- Department of Geoscience, Aarhus University, Aarhus, Denmark
The use of strontium isotopes in pre-historic mobility studies requires accurate isoscapes for evaluating whether pre-historic individuals are local to the areas in which they were buried or not. Isoscapes are often based on modern-day samples, commonly surface waters. There is, however, growing evidence that modern-day farming has a significant impact on the strontium isotopic composition of surface waters and farmed soils, mainly due to the use of agricultural lime for soil improvement. In this paper, we investigate the fate of strontium from agricultural lime in an experimentally-manipulated field in central Jutland, Denmark. Agricultural limestone was added to this field at very high rates in 2012 and 2013 to investigate CO2 storage in soils. Strontium was first measured from the site in 2014. In 2019 we reevaluated strontium concentrations and found that 80–100% of the strontium from the agricultural lime had leached out of the organic-rich topsoil, and likely seeped into the underlying groundwater and nearby surface waters. In both the sandy soils of the liming test site and farmed soils and heathland in the adjacent area, Sr exhibits a degree of mobility similar to that of calcium, which is in agreement with data for other soil types and what is predicted by the size of its hydrated ions. Strontium isotopic compositions of unfarmed heathland samples show much higher 87Sr/86Sr ratios, and so are not influenced by carbonates, suggesting that the limestone 87Sr/86Sr signature seen in the farmland and in streams and rivers in contact with this comes from agricultural lime, and not from natural carbonate relicts occasionally found in the area. This suggests that the 87Sr/86Sr signatures of the area were higher in pre-historic times, and that an isoscape map based on samples from modern-day farmland is inappropriate for application to provenance and mobility studies of pre-historic people. Thus, it is critical that the possible impact of farming is evaluated when conducting provenance and mobility studies, especially in areas with Sr-poor soils and where agricultural lime is used for soil improvement. Overlooking this can result in significant overestimation of the degree of pre-historic mobility.
Introduction
For the past 35 years, strontium isotopes have been used to elucidate the histories of our pre-historic ancestors, from analyses of their remains, food, tools and other artifacts (e.g., Ericson, 1985; Müller et al., 2003; Haak et al., 2008; Knipper et al., 2017; Madgwick et al., 2019). The strontium (Sr) method is based on the observation that 87Sr/86Sr ratios in soils vary geographically, reflecting the compositions of the soil and the underlying geology (e.g., Faure et al., 1967; Capo et al., 1998; Blum et al., 2000; Montgomery et al., 2007). Strontium is released from the substrate to the groundwater and surface waters, and becomes bioavailable, so that it is taken up in plants and animals, with no change to the average 87Sr/86Sr ratio (Blum et al., 2000; Bentley, 2006; Montgomery, 2010), making Sr a powerful tracer of the origin and migration history of people and animals during pre-historic times. In order to interpret measured 87Sr/86Sr ratios of archeological artifacts from such pre-historic individuals, these must be compared to a reference map showing the 87Sr/86Sr ratios of the bioavailable Sr in the area in which the artifacts were found. Thus, the success of the Sr method hinges on the accuracy of the reference map or isoscape. In the absence of contemporaneous samples of known geographical origin, construction of a given isoscape is based on data measured on present-day samples of surface waters, plants, animal bones, or soils (e.g., Grimstead et al., 2017; Bataille et al., 2018, 2020). A key assumption of the Sr method is that the 87Sr/86Sr signatures measured in the environment today is the same as those that would have been imparted to people during pre-historic times.
Yet there is growing evidence that modern-day farming can impact the 87Sr/86Sr ratios of the surface environment to such an extent that 87Sr/86Sr signatures measured today may be radically different than those that existed in preindustrial times, especially due to the use of agricultural lime in farming (Böhlke and Horan, 2000; Oh and Raymond, 2006; Aquilina et al., 2012; Maurer et al., 2012; Thomsen and Andreasen, 2019). This can lead to erroneous conclusions regarding the origin and mobility of pre-historic individuals, as the application of agricultural lime to low-calcareous soils can significantly lower the 87Sr/86Sr ratio of an entire watershed (Thomsen and Andreasen, 2019). In the cases of iconic Bronze Age females, The Egtved Girl and The Skrydstrup Woman, buried in central Jutland, Denmark, this effect was shown to lower the 87Sr/86Sr signatures of the local, modern watersheds from around 0.713 to 0.709, making it appear that these individuals must have come from afar, though there is no chemical or archaeological evidence to suggest that they came from anywhere other than the areas in which they were buried (Thomsen and Andreasen, 2019). Recently, the fate of Sr from agricultural lime was questioned in an article by Frei et al. (2020a), who hypothesize that Sr from agricultural lime is retained indefinitely in organic-rich farmed topsoils, and that the lime-influenced Sr isotopic signatures observed in surface waters—including lakes and rivers, comes predominantly from the dissolution of naturally-occurring carbonates in the deeper, less organic-rich parts of the soil (Frei et al., 2020a).
However, it is challenging to chemically distinguish agricultural lime from naturally-occurring limestone in a soil, as agricultural lime is nothing more than pulverized, naturally-occurring limestone, and thus is very similar to other naturally-occurring limestones. Marine limestones have the 87Sr/86Sr signature of seawater at the time that the lime was deposited (Edmond, 1992), and though the 87Sr/86Sr compositions of the oceans have changed significantly though geologic time, the variations of 87Sr/86Sr signatures in marine carbonates are very small (0.7067–0.7094—Veizer et al., 1999) compared to the variations in siliciclastic sediments and bedrock (0.703–>0.725—e.g., Hoogewerff et al., 2019; Bataille et al., 2020). One approach to studying the release of Sr from agricultural lime into the environment is using test fields where known quantities of agricultural lime have been applied to parts of the field, while other parts of the fields are used as control sites. One such test field is located in Voulund, south of the town of Ikast in central Jutland, Denmark, where the Geological Survey of Denmark and Greenland (GEUS) conducted CO2 storage in soil experiments from 2010 to 2014, by applying massive quantities of agricultural lime to farmland soil, in an attempt to increase the CO2 binding capacity of the soil. This test field was later used by Frei et al. (2020a) as “a representative site in the West Jutland sandy outwash plain” in their paper concluding that Sr from agricultural lime is retained in topsoils indefinitely, and does not contribute to the 87Sr/86Sr ratios of surface waters in contact with these. We reinvestigated this site 5 years later to study the fate of the Sr in the soils.
Methods
Massive quantities of agricultural lime were applied to parts of the test field at Voulund (56, 02, 06 N; 9, 10, 24 E) in 2012 and 2013 by Jessen et al. (2014a), as part of their experiments on CO2 storage in soils. The test field is located on a glacial outwash plain from the Weichsel ice age (Figure 1), and has been farmed for at least 100 years. The site was split into 4 quadrants (A,B,C, and D) (Jessen et al., 2014a,b), and agricultural lime was supplied at a rate of 32 t/ha to quadrants B and C in 2013, and at a rate of 4 and 16 t/ha to B & C, respectively, in 2012, for a total 36 t/ha lime added to B, and 48 t/ha lime to C. Quadrants B and C differed, in that the topsoil in C was later homogenized through tillage, while quadrant B was no-till farmed, during the course of the experiment. Quadrants A and D were used as control sites. For the duration of the experiment, barley (Hordeum vulgare) was grown in quadrants A, B, & C, and maize (Zea mays) in quadrant D. After the experiments ended in 2014, all quadrants were planted with Christmas trees (Picea abies), which are still growing in the field today. These trees have received only a minimal amount of NPK fertilizer by hand, and no agricultural lime, according to the farmer. The test field site was split from a larger field in 2010, and the rest of this field (Figure 1) has remained actively-farmed, with crop rotation every 2 years between barley (Hordeum vulgare) and potatoes (Solanum tuberosum). North of the field lies a forest and heathland (Figure 1), which has not been farmed in historical times.
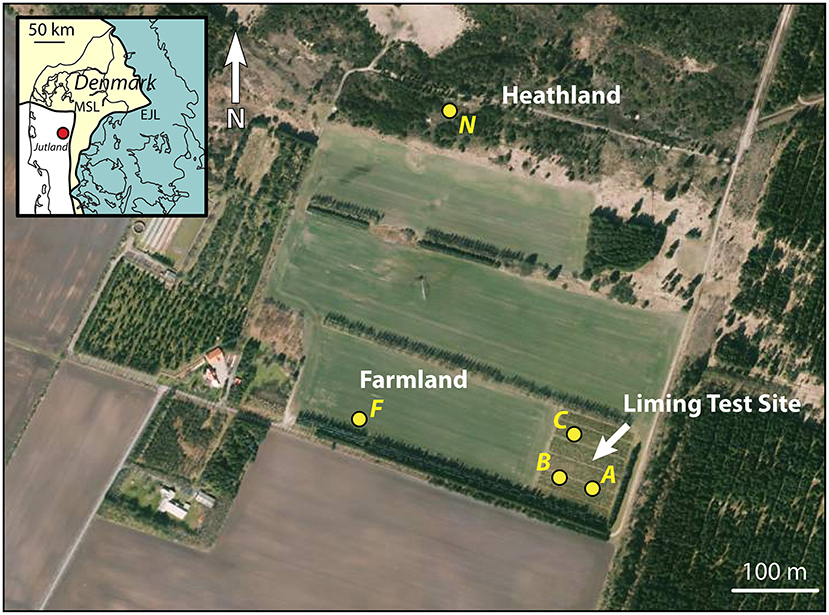
Figure 1. Orthophoto from the spring of 2019 of the liming test site at Voulund with surrounding farm- and heathland. The locations of soil profiles (A, B, & C) within the liming test site, and F & N on farm- and heathland are indicated with yellow dots. Map insert shows the locality relative to the two major ice front locations during the Weichselian Ice Age (Houmark-Nielsen, 2003). Orthophoto from Styrelsen for Dataforsyning og Effektivisering—Denmark.
Quadrants A, B, and C were selected for analysis in this study, along with a site in the currently-farmed field F, due to the similarities in soil use. Additionally, a site on the unfarmed heathland, N was selected for comparison (Figure 1). As the CO2 storage experiments focused on the mobility of major cations, Sr was not monitored continuously, but soil samples taken in March 2014 from quadrant C—the most intensively limed quadrant—was measured for Sr concentrations and 87Sr/86Sr compositions by Frei et al. (2020a), though this site certainly cannot be described as typical farmland, as is implied in Frei et al. (2020a), as the liming rates employed over the duration of the CO2 storage experiments were ca. 48 times higher than those of currently-farmed site, F, which receives around 2 t/ha every 4 years, according to the farmer. The data presented in Frei et al. (2020a) show that Sr concentrations are very low in soils below the organic-rich topsoil. This is an expected finding, as the substratum is a glacial meltwater sand, consisting predominantly of quartz, and thus has a very low cation exchange capacity. The layer of meltwater sand starts at a depth of 40–50 cm and continues to the groundwater table at a depth of around 6 m and beyond. Thus, rapid transfer of cations to the groundwater is expected, from the point that cations enter the meltwater sand in the unsaturated zone. Consequently, the depth of the soil pits in this study were limited to around 70 cm, in order to capture the transition from the organic-rich topsoil to the meltwater sand, as well as the compositions of the topmost layers of meltwater sand, whilst avoiding unnecessary large scale excavation.
A schematic drawing of the soil profiles and a photo of profile A is shown in Figure 2. Profiles A, B, C, and F each have an organic-rich topsoil layer of ca. 40 cm thickness, with a sharp boundary to the quartz-rich meltwater sand below. Profiles A and B contain slivers of meltwater sand within the organic-rich topsoil, which stem from plowing. Profile N is the only profile with developed soil horizons, consisting of a thin very organic-rich O-horizon, underlain by a bleached E-horizon of bluish-gray sand, and a B-horizon with reddish sand, and signs of iron-oxy-hydroxide accumulation, underlain by meltwater-sand at a depth of 34 cm.
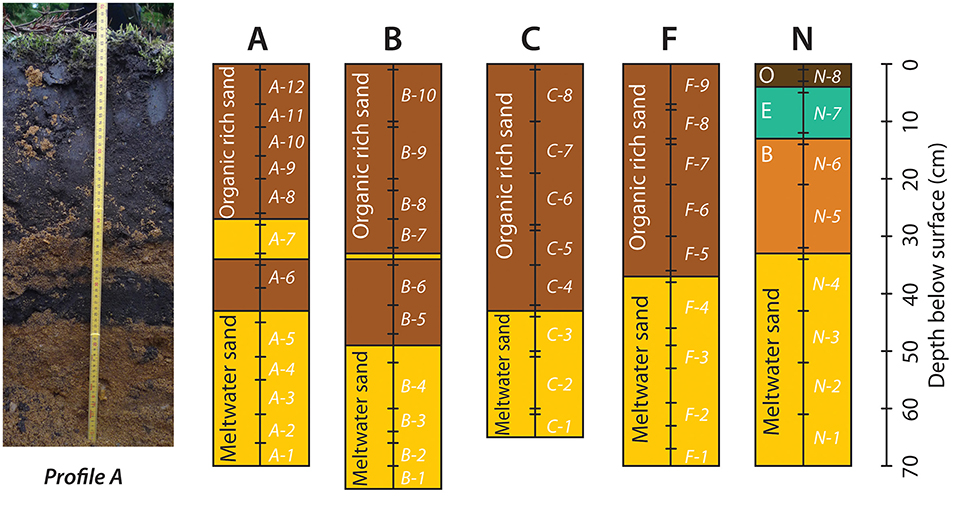
Figure 2. Photograph of soil profile A, and schematic drawings of the 5 soil profiles with lithologies and sampling depth. See Figure 1 for the position of the soil profiles. Photo by Claus Heilmann Clausen.
The soil profiles were sampled every 5–10 cm (Figure 2), and were sampled carefully, to avoid sampling the boundaries between units. A total of 49 samples were collected from the five profiles. These were supplemented by 8 soil samples from four of the locations (7 from non-farmed soils and 1 from farmed soil) in central and eastern Jutland, including the Vallerbæk Tributary of the Karup River studied in Thomsen and Andreasen (2019), such that in all, 57 samples were obtained for analysis. Samples were collected in plastic bags and dried for 2 days at 40°C. In order to examine the budget of bioavailable and easily-leachable major and trace elements from the soils, 3 aliquots of around 5 grams each were weighed out from each sample, after the samples had been dried. To the first of the 3 aliquots, 10 ml of 1.0 M ammonium nitrate (NH4NO3) were added for 2 h to extract the bioavailable fraction of cations (Willmes et al., 2018; Hoogewerff et al., 2019). To the second fraction, 10 ml of 0.2 M acetic acid (CH3COOH) was added for 1 h to extract easily leachable cations and dissolve any limestone present in the soil (Frei et al., 2020a). To the last fraction, 10 ml of MQ-water (18.2 MΩ) was added for 3 weeks to simulate rainwater percolating through the soil. All three fractions were agitated at regular intervals (every 15 min for the samples in ammonium nitrate and acetic acid, and daily for the samples in water) for the duration of the extraction. Following extraction, the leachate was pipetted from each sample, centrifuged, and analyzed for trace element analyses by quadruple ICP-MS. In addition, Sr isotopic analyses were conducted by MC-ICP-MS for soil samples from the unfarmed, heathland (Profile N), and on soil samples taken adjacent to the Vallerbæk tributary of the Karup River, where water samples had been analyzed by Thomsen and Andreasen (2019), in order to compare these with existing soil and water data, and with the data from the Voulund test site. Three soil samples were analyzed from the Vallerbæk tributary, two from either side of the brook, from where it leaves the mixed heath and forest, and enters the farmland, and one (Sample VS1) from the forest, a little removed from the brook—a site selected in order to sample an area shielded by trees from the dust blown from the farmed fields, by the predominantly westerly winds. The two samples from either side of the brook were obtained <5 m apart—one from the farmed field (Sample KF), the other from the shrubland immediately opposite the field (Sample KM).
Trace Element Analysis
The centrifuged soil leachate samples were diluted with 2% HNO3 and analyzed for selected major and trace elements by solution quadrupole ICP-MS on an Agilent 7900, at the Department of Geoscience, Aarhus University. Each set of soil leachates, ammonium nitrate, acetic acid, and water were run independently with matrix-matched multi-element standards and NIST 1643-F for calibration and quality control. Concentrations of Li, Be, Na, Mg, Al, K, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Rb, Sr, Ag, Cd, Cs, Ba, Tl, Pb, and U were determined. The concentrations of Li, Be, V, Cr, Cu, Ga, As, Ag, Cs, and U were generally below detection level for all samples. The concentration of Na, Mg, Al, K, Ca, Mn, Fe, Co, Ni, Zn, Rb, Sr, Cd, Ba, Tl, and Pb are given in Supplementary Table 1 as ppm or ppb (mg/kg or μg/kg) normalized to the dry weight of the soil sample.
Strontium Isotope Analyses
The centrifuged soil leachate samples and two samples of rainwater were dried down and digested overnight in 2 ml of aqua regia, dried down again, and dissolved in nitric acid for Sr separation chemistry. Strontium was separated from the sample matrix using Eichrom (TrisKem International) Sr spec resin and analyzed for isotopic composition using a Nu Plasma II multicollector ICP-MS (inductively coupled plasma mass spectrometer) at the Department of Geoscience, Aarhus University. The four stable isotopes of strontium (84Sr, 86Sr, 87Sr, and 88Sr) were measured simultaneously, as were isotopes of Kr, Rb, Y, and doubly charged REE, to monitor and correct for interferences. Masses 82, 83, 84, 85, 86, 87, 88, and 89 were measured simultaneously, as were half-masses 83.5, 84.5, 85.5, 86.5, and 87.5. Baselines were determined by on-peak zero, and each sample run consisted of 400 s of peak time. Data were fractionation-corrected using the exponential law and normalized to NBS SRM 987 (87Sr/86Sr = 0.71025). Samples of Holocene foraminifera (Baculogypsina sphaerulata), expected to give a modern-day seawater Sr isotope value (87Sr/86Sr = 0.70917—Dia et al., 1992) were processed along with the samples as a secondary standard. Three samples of foraminifera analyzed throughout the analytical campaign gave 87Sr/86Sr = 0.709173 ± 5 (7 ppm, 2σ). The reproducibility of individual measurements of samples is estimated at 25 ppm (2σ), based on repeat measurements of standards and samples. The Sr isotopic compositions of the analyzed samples are given in Table 1. Strontium isotopic compositions from Profile C have been found to be dominated by limestone (Frei et al., 2020a). It was therefore deemed unnecessary to repeat Sr isotopic measurements on the soils from the liming test site. The results of the strontium isotope measurements are presented in Table 1.
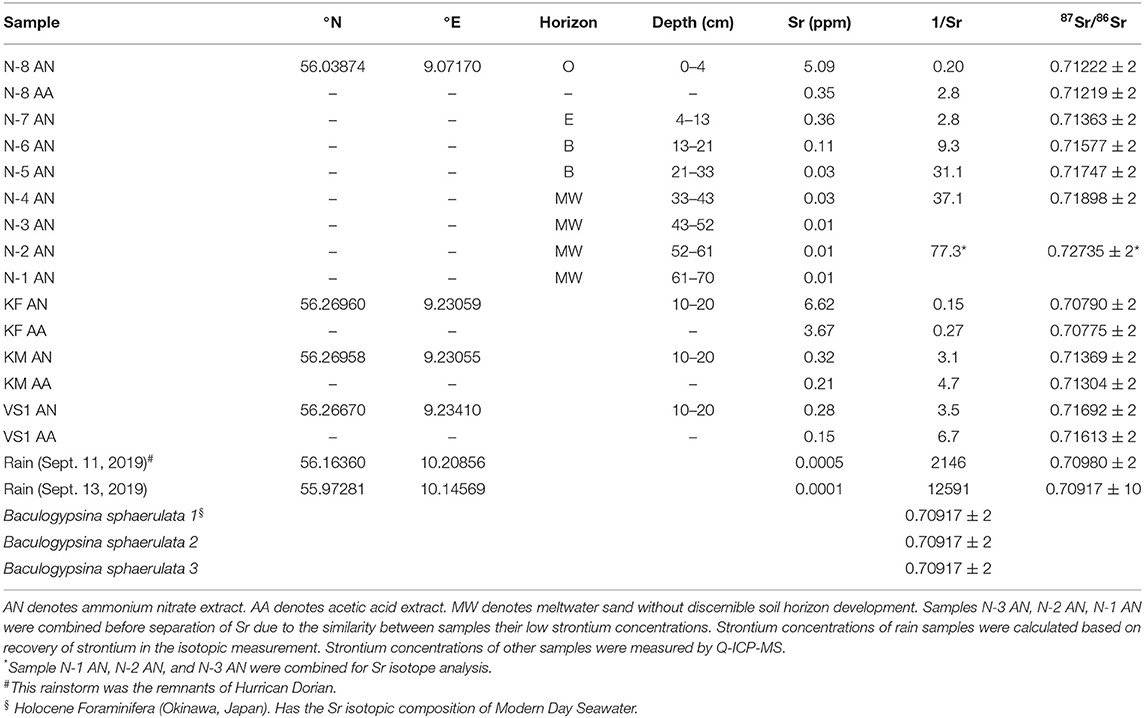
Table 1. Strontium isotopic compositions and concentrations of analyzed soil samples and samples of rainwater and samples of modern day foraminifera analyzed for quality control.
Results
The concentrations of most trace elements are highest in the (bioavailable) ammonium nitrate leach (see Supplementary Table 1), which is in good agreement with the assumption that this is the most aggressive of the leaches applied in this study (Willmes et al., 2018). Notable exceptions are the concentrations of Ca and Sr in the topmost sample from Profile B, which contained visible pieces of agricultural lime that reacted vigorously with the acetic acid. There, the Ca and Sr concentrations in the acetic acid leach are higher than in the ammonium nitrate leach. Concentrations of alkali metals and alkaline earth metals (Groups I and II of the periodic table) are generally higher in the extracts from organic-rich soils than in the extracts from sandy soils, whereas the concentrations of transition elements are similar in the organic-rich soils and the sandy ones. Calcium concentrations range from 0.3 ppm to nearly 2,300 ppm in the ammonium nitrate extracts, from 0.3 ppm to nearly 5,800 ppm in the acetic acid extracts, and 0.1 ppm to 100 ppm in the water extracts. Strontium concentrations range from 7 ppb to 8.5 ppm in the ammonium nitrate extracts, from 4 ppb to 15.5 ppm in the acetic acid extracts, and from 1 ppb to 0.4 ppm in the water extracts. The heathland profile, N exhibits the largest gradients in elemental concentrations, with high concentrations in the O-horizon, intermediate concentrations in the E- and B-horizons, and very low concentrations in the meltwater sand below the B-horizon. This is an unsurprising result, as these are the only soils that have not been mechanically mixed by tilling. The strontium isotopic compositions of the heathland soils show much greater variation than those seen in the test site soils. The 87Sr/86Sr ratios of the ammonium nitrate extracts of the heathland soils, Profile N, increase systematically with depth—from 0.7122 in the O-horizon to 0.7270 in the meltwater sand at a depth of 70 cm (Table 1). This variation is much greater than that found for the liming test site, Profile C, where the 87Sr/86Sr ratios in ammonium nitrate leachates from the meltwater sand range from 0.7081 to 0.7108 (Frei et al., 2020a), though (near total dissolution) aqua regia extracts from Profile C have strontium ratios up to 0.7267 (Frei et al., 2020a), similar to what is found in Profile N samples leached in ammonium nitrate.
Discussion
The Mobility of Strontium
When comparing the degrees of mobility for different elements in soils, it is useful looking at elemental ratios rather than concentrations, as this allows for a direct comparison of the relative mobility of pairs of elements, while avoiding the issue of dilution with inert or mostly inert phases, such as quartz in these sandy soils. In Figure 3, ratios for the mobile alkali metals and alkaline earth metals are plotted for the water and acetic acid leachates against those of the ammonium nitrate leachate for the same sample. The relative strength of the three extracts are: water < acetic acid < ammonium nitrate, such that when compared to the ammonium nitrate leach, the greatest enrichment of a more mobile element (relative to a less mobile one) is expected in the water leach. Such an enrichment is also expected in the acetic acid leach, though to a lesser degree than in the water leach. This effect is exhibited by the period 3 and 4 alkali metals, sodium (Na) and potassium (K) (Figure 3A), where the 1:1 line represents ammonium nitrate. The slope of the water extracts is significantly lower than that of the acetic acid extracts, which is significantly lower than that of the ammonium nitrate ones. Slopes <1:1 indicate that the element in the denominator is more mobile than the element in the numerator. In this case, Na is more mobile than K. The same is the case for the period 3 and 4 alkaline earth metals, magnesium (Mg) and calcium (Ca), (Figure 3B), where the slopes for the water- and acetic acid extracts are also below the 1:1 line, expressing the fact that Mg is more mobile than Ca. For the alkali metals of group 4 and 5, potassium and rubidium (Rb) (Figure 3C), the slopes for the water and acetic acid extracts are above the 1:1 line, and the slope for the water extracts is the steepest. Thus, K is more mobile than Rb. The high degree of scatter is primarily caused by the samples from Profile F—the only currently-farmed soil, and likely reflect the farmer's use of water-soluble NPK fertilizer, which has high K/Rb ratios.
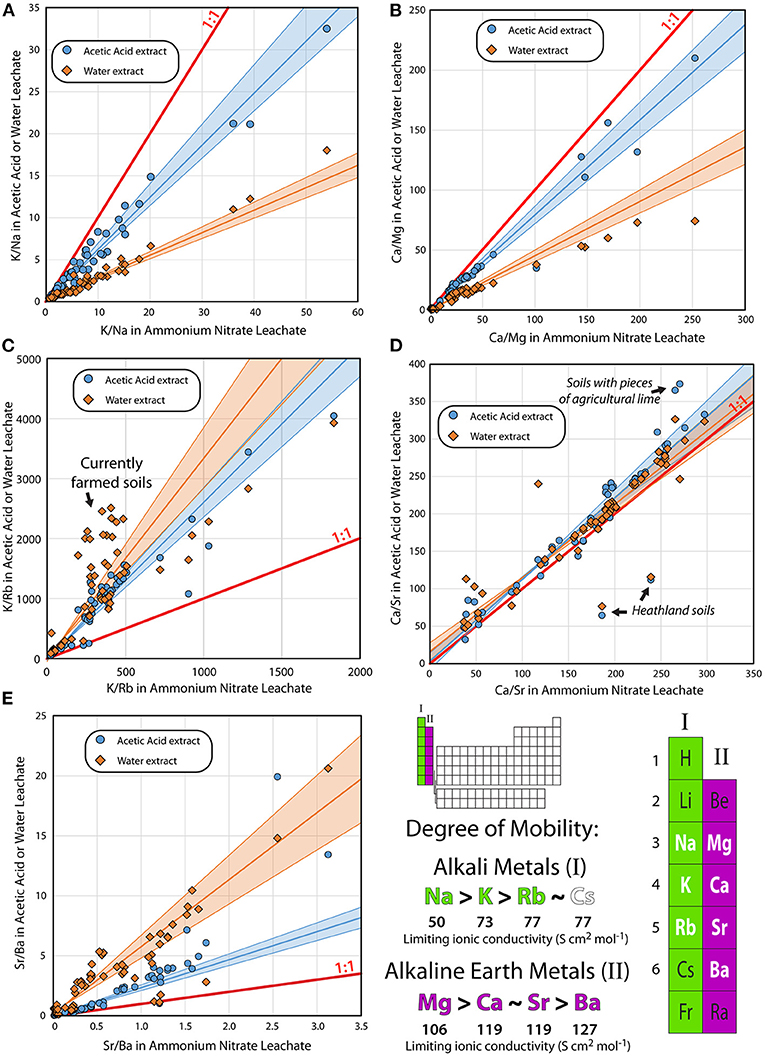
Figure 3. Relative mobility of the major alkali metals and alkaline earth metals (groups I and II in the periodic table). (A–E) Ratios in leachates of water (orange diamonds) and 0.2 M acetic acid (light blue circles) on the y-axis compared with the ratios of 1.0 M ammonium nitrate leachates of the same soil sample. Also shown are 1:1 and best fit regression lines and 95% confidence intervals [calculated using IsoplotR—Vermeesch (2018)] for the data. Slopes shallower than 1:1 indicate that the element in the numerator is more mobile in the weaker water- and acetic acid leachates than in the ammonium nitrate leach. Slopes steeper than 1:1 indicate that the element in the denominator is more mobile, whereas slopes following 1:1—including the slope of Ca/Sr—indicate that the two elements are equally mobile. Limiting ionic conductivities are from Burgess (2011).
The relationship between period 4 and 5 alkaline earth metals, calcium and strontium (Figure 3D) is different, however, in that all three extracts have the same relationship. This implies that the mobility of calcium and strontium is very similar. A few samples fall off the 1:1 line—the N-7 and N-6 samples, which represent the E- and part of the B-horizons from the Profile N heathland soil. These all have lower Ca/Sr in the water- and acetic acid extracts than in the ammonium nitrate extract, implying that Sr is released preferentially to Ca from these soils. Interestingly, this is not the case for the O-horizon, which falls on the 1:1 line. Samples containing pieces of agricultural lime fall above the 1:1 line in Figure 3D, as the acetic acid preferentially dissolves the lime, which is richer in Ca relative to Sr. Overall, the slopes for the water- and acetic acid extracts are within error of 1, showing that Sr is as mobile as Ca in the soils studied here (Figure 3E). For period 5 and 6 alkaline earth metals, strontium and barium (Ba), the slopes of the water- and acetic acid extracts are both above the 1:1 line, and the slope of the water extract is the steepest, showing that Sr is more mobile than Ba.
The retention of cations in the soil is a function of ionic charge, ionic size of the hydrated ions, the cation exchange capacity of the soil, and the redox conditions of the soil (Marcus, 2016), in a complex relationship that is also dependent on the composition of porewater and organic matter in the soils, the compositions of which may change with time. There is a strong correlation between the observed mobility in the sandy soils and the limiting ionic conductivity for the alkali metals and alkaline earth metals, which are not redox sensitive; and this is also an indicator for element mobility (Burgess, 2011). The soils studied here are from 3 different glacial outwash plains and 2 samples from a moraine; and these soils exhibit a wide range of organic content, from nearly nothing to around 5%, which is common for farmland located on glacial outwash plains in Europe and North America (Krüger et al., 2013). Otherwise, these soils can be seen as a common matrix, such that the relative mobilities of elements determined here are valid for such soils of similar types. Strontium is as mobile as calcium, and more mobile than barium in the very soils studied by Frei et al. (2020a) (Figure 3), such that there is certainly no reason to suspect that the Sr from agricultural lime will accumulate in the topsoil over time, as hypothesized by those authors, and in spite of the fact that calcium is widely regarded as a highly mobile element (e.g., Burgess, 2011). This is supported both by other studies that find significant Sr mobility in waters (e.g., Solecki, 2005; Wallace et al., 2012), and by the very similar behavior of Ca and Sr in most soil types (Smičiklas et al., 2015). The adsorbtion of Ca and Sr on DNA also appear to be similar to each other and different from the other alkaline earth metals (Long et al., 2020), which could explain the behavior observed in the organic-rich soils, where Ca and Sr appear to be equally mobile, though more work is needed to see whether this relationship is common for alkaline earth metal complexes with organic material.
The Fate of Strontium From Agricultural Lime
The liming test site in Voulund presents a unique opportunity to estimate the rate at which Sr from agricultural lime is mobilized from a soil. Frei et al. (2020a) speculated that the majority of Sr from agricultural lime is retained in the soil indefinitely, though did not provide any calculations showing how much of the Sr they measured in 2014 was there before the massive additions of agricultural lime in 2012 and 2013. By comparing the amounts and distribution of Sr within the soils of the five profiles, the rates of liming in 2012 and 2013 (Jessen et al., 2014a), and the Sr concentrations in Profile C in 2014 (Frei et al., 2020a) it is possible to construct a mass balance for the Sr in the agricultural lime applied to the liming test site in 2012 and 2013.
In Figure 4, the Sr concentrations in the five soil profiles from Voulund are shown for both the ammonium nitrate- and acetic acid leaches. Apart from the topmost part of Profile B, which contains residual agricultural lime, the Sr concentration is higher for all samples in the ammonium nitrate leach. The Sr concentration in Profile N—the heathland, is highest in the O-horizon, and decreases rapidly and systematically with depth. Perhaps surprisingly, the Sr concentration of the O-horizon in Profile N is the same as the topsoil in the heavily-limed Profile C. Profile A—the control site, and Profile F—the currently-farmed soil, exhibit similar patterns of Sr concentrations, which increase with depth in the organic-rich topsoil and decline sharply in the meltwater sand. This is indicative of Sr leaching from the uppermost topsoil. In Profile C—the limed test site with tilling, the Sr concentration is constant throughout the organic-rich topsoil, but only slightly higher than in Profiles A and F. As in the other profiles, this is followed by a sharp decrease in Sr concentration in the meltwater sand. For Profile B—the untilled, limed test site, the Sr concentration is highest at the top and decreases with depth. This is unsurprising, as there are still pieces of agricultural lime at the surface in Profile B, dissolving and releasing Sr. The differences between Profiles B and C show the effectiveness of tilling in redistributing and breaking down the agricultural lime for the release of Ca (and thus also Sr) for soil improvement. In Profile C, there are no visible pieces of agricultural lime left 6–7 years after the test site was limed with the equivalent of around 96 years' worth of agricultural lime (i.e., 48 t/ha over 2 years compared to the average rate of 2 t/ha every 4 years). The Sr and Ca are homogenously distributed within the organic-rich soil, and the Sr and Ca concentrations in the acetic acid extracts are much lower than those in the ammonium nitrate, suggesting the complete dissolution of the agricultural lime.
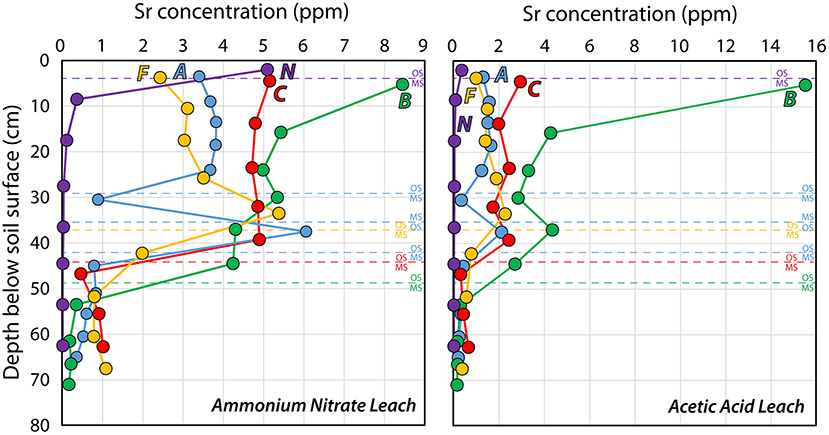
Figure 4. Strontium concentrations in ammonium nitrate- and acetic acid leachates from the five soil profiles; (A, B, C) from the liming test site, the farmland Profile F and the heathland Profile N. See Figure 1 for locations of soil profiles.
So where did the Sr go? Figure 5 shows the concentrations of Sr in ammonium nitrate- and acetic acid leaches of Profiles A, B, and C in 2019 and in Profile C in 2014 (Frei et al., 2020a). The Sr concentration in the ammonium nitrate leach was higher in the topmost layer of soil (0–10 cm) in 2014 than it was in 2019, and then was similar to that in the untilled Profile B sampled in 2019, but higher in the rest of the organic-rich soil (10–40 cm) in 2019 than it was in 2014. This again demonstrates the effectiveness of tilling in redistributing Sr in soils. For the acetic acid extracts, the concentration of Sr was much higher in 2014 than in 2019, showing that agricultural lime was present in the soil in significant quantities. Unfortunately, the resolution in the acetic acid extract data is limited for 2014, but a simple mass balance can be constructed for comparing the soil profiles, using an estimated soil density (r), which for simplicity's sake is kept constant at 1.5 g/cm3 for all depths at all profiles, the soil thickness (h), and the strontium concentration at each sampled interval (i) down to a depth of 65 cm, which is the depth of the shallowest profile (Equation 1).
Table 2 shows the weighted averages of the amounts of Sr extractable by ammonium nitrate and acetic acid for each of the five profiles measured here, to 65 cm depth, and Profile C in 2014 (Frei et al., 2020a). The control profile, A and the currently-farmed Profile F have nearly the same amount of extractable Sr by both ammonium nitrate and acetic acid. The heathland Profile N, has a much smaller amount of extractable Sr, despite having the same Sr concentration in the top of the profile. This is due to the thin O-horizon in the heathland soil, compared to that in the farmland. The heathland also has by far the smallest fraction of Sr extractable by acetic acid, which is unsurprising, as the soil has never been improved by liming or marling. The untilled Profile B has the most extractable Sr, where more is extractable with acetic acid than ammonium nitrate, due to the presence of agricultural lime. Profile C has the same amount of Sr extractable by ammonium nitrate in 2019 as it did in 2014; on the other hand, the amount of extractable Sr by acetic acid was much greater in 2014 than in 2019. The amount of extractable Sr in 2014 is based on the two available measurements from Frei et al. (2020a), and is listed here as a minimum estimate, as any attempt to inter- and/or extrapolate to the rest of the soil profile is likely to result in a severe overestimation. Based on the amount of agricultural lime added to Profile C−48 t/ha (i.e., 4.8 kg/m2–Jessen et al., 2014a) and an estimated Sr concentration in the agricultural lime of 800–1,000 mg/kg (Thomsen and Andreasen, 2019), it is calculated that Profile C received ~3.8–4.8 g/m2 of Sr in 2012 and 2013, as part of the CO2-storage experiment. The difference in Sr extractable by acetic acid between Profile C in 2014, the control site, Profile A, and the currently-farmed Profile F in 2019 is more than 4,400 mg/m2, suggesting that nearly the entire amount of Sr added during the liming experiment was bound up in the lime in March of 2014, when the samples for Frei et al. (2020a) were obtained. This is reasonable, as most of the agricultural lime was added in the fall of 2013, and the soil sampling in March 2014 was likely done before plowing and seeding that year. The difference in the amount of Sr extractable by acetic acid between Profile C in 2014 and 2019 is more than 3,900 mg/m2, suggesting that 80–100% of the Sr from the agricultural lime has been leached out of the soil, during these 5 years, as the amount of bioavailable Sr remained constant during that period. The Sr leached from the soil has very likely exchanged with bioavailable Sr in the soil, but as both have Sr isotope compositions dominated by limestone signatures, the extent of this exchange is difficult to determine. This likely occurs in a dynamic equilibrium, where amount of the input is roughly equal to that of the output, though with exchange in the inventory of Sr in the soil. Once the Sr from the agricultural lime enters the meltwater sand, it quickly ends up in the groundwater, due to the very low ion exchange capacity of the quartz-rich sand. From there, most of the Sr enters the surface waters in contact with the groundwater, including streams and rivers, imparting a lime Sr isotopic signature to these surface waters, as determined by Thomsen and Andreasen (2019).
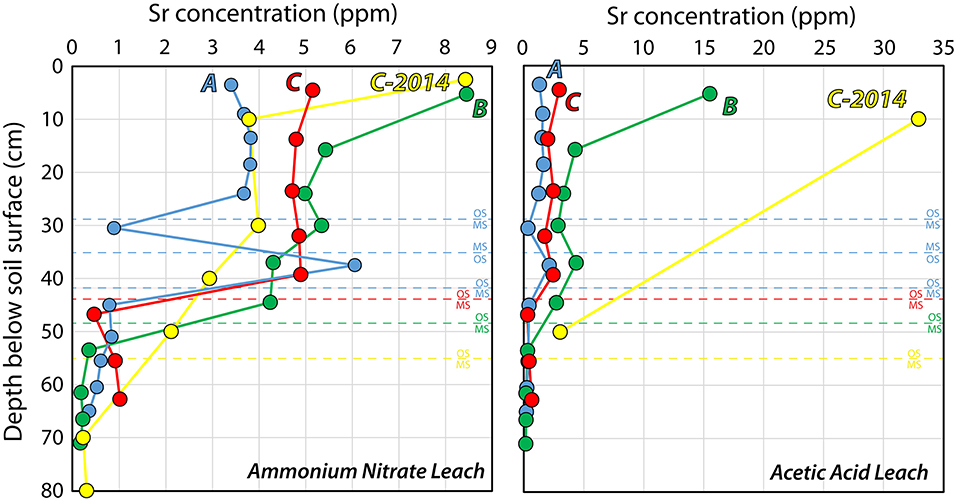
Figure 5. Strontium concentration in the three soil profiles (A, B, C) from the liming test site in ammonium nitrate- and acetic acid leaches. C (2014) shows the concentrations of Sr measured for samples from quadrant C taken in March 2014, about 6 months after the last application of agricultural lime at a rate of 32 t/ha, and 18 months after the first application of agricultural lime at a rate of 16 t/ha, in the course of CO2 storage experiments (Frei et al., 2020a).
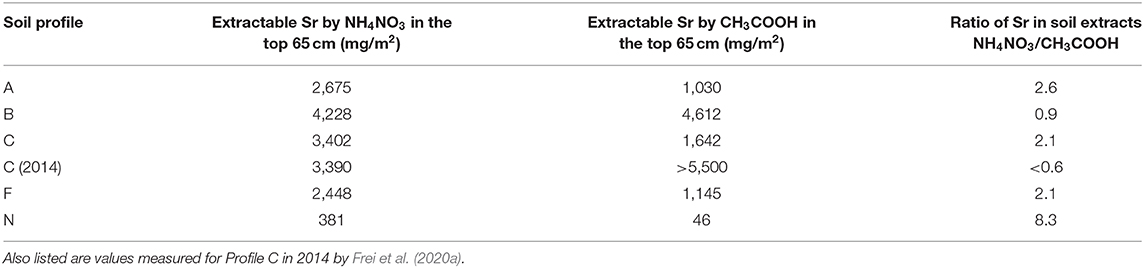
Table 2. The amount of Sr in the top 65 cm of one square meter of each soil profile that is extractable with ammonium nitrate and acetic acid, and the ratio between the two.
From the shallow groundwater, some of the Sr may be transported to deeper groundwater reservoirs (i.e., >60 m depth) and may impact the Sr isotopic composition of deeper groundwaters. Shallow groundwaters at the CO2-storage test site at Voulund have high Sr concentrations and low 87Sr/86Sr ratios (Frei et al., 2020a) dominated by lime, whereas shallow groundwaters in the vicinity of the test site (Thomsen et al., 2021) and nearby areas unaffected by farming (Jørgensen et al., 1999; Thomsen and Andreasen, 2019) have low Sr concentrations and high 87Sr/86Sr ratios, indicating that the Sr isotopic composition of the shallow groundwater at the test site is almost certainly dominated by agricultural lime. Meanwhile, deeper groundwaters have higher Sr concentrations and lower 87Sr/86Sr ratios likely dominated by natural limestone (Jørgensen et al., 1999), as do drinking water wells located 10-15 km from the test site (Frei et al., 2020b). However, as the drinking water in this area is pumped from depths of nearly 200 m, this does not reflect the surface geology, as has been argued by Frei et al. (2020b). This combined with high-degrees of lateral movement of groundwater (Danapour et al., 2019), thus renders Sr from present-day drinking waters a very poor choice for creating isoscapes for provenance studies, including pre-historic mobility studies, as has been proposed by Frei et al. (2020b), even if such waters have not been limed or otherwise treated for water hardness by municipal water works.
Strontium isotopic investigation of Profile C by Frei et al. (2020a), from samples obtained in 2014, yielded a mixing trend between a silicate endmember (having a low Sr concentration and a high 87Sr/86Sr ratio) and a lime endmember (having a high Sr concentration and a low 87Sr/86Sr ratio), but it was not possible to distinguish whether the lime endmember was agricultural lime, or whether it was residual natural of limestone found in one of the soil horizons, due to the fact that the Cretaceous Age agricultural lime, and the residual Silurian Age limestone remnants—likely deposited in the meltwater sand during the Weichselian ice age, as ice rafted material from Gotland, Sweden—have very similar Sr isotopic signatures (Frei et al., 2020a). The fact that the Sr isotopic composition of the porewaters in Frei et al. (2020a) are both lime-dominated and constant throughout the profile, suggests that the lime signature is imparted from the top of the soil through agricultural lime, rather than from occasional limestone remnants at depth. Likewise, the Silurian limestone pieces tend to be very erosion resistant (Kjær et al., 2003), and thus are unlikely to contribute much to the Sr budget. This is attested by their presence in the soil 22,000 years after deposition, compared to the agricultural lime, which dissolves in these soils within a few years. Nonetheless, Sr isotopes cannot be used to discriminate between the two sources of lime (where 87Sr/86Sr of the Cretaceous limestone = 0.7078 and 87Sr/86Sr of the local Silurian limestone = 0.7081), but an investigation of Profile N, which has never been treated with agricultural lime could resolve whether the mixing trend is due to agricultural lime or to residual natural lime.
In Figure 6, the 87Sr/86Sr compositions and reciprocal Sr concentrations are plotted for the ammonium nitrate extracts of pristine Profile N (this study) and intensively-limed Profile C (Frei et al., 2020a). The mixing trend observed for Profile N exhibits the same silicate endmember as that of Profile C, but it does not approach a limestone endmember. Rather, the 87Sr/86Sr ratio of the O-horizon (N8) is 0.7122, a value far higher than that of limestone (0.708). The linearity of the mixing line for Profile N is not perfect. In the regression, the E- and topmost sample of the B-horizon (N7 & N6) have been omitted, as these are the two samples that fall far below the 1:1 line in Figure 3D. It is unclear whether their relatively higher Sr concentration compared to 87Sr/86Sr is due to the ammonium nitrate extracts not reaching equilibrium or to mineralogical differences amongst the samples. Regardless of whether or not these samples are included, a regression of this profile that includes the O-horizon gives an intercept of 0.7122. There is no indication that there is any natural residual limestone in the heathland soil, and unsurprisingly, it is therefore also highly likely that the lime endmember in the heavily-limed Profile C is agricultural lime. One reason for the mixing relationship in the pristine Profile N could be the accumulation of Sr from rainwater in the upper parts of the soil. It would require full retention of 3,000–4,000 years of Sr from rainwater to explain the mixing, or more realistically, ca. 15% retention of Sr from rainwater for the 22,000 years since the outwash plain was formed, assuming that the Sr concentrations of rainwater determined in the course of this study and Thomsen and Andreasen (2019) are representative for the concentration of Sr in rainwater since the end of the Weichselian ice age. Alternatively, it could be that the top of Profile N is affected by windblown dust from the nearby frequently limed farmed fields, which is carried by the predominantly westerly winds. Profile N was selected for this study, due to its proximity to the liming test site; a heathland site more isolated from farmland would be useful in order to determine the relative contributions of Sr from rainwater and windblown dust from the farmland.
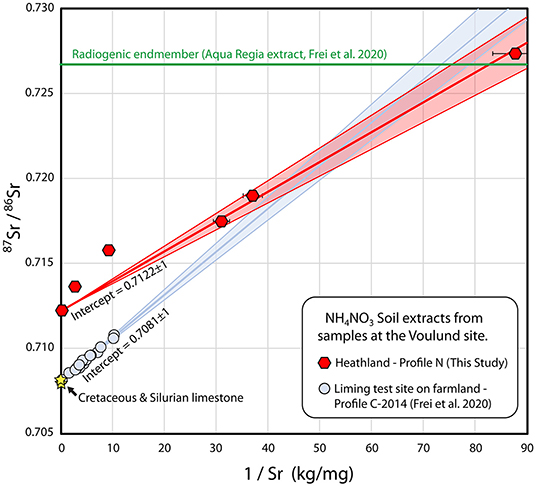
Figure 6. Strontium mixing diagram with Sr isotopic compositions of ammonium nitrate leachates from Voulund soils as a function of the reciprocal Sr concentrations. Samples from soil profile N (heathland), this study, are shown as red hexagons, and samples from soil profile C—liming test site (Frei et al., 2020a), are shown as light blue circles. Also shown is the aqua regia extract of the deepest meltwater sand sample from Profile C in 2014, Frei et al. (2020a) (shown as a green line), as the Sr concentration of this is not directly comparable to the ammonium nitrate leachate samples. The compositions of the Cretaceous limestone that sources agricultural lime, and the Silurian limestone, sporadically found locally in the glacial outwash sands are shown as yellow stars. Regression lines were calculated with IsoplotR (Vermeesch, 2018), and shaded areas indicate 2σ uncertainty envelopes.
Comparison With the Vallerbæk Tributary of the Karup River
In order to see whether the large variation in Sr isotopic compositions of the soils in Voulund is an isolated phenomenon, ammonium nitrate extracts of several soil samples from the Vallerbæk tributary of the Karup River were also analyzed. Water samples from this stream had been analyzed by Thomsen and Andreasen (2019); and 87Sr/86Sr ratios for both soil and water samples are presented in Figure 7. These samples show very different 87Sr/86Sr isotopic signatures—the sample from the field (KF) shows a signature identical to that of agricultural lime (0.7079), whereas the shrubland sample (KM) is unaffected by agricultural lime, with an 87Sr/86Sr ratio of 0.7137. This latter value is identical to that of the brook at that point. The sample from the forest (VS1) gives a much higher 87Sr/86Sr ratio of 0.7169, a signature that is not seen in the water samples of the brook, nor in the local groundwater (Thomsen and Andreasen, 2019).
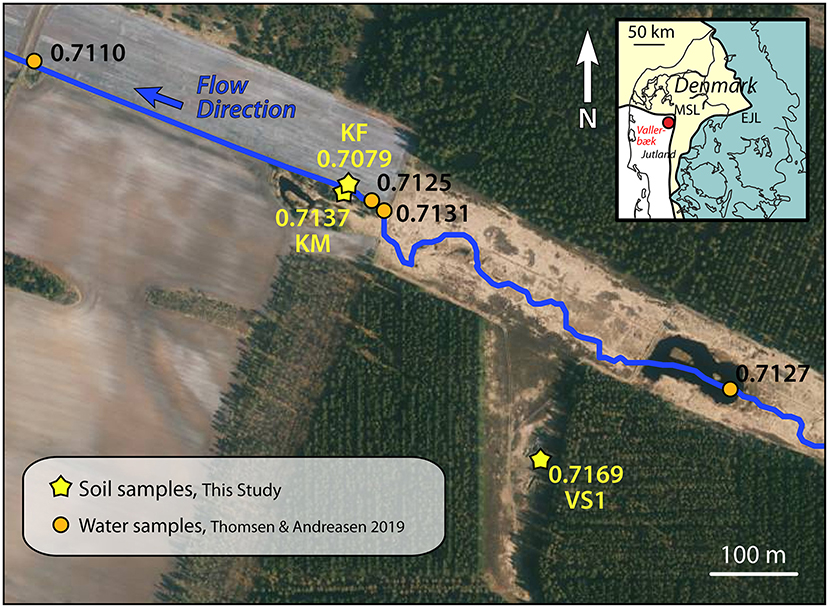
Figure 7. Orthophoto from Spring 2019 of the Vallerbæk tributary of the Karup River, where the stream enters farmland. Measurements of Sr isotopic composition in the stream water (Thomsen and Andreasen, 2019) are shown with orange dots. Measurements of the Sr isotopic composition of ammonium nitrate leachate of soil samples are shown with yellow stars. The flow direction of the stream is SE to NW. The white bands in the fields are varying proportions of quartz in the soil. Map insert shows the locality relative to the two major ice front locations during the Weichselian Ice Age (Houmark-Nielsen, 2003). Orthophoto from Styrelsen for Dataforsyning og Effektivisering—Denmark.
This, and the large variations seen in the Sr isotopic compositions of the soils at Voulund highlight one of the difficulties of using soil samples for the construction of Sr baselines—namely that a very detailed sampling grid is required to characterize each area. Conversely, the use of surface water samples (Thomsen and Andreasen, 2019; Thomsen et al., 2021) or perhaps plant samples reflects a weighted average of an area, which can be used to assess the bioavailable Sr in pre-historic times, provided agricultural areas are avoided, especially in places where soils are naturally low- to non-calcareous.
Conclusions
Our investigation into the fate of Sr from agricultural lime at a test field site on a glacial outwash plain confirms that Sr is as highly mobile as Ca is, and little is retained in organic-rich topsoils, such that Sr seeps into the underlying groundwater and nearby surface waters. In both the sandy soils of the an intensively-limed CO2 storage test site and farmed- and heathland soils adjacent to the test site, Sr exhibits a degree of mobility similar to that of Ca, as is expected, given data for other soil types (Smičiklas et al., 2015) and is what is predicted by the size of strontium's hydrated ions (Burgess, 2011). 87Sr/86Sr values from samples obtained from the pristine heathland next to the test site are significantly higher than those obtained from the test site, so the test site is unlikely to be influenced by natural relict carbonates occasionally found in the area, as was proposed by Frei et al. (2020a), confirming that the lime 87Sr/86Sr signature of farmland soils, and streams and rivers in contact with the farmland almost certainly comes from agricultural lime. This indicates that the 87Sr/86Sr signatures of the area were likely much higher, during pre-historic times, and that isoscapes based on samples from modern-day farmland or from surface waters in contact with these—like Frei and Frei's (2011) isoscape of Denmark are inappropriate for use in provenance and mobility studies of pre-historic people. And this can have significant consequences for the interpretation of pre-historic archaeological finds, as it has in these areas, including the iconic Bronze Age females, The Egtved Girl, The Skrydstrup Woman Frei et al. (2015, 2017); Thomsen and Andreasen (2019) and other individuals, discussed in Thomsen et al. (2021). Thus, it is critical that the possible impact of farming is evaluated when conducting provenance and mobility studies, especially in areas with Sr-poor soils and where agricultural lime is used for soil improvement, including the glacial outwash plains covering large parts of Europe and North America (Gillespie et al., 2003; Böse et al., 2012; Reimann et al., 2014). Overlooking this can result in significant overestimation of the degree of pre-historic mobility in an area, as it has in Denmark, where the overall mobility during pre-historic times was likely significantly lower than recently proposed (Frei et al., 2019).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ET selected the soil pits locations. RA did soil sampling in the Vallerbæk area, sample processing, and analyses as well as the initial geochemical interpretation, and drafted the manuscript. All authors did initial sampling at the Voulund site, additional sampling there was done by ET. All authors devised the study, contributed to the interpretations and the manuscript, and approved the submitted version.
Funding
This work was supported by AUFF (Aarhus University Research Foundation) NOVA grant E-2019-9-27.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Claus Heilmann Clausen is thanked for his expertise and tireless efforts in digging soil pits. Discussions on organic geochemistry with Hamed Sanei and Arka Rudra are greatly appreciated, as are discussions with Søren Munch Kristiansen on soil chemistry. Sincere thanks to Benjamin C. Bostick for sharing his insight into element mobility. The authors are grateful to Erin J. Rosenberg, who improved the manuscript. Insightful comments from two reviewers and associate guest editor Joshua Miller were very helpful in improving the presentation of the data and refining the discussion of these.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.588422/full#supplementary-material
References
Aquilina, L., Poszwa, A., Walter, C., Vergnaud, V., Pierson-Wickmann, A.-C., and Ruiz, L. (2012). Long-term effects of high nitrogen loads on cation and carbon riverine export in agricultural catchments. Environ. Sci. Technol. 46, 9447–9455 doi: 10.1021/es301715t
Bataille, C. P., Crowley, B. E., Wooller, M. J., and Bowen, G. J. (2020). Advances in global bioavailable strontium isoscapes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 555:109849. doi: 10.1016/j.palaeo.2020.109849
Bataille, C. P., von Holstein, I. C. C., Laffoon, J. E., Willmes, M., Liu, X.-M., and Davies, G. R. (2018). A bioavailable strontium isoscape for Western Europe: a machine learning approach. PLoS ONE 13:e0197386. doi: 10.1371/journal.pone.0197386
Bentley, R. A. (2006). Strontium isotopes from the earth to the archaeological skeleton: a review. J. Archaeol. Meth. Theor. 13, 135–187. doi: 10.1007/s10816-006-9009-x
Blum, J. D., Taliaferro, E. H., Wiesse, M. T., and Holmes, R. T. (2000). Changes in Sr/Ca, Ba/Ca and 87Sr/86Sr ratios between trophic levels in two forest ecosystems in the northeastern U.S.A. Biogeochemistry 49, 87–101. doi: 10.1023/A:1006390707989
Böhlke, J. K., and Horan, M. F. (2000). Strontium isotope geochemistry of groundwaters and streams affected by agriculture, Locust Grove, MD. Appl. Geochem. 15, 599–609. doi: 10.1016/S0883-2927(99)00075-X
Böse, M., Lüthgens, C., Lee, J. R., and Rose, J. (2012). Quaternary glaciations of northern Europe. Quat. Sci. Rev. 44, 1–25. doi: 10.1016/j.quascirev.2012.04.017
Burgess, J. (2011). Ions in Solution, Basic Principles of Chemical Interactions. Ellis Horwood Limited. Cambridge: Woodhead Publishing Limited.
Capo, R. C., Stewart, B. W., and Chadwick, O. A. (1998). Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma 82, 197–225. doi: 10.1016/S0016-7061(97)00102-X
Danapour, M., Højberg, A. L., Jensen, K. H., and Stisen, S. (2019). Assessment of regional inter-basin groundwater flow using both simple and highly parameterized optimization schemes. Hydrogeol. J. 27, 1929–1947. doi: 10.1007/s10040-019-01984-3
Dia, A. N., Cohen, A. S., O'Nions, R. K., and Shackleton, N. J. (1992). Seawater Sr isotope variation over the past 300 kyr and influence of global climate cycles. Nature 356, 786–788. doi: 10.1038/356786a0
Edmond, J. T. (1992). Himalayantectonics, weathering processes, and the strontium isotope record in marine limestones. Science 258, 1594–1597. doi: 10.1126/science.258.5088.1594
Ericson, J. E. (1985). Strontium isotope characterization in the study of prehistoric human ecology. J. Hum. Evol. 14, 503–514. doi: 10.1016/S0047-2484(85)80029-4
Faure, G., Crockett, J. H., and Hurley, P. M. (1967). Some aspects of strontium and calcium in the Hudson Bay and the great Lakes. Geochim. Cosmochim. Acta 81, 451–461. doi: 10.1016/0016-7037(67)90053-1
Frei, K. M., Bergerbrant, S., Sjögren, K. -G., Jørkov, M. L., Lynnerup, N., Harvig, L., et al. (2019). Mapping human mobility during the third and second millennia BC in present-day Denmark. PLoS ONE 14:e0219850. doi: 10.1371/journal.pone.0219850
Frei, K. M., and Frei, R. (2011). The geographic distribution of strontium isotopes in Danish surface waters – a base for provenance studies in archaeology, hydrology and agriculture. Appl. Geochem. 26, 326–340. doi: 10.1016/j.apgeochem.2010.12.006
Frei, K. M., Mannering, U., Kristiansen, K., Allentoft, M. E., Wilson, A. S., Skals, I., et al. (2015). Tracing the dynamic life story of a Bronze age female. Sci. Rep 5:10431. doi: 10.1038/srep10431
Frei, K. M., Villa, C., Jørkov, M. L., Allentoft, M. E., Kaul, F., Ethelberg, et al. (2017). A matter of months: high precision migration chronology of a Bronze age female. PLoS ONE 12:e0178834. doi: 10.1371/journal.pone.0178834
Frei, R., Frei, K. M., and Jessen, S. (2020a). Shallow retardation of the strontium isotope signal of agricultural liming - implications for isoscapes used in provenance studies. Sci. Total Environ. 706:135710. doi: 10.1016/j.scitotenv.2019.135710
Frei, R., Frei, K. M., Kristiansen, S. M., Jessen, S., Schullehner, J., and Hansen, B. (2020b). The link between surface water and groundwater-based drinking water – strontium isotope spatial distribution patterns and their relationships to Danish sediments. Appl. Geochem. 121:104698. doi: 10.1016/j.apgeochem.2020.104698
Gillespie, A. R., Porter, S. C., and Atwater, B. F. (2003). The Quaternary Period in the United States, Vol. 1. Amsterdam: Elsevier Science.
Grimstead, D. N., Nugent, S., and Whipple, J. (2017). Why a standardization of strontium isotope baseline environmental data is needed and recommendations for methodology. Adv. Archaeol. Pract. 5, 184–195. doi: 10.1017/aap.2017.6
Haak, W., Brandt, G., de Jong, H. N., Meyer, C., Ganslmeier, R., Heyd, V., et al. (2008). Ancient DNA, strontium isotopes, and osteological analyses shed light on social and kinship organization of the later stone age. Proc. Natl. Acad. Sci. U.S.A. 105, 18226–18231. doi: 10.1073/pnas.0807592105
Hoogewerff, J. A., Reimann, C., Ueckermann, H., Frei, R., Frei, K. M., van Aswegen, T., et al. (2019). Bioavailable 87Sr/86Sr in European soils: a baseline for provenancing studies. Sci. Total Environ. 672, 1033–1044. doi: 10.1016/j.scitotenv.2019.03.387
Houmark-Nielsen, M. (2003). Signature and timing of the kattegat ice stream: onset of the LGM-sequence in the southwestern part of the scandinavian ice sheet. Boreas 32, 227–241. doi: 10.1111/j.1502-3885.2003.tb01439.x
Jessen, S., Jakobsen, R., Postma, D., Looms, M., and Larsen, F. (2014a). Carbon Transfer Across the Vadose Zone: Inhibition by 20th Century Acid Rain?. Available online at: https://co2gs.geus.net/xpdf/WP5-Carbon_transfer_across_the_vadose_zone.pdf
Jessen, S., Postma, D., Jakobsen, R., Looms, M. C., and Larsen, F. (2014b). Inhibition of Carbon Transfer Across the Vadose Zone by 20th Century Acid Rain. Vienna: EGU General Assembly.
Jørgensen, N. O., Morthorst, J., and Holm, P. M. (1999). Strontium-isotope studies of “brown water” (organic-rich groundwater) from Denmark. Hydrogeol. J. 7, 533–539. doi: 10.1007/s100400050226
Kjær, K. H., Houmark-Nielsen, M., and Richardt, N. (2003). Ice-flow patterns and dispersal of erratics at the southwestern margin of the last scandinavian ice sheet: imprint after palaeo-ice streams. Boreas 32, 130–148. doi: 10.1111/j.1502-3885.2003.tb01434.x
Knipper, C., Mittnik, A., Massy, K., Kociumaka, C., Kucukkalipci, I., Maus, M., et al. (2017). Female exogamy and gene pool diversification at the transition from the final neolithic to the early bronze age in central Europe. Proc. Natl. Acad. Sci. U.S.A. 114, 10083–10088. doi: 10.1073/pnas.1706355114
Krüger, J., Jensen, N. H., and Greve, M. H. (2013). A statistically based mapping of the influence of geology and land use on soil pH. A case study from Denmark. Geoderma 192, 453–462. doi: 10.1016/j.geoderma.2012.08.024
Long, M. P., Alland, S., Martin, M. E., and Isborn, C. M. (2020). Molecular dynamics simulations of alkaline earth metal ions binding to DNA reveal ion size and hydration effects. Phys. Chem. Chem. Phys. 22:5584. doi: 10.1039/C9CP06844A
Madgwick, R., Lamb, A. L., Sloane, H., Nederbragt, A. J., Albarella, U., Pearson, M. P., et al. (2019). Multi-isotope analysis reveals that feasts in the stonehenge environs and across wessex drew people and animals from throughout Britain. Sci. Adv. 5:eaau6078. doi: 10.1126/sciadv.aau6078
Maurer, A. -F., Galer, S. J. G., Knipper, C., Beierlein, L., Nunn, E. V., Peters, D., et al. (2012). Bioavailable 87Sr/86Sr in different environmental samples. Effects of anthropogenic contamination and implications for isoscapes in past migration studies. Sci. Total Environ. 233, 216–229. doi: 10.1016/j.scitotenv.2012.06.046
Montgomery, J. (2010). Passports from the past: investigating human dispersals using strontium isotope analysis of tooth enamel. Ann. Hum. Biol. 37, 325–346. doi: 10.3109/03014461003649297
Montgomery, J., Evans, J. A., and Cooper, R. E. (2007). Resolving archaeological populations with Sr-isotope mixing models. Appl. Geochem. 22, 1502–1514. doi: 10.1016/j.apgeochem.2007.02.009
Müller, W., Fricke, H., Halliday, A. N., McCulloch, M. T., and Wartho, J. A. (2003). Origin and migration of the Alpine Iceman. Science 302, 862–866. doi: 10.1126/science.1089837
Oh, N. H., and Raymond, P, A. (2006). Contribution of agricultural liming to riverine bicarbonate export and CO sequestration in the Ohio river basin. Global Biogeochem. Cycles 20:3. doi: 10.1029/2005GB002565
Reimann, C., Birke, M., Demetriades, A., Filzmoser, P., and O'Connor, P, (eds). (2014). Chemistry of Europe's agricultural soils – part A: methodology and interpretation of the GEMAS data set. Geologisches Jahrbuch (Reihe B 102). Hannover: Schweizerbarth
Smičiklas, I., Jović, M., Šljivić-Ivanović, M., Mrvić, V., Cakmak, D., and Dimović, S. (2015). Correlation of Sr2+ retention and distribution with properties of different soil types. Geoderma 253–254, 21–29. doi: 10.1016/j.geoderma.2015.04.003
Solecki, J. (2005). Investigation of 85Sr adsorption on selected soils of different horizons. J. Environ. Radioact. 82 303–320. doi: 10.1016/j.jenvrad.2005.02.001
Thomsen, E., and Andreasen, R. (2019). Agricultural lime disturbs natural strontium isotope variations: implications for provenance and migration studies. Sci. Adv. 5:eaav8083. doi: 10.1126/sciadv.aav8083
Thomsen, E., Andreasen, R., and Rasmussen, T. L. (2021). Homogenous Glacial Landscapes can have high local variability of strontium isotope signatures: implications for prehistoric migration studies. Front. Ecol. Evol. 8:588318. doi: 10.3389/fevo.2020.588318
Veizer, J., Ala, D., Azmy, K., Bruckschen, P., Buhl, D., Bruhn, F., et al. (1999). Sr-87/Sr-86, delta C-13 and delta O-18 evolution of phanerozoic seawater. Chem. Geol. 161:59–88. doi: 10.1016/S0009-2541(99)00081-9
Vermeesch, P. (2018). IsoplotR: a free and open toolbox for geochronology. Geosci. Front. 9, 1479–1493. doi: 10.1016/j.gsf.2018.04.001
Wallace, S. H., Shaw, S., Morris, K., Small, J. S., Fuller, A. J., and Burke, I. T. (2012). Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: implications for 90Sr mobility at contaminated nuclear sites. Appl. Geochem. 27, 1482–1491. doi: 10.1016/j.apgeochem.2012.04.007
Keywords: 87Sr/86Sr, agricultural lime, pre-historic human mobility, element mobility, glacial deposit, soil profile
Citation: Andreasen R and Thomsen E (2021) Strontium Is Released Rapidly From Agricultural Lime–Implications for Provenance and Migration Studies. Front. Ecol. Evol. 8:588422. doi: 10.3389/fevo.2020.588422
Received: 28 July 2020; Accepted: 29 December 2020;
Published: 05 February 2021.
Edited by:
Joshua H. Miller, University of Cincinnati, United StatesReviewed by:
Lihai Hu, University of Ottawa, CanadaChris Widga, East Tennessee State University, United States
Copyright © 2021 Andreasen and Thomsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rasmus Andreasen, cmFzbXVzLmFuZHJlYXNlbkBnZW8uYXUuZGs=
 Rasmus Andreasen
Rasmus Andreasen Erik Thomsen
Erik Thomsen