- 1Department of Zoology, University of Oxford, Oxford, United Kingdom
- 2Centre for Research in Anthropology (CRIA - NOVA FCSH), Lisbon, Portugal
- 3Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Cornwall, United Kingdom
- 4Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, United States
Wild chimpanzee tool use is highly diverse and, in many cases, exhibits cultural variation: tool-use behaviours and techniques differ between communities and are passed down generations through social learning. Honey dipping – the use of sticks or leaves to extract honey from hives – has been identified across the whole species’ range. Nonetheless, there seems to be marked variation in honey dipping at a species level, with most descriptions originating from central Africa, and involving the use of complex tool sets, or even multifunctional tools. In West Africa, while honey consumption is common, in most cases tools are not used. We document, for the first time, the use of honey dipping tools in unhabituated chimpanzee (Pan troglodytes verus) communities at Cantanhez National Park (CNP), Guinea-Bissau. Over a 23-month period we employed a combination of direct (camera traps, n = 1944 camera trap days) and indirect (1000km of reconnaissance walks, collection of abandoned tools) methods to study four neighbouring communities in central CNP. Fluid dipping tools were found in three of the four communities; here we analyse 204 individual stick tools from the 70 tool-use ateliers found. In addition to documenting individual tool dimensions and raw materials, we adopt methods from primate archaeology to describe the typology of different tools based on use-wear patterns. We describe differences in tools used for different honey types, between communities, and tools and tool kits that show an unexpected degree of complexity. Our data also suggest the use of tool sets, i.e., tools with different functions used sequentially toward the same goal; as well as possible multifunction tools (pounding and dipping), never before described for western chimpanzees. Our study fills gaps in our knowledge of the wild chimpanzee cultural repertoire and highlights how chimpanzee tool manufacture and use can vary even at local scales.
Introduction
Apart from humans, chimpanzees show the greatest diversity of tool use in the animal kingdom, making and using a variety of complex tools as part of their daily lives (McGrew, 2004). In addition, different communities exhibit different tool-use repertoires (Goodall, 1986; Whiten et al., 1999; Boesch and Boesch-Achermann, 2000; McGrew, 2004; Matsuzawa et al., 2011; Pruetz et al., 2015) – a phenomenon commonly ascribed to cultural variation. West African chimpanzees, for example, are known to crack nuts using wooden or stone hammers, whereas this behaviour is entirely absent from East Africa despite the presence of the necessary resources and raw materials within East-African chimpanzees’ ranges (Boesch and Boesch-Achermann, 2000; Matsuzawa et al., 2011). How such regional differences emerge and are maintained are key questions in understanding the spread of cultural traits, and, given chimpanzees’ close evolutionary proximity to humans, are also relevant to understanding the origins of hominin technology and culture. Several different methods to identify culture have been suggested, most prominently those applying the “method of exclusion” (e.g., Whiten et al., 1999) and those positing a tri-dimensional approach to traditions (Fragaszy and Perry, 2003). The former proposes the explicit exclusion of ecological or genetic factors as drivers of inter-population behavioural variation within a species (Wrangham et al., 1994; Whiten et al., 1999), whereas the latter places the strongest emphasis on demonstrating social learning, without which a behaviour cannot be considered cultural (Fragaszy and Perry, 2003). Subsequent work argued that ecology, genetics and social learning are in fact inexorably interlinked and can all influence, to some degree, behavioural variation (Laland and Janik, 2006; Koops et al., 2013), thus leading to a useful convergence between the two main frameworks.
The past two decades have seen further refinement of these methodologies in the study of wild primates. First, while early works on chimpanzee culture conducted comparisons at a species-wide scale (Whiten et al., 1999, 2001; Schöning et al., 2008), it has been suggested that more compelling evidence for culture in chimpanzees might come from the study of behavioural variation within the same subspecies (e.g., Laland and Janik, 2006; Luncz et al., 2012). In particular, comparisons of neighbouring communities, where habitat types are similar and individuals broadly face the same ecological constraints and migrate between communities, make ecological or genetic explanations for behavioural differences less likely compared to a cultural explanation (i.e., one based on local innovation and subsequent diffusion through social learning). Illustrating this approach, Luncz et al. (2012) compared the selection of wooden and stone hammers for coula (Coula edulis) nut-cracking in three neighbouring chimpanzee communities in Taï National Park (Ivory Coast). Even though these neighbouring communities inhabit the same forest habitat and ecological variation is minimal, the study showed that there was still marked variation in hammer size and raw material preferences between communities (Luncz et al., 2012). This confirms that studying nuanced differences in details of the same behaviour between communities that exist in close proximity can yield tantalising evidence for subtle behavioural variation. Second, direct evidence for social learning being involved in the maintenance of specific behaviours has become available through observations of natural immigration and the emergence of subsequent conformity (Luncz and Boesch, 2014). Furthermore, novel social-network-based analyses have also confirmed the socially mediated diffusion of a newly invented behavioural variant in wild chimpanzees (Hobaiter et al., 2014). Taken together, these studies elegantly bridge the gap between the method of exclusion and the tri-dimensional approach to the study of animal traditions.
Honey dipping behaviour – chimpanzees’ use of sticks or leaves to extract honey from hives – has been identified across the whole species’ range (Boesch and Boesch, 1990; Tutin et al., 1995; Ohashi, 2006; Fowler and Sommer, 2007; Sanz and Morgan, 2009; McLennan, 2011). However, it is from Central Africa that most descriptions of honey dipping of arboreal and terrestrial honey from different stinging and stingless beehives seem to originate. Central African chimpanzees (Pan troglodytes troglodytes) use complex tool sets, sometimes composed of up to five tools with different functions used in sequence to gain access to and extract honey, and have even been described using multifunctional tools, i.e., tools where a single object can have different functions (Bermejo and Illera, 1999; Sanz and Morgan, 2007; Boesch et al., 2009). In East Africa, the use of stick tool sets to access honey by chimpanzees is rare. However, chimpanzees at Bulindi in Uganda use tool sets, including both digging sticks and more slender sticks to probe the stingless bees’ narrow underground entry tubes (McLennan, 2011; McLennan et al., 2019). In West Africa, honey consumption also occurs frequently, but in many cases no tools are used to extract the honey (Boesch and Boesch, 1990). This might be because this subspecies of chimpanzee (Pan troglodytes verus) feeds more frequently on the honey of stinging bees (Apis sp.) whose painful sting does not allow individuals to spend long enough near hives to use tools, meaning they must instead adopt other approaches (such as using hands only; Boesch and Boesch, 1990). Nonetheless, in the Ivory Coast the chimpanzees inhabiting Comoé National Park have been described to frequently use dipping stick tools not only to access honey but also water. These chimpanzees were even observed modifying their tools by chewing on their end to create a ‘brush tip’ prior to use (Lapuente et al., 2017). Variations in honey dipping thus appear to exist between subspecies and within the same subspecies, hence both genetic and environmental explanations might be at play. Hence, it is of particular interest to study this behaviour between neighbouring communities inhabiting similar habitats, where variation due to the latter two influences is expected to be minimal. Furthermore, we still lack a complete picture of the full chimpanzee cultural repertoire, despite long-term study across much of the species’ range, and the addition of new sites of unhabituated chimpanzee communities identifying new behaviours and behavioural variants (e.g., Sanz et al., 2004; Pruetz and Bertolani, 2007; Gruber et al., 2015; Hockings et al., 2015; Kühl et al., 2016; IUCN, 2020). Specifically, no long-term studies have yet reached the westernmost populations of the species’ distribution. Recent work in Cantanhez National Park (CNP), Guinea-Bissau, has started to fill this gap (Sousa et al., 2011; Hockings and Sousa, 2012; Bessa et al., 2015; Vieira et al., 2019; Hockings et al., 2020). For example, a 9-month study at CNP found, through the analysis of faecal samples and other indirect data, that these chimpanzees fed on wild bee honey with some degree of frequency, however no tools were ever found (Bessa et al., 2015). In more recent surveys, however, evidence of dipping tools began to emerge (E. Bersacola, personal communication), confirming the presence of the behaviour at CNP.
In the present study, we employ a combination of direct and indirect methods to systematically survey CNP for honey-dipping tools, and compare four neighbouring chimpanzee communities unhabituated to the presence of researchers. Specifically, we aim to (1) identify the presence of honey dipping tools in the four communities’ home ranges, (2) compare the characteristics of tools used to exploit different honey sources, and (3) compare the characteristics of tools across those communities that use them.
Methods
Study Site
Cantanhez National Park (CNP, N11°14.287′ W15° 02.281′) is located in the Tombali region of south-west Guinea-Bissau. CNP is a mosaic of settlements, cropland, sub-humid forest, secondary forest, mangrove, and savannah (Catarino and Palminha, 2014). There are two marked seasons in Guinea-Bissau: dry season (November to mid-May) and rainy season (mid-May to October). During 2017, annual rainfall was 2351 mm with an average temperature of 26.3°C (15.6°C min to 38.6°C max). It is estimated that there are 10-12 chimpanzee communities in CNP as a whole (Bersacola, 2019). In the forested areas of central-southern CNP, genetic, behavioural and ecological research support the presence of seven different chimpanzee communities (Hockings and Sousa, 2013; Sá, 2013; Bessa et al., 2015; Bersacola, 2019; Vieira et al., 2019; Hockings et al., 2020); these include the four studied communities: Caiquene-Cadique, Lautchandé, Madina and Cambeque. Due to the unhabituated nature of these communities, at present little is known about their community sizes and compositions; nonetheless, previous works estimate that the communities’ range between 35-60 individuals (Bessa et al., 2015; Vieira et al., 2019).
Data Collection
Data collection took place over the course of 23 consecutive months (February 2017 - December 2018). Since the main aim of this study was to assess the presence of and potential inter-group variation in honey-dipping behaviour in neighbouring chimpanzee communities, where none of the studied communities were habituated to researchers, a combination of direct and indirect methods of data collection were employed. A total of 187 reconnaissance walks (“recces”) were walked, covering just over 1,000 km. Since several neighbouring communities were being studied, five consecutive recces in each were initially performed to assess preliminary core ranging area and habitat composition. After obtaining this information, recces were walked 6 days a week. These were performed in rotation across communities, accumulating information that would help maximise data collection at each, while also ensuring that all communities were sampled equally across different months/seasons. All data were collected by JB who was accompanied by two field assistants at all times. Camera traps were set up by JB during recces and checked every two-weeks by JB or one of the trained field assistants. Supplementary Table 1 presents a summary of the cumulative study effort in each of the communities.
Resource Availability
Data on resource availability were collected during recces at each study site by following chimpanzee paths and forest trails that covered as many different habitat types as possible. This method was chosen over systematic transects in order to minimise disturbance to an already highly fragmented habitat, and to avoid opening up new trails for hunters. Honey availability was assessed during recces ad libitum: every time a hive of honey bees or stingless bees was encountered, a GPS point of its location was taken, the habitat was classified [dry forest, riparian forest, woodland, palm grove, mangroves, fallows, croplands, savannah woodland and grassland (Catarino et al., 2020)], and hive type (e.g., arboreal, subterranean) and bee species (local name and scientific name when possible) were recorded. Honey is an important subsistence resource for the local human communities, therefore, hives were usually easily located by one of the field assistants. A careful visual search was conducted to locate honey bee hives, as well as the small tubular entrances of stingless bee hives. Honey bee hives were also located through the sound of the swarm. Initially, local honey harvesters were contacted for information about the potential location of hives; other than hives that were impossible to deplete, most of the locations identified in this way were already depleted (or destroyed), or were likely to become so in the near future.
Indirect Data Collection: Home Ranges, Evidence of Honey Consumption, and Tools
Chimpanzee ranging areas were estimated using minimum bounding polygons from direct chimpanzee encounters and camera trap data, as well as indirect (e.g., nests, feeding traces, faecal samples, abandoned tools) data points, continuously collected during reconnaissance walks from February 2017 to July 2018. Additionally, the highly fragmented nature of the chimpanzees’ habitat, with human settlements, roads, cultivated areas as well as many mangrove estuaries acting as natural and artificial boundaries, was helpful when estimating the home ranges. Data points that were collected in areas where there might be overlap between communities were excluded.
Hives and their surroundings were inspected for evidence of honey consumption by chimpanzees: detached wax from the hive’s entrance, honeycomb traces, tools discarded by the hive, detached fresh green leaves (debris from tool manufacture), freshly snapped branches, or any other chimpanzee signs (e.g., prints, faecal or feeding traces).
When a tool use atelier – a location where tools were used to extract a resource, and were then left behind – was encountered, we recorded its exact location by GPS, photographed the site with the tools in situ, and photographed the individual tools. We registered the bee species associated with the tool use atelier, the species of tree in which the hive was located, whether it was an arboreal or terrestrial hive, and its distance from the ground (using a tape measure or a rangefinder depending on its height). Only sticks that showed clear signs of modification, such as stripped bark, lateral branches removed, frayed or blunt ends, or signs of honey or wax at one or both extremities, were considered tools (e.g., Hernandez-Aguilar et al., 2007; Lapuente et al., 2017). We then collected each tool, gave it a label, recorded its species, and, when possible, we measured its distance from the source by refitting (i.e., finding the presence of a scar left in a plant as a result of the chimpanzees harvesting the raw material) the tool to its original source (methods adapted from Koops et al., 2015; Pascual-Garrido, 2018). If more than one tool was found by the same hive we grouped the tools into age categories depending on colour, pliability and degree of decay (new – still green and pliable; recent – browning in colour and less pliable; old – dry appearance, no pliability/fragile, with possible signs of decay; adapted from Pascual-Garrido, 2018). Collected tools were photographed, measured (length; mid, proximal and distal diameter; length of fray), and all modifications were recorded (fragmented/detached from substrate; percentage of bark left; stripped ends; attachments removed; signs of use on extremities; bite marks; and presence of honey/wax). Additionally, we recorded use-wear patterns on the extremities, categorised into three types: brushed/frayed (significantly frayed with long and separated wood fibres), blunt/mashed (minimal to no rounding with significant fringing and lateral/backward bending of terminal wood fibres) and fragmented (broken end with sharp edges) (adapted from Heaton and Pickering, 2006; Boesch et al., 2009). In order to record whether the same hive was repeatedly exploited, the tool use sites were revisited every week, unless it could be confirmed that the hive had been depleted and the bees had abandoned it.
Remote Data Collection: Camera Trap Sampling
Since the chimpanzee communities were unhabituated to researchers’ presence, the opportunities to observe them directly were few. However, eight camera traps (Bushnell Trophy Cam HD Aggressor No-glow) provided observational data in the form of video footage. The camera traps were set up in chosen locations, on video mode, and programmed to film for 1 min when triggered by movement. These camera traps were set up in places where tools had been found and the resource (honey) had not been totally depleted (i.e., was likely to be revisited) and/or tool use behaviour was likely to take place due to the presence of beehives. To maximise the chances of capturing behaviours of interest on video, some of the cameras were moved during the study period, for example if bees had abandoned a particular hive, if water and/or salt had disabled a camera in a mangrove location, or if the chimpanzees were known to be utilising new travel routes due to seasonal changes in their habitat. 12 camera traps (three in Caiquene-Cadique, three in Cambeque, two Lautchandé and four in Madina) were operating for a cumulative total of 1923 days (399 days in Caiquene-Cadique, 648 days in Cambeque, 363 in Lautchandé and 513 in Madina), from February 2017 to December 2018 (see Supplementary Table 1).
Data Analysis
All statistical tests were performed in R (version 1.1.463), using t-tests and Chi-square tests. Given that data were based on indirect evidence, we assumed that each event was an independent event (but see section “DISCUSSION”). Data were also tested for normal distribution using the Shapiro-Wilk’s method, and for homogeneity of variance using Bartlett’s test. Two-sample t-tests and Welch’s two-sample t-tests were used when comparing datasets with equal and unequal variance, respectively.
Our principal comparisons of interest focused on the dimensions (e.g., length) and characteristics (e.g., modifications) of tools used to collect different types of honey, and of tools used to collect the same type of honey but by different communities. Given the nature of the data collected, where most hives had less than five tools found associated with them, we were not able to use a generalized linear model. Instead, we compared tool dimensions and characteristics using t-tests. For descriptive characteristics we employed Chi-square tests.
Results
Based on the 4293 direct chimpanzee encounters and camera trap data, as well as 1796 indirect data collected during reconnaissance walks, the four communities’ ranging areas surveyed in the present study were estimated using minimum convex polygons: Caiquene-Cadique 14.8 km2, Madina 19.0 km2, Cambeque 7.1 km2, and Lautchandé 8.4 km2 (Figure 1).

Figure 1. Map of the study site showing each of the study communities’ estimated core home range. CNP is marked by a red start, Caiquene-Cadique is shown in purple, Lautchandé in pink, Madina in orange and Cambeque in blue. Ranging areas during the study period were estimated using minimum bounding polygon from 4293 direct (chimpanzee encounters and camera trap data) and 1796 indirect (nests, feeding traces and faecal samples) data points, collected during reconnaissance walks from February 2017 to July 2018.
Resource Availability
All beehives were arboreal, at varying distances from the ground (15–350 cm), and could normally only be accessed by a very small opening covered with dry hard wax (see Figures 2A,C for examples). No subterranean hives were found. Three types of bees and respective hives were identified: one species of stinging honey bee (local name bagueira, scientific name Apis mellifera, hereafter BH) and two species of stingless or sweat bees, large stingless bees (local name bagueira mudo, scientific name Meliponula sp., hereafter BM) and small stingless bees (local name mosca mel, Meliplebeia sp., hereafter MM). Hive characteristics varied between and within bee species. Apis mellifera were normally found inside live trees with varying entry sizes; Meliplebeia sp. were always found in live trees with very small entries and only a single entrance tube (see Figure 2A); and Meliponula sp. were found both in dead and live trees, with generally large entries in dead trees (see Figure 2B) and generally small entries in live trees (see Figures 2C,E).

Figure 2. Beehives and tools used to extract honey by chimpanzees in CNP. (A) A small stingless bee (Meliplebeia sp.; MM) hive located in the mangrove area at Madina. The arrow points to the entrance tube of the stingless bees’ hive; the hive’s entrance covered by wax has been exposed, tools were found next to the hive. (B). A large stingless bee (Meliponula sp.; BM) hive exploited by chimpanzees in secondary open forest at Cambeque, with tools in situ (C). A large stingless bee (Meliponula sp.; BM) hive located in secondary forest at Cambeque. The arrow points to the entrance tube of the stingless bees’ hive; the hive’s entrance covered by wax has been exposed. (D) Tools found by hive (C) with two different estimated ages of use – one-day-old (new) on the left, approx. 1-week-old (recent) on the right. (E) Screenshot from camera trap footage showing a juvenile chimpanzee inserting a manufactured tool into the hive shown in (C) in Cambeque secondary open forest (tool indicated by red arrow) (see Supplementary Material 3. Observation 2).
Of all bee species, the hives of MM were most commonly encountered during recces (N = 80). They were present in all study communities but in different numbers and at different densities: 40 in Cambeque, 22 in Madina, 17 in Caiquene-Cadique and one in Lautchandé. The next most common hive was that of BH (N = 23): 10 in Madina, seven in Caiquene-Cadique, four in Cambeque, and two in Lautchandé. BM (N = 7) was only encountered six times in Cambeque and once in Madina. Supplementary Table 2 presents these densities standardized against home range size and km of recces walked.
Hives were found across different habitats. MM was almost exclusively found in the mangrove area (97.5%) with the remainder in woodland, BM mostly in woodland (71.4%) with the remainder in the mangrove area and cropland. Most BH was found in dry forest (60.9%), followed by mangrove and cropland.
Indirect Evidence of Honey Consumption Without Tools
We found indirect traces for the consumption of BH, without tools, in all four study communities. These traces consisted of honeycomb or wax that was discarded after it had been exploited. They presented distinct tooth marks or were left behind in the form of wadges.
Tools Collected
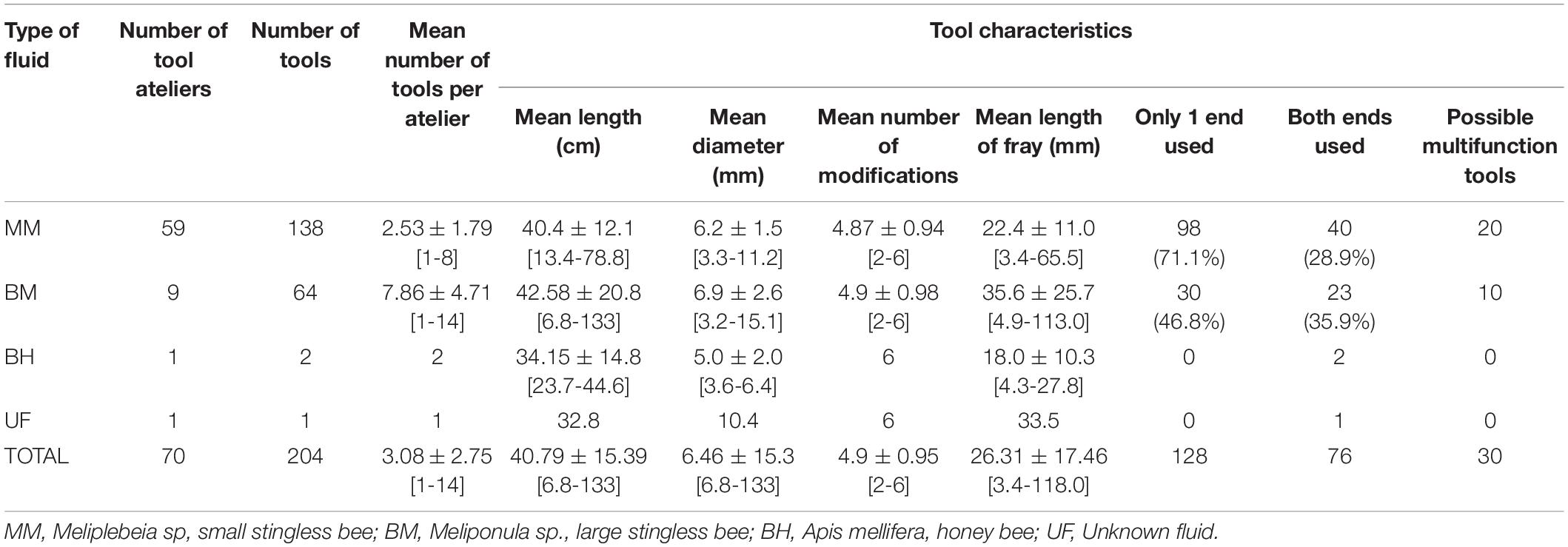
In total we collected 204 individual stick tools from 70 tool use ateliers (see Figures 2B,D for examples). Stick tools were found in three of the four study communities’ home ranges, although at different frequencies. 50% of all tools were found in Madina (N = 103) and 49% in Cambeque (N = 100), with only a single tool found in Lautchandé, and none in Caiquene-Cadique. These tools were associated with four types of fluid extraction: MM, BM, and BH honey, and, on a single occasion, an unidentified fluid (UF). Tools were mainly recovered from mangrove areas (67%), followed by closed secondary forest (30%), open secondary forest (2%) and an agricultural field (1%). In Madina we found tools associated with the extraction of MM (97%), BH (1.9%), and BM (1%), while in Cambeque tools were only associated with the extraction of BM (62%) and MM (38%). In Lautchandé the single tool found was associated with UF. By grouping tools found by the same hive into age classes as well as revisiting hives that were not depleted after the first raiding event we were able to confirm their repeated exploitation, with the use of tools, on separate occasions. One BM hive in Cambeque was successfully raided twice and on two other occasions chimpanzees attempted to exploit it without success (recorded on video). At least seven MM hives were exploited more than once by chimpanzees in Madina.
Table 1 presents descriptive statistics for all tools and tool characteristics. Overall, the mean number of tools found per atelier was 3.05 (± 2.75). Mean tool length was 40.79 (± 15.39) cm and mean mid diameter 6.46 (± 15.3) mm. 90.7% of all tools had four to six modifications. Tools were found with distinct wear patterns – brushed/frayed, blunt/mashed and fragmented (see Figures 3A–C for examples of each type of wear pattern).

Figure 3. Examples of tools found abandoned in tool use ateliers. (A) Brush tips; (B) blunt tool ends; (C) fragmented tool ends; (D) tool ends with honey residue (indicated by the red arrows); (E) potential multifunction tools with fray/brush ends and blunt/mash ends at opposite tool ends.
112 (54.9%) of 204 tools were found to have at least one frayed end and 106 (52%) tools had at least one blunt end. 14.7% (n = 30) of tools had two different wear patterns at opposite extremities, suggesting possible multifunction for those tools (Figure 3E). Sticks that had both ends fragmented or without a clear wear pattern (i.e., absence of brush or blunt end) were only considered tools if they had traces of honey on at least one of the extremities (n = 15, 7.4% tools). Honey residue was found on 132 tool extremities: on 66 (51.2%) brush tips, 36 (35.6%) blunt ends, and 30 (26.5%) fragmented ends (Figure 3D).
Of the tools with at least one frayed end, 63.4% (n = 71) had a frayed proximal end and the mean fray length was 26.31 (± 17.46) mm. Of the tools with at least one blunt end 71.7% (n = 76) had blunt distal ends. 62.4% (n = 128) of all tools had signs of wear at only one extremity, of these 56.3% (n = 72) had proximal wear patterns. 109 (53.4%) tools presented signs of honey/wax/insects and 20 (9.8%) had distinct bite marks.
Tools were made out of fresh twigs from tree species typically found no further than 5 m from the hive (mean ± SD = 0.436 ± 1.026), with most being sourced from the same tree where the hive was located. In the mangroves one species was used exclusively as raw material, the mangrove tree (Avicennia germinans). Similarly, in cropland only one species was used, the orange tree (Citrus sinensis). In dry forest, several different species were chosen as raw material, including Strombosia pustulata, Sarcocephalus latifolius, Vitex doniana, Dialium guineense, Ceiba pentandra, Antiaris toxicaria, Trichilia monodelpha and Albizia ferruginea.
Comparison of Tools by Site and by Resource Exploited
Table 1 further breaks down tools and tool characteristics by the type of fluid exploited. Of the four sources of fluid exploited, MM was associated with the highest number of tools and tool use ateliers encountered (138 and 59, respectively), followed by BM with 64 tools and 9 ateliers, BH with two tools and one atelier, and UF with a single tool recovered.
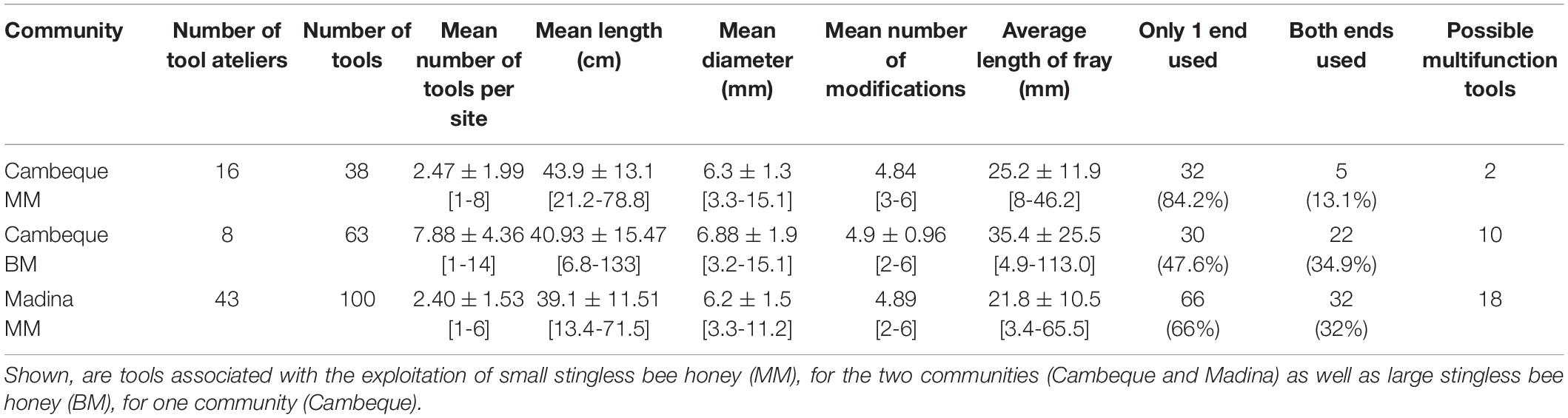
Given the low frequency of tools recovered in association with BH and UF these will not be used in subsequent comparisons. Additionally, the single tool found in Madina associated with BM extraction was not included in the comparisons. Given the structure of the dataset that remains, in which only one chimpanzee community repeatedly exploited more than one type of fluid, and only one type of fluid repeatedly exploited by more than one community, we first focus on potential differences in tool use associated with two different resources frequently consumed at one particular site, then compare tool use at two different sites associated with a single resource.
Comparison of Tools Found in Cambeque for the Extraction of BM and MM
Cambeque was the only site at which more than one type of honey (BM and MM) was frequently exploited with the use of tools (Table 2). The mean number of tools found per atelier was significantly higher for BM (7.88 ± 4.36) compared to MM (2.47 ± 1.99) (Welch’s two-sample t-test: t = 3.33, df = 8.6, p = 0.009) (Figure 4A). Mean tool length was similar for MM (43.88 ± 13.10 cm) and BM (40.93 ± 15.47 cm), confirmed by a non-significant test result (Welch’s two-sample t-test: t = 0.48, df = 97.9, p = 0.633) (Figure 4B). The mean mid diameter of BM tools was higher than that of MM tools but this difference was also not significant (6.88 ± 1.9 mm and 6.25 ± 1.31 mm, respectively; Welch’s two-sample t-test: t = 1.52, df = 95.2, p = 0.131). MM and BM tools had a similar number of modifications: on average 4.84 and 4.90 respectively. The percentage of bark left after modification was significantly lower in BM (60.47 ± 33.94%) than MM (79.45 ± 23.30%) tools (Welch’s two-sample t-test: t = 3.31, df = 96.6, p = 0.001) (Figure 4C).
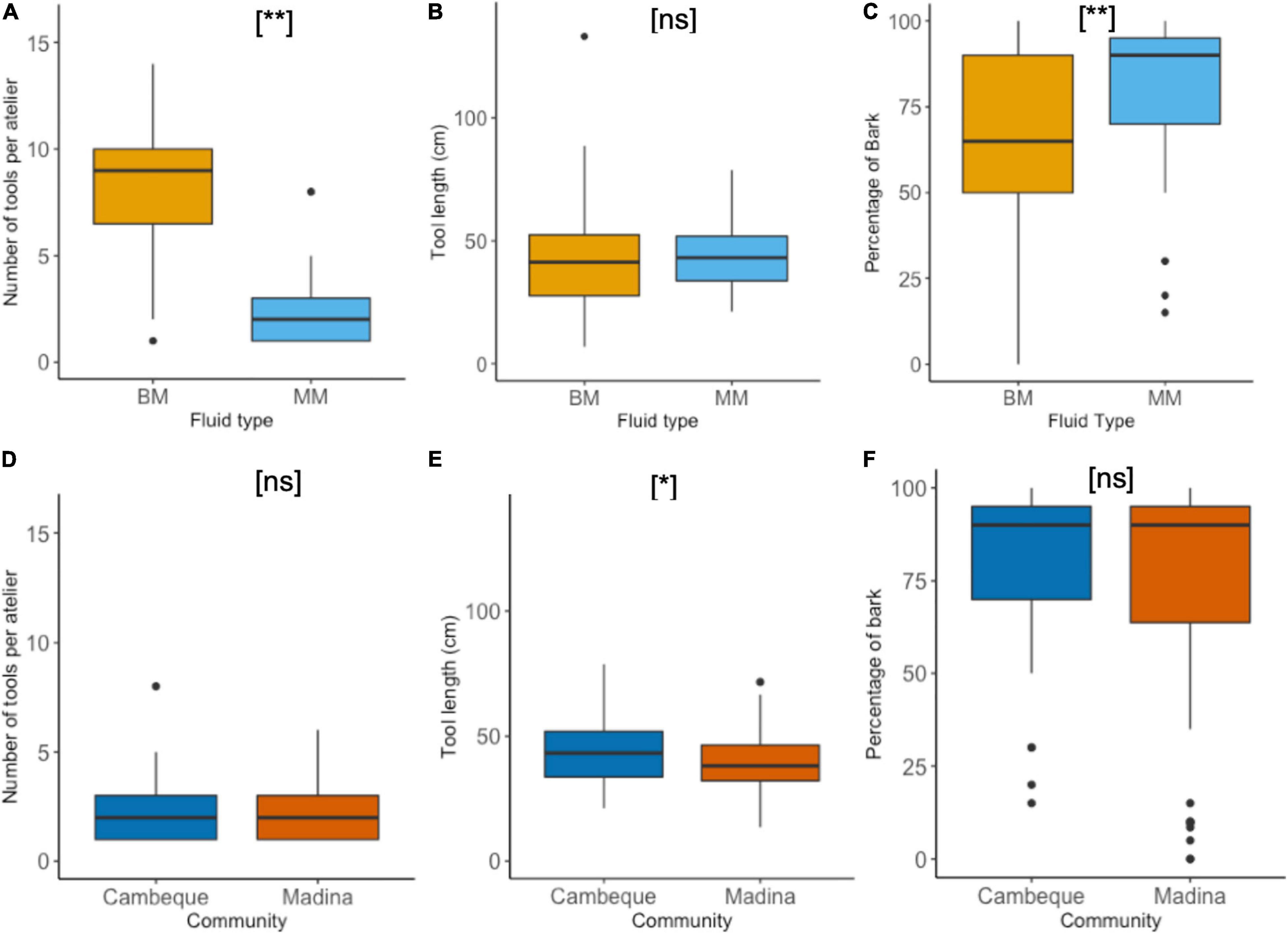
Figure 4. Honey dipping tool characteristics. Tools per atelier, tool length (cm) and percentage of bark remaining, compared between fluid types BM and MM at Cambeque (A–C) and between two neighbouring communities, Cambeque and Madina, exploiting MM (D–F). Boxplots indicate upper and lower quartile; thick horizontal lines represent median. Maximum and minimum data range indicated by whiskers; black dots show individual outliers. P-values are indicated by [ns] – p > 0.05; [*] – p ≤ 0.05; [**] – p ≤ 0.01.
Fifty one percent (n = 19) of MM tools and 49% (n = 31) of BM tools had at least one frayed end. Blunt ends were present in 47% (n = 18) and 46% (n = 29) of MM and BM tools, respectively. 17% of all BM tools and 3.2% of MM tools had fragmented ends without any further signs of wear (i.e., no blunt or frayed ends). There was a significant difference when comparing tools that had signs of wear on both ends: this was the case for 13% (n = 5) of MM tools and 35% (n = 22) of BM tools (Chi-square test: X22 = 11.34, p = 0.001). Of these tools 45% (n = 10) BM and 40% (n = 2) MM tools had different wear types at the two tool ends, i.e., they were potential multifunction tools. Finally, the average length of fray on frayed ends was significantly longer on BM tools (35.44 ± 25.48 mm) than MM tools (25.16 ± 11.91 mm) (Welch’s two-sample t-test: t = 2,09, df = 55.5, p = 0.041).
Comparison of Tools Used to Exploit MM in Cambeque and Madina
MM honey was the overall most frequently exploited resource with the use of stick tools; however, no evidence was found in Caiquene-Cadique and Lautchandé despite the presence of MM hives at those two sites. We therefore compare MM tool characteristics between the communities of Cambeque and Madina only (Table 2).
In Madina we found 43 ateliers with a total of 100 tools, while in Cambeque 16 ateliers provided 38 tools. The mean number of tools found per atelier was similar between the two communities, with 2.47 and 2.40 tools in Cambeque and Madina, respectively (two-sample t-test: t = 0.04, df = 51, p = 0.970) (Figure 4D). Mean tool length was significantly higher in Cambeque (43.9 ± 13.1 cm) compared to Madina (39.1 ± 11.53 cm) (two-sample t-test: t = 2.12, df = 136, p = 0.036) (Figure 4E), but there was no significant difference in mean mid diameter (two-sample t-test: t = 0.18, df = 136, p = 0.850). The percentage of bark left after modification was not significantly different between communities (two-sample t-test: t = 0.68, df = 136, p = 0.496) (Figure 4F). All tools found in both communities were sourced from the same tree species of mangrove (Avicennia germinans).
In Cambeque 51% (n = 19) and in Madina 58% (n = 58) of all tools had at least one frayed end. Blunt ends were present in 47% (n = 18) and 56% (n = 56) of all tools in Cambeque and Madina respectively. 5.4% of tools in Cambeque and 4.0% Madina had no signs of wear at either extremity. Tools with signs of wear at both ends were significantly more frequent in Madina (n = 32, 32%) compared to Cambeque (n = 5, 15.8%) (Chi-square test: X22 = 4.495, p = 0.034); of these 56% and 40% tools had different wear patterns at the two ends for Madina and Cambeque, respectively. The average length of fray on frayed ends was longer in Cambeque (25.16 ± 11.91mm) than Madina (21.81 ± 10.54mm), but the difference was not significant (two-sample t-test: t = 1.20, df = 82, p = 0.233).
Remote Camera Trap Observations of Tool Use and Honey Extraction
Camera traps provided footage of chimpanzees extracting or attempting to extract MM, BM, and HB. In total, 6977 videos were captured, of which 386 (5.5%) were of chimpanzees. Of the 386 chimpanzee videos, 12 (3.1%) videos provided evidence of tool use to extract MM in Madina and BM in Cambeque (Figure 2E and Supplementary Video 1). These 12 videos corresponded to seven independent events (where an event started when an individual approached a hive and finished when the individual left and did not return into the camera’s view). See Supplementary Material 3 where we describe each tool-use event.
Additionally, camera traps captured chimpanzees on three separate occasions in Caiquene-Cadique raiding a natural HB hive, by inserting their hands deep inside a tree trunk. As soon as these chimpanzees removed their hands from the active hive, swarms of bees emerged, and the chimpanzees ran off holding honeycomb. After the hive was abandoned by the bees (no more bees could be seen or heard in the camera trap footage) one individual inspected it using the same method but was seen leaving calmly and empty handed. All individuals were adult males. In Madina a natural HB hive that had been exploited the previous day by humans was checked by an adult female chimpanzee. She looked inside and inserted her hand into the tree trunk, but no honey was extracted.
Discussion
Our study examined the use of dipping tools to access different types of honey in four neighbouring chimpanzee communities in central CNP, Guinea-Bissau. This is the first evidence of honey dipping tool use by chimpanzees in Guinea Bissau. Dipping tools were found in three of the four studied communities, but only in two of these (Madina and Cambeque) were we able to positively identify the tools as honey dipping tools. These results are puzzling since honey is present in the home ranges of all four studied communities, and a previous study on the feeding ecology of CNP chimpanzees, based on indirect data collection and faecal sample analyses, have shown that honey is an important part of the diet at Caiquene-Cadique (Bessa et al., 2015), one of the communities where dipping tools were not found. This discrepancy might be due to a number of methodological, ecological or behavioural factors.
Firstly, these communities are unhabituated to human researchers – we therefore had to rely on a combination of indirect methods and camera traps to study their tool use behaviour. Thus, it is possible that tool use related to honey extraction is present at Caiquene-Cadique as well, but we simply failed to find evidence for it. In a similar vein, we were also unable to attribute tools found to specific individuals, and as such, one or a few individuals could have been responsible for the manufacturing of a large proportion of tools found at a given site (note, however, that our video footage, albeit limited, supports the idea that within a given community there are indeed different individuals making and using tools). Most of our knowledge on wild chimpanzee behaviour comes from communities that can be followed daily and their behaviour studied directly (e.g., Goodall, 1986; Boesch and Boesch-Achermann, 2000; Matsuzawa et al., 2011; McLennan et al., 2019). Hence, when studying unhabituated communities, much of the subjects’ behavioural repertoire remains inaccessible to the researcher. Nonetheless, studies on these communities are beginning to gain traction, partly because of a reduction in efforts to habituate new communities given that so many already live in fragmented habitats and in close proximity to humans, where the loss of fear of humans could be counter-productive to conservation and welfare. It is also worth noting that the number of communities already habituated to researchers is extremely low compared to the species’ total population size. In West Africa, for example, only five chimpanzee communities are fully habituated to researchers (one at Bossou, Guinea, Matsuzawa et al., 2011; three in the Taï Forest, Ivory Coast, Boesch and Boesch-Achermann, 2000; and one in Fongoli, Senegal, Pruetz and Bertolani, 2007) representing a total of approximately 200 individuals out of an estimated 52,811 (CI 17,577-96,564) chimpanzees in the region (Heinicke et al., 2019). As such, our current knowledge of chimpanzee behaviour and behavioural variation - that is biased towards those communities that can be followed - may represent only a small fraction of the full picture. On the other hand, despite the limitations of studying unhabituated communities, studies have already successfully discovered new behaviours and behavioural variation through a combination of suitable methods that did not rely on habituation (e.g. Kühl et al., 2019). Furthermore, in our research, we employed the same methodology and level of effort in each of the four studied communities, suggesting that the variation we found is unlikely to be a function of differential research effort or observational bias.
Another possible explanation for the differences we found may be rooted in differential resource availability. Honey was recorded in all the studied communities’ home ranges; however, in Lautchandé honey was not encountered frequently and chimpanzees did not seem to utilize the mangrove areas where stingless bee honey is commonly found. This might explain the low incidence of tool use (with only a single tool found) at Lautchandé. Nonetheless, honey was encountered frequently in Caiquene-Cadique, where the chimpanzees often use mangroves to access different parts of their home range and pass many stingless bee hives along their routes. This suggests that occasions for honey extraction are plentiful at Caiquene-Cadique, hence lack of opportunity cannot solely explain the complete absence of tools there.
A third, related explanation for the apparent lack of tool use for honey-extraction in Caiquene-Cadique might be that chimpanzees feed on honey from stinging honey bees (Apis mellifera) more frequently, either from natural or artificial hives (Bessa et al., 2015). To exploit this particular resource, chimpanzees have been observed employing a different approach that is quicker than tool use and therefore less likely to subject them to painful stings from the bees: they perform rapid hit-and-run raids on the hives.
Lastly, the differences in the numbers of tools recovered at the four sites might be due to variation in material culture between communities. Many studies have shown that different chimpanzee communities exhibit different behaviours, including the use of tools, even without obvious ecological or genetic determinants on these behaviours (Whiten et al., 1999; Luncz et al., 2012). This has been described as cultural variation, i.e., the emergence and maintenance of behavioural variants through local innovation and subsequent social learning. While the validity of a genetics-ecology-culture trichotomy has rightly been called into question (Laland and Hoppitt, 2003; Laland et al., 2009; Koops et al., 2013, 2014), evidence of behavioural variation even across neighbouring chimpanzee communities that share migrants and that inhabit very similar habitats has provided some of the most convincing evidence so far of cultural processes at work (Luncz et al., 2012; Koops et al., 2015; Pascual-Garrido, 2019). Nonetheless, it is also important to note that even in cases where ecological or genetic differences exist, intergroup variation in behaviour may still be attributable to culture, as long as the given behaviour is acquired at least in part by social learning. In fact, if different communities exhibit the same behavioural pattern, but these behaviours are socially learnt, they still qualify as cultural. Although we cannot state with certainty that honey dipping tools are absent in Caiquene-Cadique and Lautchandé, from the evidence gathered in this study their use certainly appears less frequent than that of Cambeque and Madina chimpanzees. Due to the spatial proximity of these four communities, and the resulting exchange of migrants and similarities in ecology and resource availability in at least three of the studied communities, we may therefore hypothesize that these differences in honey exploitation are, at least in part, cultural in nature. Nonetheless, direct evidence of social learning, as emphasised particularly strongly by the tridimensional model of animal traditions (Fragaszy and Perry, 2003), would be necessary for this hypothesis to stand. Collecting such evidence – which typically requires extended periods of direct observation – was beyond the scope of our current study.
At a more fine-grained level, when comparing tools used to exploit different types of stingless bee honey, some further notable differences were found. BM tool ateliers tended to have significantly more tools associated with each honey extraction event than did MM or BH tool ateliers, tools where both the proximal and distal ends were used were more frequently found in BM ateliers, and, when ends were frayed, the fray length tended to be significantly longer for BM tools. These differences might be due to the type of habitat where the different bee species’ hives are normally found. MM are mostly found in mangrove areas, an open landscape where shelter is limited and where humans frequently pass. Given that BM is normally found in the forest where the likelihood of encountering humans is much lower, chimpanzees might have the opportunity to spend longer periods of time exploiting them. The hives’ location and structure might be related to these differences as well. Most MM hives found were located high up in live trees and had extremely small entrances, while some BM nests were found in dead or fragile tree trunks which meant that the small hive opening could be enlarged more easily, or the trunk could be fragmented. A larger opening will allow increased access to honey and therefore more time spent at the site. This may in turn translate to more repetitions of using the same tool (thus making the fray longer), until it has to be substituted by using the other tool end (leading to more tools with both the distal and proximal ends used) and/or by manufacturing a new tool (leaving behind a higher number of tools at the site).
Additional differences were also found when comparing the tools used for MM extraction in Madina and Cambeque. In Cambeque, tools were significantly longer than in Madina, and in Madina there were significantly more tools found with both ends showing signs of use or modifications. Importantly, at both sites tools were used to exploit the same resource, the raw material used was the same (Avicennia germinans), and the hives were exclusively arboreal, located in live trees with small entryways. Hence, the differences in tools cannot easily be explained through environmental differences, and a genetic explanation is unlikely given the documented gene flow between communities (Sá, 2013). Therefore, again a cultural explanation is likely, this time pertaining to subtle differences in the characteristics of tool manufacture and use between the Cambeque and Madina communities.
When analysing the CNP chimpanzees’ dipping tool kit as a whole, some important patterns were found. Many of the tools collected had one or both ends frayed. Frayed/brush ends have been described in many other chimpanzee communities for fluid dipping (Stanford et al., 2000; Fowler and Sommer, 2007; Boesch et al., 2009; Sommer et al., 2012; Lapuente et al., 2017) or termite fishing (e.g., Sanz and Morgan, 2007). In Comoé National Park (Ivory Coast) chimpanzees have been seen biting the ends of tools to loosen the fibers creating a brush (Lapuente et al., 2017), and at Goualougo (Republic of Congo) a similar manufacture process to create brushed ends for termite fishing tools is associated with increased termite harvest (Sanz and Morgan, 2007). However, in other cases brushed ends have been linked to the fibre structure of the raw material that, when broken, might naturally form a brush (Takemoto et al., 2005). In the case of CNP we do not know if the fray is a simple by-product of use or if it is specifically added by chimpanzees prior to use. Notably, we found all three types of wear (brush, blunt and fragmented) on tools made out of multiple different species of raw material. Furthermore, all tools used to exploit MM were made out of the same raw material but exhibited all three types of wear. Taken together, these observations are more supportive of a pattern of production rather than fraying being merely a by-product of the fibre structure. Interestingly, the human communities that live alongside the CNP chimpanzees also exploit the same types of honey opportunistically: when they encounter a stingless bee hive they will often enlarge the opening of the hive, for easier access to the honey, and chimpanzee tools are sometimes encountered by these trees (JB, personal observation). Human traces, however, are clearly distinct from those of chimpanzees given that they present clean cuts made by a knife or machete on the end opposite to the brush, all side branches are sliced off, and the bark is peeled off using the same cutting tool, while the hole of the hive will also present signs of having been enlarged by a machete. As such, the likelihood that we misclassified human tools as chimpanzee tools is very low. Nonetheless, these observations raise important questions about how human activities such as honey harvesting and traditional apiculture might impact chimpanzee tool use (see Hockings et al., 2015 for similar research), and should be the focus of future research.
As tools with different wear patterns were found associated with the same hives, including subsets that were the same approximate age (see, for example, Figure 2D), it is possible they were all used during the same honey extraction episode, in a potential sequence. This suggests the use of tool sets by chimpanzees at CNP. When comparing the patterns of wear encountered in CNP with other published data it is possible that the CNP tool set has at least three types of tools. The first are exploratory probes, where very little modification is present. On some occasions (as confirmed by camera trap footage) chimpanzees simply procure a small twig, remove some side branches with leaves, and use it in a delicate motion to test if there is any honey present in the hive to collect. The second are pounding tools, where one or both tool ends present blunt or mashed ends, suggesting a pounding motion, possibly to break or separate the hard wax in the hive. Finally, the third type represent extraction tools – these have brush ends that are either a by-product of use or a deliberate modification (see Boesch et al., 2009). Given the scarcity of video evidence to date, we can only speculate that these tools serve these specific functions and that they may have been used in sequence, nonetheless the fact that tools that were made of the same raw material presented such distinct wear patterns gives us some degree of confidence that they were used for different functions. Additionally, comparing our indirect data to direct evidence collected in central Africa, where tool sets of up to five different tool types are used in sequence [e.g., Loango NP, Gabon (Boesch et al., 2009); Moukalaba-Doudou NP, Gabon (Wilfried and Yamagiwa, 2014)], strengthens our hypothesis that a similar sequence of use could be present in CNP.
Finally, we describe another characteristic of the CNP honey-dipping tool kit. In a few cases, tools showed different types of wear patterns at opposite ends, one frayed and one blunt. This suggests that these tools may have had more than one function, i.e., they were multifunctional tools, used both for pounding and for extraction. Such tools have been described for honey extraction in central Africa (at Goualougo, by Sanz and Morgan, 2009 and at Loango, by Boesch et al., 2009). Multifunctional tools were once thought to be unique to humans, manifestations of highly sophisticated and complex technology. While we now know them to also be present within the wild chimpanzee tool kit, they have never until now been described for West African chimpanzees. Indeed, the use of tools to collect honey within Western chimpanzees has been regarded as less common than among Central African chimpanzees (Boesch and Boesch, 1990; Bermejo and Illera, 1999; Ohashi, 2006; Lapuente et al., 2017). It is important to note that given the lack of direct evidence of tool manufacture, we cannot completely discount the possibility that different wear patterns on the same tool resulted from an individual re-using another individual’s tool for a different function.
Our study illustrates the importance of research on previously unstudied chimpanzee communities, including chimpanzees inhabiting human-impacted areas. They allow us to fill some gaps in our knowledge of the chimpanzee behavioural repertoire, revealing interesting new behaviours, and adding to the list of habitat types that we now know to be exploited by wild chimpanzees. CNP chimpanzees’ use of the mangrove habitat is one such example (note that previous, albeit rare, reports of mangroves being part of chimpanzee home ranges did not describe their use by the resident communities – e.g., Loango chimpanzees in Head et al., 2019). Over 67% of the tools we recovered were found in mangroves, which suggests that this habitat type may have great significance for chimpanzees inhabiting the westernmost limit of the species’ distribution. Our results also suggest potential cultural variation between neighbouring communities, and, the fact that our evidence was gathered through a combination of indirect and remote methods confirms that multifaceted methodologies are able to provide meaningful data even when studying populations where habituation is not possible or appropriate. It is clear, like in any other study of wild animal behaviour, that the longer the study continues the more we will learn about the lives and behaviour of these chimpanzees. It is, therefore, imperative that studies like ours continue long term, not only informing us about chimpanzee behaviour but also about chimpanzee behavioural variation and behavioural plasticity that might aid future conservation strategies, for example by identifying key chimpanzee resources, adaptations to anthropogenic changes, or even cryptic behaviours in response to added land pressures.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This research was reviewed and approved by the Instituto da Biodiversidade e das Áreas Protegidas (IBAP) in Guinea-Bissau. All research involving wild chimpanzees was non-invasive and purely observational, and strictly adhered to ethics guidelines detailed by the Association for the Study of Animal Behaviour (United Kingdom).
Author Contributions
JB, KH, and DB conceived the ideas and designed the methodology. JB collected and analysed the data. JB led the writing of the manuscript, all authors contributed critically to the drafts and gave final approval for publication.
Funding
JB was funded by a doctoral grant from Fundação para a Ciência e a Tecnologia, Portugal (SFRH/BD/108185/2015), and the Boise Trust Fund (University of Oxford). DB thanks the John Fell Fund (University of Oxford) for grant 122/641 to purchase camera traps. KH thanks the Darwin Initiative Funding (Grant Number: 26-018), United Kingdom.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the local communities of CNP where the research was conducted, and our local guides Mamadu Cassamá, Djibi Indjai, Iaia Camara, Adulai Camará, Idrissa Galiza and Fernando N’Dafá. We also acknowledge the Instituto da Biodiversidade e das Áreas Protegidas (IBAP) for permission to carry out this research and for support with field logistics. Thanks to Alejandra Pascual-Garrido for her guidance with analysing the tools. Thanks to Elena Bersacola for support in and out of the field.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.625303/full#supplementary-material
References
Bermejo, M., and Illera, G. (1999). Tool-set for termite-fishing and honey extraction by wild chimpanzees in the Lossi Forest. Congo. Primates 40:619. doi: 10.1007/bf02574837
Bersacola, E. (2019). Zooming in on Human-Wildlife Coexistence: Primate Community Responses in a Shared Agroforest Landscape in Guinea-Bissau. Ph.D. dissertation, Oxford Brookes University, Oxford.
Bessa, J., Sousa, C., and Hockings, K. J. (2015). Feeding ecology of chimpanzees (Pan troglodytes verus) inhabiting a forest-mangrove-savanna-agricultural matrix at Caiquene-Cadique, Cantanhez National Park, Guinea-Bissau. Am. J. Primatol. 77, 651–665. doi: 10.1002/ajp.22388
Boesch, C., and Boesch, H. (1990). Tool use and tool making in wild chimpanzees. Folia Primatol. 54, 86–99. doi: 10.1159/000156428
Boesch, C., and Boesch-Achermann, H. (2000). The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford: Oxford University Press.
Boesch, C., Head, J., and Robbins, M. M. (2009). Complex tool sets for honey extraction among chimpanzees in Loango National Park, Gabon. J. Hum. Evol. 56, 560–569. doi: 10.1016/j.jhevol.2009.04.001
Catarino, L., Frazão-Moreira, A., Bessa, J., Parathian, H., and Hockings, K. J. (2020). Plants Used by Chimpanzees and Humans in Cantanhez, Guinea-Bissau. Field Guide. Lisbon: LAE/CRIA: Environmental Anthropology and Behavioural Ecology Laboratory Centre for Research in Anthropology.
Catarino, L., and Palminha, A. (2014). Inventário Florestal do Complexo Dulombi – Boé – Tchétche: Plano de Gestão da Vegetação Florestal. UNDP-GEF Report.
Fowler, A., and Sommer, V. (2007). Subsistence technology of Nigerian chimpanzees. Int. J. Primatol. 28, 997–1023. doi: 10.1007/s10764-007-9166-0
Fragaszy, D. M., and Perry, S. (2003). “Towards a biology of traditions,” in The Biology of Traditions: Models and Evidence, eds D. M. Fragaszy and S. Perry (Cambridge: University Press).
Goodall, J. (1986). The Chimpanzees of Gombe: Patterns of Behaviour. Cambridge: Belknap Press of Harvard University Press.
Gruber, T., Poisot, T., Zuberbühler, K., Hoppitt, W., and Hobaiter, C. (2015). The spread of a novel behaviour in wild chimpanzees: new insights into the ape cultural mind. Commun. Integr. Biol. 8:e1017164. doi: 10.1080/19420889.2015.1017164
Head, J., Healy, A., and Nowak, K. (2019). “Primates of African Mangroves: ecology and conservation,” in Primates in Flooded Habitats, eds K. Nowak, A. A. Barnett, and I. Matsuda (Cambridge: University Press).
Heaton, J. L., and Pickering, T. R. (2006). Archaeological analysis does not support intentionality in the production of brushed ends on chimpanzee termiting tools. Int. J. Primatol. 27, 1619–1633. doi: 10.1007/s10764-006-9091-7
Heinicke, S., Mundry, R., Boesch, C., Amarasekaran, B., Barrie, A., Brncic, T., et al. (2019). Advancing conservation planning for western chimpanzees using IUCN SSC APES—the case of a taxon-specific database. Environ. Res. Lett. 14:064001. doi: 10.1088/1748-9326/ab1379
Hernandez-Aguilar, R. A., Moore, J., and Pickering, T. R. (2007). Savanna chimpanzees use tools to harvest the underground storage organs of plants. Proc. Natl. Acad. Sci. 104, 19210–19213. doi: 10.1073/pnas.0707929104
Hobaiter, C., Poisot, T., Zuberbühler, K., Hoppitt, W., and Gruber, T. (2014). Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 12:e1001960. doi: 10.1371/journal.pbio.1001960
Hockings, K. J., Bryson-Morrison, N., Carvalho, S., Fujisawa, M., Humle, T., McGrew, W. C., et al. (2015). Tools to tipple: ethanol ingestion by wild chimpanzees using leaf-sponges. R. Soc. Open Sci. 2:150150. doi: 10.1098/rsos.150150
Hockings, K. J., Parathian, H., Bessa, J., and Frazão-Moreira, A. (2020). Extensive overlap in the selection of wild fruits by chimpanzees and humans: implications for the management of complex social-ecological systems. Front. Ecol. Evol. 8:123.
Hockings, K. J., and Sousa, C. (2012). Differential utilization of cashew—a low-conflict crop—by sympatric humans and chimpanzees. Oryx 46, 375–381. doi: 10.1017/s003060531100130x
Hockings, K. J., and Sousa, C. (2013). Human-chimpanzee sympatry and interactions in Cantanhez National Park, Guinea-Bissau: current research and future directions. Primate Conserv. 26, 57–65. doi: 10.1896/052.026.0104
IUCN (2020). Regional Action Plan for the Conservation of Western Chimpanzees (Pan troglodytes verus) 2020–2030. Switzerland: IUCN SSC Primate Specialist Group.
Koops, K., McGrew, W. C., and Matsuzawa, T. (2013). Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees. Pan troglodytes verus? Anim. Behav. 85, 175–185. doi: 10.1016/j.anbehav.2012.10.022
Koops, K., Schöning, C., McGrew, W. C., and Matsuzawa, T. (2015). Chimpanzees prey on army ants at Seringbara, Nimba Mountains, Guinea: predation patterns and tool use characteristics. Am. J. Primatol. 77, 319–329. doi: 10.1002/ajp.22347
Koops, K., Visalberghi, E., and van Schaik, C. P. (2014). The ecology of primate material culture. Biol. Lett. 10:20140508. doi: 10.1098/rsbl.2014.0508
Kühl, H. S., Boesch, C., Kulik, L., Haas, F., Arandjelovic, M., Dieguez, P., et al. (2019). Human impact erodes chimpanzee behavioural diversity. Science 363, 1453–1455.
Kühl, H. S., Kalan, A. K., Arandjelovic, M., Aubert, F., D’Auvergne, L., Goedmakers, A., et al. (2016). Chimpanzee accumulative stone throwing. Sci. Rep. 6:22219.
Laland, K. N., and Hoppitt, W. (2003). Do animals have culture? Evol. Anthropol. Issues News Rev. 12, 150–159. doi: 10.1002/evan.10111
Laland, K. N., and Janik, V. M. (2006). The animal cultures debate. Trends Ecol. Evol. 21, 542–547. doi: 10.1016/j.tree.2006.06.005
Laland, K. N., Kendal, J. R., and Kendal, R. (2009). “Animal culture: problems and solutions,” in The Question of Animal Culture, eds B. G. Galef and K. Laland (Cambridge: Harvard University Press).
Lapuente, J., Hicks, T. C., and Linsenmair, K. E. (2017). Fluid dipping technology of chimpanzees in Comoé National Park, Ivory Coast. Am. J. Primatol. 79:e22628. doi: 10.1002/ajp.22628
Luncz, L. V., and Boesch, C. (2014). Tradition over trend: Neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. Am. J. Primatol. 76, 649–657. doi: 10.1002/ajp.22259
Luncz, L. V., Mundry, R., and Boesch, C. (2012). Evidence for cultural differences between neighboring chimpanzee communities. Curr. Biol. 22, 922–926. doi: 10.1016/j.cub.2012.03.031
Matsuzawa, T., Humle, T., and Sugiyama, Y. (2011). The Chimpanzees of Bossou and Nimba. Tokyo: Springer Science & Business Media.
McGrew, W. C. (2004). The Cultured Chimpanzee: Reflections on Cultural Primatology. Cambridge: University Press.
McLennan, M., Rohen, J., Satsias, Z., Sabiiti, T., Baruzaliire, J., and Cibot, M. (2019). ‘Customary’ use of stick tools by chimpanzees in Bulindi, Uganda: update and analysis of digging techniques from behavioural observations. Revue de Primatologie 10, 1–21.
McLennan, M. R. (2011). Tool-use to obtain honey by chimpanzees at Bulindi: new record from Uganda. Primates 52, 315–322. doi: 10.1007/s10329-011-0254-6
Ohashi, G. (2006). “Behavioural repertoire of tool use in the wild chimpanzees at Bossou,” in Cognitive Development in Chimpanzees, eds T. Matsuzawa, M. Tomonaga, and M. Tanaka (Tokyo: Springer), 439–451. doi: 10.1007/4-431-30248-4_26
Pascual-Garrido, A. (2019). Cultural variation between neighbouring communities of chimpanzees at Gombe, Tanzania. Sci. Rep. 9:8260.
Pascual-Garrido, A. (2018). Scars on plants sourced for termite fishing tools by chimpanzees: Towards an archaeology of the perishable. Am. J. Primatol. 80:e22921. doi: 10.1002/ajp.22921
Pruetz, J. D., and Bertolani, P. (2007). Savanna chimpanzees, Pan troglodytes verus, hunt with tools. Curr. Biol. 17, 412–417. doi: 10.1016/j.cub.2006.12.042
Pruetz, J. D., Bertolani, P., Ontl, K. B., Lindshield, S., Shelley, M., and Wessling, E. G. (2015). New evidence on the tool-assisted hunting exhibited by chimpanzees (Pan troglodytes verus) in a savannah habitat at Fongoli, Sénégal. R. Soc. Open Sci. 2:140507. doi: 10.1098/rsos.140507
Sá, R. M. M. (2013). Phylogeography, Conservation Genetics and Parasitology of Chimpanzees (Pan troglodytes versus) in Guinea-Bissau, West Africa. Lisboa: Universidade Nova de Lisboa, Faculdade de Ciências Sociais e Humanas.
Sanz, C., Morgan, D., and Gulick, S. (2004). New insights into chimpanzees, tools, and termites from the Congo Basin. Am. Nat. 164, 567–581. doi: 10.2307/3473169
Sanz, C. M., and Morgan, D. B. (2007). Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433. doi: 10.1016/j.jhevol.2006.11.001
Sanz, C. M., and Morgan, D. B. (2009). Flexible and persistent tool-using strategies in honey-gathering by wild chimpanzees. Int. J. Primatol. 30, 411–427. doi: 10.1007/s10764-009-9350-5
Schöning, C., Humle, T., Möbius, Y., and McGrew, W. C. (2008). The nature of culture: technological variation in chimpanzee predation on army ants revisited. J. Hum. Evol. 55, 48–59. doi: 10.1016/j.jhevol.2007.12.002
Sommer, V., Buba, U., Jesus, G., and Pascual-Garrido, A. (2012). Till the last drop. honey gathering in Nigerian chimpanzees. Ecotropica 18, 55–64.
Sousa, J., Barata, A. V., Sousa, C., Casanova, C. C., and Vicente, L. (2011). Chimpanzee oil-palm use in southern Cantanhez National Park, Guinea-Bissau. Am. J. Primatol. 73, 485–497. doi: 10.1002/ajp.20926
Stanford, C. B., Gambaneza, C., Nkurunungi, J. B., and Goldsmith, M. L. (2000). Chimpanzees in Bwindi-Impenetrable National Park, Uganda, use different tools to obtain different types of honey. Primates 41, 337–341. doi: 10.1007/bf02557602
Takemoto, H., Hirata, S., and Sugiyama, Y. (2005). The formation of the brush-sticks: modification of chimpanzees or the by-product of folding? Primates 46, 183–189. doi: 10.1007/s10329-005-0127-y
Tutin, C. E., Ham, R., and Wrogemann, D. (1995). Tool-use by chimpanzees (Pan t. troglodytes) in the Lopé Reserve, Gabon. Primates 36, 181–192. doi: 10.1007/bf02381344
Vieira, W. F., Kerry, C., and Hockings, K. J. (2019). A comparison of methods to determine chimpanzee home-range size in a forest–farm mosaic at Madina in Cantanhez National Park, Guinea-Bissau. Primates 60, 355–365. doi: 10.1007/s10329-019-00724-1
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., et al. (1999). Cultures in chimpanzees. Nature 399, 682–685.
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., et al. (2001). Charting cultural variation in chimpanzees. Behaviour 138, 1481–1516. doi: 10.1163/156853901317367717
Wilfried, E. E. G., and Yamagiwa, J. (2014). Use of tool sets by chimpanzees for multiple purposes in Moukalaba-Doudou National Park, Gabon. Primates 55, 467–472. doi: 10.1007/s10329-014-0431-5
Keywords: animal culture, behavioural variation, dipping, honey, tool use, West Africa
Citation: Bessa J, Hockings K and Biro D (2021) First Evidence of Chimpanzee Extractive Tool Use in Cantanhez, Guinea-Bissau: Cross-Community Variation in Honey Dipping. Front. Ecol. Evol. 9:625303. doi: 10.3389/fevo.2021.625303
Received: 02 November 2020; Accepted: 08 March 2021;
Published: 26 March 2021.
Edited by:
Patricia Izar, University of São Paulo, BrazilReviewed by:
Briseida Dogo Resende, University of São Paulo, BrazilKathelijne Koops, University of Cambridge, United Kingdom
Copyright © 2021 Bessa, Hockings and Biro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joana Bessa, am9hbmEuYmVzc2FAem9vLm94LmFjLnVr
†These authors have contributed equally to this work
 Joana Bessa
Joana Bessa Kimberley Hockings
Kimberley Hockings Dora Biro
Dora Biro