
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 14 July 2020
Sec. Biogeography and Macroecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00219
This article is part of the Research Topic Ecological, Behavioral and Genomic Consequences in the Rodent Family Sciuridae: Why Are Squirrels So Diverse? View all 14 articles
 Vladimir Ronkin1,2†
Vladimir Ronkin1,2† Victor Tokarsky1
Victor Tokarsky1 Nina Polchaninova1†
Nina Polchaninova1† Andrey Atemasov1
Andrey Atemasov1 Alyona Koshkina3†
Alyona Koshkina3† Galina Savchenko1,2*†
Galina Savchenko1,2*†The steppe marmot inhabits a wide range of open dry grasslands in Eurasia. Throughout this vast area, marmot habitats have undergone major changes due to human activities. Long-term ecological monitoring was conducted in the European steppe marmot settlements (Marmota bobak bobak) in Northeastern Ukraine in 2001–2019. The data obtained were compared with the observations made in M. b. schaganensis settlements in Kazakhstan during the expedition in 2017. The goals of our investigation were (1) to estimate M. bobak ecological plasticity based on general vegetation parameters of its habitats and settlement structure, (2) to relate the population density of the European subspecies to the food base of its habitats, (3) to evaluate the population response of M. b. bobak to the abandonment of cattle grazing, (4) to ascertain new ecological adaptations (if any) to the habitat changes, and (5) to reveal the steppe marmot’s status in the plant–herbivore interaction system in the grasslands of Northeastern Ukraine and Northern Kazakhstan. We have found differences in ecological features of M. b. bobak and M. b. schaganensis. The European subspecies was and continues to be a secondary pasture user. The Kazakhstan subspecies can be both secondary and primary users of the Asian dry steppes. Our studies have shown that the habitats of the European steppe marmot worsened dramatically (due to increased herbage height and cover of uneaten plant species together with litter) in comparison with those of the Kazakhstan subspecies. Presence in diverse habitats with a range of vegetation parameters as well as the differences between the settlement structures of M. b. bobak and M. b. schaganensis demonstrate the high ecological plasticity of the steppe marmot at the species level. At the same time, we have not found any new ecological adaptations that would ensure the survival of M. b. bobak settlements in modern conditions of the total cessation of cattle grazing.
In the rodent family Sciuridae, marmots (Marmota), ground squirrels (Spermophilus), and prairie dogs (Cynomys) have a pronounced adaptation to life in burrows and to feeding on live green herbs. Species of the genus Marmota are highly variable in social behavior, covering the whole range from the solitary woodchuck (M. monax) to the highly socialized Olympic (M. olympus) and hoary (M. caligata) marmots (Bibikov, 1989; Armitage, 1998, 2014; Arnold, 1990). Marmots are hibernating animals and selective foragers (Bibikov, 1989; Frase and Armitage, 1989; Bassano et al., 1996; Massemin et al., 1996; Stallman and Holmes, 2002; Armitage, 2003; Garin et al., 2008). All species prefer forbs over grasses (Armitage, 2014), many of them choose plants with succulent leaves (Sludsky et al., 1969).
Marmots have a wide altitudinal range of their distribution, covering zonal steppes, mountain and alpine tundra, and mountain deserts. A unique behavioral adaptation of the genus Marmota is plugging the entrance to their winter burrow with a compact multimeter plug (soil mixed with feces) that virtually excludes heat exchange between the internal burrow environment and the external environment (Nikol’skii, 2009). The author defines “temperature niche” as “the entire diversity of temperature conditions of marmots, taking into account the depth of the burrows, season of the year and the geographical situation of local populations.” According to Bibikov (1989), marmots are primary mountain animals. On the contrary, Nikol’skii and Rumiantsev (2012) suppose that the ancestral forms of marmots occupied a huge range thanks to the flat terrain of their primary habitats. The bobak group, which consists of M. bobak, M. baibacina, and M. kastschenkoi, is considered the youngest progressing branch of the Palearctic marmots’ evolutionary tree (Brandler and Lyapunova, 2009; Brandler et al., 2010). Based on studies of mitochondrial DNA, Brandler (2009) proposes that M. bobak evolved as a plain-steppe form in the east part of the common historical range of M. bobak and M. baibacina.
The steppe marmot inhabits a wide range of open dry grasslands in Eurasia. Throughout the vast area, marmot habitats have undergone major changes due to human activities (Kirikov, 1959). Duration of the anthropogenic effect on marmot populations varied considerably depending on the population location. In the first half of the 20th century, the European subspecies of the steppe marmot (M. b. bobak) was under threat of extinction (Bibikov et al., 1990). The major threatening factors were the plowing of most native habitats and overhunting (Zimina and Isakov, 1980; Bibikov, 1989). At the same time, the number of the Kazakhstan subspecies (M. b. schaganensis) was estimated in millions of individuals (Sludsky et al., 1969). It inhabited the boundless virgin steppes of Kazakhstan, where the main anthropogenic effects were nomadic pastoralism and hunting (Sludsky et al., 1969; Zimina and Isakov, 1980).
The number of M. b. bobak in 1940–1950 did not exceed 5,000 individuals (Bibikov et al., 1990). Then, in the early 60s, there was a rapid “rebound” of the European subspecies. The marmots colonized the vast area of gullies and river valleys transformed into intensively used cattle pastures. The main reason for that is thought to be changes in the vegetation caused by cattle grazing (Seredneva and Nesgovorov, 1977; Seredneva, 1985). In the same period of time, the Kazakhstan subspecies experienced a large-scale anthropogenic impact of “the virgin lands campaign.” In 1954–1960, 25 million hectares of virgin steppes were plowed in Kazakhstan (Brezhnev, 1978; Zimina and Isakov, 1980; Rachkovskaya and Bragina, 2012). Arable fields became the main marmot habitats, and only a part of the Kazakhstan population remained on the steppe pastures (Zimina and Isakov, 1980; Rumyantsev, 1991).
In the mid-1990s, the European subspecies accounted for about 350,000 individuals while the number of the Kazakhstan subspecies exceeded two million (Mashkin, 1997). There was a general impression that the steppe marmot had wide ecological plasticity and was capable of acquiring ecological adaptations in case of sharp changes in its natural environment (Zimina and Isakov, 1980; Bibikov et al., 1990; Rumyantsev, 1991; Mashkin, 1997). However, Rumyantsev (1991) notes that the marmots’ settlements were located mainly along the margins of arable fields at the borders with the patches of virgin steppe. In the 21st century, the habitats of both subspecies have undergone new drastic changes. In the late 1990s, huge areas of arable fields in Kazakhstan were abandoned, and the grasslands began recovering due to spontaneous succession (Brinkert et al., 2016). Some fields were subsequently reclaimed (Kamp et al., 2011; Dara et al., 2018, 2019), and the use of herbicides in arable fields increased (Kamp et al., 2011). In Ukraine, the pastures were abandoned (Ronkin et al., 2009; Ronkin and Savchenko, 2016). In Kazakhstan, areas of pasture were also decreased dramatically (Mashkin et al., 2010), but the process was highly uneven (Kamp et al., 2012). The former nomadic grazing was replaced by sedentary grazing. As a result, now the surroundings of the villages are overgrazed while the areas located further from settlements are not grazed at all (Kamp et al., 2012; Deák et al., 2018).
Long-term investigations of species-specific responses to anthropogenic and/or natural impacts contribute to the understanding of the possible trajectories and outcomes of the ecosystem evolution as a whole. Steppe ecosystems have evolved as the ecosystems of the grazing type of functioning (Abaturov, 2006), where interactions between food plants and herbivorous mammals (plant–herbivore interaction) determine animal number, distribution, and population structure (Abaturov, 2005). The loss of large wild grazers (in our studies livestock) is supposed to change ecosystem functioning. We hypothesized that large-scale and rapid environmental changes may not only influence marmot population, but also encourage development of new adaptations, which will compensate for changes in their habitats. We proceeded from the fact that the development of ecological adaptations in response to previous habitat changes has been already observed in both subspecies (Bibikov et al., 1990; Rumyantsev, 1991; Mashkin, 1997). The goals of our investigation were as follows:
(1) To estimate the M. bobak ecological plasticity based on general vegetation parameters of its habitats and settlement structure.
(2) To relate the population density of the European subspecies to the food base of its habitats.
(3) To evaluate the population response of M. b. bobak to the abandonment of cattle grazing.
(4) To ascertain new ecological adaptations (if any) to the habitat changes.
(5) To reveal the steppe marmot’s status in the plant–herbivore interaction system in the grasslands of Northeastern Ukraine and Northern Kazakhstan.
The research was carried out in Northeastern Ukraine (Kharkiv region) and Northern Kazakhstan (Kostanay and Karaganda regions) (Figure 1). The climate of Northeastern Ukraine is characterized as temperate continental with the hottest period in the second half of summer (end of June–August) and the coldest period in the second half of winter (January–February). The maximal recorded temperature during 2001–2019 was 39.4°C (July 2010, the authors’ data) and the minimal 38.3°C (January 2006, the authors’ data). An average annual rainfall ranges from 480 to 620 mm with a peak in June. The study area is desiccated with gullies and river valleys; the main soil type is chernozem. All flattened steppe plots had been transformed into arable fields by the end of the 19th century. The steppe marmot occurs in steppe gullies and floodplain meadows. Communities dominated by Festuca valesiaca agg., Poa angustifolia, and Elytrigia repens represent vegetation in the gullies while communities dominated by Elytrigia repens, Poa angustifolia, Festuca rupicola, and Festuca pratensis occupy the floodplain meadows (Ronkin and Savchenko, 2016). Chalk outcrops in the valleys of the Oskil, the Nyzhnia, and the Verkhnia Dvorichna rivers host specific plant communities of the class Helianthemo-Thymetea (Romashchenko et al., 1996). They alternate with the Festucion valesiacae communities forming the so-called chalk grasslands (Ronkin and Savchenko, 2016). In this chalky landscape, the marmot inhabits chalky steppe on the footslopes and neighboring floodplain meadows.
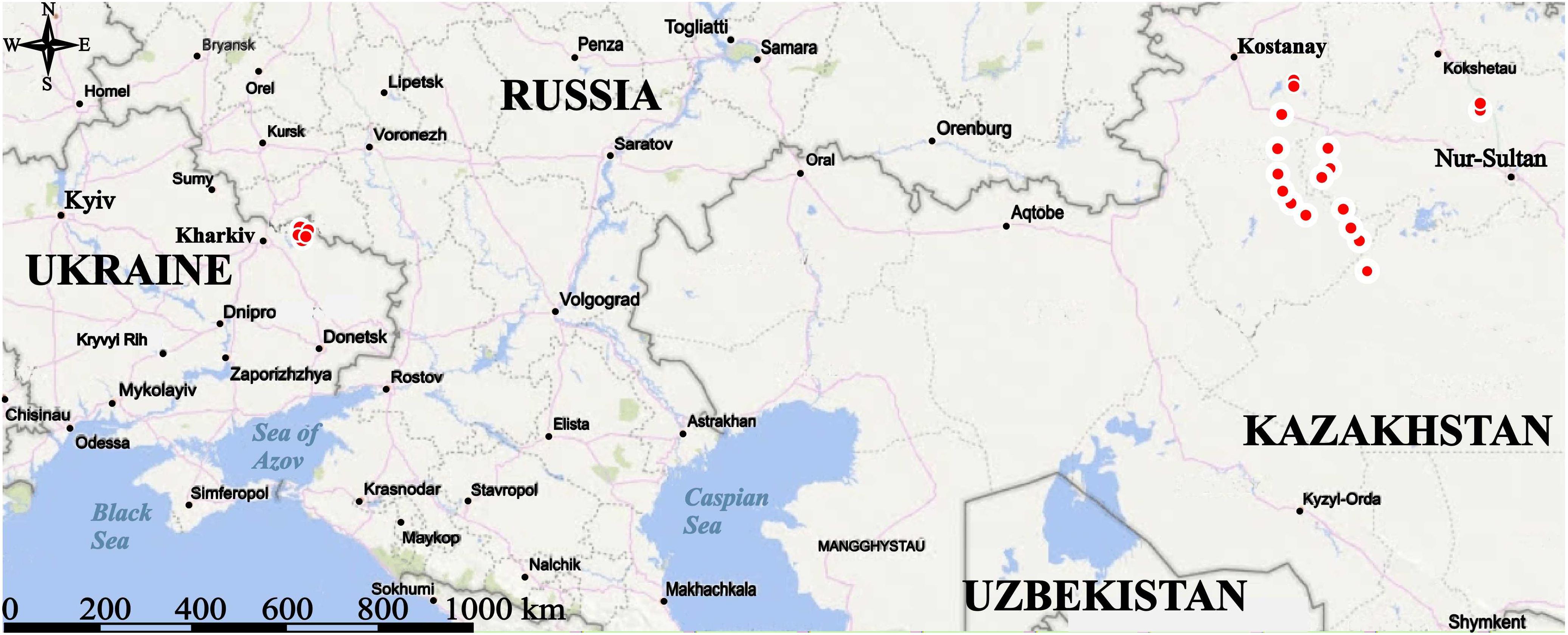
Figure 1. Location of the study areas (red circles) in Northeastern Ukraine (Kharkiv Region) and Northern Kazakhstan (Kostanay and Karaganda regions).
The climate of Northern Kazakhstan is sharply continental. The summer is usually dry and hot, but in some years, it can be cool and wet. The temperature averages 17–18°C in June and 20–21°C in July with a maximum of 40–44°C. The soil surface on certain hot days heats up to 50–60°C. In summer, there is a sharp difference in the day and night temperatures. The precipitation rate in various areas ranges from 250 to 400 mm; it decreases from north to south. Stable snow cover holds out for 5.0–5.5 months in the north and 4.0–4.5 months in the south. Approximately one third of the annual precipitation (70–100 mm) falls in winter. About 50% of the years are dry with no rains in June and/or in July. A combination of high daytime temperatures (30–35°C) and low relative humidity (30–40%) causes intense evaporation, which results in droughts. The chernozem soils are replaced in the south by the kastanozem ones.
Most of Northern Kazakhstan is located on flat sweeping landmass. Before the Soviet virgin lands campaign of the 1950s, it was covered with genuine steppes dominated by Festuca valesiaca, Stipa zalesskii, Stipa lessingiana, and Peucedanum morisonii (Rachkovskaya and Bragina, 2012; Demina and Bragina, 2014). The flat terrain determined total plowing and forming of large continuous fields in Northern Kazakhstan (2 × 2 km, the “standard” size of the Soviet virgin-land-campaign fields).
The material presented in this paper was collected in 2001–2019. A total of 20 settlements of M. b. bobak were investigated in Northeastern Ukraine (Kharkiv Region). At present, there are three types of habitats within the geographic range of the European subspecies: grazed grasslands in steppe gullies and river valleys, the same grasslands shortly after cessation of the cattle grazing, and long-abandoned pastures (Mashkin, 1997; Ronkin et al., 2009; Mashkin et al., 2010). In our study, we marked these types as “A,” “B,” and “C,” respectively. Long-term ecological monitoring was conducted in three marmot settlements in the regional landscape park Velykoburlutskyi Steppe: (1) Nesterivka, (2) Zelenyi Hai, (3) Rohozianka, and two settlements in the national nature park Dvorichanskyi: (4) Kamianka and (5) Novomlynsk. In the Velykoburlutskyi Steppe, the marmots inhabit the gully steppe on chernozem soil, and in the Dvorichanskyi, they occur in the chalky steppe and neighboring floodplain meadows. In Table 1, each habitat type and settlement is provided with a code, years of monitoring, years of vegetation sampling, and the number of sample quadrates. The grazing cessation (and, accordingly, transformation of one habitat type into another) occurred in the study settlements in different years.
The data on vegetation and settlement structure of M. b. bobak were compared with those obtained in the expedition to Northern Kazakhstan in June 2017 aimed at studying settlements of M. b. schaganensis. The expedition route passed from Nur-Sultan through Akmola and Kostanay regions to the Karaganda region; then from the northwest of the Karaganda region through the Kostanay to the north of the Akmola region (Figure 1). The habitat spectrum of the Kazakhstan subspecies is much wider than that of the European one; it includes arable fields, roadsides, grazed and/or ungrazed steppes, and grazed and/or ungrazed former croplands (Koshkina et al., 2019). Moreover, these grasslands are divided into northern and southern variants depending on their location in the geographic range. In total, the data on nine settlements of the Kazakhstan subspecies are used in this work. We named the habitat types as follows: north locality: roadsides, north locality: arable fields, north locality: grazed steppe, north locality: grazed former croplands, north locality: ungrazed former croplands, south locality: grazed steppe, south locality: ungrazed steppe, south locality: grazed former croplands, and south locality: ungrazed former croplands.
Vegetation data in M. b. bobak settlements were obtained from their foraging areas. No entire area of the marmot settlements is used for foraging activity. Despite the absence of visible reasons for ignoring (water bodies, wetlands, roads, and arable fields), there are no marmot’s paths or burrows in such areas. We detected these areas only when the whole vegetation within the settlement was short, which did not impede visual observation of foraging animals. As a rule of thumb, such vegetation state is accompanied by cattle grazing. Descriptions of typical plant communities of these unvisited areas were made to compare them with the foraging areas. Further in the text, these communities are referred to as ignored vegetation (or ignored areas).
General vegetation parameters were estimated in the quadrate 3.16 × 3.16 m (10 sq m). We estimated (1) total cover, (2) living cover of vascular plants, (3) cover of litter, (4) cover of forbs, (5) cover of grasses, and (6) herbage height. Then, we noted the cover of each vascular species if it was higher than 0.01%. To calculate parameters of the marmot food base in a given settlement (cover of food species and total cover of uneaten species together with litter) the covers of food/uneaten species were summarized. All these estimates were made in summer when all the grass layers had reached maximum growth. Herbage height was taken as the height of the main (most dense) herb layer; 11 measurements were made and then averaged.
In 2011–2019, 85 quadrates were sampled in five studied settlements of the European subspecies (Table 1). Of these, 70 quadrates were placed in the marmot foraging areas, 12 quadrates in the ignored vegetation of grazed grasslands of Nesterivka, and three quadrates in the ignored vegetation of grazed grasslands of Kamianka. After transition of one type of habitat into another (e.g., grazed grassland into the abandoned ones), new quadrates were sampled approximately at the same places. The proportion of the sampled quadrates (chernozem soil/chalky outcrops) roughly corresponds to the current ratio of the existing marmot settlements in these grasslands. In M. b. schaganensis settlements, we estimated the general vegetation parameters. In total, 62 estimates were made in nine settlements.
We performed testing on forb and grass species to compare with the marmot diet found earlier in 1989 (Ronkin and Savchenko, 2000). After grazing abandonment, the vegetation of marmot habitats changed dramatically. In 2010–2017, we continued to replenish and refine the list of food species in connection with the food base changes caused by the post-grazing succession. We recorded all plant species bitten by marmots in their natural settlements and agrocenoses. In addition, we monitored foraging preferences of free-living tame marmots to determine suitability of certain plant species as the main forage components. Two marmot families lived near our research center and were not afraid of people. During the growing season, freshly mowed plants were given to the animals twice a day in excess. We used plants defined as food species in our previous research. The fodder was heaped near a winter burrow. The plant species were considered suitable as the main forage component if the animals of all ages willingly ate a large amount of it every day. This study was carried out in accordance with the principles of the Basel Declaration and recommendations of the Law of Ukraine “On the Animal World” (Article No. 9 “Basic Requirements and Principles for the Protection, Management and Reproduction of the Animal World”), the Committee on Bioethics of Kharkiv National University named after V. N. Karazin. The protocol was approved by the Committee on Bioethics of Kharkiv National University.
The winter burrow is considered the main attribute of the marmot home range and marmot family (Seredneva, 1986; Lenti Boero, 1996, 2001). In addition, marmots have summer and auxiliary burrows (Bibikov, 1989; Mashkin, 1997; Armitage, 2014). For the mapping of winter burrows, a handheld global positioning system device (Garmin Oregon, Magellan eXplorist) and the program QGIS v.2.18.8 were used. We mapped winter burrows of 132 families of M. b. bobak in five studied settlements and measured the distances (n = 167) between the nearest winter burrows of neighboring families. This distance is a key feature of both population density and environmental conditions. Smaller average distance indicates better food conditions in the marmot settlement (Seredneva, 1986).
To estimate M. b. bobak population response to the cattle grazing cessation and to ascertain new ecological adaptations (if any) to the habitat changes, we made censuses of the number of families in a settlement before and after the pasture abandonment. We also noted the total number of all types of burrows (summer and auxiliary) used by a family of marmots. If the number of families in the settlement changed, we took repeated measurements of the distances between inhabited winter burrows. To count the burrows used by marmots, we chose a typical plot of 25.5 ha in the central part of the Nesterivka settlement and divided it into 20 × 20 m quadrates. Within these quadrates, positions of all existing burrows were outlined. This work was completed in 2000–2001. In 2001–2002, we made a census of all burrows used by each marmot family in the study plot and adjacent areas (in total, 27 home ranges). In 2002–2019, we conducted annual monitoring of the site occupancy. In case of a decrease in the number of existing families, we repeated censuses of the number of burrows used by remaining families in the same home ranges.
During the study of M. b. schaganensis, we mapped inhabited winter burrows in its typical sustainable settlements, counted the total number of burrows used by a family of marmots, and measured the distances (n = 91) between the nearest winter burrows of neighboring families in all the studied settlements excluding roadsides (see above). We considered a given settlement sustainable if the families had offspring and the number of inhabited winter burrows was much higher than the number of uninhabited ones. Then, we compared the obtained parameters in the settlements of both European and Kazakhstan subspecies. The methods applied to solve certain research goals are shown in Supplementary Figure S1.
We consider population density in the marmot settlements as a number of present families per 1 ha of a given settlement. Based on previous research (Ronkin et al., 2009), we identified three population grades in marmot settlements: normal (predicted value 0.5–2 families/ha), decreased (0.3–0.5 families/ha), and collapsed (less than 0.3 families/ha). We determined the number of burrows used by a family by the fact of visiting and by indirect signs, such as freshly excavated soil and marmot feces near the burrow entrance. Before hibernation, the marmots plug the entrances of their winter burrows with small globules made of soil and their feces. These globules as well as old grass and hay (bedding) are additional proof of burrow use.
Comparison of multiple independent samples was conducted using Kruskal–Wallis ANOVA by ranks. In most cases, the distribution in the data sets was different from normal (P < 0.05, Shapiro–Wilk and Kolmogorov–Smirnov normality tests). When comparing foraging areas within the settlements of both M. b. bobak and M. b. schaganensis, general vegetation parameters (living cover of vascular plants, cover of litter, cover of forbs, cover of grasses, and herbage height) were taken as dependent variables and the habitat type as a grouping variable. When comparing the food base in M. b. bobak settlements, the food base parameters (living cover of vascular plants, total cover of food species, total cover of uneaten species together with litter, cover of litter separately, and herbage height) were dependent variables, and the population grade (normal, decrease, and collapse) was a grouping variable. Comparison of M. b. bobak and M. b. schaganensis settlement structures was based on the distance between the nearest inhabited winter burrows as a dependent variable and the habitat type as a grouping variable. We used all the data obtained in the habitats of both M. b. bobak and M. b. schaganensis (see above) except the roadsides. Changes in the settlement structure of M. b. bobak were analyzed taking the distance between the nearest winter burrow as a dependent variable and the population grade as a grouping variable.
The U-test (Mann–Whitney) was used to determine the significance of differences in general vegetation parameters (living cover of vascular plants, cover of litter, cover of forbs, cover of grasses, and herbage height) in foraging areas. We used all the data obtained in the habitats of both M. b. bobak and M. b. schaganensis (see above) except the arable fields. Subspecies (M. b. bobak/M. b. schaganensis) was taken as a grouping variable. The U-test was also used to determine the significance of difference in distance between the nearest neighboring winter burrows in the settlements of M. bobak. Subspecies (M. b. bobak/M. b. schaganensis) was taken as a grouping variable.
The cluster analysis in the module of multivariate exploratory techniques was used for the habitat classification of M. bobak. Averaged values of the general vegetation parameters in each settlement were variables. We used all data obtained in the settlements of M. b. bobak (see above) except the ignored vegetation of chalky outcrops in Kamianka and the data on all studied settlements of M. b. schaganensis.
Statistical significance was set at P < 0.05. All the calculations were performed in the program StatSoft Statistica v8.0.
The foraging areas of M. b. bobak were characterized by a wide range of general vegetation parameters (living cover of vascular plants, cover of litter, forbs, grasses, and herbage height) due to their location in grazed and/or abandoned grasslands. The herbage height ranged from 4 cm (grazed grasslands in total) to 69 cm (grasslands of Nesterivka shortly after abandonment, communities of the loose-bunch grasses Poa angustifolia and Elytrigia repens) (Figure 2 and Supplementary Figures S2A,B). The cover of grasses had the largest range from 2% (grazed grasslands of Kamianka) to 77% (grazed grasslands of Rohozianka). The cover of litter changed in similar ranges from 1% (grazed grasslands in total) to 80% (long-abandoned grasslands of Novomlynsk, community of the loose-bunch grass Elytrigia repens). The lowest living cover of vascular plants was registered in the chalky steppe (40%, grazed grasslands of Kamianka) and that of the forbs in the communities of loose-bunch grasses (4%, grasslands of Nesterivka shortly after abandonment) (Figures 2, 3).
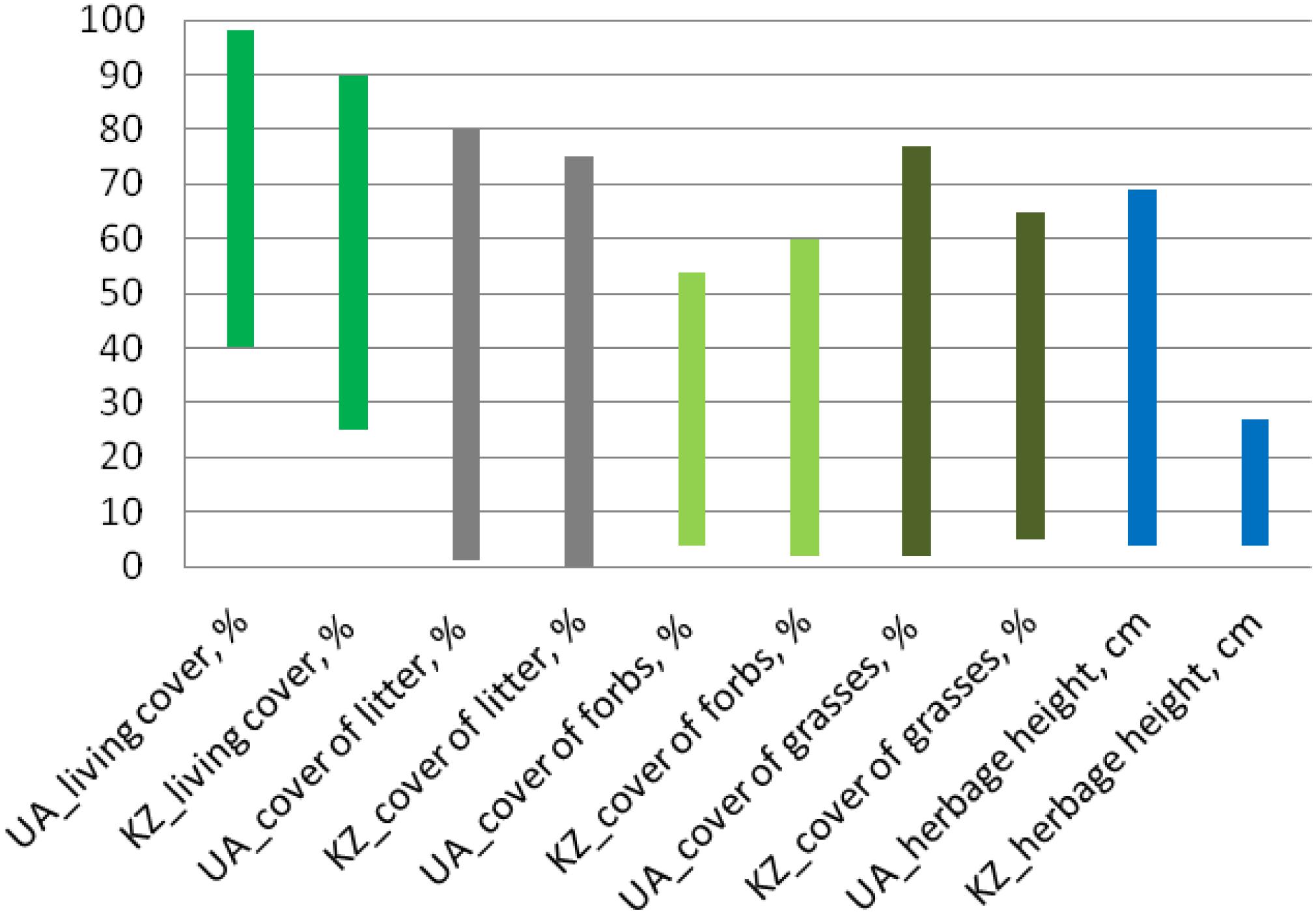
Figure 2. Ranges of general vegetation parameters (living cover of vascular plants, cover of litter, forbs, grasses, and herbage height) in foraging areas of M. b. bobak and M. b. schaganensis.
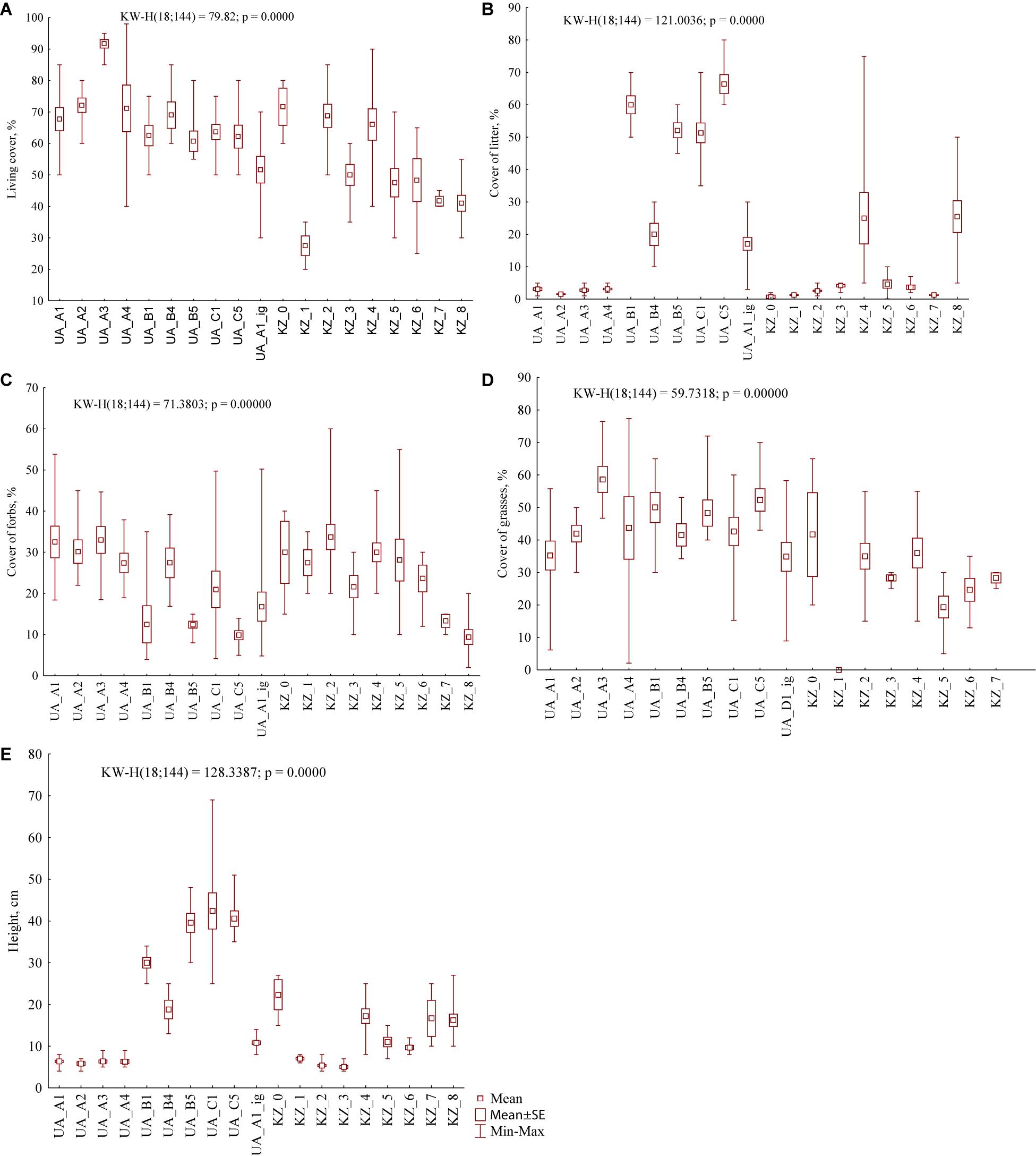
Figure 3. General vegetation parameters (A – living cover, B – litter, C – forbs, D – grasses, and E – herbage height) in foraging areas of the studied settlements of M. b. bobak (UA) and M. b. schaganensis (KZ). UA_A1, grazed grasslands of Nesterivka; UA_A2, grazed grasslands of Zelenyi Hai; UA_A3, grazed grasslands of Rohozianka; UA_A4, grazed grasslands of Kamianka; UA_B1, grasslands of Nesterivka shortly after abandonment; UA_B4, grasslands of Kamianka shortly after abandonment; UA_B5, grasslands of Novomlynsk shortly after abandonment; UA_C1, long-abandoned grasslands of Nesterivka; UA_C5, long-abandoned grasslands of Novomlynsk; KZ_0, north locality: roadsides; KZ_1, north locality: arable fields; KZ_2, north locality: grazed steppe; KZ_3, north locality: grazed former croplands; KZ_4, north locality: ungrazed former croplands; KZ_5, south locality: grazed steppe; KZ_6, south locality: ungrazed steppe; KZ_7, south locality: grazed former croplands; KZ_8, south locality: ungrazed former croplands, and UA_A1_ig, grazed grasslands of Nesterivka, ignored vegetation.
The range of vegetation parameters in foraging areas of M. b. schaganensis was similar to that of M. b. bobak except the herbage height (Figures 2, 3). The latter was 40 cm lower (4–27 cm), and its range was narrower than in the foraging areas of M. b. bobak. The cover of litter (0.1–75%) had the largest cover range. The lowest living cover of vascular plants was 25% (south locality: ungrazed steppe, communities dominated by Festuca valesiaca and Artemisia sp.), and the lowest cover of forbs was 2% (south locality: ungrazed former croplands, communities dominated by Koeleria cristata and Stipa lessingiana) (Supplementary Figures S3A,B). The living cover, the cover of litter, grasses, and herbage height in the foraging areas of the Kazakhstan subspecies, were lower than those in the foraging areas of the European subspecies (U test: U = 1,059.5, n1 = 58, n2 = 70, P < 0.0001; U = 1,499.0, n1 = 58, n2 = 70, P < 0.05; U = 877.5, n1 = 58, n2 = 70, P < 0.0001, and U = 1,548.5, n1 = 58, n2 = 70, P < 0.05, respectively).
The cluster analysis based on general vegetation parameters in the habitats of both marmot subspecies revealed three clusters (Figure 4). Although the arable fields were formally included in cluster 1, they stood apart from other habitats. The first and second clusters are characterized by low cover of litter and herbage height while the third cluster is distinguished by the high values of these parameters.
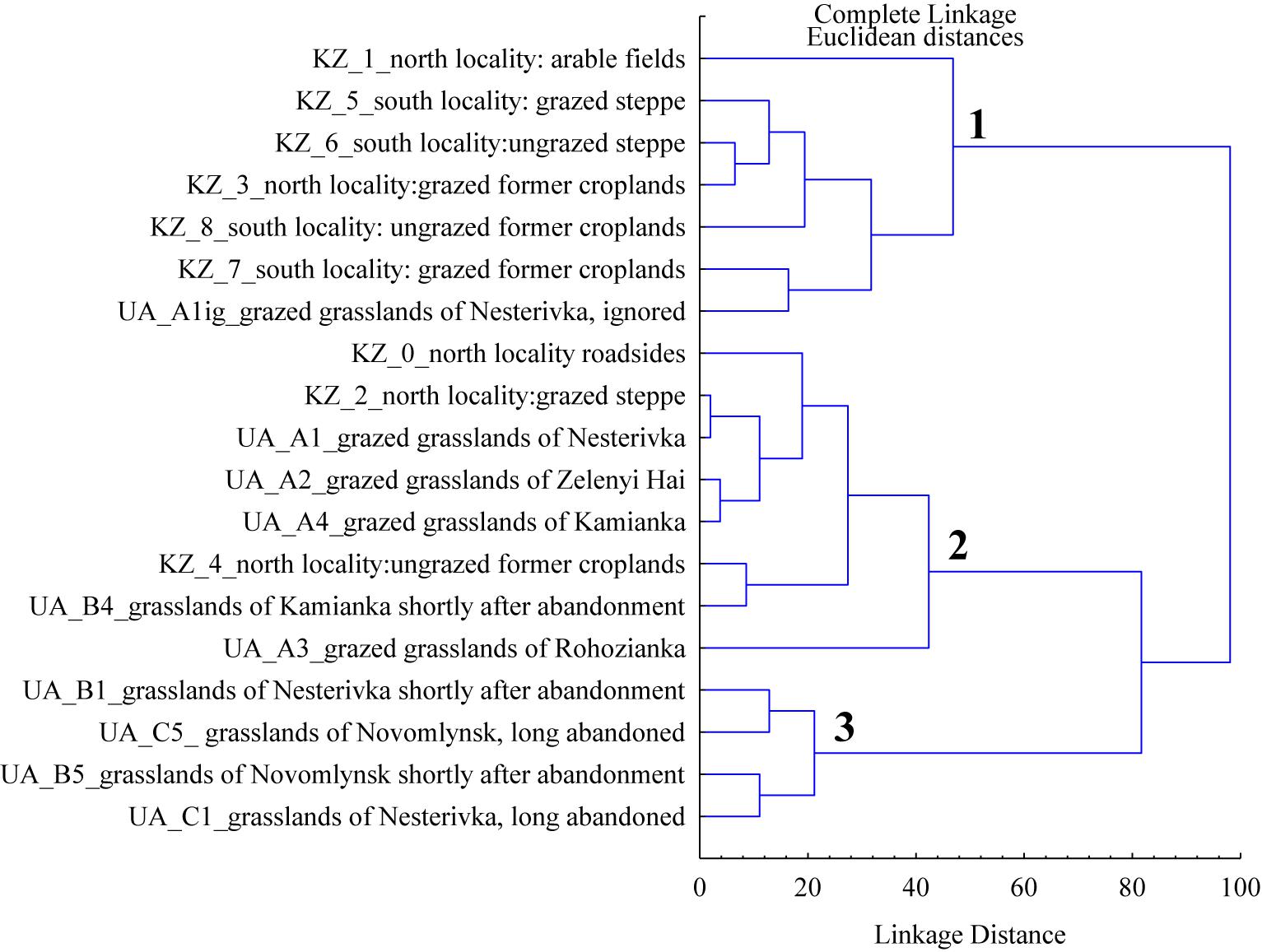
Figure 4. The clusters based on mean general vegetation parameters (living cover of vascular plants, cover of litter, cover of forbs, cover of grasses, and herbage height) for different marmot habitats of Northeastern Ukraine (UA) and Northern Kazakhstan (KZ).
We did not find inhabited marmot settlements in vast areas of former croplands covered with dense vegetation dominated by Stipa cf. zalesskii (Supplementary Figure S4A). Dense stands of Stipa pennata in Northeastern Ukraine are also avoided by marmots (Supplementary Figure S4B).
The number of burrows per family in the studied settlements of M. b. bobak varied from 15 to 49. In the settlements of M. b. schaganensis, it was much lower than in the European subspecies, ranging from one (north locality: arable fields) to seven (north locality: grazed steppe). Mean distances between the nearest winter burrows of the neighboring families of M. b. bobak ranged from 76.7 ± 24.6 m (n = 13, grazed grasslands of Kamianka) to 231.5 ± 192.0 m (n = 15, long-abandoned grasslands of Novomlynsk). These distances in M. b. schaganensis settlements varied from 120.0 ± 41.2 m (n = 6, north locality: grazed former croplands) to 227.5 ± 43.7 m (n = 6, north locality: grazed steppe) (Figure 5). The comparative analysis of all investigated settlements showed that the mean distance between the winter burrows of M. b. schaganensis is higher (172.4 ± 63.4, n = 91) than that of M. b. bobak (111.1 ± 81.9, n = 167) (U = 2,845.0, n1 = 167, n2 = 91, p < 0.001).
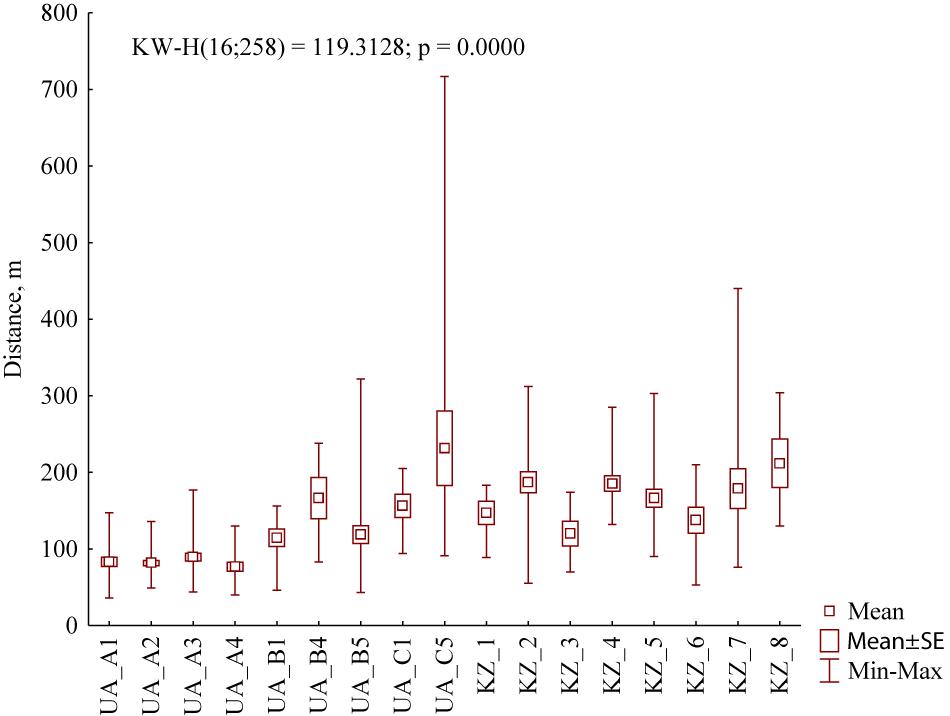
Figure 5. Mean distances between the nearest winter burrows of the neighboring families in the studied settlements of M. b. bobak and M. b. schaganensis. UA_A1, grazed grasslands of Nesterivka; UA_A2, grazed grasslands of Zelenyi Hai; UA_A3, grazed grasslands of Rohozianka; UA_A4, grazed grasslands of Kamianka; UA_B1, grasslands of Nesterivka shortly after abandonment; UA_B4, grasslands of Kamianka shortly after abandonment; UA_B5, grasslands of Novomlynsk shortly after abandonment; UA_C1, long-abandoned grasslands of Nesterivka; UA_C5, long-abandoned grasslands of Novomlynsk; KZ_1, north locality: arable fields; KZ_2, north locality: grazed steppe; KZ_3, north locality: grazed former croplands; KZ_4, north locality: ungrazed former croplands; KZ_5, south locality: grazed steppe; KZ_6, south locality: ungrazed steppe; KZ_7, south locality: grazed former croplands; KZ_8, south locality: ungrazed former croplands.
The list of plants that form the M. b. bobak food base in Northeastern Ukraine includes 41 species (Supplementary Table S1). These plants are common in various habitats of the European subspecies (Poa angustifolia, Elytrigia repens, Achillea millefolium, etc.); some of them are dominants and/or subdominants on the cattle pastures. For instance, in some grazing areas, the cover of Poa angustifolia could reach 75%. Mainly, the marmot’s food species belong to the group of mesophytic forbs. Steppe bunch grasses are absent in its fodder. Moreover, six plant species were recognized as the main diet component when feeding tame marmots (Lactuca serriola, Lactuca tatarica, Cirsium arvense, Sonchus arvensis, Medicago sativa, and Trifolium sativum). They are not typical plants of M. b. bobak habitats, but they are able to form dense monospecies stands in agricultural lands.
Food base parameters (living cover of vascular plants, total cover of food species, total cover of uneaten species together with litter, cover of litter separately, and herbage height) were related to the three population grades distinguished in the present marmot settlements: normal population, population decrease, and population collapse. The short herbage height (5–9 cm) and the low cover of litter (1–5%) accompanied normal population density. The current anthropogenic effect that provides these parameters is grazing. On the contrary, both population decrease and collapse were accompanied by the high herbage height (Kruskal–Wallis test, Í = 67.3, df = 3, P < 0.001) and the dense cover of litter (Í = 63.3, df = 3, P < 0.001) (Figures 6A,B). Such vegetation changes were observed shortly after pasture abandonment. Differences in both abovementioned parameters in the foraging and ignored areas of the normal population were statistically insignificant.
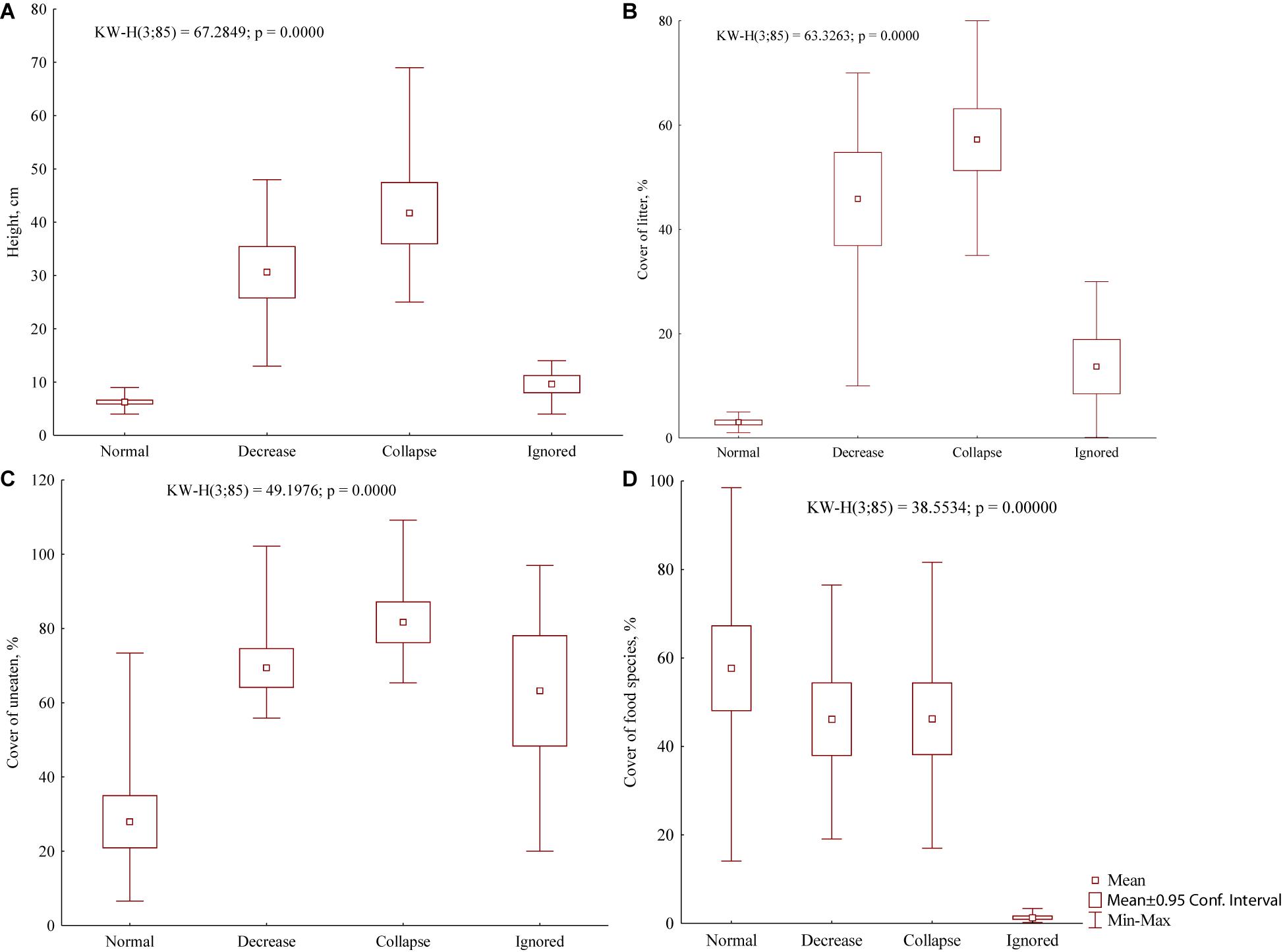
Figure 6. Vegetation characteristics of M. b. bobak habitats: height of main herb layer (A), cover of litter (B), cover of uneaten species together with litter (C), cover of food species (D) in foraging areas with three grades of population density (normal, decrease, and collapse) and in ignored vegetation with the normal population density.
The vegetation associated with the normal population differed significantly from that associated with the population decrease and the population collapse due to the low cover of uneaten species together with litter (Kruskal–Wallis test, Í = 49.2, df = 3, P < 0.001) (Figure 6C). It was also different from the ignored vegetation (Kruskal–Wallis test, Í = 38.5, df = 3, P < 0.001) due to the high cover of food species (Figure 6D). The cover range of food species in the settlements with normal population included the whole variability of this cover in the settlements with both population decrease and collapse.
According to our observations, the normal population grade is accompanied by a stable settlement structure; one marmot family uses the same winter burrow for many years in a row. We counted the total number of all types of burrows in the central part of the Nesterivka settlement (25.5 ha) (Supplementary Table S2). On average, there were 1.50 ± 1.2 burrows (mean ± SD, n = 647) per 20 × 20 m quadrate. The confidence limits for mean (95%) are quite narrow (1.41, 1.60), which indicates a fairly uniform distribution of burrows in the central part of the settlement. According to our estimates, the population uses about half of available burrows per year.
In 2001, cattle grazing was the main type of anthropogenic effect. The pastures covered nearly the whole area of the Nestrivka settlement. From 2004 to 2014, the grazing area decreased gradually, and finally, it was abandoned in 2015. Before the grazing cessation, the marmot population density corresponded to the normal grade (0.81 families/ha). An average number of burrows per family varied from 24.1 ± 8.1 (median: 24.0, n = 27, 2001) to 23.0 ± 6.4 (median: 21.0, n = 23, 2014). In 2017, the number of families dropped to 12. The population density was described as decreasing (0.48 families/ha). An average number of burrows used by each survived family grew to 27.1 ± 4.2 (median: 26.5). In the spring of 2019, there were only seven families left. The population density decreased to the grade of collapse (0.24 families/ha). A tendency to increase the number of burrows per family reversed: each family used on average 17.0 ± 2.2 burrows (median: 16.5, n = 7). Data ranking showed that the most frequent was rank of 15–21 burrows per family, and the rarest was one with more than 35 burrows per family (Figures 7A,B).
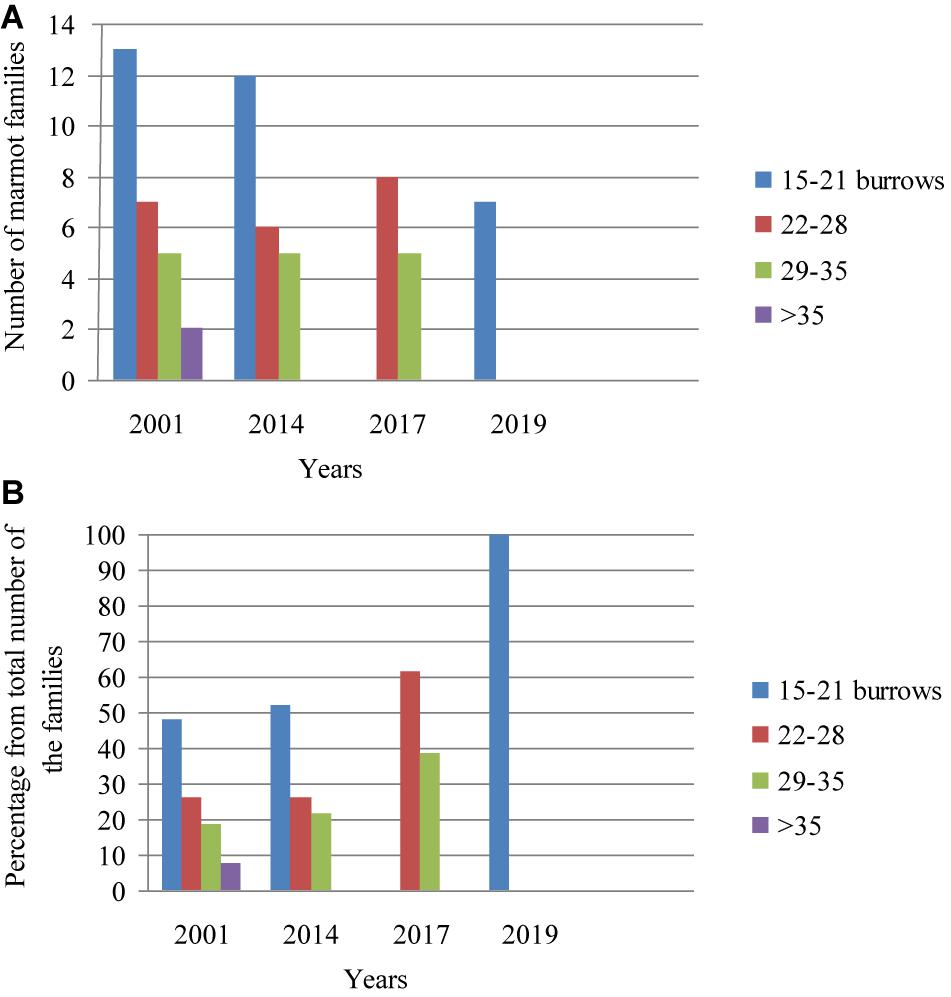
Figure 7. The ratio of marmot families using a certain number of burrows. Central part of the Nesterivka settlement. Population density grades: 2001 and 2014 – normal, 2017 – decrease, 2019 – collapse. (A) Absolute number of marmot families and (B) percentage of families.
We also investigated the other 15 marmot settlements in Northeastern Ukraine, and a sharp decrease in population density after grazing cessation was registered in all of them. Along with the population decline, the same above-described changes were recorded: (1) multiple increase in herbage height and (2) increase in the home ranges of remaining families due to occupation of the winter burrows of neighboring extinct families as well as the area around these burrows. In 2017, the cattle grazing occurred only in two monitored settlements, and its intensity decreased compared to 2001. The food base of the most families did not change, and the population density remained at the normal population grade (Zelenyi Hai: 0.95 families/ha, 2017; Rohozianka: 0.89 families/ha, 2017; in total 19 and 25 families, respectively). This proportion of abandoned and grazed marmot settlements (18 to 2) corresponds approximately to the current state of pastoral activity in Northeastern Ukraine, but there is still a tendency toward a decrease in pasture areas.
One more trait of the marmot settlement, which depends on the population density, is a distance between the nearest inhabited winter burrows. In the case of the normal population, it equaled 84.4 ± 30.9 m (n = 100). With a decrease in population density, this distance increased significantly (Figure 8): In normal populations, this parameter was 124.1 ± 64.3 m (n = 46) while in collapsed populations it was 210.0 ± 165.5 m (n = 21) (Kruskal–Wallis test, H = 40.5, df = 2, P < 0.001).
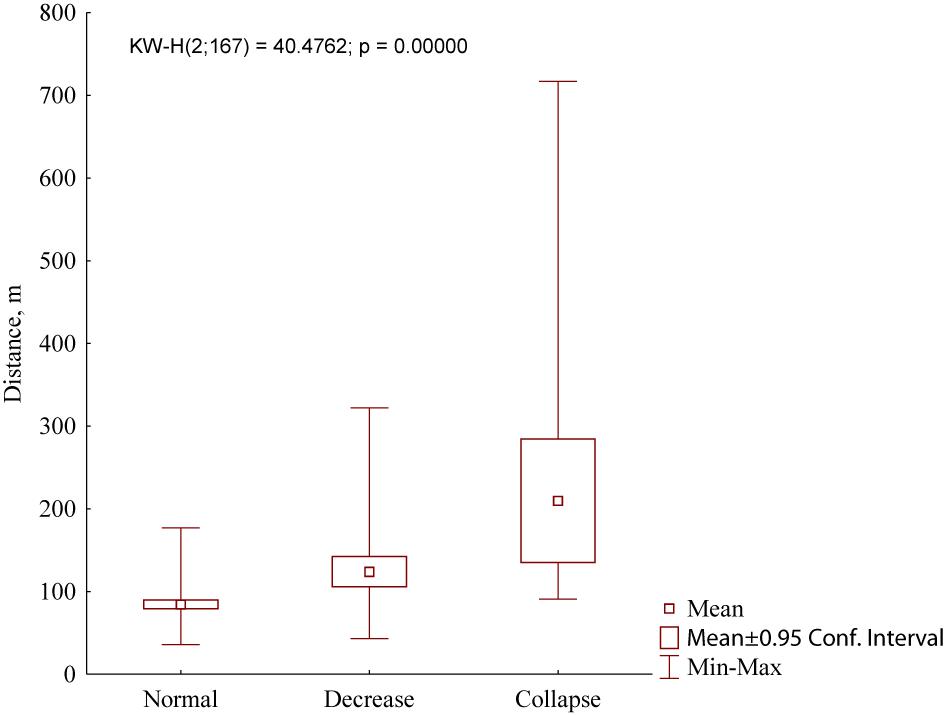
Figure 8. Distances between the nearest inhabited winter burrows in marmot settlements in Northeastern Ukraine depending on the grades of population density (normal, decrease, and collapse).
The cluster analysis (Figure 4) shows that most Kazakhstan habitats are joined into one cluster with the ignored areas of the Ukrainian grazed grasslands. The second cluster was formed by the Ukrainian grazed grasslands and a bulk of habitats in the northern sampling sites in Kazakhstan: roadsides, grazed steppes, and ungrazed former croplands. We noted earlier that the roadside vegetation responds well to the feed requirements of the European steppe marmot (Ronkin et al., 2009). Pastures of the northern sampling sites in Kazakhstan, which lay in a moister climate, are similar to the Ukrainian pastures in both appearance and basic characteristics. The ungrazed former croplands of the northern sampling sites in Kazakhstan are similar to the Ukrainian pastures as to the cover of forbs and living cover of vascular plants. The third cluster was formed by the Ukrainian shortly and long-abandoned grasslands. They are united by the large height of herbage and the dense cover of litter.
Most authors consider that peculiarities of the settlement structure reflect specificity of local foraging conditions and site topography (Sludsky et al., 1969; Bibikov, 1989; Mashkin, 1997). It was found that M. b. bobak has higher population density and lower distance between the winter burrows than M. b. schaganensis (Sludsky et al., 1969). Our data are in agreement with these observations. However, in recent decades, we have seen a decline of M. b. bobak settlements in Northeastern Ukraine that results in the increase of distances between inhabited winter burrows. Data analyses of density grading (Figure 8) revealed a sharp irregularity in the distribution of inhabited winter burrows. The low average value obtained for the Ukrainian samples is explained by the predominance of measurements in the settlements with normal density. In the studied Kazakhstan settlements, these distances were less variable, which can be addressed to greater homogeneity of living conditions in different habitats. A small number of burrows per M. b. schaganensis family, compared to a family of M. b. bobak, can also be explained by homogeneity of the environmental conditions in the habitats of the Kazakhstan subspecies. In our study, the distances between the burrows of the Kazakhstan subspecies were greater than those recorded in the first half of the 20th century: 70–440 m versus 50–80 m (Sludsky et al., 1969). The settlements with normal population density in Ukraine were the most different from all the settlements studied in Kazakhstan (e.g., UA_A1–UA_A4 and KZ_1–KZ_8, Figure 5), which reflects the difference between two types of marmot settlements: concentrated and dispersed, respectively (after Bibikov, 1989).
Interestingly, the greatest distances between inhabited winter burrows in Northern Kazakhstan were recorded in the grazed steppe (north locality, KZ_2, Figure 5) and ungrazed former croplands (south locality, KZ_8, Figure 5). In Northeastern Ukraine, the grazed habitats were characterized by the smallest distances (UA_A1–UA_A4, Figure 5). This may be partly explained by possible competition for fodder resources between marmots and livestock in the dry steppes of Kazakhstan. As a rule, distribution, quality, and abundance of the food resource determined an average distance between the nearest winter burrows. According to Seredneva (1986), the better food conditions in the marmot settlement, the less the average distance between the burrows.
Diverse habitats with differences in vegetation parameters as well as the differences between the settlement structures of M. b. bobak and M. b. schaganensis demonstrate a high ecological plasticity of the steppe marmot at the species level.
The absence of large differences in the ranges of general vegetation characteristics (herbage height and cover of litter) in the foraging and ignored areas of the European steppe marmot confirms the high food selectivity of this subspecies. When analyzing the food and uneaten plant species, the difference between the forage and ignored vegetation becomes highly significant. On the contrary, the Kazakhstan subspecies does not demonstrate high selectivity in its diet (Sludsky et al., 1969; Zimina and Isakov, 1980).
According to our data, the European subspecies ignored areas with the total cover of its food species of about 1–3%. The ignored vegetation is either dry steppe vegetation dominated by bunch grasses (Stipa capillata, Stipa pennata, and Festuca valesiaca) and xerophytic forbs (Galatella villosa) or chalky vegetation dominated by xerophytic Artemisia hololeuca and Thymus cretaceus. On the contrary, many food species in the habitats of the European steppe marmot belong to the group of mesophytic forbs. The species Lactuca serriola, Lactuca tatarica, Cirsium arvense, Sonchus arvensis, and Trifolium sativum have succulent leaves. These plants grow only in the pastures, croplands, and on the roadsides in both Ukraine and Kazakhstan.
However, the steppe marmot does not specialize in feeding on mesophytic forbs only. Its food plasticity is very wide compared to the mountain marmot species. Dry steppe grasses, xerophytic forbs, and even leaves of steppe shrubs serve as fodder plants to the Kazakhstan subspecies (Sludsky et al., 1969; Zimina and Isakov, 1980). In grazed steppes and ungrazed former croplands dominated by Stipa lessingiana, Festuca valesiaca, and Artemisia ssp., we registered families with offspring, and the juveniles looked well fed. Most of those Kazakhstan habitats are combined in one cluster with ignored vegetation of M. b. bobak (Figure 4). All of the above confirms the general opinion about the lower food selectivity of M. b. schaganensis (Sludsky et al., 1969; Bibikov et al., 1990) that provides its stable existence in the conditions unsuitable for M. b. bobak.
In 2016–2017, the current population size of M. b. schahanensis was estimated at 6.1 ± 2.4 million individuals (Koshkina et al., 2019). In Kazakhstan, its range has remained almost unchanged since the 1950s despite several drastic episodes of the land-use change (Koshkina et al., 2019). Comparison of the lists of M. b. schaganensis food plants, including crops (Sludsky et al., 1969; Zimina and Isakov, 1980; Mashkin, 1997) with a list of weeds of Kazakhstan croplands suggests that in the absence of regular herbicide treatments, forage conditions in the arable fields may be better for the marmot than that in ungrazed former croplands and dry grazed steppes. For instance, Lactuca tatarica, Cirsium arvense, and Sonchus arvensis, willingly eaten by marmots, are common weeds of the arable fields (Supplementary Figure S5). Urakchintseva (2005) estimated the weed cover in the agricultural fields not treated with herbicides as minimum as 34–48%. In our opinion, life in arable fields cannot be considered a new ecological adaptation since the field vegetation corresponds to the food requirements of the Kazakhstan subspecies, and plowing is not able to destroy their deep winter burrows (Rumyantsev, 1991). An increase in the use of herbicides in agriculture can make Kazakhstan fields unsuitable for marmots in the near future (Koshkina et al., 2019).
According to many authors, the Kazakhstan subspecies is the most independent from mesophytic forbs compared to other marmot species (Sludsky et al., 1969; Zimina and Isakov, 1980; Bibikov, 1989). It has adapted to feeding on grasses and xerophytic forbs in the Kazakhstan dry steppes (Bibikov, 1989). Currently, the European subspecies needs to adapt to changes of its habitats. Otherwise, its settlements are doomed to further decline despite a large cover of food plants. In the absence of grazing, the vegetation coarsens and gains senescence quickly. Moreover, the marmot food base is worsening due to increased herbage height and cover of uneaten plant species together with litter. A dramatic decline of the European subspecies population under total pasture abandonment encouraged us to seek possible measures alternative to grazing to restore the marmot’s habitat. Frequently repeated mowing can maintain vegetation parameters similar to those in grazed habitats during the whole season of marmot activity (Savchenko and Ronkin, 2018). It can be an effective management measure, but it requires strong and consistent dedication and enthusiasm from volunteers to mow the marmot foraging areas (Valkó et al., 2018). We are studying the possibility to preserve the gene pool of the European subspecies in Northeastern Ukraine in seminatural conditions. For this purpose, we test plants for compliance with the main forage components similar to marmot’s natural diet.
A wide range of vegetation parameters in the foraging areas of the European subspecies refers to the current period when both grazed and abandoned grasslands can be observed. However, only the range that accompanies normal population grade is suitable for animals of all ages. Vegetation transformation after grazing cessation does not meet the nutritional needs of juveniles (Savchenko and Ronkin, 2018) and results in population decrease and then collapse even though the adult nonbreeding marmots fully satisfy their food needs. Such a feature, when the needs of adults have a wider satisfaction range, corresponds well to the climate peculiarities of the steppe areas with periodic droughts. A vast fluctuation amplitude of the absolute values of environmental factors in the Kazakhstan steppes was shown in climatic data by Mordkovich (2014). Unfortunately, the food conditions become stably unsuitable for juvenile animals, which we are currently observing in Northeastern Ukraine. The range of vegetation parameters accompanying the normal population grade of the European subspecies is quite narrow. Thus, it has low ecological plasticity in food choice. A rapid population collapse after pasture abandonment does not allow the marmot to acquire adaptations to the changing food conditions.
The European subspecies formed compact dense and numerous settlements in the steppe gullies of limited size giving an example of environmental adaptation to excess food resources with limited of habitat area. According to Seredneva (1986), the number of home ranges and burrows used in a stable settlement is more or less constant. Our data concurs with this author. Lenti Boero (2003) shows that such parameters as the most used summer burrows and hibernacula, the core foraging and the feeding area, and the home range dimension are permanent and stable structural components of the alpine marmot (M. marmota) territories, and they can be inherited by different and unrelated families. Our investigation of the steppe marmot shows that, under the deterioration of food conditions and a reduction in the number of families, the deserted territories and burrows are used by the neighbors. For example, the presence of two families using more than 35 burrows in 2001 (Figure 7) is explained by the extinction of four neighboring families. The absence of the range of 15–21 burrows per family in 2017 resulted from the death of 11 out of 23 families and the use of their territories by the neighbors. Thus, with deteriorating feed conditions, the home ranges of the remaining families increase. Perhaps, it is due to the fact that the European steppe marmot retained its adaptation to occupying excess territories to ensure seasonal movements to the areas with mesophytic forbs as is described for mountain species (Bibikov, 1989).
In the conditions when undergrazing makes the herbage unsuitable for marmots, a more social behavior model, similar to the large family groups of black-tailed prairie dogs (Cynomys ludovicianus) (Hoogland, 1981, 2013; Hoogland and Brown, 2016), would suit the European steppe marmot. The large number of family groups enables prairie dogs to change the vegetation cover within their settlements. The Kazakhstan subspecies can also significantly change the vegetation around the mounds of winter burrows as the family resources include one winter burrow and one or five foraging ones (Mashkin et al., 2010; Koshkina et al., 2019). The vegetation far around the mounds is short cut due to the constant biting by the animals (Supplementary Figure S6A). Obviously, this contributes to producing a larger number of vegetative shoots, which are most valuable in terms of food quality. We did not observe any signs of the European subspecies’ ecological adaptations that resulted in concentration of their foraging activity in a small area. The vegetation remained relatively tall since the marmots did not concentrate their foraging activity around the mound (Supplementary Figure S6B).
In the climatic conditions of Northeastern Ukraine, the peak of vegetation activity occurs in May. Then, the grass matures, and the water content in the aboveground plant biomass decreases (Savchenko and Ronkin, 2018). Such course of vegetation growth is unfavorable for the European subspecies since it feeds on the succulent, constantly regrowing parts of plants. The marmot is not able to ensure plant regrowth in a sufficient part of its foraging areas. It is provided by cattle grazing. Vegetation changes under grazing impact result in alteration of dominant species. Uneaten species of Festuca and Stipa are replaced by the food species of Poa and Elytrigia. In addition, pasture herbage includes a large number of mesophytic forbs. Cessation of cattle grazing adversely affects survival of juveniles (Savchenko and Ronkin, 2018). Settlements are declining because the marmot has no time to develop new ecological adaptations. Consequently, the European steppe marmot was and continues to be a secondary pasture user. This specialization enabled it to occupy the European grasslands. According to Dinesman (1977), these grasslands were evolutionarily formed under the impact of large herbivores. After the extirpation of wild herding ungulates, the steppe marmot continued thriving in the European grasslands owing to cattle grazing. Moreover, the most favorable feeding conditions for the steppe marmot were formed at the gully bottoms, which are more humid than the steppe slopes. Both vegetation and invertebrate assemblages (Polchaninova et al., 2016) confirm the steppe-meadow character of this habitat.
On the contrary, the Kazakhstan subspecies can be both a secondary and a primary user of the Asian dry steppes. We assume that other Marmot species are also divided into primary and secondary grassland users. For example, M. camtschatica (Tokarsky and Valentsev, 1994) can stably live in the mountain meadows of Kamchatka on conditions of the absence of grazing ungulates due to a wide range of plants with succulent leaves and the long-lasting vegetative period of these meadows. The most preferred foraging areas of the marmots in the Tatra Mountains (M. marmota latirostris) are the communities of tall forbs and grasses found mainly in mesophilous habitats (Ballová and Šibík, 2015). On the contrary, ungulate grazing is necessary in M. menzbierii habitats in the Tien Shan Mountains to prevent rapid vegetation maturation and senescence (Mashkin et al., 2010). Nikol’skii and Ulak (2006) observed that M. himalayana in the Manaslu mountain range fed exclusively on livestock pastures.
In the process of evolution, marmots adapted to living in diverse countries (Bibikov, 1989; Armitage, 2014). They control the temperature of hibernation (Nikol’skii and Savchenko, 2002; Nikol’skii et al., 2005; Nikol’skii, 2009) and have a peculiar circannual rhythm (Bibikov, 1989; Armitage, 2014). Social thermoregulation acquired in the course of evolution helps the marmots to increase juvenile survival during hibernation (Arnold, 1990). However, are the marmots capable of a quick adaptation acquirement in response to sharp deterioration of the food base of their habitats? In the case of M. b. bobak, there is an additional risk that reduces its chances of a successful outcome. We suggest that, in the beginning of the 20th century, M. b. bobak genetic diversity was reduced dramatically due to a sharp population decrease and isolation of the remaining populations. Further conditions of its rebound were very similar throughout its range. Perhaps, we are now witnessing a scenario that was predicted by Gossmann et al. (2019): “If low genetic variation is a contributory factor to extinction risk, not only small but also large populations can be at risk, if their life history traps them permanently in a state of low genetic diversity.”
The food base of the European subspecies has quickly deteriorated in vast areas within the marmot range that does not give the marmot any chance to adapt to the changing conditions and to become the primary grassland user. The only real measure capable of the European subspecies conservation is a government policy aimed at restoring livestock grazing in the marmot habitats. For the Kazakhstan subspecies, we recommend avoiding herbicide use along the field margins in the areas inhabited by marmots.
Geographical peculiarities of M. bobak ecological conditions within its vast geographic range are reflected in different ecological adaptations of its subspecies. The European subspecies has taken the place of a secondary user of the pastures of herding ungulates. This allowed it to thrive in the moist pasture ecosystems after the extinction of wild herding herbivores as long as the ecosystem functioning was providing by livestock. Abandonment of the pastoral cattle breeding in the mid-1990s resulted in practically concurrent grazing cessation. Now, the forage conditions in M. b. bobak habitats got their pessimum nearly everywhere, which caused the collapse of families. The high-speed, large-scale changes of their habitats leave M. b. bobak no chance for adaptation. The Kazakhstan subspecies is capable of sustainable existence under conditions similar to the pessimum that causes extinction of the European subspecies. Adaptation to the constant environmental rigidity allows the Kazakhstan subspecies to survive in the habitats plowed in the middle of the 20th century during the virgin lands campaign. In our opinion, that was not a new ecological adaptation, but a consequence of the fact that the living conditions remained in accordance with M. b. schahanensis requirements. Modern agricultural technologies can quickly deprive the Kazakhstan subspecies of this type of habitat.
All datasets generated for this study are included in the article/Supplementary Material.
This animal study was reviewed and approved by the Committee on Bioethics of Kharkiv National University named after V. N. Karazin.
VR and GS: study design, data collection, data analysis, and manuscript writing. VT: study design and data collection. NP: manuscript writing. AA: data collection. AK: data collection and organizing of the Kazakhstan expedition. All authors contributed to the article and approved the submitted version.
The data collection in Kazakhstan was funded by the Volkswagen Foundation, project BALTRAK (Project Ref-No A112025).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Dr. Tony Gossmann as an associate editor and to reviewers for the valuable comments on initial draft. We are also thankful to Dr. S. Stolyar (University of Idaho, United States) for the English proofreading and important remarks.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00219/full#supplementary-material
Abaturov, B. D. (2005). Foraging resources, supply with food, and viability of herbivorous mammals. Zool. Zh. 84, 1251–1271.
Abaturov, B. D. (2006). Grazing type of functioning of steppe and desert ecosystems. Usp. Sovrem. Biol. 126, 435–447.
Armitage, K. B. (1998). Evolution of sociality in marmots. J. Mammal. 80, 1–10. doi: 10.2307/1383202
Armitage, K. B. (2003). Observations on plant choice by foraging yellow-bellied marmots. Oecol. Mont. 12, 25–28.
Armitage, K. B. (2014). Marmot Biology: Sociality, Individual Fitness and Population Dynamics. Cambridge: Cambridge University Press.
Arnold, W. (1990). The evolution of marmot sociality: I. Why disperse late? II. Costs and benefits of joint hibernation. Behav. Ecol. Sociobiol. 27, 229–246. doi: 10.1007/BF00164894
Ballová, Z., and Šibík, J. (2015). Microhabitat utilization of the Tatra marmot (Marmota marmota latirostris) in the Western Carpathian Mountains, Europe. Arct. Antarct. Alp. Res. 47, 169–183. doi: 10.1657/aaar0014-021
Bassano, B., Peracino, V., Pecacino, V., and Montacchini, F. (1996). “Diet composition and feeding habitats in a family group of alpine marmot (Marmota marmota) – preliminary data,” in Biodiversity in Marmots, eds M. Le Berre, R. Ramousse, and L. Le Guelte (Moscow: International marmot Network), 135–140.
Bibikov, D. I., Dezhkin, A. V., and Rumyantsev, V. Y. (1990). History and modern condition of the bobac (Marmota bobac Müll.) in Europe. Bull. Mosc. Soc. Natur. Biol. Ser. 95, 15–30.
Brandler, O. V. (2009). “Variability of the control region of the mitochondrial genome in marmots of the bobak group and the formation of their ranges,” in Proceedings of the Conference “Modern Problems of zoo– and Phylogeography on Mammals”, (Penza: Scientific Press Ltd), 19.
Brandler, O. V., and Lyapunova, E. A. (2009). Molecular phylogenies of the genus Marmota (Rodentia sciuridae): comparative analysis. Ethol. Ecol. Evol. 21, 289–298. doi: 10.1080/08927014.2009.9522484
Brandler, O. V., Lyapunova, E. A., Bannikova, A. A., and Kramerov, D. A. (2010). Phylogeny and systematics of marmots (Marmota, Sciuridae, Rodentia) inferred from inter-SINE PCR data. Russ. J. Genet. 46, 283–292. doi: 10.1134/s102279541003004x
Brinkert, A., Hölzel, N., Sidorova, T., and Kamp, J. (2016). Spontaneous steppe restoration on abandoned cropland in Kazakhstan: grazing determines successional pathways. Biodivers. Conserv. 25, 2543–2561. doi: 10.1007/s10531-015-1020-7
Dara, A., Baumann, M., Hölzel, N., Hostert, P., Kamp, J., Müller, D., et al. (2019). Post-Soviet land-use change affected fire regimes on the Eurasian steppes. Ecosystems 1–14 (in press).
Dara, A., Baumann, M., Kuemmerle, T., Pflugmacher, D., Rabe, A., Griffiths, P., et al. (2018). Mapping the timing of cropland abandonment and recultivation in northern Kazakhstan using annual Landsat time series. Remote Sens. Environ. 213, 49–60. doi: 10.1016/j.rse.2018.05.005
Deák, B., Tölgyesi, C. S., Kelemen, A., Bátori, Z., Gallé, R., Bragina, T. M., et al. (2018). The effects of micro-habitats and grazing intensity on the vegetation of burial mounds in the Kazakh steppes. Plant Ecol. Divers. 10, 509–520. doi: 10.1080/17550874.2018.1430871
Demina, O., and Bragina, T. (2014). Fundamental basis for the conservation of biodiversity of the black sea-kazakh steppes. Hacquetia. 13, 201–214. doi: 10.2478/hacq-2014-0014
Frase, B. A., and Armitage, K. B. (1989). Yellow-bellied Marmots are generalist herbivores. Ethol. Ecol. Evol. 1, 353–366. doi: 10.1080/08927014.1989.9525505
Garin, I., Aldezabal, A., Herrero, J., García-Serrano, A., and Remón, J. L. (2008). Diet selection of the Alpine marmot (Marmota m. marmota L.) in the Pyrenees. Rev. Écol. (Terre Vie.) 63, 383–390.
Gossmann, T. I., Shanmugasundram, A., Börno, S., Duvaux, L., Lemaire, C., Kuhl, H., et al. (2019). Ice-age climate adaptations trap the Alpine marmot in a state of low genetic diversity. Curr. Biol. 29, 1712–1720. doi: 10.1016/j.cub.2019.04.020
Hoogland, J. L. (1981). The evolution of coloniality in white-tailed and black-tailed prairie dogs (Sciuridae: Cynomys leucurus and C. ludovicianus). Ecology 62, 252–272. doi: 10.2307/1936685
Hoogland, J. L. (2013). Prairie dogs disperse when all close kin have disappeared. Science 339, 1205–1207. doi: 10.1126/science.1231689
Hoogland, J. L., and Brown, C. R. (2016). Prairie dogs increase fitness by killing interspecific competitors. Proc. R. Soc. B 283, 20160144. doi: 10.1098/rspb.2016.0144
Kamp, J., Siderova, T. V., Salemgareev, A. R., Urazaliyev, R. S., Donald, P. F., and Hölzel, N. (2012). Niche separation of larks (Alaudidae) and agricultural change on the drylands of the former Soviet Union. Agric. Ecosyst. Environ. 155, 41–49. doi: 10.1016/j.agee.2012.03.023
Kamp, J., Urazaliev, R., Donald, P. F., and Hölzel, N. (2011). Post-Soviet agricultural change predicts future declines after recent recovery in Eurasian steppe bird populations. Biol. Conserv. 144, 2607–2614. doi: 10.1016/j.biocon.2011.07.010
Kirikov, S. V. (1959). Changes in Wildlife in the Natural Zones of the USSR. Steppe- and Forest-Steppe Zones. Moscow: Academy of Sciences of the USSR Press.
Koshkina, A., Grigoryeva, I., Tokarsky, V., Urazaliyev, R., Kuemmerle, T., Hölzel, N., et al. (2019). Marmots from space: assessing population size and habitat use of a burrowing mammal using publicly available satellite images. RSE 6, 153–167. doi: 10.1002/rse2.138
Lenti Boero, D. (1996). “Space and resource use in Alpine marmots (Marmota marmota L.),” in Biodiversity in Marmots, eds M. Le Berre, R. Ramousse, and L. Le Guelte (Moscow: International marmot Network), 175–180.
Lenti Boero, D. (2001). Occupation of hibernacula, seasonal activity, and body size in a high altitude colony of Alpine marmots (Marmota marmota). Ethol. Ecol. Evol. 13, 209–223. doi: 10.1080/08927014.2001.9522771
Lenti Boero, D. (2003). Long-term dynamics of space and summer resource use in the alpine marmot (Marmota marmota L.). Ethol. Ecol. Evol. 15, 309–327. doi: 10.1080/08927014.2003.9522659
Mashkin, V. I., Baturin, A. L., and Kolesnikov, V. V. (2010). Ecology, Behaviour and Use of Marmots in Eurasia. Kirov: Viatskaja GSHA.
Mashkin, V. I. (1997). The European Bobak: Ecology, Conservation and Use. Kirov: Kirov Regional Press.
Massemin, S., Gibault, C., Ramousse, R., and Butet, A. (1996). First data on the alpine marmot (Marmota marmota) diet. Mammalia 60, 351–361. doi: 10.1515/mamm-1996-0302
Nikol’skii, A. A., and Rumiantsev, V. Y. (2012). Center of species diversity of Eurasian marmots (Marmota, Rodentia) in an epi-platformal orogeny area. Dokl. Biol. Sci. 445, 261–264. doi: 10.1134/S0012496612040151
Nikol’skii, A. A. (2009). The hibernation temperature niche of the marmot Marmota bobak Müller 1776. Ethol. Ecol. Evol. 21, 393–401. doi: 10.1080/08927014.2009.9522494
Nikol’skii, A. A., Belovezhets, K. I, Ronkin, V. I., and Khutorskoi, M. D. (2005). A mathematical model of the temperature conditions of mammalian burrows as exemplified by the burrow of the marmot Marmota bobak Müll., 1776. Dokl. Biol. Sci. 403, 301–302. doi: 10.1007/s10630-005-0118-6
Nikol’skii, A. A., and Savchenko, G. A. (2002). Air temperature changes in a steppe marmot burrow in the summer–autumn period. Russ. J. Ecol. 33, 109–114. doi: 10.1023/A:1014452808571
Nikol‘skii, A. A., and Ulak, A. (2006). Key factors determining the ecological niche of the Himalayan marmot, Marmota himalayana Hodgson (1841). Russ. J. Ecol. 37, 46–52. doi: 10.1134/S1067413606010085
Polchaninova, N., Savchenko, G., Drogvalenko, A., Ronkin, V., and Shabanov, D. (2016). The impact of cattle grazing on cursorial spiders (Aranei) and true bugs (Heteroptera) in steppe gullies of northeastern Ukraine. Agric. Ecosyst. Environ. 234, 65–71. doi: 10.1016/j.agee.2016.04.031
Rachkovskaya, E. I., and Bragina, T. M. (2012). “Steppes of Kazakhstan: diversity and present state,” in Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World, eds J. Marinus, J. A. Werger, and M. A. van Staalduinen (Dordrecht: Springer), 103–148. doi: 10.1007/978-94-007-3886-7_3
Romashchenko, K., Didukh, Ya., and Solomaha, V. (1996). Syntaxonomy of Helianthemo-Thymetea cl. nov. of chalk outcrops vegetation of south-eastern Ukraine. Ukrainian Phytosociologic Collection 1, 49–62.
Ronkin, V., and Savchenko, G. (2016). Flora and vegetation of dry grasslands of Northeastern Ukraine, and problems of diversity conservation. Hacquetia 15, 49–62. doi: 10.1515/hacq-2016-0013
Ronkin, V., Savchenko, G., and Tokarsky, V. (2009). The place of the steppe marmot in steppe ecosystems of Ukraine: an historical approach. Ethol. Ecol. Evol. 21, 277–284. doi: 10.1080/08927014.2009.9522482
Ronkin, V. I., and Savchenko, G. A. (2000). Adaptability of habitats for Marmota bobak (Rodentia, Sciuridae) related to plant cover structure. Zool. Zh. 79, 1229–1234.
Rumyantsev, V. Y. (1991). Marmota bobac Müll. from the arable lands of Kazakhstan. Bull. Mosc. Soc. Natur. Biol. Ser. 96, 15–28.
Savchenko, G., and Ronkin, V. (2018). Grazing, abandonment and frequent mowing influence the persistence of the steppe marmot, Marmota bobak. Hacquetia 17, 25–34. doi: 10.1515/hacq-2017-0009
Seredneva, T. A. (1985). Population density of steppe marmots and factors affecting it. Vestn. Zool. 5, 68–72.
Seredneva, T. A. (1986). Estimation of absolute population density and number of marmots (Marmota). Zool. Zh. 65, 1556–1566.
Seredneva, T. A., and Nesgovorov, A. L. (1977). Population density and productivity of the Bobak marmot (Marmota bobak) on pastures and in protected areas. Zool. Zh. 56, 1216–1225.
Sludsky, A. A., Varshavsky, S. N., Ismagilov, M. I., Kapitonov, V. I., and Shubin, I. G. (1969). Mammals of Kazakhstan: Rodents (Marmots and Ground Squirrels). Alma-Ata: Science of the Kazakh S.S.R.
Stallman, E. L., and Holmes, W. G. (2002). Selective foraging and food distribution of high-elevation yellow-bellied marmots (Marmota flaviventris). J. Mammal. 83, 576–584. doi: 10.1644/1545-1542(2002)083<0576:sfafdo>2.0.co;2
Tokarsky, V. A., and Valentsev, A. C. (1994). Distribution, biology and breeding in captivity of black-capped marmot, Marmota camtschatica (Rodentia, Sciuridae). Zool. Zh. 73, 209–222.
Urakchintseva, G. V. (2005). Ecological and Biological Substantiation of Protection of Spring Wheat Against Weeds in the Dry Steppe Zone of the Urals Region. Ph. D. Thesis, Samara state Academy of agriculture, Samara.
Valkó, O., Venn, S., Zmihorski, M., Biurrun, I., Labadessa, R., and Loos, J. (2018). The challenge of abandonment for the sustainable management of Palaearctic natural and semi-natural grasslands. Hacquetia 17, 5–16. doi: 10.1515/hacq-2017-0018
Keywords: Marmota bobak, food base, ecological plasticity, ecological adaptations, cattle grazing, abandonment
Citation: Ronkin V, Tokarsky V, Polchaninova N, Atemasov A, Koshkina A and Savchenko G (2020) Comparative Assessment of Ecological Plasticity of the Steppe Marmot Between Ukrainian and Kazakhstan Populations: Challenges of the Man-Induced Environmental Changes. Front. Ecol. Evol. 8:219. doi: 10.3389/fevo.2020.00219
Received: 19 February 2020; Accepted: 12 June 2020;
Published: 14 July 2020.
Edited by:
Toni Gossmann, Bielefeld University, GermanyReviewed by:
Orsolya Valkó, Hungarian Academy of Science, HungaryCopyright © 2020 Ronkin, Tokarsky, Polchaninova, Atemasov, Koshkina and Savchenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Galina Savchenko, c2F2Y2hnYWxhNUBnbWFpbC5jb20=
†ORCID: Vladimir Ronkin, orcid.org/0000-0003-3080-4117; Nina Polchaninova, orcid.org/0000-0003-4605-8788; Alyona Koshkina, orcid.org/0000-0002-2501-1887; Galina Savchenko, orcid.org/0000-0001-9436-7871
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.