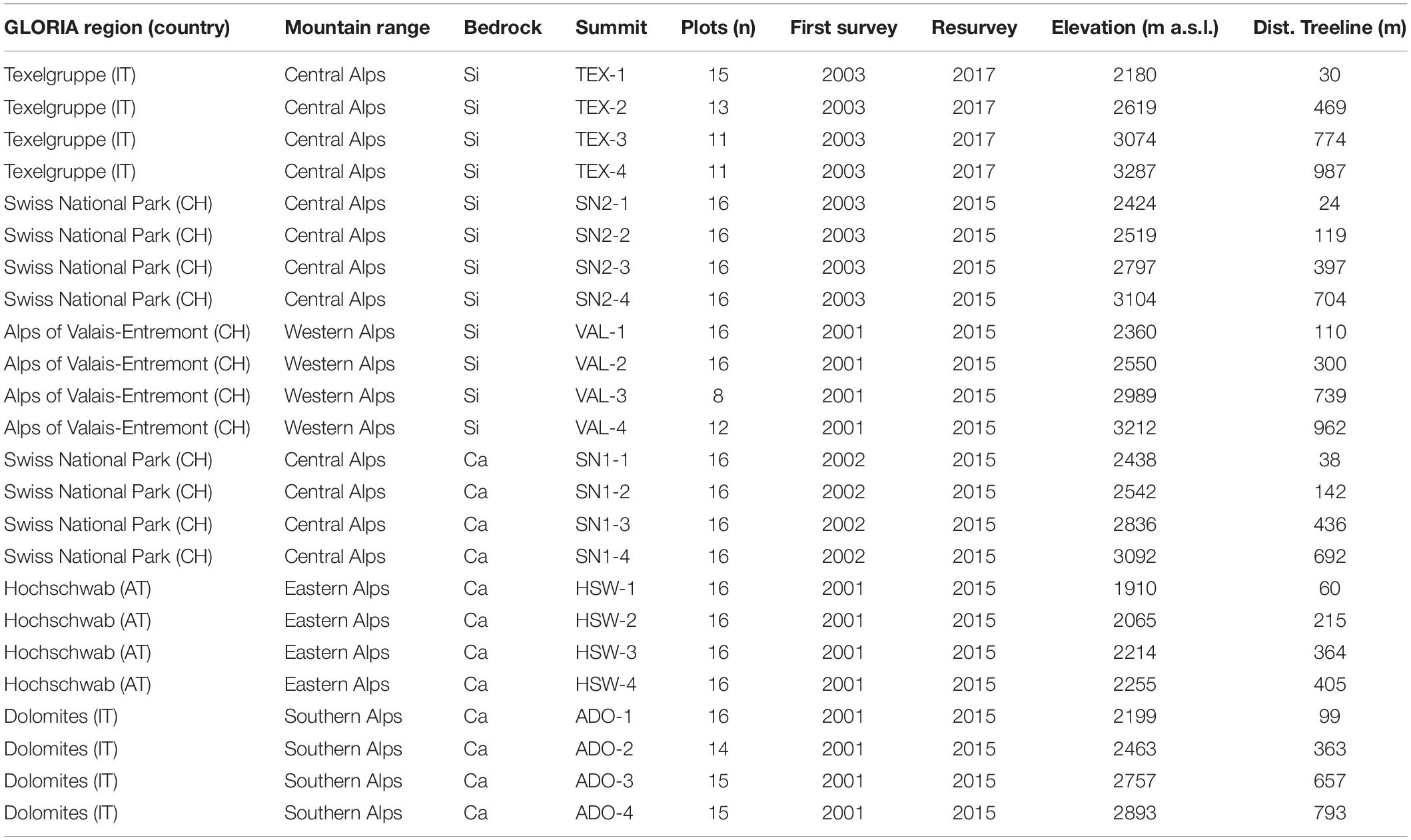
Table 1. Geographical distribution (GLORIA region, country: AT = Austria, CH = Switzerland, IT = Italy; mountain range), bedrock (Si = siliceous, Ca = calcareous), summit designation (combined of the region’s three-digit code and the summit number in ascending elevation), number of 1 m × 1 m permanent plots, year of first survey/resurvey, elevation (in m), and relative position to the treeline of the 24 inventoried summits (Dist. treeline = elevation above the treeline, in m).
Annual Chelsa climate data2 combined with data from CRU (Climate research unit3, Harris et al., 2014) were downscaled to a resolution of 100 m (see Supplementary Material for the detailed downscaling process). To investigate different temperature trends between regions or summits over the last 30 years the mean summer temperature (June, July, August) was regressed on year, allowing different trends for each region or summit. Serial autocorrelation was considered with Newey West standard errors (Newey and West, 1987).
The dependence of the percentage of the surface, covered by the four surface cover types (see study design), on (1) bedrock (siliceous, calcareous), (2) aspect (south, east, west, north), (3) elevation (distance to treeline), and (4) first survey/resurvey was investigated. Separately for each surface cover type, a mixed beta regression (with logit link) was used. A beta regression is appropriate for dependent variables that are percentages varying between zero and one (Ferrari and Cribari-Neto, 2004; Simas et al., 2010). The percentage values were transformed using the formula (Smithson and Verkuilen, 2006), where y is the percentage value of the surface cover type (divided by 100), and n is the number of observations. The variables “bedrock,” “aspect,” “distance to treeline”, and “first survey/resurvey” were used as fixed effects. In addition, summits nested in regions, i.e., distance to treeline were considered as random intercepts. All two-way interaction effects were included in the model. Thus, the final model looked like:
where g(⋅) is an appropriate link function (logit link) and E[⋅] the expectation; ytype is the transformed ratio of the specific surface cover type following the beta distribution; the intercept consists of the overall intercept β0 together with the random intercept (U0) considering the summit nested in the region; the βs capture all fixed effects and two way interaction effects; # denotes an interaction effect between the corresponding variables; ε is the remainder noise.
To visualize the trends in surface cover types, we predicted mean cover values and their 95% confidence intervals for a gradient of distance to treeline from the smallest to the highest value per bedrock type in steps of 10 m.
With the number of vascular plant species present in the 1 m × 1 m plot, trends in plant species richness were analyzed. The relationship of species richness with vascular plant cover, bedrock, aspect, and first survey/resurvey was analyzed using a general linear mixed model (GLMM) with a negative binomial distribution appropriate for count data and overdispersion. Vascular plant cover was included both as linear and quadratic term to be able to model a unimodal relationship with decreases at higher values of vascular plant cover. The relationship was allowed to be different with respect to bedrock. Random intercept and slopes per region were taken into account, as this quadratic relationship differed between regions. The model was structured thus:
where g(⋅) is the appropriate link function (log link) and E[⋅] the expectation; yindicates species richness following a negative binomial distribution; the intercept consists of the overall intercept β0 together with the random intercept variable for the region (U0); random slopes are denoted with the corresponding random variables U3 and U4; the symbol # denotes an interaction effect between the corresponding variables; ε indicates the remainder noise.
The visualization was realized as described for surface cover types; species richness and 95% confidence intervals were predicted dependent on the continuous predictor variable “vascular plant cover” in steps of 1%.
To further explore changes in richness patterns, the number of new species gained, and the number of species lost between the first survey and the resurvey were computed per plot. New species were defined as the species present in a plot in the resurvey, but not present in the first survey. Lost species were defined as species absent from a plot at the time of resurvey, but present in the first survey. New and lost species of every plot were checked and, in case of suspected determination problems (e.g., Alchemilla, Hieracium), they were merged to species aggregates to avoid pseudo-turnover. No exotic species were on the summits. The relationship between the number of new or lost species and vascular plant cover, bedrock, and aspect was analyzed using GLMMs with a negative binomial distribution to account for overdispersion. A random intercept and slope per region was taken into account. Vascular plant cover values of the first survey were used (models using the values of the vascular plant cover of the resurvey gave qualitatively the same results). The model was:
where g(⋅) is the appropriate link function (log link) and E[⋅] the expectation; y indicates number of new or the number of lost species, following the negative binomial distribution; the intercept consists of the overall intercept β0 together with the random intercept variable for the region (U0); a random slope was used (U3) for vascular plant cover (vascular_plants) to allow for regional differences; between bedrock and vascular plants an interaction effect was used (denoted by #); ε indicates the remainder noise.
The number of new or lost species was predicted dependent on the predictor variable “vascular plant cover” with a step size of 1%.
For the categorical variables “bedrock,” “aspect,” and “first survey/resurvey,” the first levels (i.e., siliceous, aspect S and first survey) were used as the reference level. For the readers’ convenience to easily obtain the appropriate P-values additionally, the models with calcareous bedrock as the reference level were computed. The results are provided in the Supplementary Tables 3, 4, 6. A significance level of 5% was used. Assumptions and residual diagnostics were checked for all models.
All analyses were performed by using R 4.0.2 (R Development Core Team, 2015) and the R packages glmmTMB (Brooks et al., 2017) for models and performance (Lüdecke et al., 2020) for calculating marginal and conditional effects. For figures we employed ggplot2 (Wickham, 2016). Moving averages (10 years) of summer temperatures were visualized by using the smooth function within the latter package.
Mean summer temperatures increased in all regions by 0.05 ± 0.01°C per year (estimate ± serial correlation robust standard error) over the period from 1986 to 2015 (Figure 1 and Supplementary Table 2, Year: p < 0.001). This finding results in an estimated temperature increase of 0.7°C over the 14 years from the first survey to the resurvey. According to the downscaled climate data, trends in mean summer temperatures did not significantly differ neither between regions nor summits.
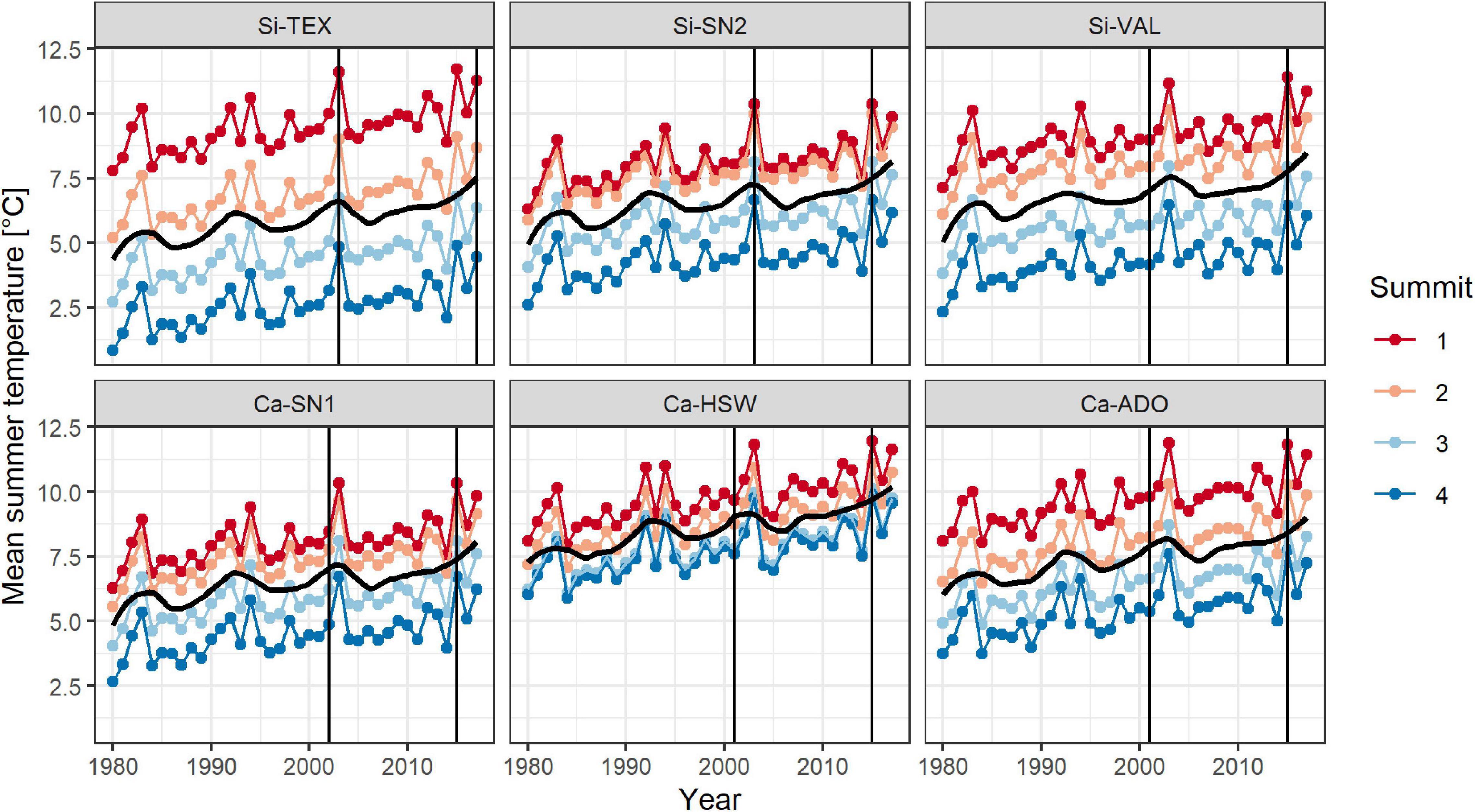
Figure 1. Trend in mean summer temperatures on each four summits (1-4, in ascending order) in the six regions (Table 1). Shown are averages of summer temperature (monthly means June, July, August) calculated with downscaled Chelsa satellite data (resolution 100 m) combined with CRU V4.03. Black trend lines represent a 10-year moving average. Vertical lines indicate the year of the first survey and the resurvey.
Neither the mean vascular plant cover nor the mean scree & rock cover differed significantly between siliceous and calcareous bedrock (Figures 2A,D and Table 2, Calcareous: p = 0.677, p = 0.138 respectively). Bare ground was significantly larger in calcareous regions (4.2 ± 0.6% mean ± standard error, see Table 3 for all mean values) than in siliceous regions (3.3 ± 0.5%; Table 2, Calcareous: p = 0.05). Generally, the proportion of litter and bare ground was low (Figures 2B,C) and poorly explained by the fixed effects of the model (Table 2, Litter cover: Marginal R2 = 0.256; Bare ground: Marginal R2 = 0.125). Surface cover types were affected by aspect, with vascular plant cover being higher at the southern and eastern plots compared to the western and northern ones (Table 2, Aspect W: p < 0.001, Aspect N: p < 0.001). Additionally, litter cover and bare ground cover were higher at southern and eastern aspects compared to western and northern ones (Table 2, Aspect W: p = 0.002, p < 0.001, Aspect N: p = 0.005, p = 0.001 respectively). The cover of scree & rock was higher at the western and northern aspects (Aspect W: p < 0.001, Aspect N: p < 0.001).
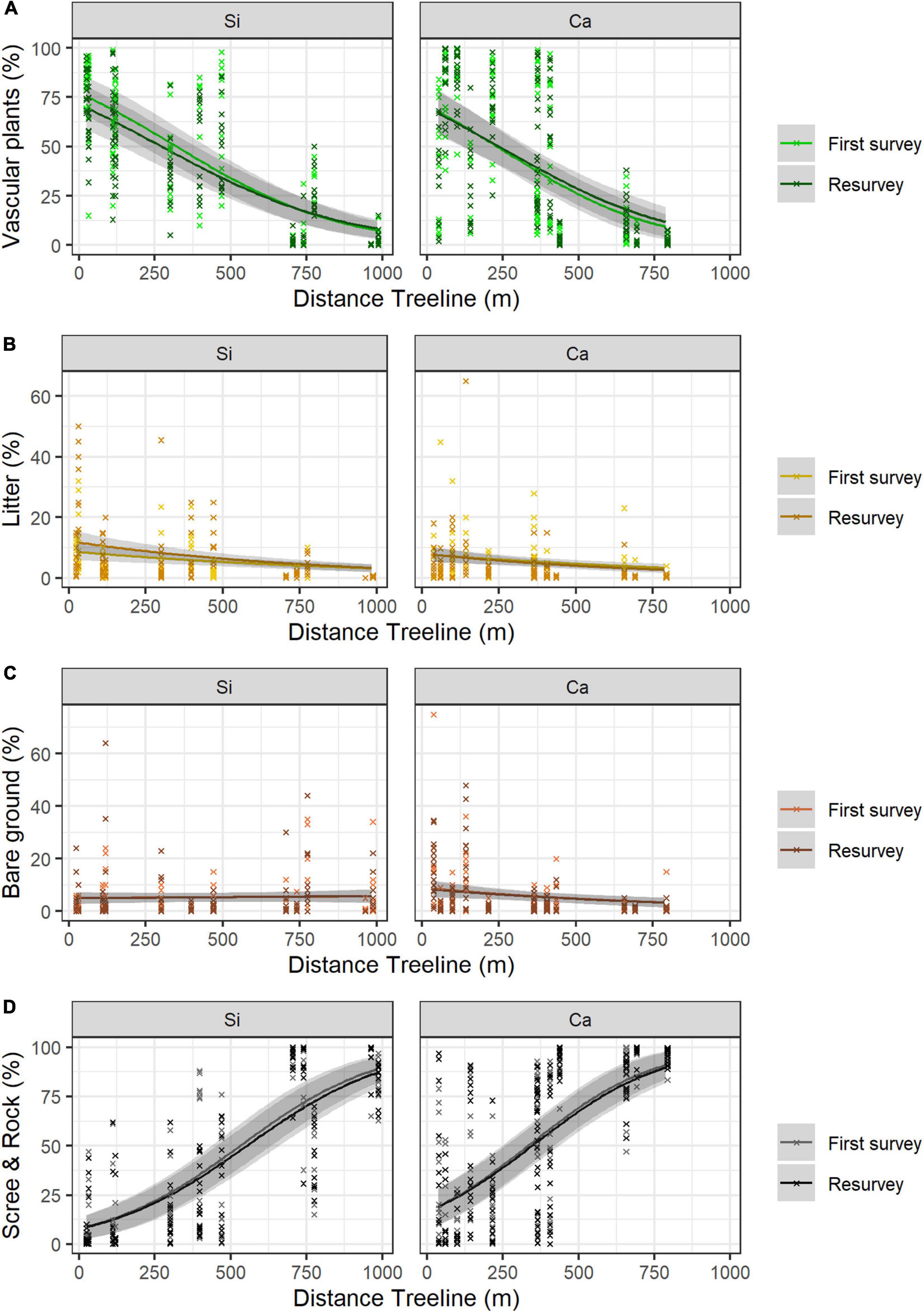
Figure 2. Proportion of surface cover types (A) vascular plants (B) litter (C) bare ground and (D) scree & rock along the elevation gradient and changes over time (first survey vs resurvey) in siliceous (Si) versus calcareous regions (Ca). The lines show the predicted cover values with 95% confidence intervals (in grey) according to GLMMs. Mean values and standard errors were calculated across aspects and random regions. Crosses represent data. Values and significance levels of the effects are given in Table 2.
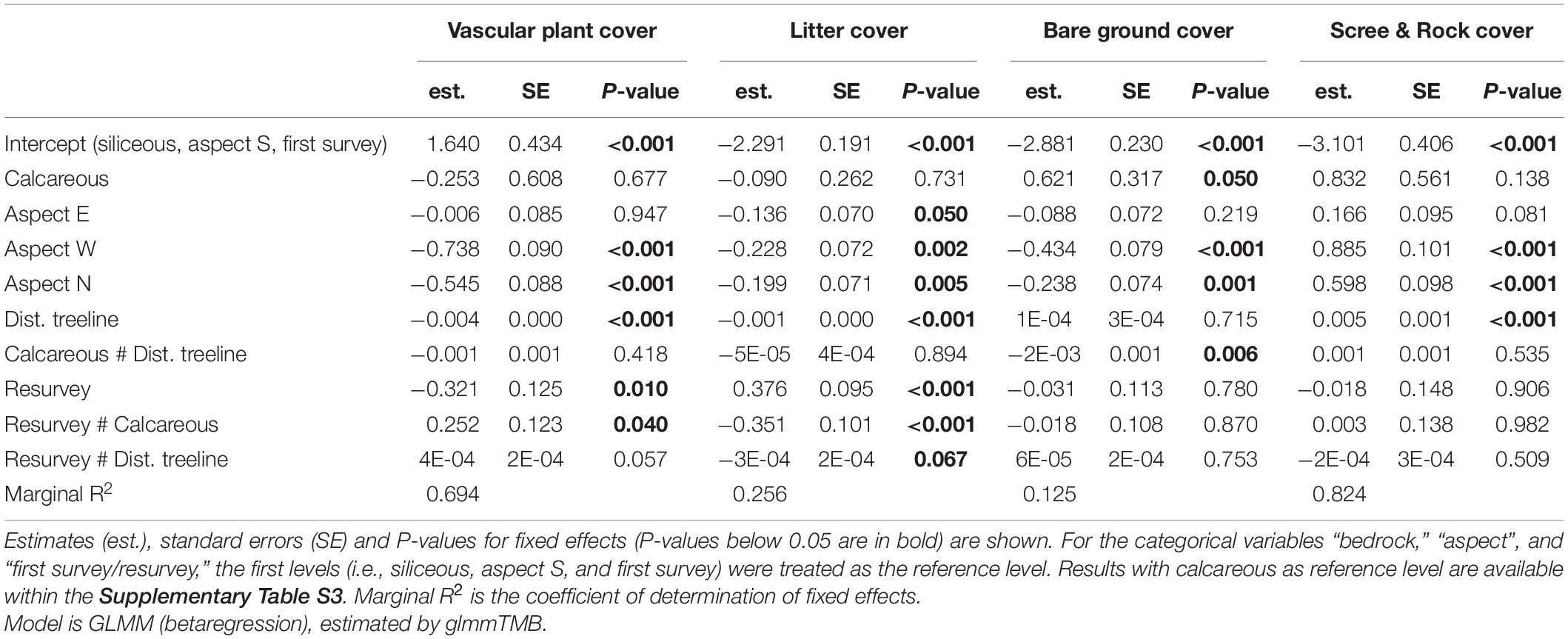
Table 2. Effects of bedrock, aspect, distance to treeline, first survey/resurvey, and selected interactions on the cover of vascular plants, litter, bare ground, and scree & rock.
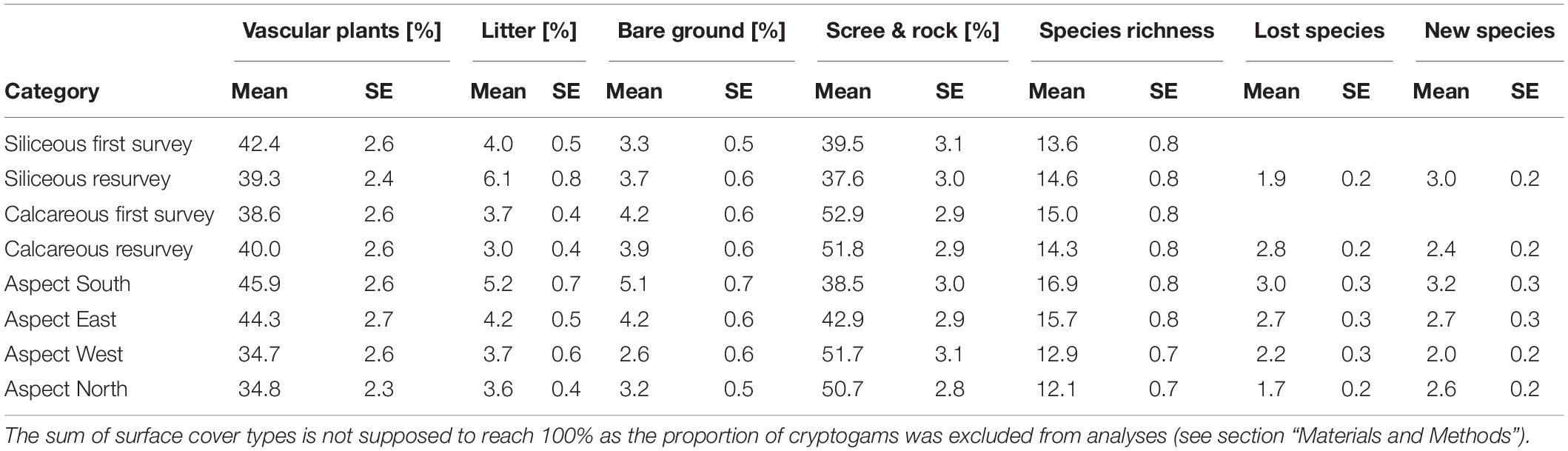
Table 3. Table of means and standard errors (SE) for all analyzed response variables: surface cover types (“vascular plant cover,” “litter cover,” “bare ground cover”), “species richness,” number of “lost species”, and “new species” for the two bedrock types during the first survey/resurvey, and according to the aspects.
Vascular plant cover decreased with increasing distance to the treeline over both bedrock types (Table 2, Dist. treeline: p < 0.001). The proportion of scree & rock increased with increasing elevation (Figure 2D and Table 2, Dist. Treeline: p < 0.001). Litter decreased with the distance to treeline (Figure 2B and Table 2, Dist. treeline: p < 0.001). Bare ground decreased with the distance to treeline only on calcareous bedrock (Supplementary Table 2, Calcareous # Dist. treeline: p = 0.006).
Differences in vascular plant cover between the first survey and the resurvey depended on bedrock types (Table 2, Resurvey # Calcareous: p = 0.040). In siliceous regions, mean vascular plant cover decreased over time from 42.2 ± 2.6% in the first survey to 39.3 ± 2.4% in the resurvey (Table 2, Resurvey: p = 0.010). In contrast, in calcareous regions, mean cover was 38.6 ± 2.6% in the first survey and this did not change significantly in the resurvey (Supplementary Table 3, Resurvey: p = 0.535). The interaction effect of first survey/resurvey and distance to treeline was marginally significant for both bedrocks (Table 2, Resurvey # Dist. treeline: p = 0.057), resulting in a stronger decrease of vascular plants cover on lower siliceous summits and a slight increase of vascular plant cover on higher calcareous summits (Figure 2A). The decrease of the vascular plant cover from the first survey to the resurvey in siliceous areas was coupled with a significant increase in litter (Table 2, Resurvey: p < 0.001) from 4.0 ± 0.5% to 6.1 ± 0.8% (Figure 2B). For bare ground and scree & rock, no significant changes over time were detected (Table 2, Resurvey: p = 0.780, p = 0.906, respectively; Figures 2C,D).
Mean species richness per 1 m × 1 m plot was lower in the siliceous regions (13.6 ± 0.8) compared to the calcareous regions (15.0 ± 0.8, Table 4, Calcareous: p = 0.033), while the differences were higher in plots with a low vascular plant cover (Figure 3). The southern and eastern aspects were more species-rich than the western (Table 4, Aspect W: p = 0.032) and the northern aspects (Table 4, Aspect N: p < 0.001).
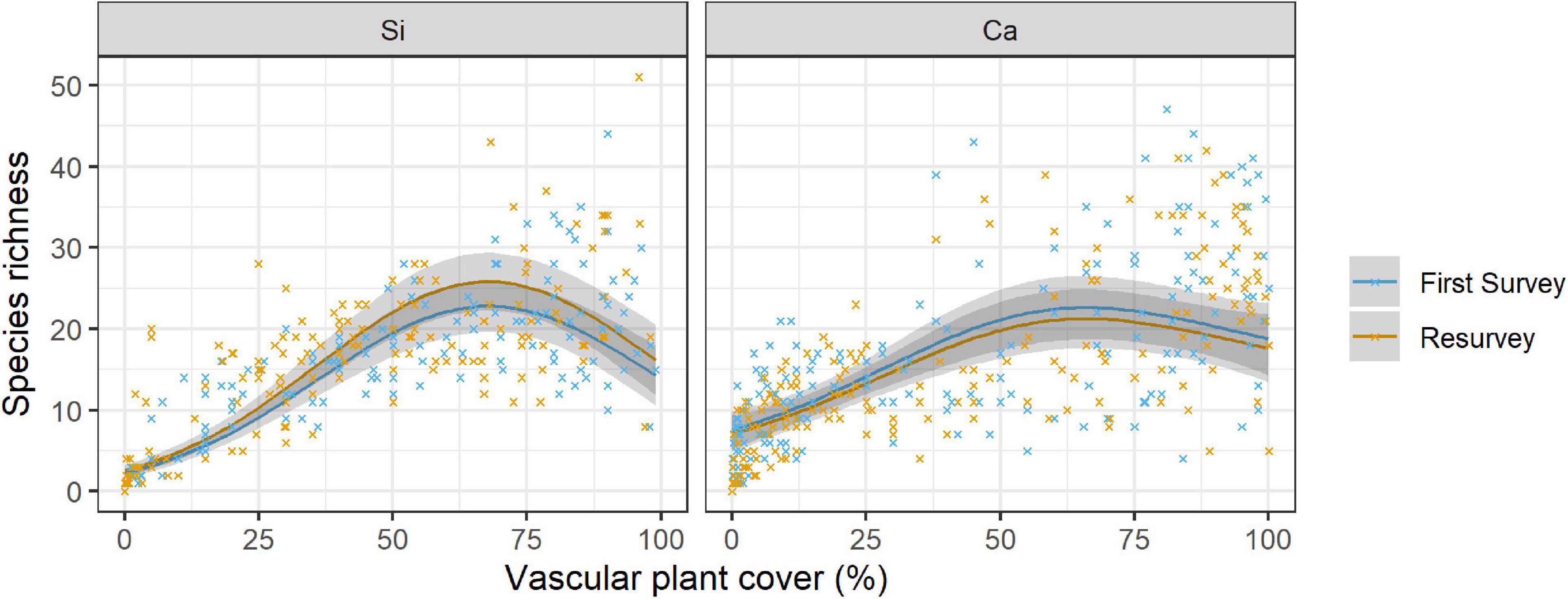
Figure 3. Relationship of species richness with vascular plant cover per 1 m × 1 m plot over time (first survey vs resurvey) on siliceous and calcareous bedrock. The lines show the predicted mean species richness and the 95% confidence interval (in grey) from a negative binomial GLMM. Mean values and standard errors were calculated across aspects and random regions. Crosses represent data. Coefficients and significance levels of effects are given in Table 4.
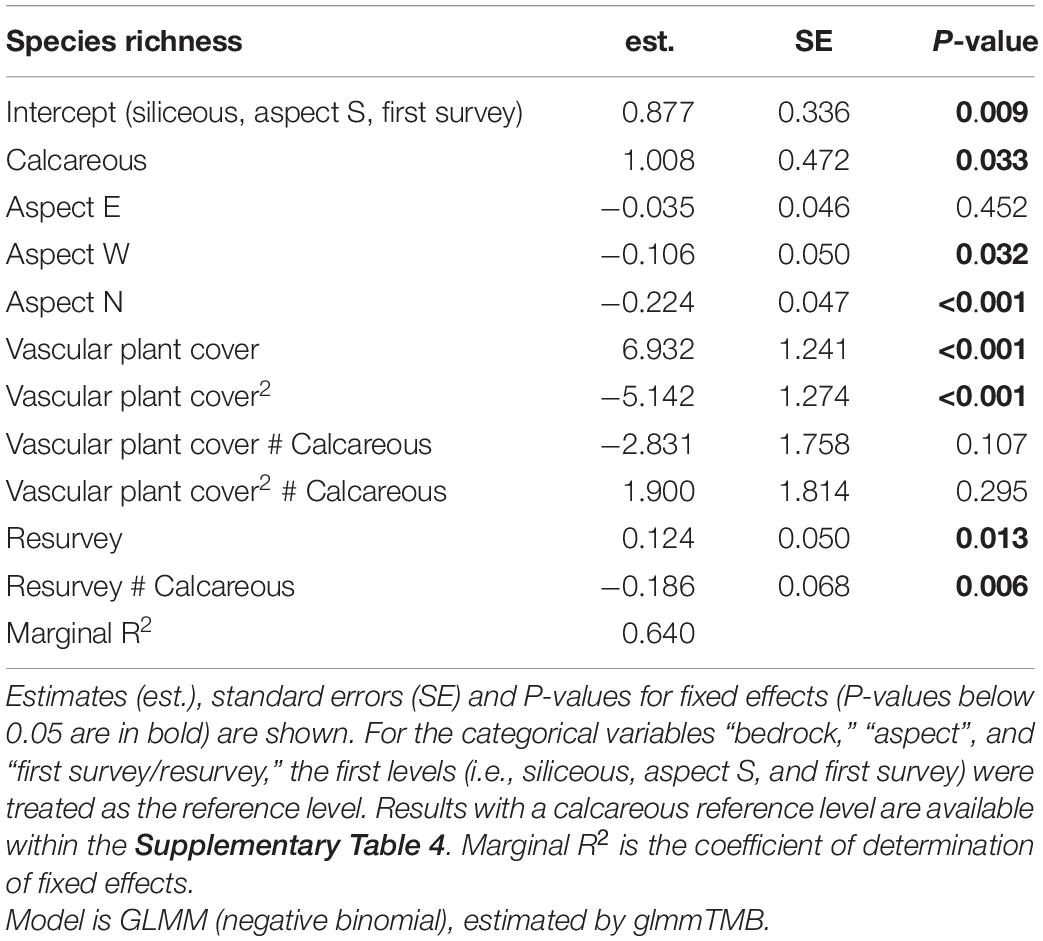
Table 4. Effects of bedrock, aspect, vascular plant cover (linear and quadratic), first survey/resurvey, and chosen interactions on species richness.
We found significant effects of the linear and the quadratic term of the vascular plant cover on species richness on both bedrock types (Table 4 and Supplementary Table 4, Vascular plant cover: p < 0.001, Vascular plant cover2: p < 0.001, p = 0.012 respectively), confirming a unimodal relationship between species richness and vascular plant cover (Figure 3). Species richness was highest at a vascular plant cover of 67% in siliceous regions and at 63% in calcareous regions (Figure 3), but the difference between bedrock types was not significant (Table 4, Vascular plant cover # Calcareous: p = 0.107, Vascular plant cover2 # Calcareous: p = 0.295). Considering the single regions, the relationships between species richness and vascular plant cover varied considerably for calcareous bedrock from unimodal to nearly linear (Supplementary Figure 2), as indicated by the relatively low marginal R2 of 0.640 (fixed effects, Table 4).
Changes in species richness over time differed significantly between siliceous and calcareous bedrock (Table 4, Resurvey # Calcareous: p = 0.006). In siliceous regions, mean species richness increased slightly but statistically significantly over time from 13.6 ± 0.8 to 14.6 ± 0.8 per plot (Table 4, Resurvey: p = 0.013, Figure 3), whereas in calcareous regions, it slightly decreased from 15.0 ± 0.8 to 14.3 ± 0.8, though not significantly (Supplementary Table 4, Resurvey: p = 0.173). In siliceous regions, the change ranged from −5 to 9 species and in calcareous regions from −17 to 10 species (Supplementary Table 5). On calcareous bedrock, the range of species richness varied among aspects from a maximum decrease per plot on eastern and western aspects (−17 and −11 species, respectively) to a maximum increase on the southern aspects (+10 species, Supplementary Table 5).
On siliceous bedrock, the number of lost species (1.9 ± 0.2) was lower than on calcareous bedrock (2.8 ± 0.2; Table 5, Calcareous: p = 0.012). In general, the number of new species was higher on the southern aspects in comparison to the western aspects (Table 5, Aspect W: p = 0.002). Species losses were highest on southern aspects; however, significant differences were found only for the northern aspect (Table 5, Aspect N: p = 0.008).
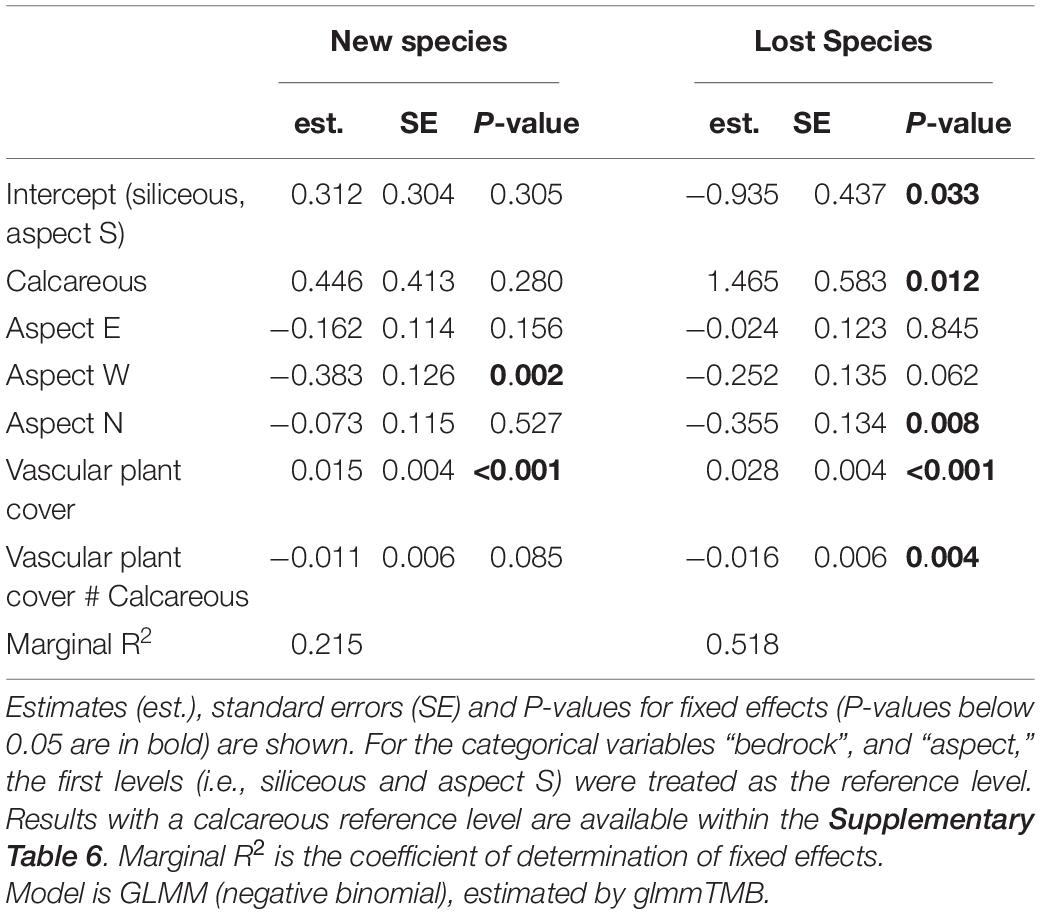
Table 5. Effects of bedrock, aspects, vascular plant cover, and the interaction between vascular plant cover and bedrock on the number of new/lost species.
In the siliceous regions, the number of both new and lost species increased with increasing vascular plant cover (Table 5, Vascular plant cover: p < 0.001). In total, the number of new species (3.0 ± 0.2) exceeded that of lost species (1.9 ± 0.2; Figure 4). In contrast, in calcareous regions, only the number of lost species increased with vascular plant cover (Supplementary Table 5, Vascular plant cover: p = 0.001), exceeding the number of new species in plots with more than 25% vascular plant cover (Figure 4). However, the relationship varied considerably among the three calcareous regions: the numbers of new and lost species were overall low in Ca-SN1, high for lost species in Ca-HSW, and higher for lost species than new species when vascular plant exceeded 55% in Ca-ADO (Supplementary Figure 3).
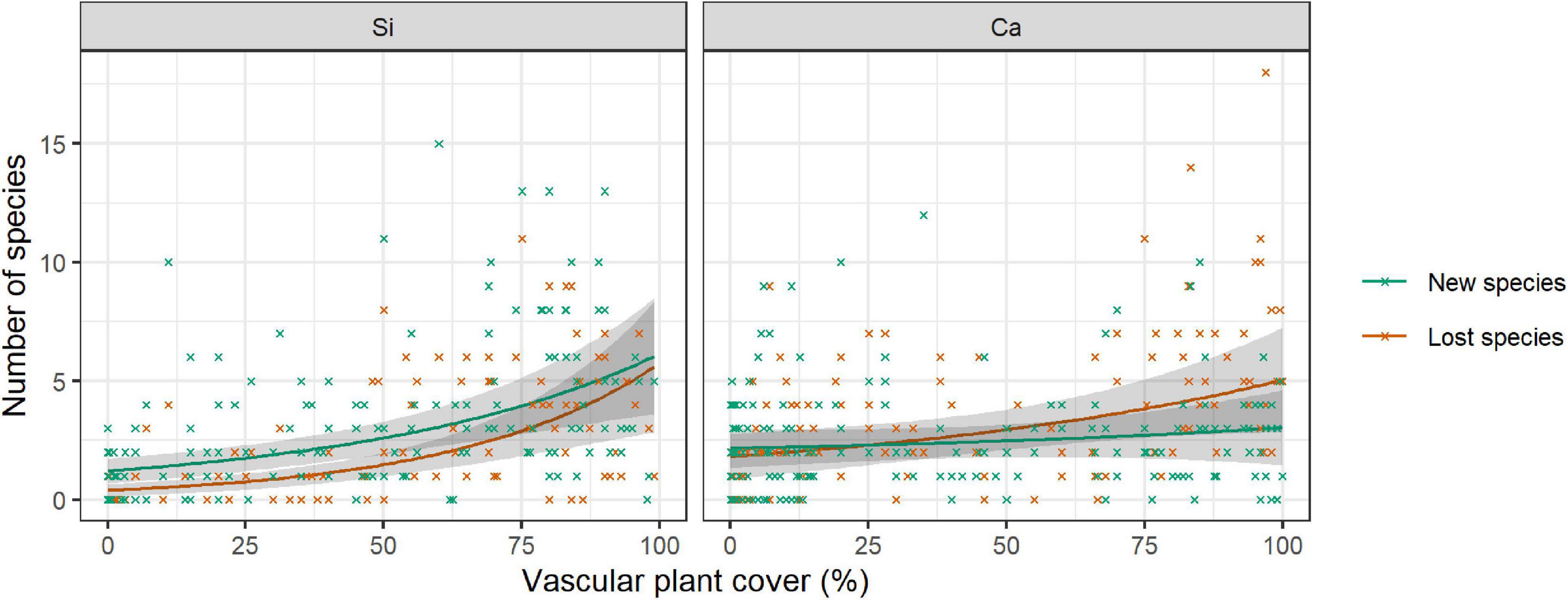
Figure 4. Number of new (green) species and lost (red) species per 1 m × 1 m plot in relation to the vascular plant cover on siliceous and calcareous bedrock. The lines show the predicted mean number of lost or new species ±95% confidence intervals (in grey) from a negative binomial GLMM. Mean values and standard errors were calculated across aspects and random regions. Crosses represent data. Values and significance levels of the effects are given in Table 5.
Our analyses of the downscaled climate data confirmed the drastic increase of summer temperature in all six studied mountain regions over the last three decades as reported from other studies (Marty and Meister, 2012; Gobiet et al., 2014; Pepin et al., 2015). As the GLORIA summits were chosen to avoid pressure from human land use (see section “Materials and Methods”), the observed changes from the first survey to the resurvey can be ascribed almost completely to the direct and indirect effects of climate change. Further, Pauli et al. (2012) and Steinbauer et al. (2018) found that increased temperature is the main driver of the observed species increase on mountain summits across Europe. Gottfried et al. (2012) found a strong correlation between the ongoing “thermophilization” of alpine plant communities and the increase of the mean daily minimum temperature of June.
Overall, we found small differences in surface cover between siliceous and calcareous bedrock. Therefore, the hypothesis (1a) that vascular plant cover and litter should prevail on siliceous bedrock, whereas bare ground, scree, and rock should dominate on calcareous bedrock could only be partially confirmed. Independently of bedrock, mean vascular plant cover decreased strongly along the elevation gradient and thus, is strongly related to the adiabatic temperature gradient (Bürli et al., 2021). Also, the proportion of scree and rock was independent of bedrock types. Nevertheless, the low bare ground cover on lower siliceous summits might indicate a lack of appropriate microhabitats for potential germination (Graae et al., 2011) in contrast to calcareous summits, where also at lower elevations small amounts of bare ground were present.
Although vascular plant cover strongly decreased with decreasing temperature along the elevation gradient, changes over time were smaller than expected from the considerable warming of 0.7°C of the mean summer temperature over the monitoring period. Using a space-for-time substitution approach, such warming would correspond to a 120 m upward shift in elevation necessary to conserve the past climatic conditions. In contrast to previous observations of vascular plant cover increases due to improved growing conditions and a longer growing season (Rogora et al., 2018), our hypothesis (1b) of an increase in vascular plant cover, more pronounced within siliceous regions, was not supported by our data. Vascular plant cover increased slightly, yet not significantly, only on the higher calcareous summits. On the lower siliceous summits mean vascular plant cover decreased by 3%, coupled with an increase of mean litter cover.
These observed decreases in plant cover on siliceous bedrock go in line with findings at Mt Schrankogel, the largest permanent plot site in the alpine-nival ecotone of the Alps on siliceous bedrock, where vascular plant cover also decreased over the last 20 years (Lamprecht et al., 2018). There, the decrease was mainly caused by a cover decrease of the cold-adapted species (Steinbauer et al., 2020), while new species from lower elevations were not able to fill the open gaps. Vascular plant cover changes, therefore, may not necessarily be connected to the worldwide observed increase of species richness on summits (Dullinger et al., 2007; Pauli et al., 2012; Steinbauer et al., 2018). This is plausible as the soil development and establishment of vegetation can take centuries to millennia (Theurillat et al., 1998; Kulonen et al., 2018) and are hampered by the steep slopes and strong erosion through gravitational processes on alpine summits. At larger spatial scales, ‘greening‘ of the European Alps was evidenced as an increase of the normalized difference vegetation index (NDVI) derived from satellite data on a spatial resolution of 30–125 m (Carlson et al., 2017; Filippa et al., 2019), which is, however, probably not comparable with the vascular plant cover changes in our plots.
Nevertheless, the slight increase of vascular plant cover on the higher calcareous summits points to a slow densification of the existing vegetation patches, either following a filling or a moving process (Grabherr et al., 1995; Gottfried et al., 1999). This increase was probably too small to be paralleled in a detectible decrease of surface cover types like bare ground or scree and rock. Additionally, it is possible that vascular plant species occupied microsites primarily covered by cryptogams, which were not considered in our analyses.
Furthermore, we found contrasting cover changes of litter between siliceous and calcareous regions. The increased proportion of litter on the lower siliceous summits and replacement of living vascular plants might be the first sign toward a more pronounced decreasing vascular plant cover in the long-term. Long-lived tussocks such as Carex curvula (Grabherr, 1989) produce a high quantity of old leaves and leaf sheaths, which decompose over a long timescale. More frequent drought events might have enhanced litter accumulation on lower siliceous summits during the last summers (Haslinger et al., 2016). The slow-decaying dead plant material can prevent colonization by new species and can negatively affect seed germination and seedling establishment (Xiong and Nilsson, 1999; Graae et al., 2011). However, effects of litter on seed germination and establishment depend on the quality and density of the dead plant material. Faster decomposing material of forbs might even be of advantage for seedlings under conditions of recurrent drought when increasing the water storing capacity or ameliorating the microclimate.
The unimodal relationship between species richness and vascular plant cover, with a peak around 65% cover (Figure 3; siliceous 67%, calcareous 63%) confirms hypothesis 2a, in line with other results describing a decrease of species richness due to increased competition for resources such as light (Grime, 1979; Rosenzweig, 1995; Grytnes, 2000; Pierce, 2014). Besides competition for light, it is likely that root competition takes place in dense alpine grasslands (Grabherr, 1989). Naud et al. (2019) showed a unimodal relationship of species richness per 1 m × 1 m plot with elevation whereby species diversity was limited by competition at low elevation and by a smaller species pool at high elevation (Moser et al., 2005). However, at the mesoscale, there is a decrease in the richness of vascular plants along the elevation gradient above the treeline (Theurillat et al., 2003).
The unimodal relationship between species richness and vascular plant cover was found in both bedrock types. However, the decrease in richness at plant cover above 65% was more pronounced in siliceous communities. All three siliceous regions showed a remarkably similar decrease in richness at plots with high vascular plant cover, pointing toward a common species richness pattern at siliceous summits in the Alps above the treeline sharing similar communities and geological characteristics (Pfiffner, 2015). Contrastingly, patterns for the calcareous regions were rather heterogeneous, and relatively high species richness could also be found at high vascular plant cover (Supplementary Figure 2). An explanation for the high heterogeneity could be that the regions were situated in different parts of the Alps (Supplementary Figure 1) with different types of limestone and varying proportions of calcium, magnesium carbonate or clay, which further impacts erosion and soil formation: (1) SN1 within the geological complex calcareous part of the Swiss Central Alps consisting of heterogenous limestone formations (Wetterstein, Dolomite) high summits and a limitation of vegetation development (no high vascular plant cover, no high species richness); (2) HSW at the eastern edge of the Northern Limestone Alps with low maximum elevations, a relatively pure calcium carbonate limestone (Wetterstein, Gutensteiner Dolomite), and a nearly monotonic increasing species richness with vascular plant cover; and finally (3) ADO within the Southern Alps dominated by Latemar limestone and Hauptdolomite showing a unimodal relationship with a high species richness maximum at high vascular plant cover.
High levels of competition in well-developed alpine grassland communities has been shown experimentally (Olsen and Klanderud, 2014) and via community analyses (Grabherr, 1989). Dense communities may be able to prevent the establishment of new species because of the presence of many long-lived, clonal species forming dense mats (Steinger et al., 1996; Theurillat and Guisan, 2001; but see discussion on species turnover below). Particularly impressive examples are Carex curvula communities with nearly no change over 32 yearsy (Windmaißer and Reisch, 2013) and communities at the Niwot Ridge, United States with minimal changes over 40 years (Naud et al., 2019). In the same way, the species-rich calcareous grassland communities with Carex sempervirens are known to be very competitive and stable (Grabherr, 1989; Gritsch et al., 2016; Matteodo et al., 2016). In comparison to the siliceous communities reaching a maximum species diversity at 65% vascular plant cover, single calcareous communities within our study reached their diversity maximum at up to 100% vascular plant cover. This goes in line with Gigon’s (1971) investigations, showing that more species can coexist at calcareous sites because dominance of competitive species is not as strong as in siliceous communities. Additionally, in the European Alps the calcareous species pool is richer than the siliceous species pool, as a result of the outer calcareous regions being less impacted by glaciations than the central, siliceous regions (Wohlgemuth, 2002; Aeschimann et al., 2012). This might enable higher species richness in calcareous plots. Our results also confirmed higher species richness on calcareous bedrock, although the mean difference of just one species was relatively small and decreased over time.
Our results showed that changes in species richness at the scale of 1 m × 1 m plots were rather small over 14 years of observation. The mean increase of species richness by one species found in siliceous plots is comparable to the changes at the alpine-nival ecotone in the siliceous Austrian Central Alps (Mt Schrankogel), where Lamprecht et al. (2018) reported a slightly higher increase of two species over 20 years. Contrastingly, changes of species richness were not significant in calcareous regions, which argues against our hypothesis 2b, suggesting a more substantial increase of species richness within calcareous regions. Probably due to the increasing numbers of lost species, species richness increase is already limited in several plots. While species richness at whole summit areas is still rapidly increasing (Matteodo et al., 2013; Unterluggauer et al., 2016; Steinbauer et al., 2018), first threats of a loss in species richness might be observed at the finer scale of 1 m × 1 m plots.
The number of new species and lost species provided additional insight to detect vegetation changes over time. So far only native species have been found on the studied summits and colonization involved only species that have been present in the region for a long time. With increasing vascular plant cover, the number of lost species increased, which confirms our hypothesis 3a. However, contrary to our expectations, the number of lost species exceeded the number of new species at the highest vascular plant cover in calcareous regions only (hypothesis 3b). This might be the first sign of diversity loss in some sites with higher vascular plant cover. Erschbamer et al. (2011) and Unterluggauer et al. (2016) for example, reported an ongoing encroachment of treeline species and grasses on the lowest summit in the calcareous region ADO. They suggested that treeline species might outcompete alpine species in the future. Eventually, due to the increased water permeability and lower water contents of soils on calcareous bedrock (Gigon, 1971), the effect of drought may be more severe in calcareous regions and lead to a higher number of lost species. Indeed, several summers (2003, 2015) within the monitoring period were dry and hot (Haslinger et al., 2016). According to predictions, the probability of increased frequency and severity of summer drought events will increase in some parts of the Alps (Gobiet et al., 2014; Haslinger et al., 2016; Spinoni et al., 2018). Together with high temperatures, which increase evapotranspiration, this might pose a serious problem for Alpine plants. However, the low resolution of the downscaled climate data and the low frequency of monitoring cycles within our study make it difficult to capture the direct effect of drought on the 1 m × 1 m plot level.
In siliceous regions, well-developed communities with a high vascular plant cover were expected to be competitive and stable. Overall, however, there was a high species turnover on siliceous bedrock, and the numbers of new and lost species both increased with increasing vascular plant cover. It seems therefore quite probable that due to the special conditions taking place on summits, a higher turnover is possible in comparison to the well-developed grasslands on homogenous slopes or flatter areas in the alpine belt (Windmaißer and Reisch, 2013; Gritsch et al., 2016; Naud et al., 2019) at least at the micro-scale of 1 m × 1 m plots. However, the observed species turnover does not mean a conversion into a new community. Most of the new species belong to the local species pool around or below the summits (Unterluggauer et al., 2016). Nevertheless, several studies have already reported a homogenization and thermophilization of the summit communities (Vittoz et al., 2008; Erschbamer et al., 2011; Gottfried et al., 2012; Pauli et al., 2012; Wipf et al., 2013; Matteodo et al., 2016; Unterluggauer et al., 2016; Lamprecht et al., 2018).
Overall, the models used in our study to predict the number of new and lost species had a comparably low marginal R2 with considerable differences between regions (Supplementary Figure 3). This is not surprising as colonizations and disappearances are rare events that are influenced by several other factors besides temperature and vascular plant cover (Graae et al., 2011), making them more prone to stochastic events occurring between plots and regions.
In their pan European GLORIA analysis, Winkler et al. (2016) clearly showed that aspects matter for species diversity on summits in temperate mountains, enabling a higher species richness on eastern and southern exposures due to warmer temperatures and better-developed soils. Therefore, it appeared straightforward to include the effect of aspect in the models in order not to omit a variable and to introduce a bias. The results of all our models confirmed the strong effect of the aspect. Winkler et al. (2016) showed that new species mostly invade the warmer southern and eastern sides. The variation between aspects might also be the reason for the large range of species richness change over time observed in the present study. Probably the higher species turnover on calcareous bedrock resulted from a higher instability of vegetation on certain aspects.
Besides the discussed factors influencing vegetation changes, grazing might be a variable that is difficult to take into account correctly. Since summits were initially chosen in areas with no or the least possible domestic grazing (Supplementary Table 1), we expect grazing effects to be small, although no quantitative data exist in this way for wild grazers. Therefore, we cannot exclude that some variation in the grazing activity may have caused some local heterogeneity or could have temporarily masked the effects of warming. For instance, the general trend of a continuous decrease of pasturing by livestock in the alpine zone of the European Alps over the last decades could have an impact on vegetation succession (Tasser and Tappeiner, 2002; Czortek et al., 2018; Frate et al., 2018). Conversely, there is a long-term trend of increasing wild ungulates (Apollonio et al., 2010). According to Steinbauer et al. (2018) land-use change may account for local variation in species richness trends, but it is unlikely at a larger scale. In a joint analysis with Mediterranean GLORIA regions, Lamprecht et al. (2019) analyzed the impact of land-use in three of our regions (ADO, HSW, VAL). Although land-use varied locally, structural equation models could not find a significant effect on species richness trends.
Observer errors, in particular visual cover estimation and wrong species identifications, are a common issue in resurveys. The relative coefficient of variation of cover estimation may reach up to 60% (Vittoz et al., 2010; Futschik et al., 2020). Wrong identification of species, leading to pseudo-turnover and pseudo-variation in species richness, may occur in 10 to 30% of the cases, according to Morrison (2016) and Futschik et al. (2020). However, Futschik et al. (2020) showed that changes in species turnover and species cover were significantly larger than pseudo-changes due to observer errors even after seven years within the tested GLORIA regions Hochschwab, Mt Schrankogel, and High Tatra. Additionally, to avoid pseudo-turnover, we checked all the new and lost species for possible misidentification. Remaining mistakes are likely to be random, and hence our extensive dataset allows us to find trends beyond potential observer errors.
Besides observer errors, several phenological variations caused by water availability, warm or cold season, and inherent population fluctuations between years in the reproductive allocations might still be apparent within our dataset and superimpose a long-term effect of climate change. As an attempt to minimize phenological variations, the first survey and resurvey were done in peak season.
The present study provides evidence that vegetation attributes such as species richness and species turnover, and the distribution of surface cover types (vascular plants, litter, bare ground, scree & rock) generally differ between calcareous and siliceous summits and show contrasting changes over 14 years of climate warming on the micro-scale of 1 m × 1 m. Surface cover types showed minor general differences between bedrock types but changed differently over time: on siliceous bedrock, vascular plant cover decreased, coupled with an increase in litter, and it marginally increased on the highest calcareous summits. Instead of the expected increase, vascular plant cover might thus even decrease at the expense of litter accumulation quite probably due to drought. Counterintuitively, this effect is more pronounced at the moment on siliceous bedrock; however, similar effects might occur in calcareous regions.
We could confirm a unimodal relationship of species richness and vascular plant cover indicating competition in communities with a high vascular plant cover. Species richness decreased more strongly beyond 65% plant cover within siliceous regions. Nevertheless, overall species richness increased over time, and the number of new species exceeds the number of lost species. Contrastingly and contrary to expected, in calcareous regions, the exceeding number of lost species might be the first sign of a negative trend in species richness with drought possibly being an increasing problem for summit vegetation. Continued monitoring will be necessary to confirm the observed trends.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LN did the analyses and prepared the manuscript. JWa supervised the analyses. MM, PU, LN, SW, CR, J-PT, AL, KS, and PV contributed GLORIA data. DM, AG, and JWe prepared the climate data and did the downscaling. BE and SW supervised all steps from analysis design to manuscript preparation. All authors contributed to the writing of the manuscript.
Fieldwork was realized with funding of the EU project no. EVK2-CT-2000-00056, the Earth System Sciences Program of the Austrian Academy of Sciences (project MEDIALPS), the Amt für Naturparke, Autonome Provinz Bozen-Südtirol, the Tiroler Wissenschaftsförderung, the Département de la culture et des sports du Valais, the Fondation Mariétan, the Société académique de Genève, the Swiss Federal Office of Education and Science, the Swiss Federal Office for the Environment, the Research Committee of the Swiss National Park, and the de Giacomi Foundation. Publication costs were thankfully reduced by 30% by Frontiers and finally covered by the publication fund of the University of Innsbruck (UIBK) and the Department of Botany of the UIBK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Georg Grabherr, Michael Gottfried, Harald Pauli, Manuela Winkler, and the whole GLORIA coordination group Vienna for supervision and database handling. We thank Josef Plattner from the Amt für Natuparke Provinz Bozen Südtirol for the transport to the field sites in the Nature Park Texelgroup, Moritz Falch, and all other helpers who made fieldwork possible. Thanks to a stay at Swiss National Park, several analyses and important steps for manuscript preparation could be realized. We thank Vera Margreiter, Anne Björkman, and Francesca Jaroszynska for advice on statistics and English writing. We thank the Herbarium of the University of Innsbruck for providing specimens for difficult taxa and determination help with the genus Hieracium.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.642309/full#supplementary-material
Aeschimann, D., Rasolofo, N., and Theurillat, J.-P. (2012). Analysis of the flora of the Alps. 4: Ecology. Candollea 67, 193–219.
Alexander, J. M., Diez, J. M., and Levine, J. M. (2015). Novel competitors shape species’ responses to climate change. Nature 525, 515–518. doi: 10.1038/nature14952
Apollonio, M., Andersen, R., and Putman, R. (2010). European Ungulates and their Management in the 21st Century. Cambridge: Camebridge University Press.
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400.
Bürli, S., Theurillat, J.-P., Winkler, M., Lamprecht, A., Pauli, H., Rixen, C., et al. (2021). A common soil temperature threshold for the upper limit of alpine grasslands in European mountains. Alp. Bot. (in press). doi: 10.1007/s00035-021-00250-1
Carlson, B. Z., Corona, M. C., Dentant, C., Bonet, R., Thuiller, W., and Choler, P. (2017). Observed long-term greening of alpine vegetation—a case study in the French Alps. Environ. Res. Lett. 12:114006. doi: 10.1088/1748-9326/aa84bd
Czortek, P., Kapfer, J., Delimat, A., Eycott, A., Grytnes, J. A., Orczewska, A., et al. (2018). Plant species composition shifts in the Tatra Mts as a response to environmental change: a resurvey study after 90 years. Folia Geobot. 53, 333–348. doi: 10.1007/s12224-018-9312-9
Dirnböck, T., Essl, F., and Rabitsch, W. (2011). Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Chang. Biol. 17, 990–996. doi: 10.1111/j.1365-2486.2010.02266.x
Dullinger, S., Gattringer, A., Thuiller, W., Moser, D., Zimmermann, N. E., Guisan, A., et al. (2012). Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change 2, 619–622. doi: 10.1038/Nclimate1514
Dullinger, S., Kleinbauer, I., Pauli, H., Gottfried, M., Brooker, R., Nagy, L., et al. (2007). Weak and variable relationships between environmental severity and small-scale co-occurrence in alpine plant communities. J. Ecol. 95, 1284–1295. doi: 10.1111/j.1365-2745.2007.01288.x
Engler, R., Randin, C. F., Thuiller, W., Dullinger, S., Zimmermann, N. E., Araujo, M. B., et al. (2011). 21st century climate change threatens mountain flora unequally across Europe. Glob. Chang. Biol. 17, 2330–2341. doi: 10.1111/j.1365-2486.2010.02393.x
Erschbamer, B., Unterluggauer, P., Winkler, E., and Mallaun, M. (2011). Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (northern Italy). Preslia 83, 387–401.
Farrer, E. C., Ashton, I. W., Spasojevic, M. J., Fu, S., Gonzalez, D. J. X., and Suding, K. N. (2015). Indirect effects of global change accumulate to alter plant diversity but not ecosystem function in alpine tundra. J. Ecol. 103, 351–360. doi: 10.1111/1365-2745.12363
Ferrari, S., and Cribari-Neto, F. (2004). Beta regression for modelling rates and proportions. J. Appl. Stat. 31, 799–815. doi: 10.1080/0266476042000214501
Filippa, G., Cremonese, E., Galvagno, M., Isabellon, M., Bayle, A., Choler, P., et al. (2019). Climatic drivers of greening trends in the Alps. Remote Sens. 11:2527. doi: 10.3390/rs11212527
Frate, L., Carranza, M. L., Evangelista, A., Stinca, A., Schaminée, J. H. J., and Stanisci, A. (2018). Climate and land use change impacts on Mediterranean high-mountain vegetation in the Apennines since the 1950s. Plant Ecol. Divers. 11, 85–96. doi: 10.1080/17550874.2018.1473521
Futschik, A., Winkler, M., Steinbauer, K., Lamprecht, A., Rumpf, S. B., Barančok, P., et al. (2020). Disentangling observer error and climate change effects in long-term monitoring of alpine plant species composition and cover. J. Veg. Sci. 31, 14–25. doi: 10.1111/jvs.12822
Gigon, A. (1971). Vergleich alpiner Rasen auf Silikat- und auf Karbonatboden. Veröff. Geobot. Inst. ETH Stiftung Rübel Zürich 48, 1–164. doi: 10.3929/ethz-a-000089178
Gigon, A. (1987). “A hierarchic approach in causal ecosystem analysis the calcifuge-calcicole problem in alpine grasslands,” in Ecology Studies, eds E. Schulze and H. Zwölfer (Berlin: Springer), 228–244.
Gobiet, A., Kotlarski, S., Beniston, M., Heinrich, G., Rajczak, J., and Stoffel, M. (2014). 21st century climate change in the European Alps – A review. Sci. Total Environ. 493, 1138–1151. doi: 10.1016/j.scitotenv.2013.07.050
Gottfried, M., Pauli, H., Futschik, A., Akhalkatsi, M., Barancok, P., Alonso, J. L. B., et al. (2012). Contitnent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115. doi: 10.1038/nclimate1329
Gottfried, M., Pauli, H., Reiter, K., and Grabherr, G. (1999). A fine-scaled predictive model for changes in species distribution patterns of high mountain plants induced by climate warming. Divers. Distrib. 5, 241–251. doi: 10.1046/j.1472-4642.1999.00058.x
Graae, B. J., Ejrnæs, R., Lang, S. I., Meineri, E., Ibarra, P. T., and Bruun, H. H. (2011). Strong microsite control of seedling recruitment in tundra. Oecologia 166, 565–576. doi: 10.1007/s00442-010-1878-8
Graae, B., Vandvik, V., Armbruster, W., Eiserhardt, W., Svenning, J.-C., Hylander, K., et al. (2018). Stay or go – how topographic complexity influences alpine plant population and community responses to climate change. Perspect. Plant Ecol. Evol. Syst. 30, 41–50. doi: 10.1016/j.ppees.2017.09.008
Grabherr, G. (1989). On community structure in high alpine grasslands. Vegetatio 83, 223–227. doi: 10.1007/BF00031694
Grabherr, G., and Mucina, L. (eds). (1993). Die Pflanzengesellschaften Österreichs. Teil II Natürliche waldfreie Vegetation. Jena, New York: G. Fischer.
Grabherr, G., Gottfried, M., Gruber, A., and Pauli, H. (1995). “Patterns and current changes in alpine plant diversity,” in Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystem Consequences, eds F. S. Chapin and C. Körner (Berlin: Springer), 167–181.
Gritsch, A., Dirnböck, T., and Dullinger, S. (2016). Recent changes in alpine vegetation differ among plant communities. J. Veg. Sci. 27, 1177–1186. doi: 10.1111/jvs.12447
Grytnes, J. A. (2000). Fine-scale vascular plant species richness in different alpine vegetation types: relationships with biomass and cover. J. Veg. Sci. 11, 87–92. doi: 10.2307/3236779
Grytnes, J. A., Kapfer, J., Jurasinski, G., Birks, H. H., Henriksen, H., Klanderud, K., et al. (2014). Identifying the driving factors behind observed elevational range shifts on European mountains. Global Ecol. Biogeogr. 23, 876–884. doi: 10.1111/geb.12170
Harris, I., Jones, P. D., Osborn, T. J., and Lister, D. H. (2014). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. Int. J. Climatol. 34, 623–642. doi: 10.1002/joc.3711
Haslinger, K., Schöner, W., and Anders, I. (2016). Future drought probabilities in the Greater Alpine Region based on COSMO-CLM experiments – Spatial patterns and driving forces. Meteorol. Z. 25, 137–148. doi: 10.1127/metz/2015/0604
Kammer, P. M., Schöb, C., and Choler, P. (2007). Increasing species richness on mountain summits: upward migration due to anthropogenic climate change or re-colonisation? J. Veg. Sci. 18, 301–306. doi: 10.1111/j.1654-1103.2007.tb02541.x
Kinzel, H. (1983). “Influence of limestone, silicates and Soil pH on vegetation,” in Physiological Plant Ecology III: Responses to the Chemical and Biological Environment, eds O. L. Lange, P. S. Nobel, C. B. Osmond, and H. Ziegler (Berlin: Springer), 201–244.
Kulonen, A., Imboden, R. A., Rixen, C., Maier, S. B., and Wipf, S. (2018). Enough space in a warmer world? Microhabitat diversity and small-scale distribution of alpine plants on mountain summits. Divers. Distrib. 24, 252–261. doi: 10.1111/ddi.12673
Lamprecht, A., Rutzinger, M., Pauli, H., Bardy-Durchhalter, M., Euller, K., Niederheiser, R., et al. (2019). Disentangling Anthropogenic Drivers of Climate Change Impacts on Alpine Plant Species: Alps vs. Mediterranean Mountains. Final Report of the Project MediAlps (Research Program ‘Earth System Sciences (ESS)’). Vienna: Austrian Academy of Sciences Press. doi: 10.1553/ESS-MEDIALPSs1
Lamprecht, A., Semenchuk, P. R., Steinbauer, K., Winkler, M., and Pauli, H. (2018). Climate change leads to accelerated transformation of high-elevation vegetation in the central Alps. New Phytol. 220, 447–459. doi: 10.1111/nph.15290
Larcher, W., Wagner, J., and Lutz, C. (1998). The effect of heat on photosynthesis, dark respiration and cellular ultrastructure of the arctic-alpine psychrophyte Ranunculus glacialis. Photosynthetica 34, 219–232. doi: 10.1023/A:1006840623763
Lüdecke, D., Makows, D., and Waggoner, P. (2020). Assessment of Regression Models Performance. R package version 0.4.4. [Online]. CRAN. Available online at: https://easystats.github.io/performance accessed January, 11, 2020).
Marcante, S., Erschbamer, B., Buchner, O., and Neuner, G. (2014). Heat tolerance of early developmental stages of glacier foreland species in the growth chamber and in the field. Plant Ecol. 215, 747–758. doi: 10.1007/s11258-014-0361-8
Marcante, S., Sierra-Almeida, A., Spindelbock, J. P., Erschbamer, B., and Neuner, G. (2012). Frost as a limiting factor for recruitment and establishment of early development stages in an alpine glacier foreland? J. Veg. Sci. 23, 858–868. doi: 10.1111/j.1654-1103.2012.01411.x
Marty, C., and Meister, R. (2012). Long-term snow and weather observations at Weissfluhjoch and its relation to other high-altitude observatories in the Alps. Theor. Appl. Clima. 110, 573–583. doi: 10.1007/s00704-012-0584-3
Matteodo, M., Ammann, K., Verrecchia, E. P., and Vittoz, P. (2016). Snowbeds are more affected than other subalpine–alpine plant communities by climate change in the Swiss Alps. Ecol. Evol. 6, 6969–6982. doi: 10.1002/ece3.2354
Matteodo, M., Wipf, S., Stockli, V., Rixen, C., and Vittoz, P. (2013). Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 8, 024043. doi: 10.1088/1748-9326/8/2/024043
Morrison, L. W. (2016). Observer error in vegetation surveys: a review. J. Plant Ecol. 9, 367–379. doi: 10.1093/jpe/rtv077
Moser, D., Dullinger, S., Englisch, T., Niklfeld, H., Plutzar, C., Sauberer, N., et al. (2005). Environmental determinants of vascular plant species richness in the Austrian Alps. J. Biogeogr. 32:1265. doi: 10.1111/j.1365-2699.2005.01265.x
Naud, L., Måsviken, J., Freire, S., Angerbjörn, A., Dalén, L., and Dalerum, F. (2019). Altitude effects on spatial components of vascular plant diversity in a subarctic mountain tundra. Ecol. Evol. 9, 4783–4795. doi: 10.1002/ece3.5081
Newey, W., and West, K. (1987). A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica 55, 703–708.
Olsen, S. L., and Klanderud, K. (2014). Biotic interactions limit species richness in an alpine plant community, especially under experimental warming. Oikos 123, 71–78. doi: 10.1111/j.1600-0706.2013.00336.x
Pauli, H., Gottfried, M., Dullinger, S., Abdaladze, O., Akhalkatsi, M., Alonso, J. L. B., et al. (2012). Recent plant diversity changes on europe’s mountain summits. Science 336, 353–355. doi: 10.1126/science.1219033
Pauli, H., Gottfried, M., Lamprecht, A., Niessner, S., Rumpf, S., Winkler, M., et al. (2015). The GLORIA Field Manual – Standard Multi-Summit Approach, Supplementary Methods and Extra Approaches, 5 Edn. Vienna: GLORIA-Coordination, Austrian Academy of Sciences & University of Natural Resources and Life Sciences. doi: 10.2777/095439
Pepin, N., Bradley, R. S., Diaz, H. F., Baraer, M., Caceres, E. B., Forsythe, N., et al. (2015). Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 5, 424–430. doi: 10.1038/nclimate2563
Pierce, S. (2014). Implications for biodiversity conservation of the lack of consensus regarding the humped-back model of species richness and biomass production. Funct. Ecol. 28, 253–257. doi: 10.1111/1365-2435.12147
Reisigl, H., and Keller, R. (1994). Alpenpflanzen im Lebensraum. Alpine Rasen Schutt- und Felsvegetation. Vegetationsökologische Informationen für Studien, Exkursionen und Wanderungen. Stuttgart; New York, NY: Gustav Fischer.
Rixen, C., and Wipf, S. (2017). “Non-equilibrium in alpine plant assemblages: shifts in Europe’s summit floras,” in High Mountain Conservation in a Changing World, eds J. Catalan, J. M. Ninot, and M. M. Aniz (Cham: Springer International Publishing), 285–303.
Rogora, M., Frate, L., Carranza, M. L., Freppaz, M., Stanisci, A., Bertani, I., et al. (2018). Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci. Total Environ. 624, 1429–1442. doi: 10.1016/j.scitotenv.2017.12.155
Rosenzweig, M. L. (1995). Species Diversity in Space and Time. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511623387
Rumpf, S. B., Hülber, K., Klonner, G., Moser, D., Schütz, M., Wessely, J., et al. (2018). Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. U.S.A. 115, 1848–1853. doi: 10.1073/pnas.1713936115
Scherrer, D., and Körner, C. (2011). Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 38, 406–416. doi: 10.1111/j.1365-2699.2010.02407.x
Simas, A. B., Barreto-Souza, W., and Rocha, A. V. (2010). Improved estimators for a general class of beta regression models. Comput. Stat. Data Anal. 54, 348–366. doi: 10.1016/j.csda.2009.08.017
Smithson, M., and Verkuilen, J. (2006). A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11, 54–71. doi: 10.1037/1082-989x.11.1.54
Spinoni, J., Vogt, J. V., Naumann, G., Barbosa, P., and Dosio, A. (2018). Will drought events become more frequent and severe in Europe? Int. J. Climatol. 38, 1718–1736. doi: 10.1002/joc.5291
Steinbauer, K., Lamprecht, A., Semenchuk, P., Winkler, M., and Pauli, H. (2020). Dieback and expansions: species-specific responses during 20 years of amplified warming in the high Alps. Alp. Bot. 130, 1–11. doi: 10.1007/s00035-019-00230-6
Steinbauer, M., Grytnes, J.-A., Jurasinski, G., Kulonen, A., Lenoir, J., Pauli, H., et al. (2018). Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234. doi: 10.1038/s41586-018-0005-6
Steinger, T., Körner, C., and Schmid, B. (1996). Long-term persistence in a changing climate: DNA analysis suggests very old ages of clones of alpine Carex curvula. Oecologia 6, 94–99. doi: 10.1007/BF00328796
Tasser, E., and Tappeiner, U. (2002). Impact of land use changes on mountain vegetation. Appl. Veg. Sci. 5, 173–184. doi: 10.1111/j.1654-109X.2002.tb00547.x
Theurillat, J. P., and Guisan, A. (2001). Potential impact of climate change on vegetation in the European Alps: a review. Clim. Change 50, 77–109. doi: 10.1023/A:1010632015572
Theurillat, J.-P., Felber, F., Geissler, P., Gobat, J.-M., Fierz, M., Fischlin, A., et al. (1998). “Sensitivity of plant and soil ecosystems of the Alps to climate change,” in Views from the Alps: Regional Perspectives on Climate Change, eds P. Cebon, U. Dahinden, C. Davies, D. Imboden, and C. C. Jäger (London: MIT Press), 225–308.
Theurillat, J.-P., Schlüssel, A., Geissler, P., Guisan, A., Velluti, C., and Wiget, L. (2003). “Vascular plant and bryophyte diversity along elevation gradients in the Alps,” in Alpine Biodiversity in Europe, eds L. Nagy, G. Grabherr, C. Körner, and D. B. A. Thompson (Heidelberg: Springer), 185–193.
Unterluggauer, P., Mallaun, M., and Erschbamer, B. (2016). The higher the summit, the higher the diversity changes – results of a long-term monitoring project in the Dolomites. Gredleriana 16, 5–34.
Virtanen, R., Dirnböck, T., Dullinger, S., Grabherr, G., Pauli, H., Staudinger, M., et al. (2003). “Patterns in the plant species richness of European high mountain vegetation,” in Alpine biodiversity in Europe, eds L. Nagy, G. Grabherr, C. Körner, and D. B. A. Thompson (Berlin: Springer), 149–172.
Vittoz, P., Bayfield, N., Brooker, R., Elston, D. A., Duff, E. I., Theurillat, J.-P., et al. (2010). Reproducibility of species lists, visual cover estimates and frequency methods for recording high-mountain vegetation. J. Veg. Sci. 21, 1035–1047. doi: 10.1111/j.1654-1103.2010.01216.x
Vittoz, P., Bodin, J., Ungricht, S., Burga, C., and Walther, G. R. (2008). One century of vegetation change on Isla Persa, a nunatak in the Bernina massif in the Swiss Alps. J. Veg. Sci. 19, 671–680. doi: 10.3170/2008-8-18434
Windmaißer, T., and Reisch, C. (2013). Long-term study of an alpine grassland: local constancy in times of global change. Alp. Bot. 123, 1–6. doi: 10.1007/s00035-013-0112-9
Winkler, D., Lubetkin, K., Carrell, A., Jabis, M., Yang, Y., and Kueppers, L. (2019). “Responses of alpine plant communities to climate warming. Microbes, Vegetation, Fauna and Soil Biogeography,” in Ecosystem Consequences of Soil Warming, ed. J. E. Mohan (London: Academic Press), 297–346.
Winkler, M., Lamprecht, A., Steinbauer, K., Hulber, K., Theurillat, J. P., Breiner, F., et al. (2016). The rich sides of mountain summits – a pan-European view on aspect preferences of alpine plants. J. Biogeogr. 43, 2261–2273. doi: 10.1111/jbi.12835
Wipf, S., Stöckli, V., Herz, K., and Rixen, C. (2013). The oldest monitoring site of the Alps revisited: accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecol. Divers. 6, 447–455. doi: 10.1080/17550874.2013.764943
Keywords: bedrock, elevation gradient, GLORIA, microscale, surface cover type, species richness, species turnover, vascular plant cover
Citation: Nicklas L, Walde J, Wipf S, Lamprecht A, Mallaun M, Rixen C, Steinbauer K, Theurillat J-P, Unterluggauer P, Vittoz P, Moser D, Gattringer A, Wessely J and Erschbamer B (2021) Climate Change Affects Vegetation Differently on Siliceous and Calcareous Summits of the European Alps. Front. Ecol. Evol. 9:642309. doi: 10.3389/fevo.2021.642309
Received: 15 December 2020; Accepted: 15 March 2021;
Published: 09 April 2021.
Edited by:
Luis Daniel Llambi, Universidad de Los Andes (Venezuela), VenezuelaReviewed by:
María Lencinas, CONICET Centro Austral de Investigaciones Científicas (CADIC), ArgentinaCopyright © 2021 Nicklas, Walde, Wipf, Lamprecht, Mallaun, Rixen, Steinbauer, Theurillat, Unterluggauer, Vittoz, Moser, Gattringer, Wessely and Erschbamer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lena Nicklas, bGVuYS5uaWNrbGFzQHVpYmsuYWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.