- 1Institute of Marine Biology, College of Life Science, National Taiwan Ocean University, Keelung, Taiwan
- 2Department of Science Education, National Taipei University of Education, Taipei City, Taiwan
- 3Department of Pathology, Mackay Memorial Hospital, New Taipei City, Taiwan
- 4Center of Excellence for Ocean Engineering, National Taiwan Ocean University, Keelung, Taiwan
- 5Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
The Danshuei River has a third largest catchment area and third longest in Taiwan. It flows through the capital, Taipei, and more than six million people live within its catchment area. Its estuary is characterized by a highly variable chemical and physical environment that is affected by the interaction of inland freshwater runoff with wastewater, and toward the coast is also influenced by the China Coastal Current and the Kuroshio Current. By collecting zooplankton bimonthly in 2014 from the surface layer (0–2 m depth) at five sites in the estuary, we were able to demonstrate that the composition of the zooplankton, and particularly its copepod fraction, varied significantly among sampling stations and months, thereby revealing seasonal succession. Fourteen higher taxa or other categories of zooplankter were identified, with the following being most common taxa: Decapoda, Copepoda (including Calanoida, Cyclopoida, and Harpacticoida), and “other larvae.” The Copepoda comprised 44 taxa (including eight only identified to genus) belonging to 3 orders, 17 families, and 29 genera, the five most abundant of which were Bestiolina n. sp. (undescribed), Corycaeus spp., Parvocalanus crassirostris, Acartia sp., and Paracalanus parvus. The highest and lowest copepod abundances were recorded in July (2557.88 inds. m–3) and January (1.3 inds. m–3), respectively. Observed changes in abundance of many kinds of copepod appeared to be significantly related to changes in physico-chemical parameters (e.g., salinity, temperature, pH, and dissolved oxygen concentration). Cluster analysis confirmed the existence of distinct copepod communities, each characterized by a preference for a different set of environmental conditions. Our comprehensive literature review of the copepod biodiversity of Taiwan’s major rivers for comparison with similar data compiled for other estuaries in the world, the first time such a review has been compiled, shows that 32 copepod taxa have been recorded from the brackish and freshwater parts of the Danshuei River. They represent 58.2% of the total number of brackish- and freshwater copepod species in Taiwan, and five of them have so far only been recorded in the Danshuei River: the calanoids Acartiella sinensis and Pseudodiaptomus forbesi, the cyclopoids Oithona fragilis and Oithona simplex, and the harpacticoid Tachidius (Tachidius) discipes.
Introduction
Estuaries are transitional zones between rivers and the sea (Meire et al., 2005; Wang et al., 2007; Telesh and Khlebovich, 2010; Shan et al., 2013). The physical and chemical aspects of their hydrography are continuously dynamic with regard to both place and time (Elliott and McLusky, 2002; Kibirige and Perissinotto, 2003). For example, the turbidity of the river water increases, and salinity decreases, in the upstream direction (Elliott and McLusky, 2002), the gradient in salinity being caused by the interplay of denser seawater and overlying riverine freshwater (Hwang et al., 2010; Telesh and Khlebovich, 2010). The estuarine biome, too, is distinctive, being characterized by low biodiversity but high abundance of the prevailing taxa, and thus by unique food webs. Estuaries provide nursery grounds for larvae and juveniles of invertebrates and fish and important foraging grounds for migratory birds (Shan et al., 2013). Terrestrial nutrient runoff into estuaries (Godhantaraman and Uye, 2003) nourishes spawning and fishing grounds that are inhabited by many commercially important species of fish and shellfish (Shan et al., 2013).
The Danshuei River is the third longest river has the third largest catchment area in Taiwan, and the largest in the north of the island (LC–FRD, 2005; Hwang et al., 2010, 2006). It has two major branches, the Xindian and Dahan rivers, and flows out into coastal waters at the boundary between the East China Sea and the Taiwan Strait (Wen et al., 2008; Cheng et al., 2011). Its estuary includes a mangrove (Kandelia candel) conservation area of about 50 hectares that was established in June, 1986 (Kao and Chang, 1998). The Danshuei River flows through Taipei City, the capital of Taiwan, and also though a portion of New Taipei City, places where more than six million people live (Lai et al., 2010). Extensive water pollution in the river’s catchment area, including the discharge of urban sewage and industrial wastewater into the Danshuei River, was formerly prevalent (Wu and Chou, 2003; Wang et al., 2014), but after a sewage treatment system was established in June, 1997, the water quality of the river improved greatly (Putri et al., 2018).
Many species that are commercially exploited by the fishing industry inhabit the Danshuei River. Among them are fishes such as Oreochromis niloticus (Cichlidae) (Chen et al., 2014), Epinephelus coioides (Serranidae), Diodon holocanthus (Diodontidae), and Pisodonophis cancrivorus (Ophichthidae); the shrimp Metapenaeus monoceros; the crabs Scylla serrata and Portunus sanguinolentus; and the bivalves Meretrix petechialis (Veneridae) (Liu, 2014) and Perna viridis (Mytilidae) (Chao, 2006). Several biological studies on, e.g., juvenile fish (Shih, 2007) and hydroids (Tseng et al., 2014) have been conducted in the Danshuei River, and a number of publications have dealt specifically with the copepods that are found there (Yu, 2005; Hwang et al., 2006, 2009, 2010; Hsiao, 2009). Some studies on the seasonal succession of the copepod community have taken place, based on four samplings per year, but such studies have focused on the fundamentally marine species in the plume and estuarine areas (Chen, 2005; Yu, 2005; Hwang et al., 2006, 2010; Hsiao, 2009) while rarely discussing the specifically brackish-water species. The temporal succession of zooplankton in this estuary’s waters thus still remains understudied. Here we make another attempt to study the intra-annual succession and distribution of the zooplankton in the estuary of the Danshuei River, with special reference to the copepods.
To do so, on a bimonthly schedule we recorded hydrographic parameters while collecting zooplankton samples in the Danshuei River’s estuary with three aims: (1) documenting the intra-annual succession of the general zooplankton assemblage, with a more specific analysis of the copepod community, (2) providing baseline taxonomic information about these subtropical freshwater and brackish-water copepods, and (3) comparing the historical records of copepods from different rivers and their estuaries in Taiwan.
Materials and Methods
Study Area and Field Sampling
Bimonthly collections were made at five selected sampling stations in the estuary of the Danshuei River in northwestern Taiwan (in New Taipei City), within the region bounded by 25°05′–25°11′N latitude and 121°25′–121°31′E longitude (Figure 1 and Table 1). Station 1 was the farthest downstream and station 5, 7.6 km away, was farthest upstream. The investigation and zooplankton collections were carried out from outside the protected area and no specific permission was required. The field studies did not involve endangered or protected species. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The estuary faces, and its waters flow into, the southwestern part of the East China Sea. Samples were collected in daytime during six cruises on January 10, March 19, May 28, July 29, September 29, and November 26, 2014. A standard NORPAC net (mouth opening diameter 0.45 m, length 1.80 m, mesh size 100 μm) was towed for 10 min at the surface (0–2 m depth), with a Hydrobios (Kiel, Germany) flowmeter mounted in the center of the net opening. The samples were immediately preserved in seawater-diluted formalin (≒ 5%) on board for later estimation of taxon composition and abundance. Before sampling, a handheld multi-parameter sensor (Model HI 9829, Hanna Instruments, Vöhringen, Germany) was used to measure the salinity, water temperature, dissolved oxygen concentration (DO), and pH at each station.
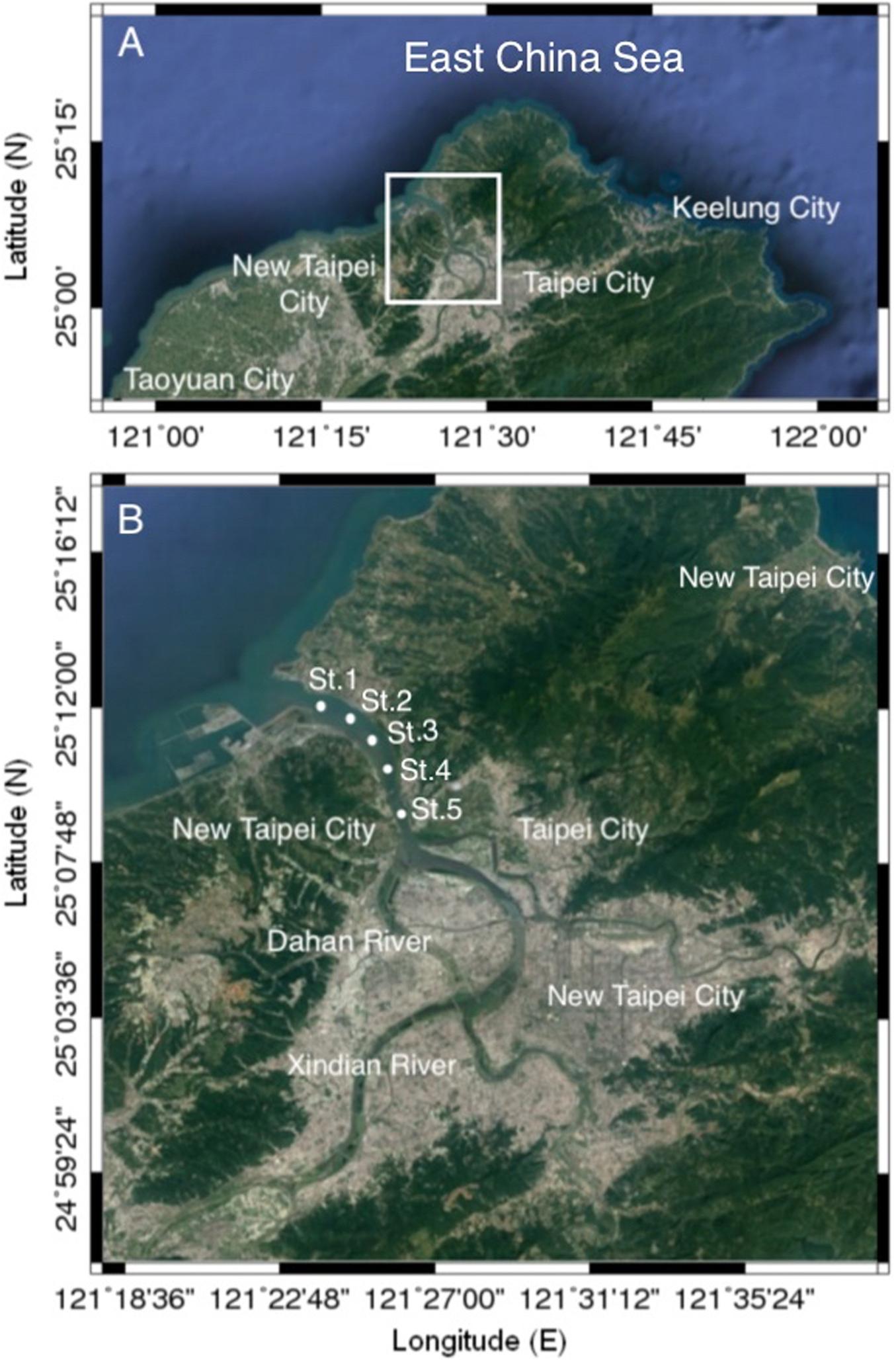
Figure 1. Maps of the study area (A) and the locations of the sampling stations (B) in the estuary of the Danshuei River in northwestern Taiwan.
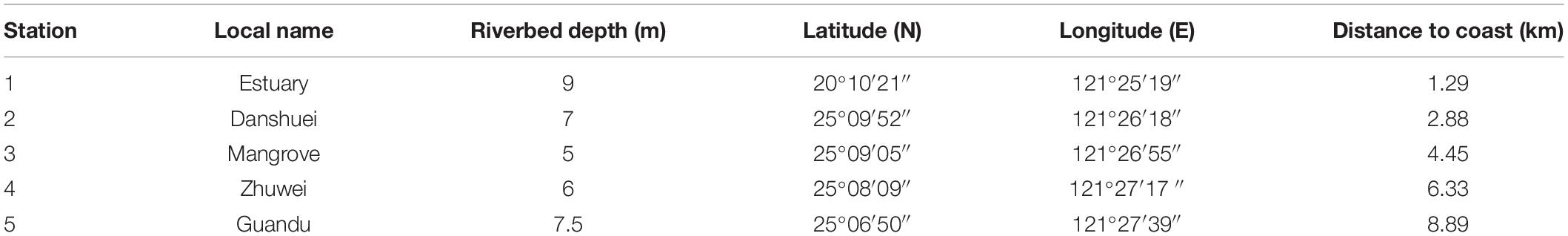
Table 1. Local name, riverbed depth (m), coordinates, and distance to coast (km) of five sampling stations in the estuarine area of the Danshuei River.
Identification and Abundance Measurements of Zooplankton
Taxonomic identification and enumeration of zooplankton were done in the laboratory at National Taiwan Ocean University. The samples were divided by a Folsom splitter until each aliquot contained approximately 400–500 specimens. Zooplankters were sorted and identified using the keys of Shen (1979) for freshwater species, and those of Chihara and Murano (1997); Walter et al. (2006), Sakaguchi and Ueda (2010); Ueda et al. (2011), Srinui et al. (2013, 2019), Lian et al. (2018), and Shih et al. (2019) for marine species. The numbers of individuals (ind.) of each zooplankton taxon were recorded (as ind. m–3), with adult copepods being identified to species. Zooplankton was sorted into 14 larger taxa or other categories. Among them, the category “Decapoda” was defined as including small shrimp as well as larvae of Macrura and Brachyura, whereas the category “Other larva” included cirriped nauplii, ophioplutei, trochophores, and aquatic insect larvae. Sexually mature copepods were also assigned to one the three taxa Calanoida, Cyclopoida, and Harpacticoida, while all immature copepods were categorized as “Copepoda copepodites.”
Images of the Sea Surface Temperature
Images of sea surface temperature (SST, source: GHRSST-PP/OSTIA, Group for High Resolution Sea Surface Temperature Pilot Project/Operational SST and Sea Ice Analysis) were obtained from the Fisheries Research Institute, Council of Agriculture, Taiwan (Figure 2). These images were used to present and track the movements of water masses and thereby perhaps explain the month-to-month succession of copepod communities.
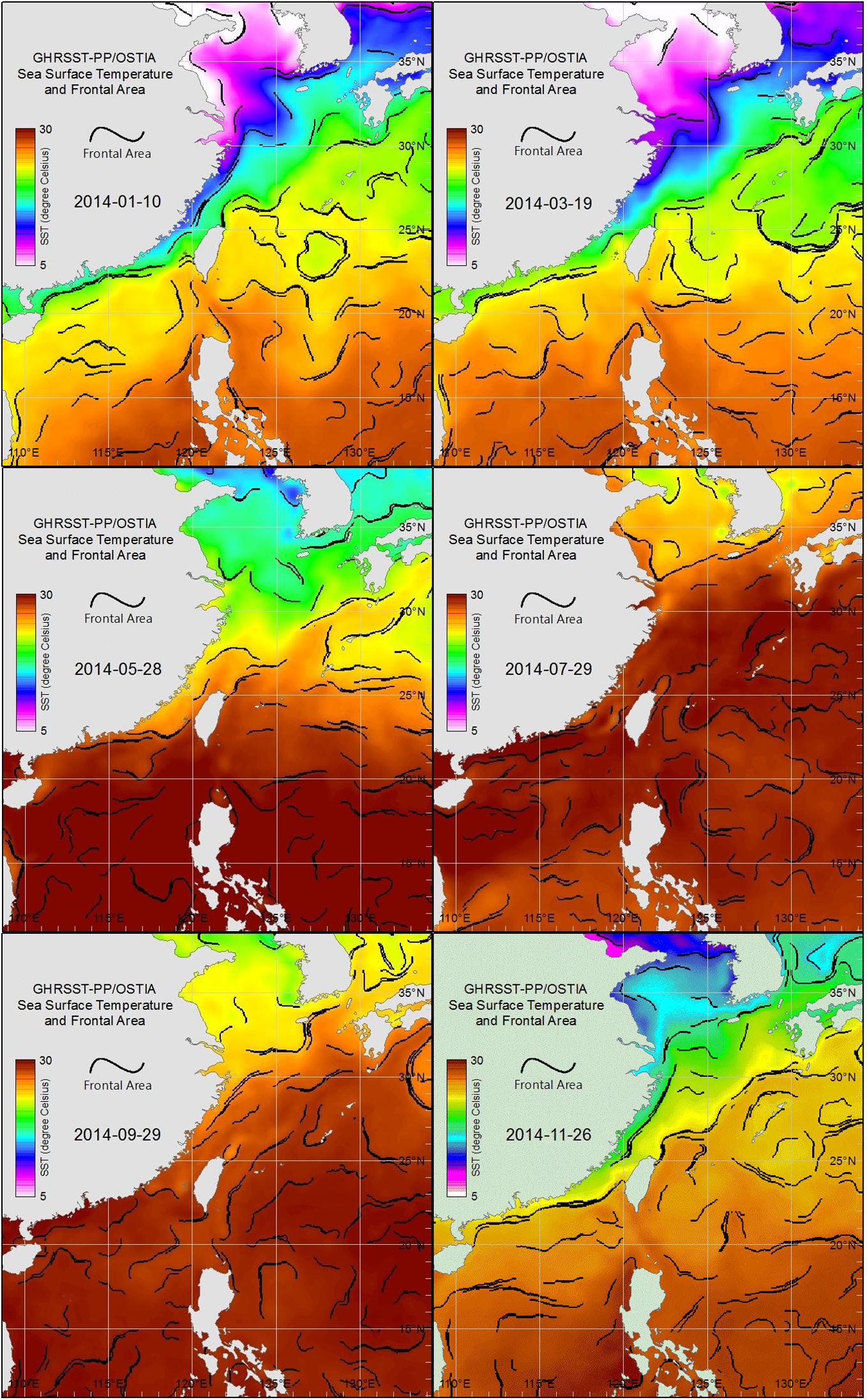
Figure 2. Sea surface water-temperature images for each bimonthly sampling date in 2014 derived from GHRSST-PP/OSTIA, showing water mass distributions in the East China Sea, Taiwan Strait, and South China Sea.
Data Analysis
To elucidate the temporal variation of copepod communities at 2-month intervals over the course of a year, the PAST software package (Paleontological Statistics: Hammer et al., 2001) was used to compare the copepod assemblages in each sample. Among the 30 samples, the top 16 most abundant copepod taxa, those with a relative abundance (RA) exceeding 1.0% (together comprising 93.44% of the total adult copepods), were used to calculate similarities before clustering and nonmetric multidimensional scaling (NMDS) analyses were done. The Bray-Curtis similarity distance was used to evaluate similarities in spatial distribution between taxa. In order to reduce the bias introduced by particularly abundant taxa, a test of the necessity for data transformation (Box and Cox, 1964) was applied prior to conducting the similarity analysis. The value (λ) of the power transformation was 0.92. In consequence, the original abundance data of copepods for all samples were log-transformed, using log (x + 1), before analysis.
A one-way analysis of variance (ANOVA) with the post hoc Tukey’s Honestly Significance Difference (HSD) test was applied to reveal differences in copepod community structure among the sampling months. Pearson’s correlation analysis was used to estimate the relationship between the abundance of copepods and various hydrographic parameters.
Results
Hydrological Structure
Sea surface temperature images (Figure 2) obtained from GHRSST-PP/OSTIA for each sampling date in January, March, and November showed the cold water mass of the China Coastal Current (CCC) extending southward from the Yellow and Bohai seas, along the coast of mainland China, and into the northern Taiwan Strait, where the zooplankton samples were collected. In contrast, similar GHRSST-PP/OSTI images for each sampling date in May, July, and September showed the northern shores of Taiwan enveloped by warm water masses of the South China Sea and Pacific Ocean (Figure 2).
Tide tables for the Danshuei River issued by Taiwan’s Central Weather Bureau (Figures 3A–F) report tidal heights relative to the local annual mean water level. During the present study, the sample collection period was limited to 09:00–16:00 on the cruise dates, and the tidal conditions each time were different. For example, low tide occurred at around 12:00 in January (Figure 3A) whereas high tide occurred then in March and July (Figures 3B,D); high tide occurred around 13:00–14:00 in September and November (Figures 3E,F), while sampling was done on an ebb tide in May (Figure 3C). The lowest (−165 cm) and highest (142 cm) water levels were recorded during the July and November cruises, respectively.
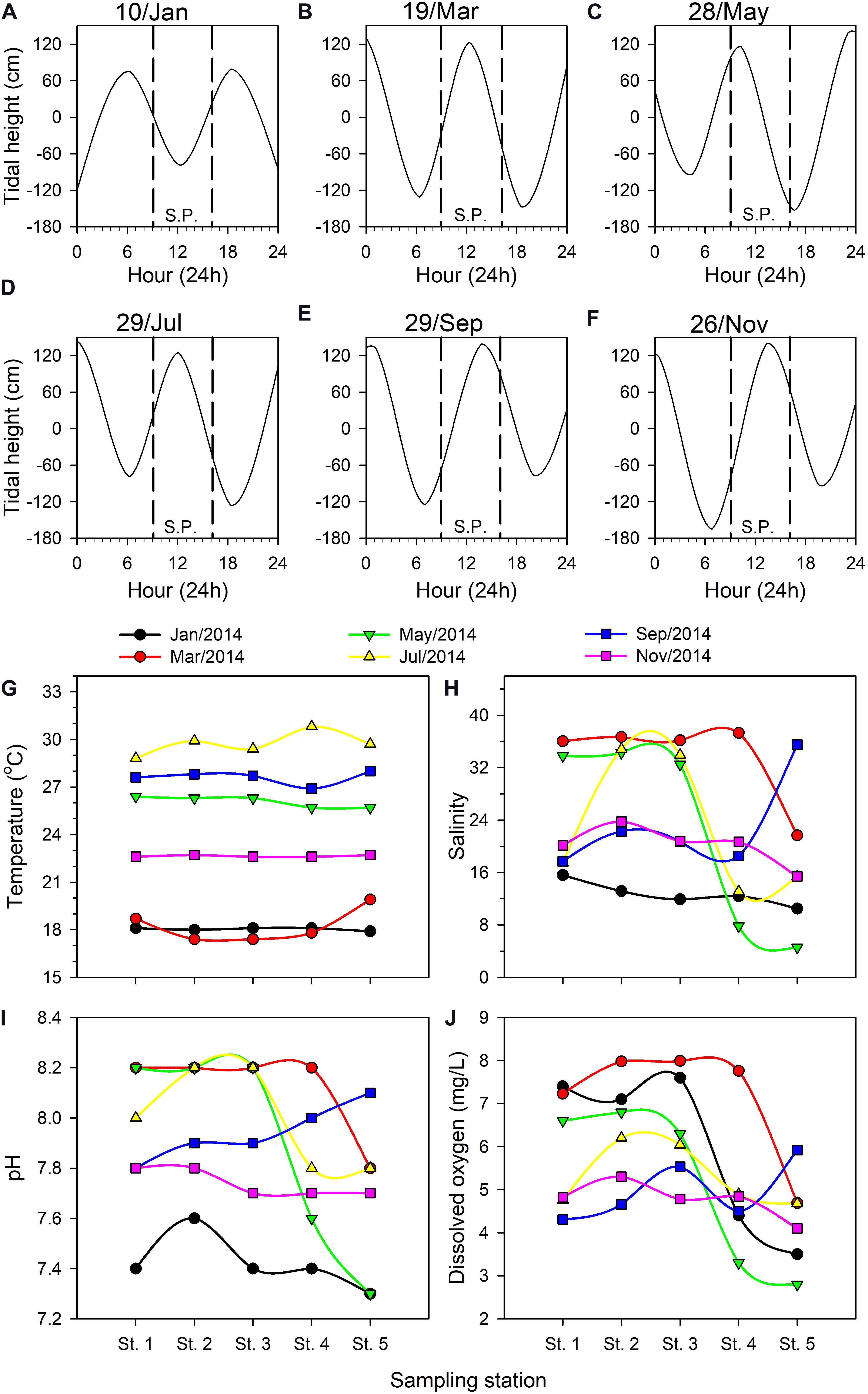
Figure 3. Tidal height (cm) fluctuations on each bimonthly sampling date in 2014 in the Danshuei River estuary: January 10 (A), March 19 (B), May 28 (C), July 29 (D), September 29 (E), and November 26 (F). Upstream-downstream variation in hydrographic parameters among the sampling stations in this estuary on each bimonthly sampling date: water temperature (G), salinity (H), pH (I), dissolved oxygen concentration (J). SP, sampling period.
Water temperature in the Danshuei River showed significant variation among sampling cruises (Figure 3G), ranging from a single-reading low of 17.4°C in March during the northeast monsoon period to a high of 30.8°C in July. The lowest average water temperature through the five stations was 18.0 ± 0.12°C in January whereas the highest was 29.39 ± 0.89°C in July (Figure 3G).
The salinity at all sampling stations was strongly affected by the tidal cycle (Figures 3A–F) and was highly dynamic on each sampling date (Figure 3H). The salinity records showed that strong intrusions of seawater reached to station 3 in May and July, station 4 in March, and station 5 in September. The cruises of July and November took place on the second and fourth days after the new moon, respectively, because of which the intrusion of seawater into the estuary should have been the strongest during these two cruises. However, because salinity was measured at the surface and because the seawater and river water were not sufficiently and uniformly mixed, the salinities recorded on these two occasions were not the highest we recorded (Figure 3H). The lowest salinity measurements were recorded at upstream station 5, with the lowest value (4.57 PSU) occurring in May, which demonstrates the relatively low influence of seawater there. Throughout the study, salinity records at station 1 were higher than at other stations excepted in September. Low salinity records at all sampling stations in January were the result of rainfall in the upper reaches of the Danshuei River’s drainage area (Figure 3H); pH records were also lower then. The lowest pH value of 7.3 was recorded twice at station 5, in January and May (Figure 3I). DO ranged from a low of 2.80 mg/L at station 5 in January to a high of 7.99 mg/L at station 3 in March (Figure 3J). Overall the changing pattern of pH and dissolved oxygen from station to station was similar during each sampling cruise (Figures 3I,J).
Pearson’s correlation analysis indicated that pH (r = 0.902, p < 0.001) and DO (r = 0.679, p < 0.001) were significantly and positively correlated with salinity. Variations in DO were significant positively correlated with pH (r = 0.514, p = 0.004), but significant negatively correlated with water temperature (r = −0.395, p = 0.031).
Zooplankton Abundance and Variation in Community Structure
A total of 14 higher-level taxa and other categories of zooplankton were identified (Supplementary Table 1), the most common of which were Decapoda, Calanoida, Cyclopoida, Harpacticoida, and “other larvae”—all were present on each sampling date. Zooplankton densities (ind. m–3) ranged from 1.53 (station 5, January) to 2902.21 (station 2, July) (Figure 4A). The total abundance of zooplankton clearly varied through the year. The number of zooplankton categories in the community on a given date was lowest at station 4 (1 taxon, January) and highest at station 2 (8 taxa, May). Taxon richness was highest in May, during the northeastern and southwestern monsoon transition period (Figure 4B), and lowest in January and November during the period of the prevailing northeastern monsoon.
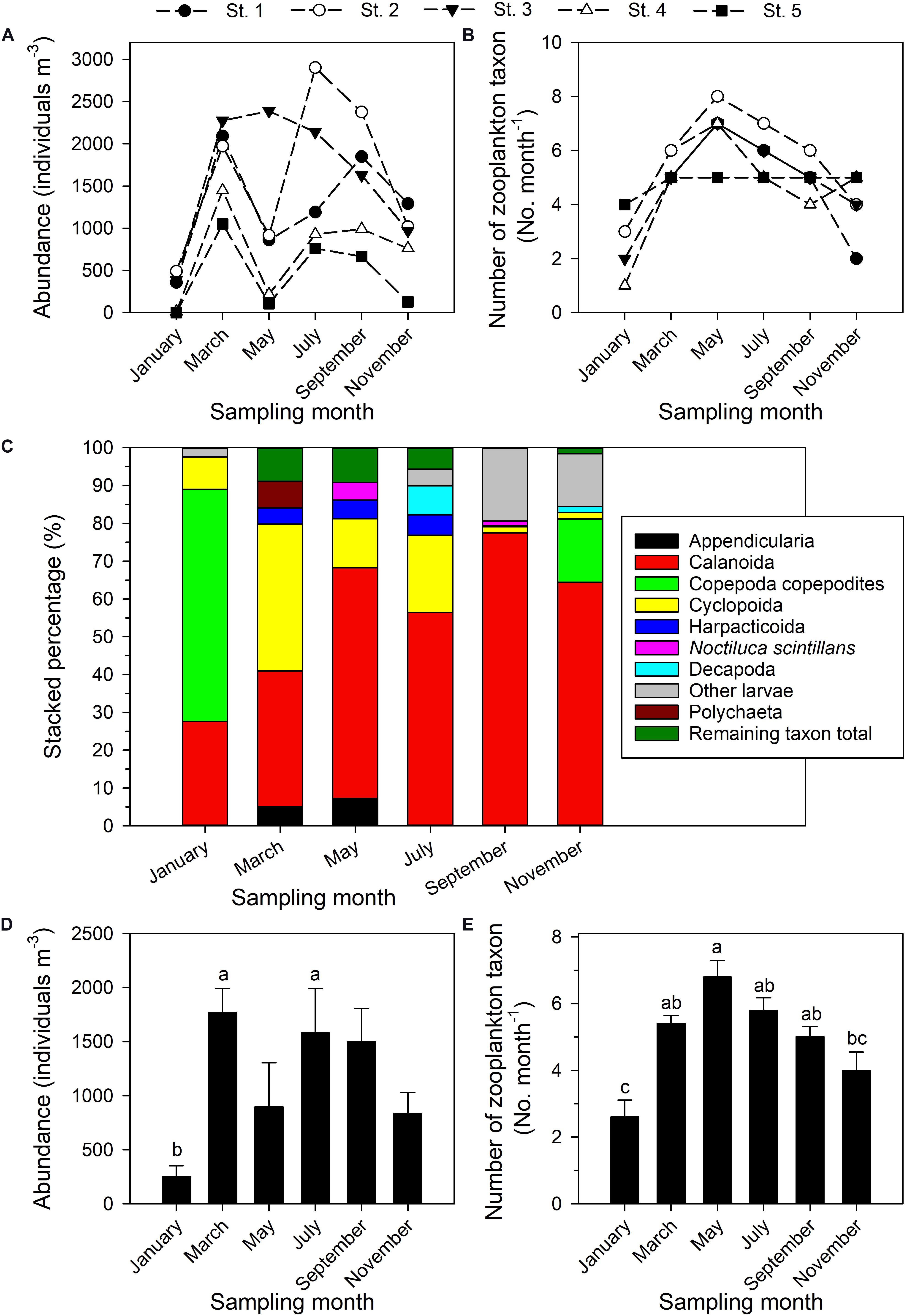
Figure 4. Total zooplankton abundance (A), number of higher taxa or other categories of zooplankton (B), relative abundances of five most dominant zooplankton taxa (C); values (mean ± standard deviation) on each bimonthly sampling date in 2014 of abundance (D) and number of higher taxa or other categories of zooplankton (E). Superscripts (a–c) denote significant differences (p < 0.05, one-way ANOVA). The remaining taxon total is the sum of the percentages of all taxa aside from the top 5.
The dominance structure within the zooplankton community changed through the year (Figure 4C). Calanoid copepods were always among the most dominant, with a contribution (RA) ranging from 27.65% (January) to 77.49% (September). Cyclopoid copepods were the second most dominant category, contributing from 1.59% (September) to 38.91% (March). Harpacticoid copepods were most highly represented in March, May, and July, with contributions of 4.21%, 4.97%, and 5.42%, respectively. Copepodites were absolutely dominant in January (61.36%), but of only secondary importance in November (16.75%). Noctiluca scintillans accounted for 4.64% of the total in May, but in other months was relatively less abundant. The taxa Appendicularia, Polychaeta, and Decapoda individually accounted for 7.28% in May, 7.08% in March, and 7.69% in July, respectively (Figure 4C), but were not otherwise major components of the community.
The ANOVA and Tukey tests conducted to compare zooplankton abundance and taxonomic category diversity among the six bimonthly sampling dates in 2014 confirmed monthly variation in total zooplankton abundance (Figure 4D), this being lowest in January, when it was significantly lower than in March (p = 0.015) and July (p = 0.04; Figure 4D). The number of higher-level zooplankton taxa and other categories encountered was significantly lower in January than in March (p = 0.001), May (p < 0.001), July (p < 0.001), or September (p = 0.007) (Figure 4E). In contrast, neither absolute abundance nor number of taxa was significantly different as annual averages among the five sampling stations (p > 0.05, one-way ANOVA).
Community Structure and Variation of Copepods
The 30 samples taken during six bimonthly research cruises in the estuary of the Danshuei River in 2014 included 44 taxa of copepods (among which eight taxa only identified to genus) belonging to 3 orders, 17 families, and 29 genera. The five most abundant taxa were Bestiolina n. sp. (an undescribed species; relative abundance (RA) in pooled samples 29.04%), Corycaeus spp. (11.17%), Parvocalanus crassirostris (10.98%), Acartia sp. (8.93%), and Paracalanus parvus parvus sensu lato (4.67%) (Supplementary Table 2). Time series values of copepod abundance (Figure 5A), species richness (Figure 5B), and rank abundance (%) (Figure 5C) from January through November revealed a spatiotemporally highly dynamic copepod community structure in this estuary. The highest copepod density was recorded at station 2 in July (2557.88 inds. m–3), followed by station 3 in May 2014 (2155.49 inds. m–3), whereas the lowest copepod density was found at station 5 in January (1.3 inds. m–3) (Figure 5A). The highest number of copepod taxa was recorded at station 5 (16 taxa) in September, whereas the lowest number (1 taxon) was recorded at station 4 in January (Figure 5B). The shapes of the rank abundance curves were considerably different among the six sampling dates, especially so for the curve representing the May cruise in which the highest number of taxa was recorded. During the cruise in January, two copepod taxa with relative abundances smaller than 1.0% (Figure 5C).
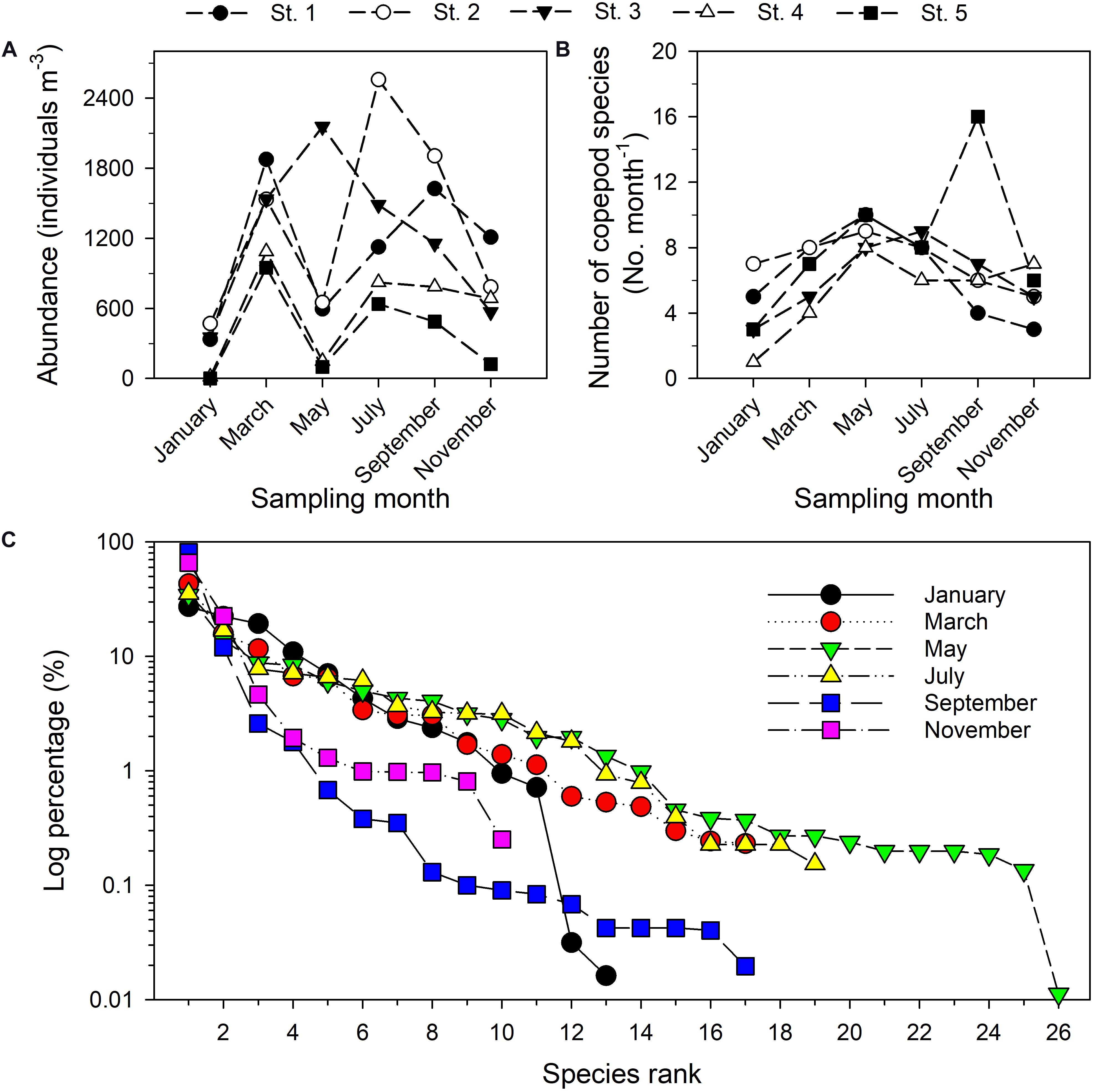
Figure 5. Total copepod abundance (A), number of distinguished copepod taxa (B), and rank abundance diagrams of planktonic copepods sampled bimonthly in 2014 in the Danshuei River estuary (C). The y-axis (percentage) is given as a log10 scale.
The relative rank abundances of the 10 most dominant copepod taxa on successive sampling dates provide evidence of faunal succession within the community (Table 2), with the ranks of the dominant taxa showing changes over time. The harpacticoid Euterpina acutifrons was in the dominant group throughout most of the year, but not in January. Acartia (Odontacartia) pacifica, Paracalanus parvus parvus sensu lato and Parvocalanus crassirostris were among the dominants in three sampling months during July–November, January–May, and May–September, respectively. Two congeneric species, Acrocalanus gibber and A. gracilis, displayed similar high abundances in March and May, but not in other months. Oithona dissimilis and Temora turbinata were found among the dominants in May and July. Other adventitiously dominant species, which displayed a short period of dominance and a subsequent rapid decline, included Ditrichocorycaeus affinis, Oithona attenuata, Oithona simplex, Oncaea venusta, Pseudodiaptomus annandalei, Tachidius (Tachidius) discipes, Tortanus (Eutortanus) dextrilobatus, and Tortanus (Eutortanus) derjugini (Table 2). Calanus sinicus, a bioindicator of cold water in the southwestern East China Sea (Figure 3G), was recorded in March (Supplementary Table 2), when the CCC reached into Taiwan’s northern waters.
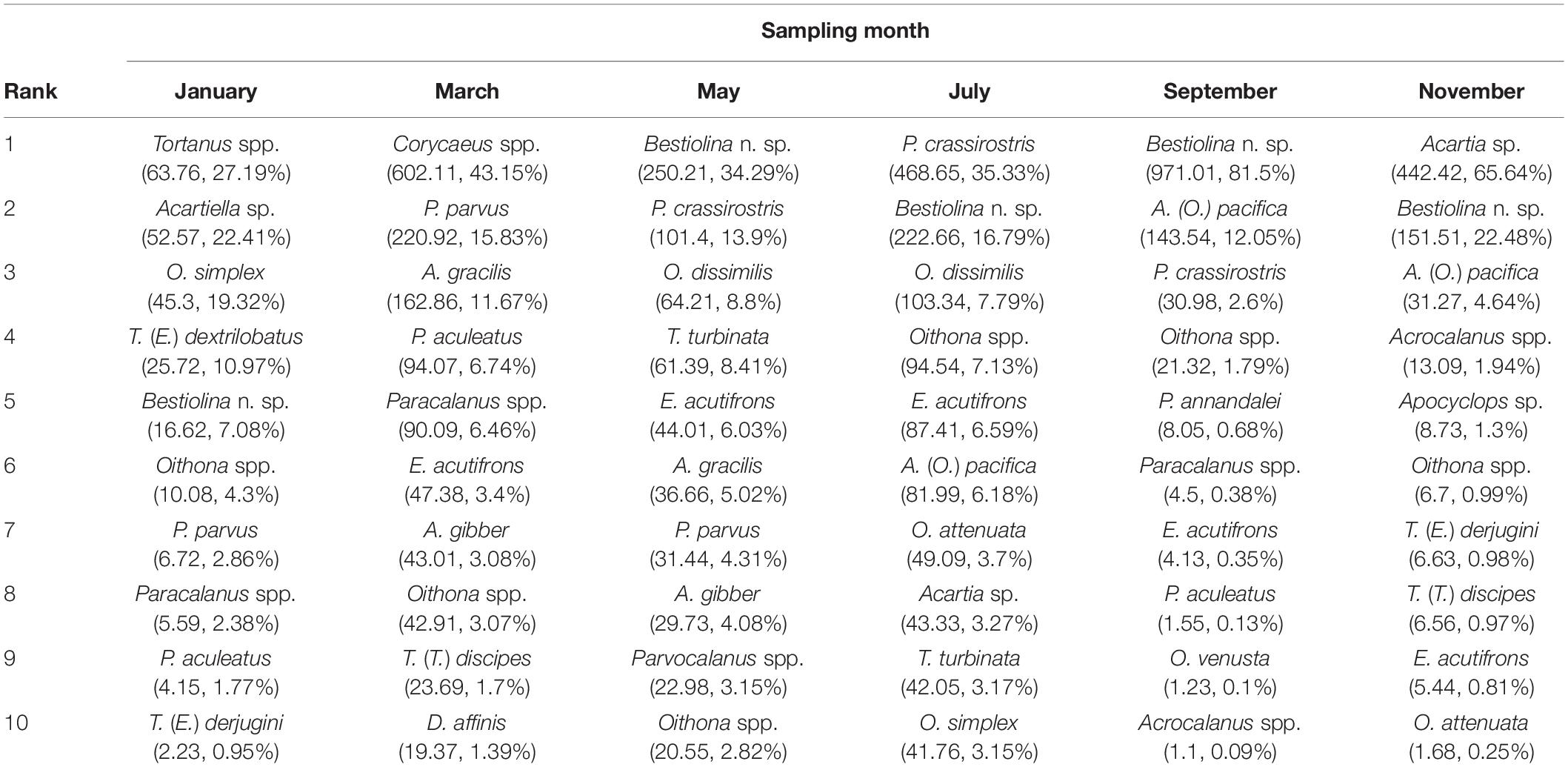
Table 2. Average density (individuals m–3), and relative abundance (RA, %) of the 10 most abundant copepod species recorded on each bimonthly sampling date in 2014.
When the Bray-Curtis similarity distance was calculated for associations among the 16 most abundant adult copepod taxa, those with similar distribution patterns formed five clusters or groups that reflected preferences for particular hydrographic conditions (Figure 6A and Table 3). Group A I, comprising Oithona simplex and Tortanus spp., occurred in three samples from January, May, and July that were characterized by high salinity (26.82 ± 11.89 PSU), high pH (8.0 ± 0.35), and high DO (6.53 ± 0.49 mg/L). Group A II, comprising the common taxa Parvocalanus crassirostris, Oithona spp., Paracalanus parvus parvus sensu lato, and Paracalanus spp., occurred in 15 samples collected throughout the year on all six cruises. The third group (Group B II) comprised four taxa, viz., Acrocalanus gibber, Acrocalanus gracilis, Paracalanus aculeatus, and Corycaeus spp., that occurred from January to September, but not in November when water temperatures were low (23.60 ± 5.07°C). The fourth group (Group C I) included three taxa, Acartia (Odontacartia) pacifica, Acartia sp., and Bestiolina n. sp., that appeared in 19 samples taken from January to July, when low salinity (20.36 ± 8.77 PSU) and low DO (5.02 ± 1.10 mg/L) were prevalent. The remaining three taxa, viz., E. acutifrons, O. dissimilis, and T. turbinata, comprising Group C II, were characterized by occurring at the highest average water temperature (26.99 ± 2.87°C). The copepod associations in group C II; among them, 12 samples were collected in May, July, and September when the water temperature was relatively high (Table 3). The present analysis demonstrates a substantial temporal succession within the copepod assemblage in the estuary of the Danshuei River, resulting at least in part from the interaction of river water and seawater (Figure 6A), and also a pattern of preference by certain groups of copepod taxa for particular environmental conditions (Table 3). Nonmetric multidimensional scaling (NMDS) demonstrated a similar distribution pattern as the cluster analysis (Figure 6B). The copepod groups among intra-annual samples were separated by cluster analyses, which further revealed and confirmed a pattern of preference for environmental conditions (Table 3).
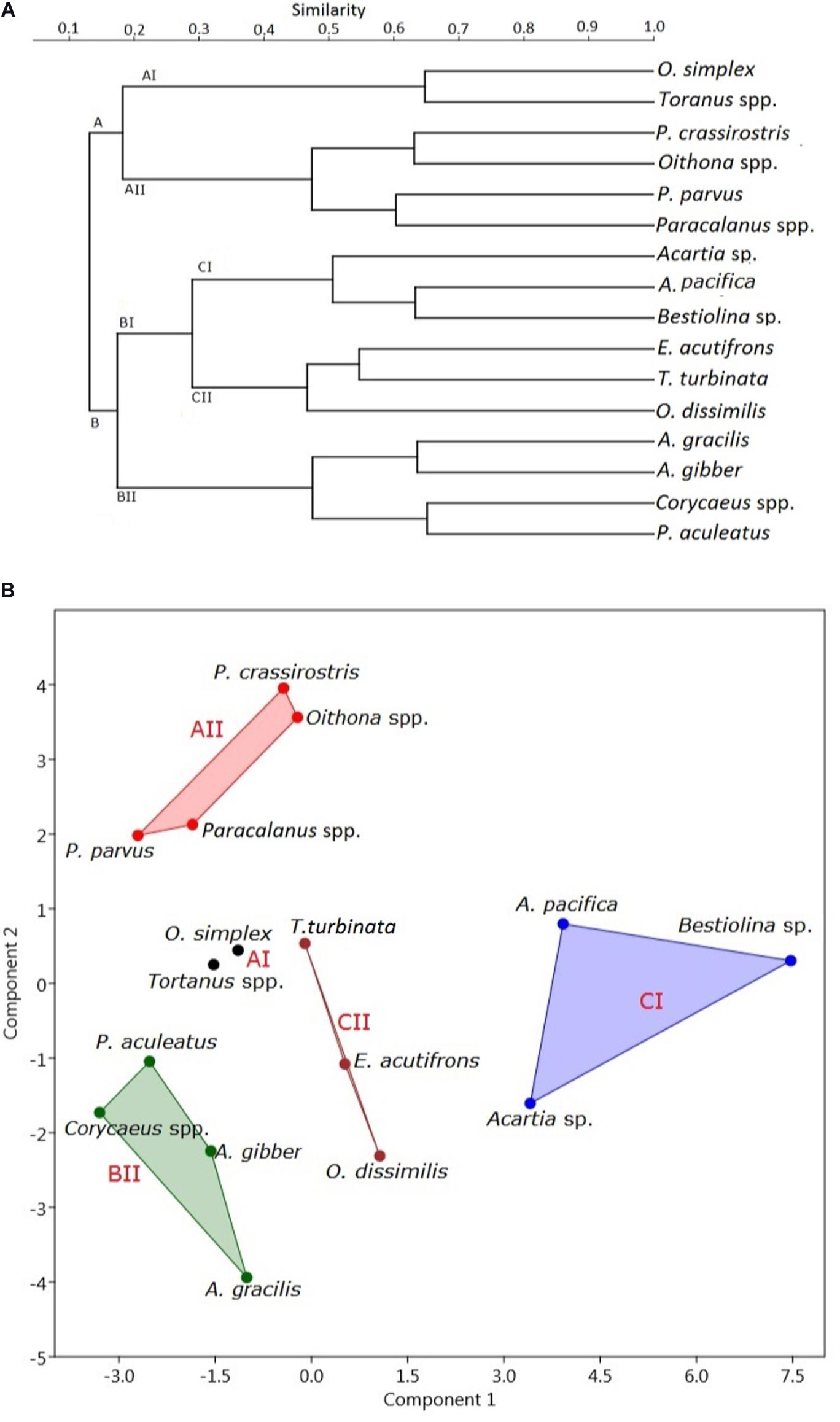
Figure 6. Cluster dendrograms of distribution plotted using the Bray–Curtis similarity distance (A) and nonmetric multidimensional scaling (B) of the 16 most abundant copepod species (comprising 93.44% of the total copepods; full names given in Supplementary Table 2) collected on six bimonthly sampling dates in 2014 in the Danshuei River estuary.
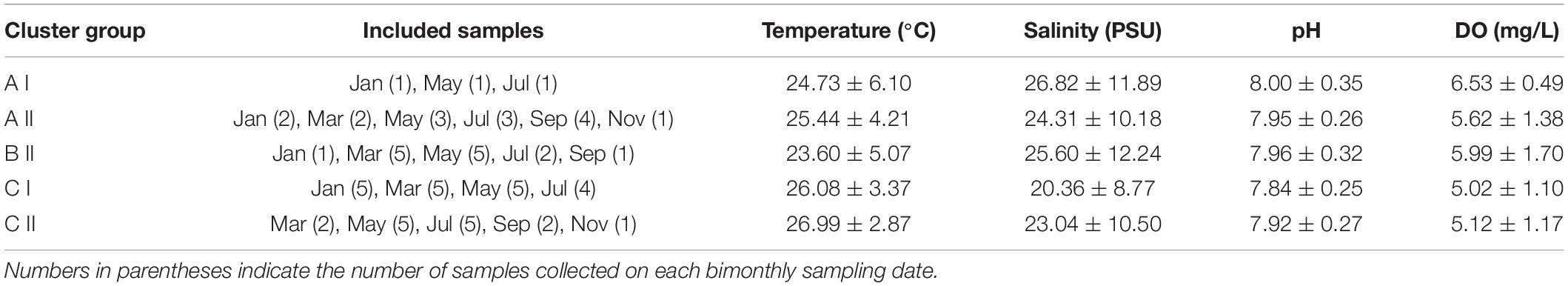
Table 3. Average (mean ± standard deviation) temperatures, salinity, pH, and dissolved oxygen concentration (DO) of copepod species occurring in the samples included in Figure 6.
Statistical Analysis
The copepod community in the study area showed a successional pattern among all six bimonthly cruises (Table 2 and Supplementary Table 2). Statistical testing to compare their temporal and spatial variability in more detail confirmed that: (1) copepod abundance showed bimonthly variation (Figures 7A,A-1); (2) total copepod abundance was significantly higher in March than in January (p = 0.038) (Figure 7B); (3) taxon number was significantly higher in May than in January (p = 0.025) (Figure 7C); (4) the richness index was not significantly different among sampling dates (p > 0.05) (Figure 7D); (5) the index of evenness was significantly lower in September than in January (p = 0.009), March (p = 0.008), and December (p = 0.01) (Figure 7E); and (6) the Shannon–Wiener diversity index was significantly higher in May than in September (p = 0.017) (Figure 7F). By contrast, the mean values of total copepod abundance and taxon number, the indices of richness and evenness, and the Shannon–Wiener diversity were not significantly different among the five sampling stations based on pooled data (p > 0.05).
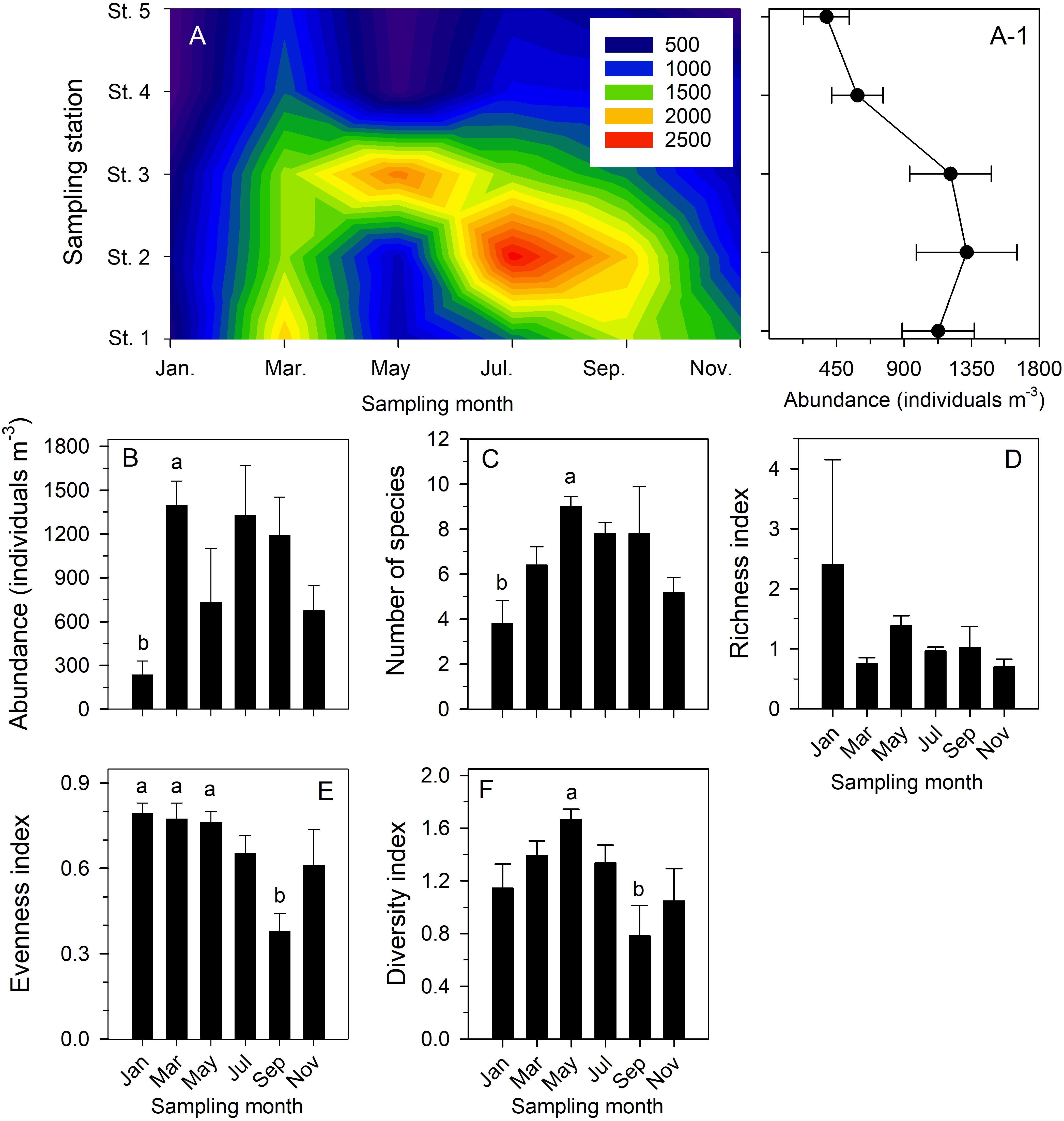
Figure 7. Contour chart showing temporal variation in total copepod abundance (individuals m− 3) of six bimonthly sample series taken in Danshuei River estuary in 2014 (A); mean abundance (±standard deviation) of total copepod abundance at each sampling station during all six bimonthly sampling cruises (A-1); and comparisons of bimonthly changes in mean (±standard deviation) abundance (B), number of species (C), and indices of richness (D), evenness (E), and Shannon–Wiener diversity (F) over the year at all stations. Various superscripts indicate significant differences (p < 0.05) confirmed by one-way analyses of variance, followed by Tukey tests.
Pearson’s correlation showed that the number of copepod taxa was significant positively correlated with sea surface temperature (SST; r = 0.49, p = 0.006) but significant negatively correlated with the evenness index (r = −0.388, p = 0.038). Salinity showed a significant positive correlation with both the number of copepod taxa (r = 0.371, p = 0.043) and total copepod abundance (r = 0.636, p < 0.001), and the latter was significant positively correlated with pH (r = 0.71, p < 0.001) and DO (r = 0.364, p = 0.048). Among the identified copepods, the abundances of P. aculeatus (r = –0.399, p = 0.03) and Corycaeus spp. (r = –0.497, p = 0.01) were negatively correlated with seawater temperature while five other taxa showed significant positive changes (p < 0.05) in abundance with seawater temperature (Table 4): A. (O.) pacifica, Bestiolina n. sp., P. crassirostris, T. turbinata, and O. dissimilis. The abundance of three taxa (A. gibber, Paracalanus spp., and T. turbinata) were positively correlated with pH (p < 0.05) while the abundance of each of the following six species was positively correlated with salinity (p < 0.05): A. gibber, A. gracilis, P. aculeatus, P. parvus, T. turbinata, and Corycaeus (D.) erythraeus (Table 4). The abundance of Sinocalanus sinensis (r = –0.358, p = 0.05) was negatively correlated with DO while seven other taxa showing significant positive (p < 0.05) changes abundance with increased DO (Table 4).
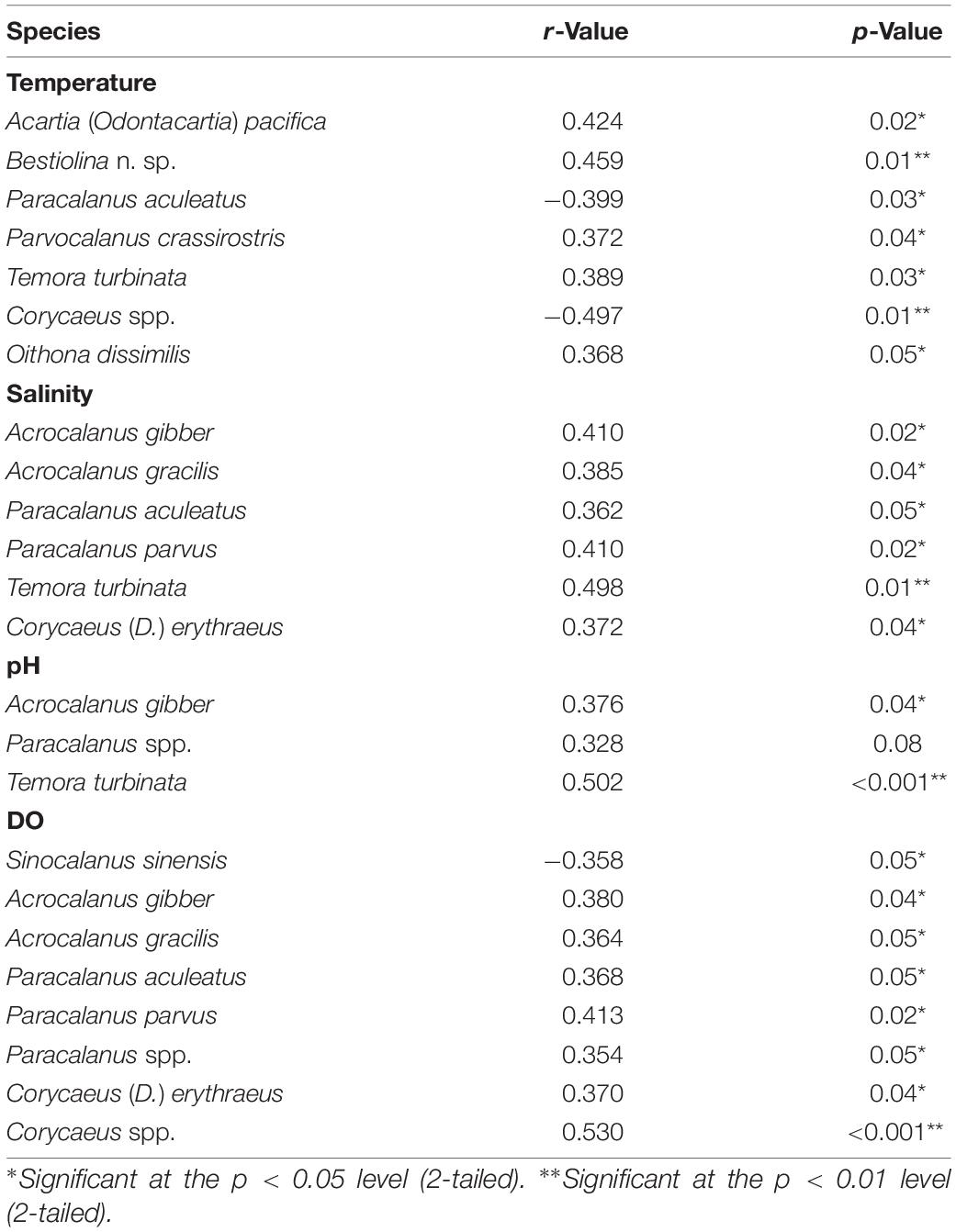
Table 4. Significant correlations between the density of copepod species and temperature, salinity, pH, and dissolved oxygen concentration (DO), as determined by Pearson’s correlation analysis.
Remarks on Brackish Water and Freshwater Copepods
Only one truly freshwater copepod species, Mongolodiaptomus birulai, was present in our samples from the Danshuei River estuary. Most of the other copepods identified in the present study, aside from the marine/brackish-water species and brackish/freshwater species mentioned below, were fully marine species, part of the zooplankton community transported by seawater intrusion into the waters of the estuary. Of the eight species that occur in both marine and brackish-water habitats—A. (O.) pacifica, S. sinensis, Pseudodiaptomus annandalei, Pseudodiaptomus inopinus, O. attenuata, O. simplex, T. (T.) discipes, and E. acutifrons (Supplementary Table 2)—the four euryhaline species were P. annandalei, P. inopinus, O. attenuata, and O. simplex. Species that occur in both fresh and brackish water accounted for 20.5% of the copepod species recorded in this study, and 11.85% of the individual adult copepods. Overall, the most abundant species was A. (O.) pacifica (42.8 ± 87.66 inds. m–3, RA: 4.63%), followed by E. acutifrons (31.39 ± 73.21 inds. m–3, RA: 3.39%) and O. simplex (16.91 ± 47.77 inds. m–3, RA: 1.83%) while the highest frequency of occurrence was recorded for A. (O.) pacifica (present in 36.7% of the samples), followed by O. attenuata and T. (T.) discipes (each 20%) (Supplementary Table 2).
Discussion
Seasonal Meteorology and Hydrological Changes
The three main hydrographic factors affecting estuarine hydrosystems are coastal currents, tidal movements, and upstream runoff. Satellite images showed that the present study area was affected by the cold water masses of the CCC, which were replaced by warm water masses from the South China Sea and the Kuroshio Current when the CCC retreated toward the coast of mainland China (Figure 2). Tidal movements occur in cycles, twice a day, and the salinity and temperature records made while sampling show that the estuarine waters were heavily influenced by tidal changes (Figure 3). Most notable was a salinity record of 35 PSU (indicating seawater intrusion) in September at station 5, 8.89 km upstream from the coast (Figure 3H). Marine zooplankton can surely intrude even higher than this in the Danshuei River by means of seawater transport.
The northeast monsoon prevails from November to March in Taiwan (Liang et al., 2003). The air temperature decreases, and rainfall in the mountains causes more freshwater flushing of the Danshuei River during that period. The monsoon thus potentially affects the hydrographic conditions of the estuary (Wyrtki, 1961; Liang et al., 2003), and may induce changes in the river’s plankton diversity (Khanna et al., 2012; Bhutiani and Khanna, 2014). The monsoon also influences the mass transport of water along the northwest coast of Taiwan, thereby also changing the structure of the marine coastal planktonic copepod communities (Hwang and Wong, 2005; Yu, 2005; Tseng et al., 2008; Hwang et al., 2014). During the present study, the present survey area along with the rest of northern Taiwan experienced two meteorological events, the moderate Typhoon Matmo (July 21–23, 2014) and the mild Typhoon Fung-Wong (September 19–22, 2014). Typhoons can affect the marine plankton’s community structure, as has been documented for phytoplankton (Chung et al., 2012; Yasuki et al., 2013; Grossmann et al., 2014), jellyfish (López-López et al., 2012; Tseng et al., 2015), and copepods (Chou et al., 2012; Beyrend-Dur et al., 2013; Grossmann et al., 2014). However, the July and September samplings in the present study both took place about a week after these typhoons had passed, long enough for any runoff-caused changes in the estuary’s zooplankton assemblages to have dissipated.
The present study’s hydrological data show clear differences in water temperature from one bimonthly sampling date to the next. Other hydrological parameters (salinity, pH, and DO), however, were subject to mixing by tidal movements and the interplay of seawater and river water, and showed no such definite long-term temporal pattern. In other words, hydrographic changes reflecting the seasonal alternation of coastal water masses were clearer than fluctuations engendered by the tidal cycle.
Dynamic Assemblages of Zooplankton and Copepods
In the present study, significant structural changes were evident in the composition of the zooplankton (Figure 4) and its copepod fraction (Table 2 and Figure 5) in different sampling months. Even so, copepods (including Calanoida, Cyclopoida, and Harpacticoida) always dominated the zooplankton, as has been reported many times previously from waters in the East China Sea (e.g., Lan et al., 2008), South China Sea (e.g., Lo et al., 2014), and coastal areas of Taiwan (e.g., Lee et al., 2018).
The composition of zooplankton in Taiwan’s northern waters is known to be affected by the CCC and the Kuroshio Current (Lan et al., 2008; Chou et al., 2012; López-López et al., 2013; Hsieh et al., 2004). The CCC, originating from the Yellow and Bohai seas, is characterized by low-temperature and low-salinity waters. It occupies the southwestern part of the East China Sea while the northeast monsoon prevails (Tseng et al., 2008). The dominant bioindicator of planktonic copepods in the CCC is the calanoid Calanus sinicus (Hwang and Wong, 2005; Lan et al., 2008). Its population size in the southern East China Sea changes in response to climate change and the monsoon (Molinero et al., 2018), and there are biogeographic indications that the CCC transports it along the coast of mainland China as far as Hainan Island in the northwestern South China Sea (Yin et al., 2011) and Kueishan Island off northeastern Taiwan (Tseng et al., 2013). During the present study, our record of C. sinicus in March (Supplementary Table 2), which corresponded to the lowest water temperature observed all year (Figure 3A), also confirmed past reports of C. sinicus in the estuary and plume areas of the Danshuei River (Hwang et al., 2006, 2009, 2010), although at a lower density than before, 3.23 ± 7.22 inds. m–3 versus 6852 ± 39039 inds. 10–3 m–3 (Hwang et al., 2006). The relatively low density was probably due to dilution of the seawater by river water in the estuary.
The waters of the Kuroshio Current, which affect the northern waters of Taiwan while the southwest monsoon prevails, are characterized by both high temperature and high salinity (Lan et al., 2008; Tseng et al., 2011b). After the seawater temperature rises, the calanoid Temora turbinata becomes the most dominant species of copepod off northern coastal Taiwan, occurring widely in both the northwestern (Chang and Fang, 2004; Tseng et al., 2008) and northeastern coastal waters (Hwang et al., 2004, 2006, 2009, 2010; Chou et al., 2012). In the present study it appeared in May, July, and September, and its abundance was ranked 4th (RA: 8.41%), 9th (RA: 3.17%) and 11th (RA: 0.08%), respectively, among all identified communities in samples collected in May, July, and September (Supplementary Table 2). Its appearance was significant positively correlated with increasing water temperature and salinity (Table 4). All the present results concerning T. turbinata are consistent the above-cited previous observations from the coastal waters of northern Taiwan (Hwang et al., 2004, 2006, 2009, 2014; Chou et al., 2012).
In the present study, several of the dominant copepod taxa appeared in the estuary of the Danshuei River only during specific periods (Table 2). For example, Parvocalanus crassirostris (RA: 10.98%) appeared in March-September, when its abundance was significantly positive correlated with water temperature and Paracalanus aculeatus was recorded in January–May and September, when its density was significant negatively correlated with seawater temperature but significantly positively correlation with salinity and dissolved oxygen. Several studies have investigated the planktonic copepod composition in various parts of the southern East China Sea for periods longer than 1 year (Hwang et al., 2004, 2006, 2009; Chou et al., 2012), but the temporal scale was seasonal (four sampling intervals per year) and the information of the change in dominant species were less accurate then here. The sampling interval in the present study was every 2 months, which has improved our understanding of the temporal occurrence of the species. Shortening the sampling interval in the Danshuei River estuary to monthly would allow an even more precise understanding of copepod community succession there.
In the copepod assemblages revealed by the present study, most of the taxa were of marine origin (Supplementary Table 2) and the densities of many copepod taxa were significantly correlated with one or more hydrographic parameters (Table 4). Although salinity and temperature (Stepien et al., 1981; Milione and Zeng, 2008), intruding seawater, rainfall, and freshwater runoff (Yu, 2005; Dahms et al., 2012, 2013; Tseng et al., 2013), tidal movements (Shih, 2007), turbidity (Islam et al., 2005), and assorted physico-chemical parameters of river water (Khanna et al., 2005; Kumar et al., 2015) are known to affect the community composition of both phytoplankton and the zooplankton, the present results show that intruding seawater is the most important transportation vector in the Danshuei River estuary. Namely, the number of taxa and the total abundance/density of copepods were both significant positively correlated with changes in salinity, most copepods in the estuary belonged to marine species, and intrusive seawater was shown to deliver marine species to at 8.89 km upstream from the coast. These results are furthermore consistent with earlier studies of zooplankton and copepods in Taiwan’s Lanyang River (Dahms et al., 2012, 2013; Tseng et al., 2016).
Copepods in Estuaries
A comparison of the present study’s results with similar data from estuaries around the world indicates a clear pattern of geographic regionalism in most cases (Table 5). The composition of the dominant taxa of copepods in estuaries around the world varies greatly. A comparison with the neighboring countries of China (Tan et al., 2004; Gao et al., 2008), Japan (Sakaguchi et al., 2011; Watanabe et al., 2014), and South Korea (Sakaguchi et al., 2011) reveals, however, a common pattern was in the changes of seasonally dominant species. Especially interesting is a study from Japan involving eight dominant copepods collected from 45 estuaries. Among them, Acartia ohtsukai, Pseudodiaptomus inopinus and Sinocalanus tenellus also occur in four estuaries in South Korea (Sakaguchi et al., 2011), but only P. inopinus appeared in the present study in Taiwan. This may be an indication that predominantly estuarine species cannot easily undergo long-distance dispersal across oceans. In different parts of the world such as India (Madhu et al., 2007; Rakhesh et al., 2013; Paul et al., 2019), Europe (Vieira et al., 2003), Africa (Kibirige and Perissinotto, 2003; Froneman, 2004; Carrasco and Perissinotto, 2015; Ounissi et al., 2016), America (Krumme and Liang, 2004; Araujo et al., 2008; Howson et al., 2017; Salvador and Bersano, 2017; Breckenridge et al., 2020), and Oceania (Rose et al., 2020), it has been shown repeatedly that differences in the composition of the dominant copepod fauna in estuaries increase with distance. The abundance and diversity of zooplankton communities showed spatial, seasonal, and interannual trends in Barnegat Bay (Howson et al., 2017), and also in the Paranagua estuary (Salvador and Bersano, 2017). Worth mentioning is that Paul et al. (2019) recorded extremely high value of zooplankton gatherings, with a density of 1,090,550 (Inds/m3) in the estuary of the Ganges River. The different mesh sizes of nets used to collect estuarine zooplankton, ranging from 60 μm (Froneman, 2004) to 500 μm (Howson et al., 2017), might also affect the composition of the dominant species (Tseng et al., 2011a). The mesh size of zooplankton nets used to collect samples in the estuary ranged from 60 μm (Froneman, 2004) to 500 μm (Howson et al., 2017). Studies using small-mesh nets have found copepodites to be the most dominant fraction in the samples (Vieira et al., 2003), but most of the literature, including the present study, has been focused on adult copepods (Table 5), mainly because of the difficulty in identifying copepodites.
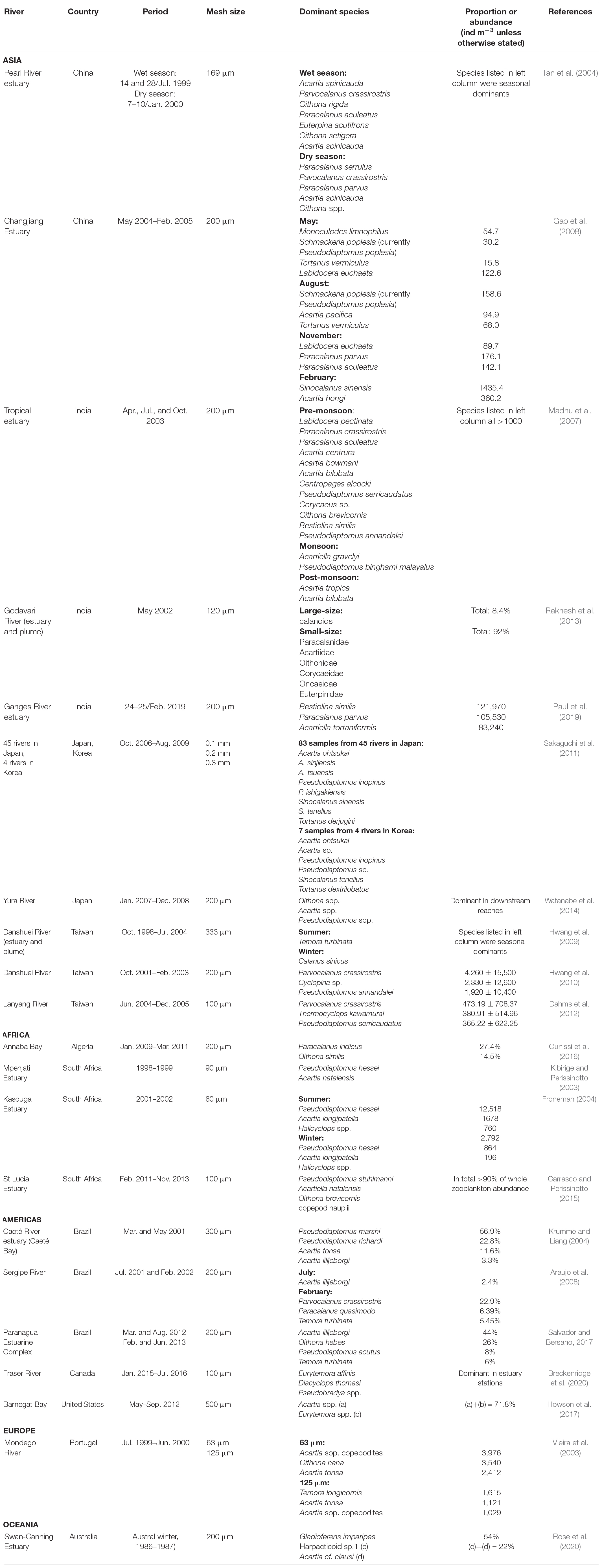
Table 5. List of the dominant copepods recorded from the downstream reaches and estuaries of rivers around the world.
Previous research on brackish-water and freshwater copepods in Taiwan has been limited (Dahms et al., 2012, 2013; Tseng et al., 2016). Nonetheless, the existing literature on the taxonomy of copepods in different rivers in Taiwan, including the Danshuei River in the island’s northwest (Chen, 2005; Yu, 2005; Hwang et al., 2006, 2009, 2010), is due for a review. The three other previously surveyed rivers in Taiwan are the Choshuei River in the west-central part of the island (Sun et al., 2014), the Gaoping River in the southwest (Yu, 2005; Mai, 2016), and the Lanyang River in the northeast (Dahms et al., 2012, 2013; Tseng et al., 2016). Most of the reports surveyed zooplankton and/or copepods in the both rivers’ estuaries and plume areas (Chen, 2005; Yu, 2005; Hwang et al., 2006, 2009, 2010; Mai, 2016), but only a few surveys were conducted in estuaries (Dahms et al., 2012, 2013; Tseng et al., 2016). The copepods studied by Chen (2005) and Sun et al. (2014) were identified only to the generic level, and Hwang et al. (2006, 2009) did not record the purely brackish-water and freshwater species.
Before the present study, 55 species of copepod from brackish water and freshwater had been recorded in Taiwan (Table 6). It is noteworthy that 32 of them were found in the Danshuei River, accounting for 58.2% of the total number of known brackish-water and freshwater species in Taiwan. Among them, five species occurred only in the Danshuei River: the calanoids A. sinensis and P. forbesi, the cyclopoids O. fragilis and O. simplex, and the harpacticoid T. (T.) discipes (Yu, 2005; Hwang et al., 2010; present study). It is worth noting that five species of brackish-water copepods were commonly recorded in all three of the other rivers: the calanoids A. (E.) southwelli, S. sinensis, and P. annandalei, the cyclopoid O. attenuata, and the harpacticoid E. acutifrons; as well as M. birulai, a freshwater calanoid copepod (Yu, 2005; Dahms et al., 2012, 2013; Sun et al., 2014; Mai, 2016) (Table 5).
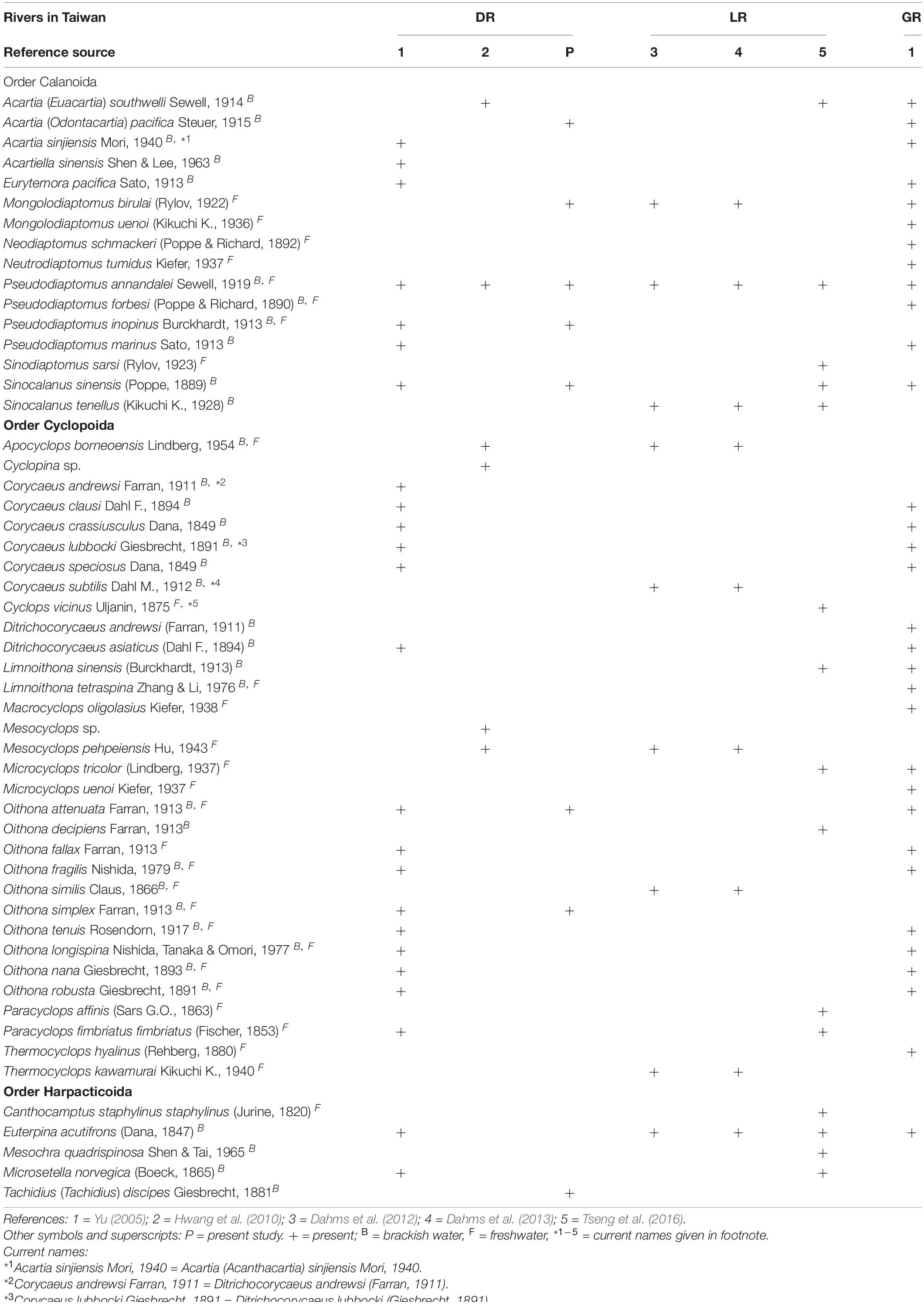
Table 6. List of previously recorded brackish-water and freshwater copepod species from the Danshuei (DR), Lanyang (LR), and Gaoping (GR) rivers in Taiwan, along with those recorded from the Danshuei River in the present study.
Conclusion
The present study has shown that the spatial and temporal patterns of zooplankton distribution in the estuary of the Danshuei River were more affected by tidal movements than by other hydrographic changes, and that the assemblages of zooplankton, and in particular copepods, underwent a clear succession pattern during the course of the year. In addition, this study provided evidence that the CCC and Kuroshio Current affect the composition and abundance of zooplankton and copepods in the Danshuei River. Tidal movements transported many coastal zooplankters far up into the estuary. Up until now there have been only scant reports on the zooplankton and copepods of the Danshuei River, based on samples taken at long intervals. The present results suggest that an increase in sampling frequency and number of sampling stations will provide better data for understanding the intra-annual changes of zooplankton composition in river estuaries.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
P-WL contributed to the manuscript preparation, data analysis and interpretation, field sampling, and manuscript review and editing. S-HH contributed to the copepod identification, and manuscript review and editing. CC contributed to the data interpretation, and manuscript review and editing. L-CT contributed to the manuscript preparation, statistical analysis, data analysis and interpretation, field sampling, and manuscript review and editing. J-SH contributed to the funding acquisition, participation in discussions, and overseeing of manuscript production as a project leader. All authors contributed to the article and approved the submitted version.
Funding
The financial support from the Ministry of Science and Technology of Taiwan to J-SH (Grant Nos. MOST 107-2621-M-019-001, MOST 108-2621-M-019-003, and MOST 109-2621-M-019-002) and Center of Excellence for Ocean Engineering (Grant No. 109J13801-51), as well as to L-CT (MOST 107-2811-M-019-004, MOST 108-2811-M-019-504, and MOST 109-2811-M-019-504). The funders had no role in the study design, data collection and analysis, or decision to publish, nor in the manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the reviewers for their invaluable, detailed suggestions and criticisms, which greatly enhanced this paper, and Dr. Mark J. Grygier for post-review English revision. We are grateful to Dr. Kandasamy Kalimuthu and Captain Wan-Yi Weng of the fishing boat Wanjing X for their assistance during the fieldwork in the Danshuei River. We are equally thankful to Mr. Chih-Ming Lin and Mrs. Hsin-Yi Lo for their assistance in the laboratory. We finally thank the Fisheries Research Institute for providing the Sea Surface Temperature images (https://en.tfrin.gov.tw/), and the National Land Surveying and Mapping Center Taiwan MAP Service (https://maps.nlsc.gov.tw/) for providing other maps.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.598274/full#supplementary-material
Supplementary File 1 | The supporting information file provides information about all figures.
References
Araujo, H. M. P., Nascimento-Vieira, D. A., Neumann-Leitaþo, S., Schwamborn, R., Lucas, A. P. O., and Alves, J. P. H. (2008). Zooplankton community dynamics in relation to the seasonal cycle and nutrient inputs in an urban tropical estuary in Brazil. Braz. J. Biol. 68, 751–762. doi: 10.1590/s1519-69842008000400009
Beyrend-Dur, D., Souissi, S., and Hwang, J.-S. (2013). Population dynamics of calanoid copepods in the subtropical mesohaline Danshuei estuary (Taiwan) and typhoon effects. Ecol. Res. 28, 771–780. doi: 10.1007/s11284-013-1052-y
Bhutiani, R., and Khanna, D. R. (2014). Qualitative studies on planktonic diversity of River Ganga at Haridwar. Biotechnol. Int. 7, 101–108.
Box, G. E. P., and Cox, D. R. (1964). An analysis of transformations. J. R. Stat. Soc. B. Stat. Methodol. 26, 211–246.
Breckenridge, J., Pakhomov, E., Emry, S., and Mahara, N. (2020). Copepod assemblage dynamics in a snowmelt-dominated estuary. Estuar. Coasts 43, 1502–1518. doi: 10.1007/s12237-020-00722-3
Carrasco, N. K., and Perissinotto, R. (2015). Zooplankton community structure during a transition from dry to wet state in a shallow, subtropical estuarine lake. Cont. Shelf Res. 111, 294–303. doi: 10.1016/j.csr.2015.08.027
Chang, W.-B., and Fang, L.-S. (2004). Temporal and spatial variations in the species composition, distribution, and abundance of copepods in Kaohsiung Harbor, Taiwan. Zool. Stud. 43, 454–463.
Chao, S.-J. (2006). The Study of Trace Metals in Perna viridis in the Danshuei area. Ph. D. Thesis, National Taiwan Ocean University, Keelung.
Chen, L.-H. (2005). Studies on the Seasonal Abundance, Temporal and Spatial Distribution of Zooplankton in Tan-Shui Estuary, Northern Taiwan. Ph. D. Thesis, National Taiwan Ocean University, Keelung.
Chen, W. L., Gwo, J. C., Wang, G. S., and Chen, C. Y. (2014). Distribution of feminizing compounds in the aquatic environment and bioaccumulation in wild tilapia tissues. Environ. Sci. Pollut. Res. Int. 21, 11349–11360. doi: 10.1007/s11356-014-3062-x
Cheng, B. Y., Liu, T. C., Shyu, G. S., Chang, T. K., and Fang, W. T. (2011). Analysis of trends in water quality: constructed wetlands in metropolitan Taipei. Water Sci. Technol. 64, 2143–2150. doi: 10.2166/wst.2011.785
Chihara, M., and Murano, M. (1997). An Illustrated Guide to Marine Plankton in Japan. Tokyo: Tokyo University Press.
Chou, C., Tseng, L.-C., Ou, C.-H., Chen, Q.-C., and Hwang, J.-S. (2012). Seasonal succession of planktonic copepods in bight environments of northeastern Taiwan. Zool. Stud. 51, 1380–1396.
Chung, C. C., Gong, G. C., and Hung, C. C. (2012). Effect of typhoon Morakot on microphytoplankton population dynamics in the subtropical Northwest Pacific. Mar. Ecol. Prog. Ser. 448, 39–49. doi: 10.3354/meps09490
Dahms, H.-U., Tseng, L.-C., Hsiao, S.-H., Chen, Q.-C., and Hwang, J.-S. (2013). A model study for an Oceania watershed: spatio-temporal changes of mesozooplankton in riverine and estuarine parts of the Lanyang River in Taiwan. Ecol. Res. 28, 345–357. doi: 10.1007/s11284-012-1026-5
Dahms, H.-U., Tseng, L.-C., Hsiao, S.-H., Chen, Q.-C., Kim, B.-R., and Hwang, J.-S. (2012). Biodiversity of planktonic copepods in the Lanyang River (northeastern Taiwan), a typical watershed of Oceania. Zool. Stud. 51, 160–174.
Elliott, M., and McLusky, D. S. (2002). The need for definitions in understanding estuaries. Estuar. Coast. Shelf Sci. 55, 815–827. doi: 10.1006/ecss.2002.1031
Froneman, P. W. (2004). Food web dynamics in a temperate temporarily open/closed estuary (South Africa). Estuar. Coast. Shelf Sci. 59, 87–95. doi: 10.1016/j.ecss.2003.08.003
Gao, Q., Xu, Z., and Zhuang, P. (2008). The relation between distribution of zooplankton and salinity in the Changjiang estuary. Chin. J. Oceanol. Limnol. 26, 178–185. doi: 10.1007/s00343-008-0178-1
Godhantaraman, N., and Uye, S.-i (2003). Geographical and seasonal variations in taxonomic composition, abundance and biomass of microzooplankton across a brackish-water lagoonal system of Japan. J. Plankton Res. 25, 465–482. doi: 10.1093/plankt/25.5.465
Grossmann, M. M., Gallager, S. M., and Mitarai, S. (2014). Continuous monitoring of near-bottom mesoplankton communities in the East China Sea during a series of typhoons. J. Oceanogr. 71, 115–124. doi: 10.1007/s10872-014-0268-y
Hammer, Ø, Harper, D. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 4–9.
Howson, U. A., Buchanan, G. A., and Nickels, J. A. (2017). Zooplankton community dynamics in a western mid-Atlantic lagoonal estuary. J. Coast. Res. 78, 141–168. doi: 10.2112/si78-012.1
Hsiao, S.-H. (2009). Copepod Species Compositions and Body Contents of Trace Metals in the Waters of Northern Taiwan. Ph. D. Dissertation, National Taiwan Ocean University, Keelung.
Hsieh, C.-H., Chiu, T.-S., and Shih, C.-t (2004). Copepod diversity and composition as indicators of intrusion of the Kuroshio Branch Current into the northern Taiwan Strait in spring 2000. Zool. Stud. 43, 393–403.
Hwang, J.-S., Kumar, R., Hsieh, C.-W., Kuo, A. Y., Souissi, S., Hsu, M.-H., et al. (2010). Patterns of zooplankton distribution along the marine, estuarine, and riverine portions of the Danshuei ecosystem in northern Taiwan. Zool. Stud. 49, 335–352.
Hwang, J.-S., López-López, L., Molinero, J.-C., Tseng, L.-C., Chen, Q.-C., and Hung, J.-J. (2014). Copepod assemblages in the northern South China Sea during inter-monsoon transition periods. J. Sea Res. 86, 43–48. doi: 10.1016/j.seares.2013.10.012
Hwang, J.-S., Souissi, S., Dahms, H.-U., Tseng, L.-C., Schmitt, F. G., and Chen, Q.-C. (2009). Rank-abundance allocations as a tool to analyze planktonic copepod assemblages off the Danshuei River estuary (Northern Taiwan). Zool. Stud. 48, 49–62.
Hwang, J.-S., Souissi, S., Tseng, L.-C., Seuront, L., Schmitt, F. G., Fang, L.-S., et al. (2006). A 5-year study of the influence of the northeast and southwest monsoons on copepod assemblages in the boundary coastal waters between the East China Sea and the Taiwan Strait. J. Plankton. Res. 28, 943–958. doi: 10.1093/plankt/fbl031
Hwang, J.-S., Tu, Y.-Y., Tseng, L.-C., Fang, L.-S., Souissi, S., Fang, T.-H., et al. (2004). Taxonomic composition and seasonal distribution of copepod assemblages from waters adjacent to Nuclear Power Plant I and II in northern Taiwan. J. Mar. Sci. Technol. 12, 380–391.
Hwang, J.-S., and Wong, C. K. (2005). The China coastal current as a driving force for transporting Calanus sinicus (Copepoda: Calanoida) from its population centers to waters off Taiwan and Hong Kong during the winter northeast monsoon period. J. Plankton Res. 27, 205–210. doi: 10.1093/plankt/fbh162
Islam, M. S., Ueda, H., and Tanaka, M. (2005). Spatial distribution and trophic ecology of dominant copepods associated with turbidity maximum along the salinity gradient in a highly embayed estuarine system in Ariake Sea, Japan. J. Exp. Mar. Biol. Ecol. 316, 101–115. doi: 10.1016/j.jembe.2004.11.001
Kao, Y. W., and Chang, K. W. (1998). Stable carbon isotope ratio and nutrient contents of the Kandelia candel mangrove populations of different growth forms. Bot. Bull. Acad. Sin. 39, 39–45.
Khanna, D. R., Bhutiani, R., and Chandra, K. S. (2005). Modeling to assess effect of light on phytoplanktonic growth dynamics: a review. Environ. Conserv. J. 6, 103–107.
Khanna, D. R., Bhutiani, R., Matta, G., Singh, V., and Bhadauriya, G. (2012). Study of planktonic diversity of river Ganga from Devprayag to Roorkee, Uttarakhand (India). Environ. Conserv. J. 13, 211–217.
Kibirige, I., and Perissinotto, R. (2003). The zooplankton community of the Mpenjati estuary, a South African temporarily open/closed system. Estuar. Coast. Shelf Sci. 58, 727–741. doi: 10.1016/s0272-7714(03)00180-x
Krumme, U., and Liang, T.-H. (2004). Tidal-induced changes in a copepod-dominated zooplankton community in a macrotidal mangrove channel in northern Brazil. Zool. Stud. 43, 404–414.
Kumar, A., Khanna, D. R., and Bhutiani, R. (2015). Seasonal variations in physico-chemical parameters of River Beas, Himachal Pradesh with special reference to planktonic population. Environ. Conserv. J. 16, 127–131. doi: 10.36953/ecj.2015.16319
Lai, D.-S., Lin, J.-B., Liu, W.-S., Pan, L.-K., Chu, K.-H., Chen, C.-Y., et al. (2010). Metal concentrations in sediments of the Tamsui River, flows through central metropolitan Taipei. Bull. Environ. Contam. Toxicol. 84, 628–634. doi: 10.1007/s00128-010-9959-2
Lan, Y.-C., Lee, M.-A., Liao, C.-H., Chen, W.-Y., Lee, D.-A., Liu, D.-C., et al. (2008). Copepod community changes in the southern East China Sea between the early and late northeasterly monsoon. Zool. Stud. 47, 61–74.
Lee, P.-W., Tseng, L.-C., and Hwang, J.-S. (2018). Comparison of mesozooplankton mortality impacted by the cooling systems of two nuclear power plants at the northern Taiwan coast, southern East China Sea. Mar. Pollut. Bull. 136, 114–124. doi: 10.1016/j.marpolbul.2018.09.003
Lian, G., Wang, Y., Sun, R., and Hwang, J.-S. (2018). Species Diversity of Marine Planktonic Copepods in China’s Sea. Beijing: China Ocean Press.
Liang, W. D., Tang, T. Y., Yang, Y. J., Ko, M. T., and Chuang, W. S. (2003). Upper-ocean currents around Taiwan. Deep Sea Res. II 50, 1085–1105. doi: 10.1016/s0967-0645(03)00011-0
Liu, J.-W. (2014). The Analysis of Benthic Macroinvertebrate and Fish Community Structure On Medial-Lower Stream of Tanshui River Basin and Application of Biotic Index as Water Quality Indicators. Ph. D. Thesis, National Hsinchu University of Education Hsinchu, Taiwan.
Lo, W.-T., Dahms, H.-U., and Hwang, J.-S. (2014). Water mass transport through the northern Bashi Channel in the northeastern South China Sea affects copepod assemblages of the Luzon Strait. Zool. Stud. 53:66.
López-López, L., Molinero, J. C., Tseng, L.-C., Chen, Q.-C., Houng, J.-W., and Hwang, J.-S. (2012). Effects of typhoons on gelatinous carnivore zooplankton off Northern Taiwan. Cah. Biol. Mar. 53, 349–355.
López-López, L., Molinero, J.-C., Tseng, L.-C., Chen, Q.-C., and Hwang, J.-S. (2013). Seasonal variability of the gelatinous carnivore zooplankton community in Northern Taiwan. J. Plankton Res. 35, 677–683. doi: 10.1093/plankt/fbt005
Madhu, N. V., Jyothibabu, R., Balachandran, K. K., Honey, U. K., Martin, G. D., Vijay, J. G., et al. (2007). Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin backwaters – India). Estuar. Coast. Shelf Sci. 73, 54–64. doi: 10.1016/j.ecss.2006.12.009
Mai, Y.-S. (2016). Assemblage Structure and Polycyclic Aromatic Hydrocarbons Accumulation of Zooplankton in the Gaoping Coastal Waters. Ph. D. Thesis, National Sun Yat-sen University, Kaohsiung.
Meire, P., Ysebaert, T., Van Damme, S., Van den Bergh, E., Maris, T., and Struyf, E. (2005). The Scheldt estuary: a description of a changing ecosystem. Hydrobiologia. 540, 1–11. doi: 10.1007/s10750-005-0896-8
Milione, M., and Zeng, C. (2008). The effects of temperature and salinity on population growth and egg hatching success of the tropical calanoid copepod, Acartia sinjiensis. Aquaculture. 275, 116–123. doi: 10.1016/j.aquaculture.2007.12.010
Molinero, J.-C., Tseng, L.-C., Abbate, C. L., Ramirez-Romero, E., and Hwang, J.-S. (2018). Interannual changes in zooplankton echo subtropical and high latitude climate effects in the southern East China Sea. PLoS One 13:e0197382. doi: 10.1371/journal.pone.0197382
Ounissi, M., Laskri, H., and Khélifi-Touhami, M. (2016). Net-zooplankton abundance and biomass from Annaba Bay (SW Mediterranean Sea) under estuarine influences. Mediterr. Mar. Sci. 17, 519–532. doi: 10.12681/mms.1474
Paul, S., Karan, S., Ghosh, S., and Bhattacharya, B. D. (2019). Hourly variation of environment and copepod community of the Ganges River estuary of India: perspectives on sampling estuarine zooplankton. Estuar. Coast. Shelf Sci. 230:106441. doi: 10.1016/j.ecss.2019.106441
Putri, M., Lou, C.-H., Syai’in, M., Ou, S.-H., and Wang, Y.-C. (2018). Long-term river water quality trends and pollution source apportionment in Taiwan. Water 10, 1–17.
Rakhesh, M., Raman, A. V., Ganesh, T., Chandramohan, P., and Dehairs, F. (2013). Small copepods structuring mesozooplankton community dynamics in a tropical estuary-coastal system. Estuar. Coast. Shelf Sci. 126, 7–22. doi: 10.1016/j.ecss.2013.03.025
Rose, T. H., Tweedley, J. R., Warwick, R. M., and Potter, I. C. (2020). Influences of microtidal regime and eutrophication on estuarine zooplankton. Estuar. Coast. Shelf Sci. 238:106689. doi: 10.1016/j.ecss.2020.106689
Sakaguchi, S. O., and Ueda, H. (2010). A new species of Pseudodiaptomus (Copepoda: Calanoida) from Japan, with notes on the closely related P. inopinus Burckhardt, 1913 from Kyushu Island. Zootaxa 2623, 52–68. doi: 10.11646/zootaxa.2623.1.2
Sakaguchi, S. O., Ueda, H., Ohtsuka, S., Soh, H. Y., and Yoon, Y. H. (2011). Zoogeography of planktonic brackish-water calanoid copepods in western Japan with comparison with neighboring Korean fauna. Plankton Benthos Res. 6, 18–25. doi: 10.3800/pbr.6.18
Salvador, B., and Bersano, J. G. F. (2017). Zooplankton variability in the subtropical estuarine system of Paranaguá Bay, Brazil, in 2012 and 2013. Estuar. Coast. Shelf Sci. 199, 1–13. doi: 10.1016/j.ecss.2017.09.019
Shan, X., Sun, P., Jin, X., Li, X., and Dai, F. (2013). Long-term changes in fish assemblage structure in the Yellow River estuary ecosystem, China. Mar. Coast. Fish. 5, 65–78. doi: 10.1080/19425120.2013.768571
Shen, J. R. (1979). Fauna Sinica: Arthropoda, Crustacea, Freshwater Copepoda. Beijing: Science Press.
Shih, C.-H. (2007). Study on Larval and Juvenile Fish Community Structure in the Lan-Yang Estuary. Ph. D. Thesis, National Taiwan Ocean University, Keelung.
Shih, C.-T., Chen, Q.-C., Lan, Y.-C., and Hsiao, S.-H. (2019). Calanoid Copepods of China Seas. Taipei City: Institute of Oceanography National Taiwan University.
Srinui, K., Nishida, S., and Ohtsuka, S. (2013). A new species of Pseudodiaptomus (Crustacea, Copepoda, Calanoida, Pseudodiaptomidae) from the Prasae River Estuary, Gulf of Thailand. Zookeys 338, 39–54. doi: 10.3897/zookeys.338.5531
Srinui, K., Ohtsuka, S., Metillo, E. B., and Nishibori, M. (2019). A new species of Acartia (Copepoda, Calanoida) from the Philippines, based on morphological and molecular analyses. Zookeys 814, 71–94. doi: 10.3897/zookeys.814.24601
Stepien, J. C., Malone, T. C., and Chervin, M. B. (1981). Copepod communities in the estuary and coastal plume of the Hudson River. Estuar. Coast. Shelf Sci. 13, 185–195. doi: 10.1016/s0302-3524(81)80075-8
Sun, H.-J., Lin, W.-R., and Wang, P.-H. (2014). Spatial structure and temporal variation of zooplankton community and ecological assessment of water quality in plain of Jhuoshuei River. Tunghai. Sci. 16, 39–56.
Tan, Y., Huang, L., Chen, Q., and Huang, X. (2004). Seasonal variation in zooplankton composition and grazing impact on phytoplankton standing stock in the Pearl River estuary, China. Cont. Shelf Res. 24, 1949–1968. doi: 10.1016/j.csr.2004.06.018
Telesh, I. V., and Khlebovich, V. V. (2010). Principal processes within the estuarine salinity gradient: a review. Mar. Pollut. Bull. 61, 149–155. doi: 10.1016/j.marpolbul.2010.02.008
Tseng, L.-C., Chou, C., Chen, Q.-C., and Hwang, J.-S. (2015). Jellyfish assemblages are related to interplay waters in the southern East China Sea. Cont. Shelf Res. 103, 33–44. doi: 10.1016/j.csr.2015.04.025
Tseng, L.-C., Dahms, H.-U., Chen, Q.-C., and Hwang, J.-S. (2013). Geospatial variability in the autumn community structure of epipelagic zooplankton in the upper layer of the northern South China Sea. Zool. Stud. 52, 1–12.
Tseng, L.-C., Dahms, H.-U., Hung, J.-J., Chen, Q.-C., and Hwang, J.-S. (2011a). Can different mesh sizes affect the results of copepod community studies? J. Exp. Mar. Biol. Ecol. 398, 47–55. doi: 10.1016/j.jembe.2010.12.007
Tseng, L.-C., Hsiao, S.-H., Sarkar, S. K., Bhattacharya, B. D., Chen, Q.-C., and Hwang, J.-S. (2016). Influence of Kuroshio water on the annual copepod community structure in an estuary in the northwest Pacific Ocean. Cont. Shelf Res. 118, 165–176. doi: 10.1016/j.csr.2016.02.018
Tseng, L.-C., Kumar, R., Chen, Q.-C., and Hwang, J.-S. (2011b). Faunal shift between two copepod congeners (Temora discaudata and T. turbinata) in the vicinity of two nuclear power plants in southern East China Sea: spatiotemporal patterns of population trajectories over a decade. Hydrobiologia 666, 301–315. doi: 10.1007/s10750-011-0616-5
Tseng, L.-C., Kumar, R., Dahms, H.-U., Chen, Q.-C., and Hwang, J.-S. (2008). Monsoon-driven succession of copepod assemblages in coastal waters of the northeastern Taiwan Strait. Zool. Stud. 47, 46–60.
Tseng, L.-C., Wu, C.-H., Twan, W.-H., Tang, Z.-C., and Hwang, J.-S. (2014). Hydroids (Cnidaria, Hydrozoa) from marine environments in Taiwan. Zool. Stud. 53, 1–7. doi: 10.11646/zootaxa.2570.1.1
Ueda, H., Yamaguchi, A., Saitoh, S.-i, Sakaguchi, S. O., and Tachihara, K. (2011). Speciation of two salinity-associated size forms of Oithona dissimilis (Copepoda: Cyclopoida) in estuaries. J. Nat. Hist. 45, 33–34.
Vieira, L., Azeiteiro, U., Ré, P., Pastorinho, R., Marques, J. C., and Morgado, F. (2003). Zooplankton distribution in a temperate estuary (Mondego estuary southern arm: Western Portugal). Acta Oecol. 24, S163–S173.
Walter, T. C., Ohtsuka, S., and Castillo, L. V. (2006). A new species of Pseudodiaptomus (Crustacea: Copepoda: Calanoida) from the Philippines, with a key to pseudodiaptomids from the Philippines and comments on the status of the genus Schmackeria. Proc. Biol. Soc. Wash. 119, 202–221. doi: 10.2988/0006-324x(2006)119[202:ansopc]2.0.co;2
Wang, C.-F., Hsu, M.-H., Liu, W.-C., Hwang, J.-S., Wu, J.-T., and Kuo, A. Y. (2007). Simulation of water quality and plankton dynamics in the Danshuei River estuary, Taiwan. J. Environ. Sci. Health A 42, 933–953. doi: 10.1080/10934520701369875
Wang, Y. B., Liu, C. W., Liao, P. Y., and Lee, J. J. (2014). Spatial pattern assessment of river water quality: implications of reducing the number of monitoring stations and chemical parameters. Environ. Monit. Assess. 186, 1781–1792. doi: 10.1007/s10661-013-3492-9
Watanabe, K., Kasai, A., Antonio, E. S., Suzuki, K., Ueno, M., and Yamashita, Y. (2014). Influence of salt-wedge intrusion on ecological processes at lower trophic levels in the Yura estuary, Japan. Estuar. Coast. Shelf Sci. 139, 67–77. doi: 10.1016/j.ecss.2013.12.018
Wen, L.-S., Jiann, K.-T., and Liu, K.-K. (2008). Seasonal variation and flux of dissolved nutrients in the Danshuei estuary, Taiwan: a hypoxic subtropical mountain river. Estuar. Coast. Shelf Sci. 78, 694–704. doi: 10.1016/j.ecss.2008.02.011
Wu, J.-T., and Chou, T.-L. (2003). Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar. Coast. Shelf. Sci. 58, 155–162. doi: 10.1016/s0272-7714(03)00070-2
Wyrtki, K. (1961). Physical Oceanography of the Southeast Asian Waters. Naga Report 2. San Diego, CA: UC San Diego Library – Scripps Digital Collection.
Yasuki, N., Suzuki, K., and Tsuda, A. (2013). Responses of lower trophic-level organisms to typhoon passage on the outer shelf of the East China Sea: an incubation experiment. Biogeosci. Disc. 10, 6605–6635.
Yin, J., Huang, L., Li, K., Lian, S., Li, C., and Lin, Q. (2011). Abundance distribution and seasonal variations of Calanus sinicus (Copepoda: Calanoida) in the northwest continental shelf of South China Sea. Cont. Shelf Res. 31, 1447–1456. doi: 10.1016/j.csr.2011.06.010
Keywords: brackish water and freshwater copepods, succession, biogeography, urban river, estuary
Citation: Lee P-W, Hsiao S-H, Chou C, Tseng L-C and Hwang J-S (2021) Zooplankton Fluctuations in the Surface Waters of the Estuary of a Large Subtropical Urban River. Front. Ecol. Evol. 9:598274. doi: 10.3389/fevo.2021.598274
Received: 24 August 2020; Accepted: 10 February 2021;
Published: 03 March 2021.
Edited by:
Roksana Majewska, North-West University, South AfricaReviewed by:
Rakesh Bhutiani, Gurukul Kangri Vishwavidyalaya, IndiaHendrik Jerling, University of Zululand, South Africa
Luiza Bielecka, University of Gdańsk, Poland
Copyright © 2021 Lee, Hsiao, Chou, Tseng and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Chun Tseng, bGljaHVuQG1haWwuYmVzdHJlZGV5ZS5vcmc=; orcid.org/0000-0003-0251-565X; Jiang-Shiou Hwang, anNod2FuZ0BtYWlsLm50b3UuZWR1LnR3
†These authors have contributed equally to this work
 Pei-Wen Lee
Pei-Wen Lee Shih-Hui Hsiao2†
Shih-Hui Hsiao2† Li-Chun Tseng
Li-Chun Tseng Jiang-Shiou Hwang
Jiang-Shiou Hwang