- 1Population and Conservation Biology Group, Department of Biology, Texas State University, San Marcos, TX, United States
- 2The Xiphophorus Genetic Stock Center, Texas State University, San Marcos, TX, United States
Human population growth and its associated effects on the environment contribute to the rapid decrease of biodiversity worldwide. Artificial light at night (ALAN) is an anthropogenic pollutant that is increasing with the spread of urbanization and may contribute to biodiversity declines. ALAN alters the migration patterns of birds, communication in frogs, and impacts reproduction, behavior, and physiology of multiple other taxa. However, most of the studies on ALAN are based on terrestrial systems, and overall, the effects of ALAN on freshwater organisms are poorly understood. We investigated how ALAN affects the physiology, behavior, and reproduction of a widespread, tolerant species of freshwater fish. Gambusia affinis are small livebearing fish often found in urban streams. We exposed groups of female G. affinis to either a natural light cycle or a constant 24-h light cycle (ALAN) in the laboratory for 60 days. In another experiment, we exposed female G. affinis to the same treatments in outdoor mesocosms for 32 days. We found that exposure to ALAN lowered glucose levels in the brain and decreased swimming activity, but had no effect on cortisol release rates, reproduction, survival, or growth. This research is strengthened by measuring multiple metrics in response to ALAN and by incorporating both a field and laboratory component which confirm similar results. These results suggest that this tolerant species of fish may behaviorally adjust to ALAN rather than modulate their endocrine stress response.
Introduction
Anthropogenic disturbances contribute to habitat loss and alteration, climate change, and increased exploitation of natural resources by humans (Dudgeon et al., 2006; Vörösmarty et al., 2010; Ellis, 2011; Helm et al., 2013). These disturbances are associated with shifts in water quality, water flow, seasonal timing, sound, and light pollution (Jenkins, 2003; Allan, 2004; Barbier, 2012; Davies et al., 2014; Swaddle et al., 2015; Shannon et al., 2016; Buxton et al., 2017; Sordello et al., 2019). Artificial light at night (ALAN) is one form of anthropogenic pollution that alters the natural light and dark cycle in an ecosystem (Swaddle et al., 2015). Light plays a key role in the ecology of organisms as a source of energy and information, a regulator of circadian rhythms, and as a cue for communication, navigation, and orientation (Gaston et al., 2012, 2017). As worldwide urbanization increases, ALAN is becoming so widespread that 83% of the global human population lives under light-polluted skies, and 40% lives in areas that are continually illuminated due to ALAN (Cinzano et al., 2001; Swaddle et al., 2015; Falchi et al., 2016). Hölker et al. (2010) showed that ALAN is increasing alongside urbanization at an average annual rate of 6%.
Global freshwater systems support 9.5% of all extant described species, and these species’ populations are declining at rates exceeding those of tropical rainforests, primarily due to anthropogenic stressors (Ricciardi and Rasmussen, 1999; Xenopoulos et al., 2005; Dudgeon et al., 2006; Balian et al., 2008). Moreover, 90% of the human population lives within 10 km of a freshwater body and 50% within 3 km, making freshwater areas the most impacted by anthropogenic disturbances, such as ALAN (Dudgeon et al., 2006; Kummu et al., 2011; Venohr et al., 2018). Aquatic organisms are affected by ALAN because they are influenced by photoperiod across life history stages, including reproduction, growth, development, and activity (Downing and Litvak, 2002; Mehner, 2012). ALAN has detrimental effects on behavior, reproduction, foraging, orientation, predation, physiology, and migration in various taxa (Longcore and Rich, 2004; Navara and Nelson, 2007; Hölker et al., 2010; Ouyang et al., 2011; Gaston et al., 2013, 2014, 2015; Davies et al., 2014), yet most studies of ALAN focus on terrestrial taxa. Of the limited studies of ALAN on aquatic organisms, there is a knowledge gap regarding the consequences of ALAN on behavior, physiology, and reproduction in aquatic species (Depledge et al., 2010; Perkin et al., 2011; Jechow and Holker, 2019).
Because ALAN can affect circadian and circannual rhythms, physiology and behavior can be altered by exposure to ALAN. Individuals of some fish species increase their activity, modify their shoaling behavior, and spend more time in open (riskier) areas under ALAN which can alter foraging and increase their risk of predation (Becker et al., 2013; Foster et al., 2016; Kurvers et al., 2018; Sanders and Gaston, 2018; Czarnecka et al., 2019). ALAN is also associated with increased blood glucose in goldfish, Carassius aurarus (Ryu et al., 2019), and impaired melatonin rhythms in European perch, Perca fluviatilis. Further, reproductive hormones (17β-estradiol and 11-ketotestosterone), along with mRNA expression of gonadotropins, were reduced in fish exposed to ALAN (Brüning et al., 2018). Together, these changes may alter survival and reproduction of fish.
Given these effects of ALAN on fish behavior and physiology, it is likely that ALAN can also affect the fish stress response. Organismal response to stressors, such as ALAN, can be quantified by measuring cortisol release rates, the primary glucocorticoid (GC) in fishes (Idler and Truscott, 1972) that is released in response to a potential stressor (Hopkins et al., 1997; Dickens and Romero, 2013; King et al., 2016; Gabor et al., 2018). When a fish encounters a stressful event, its hypothalamic-pituitary-interrenal (HPI) axis is activated, resulting in a release of cortisol into the bloodstream that induces a variable response in target organs, thus altering individual behavior and physiology to maintain homeostasis (Wendelaar Bonga, 1997; Romero, 2004). When faced with an acute stressor, this mechanism is adaptive, but prolonged exposure to a stressor can have harmful, long-term, and even fatal effects (Sapolsky et al., 2000; Romero, 2004). Elevated GCs are linked to lower survival, reproduction, and dysregulation of immune responses (Bonier et al., 2009); however, stress may have a bidirectional effect and can also enhance immunity, growth, and reproductive output (Dhabhar et al., 1995; Dhabhar and McEwen, 1996; Dhabhar and Viswanathan, 2005; Viswanathan et al., 2005; Thawley and Kolbe, 2020). Because GCs are also involved in altering other physiological processes (Sapolsky et al., 2000; Le et al., 2005; Dhabhar, 2009), if ALAN causes changes to cortisol release rates, it can indirectly affect downstream traits such as behavior, growth, and reproduction. Alternatively, organisms may behaviorally adjust to the perturbation of ALAN rather than modulate their regulation of the HPI axis.
Here, we propose to quantify the consequences of ALAN on physiology, behavior, and reproduction of Western mosquitofish (Gambusia affinis). These are small, livebearing fish native to eastern North America, but introduced globally, and found in a wide variety of environments, including urban streams (Lloyd et al., 1986; Hubbs, 2000; Pyke, 2005; Page and Burr, 2011). This species is generally found in shallow waters and forages near the surface primarily at morning and dusk, though sometimes during the day (Hess and Tarzwell, 1942; Belk and Lydeard, 1994). Unlike egg-laying species of fish, ALAN may affect gravid females and their offspring while in utero, which could alter offspring survival. Mosquitofish are considered tolerant due to their success as invaders and their ability to live in adverse conditions which could suggest that they will be less adversely affected by ALAN than other less tolerant species (Cherry et al., 1976; Lloyd et al., 1986; Pyke, 2008). We used laboratory and mesocosm experimental approaches to test the hypotheses that ALAN alters the physiology, behavior, and reproduction of female G. affinis. We performed the mesocosm experiment second to validate our laboratory experimental findings and test these questions in an ecological context.
Experiment 1: Consequences of Exposure to Alan in Aquaria on Physiological Stress, Behavior, and Fitness Correlates of Female Mosquitofish
Materials and Methods
Fish Collection and Maintenance
We collected Gambusia affinis from the Blanco River in Hays County, Texas during the breeding season (13 April 2018 and 13 June 2018) with a seine and transported them to the laboratory. We placed lux meters (Dr. Meter, model LX1330B) at the level of the water at the collection site at night and found that it was not exposed to ALAN. We placed fish in 38 L tanks and fed them ad libitum daily with ISO flake food (TetraMin®) and supplemented them three times a week with brine shrimp. One week after collection, we haphazardly placed 80 mature females individually into semi-clear 0.95 L cylindrical plastic containers fitted (stacked) into another 0.35 L cylindrical plastic container (Figure 1). We made small holes at the bottom of the upper container to allow any offspring that were born to move into the lower container (but not the adults) and avoid maternal cannibalism (following Cazan and Klerks, 2015). Each of the upper containers also had small holes on the sides to facilitate flow of conspecific cues and reduce stress of solitary confinement (personal observation) while simultaneously allowing for individual sampling. Eight of the containers were placed together in a 38 L tank filled with 28.5 L of dechlorinated tap water and gravel across the bottom.

Figure 1. Example of container setup for ALAN and control groups in the laboratory. Each container held one female Gambusia affinis in the top portion and a bottom container into which offspring could swim to escape cannibalism. Smaller holes in the top and bottom allowed for exchange of chemical cues between female focal fish.
ALAN Exposure Design and Reproduction
We conducted the experiment starting on three separate dates (21 April, 23 June, and 5 September) in the same space and using the same design each time. For each of these date “blocks” we exposed five tanks (each with the eight containers; N = 40 females), to a control treatment of 14:10 h light: dark cycle and five other tanks (N = 40 females) to the experimental treatment (ALAN) of 24:0 h light: dark cycle (14 h simulated daylight: 10 h ALAN) for 50–60 days, for a total of 15 replicates per treatment. We simulated daylight with a full spectrum white fluorescent light (MingDak) at 880 lux and ALAN using white LED lights (Utilitech) at 120 lux. Full daylight levels can reach up to 25,000 lux with illuminances up to 100,000 lux in direct sunlight (Blume et al., 2019). Light levels at night in Hays County, Texas ranged from 16 lux at dim streetlamps to 230 lux at flood lights, therefore this nighttime lux level was ecologically relevant. We hung all lights 51 cm above the tanks. After the first block, we realized that some measurements of ALAN in Hays County were higher than the lux values used in our original ALAN treatment. Consequently, we increased the daylight to 2,380 lux and ALAN to 246 lux for the next two blocks. Our measures represent the level of light reaching the water where organisms can be found because lights from bridges, roads, boardwalks, and homes next to water sources are common. Indeed, organisms in close proximity to the light source can experience light intensities greater than 100 lux (Bolton et al., 2017).
We monitored the containers daily for offspring. Females generally give live birth to all offspring in a brood within a short period as this species do not show superfetation (Turner, 1937), therefore when present, we recorded the date, number of offspring, and offspring survival (alive vs. dead) in each brood. We changed the tank water by siphoning out 3/4 of the water from the bottom (to remove feces) and replacing it with dechlorinated tap water every 2 weeks.
Water-Borne Cortisol Collection and Growth
For the first two time blocks, we collected water-borne hormones 2 days after the females had been placed in their experimental container (but light exposure had not yet begun) and thus had an opportunity to acclimate to the experimental set-up (day 0), then again on day 7, 30, and the last day of the block. The standardized methods we used for hormone collection followed Blake et al. (2014). We placed each fish in a LDPE plastic insert in a 250 ml glass beaker with 60 ml of dechlorinated water for 30 min. After that time we recorded mass (g) and standard length (SL: mm) of each fish, then returned the fish to its original container. Each hormone collection event began at 0900 h to control for natural diel fluctuations of cortisol release rates. We cleaned beakers and inserts with 95% ethanol and rinsed them with deionized water before use and handled them with non-powdered gloves to prevent contamination. Scott et al. (2008) tested this non-invasive method for establishing cortisol release rates from fish and Blake et al. (2014) validated this method of analyzing cortisol release rates from water-borne hormones using Gambusia geiseri, a close relative of G. affinis. After the last cortisol release sample was taken on the last day of the block, we assessed whether the fish were chronically stressed (as indicated by responsiveness of the HPI axis) by agitating the fish while collecting their hormones. This allowed us to measure cortisol release rates in response to an acute stressor. For this test, we placed fish into the same set-up as above and shook them for 1 min every other minute for a total of 30 min.
Shoaling Behavior
We removed N = 22 fish from both treatments after day 60 and randomly placed them into plastic containers (33.02 cm × 20.32 cm × 11.43 cm) in groups of 4, a shoal size used in previous studies with Poeciliidae (Tobler and Schlupp, 2008). We filled containers (lined on the outside with white paper towels to create contrast and track the fish easier) with 5 L of dechlorinated tap water. Groups acclimated in the containers for 30 min. We then recorded the groups under their respective treatment for ∼24 h with webcams mounted above each container using ManyCam software. Videos were taken over 2 days for logistical purposes. We analyzed videos using EthoVision XT (Noldus) and recorded the average distance moved (cm) among all four fish, time resting, and shoaling (time spent within 2 cm of each other) during day (14 h) and night hours (10 h).
Hiding Behavior
For this experiment we tested N = 29 fish which were randomly selected from both treatments from the second block of the experiment. We set up 18 L tanks with one opaque half cylinder of PVC (as a hiding place) and one clear half cylinder of PVC (which controlled for the preference to hide in a smaller place vs. out of view) which were randomly assigned to each side of the tank. We filled tanks with 1.3 L of treated tap water (just enough to cover the half cylinders). At night, we removed fish from the ALAN treatment (after day 50) and individually placed them into the testing tank located under an ALAN light and allowed the fish to acclimate under a clear, plastic 1 L container for 10 min. We covered the sides of the tanks with black paper in case the fish could still be distracted from their surroundings at night. Following acclimation, we removed the clear container and began recording with ManyCam software for 10 min. We analyzed videos using EthoVision XT (Noldus) to estimate time (s) spent “hiding” under either half cylinder and time (s) spent resting.
Cortisol Extraction, Reconstitution, and Enzyme Immunoassays
We stored water-borne hormone samples at –20°C until thawed for extractions following methods of Gabor et al. (2016). We pulled water samples through C18 solid phase extraction (SPE) columns (SepPak Vac3 cc/500 mg; Waters, Inc., Milford, MA, United States) using Tygon tubing under vacuum pressure. SPE columns were primed with 4 ml of methanol followed by 4 ml of distilled water. We then eluted columns with 4 ml methanol into borosilicate vials then evaporated the methanol by placing the vials in a 37°C water bath while under nitrogen gas. We resuspended the residue in 5% ethanol (95% lab grade) and 95% enzyme immunoassays (EIA) buffer (Cayman Chemical, Inc) to a total volume of 720 μl based on dilutions from Blake et al. (2014).
We measured cortisol release rates in duplicate for all samples using EIA kits (No. 500360, Cayman Chemical Company, Inc.). Sample absorbances were read on a spectrophotometer plate reader at 405 nm (BioTek 800XS). To obtain cortisol release rates, we multiplied cortisol concentrations (pg/ml) by the final resuspension volume (0.720 ml), divided by the SL (mm) of the individual, and 0.5 h for a final unit of pg/mm/h. Inter-plate variation was 12.35% for the laboratory experiment (five plates) while intra-plate variation ranged from 0.39 to 14.88%. For the mesocosm experiment (six plates), inter-plate variation was 11.53% and intra-plate variation ranged from 0.45 to 6.95%.
Statistical Analyses
In the laboratory experiment, we used a generalized linear mixed model (GLMM) with natural log transformed cortisol release rates (standardized for standard length −pg/mm/h) as the dependent variable, with treatment and day as the fixed effects and ID as the random effect to account for repeated measures. We did not include the blocking effect of increasing lux, as adding this variable did not significantly affect the model results. When there were significant (p < 0.05) fixed effects differences, we used post hoc Tukey’s HSD comparisons. To explore effects of ALAN on offspring survival we used a chi-square test. We used GLMM to analyze changes in mass and SL over time with treatment and time as fixed effects and individual as the random effect to account for repeated measures. To determine the effects of ALAN on shoaling behavior, we used a repeated measure ANOVA. Due to the quality of videos recorded, we were unable to analyze each shoaling video we recorded, resulting in an uneven sample size. For all other behavioral analyses, we used GLMM with SL and treatment as model effects. We used JMP Pro 14.0.0 (SAS Institute, Inc.) for all analyses.
Results
Reproduction, Growth, and Cortisol
Offspring survival did not differ significantly between treatments (χ21 = 3.07, N = 32, p = 0.08). Because so few fish had offspring, we did not statistically compare offspring number but the mean for the control was (N = 17, Mean ± SE = 13 ± 1.65) and for ALAN was (N = 15, Mean ± SE = 11.33 ± 1.26).
In the laboratory experiment, we found no significant differences in cortisol release rates between fish in the control vs. ALAN treatment (GLMM: treatment × day: F4,155 = 1.44, p = 0.224; Table 1 and Figure 2A). Fish had significantly higher agitation cortisol release rates compared to baseline after 60 days irrespective of treatment (day: F4,155 = 103.32, p < 0.0001; Figure 2A), indicating that the fish from both treatments could mount a stress response. There was no significant effect of ALAN on mass (GLMM: treatment × time: F3,460 = 0.30, p = 0.824) or SL (F3,460 = 0.25, p = 0.86) over the duration of the experiment. There were no significant random effects of individual fish (Wald p-value = 0.78).
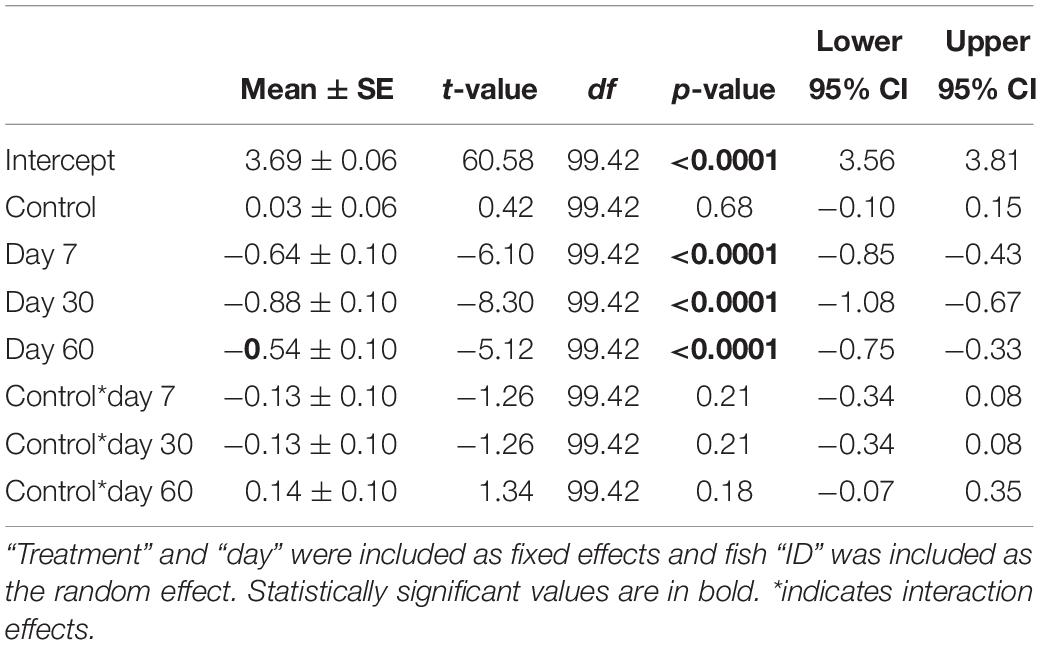
Table 1. Parameter estimates ± SE from the generalized linear mixed model for effects of ALAN on cortisol release rates of female G. affinis in the laboratory.
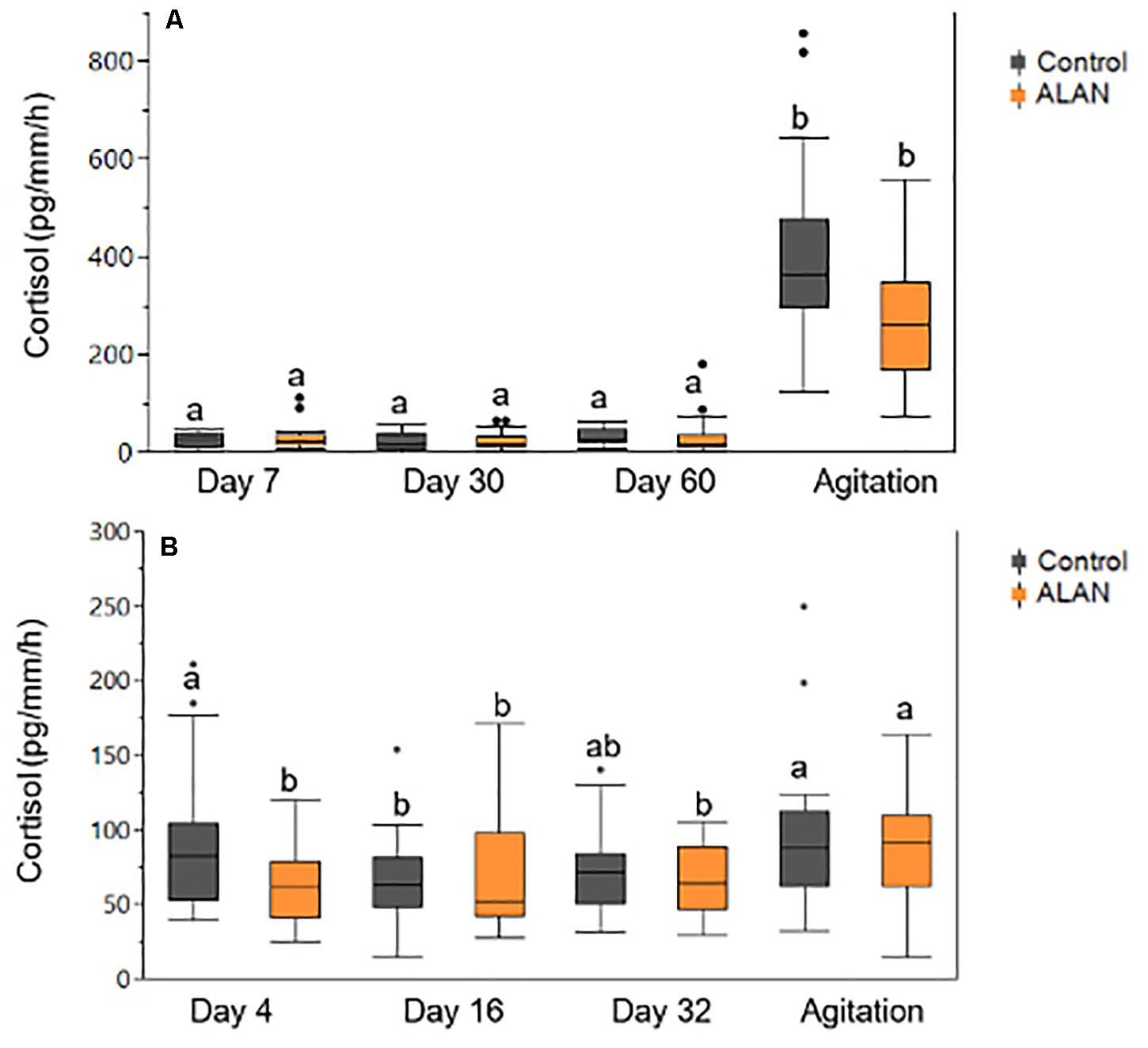
Figure 2. Cortisol release rates (pg/mm/h) obtained from female G. affinis after 30 min of baseline and agitation collection in the (A) laboratory and (B) mesocosm experiment. Agitation measures were obtained on day 60 in the lab and day 32 in mesocosms. One high agitation value was excluded from the laboratory ALAN group to increase the spread of the figure. Different lowercase letters indicate significant differences (p < 0.05) among treatment groups from Tukey’s HSD comparisons. Box plots indicate median, range, and first and third quartiles. Dots indicate outliers.
Shoaling Behavior
After being in the laboratory experiment for 59 days, female G. affinis in the ALAN treatment spent significantly less time shoaling (s) during the day than the control treatment (repeated measures ANOVA: time × treatment: F1,18 = 5.92, p = 0.026; Figure 3). Fish from both treatments moved a significantly greater distance (cm) during the day than at night (time: F1,18 = 8.05, p = 0.011).
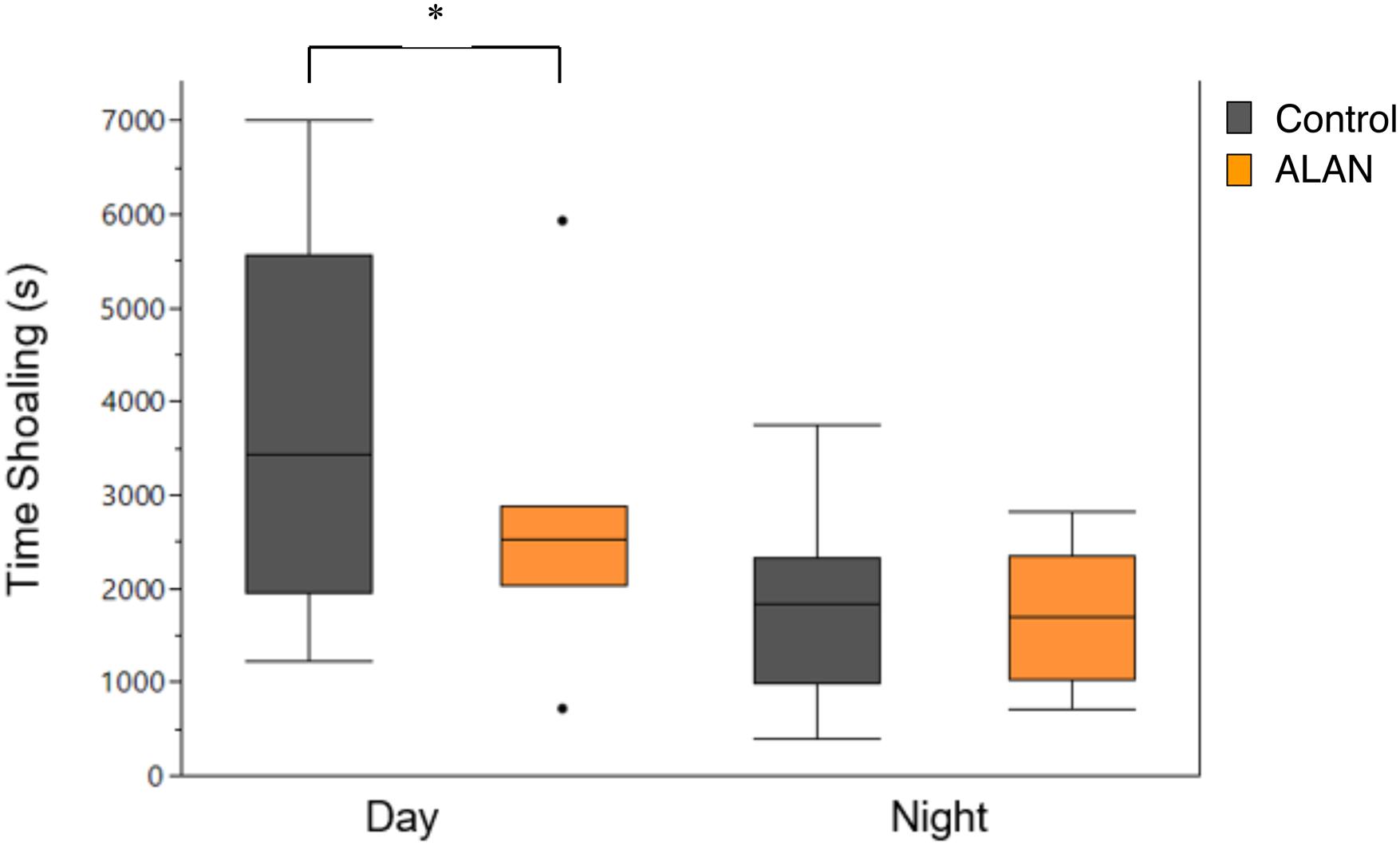
Figure 3. Time spent shoaling (s) by female G. affinis in the control (N = 15) and ALAN (N = 7) treatments in the day and night in the laboratory. Box plots indicate median, range, and first and third quartiles. Dots indicate outliers. Asterisk indicates a significant difference (p < 0.05).
Hiding Behavior
In the laboratory hiding experiment, after exposure to ALAN, fish spent significantly more time resting (LM: treatment: F1,22 = 6.93, p = 0.015; Table 2 and Figure 4) than fish in the control treatment. There was no significant effect of SL on time resting. There was no significant effect of ALAN on time spent hiding at night (F1,22 = 0.02, p = 0.896).
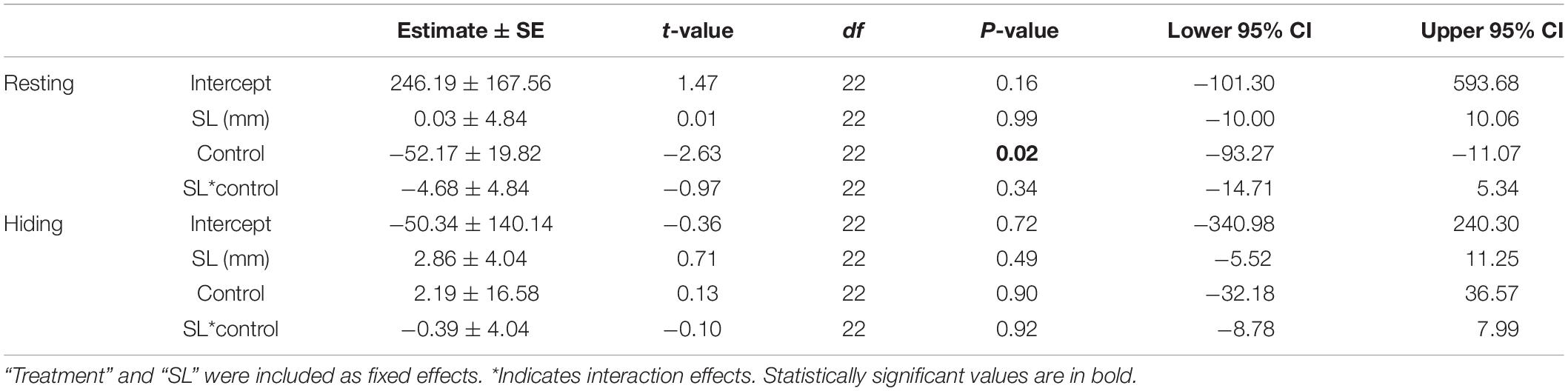
Table 2. Results from the general linear model testing the effects of ALAN on behavior of female G. affinis in the laboratory.
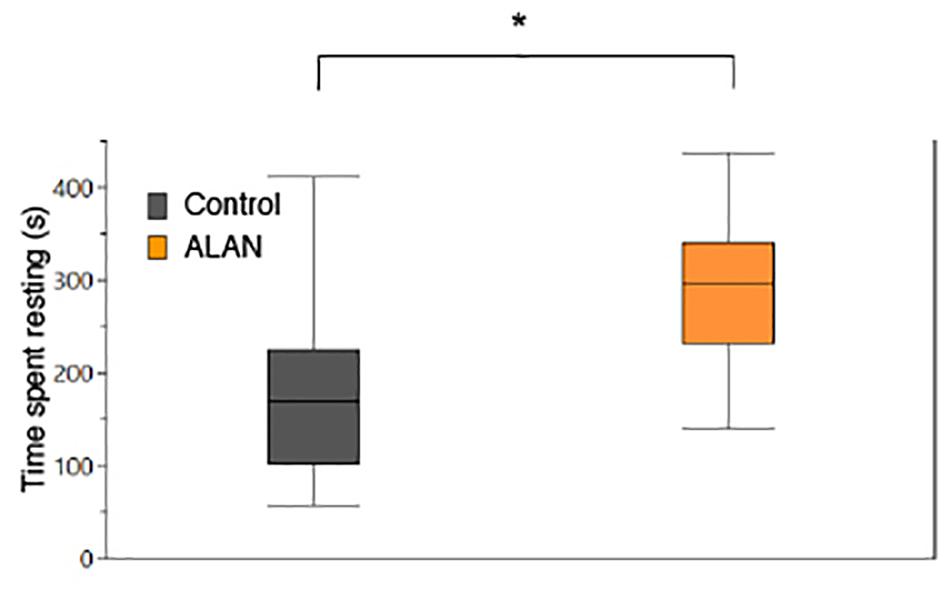
Figure 4. Mean time spent resting (s) by female G. affinis (N = 29) in the laboratory. Asterisk indicates a significant difference (p < 0.05). Box plots indicate median, range, and first and third quartiles.
Experiment 2: Consequences of Alan in Mesocosms on Physiological Stress and Behavior of Female Mosquitofish
Materials and Methods
Mesocosms set-up
We constructed 16 mesocosms using 62.45 L clear, #5 (polypropylene) plastic containers with six 5 cm holes drilled in the sides toward the top and covered with mesh for overflow water drainage while preventing fish from escaping. We cut out the center plastic of the lids and replaced it with mesh to allow light to pass through. We then placed the mesocosms outdoors underneath 60% shade cloth. On 25 June 2019, we added 48 L of water to each mesocosm. The following day we added 4 L of sediment collected from the Blanco River in Hays County, Texas, and 1 L of pond water. We added 1 L aliquots of zooplankton to each mesocosm on 29 June 2019 and 2 July 2019. We also added 16 pieces of ceramic bio media (11 BrightWater Bio Media® and five Fluval BioMax®) to each mesocosm to provide a substrate for microorganisms. Additionally, we added one sponge filter to each mesocosm to prevent the buildup of ammonia and nitrates. We added two artificial breeder plants (Penn Plax Aquarium Breeding Grass) to each mesocosm for habitat cover and to provide refuge for any offspring produced. We recorded water quality parameters (ammonia, nitrites, nitrates, total dissolved solids, temperature, conductivity, pH, and salinity) from each mesocosm twice a week. We added dechlorinated tap water to the mesocosms when it was lower than the drainage holes.
ALAN Experimental Design
From 19 June–21 June 2019 we collected 240 G. affinis from the Blanco River in Hays County, Texas using dipnets and seines, transported them to the lab, and fed them ISO flake food (TetraMin) daily. On 27 June 2019 we marked 64 females with white, red, orange, or yellow elastomer tags (N = 16 per color). On 3 July 2019, after mesocosms were established, we haphazardly selected five tagged females (for repeated measures of water-borne hormones (see below), eight non-tagged females (to provide adequate numbers of conspecifics since individuals would be removed throughout the experiment), and two males (totaling 15 fish) for placement into each mesocosm. We hung two artificial night lights (Onforu 35 W LED Flood Lights; 3,300 lumens 5,000 K) 52 cm above half of the mesocosms (below the shade cloth). Five days after placing fish into mesocosms, we turned on the artificial night lights on the experimental (ALAN) side (day 0) exposing eight mesocosms (N = 120 fish) to 24 h of light. These lights were on from 2000–0600 h and ranged from 260–280 lux at the top of the mesocosms and 155–175 lux at the surface of the water. We measured lux with a digital lux meter (Dr. Meter, model LX1330B). To prevent light from reaching the control mesocosms at night we hung a black plastic curtain in between treatment blocks. Ammonia and nitrites were not detected at any point in the mesocosms.
Water-Borne Cortisol Collection, Growth, Survival, and Glucose
We collected water samples to measure cortisol release rates from each tagged female on day 4 to capture the potential effect of treatment while giving enough time to acclimate to the mesocosms. Beginning at 0900 h we placed each fish in a LDPE plastic insert in a 250 ml glass beaker with 60 ml of spring water for 30 min (following methods above). After 30 min we collected the water sample then measured and recorded the mass (g) and standard length (SL; mm) of each fish, then returned the fish into its original mesocosm. Using the same methods, we collected baseline cortisol release rates from the same tagged individuals on days 16 and 32. Immediately after baseline cortisol release rate collection on day 32, we collected water samples to measure cortisol release rates when under an agitation treatment to test for an acute stress response (following the lab protocol above). We extracted and analyzed cortisol release rates following the same protocol as in experiment 1. On day 32 we euthanized fish in an ice slurry for 30 min then dissected 5–6 non-tagged females per mesocosm to obtain the brain, muscle, and liver tissues which were frozen until glucose analysis.
Hiding Behavior
We repeated the hiding behavioral experiment following the methods from experiment 1 (above) using tagged females from each mesocosm but performed the experiment during the day and with a 5 min acclimation period. Data were collected during the day instead of at night to examine if ALAN had effects that could be observed in daytime hours.
Glucose Extraction, Reconstitution, and Colorimetric Assays
For each mesocosm, we combined common tissue types (brain, muscle, and liver) from six fish and weighed the tissues (average weight ± SE) before adding them 400 μl of 100% ethyl alcohol. Samples where homogenized with an IKA Ultra-Turrax T25 and then centrifuged for 10 min at 10,000 rpm at 4°C. We removed the supernatant of each sample and transferred it to a new Eppendorf tube which was then placed in a vacufuge overnight (Eppendorf Vacufuge plus). We reconstituted the samples using 100 μl of 1 M phosphate buffer saline (PBS) and stored at −80°C.
We measured glucose levels following the colorimetric protocol for microplate from Bethke and Busse (2008). Duplicate samples were diluted to half by adding 25 μl of 1 M PBS per 25 μl of sample. Then 25 μl of 10 mM sodium acetate trihydrate (pH 5) and 10 μl of 150 mM PBS was added to all wells. We mixed plates then incubated them for 1 h at 40°C. After incubation, 25 μl of 150 mM PBS (pH 7.4) was added to each well, followed by 25 μl of enzyme mix. The enzyme mix was an aqueous solution containing ampliflu red, horseradish peroxidase, glucose oxidase, and 150 mM sodium phosphate buffer. Samples were measured at 560 nm in a spectrophotometer (accuSkan FC) after incubation at room temperature for 30 min every 5 min for a 20 min interval. Inter-plate variation was 10.22% and intra-plate variation ranged from 6.93 to 12.19%.
Statistical Analyses
To examine effects of ALAN on cortisol release rates in mesocosms, we performed a repeated measure generalized linear mixed model (GLMM) with natural log-transformed cortisol release rates standardized by standard length (pg/mm/h) with treatment as the fixed effect and individual nested in mesocosm as the random effect. When there were significant treatment effects, we used post hoc Tukey’s HSD comparisons. To assess effects of ALAN on G. affinis glucose levels, we used a GLMM with treatment as the fixed effect and mesocosm as the random effect. We used a GLMM to analyze changes in mass and SL over time with treatment and day as fixed effects and individual nested in mesocosm as the random effect. For behavior analyses, we used generalized linear models (LM) with SL and treatment as fixed effects and individual nested in mesocosm as the random effect. To explore differences in survival we ran a Log-Rank survival analysis. We used JMP Pro 14.0.0 (SAS Institute, Inc.) for all analyses.
Protocols and housing were approved by the Institutional Animal Care and Use Committee of Texas State University (IACUC # 83).
Results
Growth, Survival, Cortisol, and Glucose
In the mesocosm experiment, we found no significant effect of ALAN on SL (GLMM: treatment × day: F3,138 = 0.60, p = 0.619) or mass (F3,138 = 0.95, p = 0.42), however, fish in both treatments increased in SL (day: F3,138 = 16.17, p < 0.0001) and lost mass (F3,138 = 78.26, p < 0.0001) over the duration of the experiment. There were no differences in survival between treatments (Log-Rank Survival Analysis: χ2 1 = 0.89, p = 0.35). We found no significant differences in cortisol release rates between treatments across days (GLMM: treatment × day: F3,138 = 2.10, p = 0.10; Table 3 and Figure 2B). As in the lab study, cortisol release rates were higher after agitation compared to baseline for both treatments (day: F3,138 = 4.99, p = 0.003; Figure 2B), indicating the fish could mount a stress response. The random effects of mesocosm were not significant (Wald p-value = 0.12). There were significantly lower glucose levels in the brain tissues of fish from the ALAN treatment than fish from the control treatment (GLMM: treatment: F1,13 = 8.04, p = 0.014; Figure 5), but there was no effect of treatment on the glucose levels in any other tissue type (all p > 0.05; Table 4).
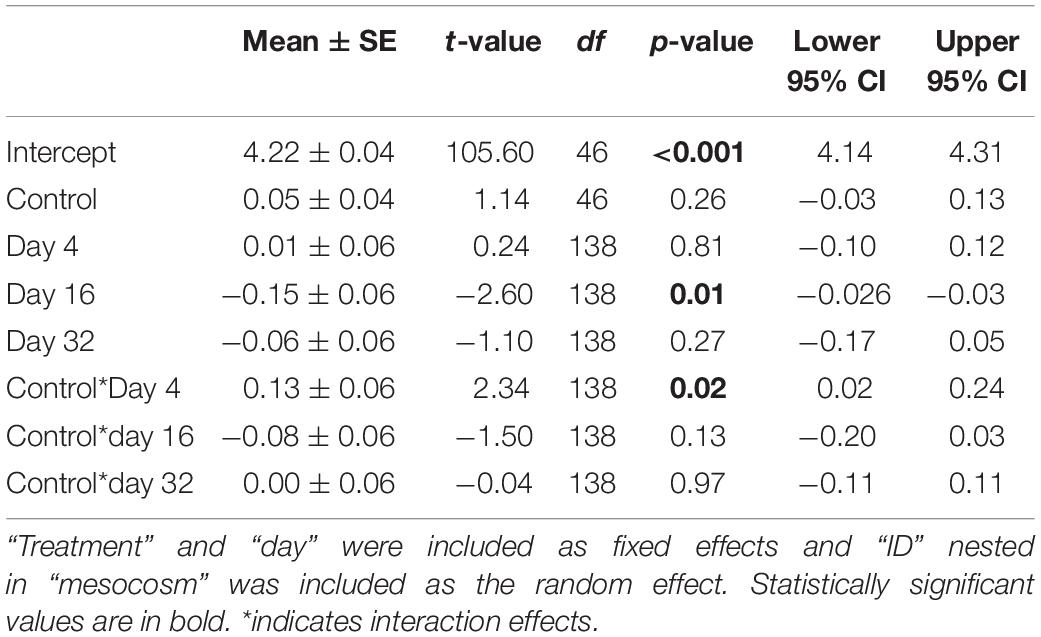
Table 3. Results from the generalized linear mixed model testing the effects of ALAN on cortisol release rates of female G. affinis in mesocosms.
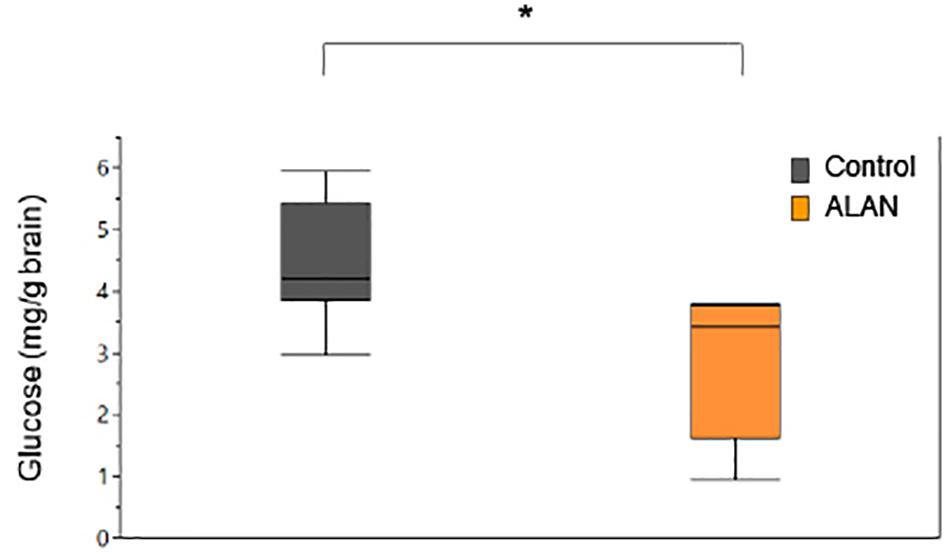
Figure 5. Brain glucose levels (mg/g brain) of female G. affinis. Asterisk indicates a significant difference (p < 0.05). Box plots indicate median, range and first and third quartiles.
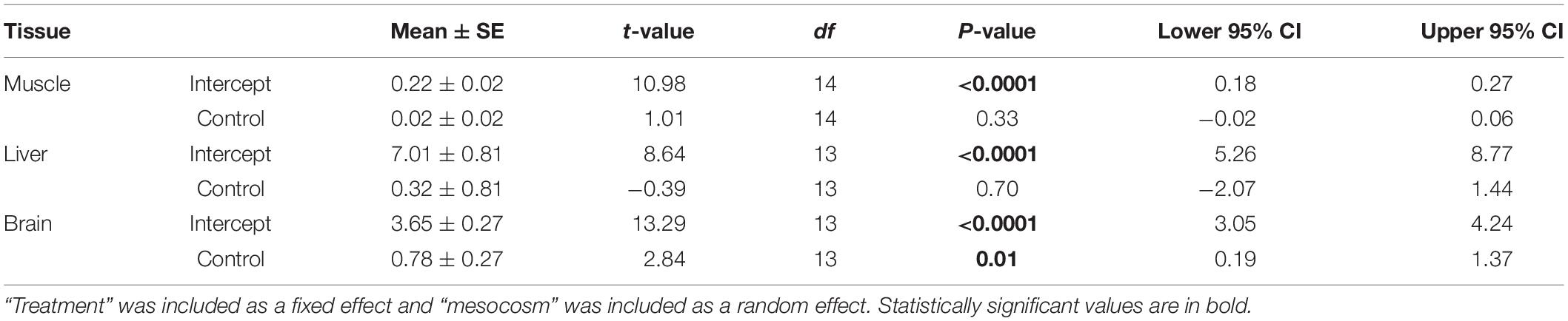
Table 4. Results from the generalized linear mixed model testing the effects of ALAN on glucose levels of female G. affinis tissues in mesocosms.
Hiding Behavior
In the mesocosm experiment, we found no significant differences in time resting, or time spent hiding (all p > 0.05; Table 5) between treatments.
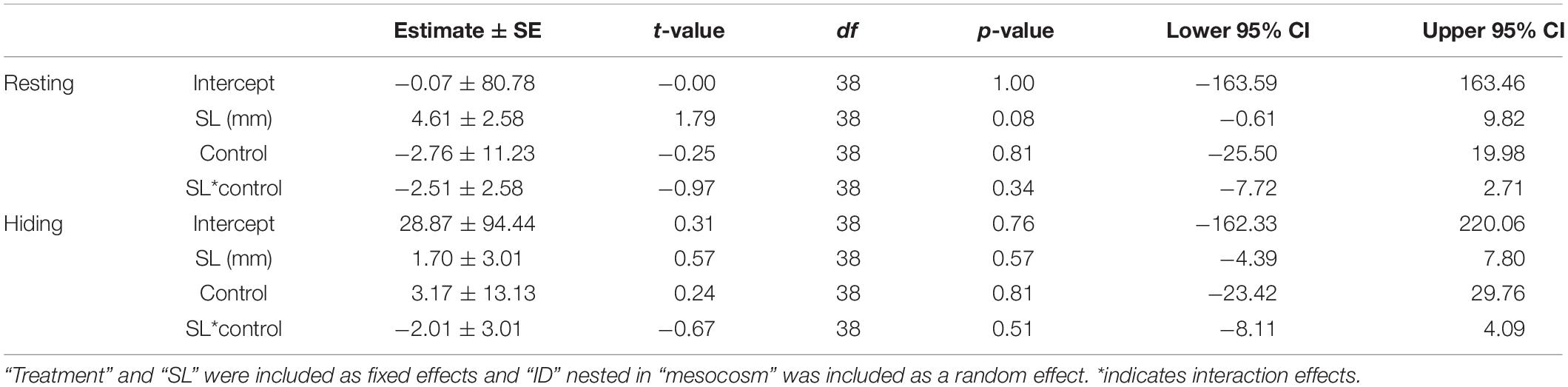
Table 5. Results from the generalized linear mixed model testing the effects of ALAN on behavior of female G. affinis in mesocosms.
Discussion
With the spread of urbanization, light pollution increases world-wide (Hölker et al., 2010; Davies et al., 2014; Falchi et al., 2016) and has the demonstrated potential to negatively impact exposed organisms by affecting their physiology, behavior, and reproduction. We found that the western mosquitofish, G. affinis, a tolerant and widespread species, responds to ALAN by decreasing their activity (both individually and around conspecifics during the day) and exhibiting reduced brain glucose levels compared to those exposed to normal light dark cycles. We did not, however, observe a change in cortisol release rates, growth, survival, or reproduction in response to ALAN. These results suggest that G. affinis may behaviorally adjust to the perturbation of ALAN rather than modulate their regulation of the HPI axis.
The lack of a cortisol response by G. affinis to ALAN may play a role in their success as invaders and establishing populations in water with varying physical properties as they may not experience the negative effects of altered cortisol release rates (Lloyd et al., 1986; Hubbs, 2000). Indeed, G. affinis copes with urban streams by flexibly altering their GC profile by reducing stress responsiveness followed by rapid negative feedback (recovery) (Kolonin et al., unpublished data). When fish from both ALAN and control groups were exposed to an agitation treatment, cortisol release rates were significantly higher than baseline levels, indicating they were capable of mounting a stress response. Therefore, the lack of a significant effect of ALAN on cortisol release rates under laboratory conditions and in mesocosms was not due to a dysregulated HPI axis, signifying that they were not chronically stressed.
In the laboratory experiment, we also measured cortisol release rates on day 2 and found that they were significantly elevated in both treatments as compared to days 0 and 7, suggesting that fish were not acclimated to the experimental setup. By day 7, cortisol release rates returned to baseline in both treatments. In the mesocosm study, we tested the hypothesis that there was a transient cortisol response to ALAN sometime between days 2 and 7 by collecting cortisol release rates on day 4; however, we still did not detect a change in cortisol release rates in response to ALAN. Our results are in concordance with other studies that found a lack of GC response to ALAN using comparable or greater lux levels. For example, Szekeres et al. (2017) found that juvenile bonefish (Albula vulpes) exhibit increased blood glucose in response to ALAN but there was no effect of ALAN on whole body cortisol. Additionally, European perch (Perca fluviatilis) exposed to ALAN had decreased melatonin production compared to the control group, but there were no differences in cortisol release rates between treatments (Brüning et al., 2015). There were no differences in corticosterone levels between control and ALAN-exposed treatments in brown anoles (Anolis sagre; Thawley and Kolbe, 2020) or wood frogs (Lithobates sylvaticus; May et al., 2019). These results suggest there are other mechanisms of coping with stressors (Ouyang et al., 2018) or ALAN may simply not elicit a GC response during the breeding season in many species (Grunst et al., 2019), including G. affinis. The species tested thus far may be more tolerant species as they are abundant, therefore testing less tolerant species might be necessary to fully understand the implications of exposure to ALAN.
Female G. affinis from mesocosms exposed to ALAN had lower glucose levels in their brain tissue compared to fish kept under control light conditions. Similarly, fish showed lower activity and daytime shoaling behavior when exposed to ALAN. Glucose provides precursors for neurotransmitter synthesis as well as ATP production in the brain, therefore the decreased levels of glucose in the brain may be associated with insufficient glucose for the required energetic demands to move (Mergenthaler et al., 2013). Lowered brain glucose could indicate that G. affinis may have diminished neuronal activity when exposed to ALAN. Experiments on the cognitive ability of fish could elucidate whether there are differences in brain function between fish exposed to ALAN and those kept under a natural light cycle.
The activity of G. affinis was overall reduced both individually at night and in shoaling during the day after exposure to ALAN. Fish moved less often at night after 50 days of exposure to ALAN in the laboratory. Because they did not spend more time hiding at night, this could put them at greater risk of predation. This may contribute to a better understanding of the mechanism behind the prior finding that fish exposed to ALAN experienced higher predation rates (O’Connor et al., 2019). This result opposes several other studies which found an increase in activity in fish (Becker et al., 2013; Foster et al., 2016; Kurvers et al., 2018; Czarnecka et al., 2019), American toads (Anaxyrus americanus), zebra finches (Taeniopygia guttata), and anoles (Anolis leachii and A. wattsi: Dananay and Benard, 2018; Batra et al., 2019; Maurer et al., 2019) after exposure to ALAN. May et al. (2019), however, found that Lithobates sylvaticus tadpoles were also less active in the day and night after exposure to ALAN and Buchanan (1993) found that gray treefrogs, Hyla chrysoscelis, reduced foraging activity at night under ALAN. These results suggest that the effects of ALAN vary by species. Female G. affinis also spent less time shoaling during the day after the same exposure to ALAN in the laboratory. Shoaling is beneficial as a defense against predation and generally results in more efficient foraging, therefore a lack of shoaling during the day could leave them more susceptible to predation and affect their ability to find food (Pitcher, 1986; Laland and Williams, 1997). In the control group, there was a clear display of diel shoaling activity where fish were more active and swam in closer proximity during the day than at night. This diel activity pattern disappeared after exposure to ALAN as fish in the treatment group shoaled the same amount during the day (and less than the control) and night. However, we cannot discount the alternative hypothesis that the treatment and control groups differ because the control group fish were in complete dark at night, without their regular lunar cycles. These results align with studies of fish where shoaling was decreased after exposure to other disturbances such as psychotropic drugs and parasites (Tobler and Schlupp, 2008; Green et al., 2012). Overall, in the laboratory, G. affinis exposed to ALAN moved less often at night and shoaled less during the day, which could leave them more susceptible to predation at all times.
Because these fish are found in highly developed urban settings, we chose to additionally run the experiment with fish exposed to slightly higher levels of light than we found in Hays County (230 vs. 246 lux). Gambusia affinis tend to be found in shallow, slow moving water (Hess and Tarzwell, 1942; Casterlin and Reynolds, 1977; Belk and Lydeard, 1994) indicating that they will experience high light levels much of the time. Further, we found that G. affinis move less at night after ALAN exposure and were not hiding, indicating that the fish are unlikely to swim away from or hide when exposed to light at night. Nonetheless, cortisol release rates were not affected by ALAN exposure.
Offspring number and survival did not differ significantly between treatments. Most females did not experience parturition during the experiment and consequently our sample size was low. Offspring counts could have been affected by cannibalism and this species can resorb embryos under suboptimal conditions (Meffe and Vrijenhoek, 1981). Offspring number and survival was not measured in mesocosms because we did not observe any offspring in either treatment during the duration of the experiment. In previous studies, continuous light cycles resulted in earlier hatching and smaller larvae size of haddock, Melanogrammus aeglefinus, embryos, complete failure of embryo hatching in the common clownfish, Amphiprion ocellaris, under a much lower lux level (26 lux), and early-stage pregnancy termination in female white rats, Rattus norvegicus Wistar (Downing and Litvak, 2002; Berbets et al., 2019; Fobert et al., 2019).
One caveat of our experiment is that we were not able to randomize the distribution of the treatments. The blocked treatments and associated spatial differences cannot be ruled out as factors in affecting our results. However, we measured several variables to ensure that there was no “location effect” including water quality measures and fish behavior. While conditions still could have differed in other non-measured variables, we did not anticipate this as all replicates were held in the same room under the same conditions and were handled similarly.
Pervasive effects of ALAN on fish behavior and physiology have previously been reported and here we show ALAN has some of these effects on a tolerant, invasive fish species. Since urbanization is on the rise mitigation efforts are necessary to minimize these impacts (Falchi et al., 2011). In areas where such efforts have already taken place, night light is successfully conserved without compromising human safety or security (Kyba et al., 2015, 2017; Steinbach et al., 2015). Additionally, few experiments have examined how various light color effects different taxa, which could potentially become a mitigation strategy. For example, melatonin rhythm was the most suppressed in European perch by green and red light and less so by blue light (Brüning et al., 2016). Expansion on studies evaluating the consequences of different spectral quality needs to be conducted to combat the negative consequences of increasing light pollution. ALAN did not compromise every variable we measured; however, the reduction of activity and brain glucose in G. affinis could have consequences for organismal fitness and in less tolerant species ALAN could have additional consequences.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Texas State University (IACUC # 83).
Author Contributions
CG, AA, and KM contributed to the conception and design of the study. KM conducted experimental testing and wrote the first draft of the manuscript. MH contributed to experimental design and analysis of glucose. All authors contributed to manuscript revision, read, and approved the final version.
Funding
This work was supported by Texas State University Graduate Research Fellowship and a Sigma Xi Grant in Aid of Research to KM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the GASP lab for assistance in collecting fish and for aid in water-borne hormone collections. We would also like to thank the editors and reviewers for helpful comments and edits.
References
Allan, J. D. (2004). Landscapes and riverscapes: The influence of land use on stream ecosystems. Ann. Rev. Ecol. Evol. Syst. 35, 257–284. doi: 10.1146/annurev.ecolsys.35.120202.110122
Balian, E. V., Segers, H., Leveque, C., and Martens, K. (2008). An introduction to the freshwater animal diversity assessment (FADA) project. Hydrobiologia 595, 3–8. doi: 10.1007/978-1-4020-8259-7_1
Barbier, E. B. (2012). Progress and challenges in valuing coastal and marine ecosystem services. Rev. Env. Econom. Policy 6, 1–19. doi: 10.1093/reep/rer017
Batra, T., Malik, I., and Kumar, V. (2019). Illuminated night alters behaviour and negatively affects physiology and metabolism in diurnal zebra finches. Env. Pollut. 254:112916. doi: 10.1016/j.envpol.2019.07.084
Becker, A., Whitfield, A. K., Cowley, P. D., Järnegren, J., and Næsje, T. F. (2013). Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 50, 43–50. doi: 10.1111/1365-2664.12024
Belk, M. C., and Lydeard, C. (1994). Effect of Gambusia holbrooki on a similar-sized, syntopic poeciliid, Heterandria formosa: Competitor or Predator? Copeia 1994, 296–302. doi: 10.2307/1446979
Berbets, A. M., Barbe, A. M., and Yuzko, O. M. (2019). Constant light exposure terminates pregnancy in rats with pineal gland dysfunction, low melatonin level and pro-inflammatory response. Melatonin Res. 2, 9–24. doi: 10.32794/mr11250038
Bethke, P. C., and Busse, J. C. (2008). Validation of a simple, colorimetric, microplate assay using Amplex red for the determination of glucose and sucrose in potato tubers and other vegetables. Am. J. Potato Res. 85, 414–421. doi: 10.1007/s12230-008-9039-x
Blake, C. A., Alberici, L., Guenther, J. E., and Gabor, C. R. (2014). Recognition and response to native and novel predators in the largespring mosquitofish. Gambusia geiseri. Ethology 121, 1–9.
Blume, C., Garbazza, C., and Spitschan, M. (2019). Effects of light on human circadian rhythms, sleep and mood. Somnologie 23, 147–156. doi: 10.1007/s11818-019-00215-x
Bolton, D., Mayer-Pinto, M., Clark, G. F., Dafforn, K. A., Brassil, W. A., Becker, A., et al. (2017). Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 576, 1–9. doi: 10.1016/j.scitotenv.2016.10.037
Bonier, F., Martin, P. R., Moore, I. T., and Wingfield, J. C. (2009). Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. doi: 10.1016/j.tree.2009.04.013
Brüning, A., Hölker, F., Franke, S., Preuer, T., and Kloas, W. (2015). Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total Environ. 511, 516–522. doi: 10.1016/j.scitotenv.2014.12.094
Brüning, A., Hölker, F., Franke, S., Kleiner, W., and Kloas, W. (2016). Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total Env. 543, 214–222. doi: 10.1016/j.scitotenv.2015.11.023
Brüning, A., Kloas, W., Preuer, T., and Hölker, F. (2018). Influence of artificially induced light pollution on the hormone system of two common fish species, perch and roach, in a rural habitat. Conserv. Physiol. 6, 1–12.
Buchanan, B. W. (1993). Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim. Behav. 45, 893–899. doi: 10.1006/anbe.1993.1109
Buxton, R. T., McKenna, M. F., Mennitt, D., Fristrup, K., Crooks, K., Angeloni, L., et al. (2017). Noise pollution is pervasive in U.S. protected areas. Science 356, 531–533. doi: 10.1126/science.aah4783
Casterlin, M. E., and Reynolds, W. W. (1977). Aspects of habitat selection in the mosquitofish Gambusia affinis. Hydrobiologia 55, 125–127. doi: 10.1007/bf00021053
Cazan, A. M., and Klerks, P. L. (2015). Effects from a short-term exposure to copper or cadmium in gravid females of the livebearer fish (Gambusia affinis). Ecotoxicol. Environ. Safety 118, 199–203. doi: 10.1016/j.ecoenv.2015.04.039
Cherry, D. S., Guthrie, R. K., Rodgers, J. H., Cairns, J., and Dickson, L. (1976). Responses of mosquitofish (Gambusia affinis) to ash effluent and thermal stress. Transac. Am. Fisheries Soc. 105, 686–694. doi: 10.1577/1548-8659(1976)105<686:romgat>2.0.co;2
Cinzano, P., Falchi, F., and Elvidge, C. D. (2001). The first world atlas of the artificial night sky brightness. North 707, 689–707. doi: 10.1046/j.1365-8711.2001.04882.x
Czarnecka, M., Kakareko, T., Jermacz, Ł, Pawlak, R., and Kobak, J. (2019). Combined effects of nocturnal exposure to artificial light and habitat complexity on fish foraging. Sci. Total Environ. 684, 14–22. doi: 10.1016/j.scitotenv.2019.05.280
Dananay, K. L., and Benard, M. F. (2018). Artificial light at night decreases metamorphic duration and juvenile growth in a widespread amphibian. Proc. R. Soc. B 285:20180367. doi: 10.1098/rspb.2018.0367
Davies, T. W., Duffy, J. P., Bennie, J., and Gaston, K. J. (2014). The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Evol. 12:347–355. doi: 10.1890/130281
Depledge, M. H., Godard-Codding, C. A. J., and Bowen, R. E. (2010). Light pollution in the sea. Mar. Pollut. Bull. 60, 1383–1385. doi: 10.1016/j.marpolbul.2010.08.002
Dhabhar, F. S. (2009). A hassle a day may keep the pathogens away: The fight-or-flight stress response and the augmentation of immune function. Integr. Comparat. Biol. 49, 215–236. doi: 10.1093/icb/icp045
Dhabhar, F. S., and McEwen, B. S. (1996). Stress-induced enhancement of antigen-specific cell-mediated immunity. J. Immunol. 156, 2608–2615.
Dhabhar, F. S., Miller, A. H., McEwen, B. S., and Spencer, R. L. (1995). Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J. Immunol. 154, 5511–5527.
Dhabhar, F. S., and Viswanathan, K. (2005). Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am. J. Physiol. 289, R738–R744.
Dickens, M. J., and Romero, L. M. (2013). A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comparat. Endocrinol. 191, 177–189. doi: 10.1016/j.ygcen.2013.06.014
Downing, G., and Litvak, M. K. (2002). Effects of light intensity, spectral composition and photoperiod on development and hatching of haddock (Melanogrammus aeglefinus) embryos. Aquaculture 213, 265–278. doi: 10.1016/s0044-8486(02)00090-x
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philosoph. Soc. 81, 163–182.
Ellis, E. C. (2011). Anthropogenic transformation of the terrestrial biosphere. Philosoph. Transac. R Soc. A 369, 1010–1035. doi: 10.1098/rsta.2010.0331
Falchi, F., Cinzano, P., Duriscoe, D., Kyba, C. C. M., Elvidge, C. D., Baugh, K., et al. (2016). The new world atlas of artificial night sky brightness. Science Advances 2, 1–26.
Falchi, F., Cinzano, P., Elvidge, C. D., Keith, D. M., and Haim, A. (2011). Limiting the impact of light pollution on human health, environment and stellar visibility. J. Env. Manag. 92, 2714–2722. doi: 10.1016/j.jenvman.2011.06.029
Fobert, E. K., Burke, da Silva, K., and Swearer, S. E. (2019). Artificial light at night causes reproductive failure in clownfish. Biol. Lett. 15:20190272. doi: 10.1098/rsbl.2019.0272
Foster, J. G., Algera, D. A., Brownscombe, J. W., Zolderdo, A. J., and Cooke, S. J. (2016). Consequences of different types of littoral zone light pollution on the parental care behaviour of a freshwater teleost fish. Water, Air Soil Pollut. 227:404.
Gabor, C. R., Davis, D. R., Kim, D. S., Zabierek, K. C., and Bendik, N. F. (2018). Urbanization is associated with elevated corticosterone in Jollyville Plateau salamanders. Ecol. Indicat. 85, 229–235. doi: 10.1016/j.ecolind.2017.10.047
Gabor, C. R., Zabierek, K. C., Kim, D. S., da Barbiano, L. A., Mondelli, M. J., Bendik, N. F., et al. (2016). A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia 104, 172–181. doi: 10.1643/ot-14-207
Gaston, K. J., Bennie, J., Davies, T. W., and Hopkins, J. (2013). The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 88, 912–927. doi: 10.1111/brv.12036
Gaston, K. J., Davies, T. W., Bennie, J., and Hopkins, J. (2012). Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 49, 1256–1266. doi: 10.1111/j.1365-2664.2012.02212.x
Gaston, K. J., Davies, T. W., Nedelec, S. L., and Holt, L. A. (2017). Impacts of artificial light at night on biological timings. Ann. Rev. Ecol. Evol. Syst. 48, 49–68. doi: 10.1146/annurev-ecolsys-110316-022745
Gaston, K. J., Duffy, J. P., Gaston, S., Bennie, J., and Davies, T. W. (2014). Human alteration of natural light cycles: causes and ecological consequences. Oecologia 176, 917–931. doi: 10.1007/s00442-014-3088-2
Gaston, K. J., Visser, M. E., and Holker, F. (2015). The biological impacts of artificial light at night: the research challenge. Philosoph. Transac.R Soc. B 370, 20140133–20140133. doi: 10.1098/rstb.2014.0133
Green, J., Collins, C., Kyzar, E. J., Pham, M., Roth, A., Gaikwad, S., et al. (2012). Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 210, 266–271. doi: 10.1016/j.jneumeth.2012.07.017
Grunst, M. L., Raap, T., Grunst, A. S., Pinxten, R., and Eens, M. (2019). Artificial light at night does not affect telomere shortening in a developing free-living songbird: A field experiment. Sci. Total Environ. 662, 266–275. doi: 10.1016/j.scitotenv.2018.12.469
Helm, B., Ben-Shlomo, R., Sheriff, M. J., Hut, R. A., Foster, R., Barnes, B. M., et al. (2013). Annual rhythms that underlie phenology: Biological time-keeping meets environmental change. Proc. R Soc. B 280:20130016. doi: 10.1098/rspb.2013.0016
Hess, A. D., and Tarzwell, C. M. (1942). The feeding habits of Gambusia affinis affnis, with special reference to the malaria mosquito, Anopheles quadrimaculatus. Am. J. Epidemiol. 35, 142–151. doi: 10.1093/oxfordjournals.aje.a118781
Hölker, F., Moss, T., Griefahn, B., Kloas, W., and Voigt, C. C. (2010). The dark side of light: A transdisciplinary research agenda for light. Ecol. Soc. 15:13.
Hopkins, W. A., Mendonça, M. T., and Congdon, J. D. (1997). Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gen. Comparat. Endocrinol. 108, 237–246. doi: 10.1006/gcen.1997.6969
Hubbs, C. (2000). Survival of Gambusia affinis in a hostile environment. Southwest. Natural. 45, 521–522. doi: 10.2307/3672601
Jechow, A., and Holker, F. (2019). How dark is a river? Artificial light at night in aquatic systems and the need for comprehensive night-time light measurements. Ecohydrology 6, 1–19. doi: 10.1142/9789812834423_0001
Kurvers, R. H. J. M., Ddrägestein, J., Hölker, F., Jechow, A., Krause, J., and Bierbach, D. (2018). Artificial light at night affects emergence from a refuge and space use in guppies. Sci. Rep. 8:14131.
King, G. D., Chapman, J. M., Cooke, S. J., and Suski, C. D. (2016). Stress in the neighborhood: Tissue glucocorticoids relative to stream quality for five species of fish. Sci. Total Environ. 547, 87–94. doi: 10.1016/j.scitotenv.2015.12.116
Kummu, M., de Moel, H., Ward, P. J., and Varis, O. (2011). How close do we live to water? a global analysis of population distance to freshwater bodies. PLoS One 6:e20578. doi: 10.1371/journal.pone.0020578
Kyba, C. C. M., Kuester, T., De Miguel, A. S., Baugh, K., Jechow, A., Hölker, F., et al. (2017). Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 3, 1–9.
Kyba, C. C. M., Tong, K. P., Bennie, J., Birriel, I., Birriel, J. J., Cool, A., et al. (2015). Worldwide variations in artificial skyglow. Sci. Rep. 5:8409.
Laland, K. N., and Williams, K. (1997). Shoaling generates social learning of foraging information in guppies. Anim. Behav. 53, 1161–1169. doi: 10.1006/anbe.1996.0318
Le, P. P., Friedman, J. R., Schug, J., Brestelli, J. E., Parker, J. B., Bochkis, I. M., et al. (2005). Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genetics 1:0159–0170. doi: 10.1371/journal.pgen.0010016
Lloyd, L. N., Arthington, A. H., and Milton, D. A. (1986). The mosquitofish—a valuable mosquito-control agent or a pest. Ecol. Exotic Plants Anim. Aust. 1986, 7–25.
Longcore, T., and Rich, C. (2004). Ecological light pollution. Front. Ecol. Environ. 2:191–198. doi: 10.1890/1540-92952004002[0191
May, D., Shidemantle, G., Melnick-Kelley, Q., Crane, K., and Hua, J. (2019). The effect of intensified illuminance and artificial light at night on fitness and susceptibility to abiotic and biotic stressors. Environ. Pollut. 251, 600–608. doi: 10.1016/j.envpol.2019.05.016
Maurer, A. S., Thawley, C. J., Fireman, A. L., Giery, S. T., and Stroud, J. T. (2019). Nocturnal activity of antiguan lizards under artificial light. Herpetol. Conserv. Biol. 14, 105–110.
Meffe, G. K., and Vrijenhoek, R. C. (1981). Starvation stress and intraovarian cannibalism in livebearers (Atheriniformes: Poeciliidae). Am. Soc. Ichthyol. Herpetol. 1981, 702–705. doi: 10.2307/1444578
Mehner, T. (2012). Diel vertical migration of freshwater fishes - proximate triggers, ultimate causes and research perspectives. Freshwater Biol. 57, 1342–1359. doi: 10.1111/j.1365-2427.2012.02811.x
Mergenthaler, P., Lindauer, U., Dienel, G. A., and Meisel, A. (2013). Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosciences 36, 587–597. doi: 10.1016/j.tins.2013.07.001
Navara, K. J., and Nelson, R. J. (2007). The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224. doi: 10.1111/j.1600-079x.2007.00473.x
O’Connor, J. J., Fobert, E. K., Besson, M., Jacob, H., and Lecchini, D. (2019). Live fast, die young: Behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar. Pollut. Bull. 146, 908–914. doi: 10.1016/j.marpolbul.2019.05.038
Ouyang, J. Q., Davies, S., and Dominoni, D. (2018). Hormonally mediated effects of artificial light at night on behavior and fitness: linking endocrine mechanisms with function. J. Exp. Biol. 221:156893.
Ouyang, J. Q., Sharp, P. J., Dawson, A., Quetting, M., and Hau, M. (2011). Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R Soc. B 278, 2537–2545. doi: 10.1098/rspb.2010.2490
Page, L. M., and Burr, B. M. (2011). Peterson field guide to freshwater fishes of North America north of Mexico. Mexico: Houghton Mifflin Harcourt.
Perkin, E. K., Hölker, F., Richardson, J. S., Sadler, J. P., Wolter, C., and Tockner, K. (2011). The influence of artificial light on stream and riparian ecosystems: Questions, challenges, and perspectives. Ecosphere 2, 1–16. doi: 10.1007/s10021-016-0056-1
Pitcher, T. J. (1986). Functions of shoaling behaviour in teleosts. In The behaviour of teleost fishes. Boston, MA: Springer, 294–337.
Pyke, G. H. (2005). A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fisher. 15, 339–365. doi: 10.1007/s11160-006-6394-x
Pyke, G. H. (2008). Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Ann. Rev. Ecol. Evol. Syst. 39, 171–191. doi: 10.1146/annurev.ecolsys.39.110707.173451
Ricciardi, A., and Rasmussen, J. B. (1999). Extinction rates of North American freshwater fauna. Conserv. Biol. 13, 1220–1222. doi: 10.1046/j.1523-1739.1999.98380.x
Ryu, H. S., Song, J. A., Park, H. S., Choi, Y. J., and Choi, C. Y. (2019). Physiological and oxidative stress response of goldfish Carassius auratus induced by a light dimming system. Fish Physiol. Biochem. 2019, 1–11.
Romero, L. M. (2004). Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. doi: 10.1016/j.tree.2004.03.008
Sanders, D., and Gaston, K. J. (2018). How ecological communities respond to artificial light at night. J. Exp. Zool. A 329, 1–7.
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000). How do glucocorticoids influence stress responses? Preparative actions. Endocrine Rev. 21, 55–89. doi: 10.1210/er.21.1.55
Scott, A. P., Hirschenhauser, K., Bender, N., Oliveira, R., Earley, R. L., Sebire, M., et al. (2008). Non-invasive measurement of steroids in fish-holding water: important considerations when applying the procedure to behaviour studies. Behaviour 145, 1307–1328. doi: 10.1163/156853908785765854
Shannon, G., McKenna, M. F., Angeloni, L. M., Crooks, K. R., Fristrup, K. M., Brown, E., et al. (2016). A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. doi: 10.1111/brv.12207
Sordello, R., De Lachapelle, F. F., Livoreil, B., and Vanpeene, S. (2019). Evidence of the environmental impact of noise pollution on biodiversity: A systematic map protocol. Env. Evid. 8, 1–7.
Steinbach, R., Perkins, C., Tompson, L., Johnson, S., Armstrong, B., Green, J., et al. (2015). The effect of reduced street lighting on road casualties and crime in England and Wales: Controlled interrupted time series analysis. J. Epidemiol. Commun. Health 69, 1118–1124. doi: 10.1136/jech-2015-206012
Swaddle, J. P., Francis, C. D., Barber, J. R., Cooper, C. B., Kyba, C. C. M., Dominoni, D. M., et al. (2015). A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. doi: 10.1016/j.tree.2015.06.009
Szekeres, P., Wilson, A. D. M., Haak, C. R., Danylchuk, A. J., Brownscombe, J. W., Elvidge, C. K., et al. (2017). Does coastal light pollution alter the nocturnal behavior and blood physiology of juvenile bonefish (Albula vulpes)? Bulletin Mar. Sci. 93, 491–505. doi: 10.5343/bms.2016.1061
Tobler, M., and Schlupp, I. (2008). Influence of black spot disease on shoaling behaviour in female western mosquitofish, Gambusia affinis (Poeciliidae, Teleostei). Environ. Biol. Fish. 81, 29–34. doi: 10.1007/s10641-006-9153-x
Thawley, C. J., and Kolbe, J. J. (2020). Artificial light at night increases growth and reproductive output in anolis lizards. Proc. R Soc. B 287:20191682. doi: 10.1098/rspb.2019.1682
Turner, C. L. (1937). Reproductive cycles and superfetation in Poeciliid fishes. Biol. Bulletin 72, 145–164. doi: 10.2307/1537249
Venohr, M., Langhans, S. D., Peters, O., Hölker, F., Arlinghaus, R., Mitchell, L., et al. (2018). The underestimated dynamics and impacts of water-based recreational activities on freshwater ecosystems. Env. Rev. 26, 199–213. doi: 10.1139/er-2017-0024
Viswanathan, K., Daugherty, C., and Dhabhar, F. S. (2005). Stress as an endogenous adjuvant: Augmentation of the immunization phase of cell-mediated immunity. Internat. Immunol. 17, 1059–1069. doi: 10.1093/intimm/dxh286
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., et al. (2010). Global threats to human water security and river biodiversity. Nature 467, 555–561. doi: 10.1038/nature09440
Keywords: Gambusia affinis, artificial light at night, corticosterone, urban stream syndrome, poecilidae, fish physiology
Citation: Miner KA, Huertas M, Aspbury AS and Gabor CR (2021) Artificial Light at Night Alters the Physiology and Behavior of Western Mosquitofish (Gambusia affinis). Front. Ecol. Evol. 9:617063. doi: 10.3389/fevo.2021.617063
Received: 13 October 2020; Accepted: 27 January 2021;
Published: 18 February 2021.
Edited by:
Jordi Figuerola, Estación Biológica de Doñana (EBD), SpainReviewed by:
Emily Fobert, The University of Melbourne, AustraliaMichael J. Pauers, Milwaukee Public Museum, United States
Copyright © 2021 Miner, Huertas, Aspbury and Gabor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea S. Aspbury, YXNwYnVyeUB0eHN0YXRlLmVkdQ==
 Krystie A. Miner
Krystie A. Miner Mar Huertas
Mar Huertas Andrea S. Aspbury
Andrea S. Aspbury Caitlin R. Gabor
Caitlin R. Gabor