
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 September 2020
Sec. Coevolution
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00262
This article is part of the Research Topic Symbiosis in a Changing Environment View all 11 articles
Microbial symbionts of marine sponges play important roles for the hosts and also for their ecosystems. The unique tolerance of marine sponges to a wide diversity of microbial symbionts allows them to acquire a wide variety of “evolutionary solutions” to environmental challenges. Ice scour is one of the main forces structuring Antarctic benthic communities, and its effect is expected to increase as further warming is projected for the Western Antarctic Peninsula (WAP). The interaction of these physical drivers may have a significant impact, shaping the microbiome of Antarctic sponges under current and future scenarios of climate change. The aim of this research was to assess how stressors, such as warming and injuries produced by ice scour, affect the microbiome of the marine Antarctic sponge Isodictya kerguelenensis under current and predicted scenarios. Individuals of I. kerguelenensis were sampled in shallow waters (10 m) off the coast of Doumer Island, Palmer Archipelago, WAP. In order to mimic the effect of tissue damage produced by ice scour, tissue samples were taken at days 0 (T0d) and 15 (T15d) from individuals placed in a control (0.5°C) and two temperature treatments (3 and 6°C). Our analysis of 16S libraries from the V4–V5 region revealed two phyla of archaea and 22 of bacteria. Proteobacteria and Bacteroidetes were the most representative in terms of both number of operational taxonomic units (OTUs) and sequence abundances. The analysis at the OTU level shows a significant interactive effect of injury and temperature. Principal coordinate analysis (PCoA) shows a clear group of uninjured sponges and three other groups of injured sponges according to temperature. Our results also show a group of OTUs that were only present in injured sponges and are potential markers of sponge damage. Our study suggests that the disturbance produced by icebergs may have a direct impact on the sponge microbiome. Future climate change scenarios with warming and increases in iceberg impacts may lead to prokaryotic symbiont disruption on sponge species, potentially having cascading effects for the host and the functional roles they play in the Antarctic ecosystem; however, the potential effects of this disruption are to be further studied.
The phylum Porifera, known as sponges, is a highly important metazoan, playing roles in three main areas: (a) impacts on the substrate (provoking bio-erosion, building and stabilizing reefs, and consolidating and regenerating the benthos substrate), (b) bentho-pelagic coupling (interfering in carbon, silicon, oxygen, and nitrogen cycles and coupling energy from the pelagic to the benthic ecosystem), and (c) sponge associations with other organisms (facilitating primary production, being directly implicated in secondary production, and providing a complex microhabitat from bacteria and archaea to other macro-invertebrates and fish) (Bell, 2008).
Sponges are the earliest diverged metazoan group still extant (van Soest et al., 2012) and continue to survive in vast numbers of marine and freshwater habitats, adapting to drastic changes in environmental factors and competing biota (Müller, 2003). According to van Soest et al. (2012), the simple body organization of sponges and relative plasticity of the cellular elements, coupled with a unique tolerance toward symbiotic microorganisms, allows for a great diversity of “evolutionary solutions” to environmental challenges. Antarctic sponges are no exception; they are important members of benthic communities and host diverse prokaryotic communities with a remarkable host specificity of the microbiome at spatial (Rodríguez-Marconi et al., 2015; Cárdenas et al., 2018a; Steinert et al., 2019) and temporal scales (Cárdenas et al., 2019). However, the number of studied species is still very limited (see Lo Giudice et al., 2019 for review).
The Western Antarctic Peninsula (WAP) is one of the areas of the planet experiencing some of the most significant environmental changes (Stenni et al., 2017; Znój et al., 2017; Siegert et al., 2019). The increase in seawater temperature constitutes a major threat to Antarctic ecosystems and recent reports have shown that some areas in the WAP seem to be more exposed to environmental variation than others, suggesting organisms inhabiting different zones might show different responses depending on the habitats where they occur (Cárdenas et al., 2018b). In addition, ice scour is considered to be a major force shaping the ecological characteristics of the Antarctic benthos in shallow waters (Gutt and Starmans, 2001; Brown et al., 2004; Barnes, 2017; Barnes et al., 2018; Lo Giudice et al., 2019; Morley et al., 2019). Disturbance produced by icebergs can affect the seabed and associated benthic communities (Gutt, 2001; Cook et al., 2005). In some areas of the WAP, up to a third of the substrate in shallow waters is disturbed by icebergs within a year (Barnes, 2017). In this regard, some research has assessed the responses of benthic organisms to damage, such as that produced by ice scour. Research on other Antarctic invertebrates has shown the existence of differences in recovering from shell damage and shell thickness in the Antarctic bivalve Laternula elliptica depending on the level of exposure to ice scour (Harper et al., 2012). In addition, a study on the transcriptomic response of shell damage in the same species (Sleight et al., 2015) revealed that different important molecular pathways were affected, switching from energy production to biomineralization during the shell repair process. Similar studies on the effect of ice scour on the ecology/physiology of other sessile Antarctic organisms, such as sponges, are yet to be conducted.
In order to improve predictions regarding the impact of climate change on Antarctic biota, it is necessary to study the synergistic effects of multiple environmental stressors because responses to a single stressor tested individually may not be the same as when they are tested at the same time (Bell and Carballo, 2017). However, only a few studies on the combined effects of pH and temperature in Antarctic invertebrates have been conducted (Ericson et al., 2012; Suckling et al., 2015; Schram et al., 2016). This is explained by difficulties associated with working in the Antarctic and the availability of facilities that are able to undertake complex laboratory experiments (Kennicutt et al., 2016).
The increase of sea-surface temperature can have negative impacts on some sponge species by negatively affecting growth and survival, producing high levels of tissue necrosis and bleaching (Bennett et al., 2017). Recent studies have reported mass mortality events due to warming in sub-Arctic sponges (Ereskovsky et al., 2019). In contrast, other sponge species are more resistant to thermal stress (Guzman and Conaco, 2016; González-Aravena et al., 2019). Several studies have also tested the effect of warming on the sponge-associated microbial community, showing contrasting results. Although some studies have demonstrated the ability of some sponge species to remain stable with no effect on their microbiome (Simister et al., 2012; Pineda et al., 2016; Cárdenas et al., 2019), in others, warming produced changes and even disruption of the microbiome, affecting sponge health (Lemoine et al., 2007; Luter et al., 2012b; Fan et al., 2013). In addition, other studies (e.g., Lesser et al., 2016) reported that, although warming produced no change in the microbiome composition in the tropical sponge Xestospongia muta, the combined stress of warming and pH decrease changed the microbiome composition. In contrast, the microbiome of the bioeroding sponge Cliona orientalis remained stable up to a 4°C increase, but a community shift occurred at a further 6°C increase, and near 32°C, the microbe composition changed significantly (Ramsby et al., 2018).
The effect of physical disturbance on the sponge microbiome remains unstudied. Studies from tropical corals have reported changes in some bacterial groups after injuries and differences between diseased and healthy sponges (Meyer et al., 2016; Shirur et al., 2016). A study on the Great Barrier Reef sponge Rhopaloeides odorabile reported that sublethal temperature stress did not produce bacterial alteration; however, a bacterial shift was observed in necrotic individuals, revealing microbiome shifts when lesions are visible (Simister et al., 2012). Regarding unhealthy and dying sponges, considerable microbiome alteration has been revealed with shifts in the abundance of some taxa (Blanquer et al., 2016; Luter et al., 2017; Belikov et al., 2019). In light of previous research from other latitudes, microbiome composition can be a good marker of holobiont health. In this regard, despite an increase of Antarctic sponge microbiome information, few studies have provided insights on holobiont health that take into account all environmental threats with which the sponge holobiont must cope at the present and in future conditions in Antarctica. In the case of the Antarctic sponge Isodictya sp., a previous transcriptomic study suggested that increased temperature could cause stress at the limits of the organism’s capacities (González-Aravena et al., 2019).
Because climate change projections suggest warming will induce further ice shelf collapse, glacier retreat, and reduction of sea ice, ice scour is expected to have increased effects on benthic organisms as more icebergs will be able to float freely in open water, reaching shallow areas (Barnes et al., 2018; Morley et al., 2019). For this reason, we tested if the interaction of increased temperature and physical injuries produced by ice scour could impact the stability of the microbiome of the Antarctic sponge Isodictya kerguelenensis (Ridley and Dendy, 1886), which is a common sponge in shallow areas around the Palmer Archipelago, WAP.
Eighteen small individuals of the Antarctic sponge Isodictya kerguelenensis (∼6–8 cm) were collected by SCUBA divers at 10 m depth in Cape Kemp, off Yelcho Research Station, Doumer Island, Palmer Archipelago, WAP (64°51′S 63°35′W), in January 2018 (Figure 1A). Samples were collected by hand and placed in plastic bags inside buckets for transportation underwater. Sponge individuals were then transported to the laboratory facility at Yelcho Research Station (INACH), where they were maintained in 140 L fiberglass tanks with unfiltered seawater at approximately 0.5°C for a week to allow acclimation to laboratory conditions. Water was changed daily, and circulation within each tank was provided by submersible aquarium pumps.
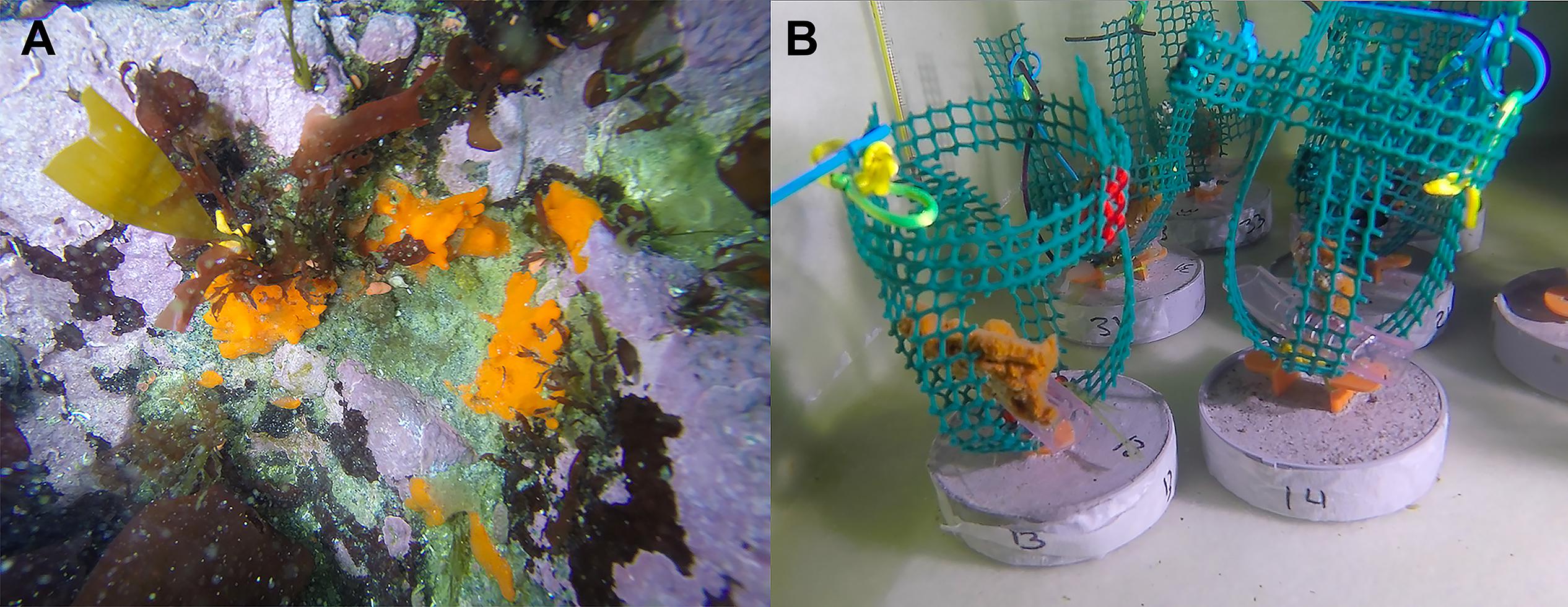
Figure 1. The Antarctic sponge Isodictya kerguelenensis. (A) Photographed in situ at 10 m depth in Cape Kemp off Yelcho Research Station, Doumer Island, WAP (64°51′S 63°35′W) in January 2017. (B) Isodictya kerguelenensis individuals maintained under experimental conditions in holding tanks at Yelcho Research Station at Doumer Island, WAP.
Experimental tanks were covered with two layers of 1-mm mesh shade cloth (fiberglass 50% neutral density screen) in order to represent light levels occurring in situ. After the acclimation period, three individuals were randomly allocated to one of three different experimental tanks (Figure 1B). The experimental design consisted of three treatments: control (0.5°C) and two heat-stress treatments at 3°C and 6°C, and these same sponges were cut to mimic the effect of ice scour (n = 3 per treatment). In order to assess the effect of increased temperature on the prokaryotic communities of I. kerguelenensis, a small piece of tissue (∼0.3 g) from each individual was obtained at day 0 (T0, before injury) and after 15 days (T15, after injury) for each temperature condition (0.5, 3, and 6°C). Sponges in the control tank were also sampled to assess for effects of tissue injuries by cutting a piece (∼0.3 g) to mimic the effect produced by ice scour on sponges. Individuals were maintained in closely controlled aquarium conditions to assess the impact of temperature increase and tissue injuries as well as the potential interactions of tissue damage with increased temperature. Previous work in the study area has shown that Antarctic sponges suffer injuries by ice scour (Cárdenas et al., 2019). Ice scour can produce partial loss of pieces of the body, or in other cases, individuals are removed entirely (Barnes, 2017). Here, we mimic sublethal effects of ice scour (partial tissue loss) to determine if even smaller injuries can provoke shifts in the prokaryotic community. A total of 18 samples were obtained at the end of the experiment, but one individual in the 3°C condition was another cryptic species. This individual was removed from the analysis, and for that reason, the 3°C (days 0 and 15) condition has only two individuals. Identification of the material used in the experiment was assisted by sponge taxonomy experts from the Museu Nacional UFRJ, Brazil.
Seawater was warmed and maintained at a set temperature with SOBO aquarium heaters (500 watt), and seawater was replaced every day from the natural Isodictya kerguelenensis environment. Temperatures used in the heat-stress treatments corresponded to unusual summer peaks recorded in shallow waters at Doumer Island (Cárdenas et al., 2018b) and the predicted temperature under the high-end 2080 IPCC scenario (IPCC, 2014), respectively.
Genomic DNA from sponge tissue for 16S rRNA gene amplicon sequencing was extracted using the Precellys© Evolution homogenizer with a Cryolys cooling unit (Bertin Technologies, Montigny-Le-Bretonneux, France) and a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Extractions were performed using both internal and external sponge tissue in order to obtain the whole bacterial community. DNA concentrations were measured using a Nanoquant spectrophotometer (Tecan, Switzerland). Ten microliters of DNA sample were sent to Dalhousie University CGEB-IMR1 for V4–V5 rRNA gene library preparation and sequencing. Primers used correspond to 517f GTGYCAGCMGCCGCGGTAA and 926r CCGYCAATTYMTTTRAGTTT (Walters et al., 2016). Samples were multiplexed using a dual indexing approach and sequenced using an Illumina Miseq with paired-end 300 + 300 bp reads. All PCR procedures, primers, and Illumina sequencing details were as described in Comeau et al. (2017). The cDNA sequences of 16 libraries were deposited at NCBI as a BioProject with accession ID PRJNA595145 with the exception of two libraries corresponding to the same individual at day 0 (before injury) and day 15 (after injury) at 3°C. These libraries were not included in the posterior analysis because this sponge individual, in fact, corresponds to a cryptic related species (González-Aravena et al., 2019).
Microbiome data was analyzed with the QIIME2 pipeline (Bolyen et al., 2018), using available plugins from the QIIME2 website2. Briefly, 16 paired-end libraries in fastq compressed files (.gz) were imported to perform merge reads from R1 and R2 libraries per sample, trimming primers and low-quality nucleotides at the end of the sequences, denoising, removing chimeras, and clustering sequences. This procedure produces three files: one with cluster sequences in a FASTA file and a second file with a table of cluster abundances. The third file contains the overall stats. Clustered and filtered sequences were then affiliated using the SILVA132 database with a 99% identity for seven levels of taxonomy. Before statistical analyses, clusters affiliated as chloroplasts and mitochondria were removed.
A rooted phylogenetic tree was created to be used for statistical diversity analyses. A core metric plugin of Qiime2 was applied to operational taxonomic unit (OTU) abundance data for each sample to obtain values for observed OTUs, Chao1, Shannon, and Simpson diversity indexes. Prokaryotic community compositions at the order level were plotted with a bar plot graphic with the most frequent prokaryotic orders. A heat map plot was created according to the OTUs’ relative abundance. A principal coordinate analysis (PCoA) was then performed to visualize the differences between samples using a Bray-Curtis distance matrix of beta diversity using the diversity core metric plugin. In order to determine the existence of OTUs present in all samples from all treatments, we examined the core OTUs that were present before and after stress exposure, selecting only OTUs present in all 16 samples from the whole OTU list. Differences in the beta diversity of the prokaryotic community between treatments at the order and OTU levels were tested with PERMANOVA analyses based on Bray-Curtis distance matrices. PERMANOVA tests also were performed (treated as a univariate measure) to test for differences in univariate measures of diversity (Sobs, Chao1, Shannon, and Simpson) among experimental treatments. Tests were performed based on Euclidean distance matrices, and Monte Carlo tests were used when the number of unique permutations was low as recommended by Anderson et al. (2008). We used PERMANOVA for univariate analyses as it allows for two-factor designs, considers an interaction term, and does not assume a normal distribution of errors. A similarity percentage analysis (SIMPER) was also performed for detecting the OTUs contributing most to the dissimilarity between experimental conditions. PERMANOVA and SIMPER analyses were performed using Primer v7 (Anderson et al., 2008).
Analysis of the prokaryotic community of 16 samples of Isodictya kerguelenensis yielded 1,905,120 paired-end reads, from which 1,555,032 reads (81.62%) were retained after filtering and denoising. Then, 1,371,028 reads (71.97%) were assembled in 685,514 contigs of which 515,163 (75.15%) were retained after chimera, chloroplast, and mitochondrial sequence removal.
A total of 648 OTUs were obtained, from which only one had no taxonomic affiliation (Supplementary File 1). The affiliation process detected one archaea phyla (Thaumarchaeota), having only two OTUs with low abundance, and 22 bacterial phyla; Proteobacteria and Bacteroidetes were the most represented for both OTUs and sequence abundance (Table 1). Other well-represented phyla for OTU frequency were Planctomycetes, Firmicutes, and Verrucomicrobia with more than 10 OTUs each. Regarding sequence frequency, other phyla that were well represented were Fusobacteria and Epsilonbacteraeota with more than 1000 sequences each.
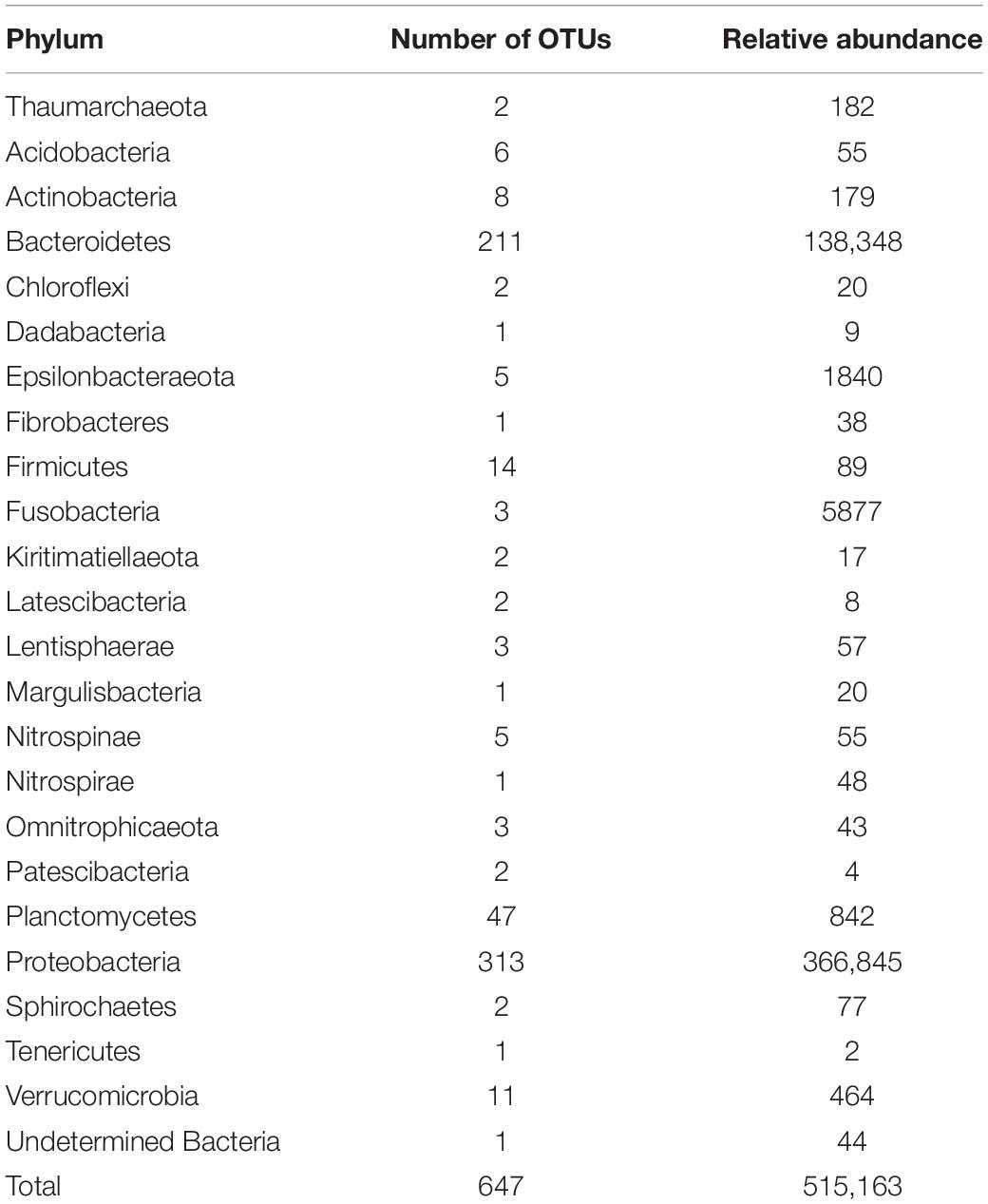
Table 1. OTU and sequence frequencies by phylum from samples of Isodictya kerguelenensis maintained under experimental conditions.
The rarefaction curves revealed that samples from treatment 3C–15d showed the highest number of observed OTUs, which was very distant from the other conditions (Supplementary Figure S1).
The effects of the experimental treatment on diversity indexes was variable (Supplementary File 2). The observed and estimated (Chao1) number of OTUs, Shannon, and Simpson were affected by injury over time (Figure 2), whereas temperature did not have an effect. However, in most cases, a significant interaction between injury and temperature was recorded (Supplementary File 2).
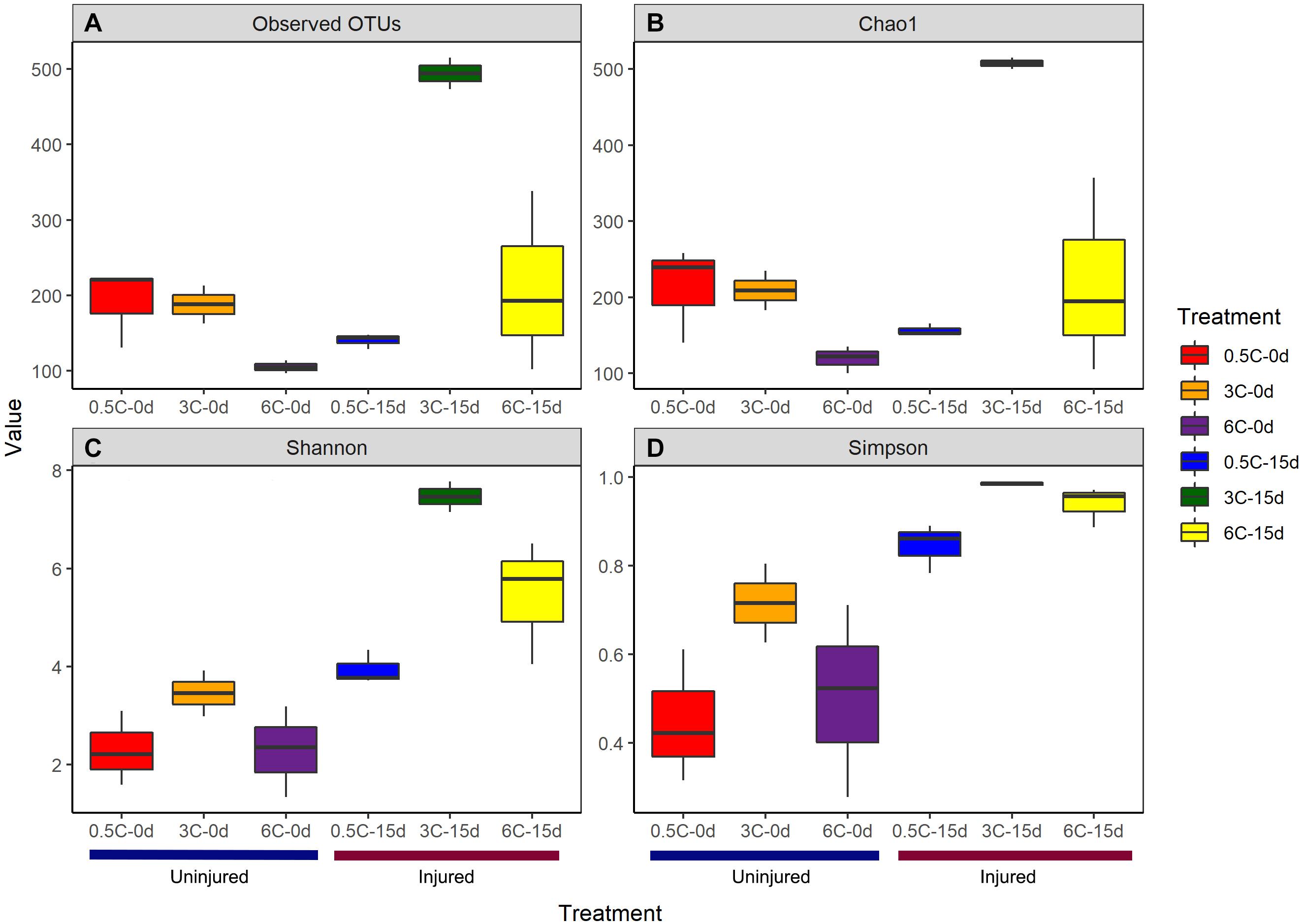
Figure 2. (A) Observed OTU richness (Sobs) (B) Estimated OTU richness (Chao1) (C) Simpson (D) Shannon diversity indices of the prokaryotic communities associated with specimens of Isodictya kerguelenensis exposed to different experimental conditions.
The PCoA showed a clear group separation between samples from T0d and T15d indicating an effect of physical damage over time, suggesting that tissue damage rather than temperature drove observed differences (Figure 3). Axis 1 explains 55.09% of the variation, separating the T0 control group from the other three groups. Axis 2 explains 14.03% of the differences, separating the injured sponges according to temperature and showing a moderate effect of temperature on microbiome composition. A significant effect of temperature (PERMANOVA F = 2.8349, R2 = 0.15, p = 0.0005) and injury (PERMANOVA F = 13.965, R2 = 0.37, p = 0.0001) and the interaction between both factors was also significant (PERMANOVA F = 2.9865, R2 = 0.16, p = 0.0003). The microbiota of the uninjured sponges (T0d) was highly dominated by an unaffiliated order of Gammaproteobacteria (66.71%) compared with samples from injured sponges (T15d; Figure 4), where most showed low relative abundance values (1.58%). In contrast, an increase in relative abundance of Altermonadales (34.92%) was recorded in injured sponges compared with uninjured sponges (4.44%; Figure 4).
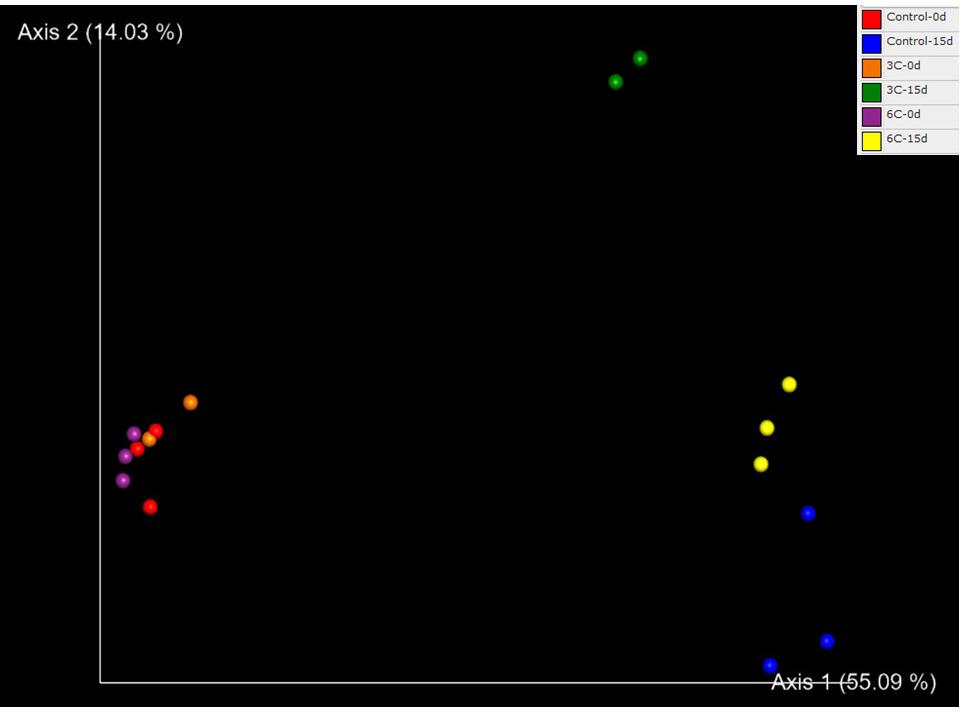
Figure 3. Principal coordinate analysis of prokaryotic OTUs associated with Isodictya kerguelenensis exposed to different experimental conditions.
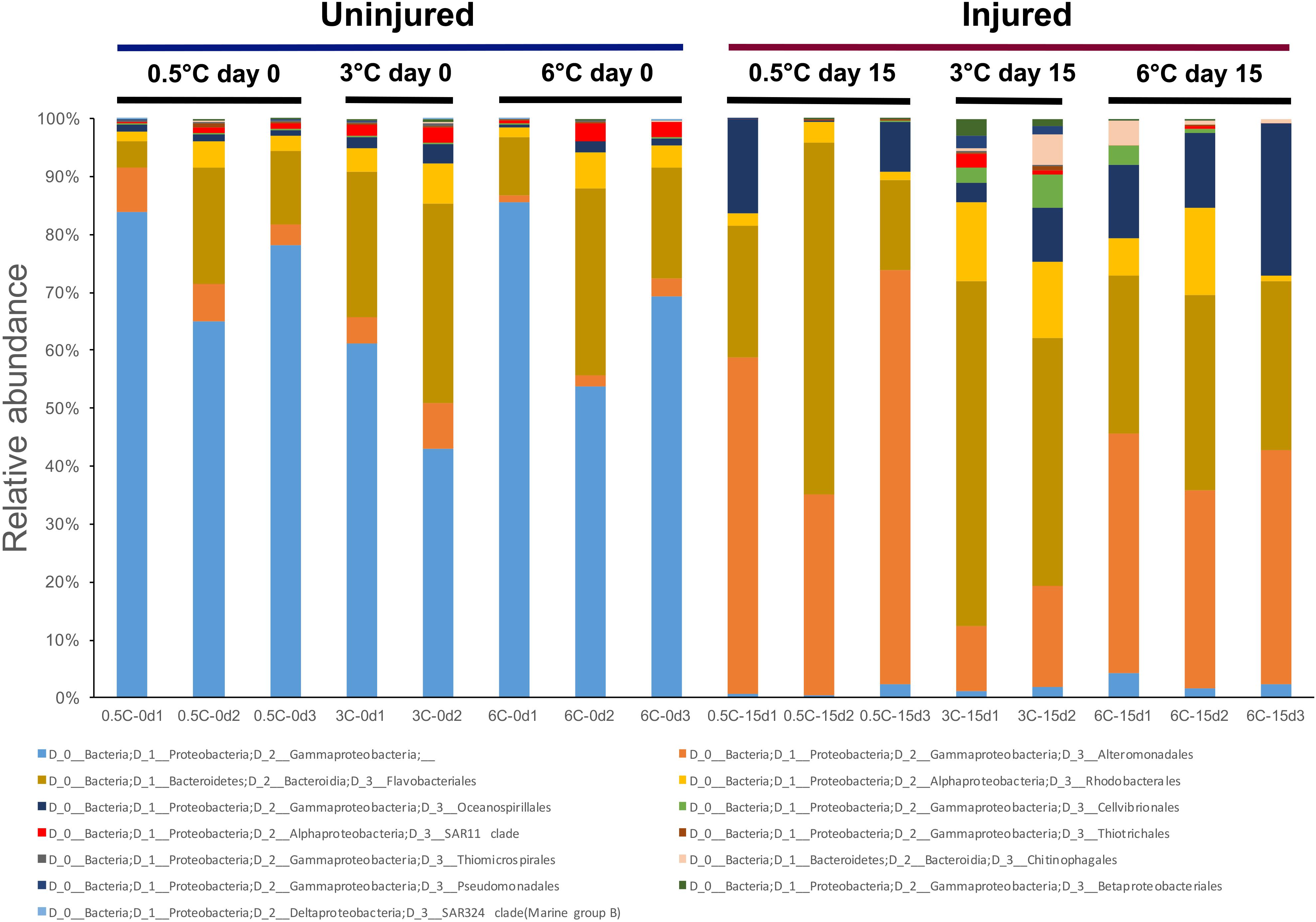
Figure 4. Relative abundance of prokaryotic groups at the order level for Isodictya kerguelenensis individuals exposed to increased temperature and physical injuries.
The core community comprised 13 OTUs (Supplementary File 3), and all of them were present among the 31 most abundant OTUs (see Supplementary Table S1). From these OTUs, 10 belonged to Proteobacteria and three to Bacteroidia. Proteobacterial OTUs belonged mainly to Alteromonadales, Rhodobacterales, and Oceanospirillales, whereas Bacteroidial OTUs belonged to Flavobacteriales.
The SIMPER analysis revealed that 40 OTUs contributed to 50% of the differences between the T0 uninjured and T15d injured samples (Supplementary File 4). From these, seven OTUs represented 25% of the differences between the two conditions, belonging to the 31 most abundant OTUs (Supplementary Table S1). From these OTUs, four were found in the core microbiome (Supplementary File 3), showing that the abundance of some core OTUs was influenced by injuries. Although OTU 631 was highly represented in healthy sponges, OTU 175 was present almost uniquely in injured individuals.
The heat map of the most representative OTUs in terms of relative abundance reveals clear differences in the abundance of several OTUs between uninjured and injured sponges (Figure 5). Among these OTUs, seven OTUs were also some the most discriminant, explaining differences between treatments according to the SIMPER analysis (Supplementary File 4). Overall, the shift in abundance shown in the heat map is highly concordant with the SIMPER analysis; the gammaproteobacterial OTU631 showed the highest abundance in uninjured sponges, reaching 83.25%, whereas its abundance was greatly reduced to 3.80% in injured sponges. Inversely, another gammaproteobacterial OTU (OTU466, Gammaproteobacteria, Alteromonadales, Colwellia genus) showed considerably higher abundance in injured sponges (48.60%) compared with uninjured sponges (2.22%). Another OTU revealed by the SIMPER analysis that showed marked differences in relative abundance between uninjured and injured sponges (as seen in the heat map) is the OTU175, which belongs to Flavobacteriaceae (genus Pseudofulvibacter).
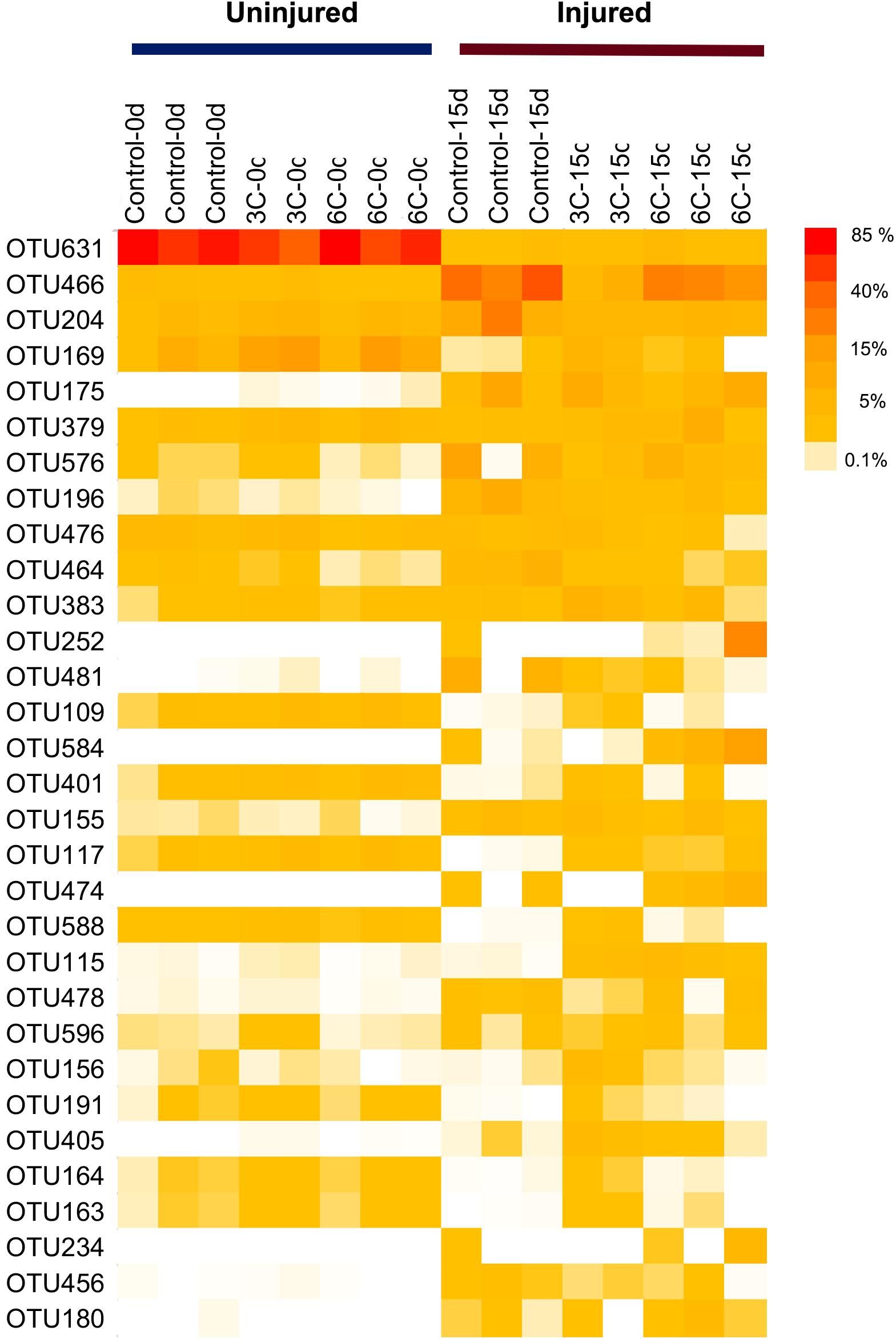
Figure 5. Heat map of the 31 most abundant prokaryotic OTUs associated with Isodictya kerguelenensis individuals and exposed to different experimental conditions.
The present study has characterized a prokaryotic community shift in the common Antarctic sponge Isodictya kerguelenensis after being experimentally exposed to tissue injury and warming. To our knowledge, this is the first study that aims to mimic the sublethal effects of ice scour, which is one of the main forces affecting Antarctic benthic communities by damaging and removing organisms from the seabed. As was observed in a previous study at Doumer Island (Cárdenas et al., 2019), sponges and other organisms are exposed to frequent disturbance produced by ice scour, especially in summer. The current study assessed the effect of small tissue damage and its potential effects on the prokaryotic community associated with a common Antarctic sponge. Our experimental study suggests that tissue injuries similar to those produced by ice scour, a common physical stressor in polar environments, may alter the sponge-associated prokaryotic communities. Current evidence suggests that, on average, almost 30% of the substrate in shallow waters in some areas of the WAP is affected by icebergs each year (Barnes, 2017), and this is expected to increase in the future as a consequence of climate change, further affecting sponges (Morley et al., 2019). This is the first study evaluating the combined effects of acute heat stress and tissue injury in an Antarctic sponge, providing valuable information about microbiome composition shifts and potential microbial markers of healthy and injured specimens of I. kerguelenensis.
The healthy sponge holobiont is considered an ecosystem that is in a state of dynamic equilibrium, but the strength and outcome of the interactions among the members of the holobiont may be affected by perturbations that challenge the healthy equilibrium. This equilibrium is a dynamic ecosystem that is characterized by its resistance and resilience (Pita et al., 2018). This study explored these characteristics using an Antarctic sponge exposed to disturbance. Our results suggest that tissue injuries on Isodictya individuals are enough to provoke a prokaryotic shift, suggesting that tissue injuries, such as those provoked by iceberg scour, can alter the dynamic equilibrium of the sponge–prokaryotes relationship.
Changes in seawater temperature have produced significant changes in sponge abundance, diseases, and mass mortality from various latitudes (Bell et al., 2015; Blanquer et al., 2016; Luter et al., 2017; Belikov et al., 2019). A recent study reported a mass mortality event in sub-Arctic sponge communities particularly affecting the sponge species Isodictya palmata and Halichondria sitiens (Ereskovsky et al., 2019); despite observing signs of illness, no further analyses were carried out.
Recent evidence suggests that, in general, sponges and their microbiome are able to cope with environmental perturbations (Gantt et al., 2017; Glasl et al., 2018) and show remarkable seasonal stability, confirming a host-specific stable association between sponges and their prokaryotic consortiums (Erwin et al., 2012; Cárdenas et al., 2018a, 2019). However, in some cases, warming can disrupt the balance of the sponge microbiome. For instance, Lesser et al. (2016) showed a significant effect of increased seawater temperature and ocean acidification on the microbiome of the tropical sponge X. muta. Similar effects have been reported for other organisms, such as mussels, when exposed to a high temperature for 3 days, producing a proliferation of opportunistic bacteria, which may promote susceptibility to disease (Li et al., 2018).
A previous study on Isodictya sp. monitored in situ during a 24-month period on Doumer Island reports high stability of the microbiome even after being exposed to abnormal summer temperatures (reaching 3°C; Cárdenas et al., 2019). In addition, a transcriptomic analysis of the effects of heat stress under laboratory conditions reports a response of Isodictya sp. at the limits of its capacity when exposed to acute heat stress (3 and 5°C; González-Aravena et al., 2019). Our experimental study further increases our knowledge of the responses of this species to environmental variation. Although we do not have clear evidence of the effect on prokaryotic composition of acute thermal stress exposure, a significant change occurred with injury induction. Interestingly, the OTUs responsible for the main differences between injured and uninjured sponges belong to Colwelliaceae and Flavobacteriaceae, being more abundant in injured sponges. The relative abundance of OTU466 (Colwelliaceae) increased from 1.31% in the uninjured sponges to 24.79% in the injured ones. For Flavobacteriaceae OTUs, the relative abundance of OTU204 increased from 3.72 to 9.14% in injured sponges, whereas OTU175 increased from 0.03 to 5.62% in injured sponges, and finally, OTU196 increased from 0.10 to 3.12%. Members of these families are related to sponge and coral diseases (Simister et al., 2012; Fan et al., 2013; Gajigan et al., 2017). Colwelliaceae OTUs have been reported as disease-related in necrotic and stressed sponges (Simister et al., 2012; Fan et al., 2013), whereas OTUs belonging to Flavobacteriaceae have been associated with diseases and stress in coral species (Gajigan et al., 2017). This suggests that tissue injuries can disrupt the holobiont balance, which may further affect their roles, potentially cascading to the benthic ecosystem; however, the roles of the symbionts and potential cascading effects are yet to be studied. Cárdenas et al. (2019) reported high stability in the microbiome of sponges after repeated sampling of the same individuals over three consecutive summers, suggesting that some sponge individuals might show some degree of resilience to increased temperature. In this regard, resilience of sponges to changes in environmental factors, such as temperature, has been extensively documented in experimental studies from other latitudes (Erwin et al., 2012; Luter et al., 2012a; Cárdenas et al., 2014; Turon et al., 2019). Previous studies highlight the capacity of Antarctic sponges to cope with environmental change as a potential key factor in their success (Morley et al., 2016; Cárdenas et al., 2019). Although our results provide the first insights into the responses of sponges to stressors, such as tissue damage and temperature, at present it is unknown if Antarctic sponges would cope in the long term with such types of stress.
The composition and diversity of sponge microbiomes is directly correlated with environmental conditions as has been shown between pristine and polluted zones (Turon et al., 2019); hence, the sponge microbiome can play an important role as an indicator of significant environmental disturbance. Changes in the sponge microbiome may even be related to mass mortalities, beginning with early disruption of the microbiome balance at the OTU level with a potential decline of sponge fitness and resistance to infections (Blanquer et al., 2016). For instance, Blanquer et al. (2016) found that bacterial diversity increased significantly in diseased tissues, which is in accordance with our results, and an increase in Shannon and Simpson diversity indices were recorded in injured sponges. In the case of our study, the alpha diversity increased after physical injuries with the apparition of particular OTUs of Alteromonadales, Flavobacteriales, and Oceanospirillales orders only in injured sponges potentially being opportunistic bacteria.
Although overall results of this study suggest a microbiome disruption by the interaction of tissue injuries and heat stress at the microbiome assembly level, it was not possible to assess the effect of these stressors over a longer period of time or to assess the existence of potential resilience. This has to be acknowledged; however, it is difficult to perform long-term experiments due to logistics constraints associated with work in Antarctica, which limits the duration of the experiments; in many cases, access to facilities is limited to a short period of time during the summer months. Future metagenomics and functional features analyses of the microbiome of I. kerguelenensis exposed to heat stress and tissue injury will be necessary to elucidate in more detail how prokaryotic functions are affected by these stressors and the potential resilience of its microbiome over a longer period of time.
Our study assessed for the first time the effects of combined climate change stressors on the microbiome of an Antarctic sponge. The results suggest that disturbance produced by icebergs may have a direct impact on the microbiome of the Antarctic sponge I. kerguelenensis with further potential effects as temperature increases. Future climate change scenarios for the WAP, including warming and increases in ice scour, may play a critical role shaping sponge symbiosis on this and other species. The potential effects of the disruption of the holobiont balance and its wider effects on the ecosystem are yet to be understood.
The datasets generated for this study can be found in NCBI BioProject, NCBI Accession No. PRJNA595145.
CC conceived the experimental design. MO carried out the experimental assays. RR carried out the sequences analyses. RR, CC, and AF carried out the statistical analyses. RR, CC, and MG-A wrote the manuscript with contribution of AF. All authors read and approved the final manuscript.
This research was funded by CONICYT/FONDECYT/INACH/INICIACION/#11150129 and the INACH Program “Marine Protected Areas.” This research contributes to the SCAR AnT-ERA and Ant-Eco programs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank D. Bravo, C. Lagger, L. Novoa, J. Levihuan, and the INACH personnel at Yelcho Antarctic Research Station for help during field and laboratory activities. We also thank F. Santa Cruz for his assistance with some of the figures and Dr. Julio Fernandez (UFRJ) for his assistance with sponge taxonomy.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00262/full#supplementary-material
FIGURE S1 | Rarefaction curves of the observed number of OTUs from Isodictya kerguelenensis at different sequencing depth by each experimental condition.
TABLE S1 | Taxonomy of the 31 most abundant OTUs (as shown in Figure 5) in samples of Isodictya kerguelenensis maintained under experimental conditions.
FILE S1 | Overall OTUs obtained with their sequence abundances.
FILE S2 | Permanova results testing the effect of treatment temperature and injuries on prokaryotic diversity indexes.
FILE S3 | Number of sequences of core OTUs in samples of Isodictya kerguelensis maintained under experimental conditions.
FILE S4 | Similarity percentages analysis (SIMPER) revealing the most important OTUs explaining dissimilarities in sponge microbiomes between experimental treatments.
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E.
Barnes, D. K. A. (2017). Iceberg killing fields limit huge potential for benthic blue carbon in Antarctic shallows. Glob. Chang. Biol. 23, 2649–2659. doi: 10.1111/gcb.13523
Barnes, D. K. A., Fleming, A., Sands, C. J., Quartino, M. L., and Deregibus, D. (2018). Icebergs, sea ice, blue carbon and Antarctic climate feedbacks. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 376:20170176. doi: 10.1098/rsta.2017.0176
Belikov, S., Belkova, N., Butina, T., Chernogor, L., Kley, A. M., Van Nalian, A., et al. (2019). Diversity and shifts of the bacterial community associated with Baikal sponge mass mortalities. PLoS One 14:e0213926. doi: 10.1371/journal.pone.0213926
Bell, J. J. (2008). The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 79, 341–353. doi: 10.1016/j.ecss.2008.05.002
Bell, J. J., McGrath, E., Biggerstaff, A., Bates, T., Cárdenas, C. A., and Bennett, H. (2015). Global conservation status of sponges. Conserv. Biol. 29, 42–53. doi: 10.1111/cobi.12447
Bell, J. J., and Carballo, J. L. (2017). “Future directions and gaps in our knowledge,” in Climate Change, Ocean Acidification and Sponges, eds J. L. Carballo and J. J. Bell Switzerland (Cham: Springer), doi: 10.1007/978-3-319-59008-0
Bennett, H. M., Altenrath, C., Woods, L., Davy, S. K., Webster, N. S., and Bell, J. J. (2017). Interactive effects of temperature and pCO2 on sponges: from the cradle to the grave. Glob. Chang. Biol. 23, 2031–2046. doi: 10.1111/gcb.13474
Blanquer, A., Uriz, M. J., Cebrian, E., and Galand, P. E. (2016). Snapshot of a bacterial microbiome shift during the early symptoms of a massive sponge die-off in the western Mediterranean. Front. Microbiol. 7:752. doi: 10.3389/fmicb.2016.00752
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C., Ghalith, G. A., et al. (2018). QIIME2: Reproducible, interactive, scalable, and extensible microbiome data science. Peer J. 6:e27295v2. doi: 10.7287/peerj.preprints.27295v2
Brown, K. M., Fraser, K. P. P., Barnes, D. K. A., and Peck, L. S. (2004). Links between the structure of an Antarctic shallow-water community and ice-scour frequency. Oecologia 141, 121–129. doi: 10.1007/s00442-004-1648-1646
Cárdenas, C. A., Bell, J. J., Davy, S. K., Hoggard, M., and Taylor, M. W. (2014). Influence of environmental variation on symbiotic bacterial communities of two temperate sponges. FEMS Microbiol. Ecol. 88, 516–527. doi: 10.1111/1574-6941.12317
Cárdenas, C. A., Font, A., Steinert, G., Rondon, R., and González-Aravena, M. (2019). Temporal stability of bacterial communities in Antarctic sponges. Front. Microbiol. 10:2699. doi: 10.3389/fmicb.2019.02699
Cárdenas, C. A., González-Aravena, M., Font, A., Hestetun, J. T., Hajdu, E., Trefault, N., et al. (2018a). High similarity in the microbiota of cold-water sponges of the Genus Mycale from two different geographical areas. Peer J. 6:e4935. doi: 10.7717/peerj.4935
Cárdenas, C. A., González-Aravena, M., and Santibañez, P. A. (2018b). The importance of local settings: within-year variability in seawater temperature at South Bay. Western Antarctic Peninsula. Peer J. 6:e4289. doi: 10.7717/peerj.4289
Comeau, A. M., Douglas, G. M., and Langille, M. G. I. (2017). Microbiome helper: a custom and streamlined workflow for microbiome research. mSystems 2, e127–e116. doi: 10.1128/msystems.00127-116
Cook, A. J., Fox, A. J., Vaughan, D. G., and Ferrigno, J. G. (2005). Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308, 541–544. doi: 10.1126/science.1104235
Ereskovsky, A., Ozerov, D. A., Pantyulin, A. N., and Tzetlin, A. B. (2019). Mass mortality event of White Sea sponges as the result of high temperature in summer 2018. Polar Biol. 42, 2313–2318. doi: 10.1007/s00300-019-02606-0
Ericson, J. A., Ho, M. A., Miskelly, A., King, C. K., Virtue, P., Tilbrook, B., et al. (2012). Combined effects of two ocean change stressors, warming and acidification, on fertilization and early development of the Antarctic echinoid Sterechinus neumayeri. Polar Biol. 35, 1027–1034. doi: 10.1007/s00300-011-1150-1157
Erwin, P. M., Pita, L., López-Legentil, S., and Turon, X. (2012). Stability of sponge-associated bacteria over large seasonal shifts in temperature and irradiance. Appl. Environ. Microbiol. 78, 7358–7368. doi: 10.1128/aem.02035-2012
Fan, L., Liu, M., Simister, R., Webster, N. S., and Thomas, T. (2013). Marine microbial symbiosis heats up: The phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J. 7, 991–1002. doi: 10.1038/ismej.2012.165
Gajigan, A. P., Diaz, L. A., and Conaco, C. (2017). Resilience of the prokaryotic microbial community of Acropora digitifera to elevated temperature. Microbiol. Open 6:e00478. doi: 10.1002/mbo3.478
Gantt, S. E., López-Legentil, S., and Erwin, P. M. (2017). Stable microbial communities in the sponge Crambe crambe from inside and outside a polluted Mediterranean harbor. FEMS Microbiol. Lett. 364:fnx105. doi: 10.1093/femsle/fnx105
Glasl, B., Smith, C. E., Bourne, D. G., and Webster, N. S. (2018). Exploring the diversity-stability paradigm using sponge microbial communities. Sci. Rep. 8:8425. doi: 10.1038/s41598-018-26641-26649
González-Aravena, M., Kenny, N. J., Osorio, M., Font, A., Riesgo, A., and Cárdenas, C. A. (2019). Warm temperatures, cool sponges: the effect of increased temperatures on the Antarctic sponge Isodictya sp. Peer J. 7:e8088. doi: 10.7717/peerj.8088
Gutt, J. (2001). On the direct impact of ice on marine benthic communities, a review. Polar Biol. 24, 553–564. doi: 10.1007/s003000100262
Gutt, J., and Starmans, A. (2001). Quantification of iceberg impact and benthic recolonisation patterns in the Weddell Sea (Antarctica). Polar Biol. 24, 615–619. doi: 10.1007/s003000100263
Guzman, C., and Conaco, C. (2016). Gene expression dynamics accompanying the sponge thermal stress response. PLoS One 11:e0165368. doi: 10.1371/journal.pone.0165368
Harper, E. M., Clark, M. S., Hoffman, J. I., Philipp, E. E. R., Peck, L. S., and Morley, S. A. (2012). Iceberg scour and shell damage in the Antarctic bivalve Laternula elliptica. PLoS One 7:e46341. doi: 10.1371/journal.pone.0046341
IPCC (2014). Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Kennicutt, M. C., Kim, Y. D., Rogan-Finnemore, M., Anandakrishnan, S., Chown, S. L., Colwell, S., et al. (2016). Delivering 21st century Antarctic and Southern Ocean science. Antarct. Sci. 28, 407–423. doi: 10.1017/S0954102016000481
Lemoine, N., Buell, N., Hill, A., and Hill, M. (2007). “Assessing the utility of sponge microbial symbiont communities as models to study global climate change: a case study with Halichondria bowerbanki,” in Porifera Research: Biodiversity, Innovation and Sustainability, eds M. Custodio, G. Lobo-Hajdu, E. Hajdu, and G. Muricy (Rio de Janeiro: Museu Nacional), 419–425.
Lesser, M. P., Fiore, C., Slattery, M., and Zaneveld, J. (2016). Climate change stressors destabilize the microbiome of the Caribbean barrel sponge, Xestospongia muta. J. Exp. Mar. Bio. Ecol. 475, 11–18. doi: 10.1016/j.jembe.2015.11.004
Li, Y. F., Yang, N., Liang, X., Yoshida, A., Osatomi, K., Power, D., et al. (2018). Elevated seawater temperatures decrease microbial diversity in the gut of Mytilus coruscus. Front. Physiol. 9:839. doi: 10.3389/fphys.2018.00839
Lo Giudice, A., Azzaro, M., and Schiaparelli, S. (2019). “Microbial Symbionts of Antarctic Marine Benthic Invertebrates,” in The Ecological Role of Micro-organisms in the Antarctic Environment, ed. S. Castro-Sowinski (Switzerland), 277–296. doi: 10.1007/978-3-030-02786-5-13
Luter, H. M., Bannister, R. J., Whalan, S., Kutti, T., Pineda, M. C., and Webster, N. S. (2017). Microbiome analysis of a disease affecting the deep-sea sponge Geodia barretti. FEMS Microbiol. Ecol. 93:fix074. doi: 10.1093/femsec/fix074
Luter, H. M., Whalan, S., and Webster, N. S. (2012a). The marine sponge Ianthella basta can recover from stress-induced tissue regression. Hydrobiologia 687, 227–235. doi: 10.1007/s10750-011-0887-x
Luter, H. M., Whalan, S., and Webster, N. S. (2012b). Thermal and sedimentation stress are unlikely causes of brown spot syndrome in the Coral Reef sponge, Ianthella basta. PLoS One 7:e39779. doi: 10.1371/journal.pone.0039779
Meyer, J. L., Rodgers, J. M., Dillard, B. A., Paul, V. J., and Teplitski, M. (2016). Epimicrobiota associated with the decay and recovery of Orbicella corals exhibiting dark spot syndrome. Front. Microbiol 7:893. doi: 10.3389/fmicb.2016.00893
Morley, S. A., Barnes, D. K. A., and Dunn, M. J. (2019). Predicting which species succeed in climate-forced polar seas. Front. Mar. Sci. 5:507. doi: 10.3389/fmars.2018.00507
Morley, S. A., Berman, J., Barnes, D. K. A., Carbonell, C., de, J., Downey, R. V., et al. (2016). Extreme phenotypic plasticity in metabolic physiology of Antarctic demosponges. Front. Ecol. Evol. 3:157. doi: 10.3389/fevo.2015.00157
Pineda, M. C., Strehlow, B., Duckworth, A., Doyle, J., Jones, R., and Webster, N. S. (2016). Effects of light attenuation on the sponge holobiont-implications for dredging management. Sci. Rep. 6:39038. doi: 10.1038/srep39038
Pita, L., Rix, L., Slaby, B. M., Franke, A., and Hentschel, U. (2018). The sponge holobiont in a changing ocean: from microbes to ecosystems. Microbiome 6:46. doi: 10.1186/s40168-018-0428-421
Ramsby, B. D., Hoogenboom, M. O., Whalan, S., and Webster, N. S. (2018). Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol. Ecol. 27, 2124–2137. doi: 10.1111/mec.14544
Ridley, S. O., and Dendy, A. (1886). Preliminary report on the Monaxonida collected by H.M.S. challenger. Ann. Mag. Nat. Hist. 18, 325–351. doi: 10.1080/00222938609459982
Rodríguez-Marconi, S., De La Iglesia, R., Díez, B., Fonseca, C. A., Hajdu, E., Trefault, N., et al. (2015). Characterization of bacterial, archaeal and eukaryote symbionts from Antarctic sponges reveals a high diversity at a three-domain level and a particular signature for this ecosystem. PLoS One 10:e0138837. doi: 10.1371/journal.pone.0138837
Schram, J. B., Schoenrock, K. M., McClintock, J. B., Amsler, C. D., and Angus, R. A. (2016). Testing antarctic resilience: The effects of elevated seawater temperature and decreased pH on two gastropod species. ICES J. Mar. Sci. 73, 739–752. doi: 10.1093/icesjms/fsv233
Shirur, K. P., Jackson, C. R., and Goulet, T. L. (2016). Lesion recovery and the bacterial microbiome in two Caribbean gorgonian corals. Mar. Biol. 163:238. doi: 10.1007/s00227-016-3008-3006
Siegert, M., Atkinson, A., Banwell, A., Brandon, M., Convey, P., Davies, B., et al. (2019). The Antarctic Peninsula under a 1.5°C global warming scenario. Front. Environ. Sci. 7:102. doi: 10.3389/fenvs.2019.00102
Simister, R., Taylor, M. W., Tsai, P., Fan, L., Bruxner, T. J., Crowe, M. L., et al. (2012). Thermal stress responses in the bacterial biosphere of the great barrier reef sponge, Rhopaloeides odorabile. Environ. Microbiol. 14, 3232–3246. doi: 10.1111/1462-2920.12010
Sleight, V. A., Thorne, M. A. S., Peck, L. S., and Clark, M. S. (2015). Transcriptomic response to shell damage in the Antarctic clam, Laternula elliptica: Time scales and spatial localisation. Mar. Genomics 20, 45–55. doi: 10.1016/j.margen.2015.01.009
Steinert, G., Wemheuer, B., Janussen, D., Erpenbeck, D., Daniel, R., Simon, M., et al. (2019). Prokaryotic diversity and community patterns in Antarctic continental shelf sponges. Front. Mar. Sci. 6:297. doi: 10.3389/fmars.2019.00297
Stenni, B., Curran, M. A. J., Abram, N. J., Orsi, A., Goursaud, S., Masson-Delmotte, V., et al. (2017). Antarctic climate variability on regional and continental scales over the last 2000 years. Clim. Past 13, 1609–1634. doi: 10.5194/cp-13-1609-2017
Suckling, C. C., Clark, M. S., Richard, J., Morley, S. A., Thorne, M. A. S., Harper, E. M., et al. (2015). Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J. Anim. Ecol. 84, 773–784. doi: 10.1111/1365-2656.12316
Turon, M., Cáliz, J., Triadó-Margarit, X., Casamayor, E. O., and Uriz, M. J. (2019). Sponges and their microbiomes show similar community metrics across impacted and well-preserved reefs. Front. Microbiol. 10:1961. doi: 10.3389/fmicb.2019.01961
van Soest, R. W. M., Boury-Esnault, N., Vacelet, J., Dohrmann, M., Erpenbeck, D., de Voogd, N. J., et al. (2012). Global diversity of sponges (Porifera). PLoS One 7:e35105. doi: 10.1371/journal.pone.0035105
Walters, W., Hyde, E. R., Berg-Lyons, D., Ackermann, G., Humphrey, G., Parada, A., et al. (2016). Improved Bacterial 16S rRNA gene (V4 and V4-5) and Fungal internal transcribed spacer Marker marker gene primers for microbial community surveys. mSystems 1, e00009–e15. doi: 10.1128/msystems.00009-15
Keywords: microbiome, Porifera, benthic communities, tissue injury, heat-stress, ice scour disturbance, sponge health
Citation: Rondon R, González-Aravena M, Font A, Osorio M and Cárdenas CA (2020) Effects of Climate Change Stressors on the Prokaryotic Communities of the Antarctic Sponge Isodictya kerguelenensis. Front. Ecol. Evol. 8:262. doi: 10.3389/fevo.2020.00262
Received: 23 December 2019; Accepted: 24 July 2020;
Published: 04 September 2020.
Edited by:
Mahasweta Saha, Plymouth Marine Laboratory, United KingdomReviewed by:
Cole G. Easson, Middle Tennessee State University, United StatesCopyright © 2020 Rondon, González-Aravena, Font, Osorio and Cárdenas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodolfo Rondon, cnJvbmRvbkBpbmFjaC5jbA==; César A. Cárdenas, Y2NhcmRlbmFzQGluYWNoLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.