- 1Department of Fisheries and Wildlife, Oregon State University, Corvallis, OR, United States
- 2National Park Service, Alaska Region, Anchorage, AK, United States
- 3Ecofish Research Ltd., Victoria, BC, Canada
- 4School of Aquatic and Fishery Sciences, University of Washington, Seattle, WA, United States
- 5Alaska Department of Fish and Game, Division of Wildlife Conservation, Juneau, AK, United States
- 6Hakai Institute, Heriot Bay, BC, Canada
- 7Department of Geography, University of Victoria, Victoria, BC, Canada
- 8Raincoast Conservation Foundation, Sidney, BC, Canada
- 9Department of Fish and Wildlife Sciences, University of Idaho, Moscow, ID, United States
- 10Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, United States
- 11Oregon Department of Fish and Wildlife, Salem, OR, United States
- 12Department of Environmental Studies, University of California, Santa Cruz, Santa Cruz, CA, United States
Apex predators play keystone roles in ecosystems through top-down control, but the effects of apex omnivores on ecosystems could be more varied because changes in the resource base alter their densities and reverberate through ecosystems in complex ways. In coastal temperate ecosystems throughout much of the Northern Hemisphere, anadromous salmon once supported abundant bear populations, but both taxa have declined or been extirpated from large parts of their former ranges with limited research on the consequences of diminished or absent interactions among species. Here we review the biogeography of bear-salmon interactions and the role of salmon-subsidized bears in (1) resource provisioning to plants and scavengers through the distribution of salmon carcasses, (2) competition among bears and other large carnivores, (3) predation of ungulate neonates, (4) seed dispersal, and (5) resource subsidies to rodents with seed-filled scats. In addition to our review of the literature, we present original data to demonstrate two community-level patterns that are currently unexplained. First, deer densities appear to be consistently higher on islands with abundant brown bears than adjacent islands with black bears and wolves, and moose calf survival is higher at low bear densities (<∼25 bears per 100 km2) but is constant across the vast majority of bear densities found in the wild (i.e., ∼>25 bears per 100 km2). Our review and empirical data highlight key knowledge gaps and research opportunities to understand the complex ecosystem effects related to bear-salmon interactions.
Introduction
Thirty years ago E.O. Wilson famously postulated that little things – invertebrates – run the world (Wilson, 1987). John Terborgh countered that in fact big things such as large-bodied ungulates and top predators run the world (Terborgh, 1988). Although nature is rarely so black and white, the “big things run the world” paradigm has been well supported by a large body of literature on the direct and indirect effects of top predators, including trophic cascade and mesopredator release theory (Ritchie and Johnson, 2009; Levi and Wilmers, 2012). This paradigm focuses on relatively simple predator-herbivore-plant and apex predator-mesopredator-prey interactions. However, nature is more complex, and some of Earth’s most iconic predators are omnivores that forage across trophic levels from plants to large herbivores, with broad ecosystem effects.
Owing to the reticulate nature of omnivorous interactions in food webs, the effects of apex omnivores on ecosystems could be more varied and widespread than those of apex predators. While apex predator populations are limited by prey availability, apex omnivores can switch among a wider array resources. This broad trophic position can lead to highly asymmetric interactions. For example, in North America, brown and American black bears (Ursus arctos and U. americanus) are often the most important predators of ungulate neonates (Supplementary Table 1; Zager and Beecham, 2006) but ungulates comprise a minor energetic resource for bears, which can subsist on alternatives such as fruit, seed mast, invertebrates, and fishes. Thus, the dynamics of taxonomically and functionally distinct plants, fishes, invertebrates, and ungulates are linked by apex omnivores that serve as hubs of interaction effects.
One reason to expect apex omnivores to have particularly strong interaction effects is the unusual abundance that they can achieve. Although many species are limited by periods of food scarcity in seasonal environments, bears at higher latitudes avoid winter food limitation by storing energetic reserves when resources are plentiful (a process referred to as “hyperphagia”) and then entering torpor (often called “winter sleep” by bear biologists) when resources are scarce. In temperate ecosystems throughout much of the Northern Hemisphere, abundant adult anadromous fishes, especially Pacific salmon (Oncorhynchus spp.; and perhaps historically Atlantic salmon, Salmo salar) support dense bear populations. Bear populations that consume more salmon have larger litters, greater body mass, and occur at much higher population densities than do bears with less access to such meat. For example, brown bears range from 5 bears per 1000 km2 in interior systems where salmon are rare or absent to over 500 bears per 1000 km2 in coastal regions where salmon are abundant (Hilderbrand et al., 1999b). Although brown bear productivity is closely tied to salmon consumption at the population level, individual bears within a population have the physiological flexibility to achieve similar body conditions whether they consume salmon or not (Mangipane et al., 2018). In rare cases, when abiotic conditions produce abundant fruit crops when salmon are available, bears can shift their diet to berries (Deacy et al., 2017).
Far fewer studies have been conducted on salmon use and population-level effects by either American or Asiatic black bears (U. thibetanus). Island systems with abundant salmon but not brown bears can contain black bears at densities exceeding 1500 bears per 1000 km2 (Peacock et al., 2011) – an order of magnitude higher than inland system densities (Supplementary Table 1). When they overlap, brown bears tend to exclude black bears from higher quality food resources (Jacoby et al., 1999) including salmon (Belant et al., 2010; Levi et al., 2015; Adams et al., 2017).
Here we review and discuss the myriad influences of the salmon resource pulse, by supporting abundant bears, on community and ecosystem processes. Specifically, we consider how salmon-supported bears can affect (1) resource subsidies to plants and vertebrate and invertebrate scavengers via the distribution of salmon carcasses, (2) food web structure via competition with other large carnivores, (3) population dynamics of ungulates via predation on neonates, (4) seed dispersal of berry-producing shrubs, and (5) food availability to small mammals in the form of seed-filled bear scats. Our goal here is to review what is known about the community ecology of the bear-salmon interactions using empirical data and ecological theory, as well as highlight key gaps in knowledge to guide future research. In so doing, we address fundamental and broader questions in ecology, management, and conservation. We additionally highlight what ecosystems have lost with the decline of the keystone bear-salmon interaction and could potentially regain if restoration efforts are successful.
Biogeography of Bear-Salmon Interactions
Among the eight bear species extant worldwide, the brown bear, American black bear, and Asiatic black bear historically overlapped substantially with salmon (both Oncorhynchus spp. and Salmo spp.) and other anadromous fishes in northern Eurasia and North America (Quinn, 2018). Spawning Pacific salmon can occur as far inland as 3000 km upriver but historically were most dense along the coast, around the Pacific rim from central California to British Columbia and Alaska, Russia, and south along the Pacific Rim to Japan (Quinn, 2018). Spawning Atlantic salmon also occurred inland in northeastern North America and in Europe and western Asia (MacCrimmon and Gots, 1979). Salmon, especially semelparous Pacific salmon that die after spawning and tend to be more abundant than the iteroparous Atlantic salmon, likely provided a rich nutrient subsidy to bears across this range. Although both genera of salmon have declined substantially, the decline of Atlantic salmon and other diadromous fishes has been most severe, being extirpated or greatly reduced in much of their original ranges in Europe and North America (Limburg and Waldman, 2009).
Substantial bear-salmon associations continue in parts of Russia (Seryodkin et al., 2016, 2017), Japan (Matsubayashi et al., 2014), Alaska (Hilderbrand et al., 1999a; Levi et al., 2015; Quinn et al., 2017), and British Columbia (Mowat et al., 2013; Adams et al., 2017; Figure 1). However, historic abundance of salmon and former widespread ranges of bears (Figure 1; Gresh et al., 2000; Proctor et al., 2012) likely led to even greater abundances of salmon-subsidized bears (Hilderbrand et al., 1996). In Hokkaido, stable isotope analysis suggests that brown bears consume much less salmon, and terrestrial meat, than a century ago (Matsubayashi et al., 2015), which has resulted in a substantial reduction in the body size of bears (Matsubayashi et al., 2016). In western North America, overall salmon production has declined from an estimated 228 million-351 million fish to 142 million-287 million fish (Gresh et al., 2000). This decline has been disproportionately felt in California, Oregon, Washington, and Idaho, which now receives only 1–1.5% of the salmon spawners in North America (Gresh et al., 2000), and most of the salmon evolutionarily significant units are now listed as threatened or endangered under the Endangered Species Act (Figure 1C). Salmon runs have also substantially declined in western Canada from estimated pre-colonization levels of 44–96 million fish to roughly 25 million fish, but remain strong in Alaska (Gresh et al., 2000). Modest Atlantic salmon runs and bears still overlap in eastern Canada and parts of Europe, but the extent of historic or modern consumption of Atlantic salmon by brown or black bears has, surprisingly, not to our knowledge been studied. While still encompassing a huge area, particularly in the Northern Pacific Rim, the range of this keystone ecological interaction is much diminished from its historical distribution (Figure 1).
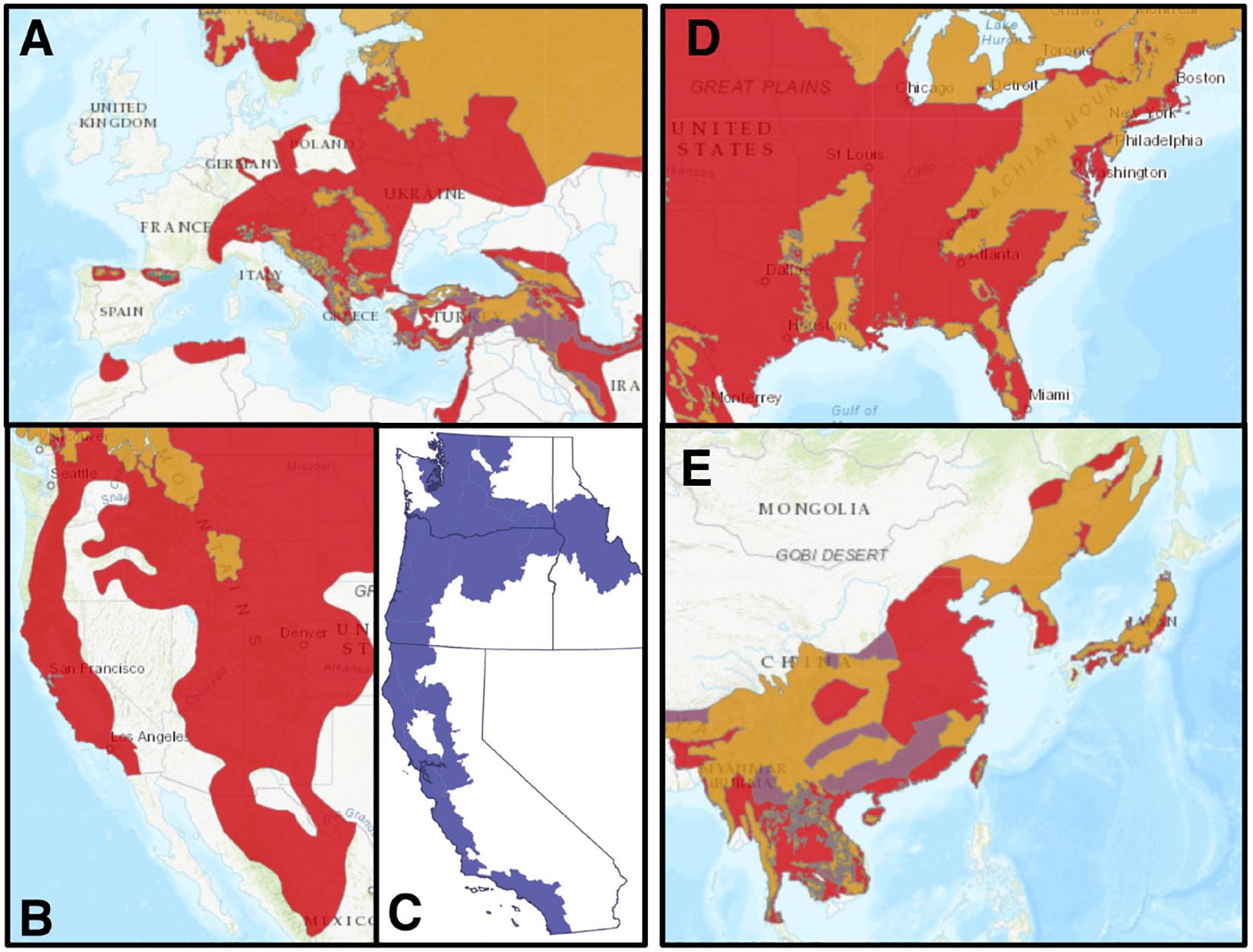
Figure 1. Extant (yellow), possibly extant (purple), and recently extirpated (red; i.e., excluding long-extirpated populations in Europe) bear population distributions from IUCN range maps (IUCN, 2020). Current and former distribution of brown bears in (A) Western Europe and the Mediterranean, and (B) Western United States and Northern Mexico. (C) Current distribution of 28 salmon evolutionarily significant units listed under the Endangered Species Act (five endangered and 23 threatened), highlighting the spatial extent of severe salmon decline. Current and former distribution of (D) American black bears in the Eastern United States and (E) Asiatic black bears in East Asia.
For both predator and prey, the bear-salmon interaction is vulnerable to anthropogenic disturbances on the land and at sea. Encroaching humans over the past couple of centuries have greatly reduced and fragmented bear distributions, while fisheries exploitation, freshwater habitat degradation, dams, and climate change threaten the viability and spatial extent of remaining salmon populations (Gustafson et al., 2007; Levi et al., 2012). An open question is how this decline in the bear-salmon interaction might affect the structure and function of ecological communities, and whether the decline of bear-salmon ecosystems can be prevented or reversed.
Bear-Distributed Salmon Carcasses as Resource Subsidies
Live and dead salmon provide a predictable pulsed resource for several hundred freshwater and terrestrial species of vertebrates and invertebrates throughout the Pacific Rim (Willson and Halupka, 1995; Cederholm et al., 1999; Gende et al., 2002; Hocking et al., 2009; Reimchen, 2017). Some aspects of the role of marine derived nutrients in salmon as resource subsidies have been previously reviewed elsewhere (Willson et al., 1998; Gende et al., 2002; Schindler et al., 2003). Here we provide an updated review with particular focus on vertebrates, invertebrates, riparian plants, and freshwater systems.
After capturing salmon in estuaries and streams, bears often move to adjacent land to feed (Figure 2). When salmon abundance is high, and stream habitat facilitates access (e.g., shallow water), bears feed selectively on energy-rich parts of fish (Gende et al., 2001), consuming as little as 25% of the salmon they kill (Lincoln and Quinn, 2018) and distributing carcass remains to vertebrate and invertebrate scavengers up to several hundred meters from waterways (Reimchen, 2000; Gende et al., 2001; Hocking and Reimchen, 2009; Levi et al., 2015). The nutrients in these carcass remains can influence all trophic levels from primary producers to large carnivores in both terrestrial and aquatic ecosystems (Helfield and Naiman, 2006; Hocking and Reynolds, 2011).
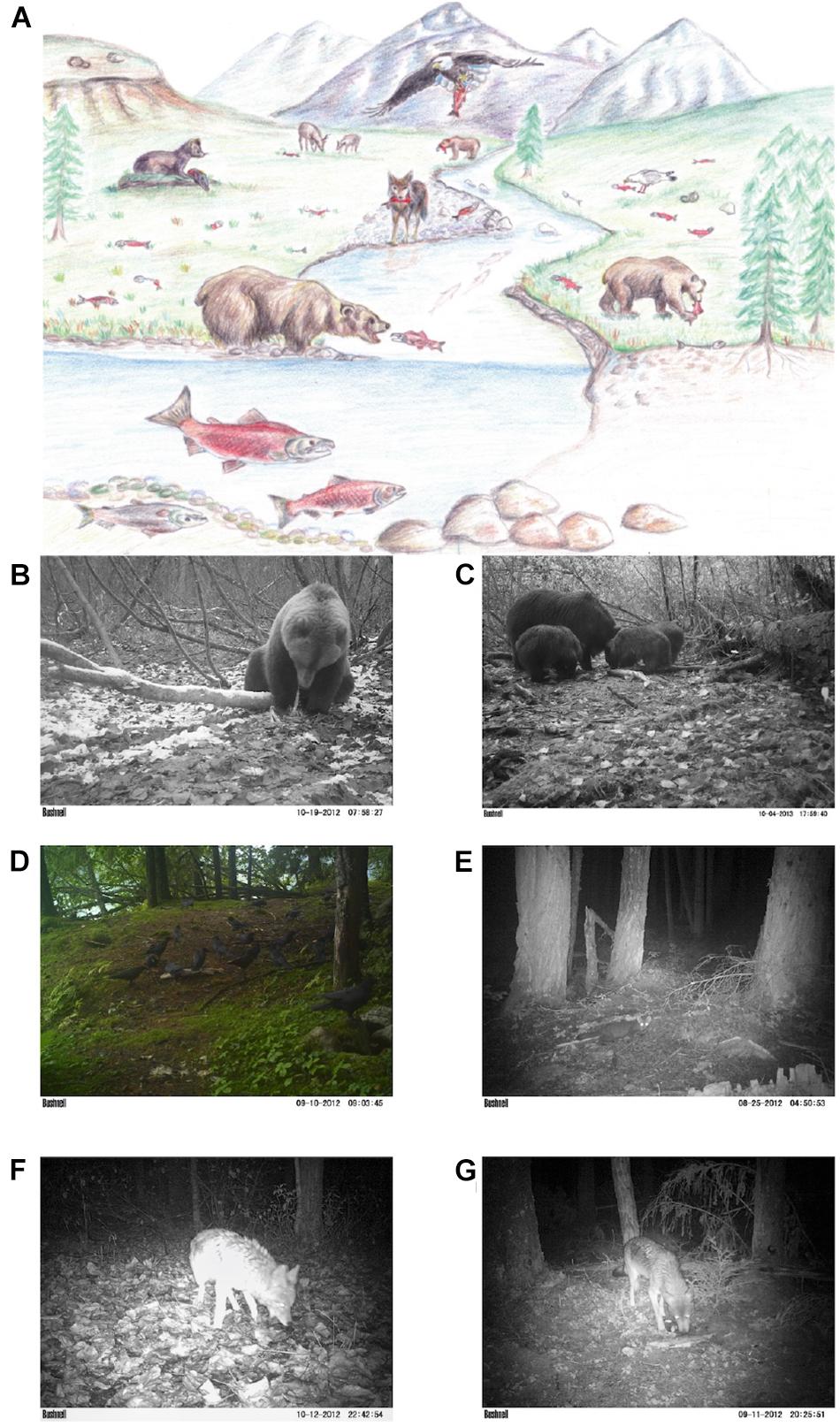
Figure 2. Mature salmon (A) reach terrestrial and aquatic systems where they are the dominant prey of bears. By leaving uneaten carcass remains in riparian areas, bears serve as vectors of salmon to terrestrial and aquatic systems, supplying nutrients and food to riparian vegetation, invertebrates, and vertebrate scavengers (Source: Levi et al., 2012). (B–G) Recorded images of wildlife consumption of individual salmon carcasses at camera trap stations from Levi et al., 2015. A wide variety of species were observed feeding on salmon carcasses, including brown bears (B,C), eagles, ravens, crows (D), mink, marten (E), coyotes (F), and wolves (G).
Vertebrate Scavengers
Cederholm et al. (1999) reported that more than 80 species of terrestrial vertebrates feed regularly on adult salmon, ranging from shrews, mink, coyotes, and marten to corvids, bald eagles, and owls. However, few of these species are large enough to successfully capture adult salmon in situ; instead relying on carcass remains left by the feeding activity of bears or by flooding (Willson and Halupka, 1995; Helfield and Naiman, 2006). Ben-David et al. (1997b) found that nearly 60% of the diets of riverine mink (Neovison vison) and nearly a quarter of the diets of coastal mink were comprised of salmon in autumn, and the timing of reproduction in female mink was shifted in salmon ecosystems so that lactation coincided with salmon carcass availability (Ben-David, 1997). Stable isotope analyses in Southeast Alaska indicated that salmon comprised a significant portion of marten (Martes americana) diet when rodents were scarce (Ben-David et al., 1997a). Behavioral, stable isotope, and fecal data from coastal British Columbia has found that wolves forage extensively on salmon with peaks on salmon consumption on islands (Darimont et al., 2003, 2008, 2009). Coyotes (Levi et al., 2015) and red foxes (Gard, 1971) have been observed both catching and scavenging salmon, but we are aware of no detailed research about the extent of salmon in their diets, the degree to which they predate or scavenge salmon, or the fitness benefits of salmon consumption. Even less is known about consumption of salmon by felids, but in 2016 a park range in Olympic National Park filmed a bobcat catching a large coho salmon (Petri, 2016), suggesting that this may be an underappreciated ecological interaction. Higher densities of passerine birds were also found in riparian forests bordering salmon-bearing streams versus non-salmon streams, perhaps because of increased densities of terrestrial invertebrates associated with salmon carcasses deposited on land by bears (Gende and Willson, 2001; Christie et al., 2008; Christie and Reimchen, 2008; Field and Reynolds, 2011). Bears themselves can also be important scavengers of salmon as well as predators. Many salmon that die of senescence or are killed and partially consumed are subsequently consumed by brown bears (Lincoln et al., 2020).
Invertebrate Scavengers
Salmon carcasses deposited on the forest floor by bears support a diverse community of terrestrial invertebrate scavengers, some of which are salmon carrion specialists. For example, a total of 60 species of invertebrates were collected from salmon carcasses from two streams in coastal British Columbia, which include obligate carrion specialists and more opportunistic scavengers or predators on salmon carcasses (Hocking et al., 2009). In coastal British Columbia and Alaska, three main groups of invertebrates dominate salmon carcass decomposition in terrestrial habitats: (1) the Diptera (flies) consisting of several dominant families, the Calliphoridae and Dryomyzidae, among others; (2) the Coleoptera (beetles), dominated by the Silphidae, Staphylinidae, and Leiodidae; and (3) parasitic Hymenoptera (wasps) (Ichneumonidae, Braconidae, Figitidae) (Meehan et al., 2005; Hocking and Reimchen, 2006; Hocking et al., 2009). All three of these salmon-specialist groups include species that could be used as indicators of intact bear-salmon interactions (Hocking and O’Regan, 2015). For example, blow flies (Calliphoridae) were the numerically dominant consumers of salmon (Meehan et al., 2005) and can time their summer emergence to the timing of sockeye salmon spawning in Alaska, which also secondarily affects the bloom timing of plant species that rely on flies as pollinators (Lisi and Schindler, 2011). Among the Coleoptera, the burying beetles (Silphidae), such as Nicrophorus investigator, exhibit specialized communal breeding of many females per carcass to produce up to 750 larvae (Hocking and Reimchen, 2006; Hocking et al., 2009). This adaptation, along with mutualistic phoretic mites that are transported by N. investigator to salmon carcasses to destroy fly eggs, enables N. investigator to compete with flies on salmon carcasses. In another trophic connection related to bears, several species of parasitic wasps (Ichneumonidae: Atractodes sp., and Braconidae: Alysia alticola) parasitize the fly larvae that consume salmon carcasses (Hocking et al., 2009). Stable isotope analysis of these invertebrates indicates that they occur at very high trophic positions (equivalent to harbor seals and walruses), suggesting that nearly all of their biomass is derived from salmon (Hocking et al., 2009).
The microbial community associated with salmon carcasses is transferred to scavenging insects, and even non-scavenging insects such as mayflies. The microbial communities differ markedly from those associated with insects in non-salmon bearing streams (Pechal and Benbow, 2016). Thus the “necrobiome” community (Benbow et al., 2013) is an important, albeit less visible, part of the connection between bears and salmon. Overall, the loss of salmon and bears has as yet unknown consequences for terrestrial scavengers and the ecosystem functions they support, but scavengers with specialized life histories associated with salmon may be good indicators of intact ecosystem processes (Hocking and O’Regan, 2015).
Riparian Plants
In small coastal streams, or in tributaries of larger rivers and lakes, bears can transfer more than 50% of the spawning salmon to streamside areas (Helfield and Naiman, 2001; Helfield and Naiman, 2006; Hocking and Reimchen, 2006; Quinn et al., 2009). This creates hotspots of salmon nutrient release that may be accessed by riparian plants (Holtgrieve et al., 2009; Hocking and Reynolds, 2012). Bears can also distribute salmon nutrients to terrestrial ecosystems through distribution of their urine and feces (Hilderbrand et al., 1999a), which is taken up by riparian plants living beside wildlife trails (Wilkinson et al., 2005). In combination with additional pathways of salmon nutrient entry into terrestrial food webs, such as flooding and hyporheic water flow, salmon subsidies facilitated by bears may ultimately affect riparian plant diversity (Wilkinson et al., 2005; Hocking and Reynolds, 2011).
Studies of the role of salmon in riparian plant nutrition have typically found increasing stable isotope ratios of heavy to light nitrogen (δ15N) in plant leaves across natural gradients in salmon spawning density or beside salmon carcass sites (Bilby et al., 2003; Bartz and Naiman, 2005; Nagasaka et al., 2006; Hocking and Reimchen, 2009; Holtgrieve et al., 2009; Hocking and Reynolds, 2012; Reimchen, 2017; Walsh et al., 2020). This suggests that salmon carcasses, which contain higher δ15N (∼12‰) than terrestrial sources of N (∼0‰), provide an important source of nitrogen to streamside plants. Bears, as the primary distributors of salmon carcasses, also indirectly cause shifts in riparian ecosystem processes such as plant physiology, productivity, and community structure. Salmon subsidies increase foliar %N, decrease foliar C/N ratio and can even increase foliar stomatal density (Hocking and Reynolds, 2011; Hocking and Reynolds, 2012; van den Top et al., 2018). At larger scales, inputs of salmon nutrients can increase the productivity of the riparian zone, increase tree growth and shift plant communities toward species adapted to nutrient-rich sites (Helfield and Naiman, 2006; Hocking and Reynolds, 2011; Reimchen, 2017). For example, in a recent multi-decade fertilization experiment where salmon carcasses were moved by researchers into the forest along a 2 km stretch of one bank of a stream but not the other, long-term salmon carcass fertilization resulted in increased tree growth on the bank that was fertilized, though the effect was small compared to other factors (Quinn et al., 2018).
Bear foraging on salmon increases marine nutrient retention in watersheds, which thus alters how plants access, use and compete for these resources. When the bear-salmon association is maintained long-term, plant community assembly may be altered such that the riparian zone is dominated by nutrient-loving species from diverse phylogenies rather than closely related species (e.g., the Ericaceae) in a phylogenetic cluster that are specifically adapted to low nutrient environments (Hocking and Reynolds, 2011; Hurteau et al., 2016). Therefore, particularly in small watersheds where floodplain processes are less dominant, bears can mediate key riparian ecosystem processes such as plant palatability and litter quality, rates of N turnover, berry production, and plant resource allocation toward stem and leaf growth rather than root development and foliar defense.
Subsidies to Freshwater Fish and Invertebrates
Whereas there has been substantial interest in how bear predation on salmon affects terrestrial consumers and riparian ecosystems, fewer studies have documented how bear predation mediates energy flows in freshwater food webs. In small streams, bears kill a substantial fraction of spawning salmon (Quinn et al., 2017) and remove salmon carcasses that would have otherwise decomposed within stream channels (Quinn et al., 2009), floodplains, or downstream lakes. This could alter the availability of salmon resources for stream dwelling fishes. Moore et al. (2008) found that stream resident fish exhibit a non-linear response to salmon abundance; egg consumption increased dramatically once salmon densities were high enough that fish began digging up each other’s nests. While egg consumption saturates for individual consumers, large pulses of egg availability provide feeding opportunities to less dominant sizes and species of fish (Bailey and Moore, 2020). Bear predation of salmon reduces their density in streams, which decreases the fraction of spawned eggs mobilized in the water column by female salmon (Moore et al., 2008). Moreover, to the extent that they can, bears preferentially kill newly arrived salmon (Gende et al., 2004) and, in the case of females, preferentially consume their eggs (Lincoln and Quinn, 2018). Consequently, bears may reduce the availability of eggs for large and small fishes.
Alternatively, bears could increase foraging opportunities for piscivorous fish. Bear-killed salmon become available for piscivorous fish earlier in the season than do salmon dying of senescence. Salmon carcasses also indirectly benefit piscivorous fish by subsidizing aquatic invertebrates such as caddisflies (Trichoptera) and stoneflies (Plecoptera) that adapt shredding mouthparts to colonize and consume salmon carcasses with higher colonization efficiency for bear-killed than senescent salmon (Minakawa and Gara, 1999; Winder et al., 2005). Bears also facilitate the colonization of salmon carcasses on land by fly and beetle larvae, which provide a “value added” trophic resource for resident fish by transforming salmon flesh depleted by the energetic requirements of migration and spawning activity into energy-dense invertebrate larvae. Fall rains wash larvae back into streams, and provide energy rich prey for stream fishes (e.g., Denton et al., 2009).
Influence of the Salmon Resource Wave
These myriad interactions are themselves influenced by spatial and temporal variability. Brown bears traverse watersheds to consume salmon from multiple populations that spawn at different times (Schindler et al., 2013; Deacy et al., 2016). When salmon runs are more synchronous, bears tend to consume only the most energy rich parts of salmon (Gende et al., 2001; Lincoln and Quinn, 2018), leaving the remainder for other components of the ecosystem. Overall, the marine-based diets and population productivities of freshwater fishes such as sculpins (Cottus spp.), Dolly Varden (Salvelinus malma), and juvenile coho salmon (O. kisutch) are positively related to increasing salmon spawning densities in streams (Bilby et al., 1998; Wipfli et al., 2003; Denton et al., 2009; Swain and Reynolds, 2015), and this may be modulated by bears, but the mechanisms are not yet clear.
Bears, Interference Competition, and Large Mammal Food Webs
Large mammal food webs can exist in a range of consistent structures, which are likely driven by the resources available in each habitat and interference competition among top predators (Figure 3). For example, in the Alexander Archipelago of western North America, some large islands, such as Admiralty, Baranof, and Chichagof, contain brown bears but neither black bears nor wolves (Canis lupus). In contrast, other islands such as many in the Alexander Archipelago of Southeast Alaska and coastal British Columbia contain black bears and wolves but not brown bears. Some outer coastal islands contain only strongly marine-oriented wolves that swim from island to island (Figure 3). The remote island complex of Haida Gwaii, BC, contains abundant salmon and a large subspecies of American black bear (Byun et al., 1997), but neither brown bears nor wolves. Similar spatial partitioning among bears occurs in east Asia where brown bears but not Asiatic black bears range over the northern islands of Sakhalin, Russia and Hokkaido, Japan, whereas Asiatic black bears but not brown bears range over the southern Japanese islands (Figure 1).
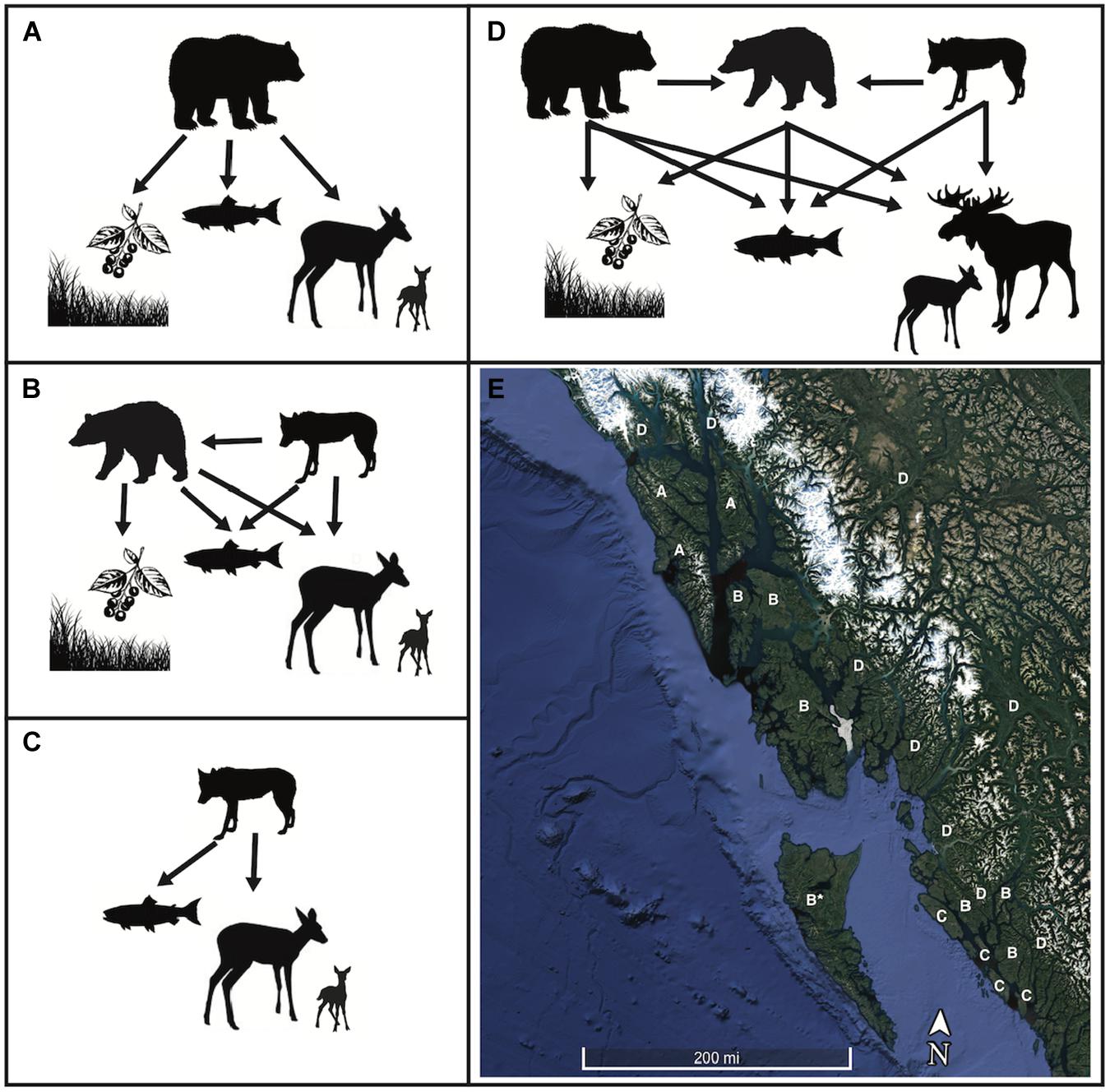
Figure 3. Food web motifs in salmon-bearing ecosystems including bears that primarily eat salmon and berries but seasonally prey on ungulate calves, and wolves that prey on ungulates and seasonally consume salmon and marine resources. These food web motifs vary across the archipelagic landscape in Southeast Alaska and British Columbia. (A) The brown bear–deer system is uncommon, but is characteristic of Admiralty, Baranof, and Chichagof islands. (B) The black bear – wolf – deer system characterizes most of the inner islands of this archipelagic landscape, while (C) wolf – ungulate systems occur on the outer islands of British Columbia. (D) Large mammal food webs on the mainland are more diverse, containing brown bears, black bears, wolves and typically moose in the north transitioning into deer to the south. (E) The spatial distribution of these large mammal food webs in Southeast Alaska and British Columbia. The remote island of Haida Gwaii is labeled “B*” because it is a unique black bear system without wolves and with deer being an introduced species.
Despite these striking spatial patterns, no experimental or observational evidence fully explains the mechanisms underlying these distributional patterns. However, one compelling potential explanation is that interference competition between salmon-supported brown bears and other top carnivores structures the predator community. Brown bears frequently kill black bears, and black bears so strongly avoid brown bears that they often forego or reduce foraging on salmon (Belant et al., 2010; Levi et al., 2015; Service et al., 2019), a primary nutritional resource in the absence of brown bears (Peacock et al., 2011; Adams et al., 2017). Whether such interference competition eliminates black bear populations on islands, and the role of salmon in mediating these interactions among bears, is now being tested by a natural experiment as brown bears increasingly colonize islands where both the archeological record and traditional and/or local ecological knowledge do not demonstrate previously persistent brown bear populations (Service et al., 2014).
Wolves and brown bears also exhibit interference competition with one another, although outcomes appear to depend on strength of participants (e.g., pack size, bear body mass) rather than fall out consistently between species (Koene et al., 2002; Gunther and Smith, 2004; Tallian et al., 2017). Although direct predation of wolf pups by brown bears has been observed (Hayes and Baer, 1992), the absence of wolves on islands with high densities of salmon-supported brown bears plausibly results from kleptoparasitism of wolf kills by brown bears in much the same way that kleptoparasitism by lions (Panthera leo) and hyenas (Crocuta crocuta) seems to negatively affect African wild dogs (Lycaon pictus) (Carbone et al., 2005; van der Meer et al., 2011). However, a challenge to the hypothesis of kleptoparasitism-based food limitation is bear hibernation behavior, which provides an open temporal niche where wolves are free from interference competition, and frequent consumption of smaller food items by wolves in the summer months (Roffler et al., 2020). Although brown bears frequently usurp wolf kills, Lewis and Lafferty (2014) observed that brown bears were tolerant of wolves while both species fed on a whale carcass over a period of months. In contrast, despite being common in their study area, black bears were never observed feeding on the whale carcass, suggesting that black bears occupy a more consistent subordinate position in this interaction web. This subordinate position is further supported by the substantial consumption of black bears by wolves in these coastal systems (estimated as high as ∼16% frequency of occurrence in several southeast Alaska study areas; Kohira and Rexstad, 1997; Roffler et al., 2020).
Why specific islands have brown bears and others have black bears and wolves is not well understood. A plausible hypothesis is that the larger home ranges and lower population densities of brown bears relative to black bears require larger islands for population persistence (perhaps exacerbated by exploitative competition for berries with black bears), and, once established, brown bears exclude black bears and wolves through interference and exploitative competition. However, an exception to this pattern is the very large Prince of Wales Island (6,674 km2), which once contained brown bears in an ice age refuge (Heaton et al., 1996), but now hosts black bears and wolves despite abundant salmon and berries. It is also possible that coexistence among brown and black bears is strongly influenced by the priority effect in which the species currently occupying the island prevents invasion of the other species (Grainger et al., 2019). If so, perhaps anthropogenic reductions in black bear density are facilitating the recent colonization of black bear islands by brown bears in British Columbia (Service et al., 2014) and Alaska (A. Crupi personal observation). Although no mechanism has strong empirical support, it is also interesting to note that both the Japanese/Russian islands and the Alexander Archipelago transition from brown bears at higher latitude islands to black bears at lower latitudes. Such exclusive structuring of large carnivore communities is less pronounced on the mainland where dispersal and niche partitioning may maintain populations of wolves and black bears despite abundant brown bears, but the mechanisms for coexistence are poorly understood. Perhaps innovative future researchers will find a way to make stronger inference about the mechanisms underlying the biogeographic variation in brown bear black bear, and wolf occupancy.
Salmon-Subsidized Bears as Predators
Predation on Ungulate Neonates
Brown and black bears can be important predators of ungulate neonates (Zager and Beecham, 2006; Supplementary Table 1), but little is known about how co-occurring salmon influence this dynamic Salmon could support higher densities of bears, and to an extent wolves (Adams et al., 2010), leading to increased predation on ungulates. However, by increasing the body size and abundance of competitively dominant brown bears, salmon could also plausibly suppress more abundant black bears and wolves. Although kleptoparasitism by brown bears might be expected to cause wolves to kill more frequently, the opposite has been observed in both Yellowstone and Scandinavia (Tallian et al., 2017). If interference competition with brown bears can reduce predation rates on ungulates by black bears and wolves, then we would predict elevated deer densities on brown bear islands where black bears and wolves do not occur (Figure 3). Such a scenario, in which brown bears competitively exclude black bears and wolves from entire island ecosystems, represents the extreme end of a competitive spectrum (i.e., maximum potential effect) where interference competition fully suppresses competitors. Accordingly, increased deer abundance on brown bear islands does not necessarily indicate that interference competition reduces predation rates where all species co-occur. However, increased deer density on brown bear islands would clearly indicate that predator community structure moderates deer density, rather than solely habitat quality and weather severity (Gilbert and Raedeke, 2004; Brinkman et al., 2011).
Extensive deer pellet surveys by Alaska Department of Fish and Game [including time series of at least 4 years from 1981 to 2016 across 52 survey locations spanning 11 distinct geographic regions (Kirchhoff and Pitcher, 1988; McCoy, 2017)] unambiguously indicate higher numbers of pellet groups (clumps of pellets representing a single fecal deposit by an individual) per plot on all three brown bear-only islands relative to black bear-wolf islands (Figure 4A). Similarly, hunters harvested deer in substantially fewer days (Alaska Department of Fish and Game Winfonet data from 1997 to 2019)1 on brown bear islands, indicating a higher catch per unit effort (Figure 4B). Should these pellet group surveys reflect deer densities (Brinkman et al., 2013), these results indicate higher deer density where only brown bears occur relative to areas with black bears and wolves. The degree to which black bears and wolves, respectively, contribute to this pattern is not clear, but research from Prince of Wales Island suggests that black bear predation can remove almost half of fawns born each year, and appears to be additive (Gilbert, 2015; Gilbert et al. in press), while the level of compensatory versus additive predation by wolves, which are a primary predator of adult deer, is not known (Farmer et al., 2006; Gilbert, 2015). What is clear is that deer densities are higher on Admiralty, Baranof, and Chichagof islands, despite them having among the highest brown bear densities in North America, with early estimates of bear density of ∼400/1000 km2 (Miller et al., 1997). This is not surprising, as even these very high brown bear densities are well below the estimated black bear density of 1500 bears/1000 km2 on nearby Kuiu Island (Peacock et al., 2011). We speculate that salmon support high densities of brown bears, which allows them to exclude wolves and even more abundant black bears on the ABC islands, which results in reduced predation and elevated densities of deer. Whether interference competition occurs among brown bears, black bears, and wolves where they co-occur is an open question with important implications for predator management geared toward increasing ungulate density.
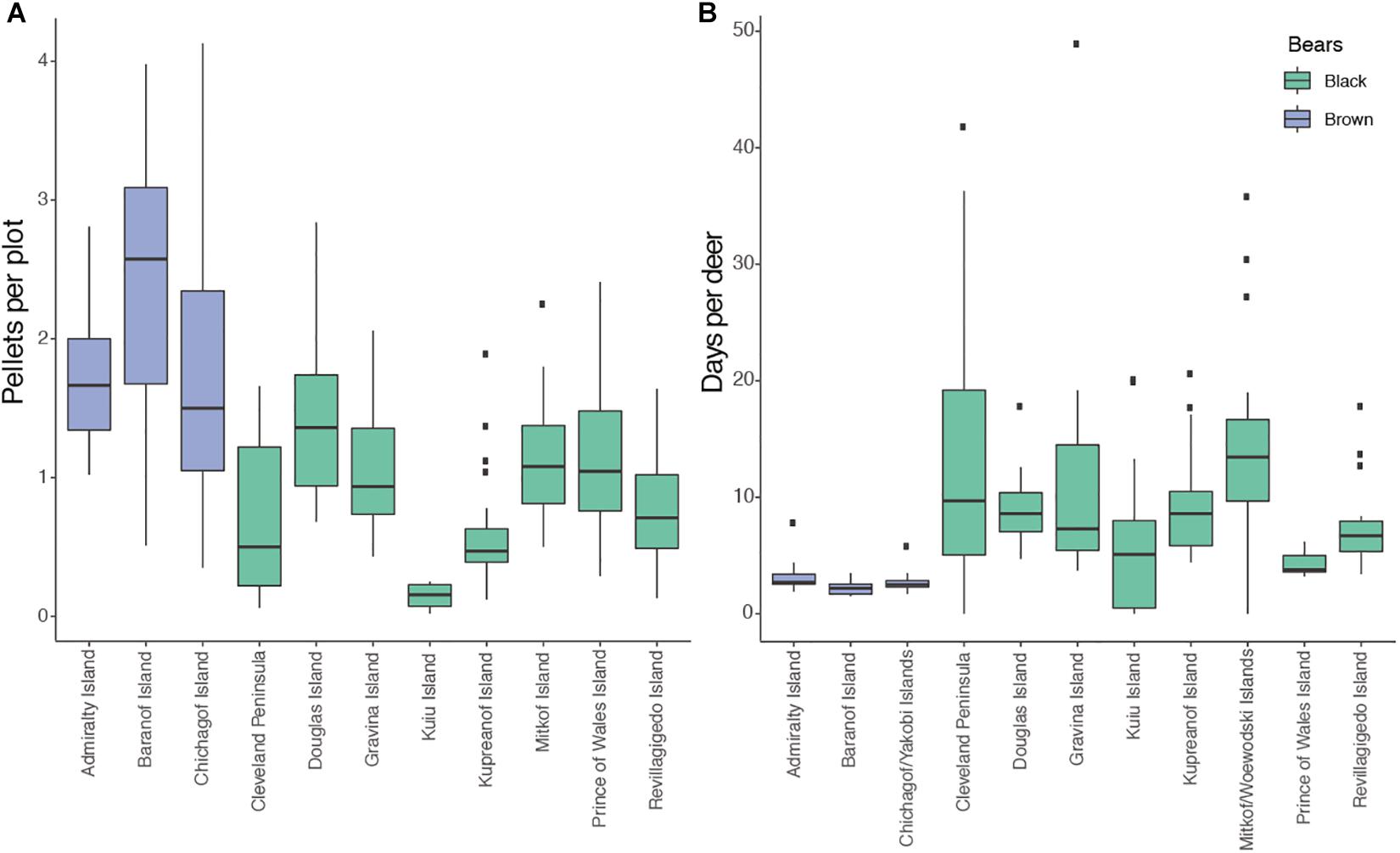
Figure 4. Indices of deer abundance from Alaska Department of Fish and Game in regions with only brown bears and those that are black bear dominated. A small brown bear population exists on the Cleveland Peninsula (annual harvest < 1 individual) and Revillagigedo (annual harvest < < 1 individual). Some additional islands have recently been colonized by brown bears. Wolves do not occur on brown bear islands. (A) Deer pellet groups per plot across 52 survey locations spanning 11 distinct geographic regions in Southeast Alaska. Data include time series of at least 4 years spanning 1981–2016. (B) Days of hunting per deer harvested from 1997 to 2019. Lower values indicate that hunters more quickly find deer, suggesting a higher density of deer.
Another open question in this system is through what mechanisms ungulates persist under apparent competition (Holt, 1977) in which salmon indirectly compete with ungulates by supporting elevated populations of their predators. It is tempting to infer that bear foraging effort is diverted away from ungulate neonates and instead allocated to feed on salmon, which are more energetically profitable due to concentrated aggregations and high nutritional value. However, the bulk of ungulate neonate mortality occurs during the first 6 weeks of life (Boertje et al., 2010), several weeks or more prior to the arrival of salmon, eliminating this diet breadth hypothesis.
The first clue as to how large herbivores persist where bears are so heavily subsidized by salmon comes from stable isotope analysis from bears across a large geographic range. Unlike in interior systems, where bears consume substantial terrestrial meat, in salmon-subsidized systems interactions between bears and ungulates are highly asymmetric; on average, bears eat little terrestrial meat on a per-capita basis (Figure 5; Mowat and Heard, 2006) but nonetheless collectively remain an important predator of ungulate neonates (Supplementary Table 1; Mowat and Heard, 2006) due to the higher bear:ungulate ratio.
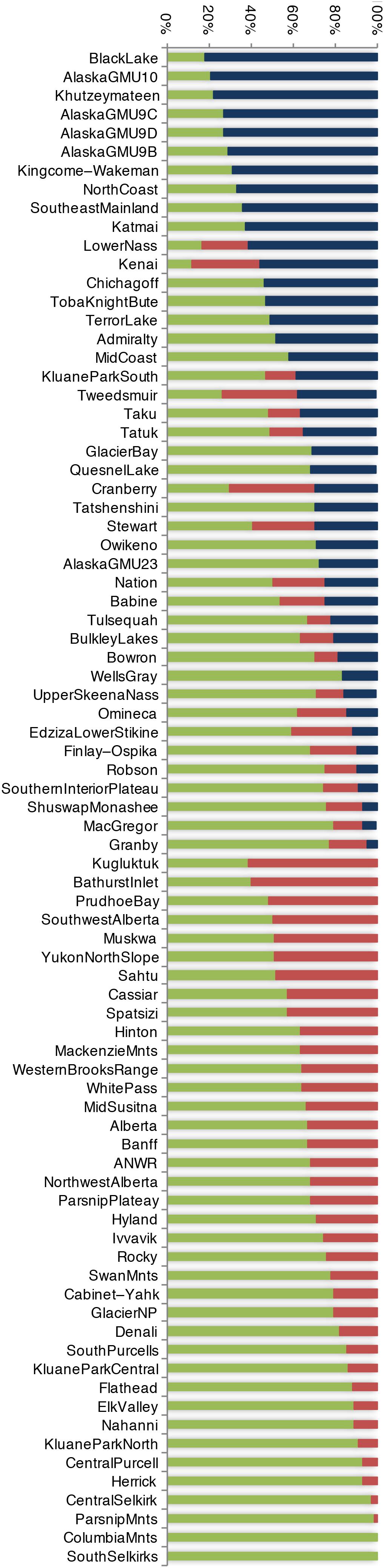
Figure 5. Percent salmon (blue), vegetation (green), and terrestrial meat (red) in the diet of 81 brown bear populations as indicated by stable isotope analysis. Some bears feed primarily on salmon, others primarily on vegetation, and some contain substantial terrestrial meat. Data originally compiled by Mowat and Heard (2006).
To explore this omnivore-salmon-ungulate relationship further, we compiled data on population density, calf survival, and causes of mortality from 18 bear and moose populations in Alaska (Supplementary Table 1). Original study methods were not necessarily consistent across areas due to local ecological conditions and logistical constraints, but, taken together, these data suggest a strongly non-linear relationship between moose calf survival and total bear (black + brown) density (Figure 6). Moose calf survival is highest at low bear density, as expected, but stabilizes at around 20% survival at total bear densities above 100 per 1000 km2 (Figure 6A). This bear density, above which no additional decline in moose calf survival is observed, is approximately 2–5 times lower than brown bear densities alone in salmon-fed coastal systems (Hilderbrand et al., 1999b) and 15 times lower than black bear densities achieved on Kuiu Island, where salmon are abundant and brown bears are absent (although they are starting to colonize), yet deer persist (Peacock et al., 2011). For moose calf survival to remain constant across this range of relatively high bear densities, a mechanism must exist by which a maximum predation rate by bears emerges.
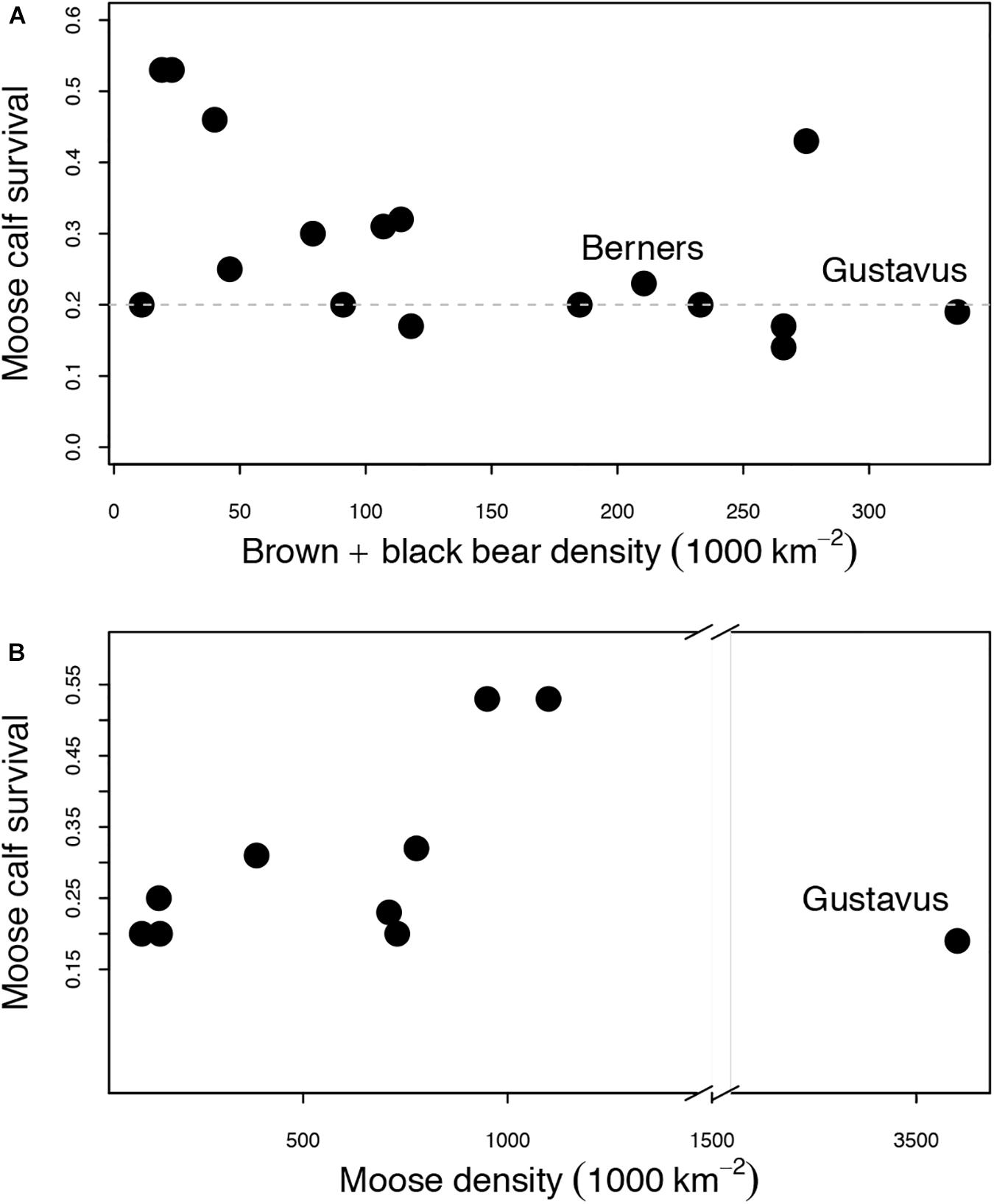
Figure 6. Empirical relationship between (A) total bear density (black + brown bear) and moose calf survival across 12 populations in Alaska, and (B) between moose density and moose calf survival (see Supplementary Table 1). Average moose calf survival stabilized near 20% over a broad range of bear densities but increases at very low bear density. Moose calf survival increased with moose density, which is counter to expectations if bear prey-switching as moose calves become rare is responsible for the invariance of calf survival to bear density. The exception is the Gustavus moose population, which is highlighted because the research occurred in the distinct context of a moose population irruption after moose first colonized the study area. Berners is highlighted because bear density is a gross underestimate with only the minimum number of black bears captured as bycatch during a brown bear survey included as part of the black bear density estimate. The true black bear density may be an order of magnitude higher (i.e., if 10% of black bears were caught as bycatch), and we postulate that Berners, which is a salmon-fed system with the highest brown bear density, has substantially higher bear density than all other study areas.
The mechanisms underlying this non-linear relationship between bear density and ungulate neonatal survival are unclear, but we suggest several potential non-exclusive mechanisms. First, interference competition among bears could reduce their per-capita predatory effectiveness as bear density increases (Supporting Information S1). Although the functional response of bears feeding on ungulate neonates has never been parameterized, a ratio-dependent functional response representing reduced kill rates at high predator densities is strongly supported for wolves preying on moose and elk (Vucetich et al., 2011).
Second, bears might cease searching for ungulate neonates once they become rare enough that it is not energetically profitable to search for them, given alternative foods, resulting in high survival of neonates below a critical local density due to diet switching behavior of optimal foragers seeking to maximize their rate of energy accumulation (Charnov, 1976; Stephens and Krebs, 1986). Experiments with diversionary feeding in Alaska illustrate this phenomenon for bears and moose. Moose carcasses deposited during the calving season diverted bear foraging effort from moose calves toward carrion, resulting in higher moose calf:cow ratios in early winter than in previous years and in untreated sites (Boertje et al., 1992). However, under this mechanism we would expect lower ungulate neonate survival at high ungulate densities, but instead we observe constant to increasing moose calf survival as ungulate densities increase, but this could be confounded if improved moose habitat quality leads to both higher moose densities and greater alternative foods for bears during and shortly after parturition when neonates are vulnerable (Supplementary Table 1 and Figure 6B).
A series of other plausible hypotheses exist. For example, female ungulate with accompanying young might forego foraging opportunities and increase vigilance as bear densities increase. Additionally, ungulate neonate survival could be limited by the availability of safe “partitioning habitat” where neonates are unlikely to be discovered or unlikely to be killed after discovery, as has been recently reported for neonatal mule deer facing predation (Hurley et al., 2020). Although the mechanism allowing for bear-ungulate coexistence is not yet clear, the data support that (1) bears do not rely on terrestrial meat in salmon systems, and (2) that hyper-abundant bears due to salmon subsidies do not appreciably reduce ungulate survival beyond the level already imposed by bears at moderate levels of abundance. There is thus, perhaps surprisingly, no evidence of bear-mediated apparent competition between salmon and ungulate neonates despite the enormous increase in bear biomass in salmon systems.
Predation on Salmon
In salmon systems, the energy requirements of brown bears are often largely derived from salmon (Figure 5). Bears are by far the dominant predator of salmon (Levi et al., 2015; Quinn, 2018), but the effects of bear predation on salmon goes beyond the numerical reduction in the number surviving to spawn to include evolutionary and ecological processes. Because bears can kill >70% of the salmon in small streams, bear predation has shaped the evolution of salmon. Where they are more easily accessible to bears, spawning salmon tend to be younger, shorter for their age, less deep-bodied (to be less conspicuous and more maneuverable in shallow water), and spawn more quickly upon arrival to spawning grounds (Quinn et al., 2001; Carlson et al., 2009).
Salmon and Seed Dispersal
A striking feature of North American temperate rainforests where salmon are abundant is the hyperdominance of fleshy-fruited shrubs requiring seed dispersal by animals. These fruit provide key seasonal nutritional resources for both brown and black bears, which, together with salmon, comprise the primary source of calories that can support high-density bear populations. Seeds readily germinate after consumption and gut passage by both bears and birds (Traveset and Willson, 1997). In a surprising result, Harrer and Levi (2018) demonstrated that bears, not birds, dispersed the bulk of devil’s club, Oplopanax horridus, seeds, the dominant berry-producing shrub in northern Southeast Alaska. In addition, most devil’s club seeds were not dispersed, suggesting that seed dispersal by bears was additive to dispersal by birds for an increase in overall seed dispersal. Brown bears dispersed substantially more seeds than did black bears prior to the arrival of salmon, after which black bears became more important seed dispersers, suggesting a complex interaction among resource bases and competing apex omnivores. Further, Shakeri et al. (2018) demonstrated that brown bears in salmon systems dispersed far more seeds, from 12 distinct plant species, than previously recognized. This included individual scats with over 80,000 devil’s club seeds and 150,000 blueberry (Vaccinium spp.) seeds. Seed contents in scats were consistent with the percent cover of berry-producing shrubs on the landscape, suggesting that perhaps bears were the most important seed disperser for a variety of berry-producing shrubs in addition to devil’s club.
Seed-dispersal services provided by bears could contribute to the uniquely high proportion of berry-producing shrubs in Southeast Alaska and British Columbia (Willson, 1991), but this depends on the unknown degree to which these plants are niche limited versus dispersal limited. It is plausible that berry-producing shrubs would dominate the forest understory regardless due to low wind conditions beneath the forest canopy, but the species composition of shrubs may be influenced by the preferences of bears. In more open systems such as recently disturbed, deglaciated, or meandering river systems, seed deposition by bears could be more consequential to the resulting plant community as fleshy-fruited plants compete for establishment in meadows or other open vegetation types. However, the relative seed dispersal services provided by bears and birds has yet to be quantified for the community of fleshy-fruited shrubs, and the importance of dispersal as a demographic bottleneck for these plants is unknown.
Resource Subsidies to Small Mammals
Seeds dispersed by the abundant bears in salmon systems are deposited in vast numbers in bear scats (Figure 7). Shakeri et al. (2018) documented extensive foraging by small mammals in seed filled bear scats, each containing an average of 114 kcal worth of digestible energy in the seeds and an unknown amount of additional energy in partially digested fruit (see also Bermejo et al., 1998). In northern Southeast Alaska, seed-filled bear scats were intensively utilized and dispersed by small mammals, primarily scatter-hoarding northwestern deer mice, Peromyscus keeni (8.5 visits per scat per day) and larder-hoarding northern red backed voles, Myodes rutilus (2.2 visits per scat per day), with visitation rates proportional to the seasonal density of each species. Scatter-hoarding mice serve an important role in secondary seed dispersal, reducing negative density dependence and allowing colonization of a greater number of microsites (Enders and Wall, 2012). In contrast, larder-hoarding voles are presumed to be seed predators. Both species are important parts of the base of the vertebrate food web.
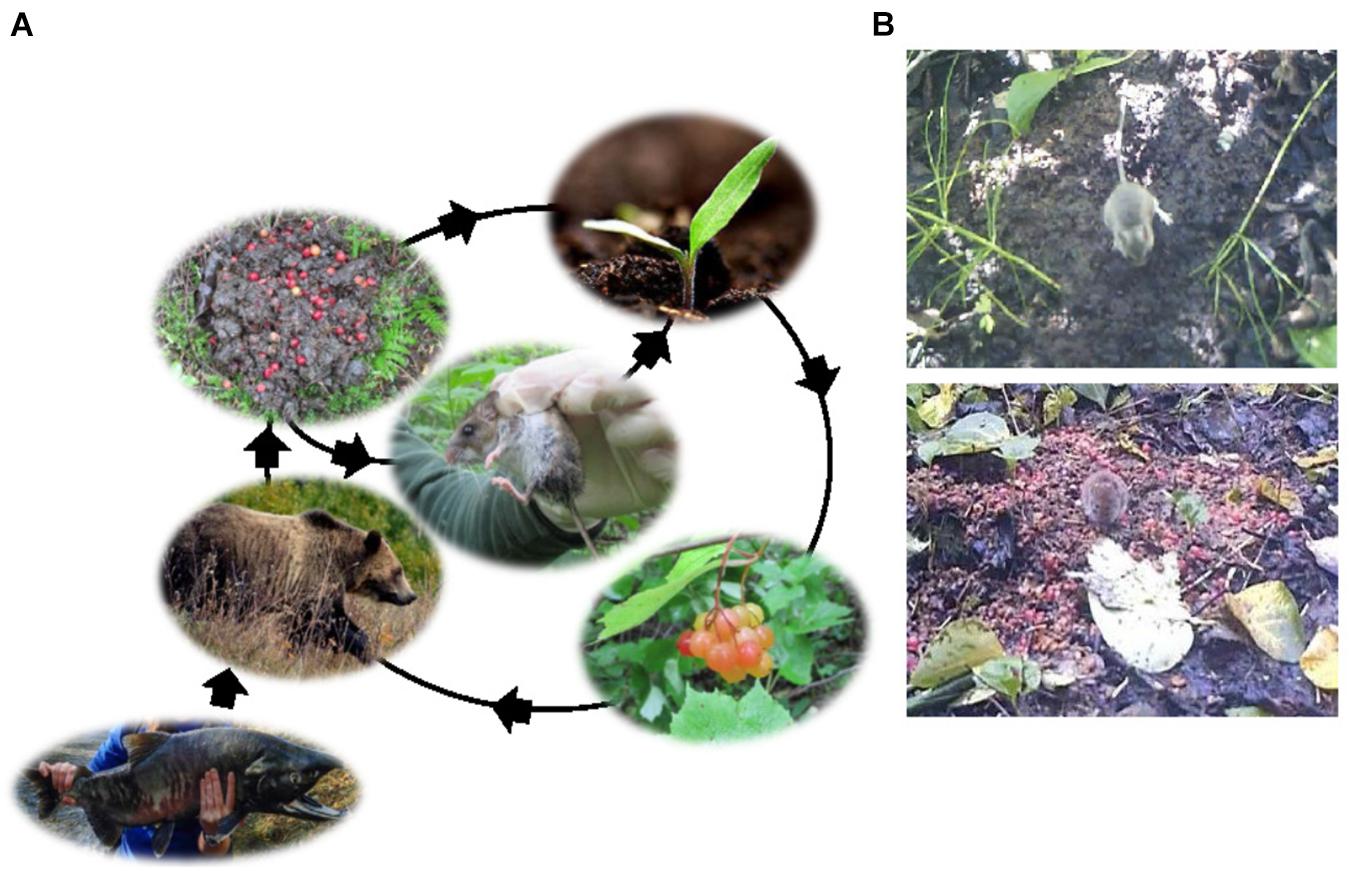
Figure 7. (A) The community relationship between salmon, brown bears, fruit, and northwestern deer mice. Salmon support bears that act as seed dispersers and provision small mammals with seed-filled bear scats. Deer mice then act as secondary seed dispersers. The northern red-backed vole (not shown) is a larder-hoarder and is not expected to significantly aid in seed dispersal of fruiting plants. (B) The northwestern deer mouse (top) and the northern red-backed vole (bottom) feeding on seed-filled bear scats (Source: Shakeri et al., 2018).
The substantial digestible energy in seed-filled bear scats may provide a significant nutritional subsidy to small mammals on a landscape scale due to the high density and defecation rate of bears during hyperphagia, the concentration of seeds into piles for efficient foraging, and the increased time period of seed availability as bears transfer seeds from shrubs to the ground. In coastal Alaska riparian areas where bears are particularly abundant, the energy in seed-filled bear scats may seasonally subsidize the energy needs of 45–65% of local deer mouse populations (Shakeri et al., 2018), presenting the possibility that this nutritional subsidy causes a numerical response in small mammals that might affect higher trophic levels.
Future Research and Conclusion
Given the enormous geographic range of large-bodied, omnivorous bears, and their current, historic, and potential future overlap with salmon [which are experiencing rapid range shifts due to climate change that are expected to intensify (Battin et al., 2007)], it is remarkable how little attention has been paid to the community ecology of the bear-salmon system. In particular, how has the widespread decline of bears and salmon (Figure 1) affected ecosystems? In addition to the topics reviewed here, there is great opportunity for a number of promising new research avenues. For example, how does the widespread consumption of Hymenopterans by bears (Swenson et al., 1999; Mattson, 2002; Auger et al., 2004; Koike, 2010) during nest raiding in coarse woody debris or mounds influence the populations of ants and wasps, influence access to Hymenopterans by smaller insectivorous animals, and influence the structure and function of coarse woody debris, which can be an important structural element for small forest carnivore rest sites, amphibians, nesting birds, and invertebrates? Or, how might climate-driven distributional shifts of bear-salmon systems toward more northern climates affect arctic ecosystems, including potential competition among polar and brown bears for salmon? Similarly, how will ecosystem succession progress as ongoing deglaciation opens new regions to bears and salmon? These phenomena may intersect with previous gazetting of strictly protected areas of “rock and ice” such as Glacier Bay National Park and Wrangell – St. Elias National Park (the largest in the United States) as they increasingly include salmon-bear habitat. This may produce large tracts of strictly protected habitat in coastal salmon systems, very little of which currently exists, with consequences for ecotourism and local economies.
Most importantly for management, how do terrestrial prey, such as ungulates, co-exist with hyper-abundant salmon despite abundant shared predators that benefit from salmon (e.g., apparent competition between salmon and terrestrial prey via salmon-supported bears)? We have reviewed some potential mechanisms for coexistence, but a number of complex bear-salmon-ungulate interactions are plausible. For instance, deciduous berry-producing shrubs can be key nutritional resources for both deer and moose. If seed dispersal is limiting, then bears may increase shrub cover by these species relative to unpalatable plants to the benefit of these herbivores. Fertilization of riparian areas by bears may also alter competition in ways that favor ungulates. For instance, alder (Alnus spp.) is an unpalatable nitrogen-fixing plant that competes for space with highly palatable willow in riparian areas. Do nitrogen subsidies by the bear-salmon interaction increase the competitive success of willow, thus increasing the nutritional carrying capacity of ungulates?
As suggested earlier, interactions among bears may also influence the survival of ungulate neonates. No research has examined the impact of black bear exclusion by brown bears on the predation rates of ungulate neonates, an important shared prey (Figure 3), but the observed impacts of mesopredator release on prey species in diverse systems worldwide (Ritchie and Johnson, 2009) suggest that it is plausible to expect that reduced densities of brown bears in salmon systems could unintentionally increase predation rates on ungulate neonates by releasing black bears, which can attain higher population densities than larger-bodied brown bears (Peacock et al., 2011) and are themselves important predators of ungulate neonates (Supplementary Table 1). These hypotheses highlight a gap in community ecology understanding: do the species responses seen in classical mesopredator release hold when the interacting species are both omnivores? Black/brown bear ecosystems could provide an excellent model for investigating this topic, which to our knowledge has not been explored.
Understanding bear-salmon-ungulate interactions is particularly important in Alaska where reducing bear abundance is a focus of management efforts designed to benefit ungulate populations in efforts to facilitate higher hunting returns by humans. For instance, over the past 15 years, liberalized hunting regulations and predator control efforts in interior Alaska have intensified with an increased focus on reducing populations of brown and black bears to increase neonate ungulate survival. Although most bear control efforts have been in relatively simple inland trophic systems without, or with limited, salmon (Miller et al., 2017), these efforts have also been implemented in some salmon-driven ecosystems (e.g., Cook Inlet), and proposed but not approved for others. Bear population reduction in interior systems has typically led to improved calf survival (Boertje et al., 2010), but it is unlikely that these results are transferable to the fundamentally different predator-prey dynamics in salmon systems where bears exist at higher density and are less reliant on ungulate neonates (Figure 5). In particular, the non-linear relationship between bear density and ungulate neonate survival (Figure 6) suggests that bear reduction programs in systems strongly subsidized by salmon are unlikely to substantially improve ungulate neonate survival unless bear densities are severely reduced. Such severe reductions in bear densities, besides being difficult to implement both practically and politically, would compromise the functional role of bears reviewed here. Further, bear-induced mortality is likely to be increasingly compensatory as ungulate populations approach nutritional carrying capacity (Zager and Beecham, 2006). For example, in southeastern Alaska, low-birthweight deer neonates were more vulnerable to bear predation and also less likely to survive to recruitment (Gilbert et al., 2015). If bear-induced mortality is compensatory, inadequate bear reductions could unintentionally reduce ungulate recruitment due to prolonged energetic costs of lactation for offspring that will die anyway, thus resulting in reduced pregnancy rates and litter sizes (Testa, 1998; Testa, 2004).
Bears are omnivores that can consume species across a wide range of taxonomic and functional groups. Their apex omnivorous trophic position ensures their strong and wide-ranging effects on food webs, which can be amplified by the salmon that allow for their hyper-abundance. Further research is needed to understand how ecosystems are influenced by the keystone bear-salmon interaction, and in so doing illuminate what has been lost as bear-salmon interactions have vanished across much of the Northern Hemisphere, what threats persist due to ongoing global change, what we maintain where this interaction persists, how we can effectively restore this interaction, and what we might gain as this interaction is restored.
Author Contributions
TL, GH, and KW conceived of the manuscript. KW collected published predator, prey, and survival data. TL conducted the analyses, made figures, and led manuscript writing with contributions from all authors. MH led writing of section on invertebrate scavenging and riparian plant subsidies. TQ contributed substantial text and literature review throughout. JA led writing of section on subsidies to freshwater fish and invertebrates. MA led section on biogeography of bear-salmon interactions. All authors reviewed and edited the final version.
Conflict of Interest
MH was employed by the company Ecofish Research Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the United States Government. We thank the many colleagues who have joined us in field work and freely shared their ideas with us.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.513304/full#supplementary-material
Footnotes
References
Adams, L. G., Farley, S. D., Stricker, C. A., Demma, D. J., Roffler, G. H., Miller, D. C., et al. (2010). Are inland wolf–ungulate systems influenced by marine subsidies of Pacific salmon? Ecol. Appl. 20, 251–262. doi: 10.1890/08-1437.1
Adams, M. S., Service, C. N., Bateman, A., Bourbonnais, M., Artelle, K. A., Nelson, T., et al. (2017). Intrapopulation diversity in isotopic niche over landscapes: spatial patterns inform conservation of bear–salmon systems. Ecosphere 8:e01843.
Auger, J., Ogborn, G. L., Pritchett, C. L., and Black, H. L. (2004). Selection of ants by the American black bear (Ursus americanos). West. N. Am. Nat. 64, 166–174.
Bailey, C. J., and Moore, J. W. (2020). Resource pulses increase the diversity of successful competitors in a multi-species stream fish assemblage. Ecosphere 11:e03211.
Bartz, K. K., and Naiman, R. J. (2005). Effects of salmon-borne nutrients on riparian soils and vegetation in southwest Alaska. Ecosystems 8, 529–545. doi: 10.1007/s10021-005-0064-z
Battin, J., Wiley, M. W., Ruckelshaus, M. H., Palmer, R. N., Korb, E., Bartz, K. K., et al. (2007). Projected impacts of climate change on salmon habitat restoration. Proc. Natl. Acad. Sci. U.S.A. 104, 6720–6725. doi: 10.1073/pnas.0701685104
Belant, J. L., Griffith, B., Zhang, Y., Follmann, E. H., and Adams, L. G. (2010). Population-level resource selection by sympatric brown and American black bears in Alaska. Polar Biol. 33, 31–40. doi: 10.1007/s00300-009-0682-6
Benbow, M., Lewis, A., Tomberlin, J., and Pechal, J. (2013). Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J. Med. Entomol. 50, 440–450. doi: 10.1603/me12194
Ben-David, M. (1997). Timing of reproduction in wild mink: the influence of spawning Pacific salmon. Can. J. Zool. 75, 376–382. doi: 10.1139/z97-047
Ben-David, M., Flynn, R., and Schell, D. (1997a). Annual and seasonal changes in diets of martens: evidence from stable isotope analysis. Oecologia 111, 280–291. doi: 10.1007/s004420050236
Ben-David, M., Hanley, T., Klein, D., and Schell, D. M. (1997b). Seasonal changes in diets of coastal and riverine mink: the role of spawning Pacific salmon. Can. J. Zool. 75, 803–811. doi: 10.1139/z97-102
Bermejo, T., Traveset, A., and Willson, M. F. (1998). Post-dispersal seed predation in the temperate rainforest of Southeast Alaska. Can. Field Nat. 112, 510–512.
Bilby, R. E., Beach, E. W., Fransen, B. R., Walter, J. K., and Bisson, P. A. (2003). Transfer of nutrients from spawning salmon to riparian vegetation in western Washington. Trans. Am. Fish. Soc. 132, 733–745. doi: 10.1577/t02-089
Bilby, R. E., Fransen, B. R., Bisson, P. A., and Walter, J. K. (1998). Response of juvenile coho salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss) to the addition of salmon carcasses to two streams in southwestern Washington. USA. Can. J. Fish. Aquatic Sci. 55, 1909–1918. doi: 10.1139/f98-094
Boertje, R. D., Grangaard, D. V., Valkenburg, P., and Dubois, S. D. (1992). Testing Socially Acceptable Methods of Managing Predation: Reducing Predation on Caribou and Moose Neonates by Diversionary Feeding of Predators, Macomb Platueau. 1990-1994. Federal Aid in Wildlife Restoration Research Progress Report. Project W-23-5, Study 1.40. Juneau: Alaska Department of Fish and Game Division of Wildlife Conservation, 1–34.
Boertje, R. D., Keech, M. A., and Paragi, T. F. (2010). Science and values influencing predator control for alaska moose management. J. Wildl. Manage. 74, 917–928. doi: 10.2193/2009-261
Brinkman, T. J., Person, D. K., Chapin Iii, F. S., Smith, W., and Hundertmark, K. J. (2011). Estimating abundance of Sitka black-tailed deer using DNA from fecal pellets. J. Wildl. Manage. 75, 232–242. doi: 10.1002/jwmg.22
Brinkman, T. J., Person, D. K., Smith, W., Chapin Iii, F. S., Mccoy, K., Leonawicz, M., et al. (2013). Using DNA to test the utility of pellet-group counts as an index of deer counts. Wildl. Soc. Bull. 37, 444–450. doi: 10.1002/wsb.270
Byun, S., Koop, B., and Reimchen, T. (1997). North American black bear mtDNA phylogeography: implications for morphology and the Haida Gwaii glacial refugium controversy. Evolution 51, 1647–1653. doi: 10.2307/2411216
Carbone, C., Frame, L., Frame, G., Malcolm, J., Fanshawe, J., Fitzgibbon, C., et al. (2005). Feeding success of African wild dogs (Lycaon pictus) in the serengeti: the effects of group size and kleptoparasitism. J. Zool. 266, 153–161. doi: 10.1017/s0952836905006710
Carlson, S. M., Rich, J., Harry, B., and Quinn, T. P. (2009). Does variation in selection imposed by bears drive divergence among populations in the size and shape of sockeye salmon? Evolution 63, 1244–1261. doi: 10.1111/j.1558-5646.2009.00643.x
Cederholm, C. J., Kundze, M. D., Murota, T., and Sibatani, A. (1999). Pacific salmon carcasses: essential contributions of nutrients and energy for freshwater and terrestrial ecosystems. Fisheries 24, 6–15. doi: 10.1577/1548-8446(1999)024<0006:psc>2.0.co;2
Charnov, E. L. (1976). Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136. doi: 10.1016/0040-5809(76)90040-x
Christie, K. S., Hocking, M. D., and Reimchen, T. E. (2008). Tracing salmon nutrients in riparian food webs: isotopic evidence in a ground-foraging passerine. Can. J. Zool. 86, 1317–1323. doi: 10.1139/z08-110
Christie, K. S., and Reimchen, T. E. (2008). Presence of salmon increases passerine density on Pacific Northwest streams. Auk 125, 51–59. doi: 10.1525/auk.2008.125.1.51
Darimont, C. T., Paquet, P. C., and Reimchen, T. E. (2008). Spawning salmon disrupt tight trophic coupling between wolves and ungulate prey in coastal British Columbia. BMC Ecol. 8:14. doi: 10.1186/1472-6785-8-14
Darimont, C. T., Paquet, P. C., and Reimchen, T. E. (2009). Landscape heterogeneity and marine subsidy generate extensive intrapopulation niche diversity in a large terrestrial vertebrate. J. Anim. Ecol. 78, 126–133. doi: 10.1111/j.1365-2656.2008.01473.x
Darimont, C. T., Reimchen, T. E., and Paquet, P. C. (2003). Foraging behaviour by gray wolves on salmon streams in coastal British Columbia. Can. J. Zool. 81, 349–353. doi: 10.1139/z02-246
Deacy, W., Leacock, W., Armstrong, J. B., and Stanford, J. A. (2016). Kodiak brown bears surf the salmon red wave: direct evidence from GPS collared individuals. Ecology 97, 1091–1098. doi: 10.1890/15-1060.1
Deacy, W. W., Armstrong, J. B., Leacock, W. B., Robbins, C. T., Gustine, D. D., Ward, E. J., et al. (2017). Phenological synchronization disrupts trophic interactions between Kodiak brown bears and salmon. Proc. Natl. Acad. Sci. 114, 10432–10437. doi: 10.1073/pnas.1705248114
Denton, K. P., Rich, H. B. Jr., and Quinn, T. P. (2009). Diet, movement, and growth of dolly varden in response to sockeye salmon subsidies. Trans. Am. Fish. Soc. 138, 1207–1219. doi: 10.1577/t09-006.1
Enders, M. S., and Wall, S. B. V. (2012). Black bears Ursus americanus are effective seed dispersers, with a little help from their friends. Oikos 121, 589–596. doi: 10.1111/j.1600-0706.2011.19710.x
Farmer, C. J., Person, D. K., and Bowyer, R. T. (2006). Risk factors and mortality of black-tailed deer in a managed forest landscape. J. Wildl. Manage. 70, 1403–1415. doi: 10.2193/0022-541x(2006)70[1403:rfamob]2.0.co;2
Field, R. D., and Reynolds, J. D. (2011). Sea to sky: impacts of residual salmon-derived nutrients on estuarine breeding bird communities. Proc. R. Soc. B Biol. Sci. 278, 3081–3088. doi: 10.1098/rspb.2010.2731
Gard, R. (1971). Brown bear predation on sockeye salmon at Karluk Lake. Alaska. J. Wildl. Manage. 35, 193–204. doi: 10.2307/3799591
Gende, S. M., Edwards, R. T., Willson, M. F., and Wipfli, M. S. (2002). Pacific salmon in aquatic and terrestrial ecosystems: Pacific salmon subsidize freshwater and terrestrial ecosystems through several pathways, which generates unique management and conservation issues but also provides valuable research opportunities. AIBS Bull. 52, 917–928. doi: 10.1641/0006-3568(2002)052[0917:psiaat]2.0.co;2
Gende, S. M., Quinn, T. P., and Willson, M. F. (2001). Consumption choice by bears feeding on salmon. Oecologia 127, 372–382. doi: 10.1007/s004420000590
Gende, S. M., Quinn, T. P., Willson, M. F., Heintz, R., and Scott, T. M. (2004). Magnitude and fate of salmon-derived nutrients and energy in a coastal stream ecosystem. J Freshw. Ecol. 19, 149–160. doi: 10.1080/02705060.2004.9664522
Gende, S. M., and Willson, M. F. (2001). Passerine densities in riparian forests of southeast Alaska: potential effects of anadromous spawning salmon. Condor 103, 624–629. doi: 10.1650/0010-5422(2001)103[0624:pdirfo]2.0.co;2
Gilbert, B. A., and Raedeke, K. J. (2004). Recruitment dynamics of black-tailed deer in the western Cascades. J. Wildl. Manage. 68, 120–128. doi: 10.2193/0022-541x(2004)068[0120:rdobdi]2.0.co;2
Gilbert, S. L. (2015). Environmental Drivers of Deer Population Dynamics and Spatial Selection in Southeast Alaska. Fairbanks, AK: University of Alaska Fairbanks.
Gilbert, S. L., Haynes, T., Lindberg, M. S., Albert, D., Kissling, M., and Person, D. K. (2015). Future Population Trends and Drivers of Change for Alexander Archipelago Wolves on and Near Prince of Wales Island, Alaska. Final Report for Cooperative Agreement: F15AC000206, Fairbanks, AK: University of Alaska Fairbanks.
Grainger, T. N., Letten, A. D., Gilbert, B., and Fukami, T. (2019). Applying modern coexistence theory to priority effects. Proc. Natl. Acad. Sci.U.S.A. 116, 6205–6210.
Gresh, T., Lichatowich, J., and Schoonmaker, P. (2000). An estimation of historic and current levels of salmon production in the Northeast Pacific ecosystem: evidence of a nutrient deficit in the freshwater systems of the Pacific Northwest. Fisheries 25, 15–21. doi: 10.1577/1548-8446(2000)025<0015:aeohac>2.0.co;2
Gunther, K. A., and Smith, D. W. (2004). Interactions between wolves and female grizzly bears with cubs in Yellowstone National Park. Ursus 15, 232–238. doi: 10.2192/1537-6176(2004)015<0232:ibwafg>2.0.co;2
Gustafson, R. G., Waples, R. S., Myers, J. M., Weitkamp, L. A., Bryant, G. J., Johnson, O. W., et al. (2007). Pacific salmon extinctions: quantifying lost and remaining diversity. Conserv. Biol. 21, 1009–1020. doi: 10.1111/j.1523-1739.2007.00693.x
Harrer, L. E., and Levi, T. (2018). The primacy of bears as seed dispersers in salmon-bearing ecosystems. Ecosphere 9, e02076.
Hayes, R., and Baer, A. (1992). Brown bear, Ursus arctos, preying upon gray wolf, Canis lupus, pack. Can. Field Nat. 107, 373–374.
Heaton, T. H., Talbot, S. L., and Shields, G. F. (1996). An ice age refugium for large mammals in the alexander archipelago, southeastern Alaska. Quat. Res. 46, 186–192. doi: 10.1006/qres.1996.0058
Helfield, J. M., and Naiman, R. J. (2001). Effects of salmon-derived nitrogen on riparian forest growth and implications for stream productivity. Ecology 82, 2403–2409. doi: 10.1890/0012-9658(2001)082[2403:eosdno]2.0.co;2
Helfield, J. M., and Naiman, R. J. (2006). Keystone interactions: salmon and bear in riparian forests of Alaska. Ecosystems 9, 167–180. doi: 10.1007/s10021-004-0063-5
Hilderbrand, G. V., Farley, S. D., Robbins, C. T., Hanley, T. A., Titus, K., and Servheen, C. (1996). Use of stable isotopes to determine diets of living and extinct bears. Can. J. Zool. 74, 2080–2088. doi: 10.1139/z96-236
Hilderbrand, G. V., Hanley, T. A., Robbins, C. T., and Schwartz, C. C. (1999a). Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121, 546–550. doi: 10.1007/s004420050961
Hilderbrand, G. V., Schwartz, C. C., Robbins, C. T., Jacoby, M. E., Hanley, T. A., Arthur, S. M., et al. (1999b). The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can. J. Zool. 77, 132–138. doi: 10.1139/z98-195
Hocking, M., and O’Regan, S. (2015). “Carrion communities as indicators in fisheries, wildlife management, and conservation,” in Carrion Ecology, Evolution, and their Applications, eds M. Eric Benbow, Jeffrey K. Tomberlin, and Aaron M. Tarone (Boca Raton: CRC Press), 495–516. doi: 10.1201/b18819-27
Hocking, M. D., and Reimchen, T. E. (2006). Consumption and distribution of salmon (Oncorhynchus spp.) nutrients and energy by terrestrial flies. Can. J. Fish. Aquatic Sci. 63, 2076–2086. doi: 10.1139/f06-110
Hocking, M. D., and Reimchen, T. E. (2009). Salmon species, density and watershed size predict magnitude of marine enrichment in riparian food webs. Oikos 118, 1307–1318. doi: 10.1111/j.1600-0706.2009.17302.x
Hocking, M. D., and Reynolds, J. D. (2011). Impacts of salmon on riparian plant diversity. Science 331, 1609–1612. doi: 10.1126/science.1201079
Hocking, M. D., and Reynolds, J. D. (2012). Nitrogen uptake by plants subsidized by Pacific salmon carcasses: a hierarchical experiment. Can. J. For. Res. 42, 908–917. doi: 10.1139/x2012-045
Hocking, M. D., Ring, R. A., and Reimchen, T. E. (2009). The ecology of terrestrial invertebrates on Pacific salmon carcasses. Ecol. Res. 24, 1091–1100. doi: 10.1007/s11284-009-0586-5
Holt, R. D. (1977). Predation, apparent competition, and the structure of prey communities. Theor. Popul. Biol. 12, 197–229. doi: 10.1016/0040-5809(77)90042-9
Holtgrieve, G. W., Schindler, D. E., and Jewett, P. K. (2009). Large predators and biogeochemical hotspots: brown bear (Ursus arctos) predation on salmon alters nitrogen cycling in riparian soils. Ecol. Res. 24, 1125–1135. doi: 10.1007/s11284-009-0591-8
Hurley, M. A., Hebblewhite, M., and Gaillard, J. M. (2020). Competition for safe real estate, not food, drives density-dependent juvenile survival in a large herbivore. Ecol. Evol. 10, 5464–5475. doi: 10.1002/ece3.6289
Hurteau, L. A., Mooers, A. Ø, Reynolds, J. D., and Hocking, M. D. (2016). Salmon nutrients are associated with the phylogenetic dispersion of riparian flowering-plant assemblages. Ecology 97, 450–460. doi: 10.1890/15-0379.1
IUCN (2020). The IUCN Red List of Threatened Species. Version 2020-2. Available online at: https://www.iucnredlist.org (accessed July 8, 2020).
Jacoby, M. E., Hilderbrand, G. V., Servheen, C., Schwartz, C. C., Arthur, S. M., Hanley, T. A., et al. (1999). Trophic relations of brown and black bears in several western North American ecosystems. J. Wildl. Manage. 63, 921–929. doi: 10.2307/3802806
Kirchhoff, M. D., and Pitcher, K. W. (1988). Evaluations of Methods for Assessing Deer Population trends in Southeast Alaska. Juneau: Alaska Department of Fish and Game, Division of Game.
Koene, P., Ardesch, J., Ludriks, A., Urff, E., Wenzelides, L., and Wittenberg, V. (2002). Interspecific and intraspecific social interactions among brown bears and wolves in an enclosure. Ursus 13, 85–93.
Kohira, M., and Rexstad, E. A. (1997). Diets of wolves, Canis lupus, in logged and unlogged forests of southeastern Alaska. Can. Field Nat. 111, 429–435.
Koike, S. (2010). Long-term trends in food habits of asiatic black bears in the misaka mountains on the pacific coast of central Japan. Mamm. Biol. 75, 17–28. doi: 10.1016/j.mambio.2009.03.008
Levi, T., Darimont, C. T., Macduffee, M., Mangel, M., Paquet, P., and Wilmers, C. C. (2012). Using grizzly bears to assess harvest and ecosystem tradeoffs in salmon fisheries. PLoS Biol. 10:e1001303. doi: 10.1371/journal.pbio.1001303
Levi, T., Wheat, R. E., Allen, J. M., and Wilmers, C. C. (2015). Differential use of salmon by vertebrate consumers: implications for conservation. PeerJ. 3:e1157. doi: 10.7717/peerj.1157
Levi, T., and Wilmers, C. C. (2012). Wolves–coyotes–foxes: a cascade among carnivores. Ecology 93, 921–929. doi: 10.1890/11-0165.1
Lewis, T. M., and Lafferty, D. J. (2014). Brown bears and wolves scavenge humpback whale carcass in Alaska. Ursus 25, 8–13. doi: 10.2192/ursus-d-14-00004.1
Limburg, K. E., and Waldman, J. R. (2009). Dramatic declines in North Atlantic diadromous fishes. BioScience 59, 955–965. doi: 10.1525/bio.2009.59.11.7
Lincoln, A. E., and Quinn, T. P. (2018). Optimal foraging or surplus killing: selective consumption and discarding of salmon by brown bears. Behav. Ecol. 30, 202–212. doi: 10.1093/beheco/ary139
Lincoln, A. E., Wirsing, A. J., and Quinn, T. P. (2020). Prevalence and patterns of scavenging by brown bears on salmon carcasses. Can. J. Zool. (in press).
Lisi, P. J., and Schindler, D. E. (2011). Spatial variation in timing of marine subsidies influences riparian phenology through a plant-pollinator mutualism. Ecosphere 2, 1–14. doi: 10.1890/ES11-00173.1
MacCrimmon, H. R., and Gots, B. L. (1979). World distribution of Atlantic salmon, salmo solar. J. Fish. Board Canada 36, 422–457. doi: 10.1139/f79-062
Mangipane, L. S., Belant, J. L., Lafferty, D. J., Gustine, D. D., Hiller, T. L., Colvin, M. E., et al. (2018). Dietary plasticity in a nutrient-rich system does not influence brown bear (Ursus arctos) body condition or denning. Polar Biol. 41, 763–772. doi: 10.1007/s00300-017-2237-6
Matsubayashi, J., Morimoto, J., Mano, T., Aryal, A., and Nakamura, F. (2014). Using stable isotopes to understand the feeding ecology of the Hokkaido brown bear (Ursus arctos) in Japan. Ursus 25, 87–98. doi: 10.2192/ursus-d-12-00015.1
Matsubayashi, J., Morimoto, J. O., Tayasu, I., Mano, T., Nakajima, M., Takahashi, O., et al. (2015). Major decline in marine and terrestrial animal consumption by brown bears (Ursus arctos). Sci. Rep. 5:9203.
Matsubayashi, J., Tayasu, I., Morimoto, J. O., and Mano, T. (2016). Testing for a predicted decrease in body size in brown bears (Ursus arctos) based on a historical shift in diet. Can. J. Zool. 94, 489–495. doi: 10.1139/cjz-2016-0046
Mattson, D. J. (2002). Consumption of wasps and bees by yellowstone grizzly bears. North. Sci. 76, 166–172.
McCoy, K. (2017). Sitka Black-Tailed Deer Pellet-Group Surveys in Southeast Alaska, 2016 Report. Juneau: Alaska Department of Fish and Game, Division of Wildlife Conservation.
Meehan, E. P., Seminet-Reneau, E. E., and Quinn, T. P. (2005). Bear predation on Pacific salmon facilitates colonization of carcasses by fly maggots. Am. Midl. Nat. 153, 142–152. doi: 10.1674/0003-0031(2005)153[0142:bpopsf]2.0.co;2
Miller, S. D., Schoen, J. W., and Schwartz, C. C. (2017). Trends in brown bear reduction efforts in Alaska, 1980–2017. Ursus 28, 135–149. doi: 10.2192/URSU-D-17-00002.1
Miller, S. D., White, G. C., Sellers, R. A., Reynolds, H. V., Schoen, J. W., Titus, K., et al. (1997). Brown and black bear density estimation in Alaska using radiotelemetry and replicated mark-resight techniques. Wildl. Monogr. 133, 1–55.
Minakawa, N., and Gara, R. I. (1999). Ecological effects of a chum salmon (Oncorhynchus keta) spawning run in a small stream of the Pacific Northwest. J. Freshw. Ecol. 14, 327–335. doi: 10.1080/02705060.1999.9663687
Moore, J. W., Schindler, D. E., and Ruff, C. P. (2008). Habitat saturation drives thresholds in stream subsidies. Ecology 89, 306–312. doi: 10.1890/07-1269.1
Mowat, G., and Heard, D. C. (2006). Major components of grizzly bear diet across North America. Can. J. Zool. 84, 473–489. doi: 10.1139/z06-016
Mowat, G., Heard, D. C., and Schwarz, C. J. (2013). Predicting grizzly bear density in western North America. PLoS One 8:e82757. doi: 10.1371/journal.pone.0082757
Nagasaka, A., Nagasaka, Y., Ito, K., Mano, T., Yamanaka, M., Katayama, A., et al. (2006). Contributions of salmon-derived nitrogen to riparian vegetation in the northwest Pacific region. J. For. Res. 11, 377–382. doi: 10.1007/s10310-006-0226-7
Peacock, E., Titus, K., Garshelis, D. L., Peacock, M. M., and Kuc, M. (2011). Mark–recapture using tetracycline and genetics reveal record-high bear density. J. Wildl. Manage. 75, 1513–1520. doi: 10.1002/jwmg.171
Pechal, J. L., and Benbow, M. E. (2016). Microbial ecology of the salmon necrobiome: evidence salmon carrion decomposition influences aquatic and terrestrial insect microbiomes. Environ. Microbiol. 18, 1511–1522. doi: 10.1111/1462-2920.13187
Petri, A. E. (2016). Bobcat vs. Salmon. Available online at: https://www.nationalgeographic.com/news/2016/12/bobcat-catches-giant-salmon-olympic-national-park-washington/: National Geographic. [Accessed November 4, 2020]
Proctor, M. F., Paetkau, D., Mclellan, B. N., Stenhouse, G. B., Kendall, K. C., Mace, R. D., et al. (2012). Population fragmentation and inter-ecosystem movements of grizzly bears in western Canada and the northern United States: fragmentation de la population et mouvements inter-ecosystèmes des Ours Grizzlis dans L’ouest du Canada et le Nord des États-Unis. Wildl. Monogr. 180, 1–46. doi: 10.1002/wmon.6
Quinn, T. P. (2018). The Behavior and Ecology of Pacific Salmon and Trout. Washington, DC: University of Washington Press.
Quinn, T. P., Carlson, S. M., Gende, S. M., and Rich, H. B. (2009). Transportation of Pacific salmon carcasses from streams to riparian forests by bears. Can. J. Zool. 87, 195–203. doi: 10.1139/z09-004
Quinn, T. P., Cunningham, C. J., and Wirsing, A. J. (2017). Diverse foraging opportunities drive the functional response of local and landscape-scale bear predation on Pacific salmon. Oecologia 183, 415–429. doi: 10.1007/s00442-016-3782-3
Quinn, T. P., Helfield, J. M., Austin, C. S., Hovel, R. A., and Bunn, A. G. (2018). A multidecade experiment shows that fertilization by salmon carcasses enhanced tree growth in the riparian zone. Ecology 99, 2433–2441. doi: 10.1002/ecy.2453
Quinn, T. P., Hendry, A. P., and Buck, G. B. (2001). Balancing natural and sexual selection in sockeye salmon: interactions between body size, reproductive opportunity and vulnerability to predation by bears. Evol. Ecol. Res. 3, 917–937.
Reimchen, T. (2000). Some ecological and evolutionary aspects of bear-salmon interactions in coastal British Columbia. Can. J. Zool. 78, 448–457. doi: 10.1139/z99-232
Reimchen, T. E. (2017). Diverse ecological pathways of salmon nutrients through an intact marine-terrestrial interface. Can. Field Nat. 131, 350–368. doi: 10.22621/cfn.v131i4.1965
Ritchie, E. G., and Johnson, C. N. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. doi: 10.1111/j.1461-0248.2009.01347.x
Roffler, G. H., Allen, J. M., Massey, A., and Levi, T. (2020). Metabarcoding of fecal DNA shows dietary diversification in wolves substitutes for ungulates in an island archipelago. Ecosphere (in press).
Schindler, D. E., Armstrong, J. B., Bentley, K. T., Jankowski, K., Lisi, P. J., and Payne, L. X. (2013). Riding the crimson tide: mobile terrestrial consumers track phenological variation in spawning of an anadromous fish. Biol. Lett. 9:20130048. doi: 10.1098/rsbl.2013.0048
Schindler, D. E., Scheuerell, M. D., Moore, J. W., Gende, S. M., Francis, T. B., and Palen, W. J. (2003). Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 1:31–37.
Service, C. N., Adams, M. S., Artelle, K. A., Paquet, P., Grant, L. V., and Darimont, C. T. (2014). Indigenous knowledge and science unite to reveal spatial and temporal dimensions of distributional shift in wildlife of conservation concern. PLoS One 9:e101595. doi: 10.1371/journal.pone.0101595
Service, C. N., Bateman, A. W., Adams, M. S., Artelle, K. A., Reimchen, T. E., Paquet, P. C., et al. (2019). Salmonid species diversity predicts salmon consumption by terrestrial wildlife. J. Anim. Ecol. 88, 392–404. doi: 10.1111/1365-2656.12932
Seryodkin, I., Paczkowski, J., Borisov, M., and Petrunenko, Y. (2017). Home ranges of brown bears on the Kamchatka peninsula and Sakhalin Island. Contemp. Probl. Ecol. 10, 599–611. doi: 10.1134/s1995425517060129
Seryodkin, I. V., Panichev, A. M., and Slaght, J. C. (2016). Geophagy by brown bears in the Russian Far East. Ursus 27, 11–17. doi: 10.2192/ursus-d-15-00014.1
Shakeri, Y. N., White, K. S., and Levi, T. (2018). Salmon-supported bears, seed dispersal, and extensive resource subsidies to granivores. Ecosphere 9:e02297. doi: 10.1002/ecs2.2297
Stephens, D. W., and Krebs, J. R. (1986). Foraging Theory. Princeton, NJ: Princeton University Press.
Swain, N. R., and Reynolds, J. D. (2015). Effects of salmon-derived nutrients and habitat characteristics on population densities of stream-resident sculpins. PLoS One 10:e0116090. doi: 10.1371/journal.pone.0116090
Swenson, J. E., Jansson, A., Riig, R., and Sandegren, F. (1999). Bears and ants: myrmecophagy by brown bears in central Scandinavia. Can. J. Zool. 77, 551–561. doi: 10.1139/z99-004
Tallian, A., Ordiz, A., Metz, M. C., Milleret, C., Wikenros, C., Smith, D. W., et al. (2017). Competition between apex predators? Brown bears decrease wolf kill rate on two continents. Proc. R. Soc. B Biol. Sci. 284:20162368. doi: 10.1098/rspb.2016.2368
Terborgh, J. (1988). The big things that run the world-a sequel to E. O. Wilson. Conserv. Biol. 2, 402–403. doi: 10.1111/j.1523-1739.1988.tb00207.x
Testa, J. W. (1998). Compensatory response to changes in calf survivorship: management consequences of a reproductive cost in moose. Alces 34, 107–115.
Testa, J. W. (2004). Population dynamics and life history trade-offs of moose (Alces alces) in south-central Alaska. Ecology 85, 1439–1452. doi: 10.1890/02-0671
Traveset, A., and Willson, M. F. (1997). Effect of birds and bears on seed germination of fleshy-fruited plants in temperate rainforests of southeast Alaska. Oikos 80, 89–95. doi: 10.2307/3546519
van den Top, G. G., Reynolds, J. D., Prins, H. H., Mattsson, J., Green, D. J., and Ydenberg, R. C. (2018). From salmon to salmonberry: the effects of salmon-derived nutrients on the stomatal density of leaves of the nitriphilic shrub Rubus spectabilis. Funct. Ecol. 32, 2625–2633. doi: 10.1111/1365-2435.13202
van der Meer, E., Moyo, M., Rasmussen, G. S., and Fritz, H. (2011). An empirical and experimental test of risk and costs of kleptoparasitism for African wild dogs (Lycaon pictus) inside and outside a protected area. Behav. Ecol. 22, 985–992. doi: 10.1093/beheco/arr079
Vucetich, J. A., Hebblewhite, M., Smith, D. W., and Peterson, R. O. (2011). Predicting prey population dynamics from kill rate, predation rate and predator–prey ratios in three wolf-ungulate systems. J. Anim. Ecol. 80, 1236–1245. doi: 10.1111/j.1365-2656.2011.01855.x
Walsh, J. C., Pendray, J. E., Godwin, S. C., Artelle, K. A., Kindsvater, H. K., Field, R. D., et al. (2020). Relationships between Pacific salmon and aquatic and terrestrial ecosystems: implications for ecosystem-based management. Ecology 101:e03060.
Wilkinson, C., Hocking, M., and Reimchen, T. (2005). Uptake of salmon-derived nitrogen by mosses and liverworts in coastal British Columbia. Oikos 108, 85–98. doi: 10.1111/j.0030-1299.2005.13277.x
Willson, M. F. (1991). Dispersal of seed by frugivorous animals in temperate forests. Rev. Chil. Hist. Nat. 64, 537–554.
Willson, M. F., Gende, S. M., and Marston, B. H. (1998). Fishes and the forest. BioScience 48, 455–462.
Willson, M. F., and Halupka, K. C. (1995). Anadromous fish as keystone species in vertebrate communities. Conserv. Biol. 9, 489–497. doi: 10.1046/j.1523-1739.1995.09030489.x
Wilson, E. O. (1987). The little things that run the world (The importance and conservation of invertebrates). Conserv. Biol. 1, 344–346. doi: 10.1111/j.1523-1739.1987.tb00055.x
Winder, M., Schindler, D. E., Moore, J. W., Johnson, S. P., and Palen, W. J. (2005). Do bears facilitate transfer of salmon resources to aquatic macroinvertebrates? Can. J. Fish. Aquatic Sci. 62, 2285–2293. doi: 10.1139/f05-136
Wipfli, M. S., Hudson, J. P., Caouette, J. P., and Chaloner, D. T. (2003). Marine subsidies in freshwater ecosystems: salmon carcasses increase the growth rates of stream-resident salmonids. Trans. Am. Fish. Soc. 132, 371–381. doi: 10.1577/1548-8659(2003)132<0371:msifes>2.0.co;2
Keywords: competition, predation, resource pulse, seed dispersal, subsidies
Citation: Levi T, Hilderbrand GV, Hocking MD, Quinn TP, White KS, Adams MS, Armstrong JB, Crupi AP, Darimont CT, Deacy W, Gilbert SL, Ripple WJ, Shakeri YN, Wheat RE and Wilmers CC (2020) Community Ecology and Conservation of Bear-Salmon Ecosystems. Front. Ecol. Evol. 8:513304. doi: 10.3389/fevo.2020.513304
Received: 23 September 2020; Accepted: 12 November 2020;
Published: 04 December 2020.
Edited by:
Laurentiu Rozylowicz, University of Bucharest, RomaniaReviewed by:
William Atlas, Wild Salmon Center, United StatesSlaven Reljic, University of Zagreb, Croatia
Copyright © 2020 Levi, Hilderbrand, Hocking, Quinn, White, Adams, Armstrong, Crupi, Darimont, Deacy, Gilbert, Ripple, Shakeri, Wheat and Wilmers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taal Levi, VGFhbC5MZXZpQE9yZWdvbnN0YXRlLmVkdQ==
 Taal Levi
Taal Levi Grant V. Hilderbrand2
Grant V. Hilderbrand2 Thomas P. Quinn
Thomas P. Quinn Sophie L. Gilbert
Sophie L. Gilbert Rachel E. Wheat
Rachel E. Wheat