- Applied Entomology Laboratory, Department of Bioresource Field Sciences, Kyoto Institute of Technology, Kyoto, Japan
Termite colonies, composed of large numbers of siblings, develop an important caste-based division of labor; individuals in these societies interact via intra- or intercaste chemical communications. For more than 50 years, termites have been known to use a variety of pheromones to perform tasks necessary for maintenance of their societies, similar to eusocial hymenopterans. Although trail-following pheromones have been chemically identified in various termites, other types of pheromones have not been elucidated chemically or functionally. In the past decade, however, chemical compositions and biological functions have been successfully identified for several types of termite pheromones; accordingly, the details of the underlying pheromone communications have been gradually revealed. In this review, we summarize both the functions of all termite pheromones identified so far and the chemical interactions among termites and other organisms. Subsequently, we argue how termites developed their sophisticated pheromone communication. We hypothesize that termites have diverted defensive and antimicrobial substances to pheromones associated in caste recognition and caste-specific roles. Furthermore, termites have repeatedly used a pre-existing pheromone or have added supplementary compounds to it in accordance with the social context, leading to multifunctionalization of pre-existing pheromones and emergence of new pheromones. These two mechanisms may enable termites to transmit various context-dependent information with a small number of chemicals, thus resulting in formation of coordinated, complex, and rational chemical communication systems.
Introduction
Termites are eusocial insects that live in colonies with large numbers of siblings, where both males and females perform largely equal roles with respect to colony maintenance. Their societies develop a caste-based division of labor; each caste is morphologically and physiologically specialized for different tasks (Eggleton, 2011). For example, reproductive castes (kings and queens) concentrate on reproduction, and soldiers and workers are engaged in other tasks necessary for colony maintenance (e.g., foraging; colony defense; nest construction; hygiene control; and caring for reproductive castes and eggs) (Eggleton, 2011).
The sophisticated colony organization of termites and other eusocial insects is characterized by an efficient communication system based mainly on pheromones. In termites and social hymenopterans, it has long been hypothesized that pheromones might be involved in all social activities (Wilson, 1965). However, termite pheromonal studies have mainly focused on trail-following pheromones and sex-pairing pheromones. A trail pheromone was first identified in the Eastern subterranean termite Reticulitermes virginicus (Rhinotermitidae) (Matsumura et al., 1968); trail-following pheromones and sex-pairing pheromones were subsequently identified in various termite species (detailed below). However, the identification of other pheromone types did not progress until the 1990s, although the existence of primer pheromones regulating differentiation into supplementary alates or soldiers had been suggested (Krishna et al., 2013a,b,c,d,e). Since the 2000s, however, several other pheromone types have been chemically and functionally identified in succession.
In this review, we first focus on the functions and components of each type of pheromone. We then discuss the origins of pheromone components; the evolutionary process of multifunctionalization; the development of intra- and intercaste communication; and the interactions among termites, pathogens, predators, and inquilines via termite pheromones.
Sex-Pairing Pheromones
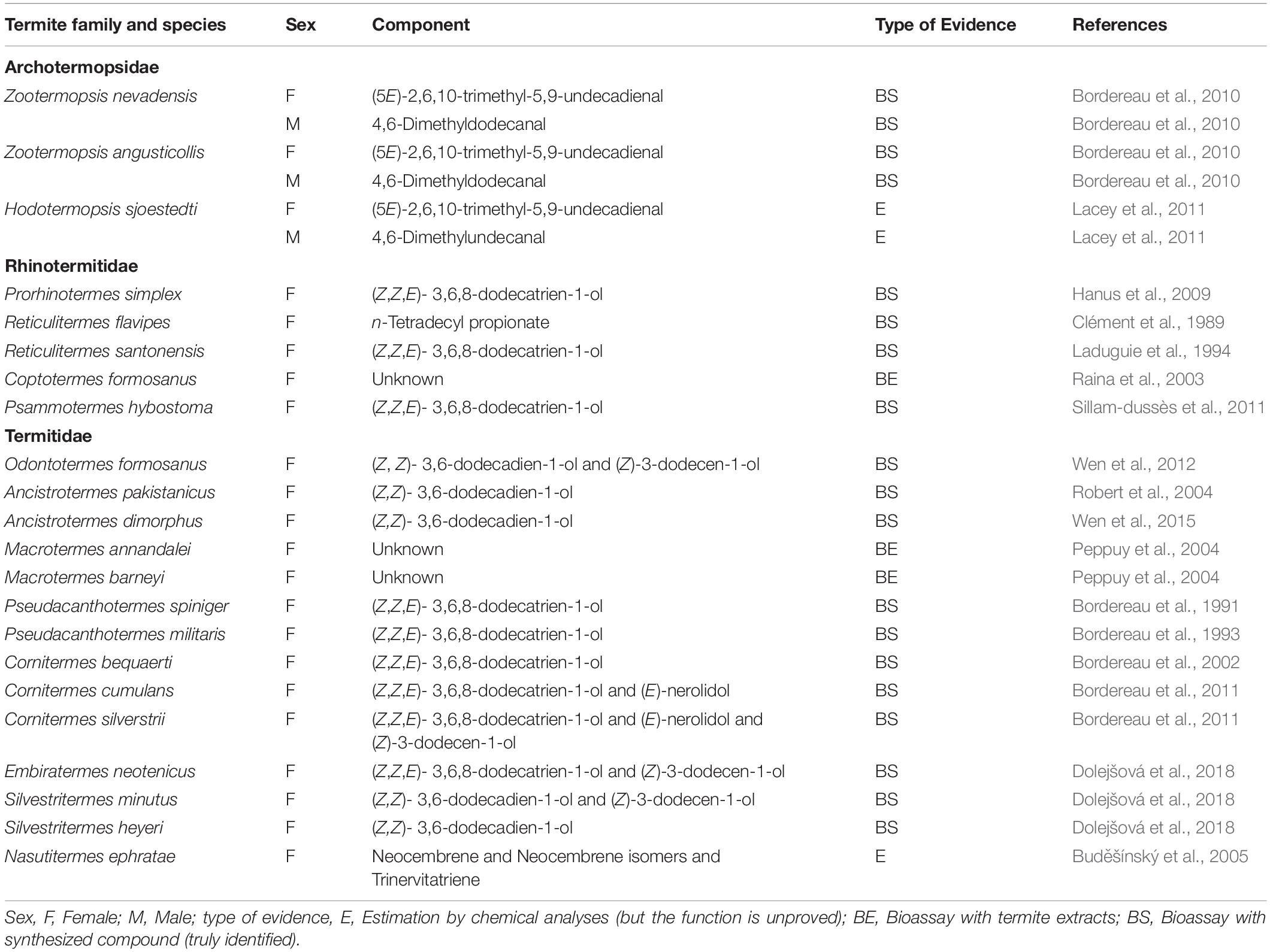
Sex-pairing pheromones in termites are used by alates (i.e., adult individuals with wings) for attracting sexual partners during mating behavior (Bordereau and Pasteels, 2011). Termite nuptial flight usually occurs once per year in a certain season, whereby virgin female and male alates come together in pairs to establish new colonies. Numerous alates disperse from their colonies by flight, then land on the ground or in trees; subsequently, they shed their wings (becoming dealates) and search for mating partners (Bordereau and Pasteels, 2011). In many species, female dealates perform calling behavior (raising the tip of the abdomen and emitting the sex-pairing pheromone from the tergal glands or the sternal gland) to attract male dealates (Bordereau and Pasteels, 2011). In a few species, such as Zootermopsis nevadensis and Zootermopsis angusticollis, both female and male dealates emit the sex-pairing pheromone (Bordereau et al., 2010). The range of action of the pheromone in natural conditions depends on the termite species; it ranges from a few centimeters (Leuthold, 1975) to a few meters (Leuthold and Bruinsma, 1977). When a male succeeds in finding the female, the male follows after it (tandem running). The leading female seeks a suitable nesting site, while the following male continues to antennate (or lick) the posterior pleural membranes of the female. During the tandem running, short-range or contact chemical stimuli by the sex-pairing pheromones play an important role in maintaining the tandem formation (Bordereau and Pasteels, 2011). After the pair of male and female dealates finds a suitable site, they establish a new colony and begin to produce offspring as the primary king and queen, respectively.
Thus far, sex-pairing pheromones have been fully identified in 17 termite species belonging to three families (Archotermopsidae, Rhinotermitidae, and Termitidae); these pheromones mainly consist of aliphatic aldehydes, alcohols, and/or diterpenes (Table 1). In Z. nevadensis and Z. angusticollis (Archotermopsidae), which belong to a relatively ancestral family (Lo and Eggleton, 2011), the female and male dealates use different compounds; the females use (5E)-2,6,10-trimethyl-5,9-undecadienal, while the males use 4,6-dimethyldodecanal (Bordereau et al., 2010). However, in more derivative termite families (Rhinotermitidae and Termitidae), only female dealates emit the pheromone, namely, (Z,Z,E)-3,6,8-dodecatrien-1-ol, which is common among many species, although some termitid species use mixtures of two or three compounds for the pheromone (Table 1). In most species, all components are required for high attraction activity of the pheromone. In Odontotermes formosanus, however, the two components act in synergy at long distance, whereas (Z,Z)-3,6-dodecadien-1-ol can act alone at a short distance (Wen et al., 2012).
The number of chemical components in termite sex-pairing pheromones varies among termite species; for example, Cornitermes bequaerti uses only (Z,Z,E)-3,6,8-dodecatrien-1-ol, whereas C. cumulans uses both (Z,Z,E)-3,6,8-dodecatrien-1-ol and (E)-nerolidol. Furthermore, C. silverstrii uses a mixture of three compounds including (Z,Z,E)-3,6,8-dodecatrien-1-ol, (E)-nerolidol, and (Z)-3-dodecen-1-ol (Table 1). The addition of species-specific minor components to a pre-existing pheromone might facilitate species recognition among sympatric species, as is often suggested of insect sex pheromones (Weiss et al., 2013; Allison and Cardé, 2016; Chen et al., 2018; Valterová et al., 2019). However, the development of species-specific pheromones among sympatric species varies considerably among species (Bordereau and Pasteels, 2011).
Trail-Following Pheromones
When foraging individuals (workers, pseudergates [individuals performing works while remaining developmentally flexible], or soldiers) find new food, they deposit trail-following pheromones from the sternal gland while returning to the nest; the pheromones elicit trail-following behavior in nestmates, leading them to the food resource (Traniello, 1981; Traniello and Busher, 1985; Czaczkes et al., 2015; Almeida et al., 2016). Trail-following pheromones are one of the most studied pheromone types, especially in ants and termites. Therefore, the biochemical, physiological, ecological, and evolutionary aspects of trail-following pheromones have been well characterized in previous studies (Wilson, 1971; Hölldobler and Wilson, 1990; Morgan, 2009; Bordereau and Pasteels, 2011; Wyatt, 2014; Czaczkes et al., 2015; Leonhardt et al., 2016).
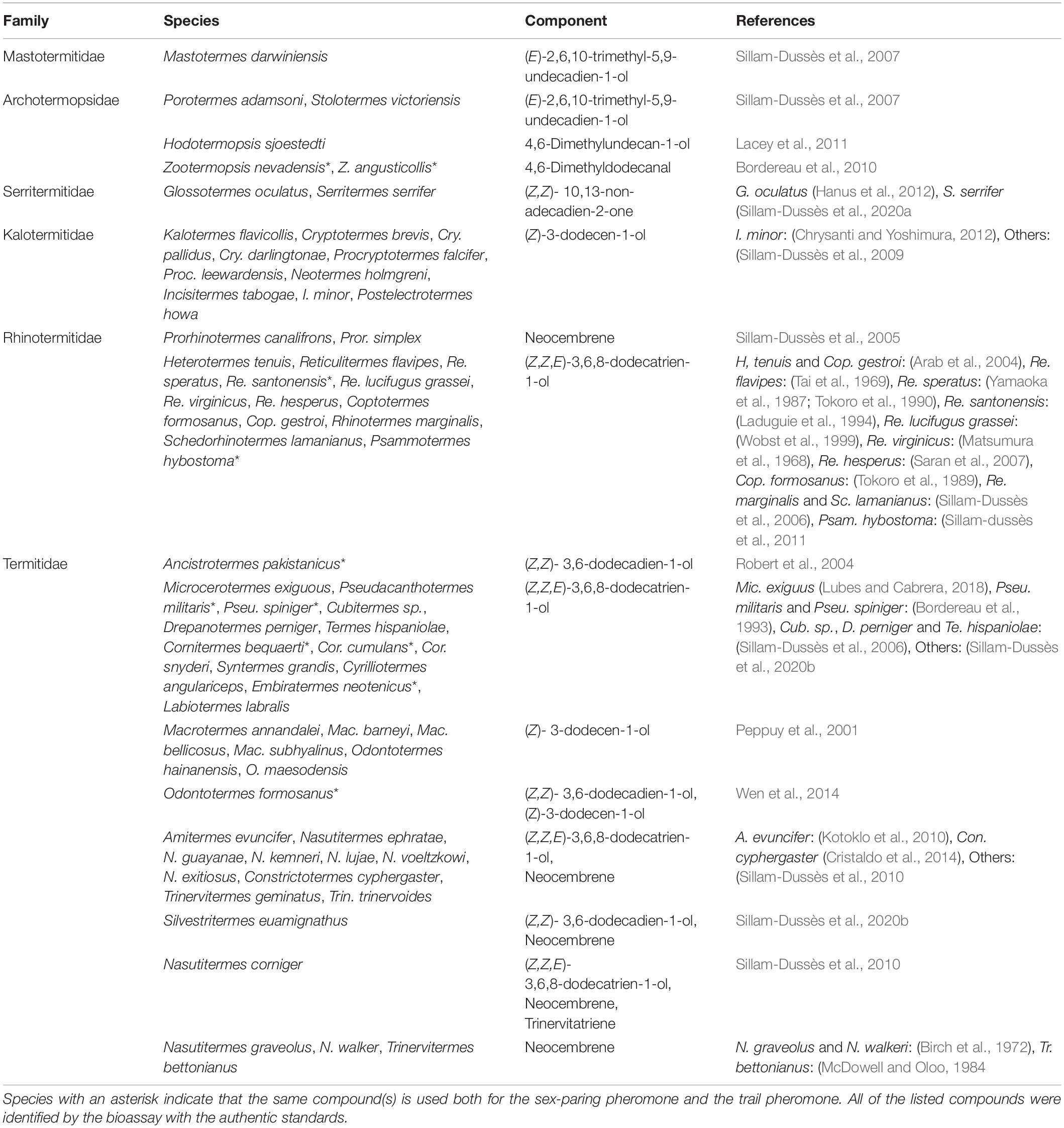
Thus far, termite trail-following pheromones are known in 68 species (Table 2). Their chemical components are often common within a taxonomical group. In particular, (Z,Z,E)-3,6,8-dodecatrien-1-ol is used in Rhinotermitidae (12 species) and Termitidae (24 species). While the trail-following pheromones of lower termite species belonging to families other than the Termitidae consist of only one compound, some higher termite species use a plurality of compounds; for example, O. formosanus uses two compounds, (Wen et al., 2014) and Nasutitermes corniger uses three compounds (Sillam-Dussès et al., 2010; Table 2). In O. formosanus, one of the compounds, (Z)-dodec-3-en-1-ol, elicits orientation behavior of termites exploring the environment, and the other (Z,Z)-dodeca-3,6-dien-1-ol has both orientation and recruitment effects on termites when food is discovered (Wen et al., 2014). Furthermore, workers of O. formosanus change the mixture ratio of the pheromone components depending on their ongoing behaviors, and the threshold of response to the pheromone components also change depending on behavioral contexts (Wen et al., 2014).
In 10 species, the constituents are completely (or partially) identical between the trail pheromone and the sex-pairing pheromone (Table 2). For example, (Z,Z,E)-3,6,8-dodecatrien-1-ol is used alone as both the trail pheromone and the sex-pairing pheromone in Reticulitermes santonensis (Laduguie et al., 1994), Pseudacanthotermes militaris (Bordereau et al., 1993), Pseudacanthotermes spiniger (Bordereau et al., 1991, 1993), and C. bequaerti (Bordereau et al., 2002; Sillam-Dussès et al., 2020b). In contrast, in C. cumulans, this compound is used alone as the trail pheromone (Sillam-Dussès et al., 2020b), but used together with (E)-nerolidol as the sex-pairing pheromone (Bordereau et al., 2011).
Aggregation Pheromones
Aggregation pheromones elicit an aggregation behavior that involves attraction of conspecific individuals from a distance, followed by arrest of those individuals at the pheromone source (Kennedy, 1978). When foraging individuals discover a new food source, they gather their nestmates to that area for exploitation and subsequent colonization of the new area. This aggregation behavior is beneficial to the individuals because it facilitates allogrooming (removal of cuticular stains and pathogens by grooming each other) and trophallaxis (exchange of nutrients, gut symbionts, and immune substances by stomodeal and proctodeal feeding), which enables workers to survive oligotrophic and microbe-rich environments (Eggleton, 2011). Thus, rapidly formed yet long-lasting aggregation is needed for foraging individuals.
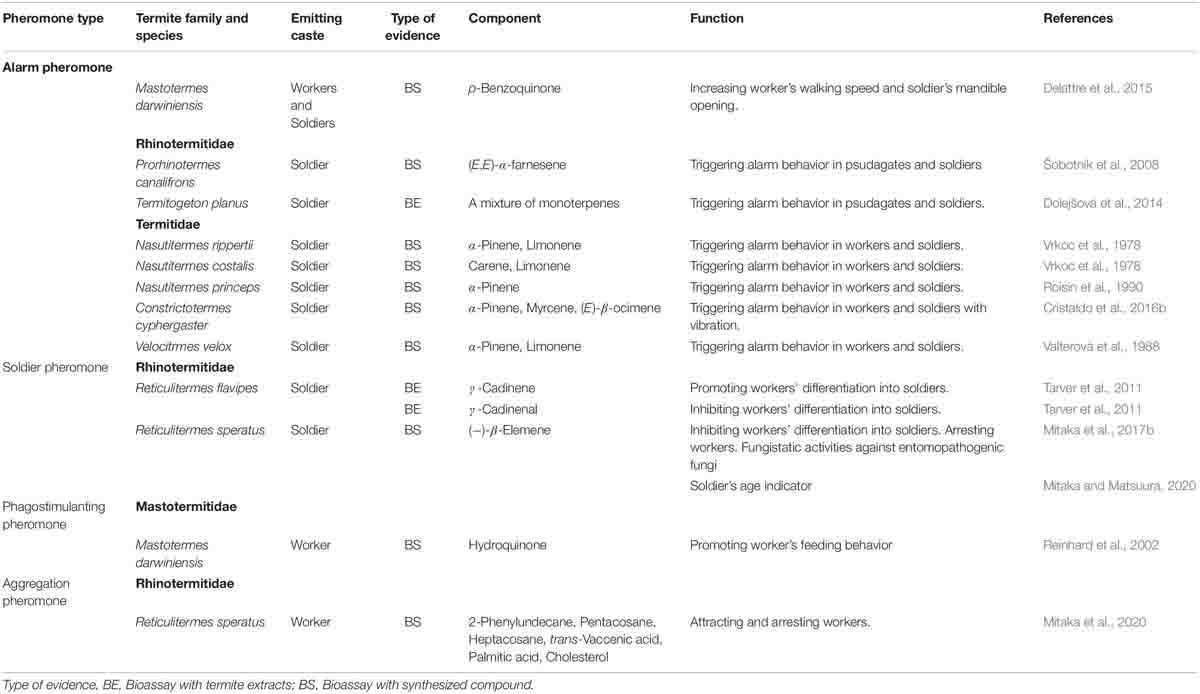
A recent study revealed that Reticulitermes speratus workers release an aggregation pheromone at their nesting site, and that this pheromone induces rapid and long-lasting aggregation of workers (Mitaka et al., 2020; Table 3). The pheromone is a mixture of 2-phenylundecane, pentacosane, heptacosane, palmitic acid, trans-vaccenic acid, and cholesterol. It is estimated that the aggregation pheromone functions as a signal to indicate nestmate and/or food presence, and as an arresting agent that causes the attracted workers to remain at the pheromone source for a long period.
Phagostimulant Pheromones
A previous study showed that hydroquinone is present in the labial glands of workers in several termite species; those findings suggested that this compound acts as a phagostimulant pheromone, which elicits worker feeding behavior, in various termites (Reinhard et al., 2002; Table 3). However, the pheromonal activity has been demonstrated only in Mastotermes darwiniensis (Reinhard et al., 2002). Therefore, the generality of termite phagostimulant pheromones has not been fully elucidated (Raina et al., 2005).
Alarm Pheromones
In termites, colony defense is performed by soldiers and workers (Roisin et al., 1990; Reinhard and Clement, 2002; Šobotník et al., 2010; Ishikawa and Miura, 2012; Yanagihara et al., 2018). Alarm pheromones elicit alarm behaviors including orientation to the pheromone source, nestmate recruitment, and attack on enemies in termite soldiers and workers (or pseudergates), although the details of the behavioral sequences are different among species. For example, in European Reticulitermes species (R. santonensis, R. lucifugus, R. grassei, and R. banyulensis), chemical alarm signals from soldier’s head induce zigzag running, antennation with nestmates, body shaking behavior, and orientation to the odor source in both soldiers and workers (Reinhard and Clement, 2002). Soldiers also perform mandible snapping and head-banging on the substrate. While workers are attracted by the odor within a few seconds, soldiers are attracted much later (Reinhard and Clement, 2002). In Nasutitermes princeps, when the alarm pheromone is secreted from soldiers, soldiers and workers are attracted by the pheromone, and then soldiers emit their sticky secretion to immobilize and incapacitate enemies. After that, older large workers eliminate the enemies (Roisin et al., 1990).
Termites use vibratory signals in combination with chemical signals, resulting in complex alarm communication. For example, in M. darwiniensis, soldiers actively face the disturbance source with repeating mandible openings and secreting defensive secretion, while workers run away from the disturbance source and spread the alarm signals to the nestmates using body vibrations (Delattre et al., 2015). Also, in R. flavipes, the alarm pheromone increases the vibratory communication among soldiers and workers (Delattre et al., 2019). Furthermore, the evoked alarm responses depending on the dose of alarm pheromone. In Constrictotermes cyphergaster, the soldiers also actively face the source of disturbance and then emit the alarm pheromone; lower doses increase body shaking movements of nestmates, and higher doses induced long-term running of them (Cristaldo et al., 2016b).
Thus far, alarm pheromones (comprising monoterpenes and/or sesquiterpenes) have been identified in six species (Table 3): p-benzoquinone in M. darwiniensis (Delattre et al., 2015), (E,E)-α-farnesene in Prorhinotermes canalifrons (Šobotník et al., 2008); α-pinene in N. princeps (Roisin et al., 1990); α-pinene, myrcene, and (E)-β-ocimene in C. cyphergaster (Cristaldo et al., 2016b); carene and limonene in Nasutitermes costalis (Vrkoc et al., 1978); and α-pinene and limonene in Nasutitermes rippertii (Vrkoc et al., 1978) and Velocitermes velox (Valterová et al., 1988). Although a mixture of terpenes is estimated to be used as the alarm pheromone in Termitogeton planus (Dolejšová et al., 2014), R. santoensis, R. grassei, R. lucifugus, R. banyulensis (Reinhard et al., 2003), and R. flavipes (Delattre et al., 2019), the compositions of the alarm pheromones has not been clarified. The secretion gland of the alarm pheromones is restricted to the frontal grand of the soldier head in Rhinotermitidae and Termitidae (Šobotník et al., 2010), but in M. darwiniensis, the alarm pheromone is secreted from the labial glands of soldiers and workers (Delattre et al., 2015).
Soldier Pheromones
In termites, soldiers are differentiated from workers (or pseudergates). For more than 35 years, it was presumed that the soldier’s head extract contains a primer pheromone that inhibits soldier differentiation (Lefeuve and Bordereau, 1984). Until recently, there was no proof of the existence of this pheromone. A previous study reported that γ-cadinene and γ-cadinenal, which were isolated from the soldiers of R. flavipes, showed stimulatory and inhibitory effects, respectively, on soldier differentiation (Tarver et al., 2011); however, these pheromonal activities were not demonstrated using authentic standards.
Recently, it was revealed that the soldiers of R. speratus secrete (−)-β-elemene as the soldier pheromone; this compound functions as the primer pheromone that inhibits soldier differentiation, as a releaser pheromone that arrests workers, and as a fungistatic agent that protects against entomopathogenic fungi (e.g., Metarhizium anisopliae and Beauveria bassiana) (Mitaka et al., 2017b; Table 3). Furthermore, the amount of (−)-β-elemene a soldier holds increases with age, resulting in functioning that serves as a signal to indicate the soldier’s age (Mitaka and Matsuura, 2020). The nests of R. speratus are divided into chambers connected by small openings; the royal chamber (i.e., location of reproductive castes) is located deep inside the nest wood. In this species, younger soldiers gather around the royal chamber to protect kings and queens, while older soldiers are distributed in the periphery of the nest wood to defend the nest entrances (Yanagihara et al., 2018). When workers decide whether to move among chambers, they examine the amount of soldier pheromone held by the soldier standing guard at the chamber entrance; they do not move to the next chamber if the amount of soldier pheromone is very small (that is, if the soldier is very young) (Mitaka and Matsuura, 2020).
Egg Recognition Pheromones
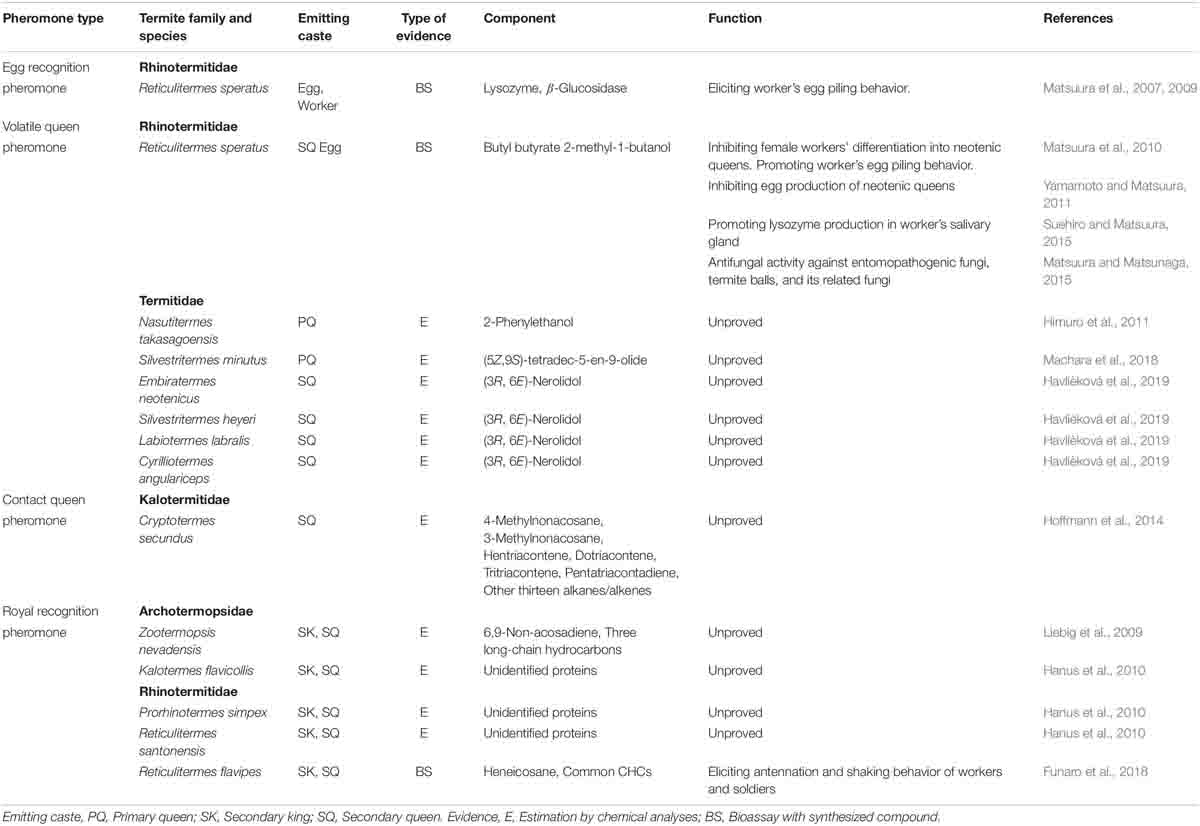
Egg protection is one of the most fundamental social activities performed by social insects (Ayasse and Paxton, 2002). In termites, workers recognize the eggs laid by queens, pile the eggs in nursery rooms, and smear their saliva on the eggs’ surfaces to protect them from desiccation and infection by pathogens (Matsuura et al., 2000, 2007). In R. speratus, lysozyme, an antibacterial enzyme expressed in the eggs and the workers’ salivary glands, is used as an egg recognition pheromone; it elicits egg-carrying and -piling behaviors in workers (Matsuura et al., 2007; Table 4). Moreover, R. speratus workers simultaneously use β-glucosidase, a cellulose-digesting enzyme expressed in the eggs, as well as in the workers’ salivary glands and hind gut, as an egg recognition pheromone (Matsuura et al., 2009; Table 4). Although each enzyme itself has sufficient pheromonal activity, the two enzymes act synergistically to elicit strong egg-carrying and -piling behaviors (Matsuura et al., 2009). It is speculated that Reticulitermes spp. commonly use lysozymes and β-glucosidase as egg recognition pheromones because of their broad cross-species activities (Matsuura et al., 2007, 2009); however, the salivary gland extracts derived from workers of Hodotermopsis sjostedti, Cryptotermes brevis, and Coptotermes formosanus also show strong pheromone activity (Matsuura et al., 2009). In addition, termites use C-type lysozymes as an egg recognition pheromone, although recent transcriptomic analyses revealed that Z. nevadensis, C. formosanus, and R. speratus also have I-type and P-type lysozymes; particularly in R. speratus, I-type and P-type lysozymes are highly expressed in soldiers (Mitaka et al., 2017a). The functions of these lysozymes are unknown; thus, further studies are needed.
Queen Pheromones
In social insects, reproduction is primarily monopolized by queens; the number of fertile queens is regulated by communication between reproductive and non-reproductive individuals, often through queen pheromones (Van Oystaeyen et al., 2014). Although the queen pheromones of social hymenopteran species, which inhibit worker differentiation into supplementary queens, have been suggested or identified for over 50 years (Van Oystaeyen et al., 2014), studies of termite queen pheromones have made little progress.
Thus far, R. speratus is the only termite species in which the queen pheromone has been identified (Matsuura et al., 2010; Table 4). The queen pheromone is a volatile pheromone that consists of butyl butyrate and 2-methyl-1-butanol (mixture ratio 2:1); it is emitted from secondary queens and eggs as an honest signal of queen presence and fertility (Matsuura et al., 2010). This pheromone has multifunctional roles: inhibition of the differentiation of workers into supplementary queens (Matsuura et al., 2010), enhancement of workers’ egg-carrying and -piling behaviors (Matsuura et al., 2010), regulation of egg production in secondary queens (Yamamoto and Matsuura, 2011), promotion of lysozyme production in workers’ salivary glands (Suehiro and Matsuura, 2015), and performance of antifungal activities against entomopathogenic and parasitic fungi (Matsuura and Matsunaga, 2015). Although 2-methyl-1-butanol is an optically active alcohol [(R)- and (S)-isomers], both enantiomers have the same pheromonal activities (Yamamoto et al., 2012). For the workers’ egg-carrying and -piling behaviors, the egg recognition pheromones (lysozyme and β-glucosidase) and the volatile queen pheromone act synergistically to enhance these behaviors (Matsuura et al., 2010).
In addition, queen-specific volatiles have been discovered in other termite species (Table 4): 2-phenylethanol in Nasutitermes takasagoensis (Himuro et al., 2011); (5Z,9S)-tetradec-5-en-9-olide in Silvestritermes minutus (Machara et al., 2018); and (3R,6E)-nerolidol in Embiratermes neotenicus, S. heyeri, Labiotermes labralis, and Cyrilliotermes angulariceps (Havlíèková et al., 2019). Furthermore, a non-volatile (contact) queen pheromone is suspected in Cryptotermes secundus; this pheromone consists of cuticular hydrocarbons (CHCs) including 4-methylnonacosane, 3-methylnonacosane, hentriacontene, dotriacontene, tritriacontene, and pentatriacontadiene [the positions of double bond(s) are unknown in all of these unsaturated hydrocarbons], and another thirteen alkanes/alkenes (Hoffmann et al., 2014). However, the pheromonal activities of these compounds have not yet been demonstrated (Table 4).
Royal Recognition Pheromones
It has long been hypothesized that when termite nestmates recognize royal castes (i.e., kings and queens), they contact the body surfaces of royals and then perceive royal-specific chemicals (i.e., royal recognition pheromones) (Korb, 2018; Hefetz, 2019). Royal recognition pheromones are predicted to be non-volatile, because volatile compounds easily saturate the colony space, and thus hamper the nestmates’ perception due to sensory habituation (Korb, 2018; Hefetz, 2019). Indeed, neotenic kings and queens of Z. nevadensis have some royal-specific CHCs, including 6,9-nonacosadiene and three long-chain hydrocarbons (Liebig et al., 2009); similarly, neotenic kings and queens of Kalotermes flavicollis, Prorhinotermes simplex, and R. santonensis have royal-specific proteins on their body surfaces (Hanus et al., 2010), although the pheromone activities of these compounds have not been demonstrated (Table 4).
A recent study revealed that one royal-specific CHC, heneicosane, functions as a royal-recognition pheromone under the presence of workers’ cuticular extract in R. flavipes (Funaro et al., 2018; Table 4). The pheromone elicits strong royal recognition behaviors (antennation and shaking of the body) in both workers and soldiers that come into contact with it (Funaro et al., 2018). Even when the pheromone is combined with foreign workers’ CHCs, the royal recognition behaviors are elicited both in workers and soldiers (Funaro et al., 2019). However, these studies analyzed the CHC profiles of the neotenic kings and queens but not of the primary ones, i.e., adult reproductives. It is therefore still unknown whether the “royal-specific” CHC would be common between primary and neotenic reproductives. Additionally, the relative proportion of each component of CHCs in termite kings and queens can change with aging (Gordon et al., 2020). Further study will be needed to elucidate how termites share the information of the royal CHC profiles among nestmates for a long period of time.
Interspecific Interaction via Termite Pheromones
Termite chemical communications are often eavesdropped or mimicked by termitophagous predators and inquilines. For example, the termite-raiding ant Odontoponera transversa eavesdrops the trail-following pheromones of fungus-growing termites (Odontoponera yunnanensis, Macrotermes yunnanensis, and Ancistrotermes dimorphus) (Wen et al., 2017). The termitophilous rove beetles (Staphylinidae) mimic CHC profiles of the host termite species: Trichopsenius frosti mimics the CHC profile of R. flavipes (Howard et al., 1980); Trichopsenius depressus, Xenistusa hexagonalis, and Philotermes howardi mimic that of R. virginicus (Howard et al., 1982); Corotoca melantho mimics that of C. cyphergaster (Rosa et al., 2018). The termite Inquilinitermes microcerus, which is an obligatory inquiline of C. cyphergaster, does not have its own trail pheromone, but this inquiline termite follows the trail pheromone of its host (Cristaldo et al., 2014). Also, C. cyphergaster responds to only its own alarm signal, while I. microcerus responds both to its own alarm signal and to an alarm signal from its host (Cristaldo et al., 2016c).
Inter-colonical chemical interactions can be affected by food resource availability and previously exposed odor. In Nasutitermes aff. coxipoenens, although the workers from colonies under low or high resource availability do not discriminate between foreign trails leading into rich and poor food resources, the workers from colonies under intermediate resource availability discriminate between foreign trails leading into rich and poor resources (Cristaldo et al., 2016a). Moreover, the individuals of N. aff. coxipoensis are attracted to allocolonial odor cues to which they were previously exposed (Ferreira et al., 2018).
The termite egg-mimicking fungus “termite ball” represents one of the most remarkable cases of fungal inquiline. Because termite nests are well sanitized by secretion of antimicrobial agents (Chen et al., 1998; Rosengaus et al., 2000, 2004; Zhao et al., 2004; Matsuura et al., 2007), it is difficult for microbes to intrude into the nest. However, the termite balls succeed in intruding termite nests by mimicking termite eggs; termite workers take care of the termite balls in a manner identical to that of eggs to prevent them from desiccation and pathogen infection (Matsuura et al., 2000). The termite balls are the sclerotia of an athelioid fungus of the genus Fibularhizoctonia (Matsuura et al., 2000), which morphologically mimics the eggs of Reticulitermes termites by matching the diameter and the smooth surface texture of the eggs (Matsuura et al., 2000; Matsuura, 2006; Yashiro and Matsuura, 2007; Ye et al., 2019). Furthermore, the termite ball chemically mimics the eggs by expressing β-glucosidase, a component of the termite egg recognition pheromone (Matsuura et al., 2009). The termite balls grow on the termite nest wood and obtain nutrition and energy by digesting cellulose contained in the wood. Accordingly, it has been speculated that the termite balls originally had the potential to produce the same substance as the termite egg recognition pheromone component; this facilitated the evolution of termite egg mimicry (Matsuura et al., 2009). Furthermore, the soldier pheromone of R. speratus, which has fungistatic activities against entomopathogenic fungi, is unable to inhibit the mycelial growth of termite balls (Mitaka et al., 2017b), suggesting that termite balls newly acquired resistance to termite fungistatic compounds (Mitaka et al., 2019). Thus far, the volatile queen pheromone of R. speratus is the only known antifungal agent that inhibits the germination and growth of termite balls (Matsuura and Matsunaga, 2015). However, the inhibitory effect of the queen pheromone differs among termite ball strains, suggesting that some termite ball strains may develop resistance even to the queen pheromone (Matsuura and Matsunaga, 2015).
Evolution of Termite Pheromones
Many termite species live inside predator- and microbe-rich habitats, such as rotten wood and soil; thus, they develop a wide variety of defensive substances including CHCs, terpenes, and antimicrobial molecules (Howard and Blomquist, 2005; Stow and Beattie, 2008; Šobotník et al., 2010; Rosengaus et al., 2011). Recent studies outlined in the above sections suggested that termites parsimoniously use these substances for pheromones involved in caste recognition and caste-specific roles.
For example, CHCs are presumed to originally have been used by insects to withstand desiccation and pathogen invasion (Blomquist and Bagnères, 2010; Menzel et al., 2017). Most insects have diversified their CHC compositions, such that the CHCs are species-specific; these CHCs often serve as species recognition cues for mating (Howard and Blomquist, 1982). In eusocial insects including termites, the compositional ratios of CHCs significantly differ between reproductive and non-reproductive castes in each colony; queens (and kings in termites) develop royal-specific CHC profiles (Van Oystaeyen et al., 2014; Hefetz, 2019). The termite kings and queens in each colony begin to produce de novo CHCs (or to increase production of a certain existing CHC component, compared to non-reproductive castes), accompanied by enhancement of juvenile hormone titer followed by sexual maturation (Brent et al., 2016). Ultimately, the royal-specific compounds are utilized as royal recognition pheromones (Le Conte et al., 2008; Leonhardt et al., 2016).
In parallel with the above CHC divergences, soldiers in Rhinotermitidae and Termitidae species developed the production ability of a variety of terpenes in the frontal glands of their heads, and they use the terpenes for defensive compounds such as repellents, poisons, antimicrobials, and sticky substances for immobilizing predators (Šobotník et al., 2010). However, some termite species use terpenes not only for such defensive substances but also for pheromones, which are associated with nestmate recruitment for colony defense and soldier differentiation (Vrkoc et al., 1978; Valterová et al., 1988; Roisin et al., 1990; Šobotník et al., 2008, 2010; Dolejšová et al., 2014; Cristaldo et al., 2016b; Mitaka et al., 2017b).
Other antimicrobial molecules are also used for chemical communication in termite societies. Workers and eggs produce the antibacterial enzyme for egg recognition pheromone (Matsuura et al., 2007), while queens use the queen-specific antifungal volatiles for the pheromone indicating the queen fertility, resulting in acquiring multifaceted roles associated with promoting egg production and survivorship, and regulating queen differentiation (Matsuura, 2012; Matsuura and Matsunaga, 2015).
Even when the same set of compounds is used, the pheromone function can change according to the emitter, and the dose. For example, in some termites, the same set of compounds is used both for sex-pairing pheromones secreted from alates and for trail-following pheromones secreted from workers (Table 2). Also, different concentrations of an alarm pheromone induce different alarm behaviors of nestmates (Cristaldo et al., 2016b).
These facts strongly suggest that multifaceted usage of the same set of compounds could have been the driving force behind sophistication of termite pheromone communication. Because the capacity of de novo biosynthesis of chemical compounds is limited and costly, evolutional pressures have led to the reuse of existing compounds for chemical communication. This phenomenon is called semiochemical parsimony (Blum, 1996), which also occurs in many other insects (Blum and Brand, 1972; Blum, 1996; Ruther et al., 2001; Allison et al., 2004; Nojima et al., 2005; de Bruijn et al., 2006; Le Conte et al., 2008; Geiselhardt et al., 2009; Chung and Carroll, 2015; Takata et al., 2019). Therefore, it has been considered that semiochemical parsimony may account for the birth of pheromones and the multifunctionalization in social insects (Blum and Brand, 1972; Matsuura, 2012). Moreover, pheromones can be developed by adding new compound(s) to pre-existing semiochemicals, according to the social context. In R. speratus, two major components of the workers’ CHCs are also used as the aggregation pheromone, in combination with another four compounds. This usage suggests that workers inform nestmates of both the presence of other nestmates and locations suitable for foraging/nesting (Mitaka et al., 2020). It is hypothesized that both the parsimonious usage of the same compound(s) and the addition of supplementary compounds to a pre-existing semiochemical depending on the social context enable termites to process considerable quantities of context-dependent information with a small number of chemicals, thus forming a coordinated and reasonable chemical communication system. However, the rationalization of chemical communications does not always induce the increase of types of pheromones in a termite society, because the obligatory inquiline termite I. microcerus takes advantage of the host’s trail and alarm pheromones instead of using its own pheromones (Cristaldo et al., 2014, 2016c). Such a usage of allelochemicals provides an extended pheromone communication to inquiline species as another level of semiochemical parsimony. Evolution of pheromone communication system may be largely affected by social structure, lifestyle, and habitat environment in termites.
Conclusion and Prospects
Ongoing development of methods and devices for chemical analyses has facilitated increased pheromone identification in recent years (Wyatt, 2014). Since the identification of the first termite pheromone [i.e., the trail pheromone of R. virginicus in 1968 (Matsumura et al., 1968)], many pheromone compounds have been identified in various termite species. In particular, the number of termite pheromone studies has been rapidly increasing since 2000 (∼84% of termite pheromone papers were published from 2000 to 2020) (Tables 1–4). Notably, trail-following pheromones are the most popular in termite pheromone studies and have been identified in 67 species; however, recent studies have made R. speratus the most well-studied species with respect to pheromones, such that five types of pheromones have been identified (as of July 2020) including the trail pheromone (Yamaoka et al., 1987; Tokoro et al., 1990), aggregation pheromone (Mitaka et al., 2020), soldier pheromone (Mitaka et al., 2017b), egg recognition pheromone (Matsuura et al., 2007, 2009), and volatile queen pheromone (Matsuura et al., 2010). Some pheromones gained multifunctional roles by parsimoniously using the same set of compounds for multiple purposes or by adding new compounds to pre-existing semiochemicals depending on the situation; these multifunctional pheromones can enable more informative communication in social insects. However, there remain predicted but unidentified pheromones, such as a king pheromone that regulates caste differentiation (Wilson, 1965) and a cement pheromone that evokes nest-building behaviors (Bonabeau et al., 1998; Mizumoto et al., 2015; Green et al., 2017). In addition, minimal research has been performed regarding the biosynthesis (Prestwich et al., 1981; Hojo et al., 2007, 2011; Blomquist, 2010; Šobotník et al., 2010; Bordereau and Pasteels, 2011; Beran et al., 2019) and molecular-level chemoreceptive mechanisms (Poulsen et al., 2014; Terrapon et al., 2014; Mitaka et al., 2016; Harrison et al., 2018) in termite pheromones. Future interdisciplinary research—including chemical ecology, genetics, physiology, and biochemistry—will provide important insights into the molecular and evolutionary mechanisms of the development of intracolonial, intercolonial, and interspecies chemical communications in termite societies.
Author Contributions
YM conducted literature retrieval. YM and TA wrote and edited the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 18J00399 (to YM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the reviewers for helpful comments regarding the manuscript.
References
Allison, J. D., Borden, J. H., and Seybold, S. J. (2004). A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14, 123–150. doi: 10.1007/s00049-004-0277-1
Allison, J. D., and Cardé, R. T. (2016). “Pheromones: reproductive isolation and evolution in moths,” in Pheromone Communication in Moths: Evolution, Behavior, And Application, eds J. D. Allison and R. T. Cardé (Oakland: University of California Press), 11–24. doi: 10.1525/9780520964433-003
Almeida, C. S., Cristaldo, P. F., Florencio, D. F., Cruz, N. G., Santos, A. A., Oliveira, A. P., et al. (2016). Combined foraging strategies and soldier behaviour in Nasutitermes aff. coxipoensis (Blattodea: Termitoidea: Termitidae). Behav. Processes 126, 76–81. doi: 10.1016/j.beproc.2016.03.006
Arab, A., Costa-Leonardo, A. M., Batista-Pereira, L. G., Dos Santos, M. G., Corrêa, A. G., and Blanco, Y. C. (2004). Trail-pheromone specificity of two sympatric termites (Rhinotermitidae) from Southeastern Brazil. Sociobiology 43, 377–387.
Ayasse, M., and Paxton, R. (2002). “Brood protection in social insects,” in Chemoecology of Insect Eggs and Egg Deposition, eds M. Hilker and T. Meiners (Oxford: Blackwell Publishing), 117–148. doi: 10.1002/9780470760253.ch5
Beran, F., Köllner, T. G., Gershenzon, J., and Tholl, D. (2019). Chemical convergence between plants and insects: biosynthetic origins and functions of common secondary metabolites. New Phytol. 223, 52–67. doi: 10.1111/nph.15718
Birch, A. J., Brown, W. V., Corrie, J. E. T., and Moore, B. P. (1972). Neocembrene-A, a termite trail pheromone. J. Chem. Soc. Perkin Trans. 1, 2653–2658. doi: 10.1039/P19720002653
Blomquist, G. J. (2010). “Biosynthesis of cuticular hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology, eds A.-G. Bagnères and G. J. Blomquist (Cambridge: Cambridge University Press), 35–52. doi: 10.1017/CBO9780511711909.004
Blomquist, G. J., and Bagnères, A.-G. (2010). “Introduction: history and overview of insect hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology, eds A.-G. Bagnères and G. J. Blomquist (Cambridge: Cambridge University Press), 3–18. doi: 10.1017/CBO9780511711909.002
Blum, M. S. (1996). Semiochemical parsimony in the Arthropoda. Annu. Rev. Entomol. 41, 353–374. doi: 10.1146/annurev.en.41.010196.002033
Blum, M. S., and Brand, J. M. (1972). Social insect phermones: their chemistry and function. Am. Zool. 12, 553–576. doi: 10.1093/icb/12.3.553
Bonabeau, E., Theraulaz, G., Deneubourg, J. L., Franks, N. R., Rafelsberger, O., Joly, J. L., et al. (1998). A model for the emergence of pillars, walls and royal chambers in termite nests. Philos. Trans. R. Soc. B Biol. Sci. 353, 1561–1576. doi: 10.1098/rstb.1998.0310
Bordereau, C., Cancello, E. M., Sémon, E., Courrent, A., and Quennedey, B. (2002). Sex pheromone identified after solid phase microextraction from tergal glands of female alates in Cornitermes bequaerti (Isoptera, Nasutitermitinae). Insectes Soc. 49, 209–215. doi: 10.1007/s00040-002-8303-1
Bordereau, C., Cancello, E. M., Sillam-Dussès, D., and Sémon, E. (2011). Sex-pairing pheromones and reproductive isolation in three sympatric Cornitermes species (Isoptera, Termitidae, Syntermitinae). J. Insect Physiol. 57, 469–474. doi: 10.1016/j.jinsphys.2011.01.010
Bordereau, C., Lacey, M. J., Sémon, E., Braekman, J., Ghostin, J., Robert, A., et al. (2010). Sex pheromones and trail-following pheromone in the basal termites Zootermopsis nevadensis (Hagen) and Z. angusticollis (Hagen) (Isoptera: Termopsidae: Termopsinae). Biol. J. Linn. Soc. 100, 519–530. doi: 10.1111/j.1095-8312.2010.01446.x
Bordereau, C., and Pasteels, J. M. (2011). Pheromones and chemical ecology of dispersal and foraging in termites. Biol. Term. 1, 279–320. doi: 10.1007/978-90-481-3977-4_11
Bordereau, C., Robert, A., Bonnard, O., and Le Quere, J.-L. (1991). (3Z,6Z,8E)-3,6,8-dodecatrien-1-ol: sex pheromone in a higher fungus-growing termite, Pseudacanthotermes spiniger (Isoptera, Macrotermitinae). J. Chem. Ecol. 17, 2177–2191. doi: 10.1007/bf00988000
Bordereau, C., Robert, A., and Laduguie, N. (1993). Détection du (Z,Z,E)-3,6,8-dodecatrien-1-ol par les ouvriers et les essaimants de deux espèces de termites champignonnistes: Pseudacanthotermes spiniger et P. militaris (Termitidae, Macrotermitinae). Actes Colloq. Insectes Sociaux 8, 145–149.
Brent, C. S., Penick, C. A., Trobaugh, B., Moore, D., and Liebig, J. (2016). Induction of a reproductive-specific cuticular hydrocarbon profile by a juvenile hormone analog in the termite Zootermopsis nevadensis. Chemoecology 26, 195–203. doi: 10.1007/s00049-016-0219-8
Buděšínský, M., Valterová, I., Sémon, E., Cancello, E., and Bordereau, C. (2005). NMR structure determination of (11E)-trinervita-1(14),2,11-triene, a new diterpene from sexual glands of termites. Tetrahedron 61, 10699–10704. doi: 10.1016/j.tet.2005.08.084
Chen, J., Henderson, G., Grimm, C. C., Lloyd, S. W., and Laine, R. A. (1998). Naphthalene in Formosan subterranean termite carton nests. J. Agric. Food Chem. 46, 2337–2339. doi: 10.1021/jf9709717
Chen, Q. H., Zhu, F., Tian, Z., Zhang, W. M., Guo, R., Liu, W., et al. (2018). Minor components play an important role in interspecific recognition of insects: a basis to pheromone based electronic monitoring tools for rice pests. Insects 9, 1–15. doi: 10.3390/insects9040192
Chrysanti, E., and Yoshimura, T. (2012). “Evaluation of trail pheromone and attractants against drywood termite Incisitermes minor (Hagen) (Blattodea: Kalotermitidae),” in Proceedings of the 9th Pacific-Rim Termite Research Group Conference, 192–198. Available online at: http://www.prtrg.org/images/proceedingsTRG-9.pdf#page=141 (accessed April 25, 2020).
Chung, H., and Carroll, S. B. (2015). Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37, 822–830. doi: 10.1002/bies.201500014
Clément, J. L., Lloyd, H., Nagnan, P., and Blum, M. S. (1989). n-Tetradecyl propionate: identification as a sex pheromone of the eastern subterranean termite Reticulitermes flavipes. Sociobiology 15, 19–24.
Cristaldo, P. F., Araújo, A. P. A., Almeida, C. S., Cruz, N. G., Ribeiro, E. J. M., Rocha, M. L. C., et al. (2016a). Resource availability influences aggression and response to chemical cues in the Neotropical termite Nasutitermes aff. coxipoensis (Termitidae: Nasutitermitinae). Behav. Ecol. Sociobiol. 70, 1257–1265. doi: 10.1007/s00265-016-2134-y
Cristaldo, P. F., DeSouza, O., Krasulová, J., Jirošová, A., Kutalová, K., Lima, E. R., et al. (2014). Mutual use of trail-following chemical cues by a termite host and its inquiline. PLoS One 9:58315. doi: 10.1371/journal.pone.0085315
Cristaldo, P. F., Jandák, V., Kutalová, K., Rodrigues, V. B., Brothánek, M., Jiøíèek, O., et al. (2016b). The nature of alarm communication in Constrictotermes cyphergaster (Blattodea: Termitoidea: Termitidae): the integration of chemical and vibroacoustic signals. Biol. Open 4, 1649–1659. doi: 10.1242/bio.014084
Cristaldo, P. F., Rodrigues, V. B., Elliot, S. L., Araújo, A. P. A., and DeSouza, O. (2016c). Heterospecific detection of host alarm cues by an inquiline termite species (Blattodea: Isoptera: Termitidae). Anim. Behav. 120, 43–49. doi: 10.1016/j.anbehav.2016.07.025
Czaczkes, T. J., Grüter, C., and Ratnieks, F. L. W. (2015). Trail pheromones: an integrative view of their role in social insect colony organization. Annu. Rev. Entomol. 60, 581–599. doi: 10.1146/annurev-ento-010814-020627
de Bruijn, P. J. A., Martijn, E., Janssen, A., and Sabelis, M. W. (2006). Pheromone-induced priming of a defensive response in Western flower thrips. J. Chem. Ecol. 32, 1599–1603. doi: 10.1007/s10886-006-9092-1
Delattre, O., Sillam-Dussès, D., Jandák, V., Brothánek, M., Rücker, K., Bourguignon, T., et al. (2015). Complex alarm strategy in the most basal termite species. Behav. Ecol. Sociobiol. 69, 1945–1955. doi: 10.1007/s00265-015-2007-9
Delattre, O., Šobotník, J., Jandák, V., Synek, J., Cvaèka, J., Jiøíèek, O., et al. (2019). Chemical and vibratory signals used in alarm communication in the termite Reticulitermes flavipes (Rhinotermitidae). Insectes Soc. 66, 265–272. doi: 10.1007/s00040-018-00682-9
Dolejšová, K., Krasulová, J., Kutalová, K., and Hanus, R. (2014). Chemical alarm in the termite Termitogeton planus (Rhinotermitidae). J. Chem. Ecol. 40, 1269–1276. doi: 10.1007/s10886-014-0515-0
Dolejšová, K., Køivánek, J., Kalinová, B., Hadravová, R., Kyjaková, P., and Hanus, R. (2018). Sex-pairing pheromones in three sympatric Neotropical termite species (Termitidae: Syntermitinae). J. Chem. Ecol. 44, 534–546. doi: 10.1007/s10886-018-0965-x
Eggleton, P. (2011). “An introduction to termites: Biology, taxonomy and functional morphology,” in Biology of Termites: A Modern Synthesis, eds D. E. Bignell, Y. Roisin, and N. Lo (New York, NY: Springer), 1–26.
Ferreira, D. V., Cristaldo, P. F., Rocha, M. L. C., Santana, D. L., Santos, L., Lima, P. S. S., et al. (2018). Attraction and vibration: effects of previous exposure and type of food resource in the perception of allocolonial odors in termites. Ethology 124, 743–750. doi: 10.1111/eth.12806
Funaro, C. F., Böröczky, K., Vargo, E. L., and Schal, C. (2018). Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes. Proc. Natl. Acad. Sci. U.S.A. 115, 3888–3893. doi: 10.1073/pnas.1721419115
Funaro, C. F., Schal, C., and Vargo, E. L. (2019). Queen and king recognition in the subterranean termite, Reticulitermes flavipes: Evidence for royal recognition pheromones. PLoS One 14:e0209810. doi: 10.1371/journal.pone.0209810
Geiselhardt, S., Schmitt, T., and Peschke, K. (2009). Chemical composition and pheromonal function of the defensive secretions in the subtribe Stizopina (Coleptera, Tenebrionidae, Opatrini). Chemoecology 19, 1–6. doi: 10.1007/s00049-008-0001-7
Gordon, J. M., Šobotník, J., and Chouvenc, T. (2020). Colony-age-dependent variation in cuticular hydrocarbon profiles in subterranean termite colonies. Ecol. Evol. 2, 1–10. doi: 10.1002/ece3.6669
Green, B., Bardunias, P., Turner, J. S., Nagpal, R., Werfel, J., and Green, B. (2017). Excavation and aggregation as organizing factors in de novo construction by mound-building termites. Proc. R. Soc. B Biol. Sci. 284:20162730. doi: 10.1098/rspb.2016.2730
Hanus, R., Luxová, A., Šobotník, J., Kalinová, B., Jiroš, P., Køeèek, J., et al. (2009). Sexual communication in the termite Prorhinotermes simplex (Isoptera, Rhinotermitidae) mediated by a pheromone from female tergal glands. Insectes Soc. 56, 111–118. doi: 10.1007/s00040-009-0005-5
Hanus, R., Šobotník, J., Krasulová, J., Jiroš, P., Žáèek, P., Kalinová, B., et al. (2012). Nonadecadienone, a new termite trail-following pheromone identified in Glossotermes oculatus (Serritermitidae). Chem. Senses 37, 55–63. doi: 10.1093/chemse/bjr065
Hanus, R., Vrkoslav, V. V., Hrdý, I., Cvacka, J., Sobotník, J., Hrdy, I., et al. (2010). Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc. R. Soc. B Biological Sci. 277, 995–1002. doi: 10.1098/rspb.2009.1857
Harrison, M. C., Jongepier, E., Robertson, H. M., Arning, N., Bitard-feildel, T., Chao, H., et al. (2018). Hemimetabolous genomes reveal molecular basis of termite eusociality. Nat. Ecol. Evol. 2, 557–566. doi: 10.1038/s41559-017-0459-1
Havlíèková, J., Dolejšová, K., Tichý, M., Vrkoslav, V., Kalinová, B., Kyjaková, P., et al. (2019). (3R,6E)-nerolidol, a fertility-related volatile secreted by the queens of higher termites (Termitidae: Syntermitinae). Zeitschr. Naturforsch. C 74, 251–264. doi: 10.1515/znc-2018-0197
Hefetz, A. (2019). The critical role of primer pheromones in maintaining insect sociality. Zeitschr. Naturforsch. C 74, 221–231. doi: 10.1515/znc-2018-0224
Himuro, C., Yokoi, T., and Matsuura, K. (2011). Queen-specific volatile in a higher termite Nasutitermes takasagoensis (Isoptera: Termitidae). J. Insect Physiol. 57, 962–965. doi: 10.1016/j.jinsphys.2011.04.012
Hoffmann, K., Gowin, J., Hartfelder, K., and Korb, J. (2014). The scent of royalty: A P450 gene signals reproductive status in a social insect. Mol. Biol. Evol. 31, 2689–2696. doi: 10.1093/molbev/msu214
Hojo, M., Matsumoto, T., and Miura, T. (2007). Cloning and expression of a geranylgeranyl diphosphate synthase gene: insights into the synthesis of termite. Insect Mol. Biol. 16, 121–131. doi: 10.1111/j.1365-2583.2007.00709.x
Hojo, M., Toga, K., Watanabe, D., Yamamoto, T., and Maekawa, K. (2011). High-level expression of the geranylgeranyl diphosphate synthase gene in the frontal gland of soldiers in Reticulitermes speratus (Isoptera: Rhinotermitidae). Arch. Insect Biochem. Physiol. 77, 17–31. doi: 10.1002/arch.20415
Howard, R. W., and Blomquist, G. J. (1982). Chemical ecology and biochemistry of insect hydrocarbons. Annu. Rev. Entomol. 27, 149–172. doi: 10.1146/annurev.en.27.010182.001053
Howard, R. W., and Blomquist, G. J. (2005). Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. doi: 10.1146/annurev.ento.50.071803.130359
Howard, R. W., McDaniel, C. A., and Blomquist, G. J. (1980). Chemical mimicry as an integrating mechanism: Cuticular hydrocarbons of a termitophile and its host. Science 210, 431–433. doi: 10.1126/science.210.4468.431
Howard, R. W., Mcdaniel, C. A., and Blomquist, G. J. (1982). Chemical mimicry as an integrating mechanism for three termitophiles associated with Reticulitermes virginicus (Banks). Psyche (Stuttg) 89, 157–167. doi: 10.1155/1982/91358
Ishikawa, Y., and Miura, T. (2012). Hidden aggression in termite workers: Plastic defensive behaviour dependent upon social context. Anim. Behav. 83, 737–745. doi: 10.1016/j.anbehav.2011.12.022
Kennedy, J. S. (1978). The concepts of olfactory ‘arrestment” and “attraction.”’. Physiol. Entomol. 3, 91–98. doi: 10.1111/j.1365-3032.1978.tb00138.x
Korb, J. (2018). Chemical fertility signaling in termites: Idiosyncrasies and commonalities in comparison with ants. J. Chem. Ecol. 44, 818–826. doi: 10.1007/s10886-018-0952-2
Kotoklo, E. A., Sillam-Dussès, D., Kétoh, G., Sémon, E., Robert, A., Bordereau, C., et al. (2010). Identification of the trail-following pheromone of the pest termite Amitermes evuncifer (Isoptera: Termitidae). Sociobiology 55, 579–588.
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013a). Treatise on the Isoptera of the World: 2. Basal Families. Bull. Am. Museum Nat. Hist. 2013, 200–623. doi: 10.1206/377.2
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013b). Treatise on the Isoptera of the World: 3. Neoisoptera Excluding Termitidae. Bull. Am. Museum Nat. Hist. 2013, 623–973. doi: 10.1206/377.3
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013c). Treatise on the Isoptera of the World: 4. Termitidae (Part One). Bull. Am. Museum Nat. Hist. 2013, 973–1495. doi: 10.1206/377.4
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013d). Treatise on the Isoptera of the world: 5. Termitidae (Part Two). Bull. Am. Museum Nat. Hist. 2013, 1495–1989. doi: 10.1206/377.5
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013e). Treatise on the isoptera of the world: 6. TERMITIDAE (Part Three), incertae sedis, taxa excluded from isoptera. Bull. Am. Museum Nat. Hist. 2013, 1989–2433. doi: 10.1206/377.6
Lacey, M. J., Sémon, E., Krasulová, J., Sillam-Dussès, D., Robert, A., Cornette, R., et al. (2011). Chemical communication in termites: syn-4,6-dimethylundecan-1-ol as trail-following pheromone, syn-4,6-dimethylundecanal and (5E)-2,6,10-trimethylundeca-5,9-dienal as the respective male and female sex pheromones in Hodotermopsis sjoestedti Isoptera. Arc. J. Insect Physiol. 57, 1585–1591. doi: 10.1016/j.jinsphys.2011.07.018
Laduguie, N., Robert, A., Bonnard, O., Vieau, F., Le Quere, J.-L., Semon, E., et al. (1994). Isolation and identification of (3Z,6Z,8E)-3,6,8-dodecatrien-1-ol in Reticulitermes santonensis Feytaud (Isoptera, Rhinotermitidae): roles in worker trail-following and in alate sex-attraction behavior. J. Insect Physiol. 40, 781–787. doi: 10.1016/0022-1910(94)90007-8
Le Conte, Y., Hefetz, A., Conte, Y., and Le Hefetz, A. (2008). Primer pheromones in social hymenoptera. Annu. Rev. Entomol. 53, 523–542. doi: 10.1146/annurev.ento.52.110405.091434
Lefeuve, P., and Bordereau, C. (1984). Soldier formation regulated by a primer pheromone from the soldier frontal gland in a higher termite, Nasutitermes lujae. Proc. Natl. Acad. Sci. U.S.A. 81, 7665–7668. doi: 10.1073/pnas.81.23.7665
Leonhardt, S. D., Menzel, F., Nehring, V., and Schmitt, T. (2016). Ecology and evolution of communication in social insects. Cell 164, 1277–1287. doi: 10.1016/j.cell.2016.01.035
Leuthold, R. H. (1975). “Orientation mediated by pheromones in social insects,” in Pheromone and Defensive Secretions in Social Insects, eds C. Noirot, P. E. Howse, and G. Masne (Dijon: Imprimerie Université de Dijon), 197–211.
Leuthold, R. H., and Bruinsma, O. (1977). Pairing behavior in Hodotermes mossambicus (Isoptera). Psyche (Stuttg) 84, 109–112. doi: 10.1155/1977/64060
Liebig, J. J., Eliyahu, D., and Brent, C. S. (2009). Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav. Ecol. Sociobiol. 63, 1799–1807. doi: 10.1007/s00265-009-0807-5
Lo, N., and Eggleton, P. (2011). “Termite phylogenetics and co-cladogenesis with symbionts,” in Biology of Termites: A Modern Synthesis, eds D. E. Bignell, Y. Roisin, and N. Lo (New York, NY: Springer), 27–50.
Lubes, G., and Cabrera, A. (2018). Identification and evaluation of (3Z,6Z,8E)-dodeca-3,6,8-trien-1-ol asthe trail following pheromone on Microcerotermes exiguus (Isoptera:Termitidae). Rev. Biol. Trop. 66, 303–311. doi: 10.15517/rbt.v66i1.27111
Machara, A., Køivánek, J., Dolejšová, K., Havlíèková, J., Bednárová, L., Hanus, R., et al. (2018). Identification and enantiodivergent synthesis of (5Z,9S)-tetradec-5-en-9-olide, a queen-specific volatile of the termite Silvestritermes minutus. J. Nat. Prod. 81, 2266–2274. doi: 10.1021/acs.jnatprod.8b00632
Matsumura, F., Coppel, H. C., and Tai, A. (1968). Isolation and identification of termite trail-following pheromone. Nature 219, 963–964. doi: 10.1038/219963a0
Matsuura, K. (2006). Termite-egg mimicry by a sclerotium-forming fungus. Proc. R. Soc. B 273, 1203–1209. doi: 10.1098/rspb.2005.3434
Matsuura, K. (2012). Multifunctional queen pheromone and maintenance of reproductive harmony in termite colonies. J. Chem. Ecol. 38, 746–754. doi: 10.1007/s10886-012-0137-3
Matsuura, K., Himuro, C., Yokoi, T., Yamamoto, Y., Vargo, E. L., and Keller, L. (2010). Identification of a pheromone regulating caste differentiation in termites. Proc. Natl. Acad. Sci. U.S.A. 107, 12963–12968. doi: 10.1073/pnas.1004675107
Matsuura, K., and Matsunaga, T. (2015). Antifungal activity of a termite queen pheromone against egg-mimicking termite ball fungi. Ecol. Res. 30, 93–100. doi: 10.1007/s11284-014-1213-7
Matsuura, K., Tamura, T., Kobayashi, N., Yashiro, T., and Tatsumi, S. (2007). The antibacterial protein lysozyme identified as the termite egg recognition pheromone. PLoS One 2:e813. doi: 10.1371/journal.pone.0000813
Matsuura, K., Tanaka, C., and Nishida, T. (2000). Symbiosis of a termite and a sclerotium-forming fungus: Sclerotia mimic termite eggs. Ecol. Res. 15, 405–414. doi: 10.1046/j.1440-1703.2000.00361.x
Matsuura, K., Yashiro, T., Shimizu, K., Tatsumi, S., and Tamura, T. (2009). Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme β-glucosidase. Curr. Biol. 19, 30–36. doi: 10.1016/j.cub.2008.11.030
McDowell, P. G., and Oloo, G. W. (1984). Isolation, identification, and biological activity of trail-following pheromone of termite Trinervitermes bettonianus (Sjöstedt) (Termitidae:Nasutitermitinae). J. Chem. Ecol. 10, 835–851. doi: 10.1007/BF00987967
Menzel, F., Blaimer, B. B., and Schmitt, T. (2017). How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B 284:20161727. doi: 10.1098/rspb.2016.1727
Mitaka, Y., Kobayashi, K., and Matsuura, K. (2017a). Caste-, sex-, and age-dependent expression of immune-related genes in a Japanese subterranean termite, Reticulitermes speratus. PLoS One 12:e0175417. doi: 10.1371/journal.pone.0175417
Mitaka, Y., Kobayashi, K., Mikheyev, A., Tin, M. M. Y., Watanabe, Y., and Matsuura, K. (2016). Caste-specific and sex-specific expression of chemoreceptor genes in a termite. PLoS One 11:e0146125. doi: 10.1371/journal.pone.0146125
Mitaka, Y., and Matsuura, K. (2020). Age-dependent increase in soldier pheromone of the termite Reticulitermes speratus. J. Chem. Ecol. 46, 483–489. doi: 10.1007/s10886-020-01182-6
Mitaka, Y., Matsuyama, S., Mizumoto, N., and Matsuura, K. (2020). Chemical identification of an aggregation pheromone in the termite Reticulitermes speratus. Sci. Rep. 10:7424. doi: 10.1038/s41598-020-64388-4
Mitaka, Y., Mori, N., and Matsuura, K. (2017b). Multi-functional roles of a soldier-specific volatile as a worker arrestant, primer pheromone and an antimicrobial agent in a termite. Proc. R. Soc. B 284:20171134. doi: 10.1098/rspb.2017.1134
Mitaka, Y., Mori, N., and Matsuura, K. (2019). A termite fungistatic compound, mellein, inhibits entomopathogenic fungi but not egg-mimicking termite ball fungi. Appl. Entomol. Zool. 54, 39–46. doi: 10.1007/s13355-018-0589-1
Mizumoto, N., Kobayashi, K., and Matsuura, K. (2015). Emergence of intercolonial variation in termite shelter tube patterns and prediction of its underlying mechanism. R. Soc. Open Sci. 2:150360. doi: 10.1098/rsos.150360
Morgan, E. D. (2009). Trail pheromones of ants. Physiol. Entomol. 34, 1–17. doi: 10.1111/j.1365-3032.2008.00658.x
Nojima, S., Coby, S., Francis, X. W., Richard, G. S., and Wendell, L. R. (2005). Identification of the sex pheromone of the German cockroach, Blattella germanica. Science 307, 1104–1106. doi: 10.1126/science.1107163
Peppuy, A., Robert, A., and Bordereau, C. (2004). Species-specific sex pheromones secreted from new sexual glands in two sympatric fungus-growing termites from northern Vietnam, Macrotermes annandalei and M. barneyi. Insectes Soc. 51, 91–98. doi: 10.1007/s00040-003-0718-9
Peppuy, A., Robert, A., Semon, E., Ginies, C., Lettere, M., Bonnard, O., et al. (2001). (Z)-dodec-3-en-1-ol, a novel termite trail pheromone identified after solid phase microextraction from Macrotermes annandalei. J. Insect Physiol. 47, 445–453. doi: 10.1016/s0022-1910(00)00135-9
Poulsen, M., Hu, H., Li, C., Chen, Z., Xu, L., Otani, S., et al. (2014). Complementary symbiont contributions to plant decomposition in a fungus-farming termite. Proc. Natl. Acad. Sci. U.S.A. 111, 14500–14505. doi: 10.1073/pnas.1319718111
Prestwich, G. D., Jones, R. W., and Collins, M. S. (1981). Terpene biosynthesis by nasute termite soldiers (Isoptera: Nasutitermitinae). Insect Biochem. 11, 331–336. doi: 10.1016/0020-1790(81)90011-1
Raina, A. K., Bland, J. M., Dickens, J. C., Park, Y. I., and Hollister, B. (2003). Premating behavior of dealates of the Formosan subterranean termite and evidence for the presence of a contact sex pheromone. J. Insect Behav. 16, 233–245. doi: 10.1023/A:1023967818906
Raina, A. K., Bland, J. M., and Osbrink, W. (2005). Hydroquinone is not a phagostimulant for the Formosan subterranean termite. J. Chem. Ecol. 31, 509–517. doi: 10.1007/s10886-005-2026-5
Reinhard, J., and Clement, J. L. (2002). Alarm reaction of European Reticulitermes termites to soldier head capsule volatiles (Isoptera, Rhinotermitidae). J. Insect Behav. 15, 95–107. doi: 10.1023/a:1014436313710
Reinhard, J., Lacey, M. J., Ibarra, F., Schroeder, F. C., Kaib, M., and Lenz, M. (2002). Hydroquinone: A general phagostimulating pheromone in termites. J. Chem. Ecol. 28, 1–14. doi: 10.1023/a:1013554100310
Reinhard, J., Quintana, A., Sreng, L., and Clément, J.-L. (2003). Chemical signals inducing attraction and alarm in European Reticulitermes termites (Isoptera, Rhinotermitidae). Sociobiology 42, 675–692.
Robert, A., Peppuy, A., Sémon, E., Boyer, F. D., Lacey, M. J., and Bordereau, C. (2004). A new C12 alcohol identified as a sex pheromone and a trail-following pheromone in termites: The diene (Z,Z)-dodeca-3,6-dien-1-ol. Naturwissenschaften 91, 34–39. doi: 10.1007/s00114-003-0481-9
Roisin, Y., Everaerts, C., Pasteels, J. M., and Bonnard, O. (1990). Caste-dependent reactions to soldier defensive secretion and chiral alarm/recruitment pheromone in Nastitermes princeps. J. Chem. Ecol. 16, 2865–2875. doi: 10.1007/bf00979479
Rosa, C. S., Cristaldo, P. F., Florencio, D. F., Marins, A., Lima, E. R., and DeSouza, O. (2018). On the chemical disguise of a physogastric termitophilous rove beetle. Sociobiology 65, 38–47. doi: 10.13102/sociobiology.v65i1.1942
Rosengaus, R. B., Lefebvre, M. L., and Traniello, J. F. A. (2000). Inhibition of fungal spore germination by Nasutitermes: Evidence for a possible antiseptic role of soldier defensive secretions. J. Chem. Ecol. 26, 21–39. doi: 10.1023/A:1005481209579
Rosengaus, R. B., Traniello, J. F. A., and Bulmer, M. S. (2011). “Ecology, behavior and evolution of disease resistance in termites,” in Biology of Termites: A Modern Synthesis, eds D. E. Bignell, Y. Roisin, and N. Lo (New York, NY: Springer), 165–192.
Rosengaus, R. B., Traniello, J. F. A., Lefebvre, M. L., and Maxmen, A. B. (2004). Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Soc. 51, 259–264. doi: 10.1007/s00040-004-0749-x
Ruther, J., Podsiadlowski, L., and Hilker, M. (2001). Quinones in cockchafers: additional function of a sex attractant as an antimicrobial agent. Chemoecology 229, 225–229. doi: 10.1007/PL00001855
Saran, R. K., Millar, J. G., and Rust, M. K. (2007). Role of (3Z,6Z,8E)-dodecatrien-1-ol in trail following, feeding, and mating behavior of Reticulitermes hesperus. J. Chem. Ecol. 33, 369–389. doi: 10.1007/s10886-006-9229-2
Sillam-dussès, D., Hanus, R., El-latif, A. O. A., Jiro, P., Sillam-Dusses, D., Hanus, R., et al. (2011). Sex pheromone and trail pheromone of the sand termite Psammotermes hybostoma. J. Chem. Ecol. 37, 179–188. doi: 10.1007/s10886-011-9910-y
Sillam-Dussès, D., Hradecký, J., Stiblik, P., da Cunha, H. F., Carrijo, T. F., Lacey, M. J., et al. (2020a). The trail-following pheromone of the termite Serritermes serrifer. Chemoecology 25:2020. doi: 10.1007/s00049-020-00324-2
Sillam-Dussès, D., Robert, A., Sémon, E., Lacey, M., and Bordereau, C. (2006). “Trail-following pheromones and phylogeny in termites,” in Proceedings of the IUSSI Congress, Washington, DC, 1712.
Sillam-Dussès, D., Sémon, E., Lacey, M. J., Robert, A., Lenz, M., and Bordereau, C. (2007). Trail-following pheromones in basal termites, with special reference to Mastotermes darwiniensis. J. Chem. Ecol. 33, 1960–1977. doi: 10.1007/s10886-007-9363-5
Sillam-Dussès, D., Sémon, E., Moreau, C., Valterová, I., Šobotník, J., Robert, A., et al. (2005). Neocembrene A, a major component of the trail-following pheromone in the genus Prorhinotermes (Insecta, Isoptera, Rhinotermitidae). Chemoecology 15, 1–6. doi: 10.1007/s00049-005-0285-9
Sillam-Dussès, D., Sémon, E., Robert, A., and Bordereau, C. (2009). (Z)-Dodec-3-en-1-ol, a common major component of the trail-following pheromone in the termites Kalotermitidae. Chemoecology 19, 103–108. doi: 10.1007/s00049-009-0017-7
Sillam-Dussès, D., Sémon, E., Robert, A., Cancello, E., Lenz, M., ValterovÁ, I., et al. (2010). Identification of multi-component trail pheromones in the most evolutionarily derived termites, the Nasutitermitinae (Termitidae). Biol. J. Linn. Soc. 99, 20–27. doi: 10.1111/j.1095-8312.2009.01348.x
Sillam-Dussès, D., Šobotník, J., Bourguignon, T., Wen, P., Sémon, E., Robert, A., et al. (2020b). Trail-following pheromones in the termite subfamily Syntermitinae (Blattodea, Termitoidae, Termitidae). J. Chem. Ecol. 46, 475–482. doi: 10.1007/s10886-020-01180-8
Šobotník, J., Hanus, R., Kalinová, B., Piskorski, R., Cvaèka, J., Bourguignon, T., et al. (2008). (E,E)-α-Farnesene, an alarm pheromone of the termite Prorhinotermes canalifrons. J. Chem. Ecol. 34, 478–486. doi: 10.1007/s10886-008-9450-2
Šobotník, J., Jirošová, A., Hanus, R., Jiros, A., Hanus, R., and Jan, S. (2010). Chemical warfare in termites. J. Insect Physiol. 56, 1012–1021. doi: 10.1016/j.jinsphys.2010.02.012
Stow, A., and Beattie, A. (2008). Chemical and genetic defenses against disease in insect societies. Brain. Behav. Immun. 22, 1009–1013. doi: 10.1016/j.bbi.2008.03.008
Suehiro, W., and Matsuura, K. (2015). Queen pheromone promotes production of salivary lysozyme by workers in a termite. Insectes Soc. 62, 193–198. doi: 10.1007/s00040-015-0396-4
Tai, A., Matsumura, F., and Coppel, H. C. (1969). Chemical identification of the trail-following pheromone for a Southern subterranean termite. J. Org. Chem. 34, 2180–2182. doi: 10.1021/jo01259a033
Takata, M., Mitaka, Y., Steiger, S., Takata, M., Mitaka, Y., Steiger, S., et al. (2019). A parental volatile pheromone triggers offspring begging in a burying beetle. iScience 19, 1260–1278. doi: 10.1016/j.isci.2019.06.041
Tarver, M. R., Schmelz, E. A., and Scharf, M. E. (2011). Soldier caste influences on candidate primer pheromone levels and juvenile hormone-dependent caste differentiation in workers of the termite Reticulitermes flavipes. J. Insect Physiol. 57, 771–777. doi: 10.1016/j.jinsphys.2011.02.015
Terrapon, N., Li, C., Robertson, H. M., Ji, L., Meng, X., Booth, W., et al. (2014). Molecular traces of alternative social organization in a termite genome. Nat. Commun. 5:3636. doi: 10.1038/ncomms4636
Tokoro, M., Takahashi, M., Tsunoda, K., and Yamaoka, R. (1989). Isolation and primary structure of trail pheromone of the termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Wood Res. 8, 29–38.
Tokoro, M., Yamaoka, R., Hayashiya, K., Takahashi, M., and Nishimoto, K. (1990). Evidence for trail-pheromone precursor in termite Reticulitermes speratus (Kolbe) (Rhinotermitidae, Isoptera). J. Chem. Ecol. 16, 2549–2557. doi: 10.1007/bf01017477
Traniello, J. F. A. (1981). Enemy deterrence in the recruitment strategy of a termite: Soldier-organized foraging in Nasutitermes costalis. Proc. Natl. Acad. Sci. U.S.A. 78, 1976–1979. doi: 10.1073/pnas.78.3.1976
Traniello, J. F. A., and Busher, C. (1985). Chemical regulation of polyethism during foraging in the neotropical termite Nasutitermes costalis. J. Chem. Ecol. 11, 319–332. doi: 10.1007/BF01411418
Valterová, I., Køeèek, J., and Vrkoc, J. (1988). Chemical composition of frontal gland secretion in soldiers of Velocitermes velox (Isoptera, Termitidae) and its biological activity. Acta Ent. Bohemoslov. 85, 241–248.
Valterová, I., Martinet, B., Michez, D., Rasmont, P., and Brasero, N. (2019). Sexual attraction: a review of bumblebee male pheromones. Zeitschr. Naturforsch. C 74, 233–250. doi: 10.1515/znc-2019-0003
Van Oystaeyen, A., Oliveira, R. C., Holman, L., van Zweden, J. S., Romero, C., Oi, C. A., et al. (2014). Conserved class of queen pheromones. Science 343, 287–291. doi: 10.1002/mrc.2761
Vrkoc, J., Krecek, J., and Hrdy, I. (1978). Monoterpenic alarm pheromones in two Nasutitermes species. Acta Entomol. Bohemoslov. 75, 1–8.
Weiss, I., Roessler, T., Hofferberth, J., Brummer, M., Ruther, J., Stoekl, J., et al. (2013). A nonspecific defensive compound evolves into a competition avoidance cue and a female sex pheromone. Nat. Commun. 4:2767. doi: 10.1038/ncomms3767
Wen, P., Ji, B. Z., Liu, S. W., Liu, C., and Sillam-Dussès, D. (2012). Sex-pairing pheromone in the Asian termite pest species Odontotermes formosanus. J. Chem. Ecol. 38, 566–575. doi: 10.1007/s10886-012-0111-0
Wen, P., Ji, B. Z., and Sillam-Dussès, D. (2014). Trail communication regulated by two trail pheromone components in the fungus-growing termite Odontotermes formosanus (Shiraki). PLoS One 9:e90906. doi: 10.1371/journal.pone.0090906
Wen, P., Mo, J., Lu, C., Tan, K., Šobotník, J., and Sillam-Dussès, D. (2015). Sex-pairing pheromone of Ancistrotermes dimorphus (Isoptera: Macrotermitinae). J. Insect Physiol. 83, 8–14. doi: 10.1016/j.jinsphys.2015.11.006
Wen, X., Wen, P., Dahlsjo, C. A. L., Sillam-dusse, D., and Šobotník, J. (2017). Breaking the cipher: ant eavesdropping on the variational trail pheromone of its termite prey. Proc. R. Soc. B 284:20170121. doi: 10.1098/rspb.2017.0121
Wilson, E. O. (1965). Chemical communication in the social insects. Science 149, 1064–1071. doi: 10.1126/science.149.3688.1064
Wobst, B., Farine, J. P., Ginies, C., Sémon, E., Robert, A., Bonnard, O., et al. (1999). (Z,Z,E)-3,6,8-dodecatrien-1-ol, a major component of trail-following pheromone in two sympatric termite species Reticulitermes lucifugus grassei and R. santonensis. J. Chem. Ecol. 25, 1305–1318. doi: 10.1023/A:1020922708599
Wyatt, T. D. (2014). Pheromones and Animal Behavior: Chemical Signals and Signatures, 2 Edn. Cambridge: Cambridge University Press.
Yamamoto, Y., Kobayashi, T., and Matsuura, K. (2012). The lack of chiral specificity in a termite queen pheromone. Physiol. Entomol. 37, 192–195. doi: 10.1111/j.1365-3032.2011.00806.x
Yamamoto, Y., and Matsuura, K. (2011). Queen pheromone regulates egg production in a termite. Biol. Lett. 7, 727–729. doi: 10.1098/rsbl.2011.0353
Yamaoka, R., Tokoro, M., and Hayashiya, K. (1987). Determination of geometric configuration in minute amounts of highly unsaturated termite trail pheromone by capillary gas chromatography in combination with mass spectrometry and fourier-transform infrared spectroscopy. J. Chromatogr. 399, 259–267. doi: 10.1016/S0021-9673(00)96128-4
Yanagihara, S., Suehiro, W., Mitaka, Y., and Matsuura, K. (2018). Age-based soldier polyethism: Old termite soldiers take more risks than young soldiers. Biol. Lett. 14:20180025. doi: 10.1098/rsbl.2018.0025
Yashiro, T., and Matsuura, K. (2007). Distribution and phylogenetic analysis of termite egg-mimicking fungi “termite balls” in Reticulitermes termites. Ann. Entomol. Soc. Am. 100, 532–538. doi: 10.1603/0013-8746(2007)100[532:dapaot]2.0.co;2
Ye, C., Li, J., Ran, Y., Rasheed, H., Xing, L., and Su, X. (2019). The nest fungus of the lower termite Reticulitermes labralis. Sci. Rep. 7, 1–7. doi: 10.1038/s41598-019-40229-x
Keywords: termite, pheromone, parsimony, antimicrobial, cuticular hydrocarbon, social context
Citation: Mitaka Y and Akino T (2021) A Review of Termite Pheromones: Multifaceted, Context-Dependent, and Rational Chemical Communications. Front. Ecol. Evol. 8:595614. doi: 10.3389/fevo.2020.595614
Received: 17 August 2020; Accepted: 14 December 2020;
Published: 11 January 2021.
Edited by:
Solange Del Carmen Issa Ponce, Simón Bolívar University, VenezuelaReviewed by:
Paulo Cristaldo, Universidade Federal Rural de Pernambuco, BrazilHélida Ferreira Cunha, State University of Goiás, Brazil
Copyright © 2021 Mitaka and Akino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuki Mitaka, eW1pdGFrYTAyQGdtYWlsLmNvbQ==
 Yuki Mitaka*
Yuki Mitaka* Toshiharu Akino
Toshiharu Akino