- Ministry of Education Key Laboratory of Ecology and Resource Use of the Mongolian Plateau & Inner Mongolia Key Laboratory of Grassland Ecology, School of Ecology and Environment, Inner Mongolia University, Hohhot, China
Examination of the mechanisms of the plant community assembly at a geographical scale is an interesting topic in ecology and biogeography, which are of great significance for the understanding of species coexistence and biodiversity conservation. But so far, only a few studies have simultaneously assessed the relative roles of multiple-scale factors in shaping the phylogenetic and functional structure of plant communities at a macroecological scale. In this study, we linked modern climate, glacial-interglacial climate change, and soil properties with the phylogenetic and functional structure of shrub and herbaceous plant communities in Inner Mongolia, China, an arid and semi-arid region. Our results showed that the functional structure of plant communities was more associated with modern climate and soil properties than the phylogenetic structure, especially for the soil properties. Modern precipitation was found in all the combinations of variables that were most closely related to the community structure in this arid and semi-arid region. These findings suggest that the phylogenetic and functional structure of biotic communities may be affected by processes at divergent spatial-temporal scales. That is, the functional structure is better linked with the modern and local factors while the phylogenetic structure is more associated with the historical and regional processes. This study highlights the importance of the associations between the different biodiversity dimensions and divergent drivers.
Introduction
How plant communities are assembled at a geographical scale is an important topic in ecology and biogeography because it could provide insight into the knowledge of species coexistence and biodiversity conservation (Webb et al., 2002; Freilich and Connolly, 2015; Daniel et al., 2019). Community assembly is codetermined by factors at divergent spatiotemporal scales (Feng et al., 2014; Blonder et al., 2018; Kubota et al., 2018). Specifically, historical processes, such as geological events and paleoclimate change, could affect the biodiversity and community structure through their effects on species speciation, migration, and extinction (Svenning et al., 2015). Modern factors, such as modern climate and local habitat filtering, could also assemble local communities by limiting the species ranges, affecting the water availability, and providing niche diversity (Currie et al., 2004; Feng et al., 2014; Stein et al., 2014). Therefore, it is important to explore how these multiple-scale factors simultaneously contributed in assembling the local communities (Fine, 2015; Pärtel et al., 2016).
Stable paleoclimate could promote high species richness by both accelerating speciation and avoiding the extinction of relict species, resulting in a relatively over-dispersed phylogenetic structure, i.e., species are more distantly related (Feng et al., 2014, 2017; Kubota et al., 2018). In contrast, the cooling and drying during glacial periods may promote a relatively clustered phylogenetic structure by filtering on the phylogenetically conserved cold tolerance (Eiserhardt et al., 2015). For example, the phylogenetic structure of forest tree communities in China and the globe is more over-dispersed in regions with stable glacial-interglacial climate (Feng et al., 2014; Kubota et al., 2018). Except for the phylogenetic structure, paleoclimate change may also affect the functional structure of local communities by filtering the regional species pools based on climate-related traits (Ordonez and Svenning, 2015; Blonder et al., 2018). For example, the functional diversity deficits of plant assemblages in Europe are positively associated with the glacial-interglacial climate instability (Ordonez and Svenning, 2015).
Modern climate, including both temperature and precipitation, has also been widely linked with biodiversity and community structure at various spatial scales and regions, providing support for many climate–based hypotheses (Currie et al., 2004; Qian et al., 2015; Feng et al., 2019). For example, the water-energy dynamics hypothesis suggests that the geographic distribution of species was codetermined by water and energy (O’Brien, 1998; Field et al., 2005). The wet and warm tropics could also promote speciation and prevent extinction by supplying enough productivity, great ecological specialization, and diverse biotic interactions, resulting in high levels of taxonomic, phylogenetic, and functional diversity (Currie et al., 2004; Ordonez and Svenning, 2015; Qian et al., 2015). Besides these climate factors, other local environmental factors also play important roles in assembling local plant communities (Stock and Verboom, 2012; Zhou et al., 2019). For example, the dominance of low-nutrient adapted plant lineages in Western Australia and South African Cape is mainly driven by the filtering of low soil fertility (Stock and Verboom, 2012). Both the phylogenetic and functional structure of plant communities in Mount Kenya vary along the elevational gradient (Zhou et al., 2019).
Although previous studies have found that both the phylogenetic and functional structure of local communities could be affected by these different spatiotemporal factors, it is also suggested that the phylogenetic structure should be mainly driven by the historical and regional processes, while the functional structure is more associated with contemporary and local factors (Feng et al., 2014; Li et al., 2019). The explanation is that phylogenetic diversity reflects the evolutionary relationships among species, which is mainly linked with the biogeographic history; while functional diversity refers to the variation in species ecological traits, which is more plastic and mainly constrained by recent and local influence (Swenson, 2013; Feng et al., 2014; Li et al., 2019). For example, the phylogenetic structure of Chinese forest tree communities is strongly associated with the glacial-interglacial climate change, while the functional structure is significantly correlated with local disturbance (Feng et al., 2014).
The shrubland, which is inadequate studied compared with grassland, covers a wide climate gradient across Inner Mongolia, from the humid open mountain areas in the east to the dry desert regions in the west (Miao et al., 2018; Guo et al., 2019). Although water availability and soil nutrient availability are the most important limitations to the herbaceous plants in this region (Bai et al., 2008; Ma et al., 2010; Guo et al., 2019), woody plants (including semi-shrubs and shrubs) may show divergent patterns with water availability because of their different strategies to environmental constraints (Moro et al., 2015; Šímová et al., 2018; Massante et al., 2019). Moreover, although the current plant growth and ecological processes are severely limited by water and nutrient availability, these regions have experienced dramatic climatic fluctuations with alternating dry and wet conditions during the Late Quaternary, which may have significant legacy on the current plant diversity and community structure (Yang et al., 2011; Tian et al., 2017). However, so far, few studies have simultaneously linked these multiple-scale factors with the phylogenetic and functional structure of both herbaceous and woody plant communities in this arid and semi-arid region. In this study, we conducted field investigations including 114 shrubland sites along the large environmental gradients in this region, and aimed to test: (1) is the phylogenetic and functional structure of plant communities shaped by divergent factors?, (2) is precipitation the dominant factor for plant community structure in this arid and semi-arid region?, and (3) do shrub and herbaceous plant communities show divergent patterns?
Materials and Methods
Study Area and Plant Investigation
We conducted surveys of the shrub communities in the Inner Mongolia Autonomous Region between June and August of 2015–2017 (Figure 1). The Inner Mongolia Autonomous Region is located in the arid and semi-arid areas of northern China. The mean annual precipitation (MAP) of study region ranges from 31 to 534 mm (from west to east) and the mean annual temperature ranges from -3.9 to 12.6°C (from east to west) (Wu et al., 2015; Miao et al., 2018).
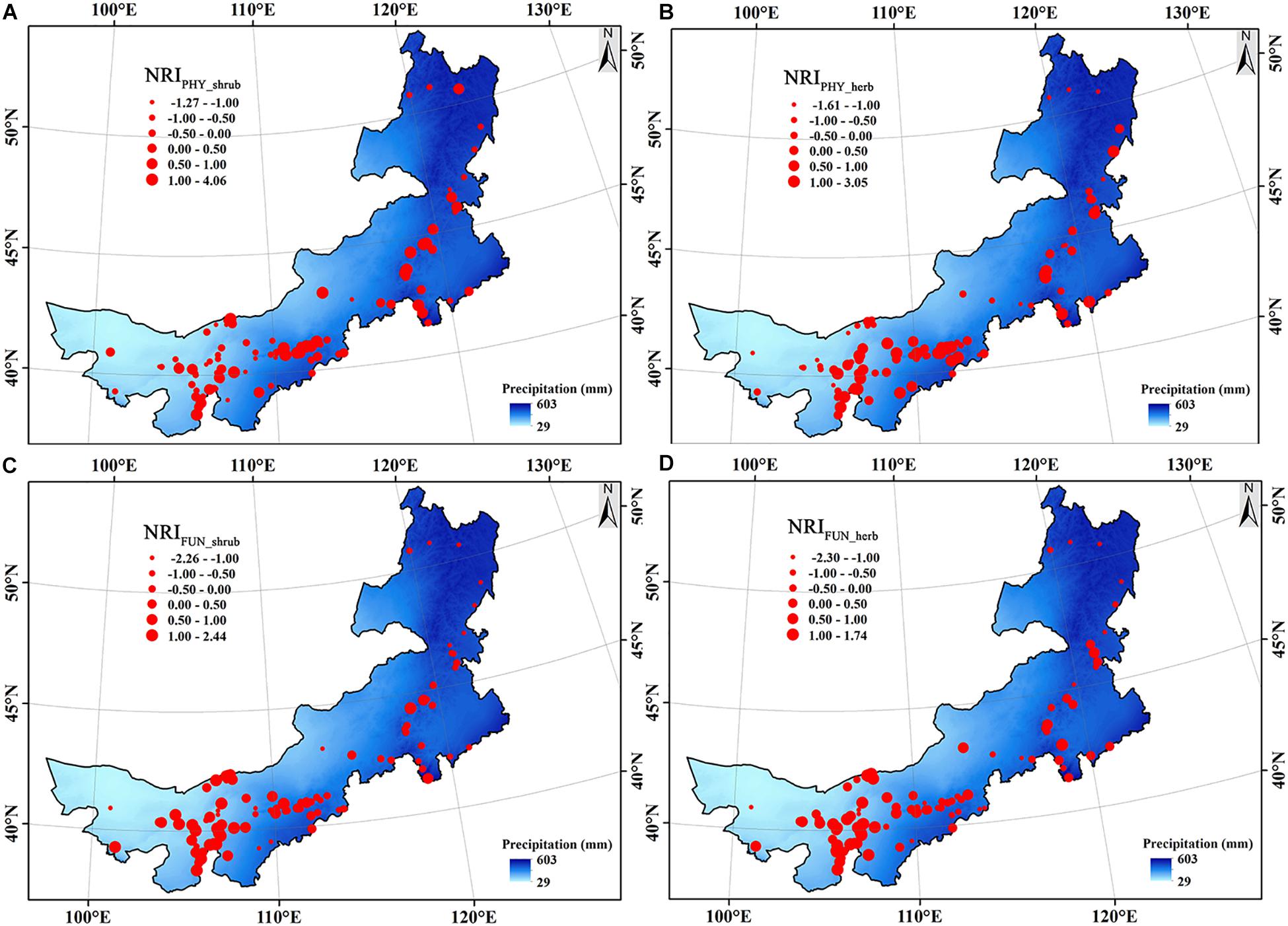
Figure 1. The spatial distribution of the phylogenetic (A,B) and functional structure index (C,D) of each site along the modern precipitation gradient. All NRI values have been standardized in this figure. (A) the phylogenetic structure of shrubs (NRIPHY_shrub), (B) the phylogenetic structure of herbs (NRIPHY_herb), (C) the functional structure of shrubs (NRIFUN_shrub), and (D) the functional structure of herbs (NRIFUN_herb).
One hundred fourteen (114) shrubland sites were investigated along a large geographic range (37°24′–53°23′N latitude, 97°12′–126°04′E longitude). At each site, we investigated shrub species in three plots of 5 m × 5 m and herbaceous species in three sub-plots (1 m × 1 m) at the diagonal of each plot. The distances between each plot within one site were 5–10 m (5 m in the mountain shrublands and 10 m in the desert sites due to the sparse distribution of shrubs). At each plot, we recorded all the species and measured the maximum height (Hmax, the distance between the upper boundary of the photosynthetic tissues on a plant and the ground) of each shrub individuals and mean Hmax for each herb species. Hmax, as a comprehensive and important trait, can reflect the ability of species to adapt to the environment, such as light competition and carbon storage capacity. And, it is also closely related to other functional traits such as leaf life, seed size, etc. (Moles et al., 2009; Long et al., 2015; Olson et al., 2018). Four hundred seventy-six (476) species were recorded (all are angiosperms), including 385 herbaceous species and 91 shrub or semi-shrub species. We used the mean value of the maximum height of a species among all the sites as the trait value of the species.
Environmental Data
At each site, we used GPS to record the longitude and latitude. The mean annual temperature (MAT), MAP, and elevation (EL) were obtained from the WorldClim database (Hijmans et al., 2005). The mean of the Community Climate System Model version 3 (Collins et al., 2006) and Model for Interdisciplinary Research on Climate version 3.2 (Hasumi and Emori, 2004) were used to represent the values of the Last Glacial Maximum (LGM) MAT and LGM MAP. MAT anomaly and MAP anomaly (present-day values minus LGM values) are indicators of paleoclimate change (Sandel et al., 2011; Kissling et al., 2012).
Three soil samples at the upper 0–20 cm were sampled with a soil core along the diagonal of each plot and mixed together. Soil samples were transported to the laboratory and air dried, and then removed the roots and stones (>2 mm). After grounded to pass through a 100–mesh sieve, soil nitrogen content (SNC) was measured using an elemental analyzer (vario MACRO cube).
Phylogenetic and Functional Structure
The relatedness index (NRI) is a measure to estimate the pairwise phylogenetic distances between co-occurring species and reflects the degree of clustering or over-dispersion of species in a community (Webb, 2000). The positive value of NRI indicates the clustering of community structure (species are more closely related/similar), while the negative value indicates over-dispersion (species are more distantly related/dissimilar).
The formula is:
where MPDobs is the observed mean phylogenetic distance (MPD) of a site, meanMPDrnd is the mean MPD of the 999 null models (null model = “taxa.labels”), and sdMPDrnd is the standard deviation of MPD of the 999 null models.
NRI index was used to calculate both the phylogenetic structure and functional structure. For the phylogenetic structure, a phylogenetic tree including 2,882 species recorded in the Key to the vascular plants of Inner Mongolia (Zhao and Zhao, 2014) was constructed in virtue of the mega-tree (Jin and Qian, 2019). To calculate the functional structure, the Hmax of the 385 herbaceous species and 91 shrub and semi-shrub species were used to build the herbaceous and shrub functional dendrograms, respectively. Euclidian distance was used to calculate the distance matrix for all species and the “complete linkage” was used for the cluster analyses. The phylogenetic tree and the functional dendrograms were then used for the following phylogenetic and functional NRI calculations for the species in each site.
Data Analyses
To unify the dimensions of all the independent and dependent variables, they were firstly standardized (mean = 0 and standard deviation = 1). The ordinary least squares models (OLS) were applied to fit the relationships between each structure index and explanatory variable.
To assess which combination of variables was most associated with each community structure, we also performed analyses with the Random Forest (RF) modeling, which could deal with the multiple correlation relationship and complex interaction between the independent variables (Cutler et al., 2007). We set up models, respectively, for all possible combinations of the six independent variables (63 combinations in total). For each model, we randomly split the data into 50% training and 50% evaluation data 1,000 times to avoid over-fitting the model. Six combinations of the variables with the highest explanatory power, which were indicated by the highest correlations between the environmental variables and phylogenetic or functional structure indices, were chosen in all the models.
The statistical analyses were performed in R 3.5.3 using the packages vegan (Oksanen et al., 2019), picante (Kembel et al., 2010), randomForest (Liaw and Wiener, 2002).
Results
Compared with the phylogenetic structure, the functional structure of both shrub and herbaceous communities showed clear patterns, i.e., functional clustering increased with decreasing precipitation from northeast to southwest (Figure 1). The ordinary least squares models showed that the MAP always occurred in the three variables most associated with the phylogenetic and functional structure of both shrub and herbaceous communities (Figure 2 and Supplementary Table S1). In addition, the MAP was relatively weakly and positively associated with the phylogenetic structure of shrub, while relatively strongly and negatively associated with the functional structure, indicating that the regions with more precipitation tend to have a clustered phylogenetic structure but over-dispersed functional structure (Figure 2). Regions with a large precipitation anomaly also tend to have a clustered phylogenetic structure but over-dispersed functional structure (Figure 2). The soil nitrogen content was only negatively correlated with the functional structure, indicating an increasing over-dispersed functional structure with increasing soil nutrient availability (Figure 2 and Supplementary Table S1).
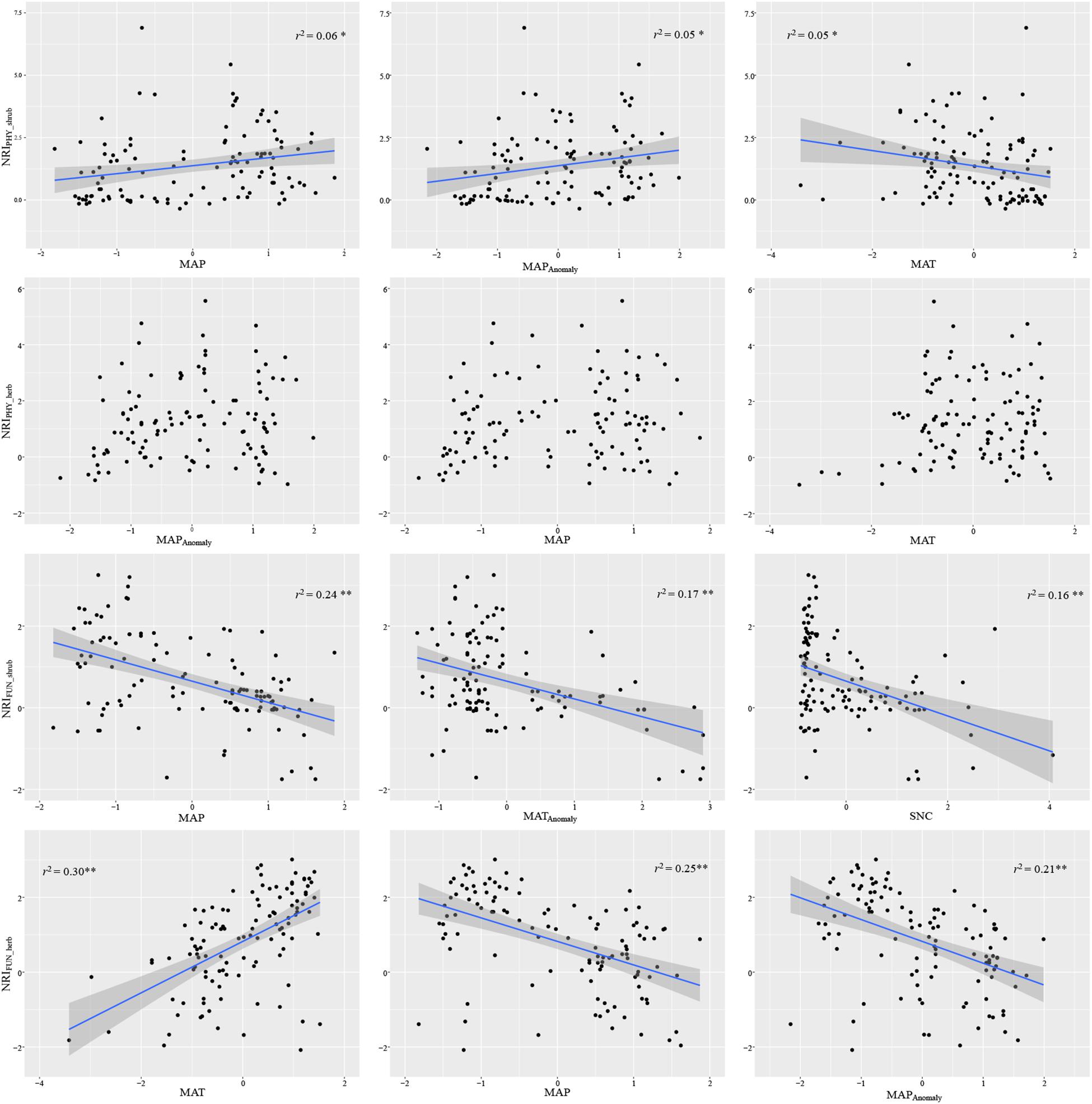
Figure 2. Scatter plots of the three most associated variables against the different structure indices. NRIPHY_shrub, phylogenetic shructure of shrub; NRIPHY_herb, phylogenetic structure of herb; NRIFUN_shrub, functional structure of shrub; NRIFUN_herb, functional structure of herb. MAT, mean annual temperature; MAP, mean annual precipitation; SNC, soil nitrogen content; MATAnomaly, MAT anomaly; MAPAnomaly, MAP anomaly.
The Random Forest modeling results showed that the functional structure of both shrub and herbaceous communities were better associated with explanatory variables than phylogenetic structure, especially for the soil nitrogen content (Figure 3). The soil nitrogen content, precipitation, and anomaly in precipitation were, again, the three variables that always occurred in the combinations of variables most associated with the phylogenetic and functional structure of plant communities (Figure 3).
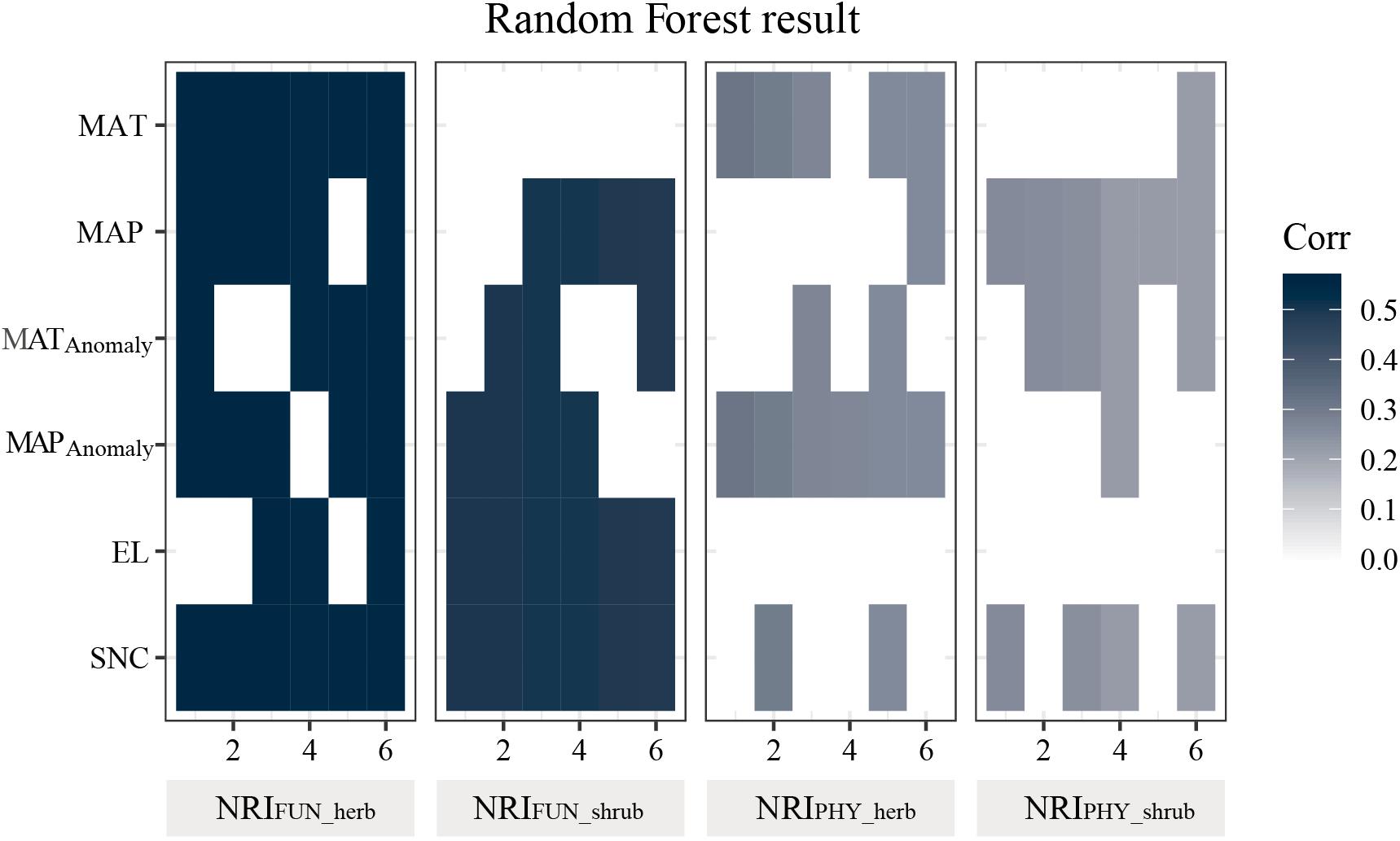
Figure 3. Six combinations of the variables with the highest explanatory power for the community structure in the Random Forest modeling. Each row represents a variable. Blue plaque indicates the environmental factors that existed in the combinations. NRIFUN_herb, functional structure of herb; NRIFUN_shrub, functional structure of shrub; NRIPHY_herb, phylogenetic structure of herb; NRIPHY_shrub, phylogenetic structure of shrub. EL, elevation; MAT, mean annual temperature; MATAnomaly means MAT anomaly; MAP, mean annual precipitation; MAPAnomaly, MAP anomaly; SNC, soil nitrogen content.
Discussion
By linking the multiple-scale drivers with the plant community structure in this arid and semi-arid region, our results showed that modern precipitation was the main factor affecting the phylogenetic and functional structure of both shrub and herbaceous communities. Soil nitrogen content mainly affects the functional structure of the shrub and herbaceous communities. Notably, compared with the phylogenetic structure, the functional structure was more associated with these drivers.
Modern Climate and Community Structure
As a major limiting factor in this arid and semi-arid region, modern precipitation was found to be the main driver of the phylogenetic and functional structure of both shrub and herbaceous communities. While previous studies showed that regions with high precipitation have an over-dispersed phylogenetic structure, e.g., vascular plant communities in Qinghai-Tibetan Plateau (Yan et al., 2013) and tree communities at global scale (Kubota et al., 2018), our results indicated that the sites with more precipitation tended to have a clustered phylogenetic structure, which is consistent with a study on woody plant communities distributed at a global scale (Massante et al., 2019). This discrepancy may be explained by the special relation between the temperature and precipitation in this region, i.e., the northeastern Inner Mongolia has a high precipitation but low temperature while the southwestern Inner Mongolia has a low precipitation but high temperature. This special climate distribution pattern makes it possible for harsh environments, featuring drought or cold, to become filters of the convergent phylogenetic structure (Kubota et al., 2018). The clustered phylogenetic structure of shrub communities with a lower temperature was consistent with previous studies (Yan et al., 2013; Feng et al., 2014). Notably, a recent study in the same region also found a weak relation between the phylogenetic structure of shrub communities and modern climate, suggesting a weak role of environmental filtering on the phylogenetic structure of plant communities in this region (Dong et al., 2019).
In contrast to the weak relations between the modern climate and phylogenetic structure, our results showed stronger and negative relations between the modern precipitation and functional structure, indicating that sites with good water conditions tended to have an over-dispersed functional structure. At the larger regional scale of China, the plant height also increases with modern temperature and precipitation (Mao et al., 2020). High precipitation in this arid and semi-arid region means high productivity, more resources, high soil nitrogen content, and soil moisture, which would promote the coexistence of more plant species and high functional diversity in height through an increased facilitation and competition for resources, such as light, soil moisture, and soil nutrition (Katabuchi et al., 2012; Spasojevic and Suding, 2012). Consistent with our findings, a study of alpine tundra in the Colorado Rocky Mountains also finds a positive relation between the functional diversity and resource availability (Spasojevic and Suding, 2012). In contrast, the increasing clustered functional structure with a less precipitation indicated a strong effect of environmental filtering on the functional structure in southwestern Inner Mongolia.
Soil Nitrogen Content and Community Structure
Being an important local driver of community structure, the soil nitrogen content at the site level was found to be only associated with the functional structure of shrub and herbaceous communities, and had no associations with the phylogenetic structure. This finding supports the idea that the functional structure should be better linked with local and contemporary drivers because the functional traits are more plastic compared with the phylogenetic relations, which are mainly shaped by historical and regional processes (Feng et al., 2014; Li et al., 2019). A recent study about shrub communities in this region also suggests that compared with the leaf width and leaf length, the height shows a low phylogenetic signal and high plasticity (Zheng et al., 2019).
The increasing over-dispersed functional structure in height with a high soil nitrogen content again suggests that high soil nutrient may promote a high functional diversity in height through the increased facilitation and competition for soil moisture and nutrition, which is essential for plants (Katabuchi et al., 2012; Spasojevic and Suding, 2012). Nitrogen and water promote the growth in twig size and number of dominant shrubs (She et al., 2016). The dominant species may occupy a wider ecological niche which results in an enhanced inter-species competition. In contrast, the increasing functional clustering with less soil nitrogen content indicates that low soil fertility may be a strong environmental filter and result in a clustered functional structure (Spasojevic and Suding, 2012; Miatto and Batalha, 2018). Our findings are consistent with previous studies on the prairie and seasonal forests in Brazil, Western Australia, and South Africa, where low soil factors limit the divergence of traits (Stock and Verboom, 2012; Miatto and Batalha, 2018). The direct effect of water and nitrogen on the functional structure may explain the high explanatory of functional structure than phylogenetic structure in our study.
Glacial-Interglacial Climate Change and Community Structure
Climate turbulence during the past glacial-interglacial periods could affect biodiversity, community assembly, and ecosystem functioning through their effects on speciation, extinction, and migration (Svenning et al., 2015; Blonder et al., 2018). Previous studies have found that regions with a large glacial-interglacial climate instability would harbor biotic assemblages with clustered phylogenetic and functional structure because of the high extinction rate and lagged immigration (Feng et al., 2014; Ordonez and Svenning, 2017; Kubota et al., 2018). Consistent with these studies, our results indicated a trend of an increasing phylogenetic clustering with a large anomaly in precipitation. The significant relations between the anomaly in precipitation and functional structure may be resulted from the high correlation (0.80) between modern precipitation and anomaly in precipitation. Alternating dry and wet climate fluctuations may also mask the process of community assembly driven by climate anomaly.
Conclusion
Being the first study simultaneously linking multiple-scale drivers with the phylogenetic and functional structure of both shrub and herbaceous communities in Inner Mongolia, China, we found that modern precipitation was the main driver of plant community structure in this arid and semiarid region. In addition, soil nutrient was only significantly associated with the plant functional structure. Notably, the plant functional structure was better explained by these drivers than the plant phylogenetic structure. Our findings highlight the importance of considering the multiple-scale drivers for the divergent dimensions of biodiversity.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
GF and WM designed the research. CS, MW, XL, CL, LZ, XZ, HM, and WM performed the field investigation. YS and GF analyzed the data. YS, GF, and WM wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Special Project of Basic Work of Science and Technology (2015FY110300) and the Inner Mongolia Autonomous Science and Technology Plan Project (No. 2019GG009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the field investigators and Dr. Bailing Miao for his assistance in drawing the graph of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.531947/full#supplementary-material
References
Bai, Y., Wu, J., Xing, Q., Pan, Q., Huang, J., Yang, D., et al. (2008). Primary production and rain use efficiency across a precipitation gradient on the Mongolia Plateau. Ecology 89, 2140–2153. doi: 10.1890/07-0992.1
Blonder, B., Enquist, B. J., Graae, B. J., Kattge, J., Maitner, B. S., Morueta-Holme, N., et al. (2018). Late quaternary climate legacies in contemporary plant functional composition. Glob. Chang. Biol. 24, 4827–4840. doi: 10.1111/gcb.14375
Collins, W. D., Bitz, C. M., Blackmon, M. L., Bonan, G. B., Bretherton, C. S., Carton, J. A., et al. (2006). The community climate system model version 3 (CCSM3). J. Clim. 19, 2122–2143. doi: 10.1175/JCLI3761.1
Currie, D. J., Mittelbach, G. G., Cornell, H. V., Field, R., Guegan, J.-F., Hawkins, B. A., et al. (2004). Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. doi: 10.1111/j.1461-0248.2004.00671.x
Cutler, D. R., Edwards, T. C., Beard, K. H., Cutler, A., Hess, K. T., Gibson, J., et al. (2007). Random forests for classification in ecology. Ecology 88, 2783–2792. doi: 10.1890/07-0539.1
Daniel, J., Gleason, J. E., Cottenie, K., and Rooney, R. C. (2019). Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos 128, 1158–1169. doi: 10.1111/oik.05987
Dong, L., Liang, C., Li, F. Y., Zhao, L., Ma, W., Wang, L., et al. (2019). Community phylogenetic structure of grasslands and its relationship with environmental factors on the Mongolian Plateau. J. Arid Land 11, 595–607. doi: 10.1007/s40333-019-0122-6
Eiserhardt, W. L., Borchsenius, F., Plum, C. M., Ordonez, A., and Svenning, J.-C. (2015). Climate-driven extinctions shape the phylogenetic structure of temperate tree floras. Ecol. Lett. 18, 263–272. doi: 10.1111/ele.12409
Feng, G., Ma, Z., Benito, B. M., Normand, S., Ordonez, A., Jin, Y., et al. (2017). Phylogenetic age differences in tree assemblages across the Northern Hemisphere increase with long-term climate stability in unstable regions. Global. Ecol. Biogeogr. 26, 1035–1042. doi: 10.1111/geb.12613
Feng, G., Mi, X. C., Bøcher, P. K., Mao, L. F., Sandel, B., Cao, M., et al. (2014). Relative roles of local disturbance, current climate and paleoclimate in determining phylogenetic and functional diversity in Chinese forests. Biogeosciences 11, 1361–1370. doi: 10.5194/bg-11-1361-2014
Feng, G., Yan, H., and Yang, X. (2019). Climate and food diversity as drivers of mammal diversity in Inner Mongolia. Ecol. Evol. 9, 2142–2148. doi: 10.1002/ece3.4908
Field, R., O’Brien, E. M., and Whittaker, R. J. (2005). Global models for predicting woody plant richness from climate: development and evaluation. Ecology 86, 2263–2277. doi: 10.1890/04-1910
Fine, P. V. A. (2015). Ecological and evolutionary drivers of geographic variation in species Diversity. Annu. Rev. Ecol. Evol. Syst. 46, 369–392. doi: 10.1146/annurev-ecolsys-112414-054102
Freilich, M. A., and Connolly, S. R. (2015). Phylogenetic community structure when competition and environmental filtering determine abundances: assessing phylogenetic community structure. Global. Ecol. Biogeogr. 24, 1390–1400. doi: 10.1111/geb.12367
Guo, Y., Schöb, C., Ma, W., Mohammat, A., Liu, H., Yu, S., et al. (2019). Increasing water availability and facilitation weaken biodiversity-biomass relationships in shrublands. Ecology 100:e02624. doi: 10.1002/ecy.2624
Hasumi, H., and Emori, S. (2004). K-1 coupled model (MIROC) description, K-1 Tech. Rep.1, 34 pp., Cent. for Clim. Syst. Res., Univ. of Tokyo, Tokyo.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., and Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. doi: 10.1002/joc.1276
Jin, Y., and Qian, H. (2019). V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359. doi: 10.1111/ecog.04434
Katabuchi, M., Kurokawa, H., Davies, S. J., Tan, S., and Nakashizuka, T. (2012). Soil resource availability shapes community trait structure in a species-rich dipterocarp forest: soil resources and community structure. J. Ecol. 100, 643–651. doi: 10.1111/j.1365-2745.2011.01937.x
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kissling, W. D., Eiserhardt, W. L., Baker, W. J., Borchsenius, F., Couvreur, T. L. P., Balslev, H., et al. (2012). Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proc. Natl. Acad. Sci. U.S.A. 109, 7379–7384. doi: 10.1073/pnas.1120467109
Kubota, Y., Kusumoto, B., Shiono, T., and Ulrich, W. (2018). Environmental filters shaping angiosperm tree assembly along climatic and geographic gradients. J. Veg. Sci. 29, 607–618. doi: 10.1111/jvs.12648
Li, Z., Jiang, X., Wang, J., Meng, X., Heino, J., and Xie, Z. (2019). Multiple facets of stream macroinvertebrate alpha diversity are driven by different ecological factors across an extensive altitudinal gradient. Ecol. Evol. 9, 1306–1322. doi: 10.1002/ece3.4841
Long, W., Schamp, B. S., Zang, R., Ding, Y., Huang, Y., and Xiang, Y. (2015). Community assembly in a tropical cloud forest related to specific leaf area and maximum species height. J. Veg. Sci. 26, 513–523. doi: 10.1111/jvs.12256
Ma, W., He, J.-S., Yang, Y., Wang, X., Liang, C., Anwar, M., et al. (2010). Environmental factors covary with plant diversity-productivity relationships among Chinese grassland sites: diversity-productivity relationships in Chinese grassland. Global. Ecol. Biogeogr. 19, 233–243. doi: 10.1111/j.1466-8238.2009.00508.x
Mao, L., Swenson, N. G., Sui, X., Zhang, J., Chen, S., Li, J., et al. (2020). The geographic and climatic distribution of plant height diversity for 19,000 angiosperms in China. Biodivers. Conserv. 29, 487–502. doi: 10.1007/s10531-019-01895-5
Massante, J. C., Götzenberger, L., Takkis, K., Hallikma, T., Kaasik, A., Laanisto, L., et al. (2019). Contrasting latitudinal patterns in phylogenetic diversity between woody and herbaceous communities. Sci. Rep. 9:6443. doi: 10.1038/s41598-019-42827-1
Miao, B., Li, Z., Liang, C., Wang, L., Jia, C., Bao, F., et al. (2018). Temporal and spatial heterogeneity of drought impact on vegetation growth on the Inner Mongolian Plateau. Rangel. J. 40, 113–128. doi: 10.1071/rj16097
Miatto, R. C., and Batalha, M. A. (2018). Are the cerrado and the seasonal forest woody floras assembled by different processes despite their spatial proximity? J. Plant Ecol. 11, 740–750. doi: 10.1093/jpe/rtx044
Moles, A. T., Warton, D. I., Warman, L., Swenson, N. G., Laffan, S. W., Zanne, A. E., et al. (2009). Global patterns in plant height. J. Ecol. 97, 923–932. doi: 10.1111/j.1365-2745.2009.01526.x
Moro, M. F., Silva, I. A., de Araújo, F. S., Nic Lughadha, E., Meagher, T. R., and Martins, F. R. (2015). The role of edaphic environment and climate in structuring phylogenetic pattern in seasonally dry tropical plant communities. PLoS One 10:e0119166. doi: 10.1371/journal.pone.0119166
O’Brien, E. (1998). Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr. 25, 379–398. doi: 10.1046/j.1365-2699.1998.252166.x
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2019). Vegan: Community Ecology Package. Available online at: https://CRAN.R-project.org/package=vegan
Olson, M. E., Soriano, D., Rosell, J. A., Anfodillo, T., Donoghue, M. J., Edwards, E. J., et al. (2018). Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. U.S.A. 115, 7551–7556. doi: 10.1073/pnas.1721728115
Ordonez, A., and Svenning, J.-C. (2015). Geographic patterns in functional diversity deficits are linked to glacial-interglacial climate stability and accessibility: glacial-interglacial legacies in functional diversity. Global. Ecol. Biogeogr. 24, 826–837. doi: 10.1111/geb.12324
Ordonez, A., and Svenning, J.-C. (2017). Consistent role of quaternary climate change in shaping current plant functional diversity patterns across European plant orders. Sci. Rep. 7:42988. doi: 10.1038/srep42988
Pärtel, M., Bennett, J. A., and Zobel, M. (2016). Macroecology of biodiversity: disentangling local and regional effects. New Phytol. 211, 404–410. doi: 10.1111/nph.13943
Qian, H., Wiens, J. J., Zhang, J., and Zhang, Y. (2015). Evolutionary and ecological causes of species richness patterns in North American angiosperm trees. Ecography 38, 241–250. doi: 10.1111/ecog.00952
Sandel, B., Arge, L., Dalsgaard, B., Davies, R. G., Gaston, K. J., Sutherland, W. J., et al. (2011). The influence of late quaternary climate-change velocity on species endemism. Science 334, 660–664. doi: 10.1126/science.1210173
She, W., Zhang, Y., Qin, S., Wu, B., and Bai, Y. (2016). Increased precipitation and nitrogen alter shrub architecture in a desert shrubland: implications for primary production. Front. Plant Sci. 7:1908. doi: 10.3389/fpls.2016.01908
Šímová, I., Violle, C., Svenning, J.-C., Kattge, J., Engemann, K., Sandel, B., et al. (2018). Spatial patterns and climate relationships of major plant traits in the New World differ between woody and herbaceous species. J. Biogeogr. 45, 895–916. doi: 10.1111/jbi.13171
Spasojevic, M. J., and Suding, K. N. (2012). Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes: functional diversity along gradients. J. Ecol. 100, 652–661. doi: 10.1111/j.1365-2745.2011.01945.x
Stein, A., Gerstner, K., and Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880. doi: 10.1111/ele.12277
Stock, W. D., and Verboom, G. A. (2012). Phylogenetic ecology of foliar N and P concentrations and N:P ratios across mediterranean-type ecosystems. Global. Ecol. Biogeogr. 21, 1147–1156. doi: 10.1111/j.1466-8238.2011.00752.x
Svenning, J.-C., Eiserhardt, W. L., Normand, S., Ordonez, A., and Sandel, B. (2015). The Influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Annu. Rev. Ecol. Evol. Syst. 46, 551–572. doi: 10.1146/annurev-ecolsys-112414-054314
Swenson, N. G. (2013). The assembly of tropical tree communities - the advances and shortcomings of phylogenetic and functional trait analyses. Ecography 36, 264–276. doi: 10.1111/j.1600-0587.2012.00121.x
Tian, F., Wang, Y., Chi, Z., Liu, J., Yang, H., Jiang, N., et al. (2017). Late quaternary vegetation and climate reconstruction based on pollen data from southeastern Inner Mongolia, China. Rev. Palaeobot. Palyno. 242, 33–42. doi: 10.1016/j.revpalbo.2017.03.003
Webb, C. O. (2000). Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Natur. 156, 145–155. doi: 10.1086/303378
Webb, C. O., Ackerly, D. D., McPeek, M. A., and Donoghue, M. J. (2002). Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448
Wu, J., Zhang, Q., Li, A., and Liang, C. (2015). Historical landscape dynamics of inner mongolia: patterns, drivers, and impacts. Landsc. Ecol. 30, 1579–1598. doi: 10.1007/s10980-015-0209-201
Yan, Y., Yang, X., and Tang, Z. (2013). Patterns of species diversity and phylogenetic structure of vascular plants on the Qinghai-Tibetan Plateau. Ecol. Evol. 3, 4584–4595. doi: 10.1002/ece3.847
Yang, X., Scuderi, L., Paillou, P., Liu, Z., Li, H., and Ren, X. (2011). Quaternary environmental changes in the drylands of China - A critical review. Q. Sci. Rev. 30, 3219–3233. doi: 10.1016/j.quascirev.2011.08.009
Zhao, Y., and Zhao, L. (2014). Key to the Vascular Plants of Inner Mongolia. Beijing: Science Press.
Zheng, Y., Dong, L., Li, Z., Zhang, J., Li, Z., Miao, B., et al. (2019). Phylogenetic structure and formation mechanism of shrub communities in arid and semiarid areas of the Mongolian Plateau. Ecol. Evol. 3:5787. doi: 10.1002/ece3.5787
Zhou, Y., Wang, S., Njogu, A. W., Ochola, A. C., Boru, B. H., Mwachala, G., et al. (2019). Spatial congruence or mismatch between phylogenetic and functional structure of seed plants along a tropical elevational gradient: different traits have different patterns. Front. Ecol. Evol. 7:100. doi: 10.3389/fevo.2019.00100
Keywords: arid and semiarid region, community assembly, functional structure, modern precipitation, phylogenetic structure, soil properties
Citation: Shi Y, Su C, Wang M, Liu X, Liang C, Zhao L, Zhang X, Minggagud H, Feng G and Ma W (2020) Modern Climate and Soil Properties Explain Functional Structure Better Than Phylogenetic Structure of Plant Communities in Northern China. Front. Ecol. Evol. 8:531947. doi: 10.3389/fevo.2020.531947
Received: 02 February 2020; Accepted: 26 August 2020;
Published: 22 September 2020.
Edited by:
Zehao Shen, Peking University, ChinaReviewed by:
Dima Chen, China Three Gorges University, ChinaLingfeng Mao, Nanjing Forestry University, China
Copyright © 2020 Shi, Su, Wang, Liu, Liang, Zhao, Zhang, Minggagud, Feng and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Feng, cWF1ZmVuZ2dhbmdAMTYzLmNvbQ==; Wenhong Ma, d2htYUBpbXUuZWR1LmNu
 Yabo Shi
Yabo Shi Chuang Su
Chuang Su Gang Feng
Gang Feng Wenhong Ma
Wenhong Ma