- 1Department of Ecology, School of Life Sciences, Nanjing University, Nanjing, China
- 2Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY, United States
Climate warming exerts profound effects on plant community composition. However, responses to climate warming are often reported at the community and functional type levels, but not at the species level. To test whether warming-induced changes are consistent among community, functional type, and species levels, we examined the warming-induced changes at different levels in an alpine meadow from 2015 to 2018. The warming was achieved by deploying six (open top) chambers [including three non-warmed chambers and three warmed chambers; 15 × 15 × 2.5 m (height) for each] that resulted in a small increase in mean annual temperature (0.3–0.5°C, varying with years) with a higher increase during the non-growing season (0.4–0.6°C) than in the growing season (0.03–0.47°C). The results show that warming increased plant aboveground biomass but did not change species richness, or Shannon diversity and evenness at the community level. At the functional type level, warming increased the relative abundance of grasses from 3 to 16%, but decreased the relative abundance of forbs from 89 to 79%; relative abundances of sedges and legumes were unchanged. However, for a given functional type, warming could result in contrasting effects on the relative abundance among species, e.g., the abundances of the forb species Geranium pylzowianum, Potentilla anserine, Euphrasia pectinate, and the sedge species Carex atrofusca increased in the warmed (compared to the non-warmed) chambers. More importantly, the difference in species identity between warmed and non-warmed chambers revealed warming-induced species loss. Specifically, four forb species were lost in both types of chambers, one additional forb species (Angelica apaensis) was lost in the non-warmed chambers, and two additional species (one forb species Saussurea stella and one sedge species Blysmus sinocompressus) were lost in the warmed chambers. Consequently, changes at the species level could not be deduced from the results at the community or functional type levels. These data indicate that species-level responses to climate changes must be more intensively studied. This work also highlights the importance of examining species identity (and not only species number) to study changes of community composition in response to climate warming.
Introduction
Global warming has exerted a profound influence on plant species diversity at the community level (Arft et al., 1999; Klein et al., 2004; Elmendorf et al., 2012; Pauli et al., 2012; Steinbauer et al., 2018), and will continue to influence ecosystem processes, functioning, and ecosystem services to human society (Hector and Bagchi, 2007; Naeem et al., 2010; Hooper et al., 2012; Tilman et al., 2014; Isbell et al., 2017; Liu et al., 2018). For example, plant species loss may significantly reduce primary productivity and plant litter decomposition rates (Hector and Bagchi, 2007; Hooper et al., 2012). Consequently, examining the responses of plant communities to warming is critical to accurately predict future changes in ecosystem properties.
Prior studies have revealed significant changes in species richness and relative abundance of plant communities in response to warming particularly in alpine and arctic ecosystems (Grabherr et al., 1994; Klein et al., 2004; Walker et al., 2006; Elmendorf et al., 2012). For example, experimental warming is reported to increase primary productivity and the relative abundance of grasses at the expense of the sedges and forbs in alpine meadows (Ganjurjav et al., 2016; Liu et al., 2018). A rapid loss of plant species has also been recorded in alpine meadows (Klein et al., 2004; Wang et al., 2012; Zhang et al., 2015, 2017). Analogous results have been obtained in arctic regions (Chapin et al., 1995; Walker et al., 2006). However, most of these reports have focused on changes in plant productivity or species diversity at the community level (Klein et al., 2004), or on changes in relative abundance at the plant functional type level (Chapin et al., 1996; Ganjurjav et al., 2016; Liu et al., 2018). Moreover, most of these studies have reported the number of species lost but not the identity of the species that were lost.
As a consequence, the effect of global warming on species diversity at the species level is incompletely understood. The paucity of information at the species level is likely to result in misleading conclusions for three reasons. First, it fosters the assumption that changes at the species level are consistent with changes at the plant functional type level. This assumption is most probably incorrect because it is unlikely that all of the species within a specific functional type will change in abundance in the same way. For example, it is possible that one species will increase its relative abundance even if the functional type to which it belongs decreases in overall abundance. Likewise, it is also possible that some species will increase in abundance while others will decrease, such that the abundance of the functional type to which they belong remains unchanged (Klanderud and Totland, 2005). Second, the assumption that changes at the species level are consistent with changes at the plant functional type level leads to the speculation that species loss occurs only in the plant functional types that decrease in relative abundance. Theoretically, this speculation is likely to be true only if the species decreasing in abundance are more likely to be lost, a speculation that thus far has not been tested. Third, in the context of global warming, it leads to the speculation that species loss is the result of warming-induced changes in species richness. This would be untrue if warming-induced changes in species identity differ among treatments. Specifically, if only species richness is considered, species loss could be masked by species invasion. Consequently, overlooking information at the species level can limit our ability to accurately predict future ecosystem functioning, particularly when the selection effect (i.e., species identity effect) of species diversity on ecosystem functioning dominates the biodiversity—ecosystem functioning relationship.
Here, we present data concerning changes in community species diversity, the relative abundance for four plant functional types (i.e., legumes, grasses, sedges, and forbs) and each plant species, and species identity in an alpine meadow in eastern Tibetan Plateau, which is considered among the most sensitive ecosystems to climate change (Piao et al., 2012; Zhang et al., 2017). We use the data to test the null hypothesis that warming-induced changes are consistent among the community level (as indicated by aboveground plant biomass, species richness, species diversity, and evenness), the functional type level, and the species level. To test this hypothesis, we examined (1) whether changes in relative abundance are consistent among different plant functional types, (2) whether changes in relative abundance are consistent among different plant species within specific plant functional types, and (3) whether species loss occurs in the functional types whose relative abundances decreased by warming.
Materials and Methods
Study Site
This study was conducted in an alpine meadow in Hongyuan County, Sichuan province, China (32°48′ N, 102°33′ E), in the eastern part of Tibetan Plateau. The elevation is ~3500 m above sea level. The climate is characterized by a short Spring and Autumn, cool mild Summer, and a long, cold Winter. The annual mean temperature is 1.7°C, with the minimum and maximum monthly mean temperature is −9.3 and 11.1°C in January and July, respectively. The annual mean precipitation is ~756 mm (but fluctuates significantly among years, mostly ranging between 450 and 900 mm), over 80% of which occurs during May to September (Cao et al., 2018).
The meadow has been intensively grazed (e.g., yak Bos grunniens, Hequ horse Equus caballus, and Tibetan sheep Ovis aries) for decades. Local residents currently use the meadow as either summer pasture (grazing in the growing season from May to September only) or winter pasture (grazing in the non-growing season from October to April only). Our experiments were performed in a winter pasture. The vegetative cover of the meadow is more than 90%, and the average plant height is ~30 cm. The vegetation is dominated by an assemblage of forbs (e.g., Saussurea nigrescens, Polygonum viviparum, Potentilla anserine, and Anemone trullifolia var. linearis), sedges (e.g., Kobresia setchwanensis and Carex spp.), and grasses (e.g., Deschampsia caespitosa, Festuca ovina, and Elymus nutans). Along with a diversity of plant species (Xiang et al., 2009), many arthropods, such as pollinators (Hu et al., 2019), herbivores (Xi et al., 2013), and dung decomposers (Wu et al., 2011) co-exist in the meadow.
Experimental Design
During October 2014, six 15 (length) × 15 (width) × 2.5 m (height) open top chambers (OTCs) were randomly deployed in a fenced, flat area of about 1.0 ha. Each OTC was large enough to include the local species pool, microhabitat variation in our study site. The sides of all OTCs were covered with thin (<0.1 mm) steel screen with a mesh size of 0.2 × 0.2 mm (Supplementary Figure 1A). Three of the chambers were additionally covered with 8 mm thick transparent tempered glass. Moreover, the roof of these three chambers was discontinuously covered by 250 0.3 m (width) transparent glass strips with an angle of 45°, with a 0.6 m space between strips (Supplementary Figure 1A), which was judged sufficient to prevent any strong airflow stack effect in the large warmed OTCs (Wilson and Tamura, 1968). Although one-third of the roof area of these chambers was covered by glass strips, these chambers are referred to as OTCs (Supplementary Figures 1A,B). All the six chambers were placed 1 m into the soil, and steel screens (with a mesh size of 0.6 × 0.6 mm) were placed 1 m into the soil along their sides to prevent rodents from entering (Supplementary Figures 1A,B). The three OTCs with transparent tempered glass are designated as “warmed chambers” and the other three (with only steel screens) as “non-warmed chambers”. It should be noted that the non-warmed chambers were constructed and installed to remove the confounding effect of the physical setting of OTCs (if any) on plant reproduction. It was observed (Xiaoli Hu, personal observation) that the steel sheets prevented arthropods entering in both the warmed and non-warmed chambers, except for a few high-flying species such as honeybees (Apis mellifera and Apis cerana) that are abundant because of apiculture in the study site (Mu et al., 2014). Consistently, plant pollination was generally not limited, as indicated by the indistinguishable difference (judged on the basis of a generalized linear model with binomial errors, P = 0.9) in seed set rate (sound seed number/total seed number) of the self-incompatible species Saussurea nigrescens among warmed chambers (0.65, n = 15, sd = 0.18), non-warmed chambers (0.63, n = 21, sd = 0.21), and field sites outside chambers (0.68, n =12, sd = 0.17). In addition, all six chambers were subject to horse grazing in the non-growing season (early October) in each study year from 2015 to 2018, making the study meadow a winter pasture (Supplementary Figure 2).
Measurements for more than 4-years using HOBO PRO (Onset Computer Corporation, USA) for air temperature and humidity and Watchdog2000 (Spectrum Technologies, Inc., USA) for soil temperature and moisture indicated that the mean annual temperature was 0.3–0.5°C higher (at 30 cm above ground surface) and 0.2–0.5°C higher (at a 5 cm soil depth) in the warmed chambers compared to the non-warmed chambers (see Table S1 and Figure S2 of Hu et al., 2020). During the non-growing season, the mean temperature was 0.4–0.6°C higher (at 30 cm above ground surface) and 0.8–1.1°C higher (at 5 cm soil depth) in the warmed compared to the non-warmed chambers, whereas temperature increases were 0.03–0.47°C and −0.2–0.8°C, respectively during the growing season (see Table S1 in Hu et al., 2020). The vapor pressure deficit, which was calculated based on air temperature and humidity measurements, was 2.6–3.7% higher in the warmed than in the non-warmed chambers during the growing seasons of 2018 and 2019. Moreover, soil moisture was 2–3% (v/v) higher at a 5 cm soil depth in the non-warmed compared to the warmed chambers (see Table S1 in Hu et al., 2020). In addition, transparency, which was calculated as inside-chamber light intensity/outside chamber light intensity (measured using light meters; TES-1336A, Taiwan, China) in the middle July of 2018, was slightly lower in the warmed chambers (94.4%, n = 45) compared to the non-warmed chambers (97.9%, n = 45) (Hu et al., 2020).
Vegetation Sampling
Each chamber was subdivided into nine 5 × 5 m subplots. Species abundance was determined during early-August each year using a quadrat sampling method (Pauli et al., 2015). Specifically, for each sampling (each year), we randomly placed five 100 × 100 cm quadrats in five of the nine subplots. Subsequently, we divided each quadrat into 10 × 10 cm grids and recorded species presence and abundance following the protocols described by Pauli et al. (2015). In order to determine whether species loss occurred during the experiment, we extensively surveyed the entire plant community within each chamber, recording the presence of all plant species. Species that did not emerge for two consecutive years were designated as “lost.”
In the middle of August of each study year when most plant species completed reproduction, all aboveground plant parts were clipped to the ground surface and harvested in 16 1 × 1 m quadrats that were randomly deployed in each chamber. The harvests were carefully sorted into four different functional types (i.e., legumes, grasses, sedges, and forbs), and then dried for 72 h at 75°C and weighed.
Data Analysis
At the community level, we determined species diversity for each quadrat by calculating Shannon diversity (i.e., Hill number 1) [i.e., )] and Shannon evenness (Hill ratio) (i.e., E = D/S), where pi is the relative abundance (species-specific abundance/total abundance per quadrat) of the ith species out of S species) (Chao et al., 2014). Generalized linear mixed models (GLMMs) were used to determine the effect of warming on aboveground plant biomass, species richness, Shannon diversity, and Shannon evenness (with Poisson errors for species richness, beta errors for Shannon evenness, and Gaussian errors for aboveground biomass and Shannon diversity). In each model, “treatment” (warmed vs. non-warmed) was set as the fixed factor, and “year” and “chamber” as random factors. GLMMs were performed using the package “lme4” (Bates et al., 2015) and “R2jags” (Su and Masanao, 2020).
At the functional type level, the differences in the relative abundance and relative biomass (i.e., plant functional type-specific aboveground plant biomass/total biomass) were determined between the warmed and non-warmed chambers, using GLMMs (with a binomial error structure) with “treatment” as the fixed factor and “year” and “chamber” as random factors. At the species level, the warming effect on relative species abundance was determined using GLMMs (with a binomial error structure) with “treatment” as the fixed factor and “year” and “chamber” as random factors. Only those species occurring in both warmed and non-warmed chambers were considered.
To further examine changes in the plant community composition during the experiment from 2015 to 2018, we conducted a two-way permutational analysis of variances (PERMANOVA) on the Bray-Curtis dissimilarity matrix including the relative abundance for all species [with log(n + 1) transformation]. The PERMANOVA was run with 999 permutations using the “adonis2” function in the R package vegan v. 2.5–4 (Oksanen et al., 2014). The dissimilarity in species compositions between warmed and non-warmed chambers was also computed using non-metric multidimensional scaling (NMDS) with Bray-Curtis distance and the “metaMDS” function in the R package vegan v. 2.5–4 (Oksanen et al., 2014).
All analyses were performed using R 3.5.3 (R Core Team, 2019).
Results
Changes at the Community Level
Experimental warming significantly increased aboveground plant biomass starting in 2016 (Figure 1). However, the difference in species richness, Shannon diversity (as indicated by the Hill number 1) and Shannon evenness between the warmed and non-warmed chambers were statistically not significant (Figure 2). Moreover, the PERMANOVA and NMDS analysis showed that the overall warming-induced change in species composition was significantly different between 2015 and 2018 (Table 1; Figure 3).
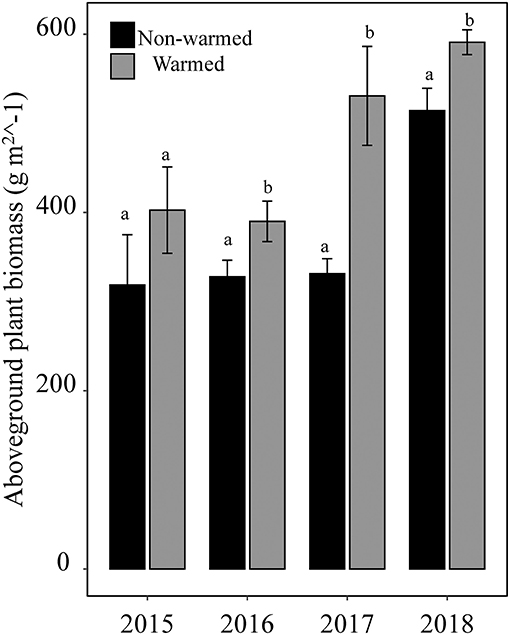
Figure 1. Aboveground biomass in both non-warmed and warmed chambers from 2015 to 2018. Different letters indicate a significant difference (P < 0.05) between warmed and non-warmed chambers for each experimental year.
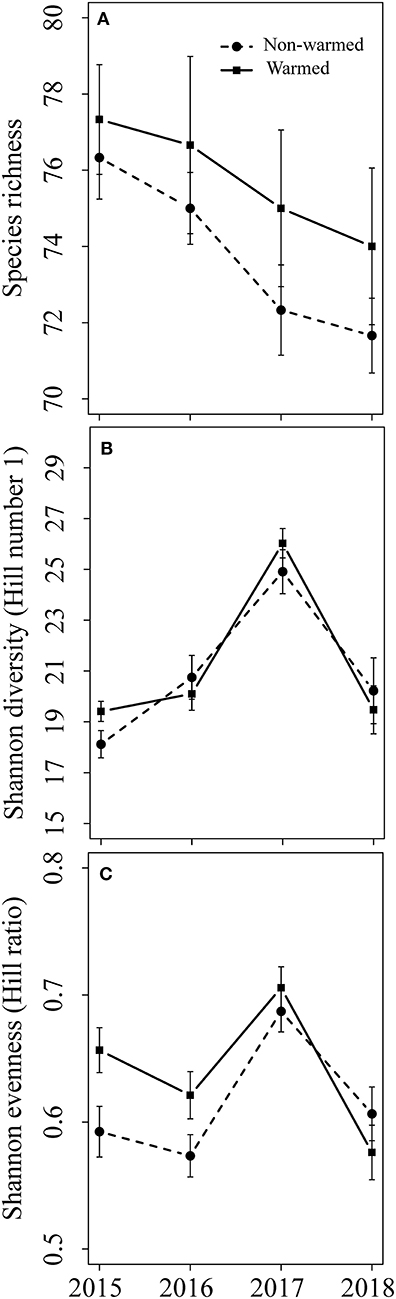
Figure 2. Plant species diversity in both non-warmed and warmed chambers from 2015 to 2018. (A) Species richness; (B) Shannon diversity (Hill number 1); (C) Shannon evenness (Hill ratio). No significant difference in each variable was found between non-warmed and warmed chambers for each study year.
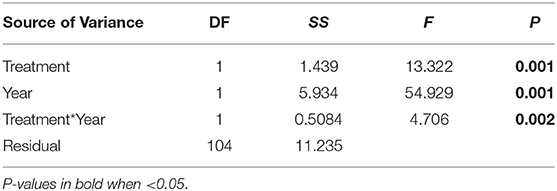
Table 1. Summary of two-way PERMANOVAs (F and P-values) on Bray-Curtis community composition from 2015 to 2018.
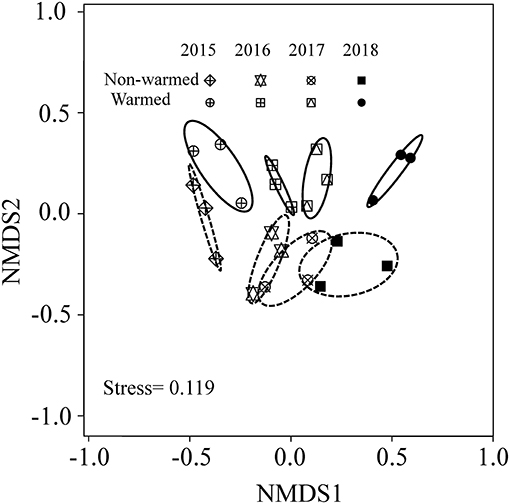
Figure 3. Results of non-metric multidimensional scaling (NMDS) showing community dissimilarity between non-warmed and warmed chambers and years (2015–2018). Dissimilarity (Bray-Curtis) was estimated using the relative abundance of each species.
Changes at the Functional Type Level
Warming-induced changes in relative abundance differed significantly among the four plant functional groups (Table 2). Warming increased the relative abundance of grasses from 3 to 16%, but decreased the relative abundance of forbs from 89 to 79% in 2018. In contrast, the relative abundance in the sedge and the legume functional groups did not significantly change (Table 2; Figure 4A). The relative biomass at the functional type level changed in a consistent way with respect to relative abundance (Table 2; Figure 4B).
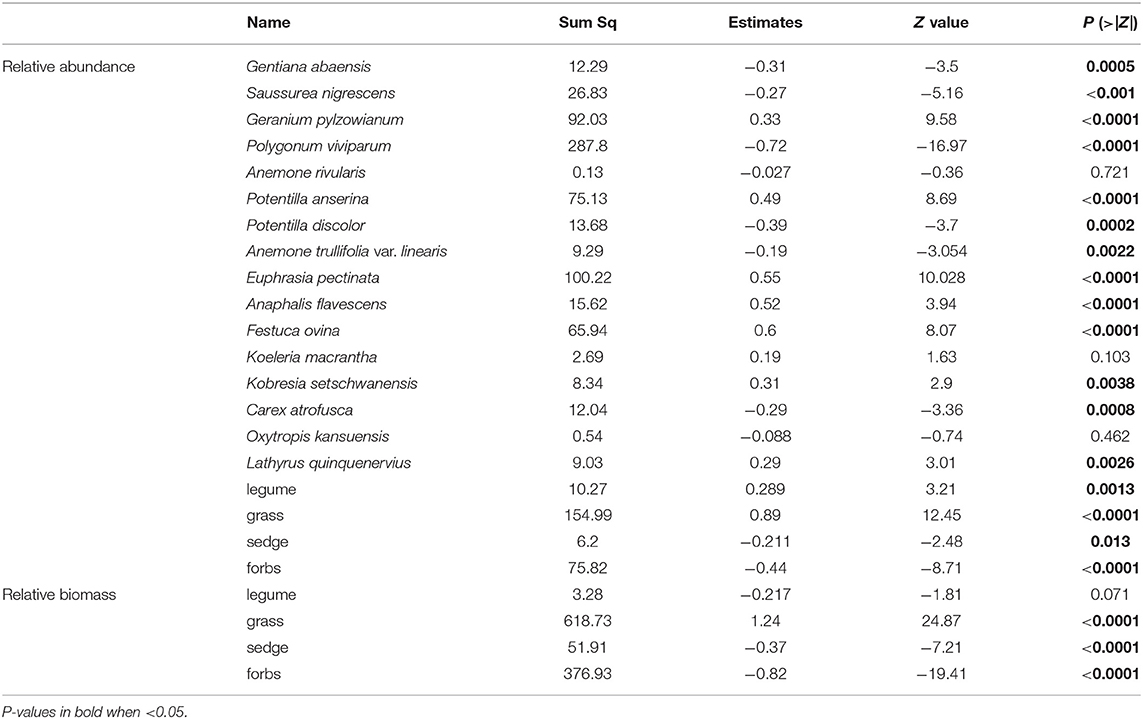
Table 2. Summary of the GLMMs showing the warming effect on the relative abundance and biomass of 16 species and four functional groups.
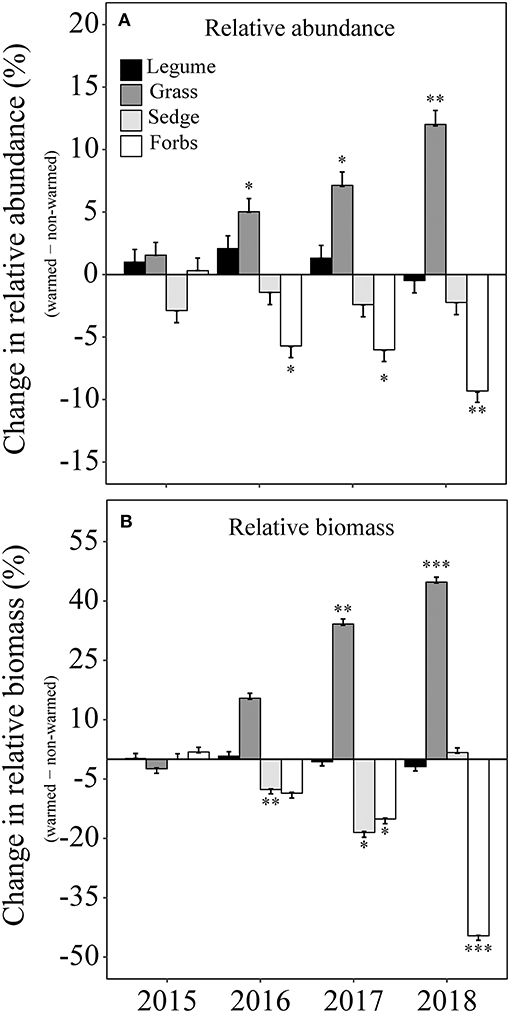
Figure 4. Difference in relative abundance (A) and relative biomass (B) between non-warmed and warmed chambers for each functional type (i.e., Legume, Grass, Sedge, and Forbs) from 2015 to 2018. Positive and negative values indicate an increase or a decrease in the warmed chambers. *P < 0.05; **P < 0.01; ***P < 0.001.
Changes at the Species Level
Species belonging to the same plant functional type showed dissimilar responses in relative abundance to warming (Table 2; Figure 5). Specifically, in the forb group, warming significantly decreased the relative abundance of Saussurea nigrescens (Table 2; Figure 5B), but significantly increased the abundance of Geranium pylzowianum, Potentilla anserine, and Euphrasia pectinate (Table 2; Figures 5C,F,I) by 2018. Similarly, for the grasses, the relative abundance of Festuca ovina significantly increased, but remained unchanged for Koeleria macrantha (Table 2; Figures 5K,L). In the case of sedges, relative abundance decreased overall. However, Carex atrofusca significantly increased relative abundance as a consequence of warming (Table 2; Figure 5N)
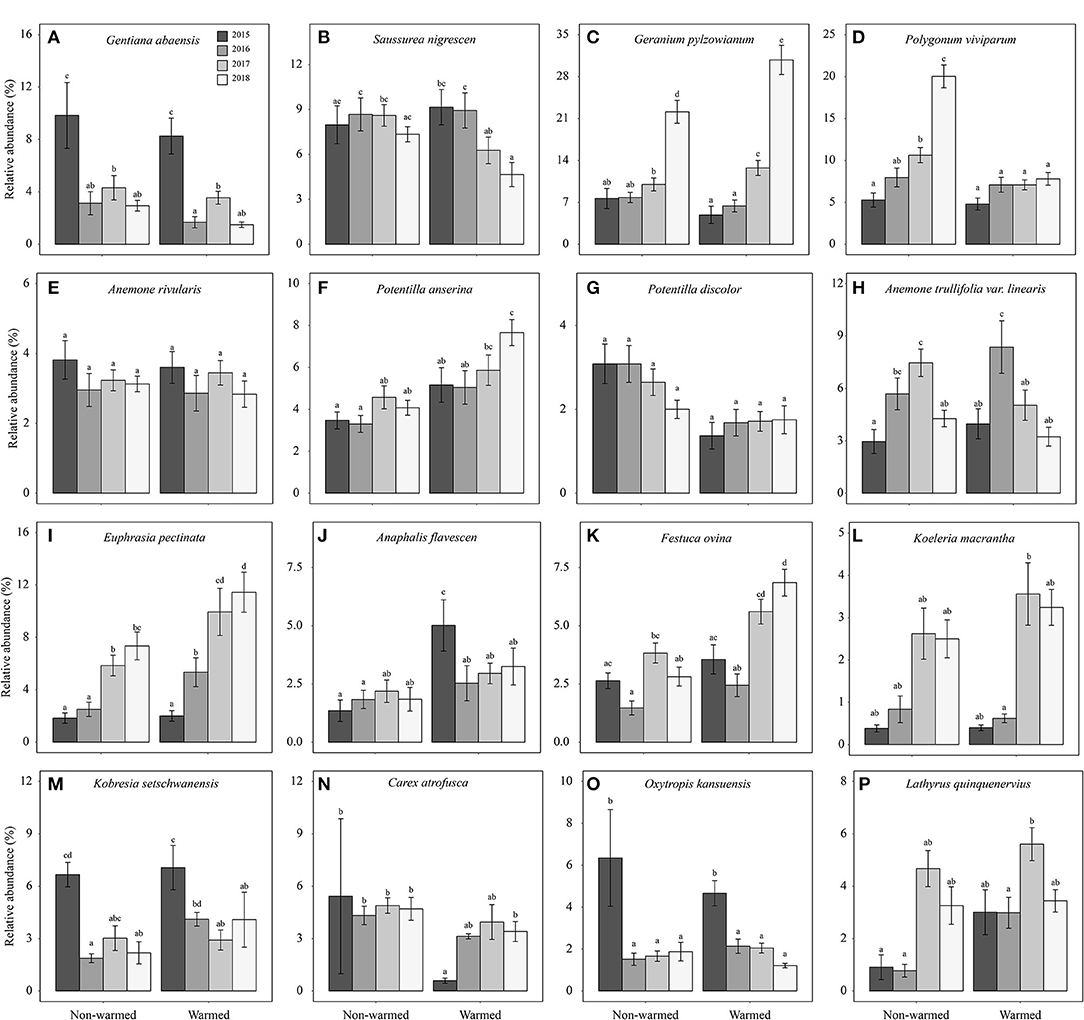
Figure 5. Relative abundance of ten forbs (Gentiana abaensis, Saussurea nigrescens, Geranium pylzowianum, Polygonum viviparum, Anemone rivularis, Potentilla anserine, Potentilla discolor, Anemone trullifolia var. linearis, Euphrasia pectinate, and Anaphalis flavescens, A–J), two grasses (Festuca ovina and Koeleria macrantha, K,L), two sedges (Kobresia setchwanensis and Carex atrofusca, M,N), and two legumes (Oxytropis kansuensis and Lathyrus quinquenervius, O,P) in both non-warmed and warmed chambers from 2015 to 2018. Error bars = 1 standard error of mean. Different letters indicate a significant difference (P < 0.05) between warmed and non-warmed chambers.
Over the study years, four forb species (i.e., Tibetia himalaica, Carum carvi, Microula sikkimensis, and Sanguisorba filiformis) were lost in both the non-warmed and warmed chambers. Two species (i.e., the perennial sedge Blysmus sinocompressus and the perennial forb species Saussurea stella) were lost in the warmed chambers, and the forb species Angelica apaensis was lost in the non-warmed chambers.
Discussion
Our results indicate that a warmed alpine meadow community has a higher primary productivity (as indicated by aboveground plant biomass), and warming-induced changes in the relative abundance differ significantly among plant functional types. These responses are consistent with previous studies addressing the effect of experimental warming on grassland communities (Arft et al., 1999; Wang et al., 2012; Zhang et al., 2015; Ganjurjav et al., 2016). Importantly, data show that species within the same plant functional type can respond differently to warming. These observations indicate that reporting changes at the community or functional type level is insufficient to predict changes at the species level. In addition, species loss occurred in both types of chambers and different species were lost in different chamber types, such that reporting a change in species richness is insufficient for describing the change at the plant community level. Thus, reporting the identity of lost species and understanding how they are lost are important when attempting to predict what species are more likely to go locally extinct under warming climatic conditions, a finding that has significance regarding conservation biology.
Although the warming-induced increase in aboveground plant biomass is consistent with previous warming studies in similar alpine meadows (Ganjurjav et al., 2016; Liu et al., 2018), changes in species diversity are less pronounced in this as opposed to other studies. For example, Chapin et al. (1995) showed that experimental warming reduced species richness by 30–50% in a tussock tundra; Klein et al. (2004) reported that warming over a period of 4 years resulted in a 26–36% decrease in species richness in Tibetan alpine meadow; and Sternberg et al. (1999) reported that manipulated warming decreased species richness by 10% in a calcareous grassland. In our study, warming did not significantly change species richness, Shannon diversity, or Shannon evenness over a 4-year period. This result contrasts with those of previous warming experiments conducted in alpine and arctic regions (Klein et al., 2004; Wang et al., 2012; Zhang et al., 2017). There are three possible explanations for why the warming-induced decrease in species richness is much smaller in our study system. First, the temperature increase in our experiment is smaller than that in other studies. Greater temperature increases often induce more pronounced changes in plant species diversity (Wang et al., 2019). Second, our experimental chambers are much larger than those used in previous studies. Large chambers might buffer the changes in species richness occurring at smaller and more homogeneous scales, i.e., larger chambers are likely to be more heterogeneous in their microclimate and micro-soil conditions compared to smaller ones (Vellend et al., 2013; Suggitt et al., 2018). Third, in the present study, the experimental control was non-warmed chambers compared to dispersal-unlimited field sites used in many other studies (e.g., Klein et al., 2004; Zhang et al., 2017). These chambers (including the warmed ones) were taller than those used in other warming studies (Klein et al., 2004, 0.4 m; Zhang et al., 2017, 0.4 m; Ganjurjav et al., 2016, 0.45 m), which could have significantly reduced or even prevented seed dispersal. Thus, seed dispersal would be equally confined in this study, whereas it was more confined in the warmed chambers than open sites in the other studies.
The difference in relative abundance among functional types reported here is also consistent with similar studies in our study region. Ganjurjav et al. (2016) and Liu et al. (2018) both reported a community shift from one dominated by forbs to one dominated by grasses in response to warming, whereas other studies indicated that sedge and legume species remain relatively unchanged in response to warming (Klanderud and Totland, 2005; Ganjurjav et al., 2016; Liu et al., 2018). These observations suggest that plant growth is normally limited by the low temperatures in alpine meadows and that increased temperatures likely increase aboveground plant biomass and plant height, which collectively increases interspecific competition for light. Tall grasses can competitively shade and exclude short forb and sedge species that tend to be rosette-like and short. Moreover, although soil moisture was on average high in our meadow study site, warming reduced soil moisture to a low level (<20% v/v) during the dry season (usually in August). This reduction in soil moisture might have limited the growth of sedge species and some forb species that are adapted to moist soil conditions (e.g., B. sinocompressus and S. stella; see also Klanderud, 2008; Little et al., 2015; Cao et al., 2017), but favored the growth of drought-tolerant species such as D. caespitosa and Festuca spp. (this study; see also Klanderud, 2008; Little et al., 2015).
Importantly, individual species differed in their responses to warming both in direction and amplitude even for those within the same plant functional type. As noted, contrasting responses to warming have been observed in all plant functional types. This observation is attributable to differences in plant traits even those within the same functional type. For example, most forb species decreased in relative abundance probably because of competitive exclusion by tall grass species. Yet, three forbs (i.e., G. pylzowianum, P. anserine, and E. pectinate) increased substantially in their relative abundance in the warmed chambers. The abundance increase in G. pylzowianum and P. anserine is consistent with previous studies (Klanderud, 2008; Li et al., 2011), and can be attributed to their belowground storage organ (tubers) and the habit of climbing growing, which allows individuals to vegetatively overgrow other plants, thereby avoiding shade compared to plants growing in non-warmed chambers. The increase in the abundance of E. pectinate is also consistent with previous findings (Nyléhn and Totland, 1999; Klanderud, 2008). The underlying mechanism for its success is that this species is an annual root hemi-parasitic forb that can absorb water and nutrients from monocot species. Consequently, it increases in abundance in tandem with the abundance of grass species. It could be argued that species with different traits should not be placed within the same functional group. However, assigning species to too many different functional groups presents additional problems. Nevertheless, it is clear that subtle differences among the species assigned to any functional species group can result in substantive differences in response to warming, whether induced experimentally or experienced under natural field conditions.
Although differences in species richness were statistically indistinguishable between warmed and non-warmed treatments, species loss was demonstrably manifest as indicated by the fact that both warmed and non-warmed chambers experienced species loss and that different species were lost in the two different types of chambers. A number of factors may contribute to this observation. For example, in both warmed and non-warmed chambers, the loss of the forb species Carum carvi and Microula sikkimensis might have been the result of reduced disturbance and increased plant density during the experiment, because these species usually grow in ruderal or bare areas. The loss of the forbs Tibetia himalaica and Sanguisorba filiformis might be due to the shading of tall grasses because these two species are short and rare. The loss of the forb species Saussurea stella and the sedge species Blysmus sinocompressus in warmed chambers may perhaps be the result of decreasing soil moisture and their competitive exclusion by tall plants. Both of these species are short and often grow in high-moisture microhabitats (Xiaoli Hu, Personal Observation; Flora of China, http://www.iplant.cn/). Finally, the loss of the forb species Angelica apaensis in non-warmed chambers may reflect its demographic stochasticity because this species is extremely rare.
In summary, the results presented here show that a small increase in mean annual temperature and a winter-biased higher temperature during the non-growing season induces significant changes in an alpine meadow plant community, as evidenced by shifts in the relative abundance and biomass of different plant functional types. The results also indicate that changes in species relative abundance and biomass are not necessarily consistent with those of plant functional types, indicating that the data from plant functional types can obscure important information about the differential responses of individual species to climate change. Perhaps more important, whether species loss occurs cannot be deduced from changes in species richness. Collectively, species-level information is necessary to accurately predict plant community responses to global change. Future studies should consider the behavior of individual species when predicting changes in ecosystem functioning or service, or making policy decisions concerning biodiversity protection and conservation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SS started the project and designed research, and drafted the manuscript. XH, WZ, and XL performed research. SS, XH, and KN analyzed data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by National Science Foundation of China (31530007, 32071605, and 31325004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Xinwei Wu, Xinqiang Xi, Liang Zhang, Fengqun Meng, Bin Lan, Rui Cao, Lei Hu, Dongbo Li, Tan Li for field and lab assistance and Qinghai-Tibetan Research Base of Southwest University of Nationalities for providing research convenience.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.569422/full#supplementary-material
References
Arft, A. M., Walker, M. D., Gurevitch, J., Alatalo, J. M., Bret-Harte, M. S., Dale, M., et al. (1999). Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecol. Monogr. 69, 491–511. doi: 10.2307/2657227
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Cao, R., Chen, Y., Wu, X., Zhou, Q., and Sun, S. (2018). The effect of drainage on CO2, CH4, and N2O emissions in the Zoige peatland: a 40-month in situ study. Mires Peat 21, 1–15. doi: 10.19189/MaP.2017.OMB.292
Cao, R., Wu, X., Yang, Y., and Xi, X. (2017). The effect of water table declines on plant biomass and species composition in the Zoige peatland: a four-year in situ field experiment. Agric. Ecosyst. Environ. 247, 389–395. doi: 10.1016/j.agee.2017.07.008
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Chapin, F. S., Bret-Harte, M., Hobbie, S., and Zhong, H. (1996). Plant functional types as predictors of transient responses of arctic vegetation to global change. J. Veg. Sci. 7, 347–358. doi: 10.2307/3236278
Chapin, F. S., Shaver, G. R., Giblin, A. E., Nadelhoffer, K. J., and Laundre, J. A. (1995). Responses of arctic tundra to experimental and observed changes in climate. Ecology 76, 694–711. doi: 10.2307/1939337
Elmendorf, S. C., Henry, G. H. R., Hollister, R. D., Björk, R. G., Bjorkman, A. D., Callaghan, T. V., et al. (2012). Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175. doi: 10.1111/j.1461-0248.2011.01716.x
Ganjurjav, H., Gao, Q., Gornish, E. S., Schwartz, M. W., Liang, Y., Cao, X., et al. (2016). Differential response of alpine steppe and alpine meadow to climate warming in the central Qinghai-Tibetan Plateau. Agric. For. Meteorol. 223, 233–240. doi: 10.1016/j.agrformet.2016.03.017
Grabherr, G., Gottfried, M., and Paull, H. (1994). Climate effects on mountain plants. Nature 369:448. doi: 10.1038/369448a0
Hector, A., and Bagchi, R. (2007). Biodiversity and ecosystem multifunctionality. Nature 448, 188–190. doi: 10.1038/nature05947
Hooper, D. U., Adair, E. C., Cardinale, B. J., Byrnes, J. E. K., Hungate, B. A., Matulich, K. L., et al. (2012). A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. doi: 10.1038/nature11118
Hu, L., Dong, Y., and Sun, S. (2019). Relative species abundance successfully predicts nestedness and interaction frequency of monthly pollination networks in an alpine meadow. PLoS One 14:e0224316. doi: 10.1371/journal.pone.0224316
Hu, X., Zhou, W., and Sun, S. (2020). Responses of plant reproductive phenology to winter-biased warming in an alpine meadow. Front. Plant. Sci. 11:534703. doi: 10.3389/fpls.2020.534703
Isbell, F., Gonzalez, A., Loreau, M., Cowles, J., Diaz, S., Hector, A., et al. (2017). Linking the influence and dependence of people on biodiversity across scales. Nature 546, 65–72. doi: 10.1038/nature22899
Klanderud, K. (2008). Species-specific responses of an alpine plant community under simulated environmental change. J. Veg. Sci. 19, 363–372. doi: 10.3170/2008-8-18376
Klanderud, K., and Totland, Ø. (2005). Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology 86, 2047–2054. doi: 10.1890/04-1563
Klein, J. A., Harte, J., and Zhao, X. (2004). Experimental warming causes large and rapid species loss, dampened by simulated grazing on the Tibetan Plateau. Ecol. Lett. 7, 1170–1179. doi: 10.1111/j.1461-0248.2004.00677.x
Li, G., Liu, Y., Frelich, L., and Sun, S. (2011). Experimental warming induces degradation of a Tibetan alpine meadow through trophic interactions. J. Appl. Ecol. 48, 659–667. doi: 10.1111/j.1365-2664.2011.01965.x
Little, C. J., Jägerbrand, A. K., Molau, U., and Alatalo, J. M. (2015). Community and species-specific responses to simulated global change in two subarctic-alpine plant communities. Ecosphere 6, 1–18. doi: 10.1890/ES14-00427.1
Liu, H., Mi, Z., Lin, L., Wang, Y., Zhang, Z., Zhang, F., et al. (2018). Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. U.S.A. 115, 4051–4056. doi: 10.1073/pnas.1700299114
Mu, J. P., Peng, Y. H., Xi, X. Q., Wu, X. W., Griffin, J. N., Niklas, K. J., et al. (2014). Domesticated honeybees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology 95, 3161–3172. doi: 10.1890/13-2055.1
Naeem, S., Bunker, D. E., and Hector, A. (2010). Biodiversity, Ecosystem Functioning, and Human Wellbeing: An Ecological and Economic Perspective. Oxford: Oxford Univ. Press.
Nyléhn, J., and Totland, Ø. (1999). Effects of temperature and natural disturbance on growth, reproduction, and population density in the alpine annual hemiparasite Euphrasia frigida. Arct. Antarct. Alp. Res. 31, 259–263. doi: 10.1080/15230430.1999.12003307
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2014). vegan: Community Ecology Package: In: R Package Version 2.5–4. Available online at: https://CRAN.R-project.org/package=vegan
Pauli, H., Gottfried, M., Dullinger, S., Abdaladze, O., Akhalkatsi, M., Alonso, J. L. B., et al. (2012). Recent plant diversity changes on Europe's mountain summits. Science 336, 353–355. doi: 10.1126/science.1219033
Pauli, H., Gottfried, M., Dullinger, S., Abdaladze, O., Akhalkatsi, M., Alonso, J. L. B., et al. (2015). The GLORIA Field Manual–Standard Multi–Summit Approach, Supplementary Methods, and Extra Approaches. Vienna: Coordination, Austrian Academy of Sciences and University of Natural Resources and Life Sciences.
Piao, S., Tan, K., Nan, H., Ciais, P., Fang, J., Wang, T., et al. (2012). Impacts of climate and CO2 changes on the vegetation growth and carbon balance of Qinghai-Tibetan grasslands over the past five decades. Glob. Planet. Change 98–99, 73–80. doi: 10.1016/j.gloplacha.2012.08.009
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Steinbauer, M. J., Grytnes, J.-A., Jurasinski, G., Kulonen, A., Lenoir, J., Pauli, H., et al. (2018). Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–247. doi: 10.1038/s41586-018-0005-6
Sternberg, M., Brown, V. K., Masters, G. J., and Clarke, I. P. (1999). Plant community dynamics in a calcareous grassland under climatechange manipulations. Plant. Ecol. 143, 29–37. doi: 10.1023/A:1009812024996
Su, Y.-S., and Masanao, Y. (2020). R2jags: Using R to Run ‘JAGS'. R Package Version 0.6-1. Available online at: https://CRAN.R-project.org/package=R2jags (accessed April 7, 2020).
Suggitt, A. J., Wilson, R. J., Isaac, N. J. B., Beale, C. M., Auffret, A. G., August, T., et al. (2018). Extinction risk from climate change is reduced by microclimatic buffering. Nat. Clim. Change 8, 713–719. doi: 10.1038/s41558-018-0231-9
Tilman, D., Isbell, F., and Cowles, J. M. (2014). Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. doi: 10.1146/annurev-ecolsys-120213-091917
Vellend, M., Baeten, L., Myers-Smith, I. H., Elmendorf, S. C., Beausejour, R., Brown, C. D., et al. (2013). Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. U.S.A. 110, 19456–19459. doi: 10.1073/pnas.1312779110
Walker, M. D., Wahren, C. H., Hollister, R. D., Henry, G. H. R., Ahlquist, L. E., Alatalo, J. M., et al. (2006). Plant community responses to experimental warming across the tundra biome. Proc. Natl. Acad. Sci. U.S.A. 103, 1342–1346. doi: 10.1073/pnas.0503198103
Wang, Q., Zhang, Z., Du, R., Wang, S., Duan, J., Iler, A. M., et al. (2019). Richness of plant communities plays a larger role than climate in determining responses of species richness to climate change. J. Ecol. 107, 1944–1955. doi: 10.1111/1365-2745.13148
Wang, S., Duan, J., Xu, G., Wang, Y., Zhang, Z., Rui, Y., et al. (2012). Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 93, 2365–2376. doi: 10.1890/11-1408.1
Wilson, A. G., and Tamura, G. T. (1968). Stack Effect and Building Design. Canadian Building Digest CBD-107. Ottawa: National Research Council Canada.
Wu, X., Duffy, J., Reich, P. B., and Sun, S. (2011). A brown-world cascade in the dung decomposer food web of an alpine meadow: effects of predator interactions and warming. Ecol. Monogr. 81, 313–328. doi: 10.1890/10-0808.1
Xi, X., Griffi, J., and Sun, S. (2013). Grasshoppers amensalistically suppress caterpillar performance and enhance plant biomass in an alpine meadow. Oikos 122, 1049–1057. doi: 10.1111/j.1600-0706.2012.00126.x
Xiang, S., Guo, R., Wu, N., and Sun, S. (2009). Current status and future prospects of Zoige Marsh in eastern Qinghai-Tibet plateau. Ecol. Eng. 35, 553–562. doi: 10.1016/j.ecoleng.2008.02.016
Zhang, C., Willis, C. G., Klein, J. A., Ma, Z., Li, J., Zhou, H., et al. (2017). Recovery of plant species diversity during long-term experimental warming of a species-rich alpine meadow community on the Qinghai-Tibet plateau. Biol. Conserv. 213, 218–224. doi: 10.1016/j.biocon.2017.07.019
Keywords: community composition, species diversity, functional group, species identity, alpine meadow
Citation: Hu X, Zhou W, Li X, Niklas KJ and Sun S (2021) Changes in Community Composition Induced by Experimental Warming in an Alpine Meadow: Beyond Plant Functional Type. Front. Ecol. Evol. 9:569422. doi: 10.3389/fevo.2021.569422
Received: 05 June 2020; Accepted: 06 January 2021;
Published: 28 January 2021.
Edited by:
Luis Daniel Llambi, Universidad de Los Andes, VenezuelaReviewed by:
Francisco Cuesta, University of the Americas, EcuadorEloisa Lasso, University of Los Andes, Colombia
Copyright © 2021 Hu, Zhou, Li, Niklas and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shucun Sun, c2hjc0BuanUuZWR1LmNu
 Xiaoli Hu
Xiaoli Hu Wenlong Zhou1
Wenlong Zhou1 Karl J. Niklas
Karl J. Niklas Shucun Sun
Shucun Sun