- 1Department of Biology and Geosciences, Graduate School of Science, Osaka City University, Osaka, Japan
- 2Research Fellow of Japan Society for the Promotion of Science, Tokyo, Japan
- 3Department of Psychology, Graduate School of Letters, Kyoto University, Kyoto, Japan
Animals adjust their behaviors based on information from multiple sources; however, the brain can effectively process limited amounts of information. Therefore, attention is restricted to a small portion of environmental stimuli. When animals process multiple information inputs, focusing on information that is deemed important improves detection probability. However, selective focus limits attention to other stimuli and associated behavioral responses. In this study, we examined how Tanganyikan cichlid, Neolamprologus furcifer, mothers selectively attack intruder fishes depending on the threat level and presence or absence of offspring. Species composition is complicated in Lake Tanganyika, and fish density is exceedingly high. Thus, parents must focus on high-threat-level intruders according to their parental care stage. Compared to females without offspring, mothers preferentially attacked carnivorous fishes farther from the nest over closer scale-eating fishes. Moreover, the percentage of females with injuries from scale-eating fish was significantly higher in those caring for offspring than those without offspring, demonstrating the cost of limited attention. Our results show that females focus on the early detection of carnivorous fishes because these predators dart in from long distances to forage eggs, fry, and juveniles, but this selective focus limits the attention placed on low-level threats. This study is the first to document the cost of limited attention in parents guarding offspring.
Introduction
Animals gather information from the environment and often respond by altering their behaviors (Shettleworth, 2010). However, the brain can process a limited amount of information at any given time. Therefore, animals pay attention to and act on a subset of the available environmental information (Dukas, 2002, 2004). Selective attention, i.e., selectively focusing attention on information that is deemed important, has been shown to improve detection probability (Dukas, 2008). Employing a selective attention strategy is beneficial to animals, especially those inhabiting complex and stimuli-rich environments (Dukas and Ellner, 1993). However, selective attention may limit other adaptive behaviors; previous studies have reported decreases in anti-predator vigilance in animals attending to feeding or conspecific intruders (e.g., Kaby and Lind, 2003; Jones and Godin, 2010; Yee et al., 2013; Hess et al., 2016; Ota, 2018).
Focusing on essential information is also crucial for parents guarding their offspring. Offspring guarding (brood defense) is recognized in many species, and successful defense strategies increase reproductive success (e.g., Ridely, 1978; Sinn et al., 2008). However, aggressive responses to non-predators incur energy costs and may provide opportunities for predators. Thus, parents should selectively attack depending on the threat level (Matsumoto and Kohda, 2004). Carnivorous fishes dart toward nests from long distances to attack eggs, fry, and juveniles; some cichlid parents selectively attack carnivorous fishes at greater distances from the nest to protect offspring (Nakano and Nagoshi, 1990; Ochi and Yanagisawa, 1998; Oliver and Watson, 1999). As the distance from the nest increases, rapid and accurate detection of predators becomes more difficult (Carlson, 1985). Selective attention toward carnivorous fishes may prevent the detection of fishes that attack from shorter distances. Limited attention constraints can be assessed by comparing the attack distances of parents guarding offspring and those without offspring, but evaluations of the attack distance of parents without offspring have been limited.
To test the cost of limited attention, we investigated the responses of an African cichlid (Neolamprologus furcifer) with and without offspring to different intruder species. N. furcifer is endemic to Lake Tanganyika, breeding and foraging for small shrimp on shaded vertical or overhanging flat surfaces of large rocks (Yanagisawa, 1987; Satoh et al., 2017, 2018). These fish display harem polygyny mating, with large males (10–13 cm standard length [SL]) visiting and monopolizing multiple females (8–10 cm SL). Females spawn clutches of ∼100 eggs within their feeding territories and care for the offspring within their breeding territories (Yanagisawa, 1987). The eggs will hatch a minimum of 3 days after spawning. Newly hatched larvae adhere to the rock surface for 4 days as they develop into 5.2-mm juvenile (Yanagisawa, 1987). Juvenile initially feed on floating plankton and begin to forage for benthic prey, mostly small shrimp, after 2 weeks (Satoh et al., 2018). Juvenile remain in the female foraging territory, where they are guarded by the female parent, for approximately 9 weeks after hatching. Juvenile begin to distance themselves from one another when they reach an SL of ∼10 mm (Yanagisawa, 1987).
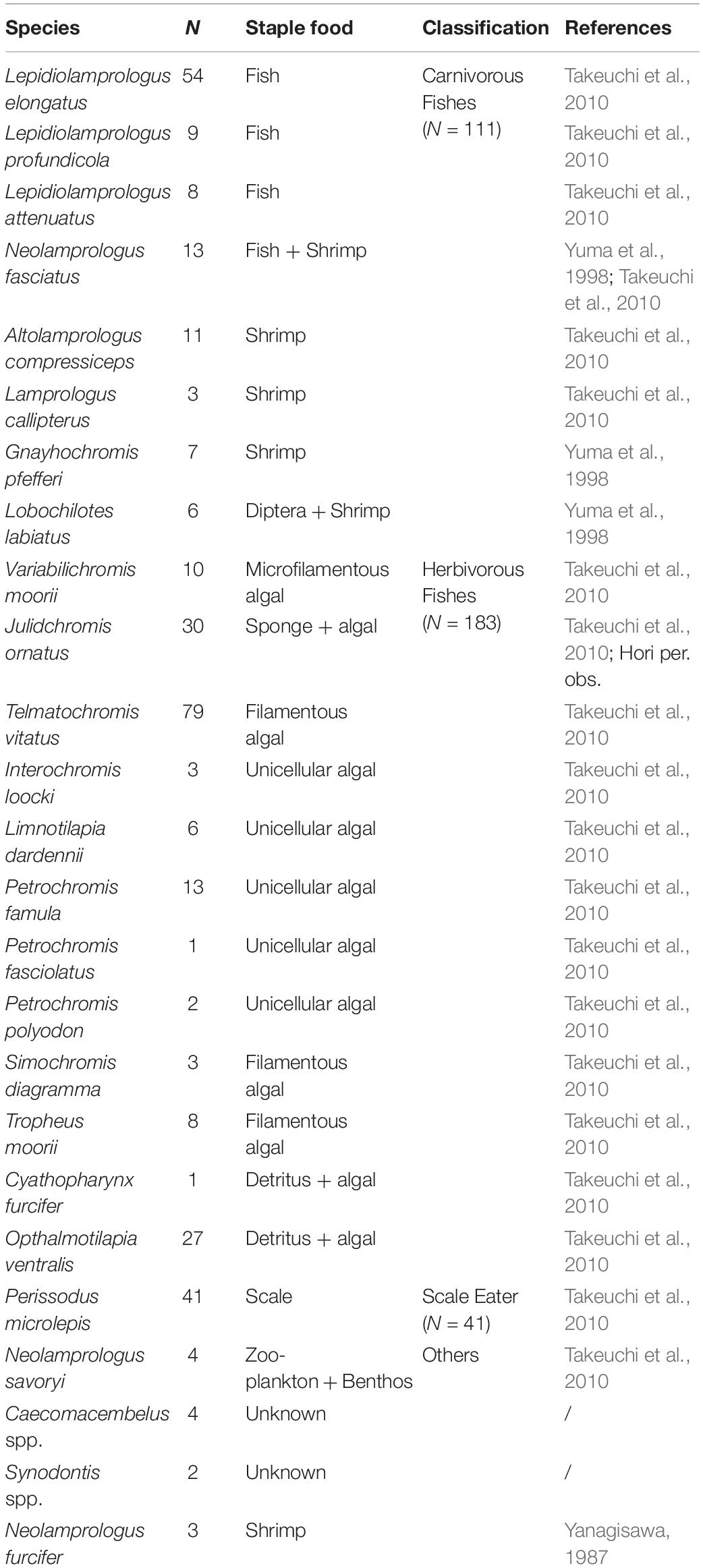
More than 250 fish species are recognized in Lake Tanganyika (Brichard, 1978). Fish density is exceedingly high and the species composition is complex, especially in the littoral zone (Hori, 1983). We measured the distance from the nest at which N. furcifer females attack intruders when faced with multiple stimuli at each parental care stage (no offspring, eggs and adhered fry, juvenile). Because N. furcifer and other Lamprologine cichlids are exposed to a large diversity of predators, we classified intruders into three categories based on feeding type, namely, carnivorous fishes, herbivorous fishes, and scale-eating fishes (see Table 1; Hori, 1983; Takeuchi et al., 2010). The threat-level varies across the categories. When females do not have offspring, carnivorous fishes such as Lepidiolamprologus elongatus and Nosopsyllus fasciatus, are the priority for attack because they compete with N. furcifer for food resources. When females are caring for offspring, the threat level of carnivorous fishes increases because these fish also attack nests and feed on eggs and juvenile (Hori, 1983; Nakano and Nagoshi, 1990). Thus, N. furcifer females are predicted to attack carnivorous fishes from further distances when guarding offspring. In contrast, scale-eating fishes such as Perissodus microlepis remain a threat, regardless of the parental care stage; this species rarely consumes eggs and juvenile, but is a direct threat to adult females (Nshombo et al., 1985). Alternatively, herbivorous fishes do not pose a threat, regardless of the parental care stage. If selective attention toward carnivorous fishes limits parental attention toward lower threat-level intruders, females guarding offspring may attack herbivorous and scale-eating fishes closer to the nest. If selective attention does not constrain attention, the attack distance toward herbivorous and scale-eating fishes will not change, regardless of the attack distance toward carnivorous fishes.
Materials and Methods
Fieldwork
Behavioral observations were recorded from October 7 to December 14, 2014, by SCUBA diving in the rocky area at Wonzye Point (depth, 3–8 m; 8.430°S, 31.080°E), near Mpulungu, Republic of Zambia. Two adjacent study areas (20 × 20 m and 20 × 50 m, divided into 2 × 2 m grids) were established at this location. All breeding N. furcifer females in these areas were identified by natural markings and body size (SL). We then haphazardly chose seven breeding females for observation (brood size: 9–63 fry). Each nest was monitored for eggs and newly hatched fry five times per week. When we discovered eggs in a nest, we estimated the spawning date based on egg coloration (Yanagisawa, 1987; Satoh, personal observation). We categorized each nest by parental care stages: egg and adhered fry stage (0–5 days after spawning (DAS), n = 4 nests), juvenile stage (≥6 DAS, n = 6 nests), and offspring absent stage (∼7 day after offspring dispersal, n = 7 nests) (Yanagisawa, 1987; Satoh et al., 2018).
We installed underwater video cameras (HDR-GWP88; Sony, Japan) to record the aggressive behavior of N. furcifer. Nests (Figure 1A) were recorded for 30 min; one camera provided a lateral view (Figure 1B), while the other simultaneously provided an overhead view (Figure 1C). These view angles are most appropriate for vertical rock surfaces (Yanagisawa, 1987). We observed the N. furcifer behavior for a total of 510 min. We concurrently identified intruder species with video recording because video identification can be difficult. Intruder fish species were classified as carnivorous, herbivorous, or scale-eating following Hori (1983) and Takeuchi et al. (2010) (Table 1).
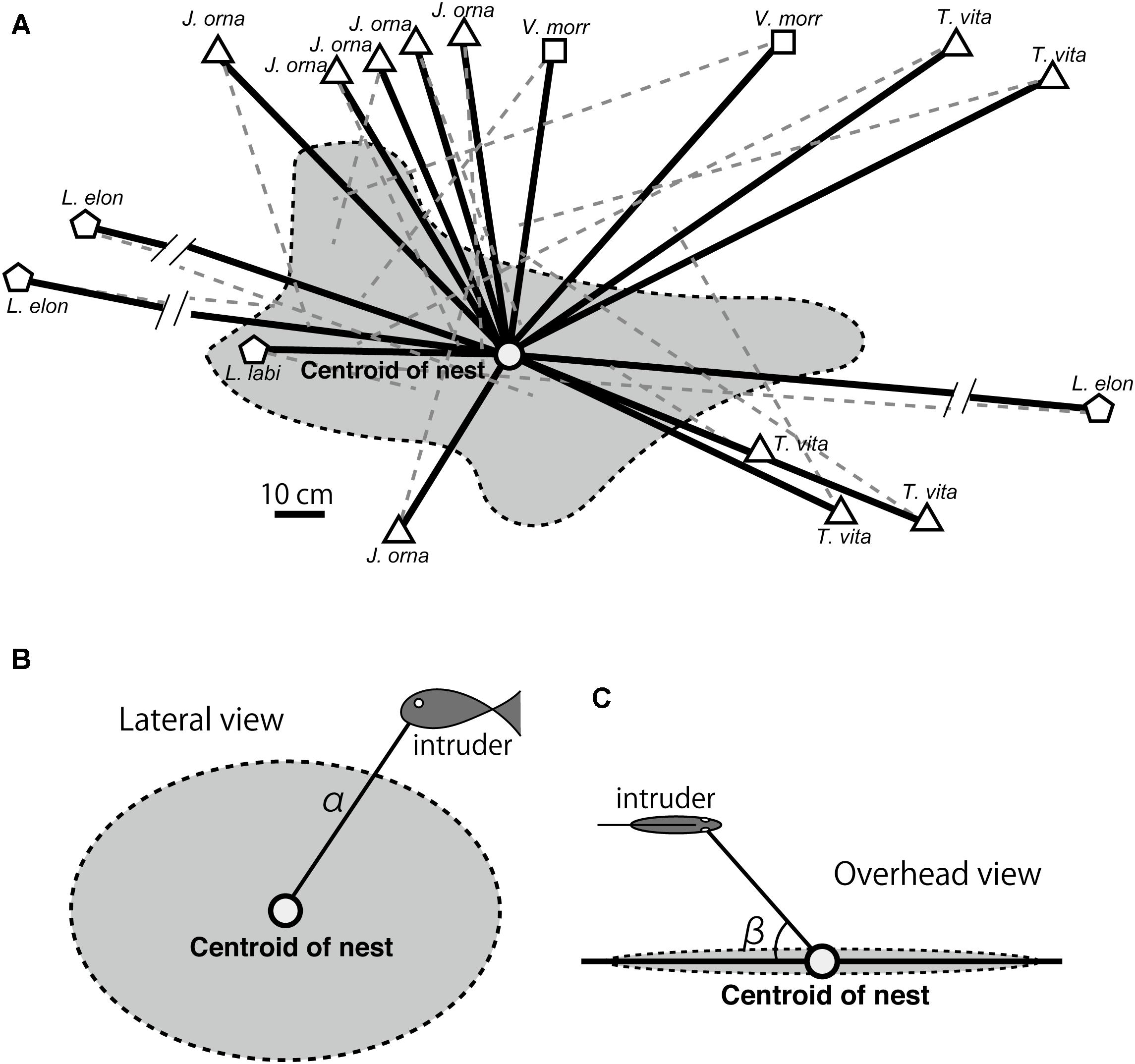
Figure 1. Neolamprologus furcifer nest depiction. The nest area is shown in gray and the center of the nest is marked with a circle. Solid black lines show attack distance; broken gray lines represent the distance between the female and the intruder when the female began to behave aggressively. (A) Depiction of Neolamprologus furcifer female brood defense behavior against Julidochromis ornatus; Variabilichromis moori; Telmatochromis vittatus; Lepidiolamprologus elongatus; Lobochilotus labiatus. (B) Depictions of the lateral and (C) overhead views of the nest recorded using video cameras. The attack distances were estimated using the following formula: attack distance = α/(cosβ).
To test whether N. furcifer adults received more attacks from scale-eating fish while caring for offspring, we haphazardly chose 14 breeding and 45 non-breeding adult females within the study area and observed whether they had wounds from scale-eating fish. The presence of scale-eating wounds can be easily distinguished by the white coloration that appears on the body surface where scales have been removed (Satoh, personal observation).
Behavioral Recording
The center of the nest was determined from the position of the eggs and adhered fry. When juvenile began free-swimming, the centroid of the area that juveniles used for forage was designated as the nest center (Satoh et al., 2018; Figure 1A). We recorded the distribution of all juvenile for 30 min to estimate the centroid of the nest. The centroid was estimated using a function in ImageJ1. The juvenile-forage-area centroid was also considered the center of the nest when offspring are absent, as the female uses the same area for foraging. The nest center position may fluctuate throughout the stages of parental care; however, the center positions did not vary greatly in this study (Satoh, personal observation).
The attack distance was defined as the distance between the center of the nest and the position of the intruder’s head when the focal female began to display territorial aggression behaviors. Video data obtained from two cameras were analyzed frame by frame, and horizontal distances α (Figure 1B) and angles β (Figure 1C) were measured using ImageJ. The attack distance was calculated from these measurements using the following equation: attack distance = α/(cosβ).
We excluded 41 events from the sequential analysis: three events in which the intruder fish showed counter-aggressive behavior toward the female nest-holder, e.g., frontal display or mouth fighting (n = 2 offspring absent; n = 1 eggs and adhered fry stage); 15 events where the correct position of the intruder fish could not be determined from the videos (n = 4 offspring absent; n = 1 eggs and adhered fry stage; n = 10 juvenile stage); 23 events in which the intruder fish invaded the nest just after the female attacked another intruder fish (n = 23 juvenile stage). For the first situation, we cannot measure correct attack distance because intruder fish approached when females dashed toward the intruder. The third situation was excluded because it was not that parents did not attack up to that point and it was difficult to treat that distance as other cases. Thus, we analyzed 336 events in this study.
Statistical Analyses
All statistical analyses were performed using R version 3.1.1 (R Core Development Team). The log-transformed attack distance (Kolmogorov–Smirnov test, D = 0.064, p = 0.565) was fitted as the response term in our linear mixed model (LMM); nest ID and observation date were fitted as random factors. The intruder fish type and parental care stage were fitted as fixed factors, and the two-way interaction between these parameters was also assessed. If females divide their attention based on the intruding species type and parental care stage, a significant two-way interaction is expected. When such an interaction was observed, we performed a post-hoc test with sequential Bonferroni correction. All analyses were two-tailed. Pearson’s chi-square test was used to determine whether the percentage of females with injuries from scale-eating fishes differed among females with and without offspring.
Results
Intruder fish were classified based on feeding type (Table 1). Among all nests, eight species of carnivorous intruders (n = 111) were identified. The carnivorous fishes included species, such as L. elongatus and N. fasciatus, that mainly feed on cichlid juvenile under parental care (Kohda et al., 1997). Herbivorous fishes were the most abundant (n = 183) and diverse (12 species) intruders, consisting of mostly Tropheini, which feed on algae and are not a threat to N. furcifer. Only one species of scale-eating intruder was identified: P. microlepis (n = 41), which snatches and feeds on scales from living fish (Hori, 1983) and is mainly a threat to N. furcifer adults. In addition, we observed aggressive interactions (n = 10) between N. furcifer and four other species that could not be categorized as carnivorous, herbivorous, or scale-eating: the cooperative-breeding cichlid Neolamprologus savoryi, spiny eel Mastacembelus spp., small catfish Synodontis sp., as well as other N. furcifer adults. These data were excluded from the sequential analysis because of the small sample size.
The two-way interaction between intruder type and parental care stage significantly affected the aggressive distance (multifactorial LMM, F4,327 = 9.476, p < 0.001); the post-hoc analysis results are presented in Table 2. First, we examined the attack distance against each category of intruders (Figure 2). The attack distance was greater against carnivorous fishes when females were caring for offspring (egg and adhered fry, juvenile stages) than when offspring were not present (p < 0.0001). The attack distance against herbivorous fishes was greater when females were caring for offspring in the egg and adhered fry stage than the offspring absent stage (p < 0.0001). The scale-eating intruder, P. microlepis, was attacked at greater distances when offspring were absent than when offspring were in the juvenile stage (p = 0.0016). Second, we compared the attack distances among parental care stages (Figure 2). When females were not caring for offspring, they did not selectively attack a specific category of intruders (p > 0.138). During the egg and adhered fry stage, the attack distances varied among each category of intruders, attacking carnivorous fishes at greater distances, followed by herbivorous fishes, and scale-eaters (p < 0.007). During the juvenile stage, females attacked carnivorous fishes at greater distances than other intruders (p < 0.0001).
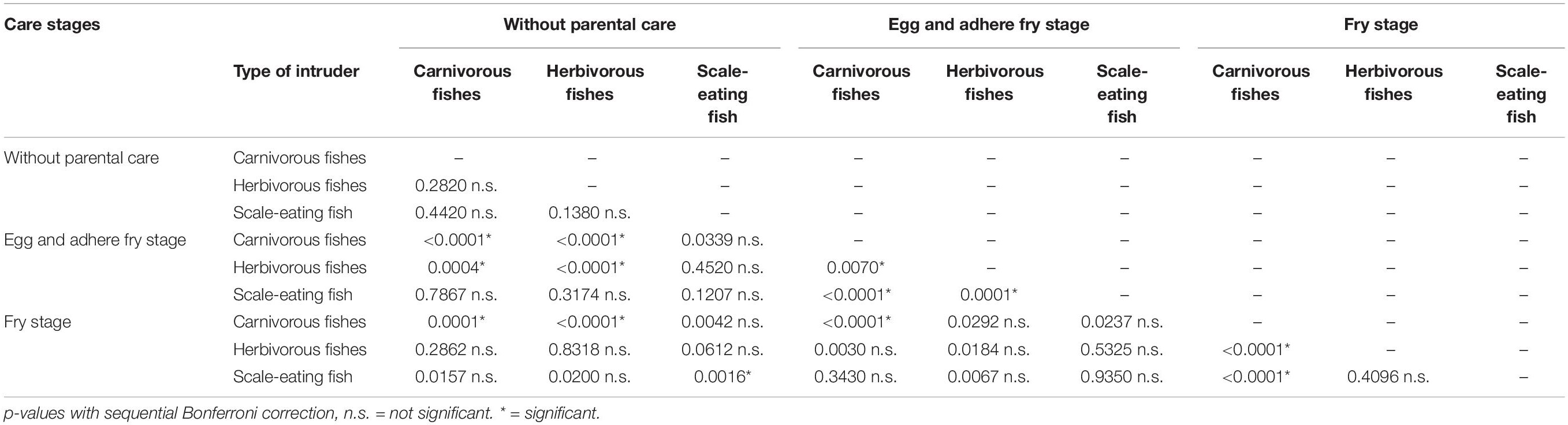
Table 2. Differences on aggressive distance between the parental care stages and type of intruder fishes.
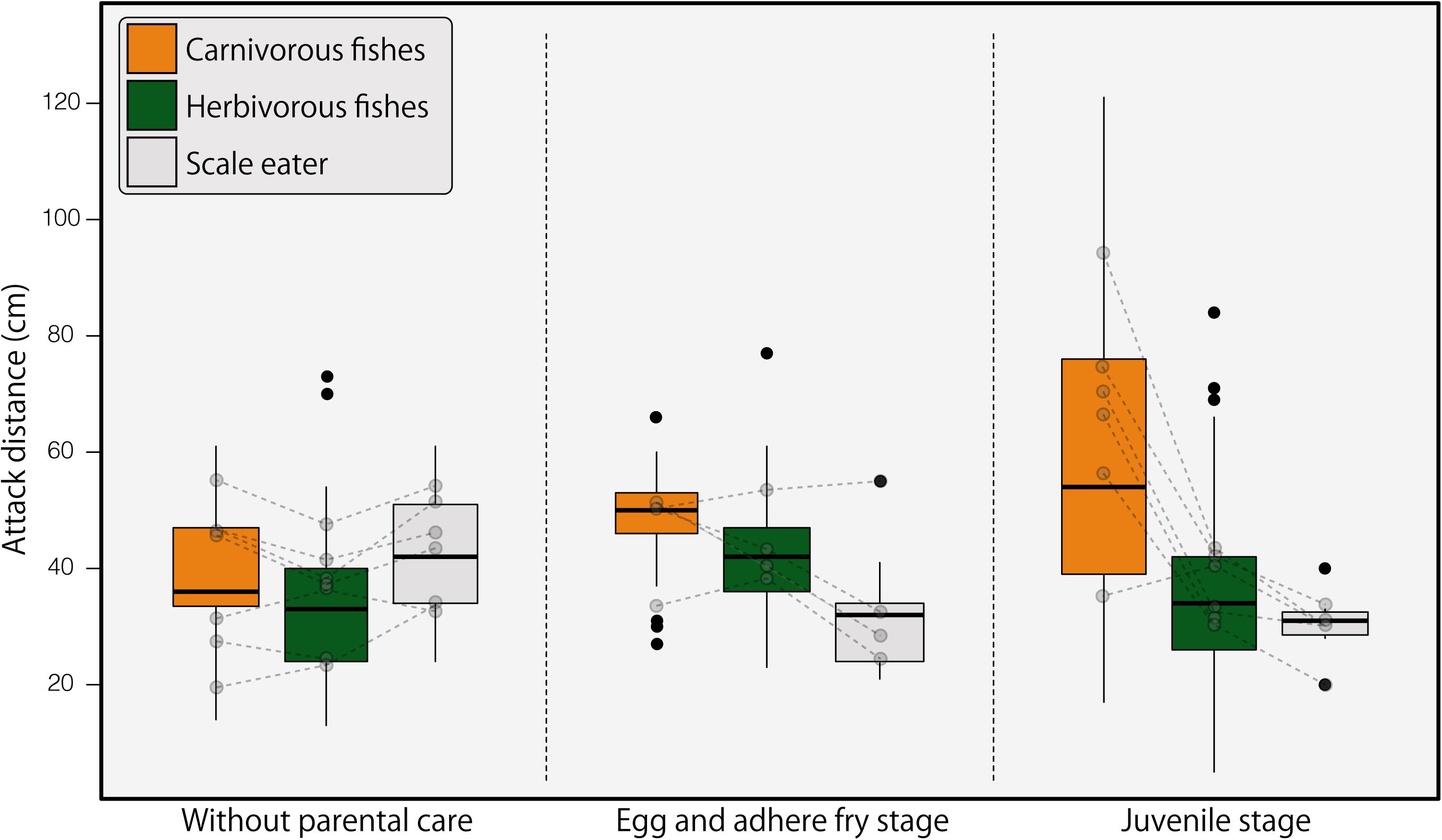
Figure 2. Attack distances of Neolamprologus furcifer females in different parental care stages against different intruder fish types. Box plots show medians and 25th and 75th percentiles; whiskers indicate the values 1.5 times the interquartile range. Black plots are outliers. Gray plot with broken line shows mean attack distance in each individual.
Finally, we found that the percentage of females with injuries caused by scale-eating fish was significantly higher when caring for offspring (28.5% of breeding female, n = 14) than without offspring (2.2% of non-breeding female, n = 45) (Pearson’s χ23 = 49.379, p < 0.001).
Discussion
Animals are surrounded by extensive environmental information; however, they are incapable of processing all available information at one time. Thus, attention must be divided, with a greater focus placed on information that is deemed important, despite the resulting constraints on access to less important information (Dukas, 2002, 2004). We assessed the constraints of limited attention by comparing the attack distances against intruders of varying threat levels among N. furcifer females guarding offspring and without offspring. Our results demonstrate that the attack distance toward carnivorous fishes by female parents increased when caring for offspring, whereas the attack distance toward scale-eating fish decreased.
Carnivorous fishes are food competitors and scale-eating fishes are a direct threat to adult females (Yanagisawa, 1987), whereas herbivorous fishes do not pose a threat to N. furcifer (Table 1). Thus, we predicted that the attack distances toward herbivorous fishes would be shorter than those toward carnivorous and scale-eating fishes. However, we found that the attack distances were similar across all categories of intruder when females were not caring for offspring and were longer toward herbivorous fishes when females were caring for egg and adhere fry. This may be caused by the fact that the cost of approaching herbivorous fish to N. furcifer is underestimated. Herbivorous fish, such as Tropheini, that browse on algae (Hori, 1983), may pose a threat because exuberant algae in nests attract the small shrimp that N. furcifer feed on (Tanaka et al., 2018). Additionally, algae in the nest are also useful for attracting the snails, Reymondia horei feeding on algae. The presence of this snail in N. furcifer nests is essential because juvenile masquerade as snails to avoid predation (Satoh et al., 2017). If snails are removed from the nest, the frequency of attacks against juvenile predators by N. furcifer mothers increases (Satoh et al., 2017), incurring a greater energetic cost. Therefore, it is beneficial for females to protect the nest from herbivorous fish during parental care to maintain the algal growth that attracts snails and shrimp.
When females were caring for offspring, attack distances toward carnivorous fishes increased compared with those toward herbivorous and scale-eating fishes. L. elongatus and N. fasciatus are the primary predators of many cichlid species fry and juvenile, often launching sudden attacks on cichlid fry and juvenile under parental care (Kohda et al., 1997). Thus, mothers need to detect and attack these intruders at greater distances to protect their offspring (Nakano and Nagoshi, 1990). It is also possible that the observed increase in attack distance may be a byproduct of offspring growth; as juvenile grow, they begin to spread out (Satoh et al., 2018), and consequently, the mothers must guard a larger area. If longer attack distances toward carnivorous fishes are attributed to the increased juvenile-foraging area, we would expect attacks against intruders to occur along the edges of the territory. However, most attacks occurred outside the territory (e.g., see Figure 1A). Thus, our results suggest that the increase in attack distance toward carnivorous fishes is mainly due to the increased threat level.
As the attack distance toward carnivorous fishes increased in females caring for offspring, the attack distance toward scale-eating fish decreased compared to those without offspring. Selective attention toward carnivorous fishes is expected to constrain attention toward other threats (Dukas, 2002, 2004). Scale-eating fish (P. microlepis) rarely eat eggs, fry, and juvenile but represent a direct threat to N. furcifer adults (Nshombo et al., 1985; Ochi et al., 1999). In fact, we observed injuries caused by scale-eating fish on a significantly higher percentage of females caring for offspring compared to those without offspring. Delayed detection of scale-eating fishes due to selective attention toward carnivorous fishes results in parental injuries; however, scale-eating fish are not a threat to eggs, fry, and juvenile (Ochi et al., 1999), and scales can be easily regenerated (Nshombo et al., 1985). Thus, females might tolerate the approach of scale-eating fishes to maintain attention on higher-threat-level intruders (Ota, 2018).
In some fish species, both male and female parents perform parental care (e.g., Nakano and Nagoshi, 1990), and one parent can compensate for the limited attention of the other parent (Sowersby et al., 2017). When males of biparental care species die or abandon their partners, females are unable to successfully drive away intruders (Lehtonen et al., 2011; Sowersby et al., 2018). In a polygynous mating system, females must focus on the highest threat-level intruder first, leaving the offspring open to other attacks (Josi et al., 2020). Our findings confirmed that N. furcifer females selectively pay attention to high-threat-level fish species to cope with limited attention and attend less or not at all to low-threat-level fish. This is supported by a comparative analysis of the brain size of African cichlid fish that revealed females of maternal care species have larger brains than females of biparental care species (Gonzalez-Voyer et al., 2009), allowing them to effectively guard offspring under limited attention constraints.
Conclusion
In conclusion, we provided evidence of the constraints of limited attention in the context of offspring guarding in the maternal care cichlid N. furcifer. The fish density in Lake Tanganyika is exceedingly high, and female parents face many intruders. If mothers attend to all intruders, superfluous fitness costs will be incurred. Thus, N. furcifer females selectively attack higher-threat-level intruders, i.e., carnivorous fishes, to reduce this cost (Nakano and Nagoshi, 1990). Selective attention on carnivorous fishes constrained attention toward other fishes, and the attack distance toward lower-threat-level species became shorter in females caring for offspring compared to those without offspring. Such selective focus allows N. furcifer females to effectively protect offspring without expending excessive energy. This study is the first to document the constraints of limited attention on parents guarding offspring. We measured attack distance to reveal the impact of attention on species with high threat level, but the frequency of attacks and time spent attacking might also be important indicators. However, it is difficult to measure attack duration in field observations, and predator models may be more effective for testing such predictions (e.g., Haley and Müller, 2002; Josi et al., 2020). Furthermore, investigating the frequency of intrusion by fishes in different feeding guilds and differential attacks by females may provide more insight into the constraints of limited attention (Nakano and Nagoshi, 1990). Our current findings do not allow us to exclude an alternative interpretation that females just needed a different amount of time to classify the intruder. Future study is needed to address this possibility.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This animal study was reviewed and approved by the Animal Care and Use Committees at Osaka City University for Advanced Studies and adhered to the ASAB/ABS guidelines for the treatment of animals in behavioral research. Our field research was conducted with the Permission for Fish Research in Lake Tanganyika from the Zambian Ministry of Agriculture, Food and Fisheries and complied with the current laws in Zambia. No permits were needed from Japanese Government for experiments involving N. furcifer.
Author Contributions
SS and MK designed the experiments. SS performed the experiments and collected and data. SS and TH analyzed the data and wrote the manuscript. MK provided critical revisions. All authors contributed critically to the drafts and gave final approval for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Carlson, A. (1985). Prey detection in the red-backed shrike (Lanius collurio): an experimental study. Anim. Behav. 33, 1243–1249. doi: 10.1016/S0003-3472(85)80184-6
Dukas, R. (2002). Behavioral and ecological consequences of limited attention. Philos. Trans. R. Soc. Lond. B 357, 1539–1547. doi: 10.1098/rstb.2002.1063
Dukas, R. (2004). Causes and consequences of limited attention. Brain. Behav. Evol. 63, 197–210. doi: 10.1159/000076781
Dukas, R. (2008). “Evolutionary biology of limited attention,” in Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain and Behavior, eds L. Tommasi, M. A. Peterson, and L. Nadel (Cambridge, MA: MIT Press), 147–162. doi: 10.7551/mitpress/9780262012935.003.0135
Dukas, R., and Ellner, S. (1993). Information processing and prey detection. Ecology 74, 1337–1346. doi: 10.2307/1940064
Gonzalez-Voyer, A., Winberg, S., and Kolm, N. (2009). Social fishes and single mothers: brain evolution in African cichlids. Proc. Biol. Sci. 276, 161–167. doi: 10.1098/rspb.2008.0979
Haley, M. P., and Müller, C. R. (2002). Territorial behavior of beaugregory damselfish (Stegastes leucostictus) in response to egg predators. J. Exp. Mar. Biol. Ecol. 273, 151–159. doi: 10.1016/S0022-0981(02)00144-2
Hess, S., Fischer, S., and Taborsky, B. (2016). Territorial aggression reduces vigilance but increases aggression towards predators in a cooperatively breeding fish. Anim. Behav. 113, 229–235. doi: 10.1016/j.anbehav.2016.01.008
Hori, M. (1983). Feeding ecology of thirteen species of Lamprologus (Teleostei; Cichlidae) coexisting at a rocky shore of Lake Tanganyika. Physiol. Ecol. Jpn. 20, 129–149.
Hori, M. (1993). Frequency-dependent natural-selection in the handedness of scale-eating cichlid fish. Science 260, 216–219. doi: 10.1126/science.260.5105.216
Jones, K. A., and Godin, J. G. J. (2010). Are fast explores slow reactors? Linking personality type and anti-predator behavior. Proc. R. Soc. Lond. B 277, 625–632. doi: 10.1098/rspb.2009.1607
Josi, D., Freudiger, A., Taborsky, M., and Frommen, J. G. (2020). Experimental predator intrusions in a cooperative breeder reveal threat-dependent task partitioning. Behav. Ecol. 31, 1369–1378. doi: 10.1093/beheco/araa094
Kaby, U., and Lind, J. (2003). What limits predator detection in blue tits (Parus caeruleus): posture, task or orientation? Behav. Ecol. Sociobiol. 54, 534–538. doi: 10.1007/s00265-003-0665-5
Kohda, M., Hori, M., and Nshombo, M. (1997). “Inter-Individual variation in foraging behavior and dimorphism,” in Fish Communities in Lake Tanganyika, eds H. Kawanabe, M. Hori, and M. Nagoshi (Kyoto: Kyoto University Press).
Lehtonen, T. K., Wong, B. B. M., Svensson, P. A., and Meyer, A. (2011). Adjustment of brood care behavior in the absence of a male in two species of Nicaraguan crater lake cichlids. Behav. Ecol. Sociobiol. 65, 613–619. doi: 10.1007/s00265-010-1062-5
Matsumoto, K., and Kohda, M. (2004). Territorial defense against various food competitors in the Tanganyikan benthophagous cichlid Neolamprologus tetracanthus. Ichthyol. Res. 51, 354–359. doi: 10.1007/s10228-004-0242-6
Nakano, S., and Nagoshi, M. (1990). Brood defense and parental roles in a biparental cichlid fish Lamprologus toae in Lake Tanganyika. Ichthyol. Res. 36, 468–476. doi: 10.1007/BF02905466
Nshombo, M., Yanagisawa, Y., and Nagoshi, M. (1985). Scale-eating in Perissodus microlepis (Cichlidae) and change of its food habitat with growth. Jpn. J. Ichthyol. 32, 66–73.
Ochi, H., Sato, Y., and Yanagisawa, Y. (1999). Obligate feeding of cichlid eggs by Caecomastacembelus zebratus in Lake Tanganyika. J. Fish Biol. 54, 450–459. doi: 10.1111/j.1095-8649.1999.tb00843.x
Ochi, H., and Yanagisawa, Y. (1998). Commensalism between cichlid fishes through differential tolerance of guarding parents toward intruders. J. Fish Biol. 52, 985–996. doi: 10.1111/j.1095-8649.1998.tb00598.x
Oliver, S. J., and Watson, E. (1999). Threat-sensitive nest defense in domino damselfish, Dascyllus albisella. Biol. Bull. 197, 244–246. doi: 10.2307/1542628
Ota, K. (2018). Fight, fatigue and flight: narrowing of attention to a threat compensates for decreased anti-predator vigilance. J. Exp. Biol. 221:jeb168047. doi: 10.1242/jeb.168047
Satoh, S., Ota, K., Awata, S., and Kohda, M. (2018). Dynamics of sibling aggression of a cichlid fish in Lake Tanganyika. Hydrobiologia 832, 201–213. doi: 10.1007/s10750-018-3768-8
Satoh, S., Takahashi, T., Tada, S., Tanaka, H., and Kohda, M. (2017). Parental females of a nest-brooding cichlid improve and benefit from the protective value of young masquerading as snails. Anim. Behav. 124, 75–82. doi: 10.1016/j.anbehav.2016.12.001
Sinn, D. L., While, G. M., and Wapstra, E. (2008). Maternal care in a social lizard: links between female aggression and offspring fitness. Anim. Behav. 76, 1249–1257. doi: 10.1016/j.anbehav.2008.06.009
Sowersby, W., Lehtonen, T. K., and Wong, B. B. M. (2017). Temporal and sex-specific patterns of breeding territory defense in a color-polymorphic cichlid fish. Hydrobiologia 791, 237–245. doi: 10.1007/s10750-016-2889-1
Sowersby, W., Lehtonen, T. K., and Wong, B. B. M. (2018). Threat sensitive adjustment of aggression by males and females in a biparental cichlid. Behav. Ecol. 29, 761–768. doi: 10.1093/beheco/ary037
Takeuchi, Y., Ochi, H., Kohda, M., Sinyinza, D., and Hori, M. (2010). A 20-year census of a rocky littoral fish community in Lake Tanganyika. Ecol. Freshw. Fish 19, 239–248. doi: 10.1111/j.1600-0633.2010.00408.x
Tanaka, H., Frommen, J. G., and Kohda, M. (2018). Helpers increase food abundance in the territory of a cooperatively breeding fish. Behav. Ecol. Sociobiol. 72:51. doi: 10.1007/s00265-018-2450-5
Yanagisawa, Y. (1987). Social organization of a polygynous cichlid Lamprologus furcifer in Lake Tanganyika. Ichthyol. Res. 34, 82–90. doi: 10.1007/BF02904148
Yee, J., Lee, J., Desowitz, A., and Blumstein, D. T. (2013). The costs of conspecifics: are social distractions or environmental distractions more salient? Ethology 119, 480–488. doi: 10.1111/eth.12085
Keywords: limited attention, offspring guarding, parental care, threat sensitivity, Neolamprologus furcifer, Lake Tanganyika
Citation: Satoh S, Hotta T and Kohda M (2021) Maternal Care-Providing Cichlid Neolamprologus furcifer Selectively Focuses on High-Threat Carnivorous Intruders, Limiting Attention to Other Threats. Front. Ecol. Evol. 9:616810. doi: 10.3389/fevo.2021.616810
Received: 13 October 2020; Accepted: 09 February 2021;
Published: 25 February 2021.
Edited by:
Elise Huchard, UMR 5554 Institut des Sciences de l’Evolution de Montpellier (ISEM), FranceReviewed by:
Cheng-Yu Li, University of Maryland, College Park, United StatesConstance O’Connor, Wildlife Conservation Society (Canada), Canada
Copyright © 2021 Satoh, Hotta and Kohda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shun Satoh, c3ltcGh5c29kb25kaXNjdXNAaWNsb3VkLmNvbQ==; Takashi Hotta, dGFrYXNpNzEyMDAwQHlhaG9vLmNvLmpw
 Shun Satoh
Shun Satoh Takashi Hotta
Takashi Hotta Masanori Kohda
Masanori Kohda