- 1Department of Environmental Science and Policy, Faculty of Science and Technology, Università degli Studi di Milano, Milan, Italy
- 2Laboratory of Subterranean Biology “Enrico Pezzoli,” Parco Regionale del Monte Barro, Galbiate, Italy
- 3Parco Regionale del Monte Barro, Galbiate, Italy
- 4University Grenoble Alpes, CNRS, Université Savoie Mont Blanc, LECA, Laboratoire d’Ecologie Alpine, Grenoble, France
In Europe, invasive freshwater crayfish are not only changing freshwater ecosystems, but they are also leading to local extinctions of native freshwater crayfish. This is particularly evident for the populations of red swamp crayfish and spiny-cheek crayfish in northern Italy, which are threatening the last and isolated populations of the white-clawed crayfish. Here, we describe the steps that accompanied a successful reintroduction of the white-clawed crayfish in an Italian stream (Park Monte Barro) that, although isolated from other freshwater sites, suffered from an illegal introduction of the spiny-cheek crayfish in 2013. After the removal of presumably all the introduced spiny-cheek crayfish individuals, we started periodical surveys (twice a year) of the stream to confirm the absence of further introductions and to monitor environmental conditions. Prior to the reintroduction of the white-clawed crayfish that started in autumn 2018, we developed an intense dissemination activity to raise awareness of white-clawed crayfish features and importance among landowners surrounding the stream, including those suspected of the introduction of the spiny-cheek crayfish: we organized public meetings and we performed seven direct visits, house to house, to the local people providing information on good practices for white-clawed crayfish conservation. From 2018 to 2020, every autumn, we reintroduced a batch of 3-month-old white-clawed crayfish juveniles, and we developed a program for the monitoring of crayfish growth and density, water quality, and direct landowners’ disturbance of the site. We detected a significant increase of the white-clawed crayfish total length (TL) from the first reintroduction (October 2018) to June 2020. In 2020, crayfish were consistently larger than in the 2019 surveys; some of them were able to breed less than 2 years after the first reintroduction. In 2020, the estimated density of large crayfish reached 0.57 individuals/m2, which is lower than the density observed prior to extinction. We did not detect any case of human disturbance of the site. Our results underline that the reintroduction actions could be more effective when the stakeholders having the greatest potential impact on the species are identified, informed, and involved as primary caretakers of the activities.
Introduction
Crayfish species are important components of freshwaters’ biodiversity, which play key roles in the food web and can provide important services (e.g., nutrient recycling and structural diversification) for aquatic ecosystems (Gherardi et al., 2003; Manenti et al., 2019b; Unger et al., 2020). Moreover, freshwater crayfish have a relevant economic and cultural value; their management, thus, has an impact on the preservation of food resources and cultural heritages (Gherardi and Souty-Grosset, 2006; Manenti et al., 2019b). This relevance for humans has heavily shaped the geographical distribution of some European species because of human activities and human-mediated translocations (Souty-Grosset et al., 1997, 2006). Along with an increasingly globalized trade, crayfish introductions outside their natural range have increased dramatically, with the growing spread of multiple species of invasive crayfish, that are threatening native biodiversity worldwide (Nishijima et al., 2017; Manenti et al., 2020).
Multiple American crayfish species are invading European freshwaters, causing an impact on freshwater ecosystems and causing several local extinctions of native freshwater crayfish species (Kouba et al., 2014; Strand et al., 2019). North American crayfish are chronic carriers of the oomycete Aphanomyces astaci, the crayfish plague pathogen, which causes this severe disease in susceptible taxa. Crayfish plague is responsible for extensive mass deaths of native European crayfish that often have a 100% rate of mortality (Svoboda et al., 2017; Caprioli et al., 2018; Martín-Torrijos et al., 2019). Extinctions of native crayfish populations caused by the crayfish plague have been reported since the mid-19th century (Alderman, 1996). The first mass mortalities occurred in northern Italy (Lombardy region) in 1859. Since then, numerous crayfish plague outbreaks have been reported throughout Europe and are still continuing today (Bland, 2017). The spread of crayfish plague initially followed the crayfish trade and the location of rearing facilities established across Europe (Souty Grosset et al., 2006). Nowadays, at least nine species of North American crayfish are well established in Europe (Kouba et al., 2014; Weiperth et al., 2017); among them, the most widespread are an astacid, the signal crayfish (Pacifastacus leniusculus), and three cambarids, the red swamp crayfish (Procambarus clarkii), the spiny-cheek crayfish (Faxonius limosus), and the marbled crayfish (Procambarus virginalis) (Kouba et al., 2014; Lo Parrino et al., 2020). With the recent observation of the cambarid Cambarellus patzcuarensis in Hungary, the Central and Western European indigenous crayfish species have been strongly outnumbered by non-indigenous species (Weiperth et al., 2017, 2020).
The patterns of crayfish invasion are complex and have tremendous effects on the spread of crayfish plague. For instance, in central Italy, multiple genotype groups of A. astaci have been identified, suggesting the existence of multiple infection sources associated with alien crayfish host species even when they are not widespread in the area (Caprioli et al., 2018). In northern Italy, populations of invasive crayfish (red swamp and spiny-cheek crayfish) are threatening the last and isolated populations of the native white-clawed crayfish (Austropotamobius pallipes) (Manenti et al., 2014). On the one hand, invasive crayfish are able to naturally disperse and colonize nearby suitable sites (Siesa et al., 2011). However, sites naturally colonized by these two alien crayfish are ecologically different from those occupied by white-clawed crayfish populations (Gil-Sanchez and Alba-Tercedor, 2006; Manenti et al., 2014; Chucholl, 2016). Both alien species, in fact, mostly select small lakes or downstream streams and rivers that are not used by the native crayfish, or from where the native crayfish disappeared long time ago because of pollution and the crayfish plague infections that occurred during the 19th century (Gherardi and Barbaresi, 2000; Ghia et al., 2013; Manenti et al., 2014). Even in catchment basins in which alien crayfish appear, the presence of barriers, such as waterfalls, can prevent the spread of invasive crayfish and crayfish plague outbreaks (Manenti et al., 2019b). On the other hand, local citizens may become, intentionally or accidentally, the main vectors of both alien crayfish and A. astaci in areas where the native crayfish still exist. This is due to the long tradition of crayfish consumption as food in Europe (Gherardi, 2011). Furthermore, in northern Italy, the white-clawed crayfish still have value as a cultural heritage, and numerous people pay attention to existing populations or sites in which they observed crayfish when they were younger (Manenti, 2006), even though this attention can be detrimental, and occasional poaching occurs (Manenti, 2006). Unfortunately, local people are often unaware of the occurrence of alien crayfish species and the disease they carry, or are unable to distinguish the invasive from native species. Unaware stakeholders can thus bring alien crayfish in sites where the native white-clawed crayfish still survive, causing their extinction due to A. astaci outbreaks (Bonelli et al., 2017). After infection, the spread of A. astaci in an astacid population cannot be stopped as A. astaci sporulation is particularly high in dying crayfish (Makkonen et al., 2013), and affected crayfish can disperse further the disease (Souty Grosset et al., 2006), even if the occurrence of unaffected refuges in the same catchment basin can allow species persistence and recovery (Kozubíková-Balcarová et al., 2014).
Nevertheless, once a native crayfish population is extinct, restoration actions are feasible if alien crayfish species are not present or, in the rarer cases, if they can be totally eradicated (Jourdan et al., 2019). A. astaci has indeed a limited life span (from a few hours to weeks) in the absence of crayfish or other suitable alternative hosts like freshwater crabs (Souty Grosset et al., 2006; Svoboda et al., 2014, 2020; Jussila et al., 2020). In sites where alien crayfish or freshwater crabs are void, the pathogen can thus disappear (Souty Grosset et al., 2006). Despite Europe-wide newscasts and internet reporting of many ongoing reintroduction actions of the white-clawed crayfish, the number of peer-reviewed studies reporting information on failure or success of reintroduction actions is surprisingly low. Published papers (Table 1) and reintroduction plans (Diéguez-Uribeondo et al., 1997; Kemp et al., 2003; Marquis, 2006) highlight water quality and the presence or absence of further crayfish plague outbreaks as key determinants of reintroduction success. Habitat preferences of the white-clawed crayfish are well known and include good water quality and high diversity of microhabitats which provide shelters for both adults and juveniles (Holdich et al., 1999). Assessing the presence of ephemeropteran communities is a simple way to identify brooks for white-clawed crayfish restocking, as mayflies are good indicators of their requirements (Grandjean et al., 2011; Jandry et al., 2014). To avoid further outbreaks of the crayfish plague, reintroductions are generally performed some years after the extinction and in places where no alien crayfish species occur (Spink and Frayling, 2000; Durlet et al., 2009). Conversely, we found no published studies on the role played by the stakeholders (fishermen, landowners) of the reintroduction sites in allowing successful crayfish recovery in Italy, even though it is increasingly evident that local stakeholders play a key role in the success of all conservation actions (Perino et al., 2019). With this paper, we describe a recent case of white-clawed crayfish reintroduction, in which a key aspect was the involvement of local stakeholders. The reintroduction action was performed in a stream of a protected area in northern Italy, that, although isolated from other freshwater sites, was affected in 2013 by the introduction of adults of the spiny-cheek crayfish (Bonelli et al., 2017); after the introduction, the whole white-clawed crayfish population that lived in the stream went extinct in less than 15 days, likely because of crayfish plague outbreak (Bonelli et al., 2017). With successive investigations, we understood that those responsible for the introduction were the local landowners and inhabitants of the nearby village, and one old landowner partially acknowledged of having introduced, with the approval of other local people, the spiny-cheek crayfish with the intention to provide “bigger individuals” to the population of white-clawed crayfish inhabiting the stream (RM pers. obs.). We thus performed an intense action of information and involvement to increase stakeholders’ awareness of white-clawed crayfish importance that we aim to describe in this paper together with all the steps that allowed the successful reintroduction of white-clawed crayfish.
The aims of this paper are to (a) describe the steps of the restoration action initiated in 2018, (b) compare freshwater characteristics before the extinction and after the reintroduction of the white-clawed crayfish, (c) assess white-clawed crayfish breeding success after the reintroduction, and (d) provide insights for successful reintroduction projects.
Materials and Methods
Study Site
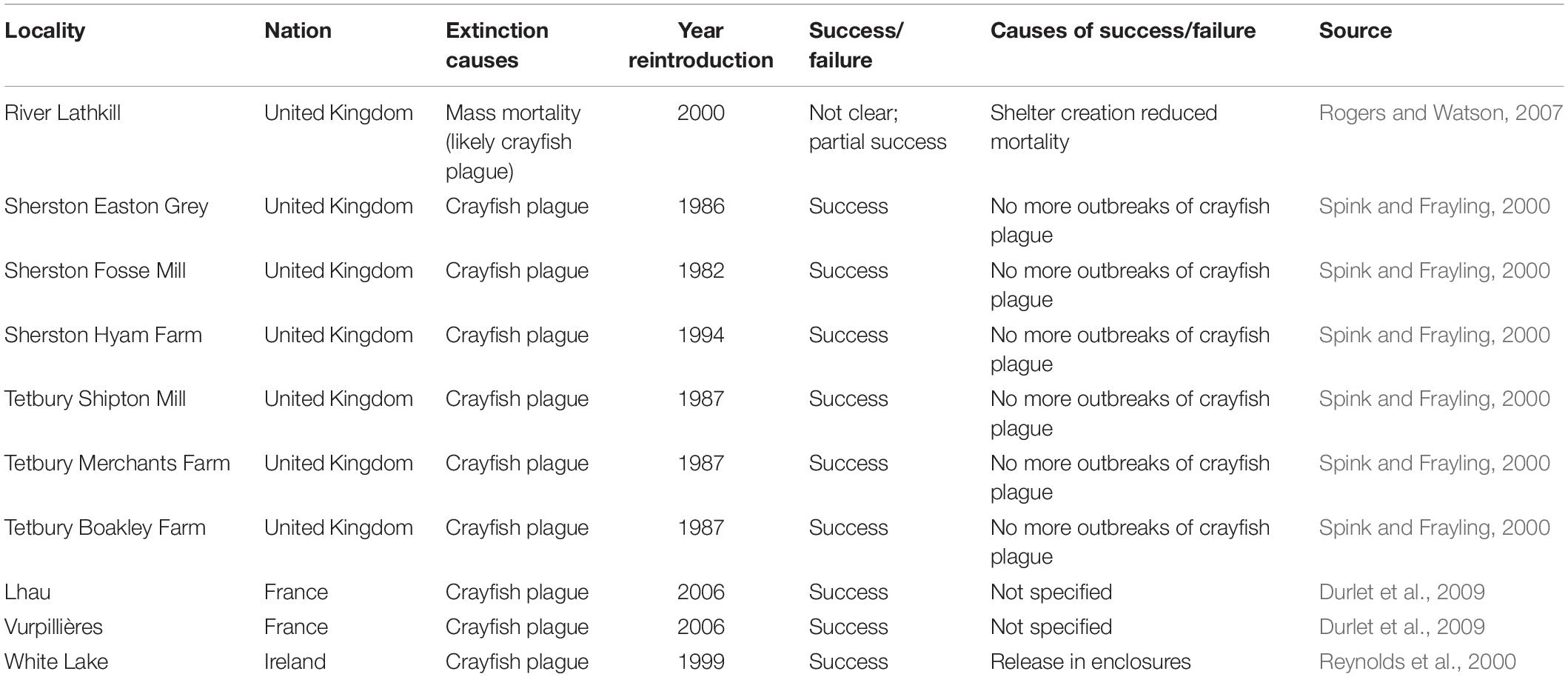
The study site is the San Michele creek within the Monte Barro Regional Park (45.84 N, 9.39 E; Figure 1), in the Lecco district (NW Italy). The creek is in a site of community importance (SCI), as defined by the European Commission Habitats Directive (92/43/EEC). The creek arises from a spring with a water flow of approximately 8 L per minute. The creek offers various microhabitats with repeated successions of small falls, riffles, and pools and the presence of two larger pools laterally connected with the creek that were built in 2009 to increase the habitat available for freshwater crayfish (Figure 1B). After 200 m, the creek continues by a 60-m-high waterfall; downstream to it, the creek becomes ephemeral and is connected with the nearby Garlate Lake and the Adda River only after a heavy rainfall. The waterfall and the irregular hydroperiod act as efficient barriers for the spread of the spiny-cheek crayfish that inhabits Garlate Lake (Bonelli et al., 2017; Manenti et al., 2019b). At 85 m upstream to the waterfall, the creek is currently difficult to access and sample because of dense vegetation. Until 2013, the creek hosted a well-structured population of white-clawed crayfish that had been periodically surveyed since 2003 (Manenti, 2006). The population belonged to the carinthiacus clade of the A. pallipes complex, which is typical for Western Lombardy (Bernini et al., 2016).
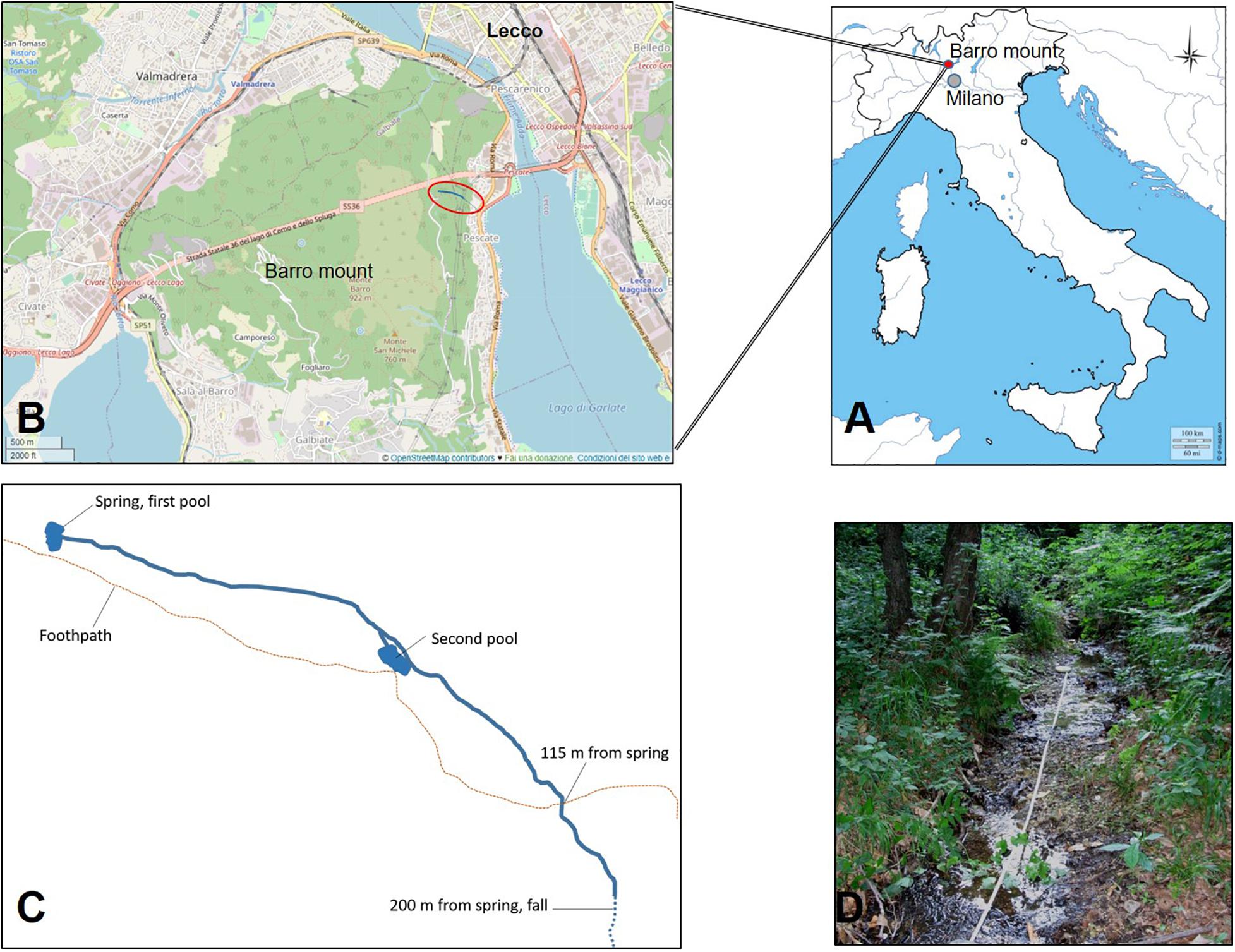
Figure 1. Location (A,B) of the San Michele stream (C,D) where white-clawed crayfish reintroduction took place. In (B), the red circle represents the location of the San Michele stream in the context of Mount Barro. (C) A schema of the San Michele stream where the main sites mentioned in the paper are indicated; the stretch between the stream spring and a waterfall with intermittent flow that makes a barrier for invasive crayfish species is 200 m long. Reintroduced white-clawed crayfish have been released in the two pools, built in 2009, and along the stream stretch 115 m long downstream from the spring. (C) Not drawn to scale.
On July 26, 2013, a mass mortality occurred among white-clawed crayfish with typical features of crayfish plague outbreaks, and many adult spiny-cheek crayfish were recorded right in the same stretch of San Michele creek (Bonelli et al., 2017). In the successive weeks, no further individuals of white-clawed crayfish were observed, but, thanks to the limited extension of the creek, it was possible to perform efficient removals of the spiny-cheek crayfish individuals (Bonelli et al., 2017). A zebra mussel (Dreissena polimorpha), which only inhabits standing waters, recorded on a spiny-cheek crayfish male (Bonelli et al., 2017) allowed us to hypothesize that spiny-cheek crayfish came from nearby localities, particularly the Lario Lake where zebra mussels are rather common. As stated above, successive investigations confirmed the intentional introduction of the alien crayfish by local landowners and inhabitants of San Michele village (RM pers. obs.).
Reintroduction Strategy and Methods
The reintroduction of white-clawed crayfish was decided by Monte Barro Park authorities and carried out as part of a larger project co-financed by the Cariplo Foundation. The reintroduction strategy has been developed in the context of the project LIFE14 IPE/IT/000018 project “LIFE IP GESTIRE 2020 – Nature Integrated Management to 2020,” activated in the Lombardy region in 2016 and involving different actions for the conservation of the white-clawed crayfish. These include the rearing of juveniles for stocking purposes, monitoring of the sites inhabited by the white-clawed crayfish, providing educational activities, and creating local task forces for interventions in case of emergencies regarding freshwater crayfish. The reintroduction performed in the park of Monte Barro has been developed in different steps with specific methodologies that are described here below.
Site Monitoring and Alien Crayfish Removal Before Reintroduction
The spiny-cheek crayfish introduced by local stakeholders were removed in summer 2013 (Bonelli et al., 2017). From autumn 2013 to autumn 2018, each year, we performed two surveys of the creek (at least one survey in each spring and at least one survey in each autumn) to assess spiny-cheek crayfish occurrence. Surveys were performed during the night (from 21:00 to 01:00). Moreover, during 2017, we assessed water quality using the extended biotic index (EBI) modified for the Italian streams (Woodiwiss, 1978; Ghetti, 1997). We compared it to EBI scores of 2005 and 2013 to verify that water quality did not change after the white-clawed crayfish extinction. EBI is a standard method used in Europe to assess stream quality; on the basis of the number and identity of taxa found, each stream has a score ranging from 1 (lowest quality: poor communities including very tolerant species) to 13 (maximum quality: the richest communities, including stenoecious species).
Stakeholders’ Control and Communication Campaign
White-clawed crayfish reintroduction was planned for autumn 2018. At the beginning of 2018, we started multiple actions of public communication to raise awareness of white-clawed crayfish features and crayfish plague and to inform on how to distinguish native from invasive crayfish species. It must be pointed out that two main categories of stakeholders exist in the park. The first includes occasional visitors that live in cities near the park; they mostly use the main footpaths of the park that do not cross the San Michele locality. The second category includes people living in the San Michele village, which are mostly represented by old persons with apparent limited educational levels and often a hostile view against wildlife management actions proposed by outsiders. During the investigations performed after the freshwater crayfish’s extinction, we observed that the old inhabitants believed themselves to be the first managers of the natural environments surrounding the village and the only ones with valid management techniques. The local practices of stream management included riverbed and stream bank cleanings that can alter shelter availability, translocations of frog clutches from unknown localities that can favor pathogen transmission, and water organic enrichment through bread and organic waste placed in the pools to feed frog tadpoles (RM pers. obs.).
To counteract this situation, first we held in February and March 2018 two meetings in the presence of biologists and forestry officers. The meetings were directed toward both park visitors and San Michele local people. The participation of the local people was limited, but park managers convinced at least the main suspect of alien crayfish introduction to participate. Second, from March to September 2018, we performed seven direct visits, house to house, to the local people. At each visit, we provided information on freshwater crayfish and explained with practical examples the risks of continuing the usual management practices performed along the stream for white-clawed crayfish once reintroduced. Each inhabitant was visited at least twice and the main suspect five times; each visit lasted at least 30 min. We also listened to their opinion and asked their collaboration in the surveillance of both territory and white-clawed crayfish health. When we held the February and March public meetings, only occasional visitors of the park and local inhabitants with negative attitude toward questionnaires participated; we thus avoided pre- and post-outreach assessments to evaluate changes in attitude of local landowners. Instead, we evaluated if the reintroduction site suffered human disturbance.
To evaluate disturbance, we placed in one of the pools connected with the creek four cylindrical stainless steel cages with a diameter of 50 cm and a mesh of 3 × 3 mm; the chosen pool was easily accessible, along the main path, but sheltered from distant sighting points. Therefore, it was in principle possible in this pool to intentionally damage objects presumably placed there by park and external authorities and alter stream bed by traditional management without being noticed from the surrounding area. Each cage circumscribed a column of water from the substrate of the pool’s bottom to the surface and was grounded in the substrate for 20 cm and at a distance of 30 cm from the pool border. The cages let water and small invertebrates flow through them but did not allow people to step on the pool’s bottom without moving them. The cages allowed us to detect possible disturbances and unplanned interventions (e.g., substrate cleaning by local people) by evaluating whether they were moved and by comparing their substrate with the substrate of the pool. Moreover, at the pool access point, we placed a small informative sign reporting that there was an ongoing action (not detailed) by an external institution (the University of Milan), which could have induced hostility and suspicion in the local people. We checked the sign position at each survey after reintroduction.
Crayfish Reintroduction
In October 2018, September 2019, and September 2020, we reintroduced in the creek altogether 568 3-month-old juveniles of white-clawed crayfish (for numbers in each year, see Table 2). These were raised in a breeding facility of the Regional Agency for the Agricultural and Forestry Services (ERSAF) and originated from 90 breeding white-clawed crayfish from different streams of the Como district whose genotype corresponds to the white-clawed crayfish clade found in Western Lombardy. For their first 3 months, juveniles were reared in two outdoor ponds with seminatural conditions and shelters. We recorded rostrum–telson total length (TL) and sex. Juveniles were reintroduced during the night in different pools of the creek over 115 m away from the springs; we avoided the 85-m-long inaccessible area directly upstream of the waterfall.
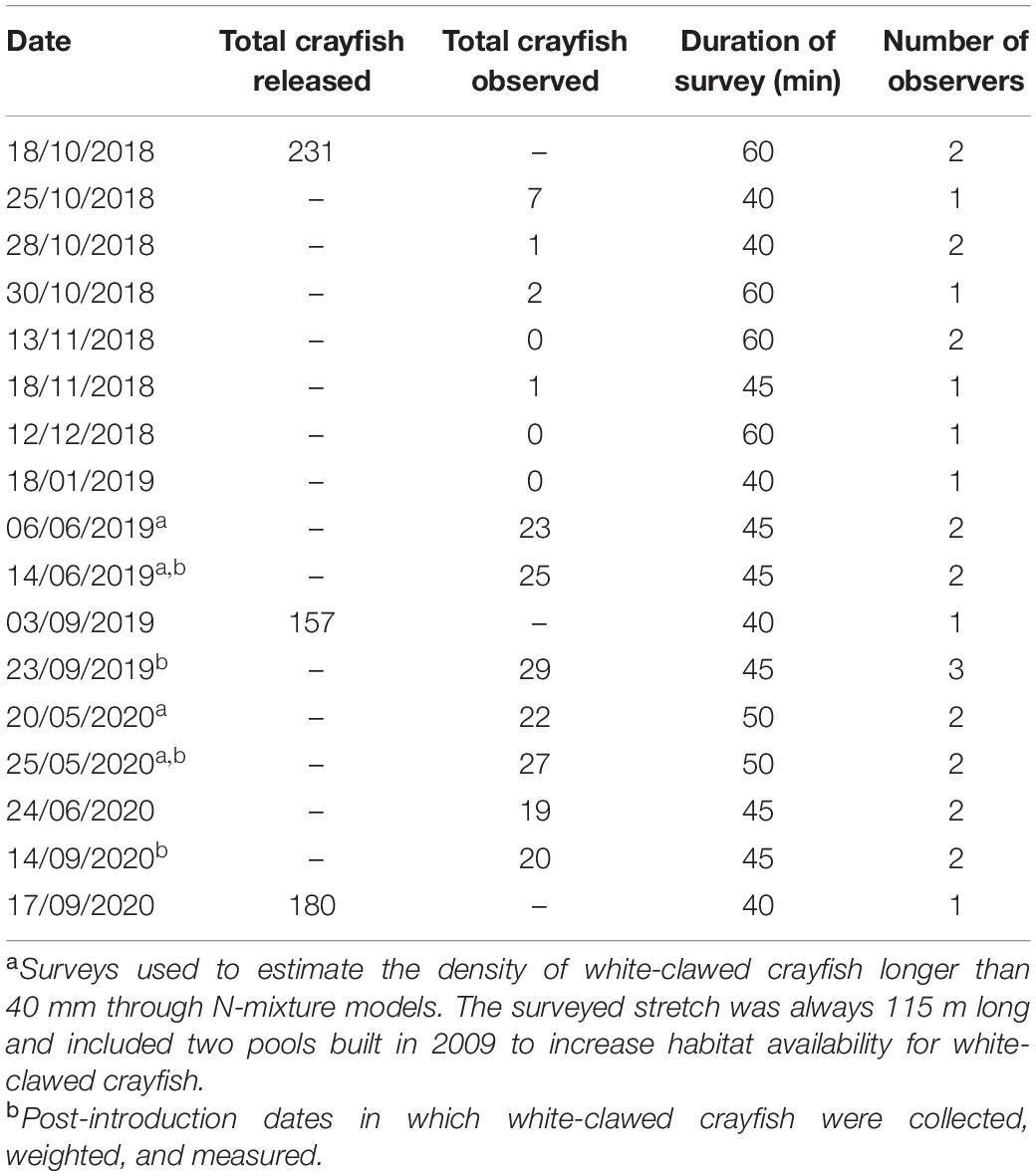
Table 2. Number of white-clawed crayfish released and observed in the successive surveys during the period October 2018–September 2020.
Post-reintroduction Monitoring
From October 2018 to September 2020, we performed periodical visual encounter surveys of the reintroduced white-clawed crayfish. We performed the surveys during nighttime at 9:00 p.m., going upstream from the final stretch before the waterfall to the spring for 115 m. We included in each survey the two pools built in 2009. Each survey was performed by one to three observers along the same stretch and lasted 40–60 min (Table 2). During the four surveys, we also captured all the detected white-clawed crayfish, measured their TL, weighed them, recorded their sex, and released them at the site of capture.
Features of the Reintroduced Population
We assessed variation of morphological features of the reintroduced white-clawed crayfish in October 2018 comparing their TL and weight during successive surveys in June 2019, September 2019, May 2020, and June 2020; we did not record individual weight at reintroduction, but only during the successive surveys.
We also compared white-clawed crayfish morphological parameters with data from a survey performed in the same site prior to 2013. In particular, we compared crayfish TL and weight recorded in May 2020 for 19 individuals with the data collected in May 2007 for 84 individuals. Both datasets were collected in a single survey date and were limited to individuals > 40 mm, which have higher detectability compared with juveniles (Arrignon, 1981).
To assess current differences in density before extinction and after the first year of reintroduction, we used the estimation obtained in May 2007 through removal samplings; in that year, we performed two successive samplings during the same night over a stream stretch with length 195 m (the 5 m of stretch before the waterfall was dry) and average width 0.47 m. During the first survey, we removed all the white-clawed crayfish with TL > 40 mm seen outside of shelters, and we placed them in two tanks outside the creek; we waited for 30 min and repeated the sampling by placing the collected individuals in two other tanks. We then measured all the individuals and released them in the creek. Subsequently, we calculated white-clawed crayfish population abundance using the method proposed by Chao and Chang (1999) to analyze removal samplings. In 2019–2020, we did not perform removal sampling to avoid excessive disturbance of the individuals, and we performed three multiple counting surveys in successive nights in order to estimate population abundance using N-mixture models (see below). The stretch surveyed in 2019–2020 was 115 m long and the average width was 0.6 m; we avoided the inaccessible stretch before the waterfall, but we included the two new pools located laterally.
Statistical Analyses
To assess variation of morphological features (TL and weight) of the white-clawed crayfish, we built linear models (LMs) using the lm function in R. We considered TL and weight as dependent variables, sampling period as a fixed factor, and sex of individuals as a covariate. We also tested the occurrence of significant interactions between period and sex. We assessed the significance of the variables using a Wald F test (Bolker et al., 2008). Subsequently, we performed Tukey’s post hoc tests to assess differences between the different periods, using the function glht of the package multcomp (Hothorn et al., 2008).
To compare crayfish TL and weight between May 2007 and May 2020, we built LMs with TL and weight as dependent variables. We considered as fixed factors the period of sampling (May 2007 or May 2020) and sex. We assessed the significance of the variables using a Wald F test (Bolker et al., 2008).
We used N-mixture models to estimate population abundance after the reintroduction. Previous studies showed that N-mixture models provide reliable estimates of abundance, with results comparable to those obtained with removal samplings (Ficetola et al., 2018). To estimate the density of white-clawed crayfish in 2019 and 2020, we used N-mixture models for closed populations. We assumed that the population was closed during two successive surveys performed during the same month (June 2019 and May 2020; Table 2); we used Akaike’s information criterion (AIC) to select the most appropriate error distribution (Poisson). The analysis was performed using the R package unmarked (Fiske and Chandler, 2011). The surveys considered for N-mixture models were performed by the same operators and had similar length (45–50 min); thus, the duration of the survey was not included as a covariate in the model. We considered for this analysis the individuals with TL > 40 mm. Density was calculated by dividing the estimated number of individuals by the area surveyed in each year. All the analyses were performed in R 3.6.3 environment.
Results
Environmental conditions remained relatively stable from 2013 to 2017. Since October 2013, no spiny-cheek crayfish were observed in the creek. The macrobenthos community both before and after the extinction showed a good degree of diversification with different bioindicator taxa typical of unpolluted small and slow-flowing streams like plecopterans of the genus Amphinemura and ephemeropterans of the genus Ecdyonurus and Paraleptophlebia. The EBI score was high and indicated a site with the highest class of water quality (Table 3). Both before and after extinction, benthic communities were dominated by amphipod crustaceans of the genus Echinogammarus.
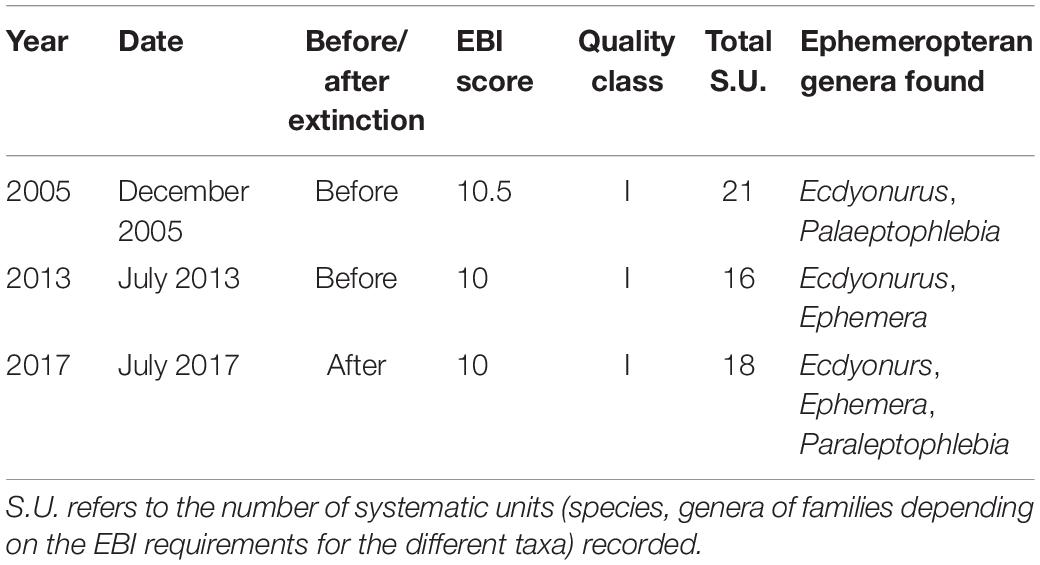
Table 3. Results of the extended biotic index (EBI) assessment in different years along the same transect of the stream where the reintroduction was conducted.
The increasing understanding and knowledge of white-clawed crayfish features and importance by local stakeholders and landowners positively influenced their attitude toward the reintroduction action. After the reintroduction, no further introductions of alien species occurred, and no disturbance actions were detected (e.g., no bread and organic waste were thrown to feed tadpoles and no cleaning of riverbed and banks occurred). The cages and the sign placed in the most accessible pool remained untouched. Moreover, the substrate showed the same layer of small organic particles and decomposed leaves in both pool and cages.
In October 2018, we reintroduced 231 3-month-old white-clawed crayfish (122 males and 109 females) ranging from 32 to 43 mm of TL. The subsequent surveys confirmed the survival of the juveniles (Table 3), even though the number of detected individuals was limited. In early summer 2019, the number of young white-clawed crayfish detected increased. LMs detected significant changes of white-clawed crayfish TL from the first reintroduction (October 2018) to June 2020 (F4,290 = 184.68, P < 0.001; Figure 2). In all the surveys, white-clawed crayfish were significantly larger than at reintroduction, and in 2020, crayfish were consistently larger than in the 2019 surveys (Tukey’s post hoc tests; Supplementary Table 1). Considering all the surveys, we detected no differences in TL between the collected males and females (F1,290 = 0.73, P = 0.39), but there was a significant interaction between period and sex with males larger than females in May 2020 (F4,290 = 4.79, P < 0.01). Weight showed significant differences between sexes, with males being significantly heavier than females (F1,68 = 8.44, P < 0.01). Furthermore, there was a significant difference of weight across periods (F3,68 = 3.25, P = 0.02), but Tukey’s post hoc tests only showed that in May 2020 white-clawed crayfish were significantly heavier than in June 2019 (t = 3.17; P = 0.01).
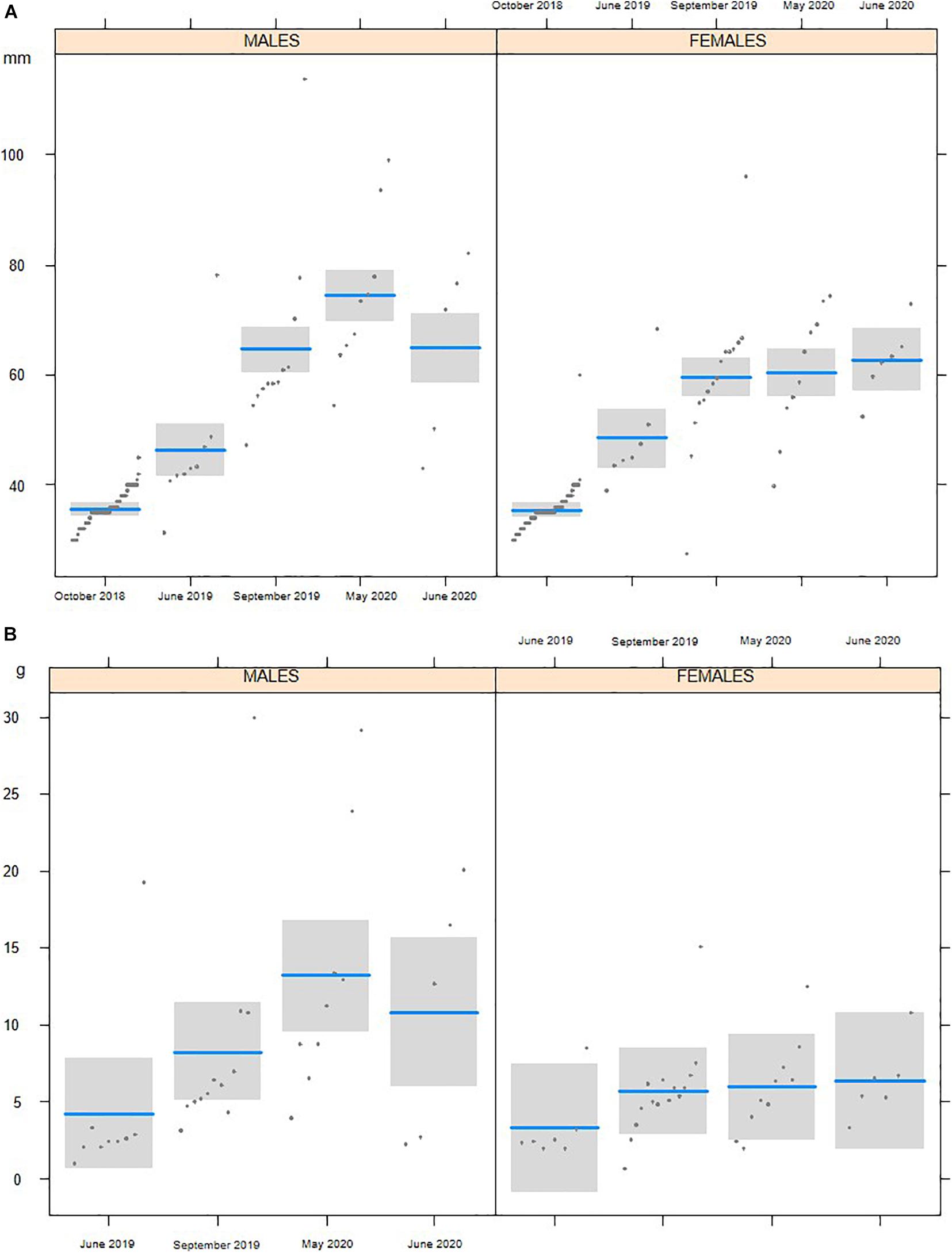
Figure 2. Total length (A) and weight (B) of the individuals of Austropotamobius pallipes collected during the surveys performed from the first reintroduction in October 2018 to June 2020.
We then compared the individuals sampled in May 2020 with the population measured before the extinction (May 2007). In May 2020, white-clawed crayfish were longer (F1,100 = 11.85, P < 0.001), but not heavier (F1,100 = 2.80, P = 0.10) than in 2007; in both periods, males were larger (F1,100 = 16.01, P < 0.001) and heavier (F1,100 = 22.21, P < 0.001) than females. Moreover, in October 2019, we detected one very large male (114 mm TL, 30 g); this individual was larger than any other white-clawed crayfish found in the stream on the same date.
Removal sampling of the population, performed in May 2007, estimated a total population of 106 individuals larger than 40 mm, which represents a density of 1.15 individuals/m2. In 2019, N-mixture models estimated a total population size of 39 individuals larger than 40 mm, i.e., a density of 0.56 individuals/m2 (95% CI 0.49–0.66). In 2020, we estimated a total population size of 40 individuals, i.e., a density of 0.57 individuals/m2 (95% CI 0.50–0.68).
The reintroduction of young white-clawed crayfish was repeated in September 2019 and in September 2020. In September 2019, we reintroduced 157 3-month-old white-clawed crayfish (73 males and 84 females) with TL ranging from 20 to 33 mm. In September 2020, we reintroduced 180 3-month-old white-clawed crayfish (76 males and 104 females) with TL ranging from 18 to 31 mm. White-clawed crayfish breeding was already recorded during the first year of reintroduction. In October 2019, we detected three females with spermatophores on their ventral side, and in May 2020, we observed a female with newly hatched crayfish still attached at the pleopods (Figure 3).

Figure 3. Different steps and structures of the white-clawed crayfish reintroduction action. (A) The breeding facility of the ERSAF organization. (B) 3-month-old white-clawed crayfish ready for the release. (C) Measurement of the 3-month-old white-clawed crayfish. (D) Introduction of the white-clawed crayfish in September 2019. (E) Grown reintroduced white-clawed crayfish female in June 2019. (F) The finding of a reintroduced female in May 2020 with a newly hatched white-clawed crayfish still attached to the pleopods. Written informed consent was obtained from the person depicted in picture (D) for the publication of any potentially identifiable images or data included in this article.
Discussion
Post-reintroduction surveys showed that white-clawed crayfish reintroduction in San Michele creek was successful; the intense dissemination activity was crucial in avoiding further introduction of alien crayfish and prevented disturbance by local stakeholders. The careful attention paid to environmental conditions prior to reintroduction enhanced the possibility to perform an effective action.
The high quality of environmental conditions of the site both before and after the reintroduction was likely a factor determining reintroduction success. EBI score remained substantially unvaried over 12 years, confirming that the stream was and remained unpolluted, providing appropriate environmental conditions for the white-clawed crayfish; even the potential organic enrichment caused by local stakeholders when feeding tadpoles did not seem to have altered water quality. In reintroduction projects dealing with freshwater fauna, insufficient water quality is a major potential cause of failure (Jourdan et al., 2019). In fact, considering all white-clawed crayfish reintroductions described in peer-reviewed literature (Table 1), unexpected pollution events affected white-clawed crayfish abundance in at least two sites where they were reintroduced after A. astaci propagation (Spink and Frayling, 2000). Our stream hosted a diversified mayfly community, with occurrence of the genus Paraleptophlebia, which is considered an indicator of favorable conditions for the white-clawed crayfish (Grandjean et al., 2011). White-clawed crayfish are good indicators of freshwater ecosystem quality and functioning (Nardi et al., 2005) and also play a fundamental role in maintaining the structure of benthic communities (Manenti et al., 2019b) especially considering their large biomass (Richman et al., 2015). Assessing how crayfish extinctions and reintroductions impact the communities and the functionality of streams and creeks could be extremely important to understand the community-wide impacts of the extinction of these keystone species (Ripple et al., 2014) and to quantify the amplitude of services provided by them.
The activities of the study, the control, and the dissemination directed toward local stakeholders are other factors that prompted reintroduction success. The geography of the reintroduction site ensures that the crayfish population is naturally isolated from surrounding populations of alien crayfish by a high natural fall and a temporary stream stretch. However, the introduction of alien crayfish carrying the crayfish plague pathogen determined the local extinction and revealed the interest in the site by local inhabitants of the nearby village (Bonelli et al., 2017). Prior to the start of reintroduction activities, we thus performed multiple dissemination actions at a local scale, targeting the inhabitants of the surrounding area. The success of this effort is confirmed by the fact that no further introductions of alien crayfish occurred, nor did we detect disturbing activities by landowners and inhabitants of the area. The cages and sign placed prominently in one of the most accessible points of the stream remained untouched, and substrate features of the pool were not different inside and outside of the cages. So far, analyses of reintroduction actions performed with white-clawed crayfish paid limited attention to the involvement of local stakeholders and the impact of outreach campaigns. Local landowners were involved in the reintroduction of the noble crayfish (Astacus astacus) in different sites of Fennoscandia, but only very limited details are available on how this involvement took place (Taugbøl, 2004; Jussila et al., 2008; Edsman and Schröder, 2009); in particular, it is reported that local landowners collaborated with local authorities in reducing the motivation for illegal stocking of alien crayfish species and performing a fast reintroduction of native crayfish, but it is not described what local stakeholders did. Our case study suggests that local practices of self-made stream management can be a threat to native crayfish. Local stakeholders of isolated hilly and mountainous areas can include people with no awareness of current threats to biodiversity and native species (Lindemann-Matthies and Bose, 2008) which act based on personal beliefs rather than proper management practices. Convincing the inhabitants to avoid activities such as inappropriate cleaning of the stream and translocations has been a hard task. In our case study, it was impossible to perform a formal assessment of the success of the outreach campaign, yet the available indicators and the success of the reintroduction action suggest they have been helpful, with potential positive effects on the long-term persistence of this isolated white-clawed crayfish population.
A certain number of white-clawed crayfish survived and at least some individuals were quickly able to successfully breed. This suggests that 3-month-old juveniles are a good choice for reintroduction actions, as they can easily adapt to new habitats and conditions (Rogers and Watson, 2007). Without intraspecific competitors, the first reintroduced juveniles quickly reached larger sizes compared with individuals comprising the extinct population. The few published studies of white-clawed crayfish reintroductions highlight that, when juveniles are introduced, shelter availability is pivotal for survival (Rogers and Watson, 2007). Most of the available studies, however, dealt with translocated adults (Reynolds et al., 2000; Durlet et al., 2009) or did not provide information on the size or age of the reintroduced white-clawed crayfish (Spink and Frayling, 2000).
In our case study, the first breeding activity was detected just 12 months after the first reintroduction, when the reintroduced individuals were in their second year of life. In two French streams where 50- to 70-mm-long white-clawed crayfish were reintroduced, breeding was recorded for the first time after 3 years (Durlet et al., 2009). The sexual maturity of the white-clawed crayfish is strongly correlated to body length (Rhodes and Holdich, 1979; Grandjean et al., 1997), and both temperature and diet strongly affect their growth, molting, and survival (Paglianti and Gherardi, 2004). The size at which the white-clawed crayfish reaches sexual maturity is usually 22–25 mm of carapace length and 50–60 mm of TL (Mason, 1975; Arrignon, 1981; Ghia et al., 2015). Usually, sexual maturity is achieved around the third or fourth year of life, and even if alleged, breeding in 2-year-old individuals has not been recorded yet (Brewis and Bowler, 1982; Mancini, 1986; Ghia et al., 2015). The very fast growth rate observed suggests that, in suitable streams without intraspecific competition, juveniles can reach maturity very quickly. Even if the species is K-selected, with a slow growth rate and long life (Ghia et al., 2015), our results underline the fact that, in the absence of further disturbances, reintroductions of white-clawed crayfish can allow the species to recover in relatively short times. Nevertheless, the present-day apparent density remains 50% lower than the one reached by the extinct population. Further surveys should allow to assess whether in the next years the population will reach the abundance observed before the extinction.
The observation of a large male in 2019 is particularly noticeable, as this individual showed the size typical of old individuals. The life span of the white-clawed crayfish may last even over 12–13 years (Mancini, 1986; Ghia et al., 2015); it is thus possible that this individual could be a survivor of the former population. Isolated cases of survival after the crayfish plague have been recorded in different species of European freshwater crayfish (Kozubíková-Balcarová et al., 2014; Jussila et al., 2016; Strand et al., 2019), but this record might also represent unauthorized attempts of reintroduction performed by the local people as we recorded for at least in one stream in the same hydrographic catchment (Manenti, 2006). In San Michele creek, our monitoring suggests that illegal introductions could happen but are unlikely, and this might indeed represent a rare case of survival from the crayfish plague outbreak.
Our results suggest that the communication campaigns addressed to stakeholders and the natural separation of the stream from environments inhabited by alien crayfish species should allow the success of the reintroduction action, with a complete recovery of the species. Even though the study site can be considered a typical ark site, i.e., a refuge site safe from non-native crayfish and crayfish plague (Kozák et al., 2011; Haddaway et al., 2012; Rosewarne et al., 2017), major concerns remain for the long-term persistence of this population. For instance, long-term isolation could expose the population to the risk of extinction because of stochastic or genetic factors. Future efforts should thus prevent extinctions in the few nearby streams where native white-clawed crayfish still survive and try to re-establish additional populations that can enable long-term persistence, for instance, by forming a metapopulation network isolated from crayfish plague outbreaks.
Successful reintroductions require accurate planning and the execution of multiple steps. Each step, from habitat assessment to dissemination activities and continued monitoring, has had a key role in the reintroduction of white-clawed crayfish. In our case study, as for most conservation programs and policies (Chazdon et al., 2017; Manenti et al., 2019a), the aim was to reverse the impacts of human actions. For this reason, a substantial part of both preliminary actions and monitoring activities were directed toward preventing the detrimental actions of stakeholders living near the reintroduction site. Reintroduction actions could be more effective when the stakeholders having the greatest potential impact on the species are identified and involved in the activities. This is especially important when reintroductions focus on animals having major cultural, gastronomic, and commercial interest.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author Contributions
RM and GFF conceived the manuscript. RM and MV planned the reintroduction. AN, BB, SC, and RM performed the surveys. All authors contributed to the writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Monitoring from 2016 to 2018 was made possible by The Mohamed bin Zayed Species Conservation Fund, project n. 162512949. Reintroduction actions were funded by the Cariplo Foundation through the project “Capitale naturale nel Monte di Brianza” of the Monte Barro Regional Park and by LIFE14 IPE/IT/000018 project “LIFE IP GESTIRE 2020 – Nature Integrated Management to 2020,” website: https://naturachevale.it/. Publication APC was covered by the University of Milan. Young crayfish for reintroductions were provided by the facility of the ERSAF organization which has two breeding capacities in Canzo (CO) and in Tignale (BS).
Acknowledgments
We are grateful to all from the LIFE IP Gestire2020 Action C6 team and to D. Ghia, G. Fea, G. Fracassi, A. Casoni, and P. Colombo. We thank Robin Whalley for proofreading the text.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.621613/full#supplementary-material
References
Alderman, D. J. (1996). Geographical spread of bacterial and fungal diseases of crustacean. Rev. Sci. Tech. Off. Int. Épizoot. 15, 603–632. doi: 10.20506/rst.15.2.943
Bernini, G., Bellati, A., Pellegrino, I., Negri, A., Ghia, D., Fea, G., et al. (2016). Complexity of biogeographic pattern in the endangered crayfish Austropotamobius italicus in northern Italy: molecular insights of conservation concern. Conserv. Genet. 17, 141–154. doi: 10.1007/s10592-015-0767-4
Bland, L. M. (2017). Global correlates of extinction risk in freshwater crayfish. Anim. Conserv. 20, 532–542. doi: 10.1111/acv.12350
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2008). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Bonelli, M., Manenti, R., and Scaccini, D. (2017). Mountain protected areas as refuges for threatened freshwater species: the detrimental effect of the direct introduction of alien species. Ecol. Mont. 9, 23–29. doi: 10.1553/eco.mont-9-2s23
Brewis, J. M., and Bowler, K. (1982). The growth of the freshwater crayfish Austropotamobius pallipes in Northumbria. Freshw. Biol. 12, 187–200. doi: 10.1111/j.1365-2427.1982.tb00613.x
Caprioli, R., Mrugała, A., Di Domenico, M., Curini, V., Giansante, C., Camma, C., et al. (2018). Aphanomyces astaci genotypes involved in recent crayfish plague outbreaks in central Italy. Dis. Aquat. Org. 130, 209–219. doi: 10.3354/dao03275
Chao, A., and Chang, S. H. (1999). An estimating function approach to the inference of catch-effort models. Environ. Ecol. Stat. 6, 313–334. doi: 10.1023/A:1009687514770
Chazdon, R. L., Brancalion, P. H. S., Lamb, D., Laestadius, L., Calmon, L., and Kumar, C. (2017). A policy-driven knowledge agenda for global forest and landscape restoration. Conserv. Lett. 10, 125–132. doi: 10.1111/conl.12220
Chucholl, C. (2016). The bad and the super-bad: prioritising the threat of six invasive alien to three imperilled native crayfishes. Biol. Invas. 18, 1967–1988. doi: 10.1007/s10530-016-1141-2
Diéguez-Uribeondo, J., Rueda, A., Castien, E., and Bascones, J. C. (1997). A plan of restoration in Navarra for the native freshwater crayfish species of Spain, Austropotamobius pallipes. Bull. Fr. Pêche Piscic. 347, 625–637. doi: 10.1051/kmae/1997056
Durlet, P., Tissot, B., Pesme, E., Marquis, A., and Besson, S. (2009). Expérience de réintroduction de deux populations d’écrevisses à pattes blanches Austropotamobius pallipes (Lereboulet, 1858). Rev. Sci. Bourgogne Nat. 10, 243–249.
Edsman, L., and Schröder, S. (2009). Action Plan for the Noble Crayfish (Astacus astacus), 2008–2013. Stockholm: Swedish Environmental Protection Agency, 5955. Report.
Ficetola, G. F., Barzaghi, B., Melotto, A., Muraro, M., Lunghi, E., Canedoli, C., et al. (2018). N-mixture models reliably estimate the abundance of small vertebrates. Sci. Rep. 8:10357. doi: 10.1038/s41598-018-28432-8
Fiske, I., and Chandler, R. B. (2011). unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43, 1–23. doi: 10.18637/jss.v043.i10
Gherardi, F. (2011). Towards a sustainable human use of freshwater crayfish (Crustacea, Decapoda, Astacidea). Knowl. Manag. Aquat. Ecosyst. 2:22. doi: 10.1051/Kmae/2011038
Gherardi, F., Acquistapace, P., Aquiloni, L., Barbaresi, S., Cioni, A., Paglianti, A., et al. (2003). The red swamp crayfish in Europe: lessons for invasion biology. Integr. Comp. Biol. 43, 1005–1005.
Gherardi, F., and Barbaresi, S. (2000). Invasive crayfish: activity patterns of Procambarus clarkii in the rice fields of the Lower Guadalquivir (Spain). Arch. Hydrobiol. 150, 153–168. doi: 10.1127/archiv-hydrobiol/150/2000/153
Gherardi, F., and Souty-Grosset, C. (2006). European crayfish as heritage species-linking research and management strategies to conservation and socio-economic development-Preface. Bull. Fr. Pêche Piscic. 38, 851–852.
Ghetti, P. F. (1997). Indice Biotico Esteso (I.B.E.): Manuale di Applicazione. Trento: Provincia Autonoma di Trento.
Ghia, D., Fea, G., Conti, A., Sacchi, R., and Nardi, P. A. (2015). Estimating age composition in Alpine native populations of Austropotamobius pallipes complex. J. Limnol. 74, 501–511. doi: 10.4081/jlimnol.2015.1139
Ghia, D., Fea, G., Sacchi, R., Di Renzo, G., Garozzo, P., Marrone, M., et al. (2013). Modelling environmental niche for the endangered crayfish Austropotamobius pallipes complex in Northern and Central Italy. Freshw.Crayfish. 19, 189–195. doi: 10.5869/fc.2013.v19-2.189
Gil-Sanchez, J., and Alba-Tercedor, J. (2006). The decline of the endangered populations of the native freshwater crayfish (Austropotamobius pallipes) in southern Spain: it is possible to avoid extinction? Hydrobiologia 559, 113–122. doi: 10.1007/s10750-005-1024-5
Grandjean, F., Jandry, J., Bardon, E., Coignet, A., Trouilhe, M. C., Parinet, B., et al. (2011). Use of Ephemeroptera as bioindicators of the occurrence of white-clawed crayfish (Austropotamobius pallipes). Hydrobiologia 671, 253–258. doi: 10.1007/s10750-011-0717-1
Grandjean, F., Romain, D., AvilaZarza, C., Bramard, M., SoutyGrosset, C., and Mocquard, J. P. (1997). Morphometry, sexual dimorphism and size at maturity of the white-clawed crayfish Austropotamobius pallipes pallipes (Lereboullet) from a wild French population at Deux-Sèvres (Decapoda, Astacidea). Crustaceana 70, 31–44. doi: 10.1163/156854097x00320
Haddaway, N. R., Mortimer, R. J. G., Christmas, M., Grahame, J. W., and Dunn, A. M. (2012). Morphological diversity and phenotypic plasticity in the threatened British white-clawed crayfish (Austropotamobius pallipes). Aquat. Conserv. 22, 220–231. doi: 10.1002/aqc.2225
Holdich, D. M., Rogers, W., and Reynolds, J. (1999). Native and alien crayfish in the British Isles. Crustac 11, 221–236. doi: 10.1201/9781315140469-14
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biomed. J. 50, 346–363. doi: 10.1002/bimj.200810425
Jandry, J., Brulin, M., Parinet, B., and Grandjean, F. (2014). Ephemeroptera communities as bioindicators of the suitability of headwater streams for restocking with white-clawed crayfish, Austropotamobius pallipes. Ecol. Indic. 46, 560–565. doi: 10.1016/j.ecolind.2014.07.005
Jourdan, J., Plath, M., Tonkin, J. D., Ceylan, M., Dumeier, A. C., Gellert, G., et al. (2019). Reintroduction of freshwater macroinvertebrates: challenges and opportunities. Biol. Rev. 94, 368–387. doi: 10.1111/brv.12458
Jussila, J., Maguire, I., Kokko, H., Tiitinen, V., and Makkonen, J. (2020). Narrow-clawed crayfish in Finland: Aphanomyces astaci resistance and genetic relationship to other selected European and Asian populations. Knowl. Manag. Aquat. Ecosyst. 421:30. doi: 10.1051/kmae/2020022
Jussila, J., Ojala, K., and Mannonen, A. (2008). Noble crayfish (Astacus astacus) reintroduction project in the river Pyhäjoki, western Finland: a case study. Freshw. Crayfish. 16, 51–56. doi: 10.5869/fc.2008.v16.51
Jussila, J., Tiitinen, V., Edsman, L., Kokko, H., and Fotedar, R. (2016). Signal crayfish in Lake Saimaa could be maladapted to the local conditions due to Aphanomyces astaci infection: a seven-year study. Freshw. Crayfish. 22, 53–60. doi: 10.5869/fc.2016.v22-1.53
Kemp, E., Birkinshaw, N., Peay, S., and Hiley, P. D. (2003). Reintroducing the White-Clawed Crayfish Austropotamobius pallipes. Peterborough: English Nature.
Kouba, A., Petrusek, A., and Kozák, P. (2014). Continental-wide distribution of crayfish species in Europe: update and maps. Knowl. Manag. Aquat. Ecosyst. 5:7. doi: 10.1051/kmae/2014007
Kozák, P., Füreder, L., Kouba, A., Reynolds, J., and Souty-Grosset, C. (2011). Current conservation strategies for European crayfish. Knowl. Manag. Aquat. Ecosyst. 1:18. doi: 10.1051/kmae/2011018
Kozubíková-Balcarová, E., Beran, L., Ďuriš, Z., Fischer, D., Horká, I., Svobodová, J., et al. (2014). Status and recovery of indigenous crayfish populations after recent crayfish plague outbreaks in the Czech Republic. Ethol. Ecol. Evol. 26, 299–319. doi: 10.1080/03949370.2014.897652
Lindemann-Matthies, P., and Bose, E. (2008). How Many Species Are There? Public Understanding and Awareness of Biodiversity in Switzerland. Hum. Ecol. 36, 731–742. doi: 10.1007/s10745-008-9194-1
Lo Parrino, E., Ficetola, G. F., Manenti, R., and Falaschi, M. (2020). Thirty years of invasion: the distribution of the invasive crayfish Procambarus clarkii in Italy. Biogeographia 35, 43–50. doi: 10.21426/B635047157
Makkonen, J., Strand, D. A., Kokko, H., Vrålstad, T., and Jussila, J. (2013). Timing and quantifying Aphanomyces astaci sporulation from the noble crayfish suffering from the crayfish plague. Vet. Microbiol. 162, 750–755. doi: 10.1016/j.vetmic.2012.09.027
Mancini, A. (1986). Astacicoltura: Allevamento e Pesca dei Gamberi d’acqua Dolce. Bologna: Edagricole.
Manenti, R. (2006). Rilievi sul Patrimonio Astacicolo Della Provincia di Lecco. Thesis, Università degli Studi di Milano, Milan.
Manenti, R., Barzaghi, B., Tonni, G., Ficetola, G. F., and Melotto, A. (2019a). Even worms matter: cave habitat restoration for a planarian species has increased prey availability but not population density. Oryx 53, 216–221. doi: 10.1017/S0030605318000741
Manenti, R., Ghia, D., Fea, G., Ficetola, G. F., Padoa-Schioppa, E., and Canedoli, C. (2019b). Causes and consequences of crayfish extinction: stream connectivity, habitat changes, alien species and ecosystem services. Freshw. Biol. 64, 284–293. doi: 10.1111/fwb.13215
Manenti, R., Bonelli, M., Scaccini, D., Binda, A., and Zugnoni, A. (2014). Austropotamobius pallipes reduction vs. Procambarus clarkii spreading: management implications. J. Nat. Conserv. 22, 586–591. doi: 10.1016/j.jnc.2014.09.001
Manenti, R., Falaschi, M., Delle Monache, D., Marta, S., and Ficetola, G. F. (2020). Network-scale effects of invasive species on spatially-structured amphibian populations. Ecography 43, 119–127. doi: 10.1111/ecog.04571
Marquis, A. (2006). Protocole de Reintroduction de l’ecrevisse a Pieds Blancs (Austropotamobius pallipes). Besançon: Universite de Franche-Comte.
Martín-Torrijos, L., Kokko, H., Makkonen, J., Jussila, J., and Diéguez-Uribeondo, J. (2019). Mapping 15 years of crayfish plague in the Iberian Peninsula: the impact of two invasive species on the endangered native crayfish. PLoS One 14:e0219223. doi: 10.1371/journal.pone.0219223
Nardi, P. A., Bernini, F., Bo, T., Bonardi, A., Fea, G., Ghia, D., et al. (2005). Status of Austropotamobius pallipes complex in the watercourses of the Alessandria province (N-W Italy). Bull. Fr. Pêche Piscic. 37, 585–598. doi: 10.1051/Kmae:2005017
Nishijima, S., Nishikawa, C., and Miyashita, T. (2017). Habitat modification by invasive crayfish can facilitate its growth through enhanced food accessibility. BMC Ecol. 17:37. doi: 10.1186/s12898-017-0147-7
Paglianti, A., and Gherardi, F. (2004). Combined effects of temperature and diet on growth and survival of young-of-year crayfish: a comparison between indigenous and invasive species. J. Crust. Biol. 24, 140–148. doi: 10.1651/C-2374
Perino, A., Pereira, H. M., Navarro, L. M., Fernandez, N., Bullock, J. M., Ceausu, S., et al. (2019). Rewilding complex ecosystems. Science 364:eaav5570. doi: 10.1126/science.aav5570
Reynolds, J., Souty-Grosset, C., Gouin, N., Devaney, S., and Grandjean, F. (2000). “Experimental restocking of native crayfish in White lake, Co. Westmeath, Ireland,” in Proceedings of the Crayfish Conference Leeds, eds D. Rogers and H. Brickland (London: Environment Agency), 123–130.
Rhodes, C. P., and Holdich, D. M. (1979). On size and sexual dimorphism in Austropotamobius pallipes (Lereboullet). Aquaculture 17, 345–358. doi: 10.1016/0044-8486(79)90089-9
Richman, N. I., Boehm, M., Adams, S. B., Alvarez, F., Bergey, E. A., Bunn, J. J. S., et al. (2015). Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140060. doi: 10.1098/Rstb.2014.0060
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the World’s largest carnivores. Science 343:151. doi: 10.1126/science.1241484
Rogers, D., and Watson, E. (2007). Increasing the chances of successful reintroduction of white-clawed crayfish (Austropotamobius pallipes) in the Peak District National Park, UK. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 3, 209–216. doi: 10.1080/17451590709618174
Rosewarne, P. J., Mortimer, R. J. G., and Dunn, A. M. (2017). Habitat use by the endangered white-clawed crayfish Austropotamobius species complex: a systematic review. Knowl. Manag. Aquat. Ecosyst. 4:9.
Siesa, M. E., Manenti, R., Padoa-Schioppa, E., De Bernardi, F., and Ficetola, G. F. (2011). Spatial autocorrelation and the analysis of invasion processes from distribution data: a study with the crayfish Procambarus clarkii. Biol. Invas. 13, 2147–2160. doi: 10.1007/s10530-011-0032-9
Souty Grosset, C., Holdich, D. M., Noël, P., Reynolds, J. D., and Haffner, P. (2006). Atlas of Crayfish in Europe. Paris: Muséum National d’Histoire Naturelle.
Souty-Grosset, C., Grandjean, F., Raimond, R., Frelon, M., Debenest, C., and Bramard, M. (1997). Conservation genetics of the white-clawed crayfish Austropotamobius pallipes: the usefulness of the mitochondrial DNA marker. Bull. Fr. Pêche Piscic. 347, 677–692. doi: 10.1051/kmae/1997055
Souty-Grosset, C., Reynolds, J., Gherardi, F., Schulz, R., Edsman, L., Füreder, L., et al. (2006). Craynet-Achievements in scientific management of European crayfish, the way forward and future challenges. Bull. Fr. Pêche Piscic. 38, 1395–1399. doi: 10.1051/Kmae:2006043
Spink, J., and Frayling, M. (2000). An assessment of post-plague reintroduced native white-clawed crayfish Austropotamobius pallipes in the Sherston Avon and Tetbury Avon. Wiltshire. Freshw. Forum 14, 59–69.
Strand, D. A., Johnsen, S. I., Rusch, J. C., Agersnap, S., Larsen, W. B., Knudsen, S. W., et al. (2019). Monitoring a Norwegian freshwater crayfish tragedy: eDNA snapshots of invasion, infection and extinction. J. Appl. Ecol. 56, 1661–1673. doi: 10.1111/1365-2664.13404
Svoboda, J., Fischer, D., Kozubíková-Balcarová, E., Št’ástková, A., Brůčková, M., Kouba, A., et al. (2020). Experimental evaluation of the potential for crayfish plague transmission through the digestive system of warm-blooded predators. J. Fish Dis. 43, 129–138. doi: 10.1111/jfd.13109
Svoboda, J., Mrugała, A., Kozubiková-Balcarová, E., and Petrusek, A. (2017). Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: a review. J. Fish Dis. 40, 127–140. doi: 10.1111/jfd.12472
Svoboda, J., Strand, D. A., Vralstad, T., Grandjean, F., Edsman, L., Kozák, P., et al. (2014). The crayfish plague pathogen can infect freshwater-inhabiting crabs. Freshw. Biol. 59, 918–929. doi: 10.1111/fwb.12315
Taugbøl, T. (2004). Reintroduction of noble crayfish Astacus astacus after crayfish plague in Norway. Bull. Fr. Pêche Piscic. 372–373, 315–328. doi: 10.1051/Kmae:2004006
Unger, S., Bodinof-Jachowski, C., Diaz, L., and Williams, L. A. (2020). Observations on habitat preference of juvenile eastern hellbender salamanders (Cryptobranchus alleganiensis). Acta Ethol. 23, 119–124. doi: 10.1007/s10211-020-00344-9
Weiperth, A., Blaha, M., Szajbert, B., Sepros, R., Banyai, Z., Patoka, J., et al. (2020). Hungary: a European hotspot of non-native crayfish biodiversity. Knowl. Manag. Aquat. Ecosyst. 421:43. doi: 10.1051/kmae/2020035
Weiperth, A., Gal, B., Kurikova, P., Blaha, M., Kouba, A., and Patoka, J. (2017). Cambarellus patzcuarensis in Hungary: the first dwarf crayfish established outside of North America. Biologia 72, 1529–1532. doi: 10.1515/biolog-2017-0159
Keywords: invertebrate, restoration, conservation, freshwater, Faxonius limosus
Citation: Manenti R, Barzaghi B, Nessi A, Cioccarelli S, Villa M and Ficetola GF (2021) Not Only Environmental Conditions but Also Human Awareness Matters: A Successful Post-Crayfish Plague Reintroduction of the White-Clawed Crayfish (Austropotamobius pallipes) in Northern Italy. Front. Ecol. Evol. 9:621613. doi: 10.3389/fevo.2021.621613
Received: 26 October 2020; Accepted: 26 January 2021;
Published: 04 March 2021.
Edited by:
Japo Jussila, University of Eastern Finland, FinlandReviewed by:
Adam Petrusek, Charles University, CzechiaWilliam Perry, Illinois State University, United States
Copyright © 2021 Manenti, Barzaghi, Nessi, Cioccarelli, Villa and Ficetola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raoul Manenti, cmFvdWxtYW5lbnRpQGdtYWlsLmNvbQ==; cmFvdWwubWFuZW50aUB1bmltaS5pdA==; Alessandro Nessi, YWxlc3NhbmRyby5uZXNzaTkzQGdtYWlsLmNvbQ==
 Raoul Manenti
Raoul Manenti Benedetta Barzaghi1
Benedetta Barzaghi1 Alessandro Nessi
Alessandro Nessi