- 1Laboratório de Etologia, Desenvolvimento e Interação Social (LEDIS), Department of Experimental Psychology, Institute of Psychology, University of São Paulo, São Paulo, Brazil
- 2Núcleo de Etologia e Evolução (NuEVo), Department of Zoology, Institute of Biology, Federal University of Bahia, Salvador, Brazil
- 3Department of Zoology, National Institute of Science and Technology in Interdisciplinary and Transdisciplinary Studies in Ecology and Evolution (INCT IN-TREE), Institute of Biology, Federal University of Bahia, Salvador, Brazil
- 4Sylvius Laboratory, Faculty of Science, Institute of Biology Leiden, Leiden University, Leiden, Netherlands
Birds communicate through acoustic variation in their songs for territorial defense and mate attraction. Noisy urban conditions often induce vocal changes that can alleviate masking problems, but that may also affect signal value. We investigated this potential for a functional compromise in a neotropical songbird: the bananaquit (Coereba flaveola). This species occurs in urban environments with variable traffic noise levels and was previously found to reduce song elaboration in concert with a noise-dependent reduction in song frequency bandwidth. Singing higher and in a narrower bandwidth may make their songs more audible in noisy conditions of low-frequency traffic. However, it was unknown whether the associated decrease in syllable diversity affected their communication. Here we show that bananaquits responded differently to experimental playback of elaborate vs. simple songs. The variation in syllable diversity did not affect general response strength, but the tested birds gave acoustically distinct song replies. Songs had fewer syllables and were lower in frequency and of wider bandwidth when individuals responded to elaborate songs compared to simple songs. This result suggests that noise-dependent vocal restrictions may change the signal value of songs and compromise their communicative function. It remains to be investigated whether there are consequences for individual fitness and how such effects may alter the diversity and density of the avian community in noisy cities.
Introduction
In the last decades, the noise levels in human-altered and natural habitats have substantially increased and affected the way birds sing (Rabin and Greene, 2002; Mennitt et al., 2015; Buxton et al., 2017). Anthropogenic noise can interfere with communication among birds because it can mask their songs through overlap in frequency and time (Brumm and Slabbekoorn, 2005; Barber et al., 2010; Parris and McCarthy, 2013). Several noise-dependent vocal changes have been reported in city birds (Brumm, 2004; Potvin and Mulder, 2013; Gil et al., 2014), which typically yield an increase in song detectability and improved efficiency of communication (Brumm and Slabbekoorn, 2005; Pohl et al., 2012). However, vocal changes may not only affect signal detectability but also signal value (Slabbekoorn and Ripmeester, 2008; Gross et al., 2010) and noise-dependent song variation may thereby involve a functional compromise (Slabbekoorn, 2013; Luther and Magnotti, 2014; Luther et al., 2016; Phillips and Derryberry, 2018). Although reports on noise-dependent song variation are widespread, tests of the potential for functional consequences for communication are still rare (see e.g., Mockford and Marshall, 2009; Ripmeester et al., 2010; Luther and Derryberry, 2012; Luther et al., 2016).
There are several ways birds change their songs by which they could counteract masking by urban noise. Several species have been found to sing higher frequencies and/or narrower-banded songs in noisier environments (Slabbekoorn and Peet, 2003; Verzijden et al., 2010; Bermúdez-Cuamatzin et al., 2011; Montague et al., 2012; LaZerte et al., 2016). As anthropogenic noise is typically biased to low-frequency bands, higher-frequency songs are more audible than lower-frequency songs (Brumm and Slabbekoorn, 2005; Nemeth and Brumm, 2010; Halfwerk et al., 2011) and concentrating all acoustic energy in a narrower band can also raise signal-to-noise ratio (Hanna et al., 2011). Birds are also reported to sing at higher amplitudes if noise levels rise and they can sing shorter or in alternating time periods when noise levels are fluctuating (Brumm, 2004; Gil et al., 2014; Gentry et al., 2017; Derryberry et al., 2017).
Although such noise-dependent changes may be successful in masking avoidance, they may also restrict the potential for communication by undermining the signaling function of the songs (Slabbekoorn and Ripmeester, 2008; Gross et al., 2010; Slabbekoorn, 2013; Luther et al., 2016). Reduction in frequency band use, for example, may restrict the use of particular syllables and limit possible syllable variation, and consequently limit song repertoire size of an individual (Montague et al., 2012; Fouda et al., 2018; Winandy et al., 2021). Song elaboration in birds may signal male size or other parental qualities (e.g., Kipper et al., 2006; Botero et al., 2009; Kagawa and Soma, 2013) and can be a good predictor of potential offspring survival and thus affect female preference (Hasselquist et al., 1996; Buchanan and Catchpole, 1997, 2000). Although, some bird species may be able to counteract song structure restrictions on song complexity (see Moseley et al., 2019), noise-dependent reduction in song elaboration in general may negatively affect signal quality and undermine information transfer about sender quality.
Potential signal value or communicative function of a song can be explored by controlled exposure to playbacks of recorded songs and by experimental manipulation of specific acoustic variation (e.g., Nelson, 1988; Slabbekoorn and ten Cate, 1998; Linhart et al., 2012). Playback of urban and rural song variation has, for example, revealed recognition of urban acoustic features in natural territories of great tits (Parus major) and European blackbirds (Turdus merula). Individual birds approach more closely, stay longer or respond vocally more quickly to playback of songs dependent on whether they are from birds from the same habitat type or similar background noise levels (Mockford and Marshall, 2009; Ripmeester et al., 2010). The potential impact of noise-dependent variation in spectral range has been tested in few studies in both male-female (Halfwerk et al., 2011; Huet des Aunay et al., 2014) and male-male communication (Luther and Magnotti, 2014; Luther et al., 2016; LaZerte et al., 2017; Phillips and Derryberry, 2018).
The bananaquit (Coereba flavoela), an abundant bird species of neotropical cities, is a good system to study the potential signal value of song elaboration. We previously showed bananaquits exhibit noise-dependent variation in song elaboration: they sing elaborate songs, rich in syllable types and syllable transitions in quiet territories and simple and repetitive songs that are poor in syllable diversity in more noisy territories (Winandy et al., 2021). They are relatively abundant across city habitats, used to human presence, and can be highly territorial to conspecific intruders (Hilty and Christie, 2018; personal observations). Consequently, bananaquits are very suitable for playback studies that demand close approach of researchers for behavioral observations and recordings.
In this study, we performed a playback exposure experiment and tested whether bananaquits responded differently to elaborate vs. simple songs. More elaborate songs were characterized by higher syllable diversity (i.e., more syllable types per song), but also by lower minimum and higher maximum frequencies (Winandy et al., 2021). In many species of birds, songs with an aggressive territorial function tend to be shorter and more repetitive than songs with a mate attraction function (Searcy and Anderson, 1986; Collins, 2004). Bananaquit territorial responses to playback may therefore be stronger to simpler songs and may also elicit a vocal response matching in song elaboration. More elaborate songs may require a wider frequency bandwidth. Consequently, we aimed at answering the following questions: (1) do simpler songs trigger stronger responses than the more elaborate songs? (2) do individuals match song elaboration? (3) do elaborate songs trigger wider frequency range songs from territory owners? This study could provide new insights into how noise pollution, through the simplification of urban songs, can alter the evolution of sexually selected signals.
Materials and Methods
Ethics
The animal study was reviewed and approved by the local “Committee of Ethics in the Use of Animals,” Federal University of Bahia – UFBA, Brazil (n°36/2016).
Study Site and Species
We conducted our playback experiment in 20 bananaquit (Coereba flaveola) territories in the city of Salvador, Bahia, Brazil (12°57′50.9″S, 38°′30′21.0″W). We tested the birds during the Brazilian summer, between February and March of 2018. This species can sing and breed throughout the year (Hilty and Christie, 2018) and territorial responsiveness does not fade during summer. The territories were located in different habitat types and traffic noise regimes: in Atlantic Forest urban parks, urban gardens and areas close or next to main avenues, with variable stands of concrete buildings and trees. We performed the experiment only during relatively quiet moments of the day for each territory, between 05H00 and 07H00 in the morning. Previous to the experiment, we assessed the noise levels of the territories throughout the morning, i.e., 05H00 to 10H00, with a sound pressure level meter, and the results are reported in Winandy et al. (2021). Noise levels rose gradually to become above 55dB(A) only after 07H00. By conducting our experiments before 07H00, before the rush hour, and by keeping the distance between playback speaker and response bird less than 14 m, we avoided possible interference of traffic noise with playback song detection, which was not our target in this study.
Bananaquits are nectarivorous songbirds that occur across the Neotropics from Mexico to Argentina and the Caribbean islands. They can be easily observed in several types of human-altered habitats, from highly urban to rural areas. They are territorial birds that sing for mate attraction and territorial defense throughout the day and year (Hilty and Christie, 2018). The singing is thought to be primarily done by males, although more research is needed about possible singing behavior in females (Riebel et al., 2019). The songs are composed of series of high-pitched syllables, which vary from complex sequences of diverse element types with high transition rates to highly repetitive series of less variable syllable types (Winandy et al., 2021). Bananquits have highly variable repertoires, and song elaboration may vary within and among individuals with behavioral context and local noise levels (Winandy et al., 2021).
Sound Recording and Analysis
Before exposing the birds to the playback, we recorded their pre-playback songs for 1 min. Usually, in 1 min of recording the bananaquits sang about 10 songs, but for some individuals we obtained less than five songs. We recorded the birds from a distance of 2–14 m, using a Tascam DR-44WL recorder connected to a Sennheiser TM (Wedemark, Germany) shotgun directional microphone (ME67 + K6). In total, we performed acoustic analyses on 11.1 ± 5.2 (mean ± SD) songs per individual. We used Raven TM PRO software, version 1.5 (Cornell Laboratory of Ornithology, Ithaca, NY, United States) for all processing of recordings and song measurements. Spectrograms settings were kept constant as: FFT length: 512, window: hann, overlap: 75%.
All song recordings, pre-playback and response songs, were first cut in shorter song sequences, separated from recorded playback stimuli, before the analyses. In this way, the observer was always blind to the origin and nature of songs in the stimuli used for the playback experiment. We used cursor placement to extract three spectral song variables (c.f. Verzijden et al., 2010; Winandy et al., 2021): minimum frequency, maximum frequency, and frequency bandwidth. The low-noise conditions during playback and the observer being blind to the stimulus type reduced the chance for observer bias or artifact effects in our spectral measurements (Verzijden et al., 2010; Brumm et al., 2017). Additionally, we counted the number of syllable types per song as a measure of song elaboration.
Playback Stimuli
We used songs of 20 bananaquits recorded at our study site in 2016 and 2017. We chose 20 song recordings varying in levels of song elaboration, from 10 individuals that sang relatively elaborate and from 10 individuals that sang relatively simple songs. Song elaboration is reflected in the number of different syllable types per song (MEAN ± SD simple = 3.1 ± 0.77, MEAN ± SD elaborate = 5.7 ± 1.6, Poisson GLM: Estimate = −0.618, N = 20, P < 0.05, Figure 1) and in the minimum and maximum song frequencies [two-way ANOVA for minimum frequency: MEAN ± SD simple = 3935.7 ± 516.5, MEAN ± SD elaborate = 2441.6 ± 590.8, F(1, 19) = 35.55, N = 20, P < 0.001, maximum frequency: MEAN ± SD simple = 12675.4 ± 494.4, MEAN ± SD elaborate = 11938.0 ± 840.4, F(1, 19) = 6.14, N = 20, P = 0.02, Figure 1]. We made sure that there were no significant differences in the length (measured in total number of syllables per song and song duration), peak frequency, syllable rate and frequency bandwidth between the two song categories [Poisson GLM for number of syllables: Estimate = 0.0366, N = 20, P = 0.7, song duration: F(1, 19) = 3.37, N = 20, P = 0.08, peak frequency: F(1, 19) = 1.21, P = 0.2, N = 20, syllable rate: F(1, 19) = 2.444, N = 20, P = 0.135, frequency bandwidth: F(1, 19) = 2.928, N = 20, P = 0.1], as they may also convey information and, for example, be indicative for motivational states of birds (Langemann et al., 2000; Ripmeester et al., 2007; Lattin and Ritchison, 2009; Linhart et al., 2012, 2013; Luther et al., 2016; Phillips and Derryberry, 2017). All the songs were high-pass filtered to remove the low-frequency background noise and normalized to an equal peak amplitude in Audacity TM v. 2.1.2 (Carnegie Mellon University, Pittsburgh, Pennsylvania, United States).
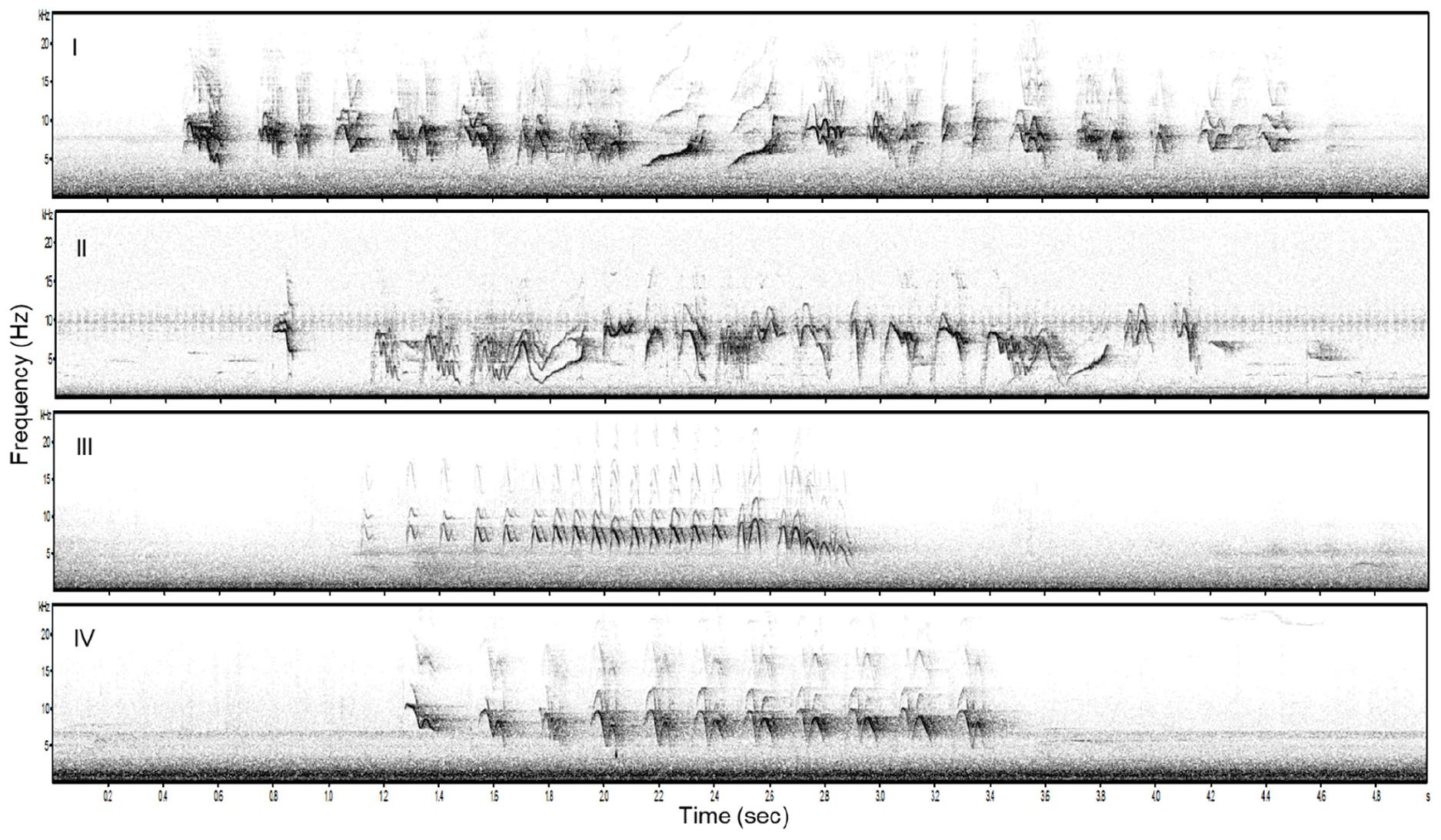
Figure 1. Examples of two elaborate (I and II) and two simple (III, IV) song stimuli used in the playback experiment.
Each playback stimulus consisted of three different songs of the same individual and song category (simple or elaborate). Songs from the same individual were only used for one stimulus and thus not in different song categories. The three songs were played back twice in the same sequence with a silent interval of 3 s between each of them (c.f. Ripmeester et al., 2010). We created in this way 10 unique exemplars of each playback stimulus: 10 simple and 10 elaborate playback stimuli.
Playback Design
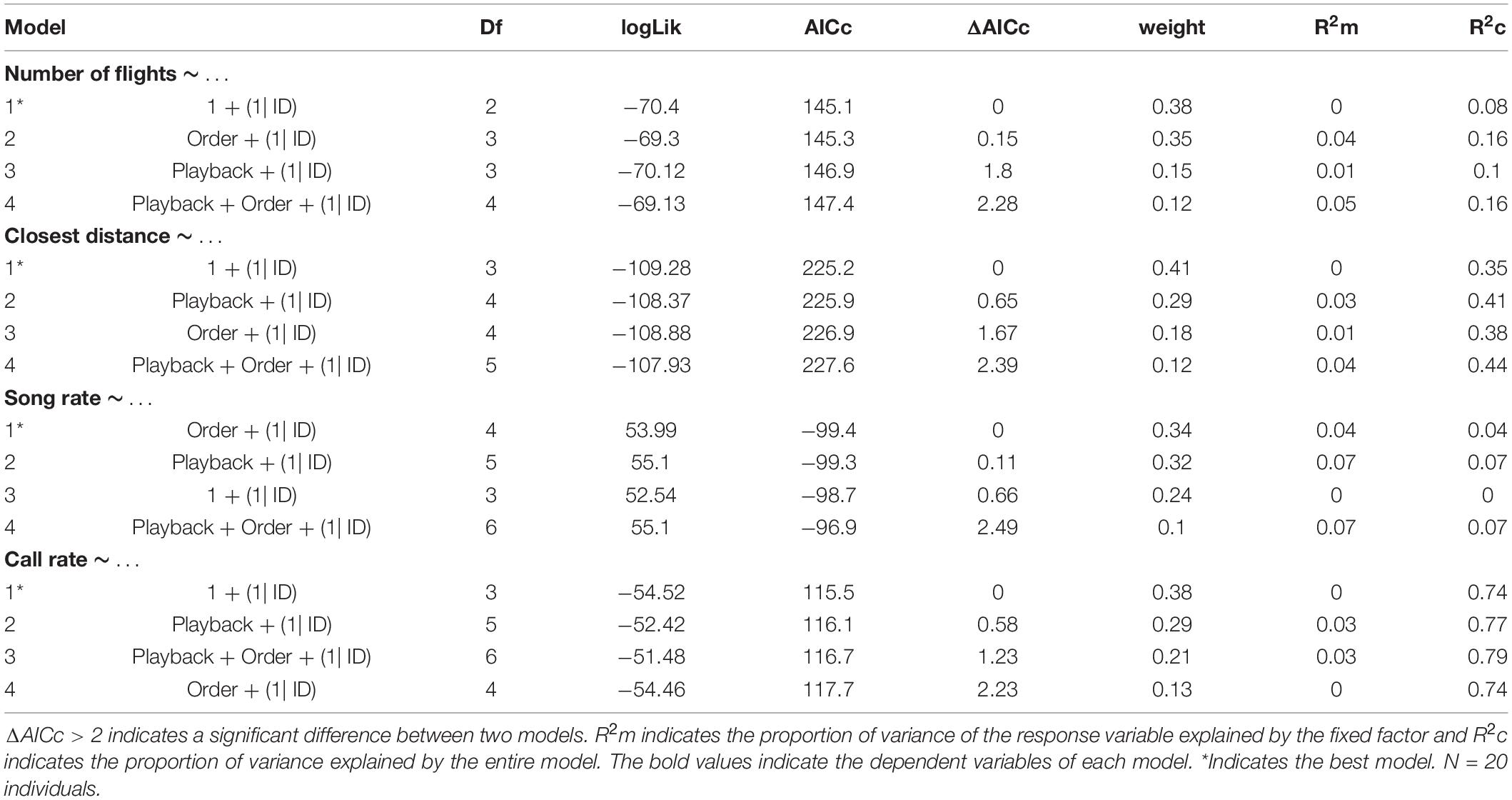
We played back the stimulus songs in bananaquit territories of actively singing birds without nearby competitors that could be agonistically interacting at the time of the experiment. These procedures were meant to reduce variation in behavioral responses related to different motivational states. We placed the ‘JBL clip 2’ loudspeaker at about 5–10 m from the focal male and the observer was positioned 5–10 m further away. We measured the amplitude of the playback with a Skill-Tec TM, SKDEC-02 (São Paulo, São Paulo, Brazil) sound pressure level meter (A-weighted, fast response, range 30–130 dB, 1 s interval) and adjusted playback levels to a volume of 70 dB(A) at a distance of 1 m from the speaker. After the start of the playback of the first song stimulus series, simple or elaborate, we scored the behavior of the focal individual for 1 min. During the playback and for 2 min after it had ended, we also recorded the songs. After the 2 min interval, we played back a song stimulus from the opposite category and recorded songs and scored response behaviors for the same periods as before (Figure 2). The order of the played back stimuli was randomized. We avoided testing direct neighbors that could have been exposed to previous playbacks. The following behaviors and song measurements were scored: number of flights over the loudspeaker, shortest distance of the focal male to the loudspeaker, number of songs, number of calls and song and call rate.
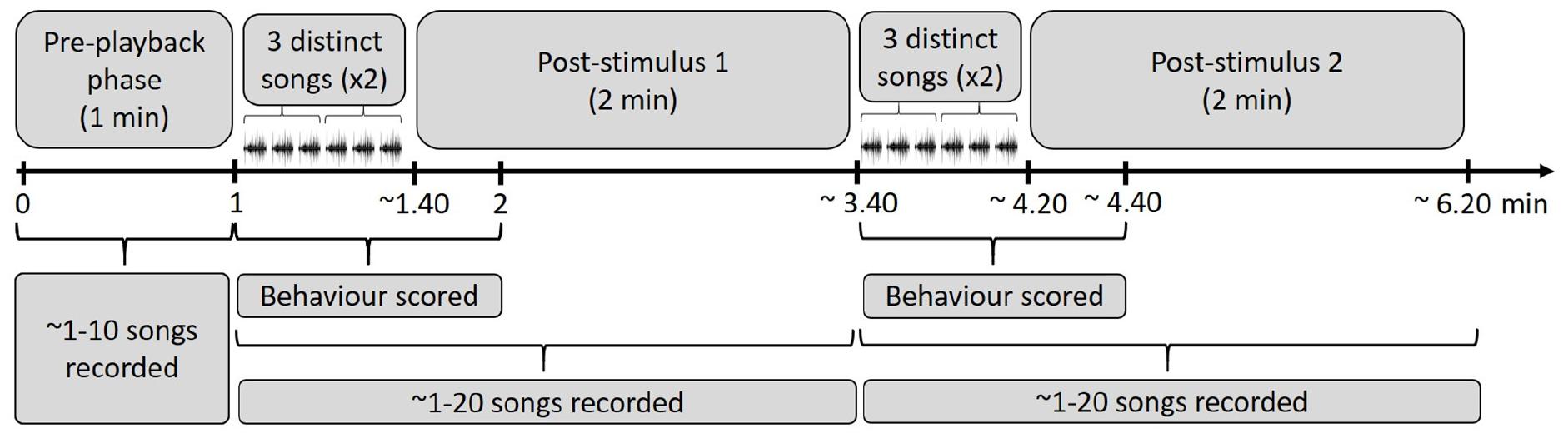
Figure 2. Time periods overview of the playback procedure in the field. A stimulus of three distinct elaborate or simple songs of the same individual was played twice after a 1 min of pre-playback recording phase. After the start of the playback of the first song stimulus series, we scored behavior for 1 min. During the playback and for 2 min after it ended, we recorded the response songs. Following that, the second stimulus was played back to the same focal individual: three distinct songs twice of the opposite stimulus category (simple or elaborate songs, depending on the order of exposure).
Statistical Analysis
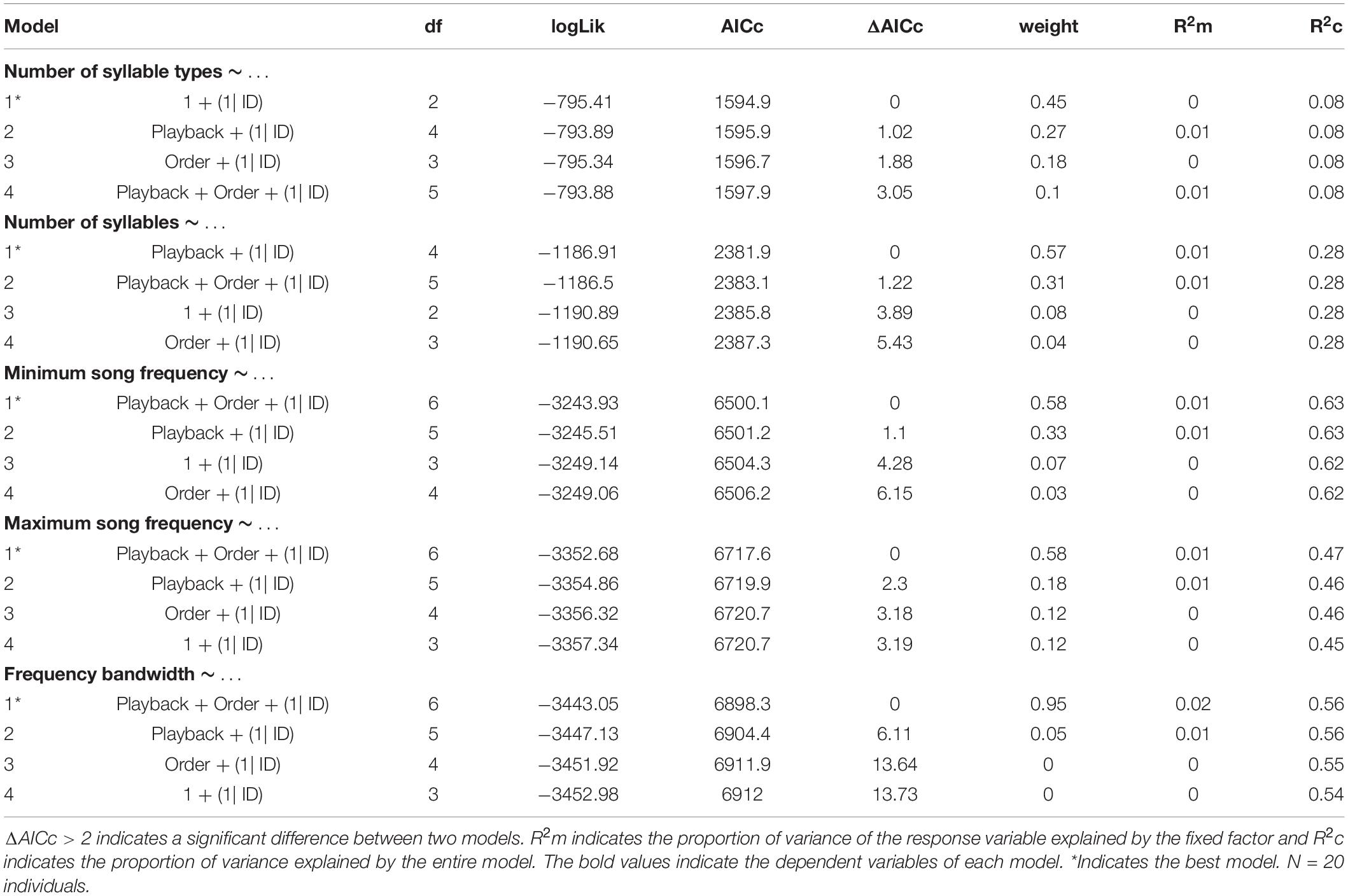
We conducted all statistical analyses in R studio software (R Core Team), using the packages lme4 (Bates et al., 2015) and MuMIn (Barton, 2016). We performed generalized linear models (GLM) and Akaike’s information criterion (AIC) model selection to find out whether the song variables and behavioral responses were affected by the stimulus type (simple vs. elaborate song playbacks) and/or by the order of the stimuli. All song measurements and behavioral responses were entered as response variables in the models. The stimulus category and playback order were entered as fixed factors in the full model and individual as random factor. We computed the statistics for all possible models, which included: (1) single predictors (stimulus category, order), (2) their additive combinations (category + order), and (3) the null models (without effect of any predictor). The response variables: number of syllable types, total number of syllables and number of flights were entered as interval variables in Poisson generalized models with log-link function.
We selected the best models based on the AICc values, considering ΔAICc > 2 a criterion for substantial difference between models (Burnham and Anderson, 2002). The model selection was made using the function dredge model selection (package MuMIn) (Barton, 2016). We calculated the marginal (R2m) and conditional (R2c) R2 values to evaluate how much the fixed effects (R2m) or the entire model (R2c) explained the variance of the response variables (Nakagawa and Schielzeth, 2013). Finally, we performed post-hoc Tukey’s tests for each response variable for which we obtained a minimal model selection. This analysis informed which pairs of playback conditions were significantly different in song or behavioral responses.
As we did in our previous correlational study, we investigated again the possible trade-off between the signal frequency reduction and the restriction in song elaboration. Therefore, we fitted linear models to test the relationship between the spectral and elaboration variables with two different datasets: one that included only the spontaneous songs sung before the start of the playback experiment and another with all songs, both the spontaneous and playback triggered songs.
Results
There was no effect of the stimulus type (elaborate vs. simple) on behavioral response strength and vocalization rate. The number of flights, the approach to the speaker, the song and call rates were all not affected by stimulus category or by the playback order (Table 1 and Figure 3). However, individuals responded in acoustically distinct ways to each playback type. Their songs had fewer syllables and were lower in frequency and wider in frequency bandwidth when they responded to the elaborate song stimuli compared to when they responded to the simple song stimuli (Figure 4).
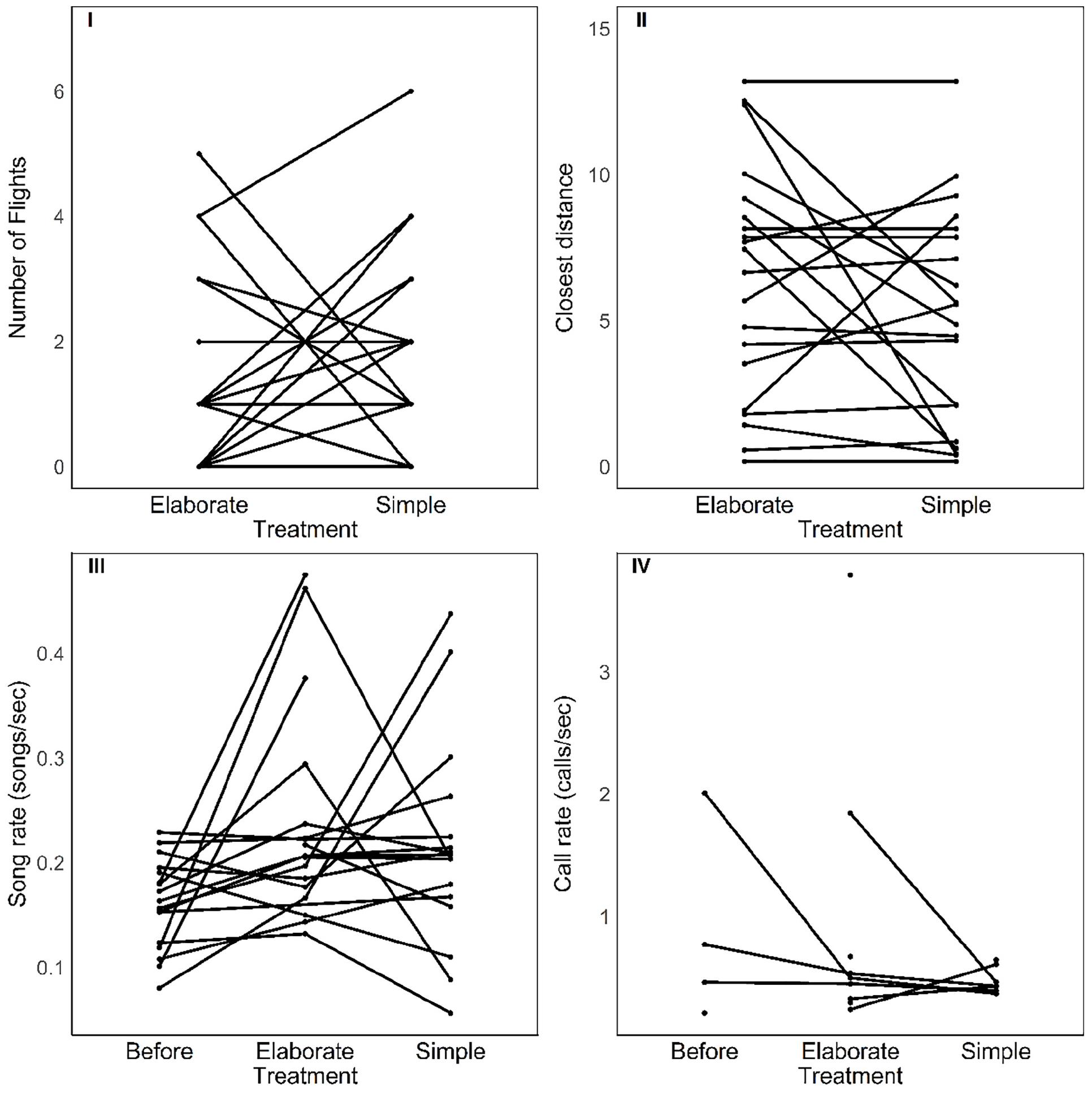
Figure 3. Strength of behavioral responses to each song playback (top) and vocalization rate (bottom) before and during each song playback. There were no significant changes between scoring periods (see text).
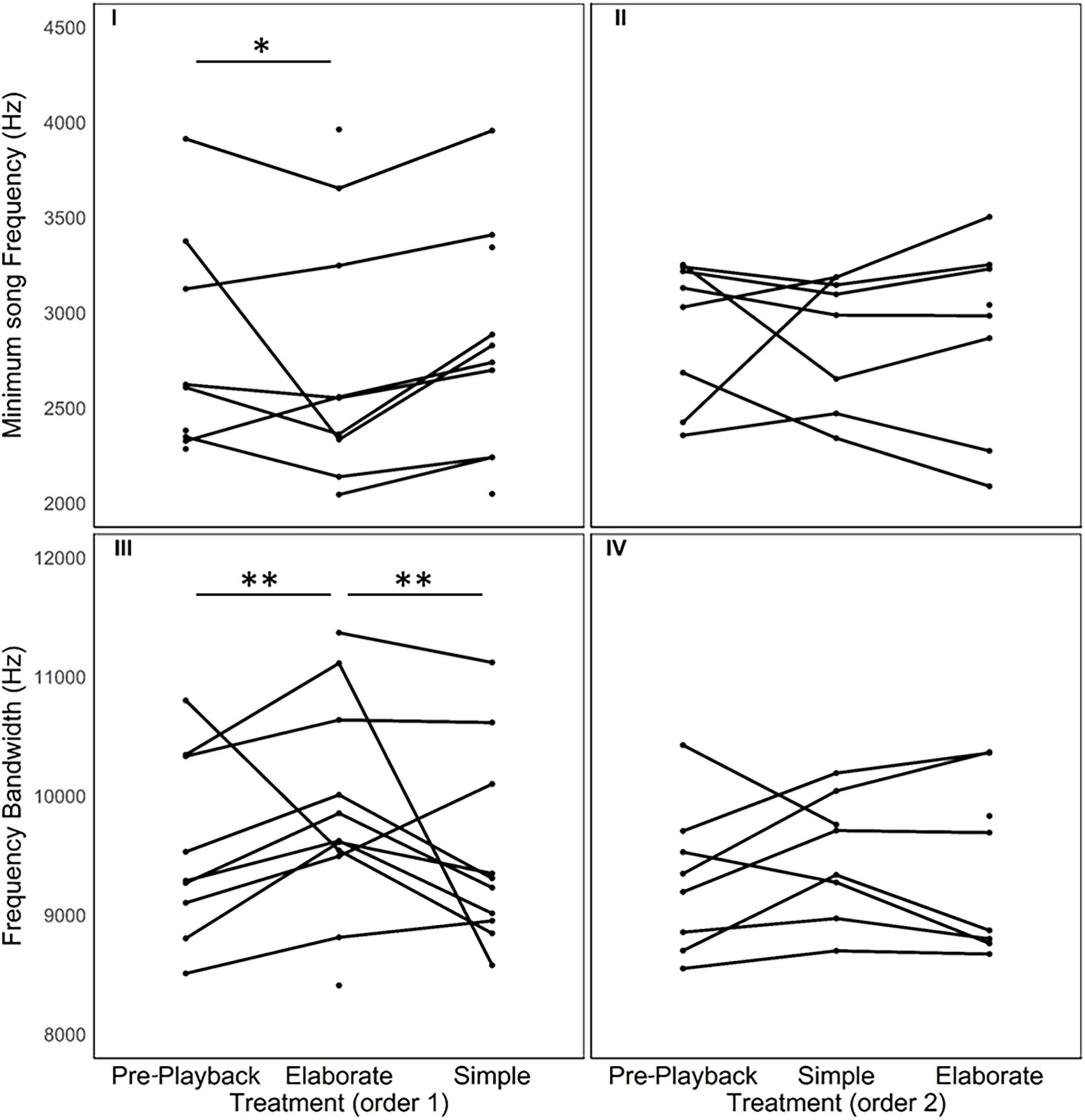
Figure 4. Spectral variation in the songs sung in response to each stimulus category. Each line connects song measures of one individual in three different periods of the playback procedure. As playback order had an effect, we provide the data in two separate sets of graphs. Individuals that were exposed first to the elaborate songs followed by the simple songs are depicted in the graphs on the left. The responses of individuals that were exposed first to the simple songs followed by the elaborate songs are depicted in the graphs on the right. * indicates statistically significant differences between the measures in two of the playback periods (*P < 0.05 and **P < 0.01).
The model selection for song variables showed that the number of syllables per song was significantly affected by the playback stimulus (Table 2). The birds sang fewer syllables per song after being exposed to the elaborate song stimulus than before the playback experiment, 12.42 syllables on average before and 11.40 on average after the elaborate playback stimulus (Table 3). The spectral variables: minimum frequency, maximum frequency and frequency bandwidth (Hz) were explained by both the song stimulus category and the order of the stimulus playback (Table 2). Regarding the order, when the elaborate stimulus was played first, the differences in the spectral responses between treatments were more pronounced (Figure 4). Birds significantly lowered the minimum frequency of their songs after being exposed to the elaborate playback (Figure 4 and Table 3). Moreover, they sang songs of significantly wider frequency bandwidth when responding to the elaborate stimulus, followed by a bandwidth decrease when exposed to the simple playback as the second stimulus (Figure 4 and Table 3).
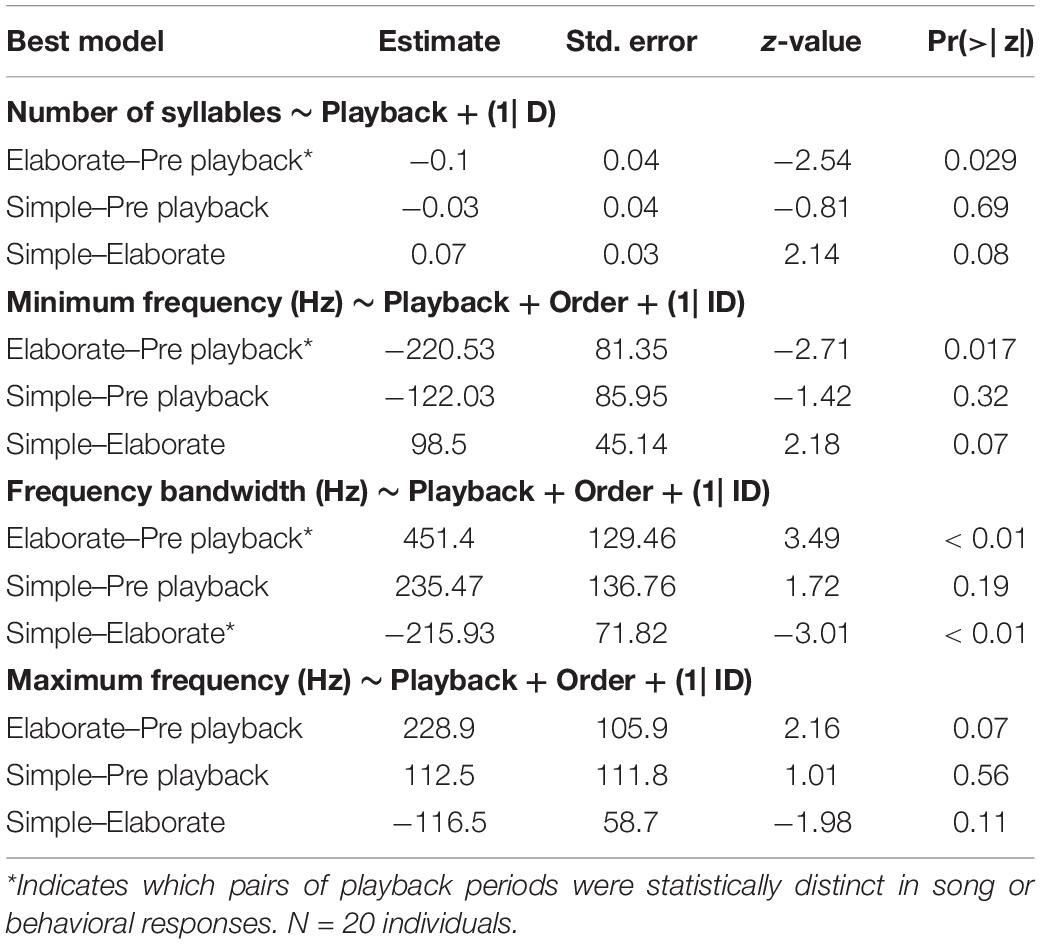
Table 3. Post-hoc tests for the song response variables where a best model with at least one fixed factor was selected.
Finally, the correlation between song elaboration and song frequency previously found for bananaquit songs was not found for the songs from the playback experiment in the current study. The number of syllable types per song and the spectral variables, minimum song frequency and frequency bandwidth, were not correlated. The correlation did not occur when we only included the spontaneous songs from the pre-playback phase [Linear model for low frequency: R2 = −0.06, F(1, 16) = 0.01, N = 17, P = 0.89; Linear model for frequency bandwidth: R2 = −0.06, F(1, 16) = 0.03, N = 17, P = 0.86] or when all songs from the playback experiment were included, i.e., for both spontaneous and playback triggered songs [Linear model for low frequency: R2 = −0.01, F(1, 53) = 0.05, N = 20, P = 0.81; Linear model for frequency bandwidth: R2 = −0.01, F(1, 53) = 0.36, N = 20, P = 0.54].
Discussion
We performed a playback exposure experiment to test whether bananaquits responded differently to elaborate vs. simple songs. We found the following answers to our questions: (1) playback of simpler songs did not trigger stronger (or weaker) behavioral responses than playback of more elaborate songs; (2) individuals did not match song elaboration to the stimulus categories, and even decreased syllable numbers in their song in response to more elaborate songs; however (3) songs triggered by elaborate song playback had a lower minimum frequency and wider frequency range compared to songs sung before the playback. The frequency bandwidth of songs sung after the elaborate song playback were also significantly wider than songs sung after simple song playback.
Song Elaboration Is Meaningful
Our current results reveal that noise-induced changes in song elaboration concern meaningful changes to territorial birds in neotropical bananaquits. Variation in responsiveness related to variation in song elaboration is in line with other studies in the literature. In simulated territorial intrusions, for example, dark-eyed juncos (Junco hyemalis) responded stronger to structurally more elaborate songs, spending longer periods closer to the playback speaker (Reichard et al., 2011). In chaffinches (Fringilla coelebs), both males and females responded stronger to more elaborate songs, i.e., signals with a higher number of different trill phrases (Leitão et al., 2006), suggesting this song parameter plays a role in both male-male competition and mate attraction. As we found an impact of song elaboration on response song variation and not on response strength, a signaling function of this song feature may be widespread but vary in content among species.
The impact of song elaboration on response song variation in our study on bananaquits concerned syllable number and spectral variation. We found no matching in elaboration, as more elaborate stimuli led to less elaborate response songs. We did not expect this, as less elaborate and more stereotypic songs can be associated with male-male interactions, while more elaborate and diverse songs can be more important for female choice (Hasselquist et al., 1996; Searcy and Beecher, 2009; Kagawa and Soma, 2013). However, we did find spectral matching in the minimum song frequency and in an increase in the frequency bandwidth when individuals responded to the elaborate playback. Similar changes in song frequency use have been found to be meaningful in other species in various ways. Frequency song matching, for example, can be an aggressive signal between rival birds during dispute (Searcy and Beecher, 2009) as reported for Kentucky warblers (Oporornis formosus, Morton and Young, 1986) and black-capped chickadees (Poecile atricapillus, Horn et al., 1992; Otter et al., 2002). Relative frequency variation among communicating birds (i.e., frequency mismatch) may be important, as shown for willow warblers (Phylloscopus trochilus, Linhart and Fuchs, 2015). Wider frequency bandwidths can also indicate higher aggressiveness, as white-crowned sparrows (Zonotrichia leucophrys nuttalli) respond less strongly to songs of restricted bandwidth (Luther et al., 2016; Phillips and Derryberry, 2018). Although we still have limited insight into the content of the message, we suggest that, according to the literature, the spectral variation and matching in bananaquit songs may also be meaningful.
The modified spectral response, in the absence of a strength in other behavioral responses, could also reflect that song elaboration plays a role in moderating territorial disputes (Slabbekoorn and ten Cate, 1996; Searcy and Nowicki, 2000; Otter et al., 2002). Graded variation in agonistic signals can convey increasing and decreasing levels of threats, before this becomes actually apparent in more overt changes in behavioral displays or approach tendencies (Searcy and Beecher, 2009). The fact that the order in which the stimuli were played influenced the escalation behavior of bananaquits in our study confirms such a possibility and warrants further exploration through playback experiments simulating dynamic changes in song elaboration (c.f. Hof and Podos, 2013).
Elaboration vs. Bandwidth as a Signal
There was an interesting discrepancy between the correlational analyses of the spectral and elaboration parameters in our previous (Winandy et al., 2021) and the current study. In the previous observational study, we found frequency bandwidth and minimum frequency to be determined by noise level, and a lower and narrower frequency range was correlated with less elaborate song. In the current experimental study, however, we found a change in bandwidth dependent on song elaboration, but song frequency range was not correlated with song elaboration. We believe that this discrepancy requires further exploration of the potential role for ambient noise in signaling bananaquits.
There are two contextual differences in the recording sets that could explain the inconsistency of the correlation: noise level during recordings and whether song was sung in response to playback. In the previous study, we recorded the birds in quiet and also in noisy conditions, while in the current study, we only recorded the birds in relatively quiet moments of the day. As we found the correlation among the song parameters only in the first study, in which noisy conditions were present, we believe that the traffic noise could be causally linked to the presence of that significant correlation. This is another indication that noisy conditions may play a role in song syllable use restriction through noise-dependent bandwidth availability. In the previous study we also only recorded spontaneous songs, while in the current study, we recorded both spontaneous and playback induced songs. However, in the current study, we found no correlation before nor after the playback. We therefore argue that motivational state is not a likely explanation for the lack of correlation between song elaboration and frequency bandwidth in the current study.
The Audibility-Signal Efficiency Trade-Off
The combination of results of the previous observational study (Winandy et al., 2021) and the current playback study allows a new perspective on the signal audibility/efficiency trade-off (Slabbekoorn and Ripmeester, 2008; Gross et al., 2010; Slabbekoorn, 2013). On the one hand, noise-dependent changes in frequency use may improve signal audibility as (1) avoiding low frequencies leaves a larger part of the song unaffected by masking low-frequency traffic noise (Nemeth and Brumm, 2010; Halfwerk et al., 2011); and (2) concentrating sound energy in a spectrally more narrow bandwidth will also improve the signal-to-noise ratio (Hanna et al., 2011). On the other hand, as song elaboration is meaningful to the birds themselves (current study), the correlation between frequency bandwidth and song elaboration under noisy conditions (previous study, Winandy et al., 2021) can be interpreted as evidence for a restriction on signal efficiency by noise-dependent song bandwidth contraction. When this signal audibility/efficiency trade-off is relaxed under relatively quiet conditions, the correlation between song frequency bandwidth and song elaboration apparently also fades.
Few studies have addressed the consequences of this trade-off between signal audibility and signal efficiency under noisy conditions. We here show for bananaquits that the noise-dependent variation in frequency use concerns biologically relevant signal variation, but for general conclusions the trade-off remains to be tested in more species. We especially need to gain insight into whether vocal changes that improve audibility actually yield any benefit to the signaler. We do know for example from a few earlier playback studies that spectral changes potentially driven by masking traffic noise affect response levels and are therefore proven to be biologically relevant (Mockford and Marshall, 2009; Ripmeester et al., 2010; Luther and Derryberry, 2012). However, we have only begun to discover the potential for reduced responsiveness to urban song features, as modified by anthropogenic noise conditions, in a mate choice context (Halfwerk et al., 2011; Huet des Aunay et al., 2014) as well as in a territorial context of male-male communication (Luther and Magnotti, 2014; LaZerte et al., 2017; Phillips and Derryberry, 2018).
Conclusion
In the present study, we showed that bananaquits recognize the variation in song elaboration as they respond with syllable adjustment and spectrally distinct songs to the variation in song elaboration in our playback stimuli. As song elaboration was shown in an earlier study to be restricted by noise-dependent song frequency bandwidth, the current results confirm that song adjustments could increase audibility through masking avoidance, but at the same time affect the signaling function. This provides another example of how the rise in anthropogenic noise levels in avian habitat may not only affect what birds sing, but also what they communicate. We still have little insight into fitness consequences of masking avoidance and changes of noise-induced adjustments in signaling content. We therefore believe that more studies are warranted into human impact on the ecology and evolution of singing birds in their acoustically altered environments due to noisy human activities worldwide.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the local ‘Committee of Ethics in the Use of Animals’, Federal University of Bahia-UFBA, Brazil (nTM36/2016).
Author Contributions
GW collected and analyzed the data and constructed the figures. All authors contributed equally to the conception and writing of the manuscript.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado da Bahia (Grant No. RED0045/2014 to HJ) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001 (Grant Nos. 1514172 and 88881.189177/2018-01 to GW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The content of this manuscript has been published as part of the thesis of the author GW (Winandy, 2019). We thank to the organization which provided funding for this study: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001, for the Ph.D. scholarship (n° 1514172) and internship grant (Programa de Doutorado Sanduíche no Exterior/Processo n° 88881.189177/2018-01) given to GW. We also thank to the Post-Graduate Program in Experimental Psychology, University of São Paulo (USP) and Núcleo de Etologia e Evolução (NuEVo), Federal University of Bahia (UFBA), for the structural and educational support. Finally, we thank all colleagues, specially the Ph.D. candidate Rilquer Mascarenhas, who helped and motivated us during field work, data collection, and processing.
References
Barber, J. R., Crooks, K. R., and Fristrup, K. M. (2010). The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189. doi: 10.1016/j.tree.2009.08.002
Barton, K. (2016). MuMIn: Multi-Model Inference. R package version 1.15.6. Available online at: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed November 10, 2018).
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). lme4: Linear Mixed-Effects Models Using Eigen and S4. R package version 1.1-9. Available online at: https://cran.r-project.org/web/packages/lme4/index.html (accessed November 10, 2018).
Bermúdez-Cuamatzin, E., Ríos-Chelén, A. A., Gil, D., and Macías Garcia, C. (2011). Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol. Lett. 7, 36–38. doi: 10.1098/rsbl.2010.0437
Botero, C. A., Boogert, N. J., Vehrencamp, S. L., and Lovette, I. J. (2009). Climatic patterns predict the elaboration of song displays in mockingbirds. Curr. Biol. 19, 1151–1155. doi: 10.1016/j.cub.2009.04.061
Brumm, H. (2004). The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440. doi: 10.1111/j.0021-8790.2004.00814.x
Brumm, H., and Slabbekoorn, H. (2005). Acoustic communication in noise. Adv. Study Behav. 35, 151–209.
Brumm, H., Zollinger, S. A., Niemelä, P. T., and Sprau, P. (2017). Measurement artefacts lead to false positives in the study of birdsong in noise. Methods Ecol. Evol. 8, 1617–1625. doi: 10.1111/2041-210x.12766
Buchanan, K. L., and Catchpole, C. K. (1997). Female choice in the sedge warbler Acrocephalus schoenobaenus multiple cues from song and territory quality. Proc. R. Soc. Lond. Ser. B Biol. Sci. 264, 521–526.
Buchanan, K. L., and Catchpole, C. K. (2000). Song as an indicator of male parental effort in the sedge warbler. Proc. R. Soc. Lond. Ser. B Biol. Sci. 267, 321–326. doi: 10.1098/rspb.2000.1003
Burnham, K. P., and Anderson, D. R. (2002). A Practical Information-Theoretic Approach: Model Selection and Multimodel Inference, 2nd Edn. New York, NY: Springer.
Buxton, R. T., McKenna, M. F., Mennitt, D., Fristrup, K., Crooks, K., Angeloni, L., et al. (2017). Noise pollution is pervasive in U.S. protected areas. Science 356, 531–533. doi: 10.1126/science.aah4783
Collins, S. (2004). “Vocal fighting and flirting: the functions of birdsong,” in Nature’s Music: the Science of Birdsong, eds P. Marler and H. Slabbekoorn (San Diego, CA: Academic Press), 33–79.
Derryberry, E. P., Gentry, K., Derryberry, G. E., Phillips, J. N., Danner, R. M., Danner, J. E., et al. (2017). White-crowned sparrow males show immediate flexibility in song amplitude but not in song minimum frequency in response to changes in noise levels in the field. Ecol. Evol. 7, 4991–5001. doi: 10.1002/ece3.3037
Fouda, L., Wingfield, J. E., Fandel, A. D., Garrod, A., Hodge, K. B., Rice, A. N., et al. (2018). Dolphins simplify their vocal calls in response to increased ambient noise. Biol. Lett. 14:20180484. doi: 10.1098/rsbl.2018.0484
Gentry, K. E., McKenna, M. F., and Luther, D. A. (2017). Evidence of suboscine song plasticity in response to traffic noise fluctuations and temporary road closures. Bioacoustics 27, 165–181. doi: 10.1080/09524622.2017.1303645
Gil, D., Honarmand, M., Pascual, J., Pérez-Mena, E., and Garcia, C. M. (2014). Birds living near airports advance their dawn chorus and reduce overlap with aircraft noise. Behav. Ecol. 26, 435–443. doi: 10.1093/beheco/aru207
Gross, K., Pasinelli, G., and Kunc, H. P. (2010). Behavioral plasticity allows short-term adjustment to a novel environment. Am. Nat. 176, 456–464. doi: 10.1086/655428
Halfwerk, W., Bot, S., Buikx, J., van der Velde, M., Komdeur, J., ten Cate, C., et al. (2011). Low-frequency songs lose their potency in noise urban conditions. Proc. Natl. Acad. Sci. U.S.A. 108, 14549–14554. doi: 10.1073/pnas.1109091108
Hanna, D., Blouin-Demers, G., Wilson, D. R., and Mennill, D. J. (2011). Anthropogenic noise affects song structure in red-winged blackbirds (Agelaius phoeniceus). J. Exp. Biol. 214, 3549–3556. doi: 10.1242/jeb.060194
Hasselquist, D., Bensch, S., and von Schantz, T. (1996). Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature 381, 229–232. doi: 10.1038/381229a0
Hilty, S., and Christie, D. A. (2018). “Bananaquit (Coereba flaveola),” in Handbook of the Birds of the World Alive, eds J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana (Barcelona: Lynx Edicions).
Hof, D., and Podos, J. (2013). Escalation of aggressive vocal signals: a sequential playback study. Proc. R. Soc. Lond. Ser. B Biol. Sci. 280:20131553. doi: 10.1098/rspb.2013.1553
Horn, A. G., Leonard, M. L., Ratcliffe, L., Shackleton, S. A., and Weisman, R. G. (1992). Frequency variation in songs of black-capped chickadees (Parus atricapillus). Auk 109, 847–852. doi: 10.2307/4088158
Huet des Aunay, G., Slabbekoorn, H., Nagle, L., Passas, F., Nicolas, P., and Draganoiu, T. I. (2014). Urban noise undermines female sexual preferences for low-frequency songs in domestic canaries. Anim. Behav. 87, 67–75. doi: 10.1016/j.anbehav.2013.10.010
Kagawa, H., and Soma, M. (2013). Song performance and elaboration as potential indicators of male quality in Java sparrows. Behav. Process. 99, 138–144. doi: 10.1016/j.beproc.2013.07.012
Kipper, S., Mundry, R., Sommer, C., Hultsch, H., and Todt, D. (2006). Song repertoire size is correlated with body measures and arrival date in common nightingales, Luscinia megarhynchos. Anim. Behav. 71, 211–217. doi: 10.1016/j.anbehav.2005.04.011
Langemann, U., Tavares, J. P., Peake, T. M., and McGregor, P. K. (2000). Response of great tits to escalating patterns of playback. Behaviour 137, 451–471. doi: 10.1163/156853900502178
Lattin, C., and Ritchison, G. (2009). Intra- and intersexual functions of singing by male blue grosbeaks: the role of within-song variation. Wilson J. Ornithol. 121, 714–721. doi: 10.1676/09-026.1
LaZerte, S. E., Slabbekoorn, H., and Otter, K. A. (2016). Learning to cope: vocal adjustment to urban noise is correlated with prior experience in black-capped chickadees. Proc. R. Soc. Lond. Ser. B Biol. Sci. 283:20161058. doi: 10.1098/rspb.2016.1058
LaZerte, S. E., Slabbekoorn, H., and Otter, K. A. (2017). Territorial black-capped chickadee males respond faster to high-than to low-frequency songs in experimentally elevated noise conditions. PeerJ 5:e3257. doi: 10.7717/peerj.3257
Leitão, A., Ten Cate, C., and Riebel, K. (2006). Within-song complexity in a songbird is meaningful to both male and female receivers. Anim. Behav. 71, 1289–1296. doi: 10.1016/j.anbehav.2005.08.008
Linhart, P., and Fuchs, R. (2015). Song pitch indicates body size and correlates with males’ response to playback in a songbird. Anim. Behav. 103, 91–98. doi: 10.1016/j.anbehav.2015.01.038
Linhart, P., Jaška, P., Petrusková, T., Petrusek, A., and Fuchs, R. (2013). Being angry, singing fast? Signalling of aggressive motivation by syllable rate in a songbird with slow song. Behav. Process. 100, 139–145. doi: 10.1016/j.beproc.2013.06.012
Linhart, P., Slabbekoorn, H., and Fuchs, R. (2012). The communicative significance of song frequency and song length in territorial chiffchaffs. Behav. Ecol. 23, 1338–1347. doi: 10.1093/beheco/ars127
Luther, D. A., and Derryberry, E. P. (2012). Birdsongs keep pace with city life: changes in song over time in an urban songbird affects communication. Anim. Behav. 83, 1059–1066. doi: 10.1016/j.anbehav.2012.01.034
Luther, D. A., and Magnotti, J. (2014). Can animals detect differences in vocalizations adjusted for anthropogenic noise? Anim. Behav. 92, 111–116. doi: 10.1016/j.anbehav.2014.03.033
Luther, D. A., Phillips, J., and Derryberry, E. P. (2016). Not so sexy in the city: urban birds adjust songs to noise but compromise vocal performance. Behav. Ecol. 27, 332–340. doi: 10.1093/beheco/arv162
Mennitt, D. J., Fristrup, K. M., and Nelson, L. (2015). A spatially explicit estimate of environmental noise exposure in the contiguous United States. J. Acoust. Soc. Am. 137, 2339–2340. doi: 10.1121/1.4920539
Mockford, E. J., and Marshall, R. C. (2009). Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. Lond. Ser. B Biol. Sci. 276, 2979–2985. doi: 10.1098/rspb.2009.0586
Montague, M. J., Danek-Gontard, M., and Kunc, H. P. (2012). Phenotypic plasticity affects the response of a sexually selected trait to anthropogenic noise. Behav. Ecol. 24, 343–348. doi: 10.1093/beheco/ars169
Morton, E. S., and Young, K. (1986). A previously undescribed method of song matching in a species with a single song ‘type’, the Kentucky warbler (Oporornis formosus). Ethology 73, 334–342. doi: 10.1111/j.1439-0310.1986.tb00813.x
Moseley, D. L., Phillips, J. N., Derryberry, E. P., and Luther, D. A. (2019). Evidence for differing trajectories of songs in urban and rural populations. Behav. Ecol. 30, 1734–1742. doi: 10.1093/beheco/arz142
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Nelson, D. A. (1988). Feature weighting in species song recognition by the field sparrow (Spizella pusilla). Behaviour 106, 158–182. doi: 10.1163/156853988x00142
Nemeth, E., and Brumm, H. (2010). Birds and anthropogenic noise: Are urban songs adaptive? Am. Nat. 176, 465–475. doi: 10.1086/656275
Otter, K. A., Ratcliffe, L., Njegovan, M., and Fotheringham, J. (2002). Importance of frequency and temporal song matching in black-capped chickadees: evidence from interactive playback. Ethology 108, 181–191. doi: 10.1046/j.1439-0310.2002.00764.x
Parris, K. M., and McCarthy, M. A. (2013). Predicting the Effect of Urban Noise on the Active Space of Avian Vocal Signals. Am. Nat. 182, 452–464. doi: 10.1086/671906
Phillips, J. N., and Derryberry, E. P. (2017). Equivalent effects of bandwidth and trill rate: support for a performance constraint as a competitive signal. Anim. Behav. 132, 209–215. doi: 10.1016/j.anbehav.2017.08.012
Phillips, J. N., and Derryberry, E. P. (2018). Urban sparrows respond to a sexually selected trait with increased aggression in noise. Sci. Rep. 8:7505.
Pohl, N. U., Leadbeater, E., Slabbekoorn, H., Klump, G. M., and Langemann, U. (2012). Great tits in urban noise benefit from high frequencies in song detection and discrimination. Anim. Behav. 83, 711–721. doi: 10.1016/j.anbehav.2011.12.019
Potvin, D. A., and Mulder, R. A. (2013). Immediate, independent adjustment of call pitch and amplitude in response to varying background noise by silvereyes (Zosterops lateralis). Behav. Ecol. 24, 1363–1368. doi: 10.1093/beheco/art075
Rabin, A. L., and Greene, C. M. (2002). Changes to acoustic communication systems in human-altered environments. J. Comp. Psychol. 116, 137–141. doi: 10.1037/0735-7036.116.2.137
Reichard, D. G., Rice, R. J., Vanderbilt, C. C., and Ketterson, E. D. (2011). Deciphering information encoded in birdsong: male songbirds with fertile mates respond most strongly to complex, low-amplitude songs used in courtship. Am. Nat. 178, 478–487. doi: 10.1086/661901
Riebel, K., Odom, K. J., Langmore, N. E., and Hall, M. L. (2019). New insights from female bird song: towards an integrated approach to studying male and female communication roles. Biol. Lett. 15:20190059. doi: 10.1098/rsbl.2019.0059
Ripmeester, E. A., De Vries, A. M., and Slabbekoorn, H. (2007). Do blackbirds signal motivation to fight with their song? Ethology 113, 1021–1028. doi: 10.1111/j.1439-0310.2007.01398.x
Ripmeester, E. A. P., Mulder, M., and Slabbekoorn, H. (2010). Habitat-dependent acoustic divergence affects playback response in urban and forest populations of the European blackbird. Behav. Ecol. 21, 876–883. doi: 10.1093/beheco/arq075
Searcy, W. A., and Anderson, M. (1986). Sexual selection and theevolution of song. Annu. Rev. Ecol. Syst. 17, 507–533.
Searcy, W. A., and Beecher, M. D. (2009). Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292. doi: 10.1016/j.anbehav.2009.08.011
Searcy, W. A., and Nowicki, S. (2000). The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Princeton, NJ: The Princeton University Press, 288.
Slabbekoorn, H. (2013). Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim. Behav. 85, 1089–1099. doi: 10.1016/j.anbehav.2013.01.021
Slabbekoorn, H., and Peet, M. (2003). Birds sing at a higher pitch in urban noise. Nature 424, 267–267. doi: 10.1038/424267a
Slabbekoorn, H., and Ripmeester, E. A. (2008). Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83. doi: 10.1111/j.1365-294x.2007.03487.x
Slabbekoorn, H., and ten Cate, C. (1996). Responses of collared doves to playback of coos. Behav. Process. 38, 169–174. doi: 10.1016/s0376-6357(96)00023-x
Slabbekoorn, H., and ten Cate, C. (1998). Perceptual tuning to frequency characteristics of territorial signals in collared doves. Anim. Behav. 56, 847–857. doi: 10.1006/anbe.1998.0887
Verzijden, M. N., Ripmeester, E. A. P., Ohms, V. R., Snelderwaard, P., and Slabbekoorn, H. (2010). Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J. Exp. Biol. 213, 2575–2581. doi: 10.1242/jeb.038299
Winandy, G. S. M. (2019). “Consequences of anthropogenic noise to song elaboration, signal value and reproductive success: field and captive studies in neotropical Bananaquits and Bengalese finches,” in Tese (Doutorado em Psicologia Experimental) – Instituto de Psicologia (São Paulo: Universidade de São Paulo), 96f.
Keywords: song elaboration, song complexity, signal quality, territoriality, syllable repertoire
Citation: Winandy GSM, Japyassú HF, Izar P and Slabbekoorn H (2021) Noise-Related Song Variation Affects Communication: Bananaquits Adjust Vocally to Playback of Elaborate or Simple Songs. Front. Ecol. Evol. 8:570431. doi: 10.3389/fevo.2020.570431
Received: 07 June 2020; Accepted: 09 October 2020;
Published: 26 April 2021.
Edited by:
David Andrew Luther, George Mason University, United StatesReviewed by:
Pierre J. Deviche, Arizona State University, United StatesDominique Potvin, University of the Sunshine Coast, Australia
Jennifer N. Phillips, California Polytechnic State University, United States
Copyright © 2021 Winandy, Japyassú, Izar and Slabbekoorn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabrielle S. M. Winandy, Z2FicmllbGxlLndpbmFuZHlAZ21haWwuY29t
 Gabrielle S. M. Winandy
Gabrielle S. M. Winandy Hilton F. Japyassú2,3
Hilton F. Japyassú2,3 Hans Slabbekoorn
Hans Slabbekoorn